Abstract
The oxidative balance of a cell is maintained by the Kelch-like ECH-associated protein 1 (KEAP1)/nuclear factor erythroid 2-related factor 2 (NRF2) pathway. This cytoprotective pathway detoxifies reactive oxygen species and xenobiotics. The role of the KEAP1/NRF2 pathway as pro-tumorigenic or anti-tumorigenic throughout stages of carcinogenesis (including initiation, promotion, progression, and metastasis) is complex. This mini review focuses on key studies describing how the KEAP1/NRF2 pathway affects cancer at different phases. The data compiled suggest that the roles of KEAP1/NRF2 in cancer are highly dependent on context; specifically, the model used (carcinogen-induced vs genetic), the tumor type, and the stage of cancer. Moreover, emerging data suggests that KEAP1/NRF2 is also important for regulating the tumor microenvironment and how its effects are amplified either by epigenetics or in response to co-occurring mutations. Further elucidation of the complexity of this pathway is needed in order to develop novel pharmacological tools and drugs to improve patient outcomes.
Keywords: cancer, initiation, metastasis, NRF2, promotion, transformation
INTRODUCTION
Carcinogenesis is an intricate and heterogeneous process that depends on cooperation among key oncogenic proteins. The mechanisms orchestrating initiation, promotion, and progression vary by the tumor type. Tumor initiation has been heavily investigated (Evans et al., 2019; Grizzi et al., 2006), as has the molecular biology of carcinogenesis (Bouvard et al., 2009; el Ghissassi et al., 2009; Grosse et al., 2009; Straif et al., 2009) and pathways of chemotherapy resistance (Alfarouk et al., 2015; Gupta et al., 2019) that contribute to cancer fitness (McCreery and Balmain, 2017). Extensive tumor heterogeneity permits the selection of distinct phylogenetic clones and consequent treatment failure (Aktipis et al., 2011; Greaves and Maley, 2012; Nowell, 1976; Worsley et al., 2016). Predictably, the heterogeneity of cancer can explain the context-dependent roles for molecules like NFE2-related factor 2 (NRF2) and Kelch-like ECH-associated protein 1 (KEAP1). As a master regulator of antioxidant responses and cellular metabolism, tumors faced with high oxidative stress and metabolic disorders benefit from constitutive NRF2 pathway activation (Wu et al., 2019). Nonetheless, the role of NRF2 throughout multi-stage carcinogenesis is complex.
Despite significant advances in the NRF2 field over the past decade (Pillai et al., 2022; Robledinos-Antón et al., 2019; Rojo de la Vega et al., 2018; Sporn and Liby, 2012; Wu et al., 2019; Zimta et al., 2019), a consensus on the precise role for NRF2 throughout carcinogenesis remains elusive. Abundant evidence confirms that NRF2 activation protects healthy cells from damaging electrophilic and oxidative stress, thus limiting genomic mutations (Gacesa et al., 2016; Loboda et al., 2016; Mukaigasa et al., 2012) and other cellular damage. This beneficial cytoprotection in normal cells supports the use of pharmacological activators of the NRF2 pathway for cancer prevention. However, these same cytoprotective mechanisms can also enhance survival of transformed cells. Indeed, a tumor-promoting role for NRF2 in cancer initiation has been reported, attributed to protection against redox stress in cells that have acquired mutations in KRAS and/or STK11 (Galan-Cobo et al., 2019). NRF2 activation can also promote chemoresistance (Purohit et al., 2021; Srivastava et al., 2022) and radiation resistance (Feng et al., 2021; Koppula et al., 2022; Matsuoka et al., 2022), as well as metastasis. These disparate findings raise questions as to whether NRF2 is an oncogene, a tumor suppressor gene, or possibly both. These apparent discordant functions may be partly explained by the diverse models used (Best et al., 2019; DeNicola et al., 2011; Ramos-Gomez et al., 2001; Satoh et al., 2013). For example, carcinogen-induced spontaneous tumor models often yield outcomes distinct from xenograft models in which NRF2 is constitutively activated, with further biological complexity found in dual KEAP1/KRAS-mutant tumors. Furthermore, there is a paucity of studies on NRF2-activated tumor–immune cell interactions, which are likely important for anti-tumor effects. Without an understanding of the tumor microenvironment, immunodeficient models may yield confounding results. This review will synthesize our current understanding of NRF2 activation throughout the stages of carcinogenesis and address its time- and context-dependent impact on tumor progression.
NRF2 AND THE STAGES OF CARCINOGENESIS
Because NRF2 activation can prevent or promote cancer depending on the phase of carcinogenesis, we will discuss implications of NRF2 activation during each of the following stages: Transformation and Initiation, Promotion and Progression, and Metastasis.
TRANSFORMATION AND INITIATION
- Transformation: “Process of converting a normal cell into a cell having some or many of the attributes of a cancer cell.”
- Initiation: “Process of changing a cell, usually in a stable fashion, so that it is able to respond subsequently to the growth-stimulatory actions of a tumor-promoting agent”; “Such a process, with the implication that the change involves a mutation.”; “The first step in multi-step tumorigenesis.”
Taken from Weinberg (2014).
As a master regulator of the oxidative stress response (Gacesa et al., 2016; Loboda et al., 2016; Mukaigasa et al., 2012), the NRF2 pathway is highly conserved in multicellular animals throughout evolution (Fuse and Kobayashi, 2017; Gacesa et al., 2016; Toyokuni et al., 2020) to defend against one of the most potent and prevalent cellular insults: oxygen. Oxidative imbalance leads to the formation of free radicals which can cause DNA damage and disruption of cellular homeostasis, leading to transformation (Klaunig et al., 1998; Toyokuni et al., 2020; Valko et al., 2006). Reactive oxygen species (ROS) can contribute to carcinogenesis directly by inducing mutations in proto-oncogenes and tumor suppressor genes and indirectly by activating kinases that induce growth-promoting cellular functions (Cerutti, 1985; Son et al., 2013; Waris and Ahsan, 2006). Activation of the NRF2 pathway induces the transcription of genes encoding antioxidant and detoxification enzymes that counteract dangerous accumulation of ROS (Kwak et al., 2003; Lee et al., 2003) and protects cells from transformation (Hao et al., 2020; Schaue et al., 2022; Wang et al., 2022b; Zhang and Gordon, 2004). Several of these protective genes include GSTP1 (Fang et al., 2020; Zhou et al., 2022), TXN, NQO1, and HMOX1 (Tonelli et al., 2018). Chronic exposure of human BEAS-2B lung epithelial cells to the carcinogen hexavalent chromium decreases KEAP1 protein levels, leading to increased basal NRF2 activity and decreased intracellular ROS (Wang et al., 2022a). Additionally, epigenetic activation of NRF2-mediated gene transcription protects mouse skin cells (Yang et al., 2018b), rat mammary cells (Singh et al., 2014), and human colorectal cells (Zuo et al., 2018) from transformation; and pharmacological activation of NRF2 blocks transformation in mouse prostate cells (Yang et al., 2018a). Conversely, inhibition of the NRF2 pathway by glucocorticoids enables the development of breast cancer (Alam et al., 2017; Giudice et al., 2022), and aberrant expression patterns of NRF2 correlate with transformation and progression of colorectal carcinoma (El-Deek et al., 2019).
Although the antioxidant activities that follow NRF2 activation largely protect against transformation, the metabolic consequences of the NRF2-mediated transcription program can have pro-cancer effects. For example, 3-nitrobenzanthrone, a compound in diesel exhaust, is metabolized to a product which forms DNA adducts and promotes mutagenicity (Enya et al., 1997). Phase II metabolic genes under transcriptional control by NRF2, including AKR1C1, AKR1C2, AKR1C3, and NQO1, enhance this bioactivation (Murray et al., 2019). On balance, however, most phase II enzymes under transcriptional control by NRF2 metabolize and inactivate a wide variety of carcinogens and toxicants (Lee and Surh, 2005).
Chronic inflammation is known to promote transformation and tumor initiation (Hanahan, 2022) and is driven by multiple signaling pathways. Uncontrolled activation of the NF-κB pathway can result in inflammatory cell damage which can lead to transformation (Naugler and Karin, 2008; Rial et al., 2012), and one way this pathway can be regulated is through NRF2/KEAP1 (Wardyn et al., 2015). The E3 ligase component KEAP1 directly suppresses NF-κB activity through ubiquitination-mediated degradation of the NF-κB activator IKKβ (Kim et al., 2010; Lee et al., 2009); NF-κB signaling is increased after NRF2 depletion (Pan et al., 2012). This negative regulation of the NF-κB pathway is complemented by other anti-inflammatory regulatory roles of NRF2 which culminate in protection from aberrant inflammation (Chi et al., 2015; Ryan et al., 2022; Suzuki et al., 2017; Thimmulappa et al., 2006). Additionally, the tumor suppressor ARF (p14ARF) decreases NRF2 activity and sensitizes damaged cells to ferroptosis, thus decreasing survival of transformed cells (Chen et al., 2017). In general, these studies suggest that NRF2 activation protects from cellular damage that would otherwise lead to the transformation of normal cells, with some exceptions in NRF2-mediated activation of carcinogens.
There is conflicting data as to whether NRF2 promotes or inhibits tumor initiation. To our knowledge, no study has demonstrated that NRF2-activating mutations alone are sufficient to initiate cancer. Attempts to create mice for assessing effects of whole-body constitutive NRF2 activity have been unsuccessful, as homozygous knockouts of KEAP1 are lethal in the post-natal period. In these mice, the esophagus and forestomach developed abnormal keratinization not present during the embryonic stages (Wakabayashi et al., 2003). Interestingly, genetically engineered mice have been created that express the most common NRF2-activating mutant in esophageal cancer, NRF2E79Q, controlled by a lox-stop-lox (LSL) motif. When crossed with KRT14-driven cre-recombinase mice, the phenotype of KEAP1 KO is recapitulated. These mice live far beyond the post-natal period, and no increase in cancer incidence was observed in this model (Bowman et al., 2020).
Carcinogen-induced models such as benzo[a]pyrene-induced models of gastric cancer or cadmium-initiated lung carcinogenesis are frequently used to investigate the protective roles of the NRF2 pathway (Ramos-Gomez et al., 2001; Wang et al., 2018). Hepatocellular carcinoma induced by diethylnitrosamine was prevented in mice with liver-specific deletion of the metabolic regulator SIRT1 which increased NRF2 pathway activation, promoting glutathione metabolism and eliminating ROS (Qiu et al., 2021). Many studies support the idea that NRF2 prevents cancer initiation if activated prior to accumulation of mutations (Ramos-Gomez et al., 2001; Satoh et al., 2013; Schaue et al., 2022; Wang et al., 2021). Specifically, pharmacological and genetic NRF2 pathway activation decreases tumor burden in vinyl carbamate- or urethane-induced models of murine lung cancer (Liby et al., 2007; Satoh et al., 2013). Satoh et al. (2013) reported decreased tumor formation in NRF2 WT mice compared to NRF2 KO mice, but the tumors that formed in NRF2 WT were larger and of higher grade, consistent with the notion that NRF2 activity can provide growth advantages to tumors that do develop. These results contrast with other models in which the loss of NRF2 exacerbated lung carcinogenesis, with higher tumor burden in NRF2 KO mice, even at late stages (Zhang et al., 2018).
NRF2 activation in genetic models has also been shown to prevent tumor development. Overexpression of the NOTCH intracellular domain in adipocytes leads to liposarcoma-like soft tissue sarcomas, but knockout of KEAP1 leading to NRF2 activation prevented tumor development through metabolic reprogramming (Chartoumpekis et al., 2018). CDDO-methyl ester, a potent NRF2 activator, delayed tumor development in BRCA1-deficient, MMTV-neu, and PyMT mouse models of breast cancers (Kim et al., 2012; Liby et al., 2008; Tran et al., 2012). Interestingly, the anti-tumor effects of NRF2 activators in these models was attributed to immune cell modulation rather than the canonical metabolic and antioxidative mechanisms described in other studies. In contrast, NRF2 activation failed to alter adenoma development in a GSTP-/-:APCMin/+ mouse model, suggesting that expression of the NRF2 transcriptional target, the phase II enzyme glutathione S-Transferase pi (GSTP), is required for NRF2-mediated protection from cancer. Tao et al. (2018) compared carcinogen-induced and genetic models of lung cancer and found that sulforaphane-mediated NRF2 activation was protective against vinyl carbamate-induced lung cancer but was ineffective in a KRASG12D genetic model. Treatment with sulforaphane prior to initiation using vinyl carbamate decreased lung tumor burden in mice, but treatment post-initiation was ineffective. Similarly, NRF2 inhibition prior to carcinogenic initiation increased the tumor number, while sulforaphane treatment of KRASG12D mice after initiation also increased tumor number. KRAS mutations, including KRASG12D, are known drivers of oncogenesis, but they also indirectly activate NRF2 through the RAF-MEK-ERK-AP1 pathway (DeNicola et al., 2011). In KRASG12D-driven pancreatic cancer, NRF2 protects tumor cells by reducing oxidative stress (DeNicola et al., 2011). The inability of NRF2 activation to delay tumorigenesis in KRASG12D-driven lung cancer could be related to ROS levels, but Tao et al. (2018) posit that constitutive KRAS activation robustly drives proliferation, thus surpassing any protective effect of NRF2 activation. Regardless, the mutational burden and timing of NRF2 activation appear to be important factors for the efficacy of prevention.
Despite numerous studies showing that NRF2 activation prevents tumor formation, a considerable body of literature reports the opposite result. In agreement with the KRAS-driven lung adenocarcinoma model described previously, tumor development and tumor burden increased following the introduction of KEAP1 mutations via inhaled Cre-adenovirus in LSL-KRASG12D/+ mice. Inflammation and macrophage numbers were reduced in these tumors, permitting tumor growth (Best et al., 2019). Supporting the immune-regulatory effect of KEAP1 mutant tumors, enhanced lung adenocarcinoma formation was found in KEAP1fl/fl;PTENfl/fl mice initiated by inhaled Cre-adenovirus, while KEAP1fl/fl or PTENfl/fl mice had no malignancy (Best et al., 2018). This model is characterized by an immunosuppressive microenvironment, although tumors regressed in response to immune checkpoint blockade.
The complexity of the story continues since many models of NRF2-mediated malignancy require co-mutation of other oncogenic drivers and/or tumor suppressors, but different combinations yield disparate results. There was no increase in tumor incidence in small cell lung cancer initiated by inhaled Cre-adenovirus in TRP53fl/fl; P16fl/fl mice when LSL-NRF2E79Q/+ was activated, although the tumor histology subtype was altered (Hamad et al., 2022). In fact, a large subset of tumors failed to express the mutant NRF2 despite recombination, and the authors concluded that it was silenced due to a deleterious effect on tumor development. Conversely, tumor burden increased when KEAP1 was deleted by CRISPR editing in vivo in a model of hepatocellular carcinoma promoted by insertional mutagenesis with a MYC transposon (Sanghvi et al., 2019). The same group reported that deglycation of NRF2 by fructosamine-3-kinase (FN3K) is required for mediation of its oncogenic function, and if FN3K is inhibited, tumors regress. These studies provide evidence for the great diversity of genetically engineered murine cancer models and how they can lead to different results. In addition, the function of accessory metabolic proteins like FN3K or co-mutations in tumor suppressors like STK11 have defined roles in tumor development. The triple mutant LSL-KRASG12D;STK11fl/fl;KEAP1fl/fl mouse lung adenocarcinoma model had poor prognosis, a more aggressive phenotype, and earlier tumor onset compared to non-KEAP1 mutant counterparts (Singh et al., 2021), mimicking observations in human lung cancer patients.
In addition to metabolic advantages afforded by constitutive NRF2 activation, NRF2 can cause enhancer remodeling of oncogenic drivers to promote tumor initiation. This remodeling can partially explain how NRF2 switches from an anti-cancer to a pro-tumorigenic phenotype (Okazaki et al., 2020). NRF2 activation also impacts cancer cell differentiation. In melanoma cells, NRF2 activation led to de-differentiation and promoted tumor formation through COX2-mediated immune evasion (Jessen et al., 2020). Increased IL-11 expression in NRF2-activated tumorigenic fibroblasts promotes cancer development, possibly through regulation of the immune system (Kitamura et al., 2017). Taken altogether, the dual roles of NRF2 in promoting or preventing the initiation of cancer remains a complex topic [see (Robertson et al., 2020) for an excellent review on NRF2 and cancer initiation] that requires carefully designed studies and prudent interpretation of data.
PROMOTION AND PROGRESSION
- Promotion: “Process that stimulates or accelerates tumor progression, usually presumed to do so without directly damaging the genomes of cells.”
- Progression: “Process of multi-step evolution of a normal cell into a tumor”; “Evolution of a benign into a malignant cancer cell”; “Evolution of a premalignant cell from a promoter-dependent to a promoter-independent state.”
Taken from Weinberg (2014).
Cytoprotective mechanisms enable cancer cells to survive their harsh environments, and NRF2 is activated after cells undergo transformation (Wu et al., 2019). Some cancer subtypes develop mutations within the NRF2 pathway, which lead to constitutive pathway activation (Taguchi and Yamamoto, 2017). Most notably, the hypoxic nature of tumors creates ROS within cancer cells that activates NRF2, regardless of mutational status (Toth and Warfel, 2017). While NRF2 activation alone is not sufficient for the initiation of cancer, it can facilitate proliferation of existing cancer cells initiated by other carcinogenic processes (Vartanian et al., 2019). Activation of oncogenes including IGF-1, KRAS, c-MYC, and others cause cellular stress which in turn activates NRF2 (Lim and Leprivier, 2019; Riis et al., 2020; Vafa et al., 2002), in part because of mitochondrial hyperactivity that occurs within rapidly proliferating cells (Sabharwal and Schumacker, 2014; Sotgia et al., 2011). However, this positive feedback loop between oncogenes such as KRAS may also provide opportunities for therapeutic intervention, exemplified by NRF2 activation sensitizing pancreatic cancer cells to glutaminase inhibition in vitro (Hamada et al., 2021).
Activation of NRF2 by direct mutation or increased oxidative stress modulates a variety of other processes facilitating progression. The direct gene targets of oncogene-mediated NRF2 activation include not only an extensive array of antioxidant genes (Kavian et al., 2018) but also genes encoding metabolic enzymes (He et al., 2020) and drug efflux pumps (Jeddi et al., 2018; del Vecchio et al., 2014) which collaborate in tumor-promoting effects ranging from increased cancer cell survival to drug resistance. Tumor cells can escape autophagy inhibition by switching to macropinocytosis, a process that is dependent on NRF2 (Su et al., 2021; Towers et al., 2019). NRF2 activation also protects cells from ferroptosis (Fiore et al., 2022; Liu et al., 2020; Nishizawa et al., 2022) and apoptosis (Niture and Jaiswal, 2012; Xie et al., 2020), allowing cancer cells to escape death. Cellular stress induced by anti-cancer drugs and radiation is alleviated by NRF2 activation, leading to therapeutic resistance (Kamble et al., 2021; Noh et al., 2021; Silva et al., 2019). However, the cancer-promoting activation of NRF2 occurs mainly as a response to the high-stress environment within tumors and therefore should be characterized as an enabler, rather than an active driver of cancer progression. For a more comprehensive evaluation of NRF2 and cancer progression, please see (Schmidlin et al., 2021) or (He et al., 2020).
METASTASIS
- Metastasis: “Malignant growth forming at one site in the body, the cells of which derive from a malignancy located elsewhere in the body.”
Taken from Weinberg (2014).
In addition to promoting cancer cell survival and progression, the ratio of HMOX1/NRF2 mRNA expression in tumors is predictive of metastasis to distant sites. The ratio in the tumor tumor tissue was lower in patients with distant metastasis (97.4%) than in those without (101%) (Chang et al., 2016). NRF2 enables metastatic dissemination through multiple mechanisms (Lignitto et al., 2019; Wiel et al., 2019). Competition for KEAP1 binding by HBXIP, part of a c-Fos complex that drives gene expression, elicits a cytoprotective effect by detoxifying ROS through NRF2 activation, enabling cancer cells to tolerate the stress encountered during metastasis (Zhou et al., 2019). A more direct pro-invasive role is mediated through an NRF2/heme oxygenase-1 (HO-1)/BACH1 axis. Constitutive NRF2 activation promotes cancer cell migration and metastasis through accumulation of the BACH1 transcriptional regulator. NRF2 upregulates expression of HO-1, increasing heme catabolism, and thereby preventing FBXO22-mediated degradation of BACH1 (Lignitto et al., 2019). In lung cancer, BACH1 accumulation promotes a transcriptional shift toward pro-metastatic gene profiles (Lignitto et al., 2019). The importance of BACH1 was corroborated by a complementary study in which chronic administration of the antioxidants N-acetylcysteine and vitamin E downregulate the NRF2 pathway. Antioxidant treatment increased tumor metastases through a metabolic switch to glycolysis that was advantageous to invading tumor cells (Wiel et al., 2019). Antioxidant treatment was a surrogate for NRF2-mediated detoxification, which increased BACH1-dependent metastases. However, in tumors with constitutive NRF2 pathway activation, it is conceivable that the BACH1-mediated mechanism for metastasis would be overactivated.
Additional evidence for the involvement of NRF2 in metastasis can be found in the promotion of the epithelial to mesenchymal transition (EMT). In glioblastoma multiforme, NRF2 acts in conjunction with p62 to activate EMT and subsequently increases tumor invasiveness (Pölönen et al., 2019). This effect was also observed in hepatocellular carcinoma with MCUR1-induced mitochondrial calcium uptake that induced NRF2/NOTCH-mediated EMT (Jin et al., 2019). Additionally, NRF2 works in conjunction with NOTCH/EMT signaling in breast cancer. NRF2 promotes the upregulation of G6PD/HIF-1α, and in turn, activates NOTCH-mediated EMT of breast cancer cells (Zhang et al., 2019). Further, in non-small cell lung cancer NRF2 activates the RhoA-ROCK1 signaling pathway that increases expression of mesenchymal-type markers and increases cell motility, which was prevented by NRF2 inhibition (Ko et al., 2021).
In combination with increased cancer cell motility through EMT, angiogenesis, a critical feature that enhances dissemination of metastatic cancer cells, is increased by NRF2 (Huang et al., 2021; Liu et al., 2021; Shahcheraghi et al., 2022). This increased angiogenesis has been attributed to NRF2-mediated stabilization of HIF-1α and activation of its transcriptional program (Ji et al., 2014; Zheng et al., 2023). Finally, there is a connection between mitochondrial stress and NRF2. Tumor cells interact with the extracellular matrix to induce intra-tumoral mechanical signaling that increases mitochondrial ROS, which in turn increases oxidative stress, and thus NRF2-mediated cytoprotection (Romani et al., 2022). Romani et al. (2022) discovered that soft extracellular metastatic niches promote NRF2-mediated chemoresistance through increased mitochondrial ROS. Activation of NRF2 is permissive for cancer cell tolerance to oxidative insults in both the invading primary tumor cells and those disseminated to distant metastatic niches.
CONCLUSIONS AND FUTURE DIRECTIONS
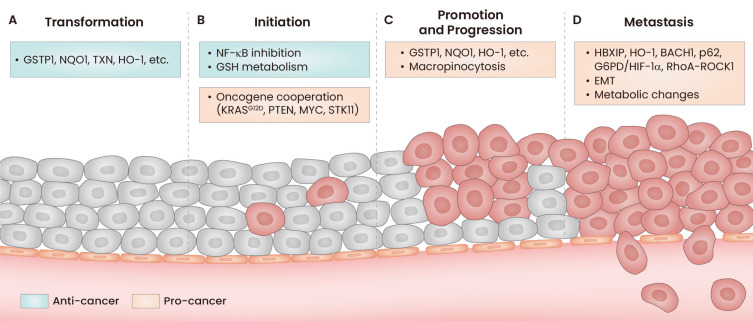
Knowledge gaps persist in defining the precise function of NRF2 in cancer transformation, initiation, promotion, and metastasis (Fig. 1). Moreover, the lack of definitive conclusions is compounded by the disparate effects observed at different stages of carcinogenesis and in the different cancer models used (genetic versus carcinogen-induced and immune competent versus immunodeficient). These observations, detailed above, lead to a primary conclusion that the functions of NRF2 are highly context dependent. Context encompasses the stage of cancer, the experimental models, and the type of cancer.
Fig. 1. NRF2 activation throughout carcinogenesis.
In the initial stages of carcinogenesis, NRF2 has anti-tumor effects through transcription of antioxidant and cytoprotective genes. As transformed cells progress, they utilize these same cytoprotective effects in cooperation with other pro-tumor mechanisms to facilitate drug resistance, cell survival, and metastasis. (A) Activation of NRF2 in the transformation stage of carcinogenesis increases cytoprotective genes that prevent cancer formation. (B) Regulation of inflammation and redox balance can prevent increases in mutational burden thereby preventing initiation, but co-occurring mutations in oncogenes or tumor suppressors increase cancer initiation by utilizing NRF2-mediated cytoprotection. (C) Transformation-preventing genes that are upregulated by NRF2 are similarly upregulated in the promotion and progression phase, although protect the existing cancer. (D) NRF2 upregulates a variety of pro-metastatic gene pathways, enabling cancer metastasis.
The prevalence of high NRF2 expression (increased transcription and translocation to the nucleus) and activation (downstream effectors) is relevant in cancers that arise in organs with high exposure to environmental insults or that function in detoxification, such as the lung, digestive system, pancreas, and liver (Gao et al., 2015; Liby et al., 2008; Pillai et al., 2022). As such, it is likely that pharmacological interventions will be first used in cancers of these organs. However, whether NRF2 activators or inhibitors are appropriate is still under investigation due to the dual roles of NRF2 in promoting and inhibiting cancer. The use of either type of pharmacological agent will likely be stage- and cancer-dependent. Currently there are 4 clinical trials targeting tumors with either NRF2 or KEAP1 mutations (Pillai et al., 2022; Yagishita et al., 2020). These pharmacological interventions mainly take advantage of the downstream metabolic vulnerabilities generated by NRF2 and KEAP1 mutations (Dinkova-Kostova and Copple, 2023). In the future it is likely that NRF2 signaling will be targeted directly, either for activation or inhibition. The NRF2 activator dimethyl fumarate is already approved for clinical use in relapsing forms of multiple sclerosis (Faissner and Gold, 2019; Schimrigk et al., 2006). The triterpenoid CDDO-Methyl ester (CDDO-Me or bardoxolone methyl), another NRF2 activator, is currently being tested in clinical trials for chronic kidney disease (Chin et al., 2018). Moreover, CDDO-Me has been tested in preclinical models of lung, pancreas, and breast cancer. Other small molecules activators, such as curcumin, resveratrol, and sulforaphane, have been tested in cancer cells and pre-clinical mouse models, alone or in combination with chemotherapies (Ashrafizadeh et al., 2020; Dinkova-Kostova et al., 2017; Farkhondeh et al., 2020; Giordano and Tommonaro, 2019; Mansouri et al., 2020; Singh et al., 2014; Tao et al., 2018).
The development of direct pharmacological inhibitors of the NRF2 protein has been hampered primarily due to the lack of a druggable binding pocket (Karunatilleke et al., 2021). NRF2 has been considered an undruggable protein, in the same category as KRAS, one of the most prevalent oncogenes in solid tumors. Development of pharmacological inhibitors or activators of NRF2 is still in its infancy, although recent advances in medicinal chemistry have led to the development of small molecules targeting NRF2 and related proteins (Bar-Peled et al., 2017). Other approaches have been developed to target NRF2-mediated transcription and DNA binding of NRF2/MAFG complexes (Simov et al., 2021). Biological insights into how the NRF2 pathway promotes or inhibits different stages of carcinogenesis provide new opportunities for drug development (Hou et al., 2023; Pouremamali et al., 2022; Robledinos-Antón et al., 2019; Zhang et al., 2021). Additionally, with distinct functions evident in different cancers, precision medicine can be used to specifically target vulnerabilities based on mutations or upregulation within the NRF2 pathway. Combination therapies, either with chemotherapy or immunotherapy, are other possible avenues to augment the effects of small molecule inhibitors of the NRF2 pathway.
Despite advances in our knowledge of the NRF2 pathway in recent years, the indirect effects of NRF2 and KEAP1 mutations in the tumor microenvironment as well as how these mutations cooperate with common co-occurring mutations, such as KRAS and STK11/LKB1, are still incompletely understood (Table 1). With the development of immunotherapy, many studies in the past decade have focused on the tumor microenvironment. NRF2 and KEAP1 mutations as well as co-occurring mutations may influence the regulation of the NRF2 pathway in the tumor microenvironment (Best et al., 2018; Cristescu et al., 2018) and consequently influence therapeutic responses to chemotherapy and/or immunotherapy (Kobayashi et al., 2016; Taguchi and Yamamoto, 2017; Taniguchi et al., 2020). Moreover, overexpression or over activation of NRF2 through epigenetic changes or by hijacking of the pathway by other tumor promoting mutations, such as KRAS and ALK, are still not well understood biologically or mechanistically. Elucidating these biological processes will likely reveal new pharmacological vulnerabilities that can improve therapeutic options for patients with aggressive cancers where NRF2 is abnormally expressed.
Table 1.
Areas requiring further investigation
| - Characterization of NRF2-activated tumor microenvironments |
| - Epigenetics of NRF2-activated tumors |
| - Implications of tumor origin and location on NRF2-mediated tumor biology |
| - Clarification on pharmacologic intervention for tumor prevention and treatment |
ACKNOWLEDGMENTS
This minireview is dedicated in memory of Michael B. Sporn, the “Father of Chemoprevention.” The work was supported by NIH R01CA226690, MTRAC for Life Sciences Innovation Hub-Mi-Kickstart Award, the Breast Cancer Research Foundation, and the MSU Discretionary Funding Initiative (all to K.T.L.). Additional funding was provided by the Barnett Rosenberg Endowed Research Assistantship (J.A.M.), Aitch Foundation (J.A.M.), Integrative Pharmacological Sciences Training Program 5T32GM142521 (C.J.O.), and DOD Career Development Award LC210240 (A.S.L.).
Footnotes
AUTHOR CONTRIBUTIONS
C.J.O. and J.A.M. surveyed the literature and wrote the main body of the manuscript. A.S.L. wrote the abstract, conclusions, and future directions. K.A.G. edited the manuscript and assisted in preparation for submission. K.T.L. provided overall direction and edited the manuscript.
CONFLICT OF INTEREST
K.T.L. is a named inventor on patents issued and filed for synthetic triterpenoids and NRF2 pathway inhibitors. Other authors have no potential conflicts of interest to disclose.
REFERENCES
- Aktipis C.A., Kwan V.S.Y., Johnson K.A., Neuberg S.L., Maley C.C. Overlooking evolution: a systematic analysis of cancer relapse and therapeutic resistance research. PLoS One. 2011;6:e26100. doi: 10.1371/journal.pone.0026100.97f9579d700e4a27ad3c04c24df48d80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M.M., Okazaki K., Nguyen L.T.T., Ota N., Kitamura H., Murakami S., Shima H., Igarashi K., Sekine H., Motohashi H. Glucocorticoid receptor signaling represses the antioxidant response by inhibiting histone acetylation mediated by the transcriptional activator NRF2. J. Biol. Chem. 2017;292:7519–7530. doi: 10.1074/jbc.M116.773960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfarouk K.O., Stock C.M., Taylor S., Walsh M., Muddathir A.K., Verduzco D., Bashir A.H.H., Mohammed O.Y., Elhassan G.O., Harguindey S., et al. Resistance to cancer chemotherapy: failure in drug response from ADME to P-gp. Cancer Cell Int. 2015;15:71. doi: 10.1186/s12935-015-0221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafizadeh M., Ahmadi Z., Mohammadinejad R., Farkhondeh T., Samarghandian S. Curcumin activates the Nrf2 pathway and induces cellular protection against oxidative injury. Curr. Mol. Med. 2020;20:116–133. doi: 10.2174/1566524019666191016150757. [DOI] [PubMed] [Google Scholar]
- Bar-Peled L., Kemper E.K., Suciu R.M., Vinogradova E.V., Backus K.M., Horning B.D., Paul T.A., Ichu T.A., Svensson R.U., Olucha J., et al. Chemical proteomics identifies druggable vulnerabilities in a genetically defined cancer. Cell. 2017;171:696–709.e23. doi: 10.1016/j.cell.2017.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best S.A., de Souza D.P., Kersbergen A., Policheni A.N., Dayalan S., Tull D., Rathi V., Gray D.H., Ritchie M.E., McConville M.J., et al. Synergy between the KEAP1/NRF2 and PI3K pathways drives non-small-cell lung cancer with an altered immune microenvironment. Cell Metab. 2018;27:935–943.e4. doi: 10.1016/j.cmet.2018.02.006. [DOI] [PubMed] [Google Scholar]
- Best S.A., Ding S., Kersbergen A., Dong X., Song J.Y., Xie Y., Reljic B., Li K., Vince J.E., Rathi V., et al. Distinct initiating events underpin the immune and metabolic heterogeneity of KRAS-mutant lung adenocarcinoma. Nat. Commun. 2019;10:4190. doi: 10.1038/s41467-019-12164-y.ac2538b3a39145fe917c923569a787a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvard V., Baan R., Straif K., Grosse Y., Secretan B., el Ghissassi F., Benbrahim-Tallaa L., Guha N., Freeman C., Galichet L., et al. A review of human carcinogens--part B: biological agents. Lancet Oncol. 2009;10:321–322. doi: 10.1016/S1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- Bowman B.M., Montgomery S.A., Schrank T.P., Simon J.M., Ptacek T.S., Tamir T.Y., Mulvaney K.M., Weir S.J., Nguyen T.T., Murphy R.M., et al. A conditional mouse expressing an activating mutation in NRF2 displays hyperplasia of the upper gastrointestinal tract and decreased white adipose tissue. J. Pathol. 2020;252:125–137. doi: 10.1002/path.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti P.A. Prooxidant states and tumor promotion. Science. 1985;227:375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- Chang L.C., Fan C.W., Tseng W.K., Chein H.P., Hsieh T.Y., Chen J.R., Hwang C.C., Hua C.C. The ratio of Hmox1/Nrf2 mRNA level in the tumor tissue is a predictor of distant metastasis in colorectal cancer. Dis. Markers. 2016;2016:8143465. doi: 10.1155/2016/8143465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoumpekis D.V., Yagishita Y., Fazzari M., Palliyaguru D.L., Rao U.N., Zaravinos A., Khoo N.K., Schopfer F.J., Weiss K.R., Michalopoulos G.K., et al. Nrf2 prevents Notch-induced insulin resistance and tumorigenesis in mice. JCI Insight. 2018;3:e97735. doi: 10.1172/jci.insight.97735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Tavana O., Chu B., Erber L., Chen Y., Baer R., Gu W. NRF2 is a major target of ARF in p53-independent tumor suppression. Mol. Cell. 2017;68:224–232.e4. doi: 10.1016/j.molcel.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi X., Yao W., Xia H., Jin Y., Li X., Cai J., Hei Z. Elevation of HO-1 expression mitigates intestinal ischemia-reperfusion injury and restores tight junction function in a rat liver transplantation model. Oxid. Med. Cell. Longev. 2015;2015:986075. doi: 10.1155/2015/986075.af783d3316f1414a82385f0ff57013a7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin M.P., Bakris G.L., Block G.A., Chertow G.M., Goldsberry A., Inker L.A., Heerspink H.J.L., O'Grady M., Pergola P.E., Wanner C., et al. Bardoxolone methyl improves kidney function in patients with chronic kidney disease stage 4 and type 2 diabetes: post-hoc analyses from bardoxolone methyl evaluation in patients with chronic kidney disease and type 2 diabetes study. Am. J. Nephrol. 2018;47:40–47. doi: 10.1159/000486398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristescu R., Mogg R., Ayers M., Albright A., Murphy E., Yearley J., Sher X., Liu X.Q., Lu H., Nebozhyn M., et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science. 2018;362:eaar3593. doi: 10.1126/science.aar3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNicola G.M., Karreth F.A., Humpton T.J., Gopinathan A., Wei C., Frese K., Mangal D., Yu K.H., Yeo C.J., Calhoun E.S., et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkova-Kostova A.T., Copple I.M. Advances and challenges in therapeutic targeting of NRF2. Trends Pharmacol. Sci. 2023;44:137–149. doi: 10.1016/j.tips.2022.12.003. [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova A.T., Fahey J.W., Kostov R.V., Kensler T.W. KEAP1 and done? Targeting the NRF2 pathway with sulforaphane. Trends Food Sci. Technol. 2017;69(Pt B):257–269. doi: 10.1016/j.tifs.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Deek H.E.M., Ahmed A.M., Mohammed R.A.A. Aberration of Nrf2-Bach1 pathway in colorectal carcinoma; role in carcinogenesis and tumor progression. Ann. Diagn. Pathol. 2019;38:138–144. doi: 10.1016/j.anndiagpath.2018.11.003. [DOI] [PubMed] [Google Scholar]
- Enya T., Suzuki H., Watanabe T., Hirayama T., Hisamatsu Y. 3-Nitrobenzanthrone, a powerful bacterial mutagen and suspected human carcinogen found in diesel exhaust and airborne particulates. Environ. Sci. Technol. 1997;31:2772–2776. doi: 10.1021/es961067i. [DOI] [Google Scholar]
- Evans J.J., Alkaisi M.M., Sykes P.H. Tumour initiation: a discussion on evidence for a "load-trigger" mechanism. Cell Biochem. Biophys. 2019;77:293–308. doi: 10.1007/s12013-019-00888-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faissner S., Gold R. Oral therapies for multiple sclerosis. Cold Spring Harb. Perspect. Med. 2019;9:a032011. doi: 10.1101/cshperspect.a032011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y., Ye J., Zhao B., Sun J., Gu N., Chen X., Ren L., Chen J., Cai X., Zhang W., et al. Formononetin ameliorates oxaliplatin-induced peripheral neuropathy via the KEAP1-NRF2-GSTP1 axis. Redox Biol. 2020;36:101677. doi: 10.1016/j.redox.2020.101677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkhondeh T., Folgado S.L., Pourbagher-Shahri A.M., Ashrafizadeh M., Samarghandian S. The therapeutic effect of resveratrol: focusing on the Nrf2 signaling pathway. Biomed. Pharmacother. 2020;127:110234. doi: 10.1016/j.biopha.2020.110234. [DOI] [PubMed] [Google Scholar]
- Feng L., Zhao K., Sun L., Yin X., Zhang J., Liu C., Li B. SLC7A11 regulated by NRF2 modulates esophageal squamous cell carcinoma radiosensitivity by inhibiting ferroptosis. J. Transl. Med. 2021;19:367. doi: 10.1186/s12967-021-03042-7.1b6d995ea709490c825d870bc81ba28c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore A., Zeitler L., Russier M., Groß A., Hiller M.K., Parker J.L., Stier L., Köcher T., Newstead S., Murray P.J. Kynurenine importation by SLC7A11 propagates anti-ferroptotic signaling. Mol. Cell. 2022;82:920–932.e7. doi: 10.1016/j.molcel.2022.02.007. [DOI] [PubMed] [Google Scholar]
- Fuse Y., Kobayashi M. Conservation of the Keap1-Nrf2 system: an evolutionary journey through stressful space and time. Molecules. 2017;22:436. doi: 10.3390/molecules22030436.7e2a8ec9b3424aa6b2d5d082b7d262a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gacesa R., Dunlap W.C., Barlow D.J., Laskowski R.A., Long P.F. Rising levels of atmospheric oxygen and evolution of Nrf2. Sci. Rep. 2016;6:27740. doi: 10.1038/srep27740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan-Cobo A., Sitthideatphaiboon P., Qu X., Poteete A., Pisegna M.A., Tong P., Chen P.H., Boroughs L.K., Rodriguez M.L.M., Zhang W., et al. LKB1 and KEAP1/NRF2 pathways cooperatively promote metabolic reprogramming with enhanced glutamine dependence in KRAS-mutant lung adenocarcinoma. Cancer Res. 2019;79:3251–3267. doi: 10.1158/0008-5472.CAN-18-3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Deeb D., Liu Y., Liu P., Zhang Y., Shaw J., Gautam S.C. CDDO-Me inhibits tumor growth and prevents recurrence of pancreatic ductal adenocarcinoma. Int. J. Oncol. 2015;47:2100–2106. doi: 10.3892/ijo.2015.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Ghissassi F., Baan R., Straif K., Grosse Y., Secretan B., Bouvard V., Benbrahim-Tallaa L., Guha N., Freeman C., Galichet L., et al. A review of human carcinogens--part D: radiation. Lancet Oncol. 2009;10:751–752. doi: 10.1016/S1470-2045(09)70213-X. [DOI] [PubMed] [Google Scholar]
- Giordano A., Tommonaro G. Curcumin and cancer. Nutrients. 2019;11:2376. doi: 10.3390/nu11102376.1bac897dd2b64acd8d1b3f2246ad8970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudice A., Aliberti S.M., Barbieri A., Pentangelo P., Bisogno I., D'Arena G., Cianciola E., Caraglia M., Capunzo M. Potential mechanisms by which glucocorticoids induce breast carcinogenesis through Nrf2 inhibition. Front. Biosci. (Landmark Ed.) 2022;27:223. doi: 10.31083/j.fbl2707223.e97b94555251464db556e2027f0c2305 [DOI] [PubMed] [Google Scholar]
- Greaves M., Maley C.C. Clonal evolution in cancer. Nature. 2012;481:306–313. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grizzi F., di Ieva A., Russo C., Frezza E.E., Cobos E., Muzzio P.C., Chiriva-Internati M. Cancer initiation and progression: an unsimplifiable complexity. Theor. Biol. Med. Model. 2006;3:37. doi: 10.1186/1742-4682-3-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse Y., Baan R., Straif K., Secretan B., el Ghissassi F., Bouvard V., Benbrahim-Tallaa L., Guha N., Galichet L., Cogliano V. A review of human carcinogens-part A: pharmaceuticals. Lancet Oncol. 2009;10:13–14. doi: 10.1016/S1470-2045(08)70286-9. [DOI] [PubMed] [Google Scholar]
- Gupta P.B., Pastushenko I., Skibinski A., Blanpain C., Kuperwasser C. Phenotypic plasticity: driver of cancer initiation, progression, and therapy resistance. Cell Stem Cell. 2019;24:65–78. doi: 10.1016/j.stem.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamad S.H., Montgomery S.A., Simon J.M., Bowman B.M., Spainhower K.B., Murphy R.M., Knudsen E.S., Fenton S.E., Randell S.H., Holt J.R., et al. TP53, CDKN2A/P16, and NFE2L2/NRF2 regulate the incidence of pure- and combined-small cell lung cancer in mice. Oncogene. 2022;41:3423–3432. doi: 10.1038/s41388-022-02348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Matsumoto R., Tanaka Y., Taguchi K., Yamamoto M., Masamune A. Nrf2 activation sensitizes K-ras mutant pancreatic cancer cells to glutaminase inhibition. Int. J. Mol. Sci. 2021;22:1870. doi: 10.3390/ijms22041870.afa9f761b7d1484b96954f5d7e57f0b2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- Hao Q., Wang M., Sun N.X., Zhu C., Lin Y.M., Li C., Liu F., Zhu W.W. Sulforaphane suppresses carcinogenesis of colorectal cancer through the ERK/Nrf2-UDP glucuronosyltransferase 1A metabolic axis activation. Oncol. Rep. 2020;43:1067–1080. doi: 10.3892/or.2020.7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F., Antonucci L., Karin M. NRF2 as a regulator of cell metabolism and inflammation in cancer. Carcinogenesis. 2020;41:405–416. doi: 10.1093/carcin/bgaa039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Z., Lockwood L., Zhang D., Occhiuto C.J., Mo L., Aldrich K.E., Stoub H.E., Gallo K.A., Liby K.T., Odom A.L. Exploring structural effects in a new class of NRF2 inhibitors. RSC Med. Chem. 2023;14:74–84. doi: 10.1039/D2MD00211F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Yang Y., Xu Y., Ma Q., Guo F., Zhao Y., Tao Y., Li M., Guo J. Nrf2/HO-1 axis regulates the angiogenesis of gastric cancer via targeting VEGF. Cancer Manag. Res. 2021;13:3155–3169. doi: 10.2147/CMAR.S292461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeddi F., Soozangar N., Sadeghi M.R., Somi M.H., Shirmohamadi M., Eftekhar-Sadat A.T., Samadi N. Nrf2 overexpression is associated with P-glycoprotein upregulation in gastric cancer. Biomed. Pharmacother. 2018;97:286–292. doi: 10.1016/j.biopha.2017.10.129. [DOI] [PubMed] [Google Scholar]
- Jessen C., Kreß J.K.C., Baluapuri A., Hufnagel A., Schmitz W., Kneitz S., Roth S., Marquardt A., Appenzeller S., Ade C.P., et al. The transcription factor NRF2 enhances melanoma malignancy by blocking differentiation and inducing COX2 expression. Oncogene. 2020;39:6841–6855. doi: 10.1038/s41388-020-01477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X., Wang H., Zhu J., Zhu L., Pan H., Li W., Zhou Y., Cong Z., Yan F., Chen S. Knockdown of Nrf2 suppresses glioblastoma angiogenesis by inhibiting hypoxia-induced activation of HIF-1α. Int. J. Cancer. 2014;135:574–584. doi: 10.1002/ijc.28699. [DOI] [PubMed] [Google Scholar]
- Jin M., Wang J., Ji X., Cao H., Zhu J., Chen Y., Yang J., Zhao Z., Ren T., Xing J. MCUR1 facilitates epithelial-mesenchymal transition and metastasis via the mitochondrial calcium dependent ROS/Nrf2/Notch pathway in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2019;38:136. doi: 10.1186/s13046-019-1135-x.ed5d8ca1857c40a68970e58299e1d31d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamble D., Mahajan M., Dhat R., Sitasawad S. Keap1-Nrf2 pathway regulates ALDH and contributes to radioresistance in breast cancer stem cells. Cells. 2021;10:83. doi: 10.3390/cells10010083.00e1a97e69a647c8bc4ca51560353b7d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunatilleke N.C., Fast C.S., Ngo V., Brickenden A., Duennwald M.L., Konermann L., Choy W.Y. Nrf2, the major regulator of the cellular oxidative stress response, is partially disordered. Int. J. Mol. Sci. 2021;22:7434. doi: 10.3390/ijms22147434.e5f5bbf48a3545ab9aedc2cec349dc18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavian N., Mehlal S., Jeljeli M., Saidu N.E.B., Nicco C., Cerles O., Chouzenoux S., Cauvet A., Camus C., Ait-Djoudi M., et al. The Nrf2-antioxidant response element signaling pathway controls fibrosis and autoimmunity in scleroderma. Front. Immunol. 2018;9:1896. doi: 10.3389/fimmu.2018.01896.58a6b67e192a4b118b0476db4f702c1f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E.H., Deng C., Sporn M.B., Royce D.B., Risingsong R., Williams C.R., Liby K.T. CDDO-Methyl ester delays breast cancer development in BRCA1-mutated mice. Cancer Prev. Res. (Phila.) 2012;5:89–97. doi: 10.1158/1940-6207.CAPR-11-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.E., You D.J., Lee C., Ahn C., Seong J.Y., Hwang J.I. Suppression of NF-kappaB signaling by KEAP1 regulation of IKKbeta activity through autophagic degradation and inhibition of phosphorylation. Cell. Signal. 2010;22:1645–1654. doi: 10.1016/j.cellsig.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Kitamura H., Onodera Y., Murakami S., Suzuki T., Motohashi H. IL-11 contribution to tumorigenesis in an NRF2 addiction cancer model. Oncogene. 2017;36:6315–6324. doi: 10.1038/onc.2017.236. [DOI] [PubMed] [Google Scholar]
- Klaunig J.E., Xu Y., Isenberg J.S., Bachowski S., Kolaja K.L., Jiang J., Stevenson D.E., Walborg E.F. The role of oxidative stress in chemical carcinogenesis. Environ. Health Perspect. 1998;106(Suppl 1):289–295. doi: 10.1289/ehp.98106s1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko E., Kim D., Min D.W., Kwon S.H., Lee J.Y. Nrf2 regulates cell motility through RhoA-ROCK1 signalling in non-small-cell lung cancer cells. Sci. Rep. 2021;11:1247. doi: 10.1038/s41598-021-81021-0.1e2ce9ed5c914ae19477f26689d4bc0f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi E.H., Suzuki T., Funayama R., Nagashima T., Hayashi M., Sekine H., Tanaka N., Moriguchi T., Motohashi H., Nakayama K., et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016;7:11624. doi: 10.1038/ncomms11624.64f68c1a2df449c4a3c17e7079f6efb6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppula P., Lei G., Zhang Y., Yan Y., Mao C., Kondiparthi L., Shi J., Liu X., Horbath A., Das M., et al. A targetable CoQ-FSP1 axis drives ferroptosis- and radiation-resistance in KEAP1 inactive lung cancers. Nat. Commun. 2022;13:2206. doi: 10.1038/s41467-022-29905-1.5fc916fe8ad34fc4b73b85133a6b2a8c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak M.K., Wakabayashi N., Itoh K., Motohashi H., Yamamoto M., Kensler T.W. Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway. Identification of novel gene clusters for cell survival. J. Biol. Chem. 2003;278:8135–8145. doi: 10.1074/jbc.M211898200. [DOI] [PubMed] [Google Scholar]
- Lee D.F., Kuo H.P., Liu M., Chou C.K., Xia W., Du Y., Shen J., Chen C.T., Huo L., Hsu M.C., et al. KEAP1 E3 ligase-mediated downregulation of NF-κB signaling by targeting IKKβ. Mol. Cell. 2009;36:131–140. doi: 10.1016/j.molcel.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.M., Calkins M.J., Chan K., Kan Y.W., Johnson J.A. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J. Biol. Chem. 2003;278:12029–12038. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]
- Lee J.S., Surh Y.J. Nrf2 as a novel molecular target for chemoprevention. Cancer Lett. 2005;224:171–184. doi: 10.1016/j.canlet.2004.09.042. [DOI] [PubMed] [Google Scholar]
- Liby K., Risingsong R., Royce D.B., Williams C.R., Yore M.M., Honda T., Gribble G.W., Lamph W.W., Vannini N., Sogno I., et al. Prevention and treatment of experimental estrogen receptor - negative mammary carcinogenesis by the synthetic triterpenoid CDDO-methyl ester and the rexinoid LG100268. Clin. Cancer Res. 2008;14:4556–4563. doi: 10.1158/1078-0432.CCR-08-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liby K., Royce D.B., Williams C.R., Risingsong R., Yore M.M., Honda T., Gribble G.W., Dmitrovsky E., Sporn T.A., Sporn M.B. The synthetic triterpenoids CDDO-methyl ester and CDDO-ethyl amide prevent lung cancer induced by vinyl carbamate in A/J mice. Cancer Res. 2007;67:2414–2419. doi: 10.1158/0008-5472.CAN-06-4534. [DOI] [PubMed] [Google Scholar]
- Lignitto L., LeBoeuf S.E., Homer H., Jiang S., Askenazi M., Karakousi T.R., Pass H.I., Bhutkar A.J., Tsirigos A., Ueberheide B., et al. Nrf2 activation promotes lung cancer metastasis by inhibiting the degradation of Bach1. Cell. 2019;178:316–329.e18. doi: 10.1016/j.cell.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J.K.M., Leprivier G. The impact of oncogenic RAS on redox balance and implications for cancer development. Cell Death Dis. 2019;10:955. doi: 10.1038/s41419-019-2192-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Lin X., Huang C. Activation of the reverse transsulfuration pathway through NRF2/CBS confers erastin-induced ferroptosis resistance. Br. J. Cancer. 2020;122:279–292. doi: 10.1038/s41416-019-0660-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Zhao S., Meng F., Wang H., Sun L., Li G., Gao F., Chen F. Nrf2 down-regulation by camptothecin favors inhibiting invasion, metastasis and angiogenesis in hepatocellular carcinoma. Front. Oncol. 2021;11:661157. doi: 10.3389/fonc.2021.661157.7e642945fa584ec0b5b4be9f32c17db0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loboda A., Damulewicz M., Pyza E., Jozkowicz A., Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016;73:3221–3247. doi: 10.1007/s00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri K., Rasoulpoor S., Daneshkhah A., Abolfathi S., Salari N., Mohammadi M., Rasoulpoor S., Shabani S. Clinical effects of curcumin in enhancing cancer therapy: a systematic review. BMC Cancer. 2020;20:791. doi: 10.1186/s12885-020-07256-8.64787addacb044dc91783307506883f0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka Y., Yoshida R., Kawahara K., Sakata J., Arita H., Nkashima H., Takahashi N., Hirayama M., Nagata M., Hirosue A., et al. The antioxidative stress regulator Nrf2 potentiates radioresistance of oral squamous cell carcinoma accompanied with metabolic modulation. Lab. Invest. 2022;102:896–907. doi: 10.1038/s41374-022-00776-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreery M.Q., Balmain A. Chemical carcinogenesis models of cancer: back to the future. Annu. Rev. Cancer Biol. 2017;1:295–312. doi: 10.1146/annurev-cancerbio-050216-122002. [DOI] [Google Scholar]
- Mukaigasa K., Nguyen L.T.P., Li L., Nakajima H., Yamamoto M., Kobayashi M. Genetic evidence of an evolutionarily conserved role for Nrf2 in the protection against oxidative stress. Mol. Cell. Biol. 2012;32:4455–4461. doi: 10.1128/MCB.00481-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J.R., de La Vega L., Hayes J.D., Duan L., Penning T.M. Induction of the antioxidant response by the transcription factor NRF2 increases bioactivation of the mutagenic air pollutant 3-nitrobenzanthrone in human lung cells. Chem. Res. Toxicol. 2019;32:2538–2551. doi: 10.1021/acs.chemrestox.9b00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naugler W.E., Karin M. NF-kappaB and cancer-identifying targets and mechanisms. Curr. Opin. Genet. Dev. 2008;18:19–26. doi: 10.1016/j.gde.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa H., Yamanaka M., Igarashi K. Ferroptosis: regulation by competition between NRF2 and BACH1 and propagation of the death signal. FEBS J. 2022 Feb 2; doi: 10.1111/febs.16382. [Epub]. https://doi.org/10.1111/febs.16382 . [DOI] [PubMed] [Google Scholar]
- Niture S.K., Jaiswal A.K. Nrf2 protein up-regulates antiapoptotic protein Bcl-2 and prevents cellular apoptosis. J. Biol. Chem. 2012;287:9873–9886. doi: 10.1074/jbc.M111.312694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh J.K., Woo S.R., Yun M., Lee M.K., Kong M., Min S., Kim S.I., Lee Y.C., Eun Y.G., Ko S.G. SOD2- and NRF2-associated gene signature to predict radioresistance in head and neck cancer. Cancer Genomics Proteomics. 2021;18:675–684. doi: 10.21873/cgp.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell P.C. The clonal evolution of tumor cell populations. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Okazaki K., Anzawa H., Liu Z., Ota N., Kitamura H., Onodera Y., Alam M.M., Matsumaru D., Suzuki T., Katsuoka F., et al. Enhancer remodeling promotes tumor-initiating activity in NRF2-activated non-small cell lung cancers. Nat. Commun. 2020;11:5911. doi: 10.1038/s41467-020-19593-0.e2fd0ce97bcb4b3aabb6a909e9e673dd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H., Wang H., Wang X., Zhu L., Mao L. The absence of Nrf2 enhances NF-κB-dependent inflammation following scratch injury in mouse primary cultured astrocytes. Mediators Inflamm. 2012;2012:217580. doi: 10.1155/2012/217580.84a4d0339c4b4a46938d98755bd831eb [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai R., Hayashi M., Zavitsanou A.M., Papagiannakopoulos T. NRF2: KEAPing tumors protected. Cancer Discov. 2022;12:625–643. doi: 10.1158/2159-8290.CD-21-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pölönen P., Jawahar Deen A., Leinonen H.M., Jyrkkänen H.K., Kuosmanen S., Mononen M., Jain A., Tuomainen T., Pasonen-Seppänen S., Hartikainen J.M., et al. Nrf2 and SQSTM1/p62 jointly contribute to mesenchymal transition and invasion in glioblastoma. Oncogene. 2019;38:7473–7490. doi: 10.1038/s41388-019-0956-6. [DOI] [PubMed] [Google Scholar]
- Pouremamali F., Pouremamali A., Dadashpour M., Soozangar N., Jeddi F. An update of Nrf2 activators and inhibitors in cancer prevention/promotion. Cell Commun. Signal. 2022;20:100. doi: 10.1186/s12964-022-00906-3.f67bcade9097433d8513da53a35211a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit V., Wang L., Yang H., Li J., Ney G.M., Gumkowski E.R., Vaidya A.J., Wang A., Bhardwaj A., Zhao E., et al. ATDC binds to KEAP1 to drive NRF2-mediated tumorigenesis and chemoresistance in pancreatic cancer. Genes Dev. 2021;35:218–233. doi: 10.1101/gad.344184.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu P., Hou W., Wang H., Lei K.K.W., Wang S., Chen W., Pardeshi L.A., Prothro K., Shukla Y., Su S.S.M., et al. Sirt1 deficiency upregulates glutathione metabolism to prevent hepatocellular carcinoma initiation in mice. Oncogene. 2021;40:6023–6033. doi: 10.1038/s41388-021-01993-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Gomez M., Kwak M.K., Dolan P.M., Itoh K., Yamamoto M., Talalay P., Kensler T.W. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rial N.S., Choi K., Nguyen T., Snyder B., Slepian M.J. Nuclear factor kappa B (NF-κB): a novel cause for diabetes, coronary artery disease and cancer initiation and promotion? Med. Hypotheses. 2012;78:29–32. doi: 10.1016/j.mehy.2011.09.034. [DOI] [PubMed] [Google Scholar]
- Riis S., Murray J.B., O'Connor R. IGF-1 signalling regulates mitochondria dynamics and turnover through a conserved GSK-3β-Nrf2-BNIP3 pathway. Cells. 2020;9:147. doi: 10.3390/cells9010147.564e7ba3f245475fa7ecef0989b481d1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H., Dinkova-Kostova A.T., Hayes J.D. NRF2 and the ambiguous consequences of its activation during initiation and the subsequent stages of tumourigenesis. Cancers (Basel) 2020;12:3609. doi: 10.3390/cancers12123609.0054e0fb7e7547368dd6916457de1b43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robledinos-Antón N., Fernández-Ginés R., Manda G., Cuadrado A. Activators and inhibitors of NRF2: a review of their potential for clinical development. Oxid. Med. Cell. Longev. 2019;2019:9372182. doi: 10.1155/2019/9372182.616f0566bc324cc8937efc0734225472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo de la Vega M., Chapman E., Zhang D.D. NRF2 and the hallmarks of cancer. Cancer Cell. 2018;34:21–43. doi: 10.1016/j.ccell.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani P., Nirchio N., Arboit M., Barbieri V., Tosi A., Michielin F., Shibuya S., Benoist T., Wu D., Hindmarch C.C.T., et al. Mitochondrial fission links ECM mechanotransduction to metabolic redox homeostasis and metastatic chemotherapy resistance. Nat. Cell Biol. 2022;24:168–180. doi: 10.1038/s41556-022-00843-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan D.G., Knatko E.V., Casey A.M., Hukelmann J.L., Dayalan Naidu S., Brenes A.J., Ekkunagul T., Baker C., Higgins M., Tronci L., et al. Nrf2 activation reprograms macrophage intermediary metabolism and suppresses the type I interferon response. iScience. 2022;25:103827. doi: 10.1016/j.isci.2022.103827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabharwal S.S., Schumacker P.T. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles' heel? Nat. Rev. Cancer. 2014;14:709–721. doi: 10.1038/nrc3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanghvi V.R., Leibold J., Mina M., Mohan P., Berishaj M., Li Z., Miele M.M., Lailler N., Zhao C., de Stanchina E., et al. The oncogenic action of NRF2 depends on de-glycation by fructosamine-3-kinase. Cell. 2019;178:807–819.e21. doi: 10.1016/j.cell.2019.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H., Moriguchi T., Takai J., Ebina M., Yamamoto M. Nrf2 prevents initiation but accelerates progression through the Kras signaling pathway during lung carcinogenesis. Cancer Res. 2013;73:4158–4168. doi: 10.1158/0008-5472.CAN-12-4499. [DOI] [PubMed] [Google Scholar]
- Schaue D., Micewicz E.D., Ratikan J.A., Iwamoto K.S., Vlashi E., McDonald J.T., McBride W.H. NRF2 mediates cellular resistance to transformation, radiation, and inflammation in mice. Antioxidants (Basel) 2022;11:1649. doi: 10.3390/antiox11091649.d93c14d74c3a4a43a4f52aa740921de6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimrigk S., Brune N., Hellwig K., Lukas C., Bellenberg B., Rieks M., Hoffmann V., Pöhlau D., Przuntek H. Oral fumaric acid esters for the treatment of active multiple sclerosis: an open-label, baseline-controlled pilot study. Eur. J. Neurol. 2006;13:604–610. doi: 10.1111/j.1468-1331.2006.01292.x. [DOI] [PubMed] [Google Scholar]
- Schmidlin C.J., Shakya A., Dodson M., Chapman E., Zhang D.D. The intricacies of NRF2 regulation in cancer. Semin. Cancer Biol. 2021;76:110–119. doi: 10.1016/j.semcancer.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahcheraghi S.H., Salemi F., Alam W., Ashworth H., Saso L., Khan H., Lotfi M. The role of NRF2/KEAP1 pathway in glioblastoma: pharmacological implications. Med. Oncol. 2022;39:91. doi: 10.1007/s12032-022-01693-0. [DOI] [PubMed] [Google Scholar]
- Silva M.M., Rocha C.R.R., Kinker G.S., Pelegrini A.L., Menck C.F.M. The balance between NRF2/GSH antioxidant mediated pathway and DNA repair modulates cisplatin resistance in lung cancer cells. Sci. Rep. 2019;9:17639. doi: 10.1038/s41598-019-54065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simov V., Altman M.D., Bianchi E., DelRizzo S., DiNunzio E.N., Feng G., Goldenblatt P., Ingenito R., Johnson S.A., Mansueto M.S., et al. Discovery and characterization of novel peptide inhibitors of the NRF2/MAFG/DNA ternary complex for the treatment of cancer. Eur. J. Med. Chem. 2021;224:113686. doi: 10.1016/j.ejmech.2021.113686. [DOI] [PubMed] [Google Scholar]
- Singh A., Daemen A., Nickles D., Jeon S.M., Foreman O., Sudini K., Gnad F., Lajoie S., Gour N., Mitzner W., et al. NRF2 activation promotes aggressive lung cancer and associates with poor clinical outcomes. Clin. Cancer Res. 2021;27:877–888. doi: 10.1158/1078-0432.CCR-20-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B., Shoulson R., Chatterjee A., Ronghe A., Bhat N.K., Dim D.C., Bhat H.K. Resveratrol inhibits estrogen-induced breast carcinogenesis through induction of NRF2-mediated protective pathways. Carcinogenesis. 2014;35:1872–1880. doi: 10.1093/carcin/bgu120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son Y., Kim S., Chung H.T., Pae H.O. Reactive oxygen species in the activation of MAP kinases. Methods Enzymol. 2013;528:27–48. doi: 10.1016/B978-0-12-405881-1.00002-1. [DOI] [PubMed] [Google Scholar]
- Sotgia F., Martinez-Outschoorn U.E., Lisanti M.P. Mitochondrial oxidative stress drives tumor progression and metastasis: should we use antioxidants as a key component of cancer treatment and prevention? BMC Med. 2011;9:62. doi: 10.1186/1741-7015-9-62.3a32f682490c41209d8a23c7862b56c6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn M.B., Liby K.T. NRF2 and cancer: the good, the bad and the importance of context. Nat. Rev. Cancer. 2012;12:564–571. doi: 10.1038/nrc3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava R., Fernández-Ginés R., Encinar J.A., Cuadrado A., Wells G. The current status and future prospects for therapeutic targeting of KEAP1-NRF2 and β-TrCP-NRF2 interactions in cancer chemoresistance. Free Radic. Biol. Med. 2022;192:246–260. doi: 10.1016/j.freeradbiomed.2022.09.023. [DOI] [PubMed] [Google Scholar]
- Straif K., Benbrahim-Tallaa L., Baan R., Grosse Y., Secretan B., el Ghissassi F., Bouvard V., Guha N., Freeman C., Galichet L., et al. A review of human carcinogens--part C: metals, arsenic, dusts, and fibres. Lancet Oncol. 2009;10:453–454. doi: 10.1016/S1470-2045(09)70134-2. [DOI] [PubMed] [Google Scholar]
- Su H., Yang F., Fu R., Li X., French R., Mose E., Pu X., Trinh B., Kumar A., Liu J., et al. Cancer cells escape autophagy inhibition via NRF2 induced macropinocytosis. Cancer Cell. 2021;39:678–693.e11. doi: 10.1016/j.ccell.2021.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Murakami S., Biswal S.S., Sakaguchi S., Harigae H., Yamamoto M., Motohashi H. Systemic activation of NRF2 alleviates lethal autoimmune inflammation in scurfy mice. Mol. Cell. Biol. 2017;37:e00063–17. doi: 10.1128/MCB.00063-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi K., Yamamoto M. The KEAP1-NRF2 system in cancer. Front. Oncol. 2017;7:85. doi: 10.3389/fonc.2017.00085.8df7e67bf7724d54b8392daf4c16dab8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi S., Elhance A., van Duzer A., Kumar S., Leitenberger J.J., Oshimori N. Tumor-initiating cells establish an IL-33-TGF-β niche signaling loop to promote cancer progression. Science. 2020;369:eaay1813. doi: 10.1126/science.aay1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao S., Rojo de la Vega M., Chapman E., Ooi A., Zhang D.D. The effects of NRF2 modulation on the initiation and progression of chemically and genetically induced lung cancer. Mol. Carcinog. 2018;57:182–192. doi: 10.1002/mc.22745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmulappa R.K., Lee H., Rangasamy T., Reddy S.P., Yamamoto M., Kensler T.W., Biswal S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J. Clin. Invest. 2006;116:984–995. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonelli C., Chio I.I.C., Tuveson D.A. Transcriptional regulation by Nrf2. Antioxid. Redox Signal. 2018;29:1727–1745. doi: 10.1089/ars.2017.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth R.K., Warfel N.A. Strange bedfellows: nuclear factor, erythroid 2-like 2 (Nrf2) and hypoxia-inducible factor 1 (HIF-1) in tumor hypoxia. Antioxidants (Basel) 2017;6:27. doi: 10.3390/antiox6020027.989139a067794b9da3efeccb1d3189e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towers C.G., Fitzwalter B.E., Regan D., Goodspeed A., Morgan M.J., Liu C.W., Gustafson D.L., Thorburn A. Cancer cells upregulate NRF2 signaling to adapt to autophagy inhibition. Dev. Cell. 2019;50:690–703.e6. doi: 10.1016/j.devcel.2019.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyokuni S., Kong Y., Cheng Z., Sato K., Hayashi S., Ito F., Jiang L., Yanatori I., Okazaki Y., Akatsuka S. Carcinogenesis as side effects of iron and oxygen utilization: from the unveiled truth toward ultimate bioengineering. Cancers (Basel) 2020;12:3320. doi: 10.3390/cancers12113320.b1a76fe01fcb4690827962a940715db9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran K., Risingsong R., Royce D., Williams C.R., Sporn M.B., Liby K. The synthetic triterpenoid CDDO-methyl ester delays estrogen receptor-negative mammary carcinogenesis in polyoma middle T mice. Cancer Prev. Res. (Phila.) 2012;5:726–734. doi: 10.1158/1940-6207.CAPR-11-0404. [DOI] [PubMed] [Google Scholar]
- Vafa O., Wade M., Kern S., Beeche M., Pandita T.K., Hampton G.M., Wahl G.M. c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: a mechanism for oncogene-induced genetic instability. Mol. Cell. 2002;9:1031–1044. doi: 10.1016/S1097-2765(02)00520-8. [DOI] [PubMed] [Google Scholar]
- Valko M., Rhodes C.J., Moncol J., Izakovic M., Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Vartanian S., Lee J., Klijn C., Gnad F., Bagniewska M., Schaefer G., Zhang D., Tan J., Watson S.A., Liu L., et al. ERBB3 and IGF1R signaling are required for Nrf2-dependent growth in KEAP1-mutant lung cancer. Cancer Res. 2019;79:4828–4839. doi: 10.1158/0008-5472.CAN-18-2086. [DOI] [PubMed] [Google Scholar]
- del Vecchio C.A., Feng Y., Sokol E.S., Tillman E.J., Sanduja S., Reinhardt F., Gupta P.B. De-differentiation confers multidrug resistance via noncanonical PERK-Nrf2 signaling. PLoS Biol. 2014;12:e1001945. doi: 10.1371/journal.pbio.1001945.fea5fd64e6e84ad395b3ca4c79149cf3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi N., Itoh K., Wakabayashi J., Motohashi H., Noda S., Takahashi S., Imakado S., Kotsuji T., Otsuka F., Roop D.R., et al. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat. Genet. 2003;35:238–245. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- Wang F., Zhang Y., Shen J., Yang B., Dai W., Yan J., Maimouni S., Daguplo H.Q., Coppola S., Gao Y., et al. The ubiquitin E3 ligase TRIM21 promotes hepatocarcinogenesis by suppressing the p62-Keap1-Nrf2 antioxidant pathway. Cell. Mol. Gastroenterol. Hepatol. 2021;11:1369–1385. doi: 10.1016/j.jcmgh.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Bayanbold K., Zhao L., Wang Y., Adamcakova-Dodd A., Thorne P.S., Yang H., Jiang B.H., Liu L.Z. Redox sensitive miR-27a/b/Nrf2 signaling in Cr(VI)-induced carcinogenesis. Sci. Total Environ. 2022a;809:151118. doi: 10.1016/j.scitotenv.2021.151118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Long F., Lin H., Wang T. Dietary phytochemicals targeting Nrf2 for chemoprevention in breast cancer. Food Funct. 2022b;13:4273–4285. doi: 10.1039/D2FO00186A. [DOI] [PubMed] [Google Scholar]
- Wang Y., Mandal A.K., Son Y.O., Pratheeshkumar P., Wise J.T.F., Wang L., Zhang Z., Shi X., Chen Z. Roles of ROS, Nrf2, and autophagy in cadmium-carcinogenesis and its prevention by sulforaphane. Toxicol. Appl. Pharmacol. 2018;353:23–30. doi: 10.1016/j.taap.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardyn J.D., Ponsford A.H., Sanderson C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015;43:621–626. doi: 10.1042/BST20150014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waris G., Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J. Carcinog. 2006;5:14. doi: 10.1186/1477-3163-5-14.1f2b137bd6394334900ef7102fce370a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R.A. The Biology of Cancer. 2nd Edition. W. W. Norton & Company; New York: 2014. [Google Scholar]
- Wiel C., le Gal K., Ibrahim M.X., Jahangir C.A., Kashif M., Yao H., Ziegler D.V., Xu X., Ghosh T., Mondal T., et al. BACH1 stabilization by antioxidants stimulates lung cancer metastasis. Cell. 2019;178:330–345.e22. doi: 10.1016/j.cell.2019.06.005. [DOI] [PubMed] [Google Scholar]
- Worsley C.M., Mayne E.S., Veale R.B. Clone wars: the evolution of therapeutic resistance in cancer. Evol. Med. Public Health. 2016;2016:180–181. doi: 10.1093/emph/eow015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Lu H., Bai Y. Nrf2 in cancers: a double-edged sword. Cancer Med. 2019;8:2252–2267. doi: 10.1002/cam4.2101.07aec071f8784b25ba750c51f7cf9914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W., Tan B., Yang Z., Yu X., Chen L., Ran D., Xu Q., Zhou X. Nrf2/ARE pathway activation is involved in negatively regulating heat-induced apoptosis in non-small cell lung cancer cells. Acta Biochim. Biophys. Sin. (Shanghai) 2020;52:439–445. doi: 10.1093/abbs/gmaa013. [DOI] [PubMed] [Google Scholar]
- Yagishita Y., Gatbonton-Schwager T.N., McCallum M.L., Kensler T.W. Current landscape of NRF2 biomarkers in clinical trials. Antioxidants (Basel) 2020;9:716. doi: 10.3390/antiox9080716.b925e72cade746ef8b6808f27e57d504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Wu R., Li W., Gao L., Yang Y., Li P., Kong A.N. The triterpenoid corosolic acid blocks transformation and epigenetically reactivates Nrf2 in TRAMP-C1 prostate cells. Mol. Carcinog. 2018a;57:512–521. doi: 10.1002/mc.22776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Yang I., Cao M., Su Z.Y., Wu R., Guo Y., Fang M., Kong A.N. Fucoxanthin elicits epigenetic modifications, Nrf2 activation and blocking transformation in mouse skin JB6 P+ cells. AAPS J. 2018b;20:32. doi: 10.1208/s12248-018-0197-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Hou Z., Aldrich K.E., Lockwood L., Odom A.L., Liby K.T. A novel Nrf2 pathway inhibitor sensitizes Keap1-mutant lung cancer cells to chemotherapy. Mol. Cancer Ther. 2021;20:1692–1701. doi: 10.1158/1535-7163.MCT-21-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Rennhack J., Andrechek E.R., Rockwell C.E., Liby K.T. Identification of an unfavorable immune signature in advanced lung tumors from Nrf2-deficient mice. Antioxid. Redox Signal. 2018;29:1535–1552. doi: 10.1089/ars.2017.7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.S., Zhang Z.G., Du G.Y., Sun H.L., Liu H.Y., Zhou Z., Gou X.M., Wu X.H., Yu X.Y., Huang Y.H. Nrf2 promotes breast cancer cell migration via up-regulation of G6PD/HIF-1α/Notch1 axis. J. Cell. Mol. Med. 2019;23:3451–3463. doi: 10.1111/jcmm.14241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Gordon G.B. A strategy for cancer prevention: stimulation of the Nrf2-ARE signaling pathway. Mol. Cancer Ther. 2004;3:885–893. doi: 10.1158/1535-7163.885.3.7. [DOI] [PubMed] [Google Scholar]
- Zheng J., Kim S.J., Saeidi S., Kim S.H., Fang X., Lee Y.H., Guillen-Quispe Y.N., Ngo H.K.C., Kim D.H., Kim D., et al. Overactivated NRF2 induces pseudohypoxia in hepatocellular carcinoma by stabilizing HIF-1α. Free Radic. Biol. Med. 2023;194:347–356. doi: 10.1016/j.freeradbiomed.2022.11.039. [DOI] [PubMed] [Google Scholar]
- Zhou J., Li X.Y., Liu Y.J., Feng J., Wu Y., Shen H.M., Lu G.D. Full-coverage regulations of autophagy by ROS: from induction to maturation. Autophagy. 2022;18:1240–1255. doi: 10.1080/15548627.2021.1984656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X.L., Zhu C.Y., Wu Z.G., Guo X., Zou W. The oncoprotein HBXIP competitively binds KEAP1 to activate NRF2 and enhance breast cancer cell growth and metastasis. Oncogene. 2019;38:4028–4046. doi: 10.1038/s41388-019-0698-5. [DOI] [PubMed] [Google Scholar]
- Zimta A.A., Cenariu D., Irimie A., Magdo L., Nabavi S.M., Atanasov A.G., Berindan-Neagoe I. The role of Nrf2 activity in cancer development and progression. Cancers (Basel) 2019;11:1755. doi: 10.3390/cancers11111755.0c3d63a431364d6baa703e75fd2c3b19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Q., Wu R., Xiao X., Yang C., Yang Y., Wang C., Lin L., Kong A.N. The dietary flavone luteolin epigenetically activates the Nrf2 pathway and blocks cell transformation in human colorectal cancer HCT116 cells. J. Cell. Biochem. 2018;119:9573–9582. doi: 10.1002/jcb.27275. [DOI] [PMC free article] [PubMed] [Google Scholar]