Abstract
Background
This phase 3 trial assessed AZD7442 (tixagevimab/cilgavimab) for post-exposure prophylaxis against symptomatic coronavirus disease 2019 (COVID-19).
Methods
Adults without prior severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection or COVID-19 vaccination were enrolled within 8 days of exposure to a SARS-CoV-2–infected individual and randomized 2:1 to a single 300-mg AZD7442 dose (one 1.5-mL intramuscular injection each of tixagevimab and cilgavimab) or placebo. Primary end points were safety and first post-dose SARS-CoV-2 reverse-transcription polymerase chain reaction (RT-PCR)–positive symptomatic COVID-19 event before day 183.
Results
A total of 1121 participants were randomized and dosed (AZD7442, n = 749; placebo, n = 372). Median (range) follow-up was 49 (5–115) and 48 (20–113) days for AZD7442 and placebo, respectively. Adverse events occurred in 162 of 749 (21.6%) and 111 of 372 (29.8%) participants with AZD7442 and placebo, respectively, mostly mild/moderate. RT-PCR–positive symptomatic COVID-19 occurred in 23 of 749 (3.1%) and 17 of 372 (4.6%) AZD7442- and placebo-treated participants, respectively (relative risk reduction, 33.3%; 95% confidence interval [CI], −25.9 to 64.7; P = .21). In predefined subgroup analyses of 1073 (96%) participants who were SARS-CoV-2 RT-PCR–negative (n = 974, 87%) or missing an RT-PCR result (n = 99, 9%) at baseline, AZD7442 reduced RT-PCR–positive symptomatic COVID-19 by 73.2% (95% CI, 27.1 to 90.1) vs placebo.
Conclusions
This study did not meet the primary efficacy end point of post-exposure prevention of symptomatic COVID-19. However, analysis of participants who were SARS-CoV-2 RT-PCR–negative or missing an RT-PCR result at baseline support a role for AZD7442 in preventing symptomatic COVID-19.
Clinical Trials Registration. NCT04625972.
Keywords: COVID-19, AZD7442, monoclonal antibodies, post-exposure prophylaxis, SARS-CoV-2
AZD7442 (tixagevimab/cilgavimab) for post-exposure prophylaxis of coronavirus disease 2019 (COVID-19) did not reduce symptomatic COVID-19 in all participants, but did so in participants who were severe acute respiratory syndrome coronavirus 2 reverse-transcription–polymerase chain reaction–negative/missing at baseline.
(See the Editorial Commentary by Davis and Li on pages 1257–9.)
Post-exposure prophylaxis comprises interventions aimed at reducing the risk of developing symptomatic disease or disease progression, as well as transmission, after pathogen exposure. Post-exposure prophylaxis is routinely recommended following exposure to viruses including influenza virus [1], human immunodeficiency virus [2], varicella-zoster virus [3], and hepatitis B virus [4].
Despite effectiveness of coronavirus disease 2019 (COVID-19) vaccines in reducing symptomatic disease, hospitalization, and mortality [5, 6], severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission continues, particularly with the emergence of highly transmissible variants [7, 8]. The risk of SARS-CoV-2 outbreak and exposure is high in settings where close indoor contact is common, such as long-term care facilities (LTCFs), healthcare facilities, and industrial and military settings, where such outbreaks have been reported even among populations with high vaccination uptake [9–11]. In these instances, additional interventions to rapidly protect individuals following SARS-CoV-2 exposure are needed, particularly for unvaccinated individuals and those at high risk of developing severe disease following infection. The median incubation period of wild-type SARS-CoV-2, from exposure to symptom onset, is estimated to be 5.1 days, and 97.5% of those who develop symptoms will do so within 11.5 days of infection [12], although this timing is likely shorter for the Omicron variant (approximately 3 days) [13]. The post-exposure period represents a potential opportunity for post-exposure prophylaxis to prevent symptomatic COVID-19.
SARS-CoV-2 neutralizing monoclonal antibodies (mAbs) represent a potential option for rapid, passive immunoprophylaxis. AZD7442 is a combination of 2 extended half-life SARS-CoV-2–neutralizing mAbs (tixagevimab and cilgavimab) derived from potent antibodies isolated from B cells from SARS-CoV-2–infected individuals. Tixagevimab and cilgavimab bind to distinct epitopes of the viral spike protein receptor-binding domain [14–17]. AZD7442 neutralizes SARS-CoV-2 and variants including Omicron [18–20] in vitro. AZD7442 significantly prevented development of symptomatic COVID-19 when used as pre-exposure prophylaxis in the phase 3 prophylaxis prevention (PROVENT) trial, protected against severe disease and death as treatment for mild to moderate COVID-19 in the phase 3 treating acute COVID-19 condition with a long half-life engineeredantibody (TACKLE) trial, and showed a 30% reduction in all-cause mortality as treatment for severe COVID-19 in the phase 3 ACTIV-3 (Therapeutics for Inpatients with COVID-19; TICO) trial, and was well tolerated across trials [21–23]. The phase 3 COVID-19 Study to Optimally Reduce Morbidity in CareHomes and Sites with Enhanced Risk (STORM CHASER) trial was conducted to assess AZD7442 for post-exposure prevention of symptomatic COVID-19 in adults within 8 days of exposure to an individual with laboratory-confirmed SARS-CoV-2 infection.
METHODS
Study Design and Participants
STORM CHASER is an ongoing, 15-month, phase 3, randomized, double-blind, placebo-controlled, multicenter study, performed at 59 sites across the United States and the United Kingdom. Data are presented from the primary analysis, assessed 30 days after the 25th primary end point event had occurred (data cutoff, 7 April 2021) and from an extended data cutoff (19 August 2021) for primary and key secondary efficacy end points, safety end points, and characterization of variants of concern.
The trial was conducted in accordance with the ethical principles derived from international guidelines, including the Declaration of Helsinki (7th revision, 2013), Council for International Organizations of Medical Sciences International Ethical Guidelines, applicable International Council for Harmonisation Good Clinical Practice Guidelines, and all applicable laws and regulations. The protocol and amendments were reviewed and approved by an institutional review board or ethics committee. All participants provided written informed consent.
Participants were aged ≥18 years with potential exposure within 8 days to a symptomatic or asymptomatic individual with laboratory-confirmed SARS-CoV-2 infection and who were at appreciable risk of imminently developing COVID-19 based on available risk assessment at time of enrollment, within a high SARS-CoV-2 exposure risk setting (Supplementary Material). Participants must not have had COVID-19 symptoms (Supplementary Table 1) within 10 days before dosing. Participants with previous laboratory-confirmed SARS-CoV-2 infection or seropositivity (based on rapid point-of-care test) at screening or receipt of COVID-19 vaccination were excluded. Full eligibility criteria are provided in the Supplementary Material. A nasopharyngeal swab was collected at screening for SARS-CoV-2 by central laboratory reverse-transcription polymerase chain reaction (RT-PCR) for future analysis.
Randomization and Masking
Participants were randomized 2:1 to a single 300-mg AZD7442 dose (one 1.5-mL injection of each mAb consecutively) or saline placebo (two 1.5-mL injections consecutively) intramuscularly on day 1 and monitored for safety for 1–4 hours post dosing. Participants were initially stratified into cohort 1 (aged ≥60 years living in LTCFs with occurrence of SARS-CoV-2 infection in another resident or staff member) or cohort 2 (aged ≥18 years exposed to SARS-CoV-2 within a prespecified high exposure risk setting; Supplementary Material). A protocol amendment (v6.0, 12 March 2021) removed caps for cohorts 1 and 2 not to exceed 80% of the total participants, as recruitment of participants from LTCFs was judged no longer feasible with the availability of COVID-19 vaccines.
Participants who received study intervention and became eligible for a COVID-19 vaccine could request to be unblinded (at any time) to consider COVID-19 vaccination and remain in the study (Supplementary Material).
Outcomes
The primary safety end point was safety and tolerability of a single intramuscular dose of AZD7442 vs placebo, assessed by adverse events (AEs), serious AEs (SAEs), medically attended AEs (MAAEs), and AEs of special interest (AESIs). The primary efficacy end point was first incidence of post-dose SARS-CoV-2 RT-PCR–positive symptomatic illness (hereafter referred to as symptomatic COVID-19) occurring before day 183, assessed 30 days after the 25th event had occurred. The day 183 time point was selected to coincide with the expected duration of the effect of AZD7442, based on modeling analyses [17].
Further details of end points and the analysis methods are provided in the Supplementary Material.
Statistical Analyses
For analysis of the primary efficacy end point, a study population of approximately 1125 participants randomized in a 2:1 ratio, assuming a 4.5% attack rate with placebo and 75% true efficacy (equating to an attack rate of 1.1% with AZD7442), was estimated to provide approximately 90% power to demonstrate the lower bound of the 2-sided 95% confidence interval (CI) for efficacy to be >0. Based on these assumptions, a minimum of 25 cases was required to demonstrate efficacy.
The primary efficacy analysis was performed using a Poisson regression model with robust variance with log of follow-up time as an offset to estimate relative risk of symptomatic COVID-19 with AZD7442 vs placebo. Efficacy was calculated as relative risk reduction (RRR) for symptomatic COVID-19. Efficacy is presented with a 2-sided CI, with statistical significance achieved if the 95% CI lower bound was >0. To support the primary analysis, a Cox proportional hazard model was fitted to the data and Kaplan–Meier curves presented for active and control groups. Deaths caused by COVID-19 and hospitalizations characterized as severe COVID-19 (Supplementary Table 2) were also considered within the definition of a primary efficacy event. Further information is detailed in the Supplementary Material.
The key secondary efficacy end point and the other secondary efficacy end points were analyzed following the same methodology as for the primary efficacy end point. Subgroup analyses are detailed in the Supplementary Material. Missing data were not imputed for the efficacy end points (Supplementary Material).
Data from participants who were unblinded to consider COVID-19 vaccination or vaccinated prior to the primary efficacy end point were included in the efficacy and safety analyses.
RESULTS
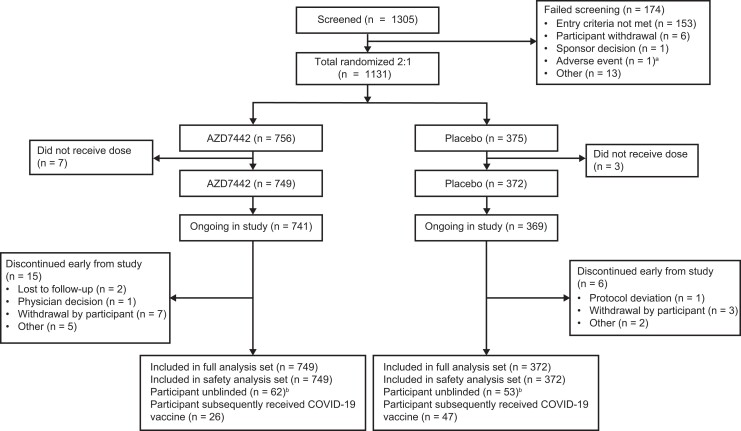
Between 2 December 2020 and 19 March 2021, 1305 participants were screened. Of these, 1131 participants had exposure to SARS-CoV-2 within 8 days and were randomized 2:1 to AZD7442 or placebo; 10 participants were not dosed, resulting in 749 participants receiving AZD7442 and 372 receiving placebo (Figure 1).
Figure 1.
Participant disposition. aThis potential participant experienced elevated blood pressure during screening and was not included in the study. bParticipants who received study intervention and became eligible for a COVID-19 vaccine could request to be unblinded to consider vaccination for COVID-19 and remain in the study. For participants who were unblinded before the primary efficacy end point, data were collected and analyzed regardless. Abbreviations: AZD7442, tixagevimab/cilgavimab; COVID-19, coronavirus disease 2019.
In the full analysis set, mean (standard deviation) age was 46 (16) years with 20% of participants aged ≥60 years and 99.4% in cohort 2 (Table 1). Most participants were SARS-CoV-2 RT-PCR–negative (n = 974, 87%) or had a missing RT-PCR status (n = 99, 9%) at baseline; 34 (4.5%) participants in the AZD7442 group and 14 (3.8%) in the placebo group were SARS-CoV-2 RT-PCR–positive. A small proportion in the AZD7442 and placebo groups (8.7% and 8.1%, respectively) were subsequently determined to be SARS-CoV-2–seropositive at baseline (based on central laboratory serum antinucleocapsid testing). Overall, 736 (66%) participants had ≥1 risk factor for severe COVID-19 (Table 1). Median (range) duration of follow-up at primary data cutoff was 49 (5–115) and 48 (20–113) days for AZD7442 and placebo, respectively. Median (range) duration of follow-up at extended data cutoff (19 August 2021) was 182 (5–249) and 178 (11–247) days for AZD7442 and placebo, respectively.
Table 1.
Participant Demographics and Baseline Clinical Characteristics: Full Analysis Set
| Characteristic | AZD7442 (Tixagevimab/Cilgavimab) (n = 749) |
Placebo (n = 372) |
Total (N = 1121) |
|---|---|---|---|
| Cohort, n (%) | |||
| ȃ1 (aged ≥60 y, living in long-term care facility) | 5 (0.7) | 2 (0.5) | 7 (0.6) |
| ȃ2 (other adults aged ≥18 y, exposed to SARS-CoV-2 within prespecified high exposure risk settings) | 744 (99.3) | 370 (99.5) | 1114 (99.4) |
| Age, mean (SD), y | 46.6 (15.7) | 46.0 (16.2) | 46.4 (15.9) |
| Age group, n (%), y | |||
| ȃ≥60 | 149 (19.9) | 75 (20.2) | 224 (20.0) |
| ȃ≥65 | 91 (12.1) | 43 (11.6) | 134 (12.0) |
| ȃ≥75 | 23 (3.1) | 16 (4.3) | 39 (3.5) |
| Female, n (%) | 373 (49.8) | 181 (48.7) | 554 (49.4) |
| Ethnicity, n (%) | |||
| ȃNot Hispanic or Latino | 299 (39.9) | 159 (42.7) | 458 (40.9) |
| ȃHispanic or Latino | 435 (58.1) | 210 (56.5) | 645 (57.5) |
| ȃUnknown/Not reported | 15 (2.0) | 3 (0.8) | 18 (1.6) |
| Race, n (%) | |||
| ȃWhite | 628 (83.8) | 315 (84.7) | 943 (84.1) |
| ȃBlack or African American | 76 (10.1) | 36 (9.7) | 112 (10.0) |
| ȃAsian | 15 (2.0) | 13 (3.5) | 28 (2.5) |
| ȃAmerican Indian/Alaska Native | 6 (0.8) | 1 (0.3) | 7 (0.6) |
| ȃNative Hawaiian/Pacific Islander | 2 (0.3) | 1 (0.3) | 3 (0.3) |
| ȃUnknown/Not reported/Multiple | 22 (2.9) | 6 (1.6) | 28 (2.5) |
| BMI, mean (SD), kg/m2 | 29.7 (6.7) | 29.9 (6.7) | 29.7 (6.7) |
| SARS-CoV-2 reverse-transcription polymerase chain reaction status, n (%) | |||
| ȃNegative | 646 (86.2) | 328 (88.2) | 974 (86.9) |
| ȃPositive | 34 (4.5) | 14 (3.8) | 48 (4.3) |
| ȃMissing data | 69 (9.2) | 30 (8.1) | 99 (8.8) |
| SARS-CoV-2 nucleocapsid antibodies serostatus at baseline, n (%) | |||
| ȃNegative | 444 (59.3) | 231 (62.1) | 675 (60.2) |
| ȃPositive | 65 (8.7) | 30 (8.1) | 95 (8.5) |
| ȃMissing data | 240 (32.0) | 111 (29.8) | 351 (31.3) |
| At risk for severe coronavirus disease 2019, n (%) | |||
| ȃAny risk factor | 492 (65.7) | 244 (65.6) | 736 (65.7) |
| ȃObesity (BMI ≥30 kg/m2) | 295 (39.4) | 162 (43.5) | 457 (40.8) |
| ȃHypertension | 184 (24.6) | 84 (22.6) | 268 (23.9) |
| ȃSmoking | 144 (19.2) | 71 (19.1) | 215 (19.2) |
| ȃDiabetes | 90 (12.0) | 38 (10.2) | 128 (11.4) |
| ȃAsthma | 49 (6.5) | 27 (7.3) | 76 (6.8) |
| ȃCancer | 24 (3.2) | 10 (2.7) | 34 (3.0) |
| ȃCardiovascular disease | 19 (2.5) | 14 (3.8) | 33 (2.9) |
| ȃChronic kidney disease | 14 (1.9) | 7 (1.9) | 21 (1.9) |
| ȃChronic obstructive pulmonary disease | 7 (0.9) | 11 (3.0) | 18 (1.6) |
| ȃChronic liver disease | 8 (1.1) | 2 (0.5) | 10 (0.9) |
| ȃImmunosuppressive treatment | 7 (0.9) | 2 (0.5) | 9 (0.8) |
| ȃSickle cell disease | 1 (0.1) | 0 | 1 (0.1) |
| ȃImmunosuppressive disease | 0 | 0 | 0 |
Data cutoff, 7 April 2021.
Abbreviations: BMI, body mass index; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation.
In the safety analysis, at the primary data cutoff, ≥ 1 AE was reported by 162 of 749 (21.6%) and 111 of 372 (29.8%) participants in the AZD7442 and placebo groups, respectively, with 95% reporting mild or moderate AEs (Table 2). The most common AEs included headache, fatigue, and cough (Supplementary Table 3). SAEs were reported by 5 (0.7%) and 3 (0.8%) participants in the AZD7442 and placebo groups, respectively; none were considered related to study intervention (Table 2, Supplementary Table 4). AESIs were reported by 4 (0.5%) and 5 (1.3%) participants in the AZD7442 and placebo groups, respectively, mostly injection site reactions (Table 2). No AEs led to study withdrawal or death.
Table 2.
Adverse Events at the Primary Data Cutoff: Safety Analysis Set
| Participants With ≥1 Event, n (%) | AZD7442 (Tixagevimab/Cilgavimab) (n = 749) |
Placebo (n = 372) |
Total (N = 1121) |
|---|---|---|---|
| AE | 162 (21.6) | 111 (29.8) | 273 (24.4) |
| ȃMild | 103 (13.8) | 74 (19.9) | 177 (15.8) |
| ȃModerate | 51 (6.8) | 31 (8.3) | 82 (7.3) |
| ȃSevere | 7 (0.9) | 6 (1.6) | 13 (1.2) |
| ȃPotentially life-threatening | 1 (0.1)a | 0 | 1 (0.1) |
| SAE | 5 (0.7) | 3 (0.8) | 8 (0.7) |
| Intervention-related SAEb | 0 | 0 | 0 |
| AE leading to study withdrawal | 0 | 0 | 0 |
| Medically attended AE | 32 (4.3) | 16 (4.3) | 48 (4.3) |
| AE of special interest | 4 (0.5) | 5 (1.3) | 9 (0.8) |
| ȃInjection-site reactionc | 4 (0.5) | 4 (1.1) | 8 (0.7) |
| ȃOther | 0 | 1 (0.3) | 1 (0.1) |
| ȃȃGeneral pruritus | 0 | 1 (0.3) | 1 (0.1) |
| Intervention-related AE of special interestb | 3 (0.4) | 5 (1.3) | 8 (0.7) |
| AE with outcome death | 0 | 0 | 0 |
Data cutoff, 7 April 2021. AEs were defined as any AE that started or worsened in severity on or after the first dose of study intervention. AEs were coded using the Medical Dictionary for Regulatory Activities, version 24.0. Participants with >1 event within a preferred term were counted once for that preferred term. Percentages are based on the number of participants in the analysis set by study intervention group (N).
Abbreviations: AE, adverse event; SAE, serious adverse event.
Potentially life-threatening event reported as overdose, not considered related to study intervention.
AEs were determined to be related to study intervention or study procedures by the investigators based on their judgment.
Includes events of injection-site pain, injection-site pruritus, other injection-site reaction, and pruritus.
Safety at the extended data cutoff was generally consistent with the primary data cutoff (Supplementary Table 5). There were 3 deaths: 2 (0.3%) participants in the AZD7442 group (metastatic lung cancer, cerebral ischemia) and 1 (0.3%) participant in the placebo group (unexplained death); none were considered related to study intervention.
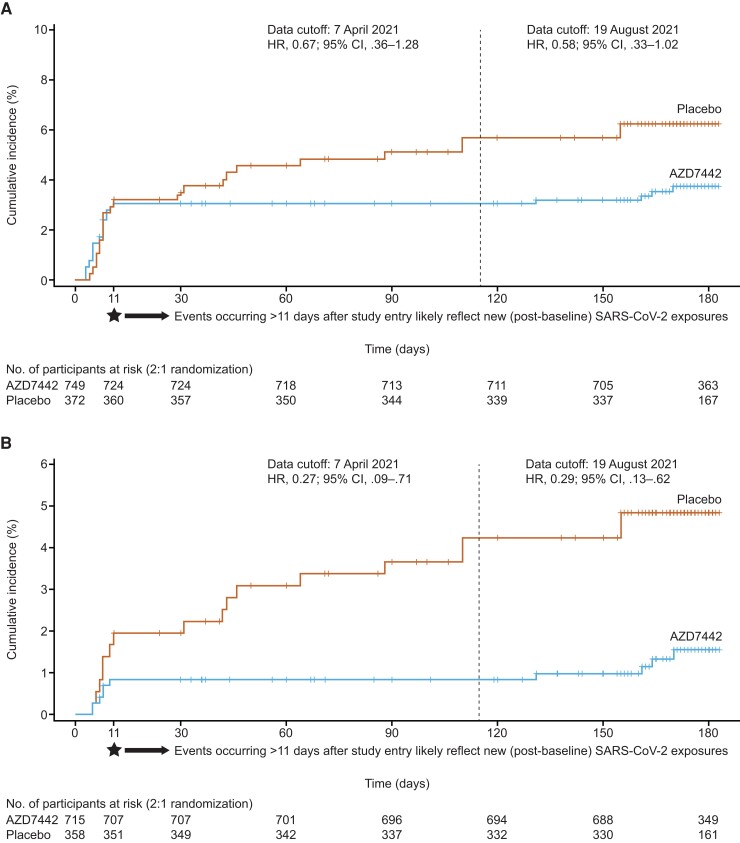
In the efficacy analysis, at the primary data cutoff, symptomatic COVID-19 developed in 23 of 749 (3.1%) participants in the AZD7442 group vs 17 of 372 (4.6%) in the placebo group (Figure 2A ). The treatment difference was not statistically significant (RRR, 33.3%; 95% CI, −25.9 to 64.7; P = .21; absolute risk reduction 1.50; 95% CI, −.76 to 4.32; P = .23). Time to first post-dose symptomatic COVID-19 before day 183 is shown in Figure 2A . Most (87.5%) events occurred within the expected post-exposure SARS-CoV-2 incubation period (11 days post-dose); all 23 events in the AZD7442 group (17 events in baseline SARS-CoV-2 RT-PCR–positive participants) and 12 events with placebo (70.6% of total events observed with placebo; 5 events in baseline SARS-CoV-2 RT-PCR–positive participants), with an additional 5 events occurring with placebo after day 11.
Figure 2.
Time to first post-dose SARS-CoV-2 reverse-transcription polymerase chain reaction (RT-PCR)–positive symptomatic illness before day 183. A, The full analysis set. B, Predefined analysis of participants who were SARS-CoV-2 RT-PCR–negative or missing an RT-PCR result at baseline. + Indicates a censored observation. Dashed line represents the primary data cutoff. HR is from the proportional hazard model with the Efron method. The 95% CI for the HR was obtained by taking 95% profile likelihood CI of the HR from the proportional hazard model. Abbreviations: AZD7442, tixagevimab/cilgavimab; CI, confidence interval; HR, hazard ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
By the extended data cutoff, symptomatic COVID-19 developed in 27 of 749 (3.6%) participants in the AZD7442 group vs 23 of 372 (6.2%) in the placebo group (RRR, 43.2%; 95% CI, .1 to 67.7; nominal P = .049). Time to first post-dose symptomatic COVID-19 at the extended data cutoff is shown in Figure 2A .
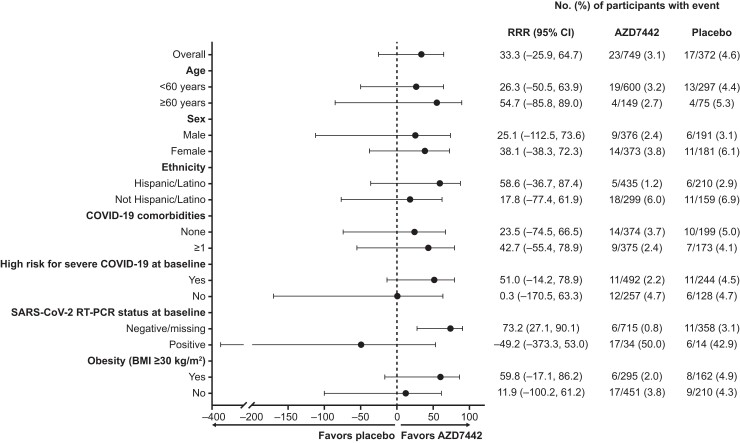
In predefined subgroup analyses among 1073 (96%) participants who were SARS-CoV-2 RT-PCR–negative or missing an RT-PCR result at baseline, symptomatic COVID-19 developed in 6 of 715 (0.8%) participants in the AZD7442 group vs 11 of 358 (3.1%) in the placebo group, resulting in an RRR of 73.2% (95% CI, 27.1 to 90.1; Figures 2B and 3) at primary data cutoff, with consistent results at the extended data cutoff (RRR, 71.5%; 95% CI, 37.5 to 87.0; Figure 2B ). Findings for other subgroup analyses at primary data cutoff were generally consistent with the overall primary analysis population (Figure 3, Supplementary Figure 1).
Figure 3.
Key predefined subgroup analysis of incidence of first post-dose SARS-CoV-2 RT-PCR–positive symptomatic illness before day 183. Data cutoff, 7 April 2021. Estimates are based on Poisson regression with robust variance or exact Poisson regression or exact conditional method for Poisson. The model includes the log of the follow-up time as an offset and a covariate for study intervention (AZD7442 vs placebo). Estimated RRR >0 ([1 – relative risk with AZD7442 vs placebo] × 100%) provides evidence in favor of AZD7442. Further subgroup analyses are shown in Supplementary Figure 1. Abbreviations: AZD7442, tixagevimab/cilgavimab; BMI, body mass index; CI, confidence interval; COVID-19, coronavirus disease 2019; RRR, relative risk reduction; RT-PCR, reverse-transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
For the key secondary end point at the primary data cutoff, severe or critical COVID-19 developed in 0 of 749 (0%) participants in the AZD7442 group and 1 of 372 (0.3%) in the placebo group (RRR not evaluable). No additional severe or critical events were reported during the extended follow-up.
Findings for the following secondary and exploratory end points are reported for the primary data cutoff only. As no deaths occurred by primary data cutoff, COVID-19–related death and all-cause mortality could not be analyzed. One (0.1%) and 2 (0.5%) participants in the AZD7442 and placebo groups, respectively, had COVID-19–related emergency department visits. There were no significant differences in post-dose seroconversion between groups, regardless of symptom status, suggesting that AZD7442 did not prevent asymptomatic SARS-CoV-2 infection (Supplementary Table 6).
SARS-CoV-2 sequencing data were available from 16 of 27 (59.2%) and 11 of 23 (47.8%) participants in the AZD7442 and placebo groups, respectively, who had symptomatic SARS-CoV-2 infection at extended follow-up. Sequencing data indicated that 12 participants were infected with the Alpha variant, and few cases (n = 3) of the Delta variant were identified (Supplementary Table 7).
In the pharmacokinetic analysis, AZD7442 reached and maintained high concentrations following dosing, as shown by geometric mean (± geometric standard deviation) serum concentrations of 18.7 (±2.0) µg/mL at day 8 and 24.9 (±1.5) µg/mL at day 58 (Supplementary Figure 2).
DISCUSSION
STORM CHASER did not meet the primary efficacy end point of reducing symptomatic COVID-19 with post-exposure prophylaxis of AZD7442 vs placebo among the full study population. However, AZD7442 was effective in preventing symptomatic COVID-19 in participants who were SARS-CoV-2 RT-PCR–negative or missing an RT-PCR result at baseline, and results suggested a protective effect over 6 months, consistent with an equivalent population in the PROVENT pre-exposure prevention study [21]. AZD7442 demonstrated a favorable safety profile and was well tolerated in STORM CHASER, consistent with other phase 3 studies, including those evaluating AZD7442 for treatment of mild to moderate COVID-19 (TACKLE) and severe COVID-19 (ACTIV-3) [21–23].
Although the impact of AZD7442 as post-exposure prophylaxis on development of severe COVID-19 (including hospitalization, respiratory failure, and death) could not be assessed in STORM CHASER due to the low number of events (1 case of severe disease in the placebo arm), the potential of AZD7442 to prevent development of severe COVID-19 was demonstrated in the phase 3 TACKLE trial, in which AZD7442 reduced the risk of developing severe COVID-19 or death vs placebo by 50.5%, 66.9%, and 88.0% among participants with mild-to-moderate COVID-19 who had been symptomatic for ≤7, ≤ 5, and ≤3 days, respectively [22]. These data also support the premise that treating earlier in the course of infection is more effective.
Eligible participants in STORM CHASER had SARS-CoV-2 exposure within 8 days of randomization, and time from SARS-CoV-2 exposure to AZD7442 administration likely impacted the primary efficacy results. At baseline, 4.5% and 3.8% of participants in the AZD7442 and placebo groups, respectively, were already SARS-CoV-2 RT-PCR–positive. However, as the date of initial SARS-CoV-2 exposure was participant reported, time from SARS-CoV-2 exposure to dosing could not be objectively confirmed or further explored. The study included a highly exposed population, so it is possible that participants may have had continuous exposure following randomization and had not started incubating prior to dosing. Furthermore, some participants had already seroconverted at baseline, based on more sensitive central laboratory serology testing for which results were not immediately available. This may be suggestive of a later disease course, though we cannot rule out the possibility that participants had a previous infection, and this likely further impacted the efficacy analysis. Analyses of early post-exposure prophylaxis within 4 days of exposure with another SARS-CoV-2 neutralizing mAb combination found greater reductions in COVID-19 among participants who were RT-PCR–negative vs RT-PCR–positive at baseline (RRRs of 81.4% and 31.5%, respectively) [24, 25]. Comparison of these studies supports that earlier treatment is more effective in preventing symptomatic COVID-19.
While this study was primarily designed to assess AZD7442 against development of COVID-19 symptoms following a reported exposure event (ie, post-exposure), the design (which assessed events up to day 183, capturing events after the expected 11-day incubation period) enabled an assessment of AZD7442 in pre-exposure prevention. Symptomatic COVID-19 events that occurred >11 days after study entry likely reflect new post-baseline exposures, and the reduction in these events with AZD7442 vs placebo over the 6-month follow-up supports a protective effect against new SARS-CoV-2 exposures owing to the extended half-life of AZD7442 [17], consistent with the results of the PROVENT study [21].
Participants could request to be unblinded to consider COVID-19 vaccination. Following unblinding, a greater proportion of participants in the placebo group than the AZD7442 group subsequently received COVID-19 vaccine (12.6% vs 3.5%, respectively). This imbalance may have biased the efficacy analysis in the assessment of pre-exposure prevention (ie, against events occurring later in the study). Therefore, the reported protective effect of AZD7442 against new SARS-CoV-2 exposures after the index exposure may be underestimated.
This study had several limitations. Efficacy results were likely affected by the period of up to 8 days allowed between exposure and randomization, which resulted in a large proportion of events in participants who were already SARS-CoV-2 RT-PCR–positive at baseline. There was a low number of symptomatic COVID-19 cases overall following index exposure, potentially due to the exclusion of the LTCFs cohort and subsequent enrollment of a younger, healthier population than was planned. Individuals living in LTCFs still remain an at-risk population in which further evaluation of post-exposure prophylaxis may be warranted, especially in the context of waning COVID-19 vaccine effectiveness, emergence of new variants, and continued SARS-CoV-2 transmission [26]. Individuals with prior receipt of a COVID-19 vaccination were not eligible to participate in STORM CHASER. However, AZD7442 has been shown to not interfere with COVID-19 vaccine immunogenicity [27], and initial evidence suggests that AZD7442 may augment pre-existing protection against SARS-CoV-2 infection in immunocompromised individuals who are fully vaccinated [28, 29]. Although efficacy of AZD7442 against Omicron could not be assessed in the present study, in vitro studies have demonstrated that AZD7442 retains neutralizing activity against the BA.1, BA.1.1, BA.2, BA.2.12.1, BA.3, BA.4, and BA.5 Omicron subvariants, with resultant potency within the half maximal inhibitory concentration (IC50) geometric mean concentration range of 4.0–806.0 ng/mL [18–20, 30–33]. Following AZD7442 administration, the resultant anti–SARS-CoV-2 neutralizing antibody titer in sera is higher than that of convalescent serum and is therefore expected to be clinically effective against these Omicron subvariants [17]. Importantly, AZD7442 retains potency against the BA.2 Omicron subvariant (IC50 of 10 ng/mL vs 4.0–9.8 ng/mL for ancestral strain vs BA.2, respectively) [20, 30], as well against BA.4/5 (IC50 40–65 ng/mL) [31, 34]. Finally, this study used a 300-mg intramuscular dose of AZD7442. To provide optimal protection against emerging variants for vulnerable populations, a revised dosage regimen of 600 mg AZD7442 (300 mg each of tixagevimab and cilgavimab) every 6 months is now recommended for pre-exposure prophylaxis of COVID-19 [30].
CONCLUSIONS
AZD7442 300 mg intramuscular administration in participants with index exposure to SARS-CoV-2 within 8 days showed favorable safety but did not meet the primary efficacy end point of reduction in symptomatic COVID-19 cases. Findings among participants from high exposure risk environments who were SARS-CoV-2 RT-PCR–negative or missing an RT-PCR result at baseline support a role for AZD7442 in prevention of symptomatic COVID-19.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. M. J. L. was international coordinating investigator for the trial. K. S., M. P., and M. E. contributed to study conceptualization. M. P. and M. E. were responsible for funding acquisition. A. T., Y. Y., S. S., C. F. H., R. H. A., R. B., E. J. K., S. I., M. P., and M. E. contributed to the study design. M. J. L., C. F. H., P. G., and M. E. supervised the trial. M. J. L., S. P., G. C. K. W. K., M. P., and M. E. contributed to project administration. A. U., C. F. H., I. M. P., S. P., R. B., and S. I. collected the data. I. M. P., P. G., and K. S. validated the data. S. T. and S. S. conducted the statistical analysis. All authors had access to all of the data in this study, contributed to data interpretation and writing and editing of the manuscript, and reviewed and approved the manuscript for submission.
Acknowledgments. The authors thank the study participants, their families, and all investigators involved in this study. Yousef Fawadleh, BSc, and Elaine Harrop, MSc, of AstraZeneca led the operational management of the study. Yee-Man Ching, PhD, of AstraZeneca facilitated author discussions, coordinated manuscript development, and critically reviewed the manuscript. Medical writing support was provided by Shaun W. Foley, BSc (Hons), ISMPP CMPP, and Rob Campbell, PhD, and editorial support was provided by Joe Alling, BSc, of Core Medica, London, UK, supported by AstraZeneca according to Good Publication Practice Guidelines (https://www.acpjournals.org/doi/10.7326/M22–1460).
Data sharing. Data underlying the findings described here may be requested in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. AstraZeneca Group of Companies allows researchers to submit a request to access anonymized patient-level clinical data, aggregate clinical or genomics data (when available), and anonymized clinical study reports through the Vivli web-based data request platform.
Disclaimer. The contents of this article are the sole responsibility of the authors and do not necessarily reflect the views, assertions, opinions, or policies of the Uniformed Services University of the Health Sciences (USU), Henry M. Jackson Foundation (HJF) for the Advancement of Military Medicine, Inc, Department of the Army, Department of the Navy, Defense Health Agency, US Department of Defense, Department of Health and Human Services, the US government, or any other government or agency. Mention of trade names, commercial products, or organizations does not imply endorsement by the US government. Some of the authors of this work are military service members or employees of the US government. This work was prepared as part of their official duties. Title 17 U.S.C. 105 provides that “copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. 101 defines a US government work as a work prepared by a military service member or employee of the US government as part of that person's official duties. The investigators have adhered to the policies for protection of human subjects as prescribed in 45 CFR 46. The sponsor was involved in the study design; collection, analysis, and interpretation of data; as well as data checking of information provided in the manuscript. Ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors.
Financial support. This work was supported by AstraZeneca and the US government. AZD7442 is being developed with support from the US government, including federal funds from the Department of Health and Human Services, Administration for Strategic Preparedness and Response, Biomedical Advanced Research and Development Authority in partnership with the Department of Defense, and Joint Program Executive Office for Chemical, Biological, Radiological, and Nuclear Defense under contract W911QY-21-9-0001.
Supplementary Material
Contributor Information
Myron J Levin, University of Colorado Denver School of Medicine, Aurora, Colorado, USA.
Andrew Ustianowski, North Manchester General Hospital, Manchester, United Kingdom.
Steven Thomas, Biometrics, Vaccines and Immune Therapies, BioPharmaceuticals Research and Development, AstraZeneca, Gaithersburg, Maryland, USA.
Alison Templeton, Biometrics, Vaccines and Immune Therapies, BioPharmaceuticals Research and Development, AstraZeneca, Cambridge, United Kingdom.
Yuan Yuan, Biometrics, Vaccines and Immune Therapies, BioPharmaceuticals Research and Development, AstraZeneca, Gaithersburg, Maryland, USA.
Seth Seegobin, Biometrics, Vaccines and Immune Therapies, BioPharmaceuticals Research and Development, AstraZeneca, Cambridge, United Kingdom.
Catherine F Houlihan, Department of Clinical Virology, University College London Hospitals National Health Service Foundation Trust, London, United Kingdom; Department of Infection and Immunity, University College London, London, United Kingdom.
Ibrahim Menendez-Perez, Project 4 Research, Miami, Florida, USA.
Simon Pollett, Infectious Disease Clinical Research Program, Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc, Bethesda, Maryland, USA.
Rosalinda H Arends, Clinical Pharmacology and Quantitative Pharmacology, Vaccines and Immune Therapies, BioPharmaceuticals Research and Development, AstraZeneca, Gaithersburg, Maryland, USA.
Rohini Beavon, Clinical Development, Vaccines and Immune Therapies, BioPharmaceuticals Research and Development, AstraZeneca, Cambridge, United Kingdom.
Kanika Dey, Clinical Development, Vaccines and Immune Therapies, BioPharmaceuticals Research and Development, AstraZeneca, Gaithersburg, Maryland, USA.
Pedro Garbes, Clinical Development, Vaccines and Immune Therapies, BioPharmaceuticals Research and Development, AstraZeneca, Gaithersburg, Maryland, USA.
Elizabeth J Kelly, Translational Medicine, Vaccines and Immune Therapies, BioPharmaceuticals Research and Development, AstraZeneca, Gaithersburg, Maryland, USA.
Gavin C K W Koh, Clinical Development, Vaccines and Immune Therapies, BioPharmaceuticals Research and Development, AstraZeneca, Cambridge, United Kingdom.
Stefan Ivanov, Clinical Development, Vaccines and Immune Therapies, BioPharmaceuticals Research and Development, AstraZeneca, Gothenburg, Sweden.
Karen A Near, Clinical Development, Vaccines and Immune Therapies, BioPharmaceuticals Research and Development, AstraZeneca, Gaithersburg, Maryland, USA.
Audrey Sharbaugh, Clinical Development, Vaccines and Immune Therapies, BioPharmaceuticals Research and Development, AstraZeneca, Durham, North Carolina, USA.
Katie Streicher, Translational Medicine, Vaccines and Immune Therapies, BioPharmaceuticals Research and Development, AstraZeneca, Gaithersburg, Maryland, USA.
Menelas N Pangalos, BioPharmaceuticals Research and Development, AstraZeneca, Cambridge, United Kingdom.
Mark T Esser, Vaccines and Immune Therapies, BioPharmaceuticals Research and Development, AstraZeneca, Gaithersburg, Maryland, USA.
References
- 1. Ikematsu H, Hayden FG, Kawaguchi K, et al. Baloxavir marboxil for prophylaxis against influenza in household contacts. N Engl J Med 2020; 383:309–20. [DOI] [PubMed] [Google Scholar]
- 2. British HIV Association . UK guideline for the use of HIV post-exposure prophylaxis. Updated 2021. Available at: https://www.bhiva.org/file/6074031a87755/PEPSE-guidelines.pdf. Accessed 22 November 2022.
- 3. Lachiewicz AM, Srinivas ML. Varicella-zoster virus post-exposure management and prophylaxis: a review. Prev Med Rep 2019; 16:101016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bader MS, McKinsey DS. Postexposure prophylaxis for common infectious diseases. Am Fam Physician 2013; 88:25–32. [PubMed] [Google Scholar]
- 5. Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on COVID-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ 2021; 373:n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pritchard E, Matthews PC, Stoesser N, et al. Impact of vaccination on new SARS-CoV-2 infections in the United Kingdom. Nat Med 2021; 27:1370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization . Tracking SARS-CoV-2 variants. Updated November 2021. Available at: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/. Accessed 27 May 2022.
- 8. de Gier B, Andeweg S, Backer JA, et al. Vaccine effectiveness against SARS-CoV-2 transmission to household contacts during dominance of Delta variant (B.1.617.2), the Netherlands, August to September 2021. Euro Surveill 2021; 26:2100977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shitrit P, Zuckerman NS, Mor O, Gottesman BS, Chowers M. Nosocomial outbreak caused by the SARS-CoV-2 Delta variant in a highly vaccinated population, Israel, July 2021. Euro Surveill 2021; 26:2100822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burugorri-Pierre C, Lafuente-Lafuente C, Oasi C, et al. Investigation of an outbreak of COVID-19 in a French nursing home with most residents vaccinated. JAMA Netw Open 2021; 4:e2125294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Petros BA, Turcinovic J, Welch NL, et al. Early introduction and rise of the Omicron Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) variant in highly vaccinated university populations. Clin Infect Dis 2023; 76:e400–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Int Med 2020; 172:577–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brandal LT, MacDonald E, Veneti L, et al. Outbreak caused by the SARS-CoV-2 Omicron variant in Norway, November to December 2021. Euro Surveill 2021; 26:2101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dong J, Zost SJ, Greaney AJ, et al. Genetic and structural basis for SARS-CoV-2 variant neutralization by a two-antibody cocktail. Nat Microbiol 2021; 6:1233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zost SJ, Gilchuk P, Chen RE, et al. Rapid isolation and profiling of a diverse panel of human monoclonal antibodies targeting the SARS-CoV-2 spike protein. Nat Med 2020; 26:1422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zost SJ, Gilchuk P, Case JB, et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature 2020; 584:443–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Loo YM, McTamney PM, Arends RH, et al. The SARS-CoV-2 monoclonal antibody combination, AZD7442, is protective in nonhuman primates and has an extended half-life in humans. Sci Transl Med 2022; 14:eabl8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. VanBlargan LA, Errico JM, Halfmann PJ, et al. An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies. Nat Med 2022; 28:490–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dejnirattisai W, Huo J, Zhou D, et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell 2022; 185:467–84.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iketani S, Liu L, Guo Y, et al. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature 2022; 604:553–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levin MJ, Ustianowski A, De Wit S, et al. Intramuscular AZD7442 (tixagevimab-cilgavimab) for prevention of Covid-19. N Engl J Med 2022; 2188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Montgomery H, Hobbs FDR, Padilla F, et al. Efficacy and safety of intramuscular administration of tixagevimab-cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2022; 10:985–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. ACTIV-3-Therapeutics for Inpatients with COVID-19 (TICO) Study Group . Tixagevimab-cilgavimab for treatment of patients hospitalised with COVID-19: a randomised, double-blind, phase 3 trial. Lancet Respir Med. 2022; 10:972–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. O’Brien MP, Forleo-Neto E, Musser BJ, et al. Subcutaneous REGEN-COV antibody combination to prevent Covid-19. N Engl J Med 2021; 385:1184–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O’Brien MP, Forleo-Neto E, Sarkar N, et al. Effect of subcutaneous casirivimab and imdevimab antibody combination vs placebo on development of symptomatic COVID-19 in early asymptomatic SARS-CoV-2 infection: a randomized clinical trial. JAMA 2022; 327:432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Singanayagam A, Hakki S, Dunning J, et al. Community transmission and viral load kinetics of the SARS-CoV-2 Delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect Dis 2022; 22:183–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nkolola JP, Yu J, Wan H, et al. A bivalent SARS-CoV-2 monoclonal antibody combination does not affect the immunogenicity of a vector-based COVID-19 vaccine in macaques. Sci Trans Med 2022; 14:eabo6160.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bar DZ, Atkatsh K, Tavarez U Erdos MR, Gruenbaum Y, Collins FS. Biotinylation by antibody recognition- a novel method for proximity labeling. BioRxiv 069187 [Preprint]. 2016. [cited 2017 Jan 12]. Available from: 10.1101/069187. [DOI] [Google Scholar]
- 29. Conte WL, Golzarri-Arroyo L. Tixagevimab and cilgavimab (Evusheld) boosts antibody levels to SARS-CoV-2 in patients with multiple sclerosis on b-cell depleters. Mult Scler Relat Disord 2022; 63:103905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. US Food and Drug Administration . Fact sheet for healthcare providers: emergency use authorization for Evusheld™ (tixagevimab co-packaged with cilgavimab). Available at: https://www.fda.gov/media/154701/download. Accessed 7 September 2022.
- 31. Tuekprakhon A, Nutalai R, Dijokaite-Guraliuc A, et al. Antibody escape of SARS-CoV-2 Omicron BA.4 and BA.5 from vaccine and BA.1 serum. Cell 2022; 185:2422–33.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhou T, Wang L, Misasi J, et al. Structural basis for potent antibody neutralization of SARS-CoV-2 variants including B.1.1.529. Science 2022; 376:eabn8897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. National Institutes of Health . OpenData Portal. SARS-CoV-2 variants & therapeutics. Therapeutic activity explorer. Available at: https://opendata.ncats.nih.gov/variant/activity. Accessed 27 May 2022.
- 34. Cao Y, Yisimayi A, Jian F, et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature 2022; 608:593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.