Abstract
Mitogen-activated protein kinase-8-interacting protein 2 (MAPK8IP2) is a scaffold protein that modulates MAPK signal cascades. Although MAPK pathways were heavily implicated in prostate cancer progression, the regulation of MAPK8IP2 expression in prostate cancer is not yet reported. We assessed MAPK8IP2 gene expression in prostate cancer related to disease progression and patient survival outcomes. MAPK8IP2 expression was analyzed using multiple genome-wide gene expression datasets derived from The Cancer Genome Atlas (TCGA) RNA-sequence project and complementary DNA (cDNA) microarrays. Multivariable Cox regressions and log-rank tests were used to analyze the overall survival outcome and progression-free interval. MAPK8IP2 protein expression was evaluated using the immunohistochemistry approach. The quantitative PCR and Western blot methods analyzed androgen-stimulated MAPK8IP2 expression in LNCaP cells. In primary prostate cancer tissues, MAPK8IP2 mRNA expression levels were significantly higher than those in the case-matched benign prostatic tissues. Increased MAPK8IP2 expression was strongly correlated with late tumor stages, lymph node invasion, residual tumors after surgery, higher Gleason scores, and preoperational serum prostate-specific antigen (PSA) levels. MAPK8IP2 upregulation was significantly associated with worse overall survival outcomes and progression-free intervals. In castration-resistant prostate cancers, MAPK8IP2 expression strongly correlated with androgen receptor (AR) signaling activity. In cell culture-based experiments, MAPK8IP2 expression was stimulated by androgens in AR-positive prostate cancer cells. However, MAPK8IP2 expression was blocked by AR antagonists only in androgen-sensitive LNCaP but not castration-resistant C4-2B and 22RV1 cells. These results indicate that MAPK8IP2 is a robust prognostic factor and therapeutic biomarker for prostate cancer. The potential role of MAPK8IP2 in the castration-resistant progression is under further investigation.
Keywords: cell cycle regulation, disease progression, mitogen-activated protein kinase-8-interacting protein 2, patient survival, prostate cancer
INTRODUCTION
In mammals, there are three primary mitogen-activated protein kinase (MAPK) cascades, namely extracellular-signal-regulated kinases (ERK), c-Jun amino-terminal kinases (JNK), and stress-activated protein kinases (SAPKs).1 All MAPK kinase cascades assemble on specific scaffold proteins as a modularized circuit to provide spatial and temporal control of the cascades.1,2,3 The scaffold proteins identified for the ERK family are kinase suppressor of ras 1/2 (KSR1/2) and late endosomal/lysosomal adaptor, MAPK and MTOR activator 3 (LAMTOR3). The JNK family scaffold proteins are mitogen-activated protein kinase-8-interacting protein 1–3 (MAPK8IP1–3), sperm-associated antigen 9 (SPAG9), and SH3 domain-containing ring finger 2 (SH3RF2, or POSH). The p38 family scaffold proteins are MAPK8IP2, SPAG9, cerebral cavernous malformation 2 (CCM2), and discs large MAGUK scaffold protein 1 (DLG1).1,2,3,4 In addition, these scaffold proteins were shown to recruit distinct cascade components, resulting in either activation or inactivation of the MAPK cascades depending on the stimuli or cell types.5,6 Other signaling molecules also modulated these scaffold proteins, providing additional layers of dynamic control for MAPK cascades.3,7,8
Due to genetic and epigenetic abnormalities in cancer cells, dysfunction or deregulation of MAPK cascades were shown to participate in cancer development and progression.4,9,10 For cancer treatment, small chemical molecules and antibodies targeting altered MAPK signaling cascades were developed.11 Particularly, aberrant expressions of several MAPK scaffold proteins were shown to correlate with patients’ survival outcomes in human cancers, such as KSR1 in breast cancer, MAPK8IP1 in glioma, MAPK8IP2 in pancreatic cancer, SPAG9 in gastric cancer, and DLG1 in endometrial cancer, indicating a potential prognostic implication.12,13,14,15,16
In recent years, high-throughput genomic and proteomic technologies have generated numerous datasets of gene expression profiles at the mRNA and protein levels. Based on this bioinformatic information, multiple molecular signatures were developed as predictive biomarkers for human cancers, including prostate cancer.17,18 However, these molecular prognostic signatures were derived from random genes that were not biologically sensible.19 We sought to explore a prognostic biomarker that holds the biological potential to drive disease progression and can be used as a therapeutic biomarker for cancer management. Our analysis revealed that MAPK8IP2 expression was significantly upregulated in prostate cancer. MAPK8IP2 upregulation was significantly correlated with disease progression and strongly associated with patient survival outcomes, including overall survival and progression-free intervals. Significantly, MAPK8IP2 expression was enhanced by androgen receptor (AR) signaling and blocked by AR antagonist in prostate cancer cells. In addition, MAPK8IP2 expression was highly associated with cell mitosis regulators, indicating a potential mechanistic role in prostate cancer progression.
MATERIALS AND METHODS
Gene expression profiles in normal and cancerous tissues
Gene expression profiles in normal and prostate cancer tissues were assessed using the RNA sequencing (RNA-seq) dataset derived from The Cancer Genome Atlas–Prostate Adenocarcinoma (TCGA-PRAD) project20 as the primary approach to compare gene expression between normal and cancerous tissues. In addition, complementary DNA (cDNA) microarray datasets on the Oncomine online platform21,22,23,24 were also used to verify the RNA-seq data. These cDNA microarray datasets were generated with Affymetrix U133 Plus 2.0 microarrays (Affymetrix, Waltham, MA, USA).
Cell type-specific gene expression in the prostate gland was assessed with the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) dataset GDS1973,25 generated from four different cell types using an antibody pull-down approach against distinct cell surface-specific markers. Immunoprecipitation-pulled down cells were collected for total RNA extracted, followed by RNA-seq analysis.
Gene expression and DNA methylation analyses in patients during disease progression
Gene expression analyses at the mRNA levels and genomic structural alteration in patients during disease progression from disease-free to relapse or between alive and deceased patients were conducted using DKFZ, MSKCC, and SU2C/PCF Dream Team datasets on the cBioportal online platform.26,27,28,29 Correlation analyses between gene expression levels and DNA methylation were also performed with the TCGA Firehose Legacy dataset on the cBioportal platform.
Gene expression levels were compared among different tumor, nodal, and metastasis (TNM) stages, Gleason scores, and serum prostate-specific antigen (PSA) levels using the TCGA-PRAD dataset. Patients were divided into high or low expression groups based on the median value of gene expression. Three additional RNA-seq datasets27,28,29 were used to verify the TCGA-PRAD dataset results. The correlations between gene expression and AR or neuroendocrine prostate cancer (NEPC) scores, PSA levels, or relapse interval were assessed using the Spearman’s coefficient analysis.
Assessment of patient survival outcomes
Patient survival outcomes, including overall survival and progression-free interval, were assessed using the TCGA-PRAD dataset with the Kaplan–Meier curve analysis. Patients were stratified into high or low expression groups using the minimum P-value approach.30 The significance of the hazard ratio (HR) was statistically analyzed using the log-rank test.
Data mining of androgen-stimulated MAPK8IP expression in LNCaP cells
Androgen stimulation of MAPK8IP1-3 expression was assessed using the NCBI GEO dataset GDS2782.31 LNCaP cells, purchased from ATCC (Manassas, VA, USA) and cultured as described in our recent publication,32 were stimulated with dihydrotestosterone (DHT; catalog #D073, Sigma-Aldrich, St. Louis, MO, USA) for 16 h in the RPMI1640 media (catalog #11875093, ThermoFisher Scientific, Waltham, MA, USA) supplied with 10% charcoal-stripped fetal bovine serum (catalog #12676029, ThermoFisher Scientific). Two hours of pretreatment was conducted with the AR antagonist bicalutamide (10 µmol l−1). Gene expression levels were assessed using the Affymetrix high-density Human Genome U133 Plus 2.0 chips. The expression levels of the actin-beta (ACTB) gene were used as the internal control.
Cell culture, reagents, real-time PCR assay, and Western blot assay
To verify androgen-stimulated MAPK8IP2 expression in LNCaP cells, we conducted a real-time PCR assay in LNCaP cells. Synthetic androgen R1881 was purchased from ICN Biomedicals (catalog #50-152311, Aurora, OH, USA) and dissolved in ethanol, as described in our previous publication.32 Serum-starved cells were stimulated with R1881 (1.0 nmol l−1) overnight, and total RNAs were extracted for quantitative real-time PCR (qPCR) analysis, as described in our previous publication.33 The first-strand cDNA was synthesized using the iScript kit (catalog #1708891, Bio-Rad, Hercules, CA, USA), and the qPCR assay was conducted on an ABI7500FAST system using the iTaq Universal SYBR Green Supermix (catalog #1725121, Bio-Rad). The ribosomal protein S18 (RPS18) gene was used as the internal control. The primers used for qPCR assays were listed as follows: MAPK8IP2 gene forward 5’-TCT TCG TCA ACA GCA CAT CTC-3’ and reverse 5’-CGG GAT GAA CCT GAA CAC AG-3’ (PrimerBank ID 117446084c2); RPS18 gene forward 5’-ATC ACC ATT ATG CAG AAT CCA CG-3’ and reverse 5’-GAC CTG GCT GTA TTT TCC ATC C-3’ (PrimerBank ID 14165467c2). The primer sequences were derived from the PrimerBank (https://pga.mgh.harvard.edu/primerbank/) and were synthesized by the IDT Inc. (Coralville, IA, USA).
AR antagonist’s effect on MAPKL8IP2 expression was evaluated in prostate cancer LNCaP, C4-2B (ATCC #CRL3315), and 22RV1 (ATCC #CRL2505) cells, as described in our previous publications.34,35 After treatment, cells were harvested in cold phosphate-buffered saline (PBS) and lysed in radioimmunoprecipitation assay (RIPA) buffer. An equal amount of cell lysate proteins was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and Western blot assay with anti-MAPK8IP2 antibody (catalog #HPA034780, Sigma-Aldrich), as described in our publication.34 Dimethyl sulfone (DMSO; catalog #20131), enzalutamide (MDV-3100; catalog #11596), and abiraterone (catalog #9002768) were purchased from Cayman Chemicals (Ann Arbor, MI, USA).
Immunohistochemistry for MAPK8IP2 expression in normal and prostate cancer tissues
Tissue microarray slides containing 140 prostate cancer and benign prostate tissue sections were gifts from Dr. Jiaoti Huang (Duke University, Durham, NC, USA). After deparaffinization and hydration, tissue slides were treated with 3% H2O2 for 15 min and then blocked with 5% bovine serum albumin (BSA) in Tris-buffered solution with Tween-20 (TBS-T) for 60 min at room temperature. The primary antibody for MAPK8IP2 was purchased from Sigma-Aldrich (catalog #HPA034780) and used at 1:40 dilution in 5% BSA/TBS-T overnight at 4°C with agitation. Visualization of the immunosignals was performed using the VECTASTAIN® Elite® ABC Universal PLUS kit (Vector Laboratories, Inc., Burlingame, CA, USA). The immunosignal index was calculated by multiplying the immune density (weak = 1, moderate = 2, and strong = 3) with the percentage positivity, as described in our recent publication.36
Data presentation and statistical analyses
Quantitative data for gene expression at the mRNA level (log2 [transcripts per million + 1]) were presented as the mean with the standard error of the mean (s.e.m.). Differences among multiple groups were analyzed using the analysis of variance (ANOVA) test. Comparisons between the two groups were conducted using a Student’s t-test. Spearman’s coefficient analysis was used for correlation evaluation. Data analysis and visualization were primarily performed using the R packages (R Foundation, Vienna, Austria) and GraphPad Prism (version 9.1.0, GraphPad Software, San Diego, CA, USA).
RESULTS
Four MAPK scaffold genes were significantly altered in prostate cancer
We first assessed the expression profiles of all MAPK pathway scaffold genes, including KSR1/2, LAMTOR3, MAPK8IP1–3, SPAG9, SH3RF2 (POSH), CCM2, and DLG1, in the normal prostate gland and prostate cancer tissues using the NCBI GEO dataset GDS197325 and the TCGA-PRAD RNA-seq dataset generated from prostate cancer tissues (n = 52) accompanied with case-matched benign prostatic tissues (n = 52). As shown in Figure 1a, these scaffold proteins were expressed at variable levels in different cell types of the normal prostate gland. Comparing to other prostatic cell types, luminal cells expressed higher levels of LAMTOR3, CCM2, and MAPK8IP2, basal cells expressed higher levels of DLG1 and SH3RF2, endothelial cells expressed higher levels of CCM2 and SPAG9, and stromal cells expressed higher levels of LAMTOR3 and CCM2. These data suggest that these scaffold proteins were expressed in a cell-specific manner.
Figure 1.
MAPK8IP2 expression is upregulated in prostate cancer. (a) Expression profiles of MAPK pathway scaffold protein genes in different prostate cell types, including basal, luminal secretory, stromal fibromuscular, and endothelial cells of the prostate. Cell types were separated with antibody-based magnetic cell sorting (MACS) against distinct cell-type-specific cluster designation (CD) antigens, as described.25 Gene expression levels were assessed using Affymetrix Human Genome U133 Plus 2.0 Array. ACTB gene expression levels were used for internal normalization. The asterisk indicates a significant difference between the basal and luminal cell types (Student’s t-test, P < 0.05). (b) Expression of MAPK pathway scaffold protein genes in prostate cancers. Gene expression levels were analyzed as a pair-wise comparison using the RNA-seq dataset from the TCGA-PRAD project in 52 cases. The asterisks indicate significant differences (**P < 0.01 and ***P < 0.001) derived from a paired t-test. (c–f) Representative microscopic images were taken from anti-MAPK8IP2 IHC staining with (c) strong signal, (d) moderate signal, and (e) weak signal in malignant tissues. (f) A section from benign prostate tissue sections showed a very weak signal. (g) Immunosignals were semiquantitated from malignant and benign tissue sections described in our recent publication.36 The error bars indicate the mean and s.e.m. The asterisk indicates a significant difference (Student’s t-test, ****P < 0.0001). RNA-seq: RNA-sequence; MAPK: mitogen-activated protein kinase; MAPK8IP2: MAPK8-interacting protein 2; IHC: immunohistochemistry; s.e.m.: standard error of the mean; TCGA-PRAD: The Cancer Genome Atlas–Prostate Adenocarcinoma; ACTB: actin-beta.
In prostate cancer tissues, four scaffold genes, KSR1, MAPK8IP1, MAPK8IP2, and CCM2, showed a significant alteration compared to the case-matched benign tissues, of which KSR1 expression was downregulated, and in contrast, the other three genes were upregulated (Figure 1b). We analyzed additional datasets generated from cDNA microarray assays.23 Our results confirmed that KSR1 and MAPK8IP2 expression was significantly altered in prostate cancer tissues compared to benign tissues (Supplementary Figure 1 (85.5KB, tif) ). MAPK8IP1 expression was only slightly increased in prostate cancer tissues compared to normal prostate tissues, and CCM2 expression did not show a statistical difference between benign and malignant prostate tissues in the cDNA microarray datasets (Supplementary Figure 1 (85.5KB, tif) ). These data demonstrated that KSR1 and MAPK8IP2 gene expression was significantly altered in prostate cancer.
We then determined if their alteration of gene expression was associated with structural alterations (mutation, deletion, or amplification) in these two genes. As shown in Supplementary Figure 2a (110.9KB, tif) , KSR1 genetic changes were seen in 1.4% of 499 cases and MAPK8IP2 genetic changes were seen in 1.6% of 499 cases in the TCGA-PRAD study cohort. A similar prevalence was also noticed in an additional 7716 patients from 21 prostate cancer studies on the cBioportal platform (Supplementary Figure 2b (110.9KB, tif) ). These results indicated that structural changes are unlikely responsible for altered gene expression.
To evaluate if MAPK8IP2 protein expression was in line with its increased mRNA levels in prostate cancer tissues, we performed an immunohistochemistry (IHC) analysis on tissue microarray (TMA) slides that hold 140 sections from malignant and benign prostate tissues, respectively. As expected, anti-MAPK8IP2 immunosignal was localized in the cytoplasmic compartment. Most prostate cancer tissues showed a significantly higher prevalence and stronger positive immunosignal (Figure 1c–1e), while the benign tissues only showed very weak immunosignals of MAPK8IP2 staining (Figure 1f). Semiquantitative analysis revealed that MAPK8IP2 immunosignal index was significantly higher in prostate cancer tissues than benign tissues (Figure 1g), consistent with the RNA-seq data of MAPK8IP2 upregulation in prostate cancer.
MAPK8IP2 upregulation is associated with clinicopathological factors in prostate cancer
To explore the clinical significance of altered gene expression, we assessed the relationship of the KSR1 and MAPK8IP2 expression with clinicopathological parameters. Patients in the TCGA-PRAD cohort were stratified into two groups (low or high expression) at the median expression value as the cutoff point. The differences in the case of numbers between these two groups were analyzed using Chi-squared or Fisher’s test. As shown in Table 1, there were significant differences in case numbers when patients were compared among different tumor stages (P < 0.001), with or without lymph node invasion (P = 0.002), postsurgery residual tumors (P = 0.009), different Gleason scores (P < 0.001), overall survival outcomes (P = 0.02), disease-specific survival (P = 0.03), and progression-free intervals (P < 0.001). There was no significant difference among patient races and distal metastasis, possibly due to only three patients with metastasis. Conversely, case numbers with high or low KSR1 expression had no significant difference in these clinicopathological parameters (Supplementary Table 1). These data strongly suggest that MAPK8IP2 upregulation but not KSR1 downregulation is tightly associated with clinicopathological parameters in prostate cancer patients.
Table 1.
Clinicopathological characteristics of the cases
| Characteristic | Low MAPK8IP2 | High MAPK8IP2 | P | Statistic | Method |
|---|---|---|---|---|---|
| Total cases (n) | 249 | 250 | |||
| T stage, n (%) | <0.001 | 19.69 | Chi-squared test | ||
| T2 | 116 (23.6) | 73 (14.8) | |||
| T3 | 126 (25.6) | 166 (33.7) | |||
| T4 | 2 (0.4) | 9 (1.8) | |||
| Excluded cases | 5 | 2 | |||
| N stage, n (%) | 0.002 | 10.04 | Chi-squared test | ||
| N0 | 181 (42.5) | 166 (39.0) | |||
| N1 | 25 (5.9) | 54 (12.7) | |||
| Excluded cases | 43 | 30 | |||
| M stage, n (%) | 0.623 | Fisher’s test | |||
| M0 | 229 (50.0) | 226 (49.3) | |||
| M1 | 1 (0.2) | 2 (0.4) | |||
| Excluded cases | 19 | 22 | |||
| Primary therapy outcome, n (%) | 0.138 | 5.52 | Chi-squared test | ||
| PD | 14 (3.2) | 14 (3.2) | |||
| SD | 13 (3.0) | 16 (3.7) | |||
| PR | 14 (3.2) | 26 (5.9) | |||
| CR | 183 (41.8) | 158 (36.1) | |||
| Excluded cases | 25 | 36 | |||
| Race, n (%) | 0.522 | 1.3 | Chi-squared test | ||
| Asian | 4 (0.8) | 8 (1.7) | |||
| Black or African American | 29 (6.0) | 28 (5.8) | |||
| White | 206 (42.6) | 209 (43.2) | |||
| Excluded cases | 10 | 5 | |||
| Age (year), n (%) | 0.303 | 1.06 | Chi-squared test | ||
| ≤60 | 118 (23.6) | 106 (21.2) | |||
| >60 | 131 (26.3) | 144 (28.9) | |||
| Residual tumor, n (%) | 0.009 | Fisher’s test | |||
| R0 | 175 (37.4) | 140 (29.9) | |||
| R1 | 61 (13.0) | 87 (18.6) | |||
| R2 | 3 (0.6) | 2 (0.4) | |||
| Excluded cases | 10 | 21 | |||
| Zone of origin, n (%) | 0.174 | Fisher’s test | |||
| Central zone | 3 (1.1) | 1 (0.4) | |||
| Overlapping/multiple zones | 46 (16.7) | 80 (29.1) | |||
| Peripheral zone | 64 (23.3) | 73 (26.5) | |||
| Transition zone | 4 (1.5) | 4 (1.5) | |||
| Excluded cases | 132 | 92 | |||
| Gleason score, n (%) | <0.001 | 27.63 | Chi-squared test | ||
| 6 | 33 (6.6) | 13 (2.6) | |||
| 7 | 140 (28.1) | 107 (21.4) | |||
| 8 | 25 (5.0) | 39 (7.8) | |||
| 9 | 50 (10.0) | 88 (17.6) | |||
| 10 | 1 (0.2) | 3 (0.6) | |||
| OS event, n (%) | 0.020 | Fisher’s test | |||
| Alive | 248 (49.7) | 241 (48.3) | |||
| Dead | 1 (0.2) | 9 (1.8) | |||
| DSS event, n (%) | 0.030 | Fisher’s test | |||
| Alive | 249 (50.1) | 243 (48.9) | |||
| Dead | 0 (0) | 5 (1.0) | |||
| Excluded cases | 0 | 2 | |||
| PFI event, n (%) | <0.001 | 10.88 | Chi-squared test | ||
| No-relapse | 217 (43.5) | 188 (37.7) | |||
| Relapsed | 32 (6.4) | 62 (12.4) | |||
| Age (year), median (IQR) | 61 (56–66) | 62 (56–66) | 0.296 | 29 444 | Wilcoxon rank-sum test |
MAPK: mitogen-activated protein kinase; MAPK8IP2: MAPK-8-interacting protein 2; OS: overall survival; IQR: interquartile range; DSS: disease-specific survival; PFI: progression-free interval; PD: progressive disease; SD: stable disease; PR: partial response; CR: complete response
Supplementary Table 1.
The relationship of kinase suppressor of ras 1 expression with clinicopathological parameters in prostate cancers
| Characteristic | Low KSR1 | High KSR1 | P | Statistic | Method |
|---|---|---|---|---|---|
| n | 249 | 250 | |||
| T stage, n (%) | 0.149 | 3.81 | Chi-squared test | ||
| T2 | 102 (20.7) | 87 (17.7) | |||
| T3 | 141 (28.7) | 151 (30.7) | |||
| T4 | 3 (0.6) | 8 (1.6) | |||
| Other unknown | 3 | 4 | |||
| N stage, n (%) | 0.280 | 1.17 | Chi-squared test | ||
| N0 | 171 (40.1) | 176 (41.3) | |||
| N1 | 33 (7.7) | 46 (10.8) | |||
| Other unknown | 45 | 28 | |||
| M stage, n (%) | 1.000 | Fisher’s test | |||
| M0 | 227 (49.6) | 228 (49.8) | |||
| M1 | 2 (0.4) | 1 (0.2) | |||
| Other unknown | 20 | 21 | |||
| Primary therapy outcome, n (%) | 0.982 | 0.17 | Chi-squared test | ||
| PD | 13 (3) | 15 (3.4) | |||
| SD | 13 (3) | 16 (3.7) | |||
| PR | 19 (4.3) | 21 (4.8) | |||
| CR | 165 (37.7) | 176 (40.2) | |||
| Other unknown | 39 | 22 | |||
| Race, n (%) | 0.494 | 1.41 | Chi-squared test | ||
| Asian | 5 (1) | 7 (1.4) | |||
| Black or African American | 32 (6.6) | 25 (5.2) | |||
| White | 202 (41.7) | 213 (44) | |||
| Other unknown | 10 | 5 | |||
| Age, n (%) | 0.756 | 0.1 | Chi-squared test | ||
| ≤60 | 114 (22.8) | 110 (22) | |||
| >60 | 135 (27.1) | 140 (28.1) | |||
| Residual tumor, n (%) | 0.481 | Fisher’s test | |||
| R0 | 155 (33.1) | 160 (34.2) | |||
| R1 | 73 (15.6) | 75 (16) | |||
| R2 | 4 (0.9) | 1 (0.2) | |||
| Other unknown | 17 | 14 | |||
| Zone of origin, n (%) | 0.192 | Fisher’s test | |||
| Central zone | 2 (0.7) | 2 (0.7) | |||
| Overlapping/multiple zones | 63 (22.9) | 63 (22.9) | |||
| Peripheral zone | 66 (24) | 71 (25.8) | |||
| Transition zone | 7 (2.5) | 1 (0.4) | |||
| Other unknown | 111 | 113 | |||
| PSA (ng/ml), n (%) | 0.858 | 0.03 | Chi-squared test | ||
| <4 | 200 (45.2) | 215 (48.6) | |||
| ≥4 | 12 (2.7) | 15 (3.4) | |||
| Other unknown | 37 | 20 | |||
| Gleason score, n (%) | 0.505 | 3.32 | Chi-squared test | ||
| 6 | 25 (5) | 21 (4.2) | |||
| 7 | 130 (26.1) | 117 (23.4) | |||
| 8 | 30 (6) | 34 (6.8) | |||
| 9 | 63 (12.6) | 75 (15) | |||
| 10 | 1 (0.2) | 3 (0.6) | |||
| OS event, n (%) | 0.544 | Fisher’s test | |||
| Alive | 243 (48.7) | 246 (49.3) | |||
| Dead | 6 (1.2) | 4 (0.8) | |||
| DSS event, n (%) | 0.216 | Fisher’s test | |||
| Alive | 244 (49.1) | 248 (49.9) | |||
| Dead | 4 (0.8) | 1 (0.2) | |||
| Other unknown | 1 | 1 | |||
| PFI event, n (%) | 0.313 | 1.02 | Chi-squared test | ||
| Tumor-free | 207 (41.5) | 198 (39.7) | |||
| Relapsed | 42 (8.4) | 52 (10.4) | |||
| Age, median (IQR) | 61 (56–66) | 61.5 (57–66) | 0.751 | 30614.5 | Wilcoxon |
The percentages were calculated based on the total case numbers used in the statistical comparison after excluding the other unknown cases. OS: overall survival; IQR: interquartile range; KSR1: kinase suppressor of ras 1; DSS: disease-specific survival; PFI: progression-free interval; PD: progressive disease; SD: stable disease; PR: partial response; CR: complete response
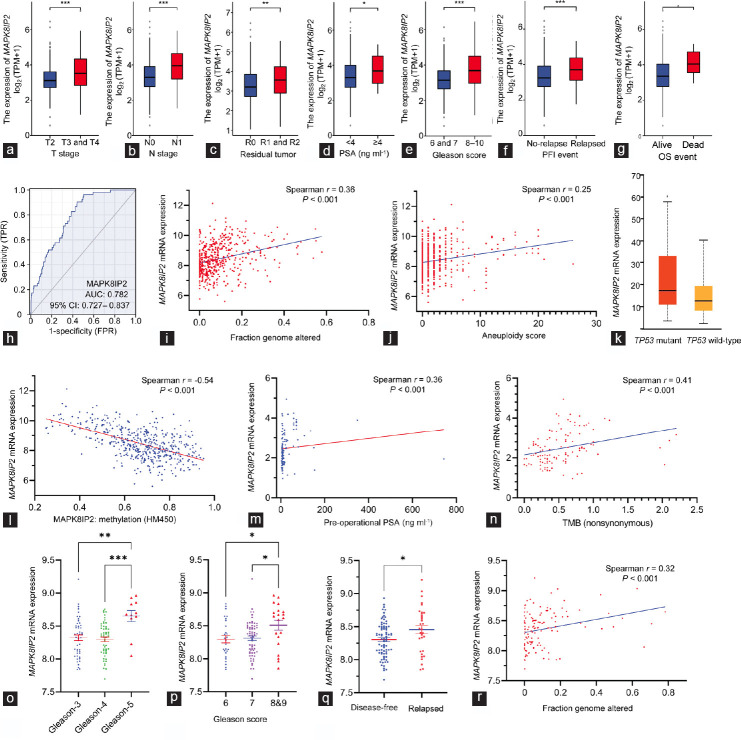
We then compared the quantitative levels of MAPK8IP2 expression in different patient groups after stratifying with clinicopathological parameters using the TCGA-PRAD dataset. Our analysis revealed that MAPK8IP2 expression was significantly higher in patients with late-stage tumors (T3 and T4 vs T2, P < 0.001; Figure 2a), lymph node invasive tumors (N1 vs N0, P < 0.001; Figure 2b), residual tumors after surgery (R1 and R2 vs R0, P < 0.01; Figure 2c), higher preoperational serum PSA level (≥4 ng ml−1 vs <4 ng ml−1, P < 0.05; Figure 2d), higher Gleason scores (6 and 7 vs 8–10, P < 0.001; Figure 2e), in progression-free vs relapsed (P < 0.001; Figure 2f), or in alive vs deceased patients (P < 0.05; Figure 2g). A receiver operating characteristic (ROC) curve analysis determined that MAPK8IP2 upregulation was a strong predictor with an area under the curve (AUC) value of 0.782 in distinguishing normal and malignant prostate tissues (Figure 2h).
Figure 2.
MAKP8IP2 expression was increased along with prostate cancer progression. (a–g) MAPK8IP2 expression levels in the TCGA-PRAG dataset were compared in different groups of clinicopathological parameters, including (a) TNM stages, (b) lymph node invasion, (c) postsurgery residual tumors, (d) presurgery serum PSA levels, (e) Gleason score, (f) progression-free incidence, and (g) overall survival. Case numbers in each subgroup are listed as follows: n = 189 for T2; n = 292 for T3; n = 11 for T4; n = 347 for N0; n = 79 for N1; n = 315 for R0; n = 148 for R1; n = 5 for R2; n = 415 for PSA <4 ng ml−1; n = 27 for PSA ≥4 ng ml−1; n = 46 for Gleason-6; n = 247 for Gleason-7; n = 64 for Gleason-8; n = 138 for Gleason-9; n = 4 for Gleason-10; n = 405 for progression-free; n = 94 for relapsed; n = 489 for alive; and n = 10 for dead. The asterisks indicate a significant difference by Wilcoxon rank-sum test. *P < 0.05; **P < 0.01; ***P < 0.001. (h) ROC analysis for the predictive potential of MAPK8IP2 upregulation in prostate cancers. (i and j) Spearman’s coefficient analysis between MAPK8IP2 expression and tumor genetic abnormalities, (i) FGA and (j) aneuploidy scores, was conducted with the RNA-seq TCGA PanCancer Atlas dataset (n = 494) downloaded from the cBioportal platform. (k) Comparison of MAPK8IP2 expression in prostate cancer with wild-type (n = 295) or mutant (n = 38) TP53 gene was conducted on the UALCAC platform. The asterisk indicates a significant difference (Student’s t-test, *P < 0.05). (l) MAPK8IP2 expression is inversely correlated with DNA methylation in prostate cancer. Spearman’s coefficient analysis was conducted using the RNA-seq dataset generated from the TCGA Firehose Legacy dataset (n = 498) downloaded from the cBioportal platform. DNA methylation data were generated using the Human Methylation-450 Bead chip. (m and n) Spearman’s coefficient analysis was performed using the DKFZ RNA-seq dataset,27 which consisted of 116 early-onset prostate cancer patients for (m) preoperation PSA analysis and (n) tumor mutation burden score. (o–r) MAPK8IP2 expression was assessed using the MSKCC RNA-seq dataset28 for the differences among different (o) Gleason primary scores, (p) Gleason sum scores, (q) disease relapses, and (r) genomic alterations. The asterisks indicate a significant difference by (o) ANOVA test, (p) Kruskal-Wallis test and (q) Student’s t-test. *P < 0.05; **P < 0.01; ***P < 0.001. Case numbers in each subgroup is listed as follow: n = 47 for Gleason-3; n = 52 for Gleason-4; n = 11 for Gleason-5; n = 27 for Gleason-6; n = 64 for Gleason-7; n = 20 for Gleason-8 and -9; n = 80 for disease-free; and n = 32 for relapsed. AUC: area under the curve; TMB: tumor mutation burden; MAPK: mitogen-activated protein kinase; MAPK8IP2: MAPK8-interacting protein 2; TCGA-PRAD: The Cancer Genome Atlas–Prostate Adenocarcinoma; TNM: tumor, nodal, and metastasis; PSA: prostate-specific antigen; ROC: receiver operating characteristic; FGA: fraction genome altered; OS: overall survival; ANOVA: analysis of variance; TP53: tumor protein p53; CI: confidence interval.
MAPK8IP2 upregulation correlates with genomic alterations in prostate cancer
We also analyzed the correlation of MAPK8IP2 expression with tumor genomic alterations using the TCGA-PRAD dataset on the cBioportal platform. Spearman’s correlation analysis revealed that MAPK8IP2 expression was strongly correlated with the scores of fraction genome altered (FGA; Figure 2i) and aneuploidy (Figure 2j). Interestingly, tumors with TP53 gene mutation showed a significantly higher level of MAPK8IP2 expression (P < 0.05), indicating a potential role of p53 in modulating MAPK8IP2 expression (Figure 2k). In addition, there was a robust correlation (Spearman r = −0.54, P < 0.001) between MAPK8IP2 expression and the methylation levels of its gene promoter region (Figure 2l), representing an epigenetic mechanism of promoter hypomethylation in MAPK8IP2 upregulation.
We also used the RNA-seq datasets derived from the DKFZ study27 and the MSKCC study28 to analyze the relationship of MAPK8IP2 expression with clinicopathological parameters. In the DKFZ cohort, MAPK8IP2 expression was significantly correlated with the preoperational serum PSA levels (P < 0.001; Figure 2m) and tumor mutation burden (TMB) index (P < 0.001; Figure 2n). In the MSKCC cohort, MAPK8IP2 expression was higher in patients with higher Gleason scores (P < 0.05; Figure 2o and 2p) and patients with relapsed diseases (P < 0.05; Figure 2q). MAPK8IP2 expression was also significantly correlated with genomic alteration (fraction genome altered [FGA] score) in prostate cancer (P < 0.001; Figure 2r). These results demonstrated a tight association of MAPK8IP upregulation with prostate cancer genetic alteration, TP53 gene mutation, promoter hypomethylation, and disease progression.
MAPK8IP2 upregulation is a robust prognostic factor for patient survival outcomes
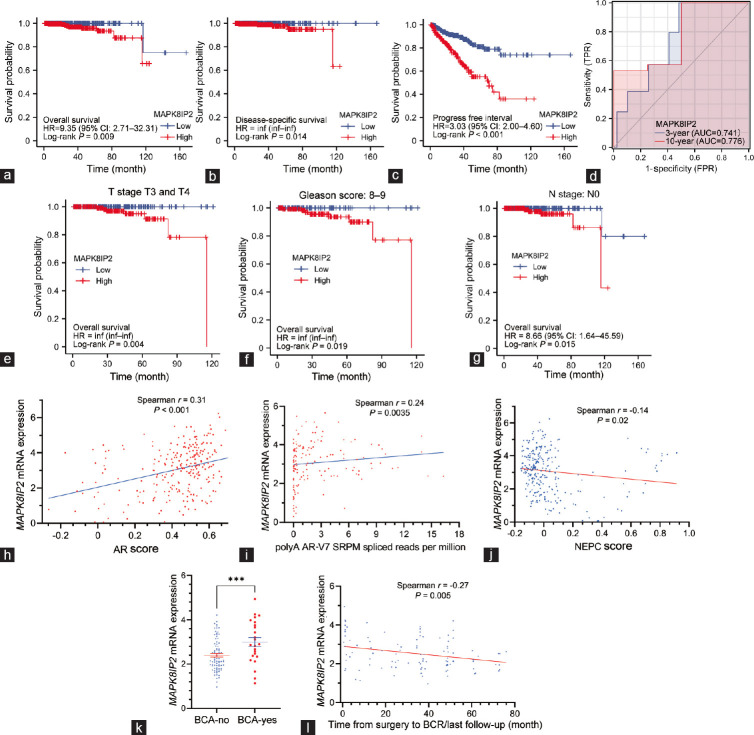
To assess the prognostic significance of MAPK8IP2 upregulation in prostate cancer, we analyzed its impact on patient survival outcomes. We stratified patients based on MAPK8IP2 expression levels with a minimum P-value approach, as previously described.37 Kaplan–Meier curve analysis revealed that MAPK8IP2 upregulation had a significantly negative impact on overall survival (P = 0.009; Figure 3a), disease-specific survival (P = 0.014; Figure 3b), and progression-free interval (P < 0.001; Figure 3c). ROC analyses determined MAPK8IP2 upregulation as a solid prognostic factor for 3- or 10-year overall survival outcomes (Figure 3d). COX regression analysis revealed that MAPK8IP2 expression had a much better prediction for patient’s overall survival (P = 0.009) than tumor stage (P = 0.165), lymph node invasion (P = 0.102), and postsurgery residue tumor incidence (P = 0.155; Supplementary Table 2).
Figure 3.
MAPK8IP2 expression is strongly associated with a worse patient survival outcome in prostate cancers. (a–c) Patient survival outcomes for (a) overall, (b) disease-specific, and (c) progression-free interval were analyzed with the Kaplan–Meier plot using a minimum P-value approach, as previously described.37 Patients were stratified using MAPK8IP2 expression levels in the TCGA-PRAD dataset. Data figures were generated using the R survminer package (version 0.4.9). The case number in each subgroup was listed at the bottom of the figure panel. (d) Time-dependent ROC analysis of overall predictive survival was conducted using the TCGA-PRAD dataset with the R-time ROC package (version 3.6.3.04) and visualized with the R-ggplot package (version 3.3.3). (e–g) Patient overall survival outcomes were analyzed in subgroups with (e) late stages, (f) higher Gleason scores, and (g) lymph node invasion-free cases. (h–j) Spearman’s coefficient analysis was conducted between MAPK8IP2 expression and (h) AR scores, (i) AR-V7 expression, and (j) NEPC score using the RNA-seq dataset generated from metastatic castration-resistant prostate cancers.29 Case numbers in the AR and NEPC score analyses were 264, and in the AR-V7 study was 146. (k) MAPK8IP2 expression was compared between patient groups with BCR (n = 24) and without BCR (n = 81) in the DKFZ RNA-seq dataset.27 The asterisk indicates a significant difference (Student’s t-test, P < 0.001). (l) Spearman’s coefficient analysis was conducted between MAPK8IP2 expression and the months from surgery to BCR (n = 105) using the DKFZ RNA-seq dataset.27 MAPK: mitogen-activated protein kinase; MAPK8IP2: MAPK8-interacting protein 2; TCGA-PRAD: The Cancer Genome Atlas–Prostate Adenocarcinoma; ROC: receiver operating characteristic; AUC: area under the curve; BCR: biochemical relapse; AR: androgen receptor; NEPC: neuroendocrine prostate cancer; inf: infinity; CI: confidence interval.
Supplementary Table 2.
COX regression analysis for patient overall survival
| Characteristics | Total (n) | HR (95% CI) | P |
|---|---|---|---|
| T stage | 492 | ||
| T2 | 189 | Reference | |
| T3 and T4 | 303 | 3.785 (2.140–6.693) | <0.001 |
| N stage | 426 | ||
| N0 | 347 | Reference | |
| N1 | 79 | 1.946 (1.202–3.150) | 0.007 |
| PSA (ng/ml) | 442 | ||
| <4 | 415 | Reference | |
| ≥4 | 27 | 4.196 (2.095–8.405) | <0.001 |
| Gleason score | 499 | ||
| 6 and 7 | 293 | Reference | |
| 10 and 9 and 8 | 206 | 4.675 (2.957–7.391) | <0.001 |
| Residual tumor | 468 | ||
| R0 | 315 | Reference | |
| R1 and R2 | 153 | 2.365 (1.566–3.570) | <0.001 |
| MAPK8IP2 | 499 | 1.519 (1.233–1.872) | <0.001 |
MAPK: mitogen-activated protein kinase; MAPK8IP2: MAPK-8-interacting protein 2; CI: confidence interval; PSA: prostate-specific antigen; HR: hazard ratio
We then analyzed the relationship of MAPK8IP2 expression with survival outcomes after stratifying patients into different subgroups based on clinicopathological parameters. MAPK8IP2 upregulation had a significantly negative impact on the overall survival of patients with late-stage (T3 and T4) tumors (P = 0.004; Figure 3e) and higher Gleason score (Gleason 8–10) tumors (P = 0.019; Figure 3f). Interestingly, MAPK8IP2 upregulation had a negative impact on overall survival outcomes only in patients without lymph node invasion (N0 diseases, P = 0.015; Figure 3g). These results indicated that MAPK8IP2 is a robust prognostic factor for late-stage, high-grade, and N0 prostate cancer patients.
MAPK8IP2 expression is associated with AR signaling in prostate cancer
Clinically, metastatic prostate cancer eventually progress into the castration-resistant stage (CRPC) after hormone deprivation therapy, and biochemical relapse (BCR) eventually occurs as a sign of AR reactivation during castration-resistant progression.38 We analyzed the correlation of MAPK8IP2 expression with CRPC progression using the RNA-seq dataset derived from the SU2C/PCF Dream Team study.29 As shown in Figure 3h, our analysis revealed a strong positive correlation between MAPK8IP2 expression and the AR score (Spearman r = 0.31, P < 0.001), a unique index reflecting AR reactivation in CRPC patients.29 Interestingly, MAPK8IP2 expression was moderately correlated with AR-V7 expression (Spearman r = 0.24, P = 0.0035; Figure 3i) but was negatively correlated with the NEPC score (Spearman r = −0.14, P = 0.02; Figure 3j), an index for neuroendocrine progression.29
We then determined if MAPK8IP2 expression was associated with BCR events in early-onset patients in the DKFZ cohort.27 As shown in Figure 3k, patients with BCR events showed a significantly higher level of MAPK8IP2 expression compared to patients without BCR (P < 0.001). Consistently, MAPK8IP2 expression was inversely correlated with the BCR-free interval after surgery (Spearman r = −0.27, P = 0.005; Figure 3l). The results verified the correlation of MAPK8IP2 expression with the AR reactivation, indicating a possible role of MAPK8IP2 expression in AR signaling.
To determine the regulatory effect of AR activation on MAPK8IP2 expression, we conducted another data mining analysis on the NCBI GEO dataset GDS2782.31 Our analysis revealed that dihydrotestosterone (DHT) stimulation significantly increased MAPK8IP2 but not MAPK8IP1 or MAPK8IP3 expression in prostate cancer LNCaP cells, which was blocked by AR antagonist bicalutamide (Figure 4a). Androgen-stimulated MAPK8IP2 expression was further confirmed in R1881-stimulated LNCaP cells using a qPCR approach (Figure 4b) and C4-2B and 22Rv1 cells in a Western blot assay (Figure 4c). Interestingly, AR antagonists (enzalutamide and abiraterone) vastly reduced MAPK8IP2 expression in androgen-sensitive LNCaP cells but not in castration-resistant C4-2B and 22Rv1 cells. These data demonstrated that MAPK8IP2 is an AR-regulated gene in prostate cancer and might be involved in the castration-resistant progression of prostate cancer. A further mechanistic investigation is underway to elucidate its role in prostate cancer progression.
Figure 4.
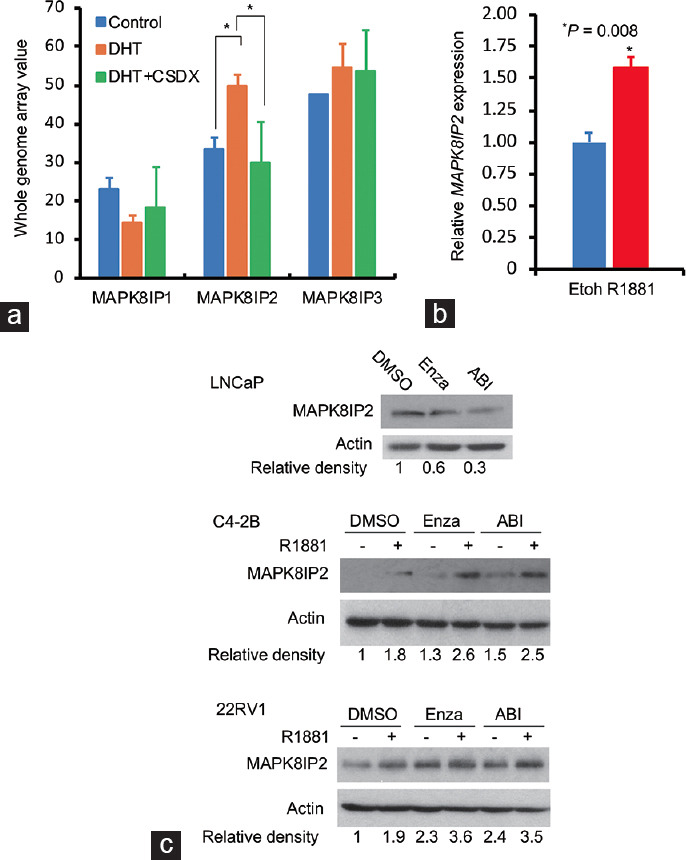
MAPK8IP2 expression is increased after androgen stimulation in AR-positive prostate cancer cells. (a) Data mining analysis was performed using the NCBI GEO dataset GDS2782.31 Prostate cancer LNCaP cells were treated with DHT (1.0 nmol l−1) for 16 h with or without Casodex (CSDX, bicalutamide) pretreatment (10 µmol l−1). Total RNAs were extracted for cDNA microarray assay on the Affymetrix Human Genome U133 Plus 2.0 Array chips. The internal normalization was conducted using the expression levels of the ACTB gene. The asterisk indicates a significant difference (Student’s t-test, P < 0.05). (b) LNCaP cells were serum-starved overnight and then treated with a synthetic androgen R1881 (1.0 nmol l−1) for 16 h in 5% charcoal-stripped FBS-supplied media. Total cellular RNAs were extracted for quantitative real-time PCR. MAPK8IP2 expression levels were normalized using the ribosomal protein RPS18 gene. The asterisk indicates a significant difference (Student’s t-test, P < 0.05). (c) LNCaP cells were treated with solvent control (DMSO), AR antagonist enzalutamide (Enza, 10 µmol l−1), or abiraterone (ABI, 10 µmol l−1) for 24 h in 5% charcoal-stripped FBS-supplied media. Cellular protein lysates were subjected to an anti-MAPK8IP2 Western blot assay. Actin blot served as the protein loading control. C4-2B and 22RV1 cells were pretreated with enzalutamide or abiraterone for 30 min, followed by R1881 (1.0 nmol l−1) treatment for 24 h in 5% charcoal-stripped FBS-supplied media. Western blot assay was conducted with an anti-MAPK8IP2 antibody, and the actin blot served as a protein loading control. The relative band density against the solvent control band was calculated using the ImageJ program after normalizing with the corresponding Actin blot band. MAPK: mitogen-activated protein kinase; MAPK8IP2: MAPK8-interacting protein 2; RPS18: ribosomal protein S18; ACTB: actin-beta; FBS: fetal bovine serum; AR: androgen receptor; DHT: dihydrotestosterone; DMSO: dimethyl sulfone; Etoh: ethanol.
MAPK8IP2 expression is associated with cellular mitotic genes in prostate cancer
To understand the potential biological role of MAPK8IP2 upregulation in prostate cancer, we conducted a transcriptome-wide correlation analysis. A total of 519 genes were identified to have a strong correlation (Spearman r > 0.3 or <−0.3) with MAPK8IP2 expression (Supplementary Table 3). Among these 519 genes, 515 genes were positively correlated with MAPK8IP2 expression. Gene enrichment analysis revealed that most of these genes were significantly clustered in cellular mitotic pathways, including cell cycle regulation and nuclear division (Figure 5a and Supplementary Table 4). Interestingly, four MAPK pathway genes (MAPK11, MAPK12, MAPK3, and MAPK8IP1) were strongly correlated with MAPK8IP2 expression (Supplementary Table 5). The significance of this strong correlation is under further investigation by our group. In contrast, four genes, including SH3RF2, steroid 5 alpha-reductase 2 (SRD5A2), FERM domain-containing kindlin 1 (FERMT1), and ADAMTS9 antisense RNA 1 (ADAMTS9-AS1), were negatively correlated with MAPK8IP2 expression (Figure 5b). Except for SH3RF2 (Figure 1b), SRD5A2, FERMT1, and ADAMTS9-AS1 were significantly downregulated in prostate cancer tissues compared to benign prostate tissues (P < 0.01; Figure 5c). Interestingly, only SRD5A2 expression significantly impacted the patient’s overall (P = 0.015), disease-specific (P = 0.001), and progression-free survival outcomes (P < 0.001), as shown in Figure 5d–5f.
Figure 5.
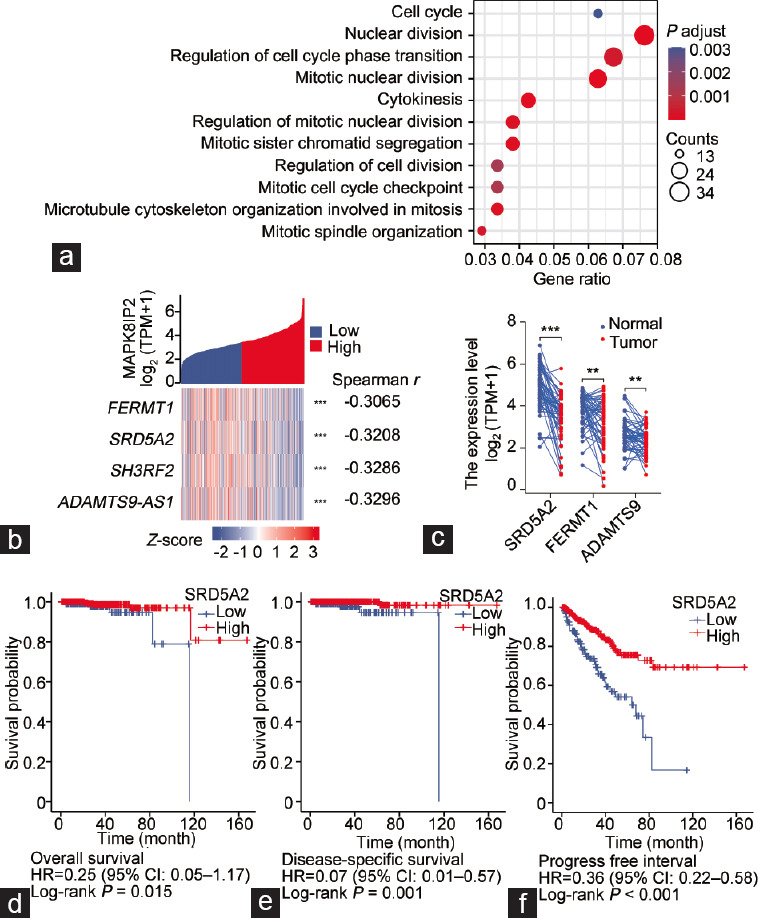
MAPK8IP2 expression is associated with cellular mitotic genes in prostate cancer cells. (a) Visualization of enriched gene clusters that were strongly correlated with MAPK8IP2 expression in the TCGA-PRAD dataset. Spearman r > 0.3, Q < 0.05. (b) Four genes were strongly and conversely correlated with MAPK8IP2 expression in the TCGA-PRAD dataset. The Spearman’s coefficients were listed on the right side. (c) Case-matched pair comparison of gene expression in prostate cancers using the TCGA-PRAD dataset. (d–f) Kaplan-Meier plot analysis of patient (d) overall survival, (e) disease-specific survival, and (f) progression-free interval using the TCGA-PRAD dataset. Patients were stratified using the SRD5A2 expression levels. Kaplan-Meier plot analysis was conducted using a minimum P-value approach, as previously described.37 MAPK: mitogen-activated protein kinase; MAPK8IP2: MAPK8-interacting protein 2; TCGA-PRAD: The Cancer Genome Atlas–Prostate Adenocarcinoma; SRD5A2: steroid 5 alpha-reductase 2.
Supplementary Table 5.
Mitogen-activated protein kinase-8-interacting protein 2 correlation with mitogen-activated protein kinase pathway component genes in prostate cancers
| gene_name | gene_id | gene_biotype | cor_pearson | p_pearson | cor_spearman | p_spearman |
|---|---|---|---|---|---|---|
| MAPK8IP1 | ENSG00000121653 | protein_coding | 0.550 | 0.000 | 0.489 | 0.000 |
| MAPK11 | ENSG00000185386 | protein_coding | 0.500 | 0.000 | 0.442 | 0.000 |
| MAPK12 | ENSG00000188130 | protein_coding | 0.448 | 0.000 | 0.403 | 0.000 |
| MAPK3 | ENSG00000102882 | protein_coding | 0.341 | 0.000 | 0.271 | 0.000 |
| MAPK9 | ENSG00000050748 | protein_coding | 0.255 | 0.000 | 0.206 | 0.000 |
| MAPK15 | ENSG00000181085 | protein_coding | 0.176 | 0.000 | 0.201 | 0.000 |
| MAPKAPK5-AS1 | ENSG00000234608 | lncRNA | 0.234 | 0.000 | 0.179 | 0.000 |
| MAPK4 | ENSG00000141639 | protein_coding | 0.179 | 0.000 | 0.168 | 0.000 |
| MAPK8IP3 | ENSG00000138834 | protein_coding | 0.160 | 0.000 | 0.145 | 0.001 |
| MAPKAPK5 | ENSG00000089022 | protein_coding | 0.192 | 0.000 | 0.131 | 0.003 |
| MAPKAP1 | ENSG00000119487 | protein_coding | 0.226 | 0.000 | 0.129 | 0.004 |
| MAPKAPK2 | ENSG00000162889 | protein_coding | 0.175 | 0.000 | 0.111 | 0.013 |
| MAPK8IP1P1 | ENSG00000262500 | processed_pseudogene | 0.083 | 0.064 | 0.101 | 0.025 |
| MAPK7 | ENSG00000166484 | protein_coding | 0.158 | 0.000 | 0.098 | 0.029 |
| MAPK8IP1P2 | ENSG00000263503 | processed_pseudogene | 0.067 | 0.132 | 0.097 | 0.030 |
| MAPK6-DT | ENSG00000259438 | lncRNA | 0.115 | 0.010 | 0.079 | 0.080 |
| MAPK14 | ENSG00000112062 | protein_coding | 0.131 | 0.003 | 0.078 | 0.081 |
| MAPKAPK5P1 | ENSG00000224498 | processed_pseudogene | 0.037 | 0.414 | 0.075 | 0.094 |
| MAPKAPK3 | ENSG00000114738 | protein_coding | 0.123 | 0.006 | 0.062 | 0.164 |
| MAPK1IP1L | ENSG00000168175 | protein_coding | 0.103 | 0.021 | 0.059 | 0.187 |
| MAPK6P1 | ENSG00000206144 | processed_pseudogene | −0.114 | 0.011 | 0.046 | 0.302 |
| MAPK1 | ENSG00000100030 | protein_coding | 0.088 | 0.050 | 0.039 | 0.387 |
| MAPKBP1 | ENSG00000137802 | protein_coding | 0.079 | 0.078 | 0.035 | 0.435 |
| MAPK6P2 | ENSG00000223488 | processed_pseudogene | 0.010 | 0.822 | 0.025 | 0.577 |
| MAPK6 | ENSG00000069956 | protein_coding | 0.032 | 0.470 | 0.004 | 0.931 |
| MAPK13 | ENSG00000156711 | protein_coding | 0.013 | 0.772 | −0.010 | 0.832 |
| MAPK6P6 | ENSG00000226389 | processed_pseudogene | 0.010 | 0.832 | −0.011 | 0.813 |
| MAPK6P5 | ENSG00000254185 | processed_pseudogene | −0.045 | 0.316 | −0.024 | 0.595 |
| MAPK8 | ENSG00000107643 | protein_coding | 0.013 | 0.767 | −0.025 | 0.571 |
| MAPK10-AS1 | ENSG00000250062 | lncRNA | −0.182 | 0.000 | −0.050 | 0.266 |
| MAPK6P3 | ENSG00000237263 | processed_pseudogene | −0.195 | 0.000 | −0.052 | 0.242 |
| MAPK10 | ENSG00000109339 | protein_coding | −0.061 | 0.172 | −0.061 | 0.171 |
| MAPK6P4 | ENSG00000215179 | processed_pseudogene | −0.128 | 0.004 | −0.082 | 0.067 |
MAPK: mitogen-activated protein kinase; MAPK8IP1: MAPK-8-interacting protein 1; cor: correlation
DISCUSSION
To explore potent prognostic factors that possess a biological relevance in prostate cancer, we conducted a comprehensive analysis of MAPK scaffold protein gene profiles. Our results showed that only MAPK8IP2 expression was significantly upregulated in prostate cancer among the ten scaffold protein genes. MAPK8IP2 upregulation was associated with higher Gleason scores, late tumor stages, lymph node invasion, postsurgery residue tumor incidences, shorter disease-free intervals, quicker biochemical relapses, and poor overall survival outcomes. Further analysis found that MAPK8IP2 upregulation was correlated with tumor genetic abnormalities, including TMB and FGA indexes, TP53 gene mutation, and promoter hypomethylation. MAPK8IP2 expression was positively associated with AR signaling score and AR-V7 expression level. Consistently, androgen stimulation significantly increased MAPK8IP2 expression in AR-positive prostate cancer cells. However, AR antagonists only suppressed MAPK8IP2 expression in androgen-sensitive but not in castration-resistant prostate cancer cells. These results demonstrated that MAPK8IP2 was significantly upregulated in prostate cancer and associated with disease progression, representing a robust prognostic factor for prostate cancer management with a strong potential for biological relevance.
MAPK8IP2 is the scaffold protein of JNK1, JNK2, and p38/SAPK modules. It recruits multiple signaling molecules including delta-like noncanonical Notch ligand 1 (DLK1), mitogen-activated protein kinase kinase kinase 10 (MAP3K10), and mitogen-activated protein kinase kinase kinase 11 (MAP3K11) as the M3Ks and mitogen-activated protein kinase kinase 7 (MAP2K7) as the M2K.2,3 In human prostate cancer LNCaP cells, JNK activity was required for phorbol ester-, thapsigargin-, or 4-HPR-induced apoptosis,39,40 which was suppressed by MAPK8IP1.40,41 Although MAPK8IP1 and MAPK8IP2 proteins were shown to facilitate JNK activation,6 MAPK8IP1 also inactivated JNK through MKP7-dependent JNK dephosphorylation.5 So far, it is not clear whether MAPK8IP2 also negatively modulates JNK activity. In addition, p38MAPK was recently identified as an upstream kinase for heat-shock protein 27 (HSP27) in facilitating AR signaling and castration-resistant progression of prostate cancer.42 Given the critical scaffolding role of MAPK8IP2 in facilitating p38MAPK activation,43,44 it is postulated that MAPK8IP2 might be involved in p38MAPK/HSP27 pathway in promoting AR signaling and prostate cancer progression. However, more mechanistic investigations are needed to verify this hypothesis.
Aberrant AR activation is the most critical driving force in prostate cancer development and progression.45 We found that MAPK8IP2 expression was increased after androgen (DHT and R1881) stimulation in AR-positive prostate cancer cells. At the same time, the AR antagonists blocked this event only in androgen-sensitive but not in castration-resistant cells. MAPK8IP2 expression was strongly correlated with the clinical biomarker PSA level and was associated with biochemical relapse, which was also a sign of AR reactivation. In CRPC tumors, MAPK8IP2 expression was strongly correlated with the AR score, a transcriptional index of AR reactivation. These results demonstrated that MAPK8IP2 expression is modulated by AR signaling in prostate cancer, although a mechanistic investigation is needed to determine its role in castration-resistant progression.
This study also found that MAPK8IP2 expression was strongly associated with cellular mitotic regulatory genes, indicating that MAPK8IP2 upregulation might potentially promote cell proliferation in prostate cancer. In contrast to more than 500 positively correlated genes, MAPK8IP2 expression was negatively and strongly associated with only four genes, JNK scaffold protein SH3RF2, steroid metabolic enzyme SRD5A2, integrin-related FERMT1, and long noncoding RNA (lncRNA) ADAMTS9-AS1. Since SH3RF2 expression was not significantly altered in malignant prostate tissues, it is unlikely to play an essential role in prostate cancer. SRD5A2 is a critical enzyme in sex hormone metabolism, especially in prostate cancer tissues.46 Previous reports showed that SRD5A2 expression was significantly reduced in primary prostate cancer46 and CRPC patients,47 consistent with our findings. In addition, androgen stimulation was reported to suppress SRD5A2 expression in prostate cancer cells,48 which might explain the negative correlation between MAPK8IP2 and SRD5A2. Finally, the lncRNA ADAMTS9-AS1 was recently reported as a tumor suppressor in prostate cancers by modulating cyclin D1.49,50 Further investigation is warranted to dissect the connection of MAPK8IP2 and ADAMTS9-AS1 in prostate cancer.
CONCLUSIONS
Our comprehensive analysis revealed that MAPK8IP2 expression in prostate cancer tissues was significantly upregulated compared with that in the benign compartments. MAPK8IP2 upregulation was strongly correlated with disease progression and patient survival outcomes. In addition, MAPK8IP2 expression increased after AR activation and tightly correlated with the AR reactivation in castration-resistant prostate cancer. These results suggest that MAPK8IP2 is an AR-modulated gene associated with prostate cancer progression. MAPK8IP2 upregulation might serve as a novel prognostic factor for prostate cancer management.
AUTHOR CONTRIBUTIONS
JH and BY Li designed the study. BY Lin analyzed the bioinformatics data. WL, JCL, and JL performed the qPCR, Western blot, and IHC assays. JH and BY Li drafted the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
Expression of KSR1, MAPK8IP1-2, and CCM2 genes in prostate cancers was analyzed using the cDNA microarray datasets derived from previous reports.22,51,52 The data figures were generated on the Oncomine platform. KSR1: kinase suppressor of ras 1; MAPK8IP1: MAPK-8-interacting protein 1; CCM2: cerebral cavernous malformation 2; cDNA: complementary DNA.
Genetic abnormalities of KSR1 and MAPK8IP2 in prostate cancers. A survey of the genetic abnormality was conducted on the cBioportal platform using the TCGA-PRAD cohort (a) and other 21 studies (b). KSR1: kinase suppressor of ras 1; MAPK8IP1: MAPK-8-interacting protein 1; TCAG-PRAD: The Cancer Genome Atlas-Prostate Adenocarcinoma.
ACKNOWLEDGMENTS
The authors are grateful for the TMA slides obtained from Dr. Jiaoti Huang at Duke University (Durham, NC, USA). The Department of Defense Prostate Cancer Research Program PC190026 project partially supported this work to Dr. Ben-Yi Li.
REFERENCES
- 1.Morrison DK. MAP kinase pathways. Cold Spring Harb Perspect Biol. 2012;4:a011254. doi: 10.1101/cshperspect.a011254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engstrom W, Ward A, Moorwood K. The role of scaffold proteins in JNK signalling. Cell Prolif. 2010;43:56–66. doi: 10.1111/j.1365-2184.2009.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhanasekaran DN, Kashef K, Lee CM, Xu H, Reddy EP. Scaffold proteins of MAP-kinase modules. Oncogene. 2007;26:3185–202. doi: 10.1038/sj.onc.1210411. [DOI] [PubMed] [Google Scholar]
- 4.Canovas B, Nebreda AR. Diversity and versatility of p38 kinase signalling in health and disease. Nat Rev Mol Cell Biol. 2021;22:346–66. doi: 10.1038/s41580-020-00322-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willoughby EA, Perkins GR, Collins MK, Whitmarsh AJ. The JNK-interacting protein-1 scaffold protein targets MAPK phosphatase-7 to dephosphorylate JNK. J Biol Chem. 2003;278:10731–6. doi: 10.1074/jbc.M207324200. [DOI] [PubMed] [Google Scholar]
- 6.Yasuda J, Whitmarsh AJ, Cavanagh J, Sharma M, Davis RJ. The JIP group of mitogen-activated protein kinase scaffold proteins. Mol Cell Biol. 1999;19:7245–54. doi: 10.1128/mcb.19.10.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van den Berg MC, van Gogh IJ, Smits AM, van Triest M, Dansen TB, et al. The small GTPase RALA controls c-Jun N-terminal kinase-mediated FOXO activation by regulation of a JIP1 scaffold complex. J Biol Chem. 2013;288:21729–41. doi: 10.1074/jbc.M113.463885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kennedy NJ, Martin G, Ehrhardt AG, Cavanagh-Kyros J, Kuan CY, et al. Requirement of JIP scaffold proteins for NMDA-mediated signal transduction. Genes Dev. 2007;21:2336–46. doi: 10.1101/gad.1563107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tam SY, Law HK. JNK in tumor microenvironment:present findings and challenges in clinical translation. Cancers (Basel) 2021;13:2196. doi: 10.3390/cancers13092196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y, et al. ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med. 2020;19:1997–2007. doi: 10.3892/etm.2020.8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braicu C, Buse M, Busuioc C, Drula R, Gulei D, et al. A comprehensive review on MAPK:a promising therapeutic target in cancer. Cancers (Basel) 2019;11:1618. doi: 10.3390/cancers11101618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benhaim L, Zhang W, Wakatsuki T, Yang D, Gerger A, et al. Genetic variants of kinase suppressors of Ras (KSR1) to predict survival in patients with ERalpha-positive advanced breast cancer. Pharmacogenomics J. 2015;15:235–40. doi: 10.1038/tpj.2014.58. [DOI] [PubMed] [Google Scholar]
- 13.Xu Y, Li R, Li X, Dong N, Wu D, et al. An autophagy-related gene signature associated with clinical prognosis and immune microenvironment in gliomas. Front Oncol. 2020;10:571189. doi: 10.3389/fonc.2020.571189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jia Y, Shen M, Zhou Y, Liu H. Development of a 12-biomarkers-based prognostic model for pancreatic cancer using multi-omics integrated analysis. Acta Biochim Pol. 2020;67:501–8. doi: 10.18388/abp.2020_5225. [DOI] [PubMed] [Google Scholar]
- 15.Miao ZF, Wang ZN, Zhao TT, Xu YY, Wu JH, et al. Overexpression of SPAG9 in human gastric cancer is correlated with poor prognosis. Virchows Arch. 2015;467:525–33. doi: 10.1007/s00428-015-1826-4. [DOI] [PubMed] [Google Scholar]
- 16.Sugihara T, Nakagawa S, Sasajima Y, Ichinose T, Hiraike H, et al. Loss of the cell polarity determinant human Discs-large is a novel molecular marker of nodal involvement and poor prognosis in endometrial cancer. Br J Cancer. 2016;114:1012–8. doi: 10.1038/bjc.2016.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X, Xu S, McClelland M, Rahmatpanah F, Sawyers A, et al. An accurate prostate cancer prognosticator using a seven-gene signature plus Gleason score and taking cell type heterogeneity into account. PLoS One. 2012;7:e45178. doi: 10.1371/journal.pone.0045178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irshad S, Bansal M, Castillo-Martin M, Zheng T, Aytes A, et al. A molecular signature predictive of indolent prostate cancer. Sci Transl Med. 2013;5:202ra122. doi: 10.1126/scitranslmed.3006408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manjang K, Yli-Harja O, Dehmer M, Emmert-Streib F. Limitations of explainability for established prognostic biomarkers of prostate cancer. Front Genet. 2021;12:649429. doi: 10.3389/fgene.2021.649429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–8. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, et al. ONCOMINE:a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6:1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varambally S, Yu J, Laxman B, Rhodes DR, Mehra R, et al. Integrative genomic and proteomic analysis of prostate cancer reveals signatures of metastatic progression. Cancer Cell. 2005;8:393–406. doi: 10.1016/j.ccr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Wallace TA, Prueitt RL, Yi M, Howe TM, Gillespie JW, et al. Tumor immunobiological differences in prostate cancer between African-American and European-American men. Cancer Res. 2008;68:927–36. doi: 10.1158/0008-5472.CAN-07-2608. [DOI] [PubMed] [Google Scholar]
- 24.Vanaja DK, Cheville JC, Iturria SJ, Young CY. Transcriptional silencing of zinc finger protein 185 identified by expression profiling is associated with prostate cancer progression. Cancer Res. 2003;63:3877–82. [PubMed] [Google Scholar]
- 25.Oudes AJ, Campbell DS, Sorensen CM, Walashek LS, True LD, et al. Transcriptomes of human prostate cells. BMC Genomics. 2006;7:92. doi: 10.1186/1471-2164-7-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, et al. The cBio cancer genomics portal:an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–4. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerhauser C, Favero F, Risch T, Simon R, Feuerbach L, et al. Molecular evolution of early-onset prostate cancer identifies molecular risk markers and clinical trajectories. Cancer Cell. 2018;34:996–1011.e8. doi: 10.1016/j.ccell.2018.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abida W, Cyrta J, Heller G, Prandi D, Armenia J, et al. Genomic correlates of clinical outcome in advanced prostate cancer. Proc Natl Acad Sci U S A. 2019;116:11428–36. doi: 10.1073/pnas.1902651116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menyhart O, Nagy A, Gyorffy B. Determining consistent prognostic biomarkers of overall survival and vascular invasion in hepatocellular carcinoma. R Soc Open Sci. 2018;5:181006. doi: 10.1098/rsos.181006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nickols NG, Dervan PB. Suppression of androgen receptor-mediated gene expression by a sequence-specific DNA-binding polyamide. Proc Natl Acad Sci U S A. 2007;104:10418–23. doi: 10.1073/pnas.0704217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao X, Thrasher JB, Pelling J, Holzbeierlein J, Sang QX, et al. Androgen stimulates matrix metalloproteinase-2 expression in human prostate cancer. Endocrinology. 2003;144:1656–63. doi: 10.1210/en.2002-0157. [DOI] [PubMed] [Google Scholar]
- 33.Zhu R, Tu Y, Chang J, Xu H, Li JC, et al. The orphan nuclear receptor gene NR0B2 is a favorite prognosis factor modulated by multiple cellular signal pathways in human liver cancers. Front Oncol. 2021;11:691199. doi: 10.3389/fonc.2021.691199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu H, Li C, Mozziconacci O, Zhu R, Xu Y, et al. Xanthine oxidase-mediated oxidative stress promotes cancer cell-specific apoptosis. Free Radic Biol Med. 2019;139:70–9. doi: 10.1016/j.freeradbiomed.2019.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang Y, Chen R, Huang Y, Li G, Huang Y, et al. Natural compound Alternol induces oxidative stress-dependent apoptotic cell death preferentially in prostate cancer cells. Mol Cancer Ther. 2014;13:1526–36. doi: 10.1158/1535-7163.MCT-13-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tao C, Liu W, Yan X, Yang M, Yao S, et al. PAQR5 expression is suppressed by TGFbeta1 and associated with a poor survival outcome in renal clear cell carcinoma. Front Oncol. 2021;11:827344. doi: 10.3389/fonc.2021.827344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J, Lichtenberg T, Hoadley KA, Poisson LM, Lazar AJ, et al. An integrated TCGA pan-cancer clinical data resource to drive high-quality survival outcome analytics. Cell. 2018;173:400–16.e11. doi: 10.1016/j.cell.2018.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Wang Y, Ci X, Choi SY, Crea F, et al. Molecular events in neuroendocrine prostate cancer development. Nat Rev Urol. 2021;18:581–96. doi: 10.1038/s41585-021-00490-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Engedal N, Korkmaz CG, Saatcioglu F. C-Jun N-terminal kinase is required for phorbol ester- and thapsigargin-induced apoptosis in the androgen responsive prostate cancer cell line LNCaP. Oncogene. 2002;21:1017–27. doi: 10.1038/sj.onc.1205167. [DOI] [PubMed] [Google Scholar]
- 40.Tawadros T, Martin D, Abderrahmani A, Leisinger HJ, Waeber G, et al. IB1/JIP-1 controls JNK activation and increased during prostatic LNCaP cells neuroendocrine differentiation. Cell Signal. 2005;17:929–39. doi: 10.1016/j.cellsig.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 41.Ikezoe T, Yang Y, Taguchi H, Koeffler HP. JNK interacting protein 1 (JIP-1) protects LNCaP prostate cancer cells from growth arrest and apoptosis mediated by 12-0-tetradecanoylphorbol-13-acetate (TPA) Br J Cancer. 2004;90:2017–24. doi: 10.1038/sj.bjc.6601834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheung S, Jain P, So J, Shahidi S, Chung S, et al. p38 MAPK inhibition mitigates hypoxia-induced AR Signaling in castration-resistant prostate cancer. Cancers (Basel) 2021;13:831. doi: 10.3390/cancers13040831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buchsbaum RJ, Connolly BA, Feig LA. Interaction of Rac exchange factors Tiam1 and Ras-GRF1 with a scaffold for the p38 mitogen-activated protein kinase cascade. Mol Cell Biol. 2002;22:4073–85. doi: 10.1128/MCB.22.12.4073-4085.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schoorlemmer J, Goldfarb M. Fibroblast growth factor homologous factors and the islet brain-2 scaffold protein regulate activation of a stress-activated protein kinase. J Biol Chem. 2002;277:49111–9. doi: 10.1074/jbc.M205520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schiewer MJ, Knudsen KE. Basic science and molecular genetics of prostate cancer aggressiveness. Urol Clin North Am. 2021;48:339–47. doi: 10.1016/j.ucl.2021.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Marks LS. 5alpha-reductase:history and clinical importance. Rev Urol. 2004;6(Suppl 9):S11–21. [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Z, Deng T, Long X, Lin X, Wu S, et al. Methylation of SRD5A2 promoter predicts a better outcome for castration-resistant prostate cancer patients undergoing androgen deprivation therapy. PLoS One. 2020;15:e0229754. doi: 10.1371/journal.pone.0229754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Audet-Walsh E, Yee T, Tam IS, Giguere V. Inverse regulation of DHT synthesis enzymes 5alpha-reductase types 1 and 2 by the androgen receptor in prostate cancer. Endocrinology. 2017;158:1015–21. doi: 10.1210/en.2016-1926. [DOI] [PubMed] [Google Scholar]
- 49.Wan J, Jiang S, Jiang Y, Ma W, Wang X, et al. Corrigendum:data mining and expression analysis of differential lncRNA ADAMTS9-AS1 in prostate cancer. Front Genet. 2020;11:361. doi: 10.3389/fgene.2020.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou Z, Wu X, Zhou Y, Yan W. Long non-coding RNA ADAMTS9-AS1 inhibits the progression of prostate cancer by modulating the miR-142-5p/CCND1 axis. J Gene Med. 2021;23:e3331. doi: 10.1002/jgm.3331. [DOI] [PubMed] [Google Scholar]
- 51.LaTulippe E, Satagopan J, Smith A, Scher H, Scardino P, et al. Comprehensive gene expression analysis of prostate cancer reveals distinct transcriptional programs associated with metastatic disease. Cancer Res. 2002;62:4499–506. [PubMed] [Google Scholar]
- 52.Grasso CS, Wu YM, Robinson DR, Cao X, Dhanasekaran SM, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487:239–43. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expression of KSR1, MAPK8IP1-2, and CCM2 genes in prostate cancers was analyzed using the cDNA microarray datasets derived from previous reports.22,51,52 The data figures were generated on the Oncomine platform. KSR1: kinase suppressor of ras 1; MAPK8IP1: MAPK-8-interacting protein 1; CCM2: cerebral cavernous malformation 2; cDNA: complementary DNA.
Genetic abnormalities of KSR1 and MAPK8IP2 in prostate cancers. A survey of the genetic abnormality was conducted on the cBioportal platform using the TCGA-PRAD cohort (a) and other 21 studies (b). KSR1: kinase suppressor of ras 1; MAPK8IP1: MAPK-8-interacting protein 1; TCAG-PRAD: The Cancer Genome Atlas-Prostate Adenocarcinoma.