Abstract
Background
Tofacitinib is an oral, small-molecule JAK inhibitor for the treatment of ulcerative colitis (UC). Using a novel electronic reporting tool, we aimed to prospectively describe the onset of tofacitinib efficacy during induction therapy in a real-world study.
Methods
Patient-reported outcome data (PROs) including the simple clinical colitis activity index (SCCAI), PRO Measurement Identification Systems (PROMIS) measures, and adverse events were collected daily for the first 14 days and at day 28 and 56. Paired t tests and P for trend were utilized to compare changes in SCCAI over time. Bivariate analyses and logistic regression models were performed to describe response (SCCAI <5) and remission (SCCAI ≤2) by clinical factors.
Results
Of all included patients (n = 96), 67% had failed ≥2 biologics, and 61.5% were on concomitant steroids. Starting at day 3, PROs showed significant and persistent decline of the mean SCCAI (−1.1, P < 000.1) including significantly lower SCCAI subscores for stool frequency (−0.3; P < .003), bleeding (−0.3; P < .0002) and urgency (−0.2; P < .001). Steroid-free remission at day 14, 28, and 56 was achieved in 25%, 30.2%, and 29.2% of patients, respectively. Neither prior biologics nor endoscopic severity were independently predictive of response or remission in multivariate models. Numeric improvements in all PROMIS measures (anxiety, depression, social satisfaction) were seen through day 56. Rates of discontinuation due to adverse events were low.
Conclusions
In this prospective real-world study, tofacitinib resulted in a rapid and persistent improvement in UC disease activity PROs. The safety findings were consistent with the established safety profile of tofacitinib.
Keywords: tofacitinib, ulcerative colitis, patient report outcomes, safety
Key Messages.
What is already known?
The oral small-molecule JAK inhibitor tofacitinib is approved for the therapy of moderate-severe ulcerative colitis (UC) based on placebo-controlled clinical studies, but there is a persistent knowledge gap in prospective real-world studies.
What is new here?
The prospectively collected real-world data of TOUR show a rapid onset of efficacy of tofacitinib by improving rectal bleeding, bowel frequency, and urgency starting as early as day 3.
How can this study help patient care?
The results of this study generated in the real world setting help providers discuss treatment options with patients with moderate-severe UC.
Introduction
Ulcerative colitis (UC) is a recurrent, chronic inflammatory bowel disease (IBD) affecting the colon with a variety of clinical symptoms, including bloody diarrhea, intense urgency, abdominal pain, weight loss, fatigue, and inflammation of joints and other organs. The chronic recurrent nature of the disease results in reduced productivity in work and school, intermittent need for hospitalizations, and as a consequence, reduced quality of life.1,2 The therapeutic options for moderate to severe UC patients are expanding, and 3 different antitumor necrosis factor (anti-TNF) agents (ie, infliximab, adalimumab, golimumab), vedolizumab or ustekinumab, and the small molecules thiopurines, tofacitinib, and ozanimod are currently approved for this indication.3,4
Tofacitinib is an oral, small-molecule JAK inhibitor, which is approved for the treatment of moderate-severe ulcerative colitis (UC). The short and long-term clinical efficacy and safety have been established in an extensive clinical trial program.5,6 Post hoc analyses of the OCTAVE 1 and 2 induction trials found early significant therapeutic effects of tofacitinib therapy by day 3, reducing stool frequency, the total number of bowel movements, and rectal bleeding.7 In contrast to results generated in randomized controlled trials used for the approval of therapeutic agents, evidence generated in the real-world setting based on results of prospective observational cohorts or interventional pragmatic trials are considered more generalizable. Patients often have more refractory disease or comorbidities in the real-world setting, which are often exclusion criteria for clinical studies.8 Several studies reporting real-world experiences with tofacitinib in patients with UC have been published so far, the vast majority of these being retrospective evaluations of treatment efficacy.9–16 The Tofacitinib Response in UC (TOUR) registry is a prospective multicenter cohort of adult patients, who initiate therapy for moderate-severe UC. The predefined outcomes of the study focus on short- and long-term efficacy and safety of tofacitinib. In contrast to the previously published cohorts, TOUR focuses on prospectively collected patient-reported outcomes (PROs) and the National Institute of Health (NIH) Patient Reported Outcome Measurement Information System (PROMIS) measures to evaluate the onset of symptom improvement, including urgency. Here, we report the results of the 56 days (8-week) induction period, which for the first time in the real-world setting aimed to describe the onset of clinical response and remission based on PROs, including urgency and incontinence. Additionally, PROs for depression, anxiety, and social satisfaction were evaluated over the induction period, along with data on adverse events and rationale for discontinuation of therapy.
Methods
Study Setting and Design
The Tofacitinib response in UC (TOUR) study is a prospective cohort study conducted in 14 sites across the United States (NCT03772145). Enrollment in the cohort started in February 2019. Patients were enrolled if there was an intent to start tofacitinib for moderate-severe UC.
Inclusion Criteria and Tofacitinib Dosing
Adult patients older than 18 years of age with UC established by usual endoscopic, histologic, and radiologic criteria, who were started on tofacitinib therapy in the setting of standard of care therapy, and who planned to be followed by the site for at least 12 months were eligible for inclusion. There were no prespecified exclusion criteria for participation in the study, except inability to use English language and lack of internet access. Tofacitinib, including dose reductions and discontinuation, was administered at the discretion of the local investigators.
Data Collection and Questionnaires
To collect all relevant patient information and PROs for this multicenter study in real-time, we developed a web-based platform to collect all patient-related information, daily PROs, and other study questionnaires (Supplemental Figure 1). Demographics including previous treatments and results of the most recent sigmoidoscopy or colonoscopy prior to tofacitinib therapy were collected during the initial office visit from sites. Specific questionnaires were sent electronically to the participating patients initially daily for 14 days after the start of tofacitinib and at days 28 and 56. If patients did not complete their data in a predefined 24-hour time interval, automatic reminder emails were generated. In case of no data entry after 36 hours, patients were contacted by phone by the site coordinator. The questionnaires included the Simple Clinical Colitis Activity Index (SCCAI) and PROMIS symptom scales for depression, anxiety, and social satisfaction. The SCCAI includes 6 variables: bowel frequency during the day and night, urgency of defecation, blood in the stool, general well-being, and extracolonic manifestations of UC.17 The SCCAI score ranges from 0-19. A score of <5 correlates with clinical response, and a score ≤2 is considered remission.18,19 Daily SCCAI stool frequency, rectal bleeding, and urgency subscores were calculated based on the daily web-based diary entry. The values were collected starting on the first day of tofacitinib therapy. Changes from baseline in each of the subscores, day and night bowel frequency, urgency, and rectal bleeding were evaluated during the first 14 days of therapy and at day 28 (week 4) and day 56 (week 8). PROMIS symptom scales were collected at the start of therapy and days 14, 28, and 56. These scales are standardized to the general population via a T score of 50 and standard deviation (SD) of 10.20 Higher T scores are associated with more of the domain, thus higher T scores for depression and anxiety are worse, whereas higher T scores for social satisfaction are better.
Outcomes and Definitions
The primary outcome for the induction phase was clinical response to tofacitinib therapy at day 56 defined by an SCCAI score of <5.21,22 The secondary outcome was clinical remission defined as an SCCAI score of ≤2.19,23 Additionally, the following adverse events were recorded: new onset of shingles, infections resulting in the need for antibiotic therapy, hospitalizations, and UC-related surgeries. If tofactinib was discontinued, reason for discontinuation was captured and patients were censored at that time as a failure.
Statistical Analysis
Continuous variables were summarized using mean values and standard deviation. Comparisons used Student’s t test or Wilcoxon rank sum. Repeated continuous measures were compared via paired t test and p for trend. Categorical variables were expressed as proportions and compared using χ2 or Fisher exact test where appropriate.
Multivariate analyses were performed using logistic regression models to compare response and remission by factors including endoscopic severity and number of prior biologics. Variables considered a priori to be related to response or remission were entered into the model. The SAS program (Cary, NC) was utilized for all analyses.
Ethical Considerations
The protocol was approved by the institutional review board or independent ethics committee at each participating center. All patients provided written informed consent.
Results
A total of 100 patients were enrolled in TOUR (for whom the provider intended to start tofacitinib; Supplemental Figure 2). Of these, 96 patients initiated tofacitinib therapy; their baseline demographics and disease characteristics are shown in Table 1. Four of the initial 100 recruited patients were not treated with tofacitinib due to colectomy (n = 2) or lack of drug approval (n = 2). Most patients had pancolitis (52%), and 94.8% (91 of 96) of patients had failed 1 anti-TNF agent, whereas 31.4 % (30 of 96) had failed ≥2 anti-TNF agents, and 58.3% (56 of 96) had failed vedolizumab. Considering all currently FDA-approved biological therapies for UC treatment, 66.7% (64 of 96) of all patients had failed ≥2 biologic medications before starting tofacitinib. Most patients had clinically active disease, whereas 18.8% (18 of 96) of patients were in remission at the start of tofacitinib therapy. Of these, 38.9% (7 of 18) were on concomitant steroids. All patients received 10 mg of tofacitinib twice daily during the initial 56 days of treatment; no dose reduction occurred during this time.
Table 1.
Characteristics of Patients in the TOUR Study (n = 96).
| Age (years, mean, range) | 37.3 | (18–76) |
| Sex (m: n, %) | 54 | 56.3% |
| Duration of disease (years; mean, range) | 8.5 | (0–50) |
| BMI (kg/m2); (mean, range) | 25.4 | (14.8–43.9) |
| Race (n,%) | ||
| White | 81 | 84.4% |
| Black/African American | 5 | 5.2% |
| Asia | 3 | 3.1% |
| Other | 5 | 5.2% |
| Unknown | 2 | 2.3% |
| Current Smoker | 2 | 2.1% |
| Site of disease (Montreal classification) | ||
| E1 | 7 | 7% |
| E2 | 38 | 40% |
| E3 | 50 | 52% |
| Unknown | 1 | 1% |
| Prior medication use (n, %) | ||
| Mesalamine | 89 | 92.7% |
| Steroids ever | 96 | 100% |
| Azathioprine/6-MP | 44 | 45.8% |
| Methotrexate (oral or sc) | 21 | 21.9 |
| Vedolizumab | 56 | 58.3% |
| Ustekinumab | 6 | 6.3% |
| Anti-TNF | 91 | 94.8% |
| Total No. prior anti-TNF | ||
| 0 | 5 | 5.2% |
| 1 | 58 | 60.4% |
| 2 | 30 | 31.4% |
| 3 | 2 | 2.1% |
| 4 | 1 | 1.0% |
| Total No. prior biologics | ||
| 0 | 4 | 4.2% |
| 1 | 28 | 29.2% |
| 2 | 35 | 36.5% |
| 3 | 24 | 25% |
| 4 | 5 | 5.2% |
| Steroid use at baseline (week 0) | 59 | 61.5% |
| SCCAI >2 | 78 | 81.3% |
| SCCAI ≤2 | 18 | 18.8% |
| Mayo endoscopy score a | ||
| 0 | 2 | 2.1% |
| 1 | 4 | 4.2% |
| 2 | 37 | 38.5% |
| 3 | 49 | 51.0% |
| Unknown | 4 | 4.2% |
aEndoscopy score prior to tofacitinib initiation (most recent colonoscopy or sigmoidoscopy reported in the system).
Response and Remission After Start of Tofacitinib on Days 3, 14, and 56
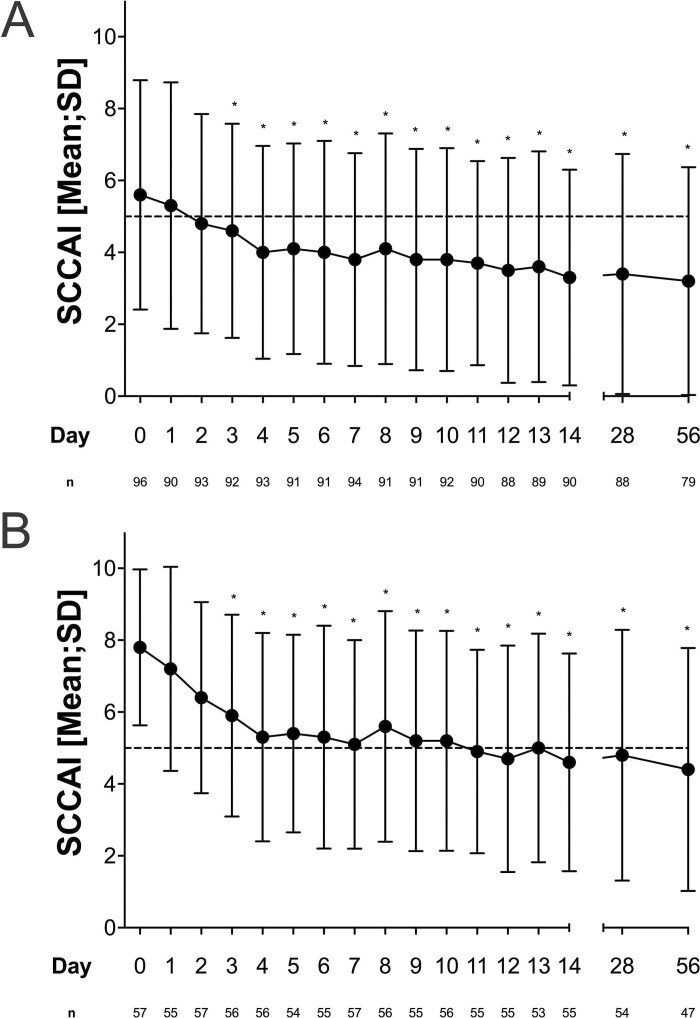
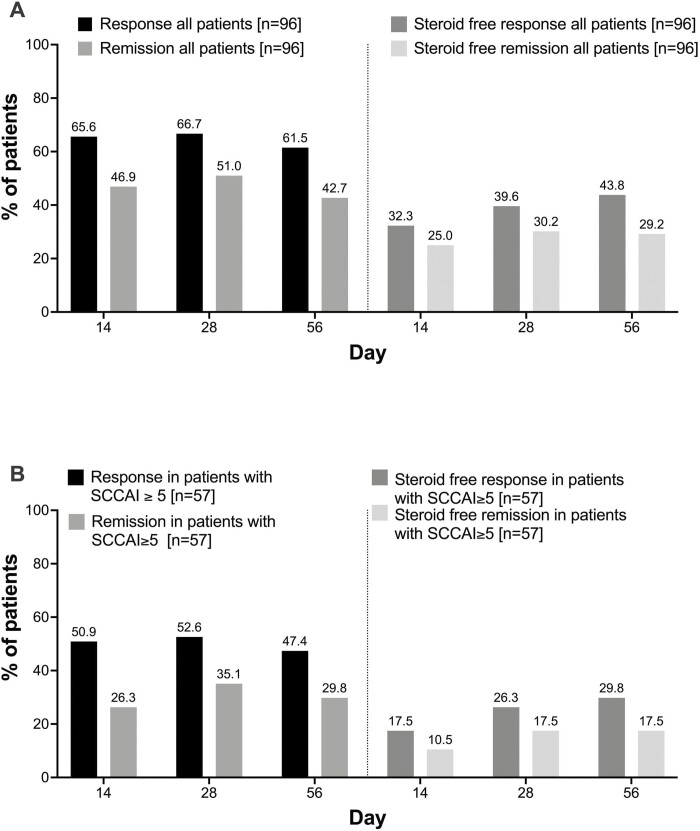
At day 3, the SCCAI decreased significantly by 1 point (5.6 ± 3.4 to 4.6 ± 2.9) in all patients. Compared with baseline, the SCCAI continued to decrease throughout the initial 14 days (Figure 1A). Because a SCCAI score <5 is already consistent with a definition of response, we additionally analyzed the group of patients with a SCCAI ≥5 at baseline (n = 57). In this group, the mean SCCAI declined at day 3 by 1.9 points (day 0, 7.8 ± 2.2; day 3, 5.9 ± 2.8; Figure 1B). On day 14, 46.9% (45 of 96) of all included patients were in remission as defined by an SCCAI score ≤2, and 65.3% (63 of 96) of patients were in response with a SCCAI score <5 (Figure 2A).Overall response and remission rates remained stable throughout day 56. Lower rates of steroid-free response and remission occurred in patients with higher disease activity (SCCAI ≥5) at baseline (Figure 2B). Subgroup analysis did not show differences in response and remission rates at day 56 based on the number of prior biologics. Significantly more patients with an endoscopic Mayo score <3 reached response and remission at earlier time points, but there was no difference in response and remission by Mayo endoscopic subscore at week 8 (Supplemental Figure 3). Multivariate analyses including number of previous exposures to biologics, disease extent, and severity of endoscopic inflammation did not find significant predictors for response or remission at week 8 (Supplemental Table 1).
Figure 1.
Decrease of SCCAI score in all patients (A) and in patients with a SCCAI ≥5 at baseline (B) during the initial 56 days of treatment with tofacitinib. The dashed line depicts a SCCAI = 5.
Figure 2.
Response (SCCAI <5) and remission (SCCAI ≤2) and steroid free-response and remission at day 14, 28 and 56.
SCCAI Subscores Stool Frequency, Rectal Bleeding, and Urgency
Stool frequency
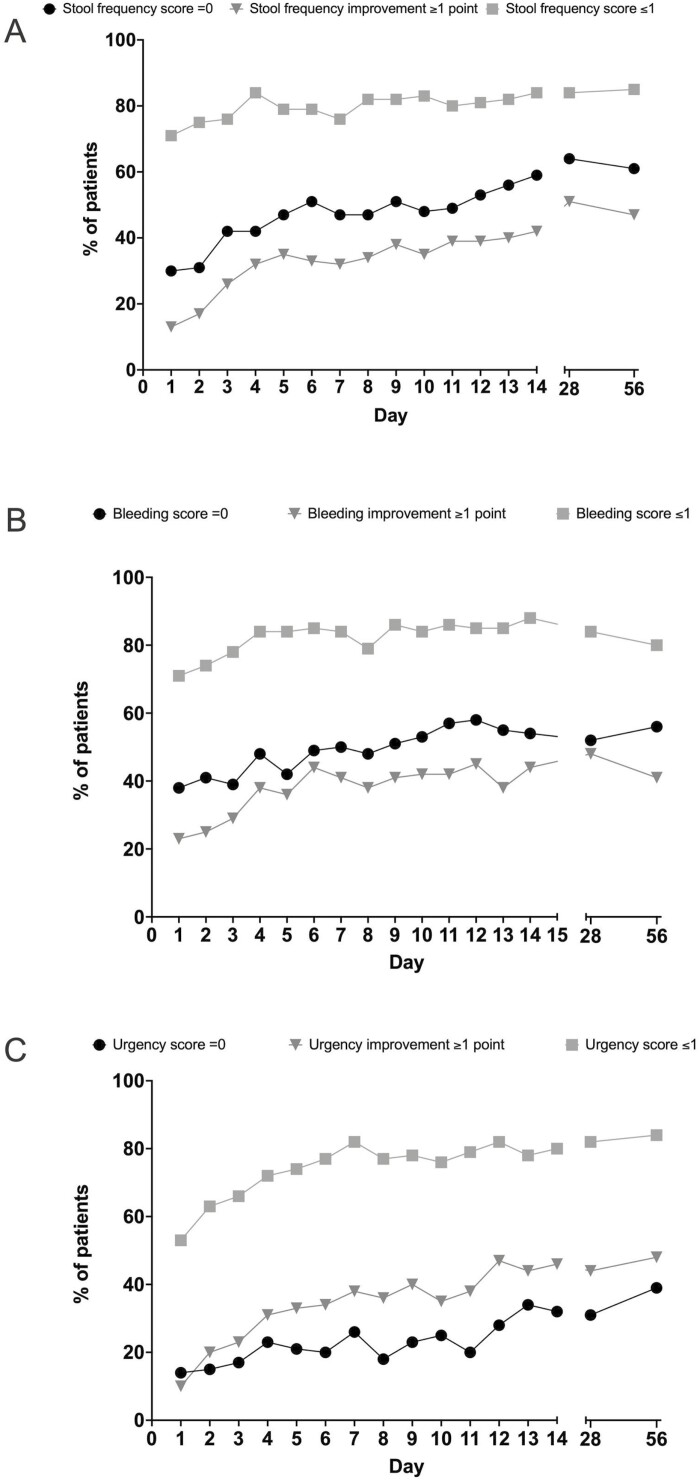
The SCCAI stool frequency subscore was reported as ≥1 by 72% (69/96) of patients at baseline, and 26% (24/92) experienced a decrease of ≥1 point at day 3 (Figure 3A). This improvement increased to 42% (38 of 90) at day 14 and remained significant in all patients completing the 56-day induction period (Table 2). The proportion of patients with a stool frequency subscore of 0 increased between baseline and day 14 from 28% (27 of 96) to 59% (53 of 90). The score remained relatively stable on days 28 and 56, with 64% (56 of 88) and 61% (48 of 79), respectively. The proportion of patients with a stool frequency >1 decreased by 13% from 28% (27 of 96) to 16% (14 of 90) by day 14 and then stabilized at 16% and 15% (14 of 88 and 12 of 79) at days 28 and 56.
Figure 3.
SCCAI subscores for stool frequency (A), bleeding (B), and urgency (C) during the initial 56 days of treatment with tofacitinib. In each panel are shown the percentage of patients with improvement of ≥1 point compared to the baseline SCCAI subscores well as the percentage of patients with a subscore of ≤1 and 0.
Table 2.
Decrease of SCCAI and its components after start of tofacitinib throughout the 56-day induction period.
| SCAAI components | Baseline (n = 96) | Day 3 (n = 92) |
Day 7 (n = 94) |
Day 14 (n = 90) |
Day 28 (n = 88) |
Day 56 (n = 79) |
|---|---|---|---|---|---|---|
| SCCAI score | 5.63 | 4.55 | 3.78 | 3.32 | 3.40 | 3.18 |
| <0.0001a | <0.0001a | <0.0001a | <0.0001a | <0.0001a | ||
| Frequency day | 1.18 | 0.93 | 0.86 | 0.66 | 0.60 | 0.61 |
| <0.0029a | <0.0006a | <0.0001a | <0.0001a | <0.0001a | ||
| Rectal bleeding | 1.20 | 0.91 | 0.70 | 0.63 | 0.68 | 0.73 |
| <0.0002a | <0.0001a | <0.0001a | <0.0001a | <0.0001a | ||
| Urgency | 1.36 | 1.21 | 0.95 | 0.89 | 0.91 | 0.81 |
| <0.0013a | <0.0001a | <0.0001a | <0.0001a | <0.0001a |
aSignificance level compared to baseline.
Thirteen patients experienced nightly bowel frequency at baseline, which was documented as resolved by 92.3% (12 of 13), 84.6% (11 of 13), 76.9% (10 of 13) at days 14, 28, and 56, respectively.
Rectal bleeding
A total of 68% (65 of 96) of patients reported a subscore of rectal bleeding ≥1, and a significant proportion (29%, 27 of 92) experienced a decrease of ≥1 point at day 3, which increased to 44% (40 of 90) at day 14 (Table 2, Figure 3B). More than half of the patients achieved a subscore of 0 on day 14 (54%, 49 of 90), day 28 (52%, 46 of 88), and day 56 (56%, 44 of 79). The proportion of patients reporting a bleeding subscore of >1 was 12% (11 of 90), 16% (14 of 88), and 20% (16 of 79) at days 14, 28, and 56, respectively.
Urgency
At baseline, 90% (86 of 96) of patients experienced symptoms of urgency (SCCAI urgency subscore of ≥1). A significant decrease in urgency of ≥1 point was reported by 23% (21 of 92) of patients at day 3 and 46% (41 of 90) at day 14 (Table 2, Figure 3C). The percentage of patients reporting an urgency subscore of 0 increased from 10% (86 of 96) at baseline to 32% (29 of 90) at day 14. At days 28 and 56, 31% (27 of 88) and 39% (31 of 79) of patients reported a score of 0. Compared with the improvement in bowel frequency and rectal bleeding, the improvement of urgency appeared prolonged (Figure 3C). An urgency subscore >1 was reported at baseline by 41% (39 of 96) of the patients. The proportion of patients with an urgency subscore >1 decreased at day 14, 28 and 56 to 20% (18 of 90), 18% (16 of 88), and 16% (13 of 79), respectively. Overall, 6.5% (6 of 92) of patients reported incontinence which resolved in 33.3% (2 of 6) at day 3.
PROMIS measures
PROMIS measures for anxiety, depression, and social satisfaction improved over the 56-day induction period. At baseline, 48%, 42%, and 50% of patients met criteria for anxiety, depression, and poor social satisfaction scores, respectively, compared with the T scores of the general population. By week 8, 42% met criteria for anxiety, 29% for depression, and 38% for poor social satisfaction. Although numerically improved, these changes did not meet statistical significance.
Adverse events and discontinuations
Adverse events resulting in the need for antibiotic therapy are listed in Table 3. Six patients (6%) were treated with antibiotics for infectious complications, all on concurrent steroid therapy. Three patients were hospitalized for UC flare (2 while being on concomitant steroid therapy). Shingles occurred in 3.1 % (3 of 96 patients). All these patients were on concomitant steroids at the time of diagnosis, and 2 of 3 of patients had received a shingles vaccine (Shingrix). Thromboembolic events were not reported during induction therapy.
Table 3.
Adverse events during 56 days of induction therapy in TOUR.
| Adverse Event | n | % |
|---|---|---|
| Hospitalization due to UC flare | 3 | 3.1% |
| Shingles | 3a | 3.1% |
| Antibiotics Bronchitis/upper respiratory infection Urinary tract infection Fever and high white blood count Severe bloating Tonsillitis Bacterial vaginitis |
6 | 6.2% |
| 1 | ||
| 1 | ||
| 1 | ||
| 1 | ||
| 1 | ||
| 1 |
*2 of 3 patients (66.7%) had been vaccinated with shingles vaccine (Shingrix).
Reasons for discontinuation of tofacitinib are listed in Table 4. After the start of tofacitinib, 5.2% (5 of 96) of patients underwent colectomy during the 56-day induction period. Tofacitinib therapy was stopped in 6.2% (6 of 96) of patients due to adverse events including headaches and worsening anxiety (n = 1); elevated liver values (n = 1); or lack of efficacy (n = 4). Overall, 3.1% (3 of 96) did not cooperate with survey completion (n = 3) or withdrew from registry (n = 1).
Table 4.
Study withdrawal and reason for withdrawal after start of tofacitinib therapy until day 56 (n = 15).
| Study withdrawal reason | n | % |
|---|---|---|
| Discontinuation of tofacitinib due to intolerance or adverse event | 2 | 13.3% |
| Discontinuation of tofacitinib due to lack of efficacy | 4 | 26.7% |
| Surgery (colectomy) | 5 | 33.3% |
| Lack of patient’s cooperation with survey completion | 3 | 20.0% |
| Patient’s request to withdraw from registry | 1 | 6.7% |
Discussion
The multicenter TOUR registry was designed to prospectively record PRO measures to evaluate the onset of symptom improvement during induction therapy with tofacitinib in real time in a real-world setting among 14 different sites in the United States. Tofacitinib therapy resulted in a rapid and significant improvement of patient-reported daily bowel frequency, bleeding, and urgency starting at day 3, with continued improvement through day 14. On day 14, nearly two-thirds of patients responded to tofacitinib therapy, and 47% of patients were in clinical remission. Steroid-free remission at day 14 was achieved by 25% of patients. Although patients reported a rather fast improvement of bowel frequency and bleeding scores, urgency scores responded slowly but consistently, continuing to improve throughout 56 days. In parallel with the PROs, the PROMIS domains of anxiety, depression, and social satisfaction improved over the initial 56 days of therapy. A dose of 10 mg of tofacitinib twice daily was overall well tolerated. Approximately 10% of patients, most of them on concomitant steroid therapy, reported mild-moderate adverse events during the induction period, including 3 cases of shingles, but no thromboembolic events.
The multicenter TOUR study used a novel electronic web-based PRO system, with direct data capture from patients while still obtaining site-based objective clinical data. This study design resulted in increased speed of the prospective data collection. The PRO system facilitated adherence to the protocol, and we were able to achieve high response rates of >95% at each data point. Our real-world findings confirm the rapid improvement of gut-associated symptoms beginning around day 3 of therapy, which was recently demonstrated in post hoc analyses of the OCTAVE studies.7 The current findings are even more remarkable if one compares the patient population of the OCTAVE studies with an anti-TNF failure rate of 50% with the patient population in TOUR.5 Before initiation of tofacitinib therapy, nearly all (95%) of the TOUR patients had failed at least 1 anti-TNF, which is consistent with the US-approved use of tofacitinib for patients with moderate to severe UC, and two-thirds had failed 2 biologics. Thus, based on the number of previous drug failures, disease severity in the TOUR patient population can be considered moderate-severe, albeit exact definitions for UC disease severity as a characterization of disease activity, disease complications, and need for therapy adjustments over time are still in development.24
Similar to the OCTAVE studies, response to tofacitinib therapy occurred regardless of the number of previously failed biologics, which is in contrast to the reduced efficacy of vedolizumab therapy in the setting of second-line therapy.25 Patients who reported higher disease activity at inclusion had lower response and remission rates in the induction period. The severity of mucosal inflammation did not impact outcomes; however, due to the pragmatic approach of the TOUR registry in most cases, the endoscopic evaluations were performed in a time frame of up to 6 months before start of tofacitinib therapy. We did not analyze disease duration or body mass index as response-modifying factors. Disease duration does not impact treatment outcome in UC compared with Crohn’s disease; and in contrast to subcutaneous biologics, body mass index has no impact on the clinical efficacy of tofacitinib.26,27
The SCCAI has been shown to correlate well with UC activity evaluations of invasive endoscopic indices and the 6-point Mayo score, excluding physician assessment and endoscopic evaluation.28,29 The exact cutoff values for mild, moderate, and severe disease activity for the SCCAI have not yet been validated, but a score of <2 to 2.5 is considered remission, and a patient-reported score ≥5 is defined as active disease.19,22,30 In the whole cohort including patients in remission at baseline, the SCCAI declined by an average of 1 point at day 3, the score decreased by 1.9 points in patients with a SCCAI score of ≥5 points. In a practical context, a decline of >1.5 points in the SCCAI correlates to a significant patient improvement such as “much improved” or “somewhat improved,” whereas patients with improvement of fewer than 1.5 points are only “slightly improved” or “about the same.”18
In contrast to the previously mentioned overall UC disease severity, the mean SCCAI score of the whole cohort at baseline corresponded more to a mild-moderate than moderate-severe disease activity. This is mostl likely due to the following reasons: (1) inclusion in TOUR was not dependent on predefined disease activity, and >60% of patients were on concomitant steroid therapy at baseline and their prior biologic therapy; (2) steroids are often initiated due to the lengthy approval process for biologics and the newer small molecules to curb ongoing disease activity; and (3) a switch in therapy in clinical practice may occur in the setting of mild-moderate disease activity and not only when a moderate-severe flare develops.
One of the novel aspects of the TOUR registry is the evaluation of the early efficacy of tofacitinib on urgency and incontinence. Several post hoc analyses of sizable placebo-controlled UC studies have been done to evaluate the early efficacy of various other therapies for several PROs (vedolizumab, ozanimod, upadacitinib, and mirikizumab). However, these analyses assess outcomes on day 14 rather than earlier time points, and except for upadacitinib, they do not include drug effects on patient-reported urgency or incontinence.25,31–33 In TOUR, an increase in stool frequency and occurrence of rectal bleeding was reported by more than two-thirds of patients. In contrast, nearly all patients (90%) documented daily urgency at baseline, and 7% of patients also experienced incontinence. Improvement of urgency was reported by one-fifth of the patients by day 3 and nearly half of the patients at day 14. The scores for rectal bleeding and bowel frequency, which are closely tied to endoscopically visible inflammation, plateaued at levels reflecting no or very mild disease activity in 80% to 85% of patients around day 14. The outcomes reported for urgency continuously improved throughout the 56 days of induction. This more continuous improvement may be due to the recently described impact of histologic healing on rectal compliance, which is only comparable to healthy controls once complete histologic resolution of inflammation is achieved.34 An urgency assessment is not part of the Mayo scoring scale, which for nearly a decade has been used as the standard assessment in UC trials conducted for the assessment and approval of investigational drugs, including the OCTAVE trials. Thus, TOUR is the first prospective study measuring the impact of tofacitinib on urgency. Patients strongly prefer a rapid onset of action in UC due to the significant impairment of daily life due to frequent bowel movements and concomitant urgency and incontinence. Urgency is one of the most frequently reported symptoms in patients with UC and a sign that patients rate as one of the most critical in need of rapid improvement.35–37 Incontinence, which is closely linked to the sensation of urgency and bowel frequency, recently was also defined as an ultimate therapeutic goal in an international expert consensus on the end points for future IBD modification trials (Selecting EndPoInts foR Disease- ModIfication Trials [SPIRIT]).38
Approximately half of the included population met clinical criteria for anxiety, depression, and reduced social satisfaction at baseline. Although these factors numerically improved over the course of induction, a significant difference was not reached at 8 weeks. This may be related to effects of concomitant medications like corticosteroids or the lack of complete response to therapy. In fact, outcomes of interventions for mood disorders are often assessed at later timepoints, such as at 180 days.39
No severe infections requiring hospitalization occurred in the first 8 weeks of therapy. All infectious adverse events in need of antibiotic therapy occurred in patients on dual therapy with tofacitinib and steroids (60% of the TOUR population). Herpes zoster (HZ) is a known adverse event of tofacitinib therapy.6 Herpes zoster infections occurred in 3% of the patients in TOUR, which is comparable to the rate seen in other real-world studies.40 Interestingly, all patients with new-onset HZ were also treated with concomitant steroids, and 2 of these also reported that they had received prior shingles vaccination with Shingrix. None of the patients stopped tofacitinib, and all continued in the study. The timing of the application of the shingles vaccine in relation to the start of tofacitinib is not known, but vaccine-induced immunity is decreased if vaccines are given in the context of steroid or immunosuppressive therapy.41 Thromboembolic events or deep venous thrombosis (DVT) were not reported during the 8-week induction period (56 days). However, tofacitinib therapy has been associated with this risk.42 Five thromboembolic events and 1 DVT, all occurring in patients on 10 mg of tofacitinib twice daily, have been reported in the UC study program, which included >1100 patients.43 Worldwide postmarketing safety surveillance experience with tofacitinib in UC also suggests a relative risk of 1.26 for vascular disorders, a term which includes thromboembolic events; however, the pathophysiological mechanism underlying this adverse event is not entirely elucidated.44
The current study has several limitations. Selection bias may have occurred because patients were required to have access to the internet for the data entries. Second, in the setting of a pragmatic trial design, we did not collect C-reactive protein or calprotectin data, which are often not measured in a standardized fashion before the start of a new therapy. Third, endoscopy was not required to confirm the status of remission, which would not have been feasible using a pragmatic real-world approach. However, the SCCAI has been shown to correlate well with endoscopic disease activity,29 and steroid-free clinical response and remission are clinically relevant end points.30
In conclusion, TOUR is the first prospective real-world study to evaluate the early therapeutic impact of tofacitinib on patient-reported outcomes in the United States, including urgency and PROMIS measures, in moderate-severe UC patients. Patients reported significant improvement in UC within 3 days of starting tofacitinib. Urgency and incontinence improved in parallel to bleeding and stool frequency in the first 14 days of therapy and then continued to improve throughout the study period. Long-term follow-up of this cohort will help to describe important further components of JAK inhibitor therapy in UC, including durability and safety of therapy.
Supplementary Material
Contributor Information
Millie D Long, University of North Carolina at Chapel Hill, Division of Gastroenterology and Hepatology, Chapel Hill, NC, USA.
Anita Afzali, Ohio State University, Division of Gastroenterology and Hepatology, Columbus, OH, USA.
Monika Fischer, Indiana University, Division of Gastroenterology and Hepatology, Indianapolis, IN, USA.
David Hudesman, NYU Langone Medical Center, New York, NY, USA.
Maisa Abdalla, University of Rochester, Division of Gastroenterology and Hepatology, Rochester, NY, USA.
Robert McCabe, MNGI Digestive Health, Minneapolis, MN, USA.
Benjamin L Cohen, Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Ryan C Ungaro, Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Will Harlan, Digestive Health Partners, Ashville, NC, USA.
John Hanson, Atrium Health Gastroenterology and Hepatology, Charlotte, NC, USA.
Gauree Konijeti, Scripps Clinic, La Jolla, CA, USA.
Steven Polyak, University of Iowa, Division of Gastroenterology and Hepatology, Iowa City, IA, USA.
Timothy Ritter, GI Alliance, Southlake, TX, USA.
Bruce Salzberg, Atlanta Gastroenterology Associates, Atlanta, GA, USA.
Jennifer Seminerio, Division of Digestive Diseases and Nutrition, University of South Florida Morsani College of Medicine, Tampa, FL, USA.
Emily English, University of North Carolina at Chapel Hill, Division of Gastroenterology and Hepatology, Chapel Hill, NC, USA.
Xian Zhang, University of North Carolina at Chapel Hill, Division of Gastroenterology and Hepatology, Chapel Hill, NC, USA.
Puza P Sharma, Pfizer Inc, New York, NY, USA.
Hans H Herfarth, MNGI Digestive Health, Minneapolis, MN, USA.
Funding
This registry is funded by Pfizer Inc.
Conflicts of Interest
M.L. has served as a consultant for AbbVie, UCB, Takeda, Janssen, Pfizer, Salix, Valeant, Lilly, Genentech, Roche, BMS, Target Pharmasolutions and has received research support from Pfizer and Takeda.
A.A. has served as a consultant for AbbVie, Takeda, Janssen, Bristol Myers Squibb, Pfizer, Eli Lilly, Gilead, DiaSorin, and TLL Pharmaceuticals; and as a speaker for AbbVie, Takeda, Janssen, Bristol Myers Squibb, and Pfizer.
M.F. has served as an advisory board member or consultant for AbbVie, Bristol Myer Squibb, Pfizer, Eli Lily, Takeda, Janssen, Scioto and as a DSMB member for Rebiotix.
D.H. has served as a consultant to Abbvie, BMS, Janssen, Pfizer, Takeda and has received research support from Janssen and Pfizer.
B.D. has served as an advisory board member or consultant for Abbvie, Celgene-Bristol Myers Squibb, Lilly, Pfizer, Sublimity Therapeutics, Takeda, TARGET RWE; CME Companies: Cornerstones, Vindico; Speaking: Abbvie; Educational Grant: Pfizer.
R.U. has served as an advisory board member or consultant for AbbVie, Janssen, Pfizer, and Takeda; research support from AbbVie, Boehringer Ingelheim, Eli Lily, and Pfizer; he is also funded by an National Institute of Health (NIH) K23 Career Development Award K23KD111995-01A1.
J,H, has served an advisory board member for Bristol Myers Squibb and for the Speakers Bureau of AbbVie and Pfizer.
G.K. has served as a consultant to Pfizer and ProciseDx and for the speakers bureau of Takeda.
S.P. has served as an advisory board member or consultant to Pfizer and Takeda; research support from Gilead, AbbVie, Seres, Bristol Myer Squibb, Pfizer, and Takeda.
T.R. has served on advisory boards for Abbive, Arena, Boeeringer Ingleheim, Bristol Myers, Ferring, Genentech, Gilead, Intercept, Iterative Scopes, Janssen, Lilly, Pfizer, Prometheus, and Takeda and as speaker for Abbvie, Bristol Myers, Janssen, Pfizer and Takeda.
J.S. has served as a consultant for AbbVie, UCB, Takeda, Janssen, Pfizer, BMS, Prometheus and has received research support from Takeda
P.S. is an employee of Pfizer Inc.
H.H.H. has served as a consultant to Alivio, BMS, Boehringer, ExeGi Pharma, Finch, Gilead, Janssen, Lycera, Merck, Otsuka, Pfizer, PureTech, and Seres; research support from Allakos, Artizan, and Pfizer.
All other authors have no conflicts to declare.
References
- 1. Ananthakrishnan AN, Higuchi LM, Huang ES, et al. . Aspirin, nonsteroidal anti-inflammatory drug use, and risk for Crohn disease and ulcerative colitis: a cohort study. Ann Intern Med. 2012;156(5):350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lichtenstein GR, Yan S, Bala M, et al. . Remission in patients with Crohn’s disease is associated with improvement in employment and quality of life and a decrease in hospitalizations and surgeries. Am J Gastroenterol. 2004;99(1):91–96. [DOI] [PubMed] [Google Scholar]
- 3. Singh S, Allegretti JR, Siddique SM, et al. . AGA Technical Review on the Management of Moderate to Severe Ulcerative Colitis. Gastroenterology 2020;158(5):1465–1496.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rubin DT, Ananthakrishnan AN, Siegel CA, et al. . ACG Clinical Guideline: Ulcerative Colitis in Adults. Am J Gastroenterol. 2019;114(3):384–413. [DOI] [PubMed] [Google Scholar]
- 5. Sandborn WJ, Su C, Sands BE, et al. . Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med. 2017;376(18):1723–1736. [DOI] [PubMed] [Google Scholar]
- 6. Sandborn WJ, Panes J, D’Haens GR, et al. . Safety of Tofacitinib for Treatment of Ulcerative Colitis, Based on 4.4 Years of Data From Global Clinical Trials. Clin Gastroenterol Hepatol. 2019;17(8):1541–1550. [DOI] [PubMed] [Google Scholar]
- 7. Hanauer S, Panaccione R, Danese S, et al. . Tofacitinib Induction Therapy Reduces Symptoms Within 3 Days for Patients With Ulcerative Colitis. Clin Gastroenterol Hepatol. 2019;17(1):139–147. [DOI] [PubMed] [Google Scholar]
- 8. Ha C, Ullman TA, Siegel CA, et al. . Patients enrolled in randomized controlled trials do not represent the inflammatory bowel disease patient population. Clin Gastroenterol Hepatol. 2012;10(9):1002–7; quiz e78; quiz e1078. [DOI] [PubMed] [Google Scholar]
- 9. Chaparro M, Garre A, Mesonero F, et al. . Tofacitinib in Ulcerative Colitis: Real-world Evidence From the ENEIDA Registry. J Crohns Colitis 2021;15(1):35–42. [DOI] [PubMed] [Google Scholar]
- 10. Honap S, Chee D, Chapman TP, et al. . Real-world Effectiveness of Tofacitinib for Moderate to Severe Ulcerative Colitis: A Multicentre UK Experience. J Crohns Colitis 2020;14(10):1385–1393. [DOI] [PubMed] [Google Scholar]
- 11. Deepak P, Alayo QA, Khatiwada A, et al. . Safety of Tofacitinib in a Real-World Cohort of Patients With Ulcerative Colitis. Clin Gastroenterol Hepatol. 2021;19(8):1592–1601.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weisshof R, Aharoni Golan M, Sossenheimer PH, et al. . Real-World Experience with Tofacitinib in IBD at a Tertiary Center. Dig Dis Sci. 2019;64(7):1945–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lair-Mehiri L, Stefanescu C, Vaysse T, et al. . Real-world evidence of tofacitinib effectiveness and safety in patients with refractory ulcerative colitis. Dig Liver Dis. 2020;52(3):268–273. [DOI] [PubMed] [Google Scholar]
- 14. Ishida N, Miyazu T, Tamura S, et al. . Real-World Efficacy and Safety Monitoring for Predicting Continuation of Tofacitinib Therapy in Patients with Ulcerative Colitis. Dig Dis Sci. 2021. doi: 10.1007/s10620-021-07233-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dalal RS, Mitri J, Goodrick H, et al. . Real-World Comparison of Tofacitinib vs Ustekinumab Among Bio-Exposed Patients With Ulcerative Colitis: A Propensity Score Analysis. Inflamm Bowel Dis. 2021;27(10):1694–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Biemans VBC, Sleutjes JAM, de Vries AC, et al. . Tofacitinib for ulcerative colitis: results of the prospective Dutch Initiative on Crohn and Colitis (ICC) registry. Aliment Pharmacol Ther. 2020;51(9):880–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Walmsley RS, Ayres RC, Pounder RE, et al. . A simple clinical colitis activity index. Gut 1998;43(1):29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Higgins PD, Schwartz M, Mapili J, et al. . Patient defined dichotomous end points for remission and clinical improvement in ulcerative colitis. Gut 2005;54(6):782–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Turner D, Seow CH, Greenberg GR, et al. . A systematic prospective comparison of noninvasive disease activity indices in ulcerative colitis. Clin Gastroenterol Hepatol. 2009;7(10):1081–1088. [DOI] [PubMed] [Google Scholar]
- 20. Kochar B, Martin CF, Kappelman MD, et al. . Evaluation of Gastrointestinal Patient Reported Outcomes Measurement Information System (GI-PROMIS) Symptom Scales in Subjects With Inflammatory Bowel Diseases. Am J Gastroenterol. 2018;113(1):72–79. [DOI] [PubMed] [Google Scholar]
- 21. Jowett SL, Seal CJ, Phillips E, et al. . Defining relapse of ulcerative colitis using a symptom-based activity index. Scand J Gastroenterol. 2003;38(2):164–171. [DOI] [PubMed] [Google Scholar]
- 22. Bennebroek Evertsz F, Nieuwkerk PT, Stokkers PC, et al. . The patient simple clinical colitis activity index (P-SCCAI) can detect ulcerative colitis (UC) disease activity in remission: a comparison of the P-SCCAI with clinician-based SCCAI and biological markers. J Crohns Colitis 2013;7(11):890–900. [DOI] [PubMed] [Google Scholar]
- 23. Marin-Jimenez I, Nos P, Domenech E, et al. . Diagnostic performance of the simple clinical colitis activity index self-administered online at home by patients with ulcerative colitis: CRONICA-UC study. Am J Gastroenterol. 2016;111(2):261–268. [DOI] [PubMed] [Google Scholar]
- 24. Peyrin-Biroulet L, Panes J, Sandborn WJ, et al. . defining disease severity in inflammatory bowel diseases: current and future directions. Clin Gastroenterol Hepatol. 2016;14(3):348–354.e17. [DOI] [PubMed] [Google Scholar]
- 25. Feagan BG, Lasch K, Lissoos T, et al. . Rapid response to vedolizumab therapy in biologic-naive patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2019;17(1):130–138.e7. [DOI] [PubMed] [Google Scholar]
- 26. Ben-Horin S, Novack L, Mao R, et al. . Efficacy of biologic drugs in short-duration versus long-duration inflammatory bowel disease: a systematic review and an individual-patient data meta-analysis of randomized controlled trials. Gastroenterology 2022;162(2):482–494. [DOI] [PubMed] [Google Scholar]
- 27. Farraye FA, Qazi T, Kotze PG, et al. . The impact of body mass index on efficacy and safety in the tofacitinib OCTAVE ulcerative colitis clinical programme. Aliment Pharmacol Ther. 2021;54(4):429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bewtra M, Brensinger CM, Tomov VT, et al. . An optimized patient-reported ulcerative colitis disease activity measure derived from the Mayo score and the simple clinical colitis activity index. Inflamm Bowel Dis. 2014;20(6):1070–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Higgins PD, Schwartz M, Mapili J, et al. . Is endoscopy necessary for the measurement of disease activity in ulcerative colitis? Am J Gastroenterol. 2005;100(2):355–361. [DOI] [PubMed] [Google Scholar]
- 30. D’Haens G, Sandborn WJ, Feagan BG, et al. . A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology 2007;132(2):763–786. [DOI] [PubMed] [Google Scholar]
- 31. Osterman MT, Longman R, Sninsky C, et al. . Rapid induction effects of ozanimod on clinical symptoms and inflammatory biomarkers in patients with moderately to severely active ulcerative colitis: results from the induction phase of TRUE NORTH. Gastroenterology 2021;160(6):SS93–SS93. [Google Scholar]
- 32. Ghosh S, Sanchez Gonzalez Y, Zhou W, et al. . upadacitinib treatment improves symptoms of bowel urgency and abdominal pain, and correlates with quality of life improvements in patients with moderate to severe ulcerative colitis. J Crohns Colitis 2021;15(12):2022–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dubinsky M, Lee SD, Panaccione R, et al. . Mirikizumab treatment improves bowel movement urgency in patients with moderately to severely active ulcerative colitis. Gastroenterology 2020;158(3):S17–S18. [Google Scholar]
- 34. Krugliak Cleveland N, Rai V, El Jurdi K, et al. . ulcerative colitis patients have reduced rectal compliance compared with non-inflammatory bowel disease controls. Gastroenterology 2022;162(1):331–333.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gray JR, Leung E, Scales J.. Treatment of ulcerative colitis from the patient’s perspective: a survey of preferences and satisfaction with therapy. Aliment Pharmacol Ther. 2009;29(10):1114–1120. [DOI] [PubMed] [Google Scholar]
- 36. Hibi T, Ishibashi T, Ikenoue Y, et al. . Ulcerative colitis: disease burden, impact on daily life, and reluctance to consult medical professionals: results from a Japanese internet survey. Inflamm Intest Dis 2020;5(1):27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rubin DT, Sninsky C, Siegmund B, et al. . International perspectives on management of inflammatory bowel disease: opinion differences and similarities between patients and physicians from the IBD GAPPS survey. Inflamm Bowel Dis. 2021;27(12):1942–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Le Berre C, Peyrin-Biroulet L, group S-I.. Selecting end points for disease-modification trials in inflammatory bowel disease: the SPIRIT consensus from the IOIBD. Gastroenterology 2021;160(5):1452–1460 e1421. [DOI] [PubMed] [Google Scholar]
- 39. Coley RY, Boggs JM, Beck A, et al. . Defining success in measurement-based care for depression: a comparison of common metrics. Psychiatr Serv. 2020;71(4):312–318. [DOI] [PubMed] [Google Scholar]
- 40. Taxonera C, Olivares D, Alba C.. Real-world effectiveness and safety of tofacitinib in patients with ulcerative colitis: systematic review with meta-analysis. Inflamm Bowel Dis. 2022;28(1):32–40. [DOI] [PubMed] [Google Scholar]
- 41. Agarwal N, Ollington K, Kaneshiro M, et al. . Are immunosuppressive medications associated with decreased responses to routine immunizations? A systematic review. Vaccine 2012;30(8):1413–1424. [DOI] [PubMed] [Google Scholar]
- 42. Ytterberg SR, Bhatt DL, Mikuls TR, et al. . Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386(4):316–326. [DOI] [PubMed] [Google Scholar]
- 43. Sandborn WJ, Lawendy N, Danese S, et al. . Safety and efficacy of tofacitinib for treatment of ulcerative colitis: final analysis of OCTAVE Open, an open-label, long-term extension study with up to 7.0 years of treatment. Aliment Pharmacol Ther. 2022;55(4):464–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rubin DT, Modesto I, Vermeire S, et al. . Worldwide post-marketing safety surveillance experience with tofacitinib in ulcerative colitis. Aliment Pharmacol Ther. 2022;55(3):302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.