Abstract
Background
Effectiveness studies with biological therapies for asthma lack standardised outcome measures. The COMSA (Core Outcome Measures sets for paediatric and adult Severe Asthma) Working Group sought to develop Core Outcome Measures (COM) sets to facilitate better synthesis of data and appraisal of biologics in paediatric and adult asthma clinical studies.
Methods
COMSA utilised a multi-stakeholder consensus process among patients with severe asthma, adult and paediatric clinicians, pharmaceutical representatives, and health regulators from across Europe. Evidence included a systematic review of development, validity and reliability of selected outcome measures plus a narrative review and a pan-European survey to better understand patients’ and carers’ views about outcome measures. It was discussed using a modified GRADE (Grading of Recommendations Assessment, Development and Evaluation) Evidence to Decision framework. Anonymous voting was conducted using predefined consensus criteria.
Results
Both adult and paediatric COM sets include forced expiratory volume in 1 s (FEV1) as z-scores, annual frequency of severe exacerbations and maintenance oral corticosteroid use. Additionally, the paediatric COM set includes the Paediatric Asthma Quality of Life Questionnaire and Asthma Control Test or Childhood Asthma Control Test, while the adult COM set includes the Severe Asthma Questionnaire and Asthma Control Questionnaire-6 (symptoms and rescue medication use reported separately).
Conclusions
This patient-centred collaboration has produced two COM sets for paediatric and adult severe asthma. It is expected that they will inform the methodology of future clinical trials, enhance comparability of efficacy and effectiveness of biological therapies, and help assess their socioeconomic value. COMSA will inform definitions of non-response and response to biological therapy for severe asthma.
Short abstract
A European multi-stakeholder working group has reached a consensus on Core Outcome Measures sets for paediatric and adult Severe Asthma (COMSA). These should inform future clinical trials and enhance comparability of findings. https://bit.ly/3yO2gB2
Introduction
Severe asthma is defined by the European Respiratory Society/American Thoracic Society (ERS/ATS) as asthma which requires treatment with high-dose inhaled corticosteroids and a second controller and/or systemic corticosteroids to prevent it from becoming “uncontrolled” or which remains “uncontrolled” despite this therapy [1]. Severe asthma affects ∼5–10% of patients with asthma [1]; however, there is variability in the prevalence estimates in children and adults [2]. It is associated with a significant impact on quality of life (QoL) [3], treatment [4, 5] and socioeconomic burden [4, 6–8]. Many patients with severe asthma miss school [9] or are unable to maintain full-time employment [10] and some fail to respond to traditional asthma treatments.
Biological therapies for severe asthma improve individual patient outcomes [11]. A series of systematic reviews reported that biologics improve asthma control and QoL, and decrease exacerbation rates and rescue medication use [12–14]. However, there is significant heterogeneity in which outcome measures are reported and what definitions are used in clinical trials. This makes it challenging to draw definite conclusions about the relative effectiveness of different biological agents, particularly given the paucity of head-to-head trials. Additionally, there are different eligibility criteria for initiating biologics in paediatric and adult patients [15, 16], and this makes comparisons between different trials difficult. Although validated and reliable outcomes or outcome measures for asthma have been recommended in the National Institutes of Health series [17–22], coreASTHMA [23], clinical asthma registries [24] and asthma trials [25], there is no agreement on what is the most appropriate Core Outcome Measures (COM) set for trials with biological therapies in severe asthma. A COM set is a minimum, standardised group of outcome measures that should be used and reported in all future clinical trials [26]. The development of a COM set requires a multi-step process involving all relevant stakeholders, including clinicians, patients and their families, to identify outcome measures that have suitable measurement properties, are most relevant and are feasible for use.
To address the need for a robust set of outcome measures for severe asthma, we aimed to develop pan-European consensus patient-centred COM sets for use in studies of biological therapies in paediatric and adult patients with severe asthma. Having standardised COM sets would enable improved reporting and synthesis of outcome measures and therefore reduce publication bias, allow meaningful comparisons of efficacy and effectiveness of different biological therapies, and improve policy and patient–doctor shared decision making.
Methods
The COMSA initiative is registered on the Core Outcome Measures in Effectiveness Trials (COMET) database (www.comet-initiative.org/Studies/Details/1698). The approach was adapted from the COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) initiative to select outcome measurement instruments for the COM set [26] and is reported in accordance with the Core Outcome Set-STAndards for Reporting (COS-STAR) statement (supplementary table S1) [27]. Approval was gained from the Ethics Committee of the University of Southampton (Southampton, UK) (ERGO 56181). This project is part of the 3TR (Taxonomy, Treatments, Targets and Remission) Consortium (https://3tr-imi.eu) funded by the European Commission's Innovative Medicines Initiative 2.
Participants for COM sets consensus process
Four key stakeholder groups were involved.
1) Paediatric and adult patient representatives with severe asthma. These included the 3TR Respiratory Adult and Youth Patient Working Groups (PWGs) as well as patient advocacy organisations including the European Lung Foundation (ELF), European Federation of Allergy and Airways Diseases Patients’ Associations (EFA), Global Allergy & Airways Patient Platform (GAAPP), and Lovexair. The ELF and EFA recruited patients and carers of patients with severe asthma from across Europe through their networks to capture a range of disease duration, unique experiences and treatments, including biological therapy. Monthly calls with the two PWGs were held throughout the project to ensure a patient-centred approach in deciding the COM set for severe asthma. At these meetings, patients and patient advocates received online training about clinical trial design, outcome selection, core outcomes, the GRADE (Grading of Recommendations, Assessment, Development and Evaluations) approach and the consensus process. Minutes and training materials were shared with PWG members after each call.
2) Paediatric and adult clinicians were invited by the lead (G.R.) and senior (E.K.) investigators, and included paediatricians, allergists, respiratory clinicians, nurses, researchers and methodologists. The selected world-leading physicians had a broad range of clinical knowledge and expertise in managing patients with severe asthma on biologics. None of the participants were involved in the development of specific outcome measurement instruments.
3) Pharmaceutical industry representatives from AstraZeneca, Sanofi, Roche and Novartis who are partners in the 3TR Consortium.
4) Regulators from European medicinal products regulatory authorities (hereafter referred to as “health regulators”). The selected health regulators had a broad range of regulatory knowledge and/or were specialised in the field of paediatric and/or adult allergology and respiratory medicine.
Overview of COM set development
Paediatric (children and adolescents aged 6–17 years) and adult (≥18 years) COM sets were developed using a similar multi-stage approach to synthesise the evidence and achieve consensus (figure 1).
FIGURE 1.
Core Outcome Measures set development process. COMSA: Core Outcome Measures for paediatric and adult Severe Asthma.
Stage 1: A systematic review to identify and appraise priority outcome measures for severe asthma
The detailed methods used to develop COM sets are provided in the systematic review [28]. In brief, Step 1 involved the generation of a list of “candidate” asthma outcome measures from a systematic literature search from the previous 2 years. Step 2 involved a modified two-round Delphi exercise among four stakeholder groups and a moderated web conference to select “key” outcome measures (rated as “critical” or “important” [29]). Step 3 involved a systematic literature search [28] to identify “initial” validation studies for the key outcome measures and compare against good measurement properties criteria using modified COSMIN methodology [30–32].
Stage 2: Capturing patients’ and carers’ views
A narrative review was undertaken by two reviewers (C.C. and C.W.) to synthesise evidence about patients’ and carers’ perceptions and opinions about outcome measures for severe asthma. Three bibliographic databases were searched from the year 2000. Full details are provided in the supplementary material.
A cross-sectional pan-European survey was conducted to gain insight in the perspectives of the wider patient population about outcome measures used for severe asthma. See the supplementary material for further details.
Stage 3: Multi-stakeholder consensus meetings
The aim of the consensus meetings for paediatric and adult outcome measures was to provide an opportunity to better understand views of different stakeholder groups, discuss key issues, resolve any disagreements and reach consensus on the final COM sets.
Initial meetings to reduce to priority outcome measures
The systematic review evidence, together with the results of a narrative review and a pan-European survey of patients’ and carers’ perceptions and preferences about outcome measures for severe asthma (supplementary material), was discussed in two initial multi-stakeholder meetings. Materials were provided 1 week before meetings. Patient-reported outcome measures (PROM) such as asthma-specific QoL, general QoL, asthma control, asthma symptoms and composite outcome measures were discussed in the first meeting followed by online voting to select eight priority PROM. Clinical and healthcare use outcome measures such as forced expiratory volume in 1 s (FEV1), fractional exhaled nitric oxide (FENO), peak expiratory flow (PEF), FEV1/forced vital capacity ratio, blood and/or sputum eosinophils, hospitalisations, exacerbations, adverse events, and oral corticosteroid (OCS) use were discussed at the second meeting followed by online voting to select four priority outcome measures [28]. Results were presented using the GRADE system [33].
Consensus meeting to decide on COM sets
Prior to the adult and paediatric consensus meetings, all participants received the agenda, reading materials, including results of the systematic review about the development and measurement properties of priority outcome measures [28], comments from previous multi-stakeholder discussions, original copies of questionnaires, results of the pan-European survey (supplementary material) and narrative review (supplementary material) as well as data from the European Academy of Allergy and Clinical Immunology (EAACI) systematic reviews [12–14] and a systematic review of real-life studies on biological therapies [34]. All materials included summaries of the results in lay language, with an additional lay glossary of terms. Participants were invited to attend optional drop-in sessions to ask questions about materials prior to the consensus meetings.
Primary consideration was given to content validity results about relevance, comprehensiveness and comprehensibility as per COSMIN guidance on selecting core outcome measurement instruments [26] as well as patient-centred literature. During previous discussions participants highlighted that the ideal outcome measures for biological trials should also have good responsiveness, established minimal clinically important difference (MCID)/minimal important difference (MID) and be relevant to severe asthma patients. Participants were invited to share their views, refine definitions, address discrepancies across stakeholders and suggest possible combinations of outcome measures.
The online consensus meetings were held on 7 June 2021 to evaluate the evidence for adult severe asthma and on 20 July 2021 for paediatric severe asthma to ratify the final COM sets. Although these meetings were initially planned to be face-to-face with all stakeholder groups, this was changed to virtual meetings due to coronavirus disease 2019 (COVID-19) public health restrictions. Each meeting was recorded to facilitate minutes and a link was shared with those participants who were not able to attend.
COM set voting
An anonymised electronic voting process was employed after the meetings. All 3TR participants received minutes, evidence discussed at the meetings and a link to an online voting form to share their views. Along with minimal demographic information, in the first round participants were asked to select up to five and six outcome measures for paediatric and adult COM sets, respectively, and rank them in the order of importance. A free-text comment box was available to provide rationale and further arguments for inclusion or exclusion of outcome measures. Votes from clinicians, researchers, pharmaceutical representatives and health regulators were included in the “academic” group, while votes from patients and patient representatives were classified into the “patient” group. Outcome measures that scored ≥70% of the panellist's groups’ (patient or academic) votes were judged to have met consensus for inclusion based on COMET guidelines and previous patient-centred COM sets [35, 36]. Several reminders were sent to improve participation in the voting.
Results of the first round were analysed and collated into a summary of votes and comments divided by stakeholder group. Prior to the next round of voting, this summary was shared with the 3TR panel (four key stakeholder groups) who were invited to provide further comments about the group of outcome measures where consensus was not achieved (<70% agreement). Subsequently, all participants were invited to take part in Round 2 (and additionally Round 3 for the adult COM set) voting for these outcome measures. A summary of all comments as well as initial voting results and evidence with comments from the meetings were included in the invitation e-mail.
Statistical analysis
All data from the pan-European survey and online voting were analysed using SPSS version 26.0 (IBM, Armonk, NY, USA). Descriptive statistics were used to describe respondent characteristics. Medians with lower and upper quartiles are presented for continuous variables given the distribution of the data. Frequency tables with percentages are provided for categorical variables. Summary tables and figures were used to represent the results.
Results
Stage 1: A systematic review to identify and appraise priority outcome measures for severe asthma
Step 1 led to the identification of 96 candidate outcome measures. These were reduced to 55 key measures in the modified Delphi exercise (Step 2). Subsequently, following the systematic literature search and multi-stakeholder meetings, eight and nine priority outcome measures were identified for adult and paediatric populations, respectively (Step 3). The validity and reliability of the priority measures (Step 4) are discussed elsewhere [28].
Stage 2: Capturing patients’ and carers’ views
Narrative review
The systematic literature search found 127 papers out of which seven papers met the inclusion criteria (supplementary figure S1). Patient perspectives were extracted about the following outcome measures: PEF monitoring [37–39], hospitalisations [3, 37, 38, 40], exacerbations [41], adverse events [3, 37, 38, 40–42] and reducing OCS use [37, 38, 40–42]. Avoiding hospitalisation, decreasing OCS use and related side-effects, and reducing the number and severity of exacerbations are treatment priorities identified by patients. More details are available in the supplementary material.
A pan-European survey
A total of 201 (87%) patients and 31 (13%) parents/carers of patients with severe asthma completed the survey. Most were female (77% and 87% patients and parents/carers, respectively), had completed university education (59% and 71%, respectively) and 54% were being treated with a biological therapy (supplementary table S2).
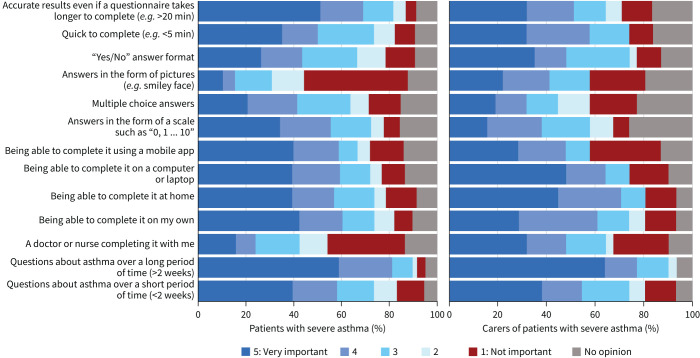
Patients and carers, respectively, identified the following characteristics in regard to filling out questionnaires as “very important”: “longer recall period, e.g. ≥2 weeks” (59% and 65%), “accurate results even if it takes longer to complete” (51% and 32%), “opportunity to complete at home” (39% and 45%) and either “using a mobile app” (40% and 29%) or “using a computer” (39% and 48%) (figure 2). Responders were willing to complete a questionnaire once every month (38% and 16%) or as often as their doctor recommends (34% and 36%). It should ideally take only 6–10 min (45% and 36%) (supplementary figure S2 and supplementary table S3).
FIGURE 2.
Patients’ and carers’ views about characteristics of questionnaires for assessment of severe asthma according to the pan-European survey.
The following characteristics of lung function tests were favoured the most and rated as “very important” in the survey by patients and carers, respectively: “accuracy of the results” (83% and 65%) and “safe to complete” (67% and 59%) (supplementary figures S3 and S4, and supplementary table S4). Further results, themes and quotes can be found in supplementary figures S5 and S6, and supplementary tables S5 and S6.
When survey respondents were asked to select only five outcomes, they ranked the following as first or second most important for patients and parents/carers, respectively: “emergency hospital admissions due to asthma” (64% and 29%), “lung function” (49% and 36%), “QoL questionnaires” (42% and 39%), “exacerbations” (40% and 40%) and “OCS use” (37% and 100%) (figure 3).
FIGURE 3.
Overall views of patients and carers about outcome measures for assessment of severe asthma according to the pan-European survey. Respondents were asked to select five outcome measures and rank their importance from 1=most important to 5=least important, for use in future severe asthma trials and clinical practice.
Stage 3: Multi-stakeholder consensus meetings
Adult COM set
A total of 35 participants comprised the multi-stakeholder panel for the adult COM set consensus meeting: 19 (54%) clinicians, nine (25%) patients and patient advocates, four (11%) health regulators, and three (9%) pharmaceutical representatives. The main discussions about the priority outcome measures are summarised in the following subsections and results of the final COM set reported at the end of the section.
Asthma-specific QoL questionnaires
Four instruments were considered: Asthma Quality of Life Questionnaire (AQLQ) [43–45], Asthma Quality of Life Questionnaire-Standardised (AQLQ-S) [45, 46], Mini Asthma Quality of Life Questionnaire (Mini-AQLQ) [45, 47] and Severe Asthma Questionnaire (SAQ) [48–50]. The SAQ had a “moderate” modified GRADE rating for development, whereas other QoL instruments were rated lower [28]. Responsiveness to change was rated “low” to “very low” for all questionnaires; MCID/MID is only reported for the AQLQ and SAQ [50], with the AQLQ MCID being quoted for the AQLQ-S and Mini-AQLQ. Patients highlighted that the Mini-AQLQ might not accurately represent the full AQLQ. The SAQ was highly endorsed as the only questionnaire developed with input from patients with severe asthma and, unlike others, includes items about fatigue and OCS side-effects. Given the novelty of the SAQ, it was suggested that the AQLQ or AQLQ-S should be considered for inclusion in the COM set to allow comparisons with results from previous studies.
Asthma control outcome measures
The Asthma Control Test (ACT) [51–53], Asthma Control Questionnaire (ACQ)-6 (symptoms and rescue medication use) [54–56] and ACQ-5 (symptoms only) [54–56] were discussed at length. None were developed with input from patients with severe asthma and were rated “very low” in terms of development. Responsiveness to change was rated “low” and “very low”, but MCID/MID data are available for all instruments. The response format of the ACQ was preferred compared with the ACT by patients, while the ACQ-6 contains an item about rescue medication use which is lacking in the ACQ-5. However, the ACQ-6 does not differentiate between the different rescue medications and their dosing; therefore, it was suggested to report it as the ACQ-5 to describe symptoms and rescue medication use separately.
Composite outcome measure
The Asthma Control and Communication Instrument (ACCI) [57] was rated “low” and “very low” for the developmental and validation process with no data about responsiveness and MCID/MID. Clinicians highlighted that it is rarely used in practice and clinical trials due to the complex scoring system.
Clinical outcome measures
Clinicians noted that FEV1 change exceeds the MID in some studies with biologics, and it is associated with mortality and future risk of exacerbations [12–14]. Reporting of FEV1 as z-scores using the Global Lung Function Initiative (GLI) predictive equations [58] was agreed by the panel.
Healthcare resource use
The ATS/ERS definition [25] of severe exacerbation defined as events requiring systemic corticosteroids for ≥3 days and/or a hospitalisation/emergency room visit for asthma requiring systemic corticosteroids was selected, with exacerbations effectively demonstrating the effectiveness of biologics for different asthma endotypes. However, the more recent ERS/EAACI statement [59] suggests the definition should be based on ≥5 days of OCS. Annual severe exacerbation frequency should be reported. Use of maintenance OCS (mOCS) defined as daily or alternate day use was considered important for inclusion by all stakeholder groups. Median (25th, 75th centiles) dose and proportion on mOCS should be reported.
Ratified COM set for adult severe asthma
The number of participants who voted in each round is listed in table 1. After the third round, five outcome measures reached the 70% consensus threshold and formed the final COM set for adults with severe asthma: SAQ, ACQ-6 (symptoms and rescue medication use reported separately), FEV1, severe exacerbations and mOCS use (figure 4, supplementary figures S7–S9 and supplementary tables S7–S9). Characteristics and availability of selected outcome measures in the adult COMSA are reported in table 2. No clear consensus was achieved on whether the AQLQ or AQLQ-S should be used in the extended COM set (COM-E). However, a suggestion was made to additionally include the AQLQ in the short term as it includes activities tailored to the patient and would enable retrospective comparisons.
TABLE 1.
Demographic information about survey respondents in the voting process to agree on the adult COMSA (Core Outcome Measures set for paediatric and adult Severe Asthma)
| Clinicians and researchers | Patient representatives | Pharmaceutical representatives | Health regulators | |||||||||
| Round 1 (n=30) | Round 2 (n=31) | Round 3 (n=26) | Round 1 (n=11) | Round 2 (n=11) | Round 3 (n=14) | Round 1 (n=3) | Round 2 (n=1) | Round 3 (n=4) | Round 1 (n=5) | Round 2 (n=4) | Round 3 (n=5) | |
| Country | ||||||||||||
| Belgium | 2 (7) | 2 (7) | 1 (4) | |||||||||
| Denmark | 1 (3) | 2 (7) | ||||||||||
| France | 2 (7) | 1 (4) | ||||||||||
| Germany | 2 (7) | 2 (7) | 1 (4) | 1 (33) | 1 (25) | 4 (80) | 3 (75) | 4 (80) | ||||
| Ireland | 2 (18) | 1 (9) | 2 (14) | |||||||||
| Italy | 2 (7) | 1 (3) | 2 (18) | 1 (9) | 2 (14) | |||||||
| Netherlands | 2 (7) | 3 (10) | 5 (19) | 1 (9) | 2 (18) | 2 (14) | ||||||
| Poland | 3 (10) | 1 (3) | 2 (8) | |||||||||
| Portugal | 1 (9) | |||||||||||
| Spain | 1 (3) | 1 (3) | 1 (9) | 1 (7) | ||||||||
| Sweden | 3 (10) | 6 (19) | 4 (15) | 2 (18) | 2 (18) | 2 (14) | 1 (33) | 1 (100) | 1 (25) | |||
| Switzerland | 1 (25) | |||||||||||
| UK | 12 (40) | 13 (42) | 12 (46) | 3 (27) | 3 (27) | 4 (29) | 1 (20) | 1 (25) | 1 (20) | |||
| USA | 1 (9) | 1 (7) | 1 (33) | 1 (25) | ||||||||
| Gender | ||||||||||||
| Male | 22 (73) | 19 (61) | 17 (65) | 2 (18) | 2 (18) | 3 (21) | 3 (100) | 1 (100) | 4 (100) | 1 (20) | 1 (25) | 1 (20) |
| Female | 8 (27) | 12 (39) | 9 (35) | 9 (82) | 9 (82) | 11 (79) | 4 (80) | 3 (75) | 4 (80) | |||
| Age group (years) | ||||||||||||
| 18–25 | 1 (3) | 1 (3) | 1 (4) | 2 (18) | 2 (18) | 2 (14) | ||||||
| 26–36 | 2 (7) | 3 (10) | 2 (8) | 2 (18) | 2 (18) | 2 (14) | ||||||
| 37–47 | 6 (20) | 8 (26) | 9 (35) | 2 (18) | 3 (27) | 4 (29) | 1 (33) | 1 (100) | 3 (75) | |||
| 48–58 | 13 (43) | 12 (39) | 10 (39) | 2 (18) | 2 (14) | 2 (67) | 1 (25) | 4 (80) | 3 (75) | 4 (80) | ||
| 59–69 | 8 (27) | 7 (23) | 3 (12) | 4 (36) | 1 (9) | 2 (14) | 1 (20) | 1 (25) | 1 (20) | |||
| 70–80 | 1 (4) | 1 (9) | 1 (9) | 2 (14) | ||||||||
| Online meeting | ||||||||||||
| Yes | 16 (53) | 16 (52) | 12 (46) | 8 (73) | 8 (73) | 10 (71) | 2 (67) | 1 (100) | 2 (50.0) | 4 (80) | 3 (75) | 4 (80) |
| No | 14 (47) | 15 (48) | 14 (54) | 3 (27) | 3 (27) | 4 (29) | 1 (33) | 2 (50.0) | 1 (20) | 1 (25) | 1 (20) | |
Data are presented as n (%); percentages are rounded to zero decimal places so totals may not add up to 100%.
FIGURE 4.
The adult Core Outcome Measures set for severe asthma clinical trials. Forced expiratory volume in 1 s should be reported as z-scores using the Global Lung Function Initiative predictive equations [58], annual severe exacerbations as per the European Respiratory Society/American Thoracic Society definition [25] and maintenance oral corticosteroid (mOCS) use defined as daily or alternate day use (median (25th, 75th centiles) dose and proportion on mOCS should be reported). The Asthma Control Questionnaire-6 should be reported as the Asthma Control Questionnaire-5 to describe symptoms and rescue medication use separately. 3TR: Taxonomy, Treatment, Targets and Remission Consortium; COMSA: Core Outcome Measures set for paediatric and adult Severe Asthma.
TABLE 2.
Characteristics of the questionnaires selected for the adult and paediatric COMSA (Core Outcome Measures set for paediatric and adult Severe Asthma)
| Scale (year) | Modes of administration | Target population | Time to complete | Patient/carer report | Recall period | Number of questions, response format(s) | Scoring method | Original language, translations# | Licence and costs |
| Questionnaires selected for the adult COMSA | |||||||||
| SAQ [48] (2018) | Self-complete; paper form |
16–78 years | 3–6 min | Patient | 2 weeks | SAQ: 16 questions: 7-point Likert scale (1=very, very difficult, 7=no problem); SAQ-global: 100-point QoL scale (0=no QoL, 100=perfect QoL) |
SAQ: average of responses (range 1–7); SAQ-global (range 0–100) |
English (UK): two validated translations; several unpublished translations | Copyrighted by University of Plymouth and University Hospitals Plymouth NHS Trust; free for non-commercial, clinical practice and research; fees may apply for funded research, healthcare organisations, commercial use |
| ACQ-6 [55]¶ symptoms and rescue medication (2001) | Self-complete; paper form; interactive web; electronic devices |
≥6 years | Not reported | Patient | 1 week | Six questions: 7-point Likert scale (0=no impairment, 6=maximum impairment) |
Average of responses: range 0–6 | English (UK): 111 translations | Copyrighted by questionnaire developer, QOL Technologies Ltd; free for non-commercial, clinical practice and research; otherwise, there is a one-time fee; electronic version requires a user fee |
| Questionnaires selected for the paediatric COMSA | |||||||||
| PAQLQ [60] (1996) | Self-complete; paper form; interviewer- administered version (≤11 years) |
7–17 years | 10–15 min at initial visit; 5–10 min at follow-ups | Patient | 1 week | 23 questions: 7-point Likert scale (1=severe impairment, 7=no impairment) |
Three subscales: average of responses; range 1–7 |
English (North America): 62 translations | Copyrighted by questionnaire developer, QOL Technologies Ltd; free for use in non-commercial, clinical practice and research; otherwise, there is a one-time fee |
| C-ACT [65] (2007) | Self-complete; paper form; web-based |
Children and carers of children aged 4–11 years | Not reported, but web-based version takes 5 min to complete | Patient and carer | 4 weeks | For children (four questions): 4-point Likert scale (0=“very bad”, 3=“very good”; including pictures of a child's face with matching expressions); for carers (three questions): 6-point Likert scale (0=“everyday”, 5=“not at all”) |
Sum of the item responses; range 0–27 (≤19 points= uncontrolled asthma) |
English (USA): 27 translations | Copyrighted by GlaxoSmithKline Ltd; free for non-commercial, clinical practice and research; fee may apply for commercial use |
| ACT [51] (2004) | Self-complete; interviewer- administered; paper form; web-based; telephone |
≥12 years | 1–2 min | Patient | 4 weeks | Five questions: 5-point scale (questions about symptoms and activities: 1=all the time, 5=not at all); patient self-rating of control: (1=not controlled at all, 5=completely controlled) |
Sum of the item responses; range 5–25 (≤19 points= uncontrolled asthma) |
English (USA): 179 translations | Copyrighted by Quality Metric Inc.; permission required for use |
SAQ: Severe Asthma Questionnaire; QoL: quality of life; ACQ: Asthma Control Questionnaire; PAQLQ: Paediatric Asthma Quality of Life Questionnaire; C-ACT: Childhood Asthma Control Questionnaire; ACT: Asthma Control Test. #: the number of translations is an estimate sourced from sites and manuals of the instruments available in English; ¶: the ACQ-6 should be reported as the ACQ-5 to describe symptoms and rescue medication use separately.
Paediatric COM set
A total of 28 participants comprised the multi-stakeholder panel for the paediatric COM consensus meeting: 13 (46%) clinicians, 12 (43%) patients and patient advocates, and three (11%) health regulators. The main discussions are summarised in the following subsections and results of the final COM set reported at the end of the section.
Asthma-specific QoL questionnaires
The Paediatric Asthma Quality of Life Questionnaire (PAQLQ) [60–63], Paediatric Asthma Quality of Life Questionnaire-Standardised (PAQLQ-S) [60, 62, 63] and Mini-Paediatric Asthma Quality of Life Questionnaire (Mini-PAQLQ) [62, 63] were reviewed. None appear to have been developed with input from patients with severe asthma. Panellists highlighted that when activities are specified (PAQLQ-S) it is easier to compare between patients, but this could be less relevant for individual patients. Responsiveness to change was rated as “low” to “very low”. The MCID for the PAQLQ is available and is used for other questionnaires. Some important concepts for severe asthma are not covered in the asthma-specific QoL questionnaires, e.g. “missed school days” and fatigue.
Asthma control outcome measures
The ACT (≥12 years) [51, 53], Childhood Asthma Control Test (C-ACT) (4–11 years) [64, 65], ACQ-7 (symptoms, rescue medication use and FEV1) [54, 56, 66, 67], ACQ-6 (symptoms and rescue medication use) [54, 56, 66] and ACQ-5 (symptoms only) (≥6 years) [54, 56, 66] were discussed. An assessment of control over 4 weeks was suggested to be advantageous. Some clinicians proposed using the ACQ-6 to harmonise the paediatric COM set with the adult COM set and facilitate transition between services. Patient advocates expressed a particular preference for the ACT and C-ACT as they both include a global question about self-rating of control.
Composite outcome measure
The Composite Asthma Severity Index (CASI) [68, 69] was deprioritised as it does not include items relating to QoL and activity limitations, and was not developed with patient input.
Clinical outcome measures
Most children aged ≥5 years can perform spirometry reliably [70]. FEV1 may not always reflect the current degree of asthma control [71]; however, clinicians suggested that low FEV1 predicts future risk of exacerbations, which is also supported by the literature [72]. Reporting of FEV1 as z-scores using the GLI predictive equations [58] was agreed by the panel. Most participants felt that FENO was a useful biomarker in understanding and managing asthma [73], although consensus was not reached for it to be one of the patient-centred COM.
Healthcare resource use
Exacerbation was ranked within the top five most important outcome measures by patients in the pan-European survey and shown to have good responsiveness to change in different biologics. The panel agreed to use annual frequency of severe exacerbations defined by the ATS/ERS definition [25].
mOCS use as per the adult COM was selected. Some clinicians thought that mOCS use was not important for children as it is used very infrequently; however, others noted that reduction in OCS use is a major criterion to assess whether a biologic has been effective. Additionally, carers in the pan-European survey indicated that OCS use is one of the most important aspects, especially due to the associated side-effects. Being treated with mOCS was selected as OCS bursts should be captured by severe exacerbations.
Ratified COM set for paediatric severe asthma
After the second round of voting, five outcome measures for paediatric severe asthma reached the 70% consensus threshold: FEV1, severe exacerbations, PAQLQ, mOCS use and ACT/C-ACT (table 3, figure 5, supplementary figures S10 and S11, and supplementary tables S10 and S11). Characteristics and availability of selected paediatric COMSA are reported in table 2.
TABLE 3.
Demographic information about survey respondents in the voting to agree on the paediatric COMSA (Core Outcome Measures set for paediatric and adult Severe Asthma)
| Clinicians and researchers | Patient representatives | Pharmaceutical representatives | Health regulators | |||||
| Round 1 (n=36) | Round 2 (n=34) | Round 1 (n=13) | Round 2 (n=9) | Round 1 (n=1) | Round 2 (n=2) | Round 1 (n=3) | Round 2 (n=3) | |
| Country of residence | ||||||||
| Denmark | 1 (3) | 1 (3) | ||||||
| France | 2 (6) | 1 (3) | ||||||
| Germany | 2 (6) | 1 (3) | 3 (100) | 3 (100) | ||||
| Ireland | 1 (8) | 1 (11) | ||||||
| Italy | 2 (6) | 2 (6) | 2 (15) | 1 (11) | ||||
| Netherlands | 4 (11) | 3 (9) | 1 (8) | |||||
| Poland | 2 (6) | 1 (3) | ||||||
| Sweden | 4 (11) | 4 (12) | 5 (39) | 3 (33) | 1 (50) | |||
| Switzerland | 1 (3) | 2 (6) | ||||||
| Turkey | 1 (3) | 1 (3) | ||||||
| UK | 17 (47) | 18 (53) | 3 (23) | 3 (33) | ||||
| USA | 1 (8) | 1 (11) | 1 (100) | 1 (50) | ||||
| Gender | ||||||||
| Male | 19 (53) | 19 (56) | 2 (15) | 1 (11) | 1 (100) | 2 (100) | ||
| Female | 17 (47) | 15 (44) | 11 (85) | 8 (89) | 3 (100) | 3 (100) | ||
| Age group (years) | ||||||||
| 12–17 | 3 (23) | 1 (11) | ||||||
| 18–25 | 1 (3) | 1 (3) | 2 (15) | 2 (22) | ||||
| 26–36 | 2 (6) | 2 (6) | 2 (15) | 2 (22) | ||||
| 37–47 | 9 (25) | 7 (21) | 3 (23) | 3 (33) | 1 (50) | |||
| 48–58 | 14 (39) | 15 (44) | 1 (8) | 1 (11) | 1 (50) | 3 (100) | 3 (100) | |
| 59–69 | 8 (22) | 7 (21) | 1 (8) | |||||
| 70–80 | 2 (6) | 2 (6) | 1 (8) | |||||
| Prefer not to say | 1 (100) | |||||||
| Online meeting | ||||||||
| Yes | 21 (58) | 21 (62) | 8 (62) | 6 (67) | 3 (100) | 2 (67) | ||
| No | 15 (42) | 13 (39) | 5 (39) | 3 (33) | 1 (100) | 2 (100) | 1 (33) | |
Data are presented as n (%); percentages are rounded to zero decimal places so totals may not add up to 100%.
FIGURE 5.
The paediatric Core Outcome Measures set for severe asthma clinical trials. Forced expiratory volume in 1 s should be reported as z-scores using the Global Lung Function Initiative predictive equations [58], annual severe exacerbations as per the European Respiratory Society/American Thoracic Society definition [25] and maintenance oral corticosteroid (mOCS) use defined as daily or alternate day use (median (25th, 75th centiles) dose and proportion on mOCS should be reported). The Childhood Asthma Control Test should be used for children 4–11 years old and the Asthma Control Test should be used for children 12–18 years old. 3TR: Taxonomy, Treatment, Targets and Remission Consortium; COMSA: Core Outcome Measures for paediatric and adult Severe Asthma.
Discussion
In this multi-step consensus process involving four key stakeholder groups, we developed adult and paediatric COM sets to standardise outcome reporting for severe asthma biological trials. Through multi-stakeholder consensus meetings and multiple rounds of voting, we identified five COM for adult and paediatric clinical trials that are important to patients, clinicians, pharmaceutical representatives and health regulators. Our recommendations were informed by data from a pan-European survey and a narrative literature review, plus the developmental and validation process including applicability for severe asthma, responsiveness to change and availability of MCID from systematic reviews.
The COM sets we present are novel since they focus specifically on severe asthma. The COMSA initiative builds on the coreASTHMA project that aimed to harmonise collection and reporting of outcomes in patients with moderate-to-severe asthma [23]. Both initiatives selected exacerbations, asthma-specific QoL and change in asthma control as core outcomes; however, COMSA aimed to select specific outcome measures to assess QoL and asthma control, and also included FEV1 and mOCS use. Furthermore, coreASTHMA included asthma-specific emergency department visits and asthma-specific hospital stay or admission. These outcomes were discussed by the COMSA panellists in multi-stakeholder discussions prior to the consensus meeting, and were excluded due to variable admission protocols and differences in healthcare settings.
Using PROM is important to understand the effect of asthma treatment on patients’ QoL and experience with biological treatment. Panellists strongly advocated the inclusion of the SAQ in the adult set; although currently validation data are only available for the UK and Portugal populations, further studies are underway to adapt the SAQ to other languages, settings and for children. The advantages of using this outcome measure were that it is the only instrument that is developed for severe asthma patients and scored well for validation and reliability. However, while the AQLQ has a longer history and experience in use, it was not specifically developed for severe asthma and does not assess side-effects of OCS use and the psychological burden for these patients.
Generic outcome measures (e.g. generic QoL instruments) were not selected, but we acknowledge they are imperative to facilitate comparisons of burden across diseases and cost-effectiveness analysis of biological therapies [74, 75]. The AQLQ would also be more appropriate for asthma studies enrolling mild, moderate and severe participants.
Identifying an asthma control instrument that would be relevant for severe asthma was noted as a challenge. The Global Initiative for Asthma 2021 report recommends using maintenance and reliever therapy (MART) for adolescents and adults with asthma at all treatment steps, and prefers the ACQ-5 as the ACQ-6 rescue question is not valid for MART [76]. However, the ACQ-6 was rated as a more relevant outcome measure for the COM set, but it should be reported as the ACQ-5 (asthma symptoms) and rescue medication use separately. Lastly, during the consensus process it was suggested that trials should record comorbidities as many patients, especially children and adolescents, have other allergic conditions and several biologics can impact on more than one disease. However, the focus of this work is severe asthma and it was suggested that separate COM should be considered for other comorbidities.
Strengths and limitations
Our study has several strengths. The COMSA was developed through a methodologically robust and multinational consensus process according to the modified guidance from the COMET initiative. It incorporated perspectives from four stakeholder groups including patients with severe asthma from across Europe. Translators were available for patients to prevent any selection bias and incorporate wider patient perspectives during meetings and online voting. Additionally, qualitative analysis of comments from the multilingual pan-European survey allowed further representation of views of patients and carers. Throughout the project, researchers collaborated with ELF and EFA representatives who have extensive experience of working with patients to ensure comprehensibility of the process. Furthermore, we used a systematic and transparent approach in assessing the development and measurement properties of priority outcome measures by applying COSMIN guidelines and synthesised the evidence using the modified GRADE approach [30–32]. Lastly, having online consensus meetings and voting allowed an interactive exchange of views from a wider range of representatives from across Europe.
We acknowledge some limitations. We aimed to develop patient-centred COM sets; however, some COM were not highly favoured from the patient perspective. Furthermore, the systematic review did not identify any validation data for the priority clinical and healthcare use measures for severe asthma, so decisions were based on expert consensus. Although a considerable number of expert clinicians, patients with severe asthma, patient representatives, pharmaceutical representatives and health regulators were involved from across Europe, it would have been useful to have included more, especially from the latter two groups. It would also have been helpful to have additional non-UK clinicians, although we had good involvement of healthcare professionals. We chose to include a relatively low number of patient representatives to ensure that we could provide them considerable support and training to allow them to provide meaningful input into the development process. This limitation was mitigated by the pan-European patient survey which widened the input of patient views. Lastly, it is important to highlight that COMSA is a minimum set only and other outcome measures could also be included by study investigators according to their research needs.
Research agenda
The development of a QoL outcome measure specifically for children and adolescents with severe asthma was identified as a major unmet need. Currently, paediatric QoL PROM do not assess all possible impairments such as anxiety and activity limitations specific to severe asthma. As highlighted by the PWG and pan-European survey, most of the questionnaires are not accessible online or via a mobile app, thus further development and validation is needed. Furthermore, there is an unmet need for long-term outcomes, and also importantly, disease-modifying outcome measures in severe asthma including disease remission.
Panellists also noted that side-effects of OCS and biologics, and adherence to therapy, should be considered as important outcome measures. Due to the lack of validated and reliable methods of collecting these data as well as data for the clinical and healthcare outcome measures for severe asthma, this was considered as a research gap. Therefore, the COMSA should be updated once new data are available. Researchers should also develop a more robust means of measuring reliever use that takes into account the different relivers such as salbutamol, terbutaline and the MART approach. Lastly, there is also a need for data specifically from paediatric studies with biologics to assess responsiveness to change of outcome measures.
Conclusions
In conclusion, we have developed evidence-based and patient-centred COM sets for paediatric and adult severe asthma biological therapy trials. The COMSA should be recommended to increase consistency in reporting of outcome measures, and to improve comparability of studies and certainty of evidence to guide policy making and clinical practice. These COM sets will inform future work for the development of definitions of response and non-response to biological therapies for severe asthma. Regular review and updates are necessary to ensure that the COM sets reflect current clinical practice. There is a need to develop an approach for monitoring implementation of these COM sets and global uptake of the agreed COM in research and practice.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00606-2022.Supplement (4.5MB, pdf)
Shareable PDF
Acknowledgements
The authors would like to thank all patients and patient representatives who participated in the 3TR Respiratory Patient Working Group: Andrea Palombo; Betty Frankemölle; Elizabeth Davin; Dominique Hamerlijnck; Breda Flood; Luciano Cattani, AsmaGrave Patients Ass.; Martine Puhl; Simona Barbaglia, Respiriamo Insieme; Francesca Pirovano; Valentina Melita; Phil Taverner; Fernando Javier Velasco Romero; Johanna Larsson; Alexandra Iderfors; Peter McQuitty; and Shane Fitch, Lovexair; and translators for patients such as Francesca Pirovano and Valentina Melita. We would also like to acknowledge the help of EFA and ELF representatives, Markaya Henderson and Pippa Powell, with recruitment of patients and management of patient activities. We would like to thank the 3TR Respiratory Work Package and COMSA Working Group, including academic clinicians and researchers such as Asger Sverrild, Bernd Schmeck, Claus Vogelmeier, Dorota Szydlowska, Eduard Monso Molas, Maciej Kupczyk, Martijn Nawijn, Michael Wilde, Nikos Lazarinis, Piotr Kuna, Salman Siddiqui, Sisse Ditlev, Therese Lapperre, Walter Canonica, Anna James, Enrico Heffler, Ian Adcock, Johan Kolmert, Lars Andersson, Åsa Wheelock, Craig Wheelock, Mahmoud Ibrahim Abdel-Aziz, Maria Mikus, Paul Brinkman, Alvar Agusti, Rosa Faner, Jadwiga Wedzicha, Gavin Donaldson, Michael Kabesch, Ricardo Fernandes, Norrice Liu and Fabio Midulla; pharmaceutical representatives such as Alix Berton, John Taylor, Judit Axmann, Veit Erpenbeck, Xavier Jaumont and Matthias Gossel; and a health regulator, Hanneke Van der Woude, for their contribution to the consensus process. We would like to acknowledge the support of the ELF in running the survey, and the ELF and EFA for dissemination of the survey among patient organisations across Europe. We also want to thank EVS Translations (UK), Ltd (Nottingham, UK) for help with translation of the survey and promotional materials into 14 different languages as well as help with translation of German and Swedish responses into English. Additionally, Lizza Hendricks (Dutch); Riccardo Guarise and Sara Manti (Italian); Katarzyna Lewandowska (Polish); Cristina Jácome (Portuguese); Oksana Viltsanyuk, Anna Konishcheva and Rustem Shaymuratov (Russian); and Laura Núñez Naveira (Spanish) assisting in translating comments from the survey into English. We would like to thank Thomy Tonia, the ERS methodologist, for valuable comments about the protocol. Finally, we would like to thank the Innovative Medicines Initiative 2 Joint Undertaking for the funding of this project.
Footnotes
The COMSA initiative is registered on the Core Outcome Measures in Effectiveness Trials (COMET) database (www.comet-initiative.org/Studies/Details/1698).
This article has editorial commentary: https://doi.org/10.1183/13993003.02058-2022 and https://doi.org/10.1183/13993003.02107-2022
Disclaimer: The content of this publication reflects only the authors’ views and the Joint Undertaking is not responsible for any use that may be made of the information it contains. B. Ahrens, S. Kaul, D. Hartenstein and V. Mahler state that the views expressed in this manuscript are the personal views of the authors and may not be understood or quoted as being made on behalf of or reflecting the position of the respective national competent authorities, the European Medicines Agency, or one of its committees or working parties.
Author contributions: G. Roberts and E. Khaleva: conceptualisation and methodology; E. Khaleva: statistical analysis of the votes; E. Khaleva: development of the survey; A. Rattu, C. Coleman and C. Williams: review of the survey; E. Khaleva: statistical analysis of the survey data and writing up; E. Khaleva and A. Rattu: thematic analysis of comments from the survey; E. Khaleva and A. Rattu: search strategies, title and abstract screening for the narrative review; C. Coleman and C. Williams: title, abstract and full-text screening and writing up of the narrative review; E. Khaleva: drafting of the original manuscript; all authors reviewed, edited and approved the manuscript.
Conflict of interest: E. Khaleva and A. Rattu declare funding from 3TR European Union Innovative Medicines Initiative 2 to their institution for the present manuscript. C. Brightling declares grants from GlaxoSmithKline, AstraZeneca, Novartis, Chiesi, Boehringer Ingelheim, Genentech, Roche, Sanofi, Mologic and 4DPharma; consulting fees from GlaxoSmithKline, AstraZeneca, Novartis, Chiesi, Boehringer Ingelheim, Genentech, Roche, Sanofi, Mologic, 4DPharma and Teva; and support from the 3TR project. G.W. Clarke declares that he is an employee of AstraZeneca; and that he holds stock or stock options in AstraZeneca. M. van den Berge declares grants from GlaxoSmithKline, AstraZeneca, Roche, Genentech and Novartis paid to the university. A. Bossios declares honoraria for lectures from GlaxoSmithKline, AstraZeneca, Teva and Novartis; support for attending meetings from AstraZeneca and Novartis; honoraria for advisory board meetings from GlaxoSmithKline, AstraZeneca, Teva, Novartis and Sanofi; being a member of the Steering Committee of SHARP, Secretary of Assembly 5 (Airway Diseases, Asthma, COPD and Chronic Cough) of the European Respiratory Society; Vice-chair of the Nordic Severe Asthma Network (NSAN). V. Ramiconi and S. Romagosa Vilarnau declare unrestricted educational grants paid to the organisation from Novartis, Pfizer, AstraZeneca, Chiesi Farmaceutici, GlaxoSmithKline, AbbVie, LeoPharma, Boehringer Ingelheim, Sanofi, Regeneron, OM Pharma, MSD, Roche and DBV Technologies; support for attending meetings from Novartis. S-E. Dahlén declares a 3TR Innovative Medicines Initiative grant; consulting fees for AstraZeneca, Cayman Co., GlaxoSmithKline, Novartis, Regeneron, Sanofi and Teva; payment for lectures from AstraZeneca and Sanofi. S. Principe declares support for provision of study materials and medical writing. G. Hedlin declares participation in advisory boards of AstraZeneca and Sanofi. F. Singer reports grants from Lung League Bern, grants from the Swiss Cystic Fibrosis Society (CFCH), personal fees from Vertex Pharmaceuticals, personal fees from Novartis, outside the submitted work. A. Deschildre reports personal consulting fees from Sanofi-Regeneron, ALK, Novartis and GlaxoSmithKline; honoraria for lectures from ALK, Boehringer Ingelheim and Novartis; support for attending meetings from AstraZeneca, Stallergènes-Greer, MEDA, Nutricia, Sanofi and Novartis, outside the submitted work; and being on the scientific committee of SFA (Société Française d'Allergologie). L.J. Fleming declares participation in advisory boards and honoraria for lectures from Sanofi, Respiri UK, AstraZeneca, Novartis and Teva, outside of the scope of this publication; all payments were made to her institution. H. Staudinger reports that he is a salaried employee of Sanofi Genzyme and owns company stock of Sanofi and Merck & Co. K.C. Pike declares consultancy fees from Novartis, Adherium and Respiri, and honoraria for a lecture from Novartis. J. Grigg declares payments from GlaxoSmithKline, OM Pharma, Omron and Novartis (advisory boards), Sanofi (for lectures) and AstraZeneca (CI clinical trial). N. Rutjes reports personal fees for advisory board work from Sanofi. G.H. Koppelman reports receiving research grants from the Lung Foundation of the Netherlands, Ubbo Emmius Foundation, H2020 European Union, Teva, GlaxoSmithKline and Vertex, outside this work (money to institution); he reports memberships of advisory boards to GlaxoSmithKline and PURE-IMS, outside this work (money to institution). D. Cunoosamy holds shares in AstraZeneca and Sanofi. A.H. Maitland-van der Zee has received research grants outside the submitted work from GlaxoSmithKline, Boehringer Ingelheim and Vertex, she is the PI of a P4O2 (Precision Medicine for more Oxygen) public private partnership sponsored by Health Holland involving many private partners that contribute in cash and/or in kind (Boehringer Ingelheim, Breathomix, Fluidda, Ortec Logiqcare, Philips, Quantib-U, Roche, Smartfish, SODAQ, Thirona, TopMD and Novartis), and she has served in advisory boards for AstraZeneca, GlaxoSmithKline and Boehringer Ingelheim with money paid to her institution. K.F. Chung has received honoraria for participating in advisory board meetings of GlaxoSmithKline, AstraZeneca, Roche, Novartis, Merck and Shionogi, regarding treatments for asthma, COPD and chronic cough, and has also been remunerated for speaking engagements for Novartis and AstraZeneca; received a MRC grant on Precision Medicine for severe asthma, EPSRC grant on air pollution and asthma, and a GlaxoSmithKline grant on mepolizumab and eosinophils in asthma. P. Nagakumar received speaker fees for talks on severe asthma from GlaxoSmithKline and Novartis. G. Brusselle declares payments from AstraZeneca, Novartis, Boehringer Ingelheim, Chiesi, Sanofi, GlaxoSmithKline and MSD, outside the submitted work. E. Hamelmann declares support from the German Ministry of Education and Research (BMBF) and German Asthma Net (GAN) eV; funding for research in severe asthma in children (CHAMP-01GL1742D) and for Severe Asthma Register. S. Vijverberg is PI of the PERMEABLE consortium. The PERMEABLE consortium is a research consortium focused on severe asthma and allergy and supported by ZonMW (456008004), the Swedish Research Council (2018-05619), the Ministry of Education, Science and Sport of the Republic of Slovenia (C3330-19-252012), and the German Ministry of Education and Research (BMBF) FKZ01KU1909A), under the frame of the ERA PerMed JTC 2018 Call. In addition, S. Vijverberg has received research funding for a project on severe paediatric asthma from the Lung Foundation Netherlands (6.2.18.244JO). A-M.M. Schoos has participated on an advisory board for ALK. B. Dahlén reports personal fees for lectures from AstraZeneca, Novartis and Sanofi; and grants from Novartis and GlaxoSmithKline, outside the submitted work; participation on an advisory board for AstraZeneca and Sanofi. A. Exley declares being a minority shareholder in GlaxoSmithKline Plc. E.A. Gaillard reports consultancy work for Boehringer Ingelheim with money paid to the institution (University of Leicester); investigator-led research grants from Circassia Group, Gilead Sciences, Chiesi Limited and Propeller Health; and has a research collaboration with Medimmune. M. Pijnenburg declares payments to her institution from Sanofi Genzyme (advisory work) and Novartis (speakers fee). E. Melén declares consulting fees from AstraZeneca, Chiesi, Novartis and Sanofi, outside the submitted work. R. Chaudhuri has received lecture fees from GlaxoSmithKline, AstraZeneca, Teva, Chiesi, Sanofi and Novartis; honoraria for advisory board meetings from GlaxoSmithKline, AstraZeneca, Teva, Chiesi and Novartis; sponsorship to attend international scientific meetings from Chiesi, Napp, Sanofi and GlaxoSmithKline, and a research grant to her institute from AstraZeneca for a UK multi-centre study. C. Pilette declares grants, consulting fees and honoraria for lectures (paid to institution) from AstraZeneca, Chiesi, GlaxoSmithKline, Novartis, Mundipharma, Teva, Sanofi and ALK. C. Porsbjerg declares grants (paid to institution), consulting fees (paid to institution and personal honoraria) and honoraria for lectures (paid to institution and personal honoraria) from AstraZeneca, GlaxoSmithKline, Novartis, Teva, Sanofi, Chiesi and ALK; participation on an advisory board (paid to institution and personal honoraria) for AstraZeneca, Novartis, Teva, Sanofi and ALK. C. Coleman and C. Williams declare funding received to support this work by the European Lung Foundation (ELF) from the European Commission's Innovative Medicines Initiative 2 Joint Undertaking under grant agreement number 831434 (3TR), and are employees of the ELF. B. Ahrens, S. Kaul, D. Hartenstein and V. Mahler declare no conflict of interest for this article and state that the views expressed in this review are the personal views of the author and may not be understood or quoted as being made on behalf of or reflecting the position of the respective national competent authorities, the European Medicines Agency or one of its committees or working parties. L.G. Heaney declares support from the 3TR; grants from industrial pharma partners Amgen, AstraZeneca, Medimmune, Janssen, Novartis, Roche/Genentech, GlaxoSmithKline and Boehringer Ingelheim; project grant funding from Medimmune, Novartis UK, Roche/Genentech and GlaxoSmithKline, outside the submitted work; payments for lectures by AstraZeneca, Novartis, Roche/Genentech, GlaxoSmithKline, Chiesi and Teva, outside the submitted work; travel funding support to international respiratory meetings by AstraZeneca, Chiesi, Novartis, Boehringer Ingelheim, Teva and GlaxoSmithKline, outside the submitted work; advisory boards for AstraZeneca, Novartis, GlaxoSmithKline, Chiesi, Teva, Theravance and Vectura, outside the submitted work. R. Djukanovic declares funding from the European Respiratory Society, Teva, GlaxoSmithKline, Novartis, Sanofi and Chiesi for the SHARP Clinical Research Collaboration; consulting fees for Synairgen; honorarium for a lecture from GlaxoSmithKline; participation on a data safety monitoring board or advisory board for Kymab (Cambridge) and shares in Synairgen outside of the submitted work. A. Bourdin declares grants from Boehringer and AstraZeneca; consulting fees and payments from Boehringer, AstraZeneca, GlaxoSmithKline, Novartis, Chiesi, Regeneron, Sanofi and Amgen outside of the submitted work. B. Karadag declares participation in a trial conducted by Sanofi (payment to institution) and attending advisory board meetings for GlaxoSmithKline (personal fees). K. Blumchen declares grants from Aimmune Therapeutics, DBV Technologies and Hipp GmbH; consulting fees from Aimmune Therapeutics, DBV Technologies and Allergy Therapeutics; payments for lectures from Aimmune Therapeutics, DBV Technologies, Novartis, Allergy Therapeutics, HAL, ALK, Allergopharma, Nutricia, Thermo Fisher Scientific, and Bausch and Lomb; personal fees for expert discussions from Novartis and Nestle; fees for attending meetings from Aimmune Therapeutics and DBV Technologies; being on data safety monitoring board of Charité, IIT. A. Gupta received speaker/advisory board fees from GlaxoSmithKline, Novartis, AstraZeneca and Boehringer Ingelheim; received research grants from GlaxoSmithKline, Novartis, AstraZeneca and Boehringer Ingelheim, paid to institution. L. Giovannini-Chami declares consulting fees from ALK, AstraZeneca and Novartis; honoraria for lectures, presentations from Novartis, ALK, Stallergènes, Sanofi and AstraZeneca; support for attending meetings from Stallergènes; participation on a data safety monitoring board or advisory board for Sanofi; being head of the Scientific Committee of the French Pediatric Pulmonology and Allergology Society. C.S. Murray has received lecture fees from GlaxoSmithKline and Novartis; received grants from Asthma UK and National Institute for Health Research; and has participated on an advisory board for Boehringer Ingelheim. G. Roberts declares European Union Innovative Medicines Initiative funding and AstraZeneca paid to the institution. Other co-authors declare no conflicts of interest for this article.
Support statement: This project has received funding from the Innovative Medicines Initiative (IMI) 2 Joint Undertaking (JU) under grant agreement number 831434 (3TR). The JU receives support from the European Union's Horizon 2020 research and innovation program and the European Federation of Pharmaceutical Industries and Associations (EFPIA). Further details about the 3TR project and IMI funding programme are available on their websites: www.3tr-imi.eu and www.imi.europa.eu. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Chung KF, Wenzel SE, Brozek JL, et al. . International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43: 343–373. doi: 10.1183/09031936.00202013 [DOI] [PubMed] [Google Scholar]
- 2.Dharmage SC, Perret JL, Custovic A. Epidemiology of asthma in children and adults. Front Pediatr 2019; 7: 246. doi: 10.3389/fped.2019.00246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foster JM, McDonald VM, Guo M, et al. . “I have lost in every facet of my life”: the hidden burden of severe asthma. Eur Respir J 2017; 50: 1700765. doi: 10.1183/13993003.00765-2017 [DOI] [PubMed] [Google Scholar]
- 4.Nordon C, Grimaldi-Bensouda L, Pribil C, et al. . Clinical and economic burden of severe asthma: a French cohort study. Respir Med 2018; 144: 42–49. doi: 10.1016/j.rmed.2018.10.002 [DOI] [PubMed] [Google Scholar]
- 5.Volmer T, Effenberger T, Trautner C, et al. . Consequences of long-term oral corticosteroid therapy and its side-effects in severe asthma in adults: a focused review of the impact data in the literature. Eur Respir J 2018; 52: 1800703. doi: 10.1183/13993003.00703-2018 [DOI] [PubMed] [Google Scholar]
- 6.Nagase H, Adachi M, Matsunaga K, et al. . Prevalence, disease burden, and treatment reality of patients with severe, uncontrolled asthma in Japan. Allergol Int 2020; 69: 53–60. doi: 10.1016/j.alit.2019.06.003 [DOI] [PubMed] [Google Scholar]
- 7.Chen W, Safari A, FitzGerald JM, et al. . Economic burden of multimorbidity in patients with severe asthma: a 20-year population-based study. Thorax 2019; 74: 1113–1119. doi: 10.1136/thoraxjnl-2019-213223 [DOI] [PubMed] [Google Scholar]
- 8.Pamuk G, Le Bourgeois M, Abou Taam R, et al. . The economic burden of severe asthma in children: a comprehensive study. J Asthma 2021; 58: 1467–1477. doi: 10.1080/02770903.2020.1802747 [DOI] [PubMed] [Google Scholar]
- 9.Moonie SA, Sterling DA, Figgs L, et al. . Asthma status and severity affects missed school days. J Sch Health 2006; 76: 18–24. doi: 10.1111/j.1746-1561.2006.00062.x [DOI] [PubMed] [Google Scholar]
- 10.Hiles SA, Harvey ES, McDonald VM, et al. . Working while unwell: workplace impairment in people with severe asthma. Clin Exp Allergy 2018; 48: 650–662. doi: 10.1111/cea.13153 [DOI] [PubMed] [Google Scholar]
- 11.Brusselle GG, Koppelman GH. Biologic therapies for severe asthma. N Engl J Med 2022; 386: 157–171. doi: 10.1056/NEJMra2032506 [DOI] [PubMed] [Google Scholar]
- 12.Agache I, Beltran J, Akdis C, et al. . Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI Guidelines – recommendations on the use of biologicals in severe asthma. Allergy 2020; 75: 1023–1042. doi: 10.1111/all.14221 [DOI] [PubMed] [Google Scholar]
- 13.Agache I, Rocha C, Beltran J, et al. . Efficacy and safety of treatment with biologicals (benralizumab, dupilumab and omalizumab) for severe allergic asthma: a systematic review for the EAACI Guidelines – recommendations on the use of biologicals in severe asthma. Allergy 2020; 75: 1043–1057. doi: 10.1111/all.14235 [DOI] [PubMed] [Google Scholar]
- 14.Agache I, Song Y, Rocha C, et al. . Efficacy and safety of treatment with dupilumab for severe asthma: a systematic review of the EAACI guidelines – recommendations on the use of biologicals in severe asthma. Allergy 2020; 75: 1058–1068. doi: 10.1111/all.14268 [DOI] [PubMed] [Google Scholar]
- 15.Bush A. Which child with asthma is a candidate for biological therapies? J Clin Med 2020; 9: 1237. doi: 10.3390/jcm9041237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bousquet J, Brusselle G, Buhl R, et al. . Care pathways for the selection of a biologic in severe asthma. Eur Respir J 2017; 50: 1701782. doi: 10.1183/13993003.01782-2017 [DOI] [PubMed] [Google Scholar]
- 17.Akinbami LJ, Sullivan SD, Campbell JD, et al. . Asthma outcomes: healthcare utilization and costs. J Allergy Clin Immunol 2012; 129: 3 Suppl., S49–S64. doi: 10.1016/j.jaci.2011.12.984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cloutier MM, Schatz M, Castro M, et al. . Asthma outcomes: composite scores of asthma control. J Allergy Clin Immunol 2012; 129: 3 Suppl., S24–S33. doi: 10.1016/j.jaci.2011.12.980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krishnan JA, Lemanske RF Jr, Canino GJ, et al. . Asthma outcomes: symptoms. J Allergy Clin Immunol 2012; 129: 3 Suppl., S124–S135. doi: 10.1016/j.jaci.2011.12.981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szefler SJ, Wenzel S, Brown R, et al. . Asthma outcomes: biomarkers. J Allergy Clin Immunol 2012; 129: 3 Suppl., S9–S23. doi: 10.1016/j.jaci.2011.12.979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tepper RS, Wise RS, Covar R, et al. . Asthma outcomes: pulmonary physiology. J Allergy Clin Immunol 2012; 129: 3 Suppl., S65–S87. doi: 10.1016/j.jaci.2011.12.986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson SR, Rand CS, Cabana MD, et al. . Asthma outcomes: quality of life. J Allergy Clin Immunol 2012; 129: 3 Suppl., S88–S123. doi: 10.1016/j.jaci.2011.12.988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tejwani V, Chang HY, Tran AP, et al. . A multistakeholder Delphi consensus core outcome set for clinical trials in moderate-to-severe asthma (coreASTHMA). Ann Allergy Asthma Immunol 2021; 127: 116–122. doi: 10.1016/j.anai.2021.03.022 [DOI] [PubMed] [Google Scholar]
- 24.Gliklich RE, Castro M, Leavy MB, et al. . Harmonized outcome measures for use in asthma patient registries and clinical practice. J Allergy Clin Immunol 2019; 144: 671–681. doi: 10.1016/j.jaci.2019.02.025 [DOI] [PubMed] [Google Scholar]
- 25.Reddel HK, Taylor DR, Bateman ED, et al. . An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med 2009; 180: 59–99. doi: 10.1164/rccm.200801-060ST [DOI] [PubMed] [Google Scholar]
- 26.Prinsen CA, Vohra S, Rose MR, et al. . How to select outcome measurement instruments for outcomes included in a “Core Outcome Set” – a practical guideline. Trials 2016; 17: 449. doi: 10.1186/s13063-016-1555-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirkham JJ, Gorst S, Altman DG, et al. . COS-STAR: a reporting guideline for studies developing core outcome sets (protocol). Trials 2015; 16: 373. doi: 10.1186/s13063-015-0913-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rattu A, Khaleva E, Brightling C, et al. . Identifying and appraising outcome measures for severe asthma: a systematic review. Eur Respir J 2023; 61: 2201231. doi: 10.1183/23120541.00000-000 [DOI] [PubMed] [Google Scholar]
- 29.Guyatt GH, Oxman AD, Kunz R, et al. . GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol 2011; 64: 395–400. doi: 10.1016/j.jclinepi.2010.09.012 [DOI] [PubMed] [Google Scholar]
- 30.Mokkink LB, de Vet HCW, Prinsen CAC, et al. . COSMIN Risk of Bias checklist for systematic reviews of Patient-Reported Outcome Measures. Qual Life Res 2018; 27: 1171–1179. doi: 10.1007/s11136-017-1765-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prinsen CAC, Mokkink LB, Bouter LM, et al. . COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual Life Res 2018; 27: 1147–1157. doi: 10.1007/s11136-018-1798-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terwee CB, Prinsen CAC, Chiarotto A, et al. . COSMIN methodology for evaluating the content validity of patient-reported outcome measures: a Delphi study. Qual Life Res 2018; 27: 1159–1170. doi: 10.1007/s11136-018-1829-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balshem H, Helfand M, Schunemann HJ, et al. . GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011; 64: 401–406. doi: 10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 34.Charles D, Shanley J, Temple SN, et al. . Real-world efficacy of treatment with benralizumab, dupilumab, mepolizumab and reslizumab for severe asthma: a systematic review and meta-analysis. Clin Exp Allergy 2022; 52: 616–627. doi: 10.1111/cea.14112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Webbe J, Brunton G, Ali S, et al. . Developing, implementing and disseminating a core outcome set for neonatal medicine. BMJ Paediatr Open 2017; 1: e000048. doi: 10.1136/bmjpo-2017-000048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williamson PR, Altman DG, Bagley H, et al. . The COMET Handbook: version 1.0. Trials 2017; 18: Suppl. 3, 280. doi: 10.1186/s13063-017-1978-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Apps LD, Chantrell S, Majd S, et al. . Patient perceptions of living with severe asthma: challenges to effective management. J Allergy Clin Immunol Pract 2019; 7: 2613–2621. doi: 10.1016/j.jaip.2019.04.026 [DOI] [PubMed] [Google Scholar]
- 38.Donald KJ, McBurney H, Browning C. Self management beliefs – attitudes and behaviour of adults with severe life threatening asthma requiring an admission to hospital. Aust Fam Physician 2005; 34: 197–200. [PubMed] [Google Scholar]
- 39.McMullen AH, Yoos HL, Kitzman H. Peak flow meters in childhood asthma: parent report of use and perceived usefulness. J Pediatr Health Care 2002; 16: 67–72. doi: 10.1067/mph.2002.116132 [DOI] [PubMed] [Google Scholar]
- 40.Hyland ME, Whalley B, Jones RC, et al. . A qualitative study of the impact of severe asthma and its treatment showing that treatment burden is neglected in existing asthma assessment scales. Qual Life Res 2015; 24: 631–639. doi: 10.1007/s11136-014-0801-x [DOI] [PubMed] [Google Scholar]
- 41.Clark VL, Gibson PG, McDonald VM. What matters to people with severe asthma? Exploring add-on asthma medication and outcomes of importance. ERJ Open Res 2021; 7: 00497-2020. doi: 10.1183/23120541.00497-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gamble J, Fitzsimons D, Lynes D, et al. . Difficult asthma: people's perspectives on taking corticosteroid therapy. J Clin Nurs 2007; 16: 59–67. doi: 10.1111/j.1365-2702.2006.01750.x [DOI] [PubMed] [Google Scholar]
- 43.Aburuz S, Gamble J, Heaney LG. Assessment of impairment in health-related quality of life in patients with difficult asthma: psychometric performance of the Asthma Quality of Life Questionnaire. Respirology 2007; 12: 227–233. doi: 10.1111/j.1440-1843.2006.01020.x [DOI] [PubMed] [Google Scholar]
- 44.Juniper EF, Guyatt GH, Ferrie PJ, et al. . Measuring quality of life in asthma. Am Rev Respir Dis 1993; 147: 832–838. doi: 10.1164/ajrccm/147.4.832 [DOI] [PubMed] [Google Scholar]
- 45.Juniper EF, Guyatt GH, Epstein RS, et al. . Evaluation of impairment of health related quality of life in asthma: development of a questionnaire for use in clinical trials. Thorax 1992; 47: 76–83. doi: 10.1136/thx.47.2.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Juniper EF, Buist AS, Cox FM, et al. . Validation of a standardized version of the Asthma Quality of Life Questionnaire. Chest 1999; 115: 1265–1270. doi: 10.1378/chest.115.5.1265 [DOI] [PubMed] [Google Scholar]
- 47.Juniper EF, Guyatt GH, Cox FM, et al. . Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J 1999; 14: 32–38. doi: 10.1034/j.1399-3003.1999.14a08.x [DOI] [PubMed] [Google Scholar]
- 48.Hyland ME, Jones RC, Lanario JW, et al. . The construction and validation of the Severe Asthma Questionnaire. Eur Respir J 2018; 52: 1800618. doi: 10.1183/13993003.00618-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hyland ME, Lanario JW, Pooler J, et al. . How patient participation was used to develop a questionnaire that is fit for purpose for assessing quality of life in severe asthma. Health Qual Life Outcomes 2018; 16: 24. doi: 10.1186/s12955-018-0851-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Masoli M, Lanario JW, Hyland ME, et al. . The Severe Asthma Questionnaire: sensitivity to change and minimal clinically important difference. Eur Respir J 2021; 57: 2100300. doi: 10.1183/13993003.00300-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nathan RA, Sorkness CA, Kosinski M, et al. . Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol 2004; 113: 59–65. doi: 10.1016/j.jaci.2003.09.008 [DOI] [PubMed] [Google Scholar]
- 52.Schatz M, Kosinski M, Yarlas AS, et al. . The minimally important difference of the Asthma Control Test. J Allergy Clin Immunol 2009; 124: 719–723. doi: 10.1016/j.jaci.2009.06.053 [DOI] [PubMed] [Google Scholar]
- 53.Schatz M, Sorkness CA, Li JT, et al. . Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol 2006; 117: 549–556. doi: 10.1016/j.jaci.2006.01.011 [DOI] [PubMed] [Google Scholar]
- 54.Juniper EF, O'Byrne PM, Guyatt GH, et al. . Development and validation of a questionnaire to measure asthma control. Eur Respir J 1999; 14: 902–907. doi: 10.1034/j.1399-3003.1999.14d29.x [DOI] [PubMed] [Google Scholar]
- 55.Juniper EF, O'Byrne PM, Roberts JN. Measuring asthma control in group studies: do we need airway calibre and rescue beta2-agonist use? Respir Med 2001; 95: 319–323. doi: 10.1053/rmed.2001.1034 [DOI] [PubMed] [Google Scholar]
- 56.Wyrwich KW, Khan SA, Navaratnam P, et al. . Validation and agreement across four versions of the asthma control questionnaire in patients with persistent asthma. Respir Med 2011; 105: 698–712. doi: 10.1016/j.rmed.2010.11.004 [DOI] [PubMed] [Google Scholar]
- 57.Patino CM, Okelo SO, Rand CS, et al. . The Asthma Control and Communication Instrument: a clinical tool developed for ethnically diverse populations. J Allergy Clin Immunol 2008; 122: 936–943. doi: 10.1016/j.jaci.2008.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Quanjer PH, Stanojevic S, Cole TJ, et al. . Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40: 1324–1343. doi: 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bourdin A, Bjermer L, Brightling C, et al. . ERS/EAACI statement on severe exacerbations in asthma in adults: facts, priorities and key research questions. Eur Respir J 2019; 54: 1900900. doi: 10.1183/13993003.00900-2019 [DOI] [PubMed] [Google Scholar]
- 60.Juniper EF, Guyatt GH, Feeny DH, et al. . Measuring quality of life in children with asthma. Qual Life Res 1996; 5: 35–46. doi: 10.1007/BF00435967 [DOI] [PubMed] [Google Scholar]
- 61.Juniper EF, Guyatt GH, Feeny DH, et al. . Minimum skills required by children to complete health-related quality of life instruments for asthma: comparison of measurement properties. Eur Respir J 1997; 10: 2285–2294. doi: 10.1183/09031936.97.10102285 [DOI] [PubMed] [Google Scholar]
- 62.Townsend M, Feeny DH, Guyatt GH, et al. . Evaluation of the burden of illness for pediatric asthmatic patients and their parents. Ann Allergy 1991; 67: 403–408. [PubMed] [Google Scholar]
- 63.Wing A, Upton J, Svensson K, et al. . The standardized and mini versions of the PAQLQ are valid, reliable, and responsive measurement tools. J Clin Epidemiol 2012; 65: 643–650. doi: 10.1016/j.jclinepi.2011.12.009 [DOI] [PubMed] [Google Scholar]
- 64.Bime C, Gerald JK, Wei CY, et al. . Measurement characteristics of the childhood Asthma-Control Test and a shortened, child-only version. NPJ Prim Care Respir Med 2016; 26: 16075. doi: 10.1038/npjpcrm.2016.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu AH, Zeiger R, Sorkness C, et al. . Development and cross-sectional validation of the Childhood Asthma Control Test. J Allergy Clin Immunol 2007; 119: 817–825. doi: 10.1016/j.jaci.2006.12.662 [DOI] [PubMed] [Google Scholar]
- 66.Juniper EF, Gruffydd-Jones K, Ward S, et al. . Asthma Control Questionnaire in children: validation, measurement properties, interpretation. Eur Respir J 2010; 36: 1410–1416. doi: 10.1183/09031936.00117509 [DOI] [PubMed] [Google Scholar]
- 67.Nguyen JM, Holbrook JT, Wei CY, et al. . Validation and psychometric properties of the Asthma Control Questionnaire among children. J Allergy Clin Immunol 2014; 133: 91–97. doi: 10.1016/j.jaci.2013.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wildfire JJ, Gergen PJ, Sorkness CA, et al. . Development and validation of the Composite Asthma Severity Index – an outcome measure for use in children and adolescents. J Allergy Clin Immunol 2012; 129: 694–701. doi: 10.1016/j.jaci.2011.12.962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krouse RZ, Sorkness CA, Wildfire JJ, et al. . Minimally important differences and risk levels for the Composite Asthma Severity Index. J Allergy Clin Immunol 2017; 139: 1052–1055. doi: 10.1016/j.jaci.2016.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gaillard EA, Kuehni CE, Turner S, et al. . European Respiratory Society clinical practice guidelines for the diagnosis of asthma in children aged 5–16 years. Eur Respir J 2021; 58: 2004173. doi: 10.1183/13993003.04173-2020 [DOI] [PubMed] [Google Scholar]
- 71.Fleming L, Murray C, Bansal AT, et al. . The burden of severe asthma in childhood and adolescence: results from the paediatric U-BIOPRED cohorts. Eur Respir J 2015; 46: 1322–1333. doi: 10.1183/13993003.00780-2015 [DOI] [PubMed] [Google Scholar]
- 72.Melen E, Guerra S, Hallberg J, et al. . Linking COPD epidemiology with pediatric asthma care: implications for the patient and the physician. Pediatr Allergy Immunol 2019; 30: 589–597. doi: 10.1111/pai.13054 [DOI] [PubMed] [Google Scholar]
- 73.Khatri SB, Iaccarino JM, Barochia A, et al. . Use of fractional exhaled nitric oxide to guide the treatment of asthma: an official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med 2021; 204: e97–e109. doi: 10.1164/rccm.202109-2093ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anderson WC 3rd, Szefler SJ. Cost-effectiveness and comparative effectiveness of biologic therapy for asthma: to biologic or not to biologic? Ann Allergy Asthma Immunol 2019; 122: 367–372. doi: 10.1016/j.anai.2019.01.018 [DOI] [PubMed] [Google Scholar]
- 75.McQueen RB, Sheehan DN, Whittington MD, et al. . Cost-effectiveness of biological asthma treatments: a systematic review and recommendations for future economic evaluations. Pharmacoeconomics 2018; 36: 957–971. doi: 10.1007/s40273-018-0658-x [DOI] [PubMed] [Google Scholar]
- 76.Reddel HK, Bacharier LB, Bateman ED, et al. . Global Initiative for Asthma Strategy 2021: executive summary and rationale for key changes. Eur Respir J 2022; 59: 2102730. doi: 10.1183/13993003.02730-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00606-2022.Supplement (4.5MB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-00606-2022.Shareable (1.2MB, pdf)