Abstract
Background
Following the introduction of the COVID-19 vaccines, there has been uncertainty as to whether receiving the COVID-19 vaccine will result in overactivation of the immune system and subsequently lead to an autoimmune disease flare.
The purpose of this study was to assess whether rheumatoid arthritis (RA) patients who received the mRNA COVID-19 vaccine are at increased risk for disease flare.
Methods
We conducted a single-center retrospective and prospective study at the Louis Stokes Cleveland VA Medical Center between 12/2021 and 2/2022. We included 100 patients with rheumatoid arthritis (RA) who were actively on immunosuppressive therapy and received three doses of the Pfizer-BioNTech vaccine. A survey questionnaire was used to collect data about their RA and if they developed symptoms post vaccination. Our primary end point was to determine incidence of flare of RA after COVID-19 vaccine. Secondary end points were to estimate the side effect profile from the vaccine, and to check if patients developed a COVID-19 infection after they received the vaccine.
Results
None of the patients reported symptoms of RA flare within two months of receiving the 3 doses of the vaccine. Most common vaccine side effects were soreness over the injection site (n = 14), headache (n = 11), fatigue(n = 7) and myalgias(n = 4). 5 patients developed a COVID-19 infection prior to receiving the vaccine, 8 after being vaccinated, 3 of the 8 within 5 months from the second dose and 5 out of the 8 within 3 months from the third vaccine dose.
Conclusion
RA patients receiving the COVID-19 Pfizer mRNA vaccine do not appear to commonly develop major symptoms, flares or side effects following the vaccine. Further research with larger numbers of patients with rheumatoid arthritis as well as those with other autoimmune disease is needed to better understand the safety and effectiveness of COVID-19 vaccine.
Keywords: COVID-19, Rheumatoid arthritis, Disease flare, Vaccine
Introduction
Initial COVID mRNA vaccine studies leading to FDA emergency use authorization excluded patients with rheumatic diseases on immunosuppressive therapy. Therefore, it has been unclear whether the COVID mRNA vaccines might provoke flares of underlying autoimmune disease or result in greater incidence of vaccine associated adverse events. Downstream consequences of this uncertainty have been proposed to contribute to vaccine hesitancy in this patient population [1], [2]. The concept of vaccine associated autoimmune disease flare is not new. Immunization against tetanus, rubella, hepatitis B, and influenza have all been proposed to contribute to flare of rheumatoid arthritis (RA), but results from small studies have been difficult to reproduce in larger controlled studies [3] In one study, approximately 7 % of patients with RA and other rheumatic diseases had a flare of their symptoms within 12 weeks of receiving the zoster vaccine recombinant adjuvanted.[4].
The purpose of our study was to assess whether patients with RA who received the mRNA COVID-19 vaccine developed a flare of their disease.
Methods
Study participants and clinical chart data extraction:
We conducted a single-center retrospective chart review and prospective questionnaire study at the Louis Stokes Cleveland VA Medical Center. Under a Cleveland VA IRB approved protocol, charts were identified for 197 patients with RA that had already received 3 doses of the COVID-19 Pfizer-BioNTech mRNA vaccine and who were actively on immunosuppressive therapy. We invited patients randomly from this list to participate in this study until achieving consent for participation from the intended sample size of 100. Our single center RA population treated in our rheumatology clinic is approximately 400. We believe we were able to capture the intended information from 25 % of our local cohort.
Baseline (before vaccine) demographics were collected from the VA medical health record, including age, sex, Race/Ethnicity, as well as the medications they were taking, including Disease-modifying antirheumatic drug (DMARD), glucocorticoid, and non-steroidal anti-inflammatory drug use. Medications were grouped into the following different classes: Conventional DMARD (cDMARD), Biologic DMARD (bDMARD), Synthetic DMARD (sDMARD), prednisone.
Other data collected were the vaccine administration dates, vaccine associated side effects and rheumatology practitioner recorded information regarding arthritis activity.
Participants were administered a phone questionnaire to further quantify patient reported peri-vaccine medication management and post vaccine related symptoms. Participants were asked about vaccine-related communication and counselling by their arthritis providers. For each specific medication that participants reported taking, they were asked if they discontinued or held for a period of time those medications (yes/no/unsure), to improve the effectiveness of a COVID-19 vaccine.
They were also asked about the occurrence of post vaccination adverse events, including headaches, fever or chills, widespread muscle/joint pain, and rash, sore arm, fatigue, nausea. Respondents also reported whether they experienced postvaccine flares of existing RA described by the patients as similar in nature to their prior RA flares) and if these flares required treatment such as NSAIDs, prednisone or whether their symptoms required emergency department or rheumatology visit. The study was conducted during the pandemic when certain restrictions were still in effect such as limiting face to face appointments, and patients fear of coming to the clinic. Thus, we relied on the patients’ self-reported disease flare, and we considered symptoms consistent with flare if patient developed worsening of their baseline disease activity, joint pain associated with swelling and morning stiffness and if symptoms were similar to their prior RA symptoms or flares.
Patients were asked if they developed any of the above symptoms after any of the three doses and up to 2 months after the last dose, hence over approximately a 9-month window period.
Data analysis
Descriptive statistics were used where the qualitative and quantitative variables were summarized by count (percentage). All analyses were performed using the R statistical system.
Our primary end point was to calculate the incidence of flare of RA after COVID-19 vaccine. This was further evaluated by asking the patients about the symptoms they had, if any, within a month from receiving the vaccine and whether the symptoms were similar to their RA flares. Secondary end points were to describe the side effect profile of the vaccine among this population, and to check if patients developed a COVID-19 infection after they received the vaccine.
Results
Baseline Characteristics (Table 1.)
Table 1.
Baseline Characteristics.
| Age (Median, range) | 73.5 (51–87) |
|---|---|
| Sex | |
| Female | 3 |
| Male | 97 |
| Race | |
| White | 79 |
| African American | 17 |
| Other | 3 |
| missing | 1 |
| Seropositivity | |
| RF | 6 |
| Anti-CCP | 12 |
| Both RF and CCP | 44 |
| Seronegative | 38 |
| Comorbidities | |
| Diabetes mellitus | 39 |
| Coronary artery disease | 23 |
| Hypertension | 72 |
| Obesity | 42 |
| On ACE inhibitors | |
| Yes | 45 |
| No | 55 |
| Rheumatoid arthritis Medications | |
| cDMARD (HCQ, MTX, SSZ, LEF) | 43 |
| Biologic DMARD (Adalimumab, Etanercept, Abatacept, Rituximab) | 16 |
| sDMARD (tofacitinib) | 2 |
| cDMARD + bDMARD | 35 |
| Prednisone | 6 |
| Compliance to Medications | |
| Yes | 95 |
| No | 5 |
| Arthritis Symptom control | |
| Good | 72 |
| Fair | 25 |
| Poorly Controlled | 3 |
| Stopped Immunosuppressive medications before or after vaccine | |
| Yes | 10 |
| No | 90 |
*HCQ: Hydroxychloroquine, MTX: Methotrexate, SSZ: Sulfasalazine, LEF: Leflunomide.
97 % of our patient’s population were male, with a median age of 73.5 years (range 51–87 years). Most of the patients had comorbidities including but not limited to hypertension, diabetes mellitus, coronary artery disease, and obesity. 62 % had seropositive RA.
All our patients were on immunosuppressive therapy (as per inclusion criteria), 43 % on conventional Disease Modifying anti- Rheumatic Drug (DMARD), 35 % on combination of conventional DMARD and biologic DMARD and 16 % on biologics only. (Table 1.).
Most of our patients received the 2nd dose 3 weeks(+/-1week) after the first dose and the 3rd dose was received after 24–28 weeks from the 2nd dose.
95 % of the patients self-reported being compliant with their immunosuppressive medications. 72 % had good symptom control. Only 10 patients stopped their immunosuppressive medications before or after the vaccine.
39 patients were contacted twice, following the first 2 doses and then after the 3rd dose, and 61 patients were contacted once after completing all three doses.
Primary end point (RA flare) (Table 2)
Table 2.
Results.
| Symptoms post vaccine | |
|---|---|
| Yes | 37 |
| No | 63 |
| Similarity of symptoms to flare | |
| Yes | 0 |
| No | 100 |
| ED visit required | |
| Yes | 0 |
| No | 100 |
| Rheumatology visit required | |
| Yes | 0 |
| No | 100 |
| COVID-19 Infection | |
| Prior to Vaccination | 5 |
| After Vaccination | 8 |
| After 3rd dose | 5 |
None of the 100 patients reported a disease flare within 2 months of completing the 3 dose Pfizer-BioNTech vaccine series (Table 2).
Three patients reported feeling that they had a disease flare at time of initial questionnaire response, but upon further questioning and chart review, their reported symptoms were interpreted to be consistent with vaccine side effects. One patient reported fatigue for a week but did not have any fever, arthralgias or joint swelling.
The second patient reported difficulty walking due to fatigue a week following the vaccine and didn’t have any symptoms after the 2nd or 3rd dose. He denied having other symptoms. The third patient reported headache and sore arm, without usual arthritis symptoms.
Secondary end point (Vaccine side effects)
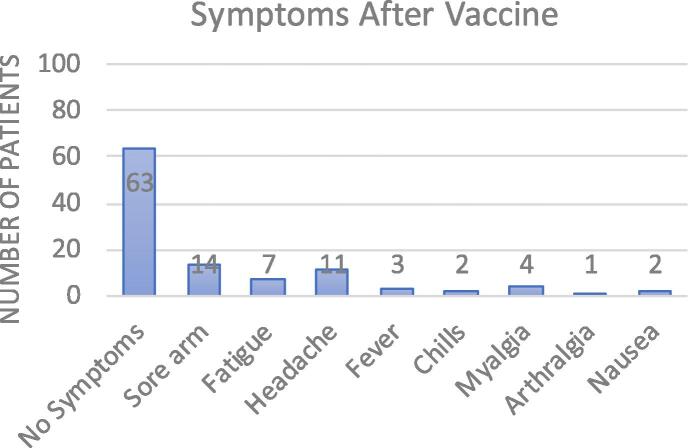
63 % of the patients did not develop any symptoms following vaccine administration.
37 % reported having symptoms. Most reported symptoms were sore arm over the injection site (n = 14), headache (n = 11), fatigue(n = 7) and myalgias(n = 4) (Fig. 1).
Fig. 1.
Symptoms after vaccine.
None of the patients required outpatient medical care for vaccine associated side effects (urgent care or rheumatology clinic care) (Table 2).
Five patients developed COVID-19 infection prior to receiving any dose of the COVID-19 vaccine. 8 patients developed COVID-19 infection after being vaccinated, 5 of the 8 within 3 months from receiving the 3rd dose.
50 % of those who stopped/held their immunosuppressive medications had vaccine associated side effects and 33 % of those who did not stop their medications had vaccine associated side effects.
Discussion
Between 12/2021 and 2/2022, a total of 100 patients who received 3 doses of the Pfizer-BioNTech COVID-19 vaccine were evaluated by questionnaire for vaccine associated RA disease flare and other vaccine associated side effects. None of them reported symptoms interpreted to be consistent with a flare of RA within 2 months of receiving the 3 dose vaccine series. These findings are in concordance with a study published by Pinte et al that concluded that patients with autoimmune diseases did not have an increased risk of flare following COVID-19 vaccination compared to patients who did not receive the vaccine[5]. Similar findings were published by Xue Li et al that looked specifically at patients with RA[6]. Published data from the EULAR Coronavirus vaccine physician-reported registry looked at the safety of the COVID-19 vaccination in 5121 patients that received Pfizer, AstraZeneca or Moderna vaccines and noted that 4.4 % of the patients reported flares of their Rheumatic disease suggesting that the risk of flare is low and probably compatible with the natural history of the diseases [7]. However there have been published results from web-based survey in New York City where 597 patients with systemic rheumatic disease (SRD) received the Pfizer vaccine and reported a typical SRD flare with 10.4 % after the first dose and 13.6 % after the second dose. Notably, the types of SRD were not reported in this study[8].There was another real-world survey conducted in China that included 434 patients with RA. In this study, 9.5 % of the responders self-reported flare after the vaccine, however the survey was completed by patients who received inactivated vaccines and not the mRNA vaccines [9]. Other published observational studies with questionnaire-based surveys had flare rates of 11 % their rheumatic and musculoskeletal disease [10]. Bixio and his colleagues prospectively looked at 77 patients with RA in clinical remission who received the Pfizer vaccine and noted that 6 patients (7.8 %) flared mostly after the second dose [11], [12].Most of the participants in our study had minimal side effects including sore arm at injection site, fatigue, myalgias and headache. The side effect profile from our cohort was similar to the studies that were conducted on patients who didn’t have autoimmune diseases and were not on immunosuppressive therapy [13], [14]. However, the frequency of reported symptoms was lower in our population overall, and the side effects were lower in those that did not stop their immunosuppressive medications compared to those who did [13], [15] This might be consistent with an overall lower immune response to the vaccine in RA patients.
8 participants (8 %) of our cohort ended up developing COVID-19 infection after the vaccine, 3 participants (3 %) after the second dose and 5 participants(5 %) within 3 months after receiving the 3rd dose, which could be consistent with a lower efficacy of the vaccine in this immunocompromised population compared to the general population [14], [16] Sun et al. reported that people with immune dysfunction such as HIV infections, RA, solid organ transplant had higher rates of COVID-19 breakthrough infection compared to people without immune dysfunction [17]. Washington state data showed that there were 21,757 vaccine breakthrough cases among more than 4.1 million vaccinated people (0.5 %) from January till August 21, 2021, a breakthrough rate lower than the percentages described here [16].
There are limitations to this study. Our study population is composed primarily of older male patients, consistent with the demographics of the VA health care system, however COVID-19 vaccination and its adverse events profile is not expected to be different between men and women. In addition, we have a small sample size and there is potential recall bias from phone surveys as there was a time gap of approximately 7 months for 61 % of the patients from the time they got the first series to the interview.
Finally, guidance regarding dosing and intervals of primary vaccine series and ACR’s COVID-19 vaccine clinical guidance were still being updated at the time of the conduction of our study. Thus, findings may not be generalizable to other populations.
In conclusion rheumatoid arthritis patients receiving the COVID-19 Pfizer vaccine do not commonly develop flares or major side effects following the vaccine. The mRNA COVID-19 vaccine appears to be safe in patients with RA. The question remains as to whether COVID-19 vaccine confers adequate immune protection and whether lack of immune protection is associated with lower rates of vaccine side effects and/or immune modulatory agents.
Key messages:
What is already known on this topic:
39 Previous studies reported possible association between vaccines and rheumatic disease flares. Following the introduction of the mRNA vaccine, published studies reported patients’ hesitancy to receive the COVID-19 vaccine due to fear from its downstream consequences.
What this study adds:
Our study shows that there were no flares in patients with RA who received the Pfizer vaccine, and the side effects were minimal.
How this study might affect research:
Patients with RA would be more inclined to take the vaccine and as a result would have better outcomes with COVID-19 infections.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
co-authors for their efforts and support Funding, grant/award info: This study was supported by VA Merit funding BX005480 and CX001791. Ethical approval information: The IRB is Cleveland VA IRB.IRB number and name for VA IRB is: Determinants of Cardiovascular Health in Rheumatic Disease: Impact of Immune Activation on Cardiovascular and Immune Health in RA. IRBNet ID #: 17046-H35.
Patient and Public Involvement: not applicable.
Data availability
Data will be made available on request.
References:
- 1.Felten R, Dubois M, Ugarte-Gil MF, et al. Cluster analysis reveals three main patterns of beliefs and intention with respect to SARS-CoV-2 vaccination in patients with autoimmune and inflammatory diseases. Rheumatology (Oxford) 2021;60:SI68–76. 10.1093/RHEUMATOLOGY/KEAB432. [DOI] [PMC free article] [PubMed]
- 2.Terracina K.A., Tan F.K. Flare of rheumatoid arthritis after COVID-19 vaccination. Lancet Rheumatol. 2021;3:e469–e470. doi: 10.1016/S2665-9913(21)00108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ray P., Black S., Shinefield H., et al. Risk of rheumatoid arthritis following vaccination with tetanus, influenza and hepatitis B vaccines among persons 15–59 years of age. Vaccine. 2011;29:6592–6597. doi: 10.1016/J.VACCINE.2011.06.112. [DOI] [PubMed] [Google Scholar]
- 4.Stevens E., Weinblatt M.E., Massarotti E., et al. Safety of the zoster vaccine recombinant adjuvanted in rheumatoid arthritis and other systemic rheumatic disease patients: a single Center’s Experience With 400 Patients. ACR Open Rheumatol. 2020;2:357–361. doi: 10.1002/ACR2.11150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinte L., Negoi F., Ionescu G.D., et al. COVID-19 Vaccine Does Not Increase the Risk of Disease Flare-Ups among Patients with Autoimmune and Immune-Mediated Diseases. J Pers Med. 2021:11. doi: 10.3390/JPM11121283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X., Tong X., Wan Yin Yeung W., et al. Two-dose COVID-19 vaccination and possible arthritis flare among patients with rheumatoid arthritis in Hong Kong. Ann Rheum Dis. 2021:1–5. doi: 10.1136/annrheumdis-2021-221571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Machado P.M., Lawson-Tovey S., Strangfeld A., et al. Safety of vaccination against SARS-CoV-2 in people with rheumatic and musculoskeletal diseases: results from the EULAR Coronavirus Vaccine (COVAX) physician-reported registry. Ann Rheum Dis Published Online First. 2021 doi: 10.1136/annrheumdis-2021-221490. [DOI] [PubMed] [Google Scholar]
- 8.Barbhaiya M., Levine J.M., Bykerk V.P., et al. Systemic rheumatic disease flares after SARS-CoV-2 vaccination among rheumatology outpatients in New York City. Ann Rheum Dis. 2021;80:1352–1354. doi: 10.1136/annrheumdis-2021-220732. [DOI] [PubMed] [Google Scholar]
- 9.Fan Y., Geng Y., Wang Y., et al. Safety and disease flare of autoimmune inflammatory rheumatic diseases: a large real-world survey on inactivated COVID-19 vaccines. Ann Rheum Dis. 2022;81:443–445. doi: 10.1136/annrheumdis-2021-221736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connolly C.M., Ruddy J.A., Boyarsky B.J., et al. Disease Flare and Reactogenicity in Patients With Rheumatic and Musculoskeletal Diseases Following Two-Dose SARS–CoV-2 Messenger RNA Vaccination. Arthritis and Rheumatol. 2022;74:28–32. doi: 10.1002/art.41924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bixio R., Bertelle D., Masia M., et al. Incidence of Disease Flare After BNT162b2 Coronavirus Disease 2019 Vaccination in Patients With Rheumatoid Arthritis in Remission. ACR Open Rheumatol. 2021;3:832–833. doi: 10.1002/acr2.11336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie Y., Liu Y., Liu Y. The flare of rheumatic disease after SARS-CoV-2 Vaccination: a Review. Front Immunol. 2022:13. doi: 10.3389/fimmu.2022.919979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beatty A.L., Peyser N.D., Butcher X.E., et al. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw Open. 2021;4:e2140364–e. doi: 10.1001/JAMANETWORKOPEN.2021.40364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu C, Lee J, Ta C, et al. A Retrospective Analysis of COVID-19 mRNA Vaccine Breakthrough Infections – Risk Factors and Vaccine Effectiveness. medRxiv Published Online First: 7 October 2021. 10.1101/2021.10.05.21264583.
- 15.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMOA2034577/SUPPL_FILE/NEJMOA2034577_PROTOCOL.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.What to Know about Breakthrough COVID-19 Cases | by Washington State Department of Health | Public Health Connection | Medium. https://medium.com/wadepthealth/what-to-know-about-breakthrough-covid-19-cases-10d3681e9991 (accessed Jul 2022).
- 17.Sun J., Zheng Q., Madhira V., et al. Association Between Immune Dysfunction and COVID-19 Breakthrough Infection After SARS-CoV-2 Vaccination in the US. JAMA Intern Med. 2022;182:153–162. doi: 10.1001/JAMAINTERNMED.2021.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.