Keywords: aging, sarcopenia, skeletal muscle mass
Abstract
Age-related skeletal muscle atrophy appears to be a muscle group-specific process, yet only a few specific muscles have been investigated and our understanding in this area is limited. This review provides a comprehensive summary of the available information on age-related skeletal muscle atrophy in a muscle-specific manner, nearly half of which comes from the quadriceps. Decline in muscle-specific size over ∼50 yr of aging was determined from 47 cross-sectional studies of 982 young (∼25 yr) and 1,003 old (∼75 yr) individuals and nine muscle groups: elbow extensors (−20%, −0.39%/yr), elbow flexors (−19%, −0.38%/yr), paraspinals (−24%, −0.47%/yr), psoas (−29%, −0.58%/yr), hip adductors (−13%, −0.27%/yr), hamstrings (−19%, −0.39%/yr), quadriceps (−27%, −0.53%/yr), dorsiflexors (−9%, −0.19%/yr), and triceps surae (−14%, −0.28%/yr). Muscle-specific atrophy rate was also determined for each of the subcomponent muscles in the hamstrings, quadriceps, and triceps surae. Of all the muscles included in this review, there was more than a fivefold difference between the least (−6%, −0.13%/yr, soleus) to the most (−33%, −0.66%/yr, rectus femoris) atrophying muscles. Muscle activity level, muscle fiber type, sex, and timeline of the aging process all appeared to have some influence on muscle-specific atrophy. Given the large range of muscle-specific atrophy and the large number of muscles that have not been investigated, more muscle-specific information could expand our understanding of functional deficits that develop with aging and help guide muscle-specific interventions to improve the quality of life of aging women and men.
INTRODUCTION
A decline in muscle mass is a well-accepted tenet associated with human aging (1), and has been investigated at the whole body, specific body region, specific muscle, and myocellular levels (2–7). Given the large number of skeletal muscles in the human body with distinct functions and contributions to overall daily activities, it is noteworthy that the number of specific muscles that have been investigated is relatively limited. In fact, muscle-specific aging studies have largely focused on the quadriceps or the vastus lateralis subcomponent of this muscle (1, 8–10). This is understandable given the ability to obtain high-quality size (via noninvasive imaging) and functional measurements of the quadriceps, coupled with the relative ease and safety of obtaining a biopsy of the vastus lateralis to perform simultaneous myocellular studies (6, 11–15). Exercise studies focused on mitigating age-related atrophy are also easily designed to focus on the quadriceps (e.g., knee extension resistance exercise or cycling) (10, 16–20). As a result, findings from the quadriceps (and vastus lateralis) investigations are generalized to the other muscles of the body with regard to the aging process and responsiveness to exercise training interventions. However, there is some evidence to suggest that age-related atrophy is muscle specific (2, 21–25) and that the responsiveness to exercise training is also muscle specific (2, 26–28).
Thus, more information is needed about an expanded number of specific muscles of the body with respect to the aging process. Muscle-specific information could improve our understanding of functional deficits that develop with aging and help guide muscle-specific interventions to improve the quality of life of aging women and men. The purpose of this review article was to provide a compilation of the current state of knowledge regarding muscle-specific atrophy in humans associated with the aging process.
LITERATURE REVIEW PARAMETERS AND SUMMARY
Numerous studies have quantified whole muscle atrophy with aging using computed tomography (CT), magnetic resonance imaging (MRI), or ultrasound. Of these, studies with cross-sectional designs that examined the human skeletal muscle cross-sectional area (CSA) or volume of generally healthy young (mean or median age of 20–29 yr) and old (mean or median age of 70–79 yr) individuals were compiled and the rate of muscle atrophy per year was determined for each muscle group. Although several studies reported muscle size comparisons between young and older (≥80 yr) individuals (29–33), these articles were excluded to allow assembling groups with a similar age across muscle groups. Studies that only reported muscle thickness, physiological CSA, or normalized muscle size (e.g., skeletal muscle index: muscle size/height2) were also excluded. Three muscle imaging methods (CT, MRI, and ultrasound) were considered equally in determining the rate of muscle loss, although ultrasound has only been validated against CT or MRI since the 1990s (34). As a result, studies on elbow extensors (anconeus, supinator, and triceps brachii), elbow flexors (biceps brachii, brachialis, brachioradialis, and pronator teres), paraspinals (erector spinae, lumbar multifidus, and quadratus lumborum), psoas (iliopsoas or psoas major), hip adductors (adductor brevis, adductor longus, adductor magnus, gracilis, pectineus, and sartorius), hamstrings (biceps femoris, semimembranosus, and semitendinosus), quadriceps femoris (rectus femoris, vastus lateralis, vastus intermedius, and vastus medialis), dorsiflexors (extensor digitorum longus, extensor hallucis longus, peroneus tertius, and tibialis anterior), and triceps surae (gastrocnemius and soleus) were included. For hamstrings, quadriceps, and triceps surae, studies that also assessed the size of subcomponent muscles within the muscle group were also compiled to determine the rate of atrophy of each muscle.
Similarly, longitudinal studies that examined human skeletal muscle CSA or volume of generally healthy older individuals were compiled and the rate of muscle atrophy per year was determined for each muscle group. The goal was to include studies near the mean or median age of the older cross-sectional cohort (i.e., 70–79 yr) either at the time of baseline measurement or longitudinal follow-up. Studies with a follow-up period of less than 1 yr were excluded. To provide comparisons across muscle groups with similar starting and longitudinal follow-up age ranges (∼65–75 yr), several studies of the quadriceps were excluded (12, 35–38). As a result, studies on paraspinals, psoas, hamstrings, and quadriceps femoris were included.
With these literature review criteria, data from 47 studies, including an accumulated total of 982 young and 1,003 old individuals were included in this review for cross-sectional comparisons between young and old individuals. Seventeen studies consisting of 717 subjects examined quadriceps, accounting for nearly 40% of the data included in this review. On the contrary, only a limited number of studies were found for each of the other eight muscle groups included in this review. Paraspinals and psoas included over 200 individuals, whereas elbow extensors and flexors only included 58 and 76 individuals, respectively. Paraspinals and psoas included more women (19% men and 17% men, respectively), whereas the other muscle groups had more men included (57%–83% men). In addition, one study for each of the four muscle groups (paraspinals, psoas, hamstrings, and quadriceps) was included for the longitudinal data on aging human skeletal muscle. All the muscle atrophy rate determinations were normalized to the sample size of each study, yet the studies with a small sample size tend to have relatively larger variability. Thus, data from muscle groups with a small total sample size should be interpreted with caution.
Cross-sectional comparisons for skeletal muscle size for the quadriceps and the other muscle groups are summarized in Tables 1 and 2, respectively. In addition, Table 3 summarizes the rate of muscle atrophy in the subcomponent muscles. Atrophy rates across all muscles ranged from −0.13%/yr to −0.66%/yr. Figure 1 presents the annualized rates of atrophy from these tables and percent changes over 50 yr of aging (∼25–75 yr) as most of the studies covered approximately this age range. Because many studies in the aging literature present an overall percent change in muscle mass, this atrophy rate per 50 yr and the rate per year will be discussed later. General muscle fiber type characteristics from separate biopsy and autopsy studies of the muscles included in this review are presented in Table 4. Sex-specific muscle atrophy rates for six muscle groups that had data to allow for this comparison are summarized in Table 5. The longitudinal studies are summarized in Table 6.
Table 1.
Summary of cross-sectional studies of human skeletal muscle aging in quadriceps femoris
| Study | Young |
Old |
ΔAge | Measurement | Method | %Δ | %Δ/yr | ||
|---|---|---|---|---|---|---|---|---|---|
| n | Age | n | Age | ||||||
| Young et al. 1984 (39) | 25 W | 20–29 | 25 W | 71–81 | 50 | CSA | US | −33 | −0.66 |
| Young et al. 1985 (40) | 12 M | 21–28 | 12 M | 70–79 | 50 | CSA | US | −25 | −0.50 |
| Overend et al. 1992 (23) | 13 M | 25 ± 5 | 12 M | 71 ± 5 | 46 | CSA | CT | −22 | −0.49 |
| Rutherford and Jones 1992 (41) | 31 W | 20–29 | 11 W | 70–82 | 50 | CSA | CT | −23 | −0.46 |
| Takahashi et al. 2006 (42) | 35 W | 21 ± 2 | 35 W | 74 ± 3 | 53 | CSA | MRI | −28 | −0.52 |
| Haus et al. 2007 (13) and (6, 7) | 10 M, 10 W | 25 ± 4 | 10 M, 12 W | 78 ± 5 | 53 | CSA/Volume | MRI | −26 | −0.49 |
| Kilgour et al. 2013 (43) | 19 M | 22 ± 2 | 33 M | 70 ± 4 | 48 | CSA | MRI | −27 | −0.57 |
| Maden-Wilkinson et al. 2013 (44) and (45–48) | 20 M | 22 ± 3 | 25 M | 72 ± 5 | 50 | Volume | MRI | −32 | −0.64 |
| 18 W | 22 ± 2 | 28 W | 72 ± 5 | 50 | −28 | −0.56 | |||
| Nilwik et al. 2013 (49) | 25 M | 23 ± 5 | 26 M | 71 ± 5 | 48 | CSA | CT | −15 | −0.31 |
| Konopka et al. 2014 (50) and (17) | 7 M | 20 ± 3 | 6 M | 74 ± 7 | 54 | CSA | MRI | −15 | −0.28 |
| Ghosh et al. 2014 (51) | 6 M, 7 W | 26 ± 4 | 8 M, 4 W | 74 ± 7 | 48 | Volume | MRI | −20 | −0.40 |
| Rudroff et al. 2014 (52) | 6 M | 26 ± 6 | 6 M | 77 ± 6 | 51 | Volume | CT | −41 | −0.81 |
| Piasecki et al. 2016 (53) | 22 M | 25 ± 5 | 20 M | 71 ± 6 | 46 | CSA | MRI | −33 | −0.71 |
| Yoshiko et al. 2017 (25) and (54, 55) | 8 M, 7 W | 21 ± 0.4 | 7 M, 8 W | 71 ± 4 | 50 | Volume | MRI | −37 | −0.74 |
| Chambers et al. 2020 (2) | 10 M | 25 ± 3 | 10 M | 75 ± 3 | 50 | CSA/Volume | MRI | −30 | −0.60 |
| 10 W | 25 ± 3 | 10 W | 75 ± 3 | 50 | −37 | −0.73 | |||
| Ogawa et al. 2021 (24) | 20 M | 23–27 | 20 M | 67–72 | 50 | CSA | MRI | −24 | −0.48 |
| Yagi et al. 2022 (56) | 17 M | 20–39 | 18 M | 60–87 | 49 | CSA | MRI | −18 | −0.36 |
| 15 W | 20–38 | 18 W | 62–84 | 46 | −20 | −0.43 | |||
| Total: 17 studies* | 353 | 24 | 364 | 73 | 50 | −0.53 | |||
Age (yr) is presented as means ± SD or range. Muscle size changes (%Δ and %Δ/yr) were averaged when the study reports both muscle cross-sectional area (CSA) and volume. CT, computed tomography; M, men; MRI, magnetic resonance imaging; US, ultrasound; W, women.
*Boldface type indicates data are total n size or average values normalized to the n size of each study. For studies listed with more than one reference, the primary reference used to generate the data in the table is listed first, followed by other references that also contain data from the same study.
Table 2.
Summary of cross-sectional studies of human skeletal muscle aging in nonquadriceps muscle groups
| Study | Young |
Old |
ΔAge | Measurement | Method | %Δ | %Δ/yr | ||
|---|---|---|---|---|---|---|---|---|---|
| n | Age | n | Age | ||||||
| Elbow extensors | |||||||||
| Klein et al. 2002 (57) and (58) | 20 M | 23 ± 3 | 10 M | 77 ± 1 | 54 | CSAa | MRI | −20 | −0.38 |
| Vidt et al. 2012 (59) and (60) | 5 M, 5 W | 29 ± 5 | 10 M, 8 W | 75 ± 4 | 47 | Volume | MRI | −19 | −0.40 |
| Total: 2 studies* | 30 | 26 | 28 | 76 | 50 | −0.39 | |||
| Elbow flexors | |||||||||
| Klein et al. 2002 (57) and (58) | 20 M | 23 ± 3 | 10 M | 77 ± 1 | 54 | CSAb | MRI | −15 | −0.28 |
| Vidt et al. 2012 (59) and (60) | 5 M, 5 W | 29 ± 5 | 10 M, 8 W | 75 ± 4 | 47 | Volume | MRI | −20 | −0.43 |
| Smart et al. 2018 (61) | 9 M | 23 ± 2 | 9 M | 77 ± 5 | 54 | CSAb | US | −26 | −0.48 |
| Total: 3 studies* | 39 | 25 | 37 | 76 | 51 | −0.38 | |||
| Paraspinals | |||||||||
| Ma et al. 2014 (62) | 21 W | 29 ± 3 | 42 W | 73 ± 4 | 44 | CSAc | MRI | −19 | −0.42 |
| Takayama et al. 2016 (63) | 20 M, 20 W | 10–29 | 30 M, 30 W | 60–88 | 55 | CSAd | MRI | −31 | −0.56 |
| Peng et al. 2020 (64) | 69 W | 27 ± 2 | 25 W | 74 ± 3 | 47 | CSAd | CT | −20 | −0.42 |
| Total: 3 studies* | 130 | 25 | 127 | 74 | 49 | −0.47 | |||
| Psoas | |||||||||
| Takahashi et al. 2006 (42) | 35 W | 21 ± 2 | 35 W | 74 ± 3 | 53 | CSAe | MRI | −37 | −0.70 |
| Ma et al. 2014 (62) | 21 W | 29 ± 3 | 42 W | 73 ± 4 | 44 | CSAe | MRI | −19 | −0.43 |
| Yagi et al. 2022 (56) | 17 M | 20–39 | 18 M | 60–87 | 49 | CSAf | MRI | −28 | −0.58 |
| 15 W | 20–38 | 19 W | 62–84 | 47 | −28 | −0.60 | |||
| Total: 3 studies* | 88 | 25 | 114 | 74 | 48 | −0.58 | |||
| Hip adductors | |||||||||
| Hogrel et al. 2015 (45) | 18 M | 24 ± 3 | 19 M | 74 ± 3 | 50 | Volume | MRI | −17 | −0.33 |
| 16 W | 24 ± 3 | 19 W | 75 ± 3 | 51 | −13 | −0.26 | |||
| Yoshiko et al. 2017 (25) | 8 M, 7 W | 21 ± 0.4 | 7 M, 8 W | 71 ± 4 | 50 | Volume | MRI | −27 | −0.55 |
| Ogawa et al. 2021 (24) | 20 M | 23–27 | 20 M | 67–72 | 50 | CSA | MRI | −14↔ | −0.28↔ |
| Total: 3 studies* | 69 | 23 | 73 | 73 | 50 | −0.27 | |||
| Hamstrings | |||||||||
| Overend et al. 1992 (23) | 13 M | 25 ± 5 | 12 M | 71 ± 5 | 46 | CSA | CT | −14↔ | −0.30↔ |
| Hogrel et al. 2015 (45) and (44, 46,47, 48) | 18 M | 24 ± 3 | 19 M | 74 ± 3 | 50 | Volume | MRI | −19 | −0.37 |
| 16 W | 24 ± 3 | 19 W | 75 ± 3 | 51 | −16 | −0.32 | |||
| Palmer and Thompson 2017 (66) | 15 M | 25 ± 3 | 15 M | 72 ± 5 | 51 | CSA | US | −17 | −0.36 |
| Yoshiko et al. 2017 (25) and (54, 55) | 8 M, 7 W | 21 ± 0.4 | 7 M, 8 W | 71 ± 4 | 50 | Volume | MRI | −28 | −0.56 |
| Ogawa et al. 2021 (24) | 20 M | 23–27 | 20 M | 67–72 | 50 | CSA | MRI | −30 | −0.60 |
| Total: 5 studies* | 97 | 23 | 100 | 72 | 49 | −0.39 | |||
| Dorsiflexors | |||||||||
| Kent-Braun et al. 2000 (67) | 11 W | 29 ± 4 | 10 W | 73 ± 6 | 44 | CSA | MRI | −11 | −0.26 |
| Hasson et al. 2011 (68) | 6 M | 27 ± 3 | 6 M | 73 ± 5 | 46 | Volume | MRI | +0.4↔ | +0.01↔ |
| 6W | 26 ± 3 | 6 W | 70 ± 5 | 44 | −10 | −0.23 | |||
| Barber et al. 2013 (69) | 10 M, 8 W | 27 ± 3 | 9 M, 7 W | 70 ± 3 | 43 | Volumeg | MRI | −22 | −0.51 |
| Christie et al. 2014 (70) | 10 M, 10 W | 24 ± 2 | 9 M, 9 W | 73 ± 6 | 50 | CSA | MRI | +3↔ | +0.06↔ |
| Power et al. 2014 (71) | 12 M | 24 ± 3 | 6 M | 79 ± 3 | 54 | CSA/Volumeg | MRI | −17 | −0.32 |
| Piasecki et al. 2016 (72) | 18 M | 26 ± 4 | 14 M | 71 ± 4 | 45 | CSAg | MRI | −11↔ | −0.25↔ |
| Total: 6 studies* | 91 | 26 | 76 | 73 | 46 | −0.19 | |||
| Triceps surae | |||||||||
| Morse et al. 2004 (73) and (74–77) | 14 M | 25 ± 5 | 21 M | 74 ± 4 | 49 | CSA/Volume | MRI | −18 | −0.36 |
| Hasson et al. 2011 (68) | 6 M | 27 ± 3 | 6 M | 73 ± 5 | 46 | Volume | MRI | −18 | −0.39 |
| 6W | 26 ± 3 | 6 W | 70 ± 5 | 44 | −19 | −0.43 | |||
| Barber et al. 2013 (69) | 10 M, 8 W | 27 ± 3 | 9 M, 7 W | 70 ± 3 | 43 | Volume | MRI | −16 | −0.38 |
| Chambers et al. 2020 (2) | 10 M | 25 ± 3 | 10 M | 75 ± 3 | 50 | Volume | MRI | −13↔ | −0.26↔ |
| 10 W | 25 ± 3 | 10 W | 75 ± 3 | 50 | −32 | −0.63 | |||
| Pinel et al. 2021 (78) | 13 M, 8 W | 25 ± 4 | 9 M, 6 W | 70 ± 2 | 46 | Volume | MRI | −1↔ | −0.03↔ |
| Total: 5 studies* | 85 | 25 | 84 | 72 | 47 | −0.28 | |||
Age (yr) is presented as means ± SD or range. Muscle size changes (%Δ and %Δ/yr) were averaged when the study reports both muscle cross-sectional area (CSA) and volume. CT, computed tomography; M, men; MRI; magnetic resonance imaging; US; ultrasound; W, women.
*Boldface type indicates data are total n size or average values normalized to the n size of each study; ↔P > 0.05, considered as zero for total muscle atrophy rate determination; atriceps brachii only; bbiceps brachii only; cerector spinae and quadratus lumborum; derector spinae and multifidus; epsoas major; filiopsoas; gtibialis anterior only. For studies listed with more than one reference, the primary reference used to generate the data in the table is listed first, followed by other references that also contain data from the same study.
Table 3.
Summary of human skeletal muscle aging in subcomponent muscles
| Muscle Group (References) | Young |
Old |
ΔAge* | Muscles | %Δ/yr* | ||
|---|---|---|---|---|---|---|---|
| n | Age* | n | Age* | ||||
| Hamstrings (45) | 18 M | 24 | 19 M | 74 | 51 | Semimembranosus | −0.40 |
| 16 W | 19 W | Semitendinosus | −0.39 | ||||
| Biceps femoris long head | −0.28 | ||||||
| Biceps femoris short head | −0.23 | ||||||
| Quadriceps (6, 45, 52) | 29 M | 24 | 30 M | 76 | 51 | Rectus femoris | −0.66 |
| 21 W | 24 W | Vastus lateralis | −0.59 | ||||
| Vastus intermedius | −0.49 | ||||||
| Vastus medialis | −0.48 | ||||||
| Triceps surae (68, 69, 74, 78) | 44 M | 26 | 36 M | 71 | 45 | Gastrocnemius | −0.41 |
| 22 W | 19 W | Soleus | −0.13 | ||||
Age is presented in years. M, men; W, women.
*Average values normalized to the n size of each study.
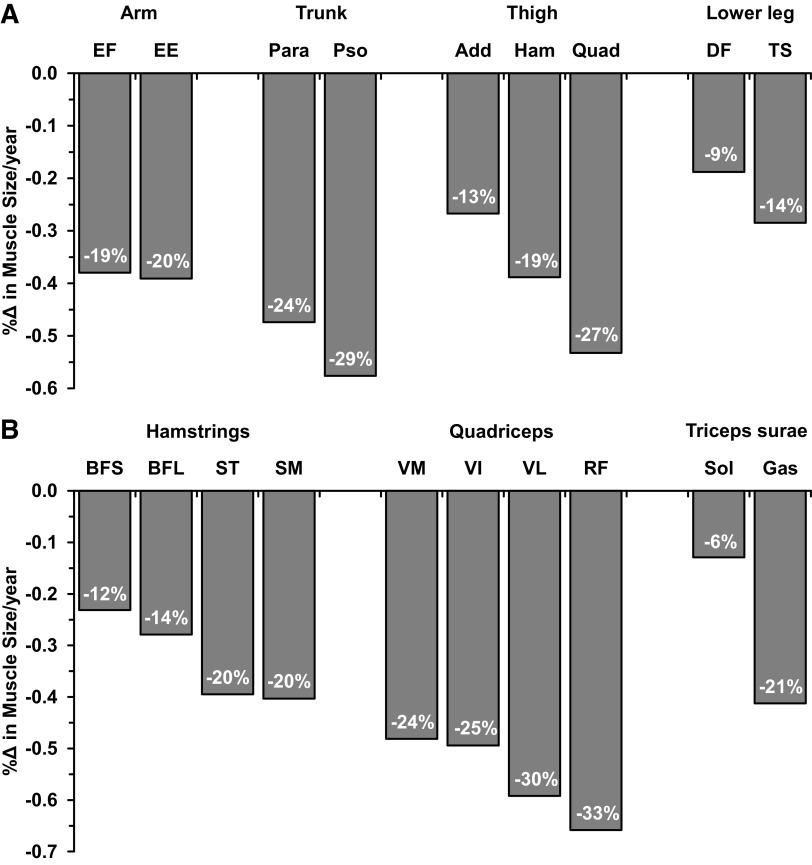
Figure 1.
Summary of skeletal muscle-specific atrophy with aging from cross-sectional studies. Muscle atrophy rates (%Δ/yr) are from Tables 1, 2 and 3. Percentage in each bar is the yearly rate multiplied by 50 to equally represent the muscle atrophy over 50 yr of aging from ∼25 yr to ∼75 yr across muscle groups. A: nine muscle groups included in this review. B: subcomponent muscles. Add, hip adductors; BFL, biceps femoris long head; BFS, biceps femoris short head; DF, dorsiflexors; EE, elbow extensors; EF, elbow flexors; Gas, gastrocnemius; Ham, hamstrings; Para, paraspinals; Pso, psoas; Quad, quadriceps; RF, rectus femoris; SM, semimembranosus; Sol, soleus; ST, semitendinosus; TS, triceps surae; VI, vastus intermedius; VL, vastus lateralis; VM, vastus medialis.
Table 4.
Human skeletal muscle fiber type distribution for muscles included in this review
| Muscle (References) | Age, yr | Muscle Fiber Type Distribution |
|
|---|---|---|---|
| Type I, % | Type II, % | ||
| Elbow extensors | |||
| Triceps brachii (79–82) | 17–40 | 36 (22–50) | 64 (50–78) |
| Elbow flexors | |||
| Biceps brachii (79–81) | 17–40 | 46 (45–46) | 54 (53–55) |
| Brachioradialis (79, 81) | 17–40 | 44 (40–47) | 56 (51–60) |
| Paraspinals | |||
| Erector spinae (81, 83–87) | 16–44 | 61 (57–66) | 39 (33–47) |
| Lumbar multifidus (83, 84, 87–89) | 17–44 | 59 (54–65) | 41 (36–46) |
| Psoas | |||
| Psoas major/Iliopsoas (81, 90) | 17–35 | 45 (40–49) | 55 (51–60) |
| Hip adductors | |||
| Adductor magnus (91) | 37–76 | 55 | 45 |
| Hamstrings | |||
| Biceps femoris short and long heads (79, 81, 92) | 17–40 | 54 (47–67) | 46 (33–53) |
| Semimembranosus (91) | 37–76 | 49 | 51 |
| Semitendinosus (93) | 17–34 | 48 | 52 |
| Quadriceps | |||
| Rectus femoris (81) | 17–30 | 38 | 62 |
| Vastus lateralis (80–82, 94–96) | 15–30 | 46 (36–59) | 54 (41–64) |
| Vastus intermedius (91, 97) | 21–83 | 51 (47–54) | 49 (46–53) |
| Vastus medialis (81) | 17–30 | 53 | 47 |
| Dorsiflexors | |||
| Tibialis anterior (79, 81, 98) | 17–40 | 74 (73–76) | 26 (24–27) |
| Triceps surae | |||
| Gastrocnemius (79, 81, 99) | 17–40 | 59 (48–70) | 41 (30–52) |
| Soleus (79–82, 94) | 17–40 | 81 (66–91) | 19 (9–35) |
Data are presented as mean and a range of the mean data when multiple studies are presented. Type II fibers included type IIa and type IIx where applicable. Where possible, references were included that represented young to middle-aged men and women to minimize any potential age influence.
Table 5.
Summary of sex-specific human skeletal muscle aging
| Muscle Group (References) | Sex | Young |
Old |
ΔAge* | %Δ/yr* | ||
|---|---|---|---|---|---|---|---|
| n | Age* | n | Age* | ||||
| Psoas (42, 56, 62) | Men | 17 | 27 | 18 | 76 | 49 | −0.58 |
| Women | 71 | 25 | 96 | 73 | 48 | −0.58 | |
| Hip adductors (24, 45) | Men | 38 | 23 | 39 | 73 | 50 | −0.16 |
| Women | 16 | 24 | 19 | 75 | 51 | −0.26 | |
| Hamstrings (23, 24, 45, 66) | Men | 66 | 23 | 66 | 72 | 49 | −0.37 |
| Women | 16 | 24 | 19 | 75 | 51 | −0.32 | |
| Quadriceps (2, 6, 23, 24, 39–44, 49, 50, 52, 53, 56) | Men | 171 | 24 | 188 | 73 | 49 | −0.52 |
| Women | 139 | 23 | 132 | 74 | 50 | −0.55 | |
| Dorsiflexors (67, 68, 71,72) | Men | 36 | 26 | 26 | 74 | 48 | −0.09 |
| Women | 17 | 28 | 16 | 72 | 44 | −0.25 | |
| Triceps surae (2, 68, 73) | Men | 30 | 25 | 37 | 74 | 49 | −0.26 |
| Women | 16 | 25 | 16 | 73 | 48 | −0.56 | |
*Average values normalized to the n size of each study.
Table 6.
Summary of longitudinal studies of human skeletal muscle aging
| Study | n | Baseline Age | Follow-Up Duration, yr | Measurement | Method | %Δ | %Δ/yr |
|---|---|---|---|---|---|---|---|
| Paraspinals | |||||||
| Murata et al. 2021 (100) | 276 M | 60–69 | 10 | CSAa | CT | −8 | −0.76 |
| 31 W | −10 | −1.01 | |||||
| Total: 1 study* | 307 | 65 | 10 | −0.79 | |||
| Psoas | |||||||
| Murata et al. 2021 (100) | 276 M | 60–69 | 10 | CSAb | CT | −9 | −0.88 |
| 31 W | −4 | −0.40 | |||||
| Total: 1 study* | 307 | 65 | 10 | −0.83 | |||
| Hamstrings | |||||||
| Frontera et al. 2000 (3) | 7 M | 65 ± 4 | 12 | CSA | CT | −15 | −1.22 |
| Total: 1 study | 7 | 65 | 12 | −1.22 | |||
| Quadriceps | |||||||
| Frontera et al. 2000 (3) | 7 M | 65 ± 4 | 12 | CSA | CT | −16 | −1.32 |
| Total: 1 study | 7 | 65 | 12 | −1.32 |
Age (yr) is presented as means ± SD, or range. CSA, cross-sectional area; CT, computed tomography; M, men; W, women.
*Data are total n size or average values normalized to the n size of each sex; aerector spinae and multifidus; bpsoas major.
MUSCLE-SPECIFIC ATROPHY WITH AGING
The main findings of this literature review are 1) nonquadriceps data for human skeletal muscle aging are limited and 2) with the available information, a diverse range of muscle atrophy rates with aging is apparent. Of the nine muscle groups available in the literature and presented here, there was more than a fivefold difference between the least (−6%, soleus) and the most (−33%, rectus femoris) atrophying muscles over the 50 yr of aging (Fig. 1). With the large number of muscles that have not been investigated, it is unknown if there are other specific muscles outside this large range. Given that nearly half of the available literature in this area is from the quadriceps, the large range of muscle-specific atrophy, and the large number of muscles that have not been investigated, it is clear that the area of aging muscle-specific atrophy needs to be expanded.
Thigh
The quadriceps, hamstrings, and hip adductors were included in this review from the thigh region. These three muscle groups cover all functional groups present in the thigh (25, 45) that are involved in different phases of various movements performed on a daily basis such as standing, walking, and stair climbing (101–107), and vigorous movements during exercise such as running, cycling, kicking, jumping, and change of direction tasks (108–114). However, the hamstrings and hip adductors represent less than 30% and 20%, respectively, of the amount of data on the quadriceps in the literature. In addition to the well-documented association between lower quadriceps strength and increased risk of falls in older men and women (115, 116), aging appears to increase the dependence on the hamstring muscles for postural stability and a greater coactivation of hamstrings and quadriceps has been associated with less occurrence of falls (117, 118). Considering the falls that lead to hip fracture occur more commonly in the lateral direction (119, 120), fall incidence is likely related to the age-related decline in hip adductor function as well (121).
Among these thigh muscles, the quadriceps appears to have the greatest rate of atrophy with a hierarchical pattern (Fig. 1): quadriceps (−27%) > hamstrings (−19%) > hip adductors (−13%). The quadriceps atrophy is also different from the loss of total skeletal muscle mass with similar age gaps reported by Janssen et al. (4) with MRI measurement (−11%) and Kyle et al. (5) with dual-energy X-ray absorptiometry measurement (−17%). This discrepancy further confirms that examination of the quadriceps does not reflect the aging process of all muscle groups. The available data on subcomponent muscles provide novel insights into muscle-specific atrophy within the same muscle groups (Table 3). In the quadriceps, the rectus femoris appears to have a greater rate of muscle atrophy than the vasti muscles. In the hamstrings, the medial muscles (semimembranosus and semitendinosus) seem more sensitive to the aging process than the lateral side of the muscle group (biceps femoris). Considering the different contribution of subcomponent muscles during various activities (106, 122, 123), understanding the relative changes in the subcomponent muscles of a muscle group during the aging process need further investigation (6).
Muscle-specific activity patterns likely influence muscle-specific aging atrophy, even in sedentary individuals, given the diverse functions across muscle groups. Electromyography (EMG) studies have shown marked differences in the EMG activity pattern, peak, and duration during simple daily movements such as normal gait, stair ambulation, and sit-to-stand movement across the thigh muscles (101–104, 106, 107). Tracking the EMG activities of various muscle groups for extended periods would provide useful information about the normal activation levels of each muscle group (124). In addition, findings from extreme inactivity provide insight into daily activity levels in a muscle-specific manner. Two months of bed rest results in atrophy of ∼17% in the quadriceps, ∼12% in the hamstrings, and ∼7% in the adductors (28, 125, 126). Thus, more than half of the quadriceps atrophy observed with 50 yr of aging occurs in only 2 mo of bed rest (27% vs. 17%), which suggests daily muscle activities significantly slow down the inactivity-induced muscle atrophy. In addition, only a relatively small amount of exercise has potent effects on age- and inactivity-related muscle atrophy (17, 28, 127–133). For example, only a few minutes a week of muscle contraction with resistance exercise has been shown to preserve muscle size in old individuals (132) or during prolonged bed rest (134, 135). Given the muscle-specific responses to exercise (2, 26–28, 134), these exercise training strategies for age-related muscle atrophy warrant investigations of more muscle groups.
Lower Leg
The triceps surae and dorsiflexors were included in this review from the lower leg region. The available information on these two muscle groups (∼20%–30% of the quadriceps data) covers the major muscles that are involved in the key functions of the lower leg for postural stability and ambulation (69, 136–141) and important contributors for running performance (142, 143). However, muscles that reside in the deep (i.e., flexor digitorum longus, flexor hallucis longus, and tibialis posterior) and the lateral lower leg (i.e., peroneus brevis and longus) have no available data, although these muscles also provide vital roles for gait and ankle stability (144, 145). Dysfunction associated with atrophy of the lower leg muscles impairs walking ability, reduces walking speed, and increases the risk of falls (65, 146–148). In addition, due to the complex network of vascular anatomy in this region, contraction of the calf muscles contributes substantially to venous return during exercise (149, 150) and it has been shown that function of the calf muscles is associated with cardiovascular health (146, 151–154).
From the available data in the literature on the lower leg muscles, there seems to be a trend toward a higher rate of muscle atrophy in the gastrocnemius than the other lower leg muscles (Fig. 1). This is supported by greater activities in the soleus and tibialis anterior than gastrocnemius during walking (106). Moreover, it has been suggested that the soleus is more active than the tibialis anterior during normal daily activities (155), which is supported by the findings of greater muscle atrophy in the soleus during bed rest (126, 156–159).
The lower leg muscles appear to be less susceptible to age-related atrophy than the thigh muscles (Fig. 1). It is well accepted that the soleus is an “antigravity muscle” that provides a sustained force for postural stability (160). Because of this, when the muscle is unloaded from gravity with space flight or microgravity simulation, it experiences approximately twice as much atrophy as the quadriceps (130, 161, 162) and this relationship is essentially reversed during the aging process (Fig. 1). In addition, it has been shown that responses to exercise training and nutrition are different between these muscle groups at the whole muscle (2, 28, 130), myocellular (27, 163–165), and molecular levels (94, 166). Thus, these muscle groups likely require different exercise programs or other intervention strategies against the aging process.
Trunk
The paraspinals and psoas were included in this review from the trunk region, both of which only contain three studies each. The paraspinals are responsible for maintaining upright position and stabilizing the spine (167) and support various daily tasks such as standing and picking up an object from the floor (168). The psoas also provides stability of the lumbar spine and femoral head (169–172) and a greater psoas size has been associated with better sprint running performance (109, 173). Although these two muscle groups make up most of the deep trunk muscle mass around the spine, data on muscles connecting the trunk and arms are lacking. For example, the latissimus dorsi and pectoralis muscles provide coordinated movement of the trunk and arms (i.e., pulling and pushing) such as opening doors, moving a shopping cart, and various strokes during swimming (174, 175). One of the most common age-related health concerns in the trunk region is low back pain, which has been reported to occur in more than half of older adults in the United States (176–179). Atrophy of the paraspinal muscles has been shown to accompany chronic low back pain (180–182). In addition, the psoas is commonly used to assess the prognosis of patients in numerous clinical settings due to its availability in various diagnostic imaging (183–185).
Somewhat greater muscle atrophy in the psoas (−29%) than in the paraspinals (−24%) follows the suggestion that muscles with more sustained work atrophy less. During unloading with spaceflight, the paraspinals experience approximately twice as much atrophy as the psoas (161). This indicates that the paraspinals are more chronically activated for spine stabilization during daily life. It has been shown that atrophy of the paraspinals may not occur until the seventh or eighth decade of life (100, 186–188), whereas the psoas has been shown to atrophy as early as the fifth decade (42, 100). This suggests that interventions to age-related muscle mass decline likely need to be muscle-specific and may need to be initiated earlier in the psoas.
Arm
The elbow extensors and flexors were included in this review from the arm region, but the data are composed of the lowest numbers of subjects among the nine muscle groups in this review (∼10% of the quadriceps data each). These two muscle groups make up all the upper arm muscles that are associated with elbow function (59) and they play major roles in cleaning, showering, and lifting heavy objects (189, 190). As the upper extremity muscles allow for performing numerous daily movements, it is not surprising that upper extremity function has been shown to be positively associated with quality of life (191, 192).
The available data suggest that age-related muscle atrophy does not seem to be different between elbow extensors (−19%) and elbow flexors (−20%). What the relative activity patterns of these muscles are over the lifespan is unclear, but the similar levels of atrophy suggest a similar decline. Other literature not included in this review provides some insight into the relative atrophy of the arm muscles. In younger aging men (68 yr) and older aging women (87 yr), measurements of quadriceps and elbow flexor muscle size in the same individuals suggest that the quadriceps atrophies more than the elbow flexors (21, 33). This notion of less atrophy in the elbow flexors is supported by observations of nearly twice as much daily muscle activity duration in the elbow flexors than quadriceps (193), and the relative activity level reduction during aging may also be smaller in the upper arm muscle groups. In addition, muscle adaptations to resistance exercise training have also shown to be different between the quadriceps and upper arm muscle groups (132, 194–196), which may be related to the motor unit number and activation in these muscle groups (197–201).
Muscle Fiber Type
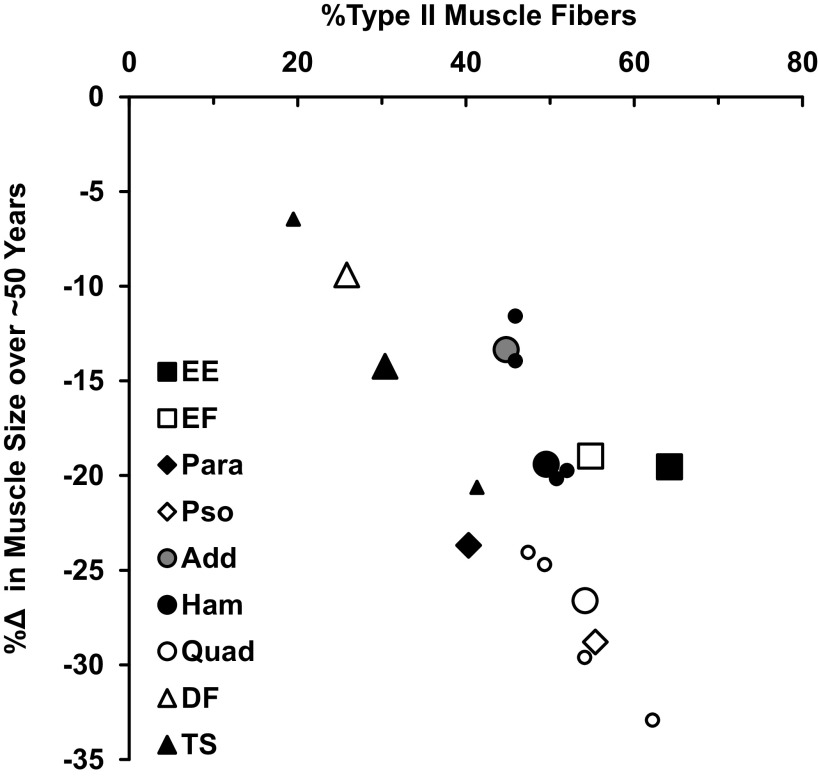
In addition to muscle activity levels, muscle fiber type may also play a role in age-related muscle atrophy (22). It is generally assumed that aging results in a higher distribution of type I skeletal muscle fibers (1, 95). In support of this, four studies on the quadriceps included in this review also reported the muscle fiber type distribution of young and old individuals (from the vastus lateralis) and all showed an increased distribution of type I muscle fibers in old men and women along with whole muscle atrophy (13, 17, 46, 49). In addition, it has been suggested that type II muscle fibers preferentially atrophy with aging (7, 49, 95). Because of these observations, sarcopenia is generally characterized by a loss of type II muscle fiber size and number (95, 96, 202). Thus, muscles with more type II fibers might be more susceptible to aging atrophy. This notion is supported by the relationship between the percentage of type II muscle fibers reported in the literature for all the muscles presented in this review compared with the percentage of age-related skeletal muscle atrophy compiled for this review (Table 4 and Fig. 2).
Figure 2.
Relationship between the percentage of type II muscle fibers and muscle atrophy over ∼50 yr of aging (r = −0.73). Muscle fiber types (%type II) are mean data from Table 4. Muscle atrophy rates (%Δ) are from Fig. 1. For hamstrings, quadriceps, and triceps surae, data for subcomponent muscles are also included as similar, but smaller symbols of the main muscle group. Add, hip adductors; DF, dorsiflexors; EE, elbow extensors; EF, elbow flexors; Ham, hamstrings; Para, paraspinals; Pso, psoas; Quad, quadriceps; TS, triceps surae.
This overall relationship seems to hold within the subcomponent muscles of a muscle group, but not across all muscle groups within a region. The four individual muscles of the quadriceps (rectus femoris, vastus lateralis, vastus intermedius, and vastus medialis), the four muscles of the hamstrings (biceps femoris short and long heads, semimembranosus, and semitendinosus), and the gastrocnemius and soleus of the triceps surae all seem to support this relationship (Fig. 2). However, this relationship does not seem to hold for the elbow extensors and flexors, which had similar rates of atrophy but somewhat different fiber type distribution. Interestingly, unlike the vastus lateralis, alterations in muscle fiber type distribution with age have not been reported in the biceps brachii with cross-sectional (203) and longitudinal (204) examinations. It is also noteworthy that those muscles that are most chronically active with typical activities of daily living [e.g., the soleus and tibialis anterior (106)] have a greater proportion of type I muscle fibers and a lower rate of age-related muscle atrophy. Overall, these data suggest that aging is a muscle-specific process even at the cellular level, and activity and fiber type are interconnected in their influence on muscle-specific atrophy with aging.
Sex Comparisons
Potential sex-related differences in the decline in muscle mass and strength with aging is an important consideration (2, 205, 206). From the currently available data (Table 5), age-related atrophy in the psoas, hamstrings, and quadriceps does not seem to be largely different between men and women. However, the hip adductors, dorsiflexors, and triceps surae do seem to atrophy more in women than men. It is important to note that, except for the quadriceps, these interpretations are based on relatively small numbers of studies and total sample size. Sex differences in skeletal muscle have been suggested to be related to sex hormones, particularly testosterone (207–209), but the muscle-specific influence of sex hormones remains to be determined (210–212). In addition, influence of age on muscle composition (i.e., lipid content) and strength of the lower leg muscle groups appears to be similar between men and women (2, 67, 213, 214). Thus, a plausible explanation for these muscle-specific sex differences is not readily apparent and requires further investigation.
Age-Related Muscle Atrophy Later in the Lifespan
The available data from longitudinal investigations of old individuals allow us to compare age-associated muscle atrophy that occurs from ∼65 yr to 75 yr in the paraspinals, psoas, hamstrings, and quadriceps (Table 6). Similar to the cross-sectional studies that examined 50 yr of aging from ∼25 yr to 75 yr, the atrophy rates are widely different across muscle groups. In addition, the greater rates of muscle atrophy in the last decade of the 50 yr age span studied in the cross-sectional examinations suggest a nonlinear decline in the size of these muscles (25–75 yr vs. 65–75 yr; paraspinals: −0.47%/yr vs. −0.79%/yr, psoas: −0.58%/yr vs. −0.83%/yr, hamstrings: −0.39%/yr vs. −1.22%/yr, quadriceps: −0.53%/yr vs. −1.32%/yr). This has also been shown in cross-sectional studies of the quadriceps that compared middle-aged to old (42, 215, 216) and old to older individuals (42, 216–218). This nonlinearity of atrophy is likely muscle specific as well. In addition, it is noteworthy that there appears to be some sex differences in the muscle atrophy rates in the trunk muscles (Table 6) that are not seen in the young to old comparison (Table 5). Given the possible differences in the onset of age-related atrophy in these muscle groups (42, 100, 186, 188, 216, 219–221), additional longitudinal investigations of older women and men (>75 yr) are warranted.
Future Directions
The existing data in the literature clearly show diverse age-related atrophy rates across muscles and support the need for future studies to expand the knowledge of muscle-specific aging. These studies should consider the time course of the aging process (i.e., the shape of the atrophy curves), exercise and other interventions, and sex while expanding on the existing muscle-specific information and investigating other important muscle groups. It is logical to pursue muscles that are associated with essential daily tasks. These include but are not limited to walking, running, cycling, stair ascending and descending, standing up from a chair, getting up from bed, bathing, dressing, carrying groceries, opening jars, eating, toileting, and lifting things from the floor. In addition, a better understanding of the influence and muscle-specific activity levels of different lifestyles (e.g., sedentary, walker, runner, cyclist, resistance exerciser) and occupations (e.g., office workers, farmers, factory workers) will aid in the future development of exercise countermeasures and other interventions to mitigate the aging process in a muscle-specific manner.
Although adaptation to exercise training is not the focus of this review, there is some evidence to suggest that the effects of aging on whole muscle size changes with an exercise intervention are also muscle specific. Available data have shown that the magnitude of hypertrophy in response to similar exercise training is different in elbow extensors, elbow flexors, hamstrings, quadriceps, and triceps surae (2, 194–196, 222–225). In addition, a few studies have shown that the adaptations to the same exercise intervention in these muscle groups differ between young and old individuals (226, 227).
Current technology for whole muscle imaging allows hundreds of human skeletal muscles to be quantified with proper reliability. Even cost-effective noninvasive imaging with ultrasound allows the examination of over 100 human skeletal muscles (34). Thus, future investigations should not be limited by the noninvasive whole muscle imaging techniques. Clearly, the nonquadriceps muscle groups included in this review require more data to draw definitive conclusions regarding the aging process. In addition, a few example regions of muscles that could be targeted for aging and intervention studies are the neck, shoulder, abdominal, gluteus, forearm, and hand. Neck and shoulder muscles provide complex functions and the musculoskeletal symptoms in this region are commonly attributed to sedentary lifestyle (228). The abdominal muscles provide trunk support and are composed of the rectus abdominis and lateral abdominals, which appear to be most activated for postural stability and balance among all the trunk muscles (229). The gluteal muscles are one of the largest muscle groups in the human body (230–232) and provide hip and pelvic stability and play essential roles in daily movements such as walking, stair climbing, and sit-to-stand motion (102, 103, 106, 107, 233, 234). A change in gluteus muscle composition has been associated with an increased risk of falls (235, 236). Forearm and hand muscles also provide essential functions in daily tasks. Hand grip strength has been used in numerous investigations and associations with various health outcomes have been shown (237–240). However, investigations into muscle-specific age-related atrophy in these muscle groups are limited (241–245). Given the variety of functions that these muscles provide, it is reasonable to hypothesize that there would be numerous muscle-specific changes with aging, which may require muscle-specific considerations for exercise and other interventions.
CONCLUSIONS
This review provides a comprehensive summary of the existing literature on age-related skeletal muscle atrophy in a muscle-specific manner. The large range of atrophy across muscle groups from the available data suggests that a single muscle group or nonmuscle-specific lean mass measurement does not likely provide the necessary information to comprehensively understand human skeletal muscle aging across the entire body. It is acknowledged that the quadriceps is functionally important and studies of this muscle have greatly expanded our understanding (and will continue to) at the whole muscle, myocellular, and molecular level. The availability of current noninvasive imaging technologies that are capable of evaluating some element of muscle composition in addition to muscle size would allow for a broader examination of skeletal muscle health assessment during the aging process. Our understanding of age-related atrophy has expanded substantially over the last several decades and will continue to grow with a more broad-ranging muscle-specific approach to skeletal muscle aging.
GRANTS
This work and our skeletal muscle imaging research have been supported by grants from the NIH under Grants AG020532, AG015833, AG000831, AG038576, AG018409, and AG015486 and National Aeronautics and Space Administration (NASA) under Grants NNJ06HF59G, EC400-NCC9-116, and NNJ04HF72G.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.N. and T.A.T. conceived and designed research; M.N. and T.A.T. prepared figures; M.N. and T.A.T. drafted manuscript; M.N., S.T., and T.A.T. edited and revised manuscript; M.N., S.T., and T.A.T. approved final version of manuscript.
REFERENCES
- 1. Larsson L, Degens H, Li M, Salviati L, Lee YI, Thompson W, Kirkland JL, Sandri M. Sarcopenia: aging-related loss of muscle mass and function. Physiol Rev 99: 427–511, 2019. doi: 10.1152/physrev.00061.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chambers TL, Burnett TR, Raue U, Lee GA, Finch WH, Graham BM, Trappe TA, Trappe S. Skeletal muscle size, function, and adiposity with lifelong aerobic exercise. J Appl Physiol (1985) 128: 368–378, 2020. doi: 10.1152/japplphysiol.00426.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol (1985) 88: 1321–1326, 2000. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- 4. Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol (1985) 89: 81–88, 2000. [Erratum in J Appl Physiol (1985) 116: 1342, 2014]. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 5. Kyle UG, Genton L, Hans D, Karsegard L, Slosman DO, Pichard C. Age-related differences in fat-free mass, skeletal muscle, body cell mass and fat mass between 18 and 94 years. Eur J Clin Nutr 55: 663–672, 2001. doi: 10.1038/sj.ejcn.1601198. [DOI] [PubMed] [Google Scholar]
- 6. Trappe TA, Lindquist DM, Carrithers JA. Muscle-specific atrophy of the quadriceps femoris with aging. J Appl Physiol (1985) 90: 2070–2074, 2001. doi: 10.1152/jappl.2001.90.6.2070. [DOI] [PubMed] [Google Scholar]
- 7. Trappe S, Gallagher P, Harber M, Carrithers J, Fluckey J, Trappe T. Single muscle fibre contractile properties in young and old men and women. J Physiol 552: 47–58, 2003. doi: 10.1113/jphysiol.2003.044966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grosicki GJ, Zepeda CS, Sundberg CW. Single muscle fibre contractile function with ageing. J Physiol 600: 5005–5026, 2022. doi: 10.1113/JP282298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koopman R, van Loon LJ. Aging, exercise, and muscle protein metabolism. J Appl Physiol (1985) 106: 2040–2048, 2009. doi: 10.1152/japplphysiol.91551.2008. [DOI] [PubMed] [Google Scholar]
- 10. Straight CR, Fedewa MV, Toth MJ, Miller MS. Improvements in skeletal muscle fiber size with resistance training are age-dependent in older adults: a systematic review and meta-analysis. J Appl Physiol (1985) 129: 392–403, 2020. doi: 10.1152/japplphysiol.00170.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bergström J. Muscle electrolytes in man. Scand J Clin Lab Invest 14: 7–110, 1962. 13862378 [Google Scholar]
- 12. Frontera WR, Reid KF, Phillips EM, Krivickas LS, Hughes VA, Roubenoff R, Fielding RA. Muscle fiber size and function in elderly humans: a longitudinal study. J Appl Physiol (1985) 105: 637–642, 2008. doi: 10.1152/japplphysiol.90332.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haus JM, Carrithers JA, Trappe SW, Trappe TA. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J Appl Physiol (1985) 103: 2068–2076, 2007. doi: 10.1152/japplphysiol.00670.2007. [DOI] [PubMed] [Google Scholar]
- 14. Karlsen A, Soendenbroe C, Malmgaard-Clausen NM, Wagener F, Moeller CE, Senhaji Z, Damberg K, Andersen JL, Schjerling P, Kjaer M, Mackey AL. Preserved capacity for satellite cell proliferation, regeneration, and hypertrophy in the skeletal muscle of healthy elderly men. FASEB J 34: 6418–6436, 2020. doi: 10.1096/fj.202000196R. [DOI] [PubMed] [Google Scholar]
- 15. Raue U, Trappe TA, Estrem ST, Qian HR, Helvering LM, Smith RC, Trappe S. Transcriptome signature of resistance exercise adaptations: mixed muscle and fiber type specific profiles in young and old adults. J Appl Physiol (1985) 112: 1625–1636, 2012. doi: 10.1152/japplphysiol.00435.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bamman MM, Ragan RC, Kim JS, Cross JM, Hill VJ, Tuggle SC, Allman RM. Myogenic protein expression before and after resistance loading in 26- and 64-yr-old men and women. J Appl Physiol (1985) 97: 1329–1337, 2004. doi: 10.1152/japplphysiol.01387.2003. [DOI] [PubMed] [Google Scholar]
- 17. Harber MP, Konopka AR, Undem MK, Hinkley JM, Minchev K, Kaminsky LA, Trappe TA, Trappe S. Aerobic exercise training induces skeletal muscle hypertrophy and age-dependent adaptations in myofiber function in young and older men. J Appl Physiol (1985) 113: 1495–1504, 2012. doi: 10.1152/japplphysiol.00786.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol (1985) 101: 531–544, 2006. doi: 10.1152/japplphysiol.01474.2005. [DOI] [PubMed] [Google Scholar]
- 19. Raue U, Slivka D, Minchev K, Trappe S. Improvements in whole muscle and myocellular function are limited with high-intensity resistance training in octogenarian women. J Appl Physiol (1985) 106: 1611–1617, 2009. doi: 10.1152/japplphysiol.91587.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Slivka D, Raue U, Hollon C, Minchev K, Trappe S. Single muscle fiber adaptations to resistance training in old (>80 yr) men: evidence for limited skeletal muscle plasticity. Am J Physiol Regul Integr Comp Physiol 295: R273–R280, 2008. doi: 10.1152/ajpregu.00093.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klitgaard H, Mantoni M, Schiaffino S, Ausoni S, Gorza L, Laurent-Winter C, Schnohr P, Saltin B. Function, morphology and protein expression of ageing skeletal muscle: a cross-sectional study of elderly men with different training backgrounds. Acta Physiol Scand 140: 41–54, 1990. doi: 10.1111/j.1748-1716.1990.tb08974.x. [DOI] [PubMed] [Google Scholar]
- 22. Miller BF, Baehr LM, Musci RV, Reid JJ, Peelor FF 3rd, Hamilton KL, Bodine SC. Muscle-specific changes in protein synthesis with aging and reloading after disuse atrophy. J Cachexia Sarcopenia Muscle 10: 1195–1209, 2019. doi: 10.1002/jcsm.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Overend TJ, Cunningham DA, Kramer JF, Lefcoe MS, Paterson DH. Knee extensor and knee flexor strength: cross-sectional area ratios in young and elderly men. J Gerontol 47: M204–M210, 1992. doi: 10.1093/geronj/47.6.m204. [DOI] [PubMed] [Google Scholar]
- 24. Ogawa M, Tanaka N, Yoshiko A, Oshida Y, Koike T, Akima H. Relationship between physical activity time and intramuscular adipose tissue content of the thigh muscle groups of younger and older men. Sci Rep 11: 19804, 2021. doi: 10.1038/s41598-021-99126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yoshiko A, Hioki M, Kanehira N, Shimaoka K, Koike T, Sakakibara H, Oshida Y, Akima H. Three-dimensional comparison of intramuscular fat content between young and old adults. BMC Med Imaging 17: 12, 2017. doi: 10.1186/s12880-017-0185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferri A, Scaglioni G, Pousson M, Capodaglio P, Van Hoecke J, Narici MV. Strength and power changes of the human plantar flexors and knee extensors in response to resistance training in old age. Acta Physiol Scand 177: 69–78, 2003. doi: 10.1046/j.1365-201X.2003.01050.x. [DOI] [PubMed] [Google Scholar]
- 27. Trappe TA, Raue U, Tesch PA. Human soleus muscle protein synthesis following resistance exercise. Acta Physiol Scand 182: 189–196, 2004. doi: 10.1111/j.1365-201X.2004.01348.x. [DOI] [PubMed] [Google Scholar]
- 28. Trappe TA, Burd NA, Louis ES, Lee GA, Trappe SW. Influence of concurrent exercise or nutrition countermeasures on thigh and calf muscle size and function during 60 days of bed rest in women. Acta Physiol (Oxf) 191: 147–159, 2007. doi: 10.1111/j.1748-1716.2007.01728.x. [DOI] [PubMed] [Google Scholar]
- 29. Gray C, MacGillivray TJ, Eeley C, Stephens NA, Beggs I, Fearon KC, Greig CA. Magnetic resonance imaging with k-means clustering objectively measures whole muscle volume compartments in sarcopenia/cancer cachexia. Clin Nutr 30: 106–111, 2011. doi: 10.1016/j.clnu.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 30. Kennedy P, Barnhill E, Gray C, Brown C, van Beek EJR, Roberts N, Greig CA. Magnetic resonance elastography (MRE) shows significant reduction of thigh muscle stiffness in healthy older adults. Geroscience 42: 311–321, 2020. doi: 10.1007/s11357-019-00147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McNeil CJ, Vandervoort AA, Rice CL. Peripheral impairments cause a progressive age-related loss of strength and velocity-dependent power in the dorsiflexors. J Appl Physiol (1985) 102: 1962–1968, 2007. doi: 10.1152/japplphysiol.01166.2006. [DOI] [PubMed] [Google Scholar]
- 32. Vandervoort AA, McComas AJ. Contractile changes in opposing muscles of the human ankle joint with aging. J Appl Physiol (1985) 61: 361–367, 1986. doi: 10.1152/jappl.1986.61.1.361. [DOI] [PubMed] [Google Scholar]
- 33. Venturelli M, Saggin P, Muti E, Naro F, Cancellara L, Toniolo L, Tarperi C, Calabria E, Richardson RS, Reggiani C, Schena F. In vivo and in vitro evidence that intrinsic upper- and lower-limb skeletal muscle function is unaffected by ageing and disuse in oldest-old humans. Acta Physiol (Oxf) 215: 58–71, 2015. doi: 10.1111/apha.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Naruse M, Trappe S, Trappe TA. Human skeletal muscle size with ultrasound imaging: a comprehensive review. J Appl Physiol (1985) 132: 1267–1279, 2022. doi: 10.1152/japplphysiol.00041.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Clark DJ, Pojednic RM, Reid KF, Patten C, Pasha EP, Phillips EM, Fielding RA. Longitudinal decline of neuromuscular activation and power in healthy older adults. J Gerontol A Biol Sci Med Sci 68: 1419–1425, 2013. doi: 10.1093/gerona/glt036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, Boudreau R, Manini TM, Nevitt M, Newman AB, Goodpaster BH. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr 90: 1579–1585, 2009. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Greig CA, Botella J, Young A. The quadriceps strength of healthy elderly people remeasured after eight years. Muscle Nerve 16: 6–10, 1993. doi: 10.1002/mus.880160103. [DOI] [PubMed] [Google Scholar]
- 38. McPhee JS, Cameron J, Maden-Wilkinson T, Piasecki M, Yap MH, Jones DA, Degens H. The contributions of fiber atrophy, fiber loss, in situ specific force, and voluntary activation to weakness in sarcopenia. J Gerontol A Biol Sci Med Sci 73: 1287–1294, 2018. doi: 10.1093/gerona/gly040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Young A, Stokes M, Crowe M. Size and strength of the quadriceps muscles of old and young women. Eur J Clin Invest 14: 282–287, 1984. doi: 10.1111/j.1365-2362.1984.tb01182.x. [DOI] [PubMed] [Google Scholar]
- 40. Young A, Stokes M, Crowe M. The size and strength of the quadriceps muscles of old and young men. Clin Physiol 5: 145–154, 1985. doi: 10.1111/j.1475-097x.1985.tb00590.x. [DOI] [PubMed] [Google Scholar]
- 41. Rutherford OM, Jones DA. The relationship of muscle and bone loss and activity levels with age in women. Age Ageing 21: 286–293, 1992. doi: 10.1093/ageing/21.4.286. [DOI] [PubMed] [Google Scholar]
- 42. Takahashi K, Takahashi HE, Nakadaira H, Yamamoto M. Different changes of quantity due to aging in the psoas major and quadriceps femoris muscles in women. J Musculoskelet Neuronal Interact 6: 201–205, 2006. [PubMed] [Google Scholar]
- 43. Kilgour AH, Gallagher IJ, MacLullich AM, Andrew R, Gray CD, Hyde P, Wackerhage H, Husi H, Ross JA, Starr JM, Chapman KE, Fearon KC, Walker BR, Greig CA. Increased skeletal muscle 11βHSD1 mRNA is associated with lower muscle strength in ageing. PLoS One 8: e84057, 2013. doi: 10.1371/journal.pone.0084057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Maden-Wilkinson TM, Degens H, Jones DA, McPhee JS. Comparison of MRI and DXA to measure muscle size and age-related atrophy in thigh muscles. J Musculoskelet Neuronal Interact 13: 320–328, 2013. [PubMed] [Google Scholar]
- 45. Hogrel JY, Barnouin Y, Azzabou N, Butler-Browne G, Voit T, Moraux A, Leroux G, Behin A, McPhee JS, Carlier PG. NMR imaging estimates of muscle volume and intramuscular fat infiltration in the thigh: variations with muscle, gender, and age. Age (Dordr) 37: 9798, 2015. doi: 10.1007/s11357-015-9798-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brocca L, McPhee JS, Longa E, Canepari M, Seynnes O, De Vito G, Pellegrino MA, Narici M, Bottinelli R. Structure and function of human muscle fibres and muscle proteome in physically active older men. J Physiol 595: 4823–4844, 2017. doi: 10.1113/JP274148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Barnouin Y, Butler-Browne G, Voit T, Reversat D, Azzabou N, Leroux G, Behin A, McPhee JS, Carlier PG, Hogrel JY. Manual segmentation of individual muscles of the quadriceps femoris using MRI: a reappraisal. J Magn Reson Imaging 40: 239–247, 2014. doi: 10.1002/jmri.24370. [DOI] [PubMed] [Google Scholar]
- 48. Maden-Wilkinson TM, McPhee JS, Rittweger J, Jones DA, Degens H. Thigh muscle volume in relation to age, sex and femur volume. Age (Dordr) 36: 383–393, 2014. doi: 10.1007/s11357-013-9571-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nilwik R, Snijders T, Leenders M, Groen BB, van Kranenburg J, Verdijk LB, van Loon LJ. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp Gerontol 48: 492–498, 2013. doi: 10.1016/j.exger.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 50. Konopka AR, Suer MK, Wolff CA, Harber MP. Markers of human skeletal muscle mitochondrial biogenesis and quality control: effects of age and aerobic exercise training. J Gerontol A Biol Sci Med Sci 69: 371–378, 2014. doi: 10.1093/gerona/glt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ghosh S, Lertwattanarak R, Garduño Jde J, Galeana JJ, Li J, Zamarripa F, Lancaster JL, Mohan S, Hussey S, Musi N. Elevated muscle TLR4 expression and metabolic endotoxemia in human aging. J Gerontol A Biol Sci Med Sci 70: 232–246, 2015. doi: 10.1093/gerona/glu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rudroff T, Kindred JH, Benson JM, Tracy BL, Kalliokoski KK. Greater glucose uptake heterogeneity in knee muscles of old compared to young men during isometric contractions detected by [(18)F]-FDG PET/CT. Front Physiol 5: 198, 2014. doi: 10.3389/fphys.2014.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Piasecki M, Ireland A, Stashuk D, Hamilton-Wright A, Jones DA, McPhee JS. Age-related neuromuscular changes affecting human vastus lateralis. J Physiol 594: 4525–4536, 2016. doi: 10.1113/JP271087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hioki M, Kanehira N, Koike T, Saito A, Takahashi H, Shimaoka K, Sakakibara H, Oshida Y, Akima H. Associations of intramyocellular lipid in vastus lateralis and biceps femoris with blood free fatty acid and muscle strength differ between young and elderly adults. Clin Physiol Funct Imaging 36: 457–463, 2016. doi: 10.1111/cpf.12250. [DOI] [PubMed] [Google Scholar]
- 55. Hioki M, Kanehira N, Koike T, Saito A, Shimaoka K, Sakakibara H, Oshida Y, Akima H. Age-related changes in muscle volume and intramuscular fat content in quadriceps femoris and hamstrings. Exp Gerontol 132: 110834, 2020. doi: 10.1016/j.exger.2020.110834. [DOI] [PubMed] [Google Scholar]
- 56. Yagi M, Taniguchi M, Tateuchi H, Hirono T, Fukumoto Y, Yamagata M, Nakai R, Yamada Y, Kimura M, Ichihashi N. Age- and sex-related differences of muscle cross-sectional area in iliocapsularis: a cross-sectional study. BMC Geriatr 22: 435, 2022. [Erratum in BMC Geriatr 22: 657, 2022]. doi: 10.1186/s12877-022-03127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Klein CS, Allman BL, Marsh GD, Rice CL. Muscle size, strength, and bone geometry in the upper limbs of young and old men. J Gerontol A Biol Sci Med Sci 57: M455–M459, 2002. doi: 10.1093/gerona/57.7.m455. [DOI] [PubMed] [Google Scholar]
- 58. Klein CS, Rice CL, Marsh GD. Normalized force, activation, and coactivation in the arm muscles of young and old men. J Appl Physiol (1985) 91: 1341–1349, 2001. doi: 10.1152/jappl.2001.91.3.1341. [DOI] [PubMed] [Google Scholar]
- 59. Vidt ME, Daly M, Miller ME, Davis CC, Marsh AP, Saul KR. Characterizing upper limb muscle volume and strength in older adults: a comparison with young adults. J Biomech 45: 334–341, 2012. doi: 10.1016/j.jbiomech.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Holzbaur KR, Murray WM, Gold GE, Delp SL. Upper limb muscle volumes in adult subjects. J Biomech 40: 742–749, 2007. doi: 10.1016/j.jbiomech.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 61. Smart RR, Baudry S, Fedorov A, Kuzyk SL, Jakobi JM. Influence of biceps brachii tendon mechanical properties on elbow flexor force steadiness in young and old males. Scand J Med Sci Sports 28: 983–991, 2018. doi: 10.1111/sms.13024. [DOI] [PubMed] [Google Scholar]
- 62. Ma HT, Griffith JF, Xu L, Leung PC. The functional muscle-bone unit in subjects of varying BMD. Osteoporos Int 25: 999–1004, 2014. doi: 10.1007/s00198-013-2482-7. [DOI] [PubMed] [Google Scholar]
- 63. Takayama K, Kita T, Nakamura H, Kanematsu F, Yasunami T, Sakanaka H, Yamano Y. New predictive index for lumbar paraspinal muscle degeneration associated with aging. Spine (Phila Pa 1976) 41: E84–E90, 2016. doi: 10.1097/BRS.0000000000001154. [DOI] [PubMed] [Google Scholar]
- 64. Peng X, Li X, Xu Z, Wang L, Cai W, Yang S, Liao W, Cheng X. Age-related fatty infiltration of lumbar paraspinal muscles: a normative reference database study in 516 Chinese females. Quant Imaging Med Surg 10: 1590–1601, 2020. doi: 10.21037/qims-19-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Maddocks M, Jones M, Snell T, Connolly B, de Wolf-Linder S, Moxham J, Rafferty GF. Ankle dorsiflexor muscle size, composition and force with ageing and chronic obstructive pulmonary disease. Exp Physiol 99: 1078–1088, 2014. doi: 10.1113/expphysiol.2014.080093. [DOI] [PubMed] [Google Scholar]
- 66. Palmer TB, Thompson BJ. Influence of age on passive stiffness and size, quality, and strength characteristics. Muscle Nerve 55: 305–315, 2017. doi: 10.1002/mus.25231. [DOI] [PubMed] [Google Scholar]
- 67. Kent-Braun JA, Ng AV, Young K. Skeletal muscle contractile and noncontractile components in young and older women and men. J Appl Physiol (1985) 88: 662–668, 2000. doi: 10.1152/jappl.2000.88.2.662. [DOI] [PubMed] [Google Scholar]
- 68. Hasson CJ, Kent-Braun JA, Caldwell GE. Contractile and non-contractile tissue volume and distribution in ankle muscles of young and older adults. J Biomech 44: 2299–2306, 2011. doi: 10.1016/j.jbiomech.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Barber LA, Barrett RS, Gillett JG, Cresswell AG, Lichtwark GA. Neuromechanical properties of the triceps surae in young and older adults. Exp Gerontol 48: 1147–1155, 2013. doi: 10.1016/j.exger.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 70. Christie AD, Tonson A, Larsen RG, DeBlois JP, Kent JA. Human skeletal muscle metabolic economy in vivo: effects of contraction intensity, age, and mobility impairment. Am J Physiol Regul Integr Comp Physiol 307: R1124–R1135, 2014. doi: 10.1152/ajpregu.00083.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Power GA, Allen MD, Booth WJ, Thompson RT, Marsh GD, Rice CL. The influence on sarcopenia of muscle quality and quantity derived from magnetic resonance imaging and neuromuscular properties. Age (Dordr) 36: 9642, 2014. doi: 10.1007/s11357-014-9642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Piasecki M, Ireland A, Coulson J, Stashuk DW, Hamilton-Wright A, Swiecicka A, Rutter MK, McPhee JS, Jones DA. Motor unit number estimates and neuromuscular transmission in the tibialis anterior of master athletes: evidence that athletic older people are not spared from age-related motor unit remodeling. Physiol Rep 4: e12987, 2016. doi: 10.14814/phy2.12987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Morse CI, Thom JM, Davis MG, Fox KR, Birch KM, Narici MV. Reduced plantarflexor specific torque in the elderly is associated with a lower activation capacity. Eur J Appl Physiol 92: 219–226, 2004. doi: 10.1007/s00421-004-1056-y. [DOI] [PubMed] [Google Scholar]
- 74. Morse CI, Thom JM, Birch KM, Narici MV. Changes in triceps surae muscle architecture with sarcopenia. Acta Physiol Scand 183: 291–298, 2005. doi: 10.1111/j.1365-201X.2004.01404.x. [DOI] [PubMed] [Google Scholar]
- 75. Morse CI, Thom JM, Reeves ND, Birch KM, Narici MV. In vivo physiological cross-sectional area and specific force are reduced in the gastrocnemius of elderly men. J Appl Physiol (1985) 99: 1050–1055, 2005. doi: 10.1152/japplphysiol.01186.2004. [DOI] [PubMed] [Google Scholar]
- 76. Thom JM, Morse CI, Birch KM, Narici MV. Triceps surae muscle power, volume, and quality in older versus younger healthy men. J Gerontol A Biol Sci Med Sci 60: 1111–1117, 2005. doi: 10.1093/gerona/60.9.1111. [DOI] [PubMed] [Google Scholar]
- 77. Thom JM, Morse CI, Birch KM, Narici MV. Influence of muscle architecture on the torque and power-velocity characteristics of young and elderly men. Eur J Appl Physiol 100: 613–619, 2007. doi: 10.1007/s00421-007-0481-0. [DOI] [PubMed] [Google Scholar]
- 78. Pinel S, Kelp NY, Bugeja JM, Bolsterlee B, Hug F, Dick TJM. Quantity versus quality: age-related differences in muscle volume, intramuscular fat, and mechanical properties in the triceps surae. Exp Gerontol 156: 111594, 2021. doi: 10.1016/j.exger.2021.111594. [DOI] [PubMed] [Google Scholar]
- 79. Dahmane R, Djordjevic S, Smerdu V. Adaptive potential of human biceps femoris muscle demonstrated by histochemical, immunohistochemical and mechanomyographical methods. Med Biol Eng Comput 44: 999–1006, 2006. [Erratum in Med Biol Eng Comput 45: 323–324, 2007]. doi: 10.1007/s11517-006-0114-5. [DOI] [PubMed] [Google Scholar]
- 80. Elder GC, Bradbury K, Roberts R. Variability of fiber type distributions within human muscles. J Appl Physiol Respir Environ Exerc Physiol 53: 1473–1480, 1982. doi: 10.1152/jappl.1982.53.6.1473. [DOI] [PubMed] [Google Scholar]
- 81. Johnson MA, Polgar J, Weightman D, Appleton D. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci 18: 111–129, 1973. doi: 10.1016/0022-510x(73)90023-3. [DOI] [PubMed] [Google Scholar]
- 82. Mittendorfer B, Andersen JL, Plomgaard P, Saltin B, Babraj JA, Smith K, Rennie MJ. Protein synthesis rates in human muscles: neither anatomical location nor fibre-type composition are major determinants. J Physiol 563: 203–211, 2005. doi: 10.1113/jphysiol.2004.077180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Agten A, Stevens S, Verbrugghe J, Eijnde BO, Timmermans A, Vandenabeele F. The lumbar multifidus is characterised by larger type I muscle fibres compared to the erector spinae. Anat Cell Biol 53: 143–150, 2020. doi: 10.5115/acb.20.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jørgensen K, Nicholaisen T, Kato M. Muscle fiber distribution, capillary density, and enzymatic activities in the lumbar paravertebral muscles of young men. Significance for isometric endurance. Spine (Phila Pa 1976) 18: 1439–1450, 1993. [PubMed] [Google Scholar]
- 85. Mannion AF, Dumas GA, Cooper RG, Espinosa FJ, Faris MW, Stevenson JM. Muscle fibre size and type distribution in thoracic and lumbar regions of erector spinae in healthy subjects without low back pain: normal values and sex differences. J Anat 190: 505–513, 1997. doi: 10.1046/j.1469-7580.1997.19040505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mannion AF, Weber BR, Dvorak J, Grob D, Müntener M. Fibre type characteristics of the lumbar paraspinal muscles in normal healthy subjects and in patients with low back pain. J Orthop Res 15: 881–887, 1997. doi: 10.1002/jor.1100150614. [DOI] [PubMed] [Google Scholar]
- 87. Thorstensson A, Carlson H. Fibre types in human lumbar back muscles. Acta Physiol Scand 131: 195–202, 1987. doi: 10.1111/j.1748-1716.1987.tb08226.x. [DOI] [PubMed] [Google Scholar]
- 88. Mazis N, Papachristou DJ, Zouboulis P, Tyllianakis M, Scopa CD, Megas P. The effect of different physical activity levels on muscle fiber size and type distribution of lumbar multifidus. A biopsy study on low back pain patient groups and healthy control subjects. Eur J Phys Rehabil Med 45: 459–467, 2009. [PubMed] [Google Scholar]
- 89. Rantanen J, Rissanen A, Kalimo H. Lumbar muscle fiber size and type distribution in normal subjects. Eur Spine J 3: 331–335, 1994. doi: 10.1007/BF02200146. [DOI] [PubMed] [Google Scholar]
- 90. Arbanas J, Klasan GS, Nikolic M, Jerkovic R, Miljanovic I, Malnar D. Fibre type composition of the human psoas major muscle with regard to the level of its origin. J Anat 215: 636–641, 2009. doi: 10.1111/j.1469-7580.2009.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Garrett WE Jr, Califf JC, Bassett FH 3rd.. Histochemical correlates of hamstring injuries. Am J Sports Med 12: 98–103, 1984. doi: 10.1177/036354658401200202. [DOI] [PubMed] [Google Scholar]
- 92. Evangelidis PE, Massey GJ, Ferguson RA, Wheeler PC, Pain MTG, Folland JP. The functional significance of hamstrings composition: is it really a “fast” muscle group? Scand J Med Sci Sports 27: 1181–1189, 2017. doi: 10.1111/sms.12786. [DOI] [PubMed] [Google Scholar]
- 93. Shalabi A, Eriksson K, Jansson E, Wredmark T. Ultrasound-guided percutaneous biopsies of the semitendinosus muscle following ACL reconstruction—a methodological description. Int J Sports Med 23: 202–206, 2002. doi: 10.1055/s-2002-23179. [DOI] [PubMed] [Google Scholar]
- 94. Carroll CC, Fluckey JD, Williams RH, Sullivan DH, Trappe TA. Human soleus and vastus lateralis muscle protein metabolism with an amino acid infusion. Am J Physiol Endocrinol Physiol 288: E479–E485, 2005. doi: 10.1152/ajpendo.00393.2004. [DOI] [PubMed] [Google Scholar]
- 95. Larsson L, Sjödin B, Karlsson J. Histochemical and biochemical changes in human skeletal muscle with age in sedentary males, age 22–65 years. Acta Physiol Scand 103: 31–39, 1978. doi: 10.1111/j.1748-1716.1978.tb06187.x. [DOI] [PubMed] [Google Scholar]
- 96. Lexell J, Taylor CC, Sjöström M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci 84: 275–294, 1988. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 97. Edgerton VR, Smith JL, Simpson DR. Muscle fibre type populations of human leg muscles. Histochem J 7: 259–266, 1975. doi: 10.1007/BF01003594. [DOI] [PubMed] [Google Scholar]
- 98. Jakobsson F, Borg K, Edström L, Grimby L. Use of motor units in relation to muscle fiber type and size in man. Muscle Nerve 11: 1211–1218, 1988. doi: 10.1002/mus.880111205. [DOI] [PubMed] [Google Scholar]
- 99. Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, Holloszy JO. Histochemical and enzymatic comparison of the gastrocnemius muscle of young and elderly men and women. J Gerontol 47: B71–B76, 1992. doi: 10.1093/geronj/47.3.b71. [DOI] [PubMed] [Google Scholar]
- 100. Murata Y, Nakamura E, Tsukamoto M, Nakagawa T, Takeda M, Kozuma M, Kadomura T, Narusawa K, Shimizu K, Uchida S, Hayashi T, Sakai A. Longitudinal study of risk factors for decreased cross-sectional area of psoas major and paraspinal muscle in 1849 individuals. Sci Rep 11: 16986, 2021. doi: 10.1038/s41598-021-96448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Goulart FR, Valls-Solé J. Patterned electromyographic activity in the sit-to-stand movement. Clin Neurophysiol 110: 1634–1640, 1999. doi: 10.1016/s1388-2457(99)00109-1. [DOI] [PubMed] [Google Scholar]
- 102. Joseph J, Watson R. Telemetering electromyography of muscles used in walking up and down stairs. J Bone Joint Surg Br 49: 774–780, 1967. doi: 10.1302/0301-620X.49B4.774. [DOI] [PubMed] [Google Scholar]
- 103. Millington PJ, Myklebust BM, Shambes GM. Biomechanical analysis of the sit-to-stand motion in elderly persons. Arch Phys Med Rehabil 73: 609–617, 1992. [PubMed] [Google Scholar]
- 104. Monster AW, Chan H, O'Connor D. Activity patterns of human skeletal muscles: relation to muscle fiber type composition. Science 200: 314–317, 1978. doi: 10.1126/science.635587. [DOI] [PubMed] [Google Scholar]
- 105. Watanabe K, Katayama K, Ishida K, Akima H. Electromyographic analysis of hip adductor muscles during incremental fatiguing pedaling exercise. Eur J Appl Physiol 106: 815–825, 2009. doi: 10.1007/s00421-009-1086-6. [DOI] [PubMed] [Google Scholar]
- 106. Winter DA, Yack HJ. EMG profiles during normal human walking: stride-to-stride and inter-subject variability. Electroencephalogr Clin Neurophysiol 67: 402–411, 1987. doi: 10.1016/0013-4694(87)90003-4. [DOI] [PubMed] [Google Scholar]
- 107. Zimmermann CL, Cook TM, Bravard MS, Hansen MM, Honomichl RT, Karns ST, Lammers MA, Steele SA, Yunker LK, Zebrowski RM. Effects of stair-stepping exercise direction and cadence on EMG activity of selected lower extremity muscle groups. J Orthop Sports Phys Ther 19: 173–180, 1994. doi: 10.2519/jospt.1994.19.3.173. [DOI] [PubMed] [Google Scholar]
- 108. Ema R, Wakahara T, Yanaka T, Kanehisa H, Kawakami Y. Unique muscularity in cyclists' thigh and trunk: a cross-sectional and longitudinal study. Scand J Med Sci Sports 26: 782–793, 2016. doi: 10.1111/sms.12511. [DOI] [PubMed] [Google Scholar]
- 109. Ema R, Sakaguchi M, Kawakami Y. Thigh and psoas major muscularity and its relation to running mechanics in sprinters. Med Sci Sports Exerc 50: 2085–2091, 2018. doi: 10.1249/MSS.0000000000001678. [DOI] [PubMed] [Google Scholar]
- 110. Hahn T, Foldspang A, Ingemann-Hansen T. Dynamic strength of the quadriceps muscle and sports activity. Br J Sports Med 33: 117–120, 1999. doi: 10.1136/bjsm.33.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kellis E. Quantification of quadriceps and hamstring antagonist activity. Sports Med 25: 37–62, 1998. [Erratum in Sports Med 25: 211, 1998]. doi: 10.2165/00007256-199825010-00004. [DOI] [PubMed] [Google Scholar]
- 112. Rand MK, Ohtsuki T. EMG analysis of lower limb muscles in humans during quick change in running directions. Gait Posture 12: 169–183, 2000. doi: 10.1016/s0966-6362(00)00073-4. [DOI] [PubMed] [Google Scholar]
- 113. Serner A, Jakobsen MD, Andersen LL, Hölmich P, Sundstrup E, Thorborg K. EMG evaluation of hip adduction exercises for soccer players: implications for exercise selection in prevention and treatment of groin injuries. Br J Sports Med 48: 1108–1114, 2014. doi: 10.1136/bjsports-2012-091746. [DOI] [PubMed] [Google Scholar]
- 114. van den Tillaar R, Solheim JAB, Bencke J. Comparison of hamstring muscle activation during high-speed running and various hamstring strengthening exercises. Int J Sports Phys Ther 12: 718–727, 2017. [PMC free article] [PubMed] [Google Scholar]
- 115. Caetano MJD, Lord SR, Brodie MA, Schoene D, Pelicioni PHS, Sturnieks DL, Menant JC. Executive functioning, concern about falling and quadriceps strength mediate the relationship between impaired gait adaptability and fall risk in older people. Gait Posture 59: 188–192, 2018. doi: 10.1016/j.gaitpost.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 116. Scott D, Stuart AL, Kay D, Ebeling PR, Nicholson G, Sanders KM. Investigating the predictive ability of gait speed and quadriceps strength for incident falls in community-dwelling older women at high risk of fracture. Arch Gerontol Geriatr 58: 308–313, 2014. doi: 10.1016/j.archger.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 117. Laughton CA, Slavin M, Katdare K, Nolan L, Bean JF, Kerrigan DC, Phillips E, Lipsitz LA, Collins JJ. Aging, muscle activity, and balance control: physiologic changes associated with balance impairment. Gait Posture 18: 101–108, 2003. doi: 10.1016/s0966-6362(02)00200-x. [DOI] [PubMed] [Google Scholar]
- 118. Palmer TB, Farrow AC, Palmer BM. Relationships between hamstring morphological characteristics and postural balance in elderly men. J Musculoskelet Neuronal Interact 20: 88–93, 2020. [PMC free article] [PubMed] [Google Scholar]
- 119. Hayes WC, Myers ER, Morris JN, Gerhart TN, Yett HS, Lipsitz LA. Impact near the hip dominates fracture risk in elderly nursing home residents who fall. Calcif Tissue Int 52: 192–198, 1993. doi: 10.1007/BF00298717. [DOI] [PubMed] [Google Scholar]
- 120. Mille ML, Johnson-Hilliard M, Martinez KM, Zhang Y, Edwards BJ, Rogers MW. One step, two steps, three steps more. … Directional vulnerability to falls in community-dwelling older people. J Gerontol A Biol Sci Med Sci 68: 1540–1548, 2013. doi: 10.1093/gerona/glt062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Johnson ME, Mille ML, Martinez KM, Crombie G, Rogers MW. Age-related changes in hip abductor and adductor joint torques. Arch Phys Med Rehabil 85: 593–597, 2004. doi: 10.1016/j.apmr.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 122. Ericson MO, Nisell R, Arborelius UP, Ekholm J. Muscular activity during ergometer cycling. Scand J Rehabil Med 17: 53–61, 1985. [PubMed] [Google Scholar]
- 123. Ericson MO, Nisell R, Ekholm J. Quantified electromyography of lower-limb muscles during level walking. Scand J Rehabil Med 18: 159–163, 1986. [PubMed] [Google Scholar]
- 124. Klein CS, Peterson LB, Ferrell S, Thomas CK. Sensitivity of 24-h EMG duration and intensity in the human vastus lateralis muscle to threshold changes. J Appl Physiol (1985) 108: 655–661, 2010. doi: 10.1152/japplphysiol.00757.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Belavý DL, Miokovic T, Armbrecht G, Rittweger J, Felsenberg D. Resistive vibration exercise reduces lower limb muscle atrophy during 56-day bed-rest. J Musculoskelet Neuronal Interact 9: 225–235, 2009. [PubMed] [Google Scholar]
- 126. Miokovic T, Armbrecht G, Felsenberg D, Belavý DL. Heterogeneous atrophy occurs within individual lower limb muscles during 60 days of bed rest. J Appl Physiol (1985) 113: 1545–1559, 2012. doi: 10.1152/japplphysiol.00611.2012. [DOI] [PubMed] [Google Scholar]
- 127. Dudley GA, Castro MJ, Rogers S, Apple DF Jr.. A simple means of increasing muscle size after spinal cord injury: a pilot study. Eur J Appl Physiol Occup Physiol 80: 394–396, 1999. doi: 10.1007/s004210050609. [DOI] [PubMed] [Google Scholar]
- 128. Giangregorio L, McCartney N. Bone loss and muscle atrophy in spinal cord injury: epidemiology, fracture prediction, and rehabilitation strategies. J Spinal Cord Med 29: 489–500, 2006. doi: 10.1080/10790268.2006.11753898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Hjeltnes N, Aksnes AK, Birkeland KI, Johansen J, Lannem A, Wallberg-Henriksson H. Improved body composition after 8 wk of electrically stimulated leg cycling in tetraplegic patients. Am J Physiol Regul Integr Comp Physiol 273: R1072–R1079, 1997. doi: 10.1152/ajpregu.1997.273.3.R1072. [DOI] [PubMed] [Google Scholar]
- 130. Ploutz-Snyder LL, Downs M, Goetchius E, Crowell B, English KL, Ploutz-Snyder R, Ryder JW, Dillon EL, Sheffield-Moore M, Scott JM. Exercise training mitigates multisystem deconditioning during bed rest. Med Sci Sports Exerc 50: 1920–1928, 2018. doi: 10.1249/MSS.0000000000001618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Scremin AM, Kurta L, Gentili A, Wiseman B, Perell K, Kunkel C, Scremin OU. Increasing muscle mass in spinal cord injured persons with a functional electrical stimulation exercise program. Arch Phys Med Rehabil 80: 1531–1536, 1999. doi: 10.1016/s0003-9993(99)90326-x. [DOI] [PubMed] [Google Scholar]
- 132. Trappe S, Williamson D, Godard M. Maintenance of whole muscle strength and size following resistance training in older men. J Gerontol A Biol Sci Med Sci 57: B138–B143, 2002. doi: 10.1093/gerona/57.4.b138. [DOI] [PubMed] [Google Scholar]
- 133. Trappe T. Influence of aging and long-term unloading on the structure and function of human skeletal muscle. Appl Physiol Nutr Metab 34: 459–464, 2009. doi: 10.1139/H09-041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Alkner BA, Tesch PA. Knee extensor and plantar flexor muscle size and function following 90 days of bed rest with or without resistance exercise. Eur J Appl Physiol 93: 294–305, 2004. doi: 10.1007/s00421-004-1172-8. [DOI] [PubMed] [Google Scholar]
- 135. Trappe S, Trappe T, Gallagher P, Harber M, Alkner B, Tesch P. Human single muscle fibre function with 84 day bed-rest and resistance exercise. J Physiol 557: 501–513, 2004. doi: 10.1113/jphysiol.2004.062166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Fukunaga T, Roy RR, Shellock FG, Hodgson JA, Day MK, Lee PL, Kwong-Fu H, Edgerton VR. Physiological cross-sectional area of human leg muscles based on magnetic resonance imaging. J Orthop Res 10: 928–934, 1992. doi: 10.1002/jor.1100100623. [DOI] [PubMed] [Google Scholar]
- 137. Louwerens JW, van Linge B, de Klerk LW, Mulder PG, Snijders CJ. Peroneus longus and tibialis anterior muscle activity in the stance phase. A quantified electromyographic study of 10 controls and 25 patients with chronic ankle instability. Acta Orthop Scand 66: 517–523, 1995. doi: 10.3109/17453679509002306. [DOI] [PubMed] [Google Scholar]
- 138. Ruiz Muñoz M, González-Sánchez M, Cuesta-Vargas AI. Tibialis anterior analysis from functional and architectural perspective during isometric foot dorsiflexion: a cross-sectional study of repeated measures. J Foot Ankle Res 8: 74, 2015. doi: 10.1186/s13047-015-0132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Scott JM, Martin DS, Ploutz-Snyder R, Caine T, Matz T, Arzeno NM, Buxton R, Ploutz-Snyder L. Reliability and validity of panoramic ultrasound for muscle quantification. Ultrasound Med Biol 38: 1656–1661, 2012. doi: 10.1016/j.ultrasmedbio.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 140. Simon SR, Mann RA, Hagy JL, Larsen LJ. Role of the posterior calf muscles in normal gait. J Bone Joint Surg Am 60: 465–472, 1978. [PubMed] [Google Scholar]
- 141. Sutherland DH, Cooper L, Daniel D. The role of the ankle plantar flexors in normal walking. J Bone Joint Surg Am 62: 354–363, 1980. [PubMed] [Google Scholar]
- 142. Kellis E, Liassou C. The effect of selective muscle fatigue on sagittal lower limb kinematics and muscle activity during level running. J Orthop Sports Phys Ther 39: 210–220, 2009. doi: 10.2519/jospt.2009.2859. [DOI] [PubMed] [Google Scholar]
- 143. Schache AG, Dorn TW, Williams GP, Brown NA, Pandy MG. Lower-limb muscular strategies for increasing running speed. J Orthop Sports Phys Ther 44: 813–824, 2014. doi: 10.2519/jospt.2014.5433. [DOI] [PubMed] [Google Scholar]
- 144. Hallinan J, Wang W, Pathria MN, Smitaman E, Huang BK. The peroneus longus muscle and tendon: a review of its anatomy and pathology. Skeletal Radiol 48: 1329–1344, 2019. doi: 10.1007/s00256-019-3168-9. [DOI] [PubMed] [Google Scholar]
- 145. Semple R, Murley GS, Woodburn J, Turner DE. Tibialis posterior in health and disease: a review of structure and function with specific reference to electromyographic studies. J Foot Ankle Res 2: 24, 2009. doi: 10.1186/1757-1146-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. McDermott MM, Ferrucci L, Guralnik J, Tian L, Liu K, Hoff F, Liao Y, Criqui MH. Pathophysiological changes in calf muscle predict mobility loss at 2-year follow-up in men and women with peripheral arterial disease. Circulation 120: 1048–1055, 2009. doi: 10.1161/CIRCULATIONAHA.108.842328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Stewart JD. Foot drop: where, why and what to do? Pract Neurol 8: 158–169, 2008. doi: 10.1136/jnnp.2008.149393. [DOI] [PubMed] [Google Scholar]
- 148. Wiszomirska I, Błażkiewicz M, Kaczmarczyk K, Brzuszkiewicz-Kuźmicka G, Wit A. Effect of drop foot on spatiotemporal, kinematic, and kinetic parameters during gait. Appl Bionics Biomech 2017: 3595461, 2017. doi: 10.1155/2017/3595461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Goldman MP, Fronek A. Anatomy and pathophysiology of varicose veins. J Dermatol Surg Oncol 15: 138–145, 1989. doi: 10.1111/j.1524-4725.1989.tb03020.x. [DOI] [PubMed] [Google Scholar]
- 150. Pollack AA, Wood EH. Venous pressure in the saphenous vein at the ankle in man during exercise and changes in posture. J Appl Physiol 1: 649–662, 1949. doi: 10.1152/jappl.1949.1.9.649. [DOI] [PubMed] [Google Scholar]
- 151. Araki CT, Back TL, Padberg FT, Thompson PN, Jamil Z, Lee BC, Duran WN, Hobson RW 2nd.. The significance of calf muscle pump function in venous ulceration. J Vasc Surg 20: 872–877, 1994. doi: 10.1016/0741-5214(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 152. Meissner MH. Lower extremity venous anatomy. Semin Intervent Radiol 22: 147–156, 2005. doi: 10.1055/s-2005-921948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Padberg FT Jr, Johnston MV, Sisto SA. Structured exercise improves calf muscle pump function in chronic venous insufficiency: a randomized trial. J Vasc Surg 39: 79–87, 2004. doi: 10.1016/j.jvs.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 154. Williams KJ, Ayekoloye O, Moore HM, Davies AH. The calf muscle pump revisited. J Vasc Surg Venous Lymphat Disord 2: 329–334, 2014. doi: 10.1016/j.jvsv.2013.10.053. [DOI] [PubMed] [Google Scholar]
- 155. Shirasawa H, Kanehisa H, Kouzaki M, Masani K, Fukunaga T. Differences among lower leg muscles in long-term activity during ambulatory condition without any moderate to high intensity exercise. J Electromyogr Kinesiol 19: e50–e56, 2009. doi: 10.1016/j.jelekin.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 156. Akima H, Kuno S, Suzuki Y, Gunji A, Fukunaga T. Effects of 20 days of bed rest on physiological cross-sectional area of human thigh and leg muscles evaluated by magnetic resonance imaging. J Gravit Physiol 4: S15–S21, 1997. [PubMed] [Google Scholar]
- 157. Akima H, Kawakami Y, Kubo K, Sekiguchi C, Ohshima H, Miyamoto A, Fukunaga T. Effect of short-duration spaceflight on thigh and leg muscle volume. Med Sci Sports Exerc 32: 1743–1747, 2000. doi: 10.1097/00005768-200010000-00013. [DOI] [PubMed] [Google Scholar]
- 158. Akima H, Ushiyama J-I, Kubo J, Fukuoka H, Kanehisa H, Fukunaga T. Effect of unloading on muscle volume with and without resistance training. Acta Astronautica 60: 728–736, 2007. doi: 10.1016/j.actaastro.2006.10.006. [DOI] [Google Scholar]
- 159. Belavý DL, Ohshima H, Rittweger J, Felsenberg D. High-intensity flywheel exercise and recovery of atrophy after 90 days bed-rest. BMJ Open Sport Exerc Med 3: e000196, 2017. doi: 10.1136/bmjsem-2016-000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Lavoie BA, Cody FW, Capaday C. Cortical control of human soleus muscle during volitional and postural activities studied using focal magnetic stimulation. Exp Brain Res 103: 97–107, 1995. doi: 10.1007/BF00241968. [DOI] [PubMed] [Google Scholar]
- 161. LeBlanc A, Lin C, Shackelford L, Sinitsyn V, Evans H, Belichenko O, Schenkman B, Kozlovskaya I, Oganov V, Bakulin A, Hedrick T, Feeback D. Muscle volume, MRI relaxation times (T2), and body composition after spaceflight. J Appl Physiol (1985) 89: 2158–2164, 2000. doi: 10.1152/jappl.2000.89.6.2158. [DOI] [PubMed] [Google Scholar]
- 162. Shackelford LC, LeBlanc AD, Driscoll TB, Evans HJ, Rianon NJ, Smith SM, Spector E, Feeback DL, Lai D. Resistance exercise as a countermeasure to disuse-induced bone loss. J Appl Physiol (1985) 97: 119–129, 2004. doi: 10.1152/japplphysiol.00741.2003. [DOI] [PubMed] [Google Scholar]
- 163. Lester BE, Standley RA, Lee JD, Fink WJ, Trappe SW, Trappe TA. Muscle-specific substrate use during cycle exercise at 1G: implications for astronaut muscle health. Aviat Space Environ Med 84: 789–796, 2013. doi: 10.3357/asem.3440.2013. [DOI] [PubMed] [Google Scholar]
- 164. Luden N, Minchev K, Hayes E, Louis E, Trappe T, Trappe S. Human vastus lateralis and soleus muscles display divergent cellular contractile properties. Am J Physiol Regul Integr Comp Physiol 295: R1593–R1598, 2008. doi: 10.1152/ajpregu.90564.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Trappe S, Creer A, Minchev K, Slivka D, Louis E, Luden N, Trappe T. Human soleus single muscle fiber function with exercise or nutrition countermeasures during 60 days of bed rest. Am J Physiol Regul Integr Comp Physiol 294: R939–R947, 2008. doi: 10.1152/ajpregu.00761.2007. [DOI] [PubMed] [Google Scholar]
- 166. Harber MP, Crane JD, Dickinson JM, Jemiolo B, Raue U, Trappe TA, Trappe SW. Protein synthesis and the expression of growth-related genes are altered by running in human vastus lateralis and soleus muscles. Am J Physiol Regul Integr Comp Physiol 296: R708–R714, 2009. doi: 10.1152/ajpregu.90906.2008. [DOI] [PubMed] [Google Scholar]
- 167. Noonan AM, Brown SHM. Paraspinal muscle pathophysiology associated with low back pain and spine degenerative disorders. JOR Spine 4: e1171, 2021. doi: 10.1002/jsp2.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Andersson GBJ, Winters JM. Role of muscle in postural tasks: spinal loading and postural stability. In: Multiple Muscle Systems: Biomechanics and Movement Organization, edited by Winters JM, Woo SLY.. New York, NY: Springer, 1990, p. 377–395. [Google Scholar]
- 169. Anderson CN. Iliopsoas: pathology, diagnosis, and treatment. Clin Sports Med 35: 419–433, 2016. doi: 10.1016/j.csm.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 170. Andersson E, Oddsson L, Grundström H, Thorstensson A. The role of the psoas and iliacus muscles for stability and movement of the lumbar spine, pelvis and hip. Scand J Med Sci Sports 5: 10–16, 1995. doi: 10.1111/j.1600-0838.1995.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 171. Penning L. Psoas muscle and lumbar spine stability: a concept uniting existing controversies. Critical review and hypothesis. Eur Spine J 9: 577–585, 2000. doi: 10.1007/s005860000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Yoshio M, Murakami G, Sato T, Sato S, Noriyasu S. The function of the psoas major muscle: passive kinetics and morphological studies using donated cadavers. J Orthop Sci 7: 199–207, 2002. doi: 10.1007/s007760200034. [DOI] [PubMed] [Google Scholar]
- 173. Sugisaki N, Kobayashi K, Tsuchie H, Kanehisa H. Associations between individual lower-limb muscle volumes and 100-m sprint time in male sprinters. Int J Sports Physiol Perform 13: 214–219, 2018. doi: 10.1123/ijspp.2016-0703. [DOI] [PubMed] [Google Scholar]
- 174. Nuber GW, Jobe FW, Perry J, Moynes DR, Antonelli D. Fine wire electromyography analysis of muscles of the shoulder during swimming. Am J Sports Med 14: 7–11, 1986. doi: 10.1177/036354658601400102. [DOI] [PubMed] [Google Scholar]
- 175. Martens J, Figueiredo P, Daly D. Electromyography in the four competitive swimming strokes: a systematic review. J Electromyogr Kinesiol 25: 273–291, 2015. doi: 10.1016/j.jelekin.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 176. Bressler HB, Keyes WJ, Rochon PA, Badley E. The prevalence of low back pain in the elderly. A systematic review of the literature. Spine (Phila PA 1976) 24: 1813–1819, 1999. doi: 10.1097/00007632-199909010-00011. [DOI] [PubMed] [Google Scholar]
- 177. de Souza IMB, Sakaguchi TF, Yuan SLK, Matsutani LA, do Espírito-Santo AS, Pereira CAB, Marques AP. Prevalence of low back pain in the elderly population: a systematic review. Clinics (Sao Paulo) 74: e789, 2019. doi: 10.6061/clinics/2019/e789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Marshall LM, Litwack-Harrison S, Makris UE, Kado DM, Cawthon PM, Deyo RA, Carlson NL, Nevitt MC. A prospective study of back pain and risk of falls among older community-dwelling men. J Gerontol A Biol Sci Med Sci 72: 1264–1269, 2017. doi: 10.1093/gerona/glw227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Patel KV, Guralnik JM, Dansie EJ, Turk DC. Prevalence and impact of pain among older adults in the United States: findings from the 2011 National Health and Aging Trends Study. Pain 154: 2649–2657, 2013. doi: 10.1016/j.pain.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Hides JA, Stokes MJ, Saide M, Jull GA, Cooper DH. Evidence of lumbar multifidus muscle wasting ipsilateral to symptoms in patients with acute/subacute low back pain. Spine (Phila Pa 1976) 19: 165–172, 1994. doi: 10.1097/00007632-199401001-00009. [DOI] [PubMed] [Google Scholar]
- 181. Hides JA, Stanton WR, McMahon S, Sims K, Richardson CA. Effect of stabilization training on multifidus muscle cross-sectional area among young elite cricketers with low back pain. J Orthop Sports Phys Ther 38: 101–108, 2008. doi: 10.2519/jospt.2008.2658. [DOI] [PubMed] [Google Scholar]
- 182. Sions JM, Elliott JM, Pohlig RT, Hicks GE. Trunk muscle characteristics of the multifidi, erector spinae, psoas, and quadratus lumborum in older adults with and without chronic low back pain. J Orthop Sports Phys Ther 47: 173–179, 2017. doi: 10.2519/jospt.2017.7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Baracos VE. Psoas as a sentinel muscle for sarcopenia: a flawed premise. J Cachexia Sarcopenia Muscle 8: 527–528, 2017. doi: 10.1002/jcsm.12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Derstine BA, Holcombe SA, Goulson RL, Ross BE, Wang NC, Sullivan JA, Su GL, Wang SC. Quantifying sarcopenia reference values using lumbar and thoracic muscle areas in a healthy population. J Nutr Health Aging 21: 180–185, 2017. doi: 10.1007/s12603-017-0983-3. [DOI] [PubMed] [Google Scholar]
- 185. Vangelov B, Bauer J, Kotevski D, Smee RI. The use of alternate vertebral levels to L3 in computed tomography scans for skeletal muscle mass evaluation and sarcopenia assessment in patients with cancer: a systematic review. Br J Nutr 127: 722–735, 2022. doi: 10.1017/S0007114521001446. [DOI] [PubMed] [Google Scholar]
- 186. Crawford RJ, Filli L, Elliott JM, Nanz D, Fischer MA, Marcon M, Ulbrich EJ. Age- and level-dependence of fatty infiltration in lumbar paravertebral muscles of healthy volunteers. AJNR Am J Neuroradiol 37: 742–748, 2016. doi: 10.3174/ajnr.A4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Fortin M, Videman T, Gibbons LE, Battié MC. Paraspinal muscle morphology and composition: a 15-yr longitudinal magnetic resonance imaging study. Med Sci Sports Exerc 46: 893–901, 2014. doi: 10.1249/MSS.0000000000000179. [DOI] [PubMed] [Google Scholar]
- 188. Stokes M, Rankin G, Newham DJ. Ultrasound imaging of lumbar multifidus muscle: normal reference ranges for measurements and practical guidance on the technique. Man Ther 10: 116–126, 2005. doi: 10.1016/j.math.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 189. Janaudis-Ferreira T, Hill K, Goldstein RS, Robles-Ribeiro P, Beauchamp MK, Dolmage TE, Wadell K, Brooks D. Resistance arm training in patients with COPD: a randomized controlled trial. Chest 139: 151–158, 2011. doi: 10.1378/chest.10-1292. [DOI] [PubMed] [Google Scholar]
- 190. Meijer K, Annegarn J, Lima Passos V, Savelberg HH, Schols AM, Wouters EF, Spruit MA. Characteristics of daily arm activities in patients with COPD. Eur Respir J 43: 1631–1641, 2014. doi: 10.1183/09031936.00082513. [DOI] [PubMed] [Google Scholar]
- 191. Keramiotou K, Anagnostou C, Kataxaki E, Galanos A, Sfikakis PP, Tektonidou MG. The impact of upper limb exercise on function, daily activities and quality of life in systemic lupus erythematosus: a pilot randomised controlled trial. RMD Open 6: e001141, 2020. doi: 10.1136/rmdopen-2019-001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Lieshout E, van de Port IG, Dijkhuizen RM, Visser-Meily JMA. Does upper limb strength play a prominent role in health-related quality of life in stroke patients discharged from inpatient rehabilitation? Top Stroke Rehabil 27: 525–533, 2020. doi: 10.1080/10749357.2020.1738662. [DOI] [PubMed] [Google Scholar]
- 193. Kern DS, Semmler JG, Enoka RM. Long-term activity in upper- and lower-limb muscles of humans. J Appl Physiol (1985) 91: 2224–2232, 2001. doi: 10.1152/jappl.2001.91.5.2224. [DOI] [PubMed] [Google Scholar]
- 194. Brown AB, McCartney N, Sale DG. Positive adaptations to weight-lifting training in the elderly. J Appl Physiol (1985) 69: 1725–1733, 1990. doi: 10.1152/jappl.1990.69.5.1725. [DOI] [PubMed] [Google Scholar]
- 195. Frontera WR, Meredith CN, O'Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol (1985) 64: 1038–1044, 1988. doi: 10.1152/jappl.1988.64.3.1038. [DOI] [PubMed] [Google Scholar]
- 196. Lexell J, Downham DY, Larsson Y, Bruhn E, Morsing B. Heavy-resistance training in older Scandinavian men and women: short- and long-term effects on arm and leg muscles. Scand J Med Sci Sports 5: 329–341, 2007. doi: 10.1111/j.1600-0838.1995.tb00055.x. [DOI] [PubMed] [Google Scholar]
- 197. Dartnall TJ, Nordstrom MA, Semmler JG. Adaptations in biceps brachii motor unit activity after repeated bouts of eccentric exercise in elbow flexor muscles. J Neurophysiol 105: 1225–1235, 2011. doi: 10.1152/jn.00854.2010. [DOI] [PubMed] [Google Scholar]
- 198. Galea V. Changes in motor unit estimates with aging. J Clin Neurophysiol 13: 253–260, 1996. doi: 10.1097/00004691-199605000-00010. [DOI] [PubMed] [Google Scholar]
- 199. Grimby G, Danneskiold-Samsøe B, Hvid K, Saltin B. Morphology and enzymatic capacity in arm and leg muscles in 78-81 year old men and women. Acta Physiol Scand 115: 125–134, 1982. doi: 10.1111/j.1748-1716.1982.tb07054.x. [DOI] [PubMed] [Google Scholar]
- 200. Landers KA, Hunter GR, Wetzstein CJ, Bamman MM, Weinsier RL. The interrelationship among muscle mass, strength, and the ability to perform physical tasks of daily living in younger and older women. J Gerontol A Biol Sci Med Sci 56: B443–B448, 2001. doi: 10.1093/gerona/56.10.b443. [DOI] [PubMed] [Google Scholar]
- 201. Narici MV, Maffulli N. Sarcopenia: characteristics, mechanisms and functional significance. Br Med Bull 95: 139–159, 2010. doi: 10.1093/bmb/ldq008. [DOI] [PubMed] [Google Scholar]
- 202. Brooks SV, Faulkner JA. Skeletal muscle weakness in old age: underlying mechanisms. Med Sci Sports Exerc 26: 432–439, 1994. [PubMed] [Google Scholar]
- 203. Klein CS, Marsh GD, Petrella RJ, Rice CL. Muscle fiber number in the biceps brachii muscle of young and old men. Muscle Nerve 28: 62–68, 2003. doi: 10.1002/mus.10386. [DOI] [PubMed] [Google Scholar]
- 204. Aniansson A, Grimby G, Hedberg M. Compensatory muscle fiber hypertrophy in elderly men. J Appl Physiol (1985) 73: 812–816, 1992. doi: 10.1152/jappl.1992.73.3.812. [DOI] [PubMed] [Google Scholar]
- 205. Doherty TJ. The influence of aging and sex on skeletal muscle mass and strength. Curr Opin Clin Nutr Metab Care 4: 503–508, 2001. doi: 10.1097/00075197-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 206. Hunter SK, Critchlow A, Enoka RM. Influence of aging on sex differences in muscle fatigability. J Appl Physiol (1985) 97: 1723–1732, 2004. doi: 10.1152/japplphysiol.00460.2004. [DOI] [PubMed] [Google Scholar]
- 207. Brown M. Skeletal muscle and bone: effect of sex steroids and aging. Adv Physiol Educ 32: 120–126, 2008. doi: 10.1152/advan.90111.2008. [DOI] [PubMed] [Google Scholar]
- 208. Herbst KL, Bhasin S. Testosterone action on skeletal muscle. Curr Opin Clin Nutr Metab Care 7: 271–277, 2004. doi: 10.1097/00075197-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 209. Roberts BM, Lavin KM, Many GM, Thalacker-Mercer A, Merritt EK, Bickel CS, Mayhew DL, Tuggle SC, Cross JM, Kosek DJ, Petrella JK, Brown CJ, Hunter GR, Windham ST, Allman RM, Bamman MM. Human neuromuscular aging: sex differences revealed at the myocellular level. Exp Gerontol 106: 116–124, 2018. doi: 10.1016/j.exger.2018.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, Bunnell TJ, Tricker R, Shirazi A, Casaburi R. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med 335: 1–7, 1996. doi: 10.1056/NEJM199607043350101. [DOI] [PubMed] [Google Scholar]
- 211. Pasiakos SM, Berryman CE, Karl JP, Lieberman HR, Orr JS, Margolis LM, Caldwell JA, Young AJ, Montano MA, Evans WJ, Vartanian O, Carmichael OT, Gadde KM, Johannsen NM, Beyl RA, Harris MN, Rood JC. Effects of testosterone supplementation on body composition and lower-body muscle function during severe exercise- and diet-induced energy deficit: a proof-of-concept, single centre, randomised, double-blind, controlled trial. EBioMedicine 46: 411–422, 2019. doi: 10.1016/j.ebiom.2019.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Lenrow DA, Holmes JH, Dlewati A, Santanna J, Rosen CJ, Strom BL. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab 84: 2647–2653, 1999. doi: 10.1210/jcem.84.8.5885. [DOI] [PubMed] [Google Scholar]
- 213. Pearson MB, Bassey EJ, Bendall MJ. The effects of age on muscle strength and anthropometric indices within a group of elderly men and women. Age Ageing 14: 230–234, 1985. doi: 10.1093/ageing/14.4.230. [DOI] [PubMed] [Google Scholar]
- 214. Vandervoort AA, Chesworth BM, Cunningham DA, Paterson DH, Rechnitzer PA, Koval JJ. Age and sex effects on mobility of the human ankle. J Gerontol 47: M17–M21, 1992. doi: 10.1093/geronj/47.1.m17. [DOI] [PubMed] [Google Scholar]
- 215. Frontera WR, Suh D, Krivickas LS, Hughes VA, Goldstein R, Roubenoff R. Skeletal muscle fiber quality in older men and women. Am J Physiol Cell Physiol 279: C611–C618, 2000. doi: 10.1152/ajpcell.2000.279.3.C611. [DOI] [PubMed] [Google Scholar]
- 216. Kasai T, Ishiguro N, Matsui Y, Harada A, Takemura M, Yuki A, Kato Y, Otsuka R, Ando F, Shimokata H. Sex- and age-related differences in mid-thigh composition and muscle quality determined by computed tomography in middle-aged and elderly Japanese. Geriatr Gerontol Int 15: 700–706, 2015. doi: 10.1111/ggi.12338. [DOI] [PubMed] [Google Scholar]
- 217. Eriksen CS, Henkel C, Svensson RB, Agergaard AS, Couppé C, Kjaer M, Magnusson SP. Lower tendon stiffness in very old compared with old individuals is unaffected by short-term resistance training of skeletal muscle. J Appl Physiol (1985) 125: 205–214, 2018. doi: 10.1152/japplphysiol.00028.2018. [DOI] [PubMed] [Google Scholar]
- 218. McCartney N, Hicks AL, Martin J, Webber CE. Long-term resistance training in the elderly: effects on dynamic strength, exercise capacity, muscle, and bone. J Gerontol A Biol Sci Med Sci 50: B97–B104, 1995. doi: 10.1093/gerona/50a.2.b97. [DOI] [PubMed] [Google Scholar]
- 219. Banks NF, Rogers EM, Jenkins NDM. Electromyographic amplitude versus torque relationships are different in young versus postmenopausal females and are related to muscle mass after controlling for bodyweight. Eur J Appl Physiol 121: 479–488, 2021. doi: 10.1007/s00421-020-04532-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Dicks ND, Kotarsky CJ, Trautman KA, Barry AM, Keith JF, Mitchell S, Byun W, Stastny SN, Hackney KJ. Contribution of protein intake and concurrent exercise to skeletal muscle quality with aging. J Frailty Aging 9: 51–56, 2020. doi: 10.14283/jfa.2019.40. [DOI] [PubMed] [Google Scholar]
- 221. Pišot R, Marusic U, Biolo G, Mazzucco S, Lazzer S, Grassi B, Reggiani C, Toniolo L, di Prampero PE, Passaro A, Narici M, Mohammed S, Rittweger J, Gasparini M, Gabrijelčič Blenkuš M, Šimunič B. Greater loss in muscle mass and function but smaller metabolic alterations in older compared with younger men following 2 wk of bed rest and recovery. J Appl Physiol (1985) 120: 922–929, 2016. doi: 10.1152/japplphysiol.00858.2015. [DOI] [PubMed] [Google Scholar]
- 222. Daly M, Vidt ME, Eggebeen JD, Simpson WG, Miller ME, Marsh AP, Saul KR. Upper extremity muscle volumes and functional strength after resistance training in older adults. J Aging Phys Act 21: 186–207, 2013. doi: 10.1123/japa.21.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Roman WJ, Fleckenstein J, Stray-Gundersen J, Alway SE, Peshock R, Gonyea WJ. Adaptations in the elbow flexors of elderly males after heavy-resistance training. J Appl Physiol (1985) 74: 750–754, 1993. doi: 10.1152/jappl.1993.74.2.750. [DOI] [PubMed] [Google Scholar]
- 224. Sipilä S, Suominen H. Effects of strength and endurance training on thigh and leg muscle mass and composition in elderly women. J Appl Physiol (1985) 78: 334–340, 1995. doi: 10.1152/jappl.1995.78.1.334. [DOI] [PubMed] [Google Scholar]
- 225. Sipilä S, Suominen H. Quantitative ultrasonography of muscle: detection of adaptations to training in elderly women. Arch Phys Med Rehabil 77: 1173–1178, 1996. doi: 10.1016/s0003-9993(96)90143-4. [DOI] [PubMed] [Google Scholar]
- 226. Létocart AJ, Mabesoone F, Charleux F, Couppé C, Svensson RB, Marin F, Magnusson SP, Grosset JF. Muscles adaptation to aging and training: architectural changes—a randomised trial. BMC Geriatr 21: 48, 2021. doi: 10.1186/s12877-020-02000-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Welle S, Totterman S, Thornton C. Effect of age on muscle hypertrophy induced by resistance training. J Gerontol A Biol Sci Med Sci 51: M270–M275, 1996. doi: 10.1093/gerona/51a.6.m270. [DOI] [PubMed] [Google Scholar]
- 228. Blangsted AK, Søgaard K, Hansen EA, Hannerz H, Sjøgaard G. One-year randomized controlled trial with different physical-activity programs to reduce musculoskeletal symptoms in the neck and shoulders among office workers. Scand J Work Environ Health 34: 55–65, 2008. doi: 10.5271/sjweh.1192. [DOI] [PubMed] [Google Scholar]
- 229. Preuss RA, Grenier SG, McGill SM. Postural control of the lumbar spine in unstable sitting. Arch Phys Med Rehabil 86: 2309–2315, 2005. doi: 10.1016/j.apmr.2005.07.302. [DOI] [PubMed] [Google Scholar]
- 230. Carbone V, Fluit R, Pellikaan P, van der Krogt MM, Janssen D, Damsgaard M, Vigneron L, Feilkas T, Koopman HF, Verdonschot N. TLEM 2.0—a comprehensive musculoskeletal geometry dataset for subject-specific modeling of lower extremity. J Biomech 48: 734–741, 2015. doi: 10.1016/j.jbiomech.2014.12.034. [DOI] [PubMed] [Google Scholar]
- 231. Ito J, Moriyama H, Inokuchi S, Goto N. Human lower limb muscles: an evaluation of weight and fiber size. Okajimas Folia Anat Jpn 80: 47–55, 2003. doi: 10.2535/ofaj.80.47. [DOI] [PubMed] [Google Scholar]
- 232. Ward SR, Eng CM, Smallwood LH, Lieber RL. Are current measurements of lower extremity muscle architecture accurate? Clin Orthop Relat Res 467: 1074–1082, 2009. doi: 10.1007/s11999-008-0594-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Cappellini G, Ivanenko YP, Poppele RE, Lacquaniti F. Motor patterns in human walking and running. J Neurophysiol 95: 3426–3437, 2006. doi: 10.1152/jn.00081.2006. [DOI] [PubMed] [Google Scholar]
- 234. Ward SR, Winters TM, Blemker SS. The architectural design of the gluteal muscle group: implications for movement and rehabilitation. J Orthop Sports Phys Ther 40: 95–102, 2010. doi: 10.2519/jospt.2010.3302. [DOI] [PubMed] [Google Scholar]
- 235. Inacio M, Ryan AS, Bair WN, Prettyman M, Beamer BA, Rogers MW. Gluteal muscle composition differentiates fallers from non-fallers in community dwelling older adults. BMC Geriatr 14: 37, 2014. doi: 10.1186/1471-2318-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Davis DL, Roberts A, Calderon R, Kim S, Ryan AS, Sanses TVD. Gluteal muscle fatty infiltration, fall risk, and mobility limitation in older women with urinary incontinence: a pilot study. Skeletal Radiol 52: 47–55, 2023. doi: 10.1007/s00256-022-04132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Bohannon RW. Grip strength: an indispensable biomarker for older adults. Clin Interv Aging 14: 1681–1691, 2019. doi: 10.2147/CIA.S194543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Celis-Morales CA, Welsh P, Lyall DM, Steell L, Petermann F, Anderson J, Iliodromiti S, Sillars A, Graham N, Mackay DF, Pell JP, Gill JMR, Sattar N, Gray SR. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: prospective cohort study of half a million UK Biobank participants. BMJ 361: k1651, 2018. doi: 10.1136/bmj.k1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Rantanen T, Masaki K, Foley D, Izmirlian G, White L, Guralnik JM. Grip strength changes over 27 yr in Japanese-American men. J Appl Physiol (1985) 85: 2047–2053, 1998. doi: 10.1152/jappl.1998.85.6.2047. [DOI] [PubMed] [Google Scholar]
- 240. Rantanen T, Guralnik JM, Foley D, Masaki K, Leveille S, Curb JD, White L. Midlife hand grip strength as a predictor of old age disability. JAMA 281: 558–560, 1999. doi: 10.1001/jama.281.6.558. [DOI] [PubMed] [Google Scholar]
- 241. Tanaka NI, Yamada M, Tanaka Y, Fukunaga T, Nishijima T, Kanehisa H. Difference in abdominal muscularity at the umbilicus level between young and middle-aged men. J Physiol Anthropol 26: 527–532, 2007. doi: 10.2114/jpa2.26.527. [DOI] [PubMed] [Google Scholar]
- 242. Valentin S, Licka T, Elliott J. Age and side-related morphometric MRI evaluation of trunk muscles in people without back pain. Man Ther 20: 90–95, 2015. [Erratum in Man Ther 20: 513, 2015]. doi: 10.1016/j.math.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Preininger B, Schmorl K, von Roth P, Winkler T, Schlattmann P, Matziolis G, Perka C, Tohtz S. A formula to predict patients' gluteus medius muscle volume from hip joint geometry. Man Ther 16: 447–451, 2011. doi: 10.1016/j.math.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 244. Preininger B, Schmorl K, von Roth P, Winkler T, Matziolis G, Perka C, Tohtz S. The sex specificity of hip-joint muscles offers an explanation for better results in men after total hip arthroplasty. Int Orthop 36: 1143–1148, 2012. doi: 10.1007/s00264-011-1411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Wang L, Yin L, Zhao Y, Su Y, Sun W, Liu Y, Yang M, Yu A, Blake GM, Cheng X, Wu X, Veldhuis A, Engelke K. Muscle density discriminates hip fracture better than computed tomography X-ray absorptiometry hip areal bone mineral density. J Cachexia Sarcopenia Muscle 11: 1799–1812, 2020. doi: 10.1002/jcsm.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]