Abstract
The circadian clock is an evolutionary molecular product that is associated with better adaptation to changes in the external environment. Disruption of the circadian rhythm plays a critical role in tumorigenesis of many kinds of cancers, including prostate cancer (PCa). Integrating circadian rhythm into PCa research not only brings a closer understanding of the mechanisms of PCa but also provides new and effective options for the precise treatment of patients with PCa. This review begins with patterns of the circadian clock, highlights the role of the disruption of circadian rhythms in PCa at the epidemiological and molecular levels, and discusses possible new approaches to PCa therapy that target the circadian clock.
Keywords: chronotherapy, circadian clock, circadian rhythm, prostate cancer
INTRODUCTION
As early as the 18th century, Mairan, a French scientist found that light did not affect the 24-h fluctuation of mimosa leaf activity, revealing the existing potential endogenous rhythms of organisms. Circadian rhythm can help reconcile a large amount of behaviors, physiological processes, and functions in the human body with circadian changes, such as the sleep–wake cycle, feeding, and other autonomic activities, as well as essential physiological activities, such as the maintenance of proper blood pressure, heart rate, basal body temperature, hormone levels, and the normal operation of cell metabolism, cell proliferation, and immune regulation.1,2,3 Therefore, unsurprisingly, circadian disruption is associated with poor health status and many diseases, such as aging,4 inflammation status,5 degenerative changes in the central nervous system,6 cardiovascular diseases,7 metabolic diseases,8 and cancers,9 including prostate cancer (PCa).10
Generally, the generation, maintenance, and regulation of circadian rhythm physiologically depend on central and peripheral clock systems as well as the coordination of rhythm input and output systems. The central clock generated in the suprachiasmatic nucleus (SCN) plays a leading role in circadian rhythm regulation, cooperating with environmental signals and output systems, such as the autonomic nervous system and the neuroendocrine system.11,12 As the secondary pacemaker of the circadian rhythm, on the one hand, peripheral clocks are controlled by various signal factors of neurohumoral regulatory systems under the central clock directly or indirectly. On the other hand, they can operate independently of the control of SCN. At the cellular level, the circadian rhythm is a 24-h oscillation consisting of a series of transcriptional and translational regulatory feedback loops. Posttranslational modifications and degradation of clock genes and clock control genes are also involved in maintaining the stabilization of homeostasis, behaviors, and other basic life activities in the human body related to better adaptation to changes in the external environment.4,13–15
MOLECULAR REGULATORY MECHANISMS OF THE CIRCADIAN CLOCK
As mentioned above, the generation and maintenance of circadian rhythm are controlled by clock genes and clock control genes. A study has shown that approximately 10% of genes in cells are rhythmically expressed.16 Circadian genes exert their effects mainly through the transcriptional translational oscillator (TTO), the main part of the classical circadian clock regulation network. TTO primarily consists of two interconnected negative feedback loops. Circadian locomotor output cycles kaput (CLOCK) and its chaperone neuronal per-arnt-sim (PAS) domain-containing protein 2 (NPAS2) form a heterodimer with aryl hydrocarbon receptor nuclear translocator-like protein 1 (ARNTL; also identified in brain and muscle as Arnt-like protein-1, BMAL1) to initiate the transcription of target genes encoding period circadian protein (PER) and cryptochrome (CRY), by directly binding to the E-box enhancer element (the sequence CACGTG) located in its upstream promoter regions in the daytime.17,18,19 With the accumulation of PER and CRY proteins, the newly formed PER and CRY protein heterodimers are transferred into the nucleus and in turn interact with CLOCK/BMAL1 heterodimers to suppress their own transcription.19 At the same time, the levels of CRY and PER proteins are decreased by ubiquitination activity and degradation of several ligases,20,21 relieving the negative feedback suppression intensity of CLOCK/BMAL1 and ultimately resuming a new TTO cycle. In addition, CLOCK/BMAL1 heterodimers can induce the expression of nuclear receptors Rev-erb α and retinoic acid receptor (RAR)-related orphan receptors (RORs). The combination of Rev-erb α or RORα with ROR binding element (RRE) in the promoter region of BMAL1 promotes or represses BMAL1 transcription, respectively, which forms the auxiliary loop of TTO.22,23 Because of these processes, the oscillation cycle of upregulation and downregulation of core circadian rhythm-regulating genes takes approximately 24 h. This oscillation not only endows the transcriptional activity of the CLOCK/BMAL1 heterodimer with a rhythmic characteristic, but also leads to the rhythmical expression of downstream clock control genes, and thus, a circadian rhythm forms.24 Furthermore, basic leucine zipper proteins, such as D-box binding protein (DBP; the direct target of BMAL1/CLOCK heterodimers), and the repressor E4 promoter-binding protein 4 (E4BP4; the direct target of REV-erb α), can also regulate PER expression via the D-box, further complicating the signal transmission network of the TTO loop.25,26
CIRCADIAN CLOCK DISRUPTION AND PCA
PCa is one of the most common cancers in men living in European and American areas and is also the main cause of death.27 According to the 2022 cancer (CA) estimated statistics, PCa ranks first in the prevalence of male malignant tumors and second in mortality, separately accounting for 27% and 11% in the USA.28 Although good progress has been made in the treatment of PCa, PCa remains one of the major health threats to men worldwide.29,30,31 Despite the relatively high morbidity and mortality, the etiology of PCa is not fully understood. However, according to some migrant studies, Asian Americans had a higher PCa rate than their counterparts living in Asia, indicating the significance of environment, lifestyle, and consequent circadian rhythm disruption in PCa formation.32,33,34 More importantly, as an age-related disease, the incidences of benign prostatic hyperplasia (BPH) and PCa increase with age, and the circadian rhythms also deteriorate with age due to many reasons, revealing a possible relationship between the circadian clock and PCa.4,35 Transurethral plasmakinetic resection of prostate (TUPKP) is one of the most mature management patterns for BPH patients with detailed and comprehensive guidelines,36,37 while PCa patients need more attention. As a supplement, increasing evidence shows the association between circadian rhythm disruption and prostate carcinogenesis as well.10 Both exogenous and endogenous factors can induce PCa risk-associated circadian disruption according to a large amount of epidemiological data and many consequent studies, including endogenous factors such as aging and alternations of the endocrine system, as well as changes in night-shift work, sleep patterns, and intermittent fasting as exogenous alterations. Figure 1 illustrates the possible link among endogenous factors, exogenous factors, circadian rhythm, and PCa.
Figure 1.
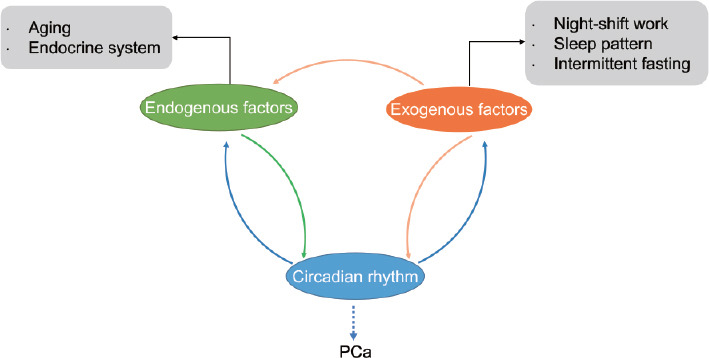
Relationship among exogenous factors, endogenous factors, circadian rhythm and PCa. Changes in endogenous factors and exogenous factors are both involved in the disruption of circadian rhythm, which is potentially related to PCa. Alternations in circadian rhythm also impact endogenous and exogenous factors. In addition, alternations in the lifestyle of exogenous factors, including night-shift work, changes in sleep patterns and intermittent fasting, play a role in aging and endocrine disturbance. PCa: prostate cancer.
Aging is closely tied to the circadian clock. Many studies have suggested that the structure and composition of the SCN are related to age. Compared with young people, the total number and volume of SCN cells decreased in elderly individuals,38,39 and the composition of SCN also changed, mainly in the decreased expression of arginine vasopressin (AVP) and vasoactive intestinal polypeptide (VIP).40,41,42 In addition, the decrease in γ-aminobutyric acid (GABA) with aging also affects synaptic transmission in the SCN to some extent, which could underlie aging-related SCN dysfunction, resulting in circadian disruption.43 This was also suggested by the deterioration of the electrical activity rhythmicity of the SCN associated with aging.44 More remarkably, although the relationship between aging and circadian gene expression has not been directly confirmed, a study found that rhythmic PER expression decreased with aging.45 In addition, it was reported that aging perhaps inhibited oscillation in circadian gene expression.46,47 In addition, the circadian rhythm also affects the aging process in turn. Aging experimental animals that received fetal SCN tissue transplantation had a longer life span, while their circadian rhythms were restored.48 In contrast, experimental animals receiving clock gene knockout showed accelerated aging phenotypes. For example, in addition to the loss of circadian rhythms, BMAL1-deficient mice also developed symptoms of premature aging, such as cataracts and organ shrinkage.49 Similarly, the life span of Drosophila lacking PER was significantly shortened.50 The interaction between circadian rhythm and aging may be mediated by sirtuin 1 (SIRT1), an nicotinamide adenine dinucleotide (NAD)-dependent protein deacetylase.4 SIRT1 is significantly elevated in human PCa, acting as an oncogene and epigenetic regulator in tumorigenesis by anti-apoptotic activity.51,52 It plays a critical role in tumor cell proliferation, invasion, migration, and drug resistance of PCa.53,54 Therefore, aging and aging-related circadian clock disruption may play an important role in the occurrence and development of PCa.
For the endocrine system, the alterations in melatonin and cortisol mainly contribute to circadian disruption. Rhythmic melatonin release is under the control of SCN and it plays a role in modulating the downstream rhythms of the main circadian clock. For example, endogenous rhythmic release of melatonin is involved in healthy sleep patterns, maintenance of basal body temperature, and physiology of inner retinal photoreceptors.55,56 Exogenous melatonin supplementation can act as a “chronobiotic” in the treatment of circadian disruption, which was also found to inhibit tumor initiation and growth in animal models and human breast cancer cell lines.55,57–59 The secretion of total melatonin decreases with age, and the rhythm of melatonin secretion also changes in elderly people compared to younger people.60,61,62 Interestingly, a study by Zeitzer et al.63 reported that very healthy older men had a melatonin rhythm similar to that of younger people, which may suggest that normal melatonin rhythms can maintain normal circadian rhythms, and thus protect against diseases, including PCa. Similar to melatonin, cortisol release is regulated by SCN and is involved in the rhythmic expression of downstream clock genes.64,65,67 Similarly, changes in cortisol rhythm are age-related, and its disruption has been linked to age-related diseases.68,69,70,71
In terms of night-shift work, studies on whether night-shift work involving disruption of circadian rhythm is related to an increased PCa risk remain contradictory. For example, a large case-control study by Barul et al.72 found no supportive result for a major role of night-shift work in PCa development. In contrast, another large case-control study conducted by Lozano-Lorca et al.73 suggested that night-shift work could increase PCa risk. Several cohort studies also showed controversial results. For instance, Behrens et al.74 reported increased risks of PCa among men involved in night-shift work, while Dickerman et al.75 found no significant relation between night-shift work and PCa risk. In general, among large clinical studies published in recent years, six suggested a link between night-shift work and PCa,73,74,76–79 while 4 studies did not75,80–82 (Table 1). Several previous meta-analyses and systematic reviews did not reach consistent conclusions about night-shift work and PCa risk.10,83 Among them, the most recent meta-analysis published in 2020 conducted by Rivera-Izquierdo et al.84 reported no association between rotating/night-shift work and PCa, as did another meta-analysis conducted by Dun et al.85 Reasons for these contradictory results may include the following: (1) the lack of a standardized definition of night-shift work and exposure assessment among different studies; (2) it is difficult to keep the baseline levels consistent in the study population, since many other factors, such as sleep–wake cycles, eating habits, and even mood changes could affect the subjects’ own circadian rhythms; and (3) each person in those studies had their own circadian rhythm, which was not equally affected by night-shift work. Moreover, some epidemiological studies also showed that changes in sleep patterns, such as sleep with low quality or short duration, were associated with increased risks of PCa.86,87 However, a systematic review including 16 epidemiological studies did not draw a definite conclusion and suggested that more studies were still needed on the potential impact of circadian disruption and sleep patterns on PCa risk.88 Furthermore, intermittent fasting seems to regulate the circadian clock and protect against carcinogenesis.89 A study by Palomar-Cros et al.90 suggested that a prolonged night-time fasting duration might be associated with a lower risk of PCa. Remarkably, a study in Drosophila suggested that the longevity extension from fasting was achieved by increasing the expression of several peripheral clock genes that regulate fat metabolism.91 Fat metabolism was confirmed to play a critical role in PCa, especially in castration-resistant PCa (CRPC).92 Presumably, we can speculate that fasting may reduce the risk of PCa, and even play a role in CRPC by regulating the circadian rhythm. Changes in the circadian clock can also affect these exogenous factors in reverse. For example, CLOCK deficiency can lead to premature cataracts,93 which affects melatonin secretion and changes sleep patterns.94 There is no consensus on the effect of different exogenous factors causing circadian rhythm disruption on PCa risk, which may be due to the existence of many endogenous factors, such as aging and endocrine disturbance mentioned above, which were not covered by previous baseline criteria for epidemiological study populations.
Table 1.
Epidemiological association between night-shift work and prostate cancer
| Study | Study type | Shift patterns | Participants | Conclusions |
|---|---|---|---|---|
| Lozano-Lorca et al.73 2020 | Case–control study | Working partly or entirely ≥3 h between 22:00 and 06:00, at least three times per month | 465 PCa cases and 410 controls | Night shifts, especially rotating night shifts, could increase PCa risk |
| Barul et al.72 2019 | Case–control study | Working ≥3 h between midnight and 05:00, ≥3 nights per month for ≥1 year | 1904 PCa cases and 1965 controls | Night-shift work was not associated with PCa development |
| Wendeu-Foyet et al.81 2018 | Case–control study | Shift starting between midnight and 06:00 Shift ending between 21:00 and 02:00 Shift starting before 00:00 and ending after 05:00 | 819 PCa cases and 879 controls | Night work was not associated with PCa |
| Behrens et al.74 2017 | Cohort study | A shift including work between 00:00 and 05:00 | 1757 men | Shift or night work was associated with increased risk of PCa |
| Åkerstedt et al.82 2017 | Cohort study | Lack of a definite definition of shifts | 12 322 male twins | Night work was not significantly associated with PCa |
| Dickerman et al.75 2016 | Cohort study | Night shifts in either a 2-shift or 3-shift pattern | 11 370 twins | Shift work was not significantly associated with PCa risk |
| Papantoniou et al.79 2015 | Case–control study | Working partly or entirely between 00:00 and 06:00, at least three times per month | 1095 PCa cases and 1388 controls | Night-shift work was associated with PCa, particularly for tumors with worse prognosis |
| Hammer et al.80 2015 | Cohort study | 3 ×12 h patterns and 4 ×12 h patterns | 12 609 rotating shift workers and 15 219 daytime workers | Both groups of workers had a higher incidence of PCa than the general population |
| Parent et al.78 2012 | Case–control study | Working between 01:00 and 02:00 for at least 6 months | 400 PCa cases and 533 controls | Night work may increase PCa risk |
| Conlon et al.77 2007 | Case–control study | Lack of a definite definition of shifts | 760 PCa cases and 1632 controls | Working full-time rotating shifts may be associated with increased risk of PCa |
| Kubo et al.76 2006 | Cohort study | Lack of a definite definition of shifts | 14 052 men | Rotating-shift was associated with increased risk of PCa |
PCa: prostate cancer
MOLECULAR MECHANISMS OF THE CIRCADIAN CLOCK IN PCA
At the cellular level, when studying exogenous factors in PCa risk, the role of circadian-associated genes in PCa should also be taken into account because they have been shown to probably regulate the influence of night-shift work in breast cancer.95,96 A variety of epidemiological studies suggested that variants of CRY1, CRY2, NPAS2, CLOCK, PER2, and RORA were associated with increased or decreased PCa risk97,98,99,100,101,102 (Table 2). Additionally, CRY2, NPAS2, RORA, and BMAL1 were found to be associated with PCa progression.103 Several studies based on epidemiological study of prostate cancer (EPICAP, a population-based case–control study in France)104 showed that ARNTL, NPAS2, and RORA were significantly associated with PCa, especially among night workers, and RORA was related to aggressive PCa.105,106 Under an equilibrated status, circadian genes may regulate and even inhibit tumor progression by regulating DNA repair, and the process of the cell cycle, and ultimately affect cell proliferation.107 In addition, circadian genes participate in inflammation and tumor immunity108 (Figure 2).
Table 2.
Genetic association between circadian genes polymorphisms and prostate cancer
| Circadian gene | SNP | PCa risk | Reference |
|---|---|---|---|
| NPAS2 | rs895521, rs1369481, rs2305160 | Decreased | 97,98,102 |
| rs17024926 | Increased | ||
| rs6542993 | Increased risk of PCa progression | ||
| BMAL1 | rs7950226 | Increased | 98,101 |
| rs142435152 | Associated | ||
| CLOCK | rs11133373 | Decreased in heterozygous variant | 98 |
| CRY1 | rs12315175, rs10778534 | Increased | 98–100 |
| rs7297614, rs1921126 | Associated with fatal PCa | ||
| CRY2 | rs2292912 | Decreased in heterozygous variant | 97,98 |
| rs1401417 | Increased | ||
| PER1 | rs885747 | Decreased in heterozygous variant | 98 |
| rs2289591 | Increased in heterozygous variant | ||
| PER2 | rs7602358 | Decreased in heterozygous variant | 98 |
| PER3 | rs1012477 | Decreased in heterozygous variant | 98,100 |
| rs228697 | Decreased | ||
| RORA | rs17191414 | Associated | 101 |
PCa: prostate cancer; SNP: single nucleotide polymorphism; NPAS2: neuronal per-arnt-sim (PAS) domain-containing protein 2; BMAL1: aryl hydrocarbon receptor nuclear translocator-like protein 1 (ARNTL, also identified in brain and muscle as Arnt-like protein-1); CLOCK: circadian locomotor output cycles kaput; CRY1: cryptochrome 1; CRY2: cryptochrome 2; PER1: period circadian protein 1; PER2: period circadian protein 2; PER3: period circadian protein 3; RORA: retinoic acid receptor (RAR)-related orphan receptor A
Figure 2.
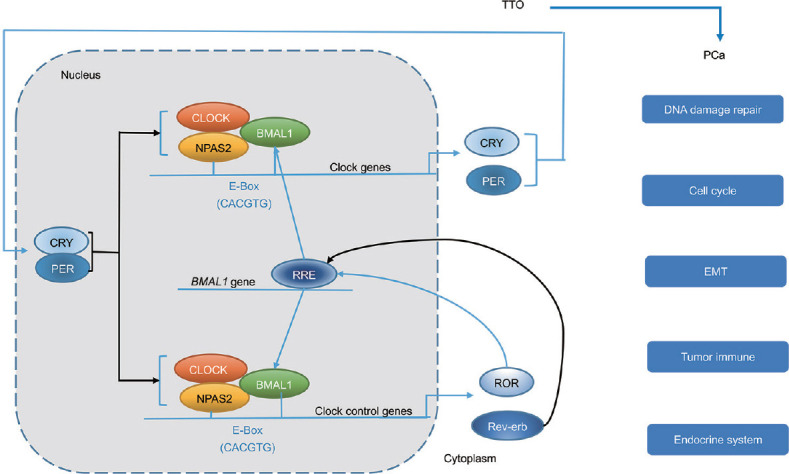
Circadian clock and PCa. The molecular organization of the circadian clock is based on the TTO, which is mainly composed of two negative feedback loops. Through the functions of TTO at the cellular level, the circadian clock eventually plays a role in DNA damage repair, the cell cycle, EMT, and the tumor immune and endocrine system of PCa. The blue arrows represent the positive effect and the black arrows represent the negative effect. TTO: transcriptional translational oscillator; PCa: prostate cancer; CLOCK: circadian locomotor output cycles kaput; BMAL1: aryl hydrocarbon receptor nuclear translocator-like protein 1 (ARNTL, also identified in brain and muscle as Arnt-like protein-1); NPAS2: neuronal per-arnt-sim (PAS) domain-containing protein 2; CRY: cryptochrome; PER: period circadian protein; ROR: retinoic acid receptor (RAR)-related orphan receptors; RRE: ROR binding element; EMT: epithelial-mesenchymal transition.
NPAS2 is suggested to play a putative role in c-myc transcriptional inhibition.109 NPAS2 is involved in the repair of DNA damage110 and cell cycle processes by regulating its diverse downstream genes.111 Until now, there have been few studies on the relationship between NPAS2 and PCa. A case–control study conducted by Chu et al.97 suggested that the NPAS2-variant A allele was related to a decreased PCa risk among men with less insulin resistance than their counterparts. Another large-scale case–control study based on Americans reported the linkage between NPAS2 variants and PCa susceptibility as well.98 Moreover, a new study conducted by Yu et al.102 revealed a potential relationship between NPAS2 expression and PCa progression. However, NPAS2 has been regarded as a prognostic biomarker of breast cancer and colorectal cancer,112,113 the silence of whose expression was reported to promote the proliferation and invasion of colorectal cancer cells,113 indicating possibly similar roles of NPAS2 in tumor development, metastasis, and prognosis in PCa.
The expression of CRY1 is predominantly regulated by ROR and REV-erb, and CRY2 is rather directly controlled by the CLOCK/BMAL1 heterodimer.114 CRY1 was found to be involved in DNA repair and the cell cycle process of PCa cells115 and its variants were associated with the prognosis of PCa.100 Previous studies on the relationship between CRY2 and PCa risk showed that different variants of CRY2 corresponded to different PCa risks.97,98 Currently, although there is only a little direct evidence linking CRY2 to PCa, studies about thyroid cancer and breast cancer have confirmed that high expression of CRY2 seems to be a protective factor against cancers.116,117 More importantly, animal experiments showed that the activation of the CLOCK/BMAL1 heterodimer caused by synchronous ablation of CRY1 and CRY2 could prevent cancer progression and improve prognosis, suggesting that the negative influence of circadian rhythm disruption might be minimized as long as the CLOCK/BMAL1 heterodimer was continuously activated.
PER1 also plays an important role in controlling DNA damage and cell growth, interacting with proteins in cell-cycle pathways as well.118 Not only was PER1 regulated by androgen in PCa cells, but overexpression of PER1 also led to significant growth inhibition and apoptosis in PCa cells.119 In addition, overexpression of PER2 was also found to result in a significant inhibition of PCa cell growth and viability,120 which was related to mPER2 to an extent, a tumor suppressor gene participating in DNA damage response regulation.109 For PER3, its low expression level stimulates BMAL1 expression, leading to the activation of the wingless/integrated (WNT)/β-catenin pathway,121 which is involved in cell proliferation and transcription.122,123 The epidemiological linkage between PCa risk and variants as well as expression of those circadian genes has been reported in many previous studies.124
Furthermore, increasing evidence shows that chronic inflammatory status is involved in prostate carcinogenesis.125 The disruption of the circadian rhythm may be related to epithelial–mesenchymal transition (EMT) induction and contribute to the formation of a proinflammatory environment at the systemic level and even in the tumor microenvironment (TME) of PCa.108 For example, the downregulation of PER2 was related to the expression of EMT-transcription factors (TFs) and increased EMT-specific cellular characteristics.126 It has been confirmed by many studies about other cancers that circadian rhythm disruption may link EMT to inflammation to favor the dissemination of cancer cells,108 which may also be one of the molecular mechanisms of prostate carcinogenesis. In addition, intrinsic circadian clock disruption of many immune cell types, such as macrophages, neutrophils, natural killer (NK) cells, T cells, and dendritic cells (DCs), affects immune escape and immune exclusion,108,127–129 thus affecting the progression of PCa.
On the other hand, owing to the close link between circadian rhythm and the endocrine system, various endocrine hormones are modulated by circadian clocks.130 Melatonin and cortisol are not only upstream regulators but also downstream targets of the circadian clock. Moreover, androgen levels are also regulated by the circadian clock. Studies in vivo have shown that androgen is important for the growth of PCa as well as normal cells.131,132 Circadian rhythm may affect PCa evolution by regulating androgen and androgen-associated productions.133,134 Melatonin and cortisol have the capacity for tumor-suppression and immunosuppression. The finding that PCa patients had lower melatonin levels than patients with BPH suggested the potential protective role of melatonin against prostatic disease progression.135 A low level of melatonin was also reported to be associated with an increased risk of PCa. Mechanistically, melatonin inhibits the accumulation of intracellular lipid droplets, as well as cell proliferation and migration. It also reduces endogenous androgen biosynthesis in CRPC mouse models by upregulating the expression of lipid metabolism-related carboxylesterase 1 (CES1).136 However, there was also a study that did not support the inhibitory effect of melatonin on PCa.137 Cortisol is another important endocrine factor for circadian rhythm modulation.68,138 The inverse relation between the melatonin/cortisol ratio and the presence and stage of PCa indicated the potential synergistic anticancer effects of melatonin and cortisol.139 Therefore, it is of great significance to understand the specific roles of melatonin and cortisol in the initiation, development, and progression of PCa, which may guide the prevention and treatment of PCa.
TREATMENT TARGETING THE CIRCADIAN CLOCK
There are also circadian changes in drug pharmacokinetics.140 The use of chronotherapy, a term generally described as using timed dosing to reach optimal therapeutic effects, has achieved good efficacy in cardiovascular diseases.141,142 When given at different times of the day, many anticancer agents show a 2–10 times variation in drug tolerability in mouse models and the time-related variations in drug tolerance have been confirmed by clinical trials of patients with cancer.140,143,144 Chronotherapy has shown clear success in PCa outcome and improved management of the disease.145 In addition to drug therapy, chronotherapy can also be applied to radiotherapy. Morning proton beam therapy for localized PCa was observed to significantly ameliorate the worsening lower urinary tract symptoms, compared with therapy around noon or late afternoon.146 Drug castration in androgen deprivation therapy (ADT) is also a common treatment for PCa patients. Although patients who lack indications for surgery and radiotherapy are treated with ADT, the majority of patients undergoing ADT therapy will progress to CRPC.147 Both epidemiological and laboratory studies suggest the importance of lipid metabolism in PCa progression and resistance to endocrine therapy148 and ADT therapy significantly changed lipid metabolism in patients with PCa.149 Circadian clock genes are involved in lipid metabolism regulation and variants of circadian genes may be associated with varying serum sex steroid levels.91,134 Therefore, ADT may aggravate circadian clock disruption and promote the progression of CRPC, and therapy targeting the circadian clock may be a new option for treating CRPC. In addition, cell proliferation regulated by circadian rhythm often shows asynchrony between normal and malignant tissues, which is also one of the theoretical bases for cancer chronotherapy.107 Despite the lack of specific studies about circadian changes and the corresponding chronotherapeutic plans of these drugs, treatment based on circadian changes in castration drugs may also be an ideal option.
Moreover, treatment strategies that involve gene editing or strict changes to a patient’s behavior and lifestyle are clearly not ideal enough for a number of reasons. Exogenous melatonin supplementation may resynchronize the circadian rhythm, helpfully providing a novel way in PCa management.120 The study of Zhou et al.136 has proven that melatonin therapy inhibits tumor growth and reverses enzalutamide resistance in CRPC animal models with a disruption of circadian rhythm. This may be because melatonin reverses the circadian rhythm disruption aggravated by ADT therapy, thus exerting its therapeutic effect on CRPC. Melatonin was also found to inhibit PCa metastasis in both in vitro and in vivo models.150 Employing transgenic adenocarcinoma of mouse prostate mice, Jung-Hynes and colleagues also demonstrated that oral melatonin intake, at human-achievable doses, significantly inhibited PCa tumorigenesis.151 Notably, in animal models, exogenous melatonin intake was found to inhibit neutrophil migration in a dose-dependent and time-dependent manner.152 Although a similar pattern has not been found in men with PCa, the amount and timing of exogenous melatonin supplementation should be carefully considered. Other pharmaceutical agents that directly target the circadian clock might also be a new option. For instance, a small molecule named longdaysin was shown to lengthen the circadian period and lead to PER1 degradation by targeting multiple kinases simultaneously.153 Another small molecule called KL001 was also found to lengthen the period of circadian clock by stabilizing CRY proteins.154 It is rather predictable that when combined with chronotherapy, these small molecules may have therapeutic potential by restoring the circadian rhythm in many biological processes in the body. Interestingly, in addition to therapeutic applications, circadian rhythm may help in surveillance of PCa metastasis. Zhu et al.155 found that daily fluctuations in circulating tumor cell (CTC) count peaked during the nocturnal active phase in rodents, confirming that CTC release may also be related to circadian rhythms.
CONCLUSIONS
As described in this review, disruption of the circadian clock strongly influences PCa initiation and development through changes in multiple regulatory pathways, including the cell cycle, EMT, tumor immunity, and the endocrine system. Although a relatively strong relationship between circadian disruption and PCa has been established, more mechanisms of how the circadian clock regulates PCa progression need to be elucidated, such as the exact molecular mechanisms of tissue-specific circadian gene expression and their impacts on prostate tumorigenesis. At the population level, after excluding other related confounding factors, the role of circadian rhythm disruption in the occurrence and development of PCa also needs further exploration. In addition, it is critical to establish reasonable chronotherapeutic strategies, especially ensuring the optimal administration time and schedule. In summary, further research on circadian rhythm could help the prevention and treatment of PCa.
AUTHOR CONTRIBUTIONS
WZZ and QYH proposed the project and wrote the manuscript. DCF revised and supplemented the manuscript. QW and LY supervised the project. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (grant No. 81974099, 82170785, 81974098, and 82170784), a program from Science and Technology Department of Sichuan Province (grant No. 21GJHZ0246), Young Investigator Award of Sichuan University 2017 (grant No. 2017SCU04A17), Technology Innovation Research and Development Project of Chengdu Science and Technology Bureau (2019-YF05-00296-SN), and Sichuan University-Panzhihua Science and Technology Cooperation Special fund (2020CDPZH-4).
REFERENCES
- 1.Abbott SM, Reid KJ, Zee PC. Circadian rhythm sleep-wake disorders. Psychiatr Clin North Am. 2015;38:805–23. doi: 10.1016/j.psc.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Smolensky MH, Hermida RC, Portaluppi F. Circadian mechanisms of 24-hour blood pressure regulation and patterning. Sleep Med Rev. 2017;33:4–16. doi: 10.1016/j.smrv.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Baxter M, Ray DW. Circadian rhythms in innate immunity and stress responses. Immunology. 2020;161:261–7. doi: 10.1111/imm.13166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hood S, Amir S. The aging clock:circadian rhythms and later life. J Clin Invest. 2017;127:437–46. doi: 10.1172/JCI90328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruan W, Yuan X, Eltzschig HK. Circadian rhythm as a therapeutic target. Nat Rev Drug Discov. 2021;20:287–307. doi: 10.1038/s41573-020-00109-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Musiek ES, Xiong DD, Holtzman DM. Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Exp Mol Med. 2015;47:e148. doi: 10.1038/emm.2014.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paschos GK, FitzGerald GA. Circadian clocks and vascular function. Circ Res. 2010;106:833–41. doi: 10.1161/CIRCRESAHA.109.211706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stubblefield JJ, Gao P, Kilaru G, Mukadam B, Terrien J, et al. Temporal control of metabolic amplitude by nocturnin. Cell Rep. 2018;22:1225–35. doi: 10.1016/j.celrep.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masri S, Sassone-Corsi P. The emerging link between cancer, metabolism, and circadian rhythms. Nature Med. 2018;24:1795–803. doi: 10.1038/s41591-018-0271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wendeu-Foyet MG, Menegaux F. Circadian disruption and prostate cancer risk:an updated review of epidemiological evidences. Cancer Epidemiol Biomarkers Prev. 2017;26:985–91. doi: 10.1158/1055-9965.EPI-16-1030. [DOI] [PubMed] [Google Scholar]
- 11.Stevens RG, Blask DE, Brainard GC, Hansen J, Lockley SW, et al. Meeting report:the role of environmental lighting and circadian disruption in cancer and other diseases. Environ Health Perspect. 2007;115:1357–62. doi: 10.1289/ehp.10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus:cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–77. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGinnis GR, Young ME. Circadian regulation of metabolic homeostasis:causes and consequences. Nat Sci Sleep. 2016;8:163–80. doi: 10.2147/NSS.S78946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang W, Ramsey KM, Marcheva B, Bass J. Circadian rhythms, sleep, and metabolism. J Clin Invest. 2011;121:2133–41. doi: 10.1172/JCI46043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roenneberg T, Merrow M. The circadian clock and human health. Curr Biol. 2016;26:R432–43. doi: 10.1016/j.cub.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Oishi K, Miyazaki K, Kadota K, Kikuno R, Nagase T, et al. Genome-wide expression analysis of mouse liver reveals CLOCK-regulated circadian output genes. J Biol Chem. 2003;278:41519–27. doi: 10.1074/jbc.M304564200. [DOI] [PubMed] [Google Scholar]
- 17.Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, et al. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science. 1994;264:719–25. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–17. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15(Spec No 2):R271–7. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- 20.Siepka SM, Yoo SH, Park J, Song W, Kumar V, et al. Circadian mutant overtime reveals F-box protein FBXL3 regulation of cryptochrome and period gene expression. Cell. 2007;129:1011–23. doi: 10.1016/j.cell.2007.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoo SH, Mohawk JA, Siepka SM, Shan Y, Huh SK, et al. Competing E3 ubiquitin ligases govern circadian periodicity by degradation of CRY in nucleus and cytoplasm. Cell. 2013;152:1091–105. doi: 10.1016/j.cell.2013.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, et al. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–60. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 23.Ueda HR, Chen W, Adachi A, Wakamatsu H, Hayashi S, et al. A transcription factor response element for gene expression during circadian night. Nature. 2002;418:534–9. doi: 10.1038/nature00906. [DOI] [PubMed] [Google Scholar]
- 24.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–46. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–62. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamajuku D, Shibata Y, Kitazawa M, Katakura T, Urata H, et al. Cellular DBP and E4BP4 proteins are critical for determining the period length of the circadian oscillator. FEBS Lett. 2011;585:2217–22. doi: 10.1016/j.febslet.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 27.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 28.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 29.Feng D, Xiong Q, Wei Q, Yang L. Cellular landscape of tumour microenvironment in prostate cancer. Immunology. 2022 doi: 10.1111/imm.13456. Doi:10.1111/imm.13456. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Feng D, Shi X, Zhang F, Xiong Q, Wei Q, et al. Energy metabolism-related gene prognostic index predicts biochemical recurrence for patients with prostate cancer undergoing radical prostatectomy. Front Immunol. 2022;13:839362. doi: 10.3389/fimmu.2022.839362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zi H, He SH, Leng XY, Xu XF, Huang Q, et al. Global, regional, and national burden of kidney, bladder, and prostate cancers and their attributable risk factors, 1990-2019. Mil Med Res. 2021;8:60. doi: 10.1186/s40779-021-00354-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsing AW, Sakoda LC, Chua S., Jr Obesity, metabolic syndrome, and prostate cancer. Am J Clin Nutr. 2007;86:s843–57. doi: 10.1093/ajcn/86.3.843S. [DOI] [PubMed] [Google Scholar]
- 33.Hsing AW, Devesa SS. Trends and patterns of prostate cancer:what do they suggest? Epidemiol Rev. 2001;23:3–13. doi: 10.1093/oxfordjournals.epirev.a000792. [DOI] [PubMed] [Google Scholar]
- 34.Lee J, Demissie K, Lu SE, Rhoads GG. Cancer incidence among Korean-American immigrants in the United States and native Koreans in South Korea. Cancer Control. 2007;14:78–85. doi: 10.1177/107327480701400111. [DOI] [PubMed] [Google Scholar]
- 35.Manoogian EN, Panda S. Circadian rhythms, time-restricted feeding, and healthy aging. Ageing Res Rev. 2017;39:59–67. doi: 10.1016/j.arr.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeng XT, Jin YH, Liu TZ, Chen FM, Ding DG, et al. Clinical practice guideline for transurethral plasmakinetic resection of prostate for benign prostatic hyperplasia (2021 Edition) Mil Med Res. 2022;9:14. doi: 10.1186/s40779-022-00371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu C, Wang DQ, Zi H, Huang Q, Gu JM, et al. Epidemiological trends of urinary tract infections, urolithiasis and benign prostatic hyperplasia in 203 countries and territories from 1990 to 2019. Mil Med Res. 2021;8:64. doi: 10.1186/s40779-021-00359-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swaab DF, Fliers E, Partiman TS. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 1985;342:37–44. doi: 10.1016/0006-8993(85)91350-2. [DOI] [PubMed] [Google Scholar]
- 39.Zhou JN, Swaab DF. Activation and degeneration during aging:a morphometric study of the human hypothalamus. Microsc Res Tech. 1999;44:36–48. doi: 10.1002/(SICI)1097-0029(19990101)44:1<36::AID-JEMT5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 40.Wu YH, Zhou JN, Van Heerikhuize J, Jockers R, Swaab DF. Decreased MT1 melatonin receptor expression in the suprachiasmatic nucleus in aging and Alzheimer's disease. Neurobiol Aging. 2007;28:1239–47. doi: 10.1016/j.neurobiolaging.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Hofman MA, Swaab DF. Alterations in circadian rhythmicity of the vasopressin-producing neurons of the human suprachiasmatic nucleus (SCN) with aging. Brain Res. 1994;651:134–42. doi: 10.1016/0006-8993(94)90689-0. [DOI] [PubMed] [Google Scholar]
- 42.Zhou JN, Hofman MA, Swaab DF. VIP neurons in the human SCN in relation to sex, age, and Alzheimer's disease. Neurobiol Aging. 1995;16:571–6. doi: 10.1016/0197-4580(95)00043-e. [DOI] [PubMed] [Google Scholar]
- 43.Palomba M, Nygård M, Florenzano F, Bertini G, Kristensson K, et al. Decline of the presynaptic network, including GABAergic terminals, in the aging suprachiasmatic nucleus of the mouse. J Biol Rhythms. 2008;23:220–31. doi: 10.1177/0748730408316998. [DOI] [PubMed] [Google Scholar]
- 44.Aujard F, Herzog ED, Block GD. Circadian rhythms in firing rate of individual suprachiasmatic nucleus neurons from adult and middle-aged mice. Neuroscience. 2001;106:255–61. doi: 10.1016/s0306-4522(01)00285-8. [DOI] [PubMed] [Google Scholar]
- 45.Nakamura TJ, Nakamura W, Tokuda IT, Ishikawa T, Kudo T, et al. Age-related changes in the circadian system unmasked by constant conditions. eNeuro. 2015;2 doi: 10.1523/ENEURO.0064-15.2015. ENEURO.0064-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hofman MA, Swaab DF. Living by the clock:the circadian pacemaker in older people. Ageing Res Rev. 2006;5:33–51. doi: 10.1016/j.arr.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 47.Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, et al. Effects of aging on central and peripheral mammalian clocks. Proc Natl Acad Sci U S A. 2002;99:10801–6. doi: 10.1073/pnas.152318499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hurd MW, Ralph MR. The significance of circadian organization for longevity in the golden hamster. J Biol Rhythms. 1998;13:430–6. doi: 10.1177/074873098129000255. [DOI] [PubMed] [Google Scholar]
- 49.Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev. 2006;20:1868–73. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krishnan N, Kretzschmar D, Rakshit K, Chow E, Giebultowicz JM. The circadian clock gene period extends healthspan in aging Drosophila melanogaster . Aging. 2009;1:937–48. doi: 10.18632/aging.100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alves-Fernandes DK, Jasiulionis MG. The role of SIRT1 on DNA damage response and epigenetic alterations in cancer. Int J Mol Sci. 2019;20:3153. doi: 10.3390/ijms20133153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huffman DM, Grizzle WE, Bamman MM, Kim JS, Eltoum IA, et al. SIRT1 is significantly elevated in mouse and human prostate cancer. Cancer Res. 2007;67:6612–8. doi: 10.1158/0008-5472.CAN-07-0085. [DOI] [PubMed] [Google Scholar]
- 53.Duan K, Ge YC, Zhang XP, Wu SY, Feng JS, et al. miR-34a inhibits cell proliferation in prostate cancer by downregulation of SIRT1 expression. Oncol Lett. 2015;10:3223–7. doi: 10.3892/ol.2015.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, Yang B, Ma B. The UCA1/miR-204/Sirt1 axis modulates docetaxel sensitivity of prostate cancer cells. Cancer Chemother Pharmacol. 2016;78:1025–31. doi: 10.1007/s00280-016-3158-8. [DOI] [PubMed] [Google Scholar]
- 55.Arendt J. Melatonin and human rhythms. Chronobiology Int. 2006;23:21–37. doi: 10.1080/07420520500464361. [DOI] [PubMed] [Google Scholar]
- 56.Pack W, Hill DD, Wong KY. Melatonin modulates M4-type ganglion-cell photoreceptors. Neuroscience. 2015;303:178–88. doi: 10.1016/j.neuroscience.2015.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anisimov VN, Popovich IG, Zabezhinski MA. Melatonin and colon carcinogenesis:I. Inhibitory effect of melatonin on development of intestinal tumors induced by 1,2-dimethylhydrazine in rats. Carcinogenesis. 1997;18:1549–53. doi: 10.1093/carcin/18.8.1549. [DOI] [PubMed] [Google Scholar]
- 58.Cini G, Coronnello M, Mini E, Neri B. Melatonin's growth-inhibitory effect on hepatoma AH 130 in the rat. Cancer Lett. 1998;125:51–9. doi: 10.1016/s0304-3835(97)00480-1. [DOI] [PubMed] [Google Scholar]
- 59.Hill SM, Blask DE. Effects of the pineal hormone melatonin on the proliferation and morphological characteristics of human breast cancer cells (MCF-7) in culture. Cancer Res. 1988;48:6121–6. [PubMed] [Google Scholar]
- 60.Kennaway DJ, Lushington K, Dawson D, Lack L, van den Heuvel C, et al. Urinary 6-sulfatoxymelatonin excretion and aging:new results and a critical review of the literature. J Pineal Res. 1999;27:210–20. doi: 10.1111/j.1600-079x.1999.tb00617.x. [DOI] [PubMed] [Google Scholar]
- 61.Zhao ZY, Xie Y, Fu YR, Bogdan A, Touitou Y. Aging and the circadian rhythm of melatonin:a cross-sectional study of Chinese subjects 30-110 yr of age. Chronobiology Int. 2002;19:1171–82. doi: 10.1081/cbi-120015958. [DOI] [PubMed] [Google Scholar]
- 62.Duffy JF, Zeitzer JM, Rimmer DW, Klerman EB, Dijk DJ, et al. Peak of circadian melatonin rhythm occurs later within the sleep of older subjects. Am J Physiol Endocrinol Metab. 2002;282:E297–303. doi: 10.1152/ajpendo.00268.2001. [DOI] [PubMed] [Google Scholar]
- 63.Zeitzer JM, Daniels JE, Duffy JF, Klerman EB, Shanahan TL, et al. Do plasma melatonin concentrations decline with age? Am J Med. 1999;107:432–6. doi: 10.1016/s0002-9343(99)00266-1. [DOI] [PubMed] [Google Scholar]
- 64.Oster H, Damerow S, Kiessling S, Jakubcakova V, Abraham D, et al. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab. 2006;4:163–73. doi: 10.1016/j.cmet.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 65.Cuesta M, Cermakian N, Boivin DB. Glucocorticoids entrain molecular clock components in human peripheral cells. FASEB J. 2015;29:1360–70. doi: 10.1096/fj.14-265686. [DOI] [PubMed] [Google Scholar]
- 66.Segall LA, Perrin JS, Walker CD, Stewart J, Amir S. Glucocorticoid rhythms control the rhythm of expression of the clock protein, Period2, in oval nucleus of the bed nucleus of the stria terminalis and central nucleus of the amygdala in rats. Neuroscience. 2006;140:753–7. doi: 10.1016/j.neuroscience.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 67.Ohmori K, Nishikawa S, Oku K, Oida K, Amagai Y, et al. Circadian rhythms and the effect of glucocorticoids on expression of the clock gene period1 in canine peripheral blood mononuclear cells. Vet J. 2013;196:402–7. doi: 10.1016/j.tvjl.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 68.Touitou Y, Sulon J, Bogdan A, Touitou C, Reinberg A, et al. Adrenal circadian system in young and elderly human subjects:a comparative study. J Endocrinol. 1982;93:201–10. doi: 10.1677/joe.0.0930201. [DOI] [PubMed] [Google Scholar]
- 69.Van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab. 1996;81:2468–73. doi: 10.1210/jcem.81.7.8675562. [DOI] [PubMed] [Google Scholar]
- 70.Breen DP, Vuono R, Nawarathna U, Fisher K, Shneerson JM, et al. Sleep and circadian rhythm regulation in early Parkinson disease. JAMA Neurol. 2014;71:589–95. doi: 10.1001/jamaneurol.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hatfield CF, Herbert J, van Someren EJ, Hodges JR, Hastings MH. Disrupted daily activity/rest cycles in relation to daily cortisol rhythms of home-dwelling patients with early Alzheimer's dementia. Brain. 2004;127:1061–74. doi: 10.1093/brain/awh129. [DOI] [PubMed] [Google Scholar]
- 72.Barul C, Richard H, Parent ME. Night-shift work and risk of prostate cancer:results from a Canadian case-control study, the prostate cancer and environment study. Am J Epidemiol. 2019;188:1801–11. doi: 10.1093/aje/kwz167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lozano-Lorca M, Olmedo-Requena R, Vega-Galindo MV, Vázquez-Alonso F, Jiménez-Pacheco A, et al. Night shift work, chronotype, sleep duration, and prostate cancer risk:CAPLIFE study. Int J Environ Res Public Health. 2020;17:6300. doi: 10.3390/ijerph17176300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Behrens T, Rabstein S, Wichert K, Erbel R, Eisele L, et al. Shift work and the incidence of prostate cancer:a 10-year follow-up of a German population-based cohort study. Scand J Work Environ Health. 2017;43:560–8. doi: 10.5271/sjweh.3666. [DOI] [PubMed] [Google Scholar]
- 75.Dickerman BA, Markt SC, Koskenvuo M, Hublin C, Pukkala E, et al. Sleep disruption, chronotype, shift work, and prostate cancer risk and mortality:a 30-year prospective cohort study of Finnish twins. Cancer Causes Control. 2016;27:1361–70. doi: 10.1007/s10552-016-0815-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kubo T, Ozasa K, Mikami K, Wakai K, Fujino Y, et al. Prospective cohort study of the risk of prostate cancer among rotating-shift workers:findings from the Japan collaborative cohort study. Am J Epidemiol. 2006;164:549–55. doi: 10.1093/aje/kwj232. [DOI] [PubMed] [Google Scholar]
- 77.Conlon M, Lightfoot N, Kreiger N. Rotating shift work and risk of prostate cancer. Epidemiology. 2007;18:182–3. doi: 10.1097/01.ede.0000249519.33978.31. [DOI] [PubMed] [Google Scholar]
- 78.Parent M, El-Zein M, Rousseau MC, Pintos J, Siemiatycki J. Night work and the risk of cancer among men. Am J Epidemiol. 2012;176:751–9. doi: 10.1093/aje/kws318. [DOI] [PubMed] [Google Scholar]
- 79.Papantoniou K, Castaño-Vinyals G, Espinosa A, Aragonés N, Pérez-Gómez B, et al. Night shift work, chronotype and prostate cancer risk in the MCC-Spain case-control study. Int J Cancer. 2015;137:1147–57. doi: 10.1002/ijc.29400. [DOI] [PubMed] [Google Scholar]
- 80.Hammer GP, Emrich K, Nasterlack M, Blettner M, Yong M. Shift work and prostate cancer incidence in industrial workers:a historical cohort study in a German chemical company. Dtsch Arztebl Int. 2015;112:463–70. doi: 10.3238/arztebl.2015.0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wendeu-Foyet MG, Bayon V, Cénée S, Trétarre B, Rébillard X, et al. Night work and prostate cancer risk:results from the EPICAP study. Occup Environ Med. 2018;75:573–81. doi: 10.1136/oemed-2018-105009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Åkerstedt T, Narusyte J, Svedberg P, Kecklund G, Alexanderson K. Night work and prostate cancer in men:a Swedish prospective cohort study. BMJ Open. 2017;7:e015751. doi: 10.1136/bmjopen-2016-015751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rao D, Yu H, Bai Y, Zheng X, Xie L. Does night-shift work increase the risk of prostate cancer?a systematic review and meta-analysis. Onco Targets Ther. 2015;8:2817–26. doi: 10.2147/OTT.S89769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rivera-Izquierdo M, Martínez-Ruiz V, Castillo-Ruiz EM, Manzaneda-Navío M, Pérez-Gómez B, et al. Shift work and prostate cancer:an updated systematic review and meta-analysis. Int J Environ Res Public Health. 2020;17:1345. doi: 10.3390/ijerph17041345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dun A, Zhao X, Jin X, Wei T, Gao X, et al. Association between night-shift work and cancer risk:updated systematic review and meta-analysis. Front Oncol. 2020;10:1006. doi: 10.3389/fonc.2020.01006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sigurdardottir LG, Valdimarsdottir UA, Mucci LA, Fall K, Rider JR, et al. Sleep disruption among older men and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2013;22:872–9. doi: 10.1158/1055-9965.EPI-12-1227-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Markt SC, Flynn-Evans EE, Valdimarsdottir UA, Sigurdardottir LG, Tamimi RM, et al. Sleep duration and disruption and prostate cancer risk:a 23-year prospective study. Cancer Epidemiol Biomarkers Prev. 2016;25:302–8. doi: 10.1158/1055-9965.EPI-14-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sigurdardottir LG, Valdimarsdottir UA, Fall K, Rider JR, Lockley SW, et al. Circadian disruption, sleep loss, and prostate cancer risk:a systematic review of epidemiologic studies. Cancer Epidemiol Biomarkers Prev. 2012;21:1002–11. doi: 10.1158/1055-9965.EPI-12-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kinouchi K, Magnan C, Ceglia N, Liu Y, Cervantes M, et al. Fasting imparts a switch to alternative daily pathways in liver and muscle. Cell Rep. 2018;25:3299–314.e6. doi: 10.1016/j.celrep.2018.11.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Palomar-Cros A, Espinosa A, Straif K, Pérez-Gómez B, Papantoniou K, et al. The association of nighttime fasting duration and prostate cancer risk:results from the multicase-control (MCC) study in Spain. Nutrients. 2021;13:2662. doi: 10.3390/nu13082662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Katewa SD, Akagi K, Bose N, Rakshit K, Camarella T, et al. Peripheral circadian clocks mediate dietary restriction-dependent changes in lifespan and fat metabolism in Drosophila. Cell Metab. 2016;23:143–54. doi: 10.1016/j.cmet.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matsushita M, Fujita K, Nonomura N. Influence of diet and nutrition on prostate cancer. Int J Mol Sci. 2020;21:1447. doi: 10.3390/ijms21041447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dubrovsky YV, Samsa WE, Kondratov RV. Deficiency of circadian protein CLOCK reduces lifespan and increases age-related cataract development in mice. Aging. 2010;2:936–44. doi: 10.18632/aging.100241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ostrin LA. Ocular and systemic melatonin and the influence of light exposure. Clin Exp Optom. 2019;102:99–108. doi: 10.1111/cxo.12824. [DOI] [PubMed] [Google Scholar]
- 95.Truong T, Liquet B, Menegaux F, Plancoulaine S, Laurent-Puig P, et al. Breast cancer risk, nightwork, and circadian clock gene polymorphisms. Endocr Relat Cancer. 2014;21:629–38. doi: 10.1530/ERC-14-0121. [DOI] [PubMed] [Google Scholar]
- 96.Rabstein S, Harth V, Justenhoven C, Pesch B, Plöttner S, et al. Polymorphisms in circadian genes, night work and breast cancer:results from the GENICA study. Chronobiol Int. 2014;31:1115–22. doi: 10.3109/07420528.2014.957301. [DOI] [PubMed] [Google Scholar]
- 97.Chu LW, Zhu Y, Yu K, Zheng T, Yu H, et al. Variants in circadian genes and prostate cancer risk:a population-based study in China. Prostate Cancer Prostatic Dis. 2008;11:342–8. doi: 10.1038/sj.pcan.4501024. [DOI] [PubMed] [Google Scholar]
- 98.Zhu Y, Stevens RG, Hoffman AE, Fitzgerald LM, Kwon EM, et al. Testing the circadian gene hypothesis in prostate cancer:a population-based case-control study. Cancer Res. 2009;69:9315–22. doi: 10.1158/0008-5472.CAN-09-0648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Markt SC, Valdimarsdottir UA, Shui IM, Sigurdardottir LG, Rider JR, et al. Circadian clock genes and risk of fatal prostate cancer. Cancer Causes Control. 2015;26:25–33. doi: 10.1007/s10552-014-0478-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lin DW, FitzGerald LM, Fu R, Kwon EM, Zheng SL, et al. Genetic variants in the LEPR, CRY1, RNASEL, IL4, and ARVCF genes are prognostic markers of prostate cancer-specific mortality. Cancer Epidemiol Biomarkers Prev. 2011;20:1928–36. doi: 10.1158/1055-9965.EPI-11-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mocellin S, Tropea S, Benna C, Rossi CR. Circadian pathway genetic variation and cancer risk:evidence from genome-wide association studies. BMC Med. 2018;16:20. doi: 10.1186/s12916-018-1010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yu CC, Chen LC, Chiou CY, Chang YJ, Lin VC, et al. Genetic variants in the circadian rhythm pathway as indicators of prostate cancer progression. Cancer Cell Int. 2019;19:87. doi: 10.1186/s12935-019-0811-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Feng D, Xiong Q, Zhang F, Shi X, Xu H, et al. Identification of a novel nomogram to predict progression based on the circadian clock and insights into the tumor immune microenvironment in prostate cancer. Front Immunol. 2022;13:777724. doi: 10.3389/fimmu.2022.777724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Menegaux F, Anger A, Randrianasolo H, Mulot C, Laurent-Puig P, et al. Epidemiological study of prostate cancer (EPICAP):a population-based case-control study in France. BMC Cancer. 2014;14:106. doi: 10.1186/1471-2407-14-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wendeu-Foyet MG, Koudou Y, Cénée S, Trétarre B, Rébillard X, et al. Circadian genes and risk of prostate cancer:findings from the EPICAP study. Int J Cancer. 2019;145:1745–53. doi: 10.1002/ijc.32149. [DOI] [PubMed] [Google Scholar]
- 106.Wendeu-Foyet MG, Cénée S, Koudou Y, Trétarre B, Rébillard X, et al. Circadian genes polymorphisms, night work and prostate cancer risk:findings from the EPICAP study. Int J Cancer. 2020;147:3119–29. doi: 10.1002/ijc.33139. [DOI] [PubMed] [Google Scholar]
- 107.Fu L, Lee CC. The circadian clock:pacemaker and tumour suppressor. Nat Rev Cancer. 2003;3:350–61. doi: 10.1038/nrc1072. [DOI] [PubMed] [Google Scholar]
- 108.Hadadi E, Acloque H. Role of circadian rhythm disorders on EMT and tumour-immune interactions in endocrine-related cancers. Endocr Relat Cancer. 2021;28:R67–80. doi: 10.1530/ERC-20-0390. [DOI] [PubMed] [Google Scholar]
- 109.Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111:41–50. doi: 10.1016/s0092-8674(02)00961-3. [DOI] [PubMed] [Google Scholar]
- 110.Hoffman AE, Zheng T, Ba Y, Zhu Y. The circadian gene NPAS2, a putative tumor suppressor, is involved in DNA damage response. Mol Cancer Res. 2008;6:1461–8. doi: 10.1158/1541-7786.MCR-07-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yi CH, Zheng T, Leaderer D, Hoffman A, Zhu Y. Cancer-related transcriptional targets of the circadian gene NPAS2 identified by genome-wide ChIP-on-chip analysis. Cancer Lett. 2009;284:149–56. doi: 10.1016/j.canlet.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xue X, Liu F, Han Y, Li P, Yuan B, et al. Silencing NPAS2 promotes cell growth and invasion in DLD-1 cells and correlated with poor prognosis of colorectal cancer. Biochem Biophys Res Commun. 2014;450:1058–62. doi: 10.1016/j.bbrc.2014.06.104. [DOI] [PubMed] [Google Scholar]
- 113.Yi C, Mu L, de la Longrais IA, Sochirca O, Arisio R, et al. The circadian gene NPAS2 is a novel prognostic biomarker for breast cancer. Breast Cancer Res Treat. 2010;120:663–9. doi: 10.1007/s10549-009-0484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dyar KA, Ciciliot S, Wright LE, Biensø RS, Tagliazucchi GM, et al. Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol Metab. 2014;3:29–41. doi: 10.1016/j.molmet.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shafi AA, McNair CM, McCann JJ, Alshalalfa M, Shostak A, et al. The circadian cryptochrome, CRY1, is a pro-tumorigenic factor that rhythmically modulates DNA repair. Nat Commun. 2021;12:401. doi: 10.1038/s41467-020-20513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mannic T, Meyer P, Triponez F, Pusztaszeri M, Le Martelot G, et al. Circadian clock characteristics are altered in human thyroid malignant nodules. J Clin Endocrinol Metab. 2013;98:4446–56. doi: 10.1210/jc.2013-2568. [DOI] [PubMed] [Google Scholar]
- 117.Cadenas C, van de Sandt L, Edlund K, Lohr M, Hellwig B, et al. Loss of circadian clock gene expression is associated with tumor progression in breast cancer. Cell Cycle. 2014;13:3282–91. doi: 10.4161/15384101.2014.954454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gery S, Komatsu N, Baldjyan L, Yu A, Koo D, et al. The circadian gene Per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol Cell. 2006;22:375–82. doi: 10.1016/j.molcel.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 119.Cao Q, Gery S, Dashti A, Yin D, Zhou Y, et al. A role for the clock gene, Per1 in prostate cancer. Cancer Res. 2009;69:7619–25. doi: 10.1158/0008-5472.CAN-08-4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jung-Hynes B, Huang W, Reiter RJ, Ahmad N. Melatonin resynchronizes dysregulated circadian rhythm circuitry in human prostate cancer cells. J Pineal Res. 2010;49:60–8. doi: 10.1111/j.1600-079X.2010.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li Q, Xia D, Wang Z, Liu B, Zhang J, et al. Circadian rhythm gene PER3 negatively regulates stemness of prostate cancer stem cells via WNT/β-catenin signaling in tumor microenvironment. Front Cell Dev Biol. 2021;9:656981. doi: 10.3389/fcell.2021.656981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.van de Wetering M, de Lau W, Clevers H. WNT signaling and lymphocyte development. Cell. 2002;109(Suppl):S13–9. doi: 10.1016/s0092-8674(02)00709-2. [DOI] [PubMed] [Google Scholar]
- 123.Lee E, Salic A, Kirschner MW. Physiological regulation of [beta]-catenin stability by Tcf3 and CK1epsilon. J Cell Biol. 2001;154:983–93. doi: 10.1083/jcb.200102074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Morales-Santana S, Morell S, Leon J, Carazo-Gallego A, Jimenez-Lopez JC, et al. An overview of the polymorphisms of circadian genes associated with endocrine ancer. Front Endocrinol. 2019;10:104. doi: 10.3389/fendo.2019.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nguyen DP, Li J, Tewari AK. Inflammation and prostate cancer:the role of interleukin 6 (IL-6) BJU Int. 2014;113:986–92. doi: 10.1111/bju.12452. [DOI] [PubMed] [Google Scholar]
- 126.Hwang-Verslues WW, Chang PH, Jeng YM, Kuo WH, Chiang PH, et al. Loss of corepressor PER2 under hypoxia up-regulates OCT1-mediated EMT gene expression and enhances tumor malignancy. Proc Natl Acad Sci U S A. 2013;110:12331–6. doi: 10.1073/pnas.1222684110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dongre A, Rashidian M, Reinhardt F, Bagnato A, Keckesova Z, et al. Epithelial-to-mesenchymal transition contributes to immunosuppression in breast carcinomas. Cancer Res. 2017;77:3982–9. doi: 10.1158/0008-5472.CAN-16-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Terry S, Savagner P, Ortiz-Cuaran S, Mahjoubi L, Saintigny P, et al. New insights into the role of EMT in tumor immune escape. Mol Oncol. 2017;11:824–46. doi: 10.1002/1878-0261.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Soundararajan R, Fradette JJ, Konen JM, Moulder S, Zhang X, et al. Targeting the interplay between epithelial-to-mesenchymal-transition and the immune system for effective immunotherapy. Cancers. 2019;11:714. doi: 10.3390/cancers11050714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gamble KL, Berry R, Frank SJ, Young ME. Circadian clock control of endocrine factors. Nat Rev Endocrinol. 2014;10:466–75. doi: 10.1038/nrendo.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Huggins C, Hodges CV. Studies on prostatic cancer.I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22:232–40. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 132.Niu Y, Xu Y, Zhang J, Bai J, Yang H, et al. Proliferation and differentiation of prostatic stromal cells. BJU Int. 2001;87:386–93. doi: 10.1046/j.1464-410x.2001.00103.x. [DOI] [PubMed] [Google Scholar]
- 133.Plymate SR, Tenover JS, Bremner WJ. Circadian variation in testosterone, sex hormone-binding globulin, and calculated non-sex hormone-binding globulin bound testosterone in healthy young and elderly men. J Androl. 1989;10:366–71. doi: 10.1002/j.1939-4640.1989.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 134.Chu LW, Zhu Y, Yu K, Zheng T, Chokkalingam AP, et al. Correlation between circadian gene variants and serum levels of sex steroids and insulin-like growth factor-I. Cancer Epidemiol Biomarkers Prev. 2008;17:3268–73. doi: 10.1158/1055-9965.EPI-08-0073. [DOI] [PubMed] [Google Scholar]
- 135.Bartsch C, Bartsch H, Schmidt A, Ilg S, Bichler KH, et al. Melatonin and 6-sulfatoxymelatonin circadian rhythms in serum and urine of primary prostate cancer patients:evidence for reduced pineal activity and relevance of urinary determinations. Clin Chim Acta. 1992;209:153–67. doi: 10.1016/0009-8981(92)90164-l. [DOI] [PubMed] [Google Scholar]
- 136.Zhou L, Zhang C, Yang X, Liu L, Hu J, et al. Melatonin inhibits lipid accumulation to repress prostate cancer progression by mediating the epigenetic modification of CES1. Clin Transl Med. 2021;11:e449. doi: 10.1002/ctm2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lozano-Lorca M, Olmedo-Requena R, Rodríguez-Barranco M, Redondo-Sánchez D, Jiménez-Pacheco A, et al. Salivary melatonin rhythm and prostate cancer:CAPLIFE study. J Urol. 2022;207:565–72. doi: 10.1097/JU.0000000000002294. [DOI] [PubMed] [Google Scholar]
- 138.Touitou Y, Sulon J, Bogdan A, Reinberg A, Sodoyez JC, et al. Adrenocortical hormones, ageing and mental condition:seasonal and circadian rhythms of plasma 18-hydroxy-11-deoxycorticosterone, total and free cortisol and urinary corticosteroids. J Endocrinol. 1983;96:53–64. doi: 10.1677/joe.0.0960053. [DOI] [PubMed] [Google Scholar]
- 139.Tai SY, Huang SP, Bao BY, Wu MT. Urinary melatonin-sulfate/cortisol ratio and the presence of prostate cancer:a case-control study. Sci Rep. 2016;6:29606. doi: 10.1038/srep29606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Levi F, Schibler U. Circadian rhythms:mechanisms and therapeutic implications. Annu Rev Pharmacol Toxicol. 2007;47:593–628. doi: 10.1146/annurev.pharmtox.47.120505.105208. [DOI] [PubMed] [Google Scholar]
- 141.Tsimakouridze EV, Alibhai FJ, Martino TA. Therapeutic applications of circadian rhythms for the cardiovascular system. Front Pharmacol. 2015;6:77. doi: 10.3389/fphar.2015.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hermida RC, Ayala DE, Smolensky MH, Mojón A, Fernández JR, et al. Chronotherapy improves blood pressure control and reduces vascular risk in CKD. Nat Rev Nephrol. 2013;9:358–68. doi: 10.1038/nrneph.2013.79. [DOI] [PubMed] [Google Scholar]
- 143.Kobayashi M, Wood PA, Hrushesky WJ. Circadian chemotherapy for gynecological and genitourinary cancers. Chronobiol Int. 2002;19:237–51. doi: 10.1081/cbi-120002600. [DOI] [PubMed] [Google Scholar]
- 144.Gorbacheva VY, Kondratov RV, Zhang R, Cherukuri S, Gudkov AV, et al. Circadian sensitivity to the chemotherapeutic agent cyclophosphamide depends on the functional status of the CLOCK/BMAL1 transactivation complex. Proc Natl Acad Sci U S A. 2005;102:3407–12. doi: 10.1073/pnas.0409897102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kaur P, Mohamed NE, Archer M, Figueiro MG, Kyprianou N. Impact of circadian rhythms on the development and clinical management of genitourinary cancers. Front Oncol. 2022;12:759153. doi: 10.3389/fonc.2022.759153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Negoro H, Iizumi T, Mori Y, Matsumoto Y, Chihara I, et al. Chronoradiation therapy for prostate cancer:morning proton beam therapy ameliorates worsening lower urinary tract symptoms. J Clin Med. 2020;9:2263. doi: 10.3390/jcm9072263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Butler W, Huang J. Neuroendocrine cells of the prostate:histology, biological functions, and molecular mechanisms. Precis Clin Med. 2021;4:25–34. doi: 10.1093/pcmedi/pbab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stoykova GE, Schlaepfer IR. Lipid metabolism and endocrine resistance in prostate cancer, and new opportunities for therapy. Int J Mol Sci. 2019;20:2626. doi: 10.3390/ijms20112626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mitsuzuka K, Kyan A, Sato T, Orikasa K, Miyazato M, et al. Influence of 1 year of androgen deprivation therapy on lipid and glucose metabolism and fat accumulation in Japanese patients with prostate cancer. Prostate Cancer Prostatic Dis. 2016;19:57–62. doi: 10.1038/pcan.2015.50. [DOI] [PubMed] [Google Scholar]
- 150.Wang SW, Tai HC, Tang CH, Lin LW, Lin TH, et al. Melatonin impedes prostate cancer metastasis by suppressing MMP-13 expression. J Cell Physiol. 2021;236:3979–90. doi: 10.1002/jcp.30150. [DOI] [PubMed] [Google Scholar]
- 151.Jung-Hynes B, Schmit TL, Reagan-Shaw SR, Siddiqui IA, Mukhtar H, et al. Melatonin, a novel Sirt1 inhibitor, imparts antiproliferative effects against prostate cancer in vitro in culture and in vivoin TRAMP model. J Pineal Res. 2011;50:140–9. doi: 10.1111/j.1600-079X.2010.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ren DL, Sun AA, Li YJ, Chen M, Ge SC, et al. Exogenous melatonin inhibits neutrophil migration through suppression of ERK activation. J Endocrinol. 2015;227:49–60. doi: 10.1530/JOE-15-0329. [DOI] [PubMed] [Google Scholar]
- 153.Hirota T, Lee JW, Lewis WG, Zhang EE, Breton G, et al. High-throughput chemical screen identifies a novel potent modulator of cellular circadian rhythms and reveals CKIαas a clock regulatory kinase. PLoS Biol. 2010;8:e1000559. doi: 10.1371/journal.pbio.1000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hirota T, Lee JW, St. John PC, Sawa M, Iwaisako K, et al. Identification of small molecule activators of cryptochrome. Science. 2012;337:1094–7. doi: 10.1126/science.1223710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhu X, Suo Y, Fu Y, Zhang F, Ding N, et al. In vivo flow cytometry reveals a circadian rhythm of circulating tumor cells. Light Sci Appl. 2021;10:110. doi: 10.1038/s41377-021-00542-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


