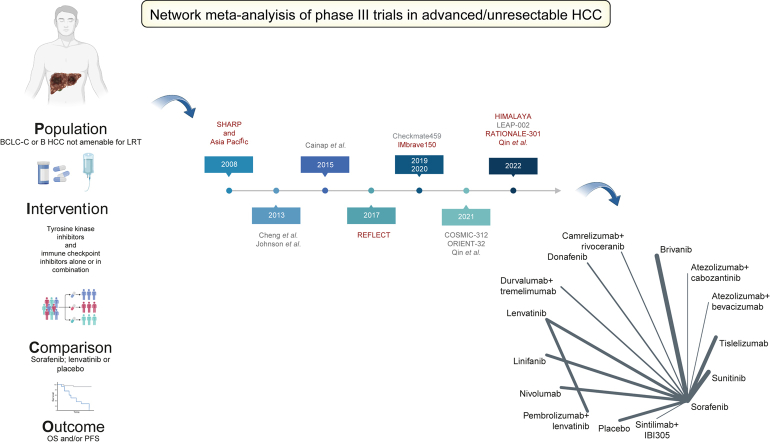
Abstract
Background & Aims
Direct comparisons across first-line regimens for advanced hepatocellular carcinoma are not available. We performed a network metanalysis of phase III of trials to compare first-line systemic treatments for hepatocellular carcinoma in terms of overall survival (OS), progression-free survival (PFS), objective response rate, disease control rate, and incidence of adverse events (AEs).
Methods
After performing a literature review from January 2008 to September 2022, we screened 6,329 studies and reviewed 3,009 studies, leading to identification of 15 phase III trials for analysis. We extracted odds ratios for objective response rate and disease control rate, relative risks for AEs, and hazard ratios (HRs) with 95% CIs for OS and PFS, and used a frequentist network metanalysis, with fixed-effect multivariable meta-regression models to estimate the indirect pooled HRs, odds ratios, relative risks, and corresponding 95% CIs, considering sorafenib as reference.
Results
Of 10,820 included patients, 10,444 received active treatment and 376 placebo. Sintilimab + IBI350, camrelizumab + rivoceranib, and atezolizumab + bevacizumab provided the greatest reduction in the risk of death compared with sorafenib, with HRs of 0.57 (95% CI 0.43–0.75), 0.62 (95% CI 0.49–0.79), and 0.66 (95% CI 0.52–0.84), respectively. Considering PFS, camrelizumab + rivoceranib and pembrolizumab + lenvatinib were associated with the greatest reduction in the risk of PFS events compared with sorafenib, with HRs of 0.52 (95% CI 0.41–0.65) and 0.52 (95% CI 0.35–0.77), respectively. Immune checkpoint inhibitor (ICI) monotherapies carried the lowest risk for all-grade and grade ≥3 AEs.
Conclusions
The combinations of ICI + anti-vascular endothelial growth factor, and double ICIs lead to the greatest OS benefit compared with sorafenib, whereas ICI + kinase inhibitor regimens are associated with greater PFS benefit at the cost of higher toxicity rates.
Impact and Implications
In the last few years, many different therapies have been studied for patients with primary liver cancer that cannot be treated with surgery. In these cases, anticancer drugs (alone or in combination) are given with the intent to keep the cancer at bay and, ultimately, to prolong survival. Among all the therapies that have been investigated, the combination of immunotherapy (drugs that boost the immune system against the cancer) and anti-angiogenic agents (drugs that act on tumoural vessels) has appeared the best to improve survival. Similarly, the combination of two types of immunotherapies that activate the immune system at different levels has also shown positive results.
Systematic Review Registration
PROSPERO CRD42022366330.
Keywords: Targeted therapy, Tyrosine kinase inhibitors, Immune checkpoint inhibitors, First-line treatment, Liver cancer
Graphical abstract
Highlights
-
•
The treatment paradigm for unresectable HCC has changed in the last few years.
-
•
The combination of atezolizumab + bevacizumab has become the new standard of care.
-
•
Durvalumab + tremelimumab has been shown to confer a survival benefit over sorafenib.
-
•
Cabozantinib + atezolizumab has been shown to confer a PFS but not OS benefit over sorafenib.
-
•
Lenvatinib + pembrolizumab does not appear to prolong PFS and OS over lenvatinib.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and the third leading cause of cancer-related death worldwide.1 After a decade of failures in phase III trials, the systemic treatment armamentarium for patients with unresectable/advanced HCC has significantly widened. Three multitargeted tyrosine kinase inhibitors (TKIs) – namely lenvatinib in the first line,2 regorafenib,3 and cabozantinib4 in sorafenib-pretreated patients – as well as the anti-vascular endothelial growth factor (VEGF) receptor-2 (VEGFR2) antibody ramucirumab in sorafenib-experienced patients with high alpha-foetoprotein5 have enabled an incremental survival benefit.
Atezolizumab + bevacizumab6,7 ushered in the era of combination immune checkpoint inhibitor (ICI) therapy, leading to the establishment of a novel first-line standard of care.8
Following the IMbrave150 trial,6 three main ICI combination regimens have been explored as first-line treatment. Anti-programmed cell death 1 (PD-1) + anti-VEGF combinations have been validated as an effective treatment option in the ORIENT-32 study.9 A dual ICI combination targeting programmed death ligand 1 (PD-L1) and the cytotoxic T-cell lymphocyte antigen 4 (CTLA-4) was proven to prolong overall survival (OS) in the HIMALAYA trial, which evaluated tremelimumab with durvalumab.10 Lastly, recent studies evaluating PD-1/PD-L1 + TKI combinations have shown variable results. The combination of the anti-PD-1 camrelizumab and rivoceranib (an anti-VEGFR2 TKI) met its primary efficacy endpoint.11 Surprisingly, the combinations of atezolizumab + cabozantinib (the COSMIC-312 trial)12 and pembrolizumab + lenvatinib (the LEAP-002 trial)13 did not meet their OS endpoints. Although atezolizumab + cabozantinib prolonged progression-free survival (PFS), making it technically a positive study,12 and pembrolizumab + lenvatinib showed some activity,13 both treatments did not improve OS vs. sorafenib and lenvatinib, respectively. Some reasons for failure of these therapies have been discussed14 but ultimately remain unclear and confirm that anti-VEGF antibodies are currently the optimal combination partner for ICI.15
Given the large number of potential therapeutic options and the lack of head-to-head comparisons, selecting the optimal first-line treatment is not trivial.8 Furthermore, ICI + TKI studies have brought more controversy than answers, owing to the lack of a synergistic effect for some of the combinations and the mismatch between efficacy data reported in phase Ib/II trials and survival extension in phase III studies.16
To support clinical decision-making in an increasingly complex setting, we directly compared efficacy and safety data of randomised controlled first-line trials for HCC using a network meta-analysis (NMA).
Materials and methods
Search strategy and selection criteria
We performed an NMA to summarise and compare available evidence on first-line systemic therapies for advanced HCC. Trials were included into the analysis if they fulfilled the following inclusion criteria: (i) phase III randomised controlled first-line trials in the palliative treatment setting, (ii) evaluate ICIs and/or TKIs and/or monoclonal antibodies alone or in combination with other systemic treatment agents, and (iii) evaluate survival (OS and/or PFS) as (co)primary endpoints. Therefore, studies evaluating loco-regional therapies as monotherapy or as combination regimen with systemic treatments as well as trials evaluating systemic treatments in a (neo)adjuvant setting were excluded. Literature research was restricted to studies published in English and conducted in MEDLINE (https://pubmed.ncbi.nlm.nih.gov), the Cochrane library (https://www.cochranelibrary.com), and Embase (www.embase.com) between 1 January 2007 and 24 September 2022. Conference abstracts published until 24 September 2022 were also retrieved from the following major scientific societies: the American Society of Clinical Oncology (ASCO), the European Society of Medical Oncology (ESMO), European Association for the Study of the Liver (EASL, and American Association for the Study of Liver Diseases (AASLD). The complete search strategy is reported in the Supplementary information. Two authors (CAMF and C-VS) performed the literature research and evaluated the eligibility of studies using the PICO (patients, interventions, comparison, and outcome) framework following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses criteria.7 Discrepancies concerning the inclusion or exclusion of studies were discussed and resolved with a third independent author (DJP). The study protocol was registered in PROSPERO, an international prospective register of systematic reviews (registration code CRD42022366330; https://www.crd.york.ac.uk/prospero/#searchadvanced).
Data analysis
All analyses were performed in R 4.1.2. using the meta and netmeta packages (R Foundation for Statistical Computing, Vienna, Austria). Primary data from included studies were extracted and entered into dedicated data collection forms. The Cochrane risk of bias assessment tool was used to evaluate seven different aspects of potential bias related to study design, conduction, and reporting of randomised controlled trials assigning a ‘low’ or ‘high’ risk of bias.17 More detailed information on the search strategy, data extraction, and data analysis is available in the Supplementary information.
We conducted subgroup analyses for OS according to HCC aetiology (HBV, HCV, and non-viral), and the presence of extrahepatic spread (EHS) and/or macrovascular invasion (MVI).
Results
Baseline characteristics
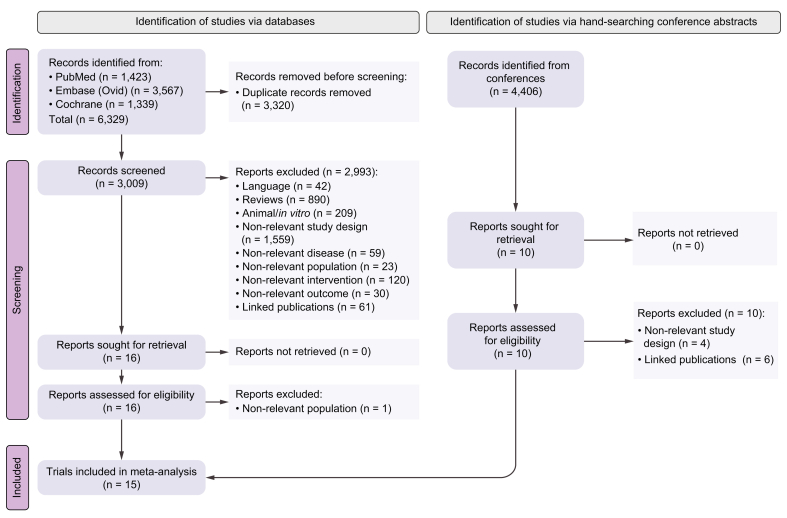
After performing a literature search in MEDLINE (https://pubmed.ncbi.nlm.nih.gov), the Cochrane library (https://www.cochranelibrary.com), and Embase (www.embase.com), according to the research strategy reported in the Supplementary information, we identified 6,329 records from January 2007 to 24 September 2022. After duplicate studies were removed, 3,009 were left for screening. Following the screening process, 2,993 reports were excluded for the reasons detailed in Fig. 1. Overall, 16 studies met the eligibility criteria, and after 1 report was excluded owing to non-relevant population of interest (patients not affected by advanced HCC), the following 15 phase III trials were selected for the analysis: SHARP,18 Asia Pacific,19 Cheng 2013,20 Johnson 2013,21 Cainap 2015,22 REFLECT,2 CheckMate 459,23 IMbrave150,7 ORIENT-32,9 HIMALAYA,10 COSMIC-312,12 Qin 2021,24 Qin 2022,11 LEAP-002,13 and RATIONALE-301.25 These tested the following, respectively: sorafenib vs. placebo (SHARP and Asia Pacific), sunitinib vs. sorafenib, brivanib vs. sorafenib, linifanib vs. sorafenib, lenvatinib vs. sorafenib, nivolumab vs. sorafenib, atezolizumab + bevacizumab vs. sorafenib, sintilimab + IBI305 vs. sorafenib, durvalumab + tremelimumab vs. sorafenib, atezolizumab + cabozantinib vs. sorafenib, donafenib vs. sorafenib, camrelizumab + rivoceranib vs. sorafenib, pembrolizumab + lenvatinib vs. lenvatinib, and tislelizumab vs. sorafenib as first-line treatments for advanced HCC.
Fig. 1.
PRISMA chart reporting the results of the research strategy.
Overall, 10,820 patients were included in the analysis, and among them, 10,444 received active treatment and 376 placebo. Sorafenib was the control arm of all the studies, except for the SHARP and Asia Pacific trials, which tested sorafenib against placebo, and the LEAP-002 trial, which adopted lenvatinib as the control arm.
Donafenib, lenvatinib, brivanib, and tislelizumab were tested for noninferiority against sorafenib, and linifanib was tested for both superiority and noninferiority; however, all the other studies were powered to detect superiority of the experimental arm against the control. The inclusion criteria appeared to be consistent between trials, patients with portal vein tumoural invasion at Vp4 were excluded by the Johnson 2013, REFLECT, ORIENT-32, HIMALAYA, LEAP-002, and RATIONALE-301 trials. Details about inclusion criteria and stratification factors are described in Table S1. All patients were required to have a preserved liver function according to Child–Pugh classification. The main differences in baseline characteristics were found to be median age, aetiology, and percentage of patients with MVI (Table 1). Median age was lower for the Asia Pacific, ORIENT-32, Qin 2021, and Qin 2022 trials. Viral aetiology was the highest in the ORIENT-32, Qin 2021, and Qin 2022 trials, reflecting the higher incidence of HBV infection in Asia. As expected, the incidence of MVI was lower in those trials excluding patients with main portal vein tumoural invasion.
Table 1.
Baseline characteristics of the population included in the trials of interest.
| Name | Arm | Median age (years) | Western region (%) | Presence of MVI (%) | EHS (%) | Viral (%) | Child–Pugh (A) (%) | ECOG 0 (%) | BCLC-C (%) |
|---|---|---|---|---|---|---|---|---|---|
| Cheng 201320 | Sunitinib | 59 | 24.1% | 78.9% | 60% | 76.0% | 99.8% | 46.8% | 87.2% |
| Sorafenib | 59 | 24.6% | 76.3% | 58.5% | 74.8% | 99.4% | 46.7% | 83.5% | |
| Johnson 201321 | Brivanib | 61 | 40% | 27% | 63% | 64% | 92% | 64% | 77% |
| Sorafenib | 60 | 35% | 27% | 62% | 66% | 92% | 61% | 78% | |
| Cainap 201522 | Linifanib | 59 | 34% | 46.3% | 59.7% | 78.8% | 93.2% | 62.8% | 84.2% |
| Sorafenib | 60 | 32.8% | 40.5% | 56.8% | 77.8% | 95.0% | 66.2% | 80.4% | |
| REFLECT2 | Lenvatinib | 63 | 33% | 23% | 61% | 72% | 99% | 64% | 78% |
| Sorafenib | 62 | 33% | 21% | 62% | 74% | 99% | 63% | 81% | |
| IMbrave1507 | Atezolizumab + bevacizumab | 64 | 60% | 38% | 63% | 70% | 100% | 62% | 82% |
| Sorafenib | 66 | 59% | 43% | 56% | 68% | 100% | 62% | 81% | |
| COSMIC-31212 | Atezolizumab + cabozabtinib | 64 | 72% | 31% | 54% | 60% | 100% | 64% | 68% |
| Sorafenib | 64 | 71% | 28% | 56% | 61% | 100% | 66% | 67% | |
| HIMALAYA10 | Durvalumab + tremelimumab | 65 | 60% | 26% | 53% | 59% | 100% | 62% | 80% |
| Durvalumab | 64 | 57% | 24% | 55% | 58% | 100% | 61% | 80% | |
| Sorafenib | 64 | 60% | 26% | 52% | 57% | 100% | 62% | 83% | |
| CheckMate- 45923 | Nivolumab | 65 | 54% | 33% | 60% | 54% | 98% | 73% | 82% |
| Sorafenib | 65 | 53% | 32% | 56% | 54% | 96% | 70% | 78% | |
| SHARP18 | Sorafenib | 65 | 100% | 36% | 53% | 48% | 95% | 54% | 82% |
| Placebo | 66 | 100% | 41% | 50% | 45% | 98% | 54% | 83% | |
| ASIA-PACIFIC19 | Sorafenib | 51 | n.a. | 36% | 69% | 81% | 97% | 25% | 95% |
| Placebo | 52 | 34% | 68% | 81% | 97% | 28% | 96% | ||
| ORIENT-329 | Sintilimab + IBI305 | 53 | n.a. | 28% | 73% | 96% | 96% | 48% | 85% |
| Sorafenib | 54 | 26% | 75% | 98% | 95% | 48% | 86% | ||
| Qin 202124 | Donafenib | 53 | n.a. | 73%∗ | 73%∗ | 91% | 99% | 39% | 87% |
| Sorafenib | 53 | 73%∗ | 73%∗ | 93% | 96% | 33% | 88% | ||
| LEAP- 00213 | Pembrolizumab + lenvatinib | 66 | 69.4% | 18% | 63% | 62.5% | 99.5% | 67.7% | 78.5% |
| Lenvatinib + placebo | 66 | 69.2% | 15% | 60.9% | 59.4% | 99.5% | 68.4% | 75.7% | |
| Qin 202211 | Camrelizumab + rivoceranib | 58 | 17.3% | 14.7% | 64.3% | 84.6% | 100% | 44.1% | 86.0% |
| Sorafenib | 56 | 17.3% | 19.2% | 66.4% | 83.4% | 100% | 42.8% | 85.2% | |
| RATIONALE-30125 | Tisilelizumab | 62 | 89% | 14.9% | 64% | 76% | 100% | 53.5% | 79.5% |
| Sorafenib | 60 | 83% | 14.8% | 59.6% | 75.9% | 100% | 54.5% | 75.9% |
BCLC-C, Barcelona Clinic Liver Cancer – stage C; ECOG, Eastern Cooperative Oncology Group; EHS, extrahepatic spread; MVI, macrovascular invasion; n.a., not applicable. ∗ presence of EHS and MVI.
The risk of bias according to the Cochrane risk of bias assessment tool showed that all the trials reported ‘low risk’ of bias in at least five out of the seven domains of interest.17 The absence of blinding of participants and personnel to the treatment arm was the reason for high risk of bias in the Cheng 2013, Cainap 2015, REFLECT, IMbrave150, COSMIC-312, HIMALAYA, CheckMate 459, ORIENT-32, and Qin 2021 trials. The absence of blinding of outcome assessment was identified as another risk of bias in the Cheng 2013, Cainap 2015, and HIMALAYA trials (Table S2).
OS and PFS
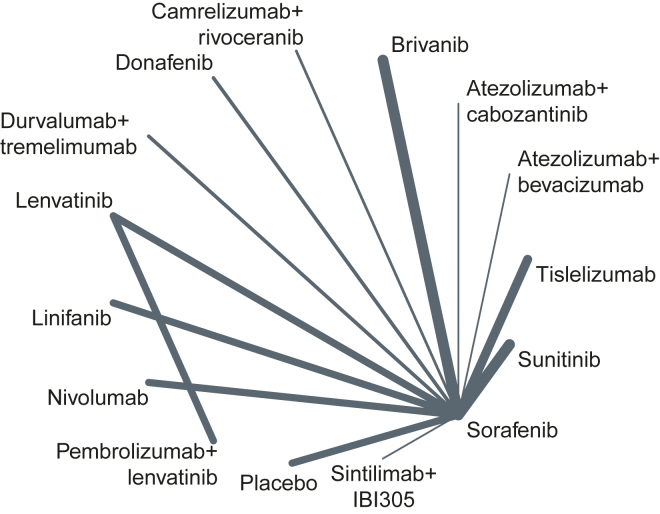
All the trials were evaluable for OS and PFS. All the trials except for the LEAP-002 trial included sorafenib as the treatment arm; the REFLECT trial compared lenvatinib with sorafenib, allowing the of use sorafenib as reference to perform the NMA (Fig. 2).
Fig. 2.
Network plot reporting the treatment arms included in the analysis.
Radiological progression was based on RECIST 1.1 in all the trials, except for the SHARP, Asia Pacific, and Cheng 2015 trials (RECIST) and the Johnson 2013 trial (modified RECIST [mRECIST]).
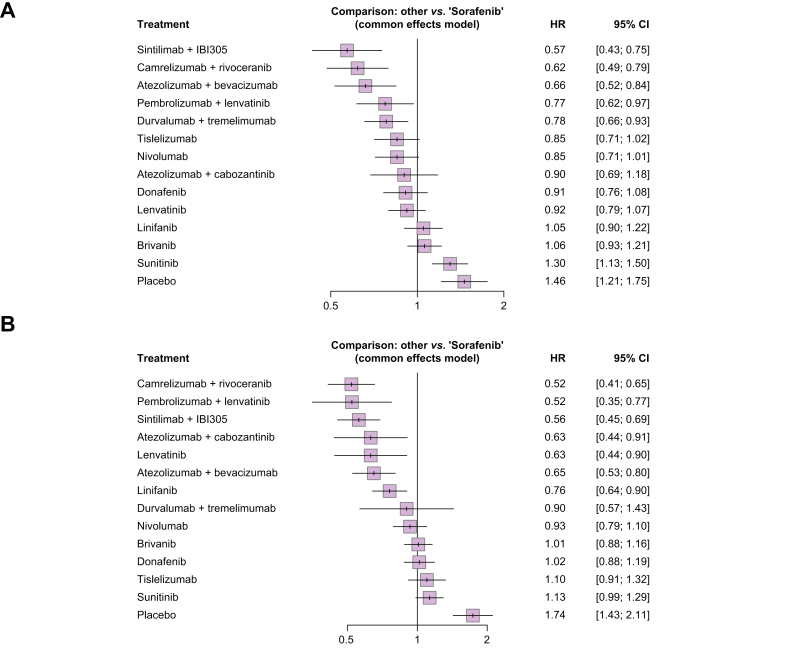
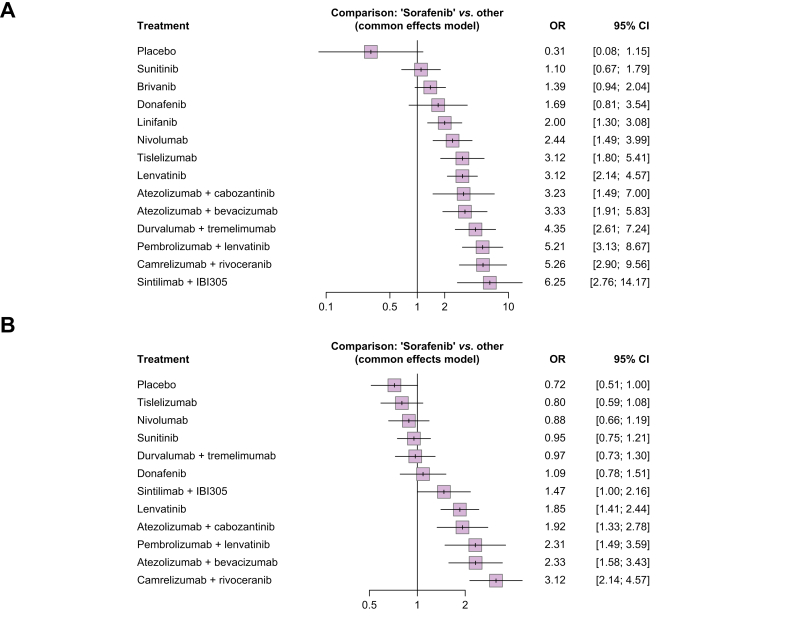
When considering sorafenib as reference, treatment with ICI + anti-VEGF agents was confirmed to provide the highest reduction in the risk of death (Fig. 3A): the association of sintilimab + IBI305 and camrelizumab + rivoceranib reduced the risk of death by 43% (hazard ratio [HR] 0.57; 95% CI 0.43–0.75) and 38% (HR 0.62; 95% CI 0.49–0.79), respectively, followed by treatment with atezolizumab + bevacizumab (HR 0.66; 95% CI 0.52–0.84). Pembrolizumab + lenvatinib and durvalumab + tremelimumab were shown to similarly reduce the risk of death compared with sorafenib, with HRs of 0.77 (95% CI 0.62–0.97) and 0.78 (95% CI 0.66–0.93), respectively. ICI monotherapies, namely nivolumab and tislelizumab, were confirmed to similarly reduce the risk of death compared with sorafenib, even if the statistically significance for superiority was not met, with HRs of 0.85 (95% CI 0.71–1.01) and 0.85 (95% CI 0.71–1.02), respectively. According to p scores, sintilimab + IBI305 and camrelizumab + rivoceranib reported a probability of 95.3% and 90.9%, respectively, to be the most effective in reducing the risk of death; atezolizumab + bevacizumab had a probability of 86.3% of reducing the risk of death (Table S3).
Fig. 3.
Forest plots.
(A) Forest plot, HRs and corresponding CIs for the association between sorafenib and mortality considering sorafenib as reference. (B) Forest plot, HRs and corresponding CIs for the association between sorafenib and progression considering sorafenib as reference. HR, hazard ratio.
As reported in Fig. 3B, camrelizumab + rivoceranib and pembrolizumab + lenvatinib performed the best in reducing the risk of PFS events compared with sorafenib, with HRs of 0.52 (95% CI 0.41–0.65) and 0.52 (95% CI 0.35–0.77), respectively. Lenvatinib and the combination of atezolizumab + cabozantinib reported the same risk reduction, with HRs of 0.63 (95% CI 0.44–0.90) and 0.63 (95% CI 0.44–0.91), respectively. ICI monotherapy and the combination of anti PD-L1 + anti-CTLA-4 were not associated with a significant reduction in the risk of progression or death compared with sorafenib. The p scores analysis showed that camrelizumab + rivoceranib has the highest probability in being the most effective in reducing the risk of progression (90.8%), followed by pembrolizumab + lenvatinib (90.0%) and sintilimab + IBI305 (85.1%) (Table S4).
As previously reported,26,27 we ran a secondary analysis to assess the reduction in the risk of death or progression associated with atezolizumab + bevacizumab vs. other treatment. The efficacy of atezolizumab + bevacizumab was confirmed to overlap with that of camrelizumab + rivoceranib (HR 1.06; 95% CI 0.75–1.51) and to be similar to that of sintilimab + IBI305 (HR 1.16; 95% CI 0.80–1.68); atezolizumab + bevacizumab was shown to be statistically superior in reducing the risk of death compared with all the TKI monotherapies; when compared with ICI monotherapies and the other combinations, the reduction in the risk of death did not reach the statistical significance (Fig. S1). In terms of PFS, atezolizumab + bevacizumab was associated with a significant reduction in the risk of PFS events compared with placebo, sunitinib, tislelizumab, donafenib, brivanib, sorafenib, and nivolumab (Fig. S2).
Objective response rate
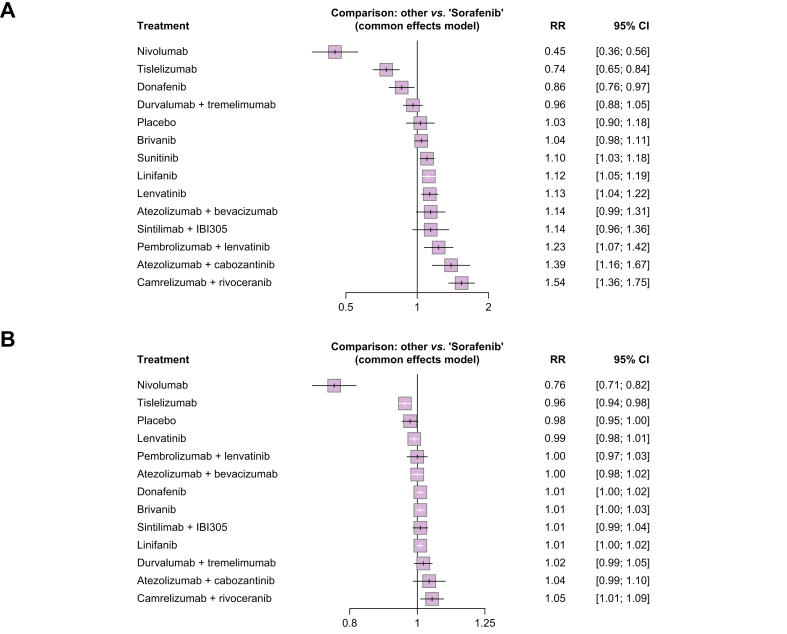
As previously reported, radiological response was assessed according to RECIST 1.1 in all the trials, except for the SHARP, Asia Pacific, and Cheng 2015 trials (RECIST) and the Johnson 2013 trial (mRECIST). Table 2 descriptively reports the objective response rate (ORR) and disease control rate (DCR) for each treatment arm. As reported in Fig. 4A, all the combination therapies were associated with a significant increase in the probability of achieving ORR (partial response or complete response), compared with sorafenib. Sintilimab + IBI305, camrelizumab + rivoceranib, and pembrolizumab + lenvatinib reported the highest probability of radiological response. When assessing the probability of all treatments in achieving DCR (partial response or complete response or stable disease), camrelizumab + rivoceranib, atezolizumab + bevacizumab, and pembrolizumab + lenvatinib were reported to increase the most the probability of achieving DCR compared with sorafenib. Among the monotherapies, only lenvatinib was associated with a significant improvement in the probability of DCR (Fig. 4B).
Table 2.
Descriptive report of efficacy outcomes.
| Study | Arm | mOS (months) (95% CI) | HR (95% CI) p value | mPFS (months) (95% CI) | HR (95% CI) p value | ORR (%) (95% CI) | DCR(%) (95% CI) |
|---|---|---|---|---|---|---|---|
| Cheng 201320 | Sunitinib | 7.9 (7.4–9.2) | 1.30 (1.1–1.5) 0.99 |
3.6 (2.8–4.1) | 1.13 (0.9–1.3) 0.88 |
6.2 | 50.0 |
| Sorafenib | 10.2 (8.9–11.4) | 3.0 (2.8–4.0) | 5.9 | 51.3 | |||
| Johnson 201321 | Brivanib | 9.9 (8.5–11.5) | 1.07 (0.9–1.2) 0.31 |
4.1 (3.1–4.2) | 1.01 (0.9–1.2) 0.85 |
12 (9–15) | 65 (61–69) |
| Sorafenib | 9.5 (8.3–10.6) | 4.2 (4.1–4.3) | 9 (7–11) | 66 (61–69) | |||
| Cainap 201522 | Linifanib | 9.1 (8.1–10.2) | 1.05 (0.9–1.2) | 5.4 (4.2–5.6) | 0.76 (0.6–0.9) 0.001 |
10.1 | n.a. |
| Sorafenib | 9.8 (8.3–11) | 4.0 (2.8–4.2) | 6.1 | n.a. | |||
| REFLECT2 | Lenvatinib | 13.6 (12.1–14.9) | 0.92 (0.8–1.1) | 7.3 (5.6–7.5) | 0.65 (0.6–0.8) <0.0001 |
18.8 (15.3–22.3) | 72.8 (68.8–76.8) |
| Sorafenib | 12.3 (10.4–13.9) | 3.6 (3.6–3.9) | 6.5 (4.3–5.14) | 59 (54.6–63.5) | |||
| IMbrave1507 | Atezolizumab + bevacizumab | 19.2 (17–23.7) | 0.66 (0.5–0.9) <0.001 |
6.9 (4.7–8.6) | 0.65 (0.53–0.81) <0.001 |
30.0 (25.0–35.0) | 74 |
| Sorafenib | 13.4 (11.4–16.9) | 4.3 (4.0–5.6) | 11.0 (7.0–17.0) | 55 | |||
| COSMIC-31212 | Atezolizumab + cabozabtinib | 15.4 (13.7–17.7) | 0.90 (0.7–1.2) 0.438 |
6.8 (5.6–8.3) | 0.63 (0.4–0.9) 0.0012 |
11.2 (8.1–14) | 78 |
| Sorafenib | 15.5 (12.1–NR) | 4.2 (2.8–7.0) | 3.7 (1.6–7.1) | 65 | |||
| HIMALAYA10 | Durvalumab + tremelimumab | 16.4 (14.2–19.6) | 0.78 (0.7–0.9) 0.0035 |
3.8 (3.7–5.3) | 0.90 (0.8–1.1) | 20.1 | 60.1 |
| Sorafenib | 13.8 (12.3–16.1) | 4.2 (3.8–5.5) | 5.1 | 60.7 | |||
| CheckMate- 45923 | Nivolumab | 16.4 (13.9–18.4) | 0.85 (0.7–1.0) 0.075 |
3.7 (3.1–3.9) | 0.93 (0.8–1.1) | 15 (12–19) | 55 |
| Sorafenib | 14.7 (11.9–17.2) | 3.8 (3.7–4.5) | 7 (5–10) | 58 | |||
| SHARP18 | Sorafenib | 10.7 (9.4–13.3) | 0.69 (0.6–0.9) <0.001 |
4.1 (3.5–4.8) | 1.1 (0.9–1.3) 0.77 |
2 | 43 |
| Placebo | 7.9 (6.8–9.1) | 4.9 (4.2–6.3) | 1 | 32 | |||
| Asia Pacific19 | Sorafenib | 6.5 (5.6–7.6) | 0.68 (0.5–0.9) 0.014 |
2.8 (2.6–3.6) | 0.57 (0.4–0.8) 0.0005 |
3.3 | 35.3 (27.7–43.6) |
| Placebo | 4.2 (3.8–5.5) | 1.4 (1.3–1.6) | 1.3 | 15.8 (8.4–26) | |||
| ORIENT-329 | Sintilimab + IBI305 | NR | 0.57 (0.4–0.8) <0.0001 |
4.6 (4.1–5.7) | 0.56 (0.5–0.7) <0.0001 |
21 (17–25) | 72 (67–77) |
| Sorafenib | 10.4 (8.5–NR) | 2.8 (2.7–3.2) | 4 (2–8) | 64 (56–71) | |||
| Qin 202124 | Donafenib | 12.0 (10.3–13.1) | 0.84 (0.7–0.9) 0.031 |
3.7 (3.0–3.7) | 0.91 (0.8–1.1) 0.057 |
4.6 | 30.8 |
| Sorafenib | 10.1 (9.2–11.9) | 3.6 (2.4–3.7) | 2.7 | 28.7 | |||
| LEAP-00213 | Lenvatinib + pembrolizumab | 21.2 (19.0–23.6) | 0.84 (0.7–0.9) 0.0227 |
8.2 (6.4–8.4) | 0.87 (0.7–1.0) 0.0466 |
26.1 (21.8–30.7) | 81.3 |
| Lenvatinib + placebo | 19.0 (17.2–21.7) | 8.0 (6.3–8.2) | 17.5 (13.9–21.6) | 78.4 | |||
| Qin 202211 | Camrelizumab + rivoceranib | 22.1 (19.1–27.2) | 0.62 (0.5–0.8) <0.0001 |
5.6 (5.5–6.3) | 0.52 (0.4–0.7) <0.0001 |
25.4 (20.3–31) | 78.3 (72.9–83.1) |
| Sorafenib | 15.2 (13.0–18.5) | 3.7 (2.8–3.7) | 5.9 (3.4–9.4) | 53.9 (47.7–59.9) | |||
| RATIONALE-30125 | Tislelizumab | 15.9 | 0.85 (0.7–1.0) 0.0398 |
2.2 | 1.1 (0.9–1.3) | 14.3 (10.8–18.5) | 41.8 |
| Sorafenib | 14.1 | 3.6 | 5.4 (3.2–8.4) | 47.3 |
DCR, disease control rate; HR, hazard ratio; mOS, median overall survival; mPFS, median progression-free survival; n.a., not available; NR, not reached; ORR, objective response rate.
Fig. 4.
Forest plots.
(A) Forest plot, ORs and corresponding 95% CIs for the association between sorafenib and radiological response, considering each treatment as reference. (B) Forest plot, ORs and corresponding 95% CIs for the association between sorafenib and disease control rate, considering each treatment as reference. OR, odds ratio.
Safety
Data about AEs was extracted by each arm of the trials of interest. AEs were reported according to NCI Common Terminology Criteria for Adverse Events (CTCAE) versions 3 (Asia Pacific, SHARP, Johnson 2013, and Cheng 2013), 4 (Cainap 2015, IMbrave150, REFLECT, CheckMate 459, and Qin 2021) and 5 (ORIENT-32, COSMIC-312, HIMALAYA, LEAP-002, Qin 2022, and RATIONALE-301). For the Asia Pacific and SHARP studies, the incidence of grade ≥3 AEs was not available. We therefore extracted relative risk (RR) for the incidence of serious AEs, defined as any AEs that were life-threatening, resulted in death, required patient hospitalisation or prolongation of hospitalisation, or resulted in a persistent or significant disability or incapacity. The overall incidence of all grade AEs of any cause was not available for the Cheng 2013 trial; therefore, it was not included in the analysis. Table 3 summarises the incidence of AEs for each treatment arm.
Table 3.
Descriptive incidence of AEs according to NCTCA.
| Study | Arm | Any grade AE, n (%) | Grade ≥3 AEs, n (%) | AEs requiring treatment discontinuation, n (%) |
|---|---|---|---|---|
| Cheng 201320 | Sunitinib | n.a. | 449 (85.4%) | 184 (35%) |
| Sorafenib | n.a. | 404 (74.5%) | 163 (30%) | |
| Johnson 201321 | Brivanib | 564 (98%) | 384 (68%) | 243 (43%) |
| Sorafenib | 569 (99%) | 371 (65.2%) | 188 (33%) | |
| Cainap 201522 | Linifanib | 508 (99.6%) | 435 (85.3%) | 185 (36.3%) |
| Sorafenib | 511 (98.5%) | 389 (75%) | 132 (25.4%) | |
| REFLECT2 | Lenvatinib | 470 (99%) | 357 (75%) | 42 (9%) |
| Sorafenib | 472 (99%) | 316 (67%) | 34 (7%) | |
| IMbrave1507 | Atezolizumab + bevacizumab | 322 (98.0%) | 230 (69.9%) | 72 (22.0%) |
| Sorafenib | 154 (99.0%) | 98 (62.8%) | 18 (12.0%) | |
| COSMIC-31212 | Atezolizumab + cabozabtinib | 93% | 63.5% | 6.1% |
| Sorafenib | 90% | 41% | 7.7 % | |
| HIMALAYA10 | Durvalumab + tremelimumab | 378 (97.4%) | 196 (50.5%) | 32 (8.2%) |
| Sorafenib | 357 (95.5%) | 196 (52.4%) | 41 (11%) | |
| Durvalumab | 345 (88.9%) | 144 (37.1%) | 16 (4.1%) | |
| CheckMate-45923 | Nivolumab | 257 (70%) | 82 (22.3%) | 27 (7.4%) |
| Sorafenib | 338 (93.1%) | 180 (49.5%) | 42 (11.6%) | |
| SHARP18 | Sorafenib | 98% | 45% | 34 (11%) |
| Placebo | 96% | 32% | 15 (5%) | |
| Asia Pacific19 | Sorafenib | 146 (98%) | 71 (serious) (47.7%) | 29 (19.5%) |
| Placebo | 71 (94.7%) | 34 (serious) (45.3%) | 10 (13.3%) | |
| ORIENT-329 | Sintilimab + IBI305 | 376 (99%) | 207 (54%) | 52 (14%) |
| Sorafenib | 181 (98%) | 87 (47%) | 11 (6%) | |
| Qin 202124 | Donafenib | 332 (100%) | 191 (57%) | 34 (10%) |
| Sorafenib | 329 (99%) | 224 (67%) | 42 (13%) | |
| LEAP-00213 | Lenvatinib + pembrolizumab | 381 (96.5%) | 247 (62.5%) | 22 (5.6%) |
| Lenvatinib + placebo | 378 (95.7%) | 227 (57.5%) | 18 (4.6%) | |
| Qin 202211 | Camrelizumab + rivoceranib | 265 (97.4%) | 220 (80.9%) | 10 (3.7%) |
| Sorafenib | 249 (92.6%) | 142 (52.8%) | 12 (4.5%) | |
| RATIONALE-30125 | Tislelizumab | 325 (96.2%) | 163 (48.2%) | 37 (10.9%) |
| Sorafenib | 324 (100%) | 212 (65.4%) | 60 (18.5%) |
AE, adverse event.
Compared with sorafenib, ICI monotherapies, namely nivolumab and tislelizumab, were associated with a significant reduction in the risk of grade ≥3 AEs, with RR of 0.45 (95% CI 0.36–0.46) and RR of 0.74 (95% CI 0.65–0.84) respectively (Fig. 5A); among the combination strategies, durvalumab + tremelimumab and atezolizumab + bevacizumab had the lowest risk of grade ≥3 AEs, whereas camrelizumab + rivoceranib was associated with the highest risk, which appeared to be similar to that of sorafenib. Pembrolizumab + lenvatinib, atezolizumab + cabozantinib, and camrelizumab + rivoceranib were reported to significantly increase the risk of grade ≥3 AEs compared with sorafenib. When considering the all-grade AEs, again nivolumab and tislelizumab reported a significant reduction in the risk of AEs, with RRs of 0.76 (95% CI 0.71–0.81) and 0.96 (95% CI 0.94–0.98) respectively (Fig. 5B).
Fig. 5.
Forest plot.
(A) Forest plot, RRs and corresponding 95% CIs for the association between sorafenib and the risk of AEs of grade 3 or higher, considering sorafenib as reference. (B) Forest plot, RR and corresponding 96% Cs for the association between sorafenib and the risk of AEs of any grade, considering sorafenib as reference. AE, adverse event; RR, relative risk.
Subgroup analyses
We performed a subgroup analysis according to the aetiology of HCC (HBV, HCV, and non-viral). As reported in Table 1, aetiology was heterogeneous among trials, reflecting the differences both in the region of origin and in the stratification factors: among the trials of interest, the Cainap 2015, HIMALAYA, COSMIC-312, and RATIONALE-301 trials were stratified according to HCC aetiology (Table S1).
Data for patients with HBV were available for all the trials. Data for HCV were available for the following trials that were therefore included in the analysis: IMbrave150, Qin 2022, COSMIC-312, RATIONALE-301, CheckMate 459, LEAP-002, HIMALAYA, Cainap 2015, and Johnson 2013. Subgroup analysis was run, including published subgroup data of the following studies: Qin 2022, HIMALAYA, RATIONALE-301, LEAP-002, IMbrave150, COSMIC-312, CheckMate 459, and REFLECT.
Overall, the subgroup analysis of patients with HBV was based on pooled data of 5,149 HBV-positive patients: 5,035 received active treatment and 114 placebo. All the combination treatments were reported to significantly reduce the risk of death compared with sorafenib in this subgroup (Fig. S3), with atezolizumab + cabozantinib being associated with the greatest reduction in the risk of death (HR 0.46; 95% CI 0.29–0.73).
In total, 1,699 patients with HCV were treated among the 10 trials reporting the specific subgroup analysis. The combination of atezolizumab + bevacizumab was the only one reporting a significant reduction in the risk of death in patients with HCV (HR 0.43; 95% CI 0.22–0.86) (Fig. S4). The non-viral subgroup included 1,594 HBV- and HCV-negative patients receiving active treatment. The non-viral subgroup analysis in the REFLECT study included only patients with alcohol-related disease. Durvalumab + tremelimumab was the only combination that was shown to provide a significant reduction in the risk of death compared with sorafenib in non-viral patients (HR 0.74; 95% CI 0.57–0.96) (Fig. S5). In patients with EHS and/or MVI, the efficacy data were similar for those reported in the whole population (Fig. S6).
Discussion
Clinicians treating HCC today face the difficult challenge of formulating treatment decisions without having credible and direct head-to-head comparisons across all the therapeutic regimens known to be effective in advanced disease. With the aim of providing practice-informing comparisons across therapies, we have performed the largest NMA exploring the various systemic therapy options studied in the first-line treatment of advanced HCC to date, including 10,820 patients receiving systemic therapy for metastatic or unresectable HCC accrued across 15 landmark phase III trials.
Our study shows that all the immunotherapy combination regimens tested in first-line phase III trials provide a significant OS advantage compared with sorafenib, with the only exception of atezolizumab + cabozantinib. Within the limitations of our NMA and accounting for unavoidable heterogeneity in eligibility criteria for each study, systematic ranking of therapeutic regimens showed a clear gradient in OS estimates that favoured VEGF-containing immunotherapy combinations over TKI monotherapy. Interestingly, our NMA demonstrates overlapping estimates of OS advantage vs. sorafenib between PD-1/VEGF containing regimens and the PD-L1/CTLA-4 combination of durvalumab + tremelimumab. In a disease where immune evasion is a key oncogenic driver, ICI-based combinations outperform monotherapy by exerting a synergistic action on hypoxia, aberrant neoangiogenesis, and immune exhaustion.28 However, accounting for the differences in the mechanism of action (MoA) explored in phase III trials, no ideal companion agent to PD-1 pathway blockade has emerged from our study, with CTLA-4 inhibition, TKIs, and VEGF inhibitors leading to largely overlapping survival estimates in comparison with sorafenib.
Whether there are intrinsic immunologic differences between selective VEGF deprivation and modulation of VEGF receptors using TKIs remains the subject of debate. It is important to emphasise that all studies testing a combination of an ICI and a pure VEGF inhibitor met their primary endpoints,7,9,11 whereas TKI-based treatment strategies failed to be proven superior to the standard of care, with the exception of rivoceranib + camrelizumab.12,13 Our NMA provides an original contribution to this debate by highlighting that the failure of PD-1 + TKI combination does not exclusively depend on differences in MoA but is to be attributed at least in part to the choice of the control arm. To this end, we show for the first time that, despite failing to show improvement against lenvatinib monotherapy, the combination of pembrolizumab + lenvatinib is superior to sorafenib, casting doubt around the therapeutic equivalence of first-line TKIs.
This aspect is of utmost importance in contemporary clinical trial design. On the one hand, the use of sorafenib as a control arm in future studies is likely to become an outdated comparator. On the other hand, the use of ‘investigator choice’ TKI, explored in certain second-line studies such as IMbrave251 (NCT04770896), might introduce unwanted heterogeneity by comparing therapies with different efficacy and immunomodulatory properties.
The only treatment strategy not involving the use of antiangiogenic agents and achieving an OS improvement compared with sorafenib is the double immune checkpoint blockade of durvalumab + the single priming dose of tremelimumab. The combination has very recently been approved by the FDA and is currently considered a potential alternative to atezolizumab + bevacizumab in the first line,8 and their different spectrum of toxicity will be key to inform the treatment choice in clinical practice.
Our study provides further insight regarding the non-linear association between PFS and OS. Intriguingly, all ICI combinations included in our NMA achieved a statistically significant PFS advantage over sorafenib, with the only notable exception of durvalumab + tremelimumab. PFS has an established role as a surrogate endpoint for patients’ survival in other oncological indications where multiple lines of therapy exist, facilitating regulatory approval of new drugs.29 Although measuring the efficacy of the single line of treatment in question, PFS is a composite endpoint that includes death and/or progression. Although a PFS threshold of 0.6 has been proposed as an optimal HR cut-off to predict for OS advantage,30 PFS remains vulnerable to the competing effect of hepatic decompensation, radiologic criteria chosen to assess progression and continuation of treatment beyond initial progression. These emerging uncertainties call for caution in the choice of endpoints in contemporary clinical trials. For novel treatment strategies including immunotherapy where a restricted group of patients may achieve long-term survival, formal testing of differences in median survival times can fall short of significance, and different endpoints have been proposed to capture the clinical benefit of ICI combinations (i.e. time to treatment failure and restricted mean survival time).31
With no clear difference emerging in terms of OS advantage across combination regimens, an aspect of increasing importance is treatment-related toxicity. Perhaps unsurprisingly, combinations of ICI + TKIs were associated with a higher risk of clinically significant toxicity compared with ICI + an anti-VEGF monoclonal antibody, with atezolizumab + cabozantinib, camrelizumab + rivoceranib, and pembrolizumab + lenvatinib being the regimens associated with the highest rates of grade ≥3 AEs. The additive toxicity profile of TKIs when combined with ICI, which often translates to symptomatic AEs, needs to be taken into consideration in evaluating the merit of each systemic therapy options in the first line. Higher rates of severe AEs could influence the likelihood of receiving a second-line treatment owing to the risk of clinical decompensation, thus playing a potential role in the apparent mismatch between PFS and OS.14
Among various combination regimens, the good tolerability of selective inhibition of the PD-1/PD-L1 checkpoint has re-ignited interest in anti-PD-1 monotherapies in view of their clinical value as noninferior therapies to sorafenib. Our NMA shows the overlap in terms of OS and PFS of both tislelizumab and nivolumab when compared against sorafenib, with both agents standing out for their association with the lowest risk of development of high-grade AEs.
Considering the favourable tolerability profile of anti-PD-1 monoclonal antibodies, these agents could find a potential role in frailer patient populations, including those with impaired liver function, who do not have contraindications to ICI but for whom clinicians would feel more comfortable in using a single agent rather than a combination strategy. Nivolumab is the only ICI prospectively tested in patients with Child–Pugh B liver dysfunction,32 with a more positive impact on quality of life compared with sorafenib.23 Evidence of its use in the first line in this special population is limited to observational studies.33
A point of intense scientific debate is the identification of differences in response to immunotherapy across various aetiologies of chronic liver disease. Preclinical evidence suggests that HCC arising on the background of non-alcoholic steatohepatitis (NASH) might be enriched with exhausted CD8+PD1+ T cells, leading to an impairment of response to ICI in murine models.34
As already suggested by the subgroup analyses conducted as part of each trial, our NMA shows that, for patients with non-viral aetiology of chronic liver disease, the only regimen confirmed to significantly improve OS, compared with sorafenib, is durvalumab + tremelimumab. However, the heterogeneity of the non-viral subgroup across trials is a significant source of bias that cannot be corrected when performing an NMA, and it prompts caution when interpreting the results of the subgroup analysis.
Although informative for future drug development strategies, our results should not be intended as practice changing. In fact, fewer than half of the studies reported outcomes in non-viral patients, therefore limiting the number of patients included in our subanalysis. In addition, the conclusions that can be drawn are limited by the heterogeneity of non-viral aetiologies, encompassing a wide range of clinical scenarios, including but not limited to NASH. Whether patients with NASH-associated HCC might display aetiology-specific therapeutic vulnerabilities,35 to date no study of ICI has ever been conducted in patients of a single disease aetiology, limiting the validity of claims that ICI may be less effective in this population. The paucity of prospective, adequately stratified studies calls for the incorporation of reproducible diagnostic criteria for non-alcoholic fatty liver disease, NASH, and alcohol excess in future trial design.
An underlying HBV infection was associated with prolonged survival for combination treatments, including atezolizumab + cabozantinib, which significantly prolonged OS against sorafenib in this subset but not in the general population. Conversely, HCV-related HCC was associated with a lower OS benefit from ICI combinations in general, with only atezolizumab + bevacizumab achieving a significant survival improvement compared with sorafenib. Increasing evidence in the literature, including this NMA, show HBV and HCV-related HCC to cluster as two distinct subgroups, with clinical, geographical, and molecular peculiarities that could partly explain the different responsiveness to ICI. For instance, CTNNB1-mutated HCC seems to gain less benefit from ICI,36 and the mutation is found more commonly in HCV.37 However, post hoc analyses of phase III clinical trials suggested that the clinical efficacy of sorafenib is enhanced in patients with HCV-related cirrhosis.38 The incomplete understanding of aetiology-specific pathobiology may be a key factor influencing the different effects of ICI and their combinations on OS across trials.
This NMA acknowledges a number of limitations, mainly as a result of its own nature of cross-trial comparison. First, this study is not meant to substitute level 1 prospective evidence, and it has been conceived pragmatically with the aim of providing additional data to aid clinical decision-making and inform future drug development strategies. The heterogeneity of the studies, including the different stratification factors, the different MoA of the interventions of interest, and the different geographic origins of the populations enrolled, could jeopardise the general clinical applicability of the results of the single studies. In particular, aside from aetiological and genetic factors, the different therapeutic approaches across continents further hamper the reliability of cross-trial comparisons. Furthermore, we acknowledge the wide time range of the trials of interest as another limitations. In fact, the prognosis of patients affected by HCC has significantly increased over time, owing to both the availability of treatment beyond the first line and the better management of AEs and concomitant risk factors. In particular, the outcomes of patients receiving sorafenib and lenvatinib have substantially improved over time, and this represents another source of bias that cannot be corrected when performing an NMA. Therefore, while attempting to disentangle the complexity of the studies with additional subgroup analyses, we recognise that these should be regarded as purely hypothesis generating given that not all the studies provided adequate and homogeneous data on patients’ aetiology.
Taken together, and despite the acknowledged limitations, our study strongly consolidates the role of immunotherapy combinations as a mainstay of treatment for advanced HCC. In a cancer diagnosis characterised by a dismal natural history, we showed how combination strategies have successfully achieved unprecedented survival results, of up to 22 months.
By providing a comprehensive analysis of the largest pool of trial participants presented to date, our NMA offers an original and up-to-date perspective on the role of first-line treatment options in advanced HCC, lending itself as a useful, evidence-based source of guidance to aid therapeutic decision-making in advanced HCC.
Financial support
AD is supported by the NIHR Imperial BRC, by grant funding from the European Association for the Study of the Liver (Andrew Burroughs Fellowship) and Cancer Research UK (RCCPDB-Nov21/100008). DJP is supported by grant funding from the Wellcome Trust Strategic Fund (PS3416), the Associazione Italiana per la Ricerca sul Cancro (AIRC MFAG 25697), the Roger Williams Institute of Hepatology – Foundation for Liver Research, and acknowledges grant support from the Cancer Treatment and Research Trust (CTRT) and infrastructural support by the Imperial Experimental Cancer Medicine Centre and the NIHR Imperial Biomedical Research Centre. AC is supported by the NIHR Imperial BRC.
Authors’ contributions
Study concept and design: CAMF, BS, AD, DJP. Acquisition of data: CAMF, C-VS, JK. Analysis and interpretation of data: CAMF, BS, AD, DJP. Statistical analysis: CAMF, LS. Acquisition of funding: NA. Study supervision: DJP. Drafting of the manuscript: CAMF, BS, AD, DJP. Critical revision of the manuscript for important intellectual content: all authors.
Data availability statement
Raw data that support the findings will be made available upon reasonable request from the corresponding author.
Conflicts of interest
DJP received lecture fees from ViiV Healthcare, Bayer Healthcare, EISAI, BMS, and Roche and travel expenses from BMS and Bayer Healthcare; consulting fees for Mina Therapeutics, DaVolterra, Mursla, IPSEN, Exact Sciences, Avamune, EISAI, Roche, and Astra Zeneca; and research funding (to institution) from MSD, GSK, and BMS. BS received travel support from AbbVie, Gilead, and Ipsen. AC received consulting fees from MSD, Astra Zeneca, Roche, and BMS. He also received speaker fees from Novartis, Astra Zeneca, and EISAI. AD received educational support for conference attendance and consultancy fees by Roche. There are no other personal or financial conflicts of interest to disclose.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100702.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Kudo M., Finn R.S., Qin S., Han K.H., Ikeda K., Piscaglia F., et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 3.Bruix J., Qin S., Merle P., Granito A., Huang Y.H., Bodoky G., et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 4.Abou-Alfa G.K., Meyer T., Cheng A.L., El-Khoueiry A.B., Rimassa L., Ryoo B.Y., et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379:54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu A.X., Kang Y.K., Yen C.J., Finn R.S., Galle P.R., Llovet J.M., et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282–296. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 6.Finn R.S., Qin S., Ikeda M., Galle P.R., Ducreux M., Kim T.Y., et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 7.Cheng A.L., Qin S., Ikeda M., Galle P.R., Ducreux M., Kim T.Y., et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862–873. doi: 10.1016/j.jhep.2021.11.030. [DOI] [PubMed] [Google Scholar]
- 8.Reig M., Forner A., Rimola J., Ferrer-Fabrega J., Burrel M., Garcia-Criado A., et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ren Z., Xu J., Bai Y., Xu A., Cang S., Du C., et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol. 2021;22:977–990. doi: 10.1016/S1470-2045(21)00252-7. [DOI] [PubMed] [Google Scholar]
- 10.Abou-Alfa G.K., Lau G., Kudo M., Chan S.L., Kelley R.K., Furuse J., et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evidence. 2022;1(8) doi: 10.1056/EVIDoa2100070. EVIDoa2100070. [DOI] [PubMed] [Google Scholar]
- 11.Qin S., Chan L.S., Gu S., Bai Y., Ren Z., Lin X., et al. Camrelizumab (C) plus rivoceranib (R) vs. sorafenib (S) as first-line therapy for unresectable hepatocellular carcinoma (uHCC): a randomized, phase III trial. [Abstract LBA35] Ann Oncol. 2022;33(Suppl. 7):S808–S869. [Google Scholar]
- 12.Kelley R.K., Rimassa L., Cheng A.L., Kaseb A., Qin S., Zhu A.X., et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23:995–1008. doi: 10.1016/S1470-2045(22)00326-6. [DOI] [PubMed] [Google Scholar]
- 13.Finn R.S., Kudo M., Merle P., Meyer T., Qin S., Ikeda M., et al. Primary results from the phase III LEAP-002 study: lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC). [Abstract LBA34] Ann Oncol. 2022;33(Suppl. 7):S808–S869. [Google Scholar]
- 14.Cabibbo G., Celsa C., D'Alessio A., Fulgenzi C.A.M., Pinato D.J. COSMIC-312: mounting immunotherapy enigmas for hepatocellular carcinoma. Lancet Oncol. 2022;23:e441. doi: 10.1016/S1470-2045(22)00497-1. [DOI] [PubMed] [Google Scholar]
- 15.Fukumura D., Kloepper J., Amoozgar Z., Duda D.G., Jain R.K. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15:325–340. doi: 10.1038/nrclinonc.2018.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finn R.S., Ikeda M., Zhu A.X., Sung M.W., Baron A.D., Kudo M., et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38:2960–2970. doi: 10.1200/JCO.20.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins J.P., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Llovet J.M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J.F., et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 19.Cheng A.L., Kang Y.K., Chen Z., Tsao C.J., Qin S., Kim J.S., et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 20.Cheng A.L., Kang Y.K., Lin D.Y., Park J.W., Kudo M., Qin S., et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31:4067–4075. doi: 10.1200/JCO.2012.45.8372. [DOI] [PubMed] [Google Scholar]
- 21.Johnson P.J., Qin S., Park J.W., Poon R.T., Raoul J.L., Philip P.A., et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31:3517–3524. doi: 10.1200/JCO.2012.48.4410. [DOI] [PubMed] [Google Scholar]
- 22.Cainap C., Qin S., Huang W.T., Chung I.J., Pan H., Cheng Y., et al. Linifanib versus sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol. 2015;33:172–179. doi: 10.1200/JCO.2013.54.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yau T., Park J.W., Finn R.S., Cheng A.L., Mathurin P., Edeline J., et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23:77–90. doi: 10.1016/S1470-2045(21)00604-5. [DOI] [PubMed] [Google Scholar]
- 24.Qin S., Bi F., Gu S., Bai Y., Chen Z., Wang Z., et al. Donafenib versus sorafenib in first-line treatment of unresectable or metastatic hepatocellular carcinoma: a randomized, open-label, parallel-controlled phase II–III trial. J Clin Oncol. 2021;39:3002–3011. doi: 10.1200/JCO.21.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin S., Kudo M., Meyer T., Finn R.S., Vogel A., Bai Y., et al. Final analysis of RATIONALE-301: randomized, phase III study of tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Ann Oncol. 2022;33(Suppl. 7):S1402–S1403. [Google Scholar]
- 26.Vogel A., Rimassa L., Sun H.C., Abou-Alfa G.K., El-Khoueiry A., Pinato D.J., et al. Comparative efficacy of atezolizumab plus bevacizumab and other treatment options for patients with unresectable hepatocellular carcinoma: a network meta-analysis. Liver Cancer. 2021;10:240–248. doi: 10.1159/000515302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fulgenzi C.A.M., D'Alessio A., Airoldi C., Scotti L., Demirtas C.O., Gennari A., et al. Comparative efficacy of novel combination strategies for unresectable hepatocellular carcinoma: a network metanalysis of phase III trials. Eur J Cancer. 2022;174:57–67. doi: 10.1016/j.ejca.2022.06.058. [DOI] [PubMed] [Google Scholar]
- 28.Llovet J.M., Castet F., Heikenwalder M., Maini M.K., Mazzaferro V., Pinato D.J., et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022;19:151–172. doi: 10.1038/s41571-021-00573-2. [DOI] [PubMed] [Google Scholar]
- 29.Belin L., Tan A., De Rycke Y., Dechartres A. Progression-free survival as a surrogate for overall survival in oncology trials: a methodological systematic review. Br J Cancer. 2020;122:1707–1714. doi: 10.1038/s41416-020-0805-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Llovet J.M., Montal R., Villanueva A. Randomized trials and endpoints in advanced HCC: role of PFS as a surrogate of survival. J Hepatol. 2019;70:1262–1277. doi: 10.1016/j.jhep.2019.01.028. [DOI] [PubMed] [Google Scholar]
- 31.Mukhopadhyay P., Ye J., Anderson K.M., Roychoudhury S., Rubin E.H., Halabi S., et al. Log-rank test vs MaxCombo and difference in restricted mean survival time tests for comparing survival under nonproportional hazards in immuno-oncology trials: a systematic review and meta-analysis. JAMA Oncol. 2022;8:1294–1300. doi: 10.1001/jamaoncol.2022.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kudo M., Matilla A., Santoro A., Melero I., Gracían A.C., Acosta-Rivera M., et al. CheckMate 040 cohort 5: a phase I/II study of nivolumab in patients with advanced hepatocellular carcinoma and Child–Pugh B cirrhosis. J Hepatol. 2021;75:600–609. doi: 10.1016/j.jhep.2021.04.047. [DOI] [PubMed] [Google Scholar]
- 33.Fessas P., Kaseb A., Wang Y., Saeed A., Szafron D., Jun T., et al. Post-registration experience of nivolumab in advanced hepatocellular carcinoma: an international study. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2020-001033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfister D., Nuñez N.G., Pinyol R., Govaere O., Pinter M., Szydlowska M., et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592:450–456. doi: 10.1038/s41586-021-03362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leslie J., Mackey J.B.G., Jamieson T., Ramon-Gil E., Drake T.M., Fercoq F., et al. CXCR2 inhibition enables NASH-HCC immunotherapy. Gut. 2022;71:2093–2106. doi: 10.1136/gutjnl-2021-326259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu A.X., Abbas A.R., de Galarreta M.R., Guan Y., Lu S., Koeppen H., et al. Molecular correlates of clinical response and resistance to atezolizumab in combination with bevacizumab in advanced hepatocellular carcinoma. Nat Med. 2022;28:1599–1611. doi: 10.1038/s41591-022-01868-2. [DOI] [PubMed] [Google Scholar]
- 37.Cancer Genome Atlas Research Network Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell. 2017;169:1327–1341. doi: 10.1016/j.cell.2017.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruix J., Raoul J.L., Sherman M., Mazzaferro V., Bolondi L., Craxi A., et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012;57:821–829. doi: 10.1016/j.jhep.2012.06.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data that support the findings will be made available upon reasonable request from the corresponding author.