Abstract
Squamous cell carcinoma (SCC) is a form of skin cancer that can be treated using a procedure known as Mohs surgery. Mohs surgery is a safe and effective procedure for eliminating SCC. This surgery requires the usage of an analgesic known as lidocaine. Additional anesthetics were also reported to be necessary for this procedure to be conducted in a manner that significantly minimizes patient harm. According to the review, it was found that SCC was treated with lidocaine as a topical analgesic outside of Mohs surgery. This review analyzes the usage of lidocaine in the treatment of SCC. It was also discovered that lidocaine, as an agent, has the potential to slow the progression of SCC, but more research is needed to see if this is truly the case. On average, it was reported that the concentration of lidocaine used in the in vivo studies was significantly higher than that in the in vitro investigations. Further exploration may be needed to verify the conclusions that were based on the analysis of the papers within the review.
Keywords: mohs surgery, xylocaine, cancer treatment, squamous cell carcinoma, lidocaine
Introduction and background
Squamous cell carcinoma (SCC), a non-melanoma skin cancer, is a keratinocyte carcinoma and is one of the most prevalent malignancies with a rising incidence (SCC of the anus incidence rates increases nearly 3% each year) [1,2]. In fact, it is anticipated that approximately 700,000 new instances of cutaneous squamous cell carcinoma (CSCC) are detected annually in the United States [3]. The accumulated exposure of skin to UV light culminates in SCC, the second most prevalent form of skin cancer. Age, cumulative sun exposure, pale skin, continuous immunosuppression, and past skin cancer diagnoses are significant SCC risk factors [3-5]. This illness is characterized by precursor lesions known as actinic keratosis, tumor growth (typically greater than 3 cm in size at stage IV SCC and less than 3 cm at earlier stages), and the potential for metastasis inside the body. SCC is responsible for the majority of non-melanoma skin cancer-related metastatic illnesses; consequently, early detection and treatment of SCC are crucial for preventing neoplastic development [4,6]. The prognosis for the majority of patients with primary SCC is favorable, and treatment is typically easy. However, a sizable proportion of malignant neoplasms may return or metastasize. On average, SCC tumors are reported to have a diameter of approximately 1.5 cm [3]. Surgical excision is the primary treatment for CSCC, with Mohs micrographic surgery becoming a preferred excisional procedure for SCC of the neck and head as well as other areas with high-risk or SCCs with high-risk features [6]. Radiation therapy (shown to have a 90% five-year cure rate) is reserved for elderly patients with SCC, for those who cannot endure surgery, or when it has been impossible to acquire clear surgical margins [6,7]. On average, patients who receive radiation therapy for SCC have a survival rate of approximately 89% if treated in the early stages [3]. Very high tumors are typically treated with adjuvant radiation following surgical intervention. Mohs surgery necessitates the use of lidocaine as an anesthetic. In vitro studies of SCC cells have also utilized lidocaine [5-6]. The objective of this review is to examine how lidocaine contributes to SCC treatment and our current understanding of the disease.
Review
Methods
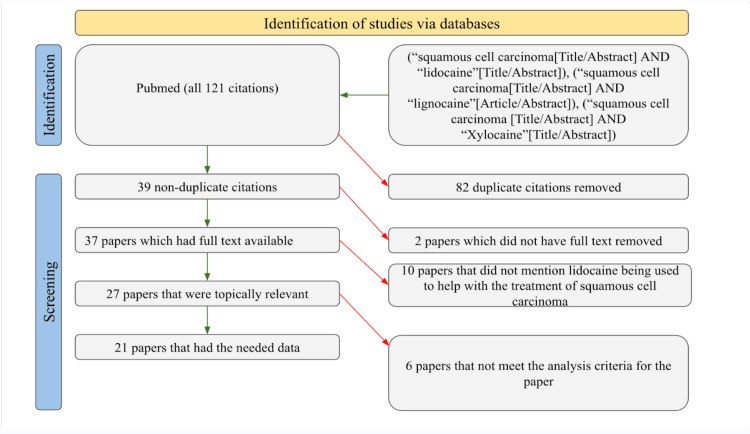
We conducted a search on PubMed to find studies about the use of lidocaine in the treatment of SCC. Exact searches were done with the keywords “squamous cell carcinoma and lidocaine,” “squamous cell carcinoma and lignocaine,” and “squamous cell carcinoma and xylocaine.” We placed no restrictions in terms of time frames in the search. The search elicited a total of 121 studies, of which 39 were not duplicates, 37 had full text available, 27 were topically relevant studies, and only 21 had the relevant information needed and fulfilled the analysis criteria of our review. The data collected from these 21 studies included the dosage of the lidocaine used, the area of anesthetic application, additional medications used, and the route of administration. Studies that did not provide sufficient information about at least two of these categories were excluded. This was done to avoid personal bias. Figure 1 provides a clear illustration of the filtering procedure used by the authors of this review.
Figure 1. PRISMA diagram representing the study selection process.
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Mohs surgery
While there are several different treatment options for SCC based on the progression of the disease, Mohs surgery, also known as Mohs micrographic surgery, is the best option in many cases due to its advantages in removing the cancerous region. Mohs surgery holds the highest cure rate of any SCC treatment [4-7]. Although there are other treatment options such as wide local excisions, electrodessication, and curettage which are viable and effective treatment options, the accepted maximum cure rate is approximately 95%, whereas Mohs is able to achieve a higher cure rate of 99% [4-7]. The reason for the discrepancy lies in the way the procedure is conducted. The physician ensures clear margins with microscopic examinations at the time of treatment versus sending the specimen to a dermatopathologist for inspection as with wide local excisions [8-10]. Thus, the entire cancerous region is removed at once, with a very minimal risk of disease progression and no additional risk of complications from a second procedure if the margins are not clear. Mohs surgery not only has the highest cure rate, but it also has the best patient outcome for SCC removal. The studies that were analyzed, as shown in Table 1, have data that also seems to support this hypothesis [8-28]. With the stage-wise protocol of tissue removal of cancerous tissue until clear margins are seen, Mohs surgery minimizes the amount of healthy tissue being removed. This precision is especially important for sensitive areas like the face and nose, where it is not only important for positive patient satisfaction but also for optimal surgical site closure to minimize scarring [6]. Further, since the procedure is done under local anesthesia, there is a reduced risk of complications that may arise from general anesthesia, making it a safer treatment option.
Table 1. The usage of lidocaine in the treatment of squamous cell carcinoma.
| Author (year) | Reported Dosage or Concentration of Lidocaine | Area of Application | Additional Pharmaceutical Medications | Route of Administration |
| Clark and Kalan (1995) [9] | 100 mg | Intravenous | Ketamine | In vivo |
| Ferreira et al. (2018) [10] | 1-5% | Applied to cells (incubation) | Doxorubicin | In vitro |
| Firoz et al. (2009) [11] | 0.50% | Injection (type unspecified) into digits | Aspirin, coumadin, plavix, and vitamin E | In vivo |
| Hakim et al. (2018) [12] | 1, 1.5, 2 mg/kg | Intravenous | Ketamine, lidocaine, mexiletine, methadone, and morphine | In vivo |
| Heller et al. (1998) [13] | 1% | Injection (type unspecified) around the treatment site | Bleomycin | In vivo |
| Ho et al. (2004) [14] | 2% | Topical (on tongue/pharynx) followed by gentle suctioning | Atropine | In vivo |
| Johnstone et al. (1995) [15] | 2.50% | Case 1: Intravenous; Case 2: Intravenous, then topical; Case 3: Intravenous, then topical; Case 4: Intravenous, then topical | Nitrous oxide isofluoride fentanyl | In vivo |
| Kintzel et al. (2018) [16] | 1 mg/minute (60 mg/hour), 0.8 mg/minute (48 mg/hour), 0.6 mg/minute (36 mg/hour), 0.6 mg/minute (36 mg/hour), 0.4 mg/minute | Intravenous | Gabapentin, methadone, ketamine acetaminophen, and pro re nata (PRN) hydromorphone | In vivo |
| Kobayashi et al. (2012) [17] | 400 μM, 676.6 μM, 735.5 μM, 811.6 μM, and 4000 μM | Applied to cells | Dibucaine tetracaine, bupivacaine, lidocaine, and procaine | In vitro |
| Krishnan and Mitragotri (2020) [18] | N/A | N/A | 5-FU, imiquimod, and ingenol mebutate | N/A |
| Lee et al. (2013) [19] | N/A | Topical | Papaverine, streptokinase, and urokinase | In vivo |
| Liu et al. (2022) [20] | 0, 1,5, and 10 mM | Applied to cells | Cisplatin | In vitro |
| Mücke et al. (2015) [21] | 20 mg/g lidocaine hydrochloride | Oral (salve) | Diclofenac and omeprazole | In vivo |
| Sercarz et al. (1995) [22] | 1% | Injection (nerve) | N/A | In vivo |
| Strickland et al. (1993) [23] | 100 mg | Intravenous | Fentanyl citrate, nitrous oxide, isoflurane fentanyl, and atropine | In vivo |
| Tartaglione et al. (2008) [24] | 10% | Topical (spray) | Nanocolloidal | In vivo |
| Thakur et al. (2012) [25] | Three subsequent sprays of 7%, followed by an intratracheal injection of 5% | Topical (spray) followed by intratracheal injection | N/A | In vivo |
| Turnbull et al. (2011) [26] | Patch 5%, jelly 2% | Topical (jelly) | Bisphosphonate, ropivacaine, fentanyl, ketamine, morphine, methadone, oxycodone, haloperidol, and mepivacaine | In vivo |
| Wang et al. (2016) [27] | 5% | Applied to the lip (assuming topical) | Cefuroxime axetil, prednisone acetate, and ketotifen fumarate | In vivo |
| Wiese et al. (1993) [29] | 0.5 ml 0.25 %, 7 μg/mL | Applied to cells: Injection (single dose) or medium with lidocaine | N/A | In vitro |
| Yasuta et al. (2014) [31] | 1% | Intradermal | Iomeprol (as a contrast agent) | In vivo |
According to the data presented in Table 1, aside from lidocaine, ketamine was the most frequently administered drug during Mohs surgery for SCC [8-28]. Low-dose perioperative ketamine has been found to alleviate postoperative pain and reduce the use of opioids when lidocaine failed to provide relief [12-15]. Increasingly, ketamine and lidocaine have been used in medicine together. Currently, ketamine is prescribed for anesthesia, pain, and intensive care. Adults and children are sedated with ketamine as the literature strongly supports the safety and efficacy of ketamine for dissociative sedation in adults and children in an effort to comfort and reduce anxiety and pain during painful or distressing procedures [9-12]. Ketamine administered to patients in the intensive care unit provides a combination of sedation and analgesia, favorable hemodynamic effects, and the ability to treat persistent bronchospasm. Small subanesthetic doses of ketamine have been administered topically or intravenously as an analgesic for the treatment of chronic pain. While ketamine has shown beneficial effects when it comes to analgesia and pain, there are certain side effects associated with its use [13-17]. Patients who received ketamine during surgery were more likely to experience hallucinations and nightmares in the recovery room and for several days following surgery. Due to its unique pharmacological benefits and newly discovered clinical properties, ketamine has multiple clinical applications. In addition to anesthesia, ketamine is now used for pain, palliative care, intensive care, and procedural sedation. It is increasingly administered in low doses and in conjunction with other drugs.
Lidocaine usage
Topical administration of lidocaine at a mean initial dosage of 1 mg/kg/h (range: 0.5-2.7 mg/kg/h) was found to be the most common route of application in the in vivo studies investigating SCC [8-29]. In these studies, lidocaine was administered topically to the tongue and pharynx, oral cavity, lip arm, or scalp [13,23-26]. Based on the areas being treated, it is reasonable to find that lidocaine was applied topically for the following reasons. One is that topical administration locally numbs the area of application. Presumably, this remains a safer route with fewer side effects (lightheadedness, dizziness, blurred vision, low blood pressure, etc.) than other types of anesthesia (e.g., intravenous or intradermal) due to its localized mechanism of action. Topical administration of lidocaine also results in a shorter duration of nerve blocking, which provides a shorter recovery time for patients [14-22,30]. As a result, topical lidocaine would be favored for shorter procedures to reduce the likelihood of severe postoperative symptoms and complications. It is important to highlight, however, that the administration route used is dependent upon the area and size of the application site. Topical lidocaine will not be preferred in all situations. Lastly, regarding the in vitro studies found, lidocaine was administered topically in all studies [8-28]. Cells were incubated with lidocaine in various concentrations to stimulate and test the effects of this anesthetic [9,16]. Logically, topical lidocaine remains the only possible route of administration for in vitro studies. Several papers analyzed within this study applied lidocaine directly to squamous cells rather than conducting an in vivo study. According to the data presented in Table 2, the variance of lidocaine concentration among in vivo studies was found to be higher than that in the in vitro studies.
Table 2. Variance and mean concentration of lidocaine.
| Group | Variance | Mean Concentration |
| In vivo | 8.704 | 3.10% |
| In vitro | 7.44 | 0.01% |
Through analysis, it can be observed that all these papers were aiming to observe the cytotoxic effects caused by the combined effects of cisplatin and lidocaine on squamous cells [9,16]. Approximately 90% of SCC cases are seen within the oral cavity [9,31]. Therefore, this paper aimed to test the effects of lidocaine through an in vitro study that applied lidocaine to cell lines from human tongue SCCs. Local anesthetics are commonly applied to tumors during head and neck surgeries; however, their effects on the oral cavity are unknown. One study compared seven local anesthetics, one of them being lidocaine, and looked at their effects on oral tumor cells relative to normal cells [16]. This study was conducted with the aim to visualize the effects of various anesthetics on the proliferation of SCC cells. One of the studies in the review also analyzed the cytotoxic effects of lidocaine on the growth of cells within head and neck SCCs. This study specifically looked at the effects of lidocaine on spindle cells and round cells found within SCC of the head and neck [27]. Future studies may be conducted to further understand the mechanisms by which lidocaine exerts its cytotoxic effects. According to the data presented in Tables 2, 3, the mean concentration of lidocaine used in the in vivo studies was significantly higher than the dosage used in in vitro studies. Concentration was compared by the same standard by converting molar to percent. This may be due to the fact that eliciting a chemical response from a human body requires a higher concentration than plated cells due to their significantly larger mass.
Table 3. Statistical significance between group means.
| Group Comparison | T-value | P-value |
| In vivo versus in vitro anesthesia concentration | 3.64 | 0.015 |
Future applications and current limitations
The results from the culmination of 21 studies suggest lidocaine use for patients with SCC undergoing Mohs surgery. There are no trends in the dosage of lidocaine as there is such a wide range. In many studies, lidocaine was combined with a secondary medication. Medications used in multiple studies include fentanyl, ketamine, nitrous oxide, cisplatin, capsaicin, and atropine. The results from this review can help physicians understand that there is a myriad of concomitantly administered drugs with lidocaine. The lack of trends gives an insight into the variability in the approach to lidocaine usage, as instead of it appearing as a standardized approach to care, it is illustrated as a patient-to-patient decision made by physicians. This review was able to effectively examine the effects of lidocaine application and Mohs surgeries. Through analysis of 21 studies, we were able to analyze the effects of different dosages and applications of lidocaine on SCC patients and cell lines. One of the limitations of this review was that several studies indicated the use of additional pharmaceutical medications, while others did not. This might introduce confounding variables between different papers. Similarly, the use of different additional medications might have resulted in different outcomes between the studies. Another limitation is that the majority of studies reported using topical or injected lidocaine, while only a few mentioned using the lidocaine orally or via application to cells. This discrepancy might account for any variable results across the many procedures. Therefore, future studies could consider this aspect and examine the different methods of lidocaine application equivalently. One way to improve this might be to conduct a review of studies that used topical, oral, cell application, intravenous, and intradermal lidocaine application equally to remove any confounding variables from the results. With these limitations in mind, lidocaine and Mohs surgery could be evaluated further. Despite the extensive study that has been undertaken on the administration and use of lidocaine on patients with SCC, new research is required to find more optimal standards that may be employed for these operations in order to avoid any side effects.
Currently, we are aware that the normal concentration of lidocaine during Mohs surgery is 1%; however, we do not have a process or procedure to follow if this concentration needs to be modified to fit the needs of a particular patient. Additional studies utilizing combination agents may be necessary to address these issues. In order to investigate the effects of various substances on the analgesic effects of lidocaine, it may be necessary to use chemicals such as ethylenediaminetetraacetic acid (EDTA) during this research. It is crucial that individuals with SCC receive an early diagnosis to minimize adverse health problems and diminish returns on treatment. This will help alleviate the discomfort that patients may experience as a result of Mohs surgery. AI software that examines dermatomes may be developed in the future to expand the number of SCC patients who are diagnosed. It is vital to conduct further studies on lidocaine's side effects to ensure the safety of SCC patients undergoing Mohs surgery.
Conclusions
This review was conducted to evaluate the usage of lidocaine as an anesthetic in the treatment and understanding of SCC. Based on the data collected from the literature, a statistical analysis was conducted, and the results suggested that the concentration of lidocaine used to create an analgesic effect on patients undergoing Mohs surgery was higher than the concentration of lidocaine that was necessary to slow the growth of SCC cells. Further investigations should be conducted to validate this finding. Additionally, more research is necessary to understand the potential side effects of using lidocaine in the treatment of SCC.
The authors have declared that no competing interests exist.
References
- 1.Sunlight exposure, pigmentation factors, and risk of nonmelanocytic skin cancer. II. Squamous cell carcinoma. Gallagher RP, Hill GB, Bajdik CD, et al. https://jamanetwork.com/journals/jamadermatology/article-abstract/556368. Arch Dermatol. 1995;131:164–169. [PubMed] [Google Scholar]
- 2.State variation in squamous cell carcinoma of the anus incidence and mortality, and association with HIV/AIDS and smoking in the United States. Damgacioglu H, Lin YY, Ortiz AP, et al. J Clin Oncol. 2022;28:0. doi: 10.1200/JCO.22.01390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Management of cutaneous tumors with Mohs micrographic surgery. Dim-Jamora KC, Perone JB. Semin Plast Surg. 2008;22:247–256. doi: 10.1055/s-0028-1095884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aging and the treatment of basal cell carcinoma. Sreekantaswamy S, Endo J, Chen A, Butler D, Morrison L, Linos E. Clin Dermatol. 2019;37:373–378. doi: 10.1016/j.clindermatol.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Squamous cell carcinoma - similarities and differences among anatomical sites. Yan W, Wistuba II, Emmert-Buck MR, Erickson HS. https://pubmed.ncbi.nlm.nih.gov/21938273/ Am J Cancer Res. 2011;1:275–300. [PMC free article] [PubMed] [Google Scholar]
- 6.Squamous cell carcinoma of the skin. Rudolph R, Zelac DE. Plast Reconstr Surg. 2004;114:82–94. doi: 10.1097/01.prs.0000138243.45735.8a. [DOI] [PubMed] [Google Scholar]
- 7.Palliative radiotherapy for skin malignancies. Vuong W, Lin J, Wei RL. Ann Palliat Med. 2017;6:165–172. doi: 10.21037/apm.2016.11.10. [DOI] [PubMed] [Google Scholar]
- 8.Optimal management of the elderly patient with head and neck cancer: issues regarding surgery, irradiation and chemotherapy. Mountzios G. World J Clin Oncol. 2015;6:7–15. doi: 10.5306/wjco.v6.i1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Effective treatment of severe cancer pain of the head using low-dose ketamine in an opioid-tolerant patient. Clark JL, Kalan GE. J Pain Symptom Manage. 1995;10:310–314. doi: 10.1016/0885-3924(95)00010-V. [DOI] [PubMed] [Google Scholar]
- 10.Effects of lidocaine and the inclusion complex with 2-hydroxypropyl-β-cyclodextrin on cell viability and proliferation of oral squamous cell carcinoma. Ferreira LE, Antunes GB, Muniz BV, et al. J Pharm Pharmacol. 2018;70:874–882. doi: 10.1111/jphp.12917. [DOI] [PubMed] [Google Scholar]
- 11.Local anesthesia using buffered 0.5% lidocaine with 1:200,000 epinephrine for tumors of the digits treated with Mohs micrographic surgery. Firoz B, Davis N, Goldberg LH. J Am Acad Dermatol. 2009;61:639–643. doi: 10.1016/j.jaad.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Case report: utility of ketamine, lidocaine, and mexiletine as nonopioid adjuvants in complex cancer-associated pain. Hakim RC, Edmonds KP, Atayee RS. J Pain Palliat Care Pharmacother. 2018;32:15–19. doi: 10.1080/15360288.2018.1463345. [DOI] [PubMed] [Google Scholar]
- 13.Treatment of cutaneous and subcutaneous tumors with electrochemotherapy using intralesional bleomycin. Heller R, Jaroszeski MJ, Reintgen DS, et al. Cancer. 1998;83:1–148. doi: 10.1002/(sici)1097-0142(19980701)83:1<148::aid-cncr20>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 14.Total airway obstruction during local anesthesia in a non-sedated patient with a compromised airway. Ho AM, Chung DC, To EW, Karmakar MK. Can J Anaesth. 2004;51:838–841. doi: 10.1007/BF03018461. [DOI] [PubMed] [Google Scholar]
- 15.Large doses of topical lidocaine during microvascular surgery are not associated with toxic blood concentrations. Johnstone RE, Wax MK, Bishop DJ, Chafin JB. Anesthesiology. 1995;82:593–596. doi: 10.1097/00000542-199502000-00034. [DOI] [PubMed] [Google Scholar]
- 16.Intravenous lidocaine administered as twice daily bolus and continuous infusion for intractable cancer pain and wound care pain. Kintzel PE, Knol JD, Roe G. J Palliat Med. 2019;22:343–347. doi: 10.1089/jpm.2018.0243. [DOI] [PubMed] [Google Scholar]
- 17.Cytotoxicity and type of cell death induced by local anesthetics in human oral normal and tumor cells. Kobayashi K, Ohno S, Uchida S, et al. https://ar.iiarjournals.org/content/32/7/2925.long. Anticancer Res. 2012;32:2925–2933. [PubMed] [Google Scholar]
- 18.Nanoparticles for topical drug delivery: potential for skin cancer treatment. Krishnan V, Mitragotri S. Adv Drug Deliv Rev. 2020;1:87–108. doi: 10.1016/j.addr.2020.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Anterograde intra-arterial urokinase injection for salvaging fibular free flap. Lee DS, Jung SI, Kim DW, et al. Arch Plast Surg. 2013;40:251–255. doi: 10.5999/aps.2013.40.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lidocaine represses proliferation and cisplatin resistance in cutaneous squamous cell carcinoma via miR-30c/SIRT1 regulation. Liu T, Jiang F, Yu LY, Wu YY. Bioengineered. 2022;13:6359–6370. doi: 10.1080/21655979.2022.2031419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clinical trial analyzing the impact of continuous defocused CO2 laser vaporisation on the malignant transformation of erosive oral lichen planus. Mücke T, Gentz I, Kanatas A, et al. J Craniomaxillofac Surg. 2015;43:1567–1570. doi: 10.1016/j.jcms.2015.06.044. [DOI] [PubMed] [Google Scholar]
- 22.Recurrent laryngeal nerve afferents and their role in laryngospasm. Sercarz JA, Nasri S, Gerratt BR, Fyfe ST, Berke GS. Am J Otolaryngol. 1995;16:49–52. doi: 10.1016/0196-0709(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 23.Prolonged QT syndrome: perioperative management. Strickland RA, Stanton MS, Olsen KD. Mayo Clin Proc. 1993;68:1016–1020. doi: 10.1016/s0025-6196(12)62277-0. [DOI] [PubMed] [Google Scholar]
- 24.The impact of superficial injections of radiocolloids and dynamic lymphoscintigraphy on sentinel node identification in oral cavity cancer: a same-day protocol. Tartaglione G, Vigili MG, Rahimi S, et al. Nucl Med Commun. 2008;29:318–322. doi: 10.1097/MNM.0b013e3282f4d399. [DOI] [PubMed] [Google Scholar]
- 25.Descriptive data on cancerous lung lesions detected by auto-fluorescence bronchoscope: a five-year study. Thakur A, Gao L, Ren H, Yang T, Chen T, Chen M. Ann Thorac Med. 2012;7:21–25. doi: 10.4103/1817-1737.91559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cutaneous nerve transection for the management of intractable upper extremity pain caused by invasive squamous cell carcinoma. Turnbull JH, Gebauer SL, Miller BL, Barbaro NM, Blanc PD, Schumacher MA. J Pain Symptom Manage. 2011;42:126–133. doi: 10.1016/j.jpainsymman.2010.10.258. [DOI] [PubMed] [Google Scholar]
- 27.Surgery combined with topical photodynamic therapy for the treatment of squamous cell carcinoma of the lip. Wang Y, Yang Y, Yang Y, Lu Y. Photodiagnosis Photodyn Ther. 2016;14:170–172. doi: 10.1016/j.pdpdt.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Intravenous lidocaine as an adjuvant for pain associated with sickle cell disease. Nguyen NL, Kome AM, Lowe DK, Coyne P, Hawks KG. J Pain Palliat Care Pharmacother. 2015;29:359–364. doi: 10.3109/15360288.2015.1082009. [DOI] [PubMed] [Google Scholar]
- 29.The effect of lidocaine on growth of cells of head and neck squamous cell carcinoma. Wiese KG, Korabiowska M, Tyrak J, Bartkowski S, Stypukowska J. J Craniomaxillofac Surg. 1993;21:157–162. doi: 10.1016/s1010-5182(05)80105-2. [DOI] [PubMed] [Google Scholar]
- 30.Torp KD, Metheny E, Simon LV. Treasure Island, FL: StatPearls Publishing; 2022. Lidocaine Toxicity. [PubMed] [Google Scholar]
- 31.Usefulness of CT-lymphography in sentinel lymph node navigation. Yasuta M, Sato S, Ishida T, Kiyohara T. Int J Clin Oncol. 2014;19:557–562. doi: 10.1007/s10147-013-0582-1. [DOI] [PubMed] [Google Scholar]