Abstract
BACKGROUND & AIM:
Tumor-microenvironment (TME) factors and cancer stem cells (CSCs) play a critical role in the aggressiveness of pancreatic cancer (PC). However, the degree to which TME factors promote stemness remains unexplored. Here, we examined whether cancer-associated fibroblasts (CAFs) promote CSC features in PC.
METHODS:
PC cells were treated long-term (30, 60, 90 days) with conditioned media (CM)-derived from normal human fibroblasts (NFs) and CAFs. The stemness features of tumorsphere formation and stemness populations, along with CSCs markers, were analyzed using 2D and 3D sodium alginate bead-based co-culture models. Immunohistochemistry and immunofluorescence staining were performed for CSCs and fibroblast markers in autochthonous KrasG12D/+; Trp53R172H/+; Pdx1-Cre (KPC) mice and human pancreatic tumors. PCR-array and gene knockdown were performed to identify the mechanism of stemness enrichment.
RESULTS:
Long-term treatment of PC cells with CAF-CM enriched stemness, as demonstrated by significantly higher CD44+, ALDH+, and AF+ populations in PC cells. Increased tumorsphere formation and elevated CSC, self-renewal, and drug-resistance markers in CAF-CM-treated PC cells were observed. In addition, CAF co-cultured with PC cells in the 3D model showed a substantial increase in stemness features. CD44 and α-SMA were positively correlated, and their expressions progressively increased from the early to late stages of KPC mice and human pancreatic tumors. OPN/SPP1 was identified as the top-differentially overexpressed gene in CAF-CM-treated PC cells, and knockdown of OPN/SPP1 significantly reduced stemness characteristics in CAF-CM-treated PC cells.
CONCLUSIONS:
Our data uncover novel insight into the interplay between CAF and enrichment of stemness population through SPP1-CD44 axis in PC.
Keywords: Pancreatic Cancer, Cancer-associated Fibroblast, Stemness, CD44, OPN/SPP1, cancer stem cells, TME, KPC
Introduction
Pancreatic ductal adenocarcinoma (PDAC), the most common form of pancreatic cancer (PC), is characterized by a desmoplastic stroma that forms the central part of the tumor microenvironment (TME)1. Fibroblasts and the extracellular matrix (ECM) are essential features of the TME. PC cells induce resident fibroblasts to acquire activated phenotypes2. Activated fibroblasts aid tumor progression and metastases3. Cancer-associated fibroblasts(CAFs) are an abundant stromal-activated fibroblast type present in the microenvironment of PDAC4. CAFs play a diverse role in the progression of cancer5, including PC6–10. A recent study demonstrated that CAFs could differentially express and secrete proteins in PC, including α-smooth muscle actin-expressing CAF known as myofibroblastic CAF (myCAFs) and interleukin 6 (IL-6)-secreting CAFs known as inflammatory CAFs (iCAFs)11. A current understanding of the multi-faceted function of CAFs has revealed that stemness factors regulate CAF phenotypes in nearby tumor cells12.
Cancer stem cells (CSCs) are a subset of tumor cells responsible for aggressiveness, drug resistance, and metastasis13. Studies have shown that CSCs play a critical role in the initiation, progression, and metastasis in PC14, 15. Distinct populations of CSCs were identified in PC using stem cell markers CD44, CD24, ESA16, CD133, and CXCR417. Our previous study identified PAF1 as a novel marker involved in maintaining and enriching pancreatic CSCs15, 18–20. Osteopontin (OPN) or secreted phosphoprotein 1 (SPP1) is a chemokine-like sialic acid-rich glycoprotein overexpressed in various cancers, including PC21–27. Studies have shown that OPN/SPP1 interacts with CD44 to induce cell signaling that modulates cell adhesion, movement, and activation of neoplastic cells leading to tumor progression and metastases28. Further, OPN/SPP1 promoted glioma stem cell-like phenotypes and shared perivascular expression patterns with CD4429.
Recently, a subpopulation of CD10 and GPR77-positive CAFs has been found to promote chemoresistance and stemness by activating NF-κB signaling in breast cancer30. Enrichment of the CD24+ population was observed in hepatocellular carcinoma (HCC) cells following treatment with CAFs by activating STAT3 signaling by IL-6 and hepatocyte growth factor (HGF), a morphogenic factor present in the CAF medium31. Begum et al. have shown that CAFs enable the synthesis of collagen type I, resulting in FAK signaling activation in PDAC cells32. Even though CAFs are a significant player in PC progression, the role of CAFs in PC stemness remains elusive. We sought to identify the molecular mechanism by which CAFs mediates stemness activation in PC progression and metastasis. This study investigated how CAFs reprogram PC stemness and promote tumorsphere formation and stemness signatures using in vitro 2D and 3D co-culture models, murine KrasG12D; Trp53R172H; Pdx-1-Cre, and human PDAC specimens. We unraveled how the OPN/SPP1-CD44 axis is modulated in CAF-CM treated PC cells to enhance the PC stemness.
Methods
Reagents and Antibodies
The reagents and antibodies used in this study are described in the supplementary Materials and Methods.
Cell Culture
Normal human fibroblasts (NFs) and CAFs were derived from fresh surgically resected normal and pancreatic tumor tissues under approved Institutional Review Board (IRB) protocol. An in-house pathologist evaluated initial pathological diagnoses of normal pancreas and PC. The tissues were minced, and the fibroblasts were isolated by differential trypsinization and subsequently transfected with human telomerase reverse transcriptase (hTERT), as shown previously33. All procedures performed in this study involving human participants followed the University of Nebraska Medical Center’s ethical standards and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
The human pancreatic cancer cell lines (CD18/HPAF and BxPC3) were received from ATCC. BxPC3 and CD18/HPAF cells were cultured in DMEM medium, supplemented with 10% fetal bovine serum and antibiotics (100 units/mL penicillin and 100 mg/mL streptomycin). Cells were incubated in a humidified incubator at 37°C, supplied with 5% CO2. All the cell lines were free of mycoplasma (as determined by MycoAlertPlus Mycoplasma Detection kit (Lonza, Walkersville, MD) and pathogenic murine viruses. For cell line authentication, short tandem repeat (STR) tests were performed in Genomics Core Facility at the University of Nebraska Medical Center34.
3D Sodium alginate bead co-culture with NFs/CAF and PC cells
BxPC3, CD18/HPAF, NFs/CAFs, and OPN/SPP1 scramble and knockdown PC cells were trypsinized, and 1×106 cells/mL cell density was resuspended with a 1:1 ratio of 4% alginate (Sigma-Aldrich, St. Louis, MO) solution in 1X PBS to provide a final concentration of 2% alginate solution35. An alginate solution containing cells was loaded into a 1 mL syringe and closed with a 22-gauge (BD Biosciences, San Jose, CA) needle, applied to a 6 well plate containing 100 mM of CaCl2 10% of FBS containing DMEM media. The alginate beads were developed by calcium cross-linking polymerization and using a syringe with or without a needle to create the various sizes of alginate beads to distinguish the cell types. Cell encapsulated beads were washed with PBS and maintained 10% of the DMEM media in the humidified incubator. After 6 and 12 days of incubation, the beads were fixed in 10% buffered formalin (Fisher Scientific, Waltham, MA) and 70% alcohol and processed for paraffin embedding for H&E and immunofluorescence staining. The images were captured using an EVOS microscope (Thermo Fisher Scientific, Waltham, MA). Then the beads were dissolved in a 0.2 M sodium citrate buffer and washed twice with PBS before cell harvesting for further immunoblot and q-RTPCR analysis.
Sphere Formation Assay
CAF-CM treated cells (30, 60, and 90 days) were seeded (3000 cells / well) in 96 well low-attachment culture plates in 100 uL of DMEM/F12 stem cell medium (Invitrogen, Grand Island, NY) supplemented with 1% B27 supplement, epidermal growth factor (20 ng/mL), and basic fibroblast growth factor (10 ng / mL). Spheres were re-seeded up to three times every 7 days to understand the self-renewal potential, and the number of spheres (> 100 mm) for each well was evaluated after 7, 14, and 21 days of cultivation. Images were captured using an EVOS microscope (Thermo Fisher Scientific, Waltham, MA).
Statistical analysis
All experiments were repeated at least three times. Data were presented as mean ± standard deviation (SD). Student’s t-test and one-way ANOVA analysis were used to analyze the variances between groups. Differences were considered statistically significant when P < 0.05 *; P < 0.01**; P < 0.001***.
Additional methods can be found in the Supplementary Methods.
Results
CAF-CM promotes the stemness and self-renewal potential in PC cells
To investigate the impact of TME on PC stemness, we generated and utilized the PC cells chronically (up to 90 days) treated with conditioned media of NFs or CAFs (Figure 1A). We first characterized the NFs and CAFs using fibroblast markers and collagen contraction assay. CAFs showed overexpression of α-SMA, Vimentin, FSP1, FAP-α, PDGFR-β, P4HB, and CD34 compared to NFs (Figure 1B, C, Supplementary Figure 1A, and B). The CAFs showed substantial contraction capacity compared to NFs in collagen contraction assay (Figure 1D and Supplementary Figure 1C), indicating that the isolated CAFs and NFs are pure populations and the CAFs phenotypically and functionally distinct as compared to NFs. To examine whether NFs-CM shows any impact on stemness, we treated PC cells (BxPC3 and CD18/HPAF) with NFs-CM 15 to 30 days, followed by stemness analysis. Western blot and flow cytometry analysis showed no significant elevation in CSC marker expressions and the percentage of AF+ and CD44+ populations in the NFs-CM exposed cells (Supplementary Figure 1D–F). Furthermore, NFs-CM treated cells showed no changes in morphological features (Supplementary Figure 2 A) and CSC associated gene expressions (Supplementary Figure 2C), suggesting that NFs-CM treatment causes no variation in stemness features in PC cells. However, CAF-CM-treated PC cells showed a time-dependent increase (30, 60, and 90 days) in the percentages of AF+ population and sphere formation potential (a measure of self-renewal) compared to basal-CM (2% FBS in media) treated cells (Figure 1E–J, Supplementary Figure 2D,E, Supplementary Figure 4 B, C). CAF-CM exposed PC cells showed changes in phenotypic characteristics (migratory and structural reprogramming), but basal media-exposed PC cells showed intact cell morphology (Supplementary Figure 2B). Additionally, a significant time-dependent increase in the percentage of the CD44+ population was observed in CAF-CM exposed cells (Figure 1K–N). We also observed a high percentage of ALDH+ population in 90 days CAF-CM exposed cells compared to respective time point control (Figure 1 O). Studies reported that the CD44 +/EPCAM+ population of primary lung cancer cells and the side population (SP) of ovarian cancer show co-expression with the high ALDH+ population. These double positive CSC populations are capable of prominent tumorigenicity and metastatic potential36, 37. Our data showed that CAF-CM exposed PC cells have over 60% of dual positive (ALDH+CD44+) CSC population. This result was proof of concept for the significant elevation of stemness in CAF-CM exposed PC cells (Supplementary Figure 2F–H). Overall, these findings indicate that CAF-CM promotes the stemness and self-renewal of PC cells.
Figure1. Exposure to CAF-CM promotes pancreatic stemness.
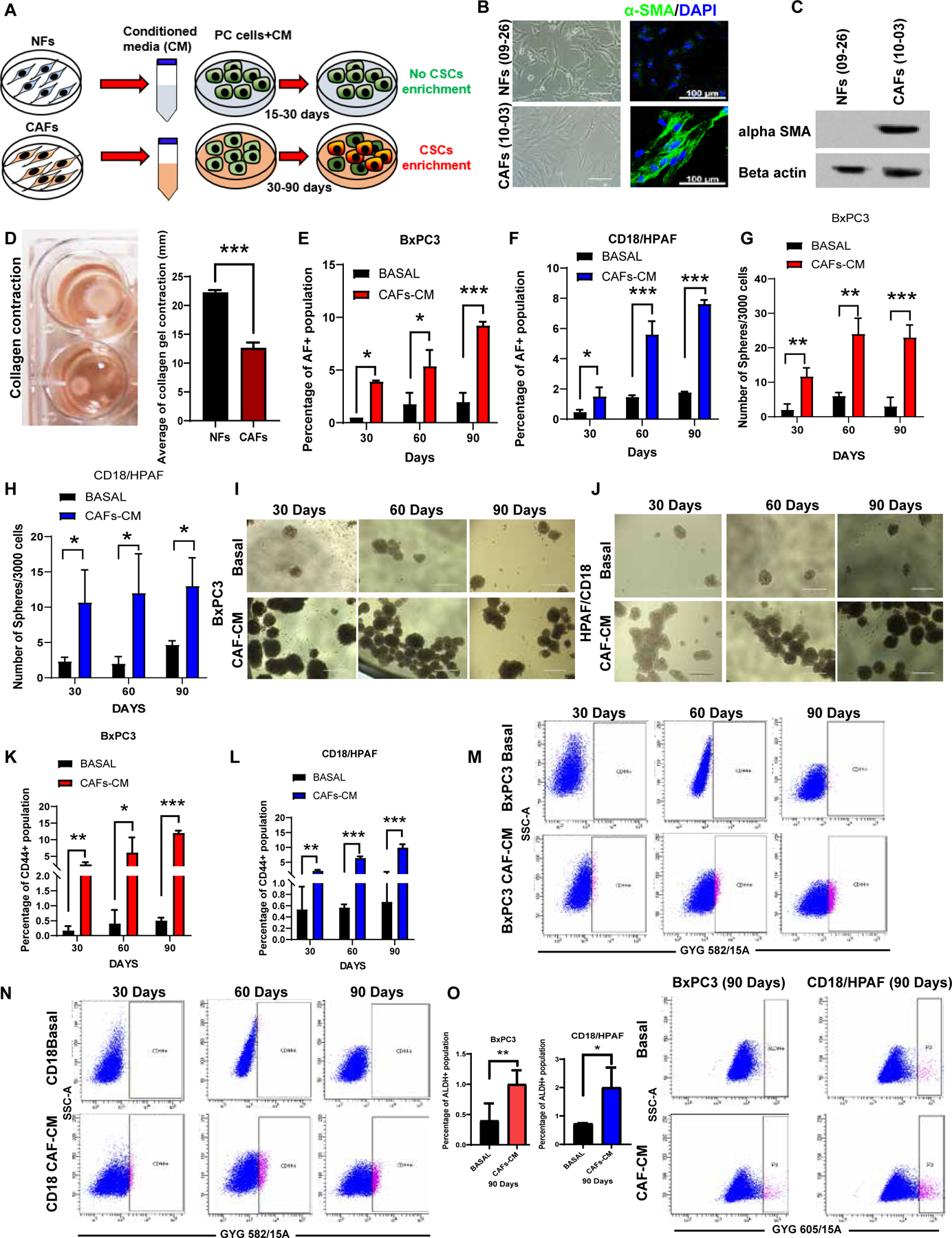
(A) Schematic diagram showing the generation of PC cells treated with NFs-CM/CAF-CM. (B) Microscopic examination of NFs (09–26) and CAFs (10–03); confocal imaging showing α-SMA (green) and DAPI nuclei staining; (C) Immunoblot analysis for α-SMA expression in NFs/CAFs. β-actin was used as a loading control. (D)Images showing collagen contraction and bar diagram represents collagen contraction (mm) (n=3); Scale bars = 100um. (E-F) Bar diagram showing the percentage of AF+ population in 30, 60, and 90-dayCAF-CM treated PC cells. (G-J) Sphere formation assay was performed on CAF-CM and basal media-incubated BxPC3, and CD18/HPAF cells; 3000/well were seeded in 96-well ultra-low attachment plates in stem cell medium. Images have shown the morphology of 7–14-days-old spheres. Scale bar = 400 um. (n=3). (K-N) Flow cytometry analysis of CD44 positive population in 30, 60, and 90-day CAF-CM-treated PC cells. The bar diagrams signify the CD44+ population in 30, 60, and 90-day CAF-CM exposed PC cells. (O) Bar diagram denoted that the percentage of the ALDH+ population in 90 days CAF-CM exposed PC cells. Representative flow cytometry plots showing ALDH positive population in 90-day CAF-CM-treated PC cells as analyzed by AldeRed assay. For all panels, data represent mean ± SD (P values were calculated by Student t-test.) *P <0 .05, **P <0 .01 ***P < 0.001.
Co-culture or mixed PC cells with CAFs in 3D sodium alginate beads increases the stemness features of PC cells.
A recent study demonstrated that the stromal cells secrete soluble factors that positively intrude on nearby epithelial tumor cells, resulting in inducing and imparting the CSC phenotype38. Hence, we reasoned that the enrichment of CSCs in tumor cells could be a consequence of TME-related factors. Firstly, we tested this assumption using the 3D sodium alginate beads co-culture method, as shown previously35. The 3D sodium alginate beads with different sizes/shapes encapsulating either CAFs or PC cells were generated (Figure 2A, B). The hematoxylin and eosin (H&E) staining of PC cell beads co-cultured with CAF beads displayed more viable prominent colonies than PC cell beads cultured alone for 12 days (Figure 2C). Further, CAF co-cultured beads containing BxPC3 and CD18/HPAF cells showed an increased CD44+ population compared to the cells alone (Figure 2D–F). To further explore the effect of CAF on pancreatic stemness, we examined stemness marker expressions by using immunoblot analysis . As shown in (Figure 2G), CAFs significantly induced a stemness signature, as demonstrated by increased protein levels of CD44, NANOG, ALDH1/2, BMI-1, SOX9, KLF4, ABCG2, PAF1, and NOTCH1.
Figure 2. Co-culture or mixed PC cells with CAFs fosters pancreatic stemness.

(A) Schematic diagram showing the strategy used to generate 3D sodium alginate beads. (B) Light microscopy images showing the cells growing in 3D beads. Scale bar = 200 um. (C) Hematoxylin and eosin (H&E) staining of sodium alginate CAFs beads alone; CAFs beads co-cultured of BxPC3 and CD18/HPAF cells beads with appropriate controls. Scale bar = 200 um. (D) The bar diagram represents the percentage of the CD44+ population in PC cell bead co-cultured with CAFs beads. (E-F) Representative flow cytometry plots of CD44+ population analysis. (G) Immunoblotting analysis of stemness markers from cells harvested after 6 days of PC/CAFs co-cultured beads or PC beads alone. β-actin used as a housekeeping control. (H, I) Bar graph shows corrected total immunofluorescence staining intensity of CD44 (red) and DAPI (blue) in 0, 2, 4, 6, and 10 days of NFs/CAFs cells mixed with/without PC cells in sodium alginate beads. Scale bar = 50um (n =3). Representative confocal images demonstrating CD44 expression upon NFs and CAFs-derived CM treatment on PC cells. Data represent mean ± SD (P values were calculated by Student t-test.) *P <0 .05, **P <0 .01, ***P < 0.001.
Additionally, we performed immunofluorescence staining of CD44 in 0, 2, 4, 6, and 10 days cultures of NFs/CAFs cells mixed with PC cells in sodium alginate beads. CD44 expression was progressively augmented in CAFs mixed with PC cells containing the alginate beads sections of 2–10 days (Figures 2H and I). Further, we explored the stemness signature genes in 6 and 12 days CAF co-cultured cells alone/mixed with PC cells of sodium alginate beads. All stemness markers were predominantly expressed in CAF co-cultured PC cells in 6- and 12-days periods (Supplementary Figure 2I and J). Additionally, we performed H&E staining on 6 days cultured NFs/CAFs mixed with PC cells and found more colonies in CAFs-mixed PC cells (Supplementary Figure 2K). The elevation of all these stemness markers, increased colonies, and progressive elevation of CD44 expression in PC cells in response to CAF co-culture or mixed together indicate the significant role of CAF in the enrichment of stemness. Overall, these data demonstrate that CAFs induce the stemness signatures in a 3D model.
Long-term exposure of CAF-CM increases key stemness signatures in PC cells
Long-term CAF-CM exposed PC cells were further analyzed for other key stemness signatures. Initially, we found that the mRNA levels of self-renewal and drug resistance genes such as CD44, ALDH1A3, KLF4, PAF1, ABCG2, NANOG, SOX9, MDR1, BMI1, ALDH1A1, and ABCG4 are significantly increased in 30, 60, and 90-day CAF-CM exposed BxPC3 cells, and CD44, ALDH1A3, KLF4, SOX9, BMI-1, PAF1, ALDH1A1, MDR1 and ABCG4 in 30, 60, and 90-day CAF-CM treated CD18/HPAF cells (Figure 3A and B). We also observed a significant overexpression of CSC and self-renewal proteins CD44, ALDH1/2, KLF4, OCT3/4, NANOG, SOX2, SOX9, PAF1, MDR1, ABCG2, and BMI-1 in CAF-CM treated PC cells (Figure 3C). In addition, 30, 60, and 90-day CAF-CM treated PC cells showed increased co-localization of CD44 and ABCG2 compared to basal media-treated PC cells (Figure 3D). The early time points of CAF-CM treatment (3, 8, and 13 days) also showed elevation of few stemness markers (CD44s, CD44V6, CD44V9, ALDH1A1, ALDH1A3, and PD2/PAF1) as compared to basal media-treated PC cells. However, other stemness markers (KLF4, BMI1, SOX9, MDR1, LIF, ABCG2, and CD133) did not increase during the early treatment phase (Supplementary Figure 4 B,C). This result suggests that the CAF-CM initially alters a specific set of stemness markers that may eventually lead to a complete stemness reprogramming with long-term CAF-CM treatment. Further, we want to test the CAF-CM enriched stemness potential in other PC cells. So, we exposed CAF-CM to MIA PaCa-2 pancreatic cancer cells. CAF-CM treated MIA PaCa-2 cells showed morphological changes (Supplementary Figure 3A). We observed that 5,10 days CAF-CM exposed MIA PaCa-2 cells enriched the stemness markers DCAML1, NANOG, OCT3/4, SOX2, SOX9, MDR1, ALDH1A3(data was not shown here). Further, CAF-CM treated MIA PaCa-2 promotes the sphere formation ability in 30 and 60 days exposure (Supplementary Figure 3 B–C). We also observed a consistent overexpression of few stemness markers (ALDH1A3 and NANOG) in 30 and 60 days CAF-CM treatment groups (Supplementary Figure 3 D–E). In addition, NANOG and DCAML1 expression levels were elevated in 60 days CAF-CM treated groups as shown by the immunoblot experiment (Supplementary Figure 3 F, G). Further, we performed the flow cytometric analysis of AF+ and ALDH+ analysis in 30,60 days CAF-CM exposed MIA PaCa-2 cells. We observed that there was no significant variation in AF+, ALDH+ population. These results suggest that CAF exposure in a less aggressive PC cell line (MIA Paca-2) also showed minimal stemness variation. These findings confirmed that the long-term exposure of CAF-CM promotes the stemness signature in PC cells.
Figure 3. CAF-CM-exposed PC cells upsurge the stemness markers.
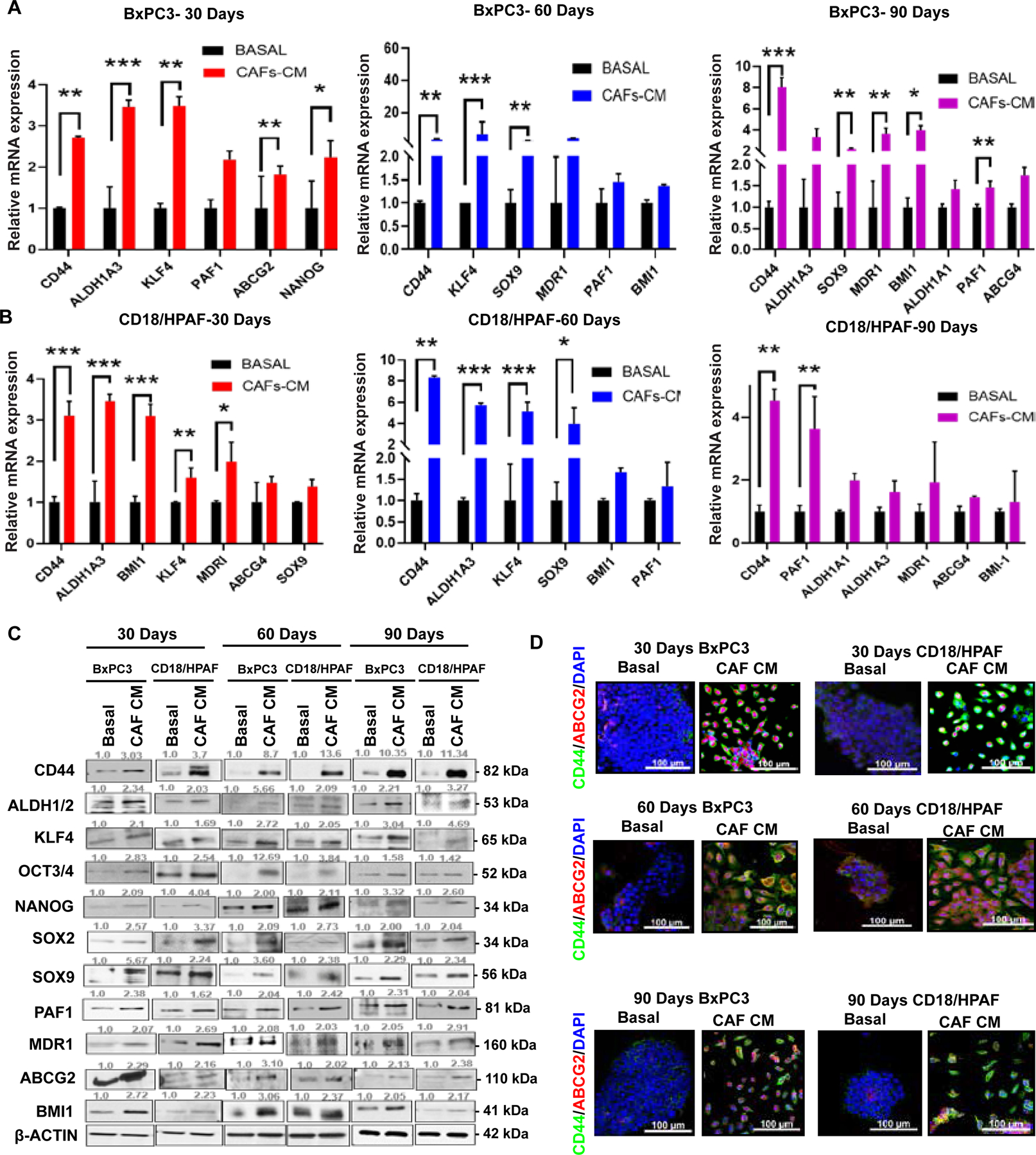
(A-B) qRT-PCR analysis of stemness marker expressions in 30, 60, and 90-day CAF-CM/Basal-treated PC cells. Data shown are normalized with β-actin expression (n =3). (C) Immunoblotting assays for stemness markers expressions in 30, 60, and 90-day CAF-CM/Basal-treated PC cells. β-actin was used as a loading control. (D) Immunofluorescence staining of 30, 60, and 90-day CAF-CM/Basal-exposed PC cells for stemness marker CD44 (Green) and ABCG2 (Red). Nuclei were stained in DAPI (blue). Scale bar = 100um. Data represent mean ± SD (P values were calculated by Student t-test.) *P <0 .05, **P <0 .01, ***P < 0.001.
CAF-CM treated PC cells exhibit enhanced proliferative, invasive potential and promote the tumorigenicity in vivo
Anchorage-independent growth is a critical feature of transformed cells and serves as a measure of in vitro tumorigenic capacity. The stem-like population retains increased proliferative and migratory potential39. Therefore, we investigated whether CAF-CM-treated PC cells acquire enhanced growth potential using colony formation assays. Our findings have shown that CAF-CM treated PC cells significantly increased the formation of a colony in anchorage-dependent fashion (plates), anchorage-independent (in soft-agar), and in vitro serial dilution (Figure 4A–D, Supplementary Figure 5 A B). Furthermore, we performed a trans-well/Boyden chamber and a wound-healing assay to assess the migration potential. CAF-CM treated PC cells showed an increased number of migrated cells than basal media-treated PC cells (Figure 4E, F). Moreover, CAF-CM-treated PC cells had a faster wound closure (within 48 hours) than the basal media-treated PC cells (Figure 4G, H, Supplementary Figure 5 C). To assess whether CAF-CM exposed PC cells show any impact on in vivo pancreatic tumorigenesis, we performed the orthotopic implantation of CAF-CM exposed CD18/HPAF cells in athymic nude mice. CAF-CM exposed PC cells significantly promoted the tumor weight (Figure 4I). CAF-CM exposed xenograft showed increased CD44 immunofluorescence staining compared to NF-CM exposed xenograft tumors (Figure 4 J, Supplementary Figure 5 M, Supplementary Figure 5 N). Further, we investigated the tumorigenic potential by subcutaneously injecting CAF-CM exposed PC cells (limiting dilution, 1000– 100, 000 cells) in the flanks of immune-compromised mice. CAF-CM exposed CD18/HPAF cells significantly increased the tumor volume and weight (Figure 4 K). These results collectively suggest that CAF plays an indispensable role in pancreatic tumorigenesis in vivo, possibly via enriching the stemness factors.
Figure 4. CAF-CM-exposed PC cells augmented cell proliferation, migration, wound healing ability and promote pancreatic tumorogenesis in in vivo model.
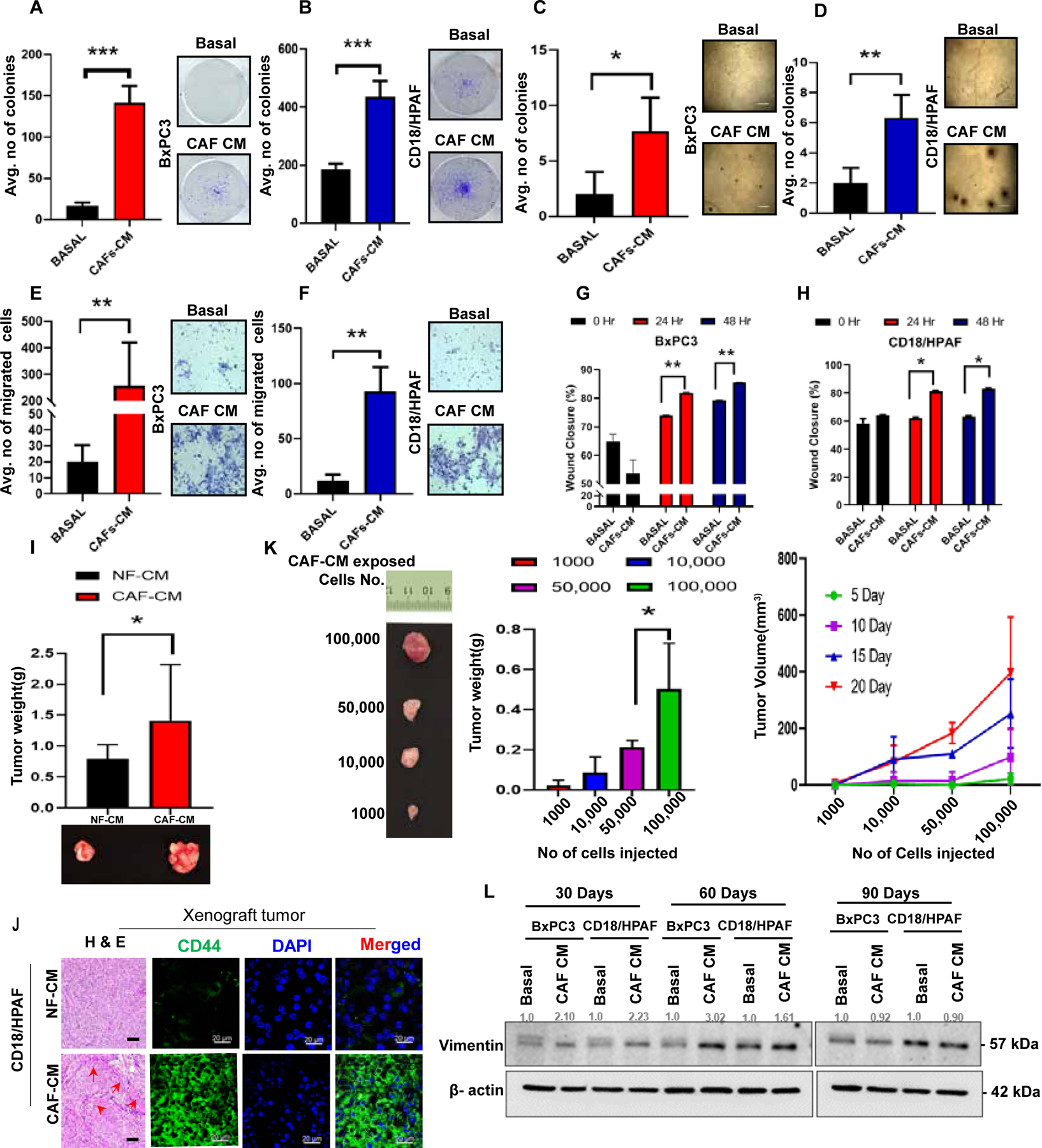
(A-B) CAF-CM treated cells were fixed with methanol and stained with 0.5% crystal violet. Scale bars=100um. The bar diagram shows the average number of colonies/well in CAF-CM/Basal-treated PC cells. (C-D) Images representing that CAF-CM-treated cells made colonies in soft agar anchorage-independent manner. The bar diagram denoted the average number of colonies/fields in CAF-CM/Basal-treated PC cells. (E-F) Representative images showing the CAF-CM exposed PC cells migrated in the inlet membrane. Scale bars=200um.The bar diagram denoted the average number of migrated cells/field in CAF-CM/Basal-treated PC cells. (G-H) The bar diagram depicting the average percentage of wound area in CAF-CM/Basal-treated PC cells. (I) Bar Graph shows the quantification of tumor weights generated using orthotopic implantation of NFs/CAFs-exposed PC cells in the pancreas of athymic nude mice. (n =4) and representative image displaying the size of each xenograft tumors. (J) Represented images displayed the H&E staining and immunofluorescence staining of CD44 (green) and DAPI (blue) in NF-CM/CAF-CM exposed PC cells xenograft tumor tissue. DAPI was used for nuclear staining; (n=3). (K) Images represented xenograft tumor of subcutaneous implantation of serially diluted CAF-CM exposed CD18/HPAF cells in athymic nude mice. (n=2). and bar graphs showing the tumor weight and tumor volume of CAF-CM exposed CD18/HPAF cells by in vivo limiting dilution assay. (L) Western blot experiment for Vimentin expression in 30, 60, and 90 days CAF-CM/Basal- treated PC cells. β-actin was used as a housekeeping control. (n=3). For all bar graphs, data represent mean ± SD (P values were calculated by Student t-test.) *P <0 .05, **P <0 .01, ***P < 0.001.
CAF-CM-treated PC cells exhibit elevation of EMT and cell cycle markers
Epithelial-to-mesenchymal transition (EMT) is a cellular program and mechanism by which epithelial cells transform into mesenchymal phenotypes and facilitate tumor progression with metastatic expansion and acquisition of stem cell-like properties. We, therefore, assessed the expression of EMT markers in CAF-CM and basal media-treated PC cells. CAF-CM exposure to PC cells altered EMT markers (E-Cadherin, N-Cadherin, Zo-1, Slug and snail proteins, and Zeb-1, Twist, Fibronectin genes) (Supplementary Figure 5 D, E, F, G), and these results well correlated with enhanced migration potential in CAF-CM-exposed PC cells. Specifically, ZEB1 promotes PC progression40 and acquisition of stemness phenotype through EMT-mediated activation of CD44s-ZEB1 regulatory loop in PC41. In line with this observation, ZEB1 expression showed significant increase in CAF-CM exposed BxPC3 and CD18/HPAF cells (Supplementary Figure 5 E, G).
Furthermore, the expression level of vimentin, a mesenchymal marker, was significantly increased in 30 and 60 days CAF-CM exposed PC cells (Figure 4L, Supplementary Figure 5 E, G). We also observed that the levels of cell cycle markers Cyclin B1 and Cyclin E increased significantly in CAF-CM-treated PC cells (Supplementary Figure 5 F). In addition, CAF-CM exposed PC cells showed significantly altered expression of cancer cell survival signaling (Phospho AKT, and with no change in total AKT) (Supplementary Figure 5 F). Next, we examined whether the enriched PC cells show reverse phenotype in the absence of conditioned media. For this, we cultured long-term CAF-CM treated cells in the presence and absence of CAF-CM. We found a decrease in stemness markers expression after in 10 days in the CAF-CM untreated cells compared to CAF-CM treated cells (Supplementary Figure 6 A, B, C). These results specify that stemness enrichment is reversible in a time-dependent fashion in the absence of CAF. All these findings indicate that treatment with CAF-CM promotes the EMT and tumorigenic markers in PC cells.
α-SMA and CD44 are co-overexpressed in KPC mice and human PDAC samples
To study the relevance of CD44 and α-SMA (a fibroblast activation marker) co-expression at different stages of disease progression in PC (Figure 5A), we used LSL-Kras (G12D/+); LSL-Trp53 (R172H/+); Pdx-1-Cre (KPC) mice autochthonous tumor tissues collected from 5, 10, 15, and 20 weeks old mice. Immunofluorescent staining of CD44 gradually increased from 10, 15,,and 20 week old KPC mice tumor tissues along with α-SMA in the stromal region (Figure 5B–C). To investigate the clinical correlation of CD44 and α-SMA, we utilized human PDAC and normal pancreas samples. Dual co-overexpression of CD44 and α-SMA were observed by immunofluorescence staining in human PDAC (stage IIA (grade 2–3), IIB (grade 2) tissues compared to normal pancreas tissue (Figure 5D–E). Immunohistochemical analyses showed CD44 staining in the apical membrane of ductal cells, and α-SMA positive staining appeared basolaterally in stromal areas (Figure 5F–G). These results indicate a strong positive correlation between CD44 and α-SMA expression in PDAC tissues. Furthermore, we examined CD44 and α-SMA expression patterns in the TGCA PDAC data set using Gepia software42. The expression of CD44 and α-SMA is associated with PC progression and increased from tumor stage I to stage II (Figure 5 H). The regression correlation revealed a positive correlation between CD44 and α-SMA in PC samples (Supplementary Figure 5 H). Also, lower overall survival is associated with higher CD44 and α-SMA co-expression in PC patients (Supplementary Figure 5 I). Overall, these results suggest that the expressions of CD44 and α-SMA progressively increased in different stages of pancreatic tumors in KPC tumors and human PDAC tissues.
Figure 5. Human/KPC in vivo models show a progressive increase in the stemness, and CAF shows at different PC stages.

(A) The schematic diagram illustrating the PC stages expression of CD44 and α-SMA in mice and human tissue samples. (B) Bar diagram represented as corrected total fluorescence intensity in different stages of KPC mice and normal mice tissues expression of CD44 and α-SMA (n=3). Scale bars=50um; 20 week-20um. (C) Representative images show the immunofluorescence staining of CD44 and α-SMA from 10, 15, and 20 weeks old KPC (KrasG12D; p53R172H; Pdx1Cre) mice tissues. (D) The Box plot representing the corrected total fluorescence intensity in PDAC and normal tissues of CD44 and α-SMA expressions (n=8). (E) Representative images show the immunofluorescence staining of CD44 and α-SMA from human PDAC stage IIA, IIB, and normal tissues. Scale bars=50um (F) The Box plot showing the H score of PDAC and normal tissues of CD44 and α-SMA expressions (n=8). (G) Immunohistochemical staining of CD44 and α-SMA from human PDAC stage IIA, IIB, and normal tissues (Scale bars=20 um). (n=8). (H) TCGA database analysis expression pattern of CD44, α-SMA in different pancreatic adenocarcinoma stages (PAAD)using the Gene Expression Profiling Interactive Analysis tool (GEPIA). Images are represented as a violin plot. CD44 expressions on different stages of PAAD, and α-SMA (ACTA2) expressions on different stages of PAAD; n(T) =179; n(N)=171.Data represent mean ± SD (P values were calculated by Student t-test.) *P <0 .05, **P <0 .01, ***P < 0.001.
OPN/SPP1 specifically overexpressed in CAF-CM enriched PC cells
We performed a PCR array to recognize receptor and ligand associated with stemness signaling molecules in CAF-CM-treated PC cells based on observations such as enrichment of stemness, tumorigenicity, invasiveness, and EMT phenotype. Our PCR array showed that 28 (receptor and ligand) genes were up-regulated in BxPC3 and CD18/HPAF-treated with CAF-CM (Figure 6A). The qRT-PCR validation of selected genes(cut off ≤ 5 fold) in 30, 60, and 90-day CAF-CM treated PC cells showed significant and consistent overexpression of OPN/SPP1 in CAF-CM treated PC cells compared to basal media-treated PC cells (Figure 6B). We also confirmed the OPN/SPP1 protein overexpression in all-time points of CAF-CM and basal media-treated PC cells (Figure 6C). Since OPN/SPP1 showed elevated expression in CAF-CM enriched PC cells, we aimed to determine its levels in PC culture supernatants. We found that the secretory level of OPN/SPP1 was significantly higher in the supernatant media of CAF-CM-treated PC cells, as detected by ELISA (Figure 6D–E). Interestingly, we also found that the supernatant media of CAFs showed significantly increased OPN/SPP1 compared to the supernatant media of NFs (Figure 6F). Also, OPN/SPP1 showed augmented expression in 10, 15-, and 20-week-old KPC mice tumors using immunofluorescence staining (Figure 6G–H). TGCA database analysis showed a strong association of OPN/SPP1 expression with PC progression in all stages (Figure 6I). The regression correlation showed a positive correlation between CD44 and SPP1 in PC samples (Supplementary Figure 5 J). Similarly, lower overall survival is associated with higher CD44 and SPP1 co-expression in PC patients (Supplementary Figure 5 K). The network analysis found that OPN/SPP1 and CD44 have cross-talk in oncogenesis (Supplementary Figure 5 L). Next, we performed immunofluorescence staining of CD44 and OPN/SPP1 in NF-CM/CAF-CM exposed CD18/HPAF implanted mouse xenograft tissues. CAF-CM treated xenograft tumor tissue sections showed increased co-localization of OPN/SPP1 and CD44 compared to NF-CM exposed PC cell xenograft tumor tissue(Supplementary Figure 5 N). These results imply that OPN/SPP1 is overexpressed exogenously and endogenously to associate with CD44 to modulate and support stemness signaling in CAF-CM treated PC cells.
Figure 6. CAF-CM subsidizes the up-regulation of OPN/SPP1 in PC cells.
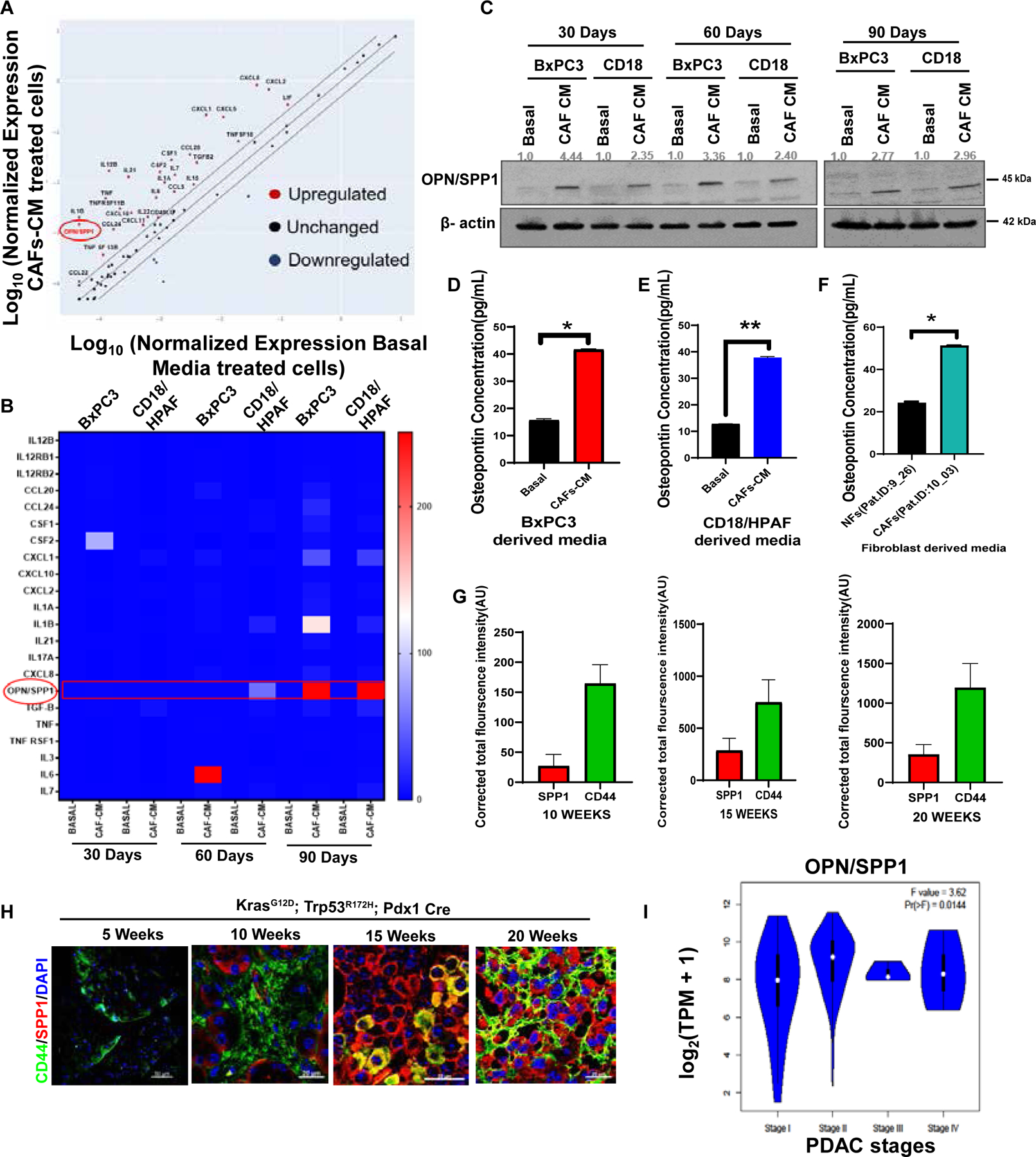
(A) PCR array for receptors and ligands molecules associated with stemness signaling from CAF-CM/Basal-exposed PC cells. (B) Heatmap of top differentially expressed genes of PCR array (cut off < 5 fold). (C) Immunoblotting of OPN/SPP1 protein expression in 30, 60, and 90-dayCAF-CM/Basal-exposed PC cells. β-actin was used as a housekeeping control. (D-F) The OPN/SPP1levels in culture media. Bar diagrams depict the OPN/SPP1levels in pg/mL in the serum-free culture media of NFs/CAF-CM-exposed cells. (G-H) Representative bar graphs and images are showing immunofluorescence staining of CD44 (green) and OPN/SPP1 (red) in 5, 10, 15, and 20 weeks old KPC (KrasG12D; p53R172H; Pdx1Cre) mice tissues. Nuclei were stained in DAPI (blue). n=3; Scale Bar= 20um. (I) Representative violin plot showing the OPN/SPP1 expressions on different stages of PAAD; n (T) =179; n (N) =171. Data represent mean ± SD (P values were calculated by Student t-test) *P <0 .05, **P <0 .01, ***P < 0.001.
Knockdown of CD44 and OPN/SPP1 in CAF-CM-treated PC cells reduced stemness population.
Next, we examined whether the OPN/SPP1-CD44 axis regulates the CAF-CM-induced stemness signature. Stable knockdowns (KD) of CD44 and OPN/SPP1 were established in BxPC3 and CD18/HPAF cells (Figure 7A), followed by the analysis of stemness characteristics. The knockdown of CD44 and OPN/SPP1 showed decreased stemness markers in CAF-CM-treated PC cells. Specifically, CD44 KD reduced KLF4, ABCG2, and OCT3/4. OPN/SPP1 KD decreased the expressions of KLF4, NANOG, OCT3/4, and ABCG2 compared to scrambled control CAF-CM-treated PC cells (Figure 7B). Further, qRT-PCR data revealed that the CD44 KD in CAF-CM treated PC cells significantly decreased mRNA expression levels of CD44, OPN/SPP1, ALDH1A1, ALDH1A3, KLF4, BMI1, and SOX2 (Supplementary Figure 7 A, B). The OPN/SPP1 KD CAF-CM treated cells significantly reduced the OPN/SPP1, ALDH1A1, ALDH1A3, CD44, KLF4, SOX2, OCT3/4, SOX9, BMI1, and NANOG (Supplementary Figure 7 C, D). Also, the silencing of CD44 and OPN/SPP1 significantly decreased the tumorsphere formation ability (Figure 7C), the percentage of CD44+, AF+ (Figure 7D–G), and ALDH+ populations (Supplementary Figure 7 E, F) in CD44 and OPN/SPP1 KD PC cells compared to the scrambled control. Further, we performed H&E staining and immunofluorescence staining of CD44 in Scramble/OPN/SPP1 knockdown PC cells with sodium alginate beads. OPN/SPP1 knockdown PC cell showed decreased colony numbers and CD44 expression compared to scramble PC cells (Supplementary Figure 7 G, H). In addition, OPN/SPP1 immunofluorescence staining in human PDAC/mouse KPC tissues showed exclusive expression in advanced tumors tissues compared to the normal pancreas (Supplementary Figure 8 A). OPN/SPP1 and CD44 knockdown cells showed decreased expression and localization of CD44, OPN/SPP1, SOX2, and MDR1 in confocal immunofluorescence analysis (Supplementary Figure 8 B–E). These overall findings confirm that both CD44 and OPN/SPP1 maintain the stemness by altering the critical stemness markers in CAF-CM-treated PC cells (Figure 7H).
Figure 7. Small interfering RNA knockdown of OPN/SPP1and CD44 in CAF-CM-induced PC cells diminished stemness features.
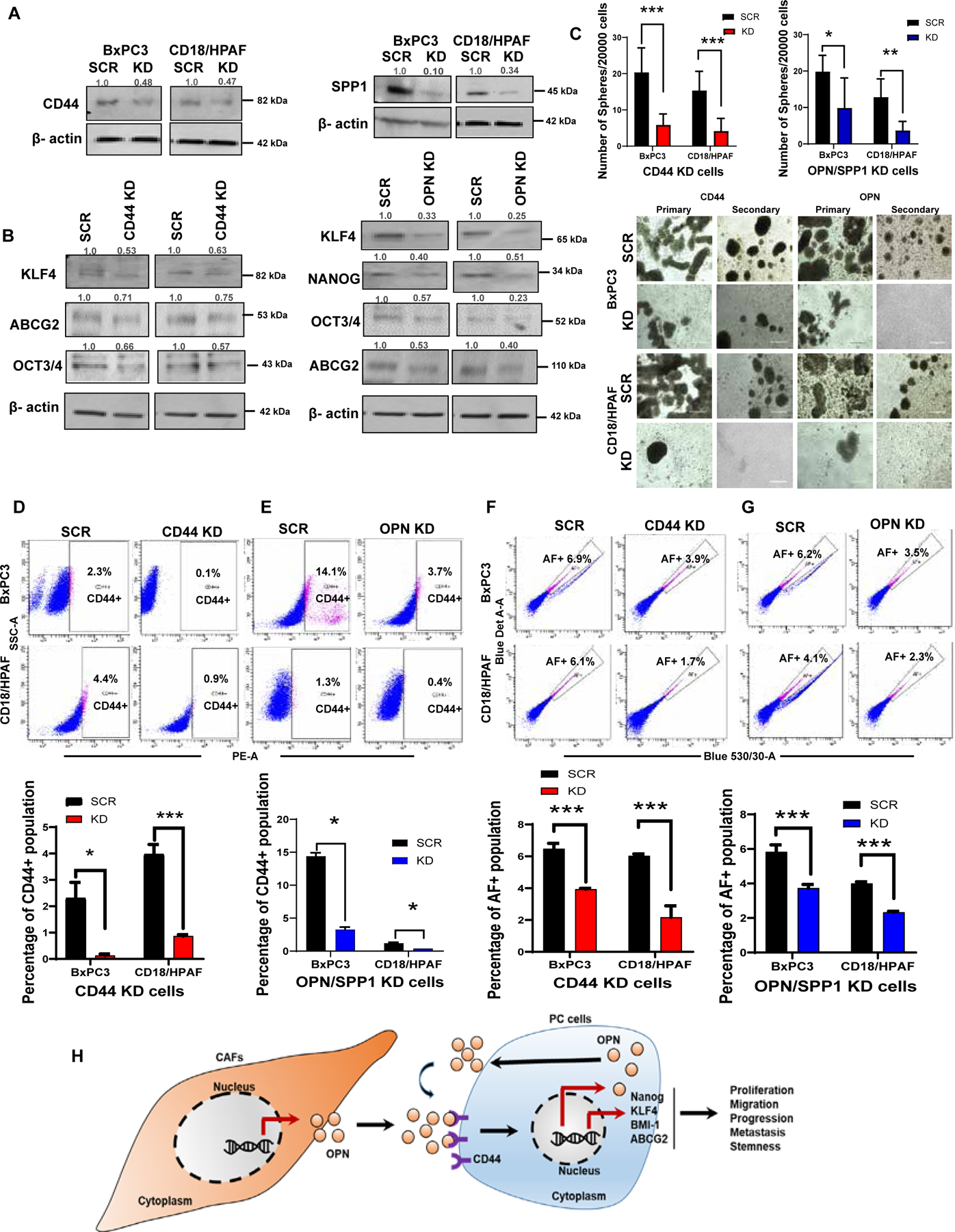
(A) Immunoblot blot analysis shows OPN/SPP1, CD44 knockdown efficiency in CAF-CM-treated PC cells. (B) Western blot analysis for stemness markers expressions in OPN/SPP1 and CD44 knockdown PC cells. β-actin was used as a housekeeping control (n=3). (C) The bar diagram showing the number of spheres per 2000 cells in OPN/SPP1 and CD44 knockdown PC cells; Sphere formation assays executed on OPN/SPP1 and CD44 knockdown PC cells; 2000/well were seeded in 96-well ultra-low attachment plates in stem cell medium; Images have shown the morphology of 7–14-days-old spheres. Scale bar = 400 um. (D-G) The bar diagram depicts the percentage of CD44+ and AF+ population in OPN/SPP1 and CD44 knockdown PC cells; Flow cytometry analysis of CD44+ and AF+ in CD44 and OPN/SPP1 knockdown PC cells. (H) Diagram illustrating how the CAFs regulate the OPN/SPP1-CD44 axis-mediated stemness enrichment in PC cells (n=3); Data represent mean ± SD (P values were calculated by Student t-test) *P <0 .05, **P <0 .01, ***P < 0.001.
Discussion
In pancreatic cancer, the desmoplastic stroma surrounds 90% of the entire tumor. Fibroblasts and extracellular matrix (ECM) in the desmoplastic stroma are the main features of pancreatic TME3. The pancreatic TME plays an essential role in drug resistance, intra-tumoral heterogeneity, and CSC phenotype, contributing to the poor survival rate43. CAFs are abundant stromal-activated fibroblast cell types in the pancreatic TME, which play a critical role in cancer progression and metastasis44. Our previous studies have shown the impact of pancreatic stemness on PC development, drug resistance, aggressiveness, and metastases15, 39, and demonstrated the effect of cigarette smoking on the enrichment of PC stemness19. In addition, several emerging studies showed the role of CAFs in the activation of the CSCs populations in different cancers30, 31, 45–47. However, the impact of CAFs on PC stemness enrichment is not well understood. Therefore, we focused on understanding the impact of the TME factor in stemness enrichment and their association with the aggressiveness of PC.
In this study, we demonstrated that CAFs facilitated the enrichment of stemness in PC cells. Long-term exposure of PC cells to CAF-CM resulted in the significant elevation of CD44+, AF+, and ALDH+ stemness populations. CAF-CM exposure also significantly increased tumorsphere formation and self-renewal potential. Moreover, CAF-CM-exposed PC cells showed increased stemness signature genes. In addition, the 3D sodium alginate bead-based co-culture48 showed the enrichment of CD44+CSC populations, a key marker for stemness and self-renewal. These findings strongly suggest the role of CAFs in the enrichment of stemness populations in PC cells.
Myofibroblasts are the vital player in the reactive tumor stroma to maintain homeostasis of tissue repair, fibrosis, and their altered expression is closely associated with cancer progression. α-SMA expressing myofibroblast (myCAFs) resides adjacent to the tumor, producing ECM components to make dense desmoplasia11. A recent study by Silva Affo et al. 2021 revealed that myCAF expresses hyaluronan synthase 2, a critical molecule that promotes the intrahepatic cholangiocarcinoma (ICC) progression50. A study showed that bone marrow-derived CAF subtype myofibroblasts promote stemness in gastric cancer cells54. However, there is a lack of studies that elucidate the role of stemness enrichment induced by the CAF subtype. Here, we showed for the first time that the myCAF enriches the stemness in human PC cells.
Similarly, recent studies indicated that a subset of CAFs promotes CSCs in breast cancer30, colorectal cancer45, hepatocellular carcinoma31, liver cancer46, and lung cancer47. Further, we observed that the expression of CD44 gradually increased along with α-SMA, a fibroblast marker55 in the progression of KrasG12D;p53R172H; Pdx1Cre mice autochthonous tumors and human PDAC tissues. Thus, our observation concludes that CAFs mediated enrichment of the CD44+ population facilitates PC progression towards metastases.
EMT reprogrammed cancer cells, which lack cell adhesive properties, detach from the primary site and attain the motile features to enable invasion, intravasation, and extravasation56. Studies have shown that cancer stemness is a hallmark of EMT reprogrammed cancer cells that respond to various environmental characteristics and recapitulate metastatic colonies in distant sites57. Interestingly, our study showed that CAF-CM exposed cancer cells promote clonogenic and metastatic properties and EMT phenotypes. These findings are consistent with the observations of other studies46, 58–60. Thus, CAF-CM promotes PC stemness, at least in part, via the induction of EMT genes and their corresponding proteins in PC cells.
Previous studies have shown that CAFs produce a surfeit of chemokines and cytokines that promote PC progression61–63. Our study identified OPN/SPP1 as a significant factor in CAF-CM-exposed PC cells, mice, and human PC tumors for the first time. Thus, the OPN/SPP1 secreted by the CAFs may be considered as a critical driver in PC progression. OPN/SPP1, commonly recognized as a multifunctional protein, accelerates cell proliferation, migration, survival, cancer progression and modulates several cancer-related signaling pathways in the TME64–66. OPN/SPP1 is secreted in various cancers to drive invasion, metastasis, and therapy resistance, including PC22. A previous study demonstrated that the glioma perivascular niche promotes stem cell features via the OPN/SPP1-CD44 signaling pathway 29 and further reported that this pathway is regulated by interferon signaling and hepatitis C virus replication in hepatic CSCs68 and bladder cancer69. To our knowledge, no documented study has been conducted reporting OPN/SPP1-CD44 axis in PC stemness enrichment. We have shown the significant role of the OPN/SPP1-CD44 axis in PC stemness enrichment. We also demonstrated that CD44/α–SMA gradually increased in expression in the various pre-neoplastic and neoplastic phases of both the genetically engineered mice pancreatic cancer and the human PDAC tissues. Finally, we have shown that the knockdown of OPN/SPP1 and CD44 in CAF-CM-exposed PC cells significantly decreases the various stem cell populations, CSCs markers, and tumorsphere formation ability. A previous study supports the concept that the silencing of CD44 significantly suppressed the development of spheroids, pluripotency, and stem cell markers in PDAC cells70, 71. Our observations suggest that OPN/SPP1 could promote stemness signature in PC cells by regulating the CD44-mediated stem cell markers NANOG, KLF4, BMI-1, and ABCG2.
In conclusion, our data demonstrate the interplay of CAFs and the enrichment of stem cells in pancreatic tumors. It provided conceptual insights into how CAFs can enrich the stemness population to encourage metastases in the TME. Our research also indicates that CAFs interact with tumor cells through the OPN/SPP1-CD44 axis to promote the stemness population in PC cells. We have shown that silencing either OPN/SPP1 or CD44 could lead to decreased stemness features. We hope that our current work has reconciled the existing discrepancy over the role of OPN/SPP1 in PC and extends the crucial understanding of how OPN/SPP1-CD44 is controlled at both expression and functional levels. In the future, this may pave the way for developing alternative PC therapeutic approaches that target the OPN/SPP1-CD44 axis, opening new avenues for advanced PC patients.
Supplementary Material
Acknowledgments:
We thank Craig Semerad, Victoria B. Smith, and Samantha Wall of the Flow Cytometry Research Facility, University of Nebraska Medical Center, for assisting with flow cytometry. We thank Janice A. Taylor and James R. Talaska of the Advanced Microscopy Core Facility at the University of Nebraska Medical Center for helping with confocal microscopy. The authors/work in this article were supported, in parts, by the following grants from the National Institutes of Health (P01 CA217798, R01 CA210637, R01 CA183459, R01 CA195586, R01 CA206444, R01 CA228524, U01 CA20046, and U01 CA210240) and the Nebraska Department of Health and Human Services LB595.
Abbreviations
- AF
autofluorescence
- ALDH
aldehyde dehydrogenase
- CSC
cancer stem cells
- EMT
epithelial-mesenchymal transition
- KPC
KrasG12D/+;Trp53R172H/+;Pdx1Cre
- PanIN
pancreatic intraepithelial neoplasia
- PC
pancreatic cancer
- PDAC
pancreatic ductal adenocarcinoma
- TME
Tumor microenvironment
- NFs
normal fibroblasts
- CAFs
cancer-associated fibroblast
- CAF-CM
cancer-associated fibroblast-conditioned media
- ECM
extracellular matrix
- OPN
Osteopontin
Footnotes
Conflicts of interest
SKB is one of the co-founders of Sanguine Diagnostics and Therapeutics, Inc. The other authors disclosed no potential conflicts of interest.
References
- 1.Ho WJ, Jaffee EM, Zheng L. The tumour microenvironment in pancreatic cancer - clinical challenges and opportunities. Nat Rev Clin Oncol 2020;17:527–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hosein AN, Brekken RA, Maitra A. Pancreatic cancer stroma: an update on therapeutic targeting strategies. Nat Rev Gastroenterol Hepatol 2020;17:487–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helms E, Onate MK, Sherman MH. Fibroblast Heterogeneity in the Pancreatic Tumor Microenvironment. Cancer Discov 2020;10:648–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalluri R The biology and function of fibroblasts in cancer. Nat Rev Cancer 2016;16:582–98. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discov 2019;18:99–115. [DOI] [PubMed] [Google Scholar]
- 6.Unterleuthner D, Neuhold P, Schwarz K, et al. Cancer-associated fibroblast-derived WNT2 increases tumor angiogenesis in colon cancer. Angiogenesis 2020;23:159–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L, Li X, Ren Y, et al. Cancer-associated fibroblasts contribute to cisplatin resistance by modulating ANXA3 in lung cancer cells. Cancer Sci 2019;110:1609–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Sun X, Mu L, et al. Cancer-Associated Fibroblasts Induce Epithelial-Mesenchymal Transition in Endometrial Cancer Cells by Regulating Pituitary Tumor Transforming Gene. Cancer Invest 2019;37:134–143. [DOI] [PubMed] [Google Scholar]
- 9.Brechbuhl HM, Finlay-Schultz J, Yamamoto TM, et al. Fibroblast Subtypes Regulate Responsiveness of Luminal Breast Cancer to Estrogen. Clin Cancer Res 2017;23:1710–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizutani Y, Kobayashi H, Iida T, et al. Meflin-Positive Cancer-Associated Fibroblasts Inhibit Pancreatic Carcinogenesis. Cancer Res 2019;79:5367–5381. [DOI] [PubMed] [Google Scholar]
- 11.Ohlund D, Handly-Santana A, Biffi G, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med 2017;214:579–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korkaya H, Liu S, Wicha MS. Breast cancer stem cells, cytokine networks, and the tumor microenvironment. J Clin Invest 2011;121:3804–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaz AP, Ponnusamy MP, Seshacharyulu P, et al. A concise review on the current understanding of pancreatic cancer stem cells. J Cancer Stem Cell Res 2014;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang VM, Ferreira RMM, Almagro J, et al. CD9 identifies pancreatic cancer stem cells and modulates glutamine metabolism to fuel tumour growth. Nat Cell Biol 2019;21:1425–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaz AP, Ponnusamy MP, Rachagani S, et al. Novel role of pancreatic differentiation 2 in facilitating self-renewal and drug resistance of pancreatic cancer stem cells. Br J Cancer 2014;111:486–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res 2007;67:1030–7. [DOI] [PubMed] [Google Scholar]
- 17.Hermann PC, Huber SL, Herrler T, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 2007;1:313–23. [DOI] [PubMed] [Google Scholar]
- 18.Karmakar S, Rauth S, Nallasamy P, et al. PAF1 Regulates Stem Cell Features of Pancreatic Cancer Cells, Independently of the PAF1 Complex, via Interactions with PHF5A and DDX3. Gastroenterology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nimmakayala RK, Seshacharyulu P, Lakshmanan I, et al. Cigarette Smoke Induces Stem Cell Features of Pancreatic Cancer Cells via PAF1. Gastroenterology 2018;155:892–908 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karmakar S, Dey P, Vaz AP, et al. PD2/PAF1 at the Crossroads of the Cancer Network. Cancer Res 2018;78:313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao H, Chen Q, Alam A, et al. The role of osteopontin in the progression of solid organ tumour. Cell Death Dis 2018;9:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolb A, Kleeff J, Guweidhi A, et al. Osteopontin influences the invasiveness of pancreatic cancer cells and is increased in neoplastic and inflammatory conditions. Cancer Biol Ther 2005;4:740–6. [DOI] [PubMed] [Google Scholar]
- 23.Chiou J, Chang YC, Tsai HF, et al. Follistatin-like Protein 1 Inhibits Lung Cancer Metastasis by Preventing Proteolytic Activation of Osteopontin. Cancer Res 2019;79:6113–6125. [DOI] [PubMed] [Google Scholar]
- 24.Xu K, Tian X, Oh SY, et al. The fibroblast Tiam1-osteopontin pathway modulates breast cancer invasion and metastasis. Breast Cancer Res 2016;18:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hahne JC, Meyer SR, Kranke P, et al. Studies on the role of osteopontin-1 in endometrial cancer cell lines. Strahlenther Onkol 2013;189:1040–8. [DOI] [PubMed] [Google Scholar]
- 26.Song JY, Lee JK, Lee NW, et al. Osteopontin expression correlates with invasiveness in cervical cancer. Aust N Z J Obstet Gynaecol 2009;49:434–8. [DOI] [PubMed] [Google Scholar]
- 27.Hui EP, Sung FL, Yu BK, et al. Plasma osteopontin, hypoxia, and response to radiotherapy in nasopharyngeal cancer. Clin Cancer Res 2008;14:7080–7. [DOI] [PubMed] [Google Scholar]
- 28.Rao G, Wang H, Li B, et al. Reciprocal interactions between tumor-associated macrophages and CD44-positive cancer cells via osteopontin/CD44 promote tumorigenicity in colorectal cancer. Clin Cancer Res 2013;19:785–97. [DOI] [PubMed] [Google Scholar]
- 29.Pietras A, Katz AM, Ekstrom EJ, et al. Osteopontin-CD44 signaling in the glioma perivascular niche enhances cancer stem cell phenotypes and promotes aggressive tumor growth. Cell Stem Cell 2014;14:357–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su S, Chen J, Yao H, et al. CD10(+)GPR77(+) Cancer-Associated Fibroblasts Promote Cancer Formation and Chemoresistance by Sustaining Cancer Stemness. Cell 2018;172:841–856 e16. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Wang R, Xiong S, et al. Cancer-associated fibroblasts promote the stemness of CD24(+) liver cells via paracrine signaling. J Mol Med (Berl) 2019;97:243–255. [DOI] [PubMed] [Google Scholar]
- 32.Begum A, McMillan RH, Chang YT, et al. Direct Interactions With Cancer-Associated Fibroblasts Lead to Enhanced Pancreatic Cancer Stem Cell Function. Pancreas 2019;48:329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee KM, Choi KH, Ouellette MM. Use of exogenous hTERT to immortalize primary human cells. Cytotechnology 2004;45:33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bian X, Yang Z, Feng H, et al. A Combination of Species Identification and STR Profiling Identifies Cross-contaminated Cells from 482 Human Tumor Cell Lines. Sci Rep 2017;7:9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Read GH, Miura N, Carter JL, et al. Three-dimensional alginate hydrogels for radiobiological and metabolic studies of cancer cells. Colloids Surf B Biointerfaces 2018;171:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masciale V, Grisendi G, Banchelli F, et al. CD44+/EPCAM+ cells detect a subpopulation of ALDH(high) cells in human non-small cell lung cancer: A chance for targeting cancer stem cells? Oncotarget 2020;11:1545–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yasuda K, Torigoe T, Morita R, et al. Ovarian cancer stem cells are enriched in side population and aldehyde dehydrogenase bright overlapping population. PLoS One 2013;8:e68187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pattabiraman DR, Weinberg RA. Tackling the cancer stem cells - what challenges do they pose? Nat Rev Drug Discov 2014;13:497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nimmakayala RK, Batra SK, Ponnusamy MP. Unraveling the journey of cancer stem cells from origin to metastasis. Biochim Biophys Acta Rev Cancer 2019;1871:50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krebs AM, Mitschke J, Lasierra Losada M, et al. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat Cell Biol 2017;19:518–529. [DOI] [PubMed] [Google Scholar]
- 41.Preca BT, Bajdak K, Mock K, et al. A self-enforcing CD44s/ZEB1 feedback loop maintains EMT and stemness properties in cancer cells. Int J Cancer 2015;137:2566–77. [DOI] [PubMed] [Google Scholar]
- 42.Tang Z, Li C, Kang B, et al. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res 2017;45:W98–W102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farrow B, Albo D, Berger DH. The role of the tumor microenvironment in the progression of pancreatic cancer. J Surg Res 2008;149:319–28. [DOI] [PubMed] [Google Scholar]
- 44.Chan TS, Shaked Y, Tsai KK. Targeting the Interplay Between Cancer Fibroblasts, Mesenchymal Stem Cells, and Cancer Stem Cells in Desmoplastic Cancers. Front Oncol 2019;9:688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sung PJ, Rama N, Imbach J, et al. Cancer-Associated Fibroblasts Produce Netrin-1 to Control Cancer Cell Plasticity. Cancer Res 2019;79:3651–3661. [DOI] [PubMed] [Google Scholar]
- 46.Zhao Z, Bai S, Wang R, et al. Cancer-associated fibroblasts endow stem-like qualities to liver cancer cells by modulating autophagy. Cancer Manag Res 2019;11:5737–5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen WJ, Ho CC, Chang YL, et al. Cancer-associated fibroblasts regulate the plasticity of lung cancer stemness via paracrine signalling. Nat Commun 2014;5:3472. [DOI] [PubMed] [Google Scholar]
- 48.Xu XX, Liu C, Liu Y, et al. Enrichment of cancer stem cell-like cells by culture in alginate gel beads. J Biotechnol 2014;177:1–12. [DOI] [PubMed] [Google Scholar]
- 49.Biffi G, Oni TE, Spielman B, et al. IL1-Induced JAK/STAT Signaling Is Antagonized by TGFbeta to Shape CAF Heterogeneity in Pancreatic Ductal Adenocarcinoma. Cancer Discov 2019;9:282–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Affo S, Nair A, Brundu F, et al. Promotion of cholangiocarcinoma growth by diverse cancer-associated fibroblast subpopulations. Cancer Cell 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hessmann E, Buchholz SM, Demir IE, et al. Microenvironmental Determinants of Pancreatic Cancer. Physiol Rev 2020;100:1707–1751. [DOI] [PubMed] [Google Scholar]
- 52.Du Y, Shao H, Moller M, et al. Intracellular Notch1 Signaling in Cancer-Associated Fibroblasts Dictates the Plasticity and Stemness of Melanoma Stem/Initiating Cells. Stem Cells 2019;37:865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu B, Wu K, Wang X, et al. Periostin secreted by cancer-associated fibroblasts promotes cancer stemness in head and neck cancer by activating protein tyrosine kinase 7. Cell Death Dis 2018;9:1082. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Zhu L, Cheng X, Shi J, et al. Cross-talk between bone marrow-derived myofibroblasts and gastric cancer cells regulates cancer stemness and promotes tumorigenesis. Oncogene 2016;35:5388–5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chaffer CL, Goetz JG. CD44 Orchestrates Metastatic Teamwork. Dev Cell 2018;47:691–693. [DOI] [PubMed] [Google Scholar]
- 56.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2002;2:442–54. [DOI] [PubMed] [Google Scholar]
- 57.Brabletz T, Jung A, Spaderna S, et al. Opinion: migrating cancer stem cells - an integrated concept of malignant tumour progression. Nat Rev Cancer 2005;5:744–9. [DOI] [PubMed] [Google Scholar]
- 58.Wang X, Zhang W, Sun X, et al. Cancer-associated fibroblasts induce epithelial-mesenchymal transition through secreted cytokines in endometrial cancer cells. Oncol Lett 2018;15:5694–5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yi Y, Zeng S, Wang Z, et al. Cancer-associated fibroblasts promote epithelial-mesenchymal transition and EGFR-TKI resistance of non-small cell lung cancers via HGF/IGF-1/ANXA2 signaling. Biochim Biophys Acta Mol Basis Dis 2018;1864:793–803. [DOI] [PubMed] [Google Scholar]
- 60.Shiga K, Hara M, Nagasaki T, et al. Cancer-Associated Fibroblasts: Their Characteristics and Their Roles in Tumor Growth. Cancers (Basel) 2015;7:2443–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Awaji M, Saxena S, Wu L, et al. CXCR2 signaling promotes secretory cancer-associated fibroblasts in pancreatic ductal adenocarcinoma. FASEB J 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuen J, Darowski D, Kluge T, et al. Pancreatic cancer cell/fibroblast co-culture induces M2 like macrophages that influence therapeutic response in a 3D model. PLoS One 2017;12:e0182039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wei L, Ye H, Li G, et al. Cancer-associated fibroblasts promote progression and gemcitabine resistance via the SDF-1/SATB-1 pathway in pancreatic cancer. Cell Death Dis 2018;9:1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.El-Tanani MK. Role of osteopontin in cellular signaling and metastatic phenotype. Front Biosci 2008;13:4276–84. [DOI] [PubMed] [Google Scholar]
- 65.Rangaswami H, Bulbule A, Kundu GC. Osteopontin: role in cell signaling and cancer progression. Trends Cell Biol 2006;16:79–87. [DOI] [PubMed] [Google Scholar]
- 66.Bandopadhyay M, Bulbule A, Butti R, et al. Osteopontin as a therapeutic target for cancer. Expert Opin Ther Targets 2014;18:883–95. [DOI] [PubMed] [Google Scholar]
- 67.Zhou Y, Dai DL, Martinka M, et al. Osteopontin expression correlates with melanoma invasion. J Invest Dermatol 2005;124:1044–52. [DOI] [PubMed] [Google Scholar]
- 68.Shirasaki T, Honda M, Yamashita T, et al. The osteopontin-CD44 axis in hepatic cancer stem cells regulates IFN signaling and HCV replication. Sci Rep 2018;8:13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ahmed M, Sottnik JL, Dancik GM, et al. An Osteopontin/CD44 Axis in RhoGDI2-Mediated Metastasis Suppression. Cancer Cell 2016;30:432–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kumazoe M, Takai M, Bae J, et al. FOXO3 is essential for CD44 expression in pancreatic cancer cells. Oncogene 2017;36:2643–2654. [DOI] [PubMed] [Google Scholar]
- 71.Molejon MI, Tellechea JI, Loncle C, et al. Deciphering the cellular source of tumor relapse identifies CD44 as a major therapeutic target in pancreatic adenocarcinoma. Oncotarget 2015;6:7408–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


