Background and Aims:
We present findings from the inaugural American College of Sports Medicine (ACSM) International Multidisciplinary Roundtable, which was convened to evaluate the evidence for physical activity as a means of preventing or modifying the course of NAFLD.
Approach and Results:
A scoping review was conducted to map the scientific literature and identify key concepts, research gaps, and evidence available to inform clinical practice, policymaking, and research. The scientific evidence demonstrated regular physical activity is associated with decreased risk of NAFLD development. Low physical activity is associated with a greater risk for disease progression and extrahepatic cancer. During routine health care visits, all patients with NAFLD should be screened for and counseled about physical activity benefits, including reduction in liver fat and improvement in body composition, fitness, and quality of life. While most physical activity benefits occur without clinically significant weight loss, evidence remains limited regarding the association between physical activity and liver fibrosis. At least 150 min/wk of moderate or 75 min/wk of vigorous-intensity physical activity are recommended for all patients with NAFLD. If a formal exercise training program is prescribed, aerobic exercise with the addition of resistance training is preferred.
Conclusions:
The panel found consistent and compelling evidence that regular physical activity plays an important role in preventing NAFLD and improving intermediate clinical outcomes. Health care, fitness, and public health professionals are strongly encouraged to disseminate the information in this report. Future research should prioritize determining optimal strategies for promoting physical activity among individuals at risk and in those already diagnosed with NAFLD.

INTRODUCTION
NAFLD remains a leading cause of morbidity and mortality globally with 25% of individuals having this condition worldwide.1–3 To date, there is no regulatory agency–approved effective drug therapy or cure, and lifestyle modification with dietary changes and increased physical activity remains the foundation of NAFLD treatment. However, most patients with NAFLD do not meet recommended amounts of weekly physical activity, and the consequences of end-stage liver disease, including liver transplantation and HCC, continue to grow.4,5
In 2012, several leading gastroenterology and hepatology societies, including the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterology Association (AGA), and the American College of Gastroenterology (ACG) released the initial clinical practice guideline for patients with NAFLD.6 In this joint document, the importance of physical activity and, in particular, exercise (defined as a subtype of physical activity that is planned, structured, and repetitive and has the goal of improvement or maintenance of physical fitness), began to be recognized.6 Following this, additional guidance from the European Association for the Study of the Liver (EASL) and independent guidance from AASLD were released in 2016 and 2018 respectively with even greater attention to leading a healthy lifestyle;7,8 yet, these guidelines remained focused on lifestyle intervention as a vehicle for weight loss rather than focusing on the potential weight-neutral benefits of regular exercise. Consequently, rates of physical activity counseling and referral to exercise specialists for tailored exercise prescription remain low in clinical practice.9,10
To further encourage physical activity in patients with NAFLD, the American College of Sports Medicine (ACSM) and their Exercise is Medicine (EIM) initiative was expanded to include NAFLD in 2018.11 However, the EIM recommendations were based largely on expert opinion. Since the original EIM guidance, there has been a substantial accumulation of new evidence supporting the role of physical activity as a means of preventing or modifying the course of NAFLD.
For these reasons, the ACSM convened the inaugural International Multidisciplinary Roundtable on NAFLD and physical activity in July 2022 with the objectives of reviewing and summarizing the biologic, epidemiological, and interventional evidence for the role of physical activity in patients with NAFLD. The purpose of this manuscript is to present the Roundtable findings and conclusions that focused on the relationship between physical activity and NAFLD in terms of (1) NAFLD pathogenesis; (2) screening, advising, and counseling patients with NAFLD about physical activity; (3) providing physical activity recommendations to patients with NAFLD, and (4) outlining future research directions.
METHODS
The ACSM International Multidisciplinary Roundtable on NAFLD was held virtually (in part due to COVID-19 restrictions) on July 7, 2022, with 21 representatives from 18 organizations globally who were invited to participate based on their clinical and research expertise. Three broad topic areas were addressed, including (1) the role of physical activity in NAFLD pathogenesis; (2) screening, advising, and counseling adult patients with NAFLD about physical activity; and (3) providing physical activity recommendations to adult patients with NAFLD. Following individual scoping reviews of the assigned topics (Supplementary Materials, http://links.lww.com/HC9/A219), each presenter provided a 20-minute overview of their topic followed by a direct question and answer session with the entire Roundtable Faculty. Herein, we provide an overview of the Roundtable presentations and discussions.
PATHOGENESIS OF NAFLD AND MECHANISMS EXPLAINING THE BENEFIT OF PHYSICAL ACTIVITY
Despite decades of research, NAFLD remains a complex, progressive disease process with many different pathogenic mechanisms and inputs.12 Regular physical activity, in particular moderate-intensity aerobic exercise training,13 can impact several pathogenic factors and may also influence gene expression.14,15 While the mechanisms by which physical activity benefits patients with NAFLD remain poorly understood and understudied in patients,16,17 regular physical activity, including exercise training, can lead to significant adaptations in not only the liver, but also in skeletal muscle and adipose tissue, each of which communicate with the liver through myokines and adipocytokines (Figure 1), and also the cardiovascular system.16,18–22
FIGURE 1.
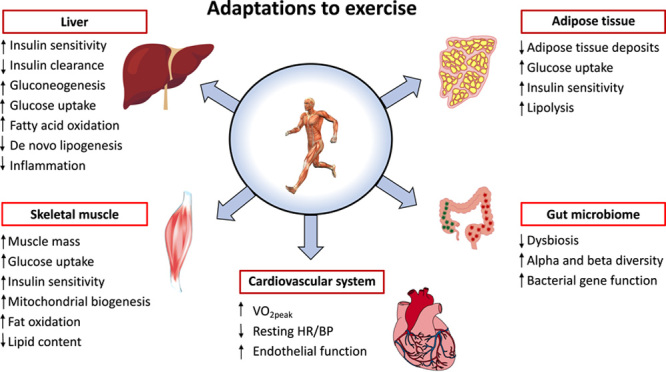
Adaptations to exercise training in patients with NAFLD. Abbreviations: BP, blood pressure; HR, heart rate; VO2peak, peak oxygen uptake.
Exercise training also impacts the microbiome and gut-liver-axis dysfunction.23 Patients with NAFLD have significant dysbiosis with an overabundance of Gram-negative bacteria, which can lead to gut barrier dysfunction and a “leaky gut.”24,25 After as little as 20 weeks of moderate-intensity aerobic exercise training, many beneficial effects have been observed on the gut-liver-axis in patients with NAFLD, including reversal of dysbiosis with the restoration of healthy bacterial balance as well as improvements in alpha (species richness) and beta diversity (species diversity).13,23 Moreover, aerobic exercise training impacts bacterial gene expression in a way that may remove many of the pathogenic factors implicated in the development of NAFLD (Figure 1).13,23
EPIDEMIOLOGICAL EVIDENCE: ASSOCIATION BETWEEN PHYSICAL ACTIVITY AND NAFLD
To date, there is a plethora of epidemiological data supporting an association between NAFLD and physical activity, including multiple large cross-sectional studies using nationwide databases.26–32 Collectively, these studies have found a consistent body of evidence that meeting or exceeding guideline-based physical activity amounts is associated with a decreased risk of incident NAFLD by roughly 50%. Perhaps more importantly, over 10+ years of follow-up, patients with NAFLD who perform regular moderate-to-vigorous physical activity have lower overall and cardiovascular disease (CVD) mortality.27 Similar trends for incident NAFLD reduction hold true when physical activity is completed in small, continuous bouts of aerobic activity of at least 10 minutes in length,33–35 provided the overall amount of physical activity completed is still the same. Unfortunately, despite this large body of epidemiological evidence, most patients with NAFLD do not meet current physical activity guidelines and spend more time pursuing sedentary behaviors.10,36,37 Specifically, patients with NAFLD walk, on average, ∼10 km less each week than those without NAFLD, and each additional hour of sedentary behavior is associated with 1.2% higher liver steatosis content.37,38
While the relationship between physical activity and risk of hepatic or extrahepatic malignancy in patients with NAFLD remains less explored, indirect evidence can be extrapolated from general population-based studies which show regular physical activity is associated with a lower risk of multiple primary cancers, including both HCC and nonhepatic cancers, which are found in greater rates in patients with NAFLD.39–41 Based on these data, it is possible to hypothesize that in NAFLD, regular physical activity could decrease the risk of developing HCC and several other extrahepatic cancers, however, at this time, there is no robust evidence directly linking those factors together and future studies are needed to confirm this.
BENEFITS OF PHYSICAL ACTIVITY
There are many well-established benefits of regular physical activity and, in particular, exercise training in patients with NAFLD and NASH, as detailed below.17 Importantly, some of these benefits may be independent of clinically significant weight loss,42,43 including improvements in liver fat (as determined by MRI), cardiovascular health, body composition, cardiorespiratory fitness, and health-related quality of life (HRQOL). Whether weight loss is required for histologic improvement with exercise training is controversial, however, in general, it is accepted that various thresholds of weight loss would be expected to lead to specific histologic changes in patients with NASH (Figure 2).44–46
FIGURE 2.
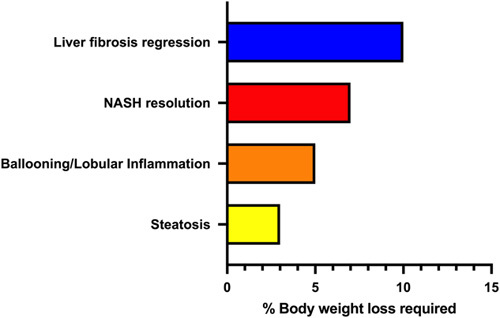
Expected change in liver histology in patients with NASH across different thresholds of body weight loss.
Reduction in liver fat content
The most widely studied and established benefit of exercise training in patients with NAFLD is an improvement in MRI-measured liver fat. Upwards of 25 studies have documented that exercise training can improve liver fat in adults with NAFLD, the majority of which appear to be independent of clinically significant weight loss.20,47–61 Importantly, these studies utilize many different types of exercise training programs, including aerobic training, resistance training, high-intensity interval training (HIIT), and aerobic and resistance training combined. Beyond this, individual studies have reported rates of liver fat reduction similar to that seen in early-phase NASH drug trials43 and at a threshold of response (a relative reduction of >30%) that may surrogate for corresponding histologic improvement in NASH activity and liver fibrosis stage.62,63
Histologic improvement (NAFLD activity score)
To date, 4 clinical trials have demonstrated exercise training alone, or in combination with dietary modification, can favorably influence liver histology in patients with NAFLD and NASH; however, these studies are limited by significant heterogeneity and sample size with <60 patients being studied in total.64–67 Following 3 months of aerobic exercise training, Hickman et al65 demonstrated steatosis and liver fibrosis stage improvement. Eckard et al66 measured change in liver histology following 6 months of moderate-intensity aerobic exercise and found no statistically significant improvement in NAFLD activity score. O’Gorman et al64 observed improvement in hepatocyte ballooning (67%) and liver fibrosis stage (58%) following 12 weeks of aerobic exercise training; however, no changes were seen in overall NAFLD activity score or 2 of its individual components in steatosis or lobular inflammation, raising questions about the clinical significance of the histologic change.68 Promrat et al67 combined 200 min/wk of unsupervised moderate-intensity aerobic exercise with a hypocaloric diet over 40 weeks and found nearly 75% of patients had NAS reduction or NASH resolution. Each of these studies that reported statistically significant changes in liver histology also observed concomitant reductions in body weight loss.64,65,67 Accordingly, it is currently unclear whether exercise training can independently improve liver histology in the absence of weight loss.
Cardiovascular health
CVD is a leading cause of death in patients with NAFLD and for this reason it remains a focus of tremendous research and clinical interest.69 The body of research about cardiovascular health and exercise in patients with NAFLD and NASH relates to the improvement of CVD biomarkers including improvements in endothelial dysfunction and changes in serum plasminogen activator inhibitor-1 concentration. Endothelial dysfunction leads to abnormal blood flow and the development of arterial plaque that over time can rupture and lead to arterial thrombosis.70,71 Independent of traditional CVD risk factors, endothelial dysfunction represents the earliest manifestation of atherosclerosis and is found globally in NAFLD.72–76 Green et al77 found a 16-week moderate-intensity aerobic exercise protocol reversed endothelial dysfunction suggesting this intervention may have benefit in the primary prevention of coronary artery disease in patients with NAFLD. Unfortunately, in their follow-up study,74 in patients who were no longer exercising 12 months after the intervention concluded, the improvement in endothelial dysfunction was no longer evident. In addition, the NASHFit study43 found a notable reduction in plasminogen activator inhibitor-1 compared with standard clinical care after 20 weeks of moderate-intensity aerobic exercise.
Change in body composition: adipose tissue volume and lean body mass
Multiple studies have demonstrated visceral adipose tissue is reduced with aerobic exercise training.43,73,78–82 Fewer studies explored the impact of exercise training on subcutaneous adipose tissue, however, the recent NASHFit study43 found a significant reduction in subcutaneous adipose tissue after 20 weeks of aerobic exercise training. Importantly, this was much more common in patients who also met the clinically significant threshold of relative reduction in MRI-measured liver fat.62,63 To date, no individual study has found a significant change in lean body mass with an exercise intervention, including those studies that used resistance training programs, albeit these were of short duration.43,83–87
Improvements in cardiorespiratory fitness
Patients with NAFLD have lower cardiorespiratory fitness (maximal oxygen uptake; VO2peak) than the general population.36,88 The majority of patients with NAFLD have a poor or very poor fitness level, independent of traditional metabolic risk factors and other hallmark predictors of fitness such as age, body weight, and sex.89 Importantly, cardiorespiratory fitness is predictive of mortality90–93 and may be associated with liver disease severity.36,94 It is widely accepted that exercise training improves cardiorespiratory fitness with multiple meta-analyses reporting a pooled increase in maximal oxygen uptake48,60,83 at the clinically significant threshold required to improve overall mortality.92 In addition, various exercise training modalities have all led to improvement in cardiorespiratory fitness including moderate-intensity aerobic exercise and HIIT.43,48,57,60,83
Improvement in HRQOL
Patients with NAFLD have low HRQOL in comparison to the general population95 and other types of chronic liver disease.96 While exercise training is known to improve HRQOL in the general population and in individuals with chronic disease, including diabetes,97,98 routine assessment of this has not traditionally been included in the design and conduct of exercise-based interventional trials outside of a recent study which demonstrated exercise training to lessen pain interference and strengthen social roles.43
Figure 3 summarizes each of the expected benefits of physical activity in patients with NAFLD.
FIGURE 3.
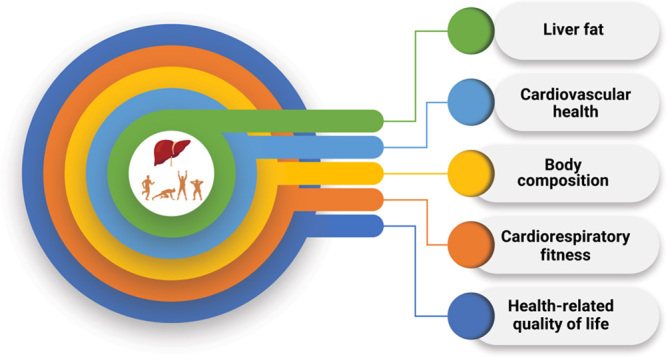
Expected benefits of physical activity in patients with NAFLD.
ASSESSMENT AND SCREENING FOR PHYSICAL ACTIVITY
Several research-based tools have been validated for the routine assessment of physical activity in the general population. These tools include the Get Active Questionnaire,99 International Physical Activity Questionnaire,100 and Physical Activity Readiness Questionnaire.101 Because these tools can be time-consuming in clinical practice, the ACSM developed the 2 question Physical Activity Vital Sign,102,103 (https://exerciseismedicine.org/wp-content/uploads/2021/04/EIM-Physical-Activity-Vital-Sign.pdf) which calculates the volume of moderate-to-vigorous physical activity completed (Table 1). Importantly, the Physical Activity Vital Sign can be readily integrated into the electronic health record and if elected, can assess the number of days per week spent performing muscle-strengthening exercises. Whether these tools are cost-effective at the population level or lead to direct improvement in clinical outcomes in patients with NAFLD remains unknown.
TABLE 1.
American College of Sports Medicine (ACSM) Physical Activity Vital Sign102,103 (https://exerciseismedicine.org/wp-content/uploads/2021/04/EIM-Physical-Activity-Vital-Sign.pdf)
| 1. On average, how many days per week do you engage in moderate to vigorous physical activity (like a brisk walk)? | —— days |
| 2. On average, how many minutes do you engage in physical activity at this level? | —— minutes |
| Total minutes per week of physical activity (multiple #1 by #2) | —— minutes per week |
Screening for barriers preventing physical activity should also be routinely performed in patients with NAFLD. While no one optimal tool has been identified, the Screening, Brief Intervention, and Referral to Treatment (SBIRT) may be considered.104 In the general population, the Centers for Disease Control and Prevention (CDC) has identified 8 common barriers to physical activity including a lack of time, social support, energy, motivation, or skill and also high cost, fear of injury, and poor weather conditions.105 Patients with NAFLD have similar self-reported barriers, although a lack of knowledge about exercise prescription in health care providers was also identified as a barrier to physical activity.10 Key unanswered questions remain about physical activity barrier assessment including what the optimal interval of assessment is and how health care providers can tackle the barriers effectively. In addition, data on the impact of assessment on clinical outcomes are lacking.
COUNSELING ABOUT PHYSICAL ACTIVITY
When counseling patients about physical activity, it is first important to understand what factors (barriers or enablers) determine whether a person engages or not in behavior. A patient’s capabilities, opportunities, and motivation all play a role.106 An understanding within and across different levels of social-ecological influence is necessary to identify which of those determinants are related to the change in behavior. When considering specific behavior change techniques and interventions for patients with NAFLD, there is a dearth of scientific literature. What exists focuses largely on validating exercise as a behavioral target for improving patient health through evidence that supervised exercise training improves fitness and NAFLD-related biomarkers of treatment response.107
When considering a recommendation for health care providers to counsel patients on exercise, there are no efficacy or comparative effectiveness trials of physician counseling about the benefits of physical activity among patients with NAFLD. However, in non-NAFLD populations with metabolic disease, physician counseling, including the importance of engaging multidisciplinary care teams to promote physical activity has been studied.108 Nevertheless, few studies have looked at the efficacy of these efforts perhaps because counseling and education alone are rarely enough to enact long-term lifestyle change. The use of patient-centered language should be encouraged within all counseling sessions. Stigmatizing language about body weight and body image should be avoided.109 Unfortunately, nearly three-fourth of patients with NAFLD reported experiencing stereotypical judgments, discrimination, shame, and social isolation at one point in time,110 which can lead to poor HRQOL and a reduction in help-seeking behavior.
Several techniques have been used to promote behavior change in patients with NAFLD. Motivational interviewing, which is a technique that is feasible to utilize within a 20-minute clinic visit, can empower patients with NAFLD to make individual health-related decisions,111 however, the majority of health care providers are not trained in this technique.112,113 Despite this lack of widespread training, motivational interviewing has been used in patients with NAFLD as a way to reduce body weight through dietary change and increased physical activity.114 Cognitive behavioral therapy is an additional technique that has also been used successfully in patients with NAFLD to increase physical activity, with gains in fitness and loss of body weight that can persist for up to 2 years.115 Lifestyle intervention based on social-cognitive theory, which explains how an individual’s motivation derives from the reciprocal interactions between a person, their behavior, and their environment that shape a person’s beliefs and goals, has also been applied to promote physical activity in patients with NAFLD.116,117 Print and web-based patient education materials have been successful modes for promoting health behavior change in patients without NAFLD,118 however, in patients with NAFLD, including those from the ACSM EIM initiative, there is a lack of evidence about the effectiveness of providing these materials. Critically, these modes are just vehicles for delivering intervention content. The degree to which that content exposes patients to evidence-based behavior change techniques will account for specific mode effects on behavior change.
Patients with advanced liver disease, including those with cirrhosis, represent a unique patient population that needs to be viewed independently of patients with earlier stage disease. A recent systematic review summarized the 11 small clinical trials limited exclusively to patients with cirrhosis, including 4 studies which used home-based interventions,119 and suggests exercise training to be feasible and safe in patients with cirrhosis, including those whose primary etiology of liver disease is NASH.120–123 In terms of efficacy, individual studies have shown regular exercise training can decrease portal hypertension,120,124 and may also improve physical performance, frailty, and HRQOL.121,122,125–127 However, these studies did not enroll patients with decompensated cirrhosis and the feasibility, safety, and efficacy of exercise training in this patient population remains unknown and we look to future and ongoing research to help answer this key question.
REFERRING A PATIENT WITH NAFLD TO AN EXERCISE SPECIALIST
Because physicians are not routinely trained in exercise prescription during their medical education, we propose that the exercise specialist should be a key member of the NAFLD treatment team. Avery et al128 performed structured interviews with health care professionals and patients with NAFLD and found the majority of physicians felt ill-equipped to address lifestyle behavior change with their patients with NAFLD, choosing to monitor rather than actively manage dietary change and physical activity. Patients felt a multidisciplinary team including an exercise specialist, rather than an individual physician, would offer personalized support with the goal of achieving long-term behavior change.
Once a patient with NAFLD is referred to an exercise specialist, a key focus of the assessment should be to evaluate the patient’s understanding of what NAFLD is and how it relates to their lifestyle behaviors. Additional factors to consider include (1) the patient’s goals for treatment; (2) measurement of current and historical levels of physical activity; (3) assessment of cardiometabolic risk factors, comorbidities, past medical history, and current medications; (4) evaluation of baseline levels of cardiorespiratory fitness as well as functional and exercise capacity; and (5) identification of barriers and facilitators to physical activity participation.
To assist the exercise specialist, the ACSM has established guidelines to determine when a medical referral is recommended before starting an exercise training program.129 These guidelines rely on the following: current exercise participation; history and symptoms of cardiovascular, metabolic, or renal disease; and the desired exercise intensity for the person who wants to initiate a physical activity program. Because the vast majority of patients with NAFLD do not exercise regularly and have a history of either metabolic or CVD, medical guidance from their treating physician may be required before starting an exercise program.
When the exercise specialist is designing a training program for patients with NAFLD, the most important consideration is how to tailor exercise for each individual, taking into account baseline capabilities, comorbidities, and personal preferences. Once an exercise program has commenced, there are many management priorities the exercise specialist should be aware of. These include targeting improvement in body weight/body composition, glycemic control, CVD risk factors (eg, blood pressure), cardiorespiratory fitness as well as functional and exercise capacity. The exercise specialist can also play a key role in counseling patients with NAFLD on alcohol intake, smoking cessation, and sleep quality and duration and may be a central person to initiate a referral to other members of the multidisciplinary care team including a dietitian or psychologist where appropriate.
CONSIDERATIONS FOR PRESCRIBING AN EXERCISE TRAINING PROGRAM
Exercise training is well-established as a key component in the clinical management of patients with NAFLD. Previous large systematic reviews, including a recent Cochrane review and those with quantitative meta-analysis, have examined the individual components of the ACSM FITT exercise prescription, including frequency, intensity, time, and type.47,57,107,130 Each has found significant heterogeneity owing to variation in the FITT principles. Across the primary literature, exercise frequency ranges from 3 to 7 days/wk; intensities that have been studied include low, moderate, moderate-vigorous, and vigorous. Time in each exercise bout is also widely variable and ranges from 20 to 60 minutes. Exercise types which have been studied include aerobic exercise training, resistance training, combined aerobic with resistance training, HIIT, and pilates. Moreover, almost all exercise interventions have been carried out under direct supervision.
Despite decades of research, no single optimal exercise prescription has been defined for patients with NAFLD. While the majority of studies have used aerobic exercise training and head-to-head direct comparisons of different exercise modalities are generally lacking, indirect evidence suggests that resistance training may have a role.20 A recent meta-analysis by Hashida et al20 found equivalent improvement in clinical outcomes when comparing similar volumes of resistance training to aerobic exercise. Importantly, resistance training required less energy consumption and can be considered in patients who cannot tolerate or participate in aerobic exercise programs. Along the same lines, Sabag et al57 performed a meta-analysis and compared HIIT to moderate-intensity aerobic exercise training and found both exercise types to reduce liver fat in similar amounts. The appropriateness of other types of physical activity including yoga and Pilates remains uncertain.131,132
While most exercise training programs for patients with NAFLD have relied on in-person supervised training, several recent studies have explored the role of telehealth as a means to increase adherence and access to exercise programs. Huber et al133 found an 8-week web-based exercise program to improve cardiorespiratory fitness, liver biochemistries, and transient elastography-measured liver fat. Motz et al134 used audiovisual telehealth technology to directly supervise a small group of patients with biopsy-proven NASH in a 20-week aerobic exercise training program. No adverse events were observed and clinical benefits exceeded those reported for traditional in-person aerobic exercise or resistance training programs. Other studies have used unsupervised, in-person resistance training135 and found that after 3 months, improvements in hepatic fat content were accompanied by favorable changes in body composition.
FUTURE DIRECTIONS—KEY KNOWLEDGE GAPS AND LIMITATIONS IN THE CURRENT SCIENTIFIC LITERATURE
Following a review of the available primary literature, the ACSM Roundtable identified key knowledge gaps in the existing scientific literature in terms of (1) the role of physical activity in NAFLD pathogenesis; (2) assessing and screening patients with NAFLD for physical activity; (3) advising and counseling patients with NAFLD about the benefits of physical activity; (4) physical activity recommendations in patients with NAFLD; and (5) referring a patient with NAFLD to an exercise specialist (Table 2).
TABLE 2.
Key knowledge gaps that remain about the relationship between NAFLD and physical activity
| Role of physical activity in NAFLD pathogenesis: |
| 1. What are the mechanisms underlying the suggested benefit of physical activity in patients with NAFLD? |
| 2. What role does the microbiome play in the association between physical activity and NAFLD? |
| Assessing and screening patients with NAFLD for physical activity: |
| 3. What is the most appropriate setting for physical activity screening and/or intervention, primary care or specialist (eg, hepatologist) clinic? |
| 4. In patients who are physically inactive, should physical function or frailty testing be routinely performed? |
| 5. Should patients with NAFLD be routinely screened for sarcopenia? |
| Advising and counseling patients with NAFLD about the benefits of physical activity: |
| 6. How can rates of lifestyle counseling be increased in the primary care and specialist setting? |
| 7. What is the minimum amount of physical activity at varying intensities that a patient with NAFLD needs to complete in order to achieve clinically meaningful benefit? |
| 8. What is the role of personalized medicine in exercise prescription in patients with NAFLD? |
| 9. Do patients with NAFLD, who are more physically active, experience greater treatment responses when exercise is prescribed in combination with pharmacologic therapy for NAFLD? |
| Physical activity recommendations in patients with NAFLD: |
| 10. What is the most health-enhancing physical activity prescription for patients with NAFLD? |
| 11. Does sustained physical activity directly lead to improvement in long-term outcomes such as cardiovascular disease events, liver and extrahepatic cancer, major adverse liver outcomes, or death? |
| 12. What are predictors (eg, genetic, physiological, psychological) of exercise response in patients with NAFLD? |
| 13. Is home-based/virtually supervised exercise as effective as supervised in-person exercise in patients with NAFLD? |
| 14. Does the addition of improved diet quality lead to greater clinically meaningful benefit of physical activity? |
| 15. Does the addition of pharmacologic obesity treatment as an adjunct to physical activity improve liver-related and non–liver-related outcomes (eg, CVD)? |
| 16. What factors are associated with patients developing motivation for physical activity, initiating an increase in physical activity and sustaining physical activity over time? |
| Referring a patient with NAFLD to an exercise specialist: |
| 17. How can suboptimal rates of referral to exercise specialists be increased? |
| 18. How can we best ascertain which patients would benefit the most from referral to an exercise specialist (eg, all patients versus those with either early stage or advanced liver disease)? |
| 19. Will engaging multiple stakeholders, including patients, lead to greater rates of exercise specialist referral? |
| 20. Will closer multidisciplinary care with an exercise specialist in the same clinical space improve patient-oriented outcomes? |
Abbreviation: CVD, cardiovascular disease.
Because liver fibrosis is closely related with long-term outcomes in patients with NASH, histologic improvement remains the goal of all NASH clinical trials.136,137 At this point in time, it is unclear whether exercise intervention can improve liver histology without clinically significant weight loss. Determining the independent impact of an exercise intervention on liver histology remains a clear, unmet need of high significance and even greater impact and should be prioritized by research funding and public health agencies as a way to answer this important question.
Many of these knowledge gaps and key unanswered questions remain either from a lack of investigation or from limitations inherent to the scientific literature where included populations across all study designs are heterogenous and often ill-defined. In fact, the majority of interventional studies include all stages of NAFLD with only 3 trials limited only to NASH.43,67,85 Moreover, there is a lack of standardization of exercise intervention, where exercise training programs vary significantly in components of FITT exercise prescription or measurement of adherence and clinical outcomes. The behavioral science literature is even more limited where the literature is largely limited to small, observational studies with a high risk of bias.
In light of these limitations, we look to future grant funding mechanisms to support the research of the highest rigor to answer each and every one of the remaining key knowledge gaps (Table 2).
CONCLUSIONS
An expert panel reviewed the published scientific evidence and came to a consensus regarding the role of physical activity in patients with NAFLD. The evidence supports that regular physical activity is associated with decreased risk of NAFLD development and that low physical activity is associated with a greater risk for disease progression and extrahepatic cancer. Physical activity screening during routine health care visits in all patients with NAFLD would seem prudent. Moreover, this visit offers a unique opportunity to open a discussion about the many benefits of regular physical activity, many of which may be weight loss independent. If weight loss does occur, this can provide additional benefits. Currently, at least 150 min/wk of moderate or 75 min/wk of vigorous-intensity physical activity are recommended for all patients with NAFLD. If a formal exercise program is to be used, the combination of aerobic plus resistance training is preferred, albeit emerging data suggests that HIIT can be considered in select patients instead. Multiple research gaps remain in this field, including the need for studies exploring the mechanistic multiorgan underpinnings of exercise’s benefit, best counseling practices, exercise dosing, and predictors of exercise response. Health care, fitness, and public health professionals are strongly encouraged to disseminate the information in this report and to encourage and support all patients with NAFLD to be as physically active as their age, abilities, and environment will allow. Future research should prioritize determining optimal strategies for promoting physical activity among individuals at risk and in those already diagnosed with NAFLD.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge Yuri Feito and Laura Young from ACSM for their contributions to organizing the RT and providing manuscript preparation support. They also acknowledge Brigid Sanner for her writing assistance.
FUNDING INFORMATION
This work was funded by the American College of Sports Medicine.
CONFLICTS OF INTEREST
Jonathan G. Stine received research funding from the NIH and also from Astra Zeneca, Galectin, Grifols Inc., Noom Inc., Novo Nordisk, and Zydus. Michelle T. Long works full-time for Novo Nordisk; however, at the time of the roundtable, she worked full-time for Boston University. David E. Conroy received research funding from the NIH, NSF, and AICR; consulting fees unrelated to this work from WW International Inc. and InsideTracker LLC. Daniel J. Cuthbertson received research funding from BMS, Novo Nordisk, and Astra Zeneca; consulting fees from Astra Zeneca and Ipsen; educational support from Perspectum. Alina M. Allen received research funding from Pfizer, Novo Nordisk, Target Pharma; consulting fees from Novo Nordisk. Matthew J. Armstrong received consulting fees from Novo Nordisk and Norgine. Vincent Wai-Sun Wong received consulting fees from AbbVie, Boehringer Ingelheim, Echosens, Gilead Sciences, Intercept, Inventiva, Novo Nordisk, Pfizer, Sagimet Biosciences, and TARGET PharmaSolutions; research funding from Gilead Sciences; co-founder of Illuminatio Medical Technology Limited. Yaron Rotman was supported by the Intramural Research Program of NIDDK; received research funding from Gilead Sciences. Shelley E. Keating received research funding from National Health and Medical Research Council (NHMRC; Early Career Fellowship 1122190), Diabetes Australia, Exercise and Sports Science Australia. The remaining authors have no conflicts to report.
Footnotes
Abbreviations: AASLD, American Association for the Study of Liver Diseases; ACG, American College of Gastroenterology; ACSM, American College of Sports Medicine; AGA, American Gastroenterology Association; BP, blood pressure; CDC, Centers for Disease Control and Prevention; CVD, cardiovascular disease; EASL, European Association for the Study of the Liver; EIM, Exercise is Medicine; FITT, frequency, intensity, time, and type; HIIT, high-intensity interval training; HRQOL, health-related quality of life; HR, heart rate; SBIRT, Screening, Brief Intervention, and Referral to Treatment; VO2peak, peak oxygen uptake.
This article is endorsed by the American Association for the Study of Liver Diseases.
Contributor Information
Jonathan G. Stine, Email: jstine@pennstatehealth.psu.edu.
Michelle T. Long, Email: mtlong@bu.edu.
Kathleen E. Corey, Email: Kathleen.Corey@MGH.HARVARD.EDU.
Robert E. Sallis, Email: Robert.E.Sallis@kp.org.
Alina M. Allen, Email: allen.alina@mayo.edu.
Matthew J. Armstrong, Email: mattyarm2010@googlemail.com.
David E. Conroy, Email: conroy@psu.edu.
Daniel J. Cuthbertson, Email: dan.cuthbertson@liverpool.ac.uk.
Andres Duarte-Rojo, Email: andres.duarte@northwestern.edu.
Kate Hallsworth, Email: kate.hallsworth@ncl.ac.uk.
Ingrid J. Hickman, Email: i.hickman@uq.edu.au.
Matthew R. Kappus, Email: matthew.kappus@duke.edu.
Shelley E. Keating, Email: s.keating@uq.edu.au.
Christopher J.A. Pugh, Email: cjpugh@cardiffmet.ac.uk.
Yaron Rotman, Email: rotmany@niddk.nih.gov.
Tracey G. Simon, Email: tgsimon@mgh.harvard.edu.
Eduardo Vilar-Gomez, Email: evilar@iu.edu.
Vincent Wai-Sun Wong, Email: wongv@cuhk.edu.hk.
Kathryn H. Schmitz, Email: schmitzk@upmc.edu.
REFERENCES
- 1.Le MH, Yeo YH, Li X, Li J, Zou B, Wu Y, et al. 2019 Global NAFLD prevalence: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2021;20:2809–2817.e28. [DOI] [PubMed] [Google Scholar]
- 2.Cheemerla S, Balakrishnan M. Global epidemiology of chronic liver disease. Clin Liver Dis (Hoboken). 2021;17:365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen AM, Therneau TM, Larson JJ, Coward A, Somers VK, Kamath PS. Nonalcoholic fatty liver disease incidence and impact on metabolic burden and death: a 20 year-community study. Hepatology. 2018;67:1726–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parikh ND, Marrero WJ, Wang J, Steuer J, Tapper EB, Konerman M, et al. Projected increase in obesity and non-alcoholic-steatohepatitis-related liver transplantation waitlist additions in the United States. Hepatology. 2019;70:487–495. [DOI] [PubMed] [Google Scholar]
- 5.Loomba R, Lim JK, Patton H, El-Serag HB. AGA clinical practice update on screening and surveillance for hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: expert review. Gastroenterology. 2020;158:1822–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126–131. [PMC free article] [PubMed] [Google Scholar]
- 7.European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. [DOI] [PubMed] [Google Scholar]
- 8.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. [DOI] [PubMed] [Google Scholar]
- 9.Anstee QM, Hallsworth K, Lynch N, Hauvespre A, Mansour E, Kozma S, et al. Real-world management of non-alcoholic steatohepatitis differs from clinical practice guideline recommendations and across regions. JHEP Rep. 2022;4:100411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stine JG, Soriano C, Schreibman I, Rivas G, Hummer B, Yoo E, et al. Breaking down barriers to physical activity in patients with nonalcoholic fatty liver disease. Dig Dis Sci. 2021;66:3604–3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American College of Sports Medicine (ACSM)—Exercise is Medicine (EIM). Being active when you have NAFLD. Accessed July 7 2022. https://www.exerciseismedicine.org/assets/page_documents/EIM_Rx%20for%20Health_NAFLD.pdf.
- 12.Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65:1038–1048. [DOI] [PubMed] [Google Scholar]
- 13.Hughes A, Dahmus J, Rivas G, Hummer B, Chen See JR, Wright JR, et al. Exercise training reverses gut dysbiosis in patients with biopsy-proven nonalcoholic steatohepatitis: a proof of concept study. Clin Gastroenterol Hepatol. 2021;19:1723–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piguet AC, Guarino M, Potaczek DP, Garn H, Dufour JF. Hepatic gene expression in mouse models of non-alcoholic fatty liver disease after acute exercise. Hepatol Res. 2019;49:637–652. [DOI] [PubMed] [Google Scholar]
- 15.Melo L, Bilici M, Hagar A, Klaunig JE. The effect of endurance training on non-alcoholic fatty liver disease in mice. Physiol Rep. 2021;9:e14926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babu AF, Csader S, Männistö V, Tauriainen MM, Pentikäinen H, Savonen K, et al. Effects of exercise on NAFLD using non-targeted metabolomics in adipose tissue, plasma, urine, and stool. Sci Rep. 2022;12:6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thorp A, Stine JG. Exercise as medicine: the impact of exercise training on nonalcoholic fatty liver disease. Curr Hepatol Rep. 2020;19:402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao H. Adipocytokines in obesity and metabolic disease. J Endocrinol. 2014;220:T47–T59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giby VG, Ajith TA. Role of adipokines and peroxisome proliferator-activated receptors in nonalcoholic fatty liver disease. World J Hepatol. 2014;6:570–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashida R, Kawaguchi T, Bekki M, Omoto M, Matsuse H, Nago T, et al. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: a systematic review. J Hepatol. 2017;66:142–152. [DOI] [PubMed] [Google Scholar]
- 21.Diniz TA, de Lima Junior EA, Teixeira AA, Biondo LA, da Rocha LAF, Valadão IC, et al. Aerobic training improves NAFLD markers and insulin resistance through AMPK-PPAR-α signaling in obese mice. Life Sci. 2020;266:118868. [DOI] [PubMed] [Google Scholar]
- 22.Shi X, Yin H, Li J, Huang C, Chen Y, Chen Z, et al. Circulating branch chain amino acids and improvement in liver fat content in response to exercise interventions in NAFLD. Sci Rep. 2021;11:13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng R, Wang L, Le S, Yang Y, Zhao C, Zhang X, et al. A randomized controlled trial for response of microbiome network to exercise and diet intervention in patients with nonalcoholic fatty liver disease. Nat Commun. 2022;13:2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou J, Tripathi M, Sinha RA, Singh BK, Yen PM. Gut microbiota and their metabolites in the progression of non-alcoholic fatty liver disease. Hepatoma Res. 2021;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loomba R, Seguritan V, Li W, Long T, Klitgord N, Bhatt A, et al. Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab. 2017;25:1054–1062.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim D, Konyn P, Cholankeril G, Ahmed A. Physical activity is associated with nonalcoholic fatty liver disease and significant fibrosis measured by FibroScan. Clin Gastroenterol Hepatol. 2022;20:e1438–e1455. [DOI] [PubMed] [Google Scholar]
- 27.Kim D, Murag S, Cholankeril G, Cheung A, Harrison SA, Younossi ZM, et al. Physical activity, measured objectively, is associated with lower mortality in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2021;19:1240–1247.e5. [DOI] [PubMed] [Google Scholar]
- 28.Zelber-Sagi S, Nitzan-Kaluski D, Goldsmith R, Webb M, Zvibel I, Goldiner I, et al. Role of leisure-time physical activity in nonalcoholic fatty liver disease: a population-based study. Hepatology. 2008;48:1791–1798. [DOI] [PubMed] [Google Scholar]
- 29.Schneider CV, Zandvakili I, Thaiss CA, Schneider KM. Physical activity is associated with reduced risk of liver disease in the prospective UK Biobank cohort. JHEP Rep. 2021;3:100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schnurr TM, Katz SF, Justesen JM, O’Sullivan JW, Saliba-Gustafsson P, Assimes TL, et al. Interactions of physical activity, muscular fitness, adiposity, and genetic risk for NAFLD. Hepatol Commun. 2022;6:1516–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwak JH, Jun DW, Lee SM, Cho YK, Lee KN, Lee HL, et al. Lifestyle predictors of obese and non-obese patients with nonalcoholic fatty liver disease: a cross-sectional study. Clin Nutr. 2018;37:1550–1557. [DOI] [PubMed] [Google Scholar]
- 32.Sung K-C, Lee M-Y, Lee J-Y, Lee S-H, Kim Y-B, Song W-J, et al. Natural course of fatty liver in 36,195 South Korean adults. Sci Rep. 2019;9:9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stine JG. Editorial: twenty minutes of moderate-to-vigorous physical activity a day keeps the NAFLD away. Aliment Pharmacol Ther. 2022;55:116–117. [DOI] [PubMed] [Google Scholar]
- 34.Tsunoda K, Kitano N, Kai Y, Jindo T, Uchida K, Arao T. Dose-response relationships of accelerometer-measured sedentary behaviour and physical activity with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2021;54:1330–1339. [DOI] [PubMed] [Google Scholar]
- 35.Long MT, Pedley A, Massaro JM, Hoffmann U, Esliger DW, Vasan RS, et al. Hepatic steatosis is associated with lower levels of physical activity measured via accelerometry. Obesity (Silver Spring). 2015;23:1259–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krasnoff JB, Painter PL, Wallace JP, Bass NM, Merriman RB. Health-related fitness and physical activity in patients with nonalcoholic fatty liver disease. Hepatology. 2008;47:1158–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hallsworth K, Thoma C, Moore S, Ploetz T, Anstee QM, Taylor R, et al. Non-alcoholic fatty liver disease is associated with higher levels of objectively measured sedentary behaviour and lower levels of physical activity than matched healthy controls. Frontline Gastroenterol. 2015;6:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bowden Davies KA, Sprung VS, Norman JA, Thompson A, Mitchell KL, Harrold JOA, et al. Physical activity and sedentary time: association with metabolic health and liver fat. Med Sci Sports Exerc. 2019;51:1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DiJoseph K, Thorp A, Harrington A, Schmitz KH, Chinchilli VM, Stine JG. Physical activity and risk of hepatocellular carcinoma: a systematic review and meta-analysis. Dig Dis Sci. 2022. doi: 10.1007/s10620-022-07601-w[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 40.Moore SC, Lee IM, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM, et al. Association of Leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med. 2016;176:816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mantovani A, Petracca G, Beatrice G, Csermely A, Tilg H, Byrne CD, et al. Non-alcoholic fatty liver disease and increased risk of incident extrahepatic cancers: a meta-analysis of observational cohort studies. Gut. 2022;71:778–788. [DOI] [PubMed] [Google Scholar]
- 42.Stine JGDK, Pattison Z, Harrington A, Chinchilli VM, Schmitz KH, Loomba R. Exercise training is associated with treatment response in liver fat content by magnetic resonance imaging independent of clinically significant body weight loss in patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Am J Gastroenterol. 2022. doi: 10.14309/ajg.0000000000002098. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stine JG, Schreibman IR, Faust AJ, Dahmus J, Stern B, Soriano C, et al. NASHFit: a randomized controlled trial of an exercise training program to reduce clotting risk in patients with NASH. Hepatology. 2021;76:172–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149:367–378.e5; quiz e14–5. [DOI] [PubMed] [Google Scholar]
- 45.Wong VW, Chan RS, Wong GL, Cheung BH, Chu WC, Yeung DK, et al. Community-based lifestyle modification programme for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol. 2013;59:536–542. [DOI] [PubMed] [Google Scholar]
- 46.Harrison SA, Fecht W, Brunt EM, Neuschwander-Tetri BA. Orlistat for overweight subjects with nonalcoholic steatohepatitis: a randomized, prospective trial. Hepatology. 2009;49:80–86. [DOI] [PubMed] [Google Scholar]
- 47.Babu AF, Csader S, Lok J, Gómez-Gallego C, Hanhineva K, El-Nezami H, et al. Positive effects of exercise intervention without weight loss and dietary changes in NAFLD-related clinical parameters: a systematic review and meta-analysis. Nutrients. 2021;13:3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baker CJ, Martinez-Huenchullan SF, D’Souza M, Xu Y, Li M, Bi Y, et al. Effect of exercise on hepatic steatosis: are benefits seen without dietary intervention? A systematic review and meta-analysis. J Diabetes. 2021;13:63–77. [DOI] [PubMed] [Google Scholar]
- 49.Battista F, Ermolao A, van Baak MA, Beaulieu K, Blundell JE, Busetto L, et al. Effect of exercise on cardiometabolic health of adults with overweight or obesity: Focus on blood pressure, insulin resistance, and intrahepatic fat—a systematic review and meta-analysis. Obes Rev. 2021;22(suppl 4):e13269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hens W, Taeyman J, Cornelis J, Gielen J, Van Gaal L, Vissers D. The effect of lifestyle interventions on excess ectopic fat deposition measured by noninvasive techniques in overweight and obese adults: a systematic review and meta-analysis. J Phys Act Health. 2016;13:671–694. [DOI] [PubMed] [Google Scholar]
- 51.Katsagoni CN, Georgoulis M, Papatheodoridis GV, Panagiotakos DB, Kontogianni MD. Effects of lifestyle interventions on clinical characteristics of patients with non-alcoholic fatty liver disease: a meta-analysis. Metabolism. 2017;68:119–132. [DOI] [PubMed] [Google Scholar]
- 52.Keating SE, Hackett DA, George J, Johnson NA. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57:157–166. [DOI] [PubMed] [Google Scholar]
- 53.Khalafi M, Symonds ME. The impact of high intensity interval training on liver fat content in overweight or obese adults: a meta-analysis. Physiol Behav. 2021;236:113416. [DOI] [PubMed] [Google Scholar]
- 54.Mohammad Rahimi GR, Attarzadeh, Hosseini SR. Effect of aerobic exercise alone or in conjunction with diet on liver function, insulin resistance and lipids in non-alcoholic fatty liver disease. Biol Res Nurs. 2022;24:259–276. [DOI] [PubMed] [Google Scholar]
- 55.Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia. 2012;55:885–904. [DOI] [PubMed] [Google Scholar]
- 56.Orci LA, Gariani K, Oldani G, Delaune V, Morel P, Toso C. Exercise-based interventions for nonalcoholic fatty liver disease: a meta-analysis and meta-regression. Clin Gastroenterol Hepatol. 2016;14:1398–1411. [DOI] [PubMed] [Google Scholar]
- 57.Sabag A, Barr L, Armour M, Armstrong A, Baker CJ, Twigg SM, et al. The effect of high-intensity interval training vs moderate-intensity continuous training on liver fat: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2022;107:862–881. [DOI] [PubMed] [Google Scholar]
- 58.Sargeant JA, Gray LJ, Bodicoat DH, Willis SA, Stensel DJ, Nimmo MA, et al. The effect of exercise training on intrahepatic triglyceride and hepatic insulin sensitivity: a systematic review and meta-analysis. Obes Rev. 2018;19:1446–1459. [DOI] [PubMed] [Google Scholar]
- 59.Smart NA, King N, McFarlane JR, Graham PL, Dieberg G. Effect of exercise training on liver function in adults who are overweight or exhibit fatty liver disease: a systematic review and meta-analysis. Br J Sports Med. 2018;52:834–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fernández T, Viñuela M, Vidal C, Barrera F. Lifestyle changes in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis. PLoS One. 2022;17:e0263931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Golabi P, Locklear CT, Austin P, Afdhal S, Byrns M, Gerber L, et al. Effectiveness of exercise in hepatic fat mobilization in non-alcoholic fatty liver disease: systematic review. World J Gastroenterol. 2016;22:6318–6327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stine JG, Munaganuru N, Barnard A, Wang JL, Kaulback K, Argo CK, et al. Change in MRI-PDFF and histologic response in patients with nonalcoholic steatohepatitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2021;19:2274–2283.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tamaki N, Munaganuru N, Jung J, Yonan AQ, Loomba RR, Bettencourt R, et al. Clinical utility of 30% relative decline in MRI-PDFF in predicting fibrosis regression in non-alcoholic fatty liver disease. Gut. 2022;71:983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O’Gorman P, Naimimohasses S, Monaghan A, Kennedy M, Melo AM, NF D, et al. Improvement in histological endpoints of MAFLD following a 12-week aerobic exercise intervention. Aliment Pharmacol Ther. 2020;52:1387–1398. [DOI] [PubMed] [Google Scholar]
- 65.Hickman IJ, Jonsson JR, Prins JB, Ash S, Purdie DM, Clouston AD, et al. Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut. 2004;53:413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eckard C, Cole R, Lockwood J, Torres DM, Williams CD, Shaw JC, et al. Prospective histopathologic evaluation of lifestyle modification in nonalcoholic fatty liver disease: a randomized trial. Therap Adv Gastroenterol. 2013;6:249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stine JG, Schmitz KH. Letter: proving the benefit of exercise intervention in non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2020;52:1424–1425. [DOI] [PubMed] [Google Scholar]
- 69.Simon TG, Roelstraete B, Khalili H, Hagström H, Ludvigsson JF. Mortality in biopsy-confirmed nonalcoholic fatty liver disease: results from a nationwide cohort. Gut. 2021;70:1375–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee SB, Park GM, Lee JY, Lee BU, Park JH, Kim BG, et al. Association between non-alcoholic fatty liver disease and subclinical coronary atherosclerosis: an observational cohort study. J Hepatol. 2018;68:1018–1024. [DOI] [PubMed] [Google Scholar]
- 71.Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension. 2011;57:363–369. [DOI] [PubMed] [Google Scholar]
- 72.Gonzalez-Paredes FJ, Hernandez Mesa G, Morales Arraez D, Marcelino Reyes R, Abrante B, Diaz-Flores F, et al. Contribution of cyclooxygenase end products and oxidative stress to intrahepatic endothelial dysfunction in early non-alcoholic fatty liver disease. PLoS One. 2016;11:e0156650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pugh CJ, Spring VS, Kemp GJ, Richardson P, Shojaee-Moradie F, Umpleby AM, et al. Exercise training reverses endothelial dysfunction in nonalcoholic fatty liver disease. Am J Physiol Heart Circ Physiol. 2014;307:H1298–H1306. [DOI] [PubMed] [Google Scholar]
- 74.Pugh CJ, Sprung VS, Jones H, Richardson P, Shojaee-Moradie F, Umpleby AM, et al. Exercise-induced improvements in liver fat and endothelial function are not sustained 12 months following cessation of exercise supervision in nonalcoholic fatty liver disease. Int J Obes (Lond). 2016;40:1927–1930. [DOI] [PubMed] [Google Scholar]
- 75.Sapmaz F, Uzman M, Basyigit S, Ozkan S, Yavuz B, Yeniova A, et al. Steatosis grade is the most important risk factor for development of endothelial dysfunction in NAFLD. Medicine (Baltimore). 2016;95:e3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thakur ML, Sharma S, Kumar A, Bhatt SP, Luthra K, Guleria R, et al. Nonalcoholic fatty liver disease is associated with subclinical atherosclerosis independent of obesity and metabolic syndrome in Asian Indians. Atherosclerosis. 2012;223:507–511. [DOI] [PubMed] [Google Scholar]
- 77.Green DJ, Maiorana A, O’Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol. 2004;561:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Keating SE, Hackett DA, Parker HM, O’Connor HT, Gerofi JA, Sainsbury A, et al. Effect of aerobic exercise training dose on liver fat and visceral adiposity. J Hepatol. 2015;63:174–182. [DOI] [PubMed] [Google Scholar]
- 79.Zhang HJ, He J, Pan LL, Ma ZM, Han CK, Chen CS, et al. Effects of moderate and vigorous exercise on nonalcoholic fatty liver disease: a randomized clinical trial. JAMA Intern Med. 2016;176:1074–1082. [DOI] [PubMed] [Google Scholar]
- 80.Bacchi E, Negri C, Targher G, Faccioli N, Lanza M, Zoppini G, et al. Both resistance training and aerobic training reduce hepatic fat content in type 2 diabetic subjects with nonalcoholic fatty liver disease (the RAED2 Randomized Trial). Hepatology. 2013;58:1287–1295. [DOI] [PubMed] [Google Scholar]
- 81.Abdelbasset WK, Tantawy SA, Kamel DM, Alqahtani BA, Elnegamy TE, Soliman GS, et al. Effects of high-intensity interval and moderate-intensity continuous aerobic exercise on diabetic obese patients with nonalcoholic fatty liver disease: a comparative randomized controlled trial. Medicine (Baltimore). 2020;99:e19471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cuthbertson DJ, Shojaee-Moradie F, Sprung VS, Jones H, Pugh CJ, Richardson P, et al. Dissociation between exercise-induced reduction in liver fat and changes in hepatic and peripheral glucose homoeostasis in obese patients with non-alcoholic fatty liver disease. Clin Sci (Lond). 2016;130:93–104. [DOI] [PubMed] [Google Scholar]
- 83.Gonzalez A, Valero-Breton M, Huerta-Salgado C, Achiardi O, Simon F, Cabello-Verrugio C. Impact of exercise training on the sarcopenia criteria in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Eur J Transl Myol. 2021;31:9630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hallsworth K, Fattakhova G, Hollingsworth KG, Thoma C, Moore S, Taylor R, et al. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut. 2011;60:1278–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Houghton D, Thoma C, Hallsworth K, Cassidy S, Hardy T, Burt AD, et al. Exercise reduces liver lipids and visceral adiposity in patients with nonalcoholic steatohepatitis in a randomized controlled trial. Clin Gastroenterol Hepatol. 2017;15:96–102.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sullivan S, Kirk EP, Mittendorfer B, Patterson BW, Klein S. Randomized trial of exercise effect on intrahepatic triglyceride content and lipid kinetics in nonalcoholic fatty liver disease. Hepatology. 2012;55:1738–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cheng S, Ge J, Zhao C, Le S, Yang Y, Ke D, et al. Effect of aerobic exercise and diet on liver fat in pre-diabetic patients with non-alcoholic-fatty-liver-disease: a randomized controlled trial. Sci Rep. 2017;7:15952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sayiner M, Stepanova M, Pham H, Noor B, Walters M, Younossi ZM. Assessment of health utilities and quality of life in patients with non-alcoholic fatty liver disease. BMJ Open Gastroenterol. 2016;3:e000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Argo CK, Stine JG, Henry ZH, Lackner C, Patrie JT, Weltman AL, et al. Physical deconditioning is the common denominator in both obese and overweight subjects with nonalcoholic steatohepatitis. Aliment Pharmacol Ther. 2018;48:290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Clausen JSR, Marott JL, Holtermann A, Gyntelberg F, Jensen MT. Midlife cardiorespiratory fitness and the long-term risk of mortality: 46 years of follow-up. J Am Coll Cardiol. 2018;72:987–995. [DOI] [PubMed] [Google Scholar]
- 91.Strasser B, Burtscher M. Survival of the fittest: VO2max, a key predictor of longevity? Front Biosci. 2018;23:1505–1516. [DOI] [PubMed] [Google Scholar]
- 92.Williams PT. Physical fitness and activity as separate heart disease risk factors: a meta-analysis. Med Sci Sports Exerc. 2001;33:754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Croci I, Coombes JS, Bucher Sandbakk S, Keating SE, Nauman J, Macdonald GA, et al. Non-alcoholic fatty liver disease: prevalence and all-cause mortality according to sedentary behaviour and cardiorespiratory fitness. The HUNT Study. Prog Cardiovasc Dis. 2019;62:127–134. [DOI] [PubMed] [Google Scholar]
- 94.Canada JM, Abbate A, Collen R, Billingsley H, Buckley LF, Carbone S, et al. Relation of hepatic fibrosis in nonalcoholic fatty liver disease to left ventricular diastolic function and exercise tolerance. Am J Cardiol. 2019;123:466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Golabi P, Otgonsuren M, Cable R, Felix S, Koenig A, Sayiner M, et al. Non-alcoholic fatty liver disease (NAFLD) is associated with impairment of Health Related Quality of Life (HRQOL). Health Qual Life Outcomes. 2016;14:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dan AA, Kallman JB, Wheeler A, Younoszai Z, Collantes R, Bondini S, et al. Health-related quality of life in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2007;26:815–820. [DOI] [PubMed] [Google Scholar]
- 97.Raafs BM, Karssemeijer EGA, Van der Horst L, Aaronson JA, Olde Rikkert MGM, Kessels RPC. Physical exercise training improves quality of life in healthy older adults: a meta-analysis. J Aging Phys Act. 2020;28:81–93. [DOI] [PubMed] [Google Scholar]
- 98.Myers VH, McVay MA, Brashear MM, Johannsen NM, Swift DL, Kramer K, et al. Exercise training and quality of life in individuals with type 2 diabetes: a randomized controlled trial. Diabetes Care 2013;36:1884–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Canadian Society for Exercise Physiology. Get Active Questionnaire; 2017.
- 100.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. [DOI] [PubMed] [Google Scholar]
- 101.Warburton DE, Jamnik VK, Bredin SS, McKenzie DC, Stone J, Shephard RJ, et al. Evidence-based risk assessment and recommendations for physical activity clearance: an introduction. Appl Physiol Nutr Metab. 2011;36(suppl 1):S1–S2. [DOI] [PubMed] [Google Scholar]
- 102.Bowen PG, Mankowski RT, Harper SA, Buford TW. Exercise is medicine as a vital sign: challenges and opportunities. Transl J Am Coll Sports Med. 2019;4:1–7. [PMC free article] [PubMed] [Google Scholar]
- 103.Sallis R. Developing healthcare systems to support exercise: exercise as the fifth vital sign. Br J Sports Med. 2011;45:473–474. [DOI] [PubMed] [Google Scholar]
- 104.Office of National Drug Control Policy (ONDCP), Substance Abuse and Mental Health Services Administration (SAMHSA). Screening, Brief Intervention, and Referral to Treatment (SBIRT); 2012.
- 105.Centers for Disease Control and Prevention (CDC). Overcoming barriers to physical activity. 2022. Accessed July 7, 2022.
- 106.Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci. 2011;6:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Buzzetti E, Linden A, Best LM, Madden AM, Roberts D, Chase TJG, et al. Lifestyle modifications for nonalcohol-related fatty liver disease: a network meta-analysis. Cochrane Database Syst Rev. 2021;6:CD013156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Arora E, Babu AS, Vidhyasagar S, Maiya GA. Physical activity promotion program on physical activity and glycemic control in prediabetes. Crit Rev Phys Rehabil Med. 2018;30:181–185. [Google Scholar]
- 109.Lazarus JV, Kakalou C, Palayew A, Karamanidou C, Maramis C, Natsiavas P, et al. A Twitter discourse analysis of negative feelings and stigma related to NAFLD, NASH and obesity. Liver Int. 2021;41:2295–2307. [DOI] [PubMed] [Google Scholar]
- 110.Carol M, Pérez-Guasch M, Solà E, Cervera M, Martínez S, Juanola A, et al. Stigmatization is common in patients with non-alcoholic fatty liver disease and correlates with quality of life. PLoS One. 2022;17:e0265153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hallsworth K, Adams LA. Lifestyle modification in NAFLD/NASH: facts and figures. JHEP Rep. 2019;1:468–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Addo SF, Maiden K, Ehrenthal DB. Awareness of the 5 A’s and motivational interviewing among community primary care providers. Del Med J. 2011;83:17–21. [PubMed] [Google Scholar]
- 113.Vallabhan MK, Kong AS, Jimenez EY, Summers LC, DeBlieck CJ, Feldstein Ewing SW. Training primary care providers in the use of motivational interviewing for youth behavior change. Res Theory Nurs Pract. 2017;31:219–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mazzotti A, Caletti MT, Brodosi L, Di Domizio S, Forchielli ML, Petta S, et al. An internet-based approach for lifestyle changes in patients with NAFLD: Two-year effects on weight loss and surrogate markers. J Hepatol. 2018;69:1155–1163. [DOI] [PubMed] [Google Scholar]
- 115.Moscatiello S, Di Luzio R, Bugianesi E, Suppini A, Hickman IJ, Di Domizio S, et al. Cognitive-behavioral treatment of nonalcoholic Fatty liver disease: a propensity score-adjusted observational study. Obesity (Silver Spring). 2011;19:763–770. [DOI] [PubMed] [Google Scholar]
- 116.Montesi L, Caselli C, Centis E, Nuccitelli C, Moscatiello S, Suppini A, et al. Physical activity support or weight loss counseling for nonalcoholic fatty liver disease? World J Gastroenterol. 2014;20:10128–10136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hallsworth K, McPherson S, Anstee QM, Flynn D, Haigh L, Avery L. Digital intervention with lifestyle coach support to target dietary and physical activity behaviors of adults with nonalcoholic fatty liver disease: systematic development process of VITALISE using intervention mapping. J Med Internet Res. 2021;23:e20491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.de Sousa D, Fogel A, Azevedo J, Padrão P. The effectiveness of web-based interventions to promote health behaviour change in adolescents: a systematic review. Nutrients. 2022;14:1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jamali T, Raasikh T, Bustamante G, Sisson A, Tandon P, Duarte-Rojo A, et al. Outcomes of exercise interventions in patients with advanced liver disease: a systematic review of randomized clinical trials. Am J Gastroenterol. 2022;117:1614–1620. [DOI] [PubMed] [Google Scholar]
- 120.Berzigotti A, Albillos A, Villanueva C, Genescá J, Ardevol A, Augustín S, et al. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: the SportDiet Study. Hepatology. 2017;65:1293–1305. [DOI] [PubMed] [Google Scholar]
- 121.Lai JC, Dodge JL, Kappus MR, Wong R, Mohamad Y, Segev DL, et al. A multicenter pilot randomized clinical trial of a home-based exercise program for patients with cirrhosis: the Strength Training Intervention (STRIVE). Am J Gastroenterol. 2020;116:717–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Duarte-Rojo A, Bloomer PM, Rogers RJ, Hassan MA, Dunn MA, Tevar AD, et al. Introducing EL-FIT (Exercise and Liver FITness): a smartphone app to prehabilitate and monitor liver transplant candidates. Liver Transpl. 2020;27:502–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Faust A, Stine JG. Leveraging the coronavirus disease 2019 pandemic: is it time to consider incorporating mobile applications into standard clinical management of the liver transplantation patient? Liver Transpl. 2021;27:479–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Macías-Rodríguez RU, Ilarraza-Lomelí H, Ruiz-Margáin A, Ponce-de-León-Rosales S, Vargas-Vorácková F, García-Flores O, et al. Changes in hepatic venous pressure gradient induced by physical exercise in cirrhosis: results of a pilot randomized open clinical trial. Clin Transl Gastroenterol. 2016;7:e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zenith L, Meena N, Ramadi A, Yavari M, Harvey A, Carbonneau M, et al. Eight weeks of exercise training increases aerobic capacity and muscle mass and reduces fatigue in patients with cirrhosis. Clin Gastroenterol Hepatol. 2014;12:1920–1926.e2. [DOI] [PubMed] [Google Scholar]
- 126.Chen HW, Ferrando A, White MG, Dennis RA, Xie J, Pauly M, et al. Home-based physical activity and diet intervention to improve physical function in advanced liver disease: a randomized pilot trial. Dig Dis Sci. 2020;65:3350–3359. [DOI] [PubMed] [Google Scholar]
- 127.Kruger C, McNeely ML, Bailey RJ, Yavari M, Abraldes JG, Carbonneau M, et al. Home exercise training improves exercise capacity in cirrhosis patients: role of exercise adherence. Sci Rep. 2018;8:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Avery L, Exley C, McPherson S, Trenell MI, Anstee QM, Hallsworth K. Lifestyle behavior change in patients with nonalcoholic fatty liver disease: a qualitative study of clinical practice. Clin Gastroenterol Hepatol. 2017;15:1968–1971. [DOI] [PubMed] [Google Scholar]
- 129.Thompson PD, Arena R, Riebe D, Pescatello LS. ACSM’s new preparticipation health screening recommendations from ACSM’s guidelines for exercise testing and prescription, ninth edition. Curr Sports Med Rep. 2013;12:215–217. [DOI] [PubMed] [Google Scholar]
- 130.Zhou BJ, Huang G, Wang W, Zhu LH, Deng YX, He YY, et al. Intervention effects of four exercise modalities on nonalcoholic fatty liver disease: a systematic review and Bayesian network meta-analysis. Eur Rev Med Pharmacol Sci. 2021;25:7687–7697. [DOI] [PubMed] [Google Scholar]
- 131.Singh AK, Kaur N, Kaushal S, Tyagi R, Mathur D, Sivapuram MS, et al. Partitioning of radiological, stress and biochemical changes in pre-diabetic women subjected to Diabetic Yoga Protocol. Diabetes Metab Syndr. 2019;13:2705–2713. [DOI] [PubMed] [Google Scholar]
- 132.Keymasi Z, Sadeghi A, Pourrazi H. Effect of pilates training on hepatic fat content and liver enzymes in men with nonalcoholic fatty liver disease. J Appl Health Stud Sport Physiol. 2017;4:49–56. [Google Scholar]
- 133.Huber Y, Pfirrmann D, Gebhardt I, Labenz C, Gehrke N, Straub BK, et al. Improvement of non-invasive markers of NAFLD from an individualised, web-based exercise program. Aliment Pharmacol Ther. 2019;50:930–939. [DOI] [PubMed] [Google Scholar]
- 134.Motz V, Faust A, Dahmus J, Stern B, Soriano C, Stine JG. Utilization of a directly supervised telehealth-based exercise training program in patients with nonalcoholic steatohepatitis: feasibility study. JMIR Form Res. 2021;5:e30239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zelber-Sagi S, Buch A, Yeshua H, Vaisman N, Webb M, Harari G, et al. Effect of resistance training on non-alcoholic fatty-liver disease a randomized-clinical trial. World J Gastroenterol. 2014;20:4382–4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65:1557–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.US Department of Health and Human Services. Food and Drug Administration. Noncirrhotic nonalcoholic steatohepatitis with liver fibrosis: developing drugs for treatment guidance for industry; 2018. Accessed July 7, 2022. https://www.fda.gov/media/119044/download.


