Abstract
Immune checkpoint inhibitors (ICIs) have revolutionized cancer therapy and now represent the mainstay of treatment for many tumor types, including triple-negative breast cancer and two agnostic registrations. However, despite impressive durable responses suggestive of an even curative potential in some cases, most patients receiving ICIs do not derive a substantial benefit, highlighting the need for more precise patient selection and stratification. The identification of predictive biomarkers of response to ICIs may play a pivotal role in optimizing the therapeutic use of such compounds. In this Review, we describe the current landscape of tissue and blood biomarkers that could serve as predictive factors for ICI treatment in breast cancer.
The integration of these biomarkers in a “holistic” perspective aimed at developing comprehensive panels of multiple predictive factors will be a major step forward towards precision immune-oncology.
Keywords: Immune checkpoint inhibitors, Predictive biomarkers, Breast cancer
Highlights
-
•
Most breast cancer (BC) patients receiving ICIs do not derive substantial benefit.
-
•
PD-L1 is an unsatisfactory predictive factor for ICI benefit in metastatic BC.
-
•
Several promising tissue and blood biomarkers may inform tailoring of ICIs in BC.
-
•
A comprehensive integration of different biomarkers is key for precision immunology.
1. Introduction
A well-established strategy to reinvigorate endogenous immune response against tumors is the pharmacological blockade of immune checkpoint molecules such as the Cytotoxic T-Lymphocyte-Associated protein 4 (CTLA-4) and the Programmed cell Death protein 1 (PD-1)/Programmed Death-Ligand 1 (PD-L1) axis using monoclonal antibodies (mAbs) named immune checkpoint inhibitors (ICIs). The employment of ICIs has revolutionized the field of cancer therapy and now represents the mainstay of treatment for many tumor types [1]. This applies also to breast cancer [2], where the blockade of PD-1/PD-L1 has entered clinical practice as a therapeutic option for both advanced- and early-stage triple-negative breast cancer (TNBC) [3], and several clinical trials are evaluating ICIs also in other breast cancer subtypes [4].
Both the anti-PD-L1 atezolizumab and the anti-PD-1 pembrolizumab have been approved in combination with chemotherapy as first-line therapy for patients with PD-L1-positive (PD-L1+) advanced-stage TNBC based on the results of the IMpassion130 [[5], [6]] and KEYNOTE-355 [[7], [8]] trials, respectively. Both trials showed a significant benefit in terms of objective response rate (ORR), progression-free survival (PFS) and overall survival (OS) from the addition of ICIs to chemotherapy in the PD-L1+ population -although significance for OS in IMpassion130 was not formally tested due to the hierarchical statistical design and the lack of significance in the intention-to-treat population.
However, in the IMpassion131 trial, which enrolled a similar population to that of IMpassion130 but employed paclitaxel instead of nab-paclitaxel as chemotherapy backbone, the addition of atezolizumab to chemotherapy did not lead to improvements in any of the endpoints [9]. Based on these findings, Roche, in consultation with the US Food and Drug Administration, decided in August 2021 to voluntarily withdraw the accelerated approval for atezolizumab in the USA, although this decision has no implication in Europe, where atezolizumab is still approved. The disappointing results of IMpassion131 well exemplify the concept that in cancer immunotherapy one size does not fit all, and the complexity of the biological processes driving the tumor–immune co-evolution is far to be fully elucidated [3].
Indeed, a substantial proportion of patients fail to respond to these therapies, either due to intrinsic or acquired resistance, revealing the implication of additional immunosuppressive pathways and suggesting the need for a precise selection of patients who would be more likely to benefit from such approaches [10].
As a matter of fact, the identification and validation of robust predictive biomarkers of response to ICIs may play a pivotal role in optimizing their therapeutic use. Moreover, since ICIs are not exempt from toxicity, a tailored use of such compounds can minimize the occurrence of potentially serious adverse events in the absence of significant clinical benefit. Overall, to fully unleash the therapeutic potential of ICIs and to ameliorate their risk-to-benefit and cost-effectiveness ratios, a bedside decision-making process based on available and innovative predictive biomarkers must be established. PD-L1 expression in the tumor is the most obvious biomarker for selecting patients who may benefit from ICIs. Nonetheless, many patients with PD-L1+ tumors actually do not benefit from ICIs and, on the other hand, PD-L1 negativity does not exclude the possibility of clinical benefit. Moreover, the clinical significance of PD-L1 positivity seems to be different across different disease settings. Indeed, other factors including both cancer cell-intrinsic and extrinsic features, such as tumor antigenicity, composition of infiltrating immune cells and spatial interactions between the various components of the tumor microenvironment may play an important role in determining sensitivity to ICIs. Here we review tissue and blood biomarkers that could serve as predictive factors for ICI treatment in breast cancer.
2. Tissue biomarkers
2.1. PD-L1 status
The assessment of PD-L1 expression in tumors by immunohistochemistry (IHC) assays is the most studied and applied biomarker for selecting patients to receive ICIs targeting the PD-1/PD-L1 axis [11].
In advanced TNBC, early phase I/II studies investigating ICIs monotherapy collectively showed that PD-L1 expression was associated with a higher probability of treatment benefit [[12], [13], [14], [15]]. Moreover, among patients with PD-L1+ tumors, some evidence suggested an increasing probability of benefit with increasing PD-L1 expression levels [12,13,16]. This latter observation was confirmed also in KEYNOTE-119, the only randomized phase III trial to date comparing pembrolizumab monotherapy with chemotherapy in patients with previously treated mTNBC, irrespective of PD-L1 expression. The trial did not meet its primary endpoint of OS in patients with PD-L1 combined positivity score (CPS) ≥10, but an exploratory post-hoc analysis in patients with PD-L1 CPS ≥20 revealed an ORR of 26.3% vs 11.5% with chemotherapy and a numerical improvement of PFS and OS [17]. By contrast, the effect of chemotherapy on outcome appeared to be independent of PD-L1 expression.
The predictive value of PD-L1 was clearly demonstrated in the aforementioned IMpassion130 [[5], [6]] and KEYNOTE-355 trials [7,8], in which the benefit derived from the addition of ICIs to chemotherapy was confined to patients with PD-L1+ tumors.
However, the implementation of PD-L1 assessment is far from being univocal and robust throughout different disease settings and ICI products, and the dichotomic classification of tumors based on PD-L1 status into either expressing or not expressing appears to be an oversimplification that does not account for the complex biological underpinnings of this biomarker [18]. Many lessons have been learnt from the extensive use of ICIs in other solid tumors.
First of all, PD-L1 can be expressed by both tumor cells and tumor-infiltrating immune cells, and different platforms (Ventana vs Dako) leveraging on different PD-L1-targeting mAb clones (e.g. SP142 for atezolizumab and 22C3 for pembrolizumab), do not show the same sensitivity and reproducibility in detecting PD-L1 expression on different cells [11]. Both Dako and Ventana platforms can provide the tumor proportional score (TPS) or tumor cell expression (TC), calculated as percentage of PD-L1-expressing tumor cells among all viable tumor cells; however, these scores do not find any direct application in ICIs therapy for TNBC.
In order to also account for PD-L1 expression on immune cells, the Ventana system can be used to generate the immune cell expression (IC) score, i.e. the percentage of tumor area infiltrated by PD-L1-expressing immune cells relative to the whole tumor area, while the Dako system provides the CPS as percentage of PD-L1-expressing tumor and immune cells relative to all (PD-L1+ and PD-L1-) viable tumor cells.
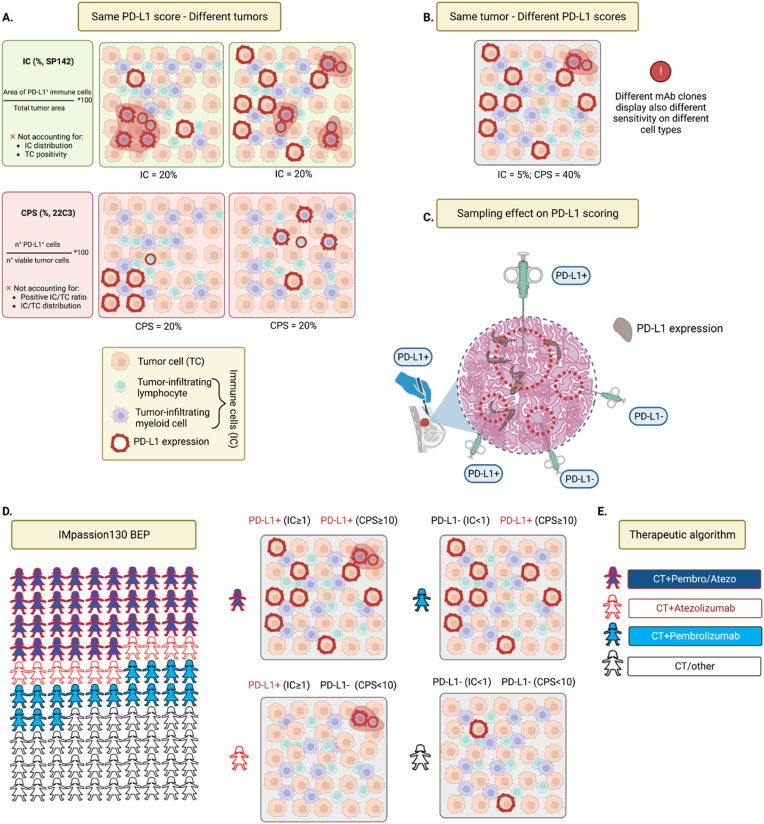
Besides different mAb clones, platforms and scoring algorithms, also the thresholds used to define positivity may be different (≥10 for CPS vs ≥ 1% for IC), and each one of these aspects may influence the definition of PD-L1 positivity and, ultimately, impact on clinical indications (Fig. 1).
Fig. 1.
Controversies of PD-L1 assessment. A) Graphical representation of different companion diagnostic PD-L1 scores for immune checkpoint treatment of advanced TNBC. The immune cell score (IC, Ventana platform, SP142 anti-PD-L1 clone), accounts for the percent total tumor area occupied by PD-L1 expressing immune cells. The combined positive score (CPS, Dako platform, 22C3 anti-PD-L1 clone), represents the percent ratio of PD-L1 expressing cells (both tumor and immune) on total viable tumor cells. IC does not account for immune cell distribution and PD-L1 expression on tumor cells. CPS lacks information regarding the cell types expressing PD-L1 and their spatial distribution. Both scores may return similar values despite significant biological differences in analyzed samples, as depicted. Graphical legend at the bottom; PD-L1 expression is represented as either red cell membrane or red shadows when considering cell numbers or tumor areas, respectively. B) Conceptual representation of intratumoural poor concordance of different PD-L1 scores. IC and CPS can provide different readouts on the same tumor sample, possibly affecting therapeutic decisions. C) Type, size, and number of tumor tissue sampling can impact PD-L1 detection. The wider the area and the higher the number of samplings, the greater the chances of PD-L1 positivity. Legend: surgical resection, surgeon hand and scalpel; Fine needle agobiopsy, small syringe; Core biopsy, big syringe; sampled areas, red-dotted area, IHC expression of PD-L1, brown areas. D) Comparison between PD-L1 assessment by SP142 (IC ≥ 1%) and 22C3 (CPS≥10) in biomarker-evaluable population of IMpassion130. Overall percentage agreement is 73% (36% both positive; 37% both negative), while 27% of patients display opposite results with the two scores, highlighting the lack of interchangeability. Representative samples of each score combinations are shown on the right. E) Suggested therapeutic algorithm following the considerations from D. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Additionally, several studies have also highlighted poor intra- and inter-assay concordance of companion diagnostic platforms in different tumor types [[19], [20], [21], [22], [23]], and some showed that both number and size of biopsy samples positively correlate with the likelihood of a proper and reproducible detection of PD-L1 expression [24].
In IMpassion130, the PD-L1 biomarker evaluated population (n = 614) was tested with both Ventana SP142 and Dako 22C3 mAbs, calculating the IC and CPS, respectively [25]. Only 73% of patients obtained concordant results (36% IC ≥ 1 and CPS ≥10; 37% IC < 1 and CPS <10), while 27% did not (10% IC ≥ 1 and CPS <10; 17% IC < 1 and CPS ≥10) (Fig. 1D). In the subgroup analysis, atezolizumab conferred no significant advantage in IC < 1 and CPS ≥10 TNBC, neither in terms of PFS nor OS [25]. This observation leads to the clinically meaningful observation that different PD-L1 IHC assessments should not be used interchangeably but, if available, both assays should be performed to tailor the most appropriate therapy to patients and avoid missing all potential candidates for ICIs therapy. Pragmatically, first-line treatment for TNBC with PD-L1 IC < 1 and CPS <10 should not include ICIs, tumors with IC ≥ 1 and CPS <10 should be treated with the combination of nab-paclitaxel and atezolizumab (where approved), and tumors with CPS ≥10 should be treated with the combination of chemotherapy and pembrolizumab (Fig. 1E).
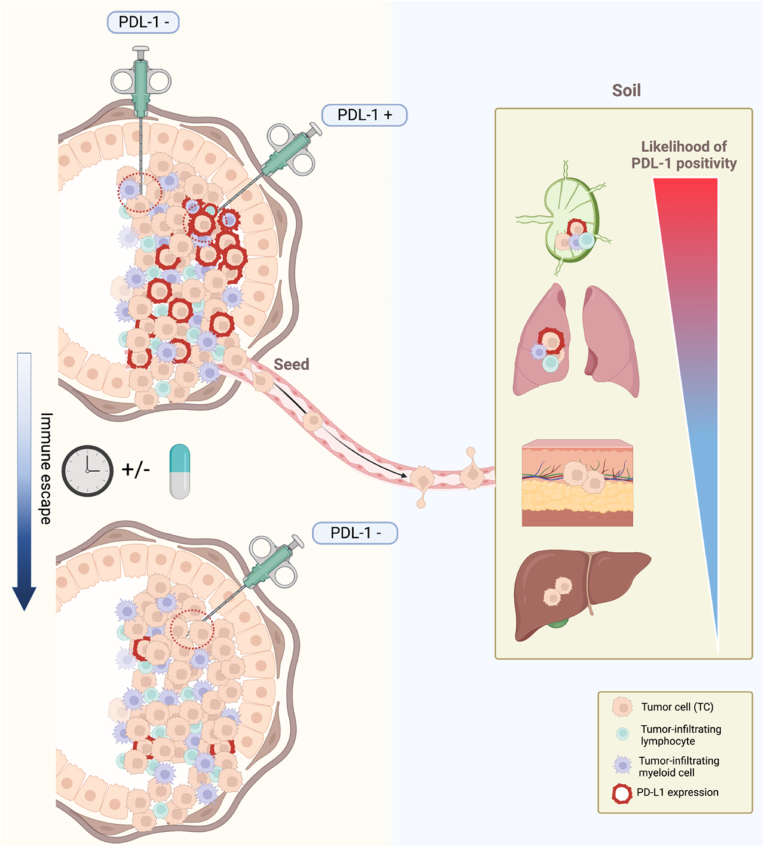
Beside analytical variables, also biological factors should be taken in account for the correct appraisal of the predictive role of PD-L1. Tumors are naturally evolving and heterogeneous, both spatially and temporally, and several studies demonstrated that this heterogeneity is reflected also in PD-L1 expression [11,[26], [27], [28], [29]]. For example, liver metastases usually present a lower lymphocytic infiltration and a higher likelihood of PD-L1 negativity, while the opposite is observed in lymph nodes [26,27]. Moreover, PD-L1 expression varies during therapy and different treatments may affect this dynamic in different ways [29] (Fig. 2).
Fig. 2.
Temporal and spatial heterogeneity of PD-L1 expression. Sources of PD-L1 assay variability through tissue biopsy encompass site of tissue sampling and dynamic changes in PD-L1 expression across the disease course. When clinically relevant, the concept of PD-L1 as a dynamic biomarker should be taken into account. Furthermore, for accurate PD-L1 detection in metastatic settings, it is crucial to consider the impact of the “soil” microenvironment in light of the tissue-specific likelihood of PD-L1 positivity.
The immunological differences between primary and metastatic breast cancer are well known [30,31]. Therefore, the different predictive significance of PD-L1 between the early and the advanced setting should not surprise [32]. The neoadjuvant KEYNOTE-173 [33], IMpassion031 [34], KEYNOTE-522 [35], GeparNuevo [36] and NeoTRIP [37] trials collectively showed that PD-L1+ tumors had a higher pCR rate than PD-L1- tumors irrespective of ICIs use, and the addition of ICIs to chemotherapy led to an increase in pCR rate compared to chemotherapy alone in both PD-L1+ and PD-L1- tumors [[34], [35], [36]], with NeoTRIP representing the only exception to this latter observation [37]. Notably, both KEYNOTE-522 [38] and GeparNuevo [39] demonstrated a statistically significant and clinically meaningful improvement in event-free survival in the immunotherapy arms without any significant difference across subgroups, including those defined according to PD-L1 expression, questioning the rightness of pCR as a surrogate endpoint to measure the role of ICIs in the neoadjuvant setting [40].
The diverse clinical significance of PD-L1 expression between early and metastatic setting and between different metastatic sites is consistent with the “seed and soil” theory and the notion that a tumor–immune co-evolution occurs during metastatic progression, moving from a more immune-activated microenvironment suitable to immunomodulation in early disease to a progressively increased immune-suppressed phenotype in metastatic disease [3] (Fig. 2).
Overall, PD-L1 remains an imperfect predictor and several sources of variability may hamper its clinical utility as a standalone biomarker. Thus, for a more comprehensive view of the tumor–immune interactions, other biomarkers are needed and should be integrated with PD-L1 in order to shed light on the biological complexity beyond ICIs sensitivity.
2.2. Tumor-infiltrating lymphocytes
Tumor-infiltrating lymphocytes (TILs) are mononuclear immune cells that infiltrate the tumor tissue, and have been described in most types of solid tumors, including breast cancer [41]. In breast cancer the majority of TILs are typically located in the tumor stroma (sTILs) and are represented by a lymphocyte population mainly comprised of cytotoxic CD8+ T cells, together with varying proportions of helper CD4+ T cells, CD19+ B cells, and NK cells [42,43]. The prevalence and clinical significance of TILs are different across breast cancer subtypes. In TNBC and HER2+ breast cancer TILs are more abundant compared to luminal tumors, and higher levels of TILs are associated with a more favorable outcome [44,45].
Early studies investigating ICI monotherapy in metastatic TNBC have shown that the clinical activity of both pembrolizumab and atezolizumab was highest in patients with CD8+ T cells- and/or sTILs-positive tumors [46,47]. In the phase I PCD4989g trial, improved ORRs (14% vs 6%) and longer PFS (HR 0.69 [0.44–0.98]) and OS (HR 0.61 [0.39–0.96]) were observed in patients with higher (above median) baseline CD8+ T-cells treated with atezolizumab monotherapy [46].
In the phase II KEYNOTE-086 study, investigating pembrolizumab monotherapy in pretreated PD-L1+/− (cohort A) and in untreated PD-L1+ (cohort B) metastatic TNBC, median TIL levels were higher in responders vs nonresponders (10% vs 5% in cohort A; 50% vs 15% in cohort B) [47]. In patients with TIL levels above vs below median, ORR was 6% vs 2% in cohort A and 39% vs 9% in cohort B. In this study, TILs significantly correlated with PD-L1 expression assessed by CPS but were independent predictors of response to pembrolizumab [47].
The KEYNOTE-119 trial corroborated the association between higher TILs and greater benefit from ICI monotherapy [48]. In this trial, TILs levels were significantly higher in responders vs nonresponders in the pembrolizumab but not in the chemotherapy arm and were significantly associated with ORR (p = 0.0004), PFS (p = 0.0002) and OS (p = 0.0003) only in the pembrolizumab arm. Median OS in the pembrolizumab and chemotherapy arm were 5.9 and 8.8 months for patients with TILs <5% (HR 1.50 [95% CI, 1.14–1.97]), and 12.5 and 11.3 months for patients with TILs ≥5% (HR 0.75 [95% CI, 0.59–0.96]), respectively. The correlation between TILs and CPS was moderate and these two biomarkers showed independent predictive value in the multivariate analysis [48].
In the IMpassion130 study, the median value of sTILs was 5% and sTIL levels were moderately correlated with PD-L1 as a continuous variable. Using a prespecified threshold of 10% or more to define sTIL positivity, patients with sTILs + tumors in the atezolizumab arm showed longer PFS and OS than those in the placebo arm. However, patients with sTILs + tumors benefited from the addition of atezolizumab to nab-paclitaxel only if their tumors were also PD-L1+ (HR for PFS: 0.54 [0.39–0.75]; HR for OS: 0.64 [0.43 to 0.96]), while no benefit was observed compared to placebo in patients with sTILs+ and PD-L1- tumors (HR for PFS: 0.92 [0.59–1.44]; HR for OS: 1.04 [0.59–1.82]), suggesting that sTILs did not provide an additional predictive value for atezolizumab benefit beyond PD-L1 27.
The substantial contribution of the host immune response to the therapeutic effects of HER2-directed monoclonal antibodies has provided a strong rationale for the investigation of immunotherapy in HER2-positive breast cancer [49]. In this context, a correlation between TILs and ICIs benefit has been shown in two separate studies [50]. In the phase Ib-II PANACEA trial investigating pembrolizumab plus trastuzumab in trastuzumab-resistant patients, TILs levels were significantly higher in responders vs nonresponders (p = 0.006) and in patients achieving disease control vs those with progressive disease (p = 0.0006) [51]. Similarly, in the phase II randomized KATE2 trial evaluating the addition of atezolizumab or placebo to TDM-1 in previously treated HER2+ metastatic breast cancer, higher TIL levels were associated with PD-L1 positivity and the addition of atezolizumab to TDM-1 was associated with a lower risk of disease progression only in patients with high TILs (HR for PFS: 0.62 [0.37–1.03] in patients with TILs ≥5% vs 1.52 [0.76–3.04] in patients with TILs <5%) [52].
The predictive role of TILs in the context of ICIs treatment has been investigated also in early-stage disease. Data about TILs in KEYNOTE-522 35 and IMpassion031 34 trials are eagerly awaited, while evidences from the KEYNOTE-173, GeparNuevo and NeoTRIP trials indicated that pre-treatment sTILs were significantly associated with pCR after neoadjuvant treatment with ICIs plus chemotherapy [29,33,36]. However, in early-stage TNBC higher levels of TILs were associated with a higher likelihood of achieving a pCR also after standard neo-/adjuvant chemotherapy [[53], [54], [55], [56]], and the results from the control arms of GeparNuevo and NeoTRIP corroborated this association. Therefore, similar to PD-L1, the use of TILs to select patients with TNBC for treatment with ICIs in the early setting is not straightforward.
Emerging evidence suggests that the specific immune cell composition and spatial distribution in the tumor microenvironment can be more informative than the simple quantification of infiltrating immune cells [57,58]. For example, data from the I-SPY 2 trial showed that the macrophage to cytotoxic T cell ratio was negatively associated with response to neoadjuvant pembrolizumab plus chemotherapy, and that the spatial distribution of CD3+ T cells in proximity to cancer cells positively correlated with pCR [59].
In the NeoTRIP trial, we showed that high density of PD-L1+/IDO + antigen presenting cells and of CD56+ neuroendocrine epithelial cells are positively associated with pCR after atezolizumab plus chemotherapy but not after chemotherapy alone (p for interaction = 0.004) [60]. Moreover, high degree of spatial connectivity between epithelial cells and specific cell phenotypes of the tumor microenvironment (e.g. CD8+/PD1+ exhausted T cells; CD8+/granzyme B + T cells; CD20+ B cells) was predictive of higher pCR rates with atezolizumab (but not with chemotherapy alone), independent of PD-L1 expression and sTIL levels [60].
Finally, the pharmacodynamic modulation of specific immune cell populations during and after ICIs treatment may add relevant predictive information for treatment benefit. In GeparNuevo, an increase of TILs in post-window samples compared with pre-treatment samples was predictive of pCR in the durvalumab arm (OR 9.36, p = 0.029) but not in the placebo arm [36]. In NeoTRIP high sTILs (≥40%) after 1 cycle of treatment showed a stronger correlation with pCR (OR 6.87, p = 0.0007) than baseline sTILs [29].
The dynamic monitoring of specific immune cell subsets during ICI therapy may also shed light on the biological mechanisms underlying treatment responses in the metastatic setting [61,62]. However, further studies are needed to better characterize the contribution of TILs dynamics in determining tumor responsiveness and patients’ outcome.
2.3. Tumor mutational burden
Somatic mutations occurring in tumor DNA are the main source of tumor-specific neoantigens which are a key target of anti-tumor immune response [63,64]. As the immunogenicity of tumor neoantigens is stochastically determined, it is reasonable to assume that the higher the number of non-synonymous mutations in a tumor, the higher the odds of generating an effective antitumor immune response after inhibition of checkpoint signals [65,66]. Indeed, tumor mutational burden (TMB), defined as the number of somatic mutations per mega-base (mut/Mb) arising in tumor-coding regions [67], is associated with neoantigen load, T-cell infiltration and expression of immune gene signatures, and accumulating evidence suggests that a high TMB may be predictive of response to ICIs across several tumor types [68,69].
This evidence led to the FDA agnostic approval of pembrolizumab for patients with metastatic TMB-high (defined as ≥10 mut/Mb) solid tumors, based on results from phase II KEYNOTE-158 trial [70]. However, breast cancer was not included in this study.
Compared to other cancers in which immunotherapy has been established for a longer time such as lung cancer or melanoma, breast cancers generally carry lower TMBs [71]. In a large study using publicly available data from 3969 patients with primary or metastatic breast cancer, the overall median TMB was of 2.63 mut/MB and only 5% of all cases were ranked as TMB-high by applying the common definition of ≥10 mut/MB [72].
In the early setting, the predictive role of TMB has not been extensively investigated. Data from the GeparNuevo trial suggested that TMB is predictive of pCR in patients with TNBC treated with neoadjuvant chemotherapy with or without immunotherapy (OR for pCR per mut/MB = 2.06 [1.33–3.20], p = 0.001). However, albeit evident in both arms, the association between pCR and TMB seemed to be stronger in the chemotherapy alone arm (OR 2.82 [1.21–6.54], p = 0.016) than in the durvalumab plus chemotherapy arm (OR 1.77 [1.00–3.13], p = 0.049) [73]. In this trial, despite a modest and not significant increase in pCR rates (from 44% to 53%, OR 1.45; p = 0.287), the addition of durvalumab to neoadjuvant chemotherapy significantly improved both distant disease-free survival (DDFS) and OS at 3 years [39]. Interestingly, an exploratory survival analysis in patients stratified according to TMB dichotomized using the upper tertile of the cohort showed that only patients with low TMB seemed to benefit from the addition of durvalumab to neoadjuvant chemotherapy (HR for DDFS = 0.23 [0.06–0.70], p = 0.02), while no differences were detected in patients with high TMB (HR = 0.95 [0.19–4.69], p = 0.95) [74].
In contrast, in the metastatic setting, current evidence suggests a positive correlation between high TMB and benefit from ICIs. An exploratory analysis of KEYNOTE-086 showed that TMB was significantly associated with ORR, PFS, and OS after adjustment for PD-L1, T-cell-inflamed gene expression profile or sTILs [16]. In IMpassion130, there was no correlation between TMB and PD-L1 status, but increasing TMB was associated with improved PFS and OS in the atezolizumab arm only in patients with PD-L1 + tumors (highest TMB quartile: HR for PFS = 0.31 [0.17–0.57]; HR for OS = 0.37 [0.15–0.90]), while no correlation between TMB and outcome was detected in patients with PD-L1– tumors [75].
In KEYNOTE-119, a potential association between TMB and clinical benefit was observed with pembrolizumab (p = 0.014 for PFS and 0.018 for OS) but not with chemotherapy (p = 0.478 for PFS and 0.906 for OS) [76]. Although limited by the small sample size (n = 26), a trend toward increased benefit with pembrolizumab vs chemotherapy was shown in patients with TMB ≥10 mut/MB (ORR 14.3% vs 8.3%; HR for OS = 0.58 [0.21–1.57]), but not in patients with TMB <10 mut/MB (n = 227, ORR 12.7% vs 12.8%; HR for OS = 0.81 [0.61–1.07]) [76].
The encouraging findings supporting a potential predictive role of TMB for ICIs provided the rationale for the design of the nonrandomized phase II TAPUR trial investigating single-agent pembrolizumab in patients with metastatic breast cancer of any subtype and high TMB (defined as TMB ≥9 mut/MB). In this cohort of heavily pre-treated patients (26 out of 28 patients with 3 or more prior systemic therapies) ORR was 21% (95% CI 8–41%), median PFS 10.6 weeks (95% CI 7.7–21.1 weeks) and median OS 30.6 weeks (95% CI, 18.3–103.3 weeks). However, no association between increasing TMB and longer PFS was found [77].
In line with these data, a retrospective analysis of 62 patients with metastatic TNBC treated with ICI mono- or combination therapy showed that patients with TMB-high tumors derived a significantly larger benefit from ICIs than patients with low TMB (OR of response = 4.32, p = 0.05; mPFS = 12.5 vs 3.7 months, p = 0.03; mOS 29.2 vs 14.2 months, p = 0.06). Again, the association between TMB and outcome was independent of PD-L1 status [78].
More recently, the single arm, phase II NIMBUS trial evaluated the efficacy of the combination of nivolumab plus ipilimumab in 30 patients with TMB-high (TMB ≥9 Mut/Mb) HER2-negative metastatic breast cancer. ORR was 16.7% and was not significantly different according to PD-L1 status and TIL levels [79]. Interestingly, patients with TMB ≥14 mut/Mb derived a much larger benefit from therapy than patients with TMB <14 mut/Mb (ORR 60% vs 8%,p = 0.02; mPFS 9.5 vs 1.4 months, HR 0.3 [0.08–1.06]; and mOS not reached vs 8.9 months, HR 0.2 [0.03–1.9]), suggesting that the optimal TMB cutoff for prediction of ICI benefit in breast cancer has not yet been well defined [79].
Collectively, these data indicate that the predictive role of TMB for ICIs benefit is far to be established, and this might be explained by both methodological and biological limitations of this biomarker. Indeed, the TMB measurement methods are not yet standardized, and different studies have used different platforms for its calculation. Several experimental factors, including sequencing depth and read length, variant calling algorithms and filters used to remove germline variants, can significantly affect TMB values [80]. Moreover, an increasing amount of evidence suggests that not all mutations are equally immunogenic and that therefore mutation quality may be even more important than mutation quantity [[80], [81], [82]]. The predictive value of the TMB could be improved by considering mutation quality or the presence of specific mutational signatures (such as the APOBEC signature [83]). Such issues highlight the limitations of implementing TMB alone to guide immunotherapy choices in breast cancer.
2.4. Gene expression signatures
A large amount of evidence supports the notion that a pre-existing anti-tumor immune response is essential -albeit not sufficient- for the efficacy of PD-1-/PD-L1–directed therapies [84]. Indeed, several studies showed that immune gene signatures reflecting an inflamed tumor microenvironment characterized by IFN-γ signaling, cytotoxic effector cells, active antigen presentation, and T cell cytokines may have a predictive role for response to ICI therapy.
One of the most well described gene signatures was the T cell-inflamed gene expression profile (GEP), which contained IFN-γ–responsive genes related to antigen presentation, chemokine expression, cytotoxic activity and was associated with clinical benefit from pembrolizumab across several cancer types, including breast cancer [85,86]. The predictive role of T cell–inflamed GEP was evaluated in patients enrolled in the KEYNOTE-086 trial and showed a statistically significant association with both PFS and OS (P < 0.001) [87]. In this trial, another signature using 37 tissue-resident memory T cell-related genes was also significantly associated with response to pembrolizumab but was highly correlated with T cell–inflamed GEP and did not add independent information [87]. Of note, T cell–inflamed GEP may provide predictive information for pembrolizumab benefit independent and complementary to that provided by TMB [88].
Another gene signature that has been shown to provide significant predictive information for ICIs benefit was the 27-gene IO-score signature [89]. This signature was evaluated in a cohort of TNBC patients enrolled in a phase I/II trial of neoadjuvant chemo-immunotherapy and showed a statistically significant predictive power for pCR with durvalumab (OR 4.13, p = 0.012) [90]. In the NeoTRIP trial, we showed that IO-score was significantly predictive of pCR in the atezolizumab plus chemotherapy arm (OR 3.64 [1.68–7.90], p = 0.001), but not in the chemotherapy alone arm (OR 1.31 [0.64–2.67] (p = 0.46) (test of interaction p = 0.029) [91]. Moreover, we also found that an early on-treatment evaluation of IO-score may add significant predictive information for pCR with atezolizumab, with assessment after 1 cycle of treatment being more informative than that at baseline. The combination of baseline and on-treatment binary IO-score defined four groups with significantly different likelihood of pCR (73.7% in positive/positive vs 15.2% in negative/negative groups, OR = 15.68, p = 0.0001), suggesting that the dynamic of IO-score may represent an early surrogate of ICIs benefit [92].
The predictive performance of immune gene signatures for ICIs response was also assessed in the GeparNuevo trial [93]. Two signatures were evaluated in this study: the GeparSixto signature - a set of immune genes that has been shown to predict response to neoadjuvant chemotherapy in triple-negative and HER2-positive breast cancer [94], and the IFNγ-signature described by Higgs and colleagues as predictive for response to durvalumab in lung and urothelial cancer [95]. Both were associated with increased pCR rates but without specificity for durvalumab. In an exploratory analysis, the authors identified seven genes related to antigen presentation and IFN signaling (HLA-A, HLA-B, TAP1, GBP1, CXCL10, STAT1, and CD38) that were significantly associated with pCR in the durvalumab arm, but not in the placebo arm [93].
In the I-SPY 2 trial, a set of 53 immune-related genes named ImPrint has been shown to predict pCR to pembrolizumab with overall sensitivity >90% and specificity >80%. Importantly, the signature appeared to be predictive for ICIs benefit also in HR + HER2-patients (sensitivity >80%, specificity >85%) [96]. Regarding TNBC, different gene expression signatures reflecting immune activation (n = 8) showed a strong correlation with response to pembrolizumab and, among them, dendritic cells and STAT1_sig/chemokine12 gene signatures were the most predictive [97]. Overall, patients with TNBC expressing the immune signatures (Immune +) had a pCR rate of 89% compared to 27% in patients without signatures expression (p = 0.0013). Moreover, gene expression signatures related to DNA repair deficiency (DRD) added further predictive information for pembrolizumab benefit (pCR 92% in Immune +/DRD + vs 20% in Immune -/DRD -) [97].
Several studies in solid tumors have shown an association between the presence of T cells displaying residency properties in the tumor microenvironment and improved outcome [87,[98], [99], [100]]. Recently, Virassamy and colleagues characterized the critical role of tissue-resident memory (TRM) T cells in mouse models of TNBC and derived a TRM-specific gene signature which was subsequently tested in the I-SPY and GeparNuevo datasets [101]. In I-SPY, higher signature expression was associated with a higher chance of achieving a pCR in both ER+/HER2-negative breast cancers and TNBC regardless of treatment, but in TNBC this association only held for patients treated with pembrolizumab. In GeparNuevo, the signature was associated with higher pCR rates and, more importantly, with excellent survival outcomes in the durvalumab arm but not in the placebo arm (p = 0.0051 for DDFS, p = 0.0052 for OS) [101].
The integration of gene expression profiling with the quantification and spatial distribution of immune cells has led to the identification of four tumor immune microenvironments (TIME), namely “immune desert” and “fully inflamed”, with homogeneously low or high numbers of CD8+ T cells, respectively, and “margin restricted” and “stroma restricted”, with compartmentalized CD8+ T cells in the tumor margins or stroma, respectively [57]. The association between TIME subtype and outcome has been investigated in a retrospective analysis of the IMpassion130 trial, which showed that the fully inflamed subtype was linked to improved PFS and OS with atezolizumab and nab-paclitaxel in PD-L1+ tumors [102]. Furthermore, an increased expression of genes related to proliferation and DNA repair pathways has also been associated with improved PFS, while increased angiogenesis, EMT, hedgehog signaling, estrogen response, and TNF signaling pathways have been associated with treatment resistance [102].
Many other gene signatures have been identified as predictors of ICIs benefit in other malignancies [[103], [104], [105], [106]], but their role in breast cancer remains to be fully elucidated.
3. Blood biomarkers
Tissue biopsy is still considered the gold standard to molecularly characterize a tumor and identify prognostic and predictive biomarkers useful to drive treatment decisions, and this also applies to immunotherapy. However, tissue biopsies are not able to fully capture spatial and temporal tumor heterogeneity, and less-invasive and more cost-effective techniques are needed to overcome such limitation [107]. In this scenario, liquid biopsies assessing blood biomarkers could help to more comprehensively address the heterogeneity of metastatic cancer and characterize the systemic immune status associated with ICIs response or resistance [108,109].
3.1. Lactate dehydrogenase
Lactate dehydrogenase (LDH) is one of the most studied blood biomarkers in cancer and has been historically considered a marker of poor prognosis [110], primarily due to its correlation with tumor burden. Besides its prognostic significance, increasing evidence suggests a role of LDH in the modulation of several biological processes, including antitumor immune response [111]. Indeed, high LDH levels may impair T cell functionality and proliferation, increase NK cells apoptosis and sustain Treg suppressor functions [111]. Consistently with evidences in other solid tumors, higher LDH levels were associated with lower ORR and shorter survival with ICIs monotherapy in patients with pretreated mTNBC [13,14,46]. However, the predictive role of LDH levels in patients treated with ICI combinations is less clear.
3.2. CD163
The hemoglobin scavenger receptor CD163 is a macrophage-specific marker upregulated by anti-inflammatory cytokines and mainly associated with M2 polarization [112]. The soluble variant of CD163 (sCD163) is constitutively present in plasma and high levels of sCD163 have been significantly associated with poor OS in patients with several cancer types [113]. Moreover, serum levels of sCD163 increased significantly in melanoma patients responding to nivolumab [114], suggesting a potential predictive role of sCD163 dynamics. Indeed, biomarker analysis of a phase II trial investigating the combination of nivolumab, paclitaxel and bevacizumab in patients with HER2-negative metastatic breast cancer revealed that patients with increased sCD163 after 1 week of treatment had significantly longer PFS compared to patients with sCD163 decrease (mPFS 18.2 vs 13.6 months, HR 0.50 [0.26–0.93], p = 0.0263), with, although not statistically significant, a similar trend in OS (p = 0.0548) [115].
3.3. Circulating tumor DNA
Circulating tumor DNA (ctDNA) analysis is a powerful research tool that can be used for several purposes in cancer, such as early detection, patient prognostication, longitudinal monitoring of treatment response and selection of mutation-directed therapies [108]. However, limited data exist about the prognostic and predictive role of ctDNA in patients receiving ICIs. In a prospective phase II trial assessing ctDNA dynamics in patients with advanced solid tumors (including TNBC) treated with pembrolizumab, Bratman and colleagues showed that baseline ctDNA levels were significantly associated with survival (lower-vs higher than-median ctDNA: HR for PFS 0.54 [0.34–0.85]; HR for OS 0.49 [0.29–0.83]), and that on-treatment ctDNA dynamics were even more informative for outcome prediction (decrease vs increase from baseline: HR for PFS 0.33 [0.19–0.58]; HR for OS 0.36 [0.18–0.71]) [116].
In the I-SPY2 trial, ctDNA clearance after 3 weeks of treatment was significantly associated with pCR (OR = 1.92, p < 0.001) and all patients who achieved pCR had no ctDNA detectable before surgery. Additionally, among patients who failed to achieve pCR, distant recurrence-free survival was significantly better in those with complete ctDNA clearance (HR 0.13; 95% CI 0.05–0.37) [117].
Genetic analysis of ctDNA may also allow for the evaluation of TMB with liquid biopsy, and some data in lung cancer suggest that high blood TMB can be predictive of benefit from immunotherapy [118]. In breast cancer, preliminary evidence suggests that a decrease in variant allele frequency of ctDNA mutations during treatment may represent a marker of response to immunotherapy [119].
3.4. Circulating tumor cells
Beyond secreted factors, studies have also examined whether the repertoire of specific cell populations in blood, including circulating tumor cells (CTCs), may provide prognostic information for ICIs benefit.
Given all the above-mentioned limitations of tissue-based PD-L1 assessment, the evaluation of PD-L1 expression on CTCs has aroused some interest in clinical research in different tumor types, including breast cancer [120,121]. Despite some conflicting results, current evidence suggests that the reduction of PD-L1+ CTCs in patients treated with ICIs may correlate with response while, on the contrary, their persistence during treatment has been associated with worse prognosis [122]. However, these studies included only few patients with breast cancer and therefore further work is needed to better elucidate the prognostic and/or predictive role of PD-L1 expression on CTCs in the context of ICI therapy.
3.5. Circulating T cells
PD-L1 expression can be found also in circulating immune cells. An exploratory analysis of the GeparNuevo trial evaluating the changes of immune cell repertoires in the blood during and after neoadjuvant treatment, revealed that the administration of durvalumab resulted in an almost complete loss of detectable PD-L1+ CD4+ and CD8+ T cells, while no effect on these cell populations was evident with placebo (t-test p < 0.05) [123]. Moreover, both baseline and on-treatment assessment of different immune cell types have been shown to be informative of treatment effects. For example, higher levels of CD4+ T cells at baseline and the expansion of γδ T cells during treatment correlated with better response to durvalumab and chemotherapy, but not to chemotherapy alone [123].
3.6. Eosinophils
Besides T lymphocytes, many other immune cell populations are implicated in anti-tumor immunity. Among these, eosinophils have been demonstrated to exert pleiotropic functions with both anti- and pro-tumorigenic significance [124]. In the context of ICIs treatment, the available data collectively suggest that eosinophils may be capable of boosting tumor immunity through both direct and indirect mechanisms, thus providing a relevant contribution to the response to cancer immunotherapy [125]. Eosinophilia has been reported to correlate with positive outcomes following ICI therapy in many tumors, including breast cancer [126]. In a phase I/II trial testing the combination of durvalumab and weekly paclitaxel in patients with mTNBC [127], an increased blood eosinophil count during treatment was significantly associated with PFS (p = 0.005), and a similar trend, although not statistically significant (p = 0.167), has been observed also for OS [126]. More recently, a longitudinal analysis of fresh blood and tumor biopsy samples obtained from mTNBC patients enrolled in the TONIC trial revealed that circulating eosinophils significantly increased in patients responding to nivolumab and that the increased expression of an eosinophil gene signature correlated with increased CD8+ T cell and IFN-g gene signatures in responders, suggesting that eosinophils may provide an active contribution to ICI response [128]. More importantly, patients with increased circulating eosinophils had longer PFS and OS [128].
3.7. Neutrophil to lymphocyte ratio
Another extensively studied cellular blood biomarker is the neutrophil to lymphocyte ratio (NLR). NLR may reflect the dynamic equilibrium between anti-tumor immune response and pro-tumorigenic inflammation [129], and several studies in different tumor types have shown that high NLR is an independent predictor of worse prognosis [[130], [131], [132]]. The clinical significance of NLR in the context of ICIs has been mainly investigated in melanoma, lung cancer and renal carcinoma [[133], [134], [135]], while data in breast cancer are scarce. In a retrospective analysis of 1714 patients with 16 different cancer types treated with ICIs, Valero and colleagues showed that higher NLR was associated with poorer OS and PFS [136]. However, in the breast cancer cohort this association did not reach statistical significance, probably due to the small sample size (n = 27) [136].
Taken together, the data presented above highlight the potential of liquid biopsy-based biomarkers to become useful tools in the management of breast cancer patients treated with ICIs. Yet, despite not being ready for prime time, the ever-growing technological advances in this field foresee an implementation of liquid biopsy in clinical practice in the next future.
4. Additional potential biomarkers
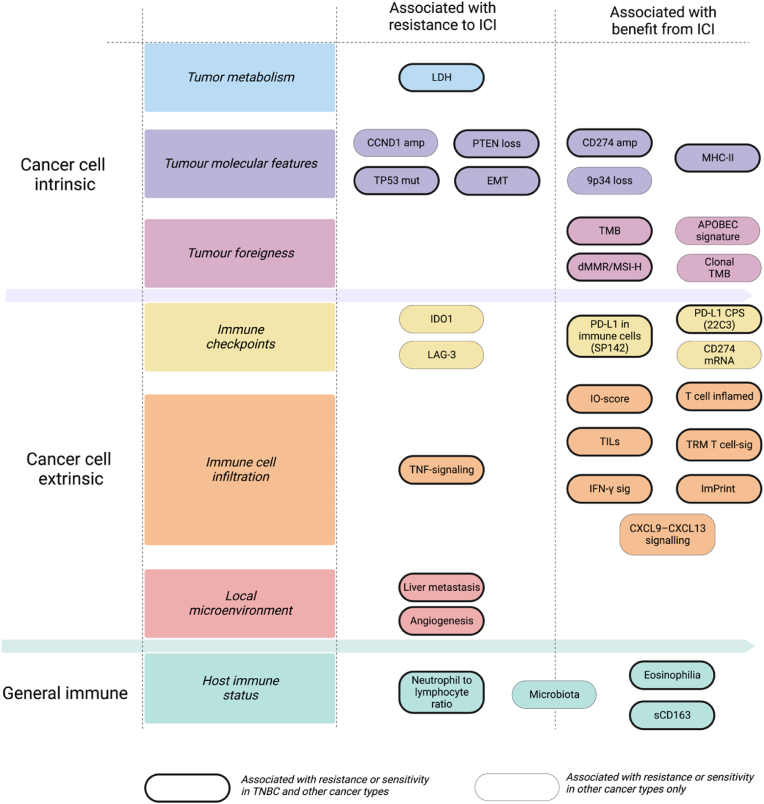
The mechanisms of action of ICIs are, at least partially, agnostic from tumor histology. Therefore, it is not surprising that several predictors of ICIs response have demonstrated a pan-cancer significance. Among these, tumor- and T cell-intrinsic biomarkers recognized also in breast cancer include, but are not limited to, CD274 (the gene encoding PD-L1) gain or amplification [137], 9q34 (TRAF2) loss [83], BRCA2 deficiency [138], POLE/POLD1 mutations [139], CCND1 amplification [83], CXCL9 expression [83] and MHC-II expression on tumor cells [60,140] (Fig. 3). Regarding CD274 gain or amplification, it is worthy to note that in the SAFIR02-BREAST IMMUNO trial, this genomic alteration was associated with increased PD-L1 expression in cancer cells but not in immune cells, suggesting a possible cancer cell-specific expression pattern [137]. However, further studies are needed to validate the role of these factors as predictive biomarkers of ICI response in breast cancer.
Fig. 3.
Landscape of biological features associated with immunotherapy response. Cancer cell intrinsic and extrinsic features, including host immunity, impact the effectiveness of immune checkpoint inhibitors targeting the PD-1/PD-L1 axis. The features reported are associated to resistance or sensitivity in either TNBC and other cancer types (bold contoured boxes) or exclusively in other cancer types (thin contoured boxes). Comprehensive assessment of such factors may be pivotal to select patients that are more likely to respond to ICI. (Adapted and updated from Bianchini et al. Nat Rev Clin Oncol 2022).
5. Conclusions
To date, PD-L1 assessment in tumor samples remains the only biomarker used to inform immunotherapy decisions in breast cancer. However, as we highlighted here, several technical and biological variables may hamper a univocal interpretation of the employed assays, and the generalization of PD-L1 as an optimal predictive biomarker for ICIs response is far from being established.
Moreover, the use of a single biomarker displays intrinsic limitations and cannot reflect temporal and spatial heterogeneity of both tumor cells and immune microenvironment. In this complex scenario, technological advances in high-throughput sequencing and single-cell spatial analyses offer the opportunity to better understand the biological mechanisms underlying tumor-immune co-evolution and will pave the way for the discovery of a multitude of novel predictive biomarkers.
The integration of these biomarkers with PD-L1 in a “holistic” perspective will be a major step towards precision immune-oncology. Research efforts to develop comprehensive panels of multiple predictive factors, including both tissue- and blood-based biomarkers, may allow clinicians to improve patient stratification with the ultimate aim of allocating individual patients to receive the most appropriate treatments.
Conflict of interest disclosures
LL has served on the advisory boards for: Lilly, Exact Sciences, Italfarmaci, AstraZeneca and Daiichi Sankyo; has received consulting fee from: Exact Sciences and Helsinn; honoraria for speakers’ bureaus from: Gilead, Exact Sciences and EISAI; support for travel, accommodations, expenses from: Lilly and Gilead.
GV has served on the advisory boards for Gilead; has received honoraria for speakers’ bureaus from Novartis, Lilly; support for travel, accommodations, expenses from: Lilly and Pfizer.
GB has received consulting fee from Roche, AstraZeneca, Novartis, MSD, Sanofi, Daiichi Sankyo, and Exact Sciences; honoraria for speakers' bureaus from Roche, Pfizer, Astra- Zeneca, Lilly, Novartis, Neopharm Israel, MSD, Chugai, Daiichi Sankyo, EISAI, and Exact Sciences; support for travel, accommodations, expenses from Roche, Pfizer, and AstraZeneca; is co-inventor of ‘European patent Application N. 12195182.6 and 12196177.5 titled “PDL-1 expression in anti-HER2 therapy” -Roche- Issued (no compensation provided); has served on the advisory boards for Pfizer, Roche, Daiichi Sankyo, Lilly, MSD, Novartis, AstraZeneca, Genomic Health, EISAI, Gilead, Seagen.
Acknowledgements
The authors received support from the Associazione Italiana per la Ricerca sul Cancro (IG2018eID21787 project grant to GB), and Fondazione Michelangelo (grants to GB).
References
- 1.Sharma P., Allison J.P. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 2.Pusztai L., Karn T., Safonov A., et al. New strategies in breast cancer: immunotherapy. Clin Cancer Res. 2016;22:2105–2110. doi: 10.1158/1078-0432.CCR-15-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bianchini G., De Angelis C., Licata L., et al. Treatment landscape of triple-negative breast cancer - expanded options, evolving needs. Nat Rev Clin Oncol. 2022;19:91–113. doi: 10.1038/s41571-021-00565-2. [DOI] [PubMed] [Google Scholar]
- 4.Jacob SL, Huppert LA, Rugo HS. Role of immunotherapy in breast cancer. JCO Oncology Practice. 2023;0 doi: 10.1200/OP.22.00483. OP.22.00483. [DOI] [PubMed] [Google Scholar]
- 5.Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Diéras V, Hegg R, Im SA, Shaw Wright G, Henschel V, Molinero L, Chui SY, Funke R, Husain A, Winer EP, Loi S, Emens LA; IMpassion130 Trial Investigators. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med. 2018 Nov 29;379(22):2108-2121. doi: 10.1056/NEJMoa1809615. Epub 2018 Oct 20. PMID: 30345906. [DOI] [PubMed]
- 6.Emens L.A., Adams S., Barrios C.H., et al. First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: IMpassion130 final overall survival analysis. Ann Oncol. 2021;32:983–993. doi: 10.1016/j.annonc.2021.05.355. [DOI] [PubMed] [Google Scholar]
- 7.Cortes J., Cescon D.W., Rugo H.S., et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817–1828. doi: 10.1016/S0140-6736(20)32531-9. [DOI] [PubMed] [Google Scholar]
- 8.Cortes J., Rugo H.S., Cescon D.W., et al. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N Engl J Med. 2022;387:217–226. doi: 10.1056/NEJMoa2202809. [DOI] [PubMed] [Google Scholar]
- 9.Miles D., Gligorov J., André F., et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann Oncol. 2021;32:994–1004. doi: 10.1016/j.annonc.2021.05.801. [DOI] [PubMed] [Google Scholar]
- 10.Jenkins R.W., Barbie D.A., Flaherty K.T. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer. 2018;118:9–16. doi: 10.1038/bjc.2017.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doroshow D.B., Bhalla S., Beasley M.B., et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat Rev Clin Oncol. 2021;18:345–362. doi: 10.1038/s41571-021-00473-5. [DOI] [PubMed] [Google Scholar]
- 12.Emens L.A., Cruz C., Eder J.P., et al. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: a phase 1 study. JAMA Oncol. 2019;5:74–82. doi: 10.1001/jamaoncol.2018.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nanda R., Chow L.Q., Dees E.C., et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol. 2016;34:2460–2467. doi: 10.1200/JCO.2015.64.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams S., Schmid P., Rugo H.S., et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30:397–404. doi: 10.1093/annonc/mdy517. [DOI] [PubMed] [Google Scholar]
- 15.Adams S., Loi S., Toppmeyer D., et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30:405–411. doi: 10.1093/annonc/mdy518. [DOI] [PubMed] [Google Scholar]
- 16.Loi S., Schmid P., Cortes J., et al. Abstract PD14-07: association between biomarkers and response to pembrolizumab in patients with metastatic triple-negative breast cancer (mTNBC): exploratory analysis from KEYNOTE-086. Cancer Res. 2021;81 PD14-07-PD14-07. [Google Scholar]
- 17.Winer E.P., Lipatov O., Im S.A., et al. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:499–511. doi: 10.1016/S1470-2045(20)30754-3. [DOI] [PubMed] [Google Scholar]
- 18.Grossman J.E., Vasudevan D., Joyce C.E., et al. Is PD-L1 a consistent biomarker for anti-PD-1 therapy? The model of balstilimab in a virally-driven tumor. Oncogene. 2021;40:1393–1395. doi: 10.1038/s41388-020-01611-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirsch F.R., McElhinny A., Stanforth D., et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the blueprint PD-L1 IHC assay Comparison project. J Thorac Oncol. 2017;12:208–222. doi: 10.1016/j.jtho.2016.11.2228. [DOI] [PubMed] [Google Scholar]
- 20.Tsao M.S., Kerr K.M., Kockx M., et al. PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of blueprint phase 2 project. J Thorac Oncol. 2018;13:1302–1311. doi: 10.1016/j.jtho.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torlakovic E., Lim H.J., Adam J., et al. Interchangeability” of PD-L1 immunohistochemistry assays: a meta-analysis of diagnostic accuracy. Mod Pathol. 2020;33:4–17. doi: 10.1038/s41379-019-0327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park Y., Koh J., Na H.Y., et al. PD-L1 testing in gastric cancer by the combined positive score of the 22C3 PharmDx and SP263 assay with clinically relevant cut-offs. Cancer Res Treat. 2020;52:661–670. doi: 10.4143/crt.2019.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torlakovic E., Albadine R., Bigras G., et al. Canadian multicenter project on standardization of programmed death-ligand 1 immunohistochemistry 22C3 laboratory-developed tests for pembrolizumab therapy in NSCLC. J Thorac Oncol. 2020;15:1328–1337. doi: 10.1016/j.jtho.2020.03.029. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y., Wu J., Deng J., et al. The detection value of PD-L1 expression in biopsy specimens and surgical resection specimens in non-small cell lung cancer: a meta-analysis. J Thorac Dis. 2021;13:4301–4310. doi: 10.21037/jtd-21-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rugo H.S., Loi S., Adams S., et al. PD-L1 immunohistochemistry assay Comparison in atezolizumab plus nab-paclitaxel-treated advanced triple-negative breast cancer. J Natl Cancer Inst. 2021;113:1733–1743. doi: 10.1093/jnci/djab108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y., Vennapusa B., Chang C.W., et al. Prevalence study of PD-L1 SP142 assay in metastatic triple-negative breast cancer. Appl Immunohistochem Mol Morphol. 2021;29:258–264. doi: 10.1097/PAI.0000000000000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emens L.A., Molinero L., Loi S., et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer: biomarker evaluation of the IMpassion130 study. J Natl Cancer Inst. 2021;113:1005–1016. doi: 10.1093/jnci/djab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sobral-Leite M., Van de Vijver K., Michaut M., et al. Assessment of PD-L1 expression across breast cancer molecular subtypes, in relation to mutation rate, BRCA1-like status, tumor-infiltrating immune cells and survival. OncoImmunology. 2018;7 doi: 10.1080/2162402X.2018.1509820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bianchini G., Huang C.S., Egle D., et al. LBA13 Tumour infiltrating lymphocytes (TILs), PD-L1 expression and their dynamics in the NeoTRIPaPDL1 trial. Ann Oncol. 2020;31:S1145–S1146. [Google Scholar]
- 30.Szekely B., Bossuyt V., Li X., et al. Immunological differences between primary and metastatic breast cancer. Ann Oncol. 2018;29:2232–2239. doi: 10.1093/annonc/mdy399. [DOI] [PubMed] [Google Scholar]
- 31.Ogiya R., Niikura N., Kumaki N., et al. Comparison of tumor-infiltrating lymphocytes between primary and metastatic tumors in breast cancer patients. Cancer Sci. 2016;107:1730–1735. doi: 10.1111/cas.13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tarantino P., Corti C., Schmid P., et al. Immunotherapy for early triple negative breast cancer: research agenda for the next decade. npj Breast Cancer. 2022;8(23) doi: 10.1038/s41523-022-00386-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmid P., Salgado R., Park Y.H., et al. Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann Oncol. 2020;31:569–581. doi: 10.1016/j.annonc.2020.01.072. [DOI] [PubMed] [Google Scholar]
- 34.Mittendorf E.A., Zhang H., Barrios C.H., et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet. 2020;396:1090–1100. doi: 10.1016/S0140-6736(20)31953-X. [DOI] [PubMed] [Google Scholar]
- 35.Schmid P., Cortes J., Pusztai L., et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382:810–821. doi: 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
- 36.Loibl S., Untch M., Burchardi N., et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Ann Oncol. 2019;30:1279–1288. doi: 10.1093/annonc/mdz158. [DOI] [PubMed] [Google Scholar]
- 37.Gianni L., Huang C.S., Egle D., et al. Pathologic complete response (pCR) to neoadjuvant treatment with or without atezolizumab in triple-negative. early high-risk and locally advanced breast cancer: NeoTRIP Michelangelo randomized study☆. Annals of Oncology. 2022;33:534–543. doi: 10.1016/j.annonc.2022.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Schmid P., Cortes J., Dent R., et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022;386:556–567. doi: 10.1056/NEJMoa2112651. [DOI] [PubMed] [Google Scholar]
- 39.Loibl S., Schneeweiss A., Huober J., et al. Neoadjuvant durvalumab improves survival in early triple-negative breast cancer independent of pathological complete response. Ann Oncol. 2022;33:1149–1158. doi: 10.1016/j.annonc.2022.07.1940. [DOI] [PubMed] [Google Scholar]
- 40.Bianchini G., Licata L., Viale G., et al. Neoadjuvant immunotherapy in triple-negative breast cancer: lesson learnt, remaining questions. Ann Oncol. 2022;33:1091–1093. doi: 10.1016/j.annonc.2022.08.088. [DOI] [PubMed] [Google Scholar]
- 41.Underwood J.C. Lymphoreticular infiltration in human tumours: prognostic and biological implications: a review. Br J Cancer. 1974;30:538–548. doi: 10.1038/bjc.1974.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salgado R., Denkert C., Demaria S., et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group. Ann Oncol. 2015;26:259–271. doi: 10.1093/annonc/mdu450. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chin Y., Janseens J., Vandepitte J., et al. Phenotypic analysis of tumor-infiltrating lymphocytes from human breast cancer. Anticancer Res. 1992;12:1463–1466. [PubMed] [Google Scholar]
- 44.Stanton S.E., Adams S., Disis M.L. Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: a systematic review. JAMA Oncol. 2016;2:1354–1360. doi: 10.1001/jamaoncol.2016.1061. [DOI] [PubMed] [Google Scholar]
- 45.Solinas C., Carbognin L., De Silva P., et al. Tumor-infiltrating lymphocytes in breast cancer according to tumor subtype: current state of the art. Breast. 2017;35:142–150. doi: 10.1016/j.breast.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 46.Emens L.A., Cruz C., Eder J.P., et al. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: a phase 1 study. JAMA Oncol. 2019;5:74–82. doi: 10.1001/jamaoncol.2018.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loi S., Adams S., Schmid P., et al. Relationship between tumor infiltrating lymphocyte (TIL) levels and response to pembrolizumab (pembro) in metastatic triple-negative breast cancer (mTNBC): results from KEYNOTE-086. Ann Oncol. 2017;28:v608. [Google Scholar]
- 48.Loi S., Winer E., Lipatov O., et al. Abstract PD5-03: relationship between tumor-infiltrating lymphocytes (TILs) and outcomes in the KEYNOTE-119 study of pembrolizumab vs chemotherapy for previously treated metastatic triple-negative breast cancer (mTNBC) Cancer Res. 2020;80 PD5-03-PD5-03. [Google Scholar]
- 49.Bianchini G., Gianni L. The immune system and response to HER2-targeted treatment in breast cancer. Lancet Oncol. 2014;15:e58–e68. doi: 10.1016/S1470-2045(13)70477-7. [DOI] [PubMed] [Google Scholar]
- 50.Agostinetto E., Montemurro F., Puglisi F., et al. Immunotherapy for HER2-positive breast cancer: clinical evidence and future perspectives. Cancers. 2022;14 doi: 10.3390/cancers14092136. 2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loi S, Giobbie-Hurder A, Gombos A, Bachelot T, Hui R, Curigliano G, Campone M, Biganzoli L, Bonnefoi H, Jerusalem G, Bartsch R, Rabaglio-Poretti M, Kammler R, Maibach R, Smyth MJ, Di Leo A, Colleoni M, Viale G, Regan MM, André F; International Breast Cancer Study Group and the Breast International Group. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b-2 trial. Lancet Oncol. 2019 Mar;20(3):371-382. doi: 10.1016/S1470-2045(18)30812-X. Epub 2019 Feb 11. PMID: 30765258. [DOI] [PubMed]
- 52.Emens L.A., Esteva F.J., Beresford M., et al. Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): a phase 2, multicentre, randomised, double-blind trial. Lancet Oncol. 2020;21:1283–1295. doi: 10.1016/S1470-2045(20)30465-4. [DOI] [PubMed] [Google Scholar]
- 53.Loi S., Drubay D., Adams S., et al. Tumor-infiltrating lymphocytes and prognosis: a pooled individual patient analysis of early-stage triple-negative breast cancers. J Clin Oncol. 2019;37:559–569. doi: 10.1200/JCO.18.01010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Denkert C., von Minckwitz G., Darb-Esfahani S., et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19:40–50. doi: 10.1016/S1470-2045(17)30904-X. [DOI] [PubMed] [Google Scholar]
- 55.Kochi M., Iwamoto T., Niikura N., et al. Tumour-infiltrating lymphocytes (TILs)-related genomic signature predicts chemotherapy response in breast cancer. Breast Cancer Res Treat. 2018;167:39–47. doi: 10.1007/s10549-017-4502-3. [DOI] [PubMed] [Google Scholar]
- 56.Ochi T., Bianchini G., Ando M., et al. Predictive and prognostic value of stromal tumour-infiltrating lymphocytes before and after neoadjuvant therapy in triple negative and HER2-positive breast cancer. Eur J Cancer. 2019;118:41–48. doi: 10.1016/j.ejca.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 57.Gruosso T., Gigoux M., Manem V.S.K., et al. Spatially distinct tumor immune microenvironments stratify triple-negative breast cancers. J Clin Invest. 2019;129:1785–1800. doi: 10.1172/JCI96313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hammerl D., Martens J.W.M., Timmermans M., et al. Spatial immunophenotypes predict response to anti-PD1 treatment and capture distinct paths of T cell evasion in triple negative breast cancer. Nat Commun. 2021;12:5668. doi: 10.1038/s41467-021-25962-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Campbell M.J., Yau C., Bolen J., et al. Abstract CT003: analysis of immune cell infiltrates as predictors of response to the checkpoint inhibitor pembrolizumab in the neoadjuvant I-SPY 2 TRIAL. Cancer Res. 2019;79 CT003-CT003. [Google Scholar]
- 60.Bianchini G., Wang X.Q., Danenberg E., et al. Abstract GS1-00: single-cell spatial analysis by imaging mass cytometry and immunotherapy response in triple-negative breast cancer (TNBC) in the NeoTRIPaPDL1 trial. Cancer Res. 2022;82 GS1-00-GS1-00. [Google Scholar]
- 61.Voorwerk L., Slagter M., Horlings H.M., et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med. 2019;25:920–928. doi: 10.1038/s41591-019-0432-4. [DOI] [PubMed] [Google Scholar]
- 62.Zhang Y., Chen H., Mo H., et al. Single-cell analyses reveal key immune cell subsets associated with response to PD-L1 blockade in triple-negative breast cancer. Cancer Cell. 2021;39:1578–1593.e8. doi: 10.1016/j.ccell.2021.09.010. [DOI] [PubMed] [Google Scholar]
- 63.DuPage M., Mazumdar C., Schmidt L.M., et al. Expression of tumour-specific antigens underlies cancer immunoediting. Nature. 2012;482:405–409. doi: 10.1038/nature10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matsushita H., Vesely M.D., Koboldt D.C., et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 2012;482:400–404. doi: 10.1038/nature10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schumacher T.N., Schreiber R.D. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 66.Yarchoan M., Johnson B.A., 3rd, Lutz E.R., et al. Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer. 2017;17:209–222. doi: 10.1038/nrc.2016.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zehir A., Benayed R., Shah R.H., et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23:703–713. doi: 10.1038/nm.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Samstein R.M., Lee C.H., Shoushtari A.N., et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51:202–206. doi: 10.1038/s41588-018-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sha D., Jin Z., Budczies J., et al. Tumor mutational burden as a predictive biomarker in solid tumors. Cancer Discov. 2020;10:1808–1825. doi: 10.1158/2159-8290.CD-20-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marabelle A., Fakih M., Lopez J., et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21:1353–1365. doi: 10.1016/S1470-2045(20)30445-9. [DOI] [PubMed] [Google Scholar]
- 71.Alexandrov L.B., Nik-Zainal S., Wedge D.C., et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barroso-Sousa R., Jain E., Cohen O., et al. Prevalence and mutational determinants of high tumor mutation burden in breast cancer. Ann Oncol. 2020;31:387–394. doi: 10.1016/j.annonc.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 73.Karn T., Denkert C., Weber K.E., et al. Tumor mutational burden and immune infiltration as independent predictors of response to neoadjuvant immune checkpoint inhibition in early TNBC in GeparNuevo. Ann Oncol. 2020;31:1216–1222. doi: 10.1016/j.annonc.2020.05.015. [DOI] [PubMed] [Google Scholar]
- 74.Karn T., Denkert C., Rey J., et al. Low TMB as predictor for additional benefit from neoadjuvant immune checkpoint inhibition in triple-negative breast cancer. J Clin Oncol. 2022;40 581-581. [Google Scholar]
- 75.Emens L.A., Molinero L., Adams S., et al. 296P Tumour mutational burden and clinical outcomes with first-line atezolizumab and nab-paclitaxel in triple-negative breast cancer: exploratory analysis of the phase III IMpassion130 trial. Ann Oncol. 2020;31:S360–S361. [Google Scholar]
- 76.Winer E.P., Lipatov O., Im S.-A., et al. Association of tumor mutational burden (TMB) and clinical outcomes with pembrolizumab (pembro) versus chemotherapy (chemo) in patients with metastatic triple-negative breast cancer (mTNBC) from KEYNOTE-119. J Clin Oncol. 2020;38 1013-1013. [Google Scholar]
- 77.Alva A.S., Mangat P.K., Garrett-Mayer E., et al. Pembrolizumab in patients with metastatic breast cancer with high tumor mutational burden: results from the targeted agent and profiling utilization registry (TAPUR) study. J Clin Oncol. 2021;39:2443–2451. doi: 10.1200/JCO.20.02923. [DOI] [PubMed] [Google Scholar]
- 78.Barroso-Sousa R., Keenan T.E., Pernas S., et al. Tumor mutational burden and PTEN alterations as molecular correlates of response to PD-1/L1 blockade in metastatic triple-negative breast cancer. Clin Cancer Res. 2020;26:2565–2572. doi: 10.1158/1078-0432.CCR-19-3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barroso-Sousa R., Li T., Reddy S., et al. Abstract GS2-10: nimbus: A phase 2 trial of nivolumab plus ipilimumab for patients with hypermutated her2-negative metastatic breast cancer (MBC) Cancer Res. 2022;82 GS2-10-GS2-10. [Google Scholar]
- 80.Chan T.A., Yarchoan M., Jaffee E., et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol. 2019;30:44–56. doi: 10.1093/annonc/mdy495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Strickler J.H., Hanks B.A., Khasraw M. Tumor mutational burden as a predictor of immunotherapy response: is more always better? Clin Cancer Res. 2021;27:1236–1241. doi: 10.1158/1078-0432.CCR-20-3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Slansky J.E., Spellman P.T. Alternative splicing in tumors - a path to immunogenicity? N Engl J Med. 2019;380:877–880. doi: 10.1056/NEJMcibr1814237. [DOI] [PubMed] [Google Scholar]
- 83.Litchfield K., Reading J.L., Puttick C., et al. Meta-analysis of tumor- and T cell-intrinsic mechanisms of sensitization to checkpoint inhibition. Cell. 2021;184:596–614.e14. doi: 10.1016/j.cell.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Savas P., Salgado R., Denkert C., et al. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat Rev Clin Oncol. 2016;13:228–241. doi: 10.1038/nrclinonc.2015.215. [DOI] [PubMed] [Google Scholar]
- 85.Ayers M., Lunceford J., Nebozhyn M., et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127:2930–2940. doi: 10.1172/JCI91190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ott P.A., Bang Y.J., Piha-Paul S.A., et al. T-Cell-Inflamed gene-expression profile, programmed Death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J Clin Oncol. 2019;37:318–327. doi: 10.1200/JCO.2018.78.2276. [DOI] [PubMed] [Google Scholar]
- 87.Loi S., Schmid P., Cortés J., et al. Abstract LB-225: RNA molecular signatures as predictive biomarkers of response to monotherapy pembrolizumab in patients with metastatic triple-negative breast cancer: KEYNOTE-086. Cancer Res. 2019;79 LB-225-LB-225. [Google Scholar]
- 88.Cristescu R., Mogg R., Ayers M., et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science. 2018;362 doi: 10.1126/science.aar3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nielsen T.J., Ring B.Z., Seitz R.S., et al. A novel immuno-oncology algorithm measuring tumor microenvironment to predict response to immunotherapies. Heliyon. 2021;7 doi: 10.1016/j.heliyon.2021.e06438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Iwase T., Blenman K.R.M., Li X., et al. A novel immunomodulatory 27-gene signature to predict response to neoadjuvant immunochemotherapy for primary triple-negative breast cancer. Cancers. 2021;13 doi: 10.3390/cancers13194839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bianchini G., Dugo M., Huang C.S., et al. LBA12 Predictive value of gene-expression profiles (GEPs) and their dynamics during therapy in the NeoTRIPaPDL1 trial. Ann Oncol. 2021;32:S1283–S1284. [Google Scholar]
- 92.Dugo M., Huang C.-S., Egle D., et al. Abstract P2-07-12: triple negative breast cancer subtypes and early dynamics of the 27-gene IO score predict pCR in the NeoTRIPaPDL1 trial. Cancer Res. 2022;82 P2-07-12-P2-07-12. [Google Scholar]
- 93.Sinn B.V., Loibl S., Hanusch C.A., et al. Immune-related gene expression predicts response to neoadjuvant chemotherapy but not additional benefit from PD-L1 inhibition in women with early triple-negative breast cancer. Clin Cancer Res. 2021;27:2584–2591. doi: 10.1158/1078-0432.CCR-20-3113. [DOI] [PubMed] [Google Scholar]
- 94.Denkert C., von Minckwitz G., Brase J.C., et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol. 2015;33:983–991. doi: 10.1200/JCO.2014.58.1967. [DOI] [PubMed] [Google Scholar]
- 95.Higgs B.W., Morehouse C.A., Streicher K., et al. Interferon gamma messenger RNA signature in tumor biopsies predicts outcomes in patients with non-small cell lung carcinoma or urothelial cancer treated with durvalumab. Clin Cancer Res. 2018;24:3857–3866. doi: 10.1158/1078-0432.CCR-17-3451. [DOI] [PubMed] [Google Scholar]
- 96.Mittempergher L., Kuilman M.M., Barcaru A., et al. The ImPrint immune signature to identify patients with high-risk early breast cancer who may benefit from PD1 checkpoint inhibition in I-SPY2. J Clin Oncol. 2022;40 514-514. [Google Scholar]
- 97.Wolf D.M., Yau C., Wulfkuhle J., et al. Redefining breast cancer subtypes to guide treatment prioritization and maximize response: predictive biomarkers across 10 cancer therapies. Cancer Cell. 2022;40:609–623.e6. doi: 10.1016/j.ccell.2022.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Boddupalli C.S., Bar N., Kadaveru K., et al. Interlesional diversity of T cell receptors in melanoma with immune checkpoints enriched in tissue-resident memory T cells. JCI Insight. 2016;1 doi: 10.1172/jci.insight.88955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ganesan A.P., Clarke J., Wood O., et al. Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nat Immunol. 2017;18:940–950. doi: 10.1038/ni.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Webb J.R., Milne K., Nelson B.H. PD-1 and CD103 are widely coexpressed on prognostically favorable intraepithelial CD8 T cells in human ovarian cancer. Cancer Immunol Res. 2015;3:926–935. doi: 10.1158/2326-6066.CIR-14-0239. [DOI] [PubMed] [Google Scholar]
- 101.Virassamy B., Caramia F., Savas P., et al. Cancer Cell; 2023. Intratumoral CD8+ T cells with a tissue-resident memory phenotype mediate local immunity and immune checkpoint responses in breast cancer. [DOI] [PubMed] [Google Scholar]
- 102.Emens L.A., Goldstein L.D., Schmid P., et al. The tumor microenvironment (TME) and atezolizumab + nab-paclitaxel (A+nP) activity in metastatic triple-negative breast cancer (mTNBC): IMpassion130. J Clin Oncol. 2021;39 1006-1006. [Google Scholar]
- 103.Jiang P., Gu S., Pan D., et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat Med. 2018;24:1550–1558. doi: 10.1038/s41591-018-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Helmink B.A., Reddy S.M., Gao J., et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577:549–555. doi: 10.1038/s41586-019-1922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dai Y., Qiang W., Lin K., et al. An immune-related gene signature for predicting survival and immunotherapy efficacy in hepatocellular carcinoma. Cancer Immunol Immunother. 2021;70:967–979. doi: 10.1007/s00262-020-02743-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.She Y., Kong X., Ge Y., et al. Immune-related gene signature for predicting the prognosis of head and neck squamous cell carcinoma. Cancer Cell Int. 2020;20(22) doi: 10.1186/s12935-020-1104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dagogo-Jack I., Shaw A.T. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15:81–94. doi: 10.1038/nrclinonc.2017.166. [DOI] [PubMed] [Google Scholar]
- 108.Wan J.C.M., Massie C., Garcia-Corbacho J., et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17:223–238. doi: 10.1038/nrc.2017.7. [DOI] [PubMed] [Google Scholar]
- 109.Hiam-Galvez K.J., Allen B.M., Spitzer M.H. Systemic immunity in cancer. Nat Rev Cancer. 2021;21:345–359. doi: 10.1038/s41568-021-00347-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Petrelli F., Cabiddu M., Coinu A., et al. Prognostic role of lactate dehydrogenase in solid tumors: a systematic review and meta-analysis of 76 studies. Acta Oncol. 2015;54:961–970. doi: 10.3109/0284186X.2015.1043026. [DOI] [PubMed] [Google Scholar]
- 111.Miholjcic T.B.S., Halse H., Bonvalet M., et al. Rationale for LDH-targeted cancer immunotherapy. Eur J Cancer. 2023;181:166–178. doi: 10.1016/j.ejca.2022.11.032. [DOI] [PubMed] [Google Scholar]
- 112.Moestrup S.K., Møller H.J. CD163: a regulated hemoglobin scavenger receptor with a role in the anti-inflammatory response. Ann Med. 2004;36:347–354. doi: 10.1080/07853890410033171. [DOI] [PubMed] [Google Scholar]
- 113.Qian S., Zhang H., Dai H., et al. Is sCD163 a clinical significant prognostic value in cancers? A systematic review and meta-analysis. Front Oncol. 2020;10 doi: 10.3389/fonc.2020.585297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fujimura T., Sato Y., Tanita K., et al. Serum level of soluble CD163 may Be a predictive marker of the effectiveness of nivolumab in patients with advanced cutaneous melanoma. Front Oncol. 2018;8:530. doi: 10.3389/fonc.2018.00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mukohara T., Ozaki Y., Kitano S., et al. Abstract P5-02-42: soluble CD163 may be a predictive biomarker of the efficacy of nivolumab plus chemotherapy in patients with HER2-negative metastatic breast cancer (WJOG9917BTR) Cancer Res. 2023;83 P5-02-42-P5-02-42. [Google Scholar]
- 116.Bratman S.V., Yang S.Y.C., Iafolla M.A.J., et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat Can (Que) 2020;1:873–881. doi: 10.1038/s43018-020-0096-5. [DOI] [PubMed] [Google Scholar]
- 117.Magbanua M.J.M., Wolf D., Renner D., et al. Abstract PD9-02: personalized ctDNA as a predictive biomarker in high-risk early stage breast cancer (EBC) treated with neoadjuvant chemotherapy (NAC) with or without pembrolizumab (P) Cancer Res. 2021;81 PD9-02-PD9-02. [Google Scholar]
- 118.Gandara D.R., Paul S.M., Kowanetz M., et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med. 2018;24:1441–1448. doi: 10.1038/s41591-018-0134-3. [DOI] [PubMed] [Google Scholar]
- 119.Araujo D.V., Wang A., Torti D., et al. Applications of circulating tumor DNA in a cohort of phase I solid tumor patients treated with immunotherapy. JNCI Cancer Spectr. 2021;5 doi: 10.1093/jncics/pkaa122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mazel M., Jacot W., Pantel K., et al. Frequent expression of PD-L1 on circulating breast cancer cells. Mol Oncol. 2015;9:1773–1782. doi: 10.1016/j.molonc.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jacot W., Mazel M., Mollevi C., et al. Clinical correlations of programmed cell Death ligand 1 status in liquid and standard biopsies in breast cancer. Clin Chem. 2020;66:1093–1101. doi: 10.1093/clinchem/hvaa121. [DOI] [PubMed] [Google Scholar]
- 122.Hofman P., Heeke S., Alix-Panabières C., et al. Liquid biopsy in the era of immuno-oncology: is it ready for prime-time use for cancer patients? Ann Oncol. 2019;30:1448–1459. doi: 10.1093/annonc/mdz196. [DOI] [PubMed] [Google Scholar]
- 123.Massa C., Karn T., Weber K., et al. Abstract PD9-04: immunological and clinical consequences of durvalumab treatment in combination to neoadjuvant chemotherapy in triple-negative breast cancer patients. Cancer Res. 2023;83 PD9-04-PD9-04. [Google Scholar]
- 124.Grisaru-Tal S., Itan M., Klion A.D., et al. A new dawn for eosinophils in the tumour microenvironment. Nat Rev Cancer. 2020;20:594–607. doi: 10.1038/s41568-020-0283-9. [DOI] [PubMed] [Google Scholar]
- 125.Grisaru-Tal S., Rothenberg M.E., Munitz A. Eosinophil–lymphocyte interactions in the tumor microenvironment and cancer immunotherapy. Nat Immunol. 2022;23:1309–1316. doi: 10.1038/s41590-022-01291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ghebeh H., Elshenawy M.A., AlSayed A.D., et al. Peripheral blood eosinophil count is associated with response to chemoimmunotherapy in metastatic triple-negative breast cancer. Immunotherapy. 2022;14:189–199. doi: 10.2217/imt-2021-0149. [DOI] [PubMed] [Google Scholar]
- 127.Ghebeh H., Al-Sayed A., Eiada R., et al. Weekly Paclitaxel given concurrently with Durvalumab has a favorable safety profile in triple-negative metastatic breast cancer. Sci Rep. 2021;11 doi: 10.1038/s41598-021-98113-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Blomberg O.S., Spagnuolo L., Garner H., et al. IL-5-producing CD4(+) T cells and eosinophils cooperate to enhance response to immune checkpoint blockade in breast cancer. Cancer Cell. 2023;41:106–123.e10. doi: 10.1016/j.ccell.2022.11.014. [DOI] [PubMed] [Google Scholar]
- 129.Faria S.S., Fernandes P.C., Jr., Silva M.J., et al. The neutrophil-to-lymphocyte ratio: a narrative review. Ecancermedicalscience. 2016;10:702. doi: 10.3332/ecancer.2016.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Templeton A.J., McNamara M.G., Šeruga B., et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 131.Azab B., Bhatt V.R., Phookan J., et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol. 2012;19:217–224. doi: 10.1245/s10434-011-1814-0. [DOI] [PubMed] [Google Scholar]
- 132.Ethier J.-L., Desautels D., Templeton A., et al. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res. 2017;19(2) doi: 10.1186/s13058-016-0794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lalani A.A., Xie W., Martini D.J., et al. Change in Neutrophil-to-lymphocyte ratio (NLR) in response to immune checkpoint blockade for metastatic renal cell carcinoma. J Immunother Cancer. 2018;6(5) doi: 10.1186/s40425-018-0315-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ferrucci P.F., Ascierto P.A., Pigozzo J., et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol. 2016;27:732–738. doi: 10.1093/annonc/mdw016. [DOI] [PubMed] [Google Scholar]
- 135.Mezquita L., Auclin E., Ferrara R., et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. 2018;4:351–357. doi: 10.1001/jamaoncol.2017.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Valero C., Lee M., Hoen D., et al. Pretreatment neutrophil-to-lymphocyte ratio and mutational burden as biomarkers of tumor response to immune checkpoint inhibitors. Nat Commun. 2021;12:729. doi: 10.1038/s41467-021-20935-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bachelot T., Filleron T., Bieche I., et al. Durvalumab compared to maintenance chemotherapy in metastatic breast cancer: the randomized phase II SAFIR02-BREAST IMMUNO trial. Nat Med. 2021;27:250–255. doi: 10.1038/s41591-020-01189-2. [DOI] [PubMed] [Google Scholar]
- 138.Samstein R.M., Krishna C., Ma X., et al. Mutations in BRCA1 and BRCA2 differentially affect the tumor microenvironment and response to checkpoint blockade immunotherapy. Nat Can (Que) 2021;1:1188–1203. doi: 10.1038/s43018-020-00139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang F., Zhao Q., Wang Y.N., et al. Evaluation of POLE and POLD1 mutations as biomarkers for immunotherapy outcomes across multiple cancer types. JAMA Oncol. 2019;5:1504–1506. doi: 10.1001/jamaoncol.2019.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gonzalez-Ericsson P.I., Wulfkhule J.D., Gallagher R.I., et al. Tumor-specific major histocompatibility-II expression predicts benefit to anti-PD-1/L1 therapy in patients with HER2-negative primary breast cancer. Clin Cancer Res. 2021;27:5299–5306. doi: 10.1158/1078-0432.CCR-21-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]