Abstract
Background
Hypertension is a highly prevalent disease in Saudi Arabia with poor control rates. Updated guidelines are needed to guide the management of hypertension and improve treatment outcomes.
Methodology
A panel of experts representing the National Heart Center (NHC) and the Saudi Heart Association (SHA) reviewed existing evidence and formulated guidance relevant to the local population, clinical practice and the healthcare system. The recommendations were reviewed to ensure scientific and medical accuracy.
Recommendations
Hypertension was defined and a new classification was proposed as relevant to the Saudi population. Recommendations on diagnosis, clinical evaluation, cardiovascular assessment were detailed, along with guidance on measurement modalities and screening/follow-up. Non-pharmacological management is the first line of hypertension treatment. Pharmacological therapy should be used appropriately as needed. Treatment priority is to control blood pressure regardless of the drug class used. The choice of treatment should be tailored to the patient profile in order to achieve treatment targets and ensure patient compliance. Recommendations were provided on pharmacological options available in Saudi Arabia, as well as guidance on the treatment of special conditions.
Conclusion
Hypertension management should be based on appropriate screening, timely diagnosis and lifestyle changes supplemented with pharmacological therapy, as needed. Clinical management should be individualized, and careful consideration should be given to special conditions and patient groups.
Keywords: Hypertension, Guidelines, Saudi Arabia
1. Introduction
Hypertension is a leading cause of morbidity and mortality, afflicting more than 1.2 billion people across the globe [1]. Despite the efficacy of blood lowering therapies and lifestyle modifications, more than half of people with hypertension remain untreated and control rates are unacceptable [1]. The prevalence and burden of hypertension is highest in middle-income and low-income countries, with the majority of deaths associated with blood pressure reported from these regions [2]. In the Middle East region, the overall prevalence of hypertension across two decades (1999–2019) was 24.36%, with an increasing trend in the development of hypertension observed with advancing age [3]. The CEPHEUS study examined individuals with hypercholesterolemia receiving lipid lowering drugs across the Middle East (including Saudi Arabia) and found 66.6% of this population have hypertension [4]. The Prospective Urban Rural Epidemiology study found that 33% of individuals from four Middle Eastern countries (Iran, Occupied Palestinian Territory, Saudi Arabia, and United Arab Emirates) suffered from hypertension, 51% of whom were unaware of their diagnosis. While 47% of people with hypertension were treated, only 19% had controlled blood pressure levels [5]. When looking specifically at findings from Saudi Arabia, the study found the highest percentage of awareness, treatment and control, and the second lowest prevalence of hypertension [5]. That being said, evidence from Saudi Arabia reflect low levels of hypertension awareness, and poor blood pressure control. In fact, 39.2–55% of individuals are unaware of their hypertension [6–8]. Most people with hypertension receive pharmacological treatment (60.8–78.9%), but overall rates of control remain below 50% (37–45%) [6,7,9] and 20.2–35.4% of people with hypertension are uncontrolled on treatment [7,9]. The rate of hypertension in Saudi Arabia has risen from 25.5% among adults in 2011 [6], to 29.2% in 2019 [7]. Unhealthy lifestyle (low physical activity) and obesity remain some of the most prevalent risk factors for hypertension in the Saudi population [6,9–11], even among younger individuals. In fact, in a population of young male Saudi university students, 61.6% suffer from hypertension and approximately 35% of the participants were obese [12]. Other common risk factors in the Saudi population are diabetes [6,9,13,14] and hypercholesterolemia [6,9,13].
Early screening and detection is therefore critically needed in Saudi Arabia in addition to national campaigns raising awareness about the disease, its risk factors and preventative measures. Updated guidelines are needed to guide the management of hypertension in Saudi Arabia and improve treatment outcomes. The National Heart Center (NHC) and the Saudi Heart Association thus outline best practices for cardiologists in the KSA to better diagnose and manage hypertension based on key opinion leaders’ expertise and available data. This project can be a step forward towards a recognized guidance for all healthcare providers in the KSA for improved evidence-based and up to date management of hypertension, with special consideration for subgroups including but not limited to older age, pregnancy, diabetes, atrial fibrillation, resistant hypertension, and hypertensive emergencies.
2. Methods
A series of meetings were conducted to review available evidence and formulate recommendations appropriate for clinical practice and local resources in Saudi Arabia. A panel of expert cardiologists based their recommendations on extant data from clinical studies and meta-analyses, international guidelines as well as local expertise. Recommendations were discussed until a consensus is reached by the panel. The guidelines followed the Saudi Heart Association methodology for guideline recommendations (Table 1).
Table 1.
Saudi Heart Association classes of recommendations.
| Color | Class | Definition |
|---|---|---|
| Recommended | The usefulness and efficacy of a particular treatment/procedure/action is supported by available evidence. | |
| Should be considered | The usefulness and efficacy of a particular treatment/procedure/action is established by favorable expert opinion on conflicting evidence. | |
| May be considered | The usefulness and efficacy of a particular treatment/procedure/action is not well established by evidence and expert opinion. | |
| Not recommended | A particular treatment/procedure/action is not useful nor effective and is potentially harmful based on available evidence and/or general agreement. |
3. Results - consensus statements
3.1. Definition and classification of hypertension
Pathophysiological hypertension is a level of blood pressure (BP) at which, if persisting, will lead to target organ damage. BP of 120/80 mmHg is considered to be normal. However, when BP exceeds 130/80 mmHg, the patient is considered to have hypertension. Diagnosis of hypertension depends on the methods of measurement (office measurement, ambulatory BP measurement and home BP measurement). BP should be measured appropriately, as outlined in Section 3.2.1. BP thresholds at which treatment is recommended are higher than the thresholds at which hypertension is diagnosed and are outlined in Table 2.
Table 2.
Definition and classification of hypertension. BP: blood pressure.
| Class | Recommendation |
|---|---|
Hypertension is diagnosed if BP, measured in an appropriate way, is:
|
|
Treatment is recommended to be considered if BP, measured in an appropriate way, is:
|
3.2. Blood pressure measurement and hypertension diagnosis
Recommendations on blood pressure measurement methods are provided in Table 3. The following sections discuss recommended practices for measuring blood pressure via conventional office measurement, as well as ambulatory and home-based measurement [15,16].
Table 3.
Recommendations for blood pressure measurement methods. ABPM: ambulatory blood pressure monitoring; HBPM: Home blood pressure monitoring.
| Class | Recommendation |
|---|---|
| Conventional office blood pressure measurement is preferred for the diagnosis of hypertension. | |
| HBPM and ABPM are complementary methods that could help confirm the diagnosis of hypertension and detect white-coat and masked hypertension. |
3.2.1. Conventional office blood pressure measurement (Auscultatory or oscillometric automatic sphygmomanometers)
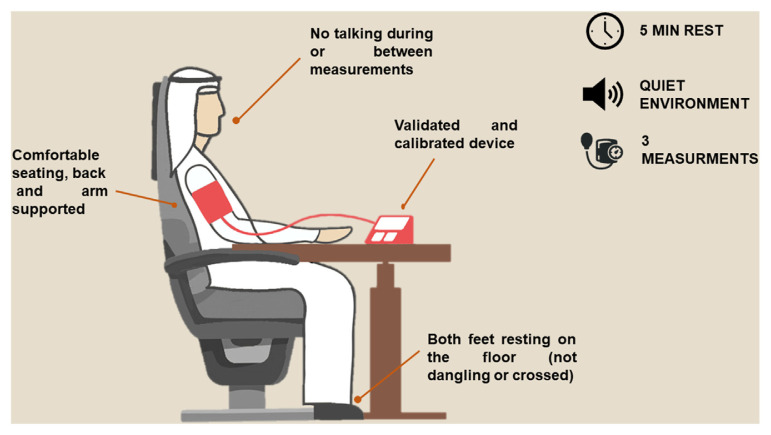
Office BP measurements have long been considered the gold standard of BP monitoring. Regardless, office measurements are frequently performed inadequately in routine practice due to insufficient attention, insufficient time or badly calibrated devices. Clear differences exist between routine office BP measurement and standardized office BP measurement. Research-grade (standardized) BP measurement yields lower BP values compared to routine office measurement [17]. The overestimation of BP with routine measurement is well documented and should be carefully addressed as it could lead to the misclassification of hypertension [18]. It is therefore of the utmost importance to measure BP in the office following a standardized approach in order to avoid misdiagnosis and inappropriate treatment, as shown in Fig. 1. Briefly, people should be seated comfortably in a quiet environment for at least 5 min before measurement. The patient’s back and arm should be supported, with both feet resting on the floor (not dangling or crossed). BP should be determined based on the average of 3 measurements, 1–2 min apart. A calibrated device should be used for BP measurement. In case of notable inconsistencies between readings (>10 mm Hg difference), additional measurements should be considered. BP should be measured in both arms. The higher value should be used as a reference in case of differences in readings. When first measuring BP, orthostatic hypotension should be excluded by measuring BP 1 min and 3 min after standing from seated position. Lying and standing BP measurement may be considered in some patient groups, such as older people, people with diabetes, and conditions associated with orthostatic hypotension.
Fig. 1.
Methodology for the measurement of office BP.
3.2.2. Out-of-office blood pressure measurement
3.2.2.1. Home blood pressure monitoring (HBPM)
Home-based BP measurement is known to have a lower diagnostic threshold for hypertension compared to office-based monitoring (see corresponding values in Table 2). Home blood pressure monitoring (HPBM) is cheap, easily accessible and could be better at predicting cardiovascular morbidity and mortality compared to conventional BP measurement [19]. It could also promote medication adherence and BP control [20,21]. However, prospective studies evaluating HPBM remain rather limited.
One study found that only 3% of people correctly measure their BP with no error [22]. Proper instructions should therefore be provided by the treating physician or the nurse in order to ensure correct HPBM. Similar to office BP measurement, home-based BP monitoring should be done with the patient seated in a quiet environment for at least 5 min prior to measurement, with legs, back and arm supported. An automatic validated BP monitor should be used to measure BP for a few days before each clinic visit. Readings should be performed twice daily (two to three readings), in the morning and the evening.
3.2.2.2. Ambulatory blood pressure monitoring (ABPM)
Ambulatory blood pressure monitoring (ABPM) is usually used to obtain an average of BP readings over a defined period, usually 24 h. ABPM also has a lower diagnostic threshold for hypertension. BP varies over the course of the day and a drop can be observed naturally during the night. It is therefore important to consider different thresholds for daytime, nighttime, and 24-h average readings (Table 2).
The role of ABPM as the method of choice in the diagnosis of hypertension continues to be debated. That being said, evidence suggests ABPM to better predict morbidity or fatal events compared to office BP [23–25]. A wealth of evidence supports the adequacy of ABPM for BP measurement [26]. Clinically relevant ABPM indexes are mainly 24 h average BP and nighttime average BP. ABPM can be used to confirm office-based readings and diagnose hypertension. It can also be used to rule out white-coat (elevated blood pressure in the office only) and masked hypertension (elevated blood pressure out-of-office only) [27].
3.3. Screening and follow-up
3.3.1. Screening
No direct trials have compared screening and no screening for hypertension. However, it is clear that early detection is associated with better outcomes based on indirect evidence from health promotion programs that was conducted in some countries [28–30]. A recent study reported alarming rates of individuals unaware of their hypertension (39.2%) [7]. Considering the high rates of missed hypertension in Saudi Arabia, wide structured screening programs should be undertaken. It is therefore recommended that all adults (>18 years of age) should be screened for hypertension. BP should be measured whenever feasible, at least at each visit to a healthcare facility. Annual screening is warranted for individuals older than 40 or those who have risk factors for hypertension. All other individuals should be screened every 3–5 years (Table 4).
Table 4.
Recommendations for screening in people with hypertension.
| Class | Recommendation |
|---|---|
| Screening for hypertension is recommended for all adults (>18 years of age) at each visit to a healthcare facility, whenever feasible. | |
| Annual screening is recommended for individuals older than 40 years of age or those who have risk factors for hypertension. | |
| Screening for the general population is recommended every 3 to 5 years. |
3.3.2. Follow-up
After treatment initiation and in case of uncontrolled BP, patient follow-up should be as frequent as possible, ideally in 1–2 months. Once BP control is achieved, people with hypertension can be seen every 3–6 months (Table 5).
Table 5.
Recommendations for follow-up schedule in people with hypertension. BP: blood pressure.
| Class | Recommendation |
|---|---|
| After therapy initiation, patient follow-up is recommended in 1 to 2 months. | |
| In people with uncontrolled BP, follow-up is recommended in 1 to 2 months after therapy adjustment. | |
| In people with controlled BP, follow-up is recommended once every 3–6 months. |
3.4. Diagnostic/clinical evaluation
Clinical evaluation aims to establish the diagnosis and grade of hypertension as well as screen for potential secondary causes of hypertension and identify factors potentially contributing to the development of hypertension (lifestyle, concomitant medications, or family history), concomitant cardiovascular (CV) risk factors (including lifestyle and family history), concomitant diseases. It also aims to establish whether there is evidence of hypertension-mediated organ damage (HMOD) or existing CV, cerebrovascular, or renal disease. Recommended medical history, physical examination and laboratory investigation are shown in Table 6.
Table 6.
Recommendation for clinical evaluation of people with suspected hypertension. BP: blood pressure; CV: cardiovascular; HMOD: Hypertension-mediated organ damage.
| Class | Recommendation |
|---|---|
| Comprehensive clinical evaluation, including medical history, physical examination and laboratory testing, is recommended to confirm the diagnosis of hypertension, identify target organ damage and potential secondary causes of hypertension | |
It is recommended that medical history documents the following:
| |
It is recommended that physical examination includes an assessment of:
|
|
| Urinary albumin to creatinine ratio is recommended for all people with diabetes and people with long-standing hypertension and established end-organ damage. | |
| A pregnancy test should be considered before initiation of behavioral management or drug therapy | |
| Echocardiography should be considered in case of electrocardiogram abnormalities or high suspicion of left ventricular dysfunction or coronary artery disease. | |
| Urinary albumin to creatinine ratio may be considered for people with suspected hypertension. | |
| Uric acid testing may be considered for people with suspected hypertension. |
3.5. Cardiovascular risk assessment
Cardiovascular risk is affected by BP levels, risk factors, HMOD and comorbidities (see Table 7). BP can be classified as shown in Table 8. Hypertension often occurs in conjunction with other CV risk factors. Cardiovascular risk factors are highly prevalent in Saudi Arabia [31]. Unhealthy lifestyle (low physical activity) and obesity are the most common risk factors for hypertension in the Saudi population across younger and older age groups [6,9–11]. Diabetes [6,9,13,14] and hypercholesterolemia [6,9,13] are also frequently observed. Metabolic syndrome was reported to have a prevalence of 30–40% in the Saudi population [32,33]. Dyslipidemia was observed in around half of Saudi people with diabetes in one study [34]. The Africa Middle East Cardiovascular Epidemiological (ACE) study was a multinational cross-sectional study which showed 68% dyslipidemia in the adult Saudi population [35]. When specifically considering cardiovascular risk factors in people with hypertension, a study in Saudi Arabia showed that 14% also have diabetes, and more than half are obese [11]. Poor physical activity is also observed in more than 70% of people with stage 1 hypertension, stage 2 hypertension or hypertensive crisis [36].
Table 7.
Recommendation for cardiovascular risk assessment.
| Class | Recommendation |
|---|---|
| Cardiovascular risk assessment should be considered for all people with hypertension not already at high or very high risk or cardiovascular disease (i.e. established cardiovascular disease, renal disease, diabetes, a markedly elevated single risk factor (e.g. cholesterol), or hypertensive left ventricular hypertrophy). |
Table 8.
Classification of BP.
| Classification | BP (MMHG) |
|---|---|
| Optimal BP | 120/80 |
| Elevated BP | 121/80–129/80 |
| Stage 1 | 130/80–139/89 |
| Stage 2 | >140/90 |
| Hypertensive crisis | >180/120a |
BP: blood pressure.
BP > 190/120 mmHg accompanied with evidence of new or worsening HMOD.
3.6. Management of hypertension
3.6.1. Non-pharmacological approaches
Non-pharmacological management is the first line of hypertension treatment (Table 9). Healthy lifestyle choices can reduce cardiovascular risk [37] and once applied in people with elevated BP, lifestyle modifications can increase the rates of BP control and enhance the effect of antihypertensive medication by further lowering BP [38].
Table 9.
Recommendation on non-pharmacological approaches. BP: blood pressure.
| Class | Recommendation |
|---|---|
| Lifestyle modification should be recommended for all people with high BP. |
Recommended lifestyle interventions include smoking cessation, reducing salt (sodium-containing salts) intake (limit to a maximum of 2–4 g daily), eating more fruit and vegetables, being physically active on a regular basis (at least 30 min of moderate dynamic exercise 5–7 days per week), avoiding use of tobacco and alcohol consumption, limiting the intake of foods high in saturated fats, eliminating/reducing trans fats in diet, limiting the intake of processed/ultraprocessed food and body weight control [39–48]. People with hypertension should also be counseled to avoid excessive use of licorice, which is common practice in Saudi Arabia [49]. Sugar-sweetened drinks should also be avoided or their intake be reduced considering their robust association with increased BP and incident hypertension [50,51].
3.6.2. Pharmacological approaches
Antihypertensive medications that can be considered for initial treatment include diuretics (thiazide and thiazide-like agents), angiotensin-converting enzyme inhibitors (ACEis), angiotens-inreceptor blockers (ARBs) and long-acting dihydropyridine calcium channel blockers (CCBs). Beta blockers might also be considered in the first line setting for specific indications, such as in younger people with hypertension with sympathetic overdrive or other compelling indications (ischemic disease, heart failure, obesity/bariatric surgery) (Table 10).
Table 10.
Recommendations for pharmacological management of hypertension. ACEi: Angiotensin-converting enzyme inhibitor; ARB: Angiotensinreceptor blocker; BP: blood pressure; CCB: calcium channel blocker; RAS: renin angiotensin system; SBP: systolic blood pressure.
| Class | Recommendation |
|---|---|
| The main target of treatment is to control BP. | |
Antihypertensive medications that can be considered for initial treatment:
|
|
| Beta blockers can be used in specific indications (young age, sympathetic overdrive, ischemic disease, heart failure, obesity/bariatric surgery). | |
| Single-pill combination treatment is recommended as initial therapy for all people with hypertension with the exception of elderly, frail or younger people with low risk. The combination of a RAS blocker with a CCB or a diuretic is preferred. | |
Monotherapy can be considered for:
|
|
| If BP control is not achieved with a two-drug combination, three-drug regimens (including a RAS blocker, a CCB, and a diuretic) should be considered. | |
| In exceptional cases where BP remains uncontrolled with all recommended drug classes, other classes of antihypertensive drugs should be considered, such as spironolactone (or other diuretics if spironolactone not tolerated), a beta blocker, an alpha blocker or direct vasodilators. |
Treatment priority is to control BP regardless of the drug class used. The choice of treatment should be tailored to the patient profile in order to achieve treatment targets and ensure patient compliance. As such, more potent medication might be considered and single–pill combinations might be favored in light of the improved medication persistence and superior BP reduction compared to multi-pill combinations [52].
Medication potency can vary, especially in different clinical settings. First-line low-dose thiazides, ACEis and CCBs might be similarly effective in improving morbidity and mortality in moderate to severe primary hypertension [53]. The effect of each drug class on cardiovascular events can be different but evidence quality remains insufficient to draw robust conclusions [54].
Some medications have higher potency than others. For example, the newest ARB azilsartanmedoxomil at thedose of 80mg seemedtobemost efficacious ARB in reducing both systolic and diastolic blood pressure in the office and on ambulatory measurement [55]. The single–pill combination of azilsartan medoxomil with chlorthalidone was also safe and effective for the reduction of BP [56].
Some medications are best used only in specific indications for the first-line management of hypertension. Beta blockers should be considered in certain patient populations where they are most beneficial, such as in younger people with sympathetic overdrive [57], ischemic heart disease [58,59], heart failure [60–63], or obesity/bariatric surgery [64]. Meta-analyses suggesting the lower efficacy of beta blockers compared to other antihypertensives are based on highly biased data [65]. It is therefore important to consider the potential differences in the efficacy of beta blocker subtypes, as well as their possible different effect in younger and older people. While second- and third-generation beta blockers are suggested to have comparable efficacy in reducing BP [66], third-generation such as nebivolol are associated with lower adverse events compared to second-generation beta blockers [67]. That being said, all drugs carry side effects. Fear of side effects should not discourage the use of any medication, such beta blockers, in the presence of compelling indications for their use and a favorable risk/benefit ratio.
3.7. Special conditions/groups
3.7.1. Resistant hypertension
The global prevalence of true-resistant hypertension is estimated to be around 10% of treated people with hypertension [68]. Resistant hypertension is defined as seated office BP > 140/90 mm Hg in people managed with three or more antihypertensive medications at optimal (or maximally tolerated) doses including a diuretic. When resistance to treatment is suspected, secondary hypertension and pseudo-resistant hypertension (poor BP measurement technique, white coat effect, nonadherence and suboptimal choices in antihypertensive therapy) should be first excluded. Diagnosis should then be confirmed with ABPM or HBPM. Once the diagnosis of resistant hypertension is confirmed, it is important that current treatment regimen be optimized, including both drug therapy and lifestyle interventions (Table 11). In fact, the TRIUMPH trial demonstrated that lifestyle modification including diet and exercise changes can lead to significant reductions in BP in people with resistant hypertension [69].
Table 11.
Recommendations for resistant hypertension. BP: blood pressure.
| Class | Recommendation |
|---|---|
| Resistant hypertension is defined as seated office BP >140/90 mm Hg in people managed with three or more antihypertensive medications at optimal (or maximally tolerated) doses including a diuretic. | |
When treatment resistance is suspected, it is recommended to:
|
|
| Sympathetic renal denervation may be considered. |
Drug regimens also carry important implications. The choice of both diuretics and ARB may affect treatment outcomes. The thiazide-like diuretics chlorthalidone and indapamide might be favored and some ARBs are more potent than others [70]. It is thus necessary to choose the right drug and the appropriate dose when managing resistant hypertension. Spironolactone is associated with significant reductions in BP compared to placebo and alternative drugs [71] and should be considered in treatment-resistant hypertension. If available, other drugs may be considered if spironolactone is not tolerated or contra-indicated are eplerenone, amiloride and doxazosin. Amiloride was shown to be as effective as spironolactone in reducing BP [72] but doxazosin was not [73].
In case of treatment failure or the presence of secondary hypertension, people with resistant hypertension should be referred to a specialized care center.
As for the use of renal sympathetic denervation for the treatment of hypertension, available evidence has been relatively contradictory. The Symplicity HTN-3 trial was the first randomized, single-blinded, sham-controlled study to compare renal sympathetic denervation to sham treatment among people with drug-resistant hypertension. While renal sympathetic denervation proved to be safe, Symplicity HTN-3 failed to meet its primary efficacy endpoint and no significant difference in BP lowering detected between the study arms [74]. Negative results were also reported in the Japan (Asian) site of the study [75], the 12-month followup analysis [76]and some other sham-controlled trials [77–79].
By contrast, the Symplicity global registry showed largely different results, reflecting renal sympathetic denervation to be a safe procedure for the sustainable reduction of blood pressure [80,81]. The safety and efficacy of renal sympathetic denervation for BP lowering is actually evident in recent randomized clinical trials and registries. This benefit is observed across patient subgroups including those with high-risk, and across device types [82]. Meta-analyses also show that renal denervation leads to significant reductions in BP in both medicated and unmedicated people with resistant hypertension [83,84]. Based on this, renal sympathetic denervation may be considered in people with treatment resistance (as defined in Table 12), and also in people with uncontrolled hypertension and high cardiovascular risk [82].
Table 12.
Recommendations for the management of hypertension with diabetes mellitus. ACEi: Angiotensin-converting enzyme inhibitor; ARB: Angiotensin-receptor blocker; BP: blood pressure; CCB: calcium channel blocker.
| Class | Recommendation |
|---|---|
| Drug treatment should be initiated at a BP ≥130/80mmHg with a treatment goal less than 130/80mmHg. | |
| Initial therapy for people with hypertension and diabetes is recommended to be an ACEi or an ARB, particularly in the presence of microalbuminuria or kidney disease. | |
| Other medications are also safe and include thiazide-like diuretics, CCBs or beta blockers (when indicated). |
3.7.2. Diabetes mellitus
People with diabetes often present with elevated BP and have a higher risk of developing cardiovascular disease [85,86]. The management of hypertension in people with concomitant diabetes ensures both vascular and renal protection by reducing macrovascular and microvascular complications as well as preventing end-stage renal disease [87,88]. It is recommended to initiate therapy when BP is equal to or higher than 130/80 mmHg and target a BP level below 130/80 mmHg [87–90] (Table 12). ACEis or ARBs are recommended especially in the presence of albuminuria or renal involvement. In fact, ACEis and ARBs are significantly more effective than other classes in reducing albuminuria and the appearance or progression of diabetic nephropathy [87]. ACEis and ARBs ensure reductions in BP and have also been shown to have significant cardioprotective effects in people with concomitant hypertension and diabetes, leading to reduced mortality (all-cause and CV) and major CV events [91,92].
CCBs and beta blockers are also safe to use for the management of people with hypertension and diabetes. The combination of a CCB and an ACEi was found to consistently lead to BP lowering as well as significantly lower cardiovascular mortality compared to other treatments [93]. Selective beta blockers are not contraindicated in people with diabetes but do not lead to comparable cardiovascular risk reduction compared to other drug classes [94,95].
3.7.3. Chronic kidney disease
The most effective pharmacological pressure-lowering therapy against end-stage kidney disease was ACEis and ARBs (renin-angiotensin system (RAS) blockers) [96,97]. The renoprotective effects of RAS blockers might be most pronounced in people with albuminuria or proteinuria [98,99]. A RAS blocker should therefore be included in the management of people with hypertension and chronic kidney disease and proteinuria or microalbuminuria (Table 13). Combination therapy with an ACEi and ARB carries a notable risk of hyperkalemia and acute kidney injury and is therefore not recommended. When more BP lowering is needed, ACEi or an ARB can be combined with other antihypertensive drugs, such as dihydropyridine CCBs [100]. Evidence on the optimal BP target in CKD is complex but generally supports clinical benefit of a target range of SBP 130–140 mmHg in both diabetic and non-diabetic CKD [101–103].
Table 13.
Recommendations for the management of hypertension with chronic kidney disease. ACEi: Angiotensin-converting enzyme inhibitor; ARB: Angiotensin-receptor blocker; BP: blood pressure.
| Class | Recommendation |
|---|---|
| An ACEi or an ARB is recommended for the first line management of people with hypertension and proteinuric chronic kidney disease (urinary protein level > 150 mg in 24 hours or albumin to creatinine ratio > 30 mg/mmol). | |
| A treatment target of SBP 130–140 mmHg is recommended in people with hypertension and CKD | |
| If needed, combination therapy should be considered to achieve BP targets. | |
| The combination of an ACEi and ARB is not recommended. |
3.7.4. Coronary artery disease
The relationship between hypertension and coronary artery disease (CAD) is a robust one, with BP lowering associated with clear reductions in the risk of CAD [104,105]. It is therefore important to use antihypertensive medication in people with concomitant CAD. Evidence supports the safety of targeting a BP level below 140/80 mmHg in people with CAD seeing as BP exceeding 140/80 mmHg is associated with increased cardiovascular risk [106,107]. A BP below 120/70 mmHg was also linked to detrimental effects [106,107]. Based on this, it is recommended that a systolic blood pressure (SBP) range 120–130 mmHg be targeted for all people with CAD if tolerated, with a higher range (130–139 mmHg) acceptable for older people. Diastolic blood pressure (DBP) should be maintained between 70 and 80 mmHg. Diastolic target level should not be decreased to less than 60 mmHg in the presence of systolic hypertension or left ventricular hypertrophy due to concerns of exacerbation of myocardial ischemia (Table 14).
Table 14.
Recommendations for the management of hypertension with coronary artery disease. ACEi: Angiotensin-converting enzyme inhibitor; ARB: Angiotensin-receptor blocker; BP: blood pressure; CAD: coronary artery disease; CCB: calcium channel blockers; DBP: diastolic blood pressure; RAS: renin angiotensin system; SBP: systolic blood pressure.
| Class | Recommendation |
|---|---|
| A Treatment target of SBP 120–130 mmHg is recommended in people with hypertension and CAD if tolerated. A higher target (130–140 mmHg) is recommended for older people (aged ≥65 years). DBP target should be lower than 80 mmHg, but higher than 70 mmHg. | |
| In the case of systolic hypertension or left ventricular hypertrophy, diastolic target level should not be decreased to less than 60mmHg when due to concerns of exacerbation of myocardial ischemia. | |
| An ACEi or an ARB is recommended for the majority of people with hypertension and CAD. | |
| Beta blockers and RAS blockers are recommended in people with a history of myocardial infarction | |
| Beta blockers and/or CCBs are recommended for people with symptomatic angina. | |
| In case of combination therapy, drug choices should be individualized. |
Beta blockers and RAS blockers (ACEis or ARBs) are preferred in people with CAD due to their positive impact on clinical outcomes post-myocardial infarction [108]. In addition to beta blockers and CCBs are also beneficial for BP lowering in people with coronary artery disease [109], especially those with symptomatic angina.
3.7.5. Heart failure
The development of heart failure is undoubtedly linked to hypertension [110]. It is recommended to provide people with heart failure with antihypertensive treatment. Antihypertensive therapy was shown to reduce the risk of incident heart failure as well as heart failure hospitalization [111,112]. Treatment should be started at a BP level higher than 140/90 mmHg, targeting SBP <130 mmHg but preferably not lower than 120 mmHg (Table 15). Comparative trials have demonstrated the efficacy of diuretics, beta-blockers and RAS blockers (ACEis or ARBs) in not only lowering BP, but also reducing heart failure risk [87]. This was shown to a lesser extend with CCBs. Nonhydropyridine CCBs are not recommended in the treatment of people with heart failure and reduced ejection fraction.
Table 15.
Recommendations for the management of hypertension with heart failure. BP: blood pressure; CCB: calcium channel blockers; RAS: reninangiotensin system.
| Class | Recommendation |
|---|---|
| BP-lowering treatment is recommended for all people with heart failure and stage 2 hypertension (BP>140/90 mmHg). Treatment threshold and target values recommended for the general population are the same for people with heart failure with reduced or preserved ejection fraction (<130mmHg but preferably not lower than 120mmHg). | |
| Treatment including a RAS blocker, and a beta-blocker and diuretic and/or mineralocorticoid receptor antagonists (if required) is recommended for people with heart failure and reduced ejection fraction. | |
| a RAS blocker in combination with a CCB or diuretic is recommended for people with left ventricular hypertrophy. | |
| Diuretics should be included in the management of people with heart failure and volume overload. | |
| Dihydropyridine CCBs may be considered in case treatment target is not achieved. | |
| Nonhydropyridine CCBs are not recommended in the treatment of people with heart failure and reduced ejection fraction. |
As for left ventricular hypertrophy, regression can be ensured with BP lowering [113], and ARBs, ACEis and CCBs lead to more effective left ventricular hypertrophy regression compared to beta blockers or diuretics [114]. It is therefore recommended to combine a RAS blocker with a CCB or diuretic for people with left ventricular hypertrophy.
3.7.6. Acute stroke and cerebrovascular disease
In the acute phase of stroke, BP can be elevated but will generally decrease with no treatment [115]. The clinical benefit of acute BP lowering after acute intracerebral hemorrhage remains uncertain and debatable. That being said, available evidence suggests that immediate reduction of BP should only be attempted in case of acute intracerebral hemorrhage and very severe hypertension (SBP ≥220 mmHg) [116,117]. As for people with acute ischemic stroke, routine BP lowering is not recommended. One exception is people who are receiving thrombolysis, in whom BP should be lowered to <180/105mmHg in case of acute ischemic stroke [118,119]. Pharmacological BP lowering may also be considered based on clinical judgment during the first 24 h after stroke onset in people with severe BP (SBP ≥220 mmHg or DBP≥120mmHg) not receiving fibrinolysis [120–123]. The SBP of people who suffered a transient ischemic attack or an ischemic stroke should be maintained at 120–130 mmHg in order to reduce the risk of future stroke [124]. As for drug choice, a RAS blocker in combination with a CCB or a thiazide-like diuretic is recommended for stroke prevention (Table 16).
Table 16.
Recommendations for the management of hypertension with acute stroke and cerebrovascular disease. BP: blood pressure; CCB: calcium channel blockers; IV: intravenous; RAS: renin-angiotensin system; SBP: systolic blood pressure.
| Class | Recommendation |
|---|---|
| Antihypertension medication is recommended immediately after transient ischemic attack and after few days of ischemic stroke. | |
| A RAS blocker in combination with a CCB or a thiazide-like diuretic is recommended for stroke prevention | |
| Immediate BP lowering should be considered intravenously to reach a target of SBP<180mmHg in people with acute intracerebral hemorrhage and SBP ≥ 220mmHg. | |
| BP should be lowered to <180/105mmHg in people with acute ischemic stroke who are eligible for IV thrombolysis. BP should be maintained in this range in the first 24 hours after thrombolysis. | |
| The SBP of people who suffered a transient ischemic attack or an ischemic stroke should be maintained at 120–130 mmHg. | |
| Antihypertensive medication may be considered to reduce BP during the first 24 hours after stroke onset in people with high BP not receiving fibrinolysis. | |
| Acute intravenous BP lowering is not recommended in people with acute intracerebral hemorrhage and SBP <220mmHg. | |
| Routine BP lowering is not recommended in people with acute ischemic stroke. |
3.7.7. Atrial fibrillation
People suffering from hypertension have a high predisposition to cardiac arrythmias, especially atrial fibrillation (AF) [125]. A higher risk of AF is even observed with elevated blood pressure levels and stage 1 hypertension [125–127]. Seeing as people with HF most frequently also suffer from hypertension and have a higher risk of stroke and heart failure, prevention with oral anticoagulation is necessary in addition to risk monitoring and bleeding prevention [125,128,129].
It is clear that antihypertensive therapy prevents new-onset HF in people with hypertension regardless of treatment modality [130]. Beta blockers or non-dihydropyridine CCBs are recommended in people with AF and high ventricular rate [128]. Beta blockers are preferred in people with reduced left ventricular systolic function considering the association of non-dihydropyridine with heart failure in these patients [128]. ACEis, ARBs, betablockers, and to a lesser extent, CCBs, have all been associated with a decreased risk of HF development [131]. ACEI/ARB therapy can reduce the occurrence of mortality and major adverse events in people with hypertension and AF [132] (Table 17).
Table 17.
Recommendations for the management of hypertension with atrial fibrillation. AF: atrial fibrillation; CCB = calcium channel blocker; CHA2DS2-VASc = Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, Stroke, Vascular disease, Age 65–74 years, Sex category (female).
| Class | Recommendation |
|---|---|
| It is recommended that people with AF are screened for hypertension. | |
| Oral anticoagulation is recommended for stroke prevention in people with concomitant AF and hypertension, and a CHA2DS2-VASc score of ≥2 in men and ≥3 in women. | |
| Hypertensive management should include a beta-blocker or non- hydropyridine CCB in case rate control is needed. |
3.7.8. Pregnancy
BP-lowering treatment threshold is higher for women who are pregnancy compared to the general population. In general, antihypertensive drugs are recommended when SBP is ≥ 150 mmHg or DBP ≥95 mmHg, with the exception of gestational hypertension alone or with pre-existing hypertension, or with subclinical or symptomatic organ damage where treatment is warranted at a lower threshold (SBP ≥140 mmHg or DBP ≥90 mmHg). Due to adverse fetal and neonatal outcomes, ACEis, ARBs, or direct renin inhibitors are not recommended during pregnancy. Treatment of choice for non-severe hypertension during pregnancy includes methyldopa, labetalol, and CCBs. These drugs have been shown to reduce the risk of severe hypertension and possibly lower the risk of adverse pregnancy outcomes [133]. As for severe hypertension, nifedipine, hydralazine, labetalol and methyldopa have been shown to be effective [134,135]. Hydralazine may be considered in the failure of other BP-lowering drugs. A pregnant woman should be admitted to the hospital in case of hypertensive emergency (SBP is ≥ 170 mmHg or DBP ≥110 mmHg). Intravenous (IV) labetalol or nicardipine and magnesium sulphate are recommended for hypertensive crisis in pregnant women [136]. IV nitroglycerin is recommended for pre-eclampsia associated with pulmonary edema. Earlier delivery is recommended in case of gestational hypertension or mild pre-eclampsia (37 weeks), particularly in case of pre-eclampsia with adverse conditions. Labetalol, methyldopa, long-acting nifedipine, enalapril, or captopril are believed to be safe in women who are lactating (Table 18).
Table 18.
Recommendations for the management of hypertension in pregnancy. ACEi: Angiotensin-converting enzyme inhibitor; ARB: Angiotensinreceptor blocker; BP: blood pressure; CCB: calcium channel blockers; DBP: diastolic blood pressure; IV: intravenous; RAS: renin-angiotensin system; SBP: systolic blood pressure.
| Class | Recommendation |
|---|---|
BP-lowering medication are recommended if:
|
|
| Treatment of choice during pregnancy includes methyldopa, labetalol, and CCBs. | |
| A pregnant woman should be admitted to the hospital in case of hypertensive emergency (SBP is ≥170 mmHg or DBP ≥110 mmHg) | |
| IV labetalol or nicardipine and magnesium are recommended for hypertensive crisis in pregnant women. | |
| IV labetalol, oral methyldopa, or nifedipine is recommended in severe hypertension | |
| IV nitroglycerin is recommended for pre-eclampsia associated with pulmonary oedema. | |
| Earlier delivery is recommended in case of gestational hypertension or mild pre-eclampsia (37 weeks), particularly in case of pre-eclampsia with adverse conditions. | |
| Labetalol, methyldopa, long-acting nifedipine, enalapril, or captopril can be used in women who are lactating. | |
| ACEis, ARBs, or direct renin inhibitors are not recommended during pregnancy. |
3.7.9. Older age (>65 years old)
Age should not be a barrier for treatment. Evidence from randomized controlled trials and meta-analyses has shown that in old and very old people, antihypertensive treatment substantially reduces CV morbidity (such as heart failure and stroke), and also mortality (CV and all-cause) [137–139]. This benefit persists even in older people who are frail [140]. Regardless, geriatric treatment should be personalized and drug therapy should only be prescribed as tolerated and at the lowest possible doses. If tolerated, a treatment target of <130 mmHg is reasonable for greater benefit on the level of cardiovascular outcomes in older people [139]. Combination therapy might not be favorable in very old people. Unless indicated, some medication should be avoided due to their association with injurious falls. These include loop diuretics and alpha blockers [141]. Whatever the drug choice, old and very old people should be closely monitored for any notable changes in BP (such as postural BP and hypotensive episodes), in addition to regularly assessing renal function (Table 19).
Table 19.
Recommendations for the management of hypertension in older age (>65 years old). BP: blood pressure.
| Class | Recommendation |
|---|---|
| It is recommended that combination therapy is used at the lowest available doses in all older people. | |
| A treatment target of SBP <130 mmHg is recommended for hypertension in older age. | |
| Drugs associated with injurious falls, such as loop diuretics and alpha blockers, should be avoided unless indicated for comorbidities. | |
| Older people should be closely monitored for postural BP and hypotensive episodes, especially if very old or frail. Monitoring should also include renal function assessments. | |
| It may be appropriate to treat very old people (≥80 years) with monotherapy. |
3.7.10. Hypertensive emergencies
A hypertensive crisis denotes severe elevations of blood pressure (>180/120 mm Hg) associated with acute target organ damage. Severe hypertension that is not associated with target organ damage constitutes a ‘hypertension urgency’ and does not necessitate admission to the hospital. Hypertensive crises are often life-threatening and require immediate hospital admission and lowering of blood pressure with intravenous therapy. The extent of organ damage can be established both by the rate of BP increase and the absolute level of BP increase. Hypertensive crises and the ensuing target organ can typically present as acute aortic dissection, acute myocardial ischemia, or acute heart failure, all of which require urgent and immediate SBP lowering. Other typical examples of hypertensive crisis include sudden severe hypertension due to pheochromocytoma and pre-eclampsia. For the management of pregnant women with severe hypertension or preeclampsia, see section 3.7.8.
In general, use of oral medication is not advised and people with hypertensive crisis should be managed with intravenous blood lowering therapy (Table 20). There is a lack of evidence from randomized controlled trials on optimal therapeutic strategies in hypertensive emergencies. Recommendations are primarily based on clinical experience. Conditions such as malignant hypertension with or without acute renal failure do not require acute blood pressure lowering. Mean arterial pressure (MAP) should instead be reduced by 20–25% over the course of several hours. Compelling conditions such as an acute coronary event, acute cardiogenic pulmonary oedema, acute aortic dissection and hypertensive encephalopathy necessitate the immediate reduction of BP in the first hour of treatment. In people with acute aortic disease (dissection or rupture), it is recommended to reduce both systolic BP to <120 mmHg and heart rate <60 beats per minute to limit aortic wall stress and disease progression. First line of treatment is Betablockers. Safe and controlled BP reduction remains the foremost concern of hypertensive crisis management. As such, it is recommended to adhere by the timescale and magnitude of BP lowering advised for each type of hypertensive emergency. Moreover, it is important to limit therapy choice to drugs with a short half-life allowing the careful titration of pharmacological BP lowering.
Table 20.
Recommendations for the management of hypertensive emergencies. MAP: mean arterial pressure; SBP: systolic blood pressure.
| Class | Recommendation |
|---|---|
| in the event of a compelling hypertensive emergency, it is recommended to admit the patient to the intensive care unit and immediately reduce blood pressure with intravenous drug therapy (except for malignant hypertension with or without acute renal failure). | |
It is recommended to immediately reduce SBP to < 140mmHg with intravenous drug therapy in the event of:
|
|
| It is recommended to immediately reduce SBP to < 120mmHg with intravenous drug therapy in addition to reducing heart rate to <60 beats/min in the event of acute aortic dissection. | |
| It is recommended to immediately reduce MAP by 20–25% with intravenous drug therapy in the case of hypertensive encephalopathy. | |
| It is recommended to reduce MAP by 20–25% over several hours in the case of malignant hypertension with or without acute renal failure. |
4. Conclusions
Almost 30% of the Saudi population suffers from hypertension. While most people receive pharmacological therapy, many remain poorly controlled. Evidence-based recommendations are therefore provided to improve hypertension management and treatment outcomes. Non-pharmacological treatment consisting of lifestyle modification and risk factor management is critical for BP control. This should be supplemented with pharmacological therapies only when needed and in appropriate patient groups. Special care should be exercised to individualize hypertension treatment in such a way as to maximize patient adherence, quality of life and treatment outcomes, taking into consideration any conditions/comorbidities that may be relevant.
Acknowledgments
The authors also thank Konoz Retaj, Saudi Arabia and Nancy Al Akkary MSc, BSc, for providing editorial and medical writing assistance for the preparation of this manuscript. This medical writing fee was funded by Hikma.
Abbreviations list
- ABPM
ambulatory blood pressure monitoring
- ACEi
Angiotensin-converting enzyme inhibitor
- AF
Atrial fibrillation
- ARB
Angiotensin-receptor blocker
- BP
blood pressure
- CAD
coronary artery disease
- CCB
calcium channel blockers
- CV
cardiovascular
- DBP
diastolic blood pressure
- HBPM
home blood pressure monitoring
- HMOD
hypertension-mediated organ damage
- IV
intravenous
- MAP
mean arterial pressure
- RAS
renin-angiotensin system
- SBP
systolic blood pressure
- SHA
Saudi Heart Association
Appendix A.
The RIGHT checklist can be found in the following link https://www.j-saudi-heart.com/cgi/editor.cgi?article=1328&window=additional_files&context=jsha.
Funding Statement
This work was supported by Hikma under PO/Grant number [20210008].
Footnotes
Conflict of interest
None declared.
Author contribution statement
Conception and design of Study: WA; Literature review: WA, AAT, MA, MA, MAB, AA, AE, HE, TH, OA; Acquisition of data: WA; Drafting of manuscript: WA, AAT, MA, MA, MAB, AA, AE, HE, TH, OA; Revising and editing the manuscript critically for important intellectual contents: WA, AAT, MA, MA, MAB, AA, AE, HE, TH, OA; Supervision of the research: WA; Research coordination and management: WA; Funding for the research: WA.
Disclosure of funding
This work was supported by Hikma under PO/Grant number [20210008].
References
- 1. Zhou B, Carrillo-Larco RM, Danaei G, Riley LM, Paciorek CJ, Stevens GA, et al. Worldwide trends in hypertension prevalence and progress in treatment and control from1990 to 2019: a pooled analysis of 1201 population-representative studies with 104million participants. Lancet. 2021;398:957–80. doi: 10.1016/S0140-6736(21)01330-1/ATTACHMENT/647D2630-0ABA-4985-B5C7-D4CA0A7F09E4/MMC1.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou B, Perel P, Mensah GA, Ezzati M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat Rev Cardiol. 2021;18:785–802. doi: 10.1038/s41569-021-00559-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Okati-Aliabad H, Ansari-Moghaddam A, Kargar S, Mohammadi M. Prevalence of hypertension and pre-hypertension in the Middle East region: a systematic review & meta-analysis. J Hum Hypertens. 2022 doi: 10.1038/S41371-021-00647-9. [DOI] [PubMed] [Google Scholar]
- 4. Arafah M, Al-Hinai AT, Al Mahmeed W, Al-Rasadi K, AlTamimi O, AlHerz S, et al. Centralized pan-Middle East Survey on the undertreatment of hypercholesterolemia: results from theCEPHEUS study in ArabianGulf countries. Angiology. 2014;65:919–26. doi: 10.1177/0003319713512414. [DOI] [PubMed] [Google Scholar]
- 5. Yusufali AM, Khatib R, Islam S, Alhabib KF, Bahonar A, Swidan HM, et al. Prevalence, awareness, treatment and control of hypertension in four Middle East countries. J Hypertens. 2017;35:1457–64. doi: 10.1097/HJH.0000000000001326. [DOI] [PubMed] [Google Scholar]
- 6. Saeed AA, Al-Hamdan NA, Bahnassy AA, Abdalla AM, Abbas MAF, Abuzaid LZ. Prevalence, awareness, treatment, and control of hypertension among Saudi adult population: a national survey. Int J Hypertens. 2011;2011:1–8. doi: 10.4061/2011/174135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aljuraiban GS, Al Slail FY, Aldhwailea SK, Badawi AA, Beaney T, Clarke J, et al. May Measurement Month 2019: an analysis of blood pressure screening results from Saudi Arabia. Eur Heart J Suppl. 2021;23:B128–30. doi: 10.1093/eurheartj/suab029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khafaji MA, Al Ghalayini KW, Sait MK, Alorri RA, Garoub T, Alharbi EA, et al. Prevalence of diabetes and hypertension among king abdulaziz university employees: data from first aid and cardiopulmonary resuscitation training program. Cureus. 2021:13. doi: 10.7759/CUREUS.20097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. El Bcheraoui C, Tuffaha M, Daoud F, Kravitz H, AlMazroa MA, Al Saeedi M, et al. Access and barriers to healthcare in the Kingdom of Saudi Arabia, 2013: findings from a national multistage survey. BMJ Open. 2015;5 doi: 10.1136/BMJOPEN-2015-007801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Al-Raddadi R, Al-Ahmadi J, Bahijri S, Ajabnoor GM, Jambi H, Enani S, et al. Gender differences in the factors associated with hypertension in non-diabetic Saudi adults-A cross-sectional study. Int J Environ Res Publ Health. 2021:18. doi: 10.3390/IJERPH182111371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aldiab A, Shubair MM, Al-Zahrani JM, Aldossari KK, Al-Ghamdi S, Househ M, et al. Prevalence of hypertension and prehypertension and its associated cardioembolic risk factors; a population based cross-sectional study in Alkharj, Saudi Arabia. BMC Publ Health. 2018;18:1327. doi: 10.1186/s12889-018-6216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Ashker S, Pednekar MS, Narake SS, Albaker W, Al-Hariri M.Blood pressure and cardio-metabolic risk profile in young Saudimales in a university setting. Medicina (Kaunas) 2021. p. 57. [DOI] [PMC free article] [PubMed]
- 13. El Bcheraoui C, Memish ZA, Tuffaha M, Daoud F, Robinson M, Jaber S, et al. Hypertension and its associated risk factors in the kingdom of Saudi Arabia, 2013 a national survey. Int J Hypertens. 20142014 doi: 10.1155/2014/564679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gutierrez J, Alloubani A, Mari M, Alzaatreh M. Cardiovascular disease risk factors: hypertension, diabetes mellitus and obesity among tabuk citizens in Saudi arabia. Open Cardiovasc Med J. 2018;12:41–9. doi: 10.2174/1874192401812010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Muntner P, Einhorn PT, Cushman WC, Whelton PK, Bello NA, Drawz PE, et al. Blood pressure assessment in adults in clinical practice and clinic-based research: JACC scientific expert panel. J Am Coll Cardiol. 2019;73:317–35. doi: 10.1016/J.JACC.2018.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stergiou GS, Palatini P, Parati G, O’Brien E, Januszewicz A, Lurbe E, et al. European Society of Hypertension practice guidelines for office and out-of-office blood pressure measurement. J Hypertens. 2021;39:1293–302. doi: 10.1097/HJH.0000000000002843.2021. [DOI] [PubMed] [Google Scholar]
- 17. Ahmad FS, Chan C, Rosenman MB, Post WS, Fort DG, Greenland P, et al. Validity of cardiovascular data from electronic sources: the multi-ethnic study of atherosclerosis and HealthLNK. Circulation. 2017;136:1207–16. doi: 10.1161/CIRCULATIONAHA.117.027436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sakhuja S, Jaeger BC, Akinyelure OP, Bress AP, Shimbo D, Schwartz JE, et al. Potential impact of systematic and random errors in blood pressure measurement on the prevalence of high office blood pressure in the United States. J Clin Hypertens. 2022;24:263–70. doi: 10.1111/JCH.14418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ward AM, Takahashi O, Stevens R, Heneghan C. Home measurement of blood pressure and cardiovascular disease: systematic review and meta-analysis of prospective studies. J Hypertens. 2012;30:449–56. doi: 10.1097/HJH.0B013E32834E4AED. [DOI] [PubMed] [Google Scholar]
- 20. McManus RJ, Mant J, Bray EP, Holder R, Jones MI, Greenfield S, et al. Telemonitoring and self-management in the control of hypertension (TASMINH2): a randomised controlled trial. Lancet (London, England) 2010;376:163–72. doi: 10.1016/S0140-6736(10)60964-6. [DOI] [PubMed] [Google Scholar]
- 21. McManus RJ, Mant J, Haque MS, Bray EP, Bryan S, Greenfield SM, et al. Effect of self-monitoring and medication self-titration on systolic blood pressure in hypertensive patients at high risk of cardiovascular disease: the TASMIN-SR randomized clinical trial. JAMA. 2014;312:799–808. doi: 10.1001/JAMA.2014.10057. [DOI] [PubMed] [Google Scholar]
- 22. Nessler K, Krztoń-Królewiecka A, Suska A, Mann MR, Nessler MB, Windak A. The quality of patients’ self-blood pressure measurements: a cross-sectional study. BMC Cardiovasc Disord. 2021:21. doi: 10.1186/S12872-021-02351-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Roush GC, Fagard RH, Salles GF, Pierdomenico SD, Reboldi G, Verdecchiaf P, et al. Prognostic impact fromclinic, daytime, and night-time systolic blood pressure in nine cohorts of 13,844 patients with hypertension. J Hypertens. 2014;32:2332–40. doi: 10.1097/HJH.0000000000000355. [DOI] [PubMed] [Google Scholar]
- 24. Parati G, Ochoa JE, Bilo G, Agarwal R, Covic A, Dekker FW, et al. Hypertension in chronic kidney disease Part 2: role of ambulatory and home blood pressure monitoring for assessing alterations in blood pressure variability and blood pressure profiles. Hypertens (Dallas, Tex 1979) 2016;67:1102–10. doi: 10.1161/HYPERTENSIONAHA.115.06896. [DOI] [PubMed] [Google Scholar]
- 25. Piper MA, Evans CV, Burda BU, Margolis KL, O’Connor E, Whitlock EP. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162:192–204. doi: 10.7326/M14-1539. [DOI] [PubMed] [Google Scholar]
- 26. Huang QF, Yang WY, Asayama K, Zhang ZY, Thijs L, Li Y, et al. Ambulatory blood pressure monitoring to diagnose and manage hypertension. Hypertension. 2021;77:254–64. doi: 10.1161/HYPERTENSIONAHA.120.14591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Viera AJ, Yano Y, Lin F-C, Simel DL, Yun J, Dave G, et al. Does this adult patient have hypertension? JAMA. 2021;326:339. doi: 10.1001/jama.2021.4533. [DOI] [PubMed] [Google Scholar]
- 28. Siu AL. Screening for high blood pressure in adults: U.S. Preventive services task force recommendation statement. Ann Intern Med. 2015;163:778–86. doi: 10.7326/M15-2223. [DOI] [PubMed] [Google Scholar]
- 29. Ostchega Y, Fryar CD, Nwankwo T, Nguyen DT. Hypertension prevalence among adults aged 18 and over: United States, 2017-2018. NCHS Data Brief. 2020;364:1–8. [PubMed] [Google Scholar]
- 30.United States Preventive Services Taskforce. Procedure manual. 2018. [Accessed 3 July 2022]. https://uspreventiveservicestaskforce.org/uspstf/about-uspstf/methods-and-processes/procedure-manual .
- 31. Alhabib KF, Batais MA, Almigbal TH, Alshamiri MQ, Altaradi H, Rangarajan S, et al. Demographic, behavioral, and cardiovascular disease risk factors in the Saudi population: results from the Prospective Urban Rural Epidemiology study (PURE-Saudi) BMC Publ Health. 2020;20:1–14. doi: 10.1186/S12889-020-09298-W/FIGURES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Al-Rubeaan K, Bawazeer N, AlFarsi Y, Youssef AM, Al-Yahya AA, AlQumaidi H, et al. Prevalence of metabolic syndrome in Saudi Arabia - a cross sectional study. BMC Endocr Disord. 2018;18 doi: 10.1186/S12902-018-0244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shin S. Prevalence of metabolic syndrome in Saudi Arabia: a meta-analysis of cross-sectional studies. 2020 [Google Scholar]
- 34. Alrasheed AA. Dyslipidemia among patients with type 1 diabetes and its associated factors in Saudi arabia: an analytical cross-sectional study. Cureus. 2022:14. doi: 10.7759/CUREUS.21923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alsheikh-Ali AA, Omar MI, Raal FJ, Rashed W, Hamoui O, Kane A, et al. Cardiovascular risk factor burden in africa and the Middle East: the africa Middle East cardiovascular epidemiological (ACE) study. PLoS One. 2014;9:e102830. doi: 10.1371/JOURNAL.PONE.0102830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yagoub Id U, Saiyed NS, Al Qahtani B, Mohammed A, Zahrani A, Birema Y, et al. Investigating the incidence and risk factors of hypertension: a multicentre retrospective cohort study in Tabuk, Saudi Arabia. PLoS One. 2022;17:e0262259. doi: 10.1371/JOURNAL.PONE.0262259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hua K, Hao G, Li W. Cardiovascular outcomes of lifestyle intervention in hypertensive patients with antihypertensive agents. Int J Cardiol. 2017;227:751–6. doi: 10.1016/J.IJCARD.2016.10.062. [DOI] [PubMed] [Google Scholar]
- 38. Treciokiene I, Postma M, Nguyen T, Fens T, Petkevicius J, Kubilius R, et al. Healthcare professional-led interventions on lifestyle modifications for hypertensive patients–asystematic review and meta-analysis. BMC Fam Pract. 2021;22:1–15. doi: 10.1186/S12875-021-01421-Z/TABLES/4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang M, Du X, Huang W, Xu Y. Ultra-processed foods consumption increases the risk of hypertension in adults: a systematic review and meta-analysis. Am J Hypertens. 2022;35:892–901. doi: 10.1093/ajh/hpac069. [DOI] [PubMed] [Google Scholar]
- 40. Elliott P, Stamler J, Nichols R, Dyer AR, Stamler R, Kesteloot H, et al. Intersalt revisited: further analyses of 24 hour sodium excretion and blood pressure within and across populations. Intersalt Cooperative Research Group. BMJ. 1996;312:1249–53. doi: 10.1136/BMJ.312.7041.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. He FJ, Li J, Macgregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev. 20132013 doi: 10.1002/14651858.CD004937.PUB2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dickinson HO, Mason JM, Nicolson DJ, Campbell F, Beyer FR, Cook JV, et al. Lifestyle interventions to reduce raised blood pressure: a systematic review of randomized controlled trials. J Hypertens. 2006;24:215–23. doi: 10.1097/01.HJH.0000199800.72563.26. [DOI] [PubMed] [Google Scholar]
- 43. Mente A, De Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169:659–69. doi: 10.1001/ARCHINTERNMED.2009.38. [DOI] [PubMed] [Google Scholar]
- 44. Estruch R, Ros E, Salas-Salvadó J, Covas M-I, Corella D, Arós F, et al. Primary prevention of cardiovascular disease with a mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378:e34. doi: 10.1056/NEJMOA1800389. [DOI] [PubMed] [Google Scholar]
- 45. Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure: a meta--analysis of randomized controlled trials. Hypertens (Dallas, Tex 1979) 2003;42:878–84. doi: 10.1161/01.HYP.0000094221.86888.AE. [DOI] [PubMed] [Google Scholar]
- 46. Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309:71–82. doi: 10.1001/JAMA.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–9. doi: 10.1056/NEJMOA1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Leitzmann MF, Park Y, Blair A, Ballard-Barbash R, Mouw T, Hollenbeck AR, et al. Physical activity recommendations and decreased risk of mortality. Arch Intern Med. 2007;167:2453–60. doi: 10.1001/ARCHINTE.167.22.2453. [DOI] [PubMed] [Google Scholar]
- 49. Penninkilampi R, Eslick EM, Eslick GD. The association between consistent licorice ingestion, hypertension and hypokalaemia: a systematic review and meta-analysis. J Hum Hypertens. 2017;31:699–707. doi: 10.1038/JHH.2017.45. [DOI] [PubMed] [Google Scholar]
- 50. Cohen L, Curhan G, Forman J. Association of sweetened beverage intakewith incident hypertension. J Gen InternMed. 2012;27:1127. doi: 10.1007/S11606-012-2069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brown IJ, Stamler J, Van Horn L, Robertson CE, Chan Q, Dyer AR, et al. Sugar-sweetened beverage, sugar intake of individuals, and their blood pressure: international study of macro/micronutrients and blood pressure. Hypertension. 2011;57:695–701. doi: 10.1161/HYPERTENSIONAHA.110.165456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Parati G, Kjeldsen S, Coca A, Cushman WC, Wang J. Adherence to single-pill versus free-equivalent combination therapy in hypertension: a systematic review and metaanalysis. Hypertens (Dallas, Tex 1979) 2021;77:692–705. doi: 10.1161/HYPERTENSIONAHA.120.15781. [DOI] [PubMed] [Google Scholar]
- 53. Wright JM, Musini VM, Gill R. First-line drugs for hypertension. Cochrane Database Syst Rev. 2018;4 doi: 10.1002/14651858.CD001841.PUB3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhu J, Chen N, Zhou M, Guo J, Zhu C, Zhou J, et al. Calcium channel blockers versus other classes of drugs for hypertension. Cochrane Database Syst Rev. 2021:10. doi: 10.1002/14651858.CD003654.PUB5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang JG, Zhang M, Feng YQ, Ma CS, Wang TD, Zhu ZM, et al. Is the newest angiotensin-receptor blocker azilsartan medoxomil more efficacious in lowering blood pressure than the older ones? A systematic review and network meta-analysis. J Clin Hypertens. 2021;23:901–14. doi: 10.1111/JCH.14227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Katsi V, Michalakeas C, Soulaidopoulos S, Antonopoulos AS, Vlachopoulos C, Tousoulis D, et al. Evaluating the safety and tolerability of azilsartan medoxomil alone or in combination with chlorthalidone in the management of hypertension: a systematic review. Curr Hypertens Rev. 2021;17:217–27. doi: 10.2174/1573402117666210112144505. [DOI] [PubMed] [Google Scholar]
- 57. Khan N, McAlister FA. Re-examining the efficacy of βblockers for the treatment of hypertension: a meta-analysis. Can Med Assoc J. 2006;174:1737. doi: 10.1503/CMAJ.060110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Brugts JJ, Bertrand M, Remme W, Ferrari R, Fox K, MacMahon S, et al. The treatment effect of an ACE-inhibitor based regimen with perindopril in relation to beta-blocker use in 29,463 patients with vascular disease: a combined analysis of individual data of ADVANCE, EUROPA and PROGRESS trials. Cardiovasc Drugs Ther. 2017;31:391. doi: 10.1007/S10557-017-6747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Peyracchia M, Errigo D, Rubin SR, Conrotto F, Dinicolantonio JJ, Omede P, et al. Beta-blocker therapy reduces mortality in patients with coronary artery disease treated with percutaneous revascularization: a meta-analysis of adjusted results. J Cardiovasc Med. 2018;19:337–43. doi: 10.2459/JCM.0000000000000662. [DOI] [PubMed] [Google Scholar]
- 60. Zhang X, Shen C, Zhai S, Liu Y, Yue WW, Han L. A metaanalysis of the effects of β-adrenergic blockers in chronic heart failure. Exp Ther Med. 2016;12:2489–96. doi: 10.3892/ETM.2016.3657/DOWNLOAD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chatterjee S, Biondi-Zoccai G, Abbate A, D’Ascenzo F, Castagno D, Van Tassell B, et al. Benefits of β blockers in patients with heart failure and reduced ejection fraction: network meta-analysis. BMJ. 2013;346 doi: 10.1136/BMJ.F55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kotecha D, Manzano L, Krum H, Rosano G, Holmes J, Altman DG, et al. Effect of age and sex on efficacy and tolerability of β blockers in patients with heart failure with reduced ejection fraction: individual patient data metaanalysis. BMJ. 2016;353 doi: 10.1136/BMJ.I1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ziff OJ, Samra M, Howard JP, Bromage DI, Ruschitzka F, Francis DP, et al. Beta-blocker efficacy across different cardiovascular indications: an umbrella review and meta-analytic assessment. BMC Med. 2020;18:1–11. doi: 10.1186/S12916-020-01564-3/FIGURES/5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Owen JG, Reisin E. Anti-hypertensive drug treatment of patients with and the metabolic syndrome and obesity: a review of evidence, meta-analysis, post hoc and guidelines publications. Curr Hypertens Rep. 2015;17 doi: 10.1007/S11906-015-0558-9. [DOI] [PubMed] [Google Scholar]
- 65. Wiysonge CS, Bradley HA, Volmink J, Mayosi BM, Opie LH. Beta-blockers for hypertension. Cochrane Database Syst Rev. 2017;1 doi: 10.1002/14651858.CD002003.PUB5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. You SC, Krumholz HM, Suchard MA, Schuemie MJ, Hripcsak G, Chen RJ, et al. Comprehensive comparative effectiveness and safety of first-line β-blocker monotherapy in hypertensive patients: a large-scale multicenter observational study. Hypertension. 2021;77:1528–38. doi: 10.1161/HYPERTENSIONAHA.120.16402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Liu JY, Guo LN, Peng WZ, Jiang Y, Wang AL, Guo XM, et al. Efficacy and safety of nebivolol in hypertensive patients: a meta-analysis of randomized controlled trials. J Int Med Res. 2020:48. doi: 10.1177/0300060520931625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Noubiap JJ, Nansseu JR, Nyaga UF, Sime PS, Francis I, Bigna JJ. Global prevalence of resistant hypertension: a meta-analysis of data from 3.2 million patients. Heart. 2019;105:98–105. doi: 10.1136/HEARTJNL-2018-313599. [DOI] [PubMed] [Google Scholar]
- 69. Blumenthal JA, Hinderliter AL, Smith PJ, Mabe S, Watkins LL, Craighead L, et al. Effects of lifestyle modification on patients with resistant hypertension: results of the TRIUMPH randomized clinical trial. Circulation. 2021;144:1212–26. doi: 10.1161/CIRCULATIONAHA.121.055329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Carey RM, Calhoun DA, Bakris GL, Brook RD, Daugherty SL, Dennison-Himmelfarb CR, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American heart association. Hypertens (Dallas, Tex 1979) 2018;72:E53–90. doi: 10.1161/HYP.0000000000000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chen C, Zhu XY, Li D, Lin Q, Zhou K. Clinical efficacy and safety of spironolactone in patients with resistant hypertension: a systematic review and meta-analysis. Medicine (Baltim) 2020;99:e21694. doi: 10.1097/MD.0000000000021694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Williams B, MacDonald TM, Morant SV, Webb DJ, Sever P, McInnes GT, et al. Endocrine and haemodynamic changes in resistant hypertension, and blood pressure responses to spironolactone or amiloride: the PATHWAY-2 mechanisms substudies. Lancet Diabetes Endocrinol. 2018;6:464–75. doi: 10.1016/S2213-8587(18)30071-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Williams B, Macdonald TM, Morant S, Webb DJ, Sever P, McInnes G, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet (London, England) 2015;386:2059–68. doi: 10.1016/S0140-6736(15)00257-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–401. doi: 10.1056/NEJMOA1402670. [DOI] [PubMed] [Google Scholar]
- 75. Kario K, Ogawa H, Okumura K, Okura T, Saito S, Ueno T, et al. SYMPLICITY HTN-Japan - first randomized controlled trial of catheter-based renal denervation in asian patients - Circ J. 2015;79:1222–9. doi: 10.1253/CIRCJ.CJ-15-0150. [DOI] [PubMed] [Google Scholar]
- 76. Bakris GL, Townsend RR, Flack JM, Brar S, Cohen SA, D’Agostino R, et al. 12-month blood pressure results of catheter-based renal artery denervation for resistant hypertension: the SYMPLICITY HTN-3 trial. J Am Coll Cardiol. 2015;65:1314–21. doi: 10.1016/J.JACC.2015.01.037. [DOI] [PubMed] [Google Scholar]
- 77. Kario K, Yokoi Y, Okamura K, Fujihara M, Ogoyama Y, Yamamoto E, et al. Catheter-based ultrasound renal denervation in patients with resistant hypertension: the randomized, controlled REQUIRE trial. Hypertens Res. 2022;45:221–31. doi: 10.1038/S41440-021-00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Desch S, Okon T, Heinemann D, Kulle K, Röhnert K, Sonnabend M, et al. Randomized sham-controlled trial of renal sympathetic denervation in mild resistant hypertension. Hypertension. 2015;65:1202–8. doi: 10.1161/HYPERTENSIONAHA.115.05283. [DOI] [PubMed] [Google Scholar]
- 79. Mathiassen ON, Vase H, Bech JN, Christensen KL, Buus NH, Schroeder AP, et al. Renal denervation in treatment-resistant essential hypertension. A randomized, SHAM-controlled, double-blinded 24-h blood pressure-based trial. J Hypertens. 2016;34:1639–47. doi: 10.1097/HJH.0000000000000977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mahfoud F, Böhm M, Schmieder R, Narkiewicz K, Ewen S, Ruilope L, et al. Effects of renal denervation on kidney function and long-term outcomes: 3-year follow-up from the Global SYMPLICITY Registry. Eur Heart J. 2019;40:3474–82. doi: 10.1093/EURHEARTJ/EHZ118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Böhm M, Mahfoud F, Ukena C, Hoppe UC, Narkiewicz K, Negoita M, et al. First report of the Global SYMPLICITY Registry on the effect of renal artery denervation in patients with uncontrolled hypertension. Hypertens (Dallas, Tex 1979) 2015;65:766–74. doi: 10.1161/HYPERTENSIONAHA.114.05010. [DOI] [PubMed] [Google Scholar]
- 82. Rodríguez-Leor O, Jaén-Águila F, Segura J, Núñez-Gil IJ, García-Touchard A, Rubio E, et al. Renal denervation for the management of hypertension. Joint position statement from the SEH-LELHA and the ACI-SEC. REC Interv Cardiol. 2022;4:39–46. doi: 10.24875/RECICE.M21000235. [DOI] [Google Scholar]
- 83. Ogoyama Y, Tada K, Abe M, Nanto S, Shibata H, Mukoyama M, et al. Effects of renal denervation on blood pressures in patients with hypertension: a systematic review and meta-analysis of randomized sham-controlled trials. Hypertens Res. 2022;45:210–20. doi: 10.1038/S41440-021-00761-8. [DOI] [PubMed] [Google Scholar]
- 84. Ahmad Y, Francis DP, Bhatt DL, Howard JP. Renal denervation for hypertension: a systematic review and metaanalysis of randomized, blinded, placebo-controlled trials. JACC Cardiovasc Interv. 2021;14:2614–24. doi: 10.1016/J.JCIN.2021.09.020. [DOI] [PubMed] [Google Scholar]
- 85. Yen FS, Wei JCC, Chiu LT, Hsu CC, Hwu CM. Diabetes, hypertension, and cardiovascular disease development. J Transl Med. 2022;20:1–12. doi: 10.1186/S12967-021-03217-2/FIGURES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018;34:575. doi: 10.1016/J.CJCA.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Thomopoulos C, Parati G, Zanchetti A. Effects of blood-pressure-lowering treatment on outcome incidence in hypertension: 10 - should blood pressure management differ in hypertensive patients with and without diabetes mellitus? Overview and meta-analyses of randomized trials. J Hypertens. 2017;35:922–44. doi: 10.1097/HJH.0000000000001276. [DOI] [PubMed] [Google Scholar]
- 88. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352 doi: 10.1136/BMJ.I717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bress AP, King JB, Kreider KE, Beddhu S, Simmons DL, Cheung AK, et al. Effect of intensive versus standard blood pressure treatment according to baseline prediabetes status: a post hoc analysis of a randomized trial. Diabetes Care. 2017;40:1401–8. doi: 10.2337/DC17-0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313:603–15. doi: 10.1001/JAMA.2014.18574. [DOI] [PubMed] [Google Scholar]
- 91. Cheng J, Zhang W, Zhang X, Han F, Li X, He X, et al. Effect of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on all-cause mortality, cardiovascular deaths, and cardiovascular events in patients with diabetes mellitus: a meta-analysis. JAMAIntern Med. 2014;174:773–85. doi: 10.1001/JAMAINTERNMED.2014.348. [DOI] [PubMed] [Google Scholar]
- 92. Hao G, Wang Z, Guo R, Chen Z, Wang X, Zhang L, et al. Effects of ACEI/ARB in hypertensive patients with type 2 diabetes mellitus: a meta-analysis of randomized controlled studies. BMC Cardiovasc Disord. 2014;14 doi: 10.1186/1471-2261-14-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Remonti LR, Dias S, Leitão CB, Kramer CK, Klassman LP, Welton NJ, et al. Classes of antihypertensive agents and mortality in hypertensive patients with type 2 diabetes-Networkmeta-analysis of randomizedtrials. J Diabet Complicat. 2016;30:1192–200. doi: 10.1016/J.JDIACOMP.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 94. Balamuthusamy S, Molnar J, Adigopula S, Arora R. Comparative analysis of beta-blockers with other antihypertensive agents on cardiovascular outcomes in hypertensive patients with diabetes mellitus: a systematic review and meta-analysis. Am J Therapeut. 2009;16:133–42. doi: 10.1097/MJT.0B013E31817FD87E. [DOI] [PubMed] [Google Scholar]
- 95. Wang G, Chen Y, Li L, Tang W, Wright JM. First-line reninangiotensin system inhibitors vs. other first-line antihypertensive drug classes in hypertensive patients with type 2 diabetes mellitus. J Hum Hypertens. 2018;32:494–506. doi: 10.1038/S41371-018-0066-X. [DOI] [PubMed] [Google Scholar]
- 96. Palmer SC, Mavridis D, Navarese E, Craig JC, Tonelli M, Salanti G, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet (London, England) 2015;385:2047–56. doi: 10.1016/S0140-6736(14)62459-4. [DOI] [PubMed] [Google Scholar]
- 97. Xie X, Liu Y, Perkovic V, Li X, Ninomiya T, Hou W, et al. Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: a bayesian network meta-analysis of randomized clinical trials. Am J Kidney Dis. 2016;67:728–41. doi: 10.1053/J.AJKD.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 98. Mishima E, Haruna Y, Arima H. Renin-angiotensin system inhibitors in hypertensive adults with non-diabetic CKD with or without proteinuria: a systematic review and metaanalysis of randomized trials. Hypertens Res. 2019;42:469–82. doi: 10.1038/S41440-018-0116-3. [DOI] [PubMed] [Google Scholar]
- 99. Wang K, Hu J, Luo T, Wang Y, Yang S, Qing H, et al. Effects of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on all-cause mortality and renal outcomes in patients with diabetes and albuminuria: a systematic review and meta-analysis. Kidney Blood Press Res. 2018;43:768–79. doi: 10.1159/000489913. [DOI] [PubMed] [Google Scholar]
- 100. Zhao HJ, Li Y, Liu SM, Sun XG, Li M, Hao Y, et al. Effect of calcium channels blockers and inhibitors of the reninangiotensin system on renal outcomes and mortality in patients suffering from chronic kidney disease: systematic review and meta-analysis. Ren Fail. 2016;38:849–56. doi: 10.3109/0886022X.2016.1165065. [DOI] [PubMed] [Google Scholar]
- 101. Tsai WC, Wu HY, Peng Y, Sen, Yang JY, Chen HY, Chiu YL, et al. Association of intensive blood pressure control and kidney disease progression in nondiabetic patients with chronic kidney disease: a systematic review and metaanalysis. JAMA Intern Med. 2017;177:792–9. doi: 10.1001/JAMAINTERNMED.2017.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, De Jong PE, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med. 2003;139 doi: 10.7326/0003-4819-139-4-200308190-00006. [DOI] [PubMed] [Google Scholar]
- 103. Sim JJ, Shi J, Kovesdy CP, Kalantar-Zadeh K, Jacobsen SJ. Impact of achieved blood pressures on mortality risk and end-stage renal disease among a large, diverse hypertension population. J Am Coll Cardiol. 2014;64:588–97. doi: 10.1016/J.JACC.2014.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Xie X, Atkins E, Lv J, Bennett A, Neal B, Ninomiya T, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet (London, England) 2016;387:435–43. doi: 10.1016/S0140-6736(15)00805-3. [DOI] [PubMed] [Google Scholar]
- 105. Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet (London, England) 2016;387:957–67. doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 106. Vidal-Petiot E, Ford I, Greenlaw N, Ferrari R, Fox KM, Tardif JC, et al. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet (London, England) 2016;388:2142–52. doi: 10.1016/S0140-6736(16)31326-5. [DOI] [PubMed] [Google Scholar]
- 107. Böhm M, Schumacher H, Teo KK, Lonn EM, Mahfoud F, Mann JFE, et al. Achieved blood pressure and cardiovascular outcomes in high-risk patients: results from ONTARGET and TRANSCEND trials. Lancet (London, England) 2017;389:2226–37. doi: 10.1016/S0140-6736(17)30754-7. [DOI] [PubMed] [Google Scholar]
- 108. Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:1245. doi: 10.1136/BMJ.B1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Jeffers BW, Robbins J, Bhambri R. Efficacy of calcium channel blockers versus other classes of antihypertensive medication in the treatment of hypertensive patients with previous stroke and/or coronary artery disease: a systematic review and meta-analysis. Am J Therapeut. 2017;24:e68–80. doi: 10.1097/MJT.0000000000000369. [DOI] [PubMed] [Google Scholar]
- 110. Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. Lancet (London, England) 2014;383:1899–911. doi: 10.1016/S0140-6736(14)60685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. JT W, JD W, PK W, JK S, KM S, MV R, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–16. doi: 10.1056/NEJMOA1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Furberg CD, Wright JT, Davis BR, Cutler JA, Alderman M, Black H, et al. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–97. doi: 10.1001/JAMA.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 113. Soliman EZ, Ambrosius WT, Cushman WC, Zhang ZM, Bates JT, Neyra JA, et al. Effect of intensive blood pressure lowering on left ventricular hypertrophy in patients with hypertension: SPRINT (systolic blood pressure intervention trial) Circulation. 2017;136:440–50. doi: 10.1161/CIRCULATIONAHA.117.028441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Fagard RH, Celis H, Thijs L, Wouters S. Regression of left ventricular mass by antihypertensive treatment: a metaanalysis of randomized comparative studies. Hypertens (Dallas, Tex 1979) 2009;54:1084–91. doi: 10.1161/HYPERTENSIONAHA.109.136655. [DOI] [PubMed] [Google Scholar]
- 115. Manning LS, Mistri AK, Potter J, Rothwell PM, Robinson TG. Short-term blood pressure variability in acute stroke: post hoc analysis of the controlling hypertension and hypotension immediately post stroke and continue or stop post-stroke antihypertensives collaborative study trials. Stroke. 2015;46:1518–24. doi: 10.1161/STROKEAHA.115.009078. [DOI] [PubMed] [Google Scholar]
- 116. Tsivgoulis G, Katsanos AH, Butcher KS, Boviatsis E, Triantafyllou N, Rizos I, et al. Intensive blood pressure reduction in acute intracerebral hemorrhage: a meta-analysis. Neurology. 2014;83:1523–9. doi: 10.1212/WNL.0000000000000917. [DOI] [PubMed] [Google Scholar]
- 117. Anderson CS, Heeley E, Huang Y, Wang J, Stapf C, Delcourt C, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368:2355–65. doi: 10.1056/NEJMOA1214609. [DOI] [PubMed] [Google Scholar]
- 118. Ahmed N, Wahlgren N, Brainin M, Castillo J, Ford GA, Kaste M, et al. Relationship of blood pressure, antihypertensive therapy, and outcome in ischemic stroke treated with intravenous thrombolysis: retrospective analysis from Safe Implementation of Thrombolysis in Stroke-International Stroke Thrombolysis Register (SITS-ISTR) Stroke. 2009;40:2442–9. doi: 10.1161/STROKEAHA.109.548602. [DOI] [PubMed] [Google Scholar]
- 119. Wu W, Huo X, Zhao X, Liao X, Wang C, Pan Y, et al. Relationship between blood pressure and outcomes in acute ischemic stroke patients administered lytic medication in the TIMS-China study. PLoS One. 2016;11 doi: 10.1371/JOURNAL.PONE.0144260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Lee M, Ovbiagele B, Hong KS, Wu YL, Lee JE, Rao NM, et al. Effect of blood pressure lowering in early ischemic stroke: meta-analysis. Stroke. 2015;46:1883–9. doi: 10.1161/STROKEAHA.115.009552. [DOI] [PubMed] [Google Scholar]
- 121. Sandset EC, Murray GD, Bath PMW, Kjeldsen SE, Berge E. Relation between change inbloodpressure in acute stroke and risk of early adverse events and poor outcome. Stroke. 2012;43:2108–14. doi: 10.1161/STROKEAHA.111.647362. [DOI] [PubMed] [Google Scholar]
- 122. Sandset EC, Bath PMW, Boysen G, Jatuzis D, Kõrv J, Lüders S, et al. The angiotensin-receptor blocker candesartan for treatment of acute stroke (SCAST): a randomised, placebo-controlled, double-blind trial. Lancet (London, England) 2011;377:741–50. doi: 10.1016/S0140-6736(11)60104-9. [DOI] [PubMed] [Google Scholar]
- 123. Jauch EC, Saver JL, Adams HP, Bruno A, Connors JJ, Buddy, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 124. Arima H, Chalmers J, Woodward M, Anderson C, Rodgers A, Davis S, et al. Lower target blood pressures are safe and effective for the prevention of recurrent stroke: the PROGRESS trial. J Hypertens. 2006;24:1201–8. doi: 10.1097/01.HJH.0000226212.34055.86. [DOI] [PubMed] [Google Scholar]
- 125. Verdecchia P, Angeli F, Reboldi G. Hypertension and atrial fibrillation. Circ Res. 2018;122:352–68. doi: 10.1161/CIRCRESAHA.117.311402. [DOI] [PubMed] [Google Scholar]
- 126. Conen D, Tedrow UB, Koplan BA, Glynn RJ, Buring JE, Albert CM. Influence of systolic and diastolic blood pressure on the risk of incident atrial fibrillation in women. Circulation. 2009;119:2146–52. doi: 10.1161/CIRCULATIONAHA.108.830042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Grundvold I, Skretteberg PT, Liestøl K, Erikssen G, Kjeldsen SE, Arnesen H, et al. Upper normal blood pressures predict incident atrial fibrillation in healthy middle-aged men: a 35-year follow-up study. Hypertens (Dallas, Tex 1979) 2012;59:198–204. doi: 10.1161/HYPERTENSIONAHA.111.179713. [DOI] [PubMed] [Google Scholar]
- 128. Freedman B, Potpara TS, Lip GYH. Stroke prevention in atrial fibrillation. Lancet (London, England) 2016;388:806–17. doi: 10.1016/S0140-6736(16)31257-0. [DOI] [PubMed] [Google Scholar]
- 129. Harskamp RE, Lucassen WAM, Lopes RD, Himmelreich JCL, Parati G, Weert HCPM. Risk of stroke and bleeding in relation to hypertension in anticoagulated patients with atrial fibrillation: a meta-analysis of randomised controlled trials. Acta Cardiol. 2022:77. doi: 10.1080/00015385.2021.1882111. [DOI] [PubMed] [Google Scholar]
- 130. Larstorp ACK, Stokke IM, Kjeldsen SE, Hecht Olsen M, Okin PM, Devereux RB, et al. Antihypertensive therapy prevents new-onset atrial fibrillation in patients with isolated systolic hypertension: the LIFE study. Blood Pres. 2019;28:317–26. doi: 10.1080/08037051.2019.1633905. [DOI] [PubMed] [Google Scholar]
- 131. Schaer BA, Schneider C, Jick SS, Conen D, Osswald S, Meier CR. Risk for incident atrial fibrillation in patients who receive antihypertensive drugs: a nested case-control study. Ann Intern Med. 2010;152:78–84. doi: 10.7326/0003-4819-152-2-201001190-00005. [DOI] [PubMed] [Google Scholar]
- 132. Xu W, Yang Y, Zhu J, Wu S, Wang J, Zhang H, et al. Impact of renin-angiotensin-aldosterone-system inhibitor drugs on mortality in patients with atrial fibrillation and hypertension. BMC Cardiovasc Disord. 2022;22:141. doi: 10.1186/S12872-022-02580-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Bone JN, Sandhu A, Abalos ED, Khalil A, Singer J, Prasad S, et al. Oral antihypertensives for nonsevere pregnancy hypertension: systematic review, network meta- and trial sequential analyses. Hypertens (Dallas, Tex 1979) 2022;79:614–28. doi: 10.1161/HYPERTENSIONAHA.121.18415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Easterling T, Mundle S, Bracken H, Parvekar S, Mool S, Magee LA, et al. Oral antihypertensive regimens (nifedipine retard, labetalol, and methyldopa) for management of severe hypertension in pregnancy: an open-label, randomised controlled trial. Lancet. 2019;394:1011–21. doi: 10.1016/S0140-6736(19)31282-6/ATTACHMENT/0C5C0CA7-F9DB-4DC6-A4D9-1A51A2542749/MMC1.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Sridharan K, Sequeira RP. Drugs for treating severe hypertension in pregnancy: a network meta-analysis and trial sequential analysis of randomized clinical trials. Br J Clin Pharmacol. 2018;84:1906–16. doi: 10.1111/BCP.13649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Duley L, Meher S, Jones L. Drugs for treatment of very high blood pressure during pregnancy. Cochrane Database Syst Rev. 20132013 doi: 10.1002/14651858.CD001449.PUB3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Briasoulis A, Agarwal V, Tousoulis D, Stefanadis C. Effects of antihypertensive treatment in patients over 65 years of age: a meta-analysis of randomised controlled studies. Heart. 2014;100:317–23. doi: 10.1136/HEARTJNL-2013-304111. [DOI] [PubMed] [Google Scholar]
- 138. Murad MH, Larrea-Mantilla L, Haddad A, Spencer-Bonilla G, Serrano V, Rodriguez-Gutierrez R, et al. Antihypertensive agents in older adults: a systematic review and meta-analysis of randomized clinical trials. J Clin EndocrinolMetab. 2019;104:1575–84. doi: 10.1210/JC.2019-00197. [DOI] [PubMed] [Google Scholar]
- 139. Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 Years: a randomized clinical trial. JAMA. 2016;315:2673–82. doi: 10.1001/JAMA.2016.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Warwick J, Falaschetti E, Rockwood K, Mitnitski A, Thijs L, Beckett N, et al. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the HYpertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med. 2015;13:1–8. doi: 10.1186/S12916-015-0328-1/TABLES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Corrao G, Mazzola P, Monzio Compagnoni M, Rea F, Merlino L, Annoni G, et al. Antihypertensive medications, loop diuretics, and risk of hip fracture in the elderly: a population-based cohort study of 81,617 Italian patients newly treated between 2005 and 2009. Drugs Aging. 2015;32:927–36. doi: 10.1007/S40266-015-0306-5. [DOI] [PubMed] [Google Scholar]