Abstract
The unprecedented economic and health impacts of the COVID-19 pandemic have shown the global necessity of mitigating the underlying drivers of zoonotic spillover events, which occur at the human–wildlife and domesticated animal interface. Spillover events are associated to varying degrees with high habitat fragmentation, biodiversity loss through land use change, high livestock densities, agricultural inputs, and wildlife hunting—all facets of food systems. As such, the structure and characteristics of food systems can be considered key determinants of modern pandemic risks. This means that emerging infectious diseases should be more explicitly addressed in the discourse of food systems to mitigate the likelihood and impacts of spillover events. Here, we adopt a scenario framework to highlight the many connections among food systems, zoonotic diseases, and sustainability. We identify two overarching dimensions: the extent of land use for food production and the agricultural practices employed that shape four archetypal food systems, each with a distinct risk profile with respect to zoonotic spillovers and differing dimensions of sustainability. Prophylactic measures to curb the emergence of zoonotic diseases are therefore closely linked to diets and food policies. Future research directions should explore more closely how they impact the risk of spillover events.
Introduction
With a death toll of more than 6·5 million people by December, 2022, the COVID-19 pandemic has unleashed unprecedented health and economic impacts on societies across the world. As the SARS-CoV-2 virus has spread globally in the past few years, the pandemic has highlighted the interconnectivity of public health with economic, ecological, and societal vulnerabilities that underscore the importance of the One Health1 and planetary health approaches. Worldwide, governments have deployed numerous strategies and measures to reduce infection rates, from social and mobility restrictions to efforts to reach herd-immunity vaccination levels. While striving for resumption of normal economic and societal activities, recent policies have expanded to better prepare for future pandemics.
Despite these expanded policies, national and international efforts still do not adequately address reducing emergent infectious disease risks nor incorporate systematic cost-benefit evaluation of putative mitigation measures.2, 3 One specific glaring omission of current policy deliberations is the global food system. This omission is concerning because of the outsized spatial dominance of agriculture over worldwide tropical and temperate ecosystems, and its consequent potential roles in zoonotic outbreaks, which reflect the complex interactions between food systems and infectious disease.4 Because food systems vary widely in production methods, product varieties, governing policies, and stakeholders (and their contribution to the various facets of sustainability), developing a general understanding of the zoonotic disease risks generated from various food systems is imperative, and will become increasingly urgent amidst growing calls for food system transformation to meet sustainability goals.5 We used a scenario framework based on an expert-driven approach to draw a qualitative blueprint of the variable impacts different agricultural production methods, land allocation patterns, and diets can have on zoonotic spillovers. We hope that this framework will foster more targeted empirical research and discourse towards broadening our understanding on how food policies and food-related interventions impact zoonotic spillovers.
Food systems and zoonotic spillovers
Zoonotic diseases are caused by pathogens of wildlife or livestock origins that spill over into human populations. They represent a major, growing subset of infectious diseases,6, 7 accounting for more than half of human-susceptible pathogens.4, 8 The incidence of zoonotic outbreaks and the diversity of their source species, whether wild or domestic, have increased in recent decades,6, 9 as has the volume of research exploring underlying mechanisms and drivers.10, 11, 12, 13 Increasing in frequency and diversity, zoonotic spillovers need to overcome a set of crucial epidemiological barriers.14 These barriers include those related to pathogen pressure (including pathogen dynamics at the host reservoir, release, and survival), exposure (the interface between the environment and the human body), and the infection outcome of exposure and transmission.
Multiple complex drivers and mechanisms, including social, ecological, environmental, and institutional factors, connect food systems to zoonotic spillover.4 Although knowledge gaps still exist, important pathways of zoonotic spillover have been identified for various emergent diseases, revealing recurring patterns. Because animal–human contact (either through direct interaction with wildlife or indirectly through vectors or intermediate, domesticated species) is a prerequisite for zoonotic spillover, any increase in the frequency of contact between humans and livestock or wildlife is a potential outbreak driver. This could include changes in human, wildlife, and vector densities as well as expanded interfaces of interaction due to land use change.4
Of particular importance is habitat fragmentation,15 which extends the contact zone between natural and agricultural land. Although the adjacency of natural and agriculturual land might improve selected ecosystem services (notably pollination and pest control), it can also increase human–wildlife contact and thus spillover risks, especially in many tropical regions.11, 12 Therefore, both the absolute extent of land transformed and its subdivision into individual plots influence the dynamics of disease emergence.16 Moreover, dividing a given land area into smaller farming plots could heighten spillover risk due to larger farmworker density required for operations, as with any factor that increases farmer density in general (eg, non-mechanised operations of commodities, such as coffee, bananas, and rubber). In addition, the increased contact between managed fragmented landscapes and natural habitats creates unique transition zones, known as anthropogenic ecotones, of biological activity and material exchange that can foster the emergence of zoonoses via enhanced mixing of species assemblages, higher host and vector abundances, and larger dispersal potential.17
Agricultural intensification has been linked with zoonotic spillover events via decreases in biodiversity,18, 19 a pattern termed the dilution effect whereby species vary in their competency to drive spillovers and higher diversity often leads to lower probability of infection. Although the dilution effect has been debated,20, 21 this pattern has been observed to persist across a diversity of ecosystems and species groups, highlighting how biodiversity losses increase the likelihood of zoonotic spillovers by increasing pathogen host proportions in species richness and total abundance.10 As a general rule of risk management based on the precautionary principle and our understanding of disease ecology, intact ecosystems tend to be healthier ones, posing lower threats to human and livestock health.22, 23
Altered biogeochemical cycles can also influence zoonotic spillovers. Particularly concerning are large-scale changes to the nitrogen and phosphorus cycles caused by intensive fertiliser application and run-offs that typically characterise industrial food and feed production. These disturbances can enhance zoonotic spillover risks due to their impacts on host or vector densities and distribution (eg, increasing mosquito efficiencies in spreading West Nile fever24), infection resistance, and pathogen virulence and abundance.4, 24, 25, 26 Water infrastructure and irrigation similarly alter the environment and shift species diversity, consequently impacting pathogen ecology and in some cases increasing the incidence of disease in livestock and people in surrounding landscapes. An example is the increased incidence of human fascioliasis due to the adaptation of liver fluke and its snail vector to large-scale irrigations in the Peruvian highlands.19, 25 Zoonotic risks have been linked to the excessive use of antibiotics at subtherapeutic doses in the livestock sector, including aquaculture, and it is predicted to increase due to growing demand for meat.27 The introduction of antimicrobials to animals facilitates the emergence of drug-resistant pathogens within the animal's microbiome that can be transferred to humans directly or via vectors unimpeded by immunity. In other cases, unmetabolised antimicrobials can build up in the environment and negatively impact the disease ecology, as evident in the large proportion of drug-resistant genes in zoonotic pathogens.28 An increase in aquatic-specific zoonoses has been facilitated by aquaculture practices that alter the complex lifecycles of these pathogens, which can include several intermediate hosts, such as snails, crustaceans, and finfish.29 While documented in wild-caught fish as well, these pathogens will probably increase as the sheer number of farmed aquatic species is predicted to increase to meet a growing demand. Similarly, pesticides—whose use for crop protection and disease vector suppression4 is increasing because of agricultural expansion and intensification—also increase zoonotic risks via the development of vector resistance to pesticides and by intensifying pathogen dynamics at the wildlife–human interface. Impacts include direct effects on the abundances of zoonotic hosts, parasites, or their natural enemies, and the susceptibility of hosts to diseases (eg, via changes to the immune system).4
Because of these observations and the growing reliance of the livestock sector on feed crops, terrestrial and aquatic animal farming has become an important pathway for zoonotic spillover across the world.30, 31, 32 Due to the prevalence of high stocking densities and genetic uniformity, farm animals can amplify zoonotic outbreaks33 with higher virulence4 by serving as intermediate hosts between wild reservoirs and humans (eg, as with highly pathogenic avian influenza34, 35 and the Nipah virus outbreaks).36 In Indigenous and local populations across Asia, Africa, and Latin America, wildlife meat is an important nutrient source,37, 38 and a major component of food sovereignty.39, 40 However, hunting and trade networks of wildlife meat put humans and animals at risk for increases in the likelihood of zoonotic spillover.41, 42, 43, 44
Constructing archetypal food systems
Based on the links between food systems and zoonotic spillovers, we categorised ten direct food-system-related drivers of zoonoses. These drivers include (1) biodiversity loss; (2) land fragmentation; (3) pesticide use; (4) water use; (5) fertiliser application; (6) antibiotics use; (7) wildlife hunting; (8) aquaculture; (9) livestock densities; and (10) farmworker densities. Because these immediate drivers reflect wider food-system characteristics (eg, institutions, economic development, policies, and food sovereignty), we briefly discuss the indirect impacts of these larger societal factors on our results in the final section of this Personal View.
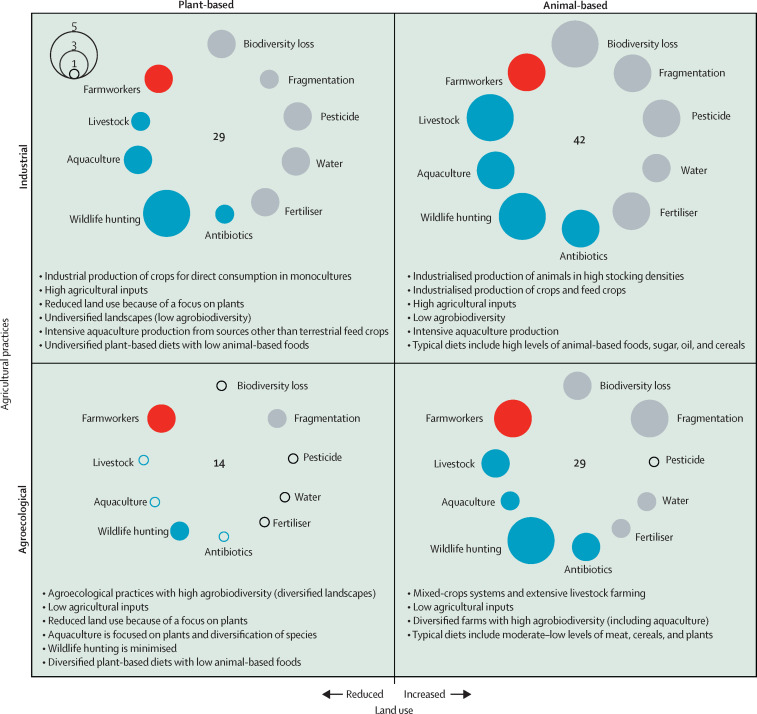
Using a scenario framework,45, 46 we selected land use extent (continuous) and agricultural practices (categorical) as the two axes that probably span the most variance in the ten food-system drivers, and yet contribute differently to sustainability. These dimensions span four archetypal food systems, each with unique structural characteristics, which facilitate exploration of four distinct global food-system narratives with varying risks and plausible interventions that can reduce zoonotic spillovers (figure ). Although we refer to these systems as scenarios, the analysis we present here is not meant to predict future developments, but rather to focus on existing typologies. Based on our expert opinion, we then ranked the potential risk for zoonotic spillover for each of the ten individual food-system drivers (on a scale of 1 [very low] to 5 [very high]) in each of the four scenarios (figure; appendix pp 1–2). Typically, overall risk is assessed as the product of an event's occurrence probability and its impact severity. Due to knowledge limitations and the complexity involved, here we assume a uniform degree of severity for all factors (ie, any spillover has the same societal impact). Consequently, in effect, we quantify risk only as the potential for occurrence. In reality, some factors are riskier than others (eg, we considered irrigation as less risky, with maximal risk across all scenarios set to 3 of 5). The overall risk of a scenario is the sum of all its individual risks, with smaller sums corresponding to low-risk scenarios and larger sums corresponding to high-risk scenarios. The four scenarios typify existing food systems and each is characterised by distinct environmental and socioeconomic impacts and degrees of zoonotic spillover risk.
Figure.
Relative zoonotic risks as a function of land use extent and agricultural practices for the four explored food system scenarios
All key food-system zoonotic spillover drivers are included in each scenario. The relative zoonotic spillover risk from each driver is depicted by the size of the circles. Grey colours indicate risks directly related to agriculture, blue highlights risks related to animal-based foods, and red indicates the risk introduced by farmworkers working within agroecosystems. The key at the top connects between the size of the circle and the relative risk score on a scale of 1 to 5 (see appendix pp 1–2 for numerical values). The overall risk score of each scenario, the sum of all individual risks within that scenario, is indicated in bold at the centre and is highest in the industrial animal-based scenario and lowest in the agroecological plant-based scenario.
The industrial animal-based scenario depicts such western industrial food systems as those of the USA or Australia, with high livestock production volume and monocultures, yielding diets high in meat, sugar, and oils. These food systems entail high spillover risks across many of the above ten explored food-system drivers, including the intense use of inputs such as water, antibiotics, pesticides, and fertilisers over large land areas.47, 48 Because the global food system is the largest single land user,49 it is also the main driver of land transformation and fragmentation, and thus of biodiversity declines.50 Consumption of large amounts of animal-based foods drives substantial land transformation and encroachment despite increased reliance on intensive industrial practices.51
Together with large animal stocking densities,52 rising meat consumption carries high zoonotic spillover potential. Because of growing demand, especially in developing countries, meat production is expected to continue rising into the future,53 further increasing the risk of zoonoses. The same holds for aquaculture, which has been the fastest growing food producing sector globally,54 and is predicted to grow as wild aquatic food sources steadily decline.55, 56 This too will increase spillover risks via agricultural intensification because of the rising reliance on terrestrial crop and animal feed inputs into aquaculture, unless other sources of aquafeed become widely available.57 Based on the underlying food-system drivers we have explored, this scenario carries the largest zoonotic spillover risk.
In the agroecological animal-based scenario, extensive production of livestock (low-density stocks and reduced transport) through agroecological practices in mixed cropping systems (eg, in southeast Asia), intensive silvopastoral systems (eg, in South America and central America58), or regenerative agriculture (eg, in the USA) relying on pastures and byproducts59, 60 would pose risks for zoonotic spillover events due to broad-scale land use and animal husbandry.61 However, this risk is lower compared with the industrial animal-based scenario due to lower livestock numbers and densities.32 Agroecological methods, including low inputs and high agrobiodiversity,62 reduce spillover risks associated with the pesticides, water, antibiotics, and biodiversity pathways. In this scenario, aquaculture is produced in polyculture systems and integrated multitrophic aquaculture,63 which focus on low-impact species, such as mollusks and seaweed, using sustainable management practices and by providing a broad range of ecosystem services.57 At the same time, and irrespective of the different farming skills needed, agroecological food production is likely to use smaller land plots and more labour,64 increasing the risk of spillover via greater habitat fragmentation (fragmentation) and increased human contact (ie, farmworkers). Terrestrial lands still dominate food production, with more limited production of aquatic foods. In this scenario, wildlife hunting delivers food security gains, but at a cost of elevated zoonotic spillover potential. Compared with the other scenarios, the agroecological animal-based scenario carries medium risk potential for zoonotic spillover.
Because animal-based foods are the largest determinant of land occupation (via crops for feed and pasture), reducing animal-based products and promoting plant-based diets (moving to the left of figure) will reduce global land use markedly for a fixed food mass,51 without necessarily compromising nutritional outcomes.65 Consequently, the industrial and agroecological plant-based scenarios (left column; figure) permit restoration of more land back to natural ecosystems, resulting in overall higher biodiversity, decreased agricultural areas, and smaller ecotones and fragmentation globally when compared with the industrial and agroecological animal-based scenarios (right column; figure). Comparatively, these food systems have lower potential for zoonotic spillovers.
The industrial plant-based scenario focuses on production of plant-based diets by intensive industrial practices. Examples of such systems include large palm oil plantations in Malaysia and Indonesia or large-scale wheat production in the USA. In this archetypal system, however, small quantities of animal-based foods are still produced by use of byproducts and waste, and in aquaculture systems with use of aquafeeds that require no terrestrial land resources, minimising land use. These aquafeeds include fishmeal, single-celled-organism proteins and oils, and seafood byproducts.57 Aquaculture species, such as unfed extractive species (eg, mollusks and algae), are also produced industrially, densely stocked. The risks of intensive agricultural practices still exist in this scenario, but are smaller compared with the industrial animal-based scenario. Furthermore, the overall landscape is poor in biodiversity, consistent with high-intensity yield maximisation. However, because intensive agriculture tends to occupy larger contiguous farms, fragmentation and thus zoonotic risks could be lower compared with smallholder agroecological farms.66 Enhanced adaptation of advanced technologies and automation solutions reduces on-farm human activity, further reducing the spillover risk to farmworkers. Conversely, because commodity production in the zoonotic-prone tropical and subtropical regions can be labour-intensive (such as in the case of bananas, cacao, or coffee), the score of this risk is medium (score 3). In the industrial plant-based scenario, wildlife hunting practices persist and their risk is high (score 5). Compared with the other scenarios, overall risk is medium.
Agroecological plant-based scenarios include contemporary agroecological farms in Latin America and Africa. This type of scenario reduces zoonotic spillover risks by minimising land use (by focusing on plant-based diets) and employing agroecological practices. Optimising agrobiodiversity10, 20 in the remaining food-producing landscapes to reduce zoonotic risk will result in landscapes that supply not only nutritionally rich food but also other important ecosystem services,67 without necessarily compromising crop yields.68 These agroecological landscapes are contrasted with specialised industrial commodity crop monocultures (ie, the industrial plant-based scenario). Aquaculture focuses on algae and on species with minimum risk to zoonotic spillovers and the ability to provide beneficial ecosystem services. Wildlife hunting is reduced via community-based solutions in zoonotic-prone regions (eg, tropical forest landscapes in southeast Asia, Africa, and Latin America) in exchange for enhanced production of alternative nutritionally rich food sources. Although reducing wildlife hunting can be equally attained with dedicated efforts in the other scenarios too, we note that reducing wildlife hunting is more likely to occur within localised agroecological systems that foster increased community involvement.69, 70, 71 Overall, these food systems have the lowest risk for zoonotic spillover events.
Prioritising policy and research directions
As calls intensify for global food-system transformation for improved sustainability, equitability, and nutrition, quantifying the full scope of the expected outcomes of such systematic shifts is crucial. The large societal impacts of the COVID-19 pandemic reveal the importance of understanding the contribution of the global food system to zoonotic risk. We argue here that different food-system typologies (eg, agricultural methods, land use, and human dietary choices) contribute differently to zoonotic spillover risk. As agricultural landscapes and human diets reflect other important food-system components (including economic development, food sovereignty, corporate dominance, and governance), the structure and function of the entire food system directly and indirectly impact zoonotic outbreaks.
Our approach conceptually highlights where these risk factors are and suggests policy directions that might alleviate them. Our hope is that follow-up empirical research will quantitatively support these conceptual insights with more nuanced detail. For example, the promotion of plant-based diets will probably be essential for zoonotic spillover risk reduction, an additional societal benefit they offer beyond their other contributions to environmental sustainability.65 Plant-based diets can potentially free large areas of currently human-appropriated lands that can be repurposed for rewilding, reducing livestock densities, and reducing agricultural lands. In many low-income and middle-income countries where a planetary health diet is unaffordable,72 and people's nutrition depends on animal-based foods and wildlife hunting for adequate intakes, focusing on other measures can be important. For example, promoting agroecological practices and increasing region-specific agrobiodiversity alongside natural habitats is beneficial for ecosystem services,73, 74, 75 including reducing zoonotic outbreaks relative to animal-based industrial type food systems.
Prophylactic measures to restrict the emergence of zoonotic diseases are closely linked to diets and food policy,32 and are location-specific and context-specific due to differing ecological and climatic conditions, as well as cultural and socioeconomic realities. Future research directions should explore more closely how they impact the risk of zoonotic outbreaks. One example includes policies that promote plant-based diets (eg, by subsidising plant-based food consumption or taxing red meat). Others might involve policies that promote agronatural landscapes and ecosystem services,76, 77 which will probably reduce zoonotic outbreaks, but additional evidence is imperative. Detailed multidisciplinary research has shown that improving hygiene measurements, and optimising the location of wet markets, can mitigate the risk of spillover events.78, 79, 80 Therefore, targeted policies should unpack the complexity of wildlife harvesting and the potential sustainability of such a practice to effectively align goals of social justice, food security, food sovereignty, and biodiversity conservation with reduced risks to zoonotic spillovers.81
Nonetheless, many knowledge gaps that connect specific agro-practices and agrobiodiversity measures directly to zoonotic risks remain and are important foci for future research. We believe that sustained government support for research is key and that future research should strive to identify region-specific low-zoonosis-risk agricultural practices that yield economically viable, nutritious, and culturally acceptable diets while simultaneously reducing zoonotic risks and explicitly promoting agrobiodiversity.
Our analysis highlights the relative contribution to zoonotic outbreaks of several archetypical food systems that might exist in isolation or in various combinations, which makes it difficult to quantify their impacts. Although our ranking of various spillover drivers builds on reviewing relevant literature and on expert opinion, it is not based on comparative empirical studies (which are not available) and has the important limitation of assuming uniform severity of spillover impacts. Further research into food-system-related spillover mechanisms, their relationships with each other and with agriculture, and the relevant spatial scale involved would probably reveal variable impacts (eg, increased virulence in particular pathways). Additionally, although our analysis focuses on the production side of food systems, other factors probably also influence zoonotic risks.82 For example, human population densities can drive encroachment of natural habitats and increase the risk of human–wildlife contact, and therefore, spillover events, independent of food-system typology. Poverty, economic interconnections, and institutional structures always influence, to varying degrees, the dynamics of land transformation, biodiversity loss, and agricultural practices that jointly impact the risks for zoonosis emergence.83 Managing global resources to strike a balance between fulfilling immediate needs and the integrity of the biosphere to provide ecosystem services and avoid associated trade-offs (such as infectious disease) in the long term is crucial.49 Future research should identify how governance can establish food policies that reduce zoonotic spillover risks, particularly by avoiding unintended consequences or rebound effects.84 Our key message is that zoonotic disease risks are inextricably linked to dietary choices and the structure and functioning of food systems, and that proposed transformation towards sustainability should carefully, sensitively, and explicitly consider these risks in their suitable societal and cultural context.
Contributors
AS designed the study. AS, TD, CK, TW, IP, JF, and CDG contributed to the literature review. AS developed the methods and TW, TD, IP, and CDG provided inputs. AS, TD, TW, IP, and CDG scored the risks. AS wrote an initial draft of the paper. All authors helped interpret the results, contributed to the writing, and reviewed the paper. All authors had full access to the data in the study.
Declaration of interests
IP acknowledges the support of grant NSF-DEB-1853261 from the National Science Foundation. All other authors declare no competing interests.
Supplementary Material
References
- 1.European Food Safety Authority. European Centre for Disease Prevention and Control The European Union One Health 2019 Zoonoses Report. EFSA J. 2021;19 doi: 10.2903/j.efsa.2021.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dobson AP, Pimm SL, Hannah L, et al. Ecology and economics for pandemic prevention. Science. 2020;369:379–381. doi: 10.1126/science.abc3189. [DOI] [PubMed] [Google Scholar]
- 3.Morens DM, Fauci AS. Emerging pandemic diseases: how we got to COVID-19. Cell. 2020;183:837. doi: 10.1016/j.cell.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rohr JR, Barrett CB, Civitello DJ, et al. Emerging human infectious diseases and the links to global food production. Nat Sustain. 2019;2:445–456. doi: 10.1038/s41893-019-0293-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Braun J, Afsana K, Fresco LO, Hassan M. Food systems: seven priorities to end hunger and protect the planet. Nature. 2021;597:28–30. doi: 10.1038/d41586-021-02331-x. [DOI] [PubMed] [Google Scholar]
- 6.Jones KE, Patel NG, Levy MA, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor LH, Latham SM, Woolhouse ME. Risk factors for human disease emergence. Philos Trans R Soc Lond B Biol Sci. 2001;356:983–989. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cutler SJ, Fooks AR, van der Poel WHM. Public health threat of new, reemerging, and neglected zoonoses in the industrialized world. Emerg Infect Dis. 2010;16:1–7. doi: 10.3201/eid1601.081467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith KF, Goldberg M, Rosenthal S, et al. Global rise in human infectious disease outbreaks. J R Soc Interface. 2014;11 doi: 10.1098/rsif.2014.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibb R, Redding DW, Chin KQ, et al. Zoonotic host diversity increases in human-dominated ecosystems. Nature. 2020;584:398–402. doi: 10.1038/s41586-020-2562-8. [DOI] [PubMed] [Google Scholar]
- 11.Wilkinson DA, Marshall JC, French NP, Hayman DTS. Habitat fragmentation, biodiversity loss and the risk of novel infectious disease emergence. J R Soc Interface. 2018;15 doi: 10.1098/rsif.2018.0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen T, Murray KA, Zambrana-Torrelio C, et al. Global hotspots and correlates of emerging zoonotic diseases. Nat Commun. 2017;8 doi: 10.1038/s41467-017-00923-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han BA, Kramer AM, Drake JM. Global patterns of zoonotic disease in mammals. Trends Parasitol. 2016;32:565–577. doi: 10.1016/j.pt.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plowright RK, Parrish CR, McCallum H, et al. Pathways to zoonotic spillover. Nat Rev Microbiol. 2017;15:502–510. doi: 10.1038/nrmicro.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curtis PG, Slay CM, Harris NL, Tyukavina A, Hansen MC. Classifying drivers of global forest loss. Science. 2018;361:1108–1111. doi: 10.1126/science.aau3445. [DOI] [PubMed] [Google Scholar]
- 16.Bloomfield LSP, McIntosh TL, Lambin EF. Habitat fragmentation, livelihood behaviors, and contact between people and nonhuman primates in Africa. Landsc Ecol. 2020;35:985–1000. [Google Scholar]
- 17.Despommier D, Ellis BR, Wilcox BA. The role of ecotones in emerging infectious diseases. EcoHealth. 2006;3:281–289. [Google Scholar]
- 18.Civitello DJ, Cohen J, Fatima H, et al. Biodiversity inhibits parasites: broad evidence for the dilution effect. Proc Natl Acad Sci USA. 2015;112:8667–8671. doi: 10.1073/pnas.1506279112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones BA, Grace D, Kock R, et al. Zoonosis emergence linked to agricultural intensification and environmental change. Proc Natl Acad Sci USA. 2013;110:8399–8404. doi: 10.1073/pnas.1208059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rohr JR, Civitello DJ, Halliday FW, et al. Towards common ground in the biodiversity-disease debate. Nat Ecol Evol. 2020;4:24–33. doi: 10.1038/s41559-019-1060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keesing F, Ostfeld RS. Impacts of biodiversity and biodiversity loss on zoonotic diseases. Proc Natl Acad Sci USA. 2021;118 doi: 10.1073/pnas.2023540118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu T, Perrings C, Shang C, et al. Protection of wetlands as a strategy for reducing the spread of avian influenza from migratory waterfowl. Ambio. 2020;49:939–949. doi: 10.1007/s13280-019-01238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glidden CK, Nova N, Kain MP, et al. Human-mediated impacts of biodiversity and the consequences for zoonotic disease spillover. Curr Biol. 2021;31:R1342–R1361. doi: 10.1016/j.cub.2021.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson PTJ, Townsend AR, Cleveland CC, et al. Linking environmental nutrient enrichment and disease emergence in humans and wildlife. Ecol Appl. 2010;20:16–29. doi: 10.1890/08-0633.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mas-Coma S, Bargues MD, Valero MA. Fascioliasis and other plant-borne trematode zoonoses. Int J Parasitol. 2005;35:1255–1278. doi: 10.1016/j.ijpara.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 26.McKenzie VJ, Townsend AR. Parasitic and infectious disease responses to changing global nutrient cycles. EcoHealth. 2007;4:384–396. [Google Scholar]
- 27.Van Boeckel TP, Brower C, Gilbert M, et al. Global trends in antimicrobial use in food animals. Proc Natl Acad Sci USA. 2015;112:5649–5654. doi: 10.1073/pnas.1503141112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dafale NA, Srivastava S, Purohit HJ. Zoonosis: an emerging link to antibiotic resistance under “One Health Approach”. Indian J Microbiol. 2020;60:139–152. doi: 10.1007/s12088-020-00860-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lima dos Santos CAM, Howgate P. Fishborne zoonotic parasites and aquaculture: a review. Aquaculture. 2011;318:253–261. [Google Scholar]
- 30.Rulli MC, D'Odorico P, Galli N, Hayman DTS. Land-use change and the livestock revolution increase the risk of zoonotic coronavirus transmission from rhinolophid bats. Nat Food. 2021;2:409–416. doi: 10.1038/s43016-021-00285-x. [DOI] [PubMed] [Google Scholar]
- 31.Morse SS, Mazet JAK, Woolhouse M, et al. Prediction and prevention of the next pandemic zoonosis. Lancet. 2021;380:1956–1965. doi: 10.1016/S0140-6736(12)61684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayek MN. The infectious disease trap of animal agriculture. Sci Adv. 2022;8 doi: 10.1126/sciadv.add6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graham JP, Leibler JH, Price LB, et al. The animal-human interface and infectious disease in industrial food animal production: rethinking biosecurity and biocontainment. Public Health Rep. 2008;123:282–299. doi: 10.1177/003335490812300309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koopmans M, Wilbrink B, Conyn M, et al. Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in the Netherlands. Lancet. 2004;363:587–593. doi: 10.1016/S0140-6736(04)15589-X. [DOI] [PubMed] [Google Scholar]
- 35.Wu T, Perrings C. The live poultry trade and the spread of highly pathogenic avian influenza: regional differences between Europe, West Africa, and Southeast Asia. PLoS One. 2018;13 doi: 10.1371/journal.pone.0208197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Epstein JH, Field HE, Luby S, Pulliam JRC, Daszak P. Nipah virus: impact, origins, and causes of emergence. Curr Infect Dis Rep. 2006;8:59–65. doi: 10.1007/s11908-006-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ripple WJ, Abernethy K, Betts MG, et al. Bushmeat hunting and extinction risk to the world's mammals. R Soc Open Sci. 2016;3 doi: 10.1098/rsos.160498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ingram DJ, Coad L, Milner-Gulland EJ, et al. Wild meat is still on the menu: progress in wild meat research, policy and practice from 2002 to 2020. Annu Rev Environ Resour. 2021;46:221–254. [Google Scholar]
- 39.Hoffman LC, Cawthorn D-M. What is the role and contribution of meat from wildlife in providing high quality protein for consumption? Anim Front. 2012;2:40–53. [Google Scholar]
- 40.Nogueira-Filho SLG, da Cunha Nogueira SS. Capybara meat: an extraordinary resource for food security in South America. Meat Sci. 2018;145:329–333. doi: 10.1016/j.meatsci.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 41.Leroy EM, Rouquet P, Formenty P, et al. Multiple Ebola virus transmission events and rapid decline of central African wildlife. Science. 2004;303:387–390. doi: 10.1126/science.1092528. [DOI] [PubMed] [Google Scholar]
- 42.Nahar N, Asaduzzaman M, Mandal UK, et al. Hunting bats for human consumption in Bangladesh. EcoHealth. 2020;17:139–151. doi: 10.1007/s10393-020-01468-x. [DOI] [PubMed] [Google Scholar]
- 43.Brashares JS, Golden CD, Weinbaum KZ, Barrett CB, Okello GV. Economic and geographic drivers of wildlife consumption in rural Africa. Proc Natl Acad Sci USA. 2011;108:13931–13936. doi: 10.1073/pnas.1011526108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ordaz-Németh I, Arandjelovic M, Boesch L, et al. The socio-economic drivers of bushmeat consumption during the West African Ebola crisis. PLoS Negl Trop Dis. 2017;11 doi: 10.1371/journal.pntd.0005450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reilly M, Willenbockel D. Managing uncertainty: a review of food system scenario analysis and modelling. Philos Trans R Soc Lond B Biol Sci. 2010;365:3049–3063. doi: 10.1098/rstb.2010.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gephart JA, Golden CD, Asche F, et al. Scenarios for global aquaculture and its role in human nutrition. Rev Fish Sci Aquacult. 2021;29:122–138. [Google Scholar]
- 47.Poore J, Nemecek T. Reducing food's environmental impacts through producers and consumers. Science. 2018;360:987–992. doi: 10.1126/science.aaq0216. [DOI] [PubMed] [Google Scholar]
- 48.Eshel G, Shepon A, Makov T, Milo R. Land, irrigation water, greenhouse gas, and reactive nitrogen burdens of meat, eggs, and dairy production in the United States. Proc Natl Acad Sci USA. 2014;111:11996–12001. doi: 10.1073/pnas.1402183111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Foley JA, Defries R, Asner GP, et al. Global consequences of land use. Science. 2005;309:570–574. doi: 10.1126/science.1111772. [DOI] [PubMed] [Google Scholar]
- 50.Díaz S, Settle J, Brondízio E. IPBES; Paris: 2019. Summary for policymakers of the Global Assessment Report on biodiversity and ecosystem services. [Google Scholar]
- 51.Alexander P, Brown C, Arneth A, Finnigan J, Rounsevell MDA. Human appropriation of land for food: the role of diet. Glob Environ Change. 2016;41:88–98. [Google Scholar]
- 52.Bar-On YM, Phillips R, Milo R. The biomass distribution on Earth. Proc Natl Acad Sci USA. 2018;115:6506–6511. doi: 10.1073/pnas.1711842115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alexandratos N, Bruinsma J. Food and Agriculture Organization of the United Nations; Rome: 2012. World agriculture towards 2030/2050: the 2012 revision. [Google Scholar]
- 54.FAO . Food and Agriculture Organization of the United Nations; Rome: 2020. The state of world fisheries and aquaculture 2020: sustainability in action. [Google Scholar]
- 55.Pauly D, Zeller D. Catch reconstructions reveal that global marine fisheries catches are higher than reported and declining. Nat Commun. 2016;7 doi: 10.1038/ncomms10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lotze HK, Tittensor DP, Bryndum-Buchholz A, et al. Global ensemble projections reveal trophic amplification of ocean biomass declines with climate change. Proc Natl Acad Sci USA. 2019;116:12907–12912. doi: 10.1073/pnas.1900194116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Naylor RL, Hardy RW, Buschmann AH, et al. A 20-year retrospective review of global aquaculture. Nature. 2021;591:551–563. doi: 10.1038/s41586-021-03308-6. [DOI] [PubMed] [Google Scholar]
- 58.Broom DM, Galindo FA, Murgueitio E. Sustainable, efficient livestock production with high biodiversity and good welfare for animals. Proc Biol Sci. 2013;280 doi: 10.1098/rspb.2013.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eshel G. Small-scale integrated farming systems can abate continental-scale nutrient leakage. PLoS Biol. 2021;19 doi: 10.1371/journal.pbio.3001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Zanten HHE, Herrero M, Van Hal O, et al. Defining a land boundary for sustainable livestock consumption. Glob Change Biol. 2018;24:4185–4194. doi: 10.1111/gcb.14321. [DOI] [PubMed] [Google Scholar]
- 61.Salaheen S, Chowdhury N, Hanning I, Biswas D. Zoonotic bacterial pathogens and mixed crop-livestock farming. Poult Sci. 2015;94:1398–1410. doi: 10.3382/ps/peu055. [DOI] [PubMed] [Google Scholar]
- 62.Wezel A, Herren BG, Kerr RB, Barrios E, Gonçalves ALR, Sinclair F. Agroecological principles and elements and their implications for transitioning to sustainable food systems. A review. Agron Sustain Dev. 2020;40:40. [Google Scholar]
- 63.Buck BH, Troell MF, Krause G, Angel DL, Grote B, Chopin T. State of the art and challenges for offshore Integrated Multi-Trophic Aquaculture (IMTA) Front Mar Sci. 2018;5:165. [Google Scholar]
- 64.Timmermann C, Félix GF. Agroecology as a vehicle for contributive justice. Agric Human Values. 2015;32:523–538. [Google Scholar]
- 65.Willett W, Rockström J, Loken B, et al. Food in the Anthropocene: the EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet. 2019;393:447–492. doi: 10.1016/S0140-6736(18)31788-4. [DOI] [PubMed] [Google Scholar]
- 66.Herrero M, Thornton PK, Power B, et al. Farming and the geography of nutrient production for human use: a transdisciplinary analysis. Lancet Planet Health. 2017;1:e33–e42. doi: 10.1016/S2542-5196(17)30007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kremen C, Merenlender AM. Landscapes that work for biodiversity and people. Science. 2018;362 doi: 10.1126/science.aau6020. [DOI] [PubMed] [Google Scholar]
- 68.Tamburini G, Bommarco R, Wanger TC, et al. Agricultural diversification promotes multiple ecosystem services without compromising yield. Sci Adv. 2020;6 doi: 10.1126/sciadv.aba1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Velden JL, Travers H, Moyo BHZ, Biggs D. Using scenarios to understand community-based interventions for bushmeat hunting and consumption in African savannas. Biol Conserv. 2020;248 [Google Scholar]
- 70.Nielsen MR. Importance, cause and effect of bushmeat hunting in the Udzungwa Mountains, Tanzania: implications for community based wildlife management. Biol Conserv. 2006;128:509–516. [Google Scholar]
- 71.Heermans B, van Rooyen J, Fynn R, Biggs D, Lewis M, McNutt J. Husbandry and herding: a community-based approach to addressing illegal wildlife trade in northern Botswana. Front Conserv Sci. 2021;2:49. [Google Scholar]
- 72.Springmann M, Clark MA, Rayner M, Scarborough P, Webb P. The global and regional costs of healthy and sustainable dietary patterns: a modelling study. Lancet Planet Health. 2021;5:e797–e807. doi: 10.1016/S2542-5196(21)00251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Remans R, Wood SA, Saha N, Anderman TL, DeFries RS. Measuring nutritional diversity of national food supplies. Glob Food Secur. 2014;3:174–182. [Google Scholar]
- 74.Karp DS, Mendenhall CD, Sandí RF, et al. Forest bolsters bird abundance, pest control and coffee yield. Ecol Lett. 2013;16:1339–1347. doi: 10.1111/ele.12173. [DOI] [PubMed] [Google Scholar]
- 75.Kremen C, Miles A. Ecosystem services in biologically diversified versus conventional farming systems: benefits, externalities, and trade-offs. Ecol Soc. 2012;17 [Google Scholar]
- 76.Perfecto I, Vandermeer J. The agroecological matrix as alternative to the land-sparing/agriculture intensification model. Proc Natl Acad Sci USA. 2010;107:5786–5791. doi: 10.1073/pnas.0905455107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gawith D, Hodge I. Focus rural land policies on ecosystem services, not agriculture. Nat Ecol Evol. 2019;3:1136–1139. doi: 10.1038/s41559-019-0934-y. [DOI] [PubMed] [Google Scholar]
- 78.Leung YHC, Lau EH, Zhang LJ, Guan Y, Cowling BJ, Peiris JS. Avian influenza and ban on overnight poultry storage in live poultry markets, Hong Kong. Emerg Infect Dis. 2012;18:1339–1341. doi: 10.3201/eid1808.111879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang R, Zhang X, Irwin DM, Shen Y. Emergence of SARS-like coronavirus poses new challenge in China. J Infect. 2020;80:350–371. doi: 10.1016/j.jinf.2020.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mellor KC, Meyer A, Elkholly DA, et al. Comparative epidemiology of highly pathogenic avian influenza virus H5N1 and H5N6 in Vietnamese live bird markets: spatiotemporal patterns of distribution and risk factors. Front Vet Sci. 2018;5:51. doi: 10.3389/fvets.2018.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cowlishaw G, Mendelson S, Rowcliffe JM. Evidence for post-depletion sustainability in a mature bushmeat market. J Appl Ecol. 2005;42:460–468. [Google Scholar]
- 82.Schmeller DS, Courchamp F, Killeen G. Biodiversity loss, emerging pathogens and human health risks. Biodivers Conserv. 2020;29:3095–3102. doi: 10.1007/s10531-020-02021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu T. The socioeconomic and environmental drivers of the COVID-19 pandemic: a review. Ambio. 2021;50:822–833. doi: 10.1007/s13280-020-01497-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Booth H, Clark M, Milner-Gulland EJ, et al. Investigating the risks of removing wild meat from global food systems. Curr Biol. 2021;31:1788–1797. doi: 10.1016/j.cub.2021.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.