NICE has accredited the process used by BSR to create its clinical guidelines. The term began on 27 February 2012 and the current renewed accreditation is valid until 31 December 2023. More information on accreditation can be viewed at www.nice.org.uk/accreditation.
Scope and purpose
Background
The rationale behind this update of the 2016 British Society for Rheumatology (BSR) guidelines on prescribing anti-rheumatic drugs in pregnancy and breastfeeding [1, 2] was described in detail in the guideline scope [3]. In brief, despite the existence of additional evidence-based guidelines on prescribing/managing rheumatic disease in pregnancy [4–7], the information contained within them requires continual review to include emerging information on the safety of new and existing drugs in pregnancy.
Chronic disease adversely affects pregnancy. Data from Mothers and Babies: Reducing Risk through Audits and Confidential Enquiries across the UK (MBRRACE-UK) reports regularly from a national programme of work conducting surveillance and investigating the causes of maternal deaths, stillbirths and infant deaths [8]. Data from 2017–19 found that 8.8 women per 100 000 died during pregnancy or up to six weeks after childbirth or the end of pregnancy, and most women who died had multiple health problems or other vulnerabilities [8]. In all decisions regarding medication choices and changes, it is important to consider the potential for deterioration in the mother's wellbeing through side effects or reduced disease control (and its adverse impact on the baby). As such, the potential benefit to the foetus from any drug changes in the mother must be balanced against the possible risks to the foetus from loss of disease control in the mother [9].
Need for guideline
There has been an appreciable increase in the number of published pregnancy exposures to biologic DMARDs (bDMARDs), and two of these drugs are now licensed for use in pregnancy. In addition, therapeutic advances in management of various inflammatory rheumatic diseases (IRDs) have led to an expansion of bDMARDs and biosimilars with different modes of action, as well as a new class of targeted synthetic DMARDs (tsDMARDs).
The continuing expansion of existing and novel DMARDs means that uncertainty remains around the use of many of these drugs in pregnancy. This uncertainty may still lead to withdrawal of treatment from pregnant women unnecessarily [10]. Discontinuation of treatment in preparation for or during early pregnancy can increase the risk of disease activity and flares during pregnancy, and are reported following discontinuation of biologics in patients with IRDs [11]. The compatibility of various immunosuppressive and disease-modifying medications relevant to rheumatic disease will be covered in this update. This updated information will provide advice for healthcare professionals and patients, to ensure more confident prescribing in these scenarios, and will highlight any medications that should be stopped and/or avoided in the reproductive age group unless highly effective contraception is used, in line with guidance issued by the Medicines and Healthcare Products Regulatory Agency (MHRA) and the Faculty of Sexual and Reproductive Healthcare [12, 13].
Objectives of guideline
To update the previous BSR guidelines on prescribing in pregnancy in rheumatic disease of the following drug categories: antimalarials; corticosteroids; conventional synthetic (cs)DMARDs and immunosuppressive therapies; bDMARDs; and tsDMARDs. The full list of medications is shown in Supplementary Data S1, available at Rheumatology online. This revised guideline was produced by systematically reviewing all evidence published since the previous guideline, to answer specific questions in relation to each drug, as follows: Should it be stopped pre-conception? Is it compatible with pregnancy? Is it compatible with breastmilk exposure? Where possible, recommendations are made regarding compatibility with paternal exposure.
Target audience
The primary audience consists of health professionals in the UK directly involved in managing patients with rheumatic disease who are (or are planning to become) pregnant and/or breastfeeding, men with rheumatic disease who are planning to conceive, and patients with rheumatic disease who have unintentionally conceived while taking these medications. This audience includes rheumatologists, rheumatology nurses/allied health professionals, rheumatology speciality trainees and pharmacists, as well as the patients themselves. The guideline will also be useful to obstetricians, obstetric physicians, midwives, renal physicians, dermatologists, gastroenterologists, respiratory physicians and general practitioners who prescribe these medications in pregnancy.
This guideline uses the terms ‘woman’, ‘maternal’ or ‘mother’ throughout. These should be taken to include people who do not identify as women but are pregnant or have given birth [14]. Where the term ‘breastfeeding’ is used in this guideline it also refers to infant breastmilk exposure via other methods (e.g. expressed breastmilk, administered via a bottle).
The areas the guideline does not cover
This guideline does not cover the management of infertility or the indications for these drugs in specific rheumatic diseases in pregnancy. Other drug categories (pain management; NSAIDs and low dose aspirin; anticoagulants; bisphosphonates; anti-hypertensives; and pulmonary vasodilators) are considered in the BSR guideline on prescribing drugs in pregnancy and breastfeeding: comorbidity medications used in rheumatology practice (https://doi.org/10.1093/rheumatology/keac552). All recommendations in this guideline were formulated by the working group on the basis of published evidence at the time of the systematic literature search, and do not necessarily refer to licensing information or Summary of Product Characteristics for individual medications.
Stakeholder involvement
This guideline was commissioned by the BSR Standards, Guidelines and Audit Working Group. A Guideline Working group (GWG) was created, consisting of a chair (I.G.), alongside representatives from relevant stakeholders shown in Supplementary Table S1, available at Rheumatology online. In accordance with BSR policy, all members of the GWG made declarations of interest, available on the BSR website.
Involvement and affiliations of stakeholder groups involved in guideline development
The GWG consisted of rheumatologists from a range of clinical backgrounds, various allied health professionals, other specialists in women’s health, lay members and representatives from the United Kingdom Teratology Information Service (UKTIS). All members of the working group contributed to the process for agreeing key questions, guideline content, recommendations and strength of agreement.
Rigour of development
Statement of scope of literature search and strategy employed
The evidence used to develop these guidelines was compiled from a systematic literature search conducted according to guidelines of Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) [15]. Studies were identified by searching MEDLINE and Embase databases from 1 January 2014 to 31 December 2020 using combinations of the key MESH and free terms: pregnancy; lactation; breastfeeding; paternal exposure; and the name of each drug. The full electronic search strategies for the MEDLINE and Embase databases are shown in Supplementary Data S2, available at Rheumatology online. Searches were not limited by disease indication; in addition to IRDs, studies in non-rheumatic diseases, such as psoriasis, inflammatory bowel disease (IBD) and organ transplantation were considered, if relevant. Additional published studies were identified through the Cochrane, LactMed (a National Library of Medicine database on drugs and lactation) and UKTIS databases (weblinks shown in Supplementary Data S2, available at Rheumatology online), and checking of reference lists from recently published national and international guidelines and systematic literature reviews. Due to the paucity of data pertaining to the use of non-TNFi biologic drugs and tsDMARDs in pregnancy and breastmilk exposure, relevant pharmaceutical companies were contacted between July and November 2021, and asked for any further available data.
Two independent reviewers screened the titles and abstracts of articles from the searches then reviewed the full texts of relevant studies, selecting articles that met inclusion criteria of: randomized and non-randomized controlled trials; cohort studies; case-control studies; and case series with more than ten participants. For medications with data on fewer than 300 pregnancy exposures, case series with more than five participants were eligible for inclusion. Conference abstracts were eligible for inclusion if they contained sufficient relevant data and there was no corresponding published manuscript. Case reports, and case series with fewer than five participants, were excluded, as were animal studies. Data extraction was performed by two reviewers. Disagreements arising during screening and extraction were resolved by group discussion, with involvement of a third reviewer where necessary.
Statement of methods used to formulate the recommendations (levels of evidence)
The working group met regularly to formalize the search strategy, review evidence, resolve disagreements and, finally, to determine recommendations. This guideline was developed in line with BSR’s Guidelines Protocol using Grading of Recommendations, Assessment, Development and Evaluations (GRADE) methodology to determine quality of evidence and strength of recommendation. Accompanying each recommendation in this guideline, in brackets, is the strength of recommendation, quality of evidence and strength of agreement (SOA).
Strength of recommendation
Using GRADE, recommendations were categorized as either strong (denoted by 1) or weak (denoted by 2), according to the balance between benefits and risks. A strong recommendation was made when the benefits clearly outweigh the risks (or vice versa). A weak recommendation denotes that the benefits are more closely balanced with the risk or more uncertain.
Quality of evidence
Using the GRADE approach, the quality of evidence was determined as either high (A), moderate (B) or low/very low (C), reflecting the confidence in the estimates of benefits or harm.
Strength of agreement
The wording of each recommendation was revised until all members were satisfied that they would score at least 80 on a scale of 1 (no agreement) to 100 (complete agreement). The 20/24 working group members with full voting rights then scored each recommendation on the same scale, and the average was calculated to generate a strength of agreement (SOA) score. Two patient representatives and data analysts expressed concern that they did not have sufficient medical knowledge of all drugs reviewed to score all recommendations; so while they fully agreed with each recommendation, they did not wish to score each one, and did not contribute to the final SOA score.
Statement of any limits of search and when the guideline will be updated
The search was conducted in January 2021. Limits were placed for English language and filters as described above. The guideline will be updated in five years.
The guideline
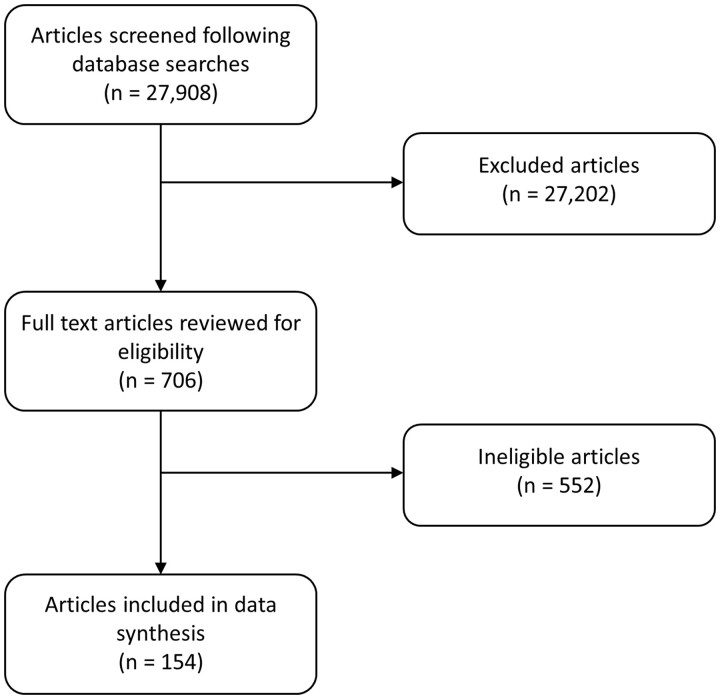
A flow diagram of study selection is shown in Fig. 1, displaying the initial number of articles screened (n = 27 908), the number of articles selected for full-length review (n = 706), and the number included in the final analysis from this updated search (n = 154). This information was then merged with the results of the previous guideline’s systematic review to give the total exposure data for each drug. The following data were extracted where possible for each medication: number of studies and study type; number of pregnancy exposures; number of live births; pregnancy duration; birth weight; maternal complications; miscarriages; number and type of congenital anomalies (where possible, congenital anomalies described in original publications were classified as major/minor according to European surveillance of congenital anomalies (EUROCAT) definitions [16]); breastmilk exposure; long-term follow-up; and paternal exposure. An overall summary of compatibility of each drug pre-conception, during pregnancy, with breastmilk exposure, and with paternal exposure is shown in Table 1. For each drug, maternal information is summarized in the text and in Tables 2 and 3, while paternal exposures and recommendations are described separately and shown in Table 4. The data synthesis strategy for Tables 2–4 is shown in Supplementary Data S3, available at Rheumatology online. Other relevant papers identified in our search that did not meet the inclusion criteria are discussed in the main text.
Figure 1.
Flow diagram of studies selected for inclusion
Table 1.
Summary of drug compatibility in pregnancy and breastmilk exposure
| Peri-conception | First trimester | Second/third trimester | Breastfeeding | Paternal exposure | |
|---|---|---|---|---|---|
| Corticosteroids | |||||
| Prednisolone | Yes | Yes | Yes | Yes | Yes |
| Antimalarials | |||||
| Hydroxychloroquine (≤400 mg/day) | Yes | Yes | Yes | Yes | Yes |
| Conventional synthetic DMARDs | |||||
| Methotrexate (≤25 mg/week) | Stop ≥1 month pre-conception | No | No | No | Yes |
| Sulfasalazine (with folic acid 5 mg/day in first trimester) | Yes | Yes | Yes | Yesa | Yesb |
| Leflunomide | No: Cholestyramine washout | No | No | No | Yes |
| Azathioprine | Yes | Yes | Yes | Yes | Yes |
| Ciclosporin | Yes | Yesc | Yesc | Yes | Yes |
| Tacrolimus | Yes | Yesc | Yesc | Yes | Yes |
| Cyclophosphamide | Exceptional circumstancesd | Exceptional circumstancesd | Exceptional circumstancesd | No | No |
| Mycophenolate mofetil | Stop ≥6 weeks pre-conception | No | No | No | Yes |
| Intravenous immunoglobulin | Yes | Yes | Yes | Yes | Yes |
| Anti-TNFα medications | |||||
| Infliximab | Yes | Yes | Yese | Yes | Yes |
| Etanercept | Yes | Yes | Yesf | Yes | Yes |
| Adalimumab | Yes | Yes | Yesg | Yes | Yes |
| Certolizumab | Yes | Yes | Yes | Yes | Yes |
| Golimumab | Yes | Yes | Yesg | Yes | Yes |
| Other biologic DMARDs | |||||
| Rituximab | Consider stopping at conceptionh | Severe disease if no alternativesh | Severe disease if no alternativesi | Yesj | Yesj |
| IL-6 inhibitors | Consider stopping at conceptionh | Severe disease if no alternativesh | Severe disease if no alternativesi | Yesj | Yesj |
| IL-1 inhibitors | Consider stopping at conceptionh | Severe disease if no alternativesh | Severe disease if no alternativesi | Yesj | Yesj |
| Abatacept | Consider stopping at conceptionh | Severe disease if no alternativesh | Severe disease if no alternativesi | Yesj | Yesj |
| Belimumab | Consider stopping at conceptionh | Severe disease if no alternativesh | Severe disease if no alternativesi | Yesj | Yesj |
| IL-17 inhibitors | Consider stopping at conceptionh | Severe disease if no alternativesh | Severe disease if no alternativesi | Yesj | Yesj |
| IL-12/23 inhibitors | Consider stopping at conceptionh | Severe disease if no alternativesh | Severe disease if no alternativesi | Yesj | Yesj |
| Targeted synthetic DMARDs | |||||
| JAK-inhibitors | Stop ≥2 weeks pre-conception | No | No | No | Yesj |
For further information and caveats, see relevant recommendations and main text in the executive summary and full guideline.
In the healthy, full-term infant only.
If conception is delayed by >12months, consider stopping sulfasalazine alongside investigation of other causes of infertility.
Suggested monitoring of maternal blood pressure, renal function, blood glucose and drug levels.
Only in cases of severe (life or organ-threatening) maternal disease.
If low risk of disease flare and stopped by 20 weeks, full-term infant can have a normal vaccination schedule.
If low risk of disease flare and stopped by 32 weeks, full-term infant can have a normal vaccination schedule.
If low risk of disease flare and stopped by 28 weeks, full-term infant can have a normal vaccination schedule.
May be considered to manage severe maternal disease if no other pregnancy-compatible drugs are suitable.
If used in third trimester, avoid live vaccinations in infant vaccination schedule until 6 months of age.
Limited evidence.
Table 2.
Summary of maternal exposure to conventional synthetic DMARDs, antimalarials and corticosteroids
| Drug | Studies (type and number) | Pregnancy exposures (exposures per trimester) | Foetal losses/total pregnancy outcomes | Pregnancy duration and birth weight | Malformations/total births | Recommendation (GRADE/Strength of agreement) |
|---|---|---|---|---|---|---|
| HCQ | 31 ct [17–25, 27, 28, 30–39, 44–53] |
|
95/936 | No significant adverse effect noted | 162/3126 Overall, no increase in rate of major malformations attributable to drug |
|
| 1 rct [29] | ||||||
| 1 nrt [26] | ||||||
| 2 cc [42, 43] | ||||||
| 3 cs [54–56] | ||||||
| 6 cr [57–62] | ||||||
| 2 sr [40, 41] | ||||||
| Pred/MP | 3 rct [84–86] |
|
70/518 | No significant adverse effect attributable to drug |
|
|
| 3 cc [43, 87, 88] | ||||||
| 22 ct [31, 46, 48, 51–53, 71, 73–81, 89–95] | ||||||
| 12 cs [55, 72, 96–105] | ||||||
| 16 cr [55, 57, 59–61, 96–117] | ||||||
| 1 Cochr [82] | ||||||
| 1 sr [83] | ||||||
| MTX | 2 cc [172, 181] | 766 | 80/479 | Insufficient data; only one study reported birthweight in a cohort of n = 23 [37], with two studies reporting pregnancy duration (n = 43) [37, 181] |
|
|
| 8 ct [37, 50, 52, 91, 179, 180, 182, 183] | (1st trimester ≥239, 2nd/3rd trimester ≥8) | |||||
| 1 cs [173] | ||||||
| 5 cr [174–178] | ||||||
| SSZ | 3 ct [46, 50, 52] | 178 | NR | No significant adverse effect noted |
|
|
| 1 cs [55] | (NR) | |||||
| 2 cr [62, 193] | ||||||
| LEF | 6 ct [50, 91, 194, 199–201] | 814 | 138/811 | No significant adverse effect noted |
|
|
| (1st ≥156, 2nd/3rd ≥24) | ||||||
| 4 cr [195–198] | ||||||
| AZA | 5 cc [88, 135, 172, 203, 204] | 1757 | 130/642 | No significant adverse effect noted |
|
|
| (1st ≥1254, 2nd/3rd ≥580) | ||||||
| 16 ct [31, 45, 50–52, 78, 90, 92, 93, 95, 205, 206, 212–215] | ||||||
| 6 cs [55, 99, 102, 173, 207, 208] | ||||||
| 2 cr [61, 107] | ||||||
| 1 sr [83] | ||||||
| CsA | 4 cc [43, 88, 135, 136] | 401 | 9/132 | Possible trend towards shorter pregnancy duration [92, 101, 136, 165] and low birth weight [88, 92, 165] |
|
|
| (1st ≥131, 2nd/3rd ≥136) | ||||||
| 8 ct [50, 51, 92, 93, 95, 182, 219, 220] | ||||||
| 3 cs [54, 101, 165] | ||||||
| TAC | 1 ct [92, 93, 219, 223, 225–231] | 515 | 108/451 | Insufficient data to confirm lack of a significant adverse effect |
|
|
| (1st ≥302, 2nd/3rd ≥135) | ||||||
| 1 cs [99] | ||||||
| 2 cr [107, 116] | ||||||
| CYC | 1 cs [102] | 20 | 2/16 | Insufficient data |
|
|
| 4 cr [106, 111, 167, 168] | (1st ≥6, 2nd/3rd ≥2) | |||||
| 1 ct [232] | ||||||
| MMF | 7 ct [92, 95, 215, 242–245] | 804 | 371/753 | Evidence of reduced pregnancy duration and birth weight |
|
|
| (1st ≥796, 2nd/3rd ≥320) | ||||||
| 3 cs [99, 208, 235] | ||||||
| 12 cr [57, 60, 113, 114, 116, 236–241] | ||||||
| IVIG | 1 cc [248] | 403 | 10/178 | No significant adverse effect noted |
|
|
| (1st ≥13, 2nd/3rd ≥77) | ||||||
| 12 ct [48, 49, 74, 79, 127, 128, 133, 249–253] | ||||||
| 1 Cochr [82] | ||||||
| 1 cs [97] | ||||||
| 3 cr [58, 110, 254] |
All studies that provided quantitative and/or qualitative information on the safety of the relevant drug in pregnancy were included; however, numerical outcome data could only be collated from papers where the relevant outcome was clearly quantified. Details of how numerical data in this table were derived are shown in Supplementary Data S3, available at Rheumatology online.
cc: case control; Cochr: Cochrane review; cr: case report; cs: case series; CsA: ciclosporin; ct: cohort; MP: methylprednisolone; NR: not reported; nrt: non-randomized trial; Pred: prednisolone; rct: randomised controlled trial; SOA: strength of agreement; sr: systematic review; TAC: tacrolimus.
Table 3.
Summary of maternal exposure to biological DMARDs and targeted synthetic DMARDs
| Drug | Studies (type and number) | Pregnancy exposures (exposures per trimester) | Foetal losses/total pregnancy outcomes | Pregnancy duration and birth weight | Malformations/total births | Recommendation (GRADE/Strength of agreement) |
|---|---|---|---|---|---|---|
| TNFi (combined data for all licenced drugs) | See individual drugs below, plus: |
|
886/4192 | No significant adverse effect noted overall |
|
|
| 28 ct [277, 278, 295–298, 301–313, 315–323] | ||||||
| 1 cs [279] | ||||||
| 4 cc [172, 299, 300, 314] | ||||||
| CZP | 2 ct [283, 284] |
|
52/567 | No significant adverse effect noted overall |
|
See recommendations above |
| 1 cs [285] | ||||||
| INF | 9 ct [50, 260–263, 291–294] |
|
255/2484 | No significant adverse effect noted overall |
|
See recommendations above |
| ETA | 5 ct [50, 52, 260, 286, 287] |
|
73/383 | No significant adverse effect noted overall |
|
See recommendations above |
| 3 cs [100, 266, 272] | ||||||
| 4 cr [108, 109, 273, 274] | ||||||
| 1 rct [288] | ||||||
| ADA | 7 ct [50, 52, 252, 261, 280–282] |
|
33/371 | No significant adverse effect noted overall |
|
See recommendations above |
| 5 cs [99, 266, 268–270] | ||||||
| 3 cr [271, 275, 276] | ||||||
| GOL | 2 ct [289, 290] |
|
34/166 | NR |
|
See recommendations above |
| RTX | 5 ct [50, 343, 344, 350, 354] | 316 | 68/293 | No significant adverse effect noted |
|
|
| 4 cs [345, 351–353] | (1st ≥13, 2nd/3rd ≥1) | |||||
| 4 cr [346–349] | ||||||
| TOC | 2 ct [358, 359] | 365 | 84/354 | No significant adverse effect attributable to drug (data limited by confounding) |
|
|
| 2 cs [356, 357] | (1st ≥46, 2nd/3rd ≥2) | |||||
| ANA | 2 ct [50, 371] | 48 | 3/43 | No significant adverse effect attributable to drug |
|
|
| 4 cs [367, 369, 370, 372] | (1st ≥25, 2nd/3rd ≥40) | |||||
| 1 cr [368] | ||||||
| CAN | 1 cs [369] | 8 (all 1st) |
1/8 | No significant adverse effect noted | 0/7 | See recommendations above |
| ABA | 1 cs [175] | 99 | 49/187 | No significant adverse effect attributable to drug (data limited by confounding) |
|
|
| 1 cr [349] | (1st ≥145, 2nd/3rd ≥10) | |||||
| 2 ct [375, 376] | ||||||
| BEL | 1 ct [380] | 66 (NR) |
18/66 | No significant adverse effect attributable to drug (data limited by confounding) |
|
|
| SEC | 2 ct [387, 388] | 244 (1st ≥161, 2nd/3rd NR) |
26/125 | No significant adverse effect noted |
|
|
| IXE | 1 ct [389] | 18 (NR) |
5/18 (spontaneous and induced) | No significant adverse effect noted |
|
See recommendations above |
| UST | 2 ct [391, 392] | 517 (1st ≥31, 2nd/3rd ≥10) |
92/517 | No significant adverse effect noted |
|
|
| 1 cs [393] | ||||||
| TOF | 1 ct [397] | 116 (all 1st, 2nd/3rd NR) |
15/72 | No significant adverse effect noted |
|
|
All studies that provided quantitative and/or qualitative information on the safety of the relevant drug in pregnancy were included; however, numerical outcome data could only be collated from papers where the relevant outcome was clearly quantified. Details of how numerical data in this table were derived are shown in Supplementary Data S3, available at Rheumatology online.
ABA: abatacept; ADA: adalimumab; ANA: anakinra; BEL: belimumab; CAN: canakinumab; cc: case control; cr: case report; cs: case series; ct: cohort; CZP: certolizumab; ETA: etanercept; GOL: golimumab; IL-1i: IL-1 inhibitors; IL-6i: IL-6 inhibitors; IL-17i: IL-17 inhibitors; INF: infliximab; IXE: ixekizumab; JAKi: Janus kinase inhibitor; NR: not reported; rct: randomized controlled trial; RTX: rituximab; SEC: secukinumab; SOA: strength of agreement; TNFi: TNF-alpha inhibitor; TOC: tocilizumab; TOF: tofacitinib; UST: ustekinumab.
Table 4.
Summary of pregnancy outcomes after paternal exposure
| Drug | Studies included (type and number) | Pregnancy exposures | Adverse pregnancy outcomes (foetal losses or malformations) | Recommendation (GRADE/Strength of agreement) |
|---|---|---|---|---|
| HCQ | 1 ct [52] | 13 | No increase | Paternal exposure to HCQ is compatible with pregnancy (GRADE 2C, SOA 99.3%) |
| 1 cs [404] | ||||
| CS | 5 ct [52, 405–407, 411] | 4507 | No increase | Paternal exposure to prednisolone is compatible with pregnancy (GRADE 1B, SOA 99.3%) |
| 2 cs [404, 408] | ||||
| SSZ | 3 ct [52, 407, 412] | 237 | No increase | Men who take SSZ may have reduced fertility. There is little evidence to suggest that SSZ should be stopped pre-conception, unless conception is delayed by >12 months when stopping SSZ should be considered along with other causes of infertility (GRADE 1C, SOA 99.0%) |
| 1 cc [409] | ||||
| LEF | 1 ct [52] | 2 | No increase | Paternal exposure to LEF is compatible with pregnancy (GRADE 2C, SOA 99.3%) |
| 1 cr [413] | ||||
| AZA | 9 ct [52, 185, 187, 216, 405–407, 414, 415] | 3282a | No increase | Paternal exposure to AZA is compatible with pregnancy (GRADE 1B, SOA 99.3%) |
| 1 cc [409] | ||||
| 2 cs [404, 408] | ||||
| MTX | 10 ct [52, 184–189, 405, 407] | 2289 | No increase | Paternal exposure to low-dose (≤25 mg/week) MTX is compatible with pregnancy (GRADE 1B, SOA 99.3%) |
| 3 cs [404, 410], 1 cr [428] | ||||
| CsA | 3 ct [185, 406, 416] | 501a | No increase | Paternal exposure to CsA is compatible with pregnancy (GRADE 1C, SOA 99.3%) |
| 2 cs [408, 410] | ||||
| TAC | 3 ct [406, 416, 417] | 41a | No increase | Paternal exposure to TAC is compatible with pregnancy (GRADE 2C, SOA 99.3%) |
| CYC | No data meeting inclusion criteria | Known to affect male fertility; evidence of an adverse impact on germ cell development and male-mediated teratogenicity from animal studies | Due to the adverse effect of CYC on male fertility, semen cryopreservation is recommended for men prior to paternal exposure (GRADE 1C, SOA 99.5%) | |
| MMF | 3 ct [185, 246, 247] | 292 | No increase | Paternal exposure to MMF is compatible with pregnancy (GRADE 2C, SOA 99.3%) |
| 3 cs [406, 416, 426] | ||||
| TNFi | 13 ct [52, 263, 293, 298, 306, 405, 412, 427, 430–434] | 751 | No increase | Paternal exposure to TNFi is compatible with pregnancy (GRADE 1C, SOA 99.3%) |
| 2 cs [404, 410] | ||||
| 2 cr [428, 429] | ||||
| 1 cc [409] | ||||
| RTX | 1 ct [343] | 11 | No increase | Paternal exposure to RTX is compatible with pregnancy (GRADE 2C, SOA 99.3%) |
| IL-6i | 1 ct [359] | 15 (TOC) | No increase | Paternal exposure to IL-6i is compatible with pregnancy (GRADE 2C, SOA 99.3%) |
| IL-1i | 1 ct [369] | 5 (ANA) | No increase | Paternal exposure to IL-1i is compatible with pregnancy (GRADE 2C, SOA 99.3%) |
| 6 (CAN) | ||||
| ABA | 1 ct [375] | 10 | No increase | Paternal exposure to ABA is compatible with pregnancy (GRADE 2C, SOA 99.3%) |
| IL-17i | 2 ct [387, 389] | 54 (SEC) | No increase | Paternal exposure to IL-17i is compatible with pregnancy (GRADE 2C, SOA 99.3%) |
| 34 (IXE) | ||||
| JAKi | 1 ct [398] | 87 (TOF) | No increase | Paternal exposure to JAKi is compatible with pregnancy (GRADE 2C, SOA 99.3%) |
All studies that provided quantitative and/or qualitative information on the safety of the relevant drug following paternal exposure were included. Details of how numerical data in this table were derived are shown in Supplementary Data S3, available at Rheumatology online.
Minimum number of pregnancy exposures to drug; additional exposures were described in some studies but could not be separated from grouped study data.
ABA: abatacept; ANA: anakinra; BEL: belimumab; CAN: canakinumab; cc: case control; cr: case report; cs: case series; CS: corticosteroids; CsA: ciclosporin; ct: cohort; IL-1i: IL-1 inhibitors; IL-6i: IL-6 inhibitors; IL-17i: IL-17 inhibitors; IXE: ixekizumab; JAKi: Janus kinase inhibitors; NR: not reported; RTX: rituximab; SEC: secukinumab; SOA: strength of agreement; TAC: tacrolimus; TNFi: TNF-alpha inhibitor; TOC: tocilizumab; TOF: tofacitinib; UST: ustekinumab.
Generic recommendations on prescribing immunomodulatory drugs and/or corticosteroids in rheumatic disease in pregnancy
Pre-conception counselling should be addressed by all healthcare professionals, with referral to professionals with relevant expertise as appropriate, to optimize disease control before pregnancy; with advice on the timing of pregnancy, and drug therapy before, during and after pregnancy, including contraception (GRADE 1A, SOA 99.5%).
If a woman is planning pregnancy, avoid pregnancy-incompatible drugs (GRADE 1A, SOA 100%).
The risks and benefits to the mother and foetus of drug treatment to control maternal disease should be discussed and clearly documented by all healthcare professionals involved in the patient’s care (GRADE 1A, SOA 99.5%).
Immunomodulatory drugs that are contraindicated in pregnancy should be switched to a pregnancy-compatible alternative in advance of conception to ensure maintenance of disease control on the new medication (GRADE 1A, SOA 100%).
When no pregnancy-compatible drugs are suitable, control of severe/life-threatening maternal disease should take priority over concerns for potential foetal outcomes (GRADE 1B, SOA 99.0%).
All biologic DMARDs may be continued throughout pregnancy if required to control active/severe maternal disease (GRADE 1B, SOA 98.5%).
Immunization schedules in infants after in-utero exposure to biologic DMARDs will depend on timing of exposure, bioavailability and persistence of the drug, mechanism of action of the drug, and live vaccines (GRADE 1C, SOA 99.5%).
Where possible, the minimum effective dose of immunomodulatory drug or corticosteroid should be used to maintain maternal disease suppression, and stopping the drug during pregnancy may be considered in women at low risk of disease flare on withdrawal of therapy (GRADE 1B, SOA 100%).
Some drugs may reduce male fertility, but paternal drug exposure in humans has not convincingly been associated with adverse foetal development or pregnancy outcome. Although the evidence is weak, men who take rheumatological medicines should be reassured about the safety of conceiving (GRADE 2C, SOA 98.4%).
Antimalarials
HCQ is the antimalarial drug most used to treat rheumatic disease and has been extensively studied in pregnancy. We identified an additional 23 studies [17–39] that, combined with the previous 23 studies [40–62], reported on (n = 4701) pregnancy exposures to HCQ, with very limited information on other antimalarials [17, 39, 63, 64]. Many of these studies were confounded by primarily reporting pregnancy outcomes in patients with SLE treated with other immunosuppressive agents, including MMF and corticosteroids, and use in anti-Ro/La positive patients in the prevention of congenital heart block (CHB). Despite these limitations, there were no appreciable adverse effects of HCQ on pregnancy duration or birth weight in the largest studies. In fact, several studies comparing HCQ-treated and untreated cohorts with rheumatic disease (mostly SLE) either found no significant difference between cohorts [17, 19, 21, 24, 28, 34, 36, 37], or significantly longer pregnancy durations and/or higher birth weight in the HCQ-treated pregnancies [18, 20, 22, 25, 27, 29, 30, 32, 33, 35]. The weighted mean for gestation across 15 studies reporting pregnancy duration in HCQ-exposed vs HCQ-unexposed pregnancies was 36.4 weeks and 34.7 weeks, respectively [18–21, 24, 27, 28, 30, 32–34, 36, 37, 43, 49]. The weighted mean for birth weight for HCQ-exposed vs HCQ-unexposed pregnancies was 2847 and 2733 g, respectively, in 10 studies reporting these outcomes [18, 20, 21, 27, 29, 33, 34, 36, 37, 43]. A total of 60 first trimester miscarriages were reported from 524 HCQ-exposed pregnancies (11.5%) in 10 studies, compared with 117 first trimester miscarriages in 718 HCQ-unexposed pregnancies (16.3%) [18–22, 27, 28, 30, 32, 33]. No specific pattern of congenital malformations was observed in association with HCQ exposure. No increased risk of adverse foetal outcomes was reported in >3229 chloroquine-exposed pregnancies in four studies [17, 39, 63, 64], including two studies where chloroquine was used as malaria prophylaxis during pregnancy; although, in these two studies, higher rates of maternal adverse events were reported, relative to the comparator (sulfadoxine-pyrimethamine). No information was found on mepacrine.
The findings for HCQ were consistent across all studies apart from a large population-based cohort study comparing HCQ-exposed (n = 2045) and HCQ-unexposed (n = 21 679) pregnancies in patients with rheumatic disease, which did not control fully for disease, comorbidity-related pregnancy risk factors, dose of corticosteroids and combination with specific immunosuppressive drugs [23]. This study found a small increase in the risk of congenital malformations associated with first trimester HCQ use, mainly oral clefts, respiratory anomalies and urinary defects, with wide confidence intervals for specific malformations. A statistically significant increase in risk, however, was only found with daily doses of ≥400 mg of HCQ. This study concluded that for most patients with autoimmune rheumatic disorders, the benefits of treatment during pregnancy will likely outweigh this risk.
Importantly, a more recent study (published after our search date) of pregnant women prospectively enrolled into MotherToBaby/Organisation of Teratology Information Specialists (OTIS) pregnancy studies, compared outcomes for HCQ-exposed pregnancies (n = 279) with disease-matched (n = 279) and healthy comparator (n = 279) HCQ-unexposed groups [65]. Reassuringly, this study found no evidence of an increased risk for structural defects or other adverse outcomes with HCQ at any dose (average 325 mg/day; range 100–800 mg/day), except for an isolated finding of reduced head circumference at birth with HCQ exposure, which was not thought to be of any clinical significance.
Therefore, advice on HCQ dosage in pregnancy relates to general guidance for reducing ophthalmic risk outside of pregnancy to a maximum of 400 mg/day, as pharmacokinetic changes in pregnancy reduce the reliability of weight-based dosing [66]. Ultimately, it is important to maintain HCQ during pregnancy, as discontinuation of this drug in pregnancy may increase risk of disease flares and foetal loss [67]. Disease flares would increase the need for alternative medications with more potential risks for mother or baby in pregnancy.
Previous studies of breastmilk exposure to HCQ were mostly limited to case reports, showing that <1% of the maternal dose of HCQ was found in breastmilk [68]. Three more recent studies of HCQ use (n = 195) confirmed very low concentrations of HCQ in breastmilk and no adverse effects on breastfed infants [36, 69, 70]. There remain limited studies of long-term outcomes in children, but no adverse immunological or clinical findings have been reported [36, 43].
Recommendations for hydroxychloroquine in pregnancy and breastmilk exposure
HCQ remains the antimalarial of choice in women planning a pregnancy with rheumatic disease in need of treatment, and should be continued during pregnancy at a dose of ≤400 mg/day (GRADE 1B, SOA 100%).
HCQ is compatible with breastmilk exposure (GRADE 1B, SOA 99.5%).
Corticosteroids
Corticosteroids used to treat rheumatic disease (prednisolone, prednisone and methylprednisolone) are metabolized in the placenta, and so 10% or less of the active drug reaches the foetus. Previously, we identified 47 studies on prednisolone and found it to be compatible with pregnancy and breastmilk exposure [1]. Studies of corticosteroid use in pregnancy were confounded by multiple concomitant medications and use in high-risk pregnancies; particularly the fluorinated steroids, which are used to prevent or treat preterm labour and complications such as foetal lung immaturity. Therefore, we searched for further evidence on corticosteroids used to treat rheumatic disease and identified additional studies: 11 on prednisolone with (n = 1218) pregnancies [31, 71–80] and one on methylprednisolone with (n = 12) pregnancies [81]. This evidence was combined with the previous studies: 47 on prednisolone (n = 1503) [43, 46, 48, 51–53, 55, 57, 59–61, 82–117]; 31 on dexamethasone (n = 11 214) [48, 54, 88, 97, 118–144]; 27 on betamethasone (n = 27 746) [118–120, 125, 126, 128, 130, 131, 140, 143, 145–162]; and 10 on general corticosteroid use (n = 785) [42, 49, 50, 54, 163–168].
Studies on the use of methylprednisolone in pregnancy were not specifically sought in the previous guideline because it is generally used as rescue therapy for severe disease. Compared with prednisolone, parenteral administration of methylprednisolone has a prolonged duration of action with similar rates of placental transfer to prednisolone [169].
Previously, we found that following prednisolone (or unspecified corticosteroid) exposure, average pregnancy duration in the majority of randomized controlled trial (RCT), case-control, cohort and case-series studies (where reported) was usually term, at ≥37 weeks [43, 51, 84, 85, 88, 92, 94, 96, 101, 102, 104, 105]. Other studies reporting ≤37-week delivery were confounded by factors such as maternal disease and concomitant medications [46, 57, 59, 60, 86, 87, 99, 106, 113, 117, 163, 165]. Birth weights followed a similar pattern and were affected by preterm deliveries and confounding factors, as described above. For instance, prednisolone exposure in those RCTs, cohorts, case-control studies and case series which reported average gestations of ≥37 weeks, average birth weights ranged from 2.6–3.4 kg [43, 46, 51, 85, 88, 92, 94, 96, 101, 104, 105]. Overall, prednisolone itself was not felt to have contributed to low birth weight (LBW) in any study [1].
High rates of maternal complications compatible with underlying disease were previously reported for prednisolone and dexamethasone, but none were specifically attributed to these medications [1]. The major congenital malformations observed with prednisolone were frequently confounded by concomitant teratogenic drug exposure, such as MMF [116], and the overall incidence was not significantly higher than in drug-free controls. Studies reporting major malformations with fluorinated steroid exposure [e.g. patent ductus arteriosus (PDA), blindness and deafness [126, 145]] did not attribute them to steroid therapy. Furthermore, in the majority of cases, the steroids were used for treatment of underlying conditions such as preterm delivery [126], where steroids were found to be beneficial in improving outcomes, or treatment of maternal autoantibody-mediated cardiomyopathy [133]. A large study analysing 832 636 live births did not show an increased risk of orofacial cleft palate with the use of corticosteroids in pregnancy [164]. foetal loss in studies of prednisolone and fluorinated steroids was attributed to underlying disease rather than steroid therapy, such as in APS [105] and complete atrio-ventricular block [170].
Most (8/11) of the additional studies on maternal prednisolone exposure that we found in our updated search did not identify any adverse effects of prednisolone use on pregnancy outcomes [31, 72–74, 76–79]. In contrast, a population-based study from Norway exploring the associations between disease activity and medications with offspring birth weight, pre-eclampsia and preterm birth in SLE found prednisolone use to be significantly associated with lower birth weight, increased risk of pre-eclampsia, and a 3-fold increase in preterm birth [71]. A conference abstract reported that continuation of high-dose glucocorticoids during 164 pregnancies increased the risks of preterm birth, low birth weight and preterm premature rupture of membranes (PPROM) at prednisolone cut-off doses of 7.5 mg, 6.7 mg, 5.0 mg per day, respectively [75]. In contrast, another conference abstract of 143 SLE pregnancies found that foetal complications were associated with prednisone >25 mg, and that low (10 mg/day) to moderate (10–24 mg/day) doses of prednisone during pregnancy were not associated with adverse foetal outcomes [77]. Similarly, the largest prospective study of SLE pregnancy outcomes did not identify prednisolone ≤20 mg/day as a risk factor for adverse pregnancy outcomes [31].
UKTIS notes that many of the studies reporting pregnancy outcomes following gestational exposure to systemic corticosteroids are limited by a lack of stratification to account for differing doses, treatment duration and steroid potencies, as well as confounding by maternal disease [171]. It concludes that preterm delivery may be associated with gestational exposure to systemic corticosteroids, and further well-controlled studies are required to address this question. Therefore, an increased risk of adverse foetal effects following use of high-dose/potency corticosteroids, or use for extended periods, cannot be ruled out.
Based on limited evidence, prednisone, prednisolone and methylprednisolone are considered compatible with breastmilk exposure [1]. There remain few breastmilk exposure studies. One study, comprising 19 pregnancy and breastmilk exposures, found that prednisone and prednisolone exhibit dose- and concentration-dependent pharmacokinetics during pregnancy, and infant exposure to these agents via breastmilk is minimal [76]. Another study of 12 patients with multiple sclerosis found the transfer of methylprednisolone into breastmilk to be low even when maternal serum concentration levels were highest at the end of an infusion, and although these levels were not considered to pose a threat to the infant, they state that mothers may choose to wait two to four h to further limit an infant’s exposure [81].
Previously, long-term follow-up studies had not reported any adverse events after prednisolone exposure in pregnancy [1]. Two additional studies did not report any adverse events from 9–12 months of post-partum follow-up of 227 non-rheumatic disease pregnancies exposed to prednisolone [78, 79].
Recommendations for corticosteroids in pregnancy and breastmilk exposure
Prednisolone is compatible with pregnancy and is the preferred corticosteroid in the treatment of maternal rheumatological disease in pregnancy and requires shared care with obstetric teams to monitor maternal blood pressure and blood glucose (GRADE 1B, SOA 100%).
Where possible, the dose of prednisolone should be <20 mg/day and tapered to the minimum effective dose to control maternal disease, in conjunction with steroid-sparing drugs compatible with pregnancy (GRADE 1C, SOA 99.5%).
Prednisolone is compatible with breastmilk exposure (GRADE 1B, SOA 100%).
Methylprednisolone has similar rates of placental transfer to prednisolone and would therefore be expected to be compatible with pregnancy and breastmilk exposure (GRADE 2C, SOA 99%).
Conventional synthetic DMARDs and immunosuppressive therapies
Methotrexate
MTX is contraindicated in pregnancy and was previously recommended to be stopped at least three months in advance of conception [68]. UKTIS considers MTX risk in pregnancy to be dependent on its use at high (>25 mg per week) or low (≤25 mg per week) dosages [171]. Rheumatology usage of MTX to treat inflammatory arthritis falls into the low-dose category and is far removed from the high doses used as a chemotherapeutic agent in the treatment of various cancers (e.g. >500 mg/m2) or as an abortifacient at 50 mg/m2. UKTIS concludes that exposure to high-dose MTX in early pregnancy confers a risk of severe embryopathy (including craniofacial defects, malformations of the digits and defects of the spine and ribs) in the foetus, and the option of termination of pregnancy should be discussed with the patient. In contrast, for exposure to lower doses of MTX prior to conception, additional foetal monitoring is advised, as well as counselling of women and their partners about the lack of available data to facilitate quantification of risk of adverse pregnancy outcomes.
Previously, we identified a high proportion of major anomalies following MTX (and other DMARD) exposure, predominantly during the first trimester of pregnancy, in 27 pregnancies from 10 studies [50, 52, 91, 172–178]. An additional 12 studies of MTX exposure in 2765 pregnancies were identified: six maternal studies [37, 179–183] and six paternal studies [184–189].
Several studies reported on the risks of pre-conceptual and pregnancy exposure to MTX. Data from the National Birth Defects Prevention Study, a US case-control study of major birth defects, reported that 4/10 113 (0.04%) mothers of foetus/infants without major birth defects (controls) had been exposed to MTX, compared with 16/27 623 (0.06%) mothers of live-born infants with a major birth defect (cases) who had been exposed to MTX [181]. The dose of MTX was not reported, but indications included a neoplasm of endocrine glands and so was presumably of high dose in at least one case. Of the 16 cases with major birth defects, 15 were exposed from three months pre-conception to the end of the first trimester.
A cohort study of 240 SLE pregnancies in whom 36.8% were exposed to MTX before and during the first trimester reported an increased risk of foetal complications [182]. A large prospective observational multicentre cohort study of 324 pregnancies exposed to MTX found an increase in the cumulative incidence of spontaneous miscarriage (42.5%) and major congenital anomalies (6.6%) among pregnancies (n = 188) exposed to a median dose of 10 mg/week of MTX after a median of 4.3 weeks post-conception [179]. This difference reached statistical significance when compared with a cohort of women without autoimmune diseases, but not when compared with a disease-matched cohort. No increased risk of miscarriage or major congenital anomaly was found in pregnancies (n = 136) exposed to a median dose of 15 mg/week of MTX that was stopped three months pre-conception.
Not all studies reported increased risks with MTX exposure. A study of pre-conception use of MTX on miscarriage rates in 114 RA pregnancies, compared with 48 MTX-unexposed RA pregnancies, did not find a statistically significant association between miscarriage and MTX use [180]. An analysis of 18 pregnancies exposed to MTX (≤20 mg/week) from up to one year pre-conception and in the first trimester found a high percentage of live-born children with no malformations [183]. Analysis of 23 first trimester exposures to low-dose MTX, identified from three United States health plan databases, did not reveal a significant increase in the risk of congenital malformations, foetal death or neonatal complications in women with chronic autoimmune disease, compared with those who received MTX before, but not during, pregnancy [37].
These studies provide some evidence that a 3-month MTX-free interval prior to conception might not be required. Therefore, unintentional exposure to low-dose MTX during the peri-conceptional period confers minimal risk in unintended pregnancy exposures, and so termination of pregnancy is not routinely recommended for MTX exposure unless it is maternally requested due to unplanned pregnancy [37, 180, 183].
Studies of MTX in breastfeeding remain very limited. Although they did not meet our inclusion criteria, we identified two case reports that found low levels of MTX in breastmilk and no adverse effects on the breastfed infants [190, 191]. LactMed describes low levels of MTX in breastmilk and conflicting expert opinion on whether it can safely be used during breastfeeding [192]. It states that withholding breastfeeding for 24 h after a weekly low-dose of MTX may decrease the infant's dose by 40%, and that if breastfeeding is undertaken during long-term, low-dose MTX use, monitoring of the infant's complete blood count and differential could be considered.
Post-partum follow-up of up to 14 months after first trimester MTX exposure was reported in three infants with long-term complications of foetal MTX syndrome, including semi lobar holoprosencephaly, cardiac abnormalities, tracheostomy and requirement for antiepileptic therapy [176, 177].
Recommendations for methotrexate in pregnancy and breastmilk exposure
MTX at any dose should be avoided in pregnancy and stopped at least one month in advance of planned conception, when it should be switched to another pregnancy-compatible drug to ensure maintenance of maternal disease suppression (GRADE 1A, SOA 98%).
In women treated with low-dose (≤25 mg/week) MTX within one month prior to conception, folic acid supplementation (5 mg/day) should be continued up to 12 weeks of pregnancy (GRADE 1B, SOA 99.5%).
In unintended pregnancy on low-dose (≤25 mg/week) MTX, there is minimal risk to the foetus; the drug should be stopped immediately, folic acid supplementation (5 mg/day) continued, and a careful evaluation of foetal risk with early referral to a foetal medicine department considered (GRADE 1C, SOA 100%).
Although only minute amounts of MTX are excreted into breastmilk, MTX cannot be recommended in breastfeeding because of theoretical risks and insufficient data on outcomes (GRADE 2C, SOA 99%).
Sulfasalazine
Previously, we recommended that SSZ is compatible with pregnancy and breastmilk exposure and can be continued with adequate folic acid supplementation (5 mg/day) [1]. This recommendation was based on six publications reporting SSZ exposure in 178 pregnancies in patients with RA, osteoporosis and ankylosing spondylitis (AS) [46, 50, 52, 55, 62, 193]. These studies contained limited information relating to miscarriage rate, pregnancy duration, birth weight or malformation rate; overall, however, there were no significant adverse effects highlighted that were considered to be directly attributable to SSZ. We did not identify any additional studies on the use of SSZ in pregnancy, breastmilk exposure or paternal exposure.
UKTIS does not identify any specific risks with SSZ exposure. It comments that although high-dose folic acid (5 mg/day) is generally recommended, no studies have investigated whether there is increased benefit of this higher dose of folic acid compared with a standard dose of 400 micrograms/day.
Minimal amounts of SSZ are expressed in breastmilk, and it can be used during breastfeeding if the infant is full term and healthy, although it should be avoided in ill, stressed or premature infants, and in infants with hyperbilirubinaemia or glucose-6-phosphate dehydrogenase deficiency [68].
Recommendations for sulfasalazine in pregnancy and breastmilk exposure
SSZ is compatible throughout pregnancy, with folic acid 5 mg/day recommended in the periconception period and during the first trimester (GRADE 1B, SOA 100%).
SSZ is compatible with breastmilk exposure in healthy, full-term infants (GRADE 1C, SOA 99.5%).
Leflunomide
Based upon limited evidence, we previously found that LEF may not be a human teratogen, but there was insufficient evidence to support its compatibility in human pregnancy, so our recommendation was that LEF is not the DMARD of choice in women planning pregnancy [1]. This recommendation was based on data from seven studies [50, 91, 194–198] reporting on 111 pregnancies exposed to LEF (discontinued in almost all cases in the first trimester, and frequently followed by a cholestyramine washout). Overall, the findings were largely reassuring, with no direct evidence of human teratogenicity.
We identified three additional studies of 703 pregnancies exposed to LEF at various stages of pregnancy, with varying exposure to washout and/or plasma testing of LEF metabolites, which did not find an increased risk of adverse pregnancy outcomes compared with the general population [199–201]. Although it was not included within our systematic review, pregnancy outcomes have been reported for teriflunomide—the principal active metabolite responsible for leflunomide’s activity in vivo—which, at recommended doses, results in a similar range of plasma concentrations to leflunomide [202]. The known outcomes from 222 pregnancy exposures to teriflunomide for relapsing forms of multiple sclerosis also found outcomes consistent with the general population [202]. Overall, these findings do not indicate a teratogenic risk of LEF in human pregnancies. The practicality of previous recommendations regarding the testing of plasma levels of teriflunomide has been questioned [199], and testing is not currently routinely available in the UK.
We did not identify any data on breastmilk exposure to LEF, and no information is available in LactMed [192].
Recommendations for leflunomide in pregnancy and breastmilk exposure
LEF may not be a human teratogen but there remains insufficient evidence to support use at the time of conception or during pregnancy (GRADE 1B, SOA 98%).
Women on LEF considering pregnancy should stop and undergo a standard cholestyramine washout procedure, and switch to alternative medication compatible with pregnancy (GRADE 1B, SOA 98.8%).
If unintended conception occurs on LEF, the drug should be stopped immediately and a standard cholestyramine washout procedure given, with early referral to a foetal medicine department considered (GRADE 1B, SOA 99%).
LEF is not recommended while breastfeeding (GRADE 1C, SOA 99.5%).
Azathioprine
Previously, we recommended that AZA is compatible with pregnancy at doses ≤2 mg/kg, with breastmilk exposure and with paternal exposure [1]. These recommendations were based on 28 studies [45, 50–52, 55, 61, 83, 88, 90, 92, 93, 95, 99, 101, 102, 107, 135, 172, 173, 203–211] in 738 AZA-exposed pregnancies, which included a wide range of diagnoses and concomitant medications, compared with 1121 disease-matched and 667 healthy controls. These data did not demonstrate an increased risk of miscarriage, preterm birth, low birth weight or congenital malformation due to AZA exposure in pregnancy.
We identified an additional nine studies of 3699 pregnancy exposures to AZA: six maternal studies [31, 78, 212–215] and three paternal studies [185, 187, 216]. Overall, the findings from maternal exposures (n = 1019) to AZA did not identify any adverse pregnancy outcomes. One study, reporting on AZA metabolism in 30 IBD pregnancies, measured active metabolites and found only 6-thioguanine nucleotide (6-TGN) but not 6-mercaptopurine (6-MP) in umbilical cord blood at delivery; no major teratogenicity was observed, although 60% of the infants had anaemia, which was suspected to be due to maternal thiopurine use [214]. Two of these studies extended follow-up to 3 months and nearly 10 years, without any adverse effects being reported. The majority of studies did not specify the mean/median dose of AZA utilized in the study populations, and there is no clear evidence regarding a dose limit. Use of AZA at an effective dose should be supported by monitoring of blood tests, following local guidelines.
Based on our previous evidence from 26 infants breastfed by mothers on AZA or 6-MP, minimal amounts of AZA were detected in breastmilk, and no adverse effects were identified [101, 209–211]. We did not identify any new studies of breastmilk exposure to AZA. LactMed states that avoiding breastfeeding for 4 h after maternal ingestion of AZA should markedly reduce the dose received by the infant in breastmilk [192]. In routine clinical practice, there is no concern in the management of solid organ transplant patients who breastfeed on this drug [217].
Recommendations for azathioprine in pregnancy and breastmilk exposure
AZA is compatible throughout pregnancy (GRADE 1B, SOA 100%).
AZA is compatible with breastmilk exposure (GRADE 2C, SOA 99.5%).
Ciclosporin
An earlier consensus document reviewed evidence from >800 human pregnancies exposed to ciclosporin (CsA) [68]. Our previous search from 2005 onwards identified a further 13 studies/reports [43, 50, 51, 54, 88, 92, 93, 95, 101, 135, 136, 165, 218] of 98 pregnancies in patients with a variety of diseases and multiple concomitant medications who had been exposed to CsA at 2–6 mg/kg during pregnancy. Reports of increased rates of preterm delivery and low birth weight were confounded by maternal disease and concomitant medications, and there was no evidence of an increased malformation risk [1]. Comorbidities, such as hypertension, pre-eclampsia and gestational diabetes mellitus, were reported at higher incidences than the general population. Based upon this evidence, CsA was considered compatible with pregnancy at the lowest effective dose, with monitoring of blood pressure, blood glucose and renal function [68]. UKTIS draws a similar conclusion [171].
We identified an additional five studies of 550 pregnancy exposures [182, 185, 219–221]. Three studies reported on maternal exposure [182, 219, 220]. A cohort study of 240 SLE pregnancies, in whom 50% were exposed to CsA before and during the first trimester, increased the risk of pancytopenia and/or pre-eclampsia in maternal outcomes [182]. A single-centre experience of outcomes of pregnancy (n = 117) following liver transplantation did not find any difference between those on CsA (n = 34) compared with tacrolimus (n = 81), and so did not attribute these outcomes to medication [219]. A study of the efficacy and safety of CsA in 29 pregnancies of patients with systemic autoimmune diseases did not find an increased risk of maternal–foetal complications, and stated that it should be continued in patients who benefit from therapy [220].
Previously identified studies described small amounts of CsA in breastmilk and almost universally undetectable blood levels in infants [68, 218], without any adverse effects reported during breastmilk exposure. We found a further study that reported low transfer of CsA and its metabolites into the breastmilk of seven post-transplant mothers in the first two days post-partum, although this study was not designed to make a corresponding assessment of drug safety [221]. LactMed recommends that breastfed infants should be monitored if CsA is used during lactation, possibly with measurement of serum levels if there is a concern for toxicity [192]. In routine clinical practice, there is no concern in the management of solid organ transplant patients who breastfeed on this drug [217].
No additional studies of long-term follow-up were identified to those found previously on 10 infants exposed to CsA in utero, which reported no complications at 11–14 months [43, 54, 135].
Recommendations for ciclosporin in pregnancy and breastmilk exposure
CsA is compatible throughout pregnancy with monitoring of maternal blood pressure, renal function, blood glucose and drug levels (GRADE 1B, SOA 100%).
CsA is compatible with breastmilk exposure (GRADE 2C, SOA 99.7%).
Tacrolimus
Based upon previous consensus [68, 222] and our previous review of six studies [92, 93, 99, 107, 116, 223] of 26 pregnancies exposed to tacrolimus and two breastmilk exposure studies [223, 224], tacrolimus was considered compatible with pregnancy and breastmilk exposure. There were complex confounding issues in many of these studies and, overall, no adverse outcomes were considered to be directly attributable to tacrolimus [1].
We found additional evidence from eight studies of 489 pregnancy exposures to tacrolimus [219, 225–231]. Studies of maternal outcomes, mostly from solid organ transplant recipients, reported varying incidences of adverse maternal-foetal outcomes, but these outcomes were confounded by transplant-associated comorbidities and concomitant immunosuppression, particularly MMF [219, 225, 227–231].
UKTIS concludes that the available data do not suggest an association between spontaneous miscarriage, congenital malformation or intrauterine death and exposure to tacrolimus during pregnancy, but data are limited and potentially confounded; therefore, an increased risk of these outcomes cannot be excluded [171].
Previously, we found studies reporting low levels of tacrolimus in umbilical cord blood and breastmilk in small numbers of breastfed infants without any adverse effects [223, 224]. These findings were confirmed in an additional study of 13 breastfed infants of mothers with SLE [225]. This study found concentrations of tacrolimus in the umbilical cord blood were lower than those in the maternal blood; the relative infant dose in breastfed infants of tacrolimus was <1%, and the level of tacrolimus in infant blood was below detectable limits. LactMed suggests that exclusively breastfed infants should be monitored [192]. In routine clinical practice, there is no concern in the management of solid organ transplant patients who breastfeed on this drug [217].
Recommendations for tacrolimus in pregnancy and breastmilk exposure
Tacrolimus is compatible throughout pregnancy with monitoring of maternal blood pressure, renal function, blood glucose and drug levels (GRADE 2B, SOA 100%).
Tacrolimus is compatible with breastmilk exposure (GRADE 2C, SOA 99.8%).
Cyclophosphamide
CYC is a known human teratogen and is gonadotoxic in men and women [68]. Our previous findings from reports of predominantly first trimester use of CYC in nine pregnancies, revealed multiple adverse outcomes in mothers with severe maternal disease and multiple concomitant medications [102, 106, 111, 167, 168]. No maternal complications of CYC were reported. The nine pregnancies ended in two first trimester miscarriages, six healthy infants and one major congenital anomaly (Klippel–Feil syndrome). Follow-up to 87–90 months in four live births reported normal development in three children [102] and the single case of Klippel–Feil syndrome [111].
We found an additional study of pregnancies (n = 11) in women with multiple sclerosis who had been exposed to CYC prior to conception [232]; 10 women had a successful delivery [five preterm delivery and one small for gestational age (SGA)], while one underwent elective termination. It should be noted, however, that the time between the last dose of CYC and conception in this study was an average of 3.7 ± 1.5 years (range 0.33–5.9 years).
Although it did not meet our inclusion criteria, one case report analysed breastmilk levels of CYC in a women with multiple sclerosis [233]. CYC levels in breastmilk samples were measured after IV CYC at a dose of 2.8 g, with relatively low levels identified in the milk. The authors reported an average relative infant dose for a period of four days that varied from 4.7% at day 1 to 0.9% at day 4.
Recommendations for cyclophosphamide in pregnancy and breastmilk exposure
CYC is a known teratogen and gonadotoxic, and therefore should only be considered in pregnancy in cases of severe life/organ-threatening maternal disease when there is appreciable risk of maternal and foetal morbidity and mortality without this therapy (GRADE 1B, SOA 99.5%).
CYC is not recommended while breastfeeding (GRADE 2C, SOA 100%).
Mycophenolate mofetil
MMF is a known teratogen and is recommended to be stopped at least 6 weeks before a planned pregnancy [68, 222]. It is rapidly absorbed following oral administration and hydrolysed to form the active ingredient, mycophenolic acid (MPA). This active metabolite has a mean apparent half-life of 17 h after a 1 g oral dose of MMF, and undergoes enterohepatic circulation, with a secondary plasma peak at 6–12 h after an oral or intravenous dose [234].
We previously reviewed data from 16 studies/reports [57, 60, 92, 95, 99, 113, 114, 116, 208, 235–241] of 90 pregnancies exposed to MMF, mostly from renal transplant patients in whom there was concomitant exposure to prednisolone and tacrolimus. Increased rates of premature delivery, low birth weight and major congenital malformations were reported, including malformations typical for the previously described MMF embryopathy (including cleft lip and/or palate, microtia with aural atresia, micrognathia and ocular anomalies) [1].
Our updated search found eight further studies of 934 pregnancy exposures to MMF: five maternal exposure studies [215, 242–245] and three paternal exposure studies [185, 246, 247]. The five studies of maternal exposure in pregnancies (n = 714) all reported increased risks of miscarriage and birth defects, with 351 foetal losses, eight stillbirths and 38 cases of congenital malformation [215, 242–245]. These studies were mostly of first trimester exposure, with three including second and third trimester exposures. One study, however, found that following discontinuation of MMF within 6 weeks of conception, outcomes including the rates of birth defects and miscarriages were similar to pregnancies not exposed to MMF [245].
UKTIS describes the increased risks of first trimester pregnancy loss, as well as major congenital anomalies, and states that women of childbearing potential who are prescribed MMF or MPA should be informed of the associated risks to the foetus and, therefore, the importance of adequate contraception. It notes that European Medicines Agency guidelines for male and female patients, published in October 2015 following a periodic safety update review, recommend additional measures to prevent foetal exposure to MMF and should be read prior to prescribing MMF [171].
As in our previous search, we did not identify any data on breastmilk exposure. Similarly, LactMed reports that no information is available on the excretion of MMF into breastmilk, and that a few infants have reportedly been breastfed during MMF therapy with no adverse effects reported [192].
We did not identify any additional long-term follow-up data to that previously found of one case of ‘small for age’ with otherwise normal development [57], and another study reporting on 3/6 exposed children (one with normal development, one who required hearing aids, and one who had motor and speech delay) [99].
Recommendations for mycophenolate mofetil in pregnancy and breastmilk exposure
MMF remains contraindicated during pregnancy, and should be avoided in women planning pregnancy or switched to a pregnancy-compatible alternative at least 6 weeks before attempting to conceive (GRADE 1B, SOA 100%).
In cases of unintended conception, switch MMF to a pregnancy-compatible alternative and refer to local experts for further advice and risk assessment (GRADE 1B, SOA 100%).
MMF is not recommended while breastfeeding (GRADE 2C, SOA 99.7%).
Intravenous immunoglobulin
IVIG is considered to be compatible with pregnancy and breastmilk exposure [68, 222]. Our previous review of data found 16 studies/reports [48, 49, 58, 82, 97, 110, 127, 128, 133, 248–254] of 336 pregnancies in which IVIG was used, mostly in APS or in the prevention of CHB in anti-Ro/La positive mothers. The studies identified were focused on therapeutic efficacy rather than the safety of IVIG; hence, all outcomes were confounded by use in patients with high-risk pregnancies and multiple concomitant medications. Overall, the number and type of maternal and foetal complications observed were compatible with known effects of the underlying maternal disease on pregnancy, rather than being specific to IVIG. The studies reviewed did not raise any new concerns to question the accepted safety of IVIG in pregnancy [1].
We identified two further studies of 67 exposures to IVIG to treat immune thrombocytopenia (ITP) in pregnancy [74, 79]. One study found that glucocorticoids increased the risk of maternal hypertension, while the addition of IVIG to corticosteroid regimes did not adversely affect pregnancy outcomes [79]. The other study found comparable benefits of IVIG compared with corticosteroids in treating ITP in pregnancy, compared with no treatment, and similar neonatal outcomes between the treatment groups [74]. UKTIS does not report on IVIG.
None of the studies we previously or recently identified addressed the use of IVIG in breastmilk exposure or with paternal exposure. LactMed states that immunoglobulin is a normal component of breastmilk, and data from two mothers indicate that IgG concentrations in milk are normal or higher, and IgM levels in milk are normal or lower, during IVIG therapy [192].
Recommendations for intravenous immunoglobulin in pregnancy and breastmilk exposure
IVIG is compatible with pregnancy (GRADE 1B, SOA 99.5%).
IVIG is compatible with breastmilk exposure (GRADE 2C, SOA 100%).
Biologic DMARDs
Biological therapies are commonly used as second-line agents to treat various forms of IRDs. They are recombinant proteins, most commonly monoclonal IgG1 antibodies directed against specific targets, or fusion proteins containing the Fc portion of IgG1 joined to receptor-blocking proteins. The presence of the Fc region of IgG1 in most of these biologic drugs is required for their active placental transfer, which accelerates by active transport from the second trimester onwards. Biologic drugs are often given alongside other DMARDs, and decisions regarding continuation of treatment should be taken for each drug independently. Although the evidence base for biosimilar use in pregnancy and breastmilk exposure is more limited than for originator biologics, they would be expected to have comparable effects. Therefore, for pragmatic reasons, our recommendations are applicable to equivalent licensed biosimilars.
Five studies were identified that assessed the impact of biologic drugs as a whole on pregnancy outcomes [255–259]. These studies included a total of 379 pregnancies in women with autoimmune disease on predominantly anti-TNFα drugs, but also rituximab (RTX), abatacept (ABA), tocilizumab (TCZ), ustekinumab (UST) and anakinra. Separate birth outcomes for specific medications were not reported. Overall, the authors of these studies found no increased risk of miscarriage, stillbirth or congenital anomalies in biologic-exposed patients. One study that reported outcomes for 120 pregnancies in women with autoimmune diseases (predominantly RA and IBD) found a slightly increased risk of prematurity and a trend towards low birth weight in the biologic-exposed group compared with those not exposed [257]. However, once statistical modelling had been performed to correct for confounding by indication and proxies of unmeasured confounders, no association was found between biologic exposure and birth weight or gestational age. In addition, the same authors found that biologic use was not associated with an increased risk of serious infections in mothers, during post-partum, or in infants during the first year of life [256].
Anti-TNFα drugs
Five biologic agents that inhibit TNFα (TNFi) are currently licensed to treat IRDs: etanercept (ETA), infliximab (INF), adalimumab (ADA), golimumab (GOL) and certolizumab pegol (CZP). Three of these drugs (INF, ADA and GOL) are monoclonal IgG1 directed against TNFα, one (ETA) is a fusion protein of the TNF receptor joined to the Fc region of IgG1, while CZP is an antigen-binding fragment (Fab′) of a monoclonal anti-TNFα antibody which lacks the Fc region of IgG1 and has been conjugated with polyethylene glycol (PEG). These drugs have different half-lives, bioavailability and rates of placental transfer, which are relevant when considering their potential use in pregnancy.
Initial 2006/8 consensus recommendations advised avoidance of ETA, INF and ADA in pregnancy and breastfeeding due to a lack of evidence rather than evidence of harm [68, 222]. Previously, we reviewed outcome data from TNFi-exposed pregnancies (n = 706) of patients with predominantly IBD but also rheumatic disease and non-autoimmune-mediated recurrent spontaneous miscarriage, compared with (n = 399) disease and (n = 170) healthy control pregnancies [50, 52, 99, 100, 108, 109, 172, 173, 252, 260–279]. There were multiple confounders of concomitant therapies (including MTX, LEF and MMF) and active inflammatory disease. Overall, these studies did not describe an increased incidence of adverse effects upon miscarriage rates, pregnancy duration, birth weight, foetal death or congenital malformation that was attributable to ETA, INF, ADA or CZP. At that time, there was limited information on placental transfer, and no published studies of GOL in human pregnancy or breastmilk exposure.
TNFi exposure in pregnancy and with breastmilk exposure has been extensively studied since our last search. We identified an additional 50 studies, reporting 12 491 pregnancy exposures to TNFi, including INF (n ≥ 5377), ADA (n ≥ 2797), ETA (n ≥ 2210), CZP (n ≥ 776) and GOL (n ≥ 196) [37, 255–259, 280–323]. Many studies reported combined outcomes for exposure to different TNFi agents. The majority of studies of maternal exposure did not report an increased risk of preterm birth, miscarriage, low birth weight or congenital malformations [37, 255, 257–259, 280–286, 288–291, 294, 296, 298–302, 304–308, 314, 316–318, 320–323].
Different adverse outcomes were reported in some studies, however. A study of ETA found that the proportion of infants with major birth defects was higher (9.4% vs 3.5%, respectively) in ETA-exposed pregnancies (n = 370) than in pregnancies of disease-matched, non-exposed women (n = 164) [287]; however, the lack of a specific pattern of birth defects and the expected minimal placental transfer of ETA in early pregnancy did not support the biologic plausibility of a drug-related effect. A study reporting a lower live birth rate in INF-exposed pregnancies (n = 99) in women with Crohn’s disease considered their findings to be confounded by more severe disease in those patients exposed to INF and increased exposure to other immunosuppressive agents [293]. A population-based study of TNFi-exposed pregnancies (n = 1027) found increased risks of preterm birth, caesarean section and SGA babies in comparison with TNFi-unexposed pregnancies (n = 9399) [295]; however, the authors noted that these associations may have been related to underlying disease activity rather than agent-specific effects, due to diverse findings across disease groups.
A retrospective cohort study of TNFi-exposed pregnancies (n = 1457) in women with IBD found TNFi exposure to be an independent risk factor for maternal complications and infections when compared with TNFi-unexposed pregnancies (n = 9818) [303]. In this study, TNFi exposure did not associate with congenital malformations or an increased risk of infection in children during the first year of life. Furthermore, there was no difference in the risk of complications in women exposed to TNFi during the third trimester, relative to cessation before week 24, although disease relapses were more common in those stopping TNFi prior to the third trimester. A study of 4961 pregnant women with autoimmune inflammatory conditions found similar risks of serious infections in women taking steroids, csDMARDs or TNFi during pregnancy, but found that higher doses of steroids were an independent risk factor for serious infections in pregnancy [323].
A registry-based study from Denmark and Sweden reported a non-statistically significant higher risk of having children with birth defects in women with RA, AS, psoriatic arthritis (PsA), IBD or psoriasis who had received TNFi during pregnancy (n = 683), relative to women with chronic inflammatory disease but without TNFi exposure (n = 21 549) [310]; however, the heterogeneity of observed birth defects went against a common aetiology. A prospective cohort study of TNFi pregnancy exposures (n = 495) in women with chronic inflammatory disease (RA, AS, PsA, psoriasis and IBD) found prenatal TNFi exposure to be associated with an increased risk of birth defects without a distinct pattern of malformations, when compared with non-disease-matched, TNFi-unexposed controls (n = 1532) [312]. An increased risk of preterm births and reduced birth weight, but not spontaneous miscarriage, was also noted. The authors concluded that, although TNFi may carry a risk of adverse pregnancy outcomes of moderate clinical relevance, they may remain a treatment option, considering the impact of inadequately controlled disease on the mother and unborn child.
A small number of studies specifically compared risks between TNFi agents. In one study, INF was found to be associated with a greater risk for preterm births relative to ETA, and a higher prevalence of severely SGA babies relative to ETA and ADA, in pregnant women with RA, AS, PsA or psoriasis. In IBD, however, the risk of preterm births and SGA babies did not differ between INF and ADA [295]. In a study of individual safety reports in pregnant IBD patients exposed to TNFi (n = 783), the odds for maternal or foetal adverse events were found to be lower for CZP monotherapy, but not for INF or ADA monotherapy, when compared with an aminosalicylate monotherapy comparator in multi-level regression models [316]. In another study, the risk of birth defects did not differ significantly between ADA, INF or ETA-exposed women with chronic inflammatory diseases [310].
Several studies specifically compared outcomes for pregnancies exposed to TNFi during late vs early trimesters [37, 280–282, 284, 289, 291, 292, 295–297, 303, 305, 308, 309, 317], the majority of which reported no significant concerns with late trimester exposure. In a study of INF-exposed pregnancies (n = 1850) in women with IBD, RA, AS, PsA and psoriasis, frequencies of congenital abnormalities and other adverse birth and infant outcomes (including neonatal infections) were similar when comparing first and third trimester exposure [291]. In a study comparing early discontinuation of INF (>90 days before delivery; n = 68) to late discontinuation (<90 days before delivery; n = 318) in pregnancies of women with IBD, early discontinuation was associated with increased disease flares, more steroid usage and more preterm births than late discontinuation [292]. Rates of other adverse outcomes, including congenital malformations and infant respiratory infections, were similar between these groups. A further study of TNFi-exposed pregnancies (n = 153) in women with IBD reported that continuation of TNFi after gestational week 30, relative to cessation before week 30, was independently associated with modestly lower birth weights in multivariate regression models after adjustment for disease activity, but not other adverse infant outcomes [297].
Different rates of placental transfer of TNFi and timing of drug exposure in the second and/or third trimester of pregnancy influenced previous advice regarding avoidance of live vaccines in the first 7 months of life. Previously, a small cohort study found CZP (a PEGylated Fab’ fragment, lacking the Fc region) to have minimal rates of placental transfer compared with INF and ADA [270], and two case reports demonstrated very low rates of placental transfer of ETA administered throughout pregnancy [108, 274]. Therefore, previous recommendations described discontinuation of ADA and ETA at the end of the second trimester to ensure negligible or no drug is detectable in cord blood at delivery. For INF, due to its prolonged bioavailability and higher rate of placental transfer, it was recommended to be stopped earlier in pregnancy (at 16 weeks) for it to be undetectable in cord blood at delivery.
Since the last guideline, we found increased data demonstrating different rates of placental transfer of TNFi [283, 285, 302, 308, 309]. In a study of pregnant women with IBD exposed to INF (n = 44) or ADA (n = 36), the median time to drug clearance was 4 months for ADA and 7 months for INF [308]. In this study, continuation of TNFi in the third trimester did not increase the risk of childhood infection, relative to discontinuation before the third trimester. In a prospective study of ADA (n = 58) and INF-exposed pregnancies (n = 73) in women with IBD, cord blood samples showed significantly higher levels of INF than ADA at birth [302]. In this study, placental transfer of INF increased exponentially over the third trimester, while ADA transportation was limited and increased in a linear fashion. Maternal and birth outcomes were comparable between these groups, as were one-year infant health outcomes, including infection and adverse reactions to vaccinations. A study of infants (n = 14) with third trimester maternal exposure to CZP found minimal rates of placental transfer, supporting continuation of this treatment during pregnancy [283]. Recent data, published after our literature search and highlighted by UCB Pharma during public consultation, reported no signal for adverse pregnancy outcomes following maternal CZP exposure in a large (n = 1425) cohort [324].
UKTIS reports that studies that investigate the use of TNFi (ADA, CZP, ETA and INF only) during pregnancy have not found an overall increased risk of congenital malformation for these therapies as a class; there is also no compelling evidence of an increased risk for spontaneous miscarriage, intrauterine death or adverse neurodevelopmental outcomes; however, data are currently too limited to exclude adverse effects on the foetus. LBW and preterm birth have been associated with in-utero TNFi exposure in some studies but are confounded by maternal disease. UKTIS also states that there are theoretical concerns that the use of immunosuppressant antibodies, which actively cross the placenta, may result in neonatal or infant immunosuppression and increase the risk of infection; therefore, a delay is advised in administration of live vaccines to infants of: 5 months after last dose of ADA; 16 weeks after last dose of ETA; and until 6 months of age after in-utero INF. In contrast, as CZP is minimally transferred across the placenta, it is unlikely that infants born to women who used CZP in pregnancy would experience sufficient levels of TNFα inhibition to significantly inhibit their immune response [171].
Previously, we found case reports and case series reporting detection of ADA in breastmilk but not in infant serum [269], and detection of ETA and INF in breastmilk in some [108, 267, 273, 274] but not all studies [265], with no adverse effects detectable in any of these breastfed infants. We found nine additional studies of TNFi reporting on 133 breastmilk exposures [283, 285, 300, 307, 308, 317, 319, 325, 326]. Overall, these studies did not find any adverse effects of TNFi. A study of mothers (n = 17) breastfeeding while taking CZP found minimal transfer of this drug into breastmilk [325]. Another study reported low or undetectable concentrations of INF, ADA, CZP, GOL and UST in 72 breastmilk samples [326]. In this study, breastfed infants on biologics (n = 243), thiopurines (n = 102) or combination therapy (n = 67) were found to have similar risks of infection and rates of milestone achievement compared with infants unexposed to these drugs via breastmilk or not breastfed. LactMed states that: CZP is excreted into breastmilk in some, but not all, women in small amounts; INF is usually either not detectable in breastmilk or detectable at very low levels; ETA, ADA and GOL are minimally excreted into breastmilk, with all TNFi being predicted to be poorly absorbed by the infant due to large molecular weight of each drug [192]. While some evidence suggests that IgG antibodies may not be digested by the gut in the early neonatal period [327, 328], other studies demonstrate marked digestion of IgG by the infant gut [329, 330].
Previously, we found long-term follow-up data in children exposed in utero to ETA [108, 266, 270, 272–274], ADA [269] and CZP [270]. We found an additional 16 studies reporting long-term follow-up data after exposure to TNFi [256, 291, 292, 299–303, 307–309, 317, 319–322]. In one study, 196 children with intrauterine exposure to TNFi (ADA, n = 81; INF, n = 115) for maternal IBD were followed up for 5 years, finding no association with long-term adverse health outcomes (including childhood infections and vaccination adverse reactions) when compared with TNFi-unexposed controls [296]. This study included women continuing TNFi during the third trimester, where no increased risk of infection was noted in their offspring. In a retrospective cohort study of children (n = 388) exposed to TNFi (ADA, n = 164; INF, n = 223; CZP, n = 1) in utero for maternal IBD, the incidence of severe infections was similar to TNFi-unexposed children of IBD mothers after median follow-up of 5 years [301]. Similarly, in another study, the risk of serious or opportunistic infections during the first year of life in live-born infants (n = 229) exposed to ADA during pregnancy in women with RA or IBD was not significantly different to disease-matched, non-exposed controls and healthy controls [281]. In this study, the risk of infection remained similar when restricting to infants exposed to ADA during the third trimester. Two additional studies, which were not included in our final analysis because they did not report primarily on pregnancy or breastmilk exposure outcomes, described infections in the first 3 years of life in children after in-utero exposure to TNFi (predominantly ETA, ADA and INF) and/or csDMARDs for IRDs, psoriasis and IBD. One study of 493 children exposed in utero to TNFi reported an increased risk of some site-specific infections but not other adverse outcomes within the first year of life only [331]. The other study of 1027 children demonstrated a slightly increased risk of paediatric infections associated with both TNFi and csDMARDs in the first and second year after birth; however, the authors noted that this association was present regardless of third trimester exposure and could also have been confounded by disease severity [332].
Studies of vaccine safety and efficacy were not specifically sought through our systematic literature search but are relevant to consider, because most guidance recommends avoidance of live vaccines up to 6–12 months post-partum in infants exposed to bDMARDs in the second/third trimester [1, 333]. This advice is heavily influenced by the finding that placental transfer of bDMARDs can lead to persistence of drug levels by up to 12 months following in-utero exposure, with a median clearance time of 6 months and longest clearance times for INF. In addition, a fatal case of disseminated TB-like disease had been reported in a 4-month-old infant who had received Bacille Calmette et Guerin (BCG) vaccination following in-utero exposure to INF [334]. This guidance impacts on rotavirus vaccination and, if indicated, the BCG vaccine, while the measles, mumps and rubella (MMR) vaccine (typically given at 12 months) is not affected. These restrictions significantly impact on rotavirus only, since the BCG vaccine may easily be deferred to be given later in life, while the rotavirus course of vaccination must be completed by 24 weeks of age due to risk of intussusception [335]. Currently, the restrictions may be avoided by discontinuing TNFi in the second or early third trimester, several half-lives prior to delivery.
Rotavirus continues to be a major cause of acute gastroenteritis in young children and has been estimated to result in >500 000 deaths and 2.4 million hospital admissions worldwide [336]. The rotavirus vaccine is over 85% effective at protecting against severe rotavirus gastroenteritis in the first two years of life [335]. Although current UK guidance recommends avoidance of rotavirus vaccination in infants of mothers exposed to biologics during pregnancy [337], it refers readers to the green book [335]. This text states that, although the vaccine is a live attenuated virus, with the exception of severe combined immunodeficiency (SCID), the benefit from vaccination may exceed risk in other forms of immunosuppression [335].
There are increasing reports of the safe use of rotavirus vaccination in infants following perinatal exposure to bDMARDs, including INF. A systematic review described cohort studies and case reports of infants (n = 54) of mothers who received antenatal bDMARDs (mostly TNFi, including INF) who then received rotavirus vaccine without significant adverse effects [336]. The authors of that review recommended that otherwise healthy newborns with a history of perinatal exposure to bDMARDS should receive rotavirus vaccinations as per the recommended schedule, while the BCG vaccine should be withheld in the first year of life. Not all consensus review articles have reached the same conclusion, however, with some recommending avoidance of both rotavirus and BCG vaccinations in infants exposed to bDMARDs in utero in the first 6 months of life unless levels of biological drugs are undetectable [338], or avoidance of live vaccines until 6–12 months of age in infants exposed to biologics that may cross the placenta at clinically significant levels [339].
A systematic review published in abstract form after our search date evaluated vaccine safety in infants exposed to bDMARDs or tsDMARDs in pregnancy [340]. It identified in-utero exposures to ADA (n = 326), CZP (n = 18), ETA (n = 1), INF (n = 408), GOL (n = 1), RTX (n = 1), TCZ (n = 3), UST (n = 1) and no tsDMARD exposures in mostly IBD (n = 849) pregnancies. Infant vaccination included: BCG (n = 111) and/or rotavirus (n = 48) in the first year of life (many <6 months); and MMR at 12 months (n = 590), 6–9 months (n = 12) and at 1, 2 or 4 months (n = 3). Adverse events with BCG vaccination included one death, two large local skin reactions, and one infant with axillary lymphadenopathy. A freedom of information request to the MHRA revealed four further suspected fatal BCG infections in infants exposed to TNFi in utero (INF, n = 2; ADA, n = 1; and unspecified TNFi, n = 1). Adverse effects noted in infants given rotavirus vaccination were mild and at similar frequency to those in biologic-unexposed infants. No complications were reported with MMR vaccination. Overall, the most evidence of clinically harmful effects was found after administration of BCG to infants <3 months of age and after in-utero exposure to INF. In contrast, outcomes following rotavirus (mostly <6 months) and MMR (mostly at a year) vaccinations were reassuring. Notably, disseminated rotavirus infection has not been reported.
Other systematic reviews have also evaluated vaccine efficacy, and report adequate vaccination response (measured by antibody levels) following non-live vaccination in infants exposed to TNFi [336], although there are conflicting reports, with low antibody responses to the Haemophilus influenzae type-B vaccine reported in some infants exposed to INF or ADA [338].
Although our literature search did not assess evidence relating to the peri-operative use of TNFi or other bDMARDs (for example, in the context of caesarean sections), relevant guidance can be found in other BSR guidelines [341].
Recommendations for anti-TNFα medications in pregnancy and breastmilk exposure
Women with no/low disease activity established on a TNFi with known placental transfer (INF, ADA, GOL) do not need to be switched to an alternative TNFi with established minimal placental transfer (CZP) either before or during pregnancy (GRADE 1B, SOA 100%).
CZP is compatible with all three trimesters of pregnancy, has no to minimal placental transfer compared with other TNFi, and does not require any alteration to the infant vaccination schedule (GRADE 1B, SOA 100%).
Women considered to have low risk of disease flare on withdrawal of TNFi in pregnancy could stop INF at 20 weeks, ADA and GOL at 28 weeks, and ETA at 32 weeks so that a full-term infant can have a normal vaccination schedule, with rotavirus vaccination at 8 weeks as per the UK schedule (GRADE 1B, SOA 99.5%).
INF, ADA, ETA or GOL may be continued throughout pregnancy to maintain maternal disease control; in these circumstances, live vaccines should be avoided in infants until they are 6 months of age (GRADE 1B, SOA 100%).
If a TNFi is stopped in pregnancy, it can be restarted as soon as practical post-partum in the absence of infections or surgical complications, regardless of breastfeeding status, to ensure control of maternal disease (GRADE 1C, SOA 100%).
TNFi are compatible with breastmilk exposure (GRADE 1C, SOA 100%).
Other biologic DMARDs (non-TNFi)
Data relating to non-TNFi biologic use in pregnancy remain scarce, and in this guideline updated case reports and small case series were excluded for consistency. A recent systematic review summarized all available data (including case reports) on non-TNFi bDMARDs and tsDMARDs and did not identify any adverse safety signals [342].
Rituximab
Previously, we found insufficient evidence to be confident that RTX is compatible with pregnancy and recommended it should be stopped 6 months before conception, although there were no direct reports of teratogenicity and only second/third trimester exposure was associated with neonatal B-cell depletion. RTX is a monoclonal IgG1 that actively crosses the placenta from 16 weeks of pregnancy onwards. Previously, we found eight studies [50, 343–349] on 173 RTX-exposed pregnancies that met our inclusion criteria. Overall, these studies reported reassuring pregnancy outcomes, but found low B cells at birth in infants exposed to RTX in the second/third trimester, no human breastmilk exposure studies were identified [1].
We identified five further studies of 143 RTX pregnancy exposures [350–354] in addition to eight exposures in studies that reported on combined outcomes of multiple biologic-exposed pregnancies (see ‘Biologic DMARDs’ above). Four studies (n = 135) of mostly pre-conception or first trimester maternal exposure did not report an increased risk of preterm birth, miscarriage, LBW or congenital anomaly [350–352, 354]. An additional cohort study of eight mothers treated with one to four cycles of RTX during pregnancy (n = 6 for diffuse large B-cell lymphoma; n = 2 for SLE) found a high rate of preterm birth and intrauterine infections, but noted the potential for confounding by the underlying disease and concomitant medications [353].
UKTIS reports that there is insufficient evidence to assess whether the risk for spontaneous miscarriage, congenital malformation, birth weight, intrauterine death or adverse neurodevelopmental outcomes is increased following exposure to RTX in utero. Due to a lack of data on the effects of RTX on the neonatal/infant immune system, it recommends delaying administration of live vaccines following in-utero exposure to RTX, although no specific timescale is given [171].
There were three studies of breastmilk exposure (n = 23) to RTX [352, 354, 355]. One study detected minimal transfer of RTX into the breastmilk of nine breastfeeding mothers, with a relative infant dose of rituximab well below theoretically acceptable levels (<0.4%) [355]. No adverse effects attributable to RTX exposure were reported in the breastfed infants, and the drug was considered to have an acceptable benefit-to-risk ratio, supporting both maternal treatment and breastmilk exposure. LactMed states that the amount of RTX in breastmilk is very low and absorption is unlikely because it is a protein with a high molecular weight that is likely to be partially destroyed in the infant’s gastrointestinal tract; thus, absorption by the infant is probably minimal [192].
Vaccine efficacy after prenatal exposure to RTX is much less studied, and systematic reviews have identified adequate non-live vaccination outcomes from 5/6 RTX-exposed infants, with low immunity to diphtheria detected in one 11-month-old infant exposed to RTX at conception [336, 338].
Recommendations for rituximab in pregnancy and breastmilk exposure
Limited evidence has not shown RTX to be teratogenic; however, there remains insufficient evidence to be confident that it is compatible with pregnancy. Consider stopping the drug at conception (GRADE 2C, SOA 99.3%).
RTX may be considered to manage severe maternal disease in pregnancy if no other pregnancy-compatible drugs are suitable (GRADE 2C, SOA 99.7%).
If RTX is used to treat severe maternal disease in the third trimester, it is currently recommended to avoid all live vaccines in the infant vaccination schedule until 6 months of age (GRADE 2C, SOA 98.7%).
Based on limited evidence, maternal treatment with RTX is compatible with breastmilk exposure (GRADE 2C, SOA 99.5%).
Interleukin-6 inhibitors
Tocilizumab
Due to insufficient data, we previously recommended that TCZ be stopped at least three months pre-conception, but that unintentional exposure early in the first trimester is unlikely to be harmful due to its IgG1 structure. This limited data came from outcomes of 33 pregnancies in 32 patients, published in abstract form [356]. We identified three further studies of 365 pregnancy exposures to TCZ [357–359] in addition to four exposures in the papers described above which reported combined outcomes of multiple biologic-exposed pregnancies (see ‘DMARDs’ above). These studies of mainly first trimester exposure did not find increased rates of congenital abnormalities in patients with rheumatic disease. Two studies commented on a higher than background rate of spontaneous miscarriage [357, 359], and could not exclude an effect on birth weight and risk of prematurity, while acknowledging potential confounding factors. For example, stopping effective treatment early in pregnancy could destabilize the rheumatic disease, with adverse consequences for the pregnancy.
UKTIS notes a small number of studies lacking comparator groups that are confounded by maternal disease, and states that there is currently no compelling evidence that TCZ is teratogenic or fetotoxic. Due to lack of data on effects on the neonatal/infant immune system, it recommends that live vaccines are avoided until the infant is 6 months of age [171].
One study of n = 2 breastmilk exposure to TCZ did not report any adverse effects attributable to the drug [360]. Saito et al. have described a total of four cases of babies exposed to TCZ via breastmilk with no complications in the infants (three publications which did not meet our inclusion criteria) [360–362]. Concentrations of TCZ were measured in the breastmilk in these studies and two additional cases reports, with no clinical adverse effects reported [363, 364]. Levels in the breastmilk were found to peak on day three following the infusion [362], but were significantly lower than the corresponding maternal serum concentrations in all of these cases, ranging from 11% in colostrum [364], down to as low as 1:2000 [360, 363].
Sarilumab
No papers were found that met our inclusion criteria. Sanofi provided data on 13 patients who became pregnant in the sarilumab and DMARD long-term safety population, of whom seven had a miscarriage [365]. In addition, two male patients fathered two healthy children.
Recommendations for IL-6 inhibitors in pregnancy and breastmilk exposure
Limited evidence has not shown IL-6 inhibitors (IL-6i) to be teratogenic; however, there remains insufficient evidence to be confident that they are compatible with pregnancy. Consider stopping the drug at conception. Any exposure, however, during pregnancy is unlikely to be harmful (GRADE 2C, SOA 99.7%).
IL-6i may be considered to manage severe maternal disease in pregnancy if no other pregnancy-compatible drugs are suitable (GRADE 2C, SOA 100%).
If IL-6i are used to treat severe maternal disease in the third trimester, it is currently recommended to avoid all live vaccines in the infant vaccination schedule until 6 months of age (GRADE 2C, SOA 99.3%).
Based on limited evidence, maternal treatment with IL-6i is compatible with breastmilk exposure (GRADE 2C, SOA 100%).
Interleukin-1 inhibitors
Anakinra
Anakinra is a recombinant form of human interleukin-1 receptor antagonist (IL-1Ra) with a high molecular weight which was not found to cross ex-vivo full-term human placentae [366]. Previously, there was insufficient information evidence on which to base a recommendation for anakinra in pregnancy, but unintentional exposure in the first trimester was considered unlikely to be harmful [1]. This limited evidence came from reports on five pregnancies in three studies/reports, with no evidence of harm [50, 367, 368].
Subsequently, we identified four studies of 43 pregnancy exposures to anakinra [369–372], in addition to one exposure in the papers described above which reported combined outcomes of multiple biologic-exposed pregnancies (see ‘Biologic DMARDs’ above). These exposures were mostly in patients with periodic fevers and severe maternal disease. Overall, outcomes were reassuring, although two congenital renal anomalies and two cases of oligohydramnios (which can be linked to foetal renal anomalies) were reported [369, 371, 372]. It was unclear, however, whether the renal abnormalities were associated with antenatal anakinra use or maternal hyperthermia or both. Given the significant beneficial effects of anakinra in suppressing maternal disease with limited pregnancy-compatible options, it was considered a safe alternative in managing disease in women with periodic fever in pregnancy. UKTIS does not report on anakinra.
Two studies of n = 12 breastmilk exposures to anakinra did not report any adverse effects attributable to the drug [369, 371]. LactMed states that IL-1Ra is a normal component of human milk, possibly as an anti-inflammatory agent, and that several infants have been breastfed during maternal anakinra therapy with no obvious adverse effects. If anakinra is required by the mother, it is not a reason to discontinue breastfeeding [192].
Canakinumab
Canakinumab is a human monoclonal antibody to IL-1. There is, therefore, the potential for active transport across the placenta from the second trimester onwards.
One paper reporting canakinumab use from pre-conception in eight pregnancies (stopped during first trimester in 5/8 pregnancies) met our inclusion criteria [369]. There were seven live births, all of whom were healthy, full term and normal birth weight. One mother with refractory Cogan syndrome had an early miscarriage at 6 weeks (after a miscarriage the previous year while on anakinra).
In addition, Novartis supplied information from analyses of the Novartis Global Safety Database, which included 76 maternal and nine paternal exposures [373]. There were 47 known pregnancy outcomes, with 27 healthy newborns, one preterm birth (with meconium staining not thought likely to be related to canakinumab exposure), nine spontaneous miscarriages (including two miscarriages related to paternal exposure in one father) and three elective terminations. Seven other adverse events were reported: one case of congenital pyelocaliectasis following paternal exposure to canakinumab; one case of congenital musculoskeletal abnormality following maternal exposure, with insufficient data on other contributory factors; one case of inherited genetic disease with neonatal RSV infection and pyrexia following infant vaccinations; and four adverse events very unlikely to relate to canakinumab exposure (one newborn non-serious hypotension and three cases of likely inherited disease: Muckle–Wells syndrome and CAPS).
One cohort study reported breastmilk exposure in four infants whose mothers were prescribed regular canakinumab, with no reported serious infections or developmental abnormalities [369].
Recommendations for IL-1 inhibitors in pregnancy and breastmilk exposure
Limited evidence has not shown IL-1 inhibitors (IL-1i) to be teratogenic; however, there remains insufficient evidence to be confident that they are compatible with pregnancy. Consider stopping the drug at conception. Any exposure, however, during pregnancy is unlikely to be harmful (GRADE 2C, SOA 99.8%).
IL-1i may be considered to manage severe maternal disease in pregnancy if no other pregnancy-compatible drugs are suitable (GRADE 2C, SOA 100%).
If IL-1i are used to treat severe maternal disease in the third trimester, it is currently recommended to avoid all live vaccines in the infant vaccination schedule until 6 months of age (GRADE 2C, SOA 99.3%).
Based on limited evidence, maternal treatment with IL-1i is compatible with breastmilk exposure (GRADE 2C, SOA 100%).
Abatacept
ABA is a fusion protein containing the Fc region of IgG1 fused to the extracellular domain of CTLA-4; therefore, it is able to cross the placental barrier from approximately week 16. Previously, there was insufficient data to recommend ABA in pregnancy, but unintentional exposure early in the first trimester was considered unlikely to be harmful [1]. This conclusion was based on reports from 11 pregnancy exposures (and a further eight exposures reported in abstract form only), many of which were confounded by concomitant MTX [175, 349, 374].
We identified two further studies of at least 196 pregnancy exposures to ABA (with some overlap of data) [375, 376], in addition to three exposures in the papers described above which reported combined outcomes of multiple biologic-exposed pregnancies (see ‘Biologic DMARDs’ above). The data from these studies did not suggest an increased risk of adverse pregnancy outcomes with ABA, and many of the congenital abnormalities were considered by the authors to be associated with concomitant use of other teratogenic DMARDs. The apparently high rate of spontaneous miscarriage (total ≥48/184) is difficult to interpret due to confounding by indication and other medications such as MTX, as well as detection bias of early miscarriages during the close monitoring of clinical trials [375]. The authors concluded that ABA should only be used during pregnancy if the benefit to the mother justifies potential risk to the foetus. UKTIS does not report on ABA.
Although it did not meet our inclusion criteria, we identified one case report of breastmilk exposure to ABA, with no adverse effects, in which ABA was secreted into breastmilk at levels 1/200–1/300 of those in serum [377]. LactMed describes one case report that reported concentrations of ABA in milk were very low and did not appear to affect the breastfed infant, concluding that if ABA is required by the mother, it is not a reason to discontinue breastfeeding, although alternative drugs may be preferred [192].
Recommendations for abatacept in pregnancy and breastmilk exposure
Limited evidence has not shown ABA to be teratogenic; however, there remains insufficient evidence to be confident that it is compatible with pregnancy. Consider stopping the drug at conception. Any exposure, however, during pregnancy is unlikely to be harmful (GRADE 2C, SOA 99.3%).
ABA may be considered to manage severe maternal disease in pregnancy if no other pregnancy-compatible drugs are suitable (GRADE 2C, SOA 99.3%).
If ABA is used to treat severe maternal disease in the third trimester, it is currently recommended to avoid all live vaccines in the infant vaccination schedule until 6 months of age (GRADE 2C, SOA 99.3%).
Based on limited evidence, maternal treatment with ABA is compatible with breastmilk exposure (GRADE 2C, SOA 99.5%).
Belimumab
Belimumab (BEL) is a fully humanized monoclonal IgG1 that inhibits B-cell activating factor. Previously, our systematic search did not identify any publications that met our search criteria on use of BEL in human pregnancy, although we found reference to pregnancy outcomes from placebo-controlled, phase 2 and 3 studies in 83 SLE pregnancies [378], and BEL pregnancy registry data describing known outcomes from 118 SLE pregnancies [379]. Overall, these studies did not identify any pattern of adverse effects in pregnancy directly attributable to BEL.
We subsequently identified one study of 66 BEL pregnancy exposures [380]. Total foetal losses in BEL-treated subjects were similar to background estimates in SLE patients (∼25%), although data remain limited. Another six studies, which did not meet our search criteria [381–386], reported an additional 124 pregnancies exposed to BEL. There was only one reported congenital abnormality: a case report of mild Ebstein’s anomaly in a baby following successful control of SLE (with previous lupus nephritis and APS, previously only controlled on MMF) [381]. Two case series (26 pregnancy exposures) reported preterm delivery in 12/22 live births, with six babies being SGA [385, 386]. However, these cohorts included patients with complex rheumatic disease (SLE and APS), including some patients with previous lupus nephritis and active disease at conception [385], and a high average maternal age and rate of previous pregnancy losses [386]. Both authors concluded that, while careful consideration and further research is required, BEL could be a reasonable treatment option for patients with SLE requiring treatment in pregnancy. UKTIS does not report on BEL.
Although they did not meet our inclusion criteria, we identified two cases of breastmilk exposure while on BEL [381, 382]. No neonatal outcomes, adverse or otherwise, were reported in one case [381]. In the other case, there were no adverse effects, and BEL was secreted into breastmilk at levels 1/200–1/500 of those in serum [382]. LactMed states that, based on preliminary information that BEL levels in breastmilk are very low and infant absorption is probably minimal, if BEL is required by the mother, it is not a reason to discontinue breastfeeding, although caution is required, especially while nursing a newborn or preterm infant [192].
Recommendations for belimumab in pregnancy and breastmilk exposure
Limited evidence has not shown BEL to be teratogenic; however, there remains insufficient evidence to be confident that it is compatible with pregnancy. Consider stopping the drug at conception. Any exposure, however, during pregnancy is unlikely to be harmful (GRADE 2C, SOA 99.3%).
BEL may be considered to manage severe maternal disease in pregnancy if no other pregnancy-compatible drugs are suitable (GRADE 2C, SOA 99.5%).
If BEL is used to treat severe maternal disease in the third trimester, it is currently recommended to avoid all live vaccines in the infant vaccination schedule until 6 months of age (GRADE 2C, SOA 98.8%).
Based on limited evidence, maternal treatment with BEL is compatible with breastmilk exposure (GRADE 2C, SOA 99.5%).
Interleukin-17 inhibitors
Interleukin-17 inhibitors (IL-17i) were not in general use and so not considered in our previous search. Secukinumab has an IgG1 structure, which may theoretically suggest increased transplacental transfer compared with the IgG4 structure of ixekizumab.
We found three studies of mostly first trimester pregnancy exposures to secukinumab (n = 244) and ixekizumab (n = 18) [387–389]. The authors did not report any increased incidence of adverse outcomes directly attributable to the drugs, although information, particularly for ixekizumab, remains very limited.
In addition, Novartis provided data from a search of their Global Safety Database, relating to 298 reports of maternal pregnancy exposures and 90 paternal exposures to secukinumab, mostly during the first trimester [390]. No outcome data were provided, but analysis within Novartis did not reveal any new safety information. UKTIS does not report on these drugs.
There were no data on breastmilk exposure to IL-17i identified in our search or in LactMed. Data provided from the Novartis Global Safety Database identified six breastmilk exposures to secukinumab, including one report of newborn pyrexia during breastfeeding [390]. The data in all cases were too limited to draw conclusions, but no new safety concerns were inferred.
Recommendations for interleukin-17 inhibitors in pregnancy and breastmilk exposure
Limited evidence has not shown IL-17i to be teratogenic; however, there remains insufficient evidence to be confident that they are compatible with pregnancy. Consider stopping the drug at conception. Any exposure, however, during pregnancy is unlikely to be harmful (GRADE 2C, SOA 99.3%).
IL-17i may be considered to manage severe maternal disease in pregnancy if no other pregnancy-compatible drugs are suitable (GRADE 2C, SOA 99%).
If IL-17i are used to treat severe maternal disease in the third trimester, it is currently recommended to avoid all live vaccines in the infant vaccination schedule until 6 months of age (GRADE 2C, SOA 99.3%).
Based on limited evidence, maternal treatment with IL-17i is compatible with breastmilk exposure (GRADE 2C, SOA 99.5%).
Interleukin-12/23 inhibitors
UST is an interleukin-12/23 inhibitor (IL-12/23i) with an IgG1 structure. UST was not in general use and not considered in our previous search. We found three studies of mostly first and second trimester pregnancy exposures to UST (n = 517) for maternal psoriasis, PsA and IBD [391–393]. Overall, these data showed that the rates of live births, spontaneous miscarriages and congenital anomalies were consistent with the general population and TNFi-exposed pregnancies.
Similarly, UKTIS did not identify any specific drug-related risk but was unable to provide a reliable evidence-based evaluation of risk due to limited information [171].
Although they did not meet our inclusion criteria, we identified two reports of exposure to UST during breastmilk exposure [326, 394]. In one case report, the trough level of UST in breastmilk after restarting this drug post-partum in a Crohn’s disease patient was initially in the same range as the corresponding serum trough level, and then decreased during maintenance therapy [394]. In another study assessing breastfeeding mothers taking a range of biologic medication for IBD, UST was detected at low levels in 4/6 mothers taking this medication [326]. Overall, in this study, breastfed infants of mothers on biologics were found to have similar risks of infection and rates of milestone achievement compared with non-breastfed infants or infants unexposed to these drugs. LactMed states that UST is either not detectable or found at very low levels in breastmilk and infant absorption is probably minimal; as such, if UST is required by the mother, it is not a reason to discontinue breastfeeding, although caution is required especially while nursing a newborn or preterm infant [192].
Recommendations for interleukin-12/23 inhibitors in pregnancy and breastmilk exposure
Limited evidence has not shown UST to be teratogenic; however, there remains insufficient evidence to be confident that it is compatible with pregnancy. Consider stopping the drug at conception. Any exposure, however, during pregnancy is unlikely to be harmful (GRADE 2C, SOA 99.3%).
UST may be considered to manage severe maternal disease in pregnancy if no other pregnancy-compatible drugs are suitable (GRADE 2C, SOA 98.8%).
If UST is used to treat severe maternal disease in the third trimester, it is currently recommended to avoid all live vaccines in the infant vaccination schedule until 6 months of age (GRADE 2C, SOA 99.3%).
Based on limited evidence, maternal treatment with UST is compatible with breastmilk exposure (GRADE 2C, SOA 99.5%).
Anifrolumab
Anifrolumab is a fully human, IgG1κ monoclonal antibody to type I interferon receptor subunit 1. Although it is not currently licensed for use in the UK, anifrolumab is approved for use in other countries, including FDA approval to treat moderate-to-severe SLE in the USA [395]. Although there is no published evidence on the use of this drug in pregnancy, breastmilk or paternal exposure, further information was provided by AstraZeneca on data from completed trials. Despite the mandatory contraception use in anifrolumab clinical trials, pregnancies have been reported in 20 SLE patients receiving anifrolumab in studies completed by December 2021. Patients who became pregnant during studies had to immediately discontinue the investigational product. No anifrolumab-associated congenital abnormalities or anifrolumab-associated adverse events have been observed in the clinical trials. Current data on anifrolumab pregnancy exposure are insufficient to inform about potential drug-related risks and therefore post-authorisation pregnancy studies are planned.
UKTIS does not report on anifrolumab. Although there is no information regarding breastmilk exposure to anifrolumab, LactMed states that due to its large molecular weight, the amount in milk is likely to be very low, and it is also likely to be partially destroyed in the infant's gastrointestinal tract with minimal absorption by the infant [192]. Based on this limited evidence, we have not made any recommendations on this drug.
Targeted synthetic DMARDs
Data relating to tsDMARD use in pregnancy remain scarce, and in this guideline, updated case reports and small case series were excluded for consistency. A recent systematic review summarized all available data including case reports and raised no additional safety concerns [342]. No studies were found relating to apremilast use in pregnancy, and therefore we were unable to make any recommendations.
JAK inhibitors
Tofacitinib (TOF), baricitinib (BAR) and upadacitinib (UPA) are oral Janus kinase inhibitors (JAKi) which were not in general use and so not considered in our previous search. They are small molecule inhibitors of low molecular weight which could theoretically cross the placenta.
JAKi have a short half-life (∼3 h for TOF), although biological changes can persist for longer (for example, dose-dependent reductions in NK cells and CRP do not appear to reverse before 2 weeks after discontinuation) [396].
We identified three published reports of pregnancies during RCTs and post-marketing surveillance reports for TOF, but due to probable overlap, only the later 2018 paper was included (further updates were published in abstract form in 2020) [397–399]. This included 116 pregnancy exposures to TOF, with the known reported outcomes including 15/72 first trimester miscarriages, and major malformations in 2/44 live births (one pulmonary valve stenosis and one ventricular septal defect), which was in line with background risks in the general population [397].
Pfizer medical information supplied further details on pregnancies reported during the TOF clinical trials programmes [400]. The RA and psoriasis trials included a total of 60 maternal exposures during the first trimester with 51 known outcomes, with concomitant MTX use either before or during pregnancy in at least 19 of these cases [399]. There were 28 healthy newborns, two premature infants, and one congenital malformation where the mother was also taking losartan. In these trials, there were nine spontaneous miscarriages, nine medical terminations, and one elective termination. In the ulcerative colitis (UC) clinical trial programmes, there were 15 maternal exposures with 13 known outcomes (nine healthy newborns, two spontaneous miscarriages and two medical terminations) [397, 398]. In the PsA programmes, there were a further four maternal and three paternal exposures with six known outcomes (three healthy newborns, two spontaneous miscarriages and one medical termination) [397]. Post-marketing reports up to 2017 were included in the analysis by Mahadevan et al. with 42 (predominantly first trimester) maternal exposures and three paternal exposures, with only 12 known outcomes (seven healthy newborns, one congenital malformation, three spontaneous miscarriages and one medical termination) [397].
Although not eligible for inclusion, we found one case report of an RA pregnancy exposed to BAR from pre-conception up to 17 weeks gestation, with a healthy infant delivered at 38 weeks gestation by caesarean section [401]. In addition, Eli Lilly provided data on 36 maternal pregnancies during the BAR clinical trials programme, with 25 known outcomes (trimester and duration unspecified) [402]. Many were taking concomitant medications, including MTX (n = 14). Outcomes included 13 healthy newborns (including three born preterm), seven spontaneous miscarriages (with MTX exposure in six of these), and five elective terminations. In addition, there have been 22 post-marketing reports of pregnancy exposures to BAR, with four known outcomes as of January 2021, including three healthy newborns (including one preterm), and one spontaneous miscarriage at 13 weeks in a 36-year-old mother (early first trimester exposure to BAR with concomitant medications for RA including HCQ, GOL, prednisolone, MTX, folic acid and ibuprofen) [402].
UKTIS does not reported on JAKi. We found no evidence relating to breastmilk exposure to JAKi. Given that they are small molecules and likely to transfer into breastmilk, they should be avoided. LactMed states that no information is available on the use of TOF or BAR during breastmilk exposure, and alternate drugs are preferred, especially while nursing a newborn or preterm infant [192].
There were no studies identified that met our inclusion criteria for UPA. Data provided by AbbVie included 54 maternal pregnancies inadvertently exposed to UPA in the month prior to conception or during the first trimester of pregnancy during the clinical trials programme [403]. The 41 known outcomes included 17 healthy live births (including two premature deliveries), 14 spontaneous miscarriages (10 of whom were on concomitant MTX), nine elective terminations (all with no reported foetal defects) and one ectopic pregnancy. Filgotinib was NICE-approved after our search window, and so was not included in our search.
Recommendations for JAK inhibitors in pregnancy and breastmilk exposure
There are insufficient data to make a recommendation on JAKi use during pregnancy and they should be stopped at least two weeks before planned conception (GRADE 2C, SOA 99.5%).
There are insufficient data to recommend JAKi in breastfeeding and, given they are likely to transfer into breastmilk, they should be avoided (GRADE 2C, SOA 99.5%).
Paternal exposure
Hydroxychloroquine
For HCQ, no additional paternal exposures were identified to the previously identified cohort study [52] and case series [404] of 13 pregnancies after paternal exposure to HCQ, which did not find any increased risk of adverse foetal outcomes.
Corticosteroids
Previously, four cohort studies [52, 405–407] and two case series [404, 408] reported on outcomes from ≥2127 pregnancies after paternal exposure to prednisolone, and a case-control study [409] and a case series [410] reported on outcomes from (n = 4) pregnancies after paternal exposure to methylprednisolone. Overall, the quality of these studies was low, but reassuringly they did not identify an increased risk of adverse foetal outcomes. Since then, an additional study of 2380 paternal exposures to corticosteroids did not identify any statistically significant increase in adverse birth outcomes [411].
Methotrexate
Previous low-quality evidence from outcomes of pregnancies after paternal exposure (n = 263) to predominantly low-dose MTX did not find any adverse effects [1]. We identified an additional six studies of paternal exposures to MTX (n = 2026) within three months of conception that similarly found no increased risk of adverse foetal outcomes when compared with MTX-unexposed controls (n = 4 700 599) [184–189]. An additional study examined foetal outcomes with paternal MTX (and other DMARD) use compared with abatacept; however, outcomes for individual drugs were not reported [375].
Sulfasalazine
Our previous review of three cohort [52, 407, 412] and one case-control study [409] reporting on 237 pregnancies after paternal exposure to SSZ did not find an increased risk of adverse foetal outcomes, although the quality of evidence was low. SSZ may also affect male fertility, with oligospermia, reduced sperm motility and increased proportions of abnormal sperm previously reported [1]. No further studies of paternal SSZ exposure were identified in our search.
Leflunomide
Previously, we identified a cohort study [52] and case report [413] describing outcomes from (n = 2) pregnancies after paternal exposure to LEF within three months of conception, and subsequent pregnancy exposure (with intercourse without a condom) in at least one case with no reported washout. No adverse foetal outcomes were observed. An additional study was identified examining foetal outcomes with paternal leflunomide (and other DMARD) use compared with abatacept; however, outcomes for individual drugs were not reported [375].
Azathioprine
In addition to the previous 602 paternal exposures [52, 404–409, 414, 415], we identified three studies of n = 2680 pregnancies after paternal exposure to AZA [185, 187, 216]. Overall, no increased risk of adverse foetal outcomes was observed.
Ciclosporin
In addition to previous studies [406, 408, 410, 416] on outcomes from pregnancies (n ≥ 254) after paternal exposure to CsA, we found a Danish population-based cohort study of birth outcomes in 247 children fathered by men treated with CsA before conception [185]. Overall, these studies did not identify an increased risk of adverse foetal outcomes with paternal exposure to CsA.
Tacrolimus
No additional paternal studies were found to complement previous findings reporting outcomes from pregnancies (n ≥ 120) after paternal exposure to tacrolimus, which did not identify an increased risk of adverse foetal outcomes [406, 416, 417].
Cyclophosphamide
No new studies of paternal exposure to CYC were identified. In addition to a potential long-term impact on spermatogenesis (and hence fertility) in men [418], there is evidence of an adverse impact on germ cell development and male-mediated teratogenicity from animal studies [419–422], although this has not been proven in humans [423–425].
Mycophenolate mofetil
In addition to the three previous studies [406, 416, 426] of paternal exposures to MMF (n ≥ 72), we found three additional studies of pregnancies (n = 220) after paternal exposure [185, 246, 247]. Overall, the quality of these studies was low, but they did not identify an increased risk of adverse foetal outcomes.
Intravenous immunoglobulin
There is no evidence relating to paternal exposure but based on maternal compatibility it is unlikely to be harmful.
Anti-TNFα drugs
Previously, we found five cohort studies [52, 263, 405, 412, 427], two case series [404, 410], two case reports [428, 429] and a case-control study [409] that reported on outcomes from pregnancies (n = 131) after paternal exposure to INF, ETA and ADA. Overall, the quality of these studies was deemed to be low, but they did not identify an increased risk of adverse foetal outcomes. We identified an additional eight cohort studies reporting outcomes from 620 pregnancies after paternal exposure to TNFi [293, 298, 306, 430–434], with no significant findings to suggest adverse foetal outcomes were more likely after TNFi exposure.
Rituximab
We did not identify any further paternal exposures to the 11 found previously [343], which did not identify any problems in relation to paternal exposure to RTX.
IL-6 Inhibitors
Two studies of n = 15 paternal exposures to TCZ did not find any drug-related effects [357, 359].
IL-1 Inhibitors
One study of n = 5 paternal exposures to anakinra did not find any drug-related effects [369]. Youngstein et al. reported on five healthy pregnancies to three fathers on long-term canakinumab treatment [369].
Abatacept
In addition to the previous case of one healthy pregnancy following paternal ABA exposure [374], a study of clinical trial and post-marketing data submitted to the manufacturer (up to 2014) reported 10 paternal ABA exposures, resulting in nine healthy live births with one elective termination [375].
IL-17 inhibitors
Two studies of paternal exposure to secukinumab (n = 54) and ixekizumab (n = 34) did not report any adverse drug-related effects [387, 389].
JAK-inhibitors
There were 87 paternal exposures to TOF in a study included in our search [397]. Further details provided by Pfizer reported paternal exposures to TOF in the PsA clinical trials (n = 3) and post-marketing reports (n = 3), described above. During the RA and psoriasis clinical trials programmes, 66 men were exposed to TOF with 45 known outcomes, including 37 normal healthy newborns, two premature deliveries (with one subsequent neonatal death) and six spontaneous miscarriages [400]. In the UC clinical trial programmes, there were 19 paternal exposures with 17 known outcomes (15 healthy newborns including one healthy preterm delivery at 34 weeks, and two spontaneous miscarriages) [398]. Data provided by Eli Lilly included eight paternal exposures to BAR in the clinical trials programmes, and outcomes included six full-term healthy newborns and two spontaneous miscarriages [402].
No data were found relating to paternal exposure to BEL, IL-12/23i or anifrolumab.
Recommendations for paternal exposure to immunomodulatory drugs
Due to the adverse effect of CYC on male fertility, semen cryopreservation is recommended for men prior to paternal exposure (GRADE 1C, SOA 99.5%).
Men who take SSZ may have reduced fertility. There is little evidence to suggest that SSZ should be stopped pre-conception, unless conception is delayed by more than 12 months when stopping SSZ should be considered along with other causes of infertility (GRADE 1C, SOA 99.0%).
Paternal exposure to the following anti-rheumatic medication is compatible with pregnancy: prednisolone, low-dose (≤25 mg/week) MTX, AZA (GRADE 1B); TNFi, cyclosporin (GRADE 1C); HCQ, LEF, tacrolimus, MMF, IVIG, RTX, IL-6i, IL-1i, ABA, BEL, IL-17i, UST and JAKi (GRADE 2C, SOA 99.3%).
Applicability and utility
Implementation
Awareness of these guidelines will aid clinical practitioners and patients in decision making and will be raised through presentation at local, regional and national meetings. No barriers to implementation of these guidelines are anticipated.
Key standards of care
Ideally, patients with rheumatic disease should receive tailored pre-pregnancy counselling and then be reviewed during pregnancy and the four-month post-partum period by clinical practitioners with expertise in the management of rheumatic disease in pregnancy, in addition to their routine obstetric care. They should have access to written information on relevant medications in pregnancy and breastfeeding that is accurate and allows them to make informed decisions regarding compatibility of certain drugs in pregnancy.
Future research agenda
The limitation of current evidence highlights the need for a national pregnancy registry for patients with rheumatic disease, as currently exists for women with epilepsy. All women with rheumatic disease who become pregnant would be eligible to register, whether or not they are on anti-rheumatic treatment. The prospective pregnancy outcome data would then be published to display information on outcomes such as miscarriage and congenital anomalies in patients treated with anti-rheumatic therapy. These data would also be used to answer specific questions such as the most suitable time to stop MTX pre-conception. Data relating to the impact of paternal exposure to these drugs (both fertility and male-mediated teratogenicity), as well as breastmilk exposure, are particularly limited, and further research in these areas is urgently required. Other research questions include: should bDMARDs with known placental transfer be stopped or switched before/during pregnancy; are tsDMARDs compatible with pregnancy; is it safe to give certain live vaccines to infants ≤6 months after in-utero exposure to bDMARDs with known placental transfer in the third trimester of pregnancy?
Mechanism for audit of the guideline
An audit pro forma to assess compliance with these guidelines is shown in the audit tool in Supplementary Data S5, available at Rheumatology online.
Supplementary data
Supplementary data are available at Rheumatology online.
Supplementary Material
Acknowledgements
A full list of Standards, Audit and Guidelines Working Group members can be found in Supplementary Data S6, available at Rheumatology online. All contributing members are named in the authorship list.
Contributor Information
Mark D Russell, Centre for Rheumatic Diseases, King’s College London, London, UK.
Mrinalini Dey, Institute of Life Course and Medical Sciences, University of Liverpool, Liverpool, UK.
Julia Flint, Department of Rheumatology, Robert Jones and Agnes Hunt Orthopaedic Hospital NHS Foundation Trust, Shropshire, UK.
Philippa Davie, Centre for Rheumatic Diseases, King’s College London, London, UK.
Alexander Allen, Clinical Affairs, British Society for Rheumatology, London, UK.
Amy Crossley, Patient Representative, UK.
Margreta Frishman, Rheumatology, North Middlesex University Hospital NHS Trust, London, UK.
Mary Gayed, Rheumatology, Sandwell and West Birmingham Hospitals, Birmingham, UK.
Kenneth Hodson, UK Teratology Information Service, UK.
Munther Khamashta, Lupus Research Unit, Division of Women’s Health, King's College London, London, UK.
Louise Moore, Rheumatic and Musculoskeletal Disease Unit, Our Lady’s Hospice and Care Service, Dublin, Ireland.
Sonia Panchal, Department of Rheumatology, South Warwickshire NHS Foundation Trust, Warwickshire, UK.
Madeleine Piper, Royal National Hospital for Rheumatic Diseases, Royal United Hospital, Bath, UK.
Clare Reid, Patient Representative, UK.
Katherine Saxby, Pharmacy, University College London Hospitals NHS Foundation Trust, London, UK.
Karen Schreiber, Thrombosis and Haemostasis, Guy's and St Thomas' NHS Foundation Trust, London, UK; Department of Rheumatology, Danish Hospital for Rheumatic Diseases, Sonderborg, Denmark; Department of Regional Health Research (IRS), University of Southern Denmark, Odense, Denmark.
Naz Senvar, Obstetrics and Gynaecology, St George's University Hospitals NHS Foundation Trust, London, UK.
Sofia Tosounidou, Lupus UK Centre of Excellence, Sandwell and West Birmingham Hospitals NHS Trust, Birmingham, UK.
Maud van de Venne, Obstetrics & Gynaecology, Frimley Park Hospital, Surrey, UK.
Louise Warburton, Primary Care and Health Sciences, Keele University, Keele, UK.
David Williams, Obstetrics, University College London Hospitals NHS Foundation Trust, London, UK.
Chee-Seng Yee, Department of Rheumatology, Doncaster and Bassetlaw Teaching Hospitals NHS Foundation Trust, Doncaster, UK.
Caroline Gordon, Rheumatology Research Group, Institute of Inflammation and Ageing, University of Birmingham, Birmingham, UK.
Ian Giles, Centre for Rheumatology, Division of Medicine, University College London, London, UK.
BSR Standards, Audit and Guidelines Working Group:
Ian Giles, Ed Roddy, Kate Armon, Lauren Astell, Caroline Cotton, Alan Davidson, Sarah Fordham, Claire Jones, Christopher Joyce, Anoop Kuttikat, Zoe McLaren, Karen Merrison, Devesh Mewar, Amanda Mootoo, and Emma Williams
Data availability statement
All relevant data produced during the guideline development process are presented in the guideline or in the accompanying supplementary material.
Funding
No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: All authors have made declarations of interest in line with BSR policy (see Supplementary Material, available at Rheumatology online). L.M. has received honoraria from UCB and educational funding support from Abbvie and Pfizer. C.G. has received honoraria from Abbvie, Alumis, Amgen, Astra-Zeneca, MGP, Sanofi and UCB. C.-S.Y. has received honoraria from Amgen. K.Sc. has received educational support from UCB and honoraria from UCB and Thermo-Fisherworks and a Novo Nordic Foundation research grant. L.W. has received honorarium from Novartis. M.R. has received educational funding support from Lilly, Pfizer, Janssen and UCB and honoraria from Lilly, Menarini and Biogen. S.T. has received honoraria from UCB. I.G. has received honoraria and unrestricted research grants from UCB. K.H. has received honoraria and educational support from UCB. M.G. has received honoraria from UCB, Lily and Abbvie. M.K. is an employee of GSK and gave independent expert advice to the Working Group. The remaining authors have declared no conflicts of interest.
References
- 1. Flint J, Panchal S, Hurrell A. et al. BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding-Part I: standard and biologic disease modifying anti-rheumatic drugs and corticosteroids. Rheumatology 2016;55:1693–7. [DOI] [PubMed] [Google Scholar]
- 2. Flint J, Panchal S, Hurrell A. et al. BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding-Part II: analgesics and other drugs used in rheumatology practice. Rheumatology 2016;55:1698–702. [DOI] [PubMed] [Google Scholar]
- 3. Giles I, Allen A, Crossley A. et al. Prescribing anti-rheumatic drugs in pregnancy and breastfeeding - the BSR guideline scope. Rheumatology 2021;60:3565–9. [DOI] [PubMed] [Google Scholar]
- 4. Gotestam Skorpen C, Hoeltzenbein M, Tincani A. et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis 2016;75:795–810. [DOI] [PubMed] [Google Scholar]
- 5. Andreoli L, Bertsias GK, Agmon-Levin N. et al. EULAR recommendations for women's health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Ann Rheum Dis 2017;76:476–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gordon C, Amissah-Arthur MB, Gayed M. et al. The British Society for Rheumatology guideline for the management of systemic lupus erythematosus in adults. Rheumatology 2018;57:e1–e45. [DOI] [PubMed] [Google Scholar]
- 7. Sammaritano LR, Bermas BL, Chakravarty EE. et al. 2020 American college of rheumatology guideline for the management of reproductive health in rheumatic and musculoskeletal diseases. Arthritis Rheumatol 2020;72:529–56. [DOI] [PubMed] [Google Scholar]
- 8. Maternal Newborn and Infant Clinical Outcome Review Programme. Saving Lives, Improving Mothers’ Care: Lessons learned to inform maternity care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2017–19. 2021. https://www.npeu.ox.ac.uk/assets/downloads/mbrrace-uk/reports/maternal-report-2021/MBRRACE-UK_Maternal_Report_2021_-_FINAL_-_WEB_VERSION.pdf (1 September 2022, date last accessed).
- 9. Thorne I, Girling J.. Pre-pregnancy care and contraception - the two-sided coin of reproductive health and safe prescribing. Obstet Med 2021;14:127–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dewulf L. Medicines in pregnancy-women and children first? time for a coalition to address a substantial patient need. Ther Innov Regul Sci 2013;47:528–32. [DOI] [PubMed] [Google Scholar]
- 11. van den Brandt S, Zbinden A, Baeten D. et al. Risk factors for flare and treatment of disease flares during pregnancy in rheumatoid arthritis and axial spondyloarthritis patients. Arthritis Res Ther 2017;19:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Medicines and Healthcare Products Regulatory Agency. Medicines with teratogenic potential: what is effective contraception and how often is pregnancy testing needed? 2019. https://www.gov.uk/drug-safety-update/medicines-with-teratogenic-potential-what-is-effective-contraception-and-how-often-is-pregnancy-testing-needed (1 September 2022, date last accessed).
- 13. Faculty of Sexual and Reproductive Healthcare. MHRA issues guidance on contraception for women taking medicines that might increase risk of birth defects. 2019. fsrh.org/news/mhra-contraception-drugs-birth-defects-fsrh-guidance/ (1 September 2022, date last accessed).
- 14. NICE. Postnatal care. 2021. https://www.nice.org.uk/guidance/ng194/resources/postnatal-care-pdf-66142082148037 (1 September 2022, date last accessed).
- 15. Liberati A, Altman DG, Tetzlaff J. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. European Network of Population-Based Registries for the Epidemiological Surveillance of Congenital Anomalies. EUROCAT Guide 1.4 and Reference Documents. 2018. https://eu-rd-platform.jrc.ec.europa.eu/sites/default/files/Full_Guide_1_4_version_28_DEC2018.pdf (1 September 2022, date last accessed).
- 17. Andersson NW, Skov L, Andersen JT.. foetal safety of chloroquine and hydroxychloroquine use during pregnancy: a nationwide cohort study. Rheumatology 2021;60:2317–26. [DOI] [PubMed] [Google Scholar]
- 18. Canti V, Scarrone M, De Lorenzo R. et al. Low incidence of intrauterine growth restriction in pregnant patients with systemic lupus erythematosus taking hydroxychloroquine. Immunol Med 2021;44:204–10. [DOI] [PubMed] [Google Scholar]
- 19. Baalbaki S, Szychowski JM, Tang Y, Wetta L, Subramaniam A.. Systemic lupus erythematosus: perinatal outcomes in patients treated with and without hydroxychloroquine. Ochsner J 2020;20:362–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abd Rahman R, Min Tun K, Kamisan Atan I. et al. New benefits of hydroxychloroquine in pregnant women with systemic lupus erythematosus: a retrospective study in a tertiary centre. Rev Bras Ginecol Obstet 2020;42:705–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beksac MS, Donmez HG.. Impact of hydroxychloroquine on the gestational outcomes of pregnant women with immune system problems that necessitate the use of the drug. J Obstet Gynaecol Res 2021;47:570–5. [DOI] [PubMed] [Google Scholar]
- 22. Haase I, Brinks R, Schneider M, Fischer-Betz R.. AB0375 Safety and beneficial effects of hydroxychloroquine on pregnancy outcomes in women with systemic lupus erythematosus. Ann Rheum Dis 2020;79:1487–8. [Google Scholar]
- 23. Huybrechts KF, Bateman BT, Zhu Y. et al. Hydroxychloroquine early in pregnancy and risk of birth defects. Am J Obstet Gynecol 2021;224:290 e1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu Y, Zhang Y, Wei Y, Yang H.. Effect of hydroxychloroquine on preeclampsia in lupus pregnancies: a propensity score-matched analysis and meta-analysis. Arch Gynecol Obstet 2021;303:435–41. [DOI] [PubMed] [Google Scholar]
- 25. Shaharir SS, Maulana SA, Shahril NS. et al. Adverse pregnancy outcomes among multi-ethnic systemic lupus erythematosus patients in Malaysia. Lupus 2020;29:1305–13. [DOI] [PubMed] [Google Scholar]
- 26. Izmirly P, Kim M, Friedman DM. et al. Hydroxychloroquine to prevent recurrent congenital heart block in fetuses of anti-SSA/Ro-positive mothers. J Am Coll Cardiol 2020;76:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Seo MR, Chae J, Kim YM. et al. Hydroxychloroquine treatment during pregnancy in lupus patients is associated with lower risk of preeclampsia. Lupus 2019;28:722–30. [DOI] [PubMed] [Google Scholar]
- 28. Mollerach FB, Scolnik M, Catoggio LJ, Rosa J, Soriano ER.. Causes of foetal third-degree atrioventricular block and use of hydroxychloroquine in pregnant women with Ro/La antibodies. Clin Rheumatol 2019;38:2211–7. [DOI] [PubMed] [Google Scholar]
- 29. Liu E, Liu Z, Zhou Y.. Feasibility of hydroxychloroquine adjuvant therapy in pregnant women with systemic lupus erythematosus. Biomed Res-Tokyo 2018;29:980–3. [Google Scholar]
- 30. Ruffatti A, Tonello M, Hoxha A. et al. Effect of additional treatments combined with conventional therapies in pregnant patients with high-risk antiphospholipid syndrome: a multicentre study. Thromb Haemost 2018;118:639–46. [DOI] [PubMed] [Google Scholar]
- 31. Buyon JP, Kim MY, Guerra MM. et al. Predictors of pregnancy outcome in a prospective, multiethnic cohort of lupus patients. Ann Intern Med 2015;163:153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kroese SJ, de Hair MJH, Limper M. et al. Hydroxychloroquine use in lupus patients during pregnancy is associated with longer pregnancy duration in preterm births. J Immunol Res 2017;2017:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sciascia S, Hunt BJ, Talavera-Garcia E. et al. The impact of hydroxychloroquine treatment on pregnancy outcome in women with antiphospholipid antibodies. Am J Obstet Gynecol 2016;214:273.e1–e8. [DOI] [PubMed] [Google Scholar]
- 34. Mekinian A, Lazzaroni MG, Kuzenko A. et al. The efficacy of hydroxychloroquine for obstetrical outcome in anti-phospholipid syndrome: data from a European multicenter retrospective study. Autoimmun Rev 2015;14:498–502. [DOI] [PubMed] [Google Scholar]
- 35. Leroux M, Desveaux C, Parcevaux M. et al. Impact of hydroxychloroquine on preterm delivery and intrauterine growth restriction in pregnant women with systemic lupus erythematosus: a descriptive cohort study. Lupus 2015;24:1384–91. [DOI] [PubMed] [Google Scholar]
- 36. Gayed M, Khamashta M, Culliford D. et al. O58. Longterm outcomes of children born to mothers with SLE exposed to hydroxychloroquine in pregnancy. Rheumatology 2014;53:i55–i. [Google Scholar]
- 37. Cooper WO, Cheetham TC, Li DK. et al. Brief report: risk of adverse foetal outcomes associated with immunosuppressive medications for chronic immune-mediated diseases in pregnancy. Arthritis Rheumatol 2014;66:444–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Luo Y, Zhang L, Fei Y. et al. Pregnancy outcome of 126 anti-SSA/Ro-positive patients during the past 24 years–a retrospective cohort study. Clin Rheumatol 2015;34:1721–8. [DOI] [PubMed] [Google Scholar]
- 39. Barsalou J, Jaeggi E, Laskin CA. et al. Prenatal exposure to antimalarials decreases the risk of cardiac but not non-cardiac neonatal lupus: a single-centre cohort study. Rheumatology 2017;56:1552–9. [DOI] [PubMed] [Google Scholar]
- 40. Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA.. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis 2010;69:20–8. [DOI] [PubMed] [Google Scholar]
- 41. Sperber K, Hom C, Chao CP, Shapiro D, Ash J.. Systematic review of hydroxychloroquine use in pregnant patients with autoimmune diseases. Pediatr Rheumatol Online J 2009;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Izmirly PM, Kim MY, Llanos C. et al. Evaluation of the risk of anti-SSA/Ro-SSB/La antibody-associated cardiac manifestations of neonatal lupus in fetuses of mothers with systemic lupus erythematosus exposed to hydroxychloroquine. Ann Rheum Dis 2010;69:1827–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Motta M, Ciardelli L, Marconi M. et al. Immune system development in infants born to mothers with autoimmune disease, exposed in utero to immunosuppressive agents. Am J Perinatol 2007;24:441–7. [DOI] [PubMed] [Google Scholar]
- 44. Cimaz R, Meregalli E, Biggioggero M. et al. Alterations in the immune system of children from mothers treated with immunosuppressive agents during pregnancy. Toxicol Lett 2004;149:155–62. [DOI] [PubMed] [Google Scholar]
- 45. Colvin L, Slack-Smith L, Stanley FJ, Bower C.. Linking a pharmaceutical claims database with a birth defects registry to investigate birth defect rates of suspected teratogens. Pharmacoepidemiol Drug Saf 2010;19:1137–50. [DOI] [PubMed] [Google Scholar]
- 46. de Man YA, Hazes JM, van der Heide H. et al. Association of higher rheumatoid arthritis disease activity during pregnancy with lower birth weight: results of a national prospective study. Arthitis Rheum 2009;60:3196–206. [DOI] [PubMed] [Google Scholar]
- 47. Diav-Citrin O, Blyakhman S, Shechtman S, Ornoy A.. Pregnancy outcome following in utero exposure to hydroxychloroquine: a prospective comparative observational study. Reprod Toxicol 2013;39:58–62. [DOI] [PubMed] [Google Scholar]
- 48. Friedman DM, Llanos C, Izmirly PM. et al. Evaluation of fetuses in a study of intravenous immunoglobulin as preventive therapy for congenital heart block: results of a multicenter, prospective, open-label clinical trial. Arthritis Rheum 2010;62:1138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Izmirly PM, Costedoat-Chalumeau N, Pisoni CN. et al. Maternal use of hydroxychloroquine is associated with a reduced risk of recurrent anti-SSA/Ro-antibody-associated cardiac manifestations of neonatal lupus. Circulation 2012;126:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kuriya B, Hernandez-Diaz S, Liu J. et al. Patterns of medication use during pregnancy in rheumatoid arthritis. Arthritis Care Res 2011;63:721–8. [DOI] [PubMed] [Google Scholar]
- 51. Motta M, Tincani A, Faden D. et al. Follow-up of infants exposed to hydroxychloroquine given to mothers during pregnancy and lactation. J Perinatol 2005;25:86–9. [DOI] [PubMed] [Google Scholar]
- 52. Viktil KK, Engeland A, Furu K.. Outcomes after anti-rheumatic drug use before and during pregnancy: a cohort study among 150,000 pregnant women and expectant fathers. Scand J Rheumatol 2012;41:196–201. [DOI] [PubMed] [Google Scholar]
- 53. Lockshin MD, Kim M, Laskin CA. et al. Prediction of adverse pregnancy outcome by the presence of lupus anticoagulant, but not anticardiolipin antibody, in patients with antiphospholipid antibodies. Arthritis Rheum 2012;64:2311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cimaz R, Meregalli E, Biggioggero M. et al. Response to tetanus vaccination in infants exposed in utero to immunosuppressants for maternal autoimmune disorders. Lupus 2007;16:129–32. [DOI] [PubMed] [Google Scholar]
- 55. Losada I, Sartori L, Di Gianantonio E. et al. Bisphosphonates in patients with autoimmune rheumatic diseases: can they be used in women of childbearing age? Autoimmun Rev 2010;9:547–52. [DOI] [PubMed] [Google Scholar]
- 56. Renault F, Flores-Guevara R, Renaud C. et al. Visual neurophysiological dysfunction in infants exposed to hydroxychloroquine in utero. Acta Paediatr 2009;98:1500–3. [DOI] [PubMed] [Google Scholar]
- 57. Anderka MT, Lin AE, Abuelo DN, Mitchell AA, Rasmussen SA.. Reviewing the evidence for mycophenolate mofetil as a new teratogen: case report and review of the literature. Am J Med Genet A 2009;149A:1241–8. [DOI] [PubMed] [Google Scholar]
- 58. David AL, Ataullah I, Yates R. et al. Congenital foetal heart block: a potential therapeutic role for intravenous immunoglobulin. Obstet Gynecol 2010;116Suppl 2:543–7. [DOI] [PubMed] [Google Scholar]
- 59. El Sebaaly Z, Charpentier B, Snanoudj R.. foetal malformations associated with mycophenolate mofetil for lupus nephritis. Nephrol Dial Transplant 2007;22:2722. [DOI] [PubMed] [Google Scholar]
- 60. Huang SY, Chueh HY, Shaw SW, Shih JC, Cheng PJ.. Sonographic diagnosis of foetal malformations associated with mycophenolate mofetil exposure in utero. Am J Obstet Gynecol 2008;199:e6-8–e8. [DOI] [PubMed] [Google Scholar]
- 61. Streit M, Speich R, Fischler M, Ulrich S.. Successful pregnancy in pulmonary arterial hypertension associated with systemic lupus erythematosus: a case report. J Med Case Rep 2009;3:7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Guo YY, Yang LL, Cui HD, Zhao S, Zhang N.. Coexisting ankylosing spondylitis and rheumatoid arthritis: a case report with literature review. Chin Med J (Engl) 2011;124:3430–2. [PubMed] [Google Scholar]
- 63. Divala TH, Mungwira RG, Mawindo PM. et al. Chloroquine as weekly chemoprophylaxis or intermittent treatment to prevent malaria in pregnancy in Malawi: a randomised controlled trial. Lancet Infect Dis 2018;18:1097–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kimani J, Phiri K, Kamiza S. et al. Efficacy and safety of azithromycin-chloroquine versus sulfadoxine-pyrimethamine for intermittent preventive treatment of plasmodium falciparum malaria infection in pregnant women in Africa: an open-label, randomized trial. PLoS One 2016;11:e0157045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chambers CD, Johnson DL, Xu R. et al. Birth outcomes in women who have taken hydroxycholoroquine in pregnancy: a prospective cohort study. Arthritis Rheumatol 2022;74:711–24. [DOI] [PubMed] [Google Scholar]
- 66. Feghali M, Venkataramanan R, Caritis S.. Pharmacokinetics of drugs in pregnancy. Semin Perinatol 2015;39:512–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Georgiou PE, Politi EN, Katsimbri P, Sakka V, Drosos AA.. Outcome of lupus pregnancy: a controlled study. Rheumatology 2000;39:1014–9. [DOI] [PubMed] [Google Scholar]
- 68. Ostensen M, Khamashta M, Lockshin M. et al. Anti-inflammatory and immunosuppressive drugs and reproduction. Arthritis Res Ther 2006;8:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Peng W, Liu R, Zhang L. et al. Breast milk concentration of hydroxychloroquine in Chinese lactating women with connective tissue diseases. Eur J Clin Pharmacol 2019;75:1547–53. [DOI] [PubMed] [Google Scholar]
- 70. Liu R, Zhang L, Mei D, Du X.. Excretion of hydroxychloroquine in milk of lactating patients. Pharmacotherapy 2016;36: e261. [Google Scholar]
- 71. Skorpen CG, Lydersen S, Gilboe IM. et al. Influence of disease activity and medications on offspring birth weight, pre-eclampsia and preterm birth in systemic lupus erythematosus: a population-based study. Ann Rheum Dis 2018;77:264–9. [DOI] [PubMed] [Google Scholar]
- 72. Kaur N, Kendall G, Saravanamuttu M, Ghosh B, Mohiyiddeen L.. A case series of prednisolone to treat unexplained recurrent miscarriage. J Assist Reprod Genet 2019;36:2611. [Google Scholar]
- 73. Skuladottir H, Wilcox AJ, Ma C. et al. Corticosteroid use and risk of orofacial clefts. Birth Defects Res A Clin Mol Teratol 2014;100:499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sun D, Shehata N, Ye XY. et al. Corticosteroids compared with intravenous immunoglobulin for the treatment of immune thrombocytopenia in pregnancy. Blood 2016;128:1329–35. [DOI] [PubMed] [Google Scholar]
- 75. Shimada H, Wakiya R, Mansour MMF. et al. FRI0209 Low-dose glucocorticoid could affect adverse pregnancy outcomes, especially in preterm birth, light-for-date newborns, preterm premature rupture of membrane in connective tissue disease patients. Ann Rheum Dis 2019;78(Suppl 2):783–4. [Google Scholar]
- 76. Ryu RJ, Easterling TR, Caritis SN. et al. Prednisone pharmacokinetics during pregnancy and lactation. J Clin Pharmacol 2018;58:1223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Miranda D, Saavedra MA, Gomez E. et al. Impact of glucocorticoid dose on maternal and foetal outcomes in lupus pregnancies. Arthritis Rheumatol 2014;66:S1169. [Google Scholar]
- 78. Ban L, Tata LJ, Fiaschi L, Card T.. Limited risks of major congenital anomalies in children of mothers with IBD and effects of medications. Gastroenterology 2014;146:76–84. [DOI] [PubMed] [Google Scholar]
- 79. Xu X, Liang MY, Dou S, Wang JL, Zhang XH.. Evaluation of glucocorticoid compared with immunoglobulin therapy of severe immune thrombocytopenia during pregnancy: response rate and complication. Am J Reprod Immunol 2018;80:e13000. [DOI] [PubMed] [Google Scholar]
- 80. Palmsten K, Bandoli G, Vazquez-Benitez G. et al. Oral corticosteroid use during pregnancy and risk of preterm birth. Rheumatology 2020;59:1262–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zengin Karahan S, Boz C, Terzi M. et al. Methylprednisolone concentrations in breast milk and serum of patients with multiple sclerosis treated with IV pulse methylprednisolone. Clin Neurol Neurosurg 2020;197:106118. [DOI] [PubMed] [Google Scholar]
- 82. Empson M, Lassere M, Craig J, Scott J.. Prevention of recurrent miscarriage for women with antiphospholipid antibody or lupus anticoagulant. Cochrane Database Syst Rev 2005;2005:CD002859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kardos M, Levine D, Gurcan HM, Ahmed RA.. Pemphigus vulgaris in pregnancy: analysis of current data on the management and outcomes. Obstet Gynecol Surv 2009;64:739–49. [DOI] [PubMed] [Google Scholar]
- 84. Goetzl L, Zighelboim I, Badell M. et al. Maternal corticosteroids to prevent intrauterine exposure to hyperthermia and inflammation: a randomized, double-blind, placebo-controlled trial. Am J Obstet Gynecol 2006;195:1031–7. [DOI] [PubMed] [Google Scholar]
- 85. Fawzy M, Shokeir T, El-Tatongy M. et al. Treatment options and pregnancy outcome in women with idiopathic recurrent miscarriage: a randomized placebo-controlled study. Arch Gynecol Obstet 2008;278:33–8. [DOI] [PubMed] [Google Scholar]
- 86. van Runnard Heimel PJ, Huisjes AJ, Franx A. et al. A randomised placebo-controlled trial of prolonged prednisolone administration to patients with HELLP syndrome remote from term. Eur J Obstet Gynecol Reprod Biol 2006;128:187–93. [DOI] [PubMed] [Google Scholar]
- 87. van Runnard Heimel PJ, Schobben AF, Huisjes AJ, Franx A, Bruinse HW.. The transplacental passage of prednisolone in pregnancies complicated by early-onset HELLP syndrome. Placenta 2005;26:842–5. [DOI] [PubMed] [Google Scholar]
- 88. Hussein SZ, Jacobsson LT, Lindquist PG, Theander E.. Pregnancy and foetal outcome in women with primary Sjogren's syndrome compared with women in the general population: a nested case-control study. Rheumatology 2011;50:1612–7. [DOI] [PubMed] [Google Scholar]
- 89. Egerman RS, Ramsey RD, Kao LW. et al. Hypertensive disease in pregnancies complicated by systemic lupus erythematosus. Am J Obstet Gynecol 2005;193:1676–9. [DOI] [PubMed] [Google Scholar]
- 90. Clowse ME, Magder L, Witter F, Petri M.. Hydroxychloroquine in lupus pregnancy. Arthritis Rheum 2006;54:3640–7. [DOI] [PubMed] [Google Scholar]
- 91. Cassina M, Johnson DL, Robinson LK. et al. Pregnancy outcome in women exposed to leflunomide before or during pregnancy. Arthritis Rheum 2012;64:2085–94. [DOI] [PubMed] [Google Scholar]
- 92. Perales-Puchalt A, Vila Vives JM, López Montes J, Diago Almela VJ, Perales A.. Pregnancy outcomes after kidney transplantation-immunosuppressive therapy comparison. J Matern foetal Neonatal Med 2012;25:1363–6. [DOI] [PubMed] [Google Scholar]
- 93. Terrabuio DR, Abrantes-Lemos CP, Carrilho FJ, Cancado EL.. Follow-up of pregnant women with autoimmune hepatitis: the disease behavior along with maternal and foetal outcomes. J Clin Gastroenterol 2009;43:350–6. [DOI] [PubMed] [Google Scholar]
- 94. Chi C-C, Wang S-H, Charles-Holmes R. et al. Pemphigoid gestationis: early onset and blister formation are associated with adverse pregnancy outcomes. Br J Dermatol 2009;160:1222–8. [DOI] [PubMed] [Google Scholar]
- 95. Ghafari A, Sanadgol H.. Pregnancy after renal transplantation: ten-year single-center experience. Transplant Proc 2008;40:251–2. [DOI] [PubMed] [Google Scholar]
- 96. Brucato A, Imazio M, Curri S, Palmieri G, Trinchero R.. Medical treatment of pericarditis during pregnancy. Int J Cardiol 2010;144:413–4. [DOI] [PubMed] [Google Scholar]
- 97. Pisoni CN, Brucato A, Ruffatti A. et al. Failure of intravenous immunoglobulin to prevent congenital heart block: findings of a multicenter, prospective, observational study. Arthritis Rheum 2010;62:1147–52. [DOI] [PubMed] [Google Scholar]
- 98. Makino S, Yonemoto H, Itoh S, Takeda S.. Effect of steroid administration and plasmapheresis to prevent foetal congenital heart block in patients with systemic lupus erythematosus and/or Sjogren's syndrome. Acta Obstet Gynecol Scand 2007;86:1145–6. [DOI] [PubMed] [Google Scholar]
- 99. Merlob P, Stahl B, Klinger G.. Tetrada of the possible mycophenolate mofetil embryopathy: a review. Reprod Toxicol 2009;28:105–8. [DOI] [PubMed] [Google Scholar]
- 100. Natsumi I, Matsukawa Y, Miyagawa K. et al. Successful childbearing in two women with rheumatoid arthritis and a history of miscarriage after etanercept treatment. Rheumatol Int 2013;33:2433–5. [DOI] [PubMed] [Google Scholar]
- 101. Moretti ME, Verjee Z, Ito S, Koren G.. Breast-feeding during maternal use of azathioprine. Ann Pharmacother 2006;40:2269–72. [DOI] [PubMed] [Google Scholar]
- 102. Lannes G, Elias FR, Cunha B. et al. Successful pregnancy after cyclophosphamide therapy for lupus nephritis. Arch Gynecol Obstet 2011;283(Suppl 1):61–5. [DOI] [PubMed] [Google Scholar]
- 103. Bramham K, Thomas M, Nelson-Piercy C, Khamashta M, Hunt BJ.. First-trimester low-dose prednisolone in refractory antiphospholipid antibody-related pregnancy loss. Blood 2011;117:6948–51. [DOI] [PubMed] [Google Scholar]
- 104. Mecacci F, Bianchi B, Pieralli A. et al. Pregnancy outcome in systemic lupus erythematosus complicated by anti-phospholipid antibodies. Rheumatology 2009;48:246–9. [DOI] [PubMed] [Google Scholar]
- 105. El-Haieg DO, Zanati MF, El-Foual FM.. Plasmapheresis and pregnancy outcome in patients with antiphospholipid syndrome. Int J Gynaecol Obstet 2007;99:236–41. [DOI] [PubMed] [Google Scholar]
- 106. Adam FU, Torun D, Bolat F. et al. Acute renal failure due to mesangial proliferative glomerulonephritis in a pregnant woman with primary Sjogren's syndrome. Clin Rheumatol 2006;25:75–9. [DOI] [PubMed] [Google Scholar]
- 107. Alsuwaida A. Successful management of systemic lupus erythematosus nephritis flare-up during pregnancy with tacrolimus. Mod Rheumatol 2011;21:73–5. [DOI] [PubMed] [Google Scholar]
- 108. Murashima A, Watanabe N, Ozawa N, Saito H, Yamaguchi K.. Etanercept during pregnancy and lactation in a patient with rheumatoid arthritis: drug levels in maternal serum, cord blood, breast milk and the infant's serum. Ann Rheum Dis 2009;68:1793–4. [DOI] [PubMed] [Google Scholar]
- 109. Hemmati I, Stephanie E, Shojania K.. Coarctation of the aorta in an infant exposed to etanercept in utero. J Rheumatol 2009;36:2848. [DOI] [PubMed] [Google Scholar]
- 110. Kwak-Kim J, Lee SK, Gilman-Sachs A.. Elevated Th1/Th2 cell ratios in a pregnant woman with a history of RSA, secondary Sjogren's syndrome and rheumatoid arthritis complicated with one foetal demise of twin pregnancy. Am J Reprod Immunol 2007;58:325–9. [DOI] [PubMed] [Google Scholar]
- 111. Lazalde B, Grijalva-Flores J, Guerrero-Romero F.. Klippel-Feil syndrome in a boy exposed inadvertently to cyclophosphamide during pregnancy: a case report. Birth Defects Res A Clin Mol Teratol 2012;94:249–52. [DOI] [PubMed] [Google Scholar]
- 112. Mutsukura K, Nakamura H, Iwanaga N. et al. Successful treatment of a patient with primary Sjogren's syndrome complicated with pericarditis during pregnancy. Intern Med 2007;46:1143–7. [DOI] [PubMed] [Google Scholar]
- 113. Schoner K, Steinhard J, Figiel J, Rehder H.. Severe facial clefts in acrofacial dysostosis: a consequence of prenatal exposure to mycophenolate mofetil? Obstet Gynecol 2008;111:483–6. [DOI] [PubMed] [Google Scholar]
- 114. Somalanka S, Tawil M, Baikunje S.. Oesophageal anomaly in a newborn after maternal exposure to mycofenolate mofetil. BMJ Case Rep 2009;2009:bcr04.2009.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Watanabe R, Shirai T, Tajima Y. et al. Pregnancy-associated thrombotic thrombocytopenic purpura with anti-centromere antibody-positive Raynaud's syndrome. Intern Med 2010;49:1229–32. [DOI] [PubMed] [Google Scholar]
- 116. Parisi MA, Zayed H, Slavotinek AM, Rutledge JC.. Congenital diaphragmatic hernia and microtia in a newborn with mycophenolate mofetil (MMF) exposure: phenocopy for Fryns syndrome or broad spectrum of teratogenic effects? Am J Med Genet A 2009;149A:1237–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Noh JJ, Park CH, Jo MH, Kwon JY.. Rupture of an unscarred uterus in a woman with long-term steroid treatment for systemic lupus erythematosus. Obstet Gynecol 2013;122:472–5. [DOI] [PubMed] [Google Scholar]
- 118. Brownfoot FC, Gagliardi DI, Bain E, Middleton P, Crowther CA.. Different corticosteroids and regimens for accelerating foetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 2013;8:CD006764. [DOI] [PubMed] [Google Scholar]
- 119. Mackeen AD, Seibel-Seamon J, Grimes-Dennis J, Baxter JK, Berghella V.. Tocolytics for preterm premature rupture of membranes. Cochrane Database Syst Rev 2011;CD007062. [DOI] [PubMed] [Google Scholar]
- 120. Roberge S, Lacasse Y, Tapp S. et al. Role of foetal sex in the outcome of antenatal glucocorticoid treatment to prevent respiratory distress syndrome: systematic review and meta-analysis. J Obstet Gynaecol Can 2011;33:216–26. [DOI] [PubMed] [Google Scholar]
- 121. Woudstra DM, Chandra S, Hofmeyr GJ, Dowswell T.. Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy. Cochrane Database Syst Rev 2010;CD008148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kavanagh J, Kelly AJ, Thomas J.. Corticosteroids for cervical ripening and induction of labour. Cochrane Database Syst Rev 2006;CD003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Katz L, de Amorim MM, Figueiroa JN, Pinto e Silva JL.. Postpartum dexamethasone for women with hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome: a double-blind, placebo-controlled, randomized clinical trial. Am J Obstet Gynecol 2008;198:283 e1–8. [DOI] [PubMed] [Google Scholar]
- 124. Fesslova V, Vignati G, Brucato A. et al. The impact of treatment of the fetus by maternal therapy on the foetal and postnatal outcomes for fetuses diagnosed with isolated complete atrioventricular block. Cardiol Young 2009;19:282–90. [DOI] [PubMed] [Google Scholar]
- 125. Ferguson S, Allen VM, Craig C, Allen AC, Dodds L.. Timing of indicated delivery after antenatal steroids in preterm pregnancies with severe hypertension. Hypertens Pregnancy 2009;28:63–75. [DOI] [PubMed] [Google Scholar]
- 126. Carlo WA, McDonald SA, Fanaroff AA. et al. Association of antenatal corticosteroids with mortality and neurodevelopmental outcomes among infants born at 22 to 25 weeks' gestation. JAMA 2011;306:2348–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Cuneo BF, Lee M, Roberson D. et al. A management strategy for foetal immune-mediated atrioventricular block. J Matern foetal Neonatal Med 2010;23:1400–5. [DOI] [PubMed] [Google Scholar]
- 128. Eliasson H, Sonesson SE, Sharland G. et al. Isolated atrioventricular block in the fetus: a retrospective, multinational, multicenter study of 175 patients. Circulation 2011;124:1919–26. [DOI] [PubMed] [Google Scholar]
- 129. Friedman DM, Kim MY, Copel JA. et al. Utility of cardiac monitoring in fetuses at risk for congenital heart block: the PR Interval and Dexamethasone Evaluation (PRIDE) prospective study. Circulation 2008;117:485–93. [DOI] [PubMed] [Google Scholar]
- 130. Hayes EJ, Paul DA, Stahl GE. et al. Effect of antenatal corticosteroids on survival for neonates born at 23 weeks of gestation. Obstet Gynecol 2008;111:921–6. [DOI] [PubMed] [Google Scholar]
- 131. Kamath-Rayne BD, DeFranco EA, Marcotte MP.. Antenatal steroids for treatment of foetal lung immaturity after 34 weeks of gestation: an evaluation of neonatal outcomes. Obstet Gynecol 2012;119:909–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Liu J, Feng ZC, Li J, Wang Q.. Antenatal dexamethasone has no adverse effects on child physical and cognitive development: a long-term cohort follow-up investigation. J Matern foetal Neonatal Med 2012;25:2369–71. [DOI] [PubMed] [Google Scholar]
- 133. Trucco SM, Jaeggi E, Cuneo B. et al. Use of intravenous gamma globulin and corticosteroids in the treatment of maternal autoantibody-mediated cardiomyopathy. J Am Coll Cardiol 2011;57:715–23. [DOI] [PubMed] [Google Scholar]
- 134. Rein AJ, Mevorach D, Perles Z. et al. Early diagnosis and treatment of atrioventricular block in the fetus exposed to maternal anti-SSA/Ro-SSB/La antibodies: a prospective, observational, foetal kinetocardiogram-based study. Circulation 2009;119:1867–72. [DOI] [PubMed] [Google Scholar]
- 135. Biggioggero M, Borghi MO, Gerosa M. et al. Immune function in children born to mothers with autoimmune diseases and exposed in utero to immunosuppressants. Lupus 2007;16:651–6. [DOI] [PubMed] [Google Scholar]
- 136. Meregalli E, Biggioggero M, Borghi O, Meroni P, Cimaz R.. In vivo effects of maternal immunosuppression during pregnancy on the immune function of newborn infants. Arh Hig Rada Toksikol 2005;56:151–6. [PubMed] [Google Scholar]
- 137. Hirvikoski T, Nordenstrom A, Lindholm T. et al. Long-term follow-up of prenatally treated children at risk for congenital adrenal hyperplasia: does dexamethasone cause behavioural problems? Eur J Endocrinol 2008;159:309–16. [DOI] [PubMed] [Google Scholar]
- 138. Meyer-Bahlburg HF, Dolezal C, Haggerty R, Silverman M, New MI.. Cognitive outcome of offspring from dexamethasone-treated pregnancies at risk for congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Eur J Endocrinol 2012;167:103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. New MI, Abraham M, Yuen T, Lekarev O.. An update on prenatal diagnosis and treatment of congenital adrenal hyperplasia. Semin Reprod Med 2012;30:396–9. [DOI] [PubMed] [Google Scholar]
- 140. Shanks A, Gross G, Shim T. et al. Administration of steroids after 34 weeks of gestation enhances foetal lung maturity profiles. Am J Obstet Gynecol 2010;203:47 e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Brucato A, Astori MG, Cimaz R. et al. Normal neuropsychological development in children with congenital complete heart block who may or may not be exposed to high-dose dexamethasone in utero. Ann Rheum Dis 2006;65:1422–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Adams LL, Gungor S, Salim M, Harman CR, Baschat AA.. Regression of foetal heart block and myocardial echogenicity with steroid therapy in maternal Sjogren's syndrome. Ultrasound Obstet Gynecol 2008;32:839–40. [DOI] [PubMed] [Google Scholar]
- 143. Carbonne B, Mace G, Cynober E, Milliez J, Cabane J.. Successful pregnancy with the use of nitric oxide donors and heparin after recurrent severe preeclampsia in a woman with scleroderma. Am J Obstet Gynecol 2007;197:e6–7. [DOI] [PubMed] [Google Scholar]
- 144. Claus R, Hickstein H, Kulz T. et al. Identification and management of fetuses at risk for, or affected by, congenital heart block associated with autoantibodies to SSA (Ro), SSB (La), or an HsEg5-like autoantigen. Rheumatol Int 2006;26:886–95. [DOI] [PubMed] [Google Scholar]
- 145. Crowther CA, McKinlay CJ, Middleton P, Harding JE.. Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes. Cochrane Database Syst Rev 2015;2015:CD003935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Roberts D, Brown J, Medley N, Dalziel S.. Antenatal corticosteroids for accelerating foetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 2017;3:CD004454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Sotiriadis A, Makrydimas G, Papatheodorou S, Ioannidis JP, McGoldrick E.. Corticosteroids for preventing neonatal respiratory morbidity after elective caesarean section at term. Cochrane Database Syst Rev 2018;8:CD006614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Marti-Carvajal AJ, Pena-Marti GE, Comunian-Carrasco G, Cochrane Pregnancy and Childbirth Group. Medical treatments for idiopathic thrombocytopenic purpura during pregnancy. Cochrane Database Syst Rev 2009;CD007722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Crowther CA, Haslam RR, Hiller JE, Doyle LW, Robinson JS.. Australasian Collaborative Trial of Repeat Doses of Steroids Study G. Neonatal respiratory distress syndrome after repeat exposure to antenatal corticosteroids: a randomised controlled trial. Lancet 2006;367:1913–9. [DOI] [PubMed] [Google Scholar]
- 150. Murphy KE, Willan AR, Hannah ME. et al. Effect of antenatal corticosteroids on foetal growth and gestational age at birth. Obstet Gynecol 2012;119:917–23. [DOI] [PubMed] [Google Scholar]
- 151. Porto AM, Coutinho IC, Correia JB, Amorim MM.. Effectiveness of antenatal corticosteroids in reducing respiratory disorders in late preterm infants: randomised clinical trial. BMJ 2011;342:d1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Ozer A, Kanat-Pektas M, Ozer S. et al. The effects of betamethasone treatment on clinical and laboratory features of pregnant women with HELLP syndrome. Arch Gynecol Obstet 2009;280:65–70. [DOI] [PubMed] [Google Scholar]
- 153. Balci O, Ozdemir S, Mahmoud AS, Acar A, Colakoglu MC.. The effect of antenatal steroids on foetal lung maturation between the 34th and 36th week of pregnancy. Gynecol Obstet Invest 2010;70:95–9. [DOI] [PubMed] [Google Scholar]
- 154. Blickstein I, Reichman B, Lusky A, Shinwell ES, Israel Neonatal Network. Plurality-dependent risk of severe intraventricular hemorrhage among very low birth weight infants and antepartum corticosteroid treatment. Am J Obstet Gynecol 2006;194:1329–33. [DOI] [PubMed] [Google Scholar]
- 155. Giannubilo SR, Shkara VA, Tranquilli AL.. Effect of betametasone administration on platelet count in thrombocytopenic and normal pregnant women. Arch Gynecol Obstet 2006;274:130–2. [DOI] [PubMed] [Google Scholar]
- 156. Henderson JJ, Hartmann PE, Newnham JP, Simmer K.. Effect of preterm birth and antenatal corticosteroid treatment on lactogenesis II in women. Pediatrics 2008;121:e92-100–e100. [DOI] [PubMed] [Google Scholar]
- 157. Bontis N, Vavilis D, Tsolakidis D, Goulis DG. et al. Comparison of single versus multiple courses of antenatal betamethasone in patients with threatened preterm labor. Clin Exp Obstet Gynecol 2011;38:165–7. [PubMed] [Google Scholar]
- 158. Carreno CA, Refuerzo JS, Holland MG, Ramin SM. et al. The frequency of prior antenatal corticosteroid therapy in late preterm birth pregnancies. Am J Perinatol 2011;28:767–72. [DOI] [PubMed] [Google Scholar]
- 159. Hjalmarson O, Sandberg KL.. Effect of antenatal corticosteroid treatment on lung function in full-term newborn infants. Neonatology 2011;100:32–6. [DOI] [PubMed] [Google Scholar]
- 160. Wang YC, Tseng HI, Yang SN. et al. Effects of antenatal corticosteroids on neonatal outcomes in very-low-birth-weight preterm newborns: a 10-year retrospective study in a medical center. Pediatr Neonatol 2012;53:178–83. [DOI] [PubMed] [Google Scholar]
- 161. Stalnacke J, Diaz Heijtz R, Norberg H. et al. Cognitive outcome in adolescents and young adults after repeat courses of antenatal corticosteroids. J Pediatr 2013;163:441–6. [DOI] [PubMed] [Google Scholar]
- 162. Battin M, Bevan C, Harding J.. Growth in the neonatal period after repeat courses of antenatal corticosteroids: data from the ACTORDS randomised trial. Arch Dis Childhood foetal Neonatal Ed 2012;97:F99–105. [DOI] [PubMed] [Google Scholar]
- 163. Vigil-De Gracia P, Reyes Tejada O, Calle Minaca A. et al. Expectant management of severe preeclampsia remote from term: the MEXPRE Latin Study, a randomized, multicenter clinical trial. Am J Obstet Gynecol 2013;209:425.e1–8. [DOI] [PubMed] [Google Scholar]
- 164. Hviid A, Molgaard-Nielsen D.. Corticosteroid use during pregnancy and risk of orofacial clefts. Canad Med Assoc J 2011;183:796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Branche J, Cortot A, Bourreille A. et al. Cyclosporine treatment of steroid-refractory ulcerative colitis during pregnancy. Inflamm Bowel Dis 2009;15:1044–8. [DOI] [PubMed] [Google Scholar]
- 166. Unterberger I, Trinka E, Engelhardt K. et al. Linear scleroderma “en coup de sabre” coexisting with plaque-morphea: neuroradiological manifestation and response to corticosteroids. J Neurol Neurosurg Psychiatry 2003;74:661–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Celikbilek M, Elsurer R, Afsar B. et al. Mixed connective tissue disease: a case with scleroderma renal crisis following abortion. Clin Rheumatol 2007;26:1545–7. [DOI] [PubMed] [Google Scholar]
- 168. Aslan E, Tarim E, Kilicdag E, Simsek E.. Sjogren's syndrome diagnosed in pregnancy: a case report. J Reprod Med 2005;50:67–70. [PubMed] [Google Scholar]
- 169. Pfizer. Summary of product characteristics. Methylprednisolone. 2013. https://www.medicines.org.uk/emc/product/3067/ (1 September 2022, date last accessed).
- 170. Jaeggi ET, Fouron JC, Silverman ED. et al. Transplacental foetal treatment improves the outcome of prenatally diagnosed complete atrioventricular block without structural heart disease. Circulation 2004;110:1542–8. [DOI] [PubMed] [Google Scholar]
- 171. UK Teratology Information Service. UKTIS. http://www.uktis.org/ (1 September 2022, date last accessed).
- 172. Casanova MJ, Chaparro M, Domenech E. et al. Safety of thiopurines and anti-TNF-alpha drugs during pregnancy in patients with inflammatory bowel disease. Am J Gastroenterol 2013;108:433–40. [DOI] [PubMed] [Google Scholar]
- 173. Zelinkova Z, de Haar C, de Ridder L. et al. High intra-uterine exposure to infliximab following maternal anti-TNF treatment during pregnancy. Aliment Pharmacol Ther 2011;33:1053–8. [DOI] [PubMed] [Google Scholar]
- 174. Lorenz LB, Knudtson EJ.. Anomalies in a fetus exposed to methotrexate in the first trimester. Am J Pharmacol Toxicol 2007;2:146–7. [Google Scholar]
- 175. Westhovens R, Robles M, Ximenes AC. et al. Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Ann Rheum Dis 2009;68:1870–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Corona-Rivera JR, Rea-Rosas A, Santana RA. et al. Holoprosencephaly and genitourinary anomalies in foetal methotrexate syndrome. Am J Med Genet A 2010;152a:1741–6. [DOI] [PubMed] [Google Scholar]
- 177. Piggott KD, Sorbello A, Riddle E, DeCampli W.. Congenital cardiac defects: a possible association of aminopterin syndrome and in utero methotrexate exposure? Pediatr Cardiol 2011;32:518–20. [DOI] [PubMed] [Google Scholar]
- 178. Neeman N, Aronson MD, Schulze JE, Shmerling RH.. Improving pregnancy counseling for women with rheumatoid arthritis taking methotrexate. Am J Med 2009;122:998–1000. [DOI] [PubMed] [Google Scholar]
- 179. Weber-Schoendorfer C, Chambers C, Wacker E. et al. Pregnancy outcome after methotrexate treatment for rheumatic disease prior to or during early pregnancy: a prospective multicenter cohort study. Arthritis Rheumatol 2014;66:1101–10. [DOI] [PubMed] [Google Scholar]
- 180. Brouwer J, Laven JS, Hazes JM, Dolhain RJ.. Brief report: miscarriages in female rheumatoid arthritis patients: associations with serologic findings, disease activity, and antirheumatic drug treatment. Arthritis Rheumatol 2015;67:1738–43. [DOI] [PubMed] [Google Scholar]
- 181. Dawson AL, Riehle-Colarusso T, Reefhuis J, Arena JF, National Birth Defects Prevention Study. Maternal exposure to methotrexate and birth defects: a population-based study. Am J Med Genet A 2014;164A:2212–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Rajaei E, Shahbazian N, Rezaeeyan H. et al. The effect of lupus disease on the pregnant women and embryos: a retrospective study from 2010 to 2014. Clin Rheumatol 2019;38:3211–5. [DOI] [PubMed] [Google Scholar]
- 183. Vázquez ER, Martín CA, Arteaga LC, Ferrer MAA.. Outcomes of pregnancies exposed to methotrexate . Basic Clin Pharmacol Toxicol 2018;123:24–64. [Google Scholar]
- 184. Winter RW, Larsen MD, Magnussen B. et al. Birth outcomes after preconception paternal exposure to methotrexate: a nationwide cohort study. Reprod Toxicol 2017;74:219–23. [DOI] [PubMed] [Google Scholar]
- 185. Egeberg A, Gislason GH, Nast A.. Birth outcomes in children fathered by men treated with immunosuppressant drugs before conception - A Danish population-based cohort study. J Invest Dermatol 2017;137:1790–2. [DOI] [PubMed] [Google Scholar]
- 186. Andersen J, Askaa B, Jensen T. et al. P06 Paternal exposure to methotrexate and the risk of miscarriage – a register based nationwide cohort study. Arch Dis Childhood 2019;104:e19–20. [Google Scholar]
- 187. Friedman S, Larsen MD, Magnussen B. et al. Paternal use of azathioprine/6-mercaptopurine or methotrexate within 3 months before conception and long-term health outcomes in the offspring-a nationwide cohort study. Reprod Toxicol 2017;73:196–200. [DOI] [PubMed] [Google Scholar]
- 188. Weber-Schoendorfer C, Hoeltzenbein M, Wacker E, Meister R, Schaefer C.. No evidence for an increased risk of adverse pregnancy outcome after paternal low-dose methotrexate: an observational cohort study. Rheumatology 2014;53:757–63. [DOI] [PubMed] [Google Scholar]
- 189. Eck LK, Jensen TB, Mastrogiannis D. et al. Risk of adverse pregnancy outcome after paternal exposure to methotrexate within 90 days before pregnancy. Obstetr Gynecol 2017;129:707–14. [DOI] [PubMed] [Google Scholar]
- 190. Baker T, Datta P, Rewers-Felkins K, Hale TW.. High-dose methotrexate treatment in a breastfeeding mother with placenta accreta: a case report. Breastfeed Med 2018;13:450–2. [DOI] [PubMed] [Google Scholar]
- 191. Thorne JC, Nadarajah T, Moretti M, Ito S.. Methotrexate use in a breastfeeding patient with rheumatoid arthritis. J Rheumatol 2014;41:2332. [DOI] [PubMed] [Google Scholar]
- 192. Drugs and Lactation Database. LactMed. https://www.ncbi.nlm.nih.gov/books/NBK501922/ (1 September 2022, date last accessed).
- 193. Sng BL, Shah MK.. Regional anaesthesia for Caesarean section in an ankylosing spondylitic patient with twin pregnancy. Eur J Anaesthesiol 2008;25:767–9. [DOI] [PubMed] [Google Scholar]
- 194. Chambers CD, Johnson DL, Robinson LK. et al. Birth outcomes in women who have taken leflunomide during pregnancy. Arthritis Rheum 2010;62:1494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Neville CE, McNally J.. Maternal exposure to leflunomide associated with blindness and cerebral palsy. Rheumatology 2007;46:1506. [DOI] [PubMed] [Google Scholar]
- 196. Heine K, Poets CF.. A pair of twins born after maternal exposure to leflunomide. J Perinatol 2008;28:841–2. [DOI] [PubMed] [Google Scholar]
- 197. Sayarlioglu M, Sahin M, Cetin GY, Avan R, Cerit M.. Maternal exposure to leflunomide and methotrexate in a patient with adult-onset Still's disease. Rheumatology 2010;49:1787–9. [DOI] [PubMed] [Google Scholar]
- 198. Hajdyla-Banas I, Banas T, Rydz-Stryszowska I. et al. Pregnancy course and neonatal outcome after exposure to leflunomide–2 cases report and review of literature. Przegl Lek 2009;66:1069–71. [PubMed] [Google Scholar]
- 199. Weber-Schoendorfer C, Beck E, Tissen-Diabate T, Schaefer C.. Leflunomide - a human teratogen? A still not answered question. An evaluation of the German Embryotox pharmacovigilance database. Reprod Toxicol 2017;71:101–7. [DOI] [PubMed] [Google Scholar]
- 200. Berard A, Zhao JP, Shui I, Colilla S.. Leflunomide use during pregnancy and the risk of adverse pregnancy outcomes. Ann Rheum Dis 2018;77:500–9. [DOI] [PubMed] [Google Scholar]
- 201. Henson LJ, Afsar S, Davenport L. et al. Pregnancy outcomes in patients treated with leflunomide, the parent compound of the multiple sclerosis drug teriflunomide. Reprod Toxicol 2020;95:45–50. [DOI] [PubMed] [Google Scholar]
- 202. Vukusic S, Coyle PK, Jurgensen S. et al. Pregnancy outcomes in patients with multiple sclerosis treated with teriflunomide: linical study data and 5 years of post-marketing experience. Mult Scler 2020;26:829–36. [DOI] [PubMed] [Google Scholar]
- 203. Goldstein LH, Dolinsky G, Greenberg R. et al. Pregnancy outcome of women exposed to azathioprine during pregnancy. Birth Defects Res A Clin Mol Teratol 2007;79:696–701. [DOI] [PubMed] [Google Scholar]
- 204. Fischer-Betz R, Specker C, Brinks R, Aringer M, Schneider M.. Low risk of renal flares and negative outcomes in women with lupus nephritis conceiving after switching from mycophenolate mofetil to azathioprine. Rheumatology 2013;52:1070–6. [DOI] [PubMed] [Google Scholar]
- 205. Shim L, Eslick GD, Simring AA, Murray H, Weltman MD.. The effects of azathioprine on birth outcomes in women with inflammatory bowel disease (IBD). J Crohns Colitis 2011;5:234–8. [DOI] [PubMed] [Google Scholar]
- 206. de Meij TG, Jharap B, Kneepkens CM. et al. Long-term follow-up of children exposed intrauterine to maternal thiopurine therapy during pregnancy in females with inflammatory bowel disease. Aliment Pharmacol Ther 2013;38:38–43. [DOI] [PubMed] [Google Scholar]
- 207. Triantafillidis JK, Malgarinos G, Gikas A. et al. Pregnancy and inflammatory bowel disease in Greece: A prospective study of seven cases in a single hospital setting. Ann Gastroenterol 2007;20:1. [Google Scholar]
- 208. Rosner I, Haddad A, Boulman N. et al. Pregnancy in rheumatology patients exposed to anti-tumour necrosis factor (TNF)-alpha therapy. Rheumatology 2007;46:1508. [DOI] [PubMed] [Google Scholar]
- 209. Christensen LA, Dahlerup JF, Nielsen MJ, Fallingborg JF, Schmiegelow K.. Azathioprine treatment during lactation. Aliment Pharmacol Ther 2008;28:1209–13. [DOI] [PubMed] [Google Scholar]
- 210. Sau A, Clarke S, Bass J. et al. Azathioprine and breastfeeding: is it safe? BJOG 2007;114:498–501. [DOI] [PubMed] [Google Scholar]
- 211. Gardiner SJ, Gearry RB, Roberts RL. et al. Exposure to thiopurine drugs through breast milk is low based on metabolite concentrations in mother-infant pairs. Br J Clin Pharmacol 2006;62:453–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Mahadevan U, Martin CF, Chambers C. et al. 1 Achievement of developmental milestones among offspring of women with inflammatory bowel disease: the PIANO registry. Gastroenterology 2014;146:S-1. [Google Scholar]
- 213. Saavedra MÁ, Sánchez A, Morales S, Ángeles U, Jara LJ.. Azathioprine during pregnancy in systemic lupus erythematosus patients is not associated with poor foetal outcome. Clin Rheumatol 2015;34:1211–6. [DOI] [PubMed] [Google Scholar]
- 214. Jharap B, de Boer NKH, Stokkers P. et al. Intrauterine exposure and pharmacology of conventional thiopurine therapy in pregnant patients with inflammatory bowel disease. Gut 2014;63:451–7. [DOI] [PubMed] [Google Scholar]
- 215. Thai TN, Sarayani A, Wang X. et al. Risk of pregnancy loss in patients exposed to mycophenolate compared to azathioprine: a retrospective cohort study. Pharmacoepidemiol Drug Saf 2020;29:716–24. [DOI] [PubMed] [Google Scholar]
- 216. Norgard BM, Magnussen B, Larsen MD, Friedman S.. Reassuring results on birth outcomes in children fathered by men treated with azathioprine/6-mercaptopurine within 3 months before conception: a nationwide cohort study. Gut 2017;66:1761–6. [DOI] [PubMed] [Google Scholar]
- 217. Chandra A, Midtvedt K, Åsberg A, Eide IA.. Immunosuppression and Reproductive Health After Kidney Transplantation. Transplantation 2019;103:e325–33. [DOI] [PubMed] [Google Scholar]
- 218. Osadchy A, Koren G.. Cyclosporine and lactation: when the mother is willing to breastfeed. Ther Drug Monit 2011;33:147–8. [DOI] [PubMed] [Google Scholar]
- 219. Westbrook RH, Yeoman AD, Agarwal K. et al. Outcomes of pregnancy following liver transplantation: the King's College Hospital experience. Liver Transpl 2015;21:1153–9. [DOI] [PubMed] [Google Scholar]
- 220. Reggia R, Bazzani C, Andreoli L. et al. The efficacy and safety of cyclosporin A in Pregnant Patients with Systemic Autoimmune Diseases. Am J Reprod Immunol 2016;75:654–60. [DOI] [PubMed] [Google Scholar]
- 221. Kociszewska-Najman B, Mazanowska N, Borek-Dzieciol B. et al. Low content of cyclosporine A and its metabolites in the colostrum of post-transplant mothers. Nutrients 2020;12:2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Ostensen M, Lockshin M, Doria A. et al. Update on safety during pregnancy of biological agents and some immunosuppressive anti-rheumatic drugs. Rheumatology 2008;47(Suppl 3):iii28–31. [DOI] [PubMed] [Google Scholar]
- 223. Zheng S, Easterling TR, Hays K. et al. Tacrolimus placental transfer at delivery and neonatal exposure through breast milk. Br J Clin Pharmacol 2013;76:988–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Bramham K, Chusney G, Lee J, Lightstone L, Nelson-Piercy C.. Breastfeeding and tacrolimus: serial monitoring in breast-fed and bottle-fed infants. Clin J Am Soc Nephrol 2013;8:563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Hiramatsu Y, Yoshida S, Kotani T. et al. Changes in the blood level, efficacy, and safety of tacrolimus in pregnancy and the lactation period in patients with systemic lupus erythematosus. Lupus 2018;27:2245–52. [DOI] [PubMed] [Google Scholar]
- 226. Kociszewska-Najman B, Mazanowska N, Pietrzak B. et al. Low transfer of tacrolimus and its metabolites into colostrum of graft recipient mothers. Nutrients 2018;10:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Nakagawa K, Kwak-Kim J, Hisano M. et al. Obstetric and perinatal outcome of the women with repeated implantation failures or recurrent pregnancy losses who received pre- and post-conception tacrolimus treatment. Am J Reprod Immunol 2019;82:e13142. [DOI] [PubMed] [Google Scholar]
- 228. Aktürk S, Çelebi ZK, Erdoğmuş Ş. et al. Pregnancy after kidney transplantation: outcomes, tacrolimus doses, and trough levels. Transplant Proc 2015;47:1442–4. [DOI] [PubMed] [Google Scholar]
- 229. Punnoose LR, Coscia LA, Armenti DP, Costantinescu S, Moritz MJ.. Pregnancy outcomes in 91 female heart transplant recipients. J Heart Lung Transplant 2018;37:S93–S94. [DOI] [PubMed] [Google Scholar]
- 230. Nakagawa K, Kwak-Kim J, Ota K. et al. Immunosuppression with tacrolimus improved reproductive outcome of women with repeated implantation failure and elevated peripheral blood TH1/TH2 cell ratios. Am J Reprod Immunol 2015;73:353–61. [DOI] [PubMed] [Google Scholar]
- 231. Sass N, Sato JL, Facca TA. et al. Tacrolimus as the first choice of immunosuppressive therapy in kidney transplantation pregnant did not reduce significantly maternal and perinatal risks. A preliminary analysis. Pregnancy Hypertens 2015;5:110–1. [Google Scholar]
- 232. Patti F, Messina S, D'Amico E, Lo Fermo S, Zappia M.. Pregnancy outcomes in multiple sclerosis patients previously treated with cyclophosphamide. Acta Neurol Scand 2014;130:e41-4–e44. [DOI] [PubMed] [Google Scholar]
- 233. Fierro ME, Datta P, Rewers-Felkins K. et al. Cyclophosphamide use in multiple sclerosis: levels detected in human milk. Breastfeed Med 2019;14:128–30. [DOI] [PubMed] [Google Scholar]
- 234. Jeong H, Kaplan B.. Therapeutic monitoring of mycophenolate mofetil. Clin J Am Soc Nephrol CJASN 2007;2:184–91. [DOI] [PubMed] [Google Scholar]
- 235. Sifontis NM, Coscia LA, Constantinescu S. et al. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation 2006;82:1698–702. [DOI] [PubMed] [Google Scholar]
- 236. Ang GS, Simpson SA, Reddy AR.. Mycophenolate mofetil embryopathy may be dose and timing dependent. Am J Med Genet A 2008;146A:1963–6. [DOI] [PubMed] [Google Scholar]
- 237. Lin AE, Singh KE, Strauss A. et al. An additional patient with mycophenolate mofetil embryopathy: cardiac and facial analyses. Am J Med Genet A 2011;155A:748–56. [DOI] [PubMed] [Google Scholar]
- 238. Perez-Aytes A, Ledo A, Boso V. et al. In utero exposure to mycophenolate mofetil: a characteristic phenotype? Am J Med Genet A 2008;146a:1–7. [DOI] [PubMed] [Google Scholar]
- 239. Pergola PE, Kancharla A, Riley DJ.. Kidney transplantation during the first trimester of pregnancy: immunosuppression with mycophenolate mofetil, tacrolimus, and prednisone. Transplantation 2001;71:994–7. [DOI] [PubMed] [Google Scholar]
- 240. Velinov M, Zellers N.. The foetal mycophenolate mofetil syndrome. Clin Dysmorphol 2008;17:77–8. [DOI] [PubMed] [Google Scholar]
- 241. Andrade Vila JH, da Silva JP, Guilhen CJ, Baumgratz JF, da Fonseca L.. Even low dose of mycophenolate mofetil in a mother recipient of heart transplant can seriously damage the fetus. Transplantation 2008;86:369–70. [DOI] [PubMed] [Google Scholar]
- 242. Moritz MJ, Coscia L, Armenti DP, Constantinescu S.. Pregnancy outcomes in female solid organ transplant recipients with exposure to mycophenolic acid products. Transplantation 2016;100: S395. [Google Scholar]
- 243. Coscia L, Daly T, Nathan HM. et al. Pregnancy outcomes in 1164 female kidney transplant recipients. Transplantation 2020;104:S573. [Google Scholar]
- 244. Coscia L, Armenti D, Patel P, Constantinescu S, Moritz M.. Outcomes of pregnancies in female liver transplant recipients. Am J Transplant 2018;18(Suppl 4):244. [Google Scholar]
- 245. Sifontis N, Coscia L, Armenti D, Constantinescu S, Moritz M.. What if mycophenolic acid product is discontinued less than 6 weeks before pregnancy? Am J Transplant 2016;16(Suppl 3):D265. [Google Scholar]
- 246. Midtvedt K, Bergan S, Reisæter AV, Vikse BE, Åsberg A.. Exposure to mycophenolate and fatherhood. Transplantation 2017;101:e214–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Lopez-Lopez I, Rodelo-Haad C, Agüera ML. et al. Administration of mycophenolic acid is not associated with malformations in descendants from kidney transplanted males. PLoS One 2018;13:e0202589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Perricone R, De Carolis C, Kroegler B. et al. Intravenous immunoglobulin therapy in pregnant patients affected with systemic lupus erythematosus and recurrent spontaneous abortion. Rheumatology 2008;47:646–51. [DOI] [PubMed] [Google Scholar]
- 249. Jeremic K, Pervulov M, Gojnic M. et al. Comparison of two therapeutic protocols in patients with antiphospholipid antibodies and recurrent miscarriages. Vojnosanit Pregl 2005;62:435–9. [DOI] [PubMed] [Google Scholar]
- 250. Heilmann L, Schorch M, Hahn T. et al. Pregnancy outcome in women with antiphospholipid antibodies: report on a retrospective study. Semin Thromb Hemost 2008;34:794–802. [DOI] [PubMed] [Google Scholar]
- 251. Routsias JG, Kyriakidis NC, Friedman DM. et al. Association of the idiotype:antiidiotype antibody ratio with the efficacy of intravenous immunoglobulin treatment for the prevention of recurrent autoimmune-associated congenital heart block. Arthritis Rheum 2011;63:2783–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Winger EE, Reed JL, Ashoush S. et al. Birth defect rates in women using Adalimumab (Humira((R))) to treat immunologic-based infertility in IVF patients. Am J Reprod Immunol 2011;66:237–41. [DOI] [PubMed] [Google Scholar]
- 253. Dendrinos S, Sakkas E, Makrakis E.. Low-molecular-weight heparin versus intravenous immunoglobulin for recurrent abortion associated with antiphospholipid antibody syndrome. Int J Gynaecol Obstet 2009;104:223–5. [DOI] [PubMed] [Google Scholar]
- 254. Chang P, Millar D, Tsang P. et al. Intravenous immunoglobulin in antiphospholipid syndrome and maternal floor infarction when standard treatment fails: a case report. Am J Perinatol 2006;23:125–9. [DOI] [PubMed] [Google Scholar]
- 255. Pontikaki I, Gerosa M, Argolini LM. et al. FRI0569 What does it mean to become pregnant with juvenile idiopathic arthritis? A monocentric experience in a tertiary centre of milan dedicated to young adults affected by jia. Ann Rheum Dis 2019;78(Suppl 2):980. [Google Scholar]
- 256. Tsao NW, Lynd LD, Sayre EC. et al. Use of biologics during pregnancy and risk of serious infections in the mother and baby: a Canadian population-based cohort study. BMJ Open 2019;9:e023714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Tsao NW, Sayre EC, Hanley G. et al. Risk of preterm delivery and small-for-gestational-age births in women with autoimmune disease using biologics before or during pregnancy: a population-based cohort study. Ann Rheum Dis 2018;77:869–74. [DOI] [PubMed] [Google Scholar]
- 258. Bazzani C, Scrivo R, Andreoli L. et al. Prospectively-followed pregnancies in patients with inflammatory arthritis taking biological drugs: an Italian multicentre study. Clin Exp Rheumatol 2015;33:688–93. [PubMed] [Google Scholar]
- 259. Strangfeld A, Pattloch D, Spilka M. et al. OP0017 pregnancies in patients with rheumatoid arthritis: treatment decisions, course of the disease, and pregnancy outcomes. Ann Rheum Dis 2015;74:70.2–1. [Google Scholar]
- 260. Carter JD, Ladhani A, Ricca LR, Valeriano J, Vasey FB.. A safety assessment of tumor necrosis factor antagonists during pregnancy: a review of the Food and Drug Administration database. J Rheumatol 2009;36:635–41. [DOI] [PubMed] [Google Scholar]
- 261. Schnitzler F, Fidder H, Ferrante M. et al. Outcome of pregnancy in women with inflammatory bowel disease treated with antitumor necrosis factor therapy. Inflamm Bowel Dis 2011;17:1846–54. [DOI] [PubMed] [Google Scholar]
- 262. Arguelles-Arias F, Castro-Laria L, Barreiro-de Acosta M. et al. Is safety infliximb during pregnancy in patients with inflammatory bowel disease? Rev Esp Enferm Dig 2012;104:59–64. [DOI] [PubMed] [Google Scholar]
- 263. Lichtenstein GR, Feagan BG, Cohen RD. et al. Serious infection and mortality in patients with Crohn's disease: more than 5 years of follow-up in the TREAT registry. Am J Gastroenterol 2012;107:1409–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Paschou S, Voulgari PV, Vrabie IG, Saougou IG, Drosos AA.. Fertility and reproduction in male patients with ankylosing spondylitis treated with infliximab. J Rheumatol 2009;36:351–4. [DOI] [PubMed] [Google Scholar]
- 265. Kane S, Ford J, Cohen R, Wagner C.. Absence of infliximab in infants and breast milk from nursing mothers receiving therapy for Crohn's disease before and after delivery. J Clin Gastroenterol 2009;43:613–6. [DOI] [PubMed] [Google Scholar]
- 266. Berthelot JM, De Bandt M, Goupille P. et al. Exposition to anti-TNF drugs during pregnancy: outcome of 15 cases and review of the literature. Joint Bone Spine 2009;76:28–34. [DOI] [PubMed] [Google Scholar]
- 267. Ben-Horin S, Yavzori M, Kopylov U. et al. Detection of infliximab in breast milk of nursing mothers with inflammatory bowel disease. J Crohns Colitis 2011;5:555–8. [DOI] [PubMed] [Google Scholar]
- 268. Zelinkova Z, van der Ent C, Bruin KF. et al. Effects of discontinuing anti-tumor necrosis factor therapy during pregnancy on the course of inflammatory bowel disease and neonatal exposure. Clin Gastroenterol Hepatol 2013;11:318–21. [DOI] [PubMed] [Google Scholar]
- 269. Fritzsche J, Pilch A, Mury D, Schaefer C, Weber-Schoendorfer C.. Infliximab and adalimumab use during breastfeeding. J Clin Gastroenterol 2012;46:718–9. [DOI] [PubMed] [Google Scholar]
- 270. Mahadevan U, Wolf DC, Dubinsky M. et al. Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2013;11:286–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Wibaux C, Andrei I, Paccou J. et al. Pregnancy during TNFalpha antagonist therapy: beware the rifampin-oral contraceptive interaction. Report of two cases. Joint Bone Spine 2010;77:268–70. [DOI] [PubMed] [Google Scholar]
- 272. Scioscia C, Scioscia M, Anelli MG. et al. Intentional etanercept use during pregnancy for maintenance of remission in rheumatoid arthritis. Clin Exp Rheumatol 2011;29:93–5. [PubMed] [Google Scholar]
- 273. Keeling S, Wolbink GJ.. Measuring multiple etanercept levels in the breast milk of a nursing mother with rheumatoid arthritis. J Rheumatol 2010;37:1551. [DOI] [PubMed] [Google Scholar]
- 274. Berthelsen BG, Fjeldsoe-Nielsen H, Nielsen CT, Hellmuth E.. Etanercept concentrations in maternal serum, umbilical cord serum, breast milk and child serum during breastfeeding. Rheumatology 2010;49:2225–7. [DOI] [PubMed] [Google Scholar]
- 275. Jurgens M, Brand S, Filik L. et al. Safety of adalimumab in Crohn's disease during pregnancy: case report and review of the literature. Inflamm Bowel Dis 2010;16:1634–6. [DOI] [PubMed] [Google Scholar]
- 276. Dessinioti C, Stefanaki I, Stratigos AJ. et al. Pregnancy during adalimumab use for psoriasis. J Eur Acad Dermatol Venereol 2011;25:738–9. [DOI] [PubMed] [Google Scholar]
- 277. Winger EE, Reed JL.. Treatment with tumor necrosis factor inhibitors and intravenous immunoglobulin improves live birth rates in women with recurrent spontaneous abortion. Am J Reprod Immunol 2008;60:8–16. [DOI] [PubMed] [Google Scholar]
- 278. Verstappen SM, King Y, Watson KD, Symmons DP, Hyrich KL;. BSRBR Control Centre Consortium, BSR Biologics Register. BSRBR Control Centre Consortium BSRBR. Anti-TNF therapies and pregnancy: outcome of 130 pregnancies in the British Society for Rheumatology Biologics Register. Ann Rheum Dis 2011;70:823–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Bortlik M, Machkova N, Duricova D. et al. Pregnancy and newborn outcome of mothers with inflammatory bowel diseases exposed to anti-TNF-alpha therapy during pregnancy: three-center study. Scand J Gastroenterol 2013;48:951–8. [DOI] [PubMed] [Google Scholar]
- 280. Kawai Y, Tsuchiya T, Aoki S.. Pregnancy outcomes of patients exposed to adalimumab in Japan. Dig Dis 2019;37:123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Chambers CD, Johnson DL, Xu R. et al. Birth outcomes in women who have taken adalimumab in pregnancy: a prospective cohort study. PLoS One 2019;14:e0223603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Burmester GR, Landewe R, Genovese MC. et al. Adalimumab long-term safety: infections, vaccination response and pregnancy outcomes in patients with rheumatoid arthritis. Ann Rheum Dis 2017;76:414–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Mariette X, Forger F, Abraham B. et al. Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis 2018;77:228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Clowse MEB, Scheuerle AE, Chambers C. et al. Pregnancy outcomes after exposure to certolizumab pegol: updated results from a pharmacovigilance safety database. Arthritis Rheumatol 2018;70:1399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Forger F, Zbinden A, Villiger PM.. Certolizumab treatment during late pregnancy in patients with rheumatic diseases: low drug levels in cord blood but possible risk for maternal infections. A case series of 13 patients. Joint Bone Spine 2016;83:341–3. [DOI] [PubMed] [Google Scholar]
- 286. Carman WJ, Accortt NA, Anthony MS, Iles J, Enger C.. Pregnancy and infant outcomes including major congenital malformations among women with chronic inflammatory arthritis or psoriasis, with and without etanercept use. Pharmacoepidemiol Drug Saf 2017;26:1109–18. [DOI] [PubMed] [Google Scholar]
- 287. Chambers CD, Johnson DL, Luo Y, Xu R, Jones KL.. Pregnancy outcome in women treated with etanercept: An update on the OTIS autoimmune diseases in pregnancy project. Pharmacoepidemiol Drug Saf 2015;24(Suppl 1):402. [Google Scholar]
- 288. Fu J, Li L, Qi L, Zhao L.. A randomized controlled trial of etanercept in the treatment of refractory recurrent spontaneous abortion with innate immune disorders. Taiwan J Obstet Gynecol 2019;58:621–5. [DOI] [PubMed] [Google Scholar]
- 289. Otero-Lobato M, Esslinger S, Gabriel S. et al. SAT0117 Trimester exposure and pregnancy outcomes in women exposed to golimumab – results from the company pharmacovigilance database. Ann Rheum Dis 2020;79:992. [Google Scholar]
- 290. Lau A, Clark M, Harrison DD. et al. THU0153 pregnancy outcomes in women exposed to the tumor necrosis factor inhibitor golimumab . Ann Rheum Dis 2014;73:232–233. [Google Scholar]
- 291. Geldhof A, Slater J, Clark M, Chandran U, Coppola D.. Exposure to infliximab during pregnancy: post-marketing experience. Drug Saf 2020;43:147–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Truta B, Leeds IL, Canner JK. et al. Early discontinuation of infliximab in pregnant women with inflammatory bowel disease. Inflamm Bowel Dis 2020;26:1110–1117. [DOI] [PubMed] [Google Scholar]
- 293. Lichtenstein GR, Feagan BG, Mahadevan U. et al. Pregnancy outcomes reported during the 13-year TREAT registry: a descriptive report. Am J Gastroenterol 2018;113:1678–1688. [DOI] [PubMed] [Google Scholar]
- 294. Kolar M, Duricova D, Bortlik M. et al. P614 Pregnancy outcomes in women with IBD treated with biosimilar infliximab. J Crohn's Colitis 2018;12:S419–S420. [Google Scholar]
- 295. Broms G, Kieler H, Ekbom A. et al. Anti-TNF treatment during pregnancy and birth outcomes: a population-based study from Denmark, Finland, and Sweden. Pharmacoepidemiol Drug Saf 2020;29:316–327. [DOI] [PubMed] [Google Scholar]
- 296. Kanis SL, Modderman S, Escher JC. et al. Health outcomes of 1000 children born to mothers with inflammatory bowel disease in their first 5 years of life. Gut 2021;70:1266–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Julsgaard M, Hvas CL, Gearry RB. et al. Anti-TNF therapy in pregnant women with inflammatory bowel disease: effects of therapeutic strategies on disease behavior and birth outcomes. Inflamm Bowel Dis 2020;26:93–102. [DOI] [PubMed] [Google Scholar]
- 298. Drechsel P, Studemann K, Niewerth M. et al. Pregnancy outcomes in DMARD-exposed patients with juvenile idiopathic arthritis-results from a JIA biologic registry. Rheumatology 2020;59:603–612. [DOI] [PubMed] [Google Scholar]
- 299. Moens A, van der Woude CJ, Julsgaard M. et al. Pregnancy outcomes in inflammatory bowel disease patients treated with vedolizumab, anti-TNF or conventional therapy: results of the European CONCEIVE study. Aliment Pharmacol Ther 2020;51:129–138. [DOI] [PubMed] [Google Scholar]
- 300. Andreoli L, Gerardi M, Bazzani C. et al. Long-term outcome of children born to mothers with chronic arthritis and exposed to TNF-inhibitors during pregnancy: a case-control study [abstract-2429]. Arthritis Rheumatol 2018;70(Suppl 10). [Google Scholar]
- 301. Chaparro M, Verreth A, Lobaton T. et al. Long-term safety of in utero exposure to anti-TNFalpha drugs for the treatment of inflammatory bowel disease: results from the multicenter European TEDDY study. Am J Gastroenterol 2018;113:396–403. [DOI] [PubMed] [Google Scholar]
- 302. Kanis SL, de Lima-Karagiannis A, van der Ent C, Rizopoulos D, van der Woude CJ.. Anti-TNF levels in cord blood at birth are associated with anti-TNF Type. J Crohn's Colitis 2018;12:939–47. [DOI] [PubMed] [Google Scholar]
- 303. Luu M, Benzenine E, Doret M. et al. Continuous anti-TNFalpha use throughout pregnancy: possible complications for the mother but not for the fetus. A retrospective cohort on the French National Health Insurance Database (EVASION). Am J Gastroenterol 2018;113:1669–77. [DOI] [PubMed] [Google Scholar]
- 304. Tsao NW, Hanley G, Sadatsafavi M. et al. Risk of major congenital malformations associated with exposure to biologics before or during pregnancy: a population-based cohort study [abstract-14]. J Rheumatol 2018;45. https://www.jrheum.org/content/jrheum/45/7/964.full.pdf. [Google Scholar]
- 305. Kammerlander H, Nielsen J, Knudsen T. et al. Anti-TNF-alpha use during the third trimester of pregnancy in women with moderate-severe inflammatory bowel disease and the risk of preterm birth and low birth weight. Inflamm Bowel Dis 2017;23:1916–23. [DOI] [PubMed] [Google Scholar]
- 306. Hoxha A, Calligaro A, Poi ED. et al. Pregnancy and foetal outcomes following anti-tumor necrosis factor alpha therapy: a prospective multicentre study. Joint Bone Spine 2017;84:169–73. [DOI] [PubMed] [Google Scholar]
- 307. Dall'ara F, Reggia R, Bazzani C. et al. FRI0176 safety of anti-TNF alfa agents during pregancy and breastfeeding: longterm follow up of exposed children in a case-series of mothers with chronic arthritides. Ann Rheum Dis 2016;75:493.1. [Google Scholar]
- 308. Julsgaard M, Christensen LA, Gibson PR. et al. Concentrations of adalimumab and infliximab in mothers and newborns, and effects on infection. Gastroenterology 2016;151:110–119. [DOI] [PubMed] [Google Scholar]
- 309. de Lima A, Zelinkova Z, van der Ent C, Steegers EA, van der Woude CJ.. Tailored anti-TNF therapy during pregnancy in patients with IBD: maternal and foetal safety. Gut 2016;65:1261–8. [DOI] [PubMed] [Google Scholar]
- 310. Broms G, Granath F, Ekbom A. et al. Low risk of birth defects for infants whose mothers are treated with anti-tumor necrosis factor agents during pregnancy. Clin Gastroenterol Hepatol 2016;14:234–41. [DOI] [PubMed] [Google Scholar]
- 311. Komoto S, Motoya S, Nishiwaki Y. et al. Pregnancy outcome in women with inflammatory bowel disease treated with anti-tumor necrosis factor and/or thiopurine therapy: a multicenter study from Japan. Intest Res 2016;14:139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Weber-Schoendorfer C, Oppermann M, Wacker E. et al. Pregnancy outcome after TNF-alpha inhibitor therapy during the first trimester: a prospective multicentre cohort study. Br J Clin Pharmacol 2015;80:727–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Kimyon G, Zengin O, Kısacık B, Onat AM.. AB1121 Anti-tnf drugs may result to elevated abortion rates in late pregnancy. Ann Rheum Dis 2015;74:1276.1–1276. [Google Scholar]
- 314. Agosti M, Andreoli L, Bazzani C. et al. SAT0159 long-term follow-up of children born to mothers with chronic arthritides and exposed in utero to anti-TNF-alpha agents: a case-control study. Ann Rheum Dis 2015;74:709.3–710. [Google Scholar]
- 315. Diav-Citrin O, Otcheretianski-Volodarsky A, Shechtman S, Ornoy A.. Pregnancy outcome following gestational exposure to TNF-alpha-inhibitors: a prospective, comparative, observational study. Reprod Toxicol 2014;43:78–84. [DOI] [PubMed] [Google Scholar]
- 316. Deepak P, Stobaugh DJ.. Maternal and foetal adverse events with tumour necrosis factor-alpha inhibitors in inflammatory bowel disease. Aliment Pharmacol Ther 2014;40:1035–43. [DOI] [PubMed] [Google Scholar]
- 317. Seirafi M, de Vroey B, Amiot A. et al. Factors associated with pregnancy outcome in anti-TNF treated women with inflammatory bowel disease. Aliment Pharmacol Ther 2014;40:363–73. [DOI] [PubMed] [Google Scholar]
- 318. Giacuzzo S, Padovan M, Capucci R, Barbieri MG, Govoni M.. FRI0090 pregnancy outcome of mothers with rheumatic diseases exposed to biological agent during pregnancy: a single-centre study. Ann Rheum Dis 2014;73:414.2–414.23355079 [Google Scholar]
- 319. Bortlik M, Duricova D, Machkova N. et al. Impact of anti-tumor necrosis factor alpha antibodies administered to pregnant women with inflammatory bowel disease on long-term outcome of exposed children. Inflamm Bowel Dis 2014;20:495–501. [DOI] [PubMed] [Google Scholar]
- 320. Duricova D, Dvorakova E, Hradsky O. et al. Safety of anti-TNF-alpha therapy during pregnancy on long-term outcome of exposed children: a controlled, multicenter observation. Inflamm Bowel Dis 2019;25:789–796. [DOI] [PubMed] [Google Scholar]
- 321. Vinet E, De Moura C, Pineau CA. et al. Serious infections in rheumatoid arthritis offspring exposed to tumor necrosis factor inhibitors: a cohort study. Arthritis Rheumatol 2018;70:1565–1571. [DOI] [PubMed] [Google Scholar]
- 322. Truta B, Canner JK, Efron J, Safar B.. The effect of intrauterine exposure to biologics: two years follow up. Gastroenterology 2016;150:S773. [Google Scholar]
- 323. Desai RJ, Bateman BT, Huybrechts KF. et al. Risk of serious infections associated with use of immunosuppressive agents in pregnant women with autoimmune inflammatory conditions: cohort study. BMJ 2017;356:j895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Clowse M, Fischer-Betz R, Nelson-Piercy C. et al. Pharmacovigilance pregnancy data in a large population of patients with chronic inflammatory disease exposed to certolizumab pegol. Ther Adv Musculoskelet Dis 2022;14:1759720X221087650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Clowse ME, Forger F, Hwang C. et al. Minimal to no transfer of certolizumab pegol into breast milk: results from CRADLE, a prospective, postmarketing, multicentre, pharmacokinetic study. Ann Rheum Dis 2017;76:1890–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Matro R, Martin CF, Wolf D, Shah SA, Mahadevan U.. Exposure concentrations of infants breastfed by women receiving biologic therapies for inflammatory bowel diseases and effects of breastfeeding on infections and development. Gastroenterology 2018;155:696–704. [DOI] [PubMed] [Google Scholar]
- 327. Walthall K, Cappon GD, Hurtt ME, Zoetis T.. Postnatal development of the gastrointestinal system: a species comparison. Birth Defects Res B Dev Reprod Toxicol 2005;74:132–56. [DOI] [PubMed] [Google Scholar]
- 328. Weström B, Arévalo Sureda E, Pierzynowska K, Pierzynowski SG, Pérez-Cano F-J.. The immature gut barrier and its importance in establishing immunity in newborn mammals. Front Immunol 2020;11:1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Demers-Mathieu V, Underwood MA, Beverly RL, Nielsen SD, Dallas DC.. Comparison of human milk immunoglobulin survival during gastric digestion between preterm and term infants. Nutrients 2018;10:631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Pieri M, Nicolaidou V, Paphiti I. et al. Survival of vaccine-induced human milk SARS-CoV-2 IgG and IgA immunoglobulins across simulated human infant gastrointestinal digestion. Nutrients 2022;14:3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Norgard BM, Nielsen J, Friedman S.. In utero exposure to thiopurines/anti-TNF agents and long-term health outcomes during childhood and adolescence in Denmark. Aliment Pharmacol Ther 2020;52:829–842. [DOI] [PubMed] [Google Scholar]
- 332. Bröms G, Kieler H, Ekbom A. et al. Paediatric infections in the first 3 years of life after maternal anti-TNF treatment during pregnancy. Aliment Pharmacol Ther 2020;52:843–854. [DOI] [PubMed] [Google Scholar]
- 333. Mahadevan U, Robinson C, Bernasko N. et al. Inflammatory bowel disease in pregnancy clinical care pathway: a report from the American Gastroenterological Association IBD Parenthood Project Working Group. Gastroenterology 2019;156:1508–1524. [DOI] [PubMed] [Google Scholar]
- 334. Cheent K, Nolan J, Shariq S. et al. Case Report: fatal case of disseminated BCG infection in an infant born to a mother taking infliximab for Crohn's disease. J Crohns Colitis 2010;4:603–5. [DOI] [PubMed] [Google Scholar]
- 335. UK Health Security Agency. UK immunisation schedule: the green book. 2019. https://www.gov.uk/government/collections/immunisation-against-infectious-disease-the-green-book (1 September 2022, date last accessed).
- 336. Berkhout A, Clark JE, Wen SC.. In utero exposure to biologic disease-modifying anti-rheumatic drugs and effects to the infant: infectious complications, vaccine response, and safety of live vaccine administration. Expert Rev Vaccines 2019;18:495–504. [DOI] [PubMed] [Google Scholar]
- 337. Public Health England. The rotavirus vaccination programme: Information for healthcare practitioners. 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1018732/Rotavirus_information__for_healthcare_practitioners_Sept_2021.pdf (1 September 2022, date last accessed).
- 338. Ling J, Koren G.. Challenges in vaccinating infants born to mothers taking immunoglobulin biologicals during pregnancy. Expert Rev Vaccines 2016;15:239–56. [DOI] [PubMed] [Google Scholar]
- 339. Pham-Huy A, Sadarangani M, Huang V. et al. From mother to baby: antenatal exposure to monoclonal antibody biologics. Expert Rev Clin Immunol 2019;15:221–229. [DOI] [PubMed] [Google Scholar]
- 340. Goulden B, Chua N, Parker E, Giles I.. P202 Administering live vaccines to infants exposed to biologic and targeted synthetic DMARDs in-utero for maternal treatment of rheumatic disease: a systematic review of the literature [abstract-P202]. Rheumatology 2021;60. [Google Scholar]
- 341. Holroyd CR, Seth R, Bukhari M. et al. The British Society for Rheumatology biologic DMARD safety guidelines in inflammatory arthritis – executive summary. Rheumatology 2019;58:220–226. [DOI] [PubMed] [Google Scholar]
- 342. Nguyen H, Ahmed K, Luo W, Flint J, Giles I.. A systematic review of the safety of non-TNF inhibitor biologic and targeted synthetic drugs in rheumatic disease in pregnancy. Semin Arthritis Rheum 2021;51:1205–1217. [DOI] [PubMed] [Google Scholar]
- 343. Chakravarty EF, Murray ER, Kelman A, Farmer P.. Pregnancy outcomes after maternal exposure to rituximab. Blood 2011;117:1499–506. [DOI] [PubMed] [Google Scholar]
- 344. Pendergraft WF 3rd, McGrath MM, Murphy AP, Murphy P. et al. foetal outcomes after rituximab exposure in women with autoimmune vasculitis. Ann Rheum Dis 2013;72:2051–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Sangle SR, Lutalo PM, Davies RJ, Khamashta MA, D'Cruz DP.. B-cell depletion therapy and pregnancy outcome in severe, refractory systemic autoimmune diseases. J Autoimmun 2013;43:55–59. [DOI] [PubMed] [Google Scholar]
- 346. Ton E, Tekstra J, Hellmann PM, Nuver-Zwart IH, Bijlsma JW.. Safety of rituximab therapy during twins' pregnancy. Rheumatology 2011;50:806–8. [DOI] [PubMed] [Google Scholar]
- 347. Alkaabi JK, Alkindi S, Riyami NA. et al. Successful treatment of severe thrombocytopenia with romiplostim in a pregnant patient with systemic lupus erythematosus. Lupus 2012;21:1571–4. [DOI] [PubMed] [Google Scholar]
- 348. Gualtierotti R, Ingegnoli F, Meroni PL.. Pre-conceptional exposure to rituximab: comment on the article by Ojeda-Uribe et al. Clin Rheumatol 2013;32:727–8. [DOI] [PubMed] [Google Scholar]
- 349. Ojeda-Uribe M, Afif N, Dahan E. et al. Exposure to abatacept or rituximab in the first trimester of pregnancy in three women with autoimmune diseases. Clin Rheumatol 2013;32:695–700. [DOI] [PubMed] [Google Scholar]
- 350. Winthrop KL, Saag K, Cascino MD. et al. Long-term safety of rituximab in rheumatoid arthritis: analysis from the SUNSTONE registry. Arthritis Care Res 2019;71:993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. De Cock D, Birmingham L, Watson KD. et al. Pregnancy outcomes in women with rheumatoid arthritis ever treated with rituximab. Rheumatology 2017;56:661–663. [DOI] [PubMed] [Google Scholar]
- 352. Seyed Ahadi M, Sahraian MA, Baghbanian SM. et al. Pregnancy outcome in patients with multiple sclerosis treated with Rituximab: a case-series study. Mult Scler Relat Disord 2021;47:102667. [DOI] [PubMed] [Google Scholar]
- 353. Zagorodnikova K, Monosova K, Ivanov V, Zaritskey A.. #3 Pregancy and infant outcomes after maternal exposure to rituximab. Reproduct Toxicol 2019;88:134. [Google Scholar]
- 354. Smith JB, Hellwig K, Fink K. et al. Rituximab, MS, and pregnancy. Neurol Neuroimmunol Neuroinflamm 2020;7:e734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Krysko KM, LaHue SC, Anderson A. et al. Minimal breast milk transfer of rituximab, a monoclonal antibody used in neurological conditions. Neurol Neuroimmunol Neuroinflamm 2020;7:e637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Rubbert-Roth A, Goupille P, Moosavi S, Hou A.. First experiences with pregnancies in RA patients (pts) receiving tocilizumab (TCZ) therapy. Arthritis Rheum 2010;62:384. [Google Scholar]
- 357. Weber-Schoendorfer C, Schaefer C.. Pregnancy outcome after tocilizumab therapy in early pregnancy-a case series from the German Embryotox Pharmacovigilance Center. Reprod Toxicol 2016;60:29–32. [DOI] [PubMed] [Google Scholar]
- 358. Nakajima K, Watanabe O, Mochizuki M. et al. Pregnancy outcomes after exposure to tocilizumab: a retrospective analysis of 61 patients in Japan. Mod Rheumatol 2016;26:667–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Hoeltzenbein M, Beck E, Rajwanshi R. et al. Tocilizumab use in pregnancy: analysis of a global safety database including data from clinical trials and post-marketing data. Semin Arthritis Rheum 2016;46:238–245. [DOI] [PubMed] [Google Scholar]
- 360. Saito J, Yakuwa N, Takai C. et al. Tocilizumab concentrations in maternal serum and breast milk during breastfeeding and a safety assessment in infants: a case study. Rheumatology 2018;57:1499–1501. [DOI] [PubMed] [Google Scholar]
- 361. Saito J, Yakuwa N, Kaneko K. et al. Tocilizumab drug levels during pregnancy and lactation: A woman who discontinued tocilizumab therapy until the end of the first trimester and resumed it after birth. Obstetr Med 2021;14:260–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Saito J, Yakuwa N, Kaneko K. et al. Tocilizumab during pregnancy and lactation: drug levels in maternal serum, cord blood, breast milk and infant serum. Rheumatology 2019;58:1505–1507. [DOI] [PubMed] [Google Scholar]
- 363. Moriyama M, Wada Y, Minamoto T. et al. Unexpectedly lower proportion of placental transferred tocilizumab relative to whole immunoglobulin G: a case report. Scand J Rheumatol 2020;49:165–166. [DOI] [PubMed] [Google Scholar]
- 364. Tada Y, Sakai M, Nakao Y. et al. Placental transfer of tocilizumab in a patient with rheumatoid arthritis. Rheumatology 2019;58:1694–1695. [DOI] [PubMed] [Google Scholar]
- 365. Sanofi. Data on File. 2021.
- 366. Zaretsky MV, Alexander JM, Byrd W, Bawdon RE.. Transfer of inflammatory cytokines across the placenta. Obstetr Gynecol 2004;103:546–50. [DOI] [PubMed] [Google Scholar]
- 367. Fischer-Betz R, Specker C, Schneider M.. Successful outcome of two pregnancies in patients with adult-onset Still's disease treated with IL-1 receptor antagonist (anakinra). Clin Exp Rheumatol 2011;29:1021–3. [PubMed] [Google Scholar]
- 368. Berger CT, Recher M, Steiner U, Hauser TM.. A patient's wish: anakinra in pregnancy. Ann Rheum Dis 2009;68:1794–5. [DOI] [PubMed] [Google Scholar]
- 369. Youngstein T, Hoffmann P, Gul A. et al. International multi-centre study of pregnancy outcomes with interleukin-1 inhibitors. Rheumatology 2017;56:2102–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370. Ozdogan H, Ugurlu S, Ergezen B.. How safe it is to treat pregnant FMF patients with Anakinra? Pediatr Rheumatol 2015;13:P124. [Google Scholar]
- 371. Smith CJF, Chambers CD.. Five successful pregnancies with antenatal anakinra exposure. Rheumatology 2018;57:1271–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. Chang Z, Spong CY, Jesus AA. et al. Anakinra use during pregnancy in patients with cryopyrin-associated periodic syndromes (CAPS). Arthritis Rheumatol 2014;66:3227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373. Novartis. Data on File. 2021.
- 374. Pham T, Bachelez H, Berthelot JM. et al. Abatacept therapy and safety management. Joint Bone Spine 2012;79:3–84. [DOI] [PubMed] [Google Scholar]
- 375. Kumar M, Ray L, Vemuri S, Simon TA.. Pregnancy outcomes following exposure to abatacept during pregnancy. Semin Arthritis Rheum 2015;45:351–6. [DOI] [PubMed] [Google Scholar]
- 376. Yu H, Angelini K, Dominique ASimon TA.. Pregnancy outcomes in patients with rheumatoid arthritis treated with abatacept – review of a safety database [abstract-2553]. Arthritis Rheumatol 2016;68. https://acrabstracts.org/abstract/pregnancy-outcomes-in-patients-with-rheumatoid-arthritis-treated-with-abatacept-review-of-a-safety-database/. [Google Scholar]
- 377. Saito J, Yakuwa N, Takai C. et al. Abatacept concentrations in maternal serum and breast milk during breastfeeding and an infant safety assessment: a case study. Rheumatology 2019;58:1692–1694. [DOI] [PubMed] [Google Scholar]
- 378. Peart E, Clowse ME.. Systemic lupus erythematosus and pregnancy outcomes: an update and review of the literature. Curr Opin Rheumatol 2014;26:118–23. [DOI] [PubMed] [Google Scholar]
- 379. Medical Information, GlaxoSmithKline. 2015.
- 380. Powell M, Hill D, Eudy A, Landy H, Petri M.. OP0041 Pregnancy outcomes for systemic lupus erythematosus (SLE) subjects with conception during belimumab intravenous (IV) and subcutaneous (SC) placebo-controlled clinical trials and long term extension trials. Annals of the Rheumatic Diseases 2014;73:75.3–76.23912798 [Google Scholar]
- 381. Danve A, Perry L, Deodhar A.. Use of belimumab throughout pregnancy to treat active systemic lupus erythematosus: a case report. Semin Arthritis Rheum 2014;44:195–7. [DOI] [PubMed] [Google Scholar]
- 382. Saito J, Yakuwa N, Ishizuka T. et al. Belimumab concentrations in maternal serum and breast milk during breastfeeding and the safety assessment of the infant: a case study. Breastfeed Med 2020;15:475–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383. Bitter H, Bendvold AN, Østensen ME.. Lymphocyte changes and vaccination response in a child exposed to belimumab during pregnancy. Ann Rheum Dis 2018;77:1692–1693. [DOI] [PubMed] [Google Scholar]
- 384. Sandhu VK, Wallace DJ, Weisman MH.. Monoclonal antibodies, systemic lupus erythematosus, and pregnancy: insights from an open-label study. J Rheumatol 2015;42:728–30. [DOI] [PubMed] [Google Scholar]
- 385. Crisafulli F, Gerardi MC, Moschetti L. et al. POS0702 Pregnancy in SLE patients treated with belimumab: experience from 3 italian centers. Ann Rheum Dis 2021;80:600.2–601. [Google Scholar]
- 386. Kao J-H, Lan T-Y, Lu C-H. et al. Pregnancy outcomes in patients treated with belimumab: report from real-world experience. Semin Arthritis Rheum 2021;51:963–968. [DOI] [PubMed] [Google Scholar]
- 387. Warren RB, Reich K, Langley RG. et al. Secukinumab in pregnancy: outcomes in psoriasis, psoriatic arthritis and ankylosing spondylitis from the global safety database. Br J Dermatol 2018;179:1205–1207. [DOI] [PubMed] [Google Scholar]
- 388. Meroni M, Generali E, Guidelli GM. et al. THU0319 Overall safety of 7-week secukinumab exposure during pregnancy in women with psoriatic arthritis. Ann Rheum Dis 2018;77(Suppl 2):377–8. [Google Scholar]
- 389. Feldman S, Pangallo B, Xu W. et al. Ixekizumab and pregnancy outcome [abstract-AB419]. J Am Acad Dermatol 2017;76. https://www.jaad.org/article/S0190-9622(17)32021-2/fulltext. [Google Scholar]
- 390. Novartis. Data on File. 2019.
- 391. Tikhonov I, Volger S, Lin C, Lin C, Geldhof A.. Pregnancy outcomes in women with psoriasis, psoriatic arthritis, Crohn disease and ulcerative colitis treated with ustekinumab. J Am Acad Dermatol 2020;83:AB74. [Google Scholar]
- 392. Wils P, Seksik P, Stefanescu C. et al. Safety of ustekinumab or vedolizumab in pregnant inflammatory bowel disease patients: a multicentre cohort study. Aliment Pharmacol Ther 2021;53:460–70. [DOI] [PubMed] [Google Scholar]
- 393. Watson N, Wu K, Farr P, Reynolds NJ, Hampton PJ.. Ustekinumab exposure during conception and pregnancy in patients with chronic plaque psoriasis: a case series of 10 pregnancies. Br J Dermatol 2019;180:195–196. [DOI] [PubMed] [Google Scholar]
- 394. Klenske E, Osaba L, Nagore D. et al. Drug levels in the maternal serum, cord blood and breast milk of a ustekinumab-treated patient with crohn's disease. J Crohns Colitis 2019;13:267–269. [DOI] [PubMed] [Google Scholar]
- 395. Burki TK. FDA approval for anifrolumab in patients with lupus. Lancet Rheumatol 2021;3:E689. [Google Scholar]
- 396. Electronic Medicines Compendium. XELJANZ 5mg film-coated tablets. 2021. https://www.medicines.org.uk/emc/medicine/33167 (1 September 2022, date last accessed).
- 397. Mahadevan U, Dubinsky MC, Su C. et al. Outcomes of pregnancies with maternal/paternal exposure in the tofacitinib safety databases for ulcerative colitis. Inflamm Bowel Dis 2018;24:2494–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. Mahadevan U, Baumgart DC, Dubinsky MC. et al. S0847 pregnancy outcomes in the tofacitinib ulcerative colitis OCTAVE studies: an update as of February 2020. Am J Gastroenterol 2020;115:S437–8. [Google Scholar]
- 399. Clowse ME, Feldman SR, Isaacs JD. et al. Pregnancy outcomes in the tofacitinib safety databases for rheumatoid arthritis and psoriasis. Drug Saf 2016;39:755–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400. Pfizer. Data on File. 2021.
- 401. Costanzo G, Firinu D, Losa F. et al. Baricitinib exposure during pregnancy in rheumatoid arthritis. Ther Adv Musculoskelet Dis 2020;12:1759720X19899296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402. Lilly E. Data on File. 2021.
- 403. AbbVie. Data on File. 2021.
- 404. Beghin D, Cournot MP, Vauzelle C, Elefant E.. Paternal exposure to methotrexate and pregnancy outcomes. J Rheumatol 2011;38:628–32. [DOI] [PubMed] [Google Scholar]
- 405. Lee CY, Jin C, Mata AM. et al. A pilot study of paternal drug exposure: the Motherisk experience. Reprod Toxicol 2010;29:353–60. [DOI] [PubMed] [Google Scholar]
- 406. Xu LG, Wang HW, Peng WL. et al. Marital status and fertility of 185 male renal transplant recipients in China. J Androl 2008;29:618–21. [DOI] [PubMed] [Google Scholar]
- 407. Engeland A, Bjorge T, Daltveit AK. et al. Effects of preconceptional paternal drug exposure on birth outcomes: cohort study of 340 000 pregnancies using Norwegian population-based databases. Br J Clin Pharmacol 2013;75:1134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408. Xu L, Han S, Liu Y. et al. The influence of immunosuppressants on the fertility of males who undergo renal transplantation and on the immune function of their offspring. Transpl Immunol 2009;22:28–31. [DOI] [PubMed] [Google Scholar]
- 409. Teruel C, Lopez-San Roman A, Bermejo F. et al. Outcomes of pregnancies fathered by inflammatory bowel disease patients exposed to thiopurines. Am J Gastroenterol 2010;105:2003–8. [DOI] [PubMed] [Google Scholar]
- 410. Saougou I, Markatseli TE, Papagoras C. et al. Fertility in male patients with seronegative spondyloarthropathies treated with infliximab. Joint Bone Spine 2013;80:34–7. [DOI] [PubMed] [Google Scholar]
- 411. Larsen MD, Friedman S, Magnussen B, Norgard BM.. Birth outcome of children fathered by men treated with systemic corticosteroids during the conception period - a cohort study based on nationwide data. Basic Clin Pharmacol Toxicol 2018;122:133–138. [DOI] [PubMed] [Google Scholar]
- 412. Sato A, Naganuma M, Asakura K. et al. Conception outcomes and opinions about pregnancy for men with inflammatory bowel disease. J Crohns Colitis 2010;4:183–8. [DOI] [PubMed] [Google Scholar]
- 413. De Santis M, Straface G, Cavaliere A, Carducci B, Caruso A.. Paternal and maternal exposure to leflunomide: pregnancy and neonatal outcome. Ann Rheum Dis 2005;64:1096–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414. Hellwig K, Haghikia A, Gold R.. Parenthood and immunomodulation in patients with multiple sclerosis. J Neurol 2010;257:580–3. [DOI] [PubMed] [Google Scholar]
- 415. Hoeltzenbein M, Weber-Schoendorfer C, Borisch C. et al. Pregnancy outcome after paternal exposure to azathioprine/6-mercaptopurine. Reprod Toxicol 2012;34:364–9. [DOI] [PubMed] [Google Scholar]
- 416. Armenti VT, Constantinescu S, Moritz MJ, Davison JM.. Pregnancy after transplantation. Transplant Rev 2008;22:223–40. [DOI] [PubMed] [Google Scholar]
- 417. Ecevit C, Unal F, Baran M, Aydogdu S.. Parenthood in pediatric liver transplant patients. Pediatr Transplant 2012;16:346–9. [DOI] [PubMed] [Google Scholar]
- 418. Green DM, Liu W, Kutteh WH. et al. Cumulative alkylating agent exposure and semen parameters in adult survivors of childhood cancer: a report from the St Jude Lifetime Cohort Study. Lancet Oncol 2014;15:1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419. Ghafouri-Fard S, Shoorei H, Abak A. et al. Effects of chemotherapeutic agents on male germ cells and possible ameliorating impact of antioxidants. Biomed Pharmacother 2021;142:112040. [DOI] [PubMed] [Google Scholar]
- 420. Meistrich ML. Risks of genetic damage in offspring conceived using spermatozoa produced during chemotherapy or radiotherapy. Andrology 2020;8:545–558. [DOI] [PubMed] [Google Scholar]
- 421. Medrano JV, Hervas D, Vilanova-Perez T. et al. Histologic analysis of testes from prepubertal patients treated with chemotherapy associates impaired germ cell counts with cumulative doses of cyclophosphamide, ifosfamide, cytarabine, and asparaginase. Reprod Sci 2021;28:603–613. [DOI] [PubMed] [Google Scholar]
- 422. Ozolins TR. Cyclophosphamide and the Teratology Society: an awkward marriage. Birth Defects Res B Dev Reprod Toxicol 2010;89:289–99. [DOI] [PubMed] [Google Scholar]
- 423. Green DM, Whitton JA, Stovall M. et al. Pregnancy outcome of partners of male survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol 2003;21:716–21. [DOI] [PubMed] [Google Scholar]
- 424. Mouyis M, Flint JD, Giles IP.. Safety of anti-rheumatic drugs in men trying to conceive: A systematic review and analysis of published evidence. Semin Arthritis Rheum 2019;48:911–920. [DOI] [PubMed] [Google Scholar]
- 425. Micu MC, Ostensen M, Villiger PM, Micu R, Ionescu R.. Paternal exposure to antirheumatic drugs-What physicians should know: review of the literature. Semin Arthritis Rheum 2018;48:343–355. [DOI] [PubMed] [Google Scholar]
- 426. Jones A, Clary MJ, McDermott E. et al. Outcomes of pregnancies fathered by solid-organ transplant recipients exposed to mycophenolic acid products. Prog Transplant 2013;23:153–7. [DOI] [PubMed] [Google Scholar]
- 427. Katz JA, Antoni C, Keenan GF. et al. Outcome of pregnancy in women receiving infliximab for the treatment of Crohn's disease and rheumatoid arthritis. Am J Gastroenterol 2004;99:2385–92. [DOI] [PubMed] [Google Scholar]
- 428. Lamboglia F, D'Inca R, Oliva L, Bertomoro P, Sturniolo GC.. Patient with severe Crohn's disease became a father while on methotrexate and infliximab therapy. Inflamm Bowel Dis 2009;15:648–9. [DOI] [PubMed] [Google Scholar]
- 429. Rezvani A, Ozaras N.. Infertility improved by etanercept in ankylosing spondylitis. Indian J Pharmacol 2008;40:276–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430. Clowse ME, Wolf DC, Forger F. et al. Pregnancy outcomes in subjects exposed to certolizumab pegol. J Rheumatol 2015;42:2270–8. [DOI] [PubMed] [Google Scholar]
- 431. Wallenius M, Lie E, Daltveit AK. et al. No excess risks in offspring with paternal preconception exposure to disease-modifying antirheumatic drugs. Arthritis Rheumatol 2015;67:296–301. [DOI] [PubMed] [Google Scholar]
- 432. Uyaroglu OA, Seyhoglu E, Erden A. et al. Pregnancy outcomes in partners of male ankylosing spondylitis patients treated with anti-tumour necrosis factor-alpha biologics: real-life results from a single-centre cross-sectional study. Rheumatol Int 2020;40:1501–1507. [DOI] [PubMed] [Google Scholar]
- 433. Micu MC, Ostensen M, Bojincă V. et al. Pregnancy outcomes in couples with males exposed to longterm anti-tumor necrosis factor-alpha inhibitor therapies: a prospective study. J Rheumatol 2019;46:1084–1088. [DOI] [PubMed] [Google Scholar]
- 434. Larsen MD, Friedman S, Magnussen B, Norgard BM.. Birth outcomes in children fathered by men treated with anti-TNF-alpha agents before conception. Am J Gastroenterol 2016;111:1608–1613. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data produced during the guideline development process are presented in the guideline or in the accompanying supplementary material.