Abstract
PHYTOCHROME-INTERACTING FACTORs (PIFs) repress photosynthetic genes partly by upregulating REPRESSOR OF PHOTOSYNTHETIC GENES 1 (RPGE1) and RPGE2. However, it is unknown how RPGEs inhibit gene expression at the molecular level. Here, we show that Arabidopsis (Arabidopsis thaliana) RPGE overexpression lines display extensive similarities to the golden2-like 1 (glk1)/glk2 double mutant at the phenotypic and transcriptomic levels, prompting us to hypothesize that there is a close molecular relationship between RPGEs and chloroplast development-regulating GLK transcription factors. Indeed, we found that RPGE1 disrupts the homodimerization of GLK1 by interacting with its dimerization domain and debilitates the DNA-binding activity of GLK1. The interaction was not restricted to the Arabidopsis RPGE1-GLK1 pair, but rather extended to RPGE-GLK homolog pairs across species, providing a molecular basis for the pale green leaves of Arabidopsis transgenic lines expressing a rice (Oryza sativa) RPGE homolog. Our discovery of RPGE-GLK regulatory pairs suggests that any condition leading to an increase in RPGE levels would decrease the expression levels of GLK target genes. Consistently, we found that shade, which upregulates the RPGE mRNA by stabilizing PIFs, represses the expression of photosynthetic genes partly by inhibiting the DNA-binding activity of GLK1. Taken together, these results indicate that RPGE-GLK regulatory pairs regulate photosynthetic gene expression downstream of PIFs.
Shade represses photosynthetic genes by increasing the expression of a small protein that disrupts the DNA binding of a key transcription factor.
Introduction
Phytochromes (phys) are red and far-red light photoreceptors that constitute a set of plant photoreceptors together with 3 blue light photoreceptors (cryptochromes, phototropins, and zeitlupe), and the UV light photoreceptor, UV RESISTANCE LOCUS 8 (UVR8) (reviewed in Galvao and Fankhauser, 2015). Different plant species possess different numbers of phy (Li et al., 2015): For example, Arabidopsis (Arabidopsis thaliana) possesses 5 phys (phyA to phyE) (Clack et al., 1994) and rice (Oryza sativa) possesses 3 phys (phyA to phyC) (Takano et al., 2005); in both cases, phyA and phyB are the major phys responsible for promoting light responses. Active phys promote seed germination, seedling photomorphogenesis, and chloroplast development, but repress shade avoidance response and leaf senescence (reviewed in Franklin and Quail, 2010 and Galvao and Fankhauser, 2015). Phys regulate light responses partly by interacting with and inhibiting the phytochrome-interacting factors (PIFs), which are a group of basic helix-loop-helix (bHLH) transcription factors that repress light responses in the dark (reviewed in Leivar and Quail, 2011, and Lee and Choi, 2017). Eight different PIFs have been characterized for their ability to regulate various light responses downstream of phys, either redundantly or distinctively, in Arabidopsis (Oh et al., 2020, reviewed in Pham et al., 2017).
Phy-PIF regulatory pairs regulate chloroplast development by regulating photosynthetic genes (reviewed in Kobayashi and Masuda, 2016; Cackett et al., 2022). In the dark, plastids develop as etioplasts that have poorly developed thylakoids and a prolamellar body, which is a semi-crystalline lattice made of protochlorophyllide, protochlorophyllide oxidoreductase (POR), and membranes (reviewed in Solymosi and Schoefs, 2010). When etiolated seedlings are exposed to light, etioplasts transform to chloroplasts via processes including the POR-mediated and light-driven conversion of protochlorophyllide to chlorophyllide, the development of thylakoids with stacked grana, and the expansion of photosynthetic capacity by increasing levels of photosynthetic components (e.g. photosynthetic pigments, light-harvesting complexes, and Calvin cycle enzymes). The transition of etioplasts to chloroplasts enhances a slew of chloroplast metabolic processes, including sugar and amino acid metabolisms geared toward photoautotrophic growth and development (reviewed in Cackett et al., 2022). Phy-PIF regulatory pairs play key roles in regulating seedling photomorphogenesis, as evidenced by the failure of a phy quintuple mutant to deetiolate in red light (Strasser et al., 2010; Hu et al., 2013) and the failure of a pif multiple mutant to etiolate in the dark (Leivar et al., 2008; Shin et al., 2009). Chlorophyll biosynthesis is promoted by active phys and repressed by PIFs, as supported by the hypoaccumulation of chlorophyll in phy multiple mutants (Hu et al., 2013) and the hyperaccumulation of chlorophyll intermediates in pif mutants in the dark (Huq et al., 2004; Shin et al., 2009; Stephenson et al., 2009). Transcriptomic analyses further showed that a large portion of PIF-regulated genes are photosynthetic genes, including chlorophyll biosynthetic genes, light-harvesting complex genes, and Calvin cycle genes (Leivar et al., 2009; Shin et al., 2009). However, genome-wide binding site analysis of PIFs indicated that only a few photosynthetic genes are direct target genes of PIF, suggesting that PIFs indirectly regulate the majority of photosynthetic genes (Oh et al., 2009; Hornitschek et al., 2012; Zhang, 2013).
The members of the GOLDEN2-LIKE (GLK) transcription factor family regulate chloroplast development downstream of phy-PIF regulatory pairs (Song et al., 2014; Martín et al., 2016). The 2 GLKs in land plants, GLK1 and GLK2, are GARP (Golden2, ARR-B, Psr1) family transcription factors that possess a DNA-binding Myb-related domain and a dimerizing GCT domain (Rossini et al., 2001, Fitter, 2002). GLKs directly bind to the promoters of various photosynthetic genes through a CCAATC core sequence, and thereby activate their mRNA expression (Waters et al., 2009). Consistent with the actions of GLK1 and GLK2 as key transcription factors for photosynthetic genes in land plants, glk1/glk2 double mutants (glk1/2) develop pale green leaves in Arabidopsis, Oryza sativa, tomato (Solanum lycopersicum), and moss (Physcomitrium patens), reflecting that their chloroplasts have fewer unstacked thylakoid grana and reduced chlorophyll levels (reviewed in Cackett et al., 2022). Conversely, GLK overexpression lines produce dark green leaves in Arabidopsis, reflecting that their chloroplasts have larger thylakoid grana and increased chlorophyll levels. GATA, NITRATE-INDUCIBLE, CARBON-METABOLISM-INVOLVED (GNC) and its homolog, CYTOKININ-RESPONSIVE GATA1 (CGA1/GNC-LIKE; GNL), represent another family of transcription factors that promote chloroplast development (Bi, 2005; Mara and Irish, 2008). GNCs are GATA-type transcription factors that bind to the promoters of many different genes, including the brassinosteroid (BR) biosynthetic genes, DWARF3 (DWF3), DWF4, and DWF7, through a GATC core sequence and subsequently regulate their mRNA expression (Zubo et al., 2018). Similar to the situation described for GLKs, the gnc/cga1 double mutant develops slightly pale green leaves with small chloroplasts and reduced chlorophyll levels, while GNC overexpression lines develop chloroplasts even in the epidermis and root cortex (Mara and Irish, 2008; Hudson et al., 2011). Analysis of the glk1/glk2/gnc/cga1 quadruple mutant further showed that although the 2 transcription factors have similar functions, GLKs primarily contribute to the expansion of thylakoids while GNCs more strongly contribute to chloroplast division (Zubo et al., 2018). Phy-PIF regulatory pairs regulate chloroplast development partly through GLKs and GNCs, as PIFs directly bind to the promoters of both GLKs and GNCs to repress their mRNA expression levels (Richter et al., 2010; Song et al., 2014).
GLKs and GNCs, however, are not the sole regulators of chloroplast development downstream of phy-PIF regulatory pairs. We previously showed that REPRESSOR OF PHOTOSYNTHETIC GENES 1 (RPGE1) represses the expression of photosynthetic genes downstream of PIFs, and its overexpression causes pale leaves in Arabidopsis (Kim et al., 2016). The RPGE family has 4 members (RPGE1 to RPGE4) in Arabidopsis. RPGEs lack any identified functional protein domain, making it difficult to predict their molecular functions. In the present study, we investigated how RPGE represses photosynthetic genes at the molecular level. We found that RPGE represses photosynthetic genes downstream of phy-PIF regulatory pairs by disrupting the dimerization of GLK and inhibiting its DNA-binding activity.
Results
RPGEs and GLKs antagonistically regulate chlorophyll biosynthesis
We previously reported that PIFs activate the mRNA expression of RPGE1 and RPGE2 by directly binding to their promoters, and transgenic lines overexpressing RPGE1 and RPGE2 under the control of the 35S promoter (RPGE1-OX, RPGE2-OX) develop pale green leaves (Kim et al., 2016). RPGEs belong to a small protein family composed of 4 members (RPGE1 to RPGE4) in Arabidopsis. RPGE homologs are found not only in land plants, such as liverwort (Marchantia polymorpha), rice, and tomato, but also in charophytes, such as Chara braunii and Klebsormidium nitens (Supplemental Figure 1A). The presence of RPGE homologs in streptophytes suggests that RPGEs are important regulators of chloroplast development. Interestingly, streptophytes that have RPGE homologs also possess GLK homologs (Supplemental Figure 1B), which are known as the master regulators of chloroplast development. This co-existence of RPGE-GLK pairs led us to speculate that RPGEs and GLKs might be functionally related.
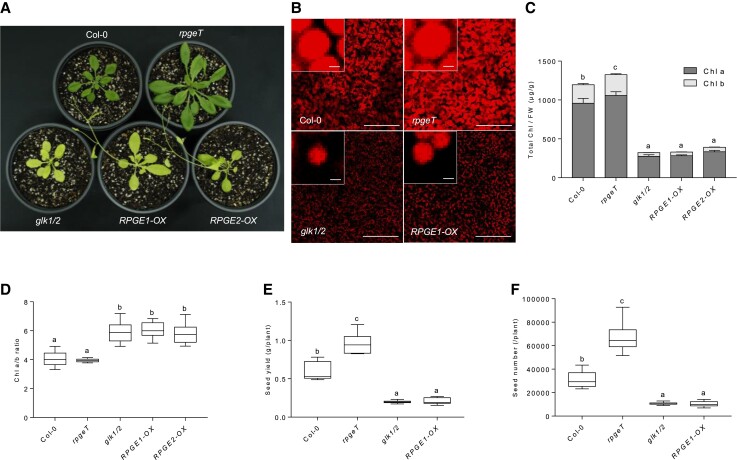
To investigate this hypothesis, we systematically compared the phenotypes of RPGE-OXs and the glk1/glk2 double mutant (glk1/2). Since RPGE1-OX and RPGE2-OX have virtually identical phenotypes, we will hereinafter refer to them as RPGE-OX without distinguishing RPGE1 and RPGE2, unless otherwise indicated. We also generated a rpge1/rpge2/rpge3 triple mutant (rpgeT) using the CRISPR-Cas9 system (Supplemental Figure 2) and included the rpgeT mutant for the comparison. We found that RPGE-OX develops pale green leaves strikingly similar to those of the glk1/2 mutant in white light (Figure 1A). Consistent with their pale green leaves, RPGE-OX and the glk1/2 mutant emit weaker chlorophyll autofluorescence (which reflects the amount of chlorophyll) than wild-type plants; in contrast, the rgpeT mutant emits stronger autofluorescence (Figure 1B). Consistently, RPGE-OX and the glk1/2 mutant have lower chlorophyll levels than wild-type, while the rpgeT mutant has a higher chlorophyll level than wild-type (Figure 1C). Among the chlorophylls, chlorophyll b is proportionally more reduced than chlorophyll a in RPGE-OX and the glk1/2 mutant (Figure 1D). The glk1/2 mutant is known to have a lower maximal possible value for chlorophyll fluorescence (Fm) and a lower minimum value for chlorophyll fluorescence (F0) values than wild-type. Consistently, RPGE-OX and the glk1/2 mutant have lower Fm and F0 but higher maximum quantum yield of PSII chemistry (Fv/Fm) than wild-type (Table 1).
Figure 1.
RPGE overexpression lines show phenotypes similar to those of the glk1/glk2 double mutant. A, Pale green leaves in RPGE-OXs and the glk1/2 mutant. Plants were grown in a long-day condition (LD; 16 hours light/8 hours dark; white light, 100 µmol m−2s) at 22°C for 30 days. RPGE1-OX and RPGE2-OX indicate transgenic lines expressing RPGE1 and RPGE2, respectively, under the 35S promoter. rpgeT: rpge1/rpge2/rpge3 triple mutant. glk1/2: glk1/glk2 double mutant. B–D, Chlorophyll related-phenotypes of RPGE-OXs, the rpgeT mutant, and the glk1/2 mutant. Seedlings were grown in continuous white light (WLc; 50 µmol m−2s) at 22°C for 7 days. B, Decreased chlorophyll autofluorescence of RPGE-OXs and the glk1/2 mutant. Autofluorescence images were taken from cotyledons under a confocal microscope with the same exposure time. Scale bar = 200 µm. Insets present magnified views of a single chloroplast (scale bar = 2 µm). C, Decreased chlorophyll levels of RPGE-OXs and the glk1/2 mutant. Chlorophyll levels were measured from whole seedlings and normalized by the fresh weight. Letters indicate statistical significance, which was determined by ANOVA with Tukey's HSD post-hoc test for multiple comparisons (P < 0.01). Error bars indicate SD (n = 3 biological replicates). D, Preferential increase of chlorophyll a to chlorophyll b ratio (Chl a/b) in RPGE-OXs and the glk1/2 mutant. Letters indicate statistical significance, as determined by ANOVA with Tukey's HSD post-hoc test for multiple comparisons (P < 0.01). The borders of the boxes indicate the 25th and 75th percentiles. The horizontal line indicates the median, and the whiskers indicates min to max values. E, Decreased seed yield per plant in RPGE-OXs and the glk1/2 mutant. Letters indicate statistical significance, as determined by ANOVA with Tukey's HSD post-hoc test for multiple comparisons (P < 0.01). The borders of the boxes indicate the 25th and 75th percentiles. The horizontal line indicates the median, and the whiskers indicates min to max values. F, Decreased seed numbers per plant in RPGE-OXs and the glk1/2 mutant. Letters indicate statistical significance, as determined by ANOVA with Tukey's HSD post-hoc test for multiple comparisons (P < 0.01). The borders of the boxes indicate the 25th and 75th percentiles. The horizontal line indicates the median, and the whiskers indicates min to max values.
Table 1.
The maximum quantum efficiency of photosystem II (Fv/Fm). Plants were grown in long-day conditions (16 hours light/8 hours dark; white light, 100 µmol m−2s) at 22°C for 30 days, and the 5th and 6th leaves were used for the measurement. Fv/Fm: maximum quantum efficiency of photosystem II, F0: minimum value for chlorophyll fluorescence, Fm: maximal possible value for chlorophyll fluorescence. Letters (a,b,c) indicate statistical significance, as determined by ANOVA with Tukey's HSD post-hoc test for multiple comparisons (P < 0.01). The ± values indicate SD (Col-0: 9 leaves, rpgeT: 7 leaves, glk1/2: 10 leaves, and RPGE1-OX: 8 leaves)
| Col-0 | rpgeT | glk1/2 | RPGE1-OX | |
|---|---|---|---|---|
| Fv/Fm | 0.848 ± 0.003a | 0.845 ± 0.006a | 0.853 ± 0.003b | 0.866 ± 0.005c |
| F 0 | 400.667 ± 9.887 | 422.571 ± 11.998 | 176.625 ± 8.568 | 228.1 ± 16.441 |
| Fm | 2641.667 ± 72.102 | 2737.429 ± 138.327 | 1209.25 ± 72.302 | 1706.8 ± 129.193 |
| (n = 9) | (n = 7) | (n = 10) | (n = 8) |
The pale green leaves and low Fm and F0 values suggested that RPGE-OX and the glk1/2 mutant are likely to have lower seed yields than wild-type, as the enhancement of photosynthesis has been shown to improve crop-seed yields (Li et al., 2020, reviewed in Long et al., 2015; Ort et al., 2015). To determine seed yields, we grew plants individually in pots under a long-day (LD; 16 hours light/8 hours dark) cycle in a growth room, and harvested the seeds. Both RPGE-OX and the glk1/2 mutant have lower total seed yields per plant than wild-type, whereas the rpgeT mutant has a higher seed yield (Figure 1E). The differences in total seed yields are largely due to differences in the seed number per plant (Figure 1F). Taken together, these results indicate that RPGE-OX and the glk1/2 mutant are similar in having pale green leaves, low chlorophyll levels, and low seed yields.
RPGEs and GLKs antagonistically regulate photosynthetic gene expression
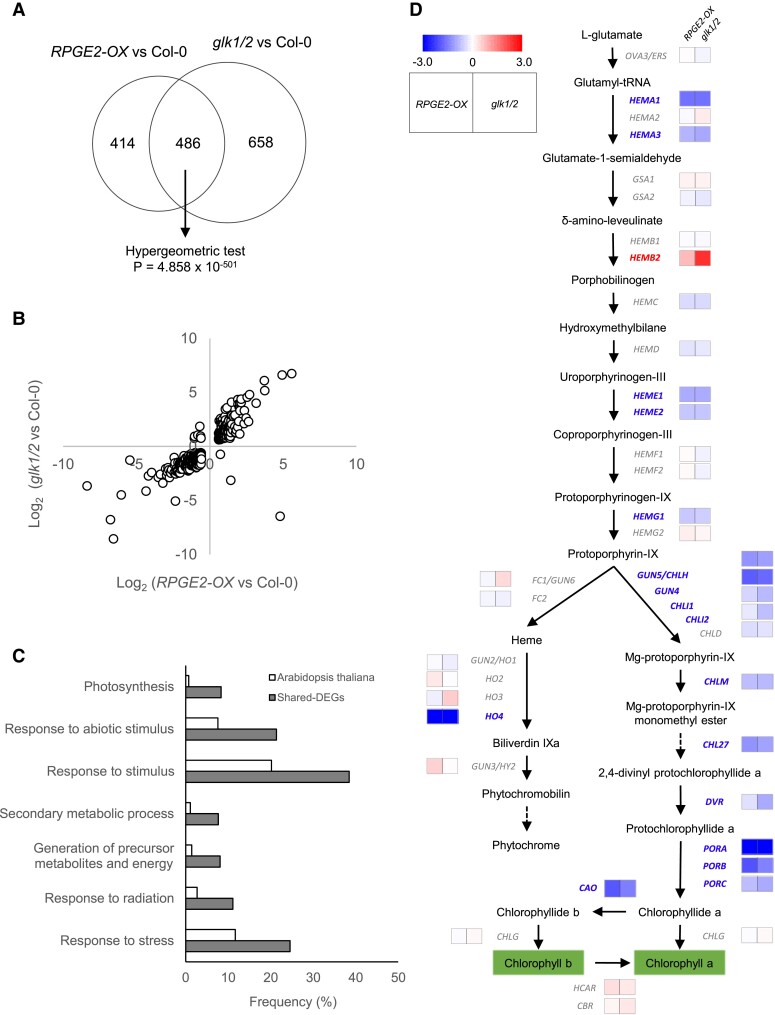
We further investigated the functional relationship between RPGEs and GLKs by analyzing transcriptomes of RPGE2-OX, the glk1/2 mutant, and Col-0 seedlings that are grown in continuous white light for 7 days. Since phenotypic similarities are clearly seen in white light-grown plants, we performed the transcriptome analysis with white-light grown seedlings. We identified 900 differentially expressed genes (DEGs, 1.5-fold and false discovery rate [FDR] 0.05) when comparing RPGE2-OX and Col-0, and 1,144 DEGs when comparing the glk1/2 mutant and Col-0 (Figure 2A). The 2 sets of DEGs show significant overlap (486 shared DEGs, hypergeometric test, P = 4.858 × 10−501) (Figure 2A), indicating that RPGE2 and GLKs co-regulate a set of genes. A dot plot analysis shows that, of the shared DEGs, 477 are altered in the same direction with a high correlation coefficient (r = 0.8597) (Figure 2B). Since the transcriptomes of RPGE2-OX and the glk1/2 mutant were used for the comparison, this expression pattern indicates that RPGE2 and GLKs antagonistically regulate the expression levels of the shared DEGs.
Figure 2.
RPGE2-OX and the glk1/2 mutant possess similar transcriptomes characterized by the repression of chlorophyll biosynthetic and photosynthetic genes. A, A Venn diagram showing the significant overlap between DEGs of the RPGE2 (RPGE2-OX vs Col-0) and glk1/2 (glk1/2 vs Col-0) mutants. Transcriptome analyses were performed with seedlings grown under continuous white light (50 µmol m−2s) for 7 days. The hypergeometric test was used to test the statistical significance of the overlap between the 2 sets of DEGs. B, Co-regulation of the shared DEGs. Log2(Fold Change) of the shared DEGs identified in (A) is plotted. C, Gene ontology (GO) analysis of the shared DEGs showing enrichment for photosynthesis-related terms. The top 7 enriched biological processes are ranked by their P-values. Black bars indicate the percentage of the shared DEG genes belonging to each GO category, while the white bars indicate the percentage of all Arabidopsis genes belonging to each GO term. D, Reduced expression of chlorophyll biosynthetic genes mapped to the tetrapyrrole biosynthetic pathway. The log2 expression fold change of each gene in RPGE2-OX and the glk1/2 mutant compared with wild-type (Col-0) are indicated by colored squares next to the gene name. The colored bar indicates the log2 expression fold change.
We next performed a gene ontology (GO) analysis of the 486 shared DEGs to examine which biological processes are co-regulated by RPGE2 and GLKs. We found that the co-repressed DEGs are enriched for photosynthesis and related biological processes (Figure 2C, Supplemental Figure 3), which is in line with the pale green leaves seen in RPGE-OX and the glk1/2 mutant. We classified photosynthesis-related genes into a few different functional categories and further examined their expression patterns. The heatmap showed that consistent with the low chlorophyll levels, the majority of chlorophyll biosynthetic genes (e.g. HEMA1 and PORA) are repressed in both RPGE2-OX and the glk1/2 mutant (Figure 2D). Our RT-qPCR analysis further corroborated that HEMA1 and PORA are repressed in RPGE-OX and the glk1/2 mutant, but activated in the rpgeT mutant (Supplemental Figure 4). Both RPGE-OX and the glk1/2 mutant have high chlorophyll a/chlorophyll b (chl a/chl b) ratios (Figure 1D). Consistently, CHLOROPHYLLIDE A OXYGENASE (CAO), which converts chl a to chl b is repressed in both RPGE-OX and the glk1/2 mutant. Unlike these chlorophyll synthetic genes, chlorophyll catabolic genes (e.g. NON-YELLOWING 1 (NYE1) and PHEOPHYTINASE (PPH)) are not found to be significantly regulated by RPGEs and GLKs (Supplemental Figure 5, A and B). This indicates that the pale green color of the leaves is largely due to decreased chlorophyll synthesis rather than increased chlorophyll degradation. Among other photosynthesis-related genes, the majority of nuclear-encoded photosystem I (PSI) and PSII genes (e.g. PSI SUBUNIT E-1 (PSAE-1), PSII SUBUNIT Q-2 (PSBQ-2)), and light harvesting complex genes (e.g. PSI LIGHT HARVESTING COMPLEX GENE 1 (LHCA1), LHCA2, LHCA3, PSII LIGHT HARVESTING COMPLEX GENE 2.4 (LHCB2.4), LHCB3, and LHCB4.1) are also repressed in both RPGE-OX and the glk1/2 mutant (Supplemental Data S2). RT-qPCR analysis further confirms that LHCA1, LHCA2, LHCB3, and LHCB4.1 are repressed in RPGE-OX and the glk1/2 mutant, but activated in the rpgeT mutant (Supplemental Figure 4). Taken together, these results indicate that RPGEs and GLKs antagonistically regulate many photosynthesis-related genes.
RPGE proteins interact with GLK proteins
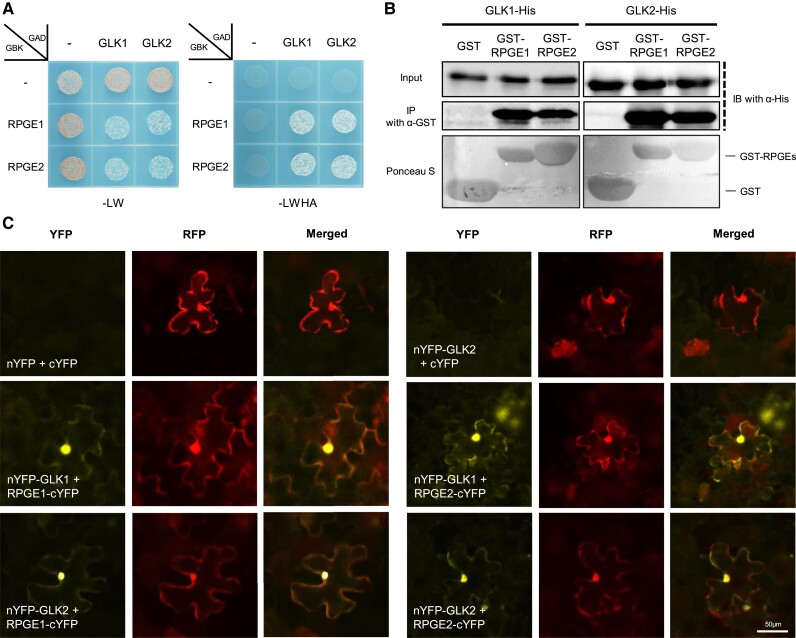
The phenotypic similarity between RPGE-OX and the glk1/2 mutant may reflect the inhibition of GLKs by RPGEs or the activation of RPGEs by GLKs. The mRNA expression analysis indicates that this phenotypic similarity is not due to the downregulation of GLK mRNA expression in RPGE-OX or the upregulation of RPGE mRNA expression in the glk1/2 mutant (Supplemental Figure 6). On the contrary, GLK2 mRNA levels are increased in RPGE-OXs and RPGE mRNA levels are decreased in the glk1/2 mutant, suggesting the presence of a feedback regulation between GLKs and RPGEs. We thus questioned whether RPGEs inhibit GLKs through a protein–protein interaction. We examined whether RPGE proteins interact with GLK proteins. RPGE proteins interact with GLK proteins in a yeast 2-hybrid assay (Figure 3A). Recombinant GST-tagged RPGEs, but not GST alone, also interact with recombinant His-tagged GLKs in vitro (Figure 3B). A bimolecular fluorescence complementation (BiFC) assay further shows the interaction between RPGEs and GLKs both in the nucleus and the cytosol in vivo (Figure 3C). Taken together, these results indicate that RPGEs interact with GLKs both in vitro and in vivo.
Figure 3.
RPGEs interact with GLKs. A, Interaction between RPGEs and GLKs shown by a yeast 2-hybrid assay. RPGEs were cloned into a GAL4 DNA-binding domain plasmid (GBK) and GLKs were cloned into a GAL4 DNA activation domain plasmid (GAD). Empty vectors used as negative controls are indicated by—signs. Transformed yeasts were grown on a synthetic dropout medium lacking either Leu and Trp (-LW) or Leu, Trp, His, and Ade (-LWHA). B, In vitro binding assay showing the interaction between RPGEs and GLKs. His-tagged GLKs were precipitated with resin-bound GST-tagged RPGEs and the precipitated proteins were immunoblotted with His antibody (a-His) or GST antibody (a-GST). The lower panel shows sepharose-bound GST or GST-RPGE proteins stained with Ponceau S. C, Bimolecular fluorescence complementation (BiFC) assay showing the interaction between RPGE and GLK2 in N. benthamiana. N-yellow fluorescent protein (nYFP) was fused to the N-terminus of GLK1 or GLK2 (nYFP-GLK1, nYFP-GLK2), and cYFP was fused to the C-terminus of RPGE1 or RPGE2 (RPGE1-cYFP, RPGE2-cYFP). Red fluorescent protein (RFP) was co-expressed as a positive control for transfection. YFP and RFP signals were imaged with an epifluorescence microscope. Bar = 50 µm.
RPGE1 inhibits the transcription factor activity of GLK1
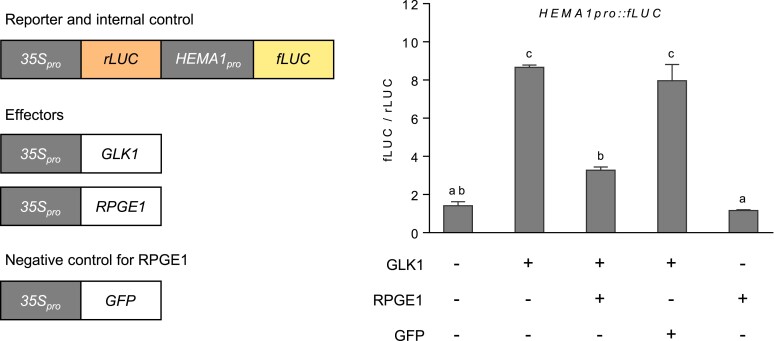
The interaction between RPGEs and GLKs led us to speculate that RPGEs could inhibit GLK transcription factor activity, given that GLKs are known to regulate gene expression by directly binding to the promoters of various photosynthesis-related genes. To investigate this possibility, we performed luciferase-based transcription factor activity assays in Arabidopsis protoplasts (Figure 4). We used HEMA1 promoter-driven firefly luciferase (fLUC) as a reporter and 35S promoter-driven GLK1, RPGE1, or GFP as effectors. We also included 35S promoter-driven renilla luciferase (rLUC) as an expression control. The assay shows that the relative expression of firefly luciferase (fLUC/rLUC) is about 6-folds higher with the GLK1, supporting the notion that GLK1 activates the expression of HEMA1 promoter-driven fLUC. The co-expression of RPGE1 drastically decreases the fLUC/rLUC ratio, whereas the co-expression of GFP does not. These results indicate that RPGE1 inhibits the transactivation activity of GLK1.
Figure 4.
RPGE1 inhibits the transactivation activity of GLK1. GLK1 was introduced to Arabidopsis protoplasts with or without RPGE1 and GFP. The reporter genes, HEMA1pro-driven firefly luciferase (fLUC) and the 35Spro-driven Renilla luciferase (rLUC) gene, were also introduced. The transactivation activity was measured as the fLUC/rLUC ratio. Error bars indicate SE (n = 3 biological replicates). Letters indicate statistical significance, as determined by ANOVA with Tukey's HSD post-hoc test for multiple comparisons (P < 0.01).
RPGE1 inhibits the DNA-binding activity of GLK1 by disrupting its dimerization
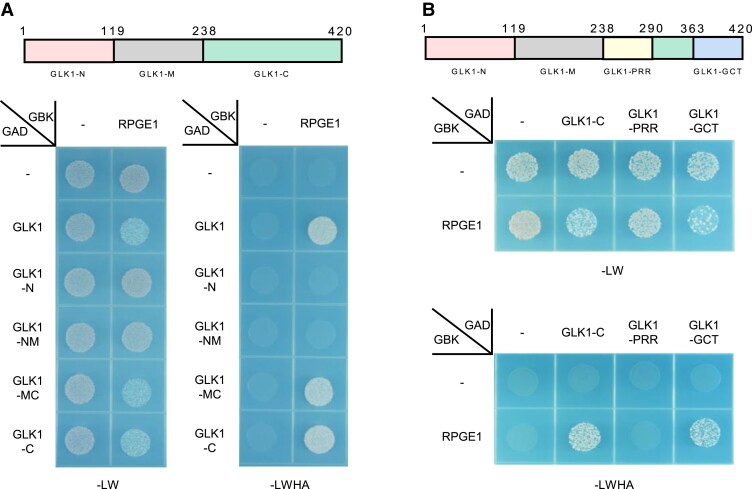
We next investigated how RPGE1 inhibits the transactivation activity of GLK1. We hypothesized that RPGE1 might interact with and block the transactivation domain of GLK1. Alternatively, RPGE1 might inhibit the DNA-binding activity of GLK1 by directly interacting with its DNA-binding domain or by disrupting its dimerization. To gauge which mechanism is more likely, we performed yeast 2-hybrid assays with truncated forms of GLK1 to identify an interacting region. We considered full-length GLK1 to comprise 3 domains: the N-domain (1–118 a.a., GLK1-N), which includes a transactivation domain (Supplemental Figure 7A); the M-domain (119–237 a.a., GLK1-M), which includes a DNA-binding domain; and the C-domain (238–420 a.a., GLK1-C), which includes a dimerization domain (Supplemental Figure 7B). The yeast 2-hybrid assays show that RPGE1 interacts with any truncated form of GLK1 that includes the C-domain (GLK1, GLK1-MC, and GLK1-C), but not with truncated forms lacking the C-domain (GLK1-N, GLK1-NM) (Figure 5A). GLK1-C can be further divided into the PRR (238–290 a.a., proline-rich region) domain and the GCT (363–420 a.a., GLK C-terminal) domain, of which the GCT domain is a dimerization domain (Rossini et al., 2001). RPGE1 interacts with the GCT domain but not with the PRR domain (Figure 5B). These results indicate that RPGE1 interacts with the dimerization domain rather than the transcription activation domain or the DNA-binding domain of GLK1.
Figure 5.
RPGE1 binds to the dimerization domain of GLK1. A, Interaction between RPGE1 and the GLK1-C domain, as shown by yeast 2-hybrid assay. RPGEs were cloned into the GAL4 DNA-binding domain plasmid (GBK) and GLK domains were cloned into the GAL4 DNA activation domain plasmid (GAD). Empty vectors used as negative controls are indicated by—signs. Transformed yeasts were grown on a synthetic dropout medium lacking either Leu and Trp (-LW) or Leu, Trp, His, and Ade (-LWHA). Upper diagram shows truncation scheme that divides GLK1 into 3 domains (GLK-N, GLK1-N, GLK1-C). B, Interaction between RPGE1 and the dimerization domain (GCT domain) of GLK1, as shown by yeast 2-hybrid assay. Notations are identical to those in (A). Upper diagram shows the scheme for further truncation of the GLK1-C domain.
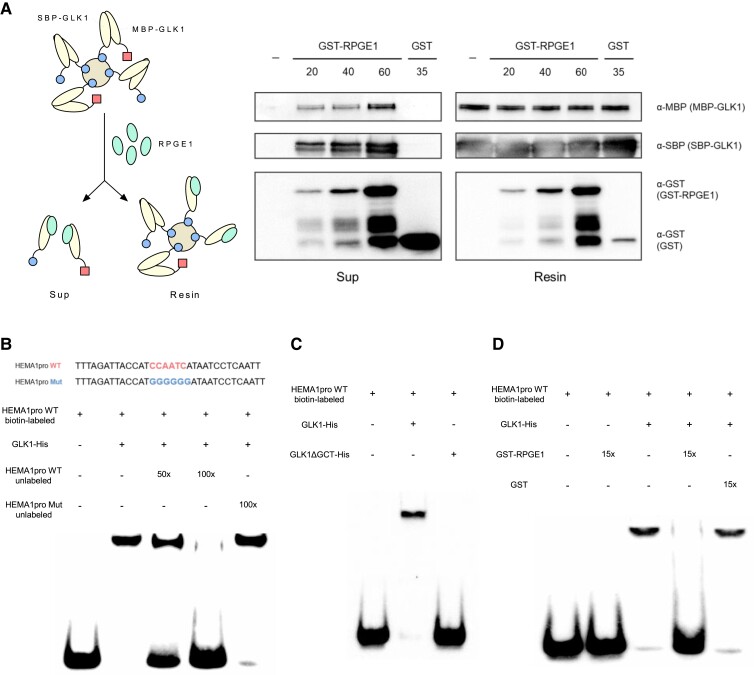
The interaction of RPGE1 with the GCT domain suggests that RPGE1 could disrupt the dimerization of GLK1. We thus examined whether RPGE1 disrupts the dimerization of GLK1 in vitro. For the assay, we co-expressed MBP-tagged GLK1 and SBP-tagged GLK1 in E. coli and used streptavidin sepharose resin to collect both SBP-GLK1/SBP-GLK1 homodimers and SBP-GLK1/MBP-GLK1 heterodimers. After extensive washing, we added GST-tagged RPGE1 to the GLK1-bound resin and determined if the addition of RPGE1 dissociated the GLK1 dimers. We found that the addition of an increasing amount of GST-RPGE1 leads to the elution of increasing amounts of both SBP-GLK1 and MBP-GLK1, whereas buffer alone or GST protein does not (Figure 6A). We further speculated that if RPGE1 disrupts the dimerization of GLK1 by stably binding to its dimerization domain, then GST-RPGE1 would be associated with both eluted and resin-bound GLK1. Consistently, we also detected GST-RPGE1 in the resin-bound GLK1 (Figure 6A). These results suggest that RPGE1 disrupts the dimerization of GLK1 by binding to its dimerization domain.
Figure 6.
RPGE1 inhibits the DNA binding of GLK1 by disrupting its dimerization. A, The disruption of Arabidopsis GLK1 dimer by Arabidopsis RPGE1 in vitro. A diagram in the left indicates the experimental scheme. RPGE1 (GST-RPGE1-His) was added to streptavidin resin-bound GLK1 dimers of SBP-GLK1/SBP-GLK1 or SBP-GLK1/MBP-GLK1. After elution, immunoblotting with the appropriate antibodies (α-GST, α-SBP, and α-MBP) was used to detect each protein in the supernatant (Sup) and resin. The addition of buffer only or GST instead of GST-RPGE1-His is indicated by the minus (−) sign and GST, respectively. B, The binding of Arabidopsis GLK1 to a HEMA1 promoter fragment, as determined by electrophoretic mobility shift assay (EMSA). Upper panel shows sequences of the HEMA1 WT and mutant probes. EMSA was performed with biotin-labeled HEMA1 probe. Competition for the binding was tested with 50 × or 100 × of unlabeled WT probe or 100 × of unlabeled mutant probe. C, The failure of the DNA binding of the dimerization domain-deleted Arabidopsis GLK1 (GLK1ΔGCT). The EMSA was performed as in (B) except in the presence of Arabidopsis GLK1ΔGCT. D, Inhibition of the DNA binding of Arabidopsis GLK1 by Arabidopsis RPGE1. EMSA was performed as described in (B) except in the presence of Arabidopsis GST-RPGE1 or GST. GST or GST-RPGE was added to a 15 × molar concentration of GLK1 protein.
The disruption of GLK1 dimerization by RPGEs may inhibit the DNA binding of GLK1. To determine whether the dimerization domain is necessary for GLK1 to bind to DNA, we performed EMSA with GLK1 and the dimerization domain-deleted GLK1 (GLK1ΔGCT). For a target DNA sequence, we used a HEMA1 promoter fragment containing a CCAATC motif known to be a binding site for GLK1 (Waters et al., 2009). The EMSA results show that GLK1 shifts the mobility of the probe and competition is seen in the presence of an unlabeled wild-type probe but not a binding-motif mutant probe (Figure 6B). When the dimerization domain of GLK1 is deleted, GLK1ΔGCT cannot bind to the probe anymore, supporting that the dimerization is necessary for the DNA binding (Figure 6C). To further investigate whether RPGE1 dissociates the DNA binding of GLK1, we next performed EMSA with GLK1 in the presence of RPGE1. EMSA further shows that the addition of GST-tagged RPGE1 decreases shifted probes, whereas the addition of GST protein does not (Figure 6D). These results support the idea that RPGE1 inhibits the DNA binding of GLK1 by disrupting the dimerization of GLK1.
A rice RPGE homolog disrupts the dimerization of Arabidopsis GLK1 and causes pale green leaves in Arabidopsis
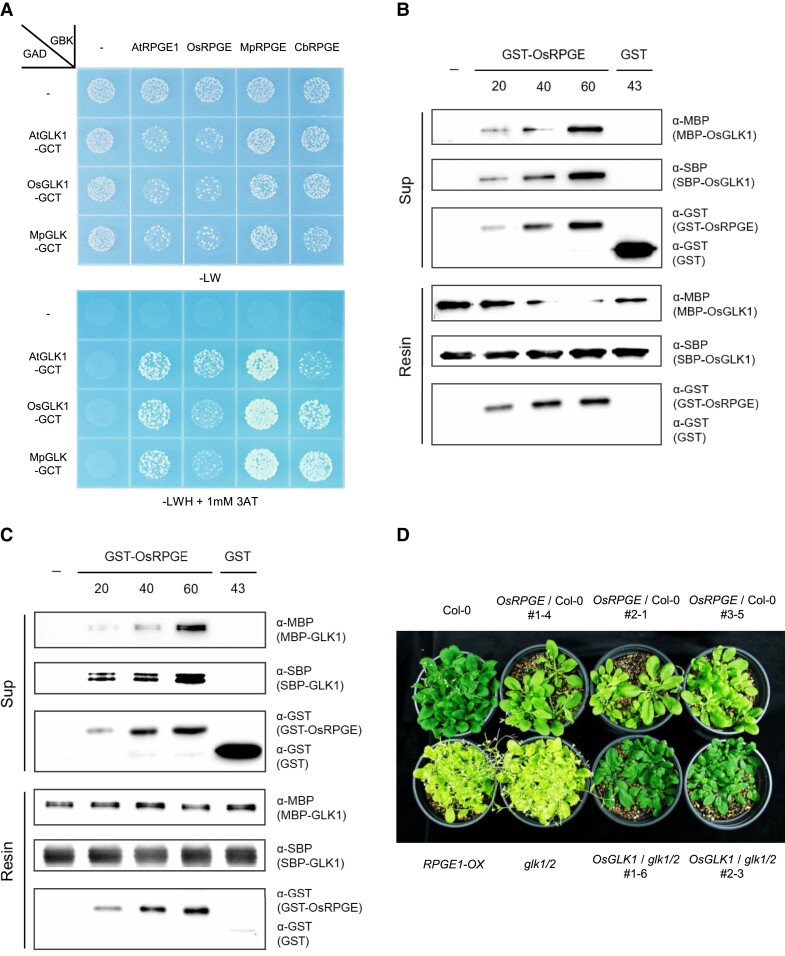
As chloroplasts are essential organelles, it is likely that conserved regulatory mechanisms evolved early to control chloroplast development. RPGE and GLK homologs are found in other land plants, including rice (Oryza sativa) and liverwort (Marchantia polymorpha), and also in charophytes, including Chara braunii (Supplemental Figure 1A). This suggests that the RPGE-GLK pair might be a conserved regulator of chloroplast development in streptophytes. We thus speculated that the RPGE homologs of these species may also interact with GLK homologs. Consistent with this hypothesis, a previous report showed that the rice RPGE homolog, DEEP GREEN PANICLE1 (DGP1, OsRPGE, Os03g0280400), interacts with OsGLK1 and OsGLK2 and inhibits transactivation activity of OsGLK1 (Zhang et al., 2021a). To test if RPGEs interact with GLKs through the dimerization domain also in other species, we performed yeast 2-hybrid assays with RPGE homologs from Arabidopsis, rice, liverwort, and Chara braunii and GLK homologs from Arabidopsis, rice, and liverwort. The yeast 2-hybrid assay further shows that RPGE homologs interact not only with the dimerization domains of the GLK homologs from the cognate species but also with those from other species (Figure 7A, Supplemental Figure 8). These findings support the idea that there are interspecies interactions between RPGEs and the dimerization domains of GLKs. We thus questioned whether the RPGE from one species would inhibit the GLK of another species by disrupting its dimerization.
Figure 7.
RPGE homologs bind to the dimerization domains of GLK homologs. A, Interspecies-wide interaction between RPGE homologs and the dimerization domains of GLK homologs RPGEs are cloned into the GAL4 DNA-binding domain plasmid (GBK) and GLK dimerization domains are cloned into the GAL4 DNA activation domain plasmid (GAD). Empty vectors used as negative controls are indicated by—signs. Transformed yeasts were grown on a synthetic dropout medium lacking either Leu and Trp (-LW) or Leu, Trp, and His with 1 mM 3-amino-1,2,4-triazole (-LWH + 1 mM 3-AT) OsRPGE (Os03g0280400), MpRPGE (Mapoly0002s0278), CbRPGE (G70694), and MpGLK (Mapoly0156s0007) B, The disruption of rice GLK1 dimerization by a rice RPGE homolog in vitro. GST-fused OsRPGE was added to streptavidin resin-bound SBP-OsGLK1/SBP-OsGLK1 and SBP-OsGLK1/MBP-OsGLK1. After the elution, the immunoblot was used to determine each protein with corresponding antibody (α-GST, α-SBP, and α-MBP) both in the supernatant (Sup) and in the resin. The addition of buffer only or GST is indicated by minus (−) sign and GST, respectively. C, The disruption of Arabidopsis GLK1 dimerization by a rice RPGE homolog in vitro. GST-fused OsRPGE was added to streptavidin resin-bound SBP-GLK1/SBP-GLK1 and SBP-GLK1/MBP-GLK1. Notations are identical to (B). D, Functional conservation of OsRPGE and OsGLK1 in Arabidopsis. Transgenic plants expressing OsRPGE in Col-0 background (OsRPGE/Col-0) develop pale green leaves, while and transgenic plants expressing OsGLK1 in the glk1/2 mutant background (OsGLK1/glk1/2) develop dark green leaves. Plants are grown in an LD condition (16 hours light/8 hours dark; white light, 100 µmol m−2s) at 22°C for 28 days.
To test this possibility, we examined whether a rice RPGE homolog (OsRPGE) could disrupt the dimerization of not only rice GLK1 (OsGLK1), but also Arabidopsis GLK1 (AtGLK1). Similar to the results obtained with AtRPGE1 and AtGLK1 (Figure 6A), the addition of increasing amounts of OsRPGE leads to the elution of increasing amounts of SBP-OsGLK1 and MBP-OsGLK1 from streptavidin resin-bound SBP-OsGLK1/SBP-OsGLK1 and SBP-OsGLK1/MBP-OsGLK1, whereas no such elution is seen with buffer alone or GST protein (Figure 7B). Similarly, the addition of increasing amounts of OsRPGE leads to the elution of increasing amounts of SBP-AtGLK1 and MBP-AtGLK1 from streptavidin resin-bound SBP-AtGLK1/SBP-AtGLK1 and SBP-AtGLK1/MBP-AtGLK1, whereas no such elution is seen with buffer alone or GST protein (Figure 7C). OsRPGE is also detected with the resin-bound AtGLK1 after elution, supporting the idea that, like AtRPGE1, OsRPGE disrupts the dimerization of AtGLK1 by stably binding to its dimerization domain. The disruption of AtGLK1 dimerization by OsRPGE suggested that overexpression of OsRPGE in Arabidopsis should cause phenotypes similar to those generated by overexpression of AtRPGE. Indeed, overexpression of OsRPGE causes pale green leaves in Arabidopsis similar to those caused by the overexpression of AtRPGE, and the overexpression of OsGLK1 also complements the pale green leaves of the Arabidopsis glk1/2 mutant (Figure 7D). Taken together, these results support the idea that RPGE-GLK regulatory pairs are conserved in interspecies.
Shade represses the expression of photosynthetic genes in part through RPGE-GLK regulatory pairs
The conservation of RPGE-GLK regulatory pairs across diverse plant species implies that this regulatory pair is likely to play important roles in regulating photosynthetic genes under various environmental conditions. We hypothesized that the shade condition might be an environmental condition in which the RPGE-GLK regulatory pairs regulate photosynthetic gene expression, given that shade stabilizes PIFs which activate the expression of their target genes, RPGE1, and RPGE2.
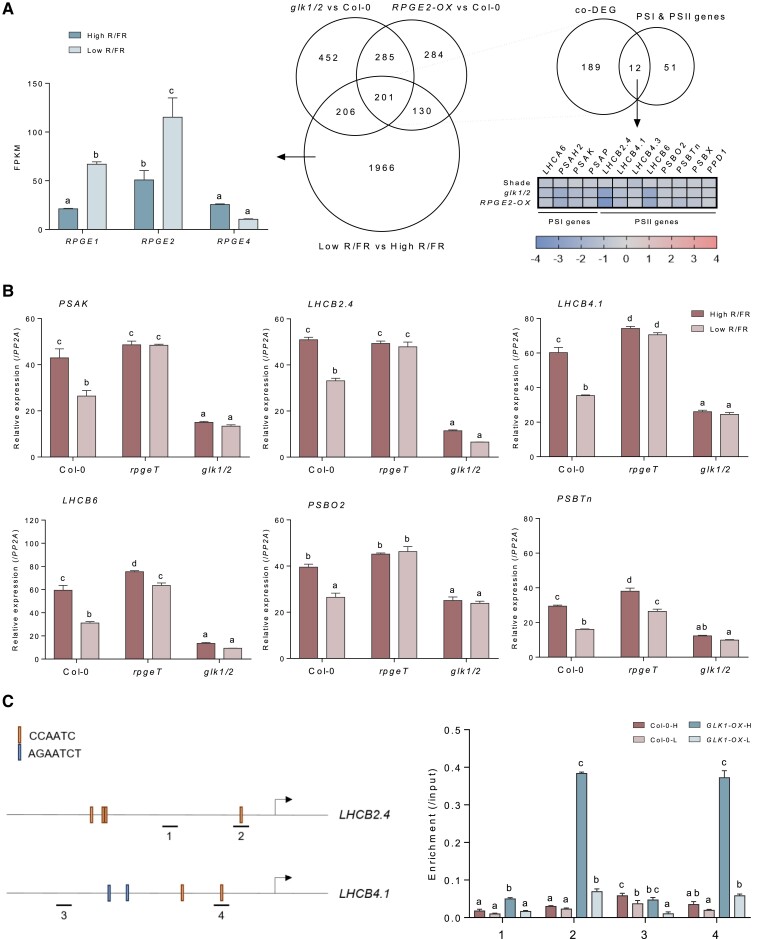
To investigate whether the RPGE-GLK regulatory pairs regulate the expression of photosynthetic genes in the shade, we first compared the DEGs shared by RPGE2-OX and the glk1/2 mutant (shared DEGs; Figure 2A) with DEGs seen under a shade condition (shade DEGs; 1.5-fold and FDR < 0.05) (Figure 8A). Indeed, the shared DEGs significantly overlap with the shade DEGs (201 genes, called co-DEGs; hypergeometric test, P = 6.1104 × 10−98), indicating that shade significantly regulates a subset of the shared DEGs. We next compared the co-DEGs with the nuclear-encoded PSI and PSII genes, and found a significant overlap (hypergeometric test, P = 1.5146 × 10−13). These overlapped photosynthetic genes are repressed in shade-treated Col-0 and in light-grown RPGE2-OX and glk1/2 plants, indicating that these genes are repressed by the shade and RPGE2, but activated by GLKs. Consistent with RPGE1 and RPGE2 being PIF target genes, the expression levels of RPGE1 and RPGE2 are increased by the shade, while that of RPGE4 is not. The RPGE3 expression is not detected in any of the 3 transcriptomes, suggesting that is very low in Arabidopsis.
Figure 8.
Shade represses the expression of photosynthetic genes through RPGE-GLK regulatory pairs. A, The repression of photosynthetic genes by shade, RPGE2-OX, and the glk1/2 mutant. The graph on the left shows the expression changes of RPGE1, RPGE2, and RPGE4 by shade in Col-0. Letters indicate statistical significance, as determined by an ANOVA with Tukey's HSD post-hoc test for multiple comparisons (P < 0.01). Error bars = SE (n = 3 biological replicates). The Venn diagram in the middle shows the overlap between DEGs observed under shade, RPGE2-OX, and the glk1/2 mutant. The DEGs of RPGE2-OX and the glk1/2 mutant are as presented in Figure 2A. The DEGs under shade are derived from a transcriptome of 4-day-old high R/FR (red light, 20 µmol m−2s; blue light, 20 µmol m−2s; far-red light, 10 µmol m−2s) light-grown Col-0 seedlings transferred to low R/FR light (red light, 2 µmol m−2s; blue light, 2 µmol m−2s; far-red light, 10 µmol m−2s) for 6 hours at 22°C. The Venn diagram on the right shows the overlap of co-DEGs for the 3 transcriptomes and the set of photosystem I (PSI) and PSII genes. The heatmap below the Venn diagram shows the log2 expression fold changes of the overlapped PSI and PSII genes under shade and in RPGE2-OX and the glk1/2 mutant. The increased expression of RPGE1 and RPGE2 in the shade transcriptome is indicated by a graph on the left. B, The RPGE-GLK regulatory pair-dependent repression of photosynthetic genes under shade. For the expression analysis, seedlings grown in high R/FR light for 4 days were transferred to low R/FR light for 2 hours at 22°C. mRNA expression levels were determined by RT-qPCR and normalized with respect to PP2A. Letters indicate statistical significance, as determined by an ANOVA with Tukey's HSD post-hoc test for multiple comparisons (P < 0.01). Error bars = SE (n = 3 biological replicates). C, The inhibition of GLK1 targeting to promoters of GLK1 target genes by shade. Diagrams show LHCB2.4 and LHCB4.1 promoters. Underline, promoter fragment for ChIP-qPCR; arrow, transcription start site. Each underline 1 or 2 indicates 595 base pair (bp) to 694 bp, or 97–196 bp upstream of LHCB2.4 transcription start site (TSS), and each underline 3 or 4 indicates 1,218 base pair (bp) to 1321 bp, or 200–297 bp upstream of LHCB4.1 TSS. ChIP assay was performed with Col-0 or transgenic seedlings expressing GLK1 (GLK1-OX) grown in high R/FR for 4 days and kept in high R/FR for additional 4 hours (-H) or transferred to low R/FR for 4 hours (-L) at 22°C. Error bars indicate SD (n = 3, biological replicates). Letters indicate statistical significance, as determined by ANOVA with Tukey's HSD post-hoc test for multiple comparisons (P < 0.01).
We examined whether shade represses the overlapped photosynthetic genes through the RPGE-GLK regulatory pairs. All 12 overlapped photosynthetic genes either have a CCAATC motif within 1 kb upstream from the start codon or have been identified as GLK1 target genes (Waters et al., 2009). From among them, we examined the expression levels of 6 genes by using RT-qPCR. Consistent with the transcriptomic data, the expression levels of these photosynthetic genes are repressed in Col-0. In the rpgeT mutant, these genes either fail to show repression under shade or show less repression under shade compared with Col-0 (Figure 8B). In contrast, these genes are expressed at low levels in the glk1/2 mutant regardless of shade. These results support the idea that shade represses a subset of nuclear-encoded photosynthetic genes in part through RPGE-GLK regulatory pairs.
We next examined if the repression of these photosynthetic genes by shade is partly due to the inhibition of GLK1 DNA-binding activity. To determine if shade decreases the targeting of GLK1 to these gene promoters, we performed chromatin immunoprecipitation (ChIP)-qPCR with GLK1-OX that expresses 35S promoter-driven GLK1. ChIP assay shows that shade strongly decreases the targeting of GLK1 to the promoters of LHCB2.4 and LHCB4.1 (Figure 8C). The decreased targeting is not due to the degradation of GLK1 protein by shade as its protein level is not reduced, but rather increased by shade (Supplemental Figure 9). Together, the results support that shade represses the expression of photosynthetic genes partly by inhibiting the DNA-binding activity of GLK1.
Discussion
RPGE proteins inhibit GLK transcription factors by inhibiting their DNA binding
We previously showed that 2 members of the RPGE family, RPGE1 and RPGE2, inhibit the expression levels of photosynthetic genes downstream of PIFs (Kim et al., 2016). The overexpression of RPGE causes pale green leaves reminiscent of those of the glk1/2 mutant. Here, we show that RPGEs act by inhibiting GLK transcription factors, which are master regulators responsible for activating the expression of many chlorophyll biosynthetic and photosynthetic genes. We found that RPGEs interact with the dimerization domains of GLKs and disrupt their dimerization to inhibit their critical DNA-binding activity. The close molecular relationship between RPGEs and GLKs is manifested in the similarities found in the transcriptomes of RPGE-OX and the glk1/2 mutant, which both exhibit repression of chlorophyll biosynthetic and photosynthetic genes consistent with their pale green leaves. Apparently, the interaction is not restricted to the RPGE-GLK regulatory pairs in Arabidopsis. We found that Arabidopsis RPGE and homologs from rice, liverwort, and Chara interact not only with their cognate GLKs but also with GLK homologs in other species, and that this interaction occurs through dimerization domains of GLK homologs. Consistent with the interspecies conservation of the interaction between RPGEs and GLKs, a rice RPGE homolog could disrupt the dimerization of not only rice GLK1 but also Arabidopsis GLK1 and cause pale green leaves when overexpressed in Arabidopsis.
The molecular intimacy between RPGEs and GLKs suggests that any condition that alters the level of RPGE is likely to alter the activity of GLK. The shade is one such example, as it increases the expression of RPGE1 and RPGE2 by stabilizing PIFs. Indeed, we found that shade decreases the targeting of GLK1 to a subset of GLK-regulated photosynthetic gene promoters and fails to repress them in the rpgeT mutant, whereas high R/FR light fails to activate the same subset of photosynthetic genes in the glk1/2 mutant. Our results support the occurrence of the following molecular events under shade (Figure 9): Shade stabilizes PIF proteins, which activates the expression of RPGE1 and RPGE2. The increased RPGEs interact with the dimerization domains of GLKs to disrupt their dimerization, which leads to the dissociation of GLKs from their target promoters, thereby repressing GLK target gene expression. The effects of GLK inhibition under shade, however, are not restricted to the activation of RPGE expression. A previous study showed that PIF4 represses the expression of GLK1 and GLK2 by directly targeting their promoters (Song et al., 2014). This suggests that shade inhibits GLKs by both repressing GLK mRNA expression and inhibiting GLK dimerization. Such a dual inhibitory mechanism would increase the robustness of regulation, implying that the inhibition of GLK activity is an important process under shade.
Figure 9.
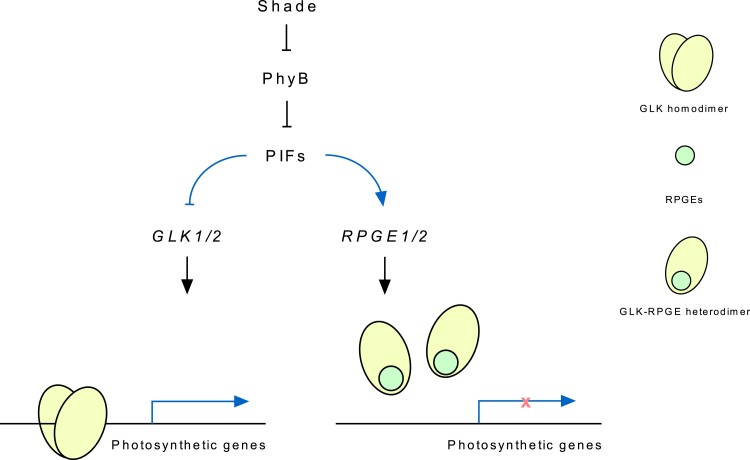
A diagram depicting the repression of photosynthetic genes under shade through RPGE-GLK regulatory pairs. Shade stabilizes PIFs by inactivating phyB. The stabilized PIFs repress the expression of GLK mRNAs by directly binding to their promoters. At the same time, PIFs also directly bind to RPGE1 and RPGE2 promoters and activate the expression of their mRNAs. The increased RPGE proteins inhibit the DNA binding of GLK proteins by binding to their GCT domain to disrupt dimerization. The reduction of GLK mRNAs and the inhibition of GLK proteins under shade ensure the shade-induced repression of GLK target photosynthetic genes. The blue lines indicate transcriptional regulation.
Our identification of RPGE-GLK regulatory pairs supports that GLK activities are regulated at multiple levels. At the mRNA level, the transcription factor, WRKY75, directly represses the expression of GLK1 and GLK2 to promote ABA-mediated leaf senescence (Zhang et al., 2022). GENOMES UNCOUPLED 1 (GUN1)-mediated retrograde signaling represses the expression of GLK1 (Tokumaru et al., 2017). Peanut HISTONE DEACETYLASE 1 (Arachis hypogaea L. HDA1; AhHDA1) represses the expression of AhGLK1 by altering its histone deacetylation levels (Liu et al., 2020). At the protein level, the protein stability of GLK is regulated. BRASSINOSTEROID INSENSITIVE 2 (BIN2) interacts with GLK1 and phosphorylates its DNA-binding domain to stabilize the GLK1 protein (Zhang et al., 2021b). The stability of GLK is also likely to be regulated by polyubiquitination. GLK1 is known to be polyubiquitinated when chloroplast biogenesis is inhibited (Tokumaru et al., 2017). The subsequent degradation of GLK1 results in the down-regulation of PhANGs, thereby preventing the accumulation of unnecessary precursor proteins in the cytosol. Consistent with the polyubiquitination of GLKs, tomato GLK2 (SlGLK2) protein was shown to be polyubiquitinated by CUL4-DDB1-DET1 ubiquitin ligase complex for proteasome-mediated degradation (Tang et al., 2016). It is curious to note that shade also stabilizes GLK1 protein (Supplemental Figure 9). However, the physiological importance of GLK1 stabilization by shade needs to be further determined. The DNA-binding activities of GLKs are also regulated. Previous studies showed that LESION-SIMULATING DISEASE1 (LSD1) directly binds to GLKs and inhibits the DNA-binding activity of GLK1, while the defense-related transcriptional coregulator, SIGMA FACTOR-BINDING PROTEIN 1 (SIB1), interacts with GLKs and interferes with the LSD1-GLK interaction to enhance the expression of nuclear-encoded photosynthetic genes in response to an increase in salicylic acid (Li et al., 2022). The DNA-binding activity of GLKs was also shown to be oppositely regulated by 2 plant viruses. The intracellular immune receptor, Rx1, directly binds with NbGlk1 (Nicotiana benthamiana GLK1) and inhibits its DNA binding. In the presence of potato virus × (PVX), the activated Rx1 allows NbGLK1 to bind to its target promoters (Townsend et al., 2018). In contrast, Turnip Yellow Mosaic Virus P69 interacts with GLKs and inhibits their DNA binding (Ni et al., 2017). At the level of transcriptional activation ability, the NAC-domain transcription factor, ORESARA 1 (ORE1), was shown to interact with GLK2 and inhibit its transcriptional activation ability to regulate leaf senescence (Rauf et al., 2013). BiFC assay shows that RPGEs interact with GLKs both in the nucleus and the cytosol (Figure 3C), which is reminiscent of the interaction between LSD1 and the dimerization domain-deleted GLK1 both in the nucleus and the cytosol (Li et al., 2022), suggesting that RPGEs may also regulate the nuclear localization of GLKs. Such the regulation of GLK activities at multiple levels imply that plants need to constantly adjust GLK activities according to developmental stages and environmental changes.
The RPGE-GLK regulatory pairs are conserved
Genome sequence analyses of various green organisms show that genes encoding RPGEs and GLKs coexist in both land plants (embryophytes) and charophytes, suggesting that RPGE-GLK regulatory pairs are present in streptophytes (embryophytes + charophytes). Their abilities to interact are also conserved, as our data show that RPGE homologs from different species interact not only with their cognate GLK homologs but also with GLKs from other species. Furthermore, we find that a rice RPGE homolog can disrupt the dimerization of not only rice GLK1 but also Arabidopsis GLK1. Consistent with its interaction with Arabidopsis GLK, overexpression of a rice RPGE homolog causes pale green leaves in Arabidopsis, and overexpression of OsGLK1 complements the Arabidopsis glk1/2 mutant, strongly suggesting that RPGE-GLK regulatory pairs are functional across species. The presence of RPGE-GLK regulatory pairs in rice is also supported by a previous report showing that the transactivation activity of OsGLK1 is inhibited by the rice RPGE homolog, DEEP GREEN PANICLE1 (DGP1, OsRPGE), in rice protoplasts (Zhang et al., 2021a). Our present data show that RPGE homologs interact with the dimerization domains of GLK homologs across species and disrupt their dimerization. Thus, we speculate that the reported ability of OsRPGE to inhibit the transactivation ability of OsGLK1 in protoplasts also likely reflects the disruption of OsGLK1 dimerization by OsRPGE.
RPGEs are among a growing number of small proteins found to play inhibitory roles by heterodimerizing DNA-binding transcription factors
Our identification that RPGEs disrupt the dimerization of GLKs indicates that RPGEs are examples of small proteins that inhibit transcription factors by disrupting their dimerization. Such regulatory small proteins have been identified in both animals and plants. In human, inhibitor of differentiation1 (ID1, 155 a.a.) inhibits the binding of E2A homodimers to the CDKN2A promoter by heterodimerizing with E2A (Zheng et al., 2004). In Arabidopsis, LITTLE ZIPPER (ZPR, 136 a.a.) heterodimerizes with REVOLUTA (REV) to inhibit its DNA binding (Wenkel et al., 2007), while PHY RAPIDLY REGULATED 1 (PAR1, 118 a.a.) does the same with PIF4 (Hao et al., 2012). Variations on this mode of regulation are also found. For example, KIDARI (KDR, 94 a.a.) heterodimerizes with and inhibits the non-transcription factor, LONG HYPOCOTYL IN FAR-RED 1 (HFR1, 292 a.a.). Since HFR1 forms a heterodimer with the transcription factor, PIF4, to inhibit its DNA binding, the heterodimerization of KDR with HFR1 increases the ability of PIF4 to bind its target promoters (Hong et al., 2013). Similarly, PACLOBUTRAZOL RESISTANCE1 (PRE1, 92 a.a.) interacts with and inhibits the non-transcription factor, bHLH1 (IBH1, 156 a.a.), which heterodimerizes with ACTIVATOR FOR CELL ELONGATION1 (ACE1, 486 a.a.), causing liberation of ACE1 from the transcriptionally inactive IBH1-ACE1 complex (Ikeda et al., 2012).
Materials and methods
Plant materials and growth conditions
Arabidopsis (Arabidopsis thaliana) plants were grown on soil under an LD condition (16 hours light/8 hours dark; white light, 100 µmol m−2s; LED light fixture) at 22°C for general growth and seed harvest. The glk1/2 mutant was obtained from the ABRC (Fitter, 2002). The rpgeT mutant was generated via CRISPR-Cas9 system (Supplemental Figure 2). Guide RNA sequences are in Supplemental Table S1. Arabidopsis RPGE-OXs were previously reported (Kim et al., 2016). For transgenic lines overexpressing OsRPGE (OsRPGE/Col-0), OsRPGE was synthesized and cloned into a pBI121-derived vector. The construct was transformed into Col-0 mediated through Agrobacterium and 3 independent transgenic lines were established. For OsGLK1-OX/glk1/2 lines, the synthesized OsGLK1 was cloned into a pBI121-derived vector and transformed into the glk1/2 mutant. Two independent lines were established. For GLK1-OX lines, the Arabidopsis GLK1 was cloned into a pBI121-derived vector containing a TAP tag (2xProteinG-SBP) and transformed into Col-0.
Phylogenetic analyses
For phylogenetic analyses, RPGE and GLK homolog sequences were retrieved from the TAIR (arabidopsis.org) for Arabidopsis thaliana, EnsemblPlants (plants.ensembl.org) for Solanum lycopersicum and Oryza sativa, MarpolBase (marchantia.info) for Marchantia polymorpha and Klebsormidium nitens, and OrcAE (bioinformatics.psb.ugent.be/orcae/) for Chara braunii. Amino acid sequences were aligned with the MUSCLE algorithm and a phylogenetic tree was inferred by the maximum likelihood method with 1,000 bootstrap repeats using the MEGA × package (Kumar et al., 2018).
Chlorophyll-related phenotypes
Seeds were surface-sterilized using a sterilization solution (0.02% (v/v) Triton X-100% and 0.4% (w/v) NaClO). The sterilized seeds were spotted on Murashige and Skoog (MS) agar plates (pH 5.7, half-strength MS medium, 0.05% (w/v) MES, and 0.8% (w/v) Phytoagar). The spotted seeds were stratified for 3 days in the dark at 4°C and transferred to continuous white light (50 µmol m−2s) for 7 days at 22°C. The chlorophyll auto-fluorescence was observed in cotyledons under a confocal microscope (laser: 561 nm, emission: 570–620 nm, HV[GaAsP]: 120) (A1 HD25, Nikon). Chlorophyll contents were measured from same conditions. Sampled seedlings were soaked in 100% ethanol to extract chlorophyll for 20 hours in the dark at 4°C. The absorbance of the extracted solution was measured at 648.6 nm (A648.6) and 664.2 nm (A664.2) by a spectrophotometer (UV-1800, Shimadzu). The chlorophyll concentration was calculated using following equations: Chlorophyll a (µg/g) = (−5.2007×A648.6 + 13.5275×A664.2)×V/W, Chlorophyll b (µg/g)= (22.4327×A648.6−7.0741×A664.2)×V/W, where V is the volume (mL) of the extracted solution and W is the fresh weight (g) of the sample.
To measure the maximum quantum efficiency of PSII Photochemistry (Fv/Fm), Arabidopsis plants were grown on soil under an LD condition (16 hours light/8 hours dark; white light, 100 µmol m−2s) for 30 days at 22°C. Fv/Fm was measured using dark-adapted 5th and 6th rosette leaves with the Plant efficiency analyzer II (PEA II, Hansatech Instruments).
RNA expression analysis
Seedlings were grown on MS agar plates (pH 5.7) with 1% (w/v) sucrose and sampled for the analysis. Total RNA was isolated using the Spectrum Plant Total RNA Kit (STRN250, Sigma-Aldrich). For the expression analysis, 2 μg of isolated RNA was reverse transcribed and gene expression levels were determined by reverse transcription quantitative PCR (CFX Connect™, Bio-Rad) with a set of specific primers (listed in Supplemental Table 1). Gene expression levels were normalized with the expression values of PP2A. For RNA-seq analysis, 1 μg of total RNA was processed for sequencing with Nextseq500. DEGs were defined based on the criteria: expression fold change > 1.5 and adjusted P-value < 0.05. All expression analyses were performed with 3 independently grown seedling samples.
Yeast 2-hybrid assay
For the yeast 2-hybrid assay, genes or gene fragments were amplified and cloned. Primer sequences are listed in Supplemental Table S1. Vector pairs for testing the interaction were transformed into the yeast (Saccharomyces cerevisiae) strain AH109 cells and selected on the yeast double drop-out media plates lacking leucine and tryptophan (-LW). Selected colonies were suspended in water and 10 μl of the diluted yeasts (OD600 = 0.1) were spotted on the triple drop-out media plate lacking leucine, tryptophan, and histidine with 1 mM 3-amino-1,2,4-triazole (-LWH + 1 mM 3-AT) or the quadruple drop-out media plate lacking leucine, tryptophan, histidine and adenine (-LWHA). Spotted plates were incubated for 3 days at 30°C.
In vitro binding assay
For the in vitro binding assay, RPGE1 and RPGE2 were amplified and cloned into the pGEX-4T1 vector (GE Healthcare) and GLK1 and GLK2 were cloned into the pET-29a (Novagen)-derived vector lacking the S-tag. The S-tag was removed by cloning an adaptor into NdeI and XhoI site of pET-29a. Primer sequences are listed in Supplemental Table S1. Vectors were transformed into an Escherichia coli strain BL21-CodonPlus-RIL line (#230240, Agilent Technologies) and recombinant proteins were purified with Glutathione Sepharose 4B (17075601, Cytiva) for N-terminal GST-tagged RPGE1 (GST-RPGE1) and GST-RPGE2 proteins or with Ni-NTA Agarose (30210, QIAGEN) for C-terminal 6xHis-tagged GLK1 (GLK1-His) and GLK2-His proteins (Supplemental Figure 10). For the purification, the following buffers were used: a lysis buffer (pH 7.4, 50 mM Tris–HCl, 20 mM NaCl, 2 mM EDTA, and 0.1% (w/v) Lysozyme), a binding buffer and a GST-tag wash buffer (pH 7.4, 50 mM Tris–HCl, 150 mM NaCl, 0.05% (v/v) Triton X-100, and 10% (v/v) glycerol), a wash buffer for His-tag purification (pH 8.0, the binding buffer, and 40 mM Imidazole), a GST-tag elution buffer (pH 8.0, 50 mM Tris–HCl, 150 mM NaCl, 30 mM reduced L-glutathione, 5 mM 1,4-Dithiothreitol, 0.05% (v/v) Triton X-100, and 10% (v/v) glycerol), and a His-tag elution buffer (pH 8.0, the binding buffer, and 250 mM Imidazole). For the in vitro binding assays between RPGEs and GLKs, 3 µg of GST-RPGEs bound to 20 μl of 50% (v/v) Glutathione Sepharose slurry were mixed with 3 µg of GLKs-His in a binding buffer (pH 7.5, 50 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 0.1% (v/v) Triton X-100, 0.05% (w/v) sodium deoxycholate and 10% (v/v) glycerol) and incubated at 4°C for 2 hours. After the incubation, the resins were washed with the binding buffer and the co-precipitated proteins were eluted in 50 μl of 1X SDS sample buffer (pH 6.8, 50 mM Tris–Cl, 2% (w/v) SDS, 0.02% (w/v) Bromophenol blue and 5% (v/v) Glycerol). The eluted GLKs-His were detected by the immunoblot assay with a His-tag antibody (#2365, Cell Signaling) and GST-RPGEs were detected with a Ponceau S solution (MB-072-0500, Rockland).
For the in vitro dimerization disruption assay, RPGE1 and OsRPGE were amplified and cloned into the pET-41a-derived vector (Novagen) and recombinant GST-tagged RPGE1 and OsRPGE proteins were purified with Glutathione Sepharose 4B (17075601, Cytiva). GLK1 was amplified and cloned into a pET-29a (Novagen)-derived vector for SBP-tagged GLK1 (SBP-GLK1) or into a pMAL-c2X vector (#75286, Addgene) for MBP-tagged GLK1 (MBP-GLK1). Primer sequences are listed in Supplemental Table S1. Recombinant proteins were purified with 250 μl of 50% (v/v) Streptavidin Sepharose (17511301, Cytiva) slurry according to the manufacturer's guideline. The resin-bound GLK1 proteins were mixed with GST-tagged RPGE1, OsRPGE, or GST in 150 µl of binding buffer (pH 7.4, 50 mM Tris–HCl, 150 mM NaCl, 0.05% (v/v) Triton X-100% and 10% (v/v) glycerol) and incubated at 4°C for 3 hours. After the incubation, the resins were centrifuged and the 100 μl of supernatant was mixed with 25 μl of 5X SDS sample buffer (Sup). The resins were washed 5 times with the binding buffer and eluted in 250 μl of 1X SDS sample buffer (Resin). MBP-GLK1, SBP-GLK1, and GST-tagged RPGEs were detected by the immunoblot assay with an anti-MBP antibody (sc-809, Santa Cruz), anti-SBP antibody (sc-101595, Santa Cruz), and anti-GST antibody (sc-138, Santa Cruz), respectively, both in the Sup and the Resin samples.
BiFC assay
For the BiFC assay, GLK2, RPGE1 and RPGE2 were cloned into pBiFCt-2in1-NC (Grefen and Blatt, 2012) expressing either the nYFP-GLK2 and RPGE1-cYFP pair or the nYFP-GLK2 and RPGE2-cYFP pair together with RFP. Primer sequences are listed in Supplemental Table S1. The vector was agroinfiltrated into Nicotiana benthamiana leaves together with the RK19 vector expressing P19. Three days after the infiltration, fluorescence signals were observed under a fluorescence microscope (BX51, Olympus) with a YFP filter (39,003—AT, Chroma, excitation filter: 495/20 nm, emission filter: 540/30 nm), an RFP filter (49,004—ET, Chroma, excitation filter: 545/25 nm, emission filter: 605/70 nm) and the laser setup (ISO200, Exposure time: 1/30 s).
Luciferase assay
The promoter region of HEMA1 (500 bp upstream from the start codon) was amplified from Col-0 genomic DNA and cloned into a pRLuc-derived vector composed of firefly luciferase (fLUC) reporter gene and 35Spro::rLUC (Renilla luciferase) gene to generate HEMA1pro::fLUC. The effector vectors were constructed by amplifying and cloning GLK1, RPGE1, or GFP gene into the pBI221 vector. Primer sequences are listed in Supplemental Table S1. The HEMA1pro::fLUC vector and the effector vectors were co-transfected into Arabidopsis protoplasts. Arabidopsis protoplasts were isolated and the vectors were transfected by the DNA-PEG-calcium transfection method as previously described (Yoo et al., 2007). Transfected protoplasts were incubated at 22°C for 16 hours under continuous dim white light (10 µmol m−2s). The Dual-Luciferase Reporter assay system (E1910, Promega) was used to measure the ratio of firefly luciferase activity to renilla luciferase activity (fLUC/rLUC). Experiments were performed 3 times with independently prepared protoplasts.
Electrophoretic mobility shift assay (EMSA)
EMSA was performed with recombinant C-terminal 6xHis-tagged GLK1 (GLK1-His) or the GCT domain-deleted GLK1 (GLK1ΔGCT-His) protein and biotin-labeled probes as previously described (Oh et al., 2007). GLK1 and GLK1ΔGCT were inserted into the pET-29a (Novagen)-derived vector lacking S-tag and recombinant proteins were purified as described above. Double-stranded biotin-labeled probes (2 nM) were incubated with 1.5 μg GLK1-His proteins in 10 μl of a binding buffer (pH 7.4, 20 mM HEPES, 100 mM KCl, 1 mM DTT, 0.005% (w/v) BSA, 0.02% (v/v) NP-40, 1.6 pmol Poly(dI-dC), 1.6 pmol Poly(dA-dT), and 10% (v/v) Glycerol) in the presence or absence of unlabeled wild-type or mutant probes (100 or 200 nM) for 30 minutes at 22°C. The competition assay was performed in the presence of increasing amounts of GST-RPGE1 or GST-OsRPGE (20, 40, or 60 μg) or GST (35 or 43 μg; equal molar amounts to 60 μg of GST-RPGE1 or GST-OsRPGE). After the incubation, the mixture was separated on 6% native polyacrylamide gel and transferred to a positively charged nylon transfer membrane (Hybond-N+, RPN203B, Cytiva) by the semi-dry transfer method. The positions of biotinylated probes were detected with the Chemiluminescent Nucleic Acid Detection Module Kit (89880, Thermo Fisher) according to the manufacturer's guideline. Probe sequences are listed in Supplemental Table S1.
ChIP assay
One thousand seedlings grown on MS agar plates (pH 5.7) with 1% (w/v) sucrose under high R/FR light (red light, 20 µmol m−2s; blue light, 20 µmol m−2s; far-red light, 10 µmol m−2s) for 4 days were kept in high R/FR for additional 4 hours or transferred to low R/FR light (red light, 2 µmol m−2s; blue light, 2 µmol m−2s; far-red light, 10 µmol m−2s) for 4 hours at 22°C. Seedlings were sampled, and ChIP assay was performed as described previously (Oh et al., 2020) except using IgG sepharose (17-0969-01, GE Healthcare) to precipitate TAP-GLK1-chromatin complex. ChIP assays were performed on 3 biological replicates using independently grown seedlings. Primer sequences are listed in Supplemental Table S1.
Immunoblot analysis
Seedlings were grown under the same conditions as for the ChIP assay before sampling. Sampled seedlings were ground in liquid nitrogen, dissolved in denaturing buffer (pH 8.0, 10 mM Tris–HCl, 177 mM NaH2PO4, 8 M urea, 1 mM PMSF, and 1 × protease inhibitor cocktail), and centrifuged to remove debris. The supernatant fractions were boiled in SDS sample buffer and protein levels were determined by immunoblot analysis using the following antibodies: anti-SBP (sc-101595, Santa Cruz), anti-TUB (T9026, Sigma-Aldrich), and goat anti-mouse IgG-HRP (LF-SA8001, AbFrontier). Luminescence was detected by the ChemiDoc XRS + System (Bio-Rad) after reacting with Clarity Max Western ECL (1705062, Bio-Rad). Band intensities were quantified with Image Lab Software (Bio-Rad).
Accession numbers
GLK1 (AT2G20570), GLK2 (AT5G44190), RPGE1 (AT5G02580), RPGE2 (AT3G28990), RPGE3 (AT3G55240), RPGE4 (AT1G10657), OVA3/ERS (AT5G64050), HEMA1 (AT1G58290), HEMA2 (AT1G09940), HEMA3 (AT2G31250), GSA1 (AT5G63570), GSA2 (AT3G48730), HEMB1/ALAD1 (AT1G69740), HEMB2 (AT1G44318), HEMC (AT5G08280), HEMD (AT2G26540), HEME1 (AT3G14930), HEME2 (AT2G40490), HEMF1 (AT1G03475), HEMF2 (AT4G03205), HEMG1 (AT4G01690), HEMG2 (AT5G14220), GUN5/CHLH (AT5G13630), GUN4 (AT3G59400), CHLI1 (AT4G18480), CHLI2 (AT5G45930), CHLD (AT1G08520), CHLM (AT4G25080), CHL27 (AT3G56940), DVR (AT5G18660), PORA (AT5G54190), PORB (AT4G27440), PORC (AT1G03630), CAO (AT1G44446), CHLG (AT3G51820), HCAR (AT1G04620), CBR (AT5G17770), GUN2/HO1 (AT2G26670), HO2 (AT2G26550), HO3 (AT1G69720), HO4 (AT1G58300), GUN3/HY2 (AT3G09150), LHCA1 (AT3G54890), LHCA2 (AT3G61470), LHCA3 (AT1G61520), LHCB2.4 (AT3G27690), LHCB3 (AT5G54270), LHCB4.1 (AT5G01530), PSAK (AT1G30380), PSBO2 (AT3G50820), PSBTn (AT3G21055), NOL (AT5G04900), NYC1 (AT4G13250), CLH1 (AT1G19670), CLH2 (AT5G43860), PPH (AT5G13800), PAO (AT3G44880), RCCR (AT4G37000), NYE1 (AT4G22920), PP2A (AT1G13320), OsGLK1 (LOC_Os06g03488), OsGLK2 (LOC_Os01g02390), SlGLK1 (Solyc07g053630), SlGLK2 (Solyc10g008160), MpGLK (Mapoly0156s0007), OsRPGE (Os03g0280400), MpRPGE (Mapoly0002s0278) and CbRPGE (G70694). Sequence data can be found in the TAIR (arabidopsis.org) for Arabidopsis thaliana, EnsemblPlants (plants.ensembl.org) for Solanum lycopersicum and Oryza sativa, MarpolBase (marchantia.info) for Marchantia polymorpha and Klebsormidium nitens, and OrcAE (bioinformatics.psb.ugent.be/orcae/) for Chara braunii. RNA-seq data can be found in National Center for Biotechnology Information (NCBI) with an accession number GSE199312 and The Sequence Read Archive (SRA) with an accession number PRJNA669623.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1 . RPGEs and GLKs are present in streptophytes.
Supplemental Figure S2 . The rpge1/rpge2/rpge3 triple mutant generated by the CRISPR/Cas9 system.
Supplemental Figure S3 . GO analysis of the shared DEGs.
Supplemental Figure S4 . Chlorophyll biosynthetic and photosynthetic genes are repressed in RPGE-OXs and the glk1/2 mutant.
Supplemental Figure S5 . The chlorophyll catabolic genes are not repressed in RPGE-OXs and the glk1/2 mutant.
Supplemental Figure S6 . The expression of RPGEs and GLKs in RPGE-OXs and glk1/2 mutant.
Supplemental Figure S7 . GLK1 possesses a transcription activation domain and a dimerization domain.
Supplemental Figure S8 . Interaction between RPGE homologs and GLK homologs from different species shown by a yeast 2-hybrid assay.
Supplemental Figure S9 . Shade does not decrease GLK1 protein levels.
Supplemental Figure S10 . Coomassie brilliant blue (CBB) staining of purified recombinant GLK proteins.
Supplemental Table S1 . Primers used in this study.
Supplemental Data Set S1 . DEGs in this study.
Supplementary Material
Acknowledgments
We thank Younil Park (Chungnam National University) for helping the measurement of Fv/Fm.
Contributor Information
Namuk Kim, Department of Biological Sciences, KAIST, Daejeon 34141, Korea.
Jinkil Jeong, Department of Biological Sciences, KAIST, Daejeon 34141, Korea.
Jeongheon Kim, Department of Biological Sciences, KAIST, Daejeon 34141, Korea.
Jeonghwa Oh, Department of Biological Sciences, KAIST, Daejeon 34141, Korea.
Giltsu Choi, Department of Biological Sciences, KAIST, Daejeon 34141, Korea.
Author contributions
N.K., J.J., and G.C. designed the experiments; N.K., J.J., J.K., and J.O. performed the experiments; N.K. and G.C. wrote the article.
Funding
This research was supported by the National Research Foundation of Korea (2018R1A3B1052617).
References
- Bi YM, Zhang Y, Signorelli T, Zhao R, Zhu T, Rothstein S (2005) Genetic analysis of Arabidopsis GATA transcription factor gene family reveals a nitrate-inducible member important for chlorophyll synthesis and glucose sensitivity. Plant J 44(4): 680–692 [DOI] [PubMed] [Google Scholar]
- Cackett L, Luginbuehl LH, Schreier TB, Lopez-Juez E, Hibberd JM (2022) Chloroplast development in green plant tissues: the interplay between light, hormone, and transcriptional regulation. New Phytol 233(5): 2000–2016 [DOI] [PubMed] [Google Scholar]
- Clack T, Mathews S, Sharrock RA (1994) The phytochrome apoprotein family in Arabidopsis is encoded by five genes: the sequences and expression of PHYD and PHYE. Plant Mol Biol 25(3): 413–427 [DOI] [PubMed] [Google Scholar]
- Fitter DW, Martin DJ, Scotland RW, Langdale JA (2002) GLK Gene pairs regulate chloroplast development in diverse plant species. Plant J 31(6): 713–727 [DOI] [PubMed] [Google Scholar]
- Franklin KA, Quail PH (2010) Phytochrome functions in Arabidopsis development. J Exp Bot 61(1): 11–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvão VC, Fankhauser C (2015) Sensing the light environment in plants: photoreceptors and early signaling steps. Curr Opin Neurobiol 34: 46–53 [DOI] [PubMed] [Google Scholar]
- Grefen C, Blatt MR (2012) A 2in1 cloning system enables ratiometric bimolecular fluorescence complementation (rBiFC). Biotechniques 53(5): 311–114 [DOI] [PubMed] [Google Scholar]
- Hao Y, Oh E, Choi G, Liang Z, Wang ZY (2012) Interactions between HLH and bHLH factors modulate light-regulated plant development. Mol Plant 5(3): 688–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SY, Seo PJ, Ryu JY, Cho SH, Woo JC, Park CM (2013) A competitive peptide inhibitor KIDARI negatively regulates HFR1 by forming nonfunctional heterodimers in Arabidopsis photomorphogenesis. Mol Cells 35(1): 25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornitschek P, Kohnen MV, Lorrain S, Rougemont J, Ljung K, López-Vidriero I, Franco-Zorrilla JM, Solano R, Trevisan M, Pradervand S, (2012) Phytochrome interacting factors 4 and 5 control seedling growth in changing light conditions by directly controlling auxin signaling. Plant J 71(5): 699–711 [DOI] [PubMed] [Google Scholar]
- Hu W, Franklin KA, Sharrock RA, Jones MA, Harmer SL, Lagarias JC (2013) Unanticipated regulatory roles for Arabidopsis phytochromes revealed by null mutant analysis. Proc Natl Acad Sci USA 110(4): 1542–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson D, Guevara D, Yaish MW, Hannam C, Long N, Clarke JD, Bi YM, Rothstein SJ (2011) GNC And CGA1 modulate chlorophyll biosynthesis and glutamate synthase (GLU1/Fd-GOGAT) expression in Arabidopsis. PLOS ONE 6(11): e26765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq E, Al-Sady B, Hudson M, Kim C, Apel K, Quail PH (2004) Phytochrome-interacting factor 1 is a critical bHLH regulator of chlorophyll biosynthesis. Science 305(5692): 1937–1941 [DOI] [PubMed] [Google Scholar]
- Ikeda M, Fujiwara S, Mitsuda N, Ohme-Takagi M (2012) A triantagonistic basic Helix-loop-Helix system regulates cell elongation in Arabidopsis. Plant Cell 24(11): 4483–4497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Jeong J, Kim J, Lee N, Kim ME, Lee S, Chang Kim S, Choi G (2016) PIF1 Regulates plastid development by repressing photosynthetic genes in the endodermis. Mol Plant 9(10): 1415–1427 [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Masuda T (2016) Transcriptional regulation of tetrapyrrole biosynthesis in Arabidopsis thaliana. Front Plant Sci 7: 1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35(6): 1547–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N, Choi G (2017) Phytochrome-interacting factor from Arabidopsis to liverwort. Curr Opin Plant Biol 35: 54–60 [DOI] [PubMed] [Google Scholar]
- Leivar P, Monte E, Oka Y, Liu T, Carle C, Castillon A, Huq E, Quail PH (2008) Multiple phytochrome-interacting bHLH transcription factors repress premature seedling photomorphogenesis in darkness. Curr Biol 18(23): 1815–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P, Quail PH (2011) PIFs: pivotal components in a cellular signaling hub. Trends Plant Sci 16(1): 19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P, Tepperman JM, Monte E, Calderon RH, Liu TL, Quail PH (2009) Definition of early transcriptional circuitry involved in light-induced reversal of PIF-imposed repression of photomorphogenesis in young Arabidopsis seedlings. Plant Cell 21(11): 3535–3553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li FW, Melkonian M, Rothfels CJ, Villarreal JC, Stevenson DW, Graham SW, Wong GK, Pryer KM, Mathews S (2015) Phytochrome diversity in green plants and the origin of canonical plant phytochromes. Nat Commun 6(1): 7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Lee KP, Liu T, Dogra V, Duan J, Li M, Xing W, Kim C (2022) Antagonistic modules regulate photosynthesis-associated nuclear genes via GOLDEN2-LIKE transcription factors. Plant Physiol 188(4): 2308–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wang P, Li J, Wei S, Yan Y, Yang J, Zhao M, Langdale JA, Zhou W (2020) Maize GOLDEN2-LIKE genes enhance biomass and grain yields in rice by improving photosynthesis and reducing photoinhibition. Commun Biol 3(1): 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Li L, Zhang B, Zeng L, Li L (2020) AhHDA1-mediated AhGLK1 promoted chlorophyll synthesis and photosynthesis regulates recovery growth of peanut leaves after water stress. Plant Sci 294: 110461 [DOI] [PubMed] [Google Scholar]
- Long SP, Marshall-Colon A, Zhu XG (2015) Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell 161(1): 56–66 [DOI] [PubMed] [Google Scholar]
- Mara CD, Irish VF (2008) Two GATA transcription factors are downstream effectors of floral homeotic gene action in Arabidopsis. Plant Physiol 147(2): 707–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín G, Leivar P, Ludevid D, Tepperman JM, Quail PH, Monte E (2016) Phytochrome and retrograde signalling pathways converge to antagonistically regulate a light-induced transcriptional network. Nat Commun 7(1): 11431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni F, Wu L, Wang Q, Hong J, Qi Y, Zhou X (2017) Turnip yellow mosaic virus P69 interacts with and suppresses GLK transcription factors to cause pale-green symptoms in Arabidopsis. Mol Plant 10(5): 764–766 [DOI] [PubMed] [Google Scholar]
- Oh E, Kang H, Yamaguchi S, Park J, Lee D, Kamiya Y, Choi G (2009) Genome-wide analysis of genes targeted by PHYTOCHROME INTERACTING FACTOR 3-LIKE5 during seed germination in Arabidopsis. Plant Cell 21(2): 403–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E, Yamaguchi S, Hu J, Yusuke J, Jung B, Paik I, Lee HS, Sun TP, Kamiya Y, Choi G (2007) PIL5, A phytochrome-interacting bHLH protein, regulates gibberellin responsiveness by binding directly to the GAI and RGA promoters in Arabidopsis seeds. Plant Cell 19(4): 1192–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Park E, Song K, Bae G, Choi G (2020) PHYTOCHROME INTERACTING FACTOR8 inhibits phytochrome A-mediated far-red light responses in Arabidopsis. Plant Cell 32(1): 186–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort DR, Merchant SS, Alric J, Barkan A, Blankenship RE, Bock R, Croce R, Hanson MR, Hibberd JM, Long SP, (2015) Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc Natl Acad Sci USA 112(28): 8529–8536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham VN, Kathare PK, Huq E (2017) Phytochromes and phytochrome interacting factors. Plant Physiol 176(2): 1025–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauf M, Arif M, Dortay H, Matallana-Ramírez LP, Waters MT, Gil Nam H, Lim PO, Mueller-Roeber B, Balazadeh S (2013) ORE1 balances leaf senescence against maintenance by antagonizing G2-like-mediated transcription. EMBO Rep 14(4): 382–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter R, Behringer C, Müller IK, Schwechheimer C (2010) The GATA-type transcription factors GNC and GNL/CGA1 repress gibberellin signaling downstream from DELLA proteins and PHYTOCHROME-INTERACTING FACTORS. Genes Dev 24(18): 2093–2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini L, Cribb L, Martin DJ, Langdale JA (2001) The maize Golden2 gene defines a novel class of transcriptional regulators in plants. Plant Cell 13(5): 1231–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, Kim K, Kang H, Zulfugarov IS, Bae G, Lee CH, Lee D, Choi G (2009) Phytochromes promote seedling light responses by inhibiting four negatively-acting phytochrome-interacting factors. Proc Natl Acad Sci USA 106(18): 7660–7665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solymosi K, Schoefs B (2010) Etioplast and etio-chloroplast formation under natural conditions: the dark side of chlorophyll biosynthesis in angiosperms. Photosynth Res 105(2): 143–166 [DOI] [PubMed] [Google Scholar]
- Song Y, Yang C, Gao S, Zhang W, Li L, Kuai B (2014) Age-triggered and dark-induced leaf senescence require the bHLH transcription factors PIF3, 4, and 5. Mol Plant 7(12): 1776–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson PG, Fankhauser C, Terry MJ (2009) PIF3 Is a repressor of chloroplast development. Proc Natl Acad Sci USA 106(18): 7654–7659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser B, Sánchez-Lamas M, Yanovsky MJ, Casal JJ, Cerdán PD (2010) Arabidopsis thaliana life without phytochromes. Proc Natl Acad Sci USA 107(10): 4776–4781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano M, Inagaki N, Xie X, Yuzurihara N, Hihara F, Ishizuka T, Yano M, Nishimura M, Miyao A, Hirochika H, (2005) Distinct and cooperative functions of phytochromes A, B, and C in the control of deetiolation and flowering in rice. Plant Cell 17(12): 3311–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Miao M, Niu X, Zhang D, Cao X, Jin X, Zhu Y, Fan Y, Wang H, Liu Y, (2016) Ubiquitin-conjugated degradation of golden 2-like transcription factor is mediated by CUL4-DDB1-based E3 ligase complex in tomato. New Phytol 209(3): 1028–1039 [DOI] [PubMed] [Google Scholar]
- Tokumaru M, Adachi F, Toda M, Ito-Inaba Y, Yazu F, Hirosawa Y, Sakakibara Y, Suiko M, Kakizaki T, Inaba T (2017) Ubiquitin-Proteasome dependent regulation of the GOLDEN2-LIKE 1 transcription factor in response to plastid signals. Plant Physiol 173(1): 524–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend PD, Dixon CH, Slootweg EJ, Sukarta OCA, Yang AWH, Hughes TR, Sharples GJ, Pålsson LO, Takken FLW, Goverse A, (2018) The intracellular immune receptor Rx1 regulates the DNA-binding activity of a Golden2-like transcription factor. J Biol Chem 293(9): 3218–3233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters MT, Wang P, Korkaric M, Capper RG, Saunders NJ, Langdale JA (2009) GLK transcription factors coordinate expression of the photosynthetic apparatus in Arabidopsis. Plant Cell 21(4): 1109–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenkel S, Emery J, Hou BH, Evans MM, Barton MK (2007) A feedback regulatory module formed by LITTLE ZIPPER and HD-ZIPIII genes. Plant Cell 19(11): 3379–3390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2(7): 1565–1572 [DOI] [PubMed] [Google Scholar]
- Zhang C, Zhang J, Tang Y, Liu K, Liu Y, Tang J, Zhang T, Yu H (2021a) DEEP GREEN PANICLE1 suppresses GOLDEN2-LIKE activity to reduce chlorophyll synthesis in rice glumes. Plant Physiol 185(2): 469–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Tan W, Yang F, Han Q, Deng X, Guo H, Liu B, Yin Y, Lin H (2021b) A BIN2-GLK1 signaling module integrates brassinosteroid and light signaling to repress chloroplast development in the dark. Dev Cell 56(3): 310–324 e7 [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhang L, Ji Y, Jing Y, Li L, Chen Y, Wang R, Zhang H, Yu D, Chen L (2022) Arabidopsis SIGMA FACTOR BINDING PROTEIN1 (SIB1) and SIB2 inhibit WRKY75 function in abscisic acid-mediated leaf senescence and seed germination. J Exp Bot 73(1): 182–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Mayba O, Pfeiffer A, Shi H, Tepperman JM, Speed TP, Quail PH (2013) A quartet of PIF bHLH factors provides a transcriptionally centered signaling hub that regulates seedling morphogenesis through differential expression-patterning of shared target genes in Arabidopsis. PLoS Genet 9(1): e1003244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Wang H, Xue L, Zhang Z, Tong T (2004) Regulation of cellular senescence and p16(INK4a) expression by Id1 and E47 proteins in human diploid fibroblast. J Biol Chem 279(30): 31524–31532 [DOI] [PubMed] [Google Scholar]
- Zubo YO, Blakley IC, Franco-Zorrilla JM, Yamburenko MV, Solano R, Kieber JJ, Loraine AE, Schaller GE (2018) Coordination of chloroplast development through the action of the GNC and GLK transcription factor families. Plant Physiol 178(1): 130–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.