Abstract
Introduction
Nintedanib is recommended for the treatment of idiopathic pulmonary fibrosis (IPF); however, treatment discontinuation due to adverse events (AEs) is common. A large-scale post-marketing surveillance study is investigating the real-world tolerability/safety of nintedanib in Japanese patients with IPF in routine clinical practice. Here, we report a 12-month interim analysis of this study.
Methods
The study included Japanese patients with IPF who started nintedanib between 31 August 2015 and 25 December 2018. The primary outcome was the frequency of adverse drug reactions (ADRs), defined as AEs for which a causal relationship with nintedanib could not be excluded. The secondary outcome was change from baseline in forced vital capacity (FVC). Outcomes were analysed in patients who stopped (‘discontinued’ subgroup) and continued (‘continued’ subgroup) nintedanib after 12 months. A multivariate analysis was performed to determine potential risk factors for treatment discontinuation.
Results
Of 5578 patients in the safety analysis set, 2795 (50.1%) discontinued nintedanib within 12 months of treatment initiation. Overall, 3767 patients (67.5%) had ADRs, with 1356 (24.3%) discontinuing nintedanib because of an ADR. Among patients in the ‘discontinued’ subgroup (n = 2795), 1442 (51.6%) discontinued because of an ADR. The most common ADRs causing discontinuation within 3 and 12 months were hepatic function abnormal (n = 137/730; 18.8%) and diarrhoea (n = 190/1442; 13.2%), respectively. At 12 months, the decrease in FVC from baseline was smaller in the ‘continued’ versus the ‘discontinued’ subgroup (adjusted mean ± standard error change − 104.4 ± 10.9 ml vs. − 311.2 ± 29.2 ml). Stage III/IV IPF and FVC < 70% predicted at baseline were risk factors for early treatment discontinuation.
Conclusion
About 50% of Japanese patients with IPF discontinued nintedanib within the first year of treatment, with worse lung function being associated with an increased risk of early treatment discontinuation.
Trial registration: ClinicalTrials.gov: NCT02607722; European Union electronic register of Post-Authorisation Studies: EUPAS10891.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-022-02411-y.
Keywords: Adverse drug reactions, Idiopathic pulmonary fibrosis, Nintedanib, Post-marketing surveillance study, Safety
Key Summary Points
| Why carry out this study? |
| Nintedanib is recommended in patients with idiopathic pulmonary fibrosis (IPF) to slow the progressive decline in lung function |
| Real-world data indicate that rates of nintedanib discontinuation are high, particularly among Japanese patients, with adverse events being the most common reason for stopping treatment |
| The primary objective of this large-scale post-marketing surveillance study was to describe the real-world safety and tolerability of nintedanib in Japanese patients with IPF as well as to identify potential risk factors for treatment discontinuation and evaluate the effect of treatment on lung function |
| What was learned from the study? |
| About 50% of Japanese patients with IPF discontinued treatment within the first year of treatment, with age ≥ 75 years, male sex, body surface area < 1.58 m2, stage III/IV IPF and forced vital capacity < 70% predicted at baseline being associated with an increased risk of early treatment discontinuation |
| Adverse drug reactions, which caused over half of the nintedanib discontinuations, should be first managed with nintedanib dose reduction or interruption rather than permanent discontinuation to prevent further worsening of lung function and clinical status |
| Nintedanib treatment should be initiated early in the disease course to maximise clinical benefit and treatment persistence |
Introduction
Idiopathic pulmonary fibrosis (IPF) affects approximately 27 in 100,000 people in Japan, with the prevalence higher in men than in women and highest in those aged 75–79 years [1]. IPF is progressive and irreversible, and the median survival time from diagnosis is 35 months in Japan [2].
Treatment options for IPF are limited, but Japanese and international guidelines recommend the antifibrotic agent pirfenidone or the tyrosine kinase inhibitor nintedanib to slow the progressive decline in lung function [3, 4]. In the INPULSIS-1 and -2 trials, nintedanib significantly slowed the annual decline in forced vital capacity (FVC) compared with placebo in patients with IPF [5]. In a prespecified pooled analysis of the INPULSIS trials, nintedanib was associated with a numerically lower risk of first acute exacerbation compared with placebo [5], and a pooled analysis of the INPULSIS and TOMORROW trials showed a significant reduction in the risk of acute IPF exacerbations with nintedanib [6]. In these studies, adverse events (AEs) that commonly occurred at a higher rate with nintedanib than with placebo were diarrhoea and elevated liver enzyme levels [5]. In patients who continued nintedanib therapy during an open-label extension of the INPULSIS trials (INPULSIS-ON), diarrhoea was the most frequent AE [7]. In a Japanese subgroup analysis of the INPULSIS trials, 75% of patients developed diarrhoea and 18.4% developed abnormal hepatic function [8]. In this subgroup analysis, diarrhoea was of mild-to-moderate severity and reversible for most patients (54/57; 94.7%), but two patients discontinued nintedanib because of diarrhoea. Overall, 25% of patients in the Japanese subgroup discontinued nintedanib because of AEs, 3.5% discontinued because of severe diarrhoea and 2.6% discontinued because of increased hepatic enzyme levels [8].
Randomised clinical trial data have limited external validity and do not always reflect the results of treatment in a real-world setting [9]. Real-world data from the UK, Greece, the USA and Germany indicate that between 11 and 26% of patients discontinue treatment with nintedanib [10–14]. Data from Asia, however, suggest that about 50% of patients who start nintedanib for IPF stop taking it within 12 months. Two Japanese studies reported that 47–51% of patients taking nintedanib for IPF discontinued treatment within 12 months and that AEs were the most common reason for discontinuation [15, 16]. Similarly, a study in Korea reported that 53% of IPF patients taking nintedanib discontinued treatment because of an AE [17].
Therefore, we undertook a large-scale prospective post-marketing surveillance study on the safety and tolerability of nintedanib, as well as persistence rates, in Japanese patients with IPF starting nintedanib treatment during routine clinical practice. The primary objective was to describe the real-world tolerability and safety of nintedanib in this setting. This study also investigated potential risk factors for treatment discontinuation and examined the effects of nintedanib on lung function. Here, we report an interim analysis of this study using data up to 12 months.
Methods
Study Design
This was a prospective, non-interventional, all-case post-marketing surveillance study (NCT02607722, EUPAS10891) to assess the safety and effectiveness of nintedanib in Japanese patients with IPF. The study was conducted at institutions in Japan with respiratory specialists who had access to nintedanib during the study period and were able to comply with the study protocol.
Patients starting treatment with nintedanib were registered by their attending physician anonymously using electronic and paper case report forms (CRFs) through a central registration system managed by the study sponsor, Boehringer Ingelheim. CRFs were completed for all patients who started nintedanib treatment by 15 October 2017 and for those who started nintedanib on or after 16 October 2017 and in whom it was judged necessary to complete a CRF by the Boehringer Ingelheim study coordinator.
The primary outcome was the frequency of patients with any suspected adverse drug reactions (ADRs), i.e. AEs for which the causal relationship with nintedanib could not be excluded by either the investigator or sponsor (or both). The secondary outcome of this study was absolute change from baseline in FVC (ml) at Week 104.
This post-marketing surveillance study was conducted under the direction of the Ministry of Health, Labour and Welfare (MHLW) and the protocol was approved by the MHLW before study initiation. Participating institutes were contracted by Boehringer Ingelheim. As per the Japanese Pharmaceutical and Medical Device Act, the study was conducted in accordance with Good Post-marketing Study Practice (GPSP) guidance. Furthermore, as per GPSP guidance, the study was exempt from protocol review and approval by ethics committees of the participating institutions and exempt from the requirement for the collection of informed consent from patients, unless such procedures were specifically required by the participating institutions.
Patients and Treatment
Patients who were diagnosed with IPF by their attending physician, according to the most recent American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Society guidelines at the time of patient enrolment [4], and started nintedanib in Japan between 31 August 2015 and 25 December 2018 were enrolled and followed for 2 years or until treatment discontinuation. IPF diagnosis at University Hospitals and Centre Hospitals involved multidisciplinary discussion, while diagnosis at the other centres was mainly dependent on the attending physician's judgement.
Nintedanib 150 mg twice daily (bid; i.e. 300 mg/day) was administered after meals in accordance with the package insert, but a dose increase or decrease was also permitted at the investigator’s discretion.
Data Collection
At enrolment, the physician recorded the site, treatment, doctor’s name, patient identification number, sex, date of birth, smoking history, height, body weight, comorbidities and baseline medications, IPF severity stage using Japanese guidelines [18], complete medical and IPF history, vital signs, baseline laboratory test results, previous treatments for IPF, start date for nintedanib treatment, reason for prescribing nintedanib, previous use of nintedanib, dose of nintedanib prescribed and any potential contraindications.
Clinical data, including actual nintedanib dosage, pulmonary function test results and AEs, were recorded at approximately Week 4, 13, 26, 39, 52, 65, 78, 91 and 104 after the initiation of nintedanib, as long as the patients continued to receive treatment. In this report, the data up to 52 weeks were evaluated.
AEs were classified by using preferred terms in the Japanese version of the Medical Dictionary for Regulatory Activities (MedDRA) version 23.0. Based on previous experience with nintedanib, special attention was paid to the occurrence of diarrhoea and hepatic function disorders; the latter was defined by abnormalities in any liver enzyme parameter (i.e. alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase and gamma glutamyl transferase), hepatic function test or blood bilirubin level or any of the following MedDRA preferred terms (PTs): hypoalbuminaemia, hyperammonaemia, hepatic encephalopathy, ascites, hepatic function abnormal, liver disorder, drug-induced liver injury, liver injury, hyperbilirubinaemia, cholestasis, hepatic cirrhosis, jaundice, hepatobiliary disease or congestive hepatopathy.
Statistical Analysis
The target sample size was 1000 patients. Based on previous clinical studies with nintedanib [19], a sample size of 1000 would have 95% power to detect any ADR in at least one patient occurring at a frequency of ≥ 0.3%. This post-marketing surveillance study was conducted as a Japanese Pharmaceutical Medical Device Act approval condition for nintedanib; this condition was removed after the target number of patients was reached.
The safety analysis set included all patients who received at least one dose of nintedanib and had at least one post-baseline study visit.
The effectiveness analysis set included all patients who received nintedanib according to the approved product label (i.e. not off-label) and had at least one post-baseline effectiveness evaluation.
Most analyses were descriptive, using mean and standard deviation (SD), median and quartiles 1 and 3 (Q1 and Q3) and frequency. For safety outcomes, incidence rates and 95% confidence intervals (CIs) were calculated. Change from baseline in effectiveness outcomes (continuous variables) were analysed using Mixed Effects Model for Repeated Measures (MMRM), with fixed effects for visit, sex, baseline age, baseline height, baseline FVC (absolute or % predicted) and baseline FVC (absolute or % predicted)-by-visit interaction and random effect for patient. These are presented using adjusted means and standard error (SE).
Two patient subgroups were analysed: (1) patients who stopped nintedanib before 12 months, classified as the ‘discontinued’ subgroup; (2) patients who were still taking nintedanib at 12 months, classified as the ‘continued’ subgroup. In these groups the odds ratio and 95% CIs for discontinuation rate were calculated by each baseline characteristic.
Logistic regression analysis was used to conduct a multivariate analysis to determine potential risk factors associated with nintedanib treatment discontinuation; the independent variables included in the model were age, sex, body surface area (BSA; < 1.58 m2 or ≥ 1.58 m2), IPF severity stage, FVC % predicted, diarrhoea (ADR) and liver dysfunction (ADR) within 12 months. For the BSA variable, the threshold of 1.58 m2 was used to define low versus high BSA subgroups because this BSA value was previously shown to have maximal sensitivity and specificity in predicting hepatotoxicity risk with nintedanib [20].
Results
Patient Disposition and Characteristics
As of 15 October 2020, 5597 patients from 1011 institutions had confirmed evaluable data. Of these, 19 patients were excluded from analysis because no visit after the registration visit was recorded (n = 18) or because they did not receive treatment with nintedanib (n = 1). Therefore, the safety analysis set included 5578 patients.
The effectiveness analysis set included 3004 patients; 2574 patients were excluded because no effectiveness data were recorded (n = 2554) and/or because nintedanib was being used off-label (i.e. IPF had not been diagnosed; n = 56).
The baseline characteristics of patients in the safety analysis set are shown in Table 1. This population included 4353 men (78.0% of patients), and 3936 patients (70.6%) were current or former smokers. Among 4273 patients whose IPF severity stage was evaluated, 1482 patients (34.7%) had stage IV IPF severity. The mean (SD) age of the cohort was 71.8 (8.1) years, and the mean (SD) weight, BMI and BSA were 59.4 (12.5) kg, 22.8 (3.9) kg/m2 and 1.62 (0.19) m2, respectively. The mean (SD) FVC at baseline was 2114.1 (717.1) ml and 69.3 (38.1) % predicted. Previous treatment for IPF had been received by 1315 patients (23.6%) and 1329 patients (23.8%) received corticosteroid treatment at baseline.
Table 1.
Baseline patient demographic and clinical characteristics and nintedanib treatment pattern
| All patients (N = 5578) | |
|---|---|
| Age, years | |
| Mean (SD) | 71.8 (8.1) |
| Median | 73.0 |
| < 75 years, n (%) | 3373 (60.5) |
| ≥ 75 years, n (%) | 2205 (39.5) |
| Sex, n (%) | |
| Male | 4353 (78.0) |
| Female | 1225 (22.0) |
| Body weight, kg | (n = 5291) |
| Mean (SD) | 59.4 (12.5) |
| Median | 59.1 |
| BMI, kg/m2 | (n = 5261) |
| Mean (SD) | 22.8 (3.9) |
| Median | 22.8 |
| BSA, m2 | (n = 5261) |
| Mean (SD) | 1.62 (0.19) |
| Median | 1.62 |
| BSA category, n (%) | |
| < 1.58 m2 | 2147 (38.5) |
| ≥ 1.58 m2 | 3114 (55.8) |
| Data missing | 317 (5.7) |
| Current or previous smoking history, n (%) | 3936 (70.6) |
| IPF severity stage, n (%) | (n = 4273) |
| I | 1059 (24.8) |
| II | 219 (5.1) |
| III | 1004 (23.5) |
| IV | 1482 (34.7) |
| Data missing | 509 (11.9) |
| FVC, ml, mean (SD) | (n = 4838) |
| Mean (SD) | 2114.1 (717.1) |
| FVC, % predicted | (n = 4410) |
| Mean (SD) | 69.3 (38.1) |
| Median | 67.0 |
| FVC % predicted category, n (%) | |
| < 70% | 2456 (44.0) |
| ≥ 70% | 1954 (35.0) |
| Data missing | 1168 (20.9) |
| Previous treatment for IPF, n (%) | 1315 (23.6) |
| Pirfenidone | 1036 (18.6) |
| Corticosteroids | 231 (4.1) |
| Immunosuppressants | 117 (2.1) |
| Baseline IPF medication,a n (%) | 1793 (32.1) |
| Corticosteroids | 1329 (23.8) |
| Pirfenidone | 205 (3.7) |
| Immunosuppressants | 305 (5.5) |
| Cyclosporine | 181 (3.2) |
| Tacrolimus | 73 (1.3) |
| Azathioprine | 41 (0.7) |
| Nintedanib treatment during observation period | |
| Initial starting dose, n (%) | |
| 100 mg bid | 736 (13.2) |
| 150 mg bid | 4686 (84.0) |
| Other | 128 (2.3) |
| Data missing | 28 (0.5) |
| Dose changes, n (%) | |
| Change from 150 → 100 mg bid | 1644/4686 (35.1) |
| Change from 100 → 150 mg bid | 173/736 (23.5) |
| Time to first dose reduction, days | (n = 1773) |
| Mean (SD) | 141.9 (151.0) |
| Median | 83.0 |
bid twice daily, BMI body mass index, BSA body surface area, IPF idiopathic pulmonary fibrosis, FVC forced vital capacity, SD standard deviation
aIncluding drugs for treatment of acute exacerbation, regardless of other treatment use
The baseline characteristics of patients who completed 12 months of treatment (‘continued’ subgroup; n = 2509) and those who did not (‘discontinued’ subgroup; n = 2795) are shown in Table 2. Two hundred seventy-four patients who were observed for < 12 months and were still on treatment were excluded from this analysis. Body weight [mean (SD) 62.1 (12.1) vs. 56.8 (12.2) kg], BMI [23.7 (3.7) vs. 22.0 (4.0) kg/m2] and BSA [1.66 (0.18) vs. 1.58 (0.19) m2] in the ‘continued’ subgroup were higher than in the ‘discontinued’ subgroup. A higher proportion of patients with stage III or IV IPF were in the ‘discontinued’ subgroup than in the ‘continued’ subgroup (54.8% vs. 45.2%), whereas a higher proportion of patients with stage I or II IPF were in the ‘continued’ subgroup than in the ‘discontinued’ subgroup (57.5% vs. 42.5%). Patients with a higher stage (III or IV) disease were more likely to discontinue treatment than those with a lower stage (I or II) disease (odds ratio 1.64; 95% CI 1.43–1.89).
Table 2.
Patient demographic and clinical characteristics at baseline, and nintedanib treatment pattern in the ‘continued’ and ‘discontinued’ subgroups. All data are n (%) unless otherwise stated
| ‘Continued’ subgroup (n = 2509) | ‘Discontinued’ subgroup (n = 2795) | OR (95% CI) | |
|---|---|---|---|
| Age, years | |||
| Mean (SD) | 71.1 (7.9) | 72.5 (8.1) | |
| Median (range) | 72.0 (17–98) | 73.0 (20–96) | |
| < 75 years (N = 3202), n (%) | 1620 (50.6) | 1582 (49.4) | Ref |
| ≥ 75 years (N = 2102), n (%) | 889 (42.3) | 1213 (57.7) | 1.40 (1.25–1.56)a |
| Sex | |||
| Male (N = 4138) | 1976 (47.8) | 2162 (52.2) | Ref |
| Female (N = 1166) | 533 (45.7) | 633 (54.3) | 1.09 (0.95–1.24) |
| Body weight, kg | (n = 2386) | (n = 2646) | |
| Mean (SD) | 62.1 (12.1) | 56.8 (12.2) | |
| Median (range) | 62.0 (28.0–154.5) | 56.3 (26.0–102.3) | |
| BMI, kg/m2 | (n = 2379) | (n = 2626) | |
| Mean (SD) | 23.7 (3.7) | 22.0 (4.0) | |
| Median (range) | 23.5 (12.4–52.6) | 22.0 (10.8–42.0) | |
| BSA, m2 | (n = 2379) | (n = 2626) | |
| Mean (SD) | 1.66 (0.18) | 1.58 (0.19) | |
| Median (range) | 1.66 (0.99–2.55) | 1.59 (1.00–2.18) | |
| ≥ 1.58 m2 (N = 2948), n (%) | 1600 (54.3) | 1348 (45.7) | Ref |
| < 1.58 m2 (N = 2057), n (%) | 779 (37.9) | 1278 (62.1) | 1.95 (1.74–2.18)a |
| Smoking history, n (%) | |||
| Current or previous smoker (N = 3747) | 1849 (49.3) | 1898 (50.7) | 0.78 (0.68–0.88)a |
| Never smoked (N = 1288) | 555 (43.1) | 733 (56.9) | Ref |
| IPF severity stage, n (%) | |||
| I (N = 1002) | 585 (58.4) | 417 (41.6) | Ref |
| II (N = 207) | 110 (53.1) | 97 (46.9) | 1.24 (0.92–1.67) |
| III (N = 960) | 511 (53.2) | 449 (46.8) | 1.23 (1.03–1.47)a |
| IV (N = 1413) | 561 (39.7) | 852 (60.3) | 2.13 (1.81–2.51)a |
| Data missing/unknown (N = 485) | 246 (50.7) | 239 (49.3) | – |
| I or II (N = 1209) | 695 (57.5) | 514 (42.5) | Ref |
| III or IV (N = 2373) | 1072 (45.2) | 1301 (54.8) | 1.64 (1.43–1.89)a |
| FVC, ml, mean (SD) | (n = 2288) | (n = 2311) | |
| Mean (SD) | 2284.2 (707.1) | 1935.0 (678.2) | |
| FVC, % predicted | (n = 2101) | (n = 2110) | |
| Mean (SD) | 73.2 (25.9) | 65.1 (47.9) | |
| Median (range) | 71.8 (19.8–903.0) | 61.9 (14.7–1810.0) | |
| < 70% (N = 2359), n (%) | 970 (41.1) | 1389 (58.9) | 2.25 (1.98–2.54)a |
| ≥ 70% (N = 1852), n (%) | 1131 (61.1) | 721 (38.9) | Ref |
| Previous IPF treatment n (%) | |||
| Yes (N = 1263) | 551 (43.6) | 712 (56.4) | 1.22 (1.07–1.38)a |
| No (N = 4014) | 1946 (48.5) | 2068 (51.5) | Ref |
| Pirfenidone | |||
| Yes (N = 1000) | 439 (43.9) | 561 (56.1) | 1.19 (1.03–1.36)a |
| No (N = 4296) | 2067 (48.1) | 2229 (51.9) | Ref |
| Corticosteroids | |||
| Yes (N = 217) | 89 (41.0) | 128 (59.0) | 1.31 (0.99–1.72) |
| No (N = 5073) | 2414 (47.6) | 2659 (52.4) | Ref |
| Immunosuppressants | |||
| Yes (N = 115) | 47 (40.9) | 68 (59.1) | 1.31 (0.90–1.90) |
| No (N = 5184) | 2460 (47.5) | 2724 (52.5) | Ref |
| Baseline IPF medication,b n (%) | |||
| Yes (N = 1722) | 709 (41.2) | 1013 (58.8) | 1.33 (1.18–1.49)a |
| No (N = 3102) | 1493 (48.1) | 1609 (51.9) | Ref |
| Corticosteroids | |||
| Yes (N = 1271) | 514 (40.4) | 757 (59.6) | 1.36 (1.19–1.55)a |
| No (N = 3642) | 1748 (48.0) | 1894 (52.0) | Ref |
| Pirfenidone | |||
| Yes (N = 202) | 69 (34.2) | 133 (65.8) | 1.75 (1.30–2.35)a |
| No (N = 5038) | 2395 (47.5) | 2643 (52.5) | Ref |
| Immunosuppressants | |||
| Yes (N = 285) | 107 (37.5) | 178 (62.5) | 1.52 (1.19–1.94)a |
| No (N = 4902) | 2338 (47.7) | 2564 (52.3) | Ref |
| Cyclosporine | |||
| Yes (N = 175) | 57 (32.6) | 118 (67.4) | 1.89 (1.37–2.61)a |
| No (N = 5078) | 2426 (47.8) | 2652 (52.2) | Ref |
| Tacrolimus | |||
| Yes (N = 64) | 28 (43.8) | 36 (56.3) | 1.15 (0.70–1.89) |
| No (N = 5187) | 2451 (47.3) | 2736 (52.7) | Ref |
| Azathioprine | |||
| Yes (N = 38) | 20 (52.6) | 18 (47.4) | 0.81 (0.43–1.53) |
| No (N = 5260) | 2484 (47.2) | 2776 (52.8) | Ref |
| Initial dose, n (%) | |||
| 100 mg bid (N = 683) | 304 (44.5) | 379 (55.5) | 1.15 (0.98–1.36) |
| 150 mg bid (N = 4480) | 2154 (48.1) | 2326 (51.9) | Ref |
| Other (N = 124) | 51 (41.1) | 73 (58.9) | 1.33 (0.92–1.90) |
| Initial dose by BSA, n (%) | |||
| 100 mg bid | |||
| BSA < 1.58 m2 (N = 339) | 133 (39.2) | 206 (60.8) | 1.72 (1.26–2.36)a |
| BSA ≥ 1.58 m2 (N = 300) | 158 (52.7) | 142 (47.3) | Ref |
| 150 mg bid | |||
| BSA < 1.58 m2 (N = 1660) | 631 (38.0) | 1029 (62.0) | 1.97 (1.74–2.23)a |
| BSA ≥ 1.58 m2 (N = 2585) | 1414 (54.7) | 1171 (45.3) | Ref |
bid twice daily, BMI body mass index, BSA body surface area, CI confidence interval, FVC forced vital capacity, IPF idiopathic pulmonary fibrosis, OR odds ratio, Ref reference, SD standard deviation
aUpper 95% CI is < 1 or lower 95% CI is > 1
bIncluding drugs for treatment of acute exacerbation
Among patients with BSA ≥ 1.58 m2, the proportion of patients in the ‘continued’ subgroup was higher than in the ‘discontinued’ subgroup (54.3% vs. 45.7%). In contrast, among patients with BSA < 1.58 m2, the proportion of patients in the ‘continued’ subgroup was lower than in the ‘discontinued’ subgroup (37.9% vs. 62.1%), suggesting that patients with a lower BSA were more likely to discontinue treatment than those with a higher BSA (odds ratio 1.95; 95% CI 1.74–2.18).
Baseline mean FVC, as an absolute or as a percentage predicted value, was lower in the ‘discontinued’ than in the ‘continued’ subgroup. Furthermore, among patients with FVC ≥ 70% predicted, the proportion of patients in the ‘continued’ subgroup was higher than in the ‘discontinued’ subgroup (61.1% vs. 38.9%). In contrast, among patients with FVC < 70% predicted, the proportion of patients in the ‘continued’ subgroup was lower than in the ‘discontinued’ subgroup (41.1% vs. 58.9%), suggesting that patients with better disease control (FVC ≥ 70% predicted) were less likely to discontinue treatment than those with an FVC < 70% predicted (odds ratio 2.25; 95% CI 1.98–2.54).
Nintedanib Treatment
Overall, 5550 patients had baseline nintedanib dose information recorded; 4686 patients (84.0%) initiated nintedanib at a dose of 150 mg bid, 736 (13.2%) started at a dose of 100 mg bid, and 128 patients (2.3%) received another dose. During 12 months of follow-up, 1644 of the 4686 patients (35.1%) who started nintedanib at 150 mg bid had a dose reduction to 100 mg bid; the median time to dose reduction was 83 days (Table 1).
There was no difference in the nintedanib starting dose between patients in the ‘continued’ and ‘discontinued’ subgroups (Table 2). In patients with BSA < 1.58 m2, the proportion of patients in the ‘discontinued’ subgroup was higher than in the ‘continued’ subgroup at both the initial dose of 100 mg bid (60.8% vs. 39.2%) and 150 mg bid (62.0% vs. 38.0%), whereas in patients with BSA ≥ 1.58 m2, the proportion of patients in the ‘discontinued’ subgroup was lower than in the ‘continued’ subgroup at both the initial dose of 100 mg bid (47.3% vs. 52.7%) and 150 mg bid (45.3% vs. 54.7%). Patients with a lower BSA were more likely to discontinue treatment, at a dose of 100 mg bid (odds ratio 1.72; 95% CI 1.26–2.36) and 150 mg bid (odds ratio 1.97; 95% CI 1.74–2.23), than those with a higher BSA.
Adverse Events
A total of 4662 patients (83.6%) developed an AE, 1242 (22.3%) had a nintedanib dose reduction because of an AE, 2276 (40.8%) discontinued nintedanib because of an AE, and 1107 patients (19.8%) died as a result of an AE (Table 3). ADRs developed in 3767 patients (67.5%), 1196 (21.4%) required a dose reduction because of an ADR, 1356 (24.3%) discontinued nintedanib because of an ADR, and 140 patients (2.5%) died as a result of an ADR (Table 3).
Table 3.
Summary of incidence of AEs and ADRs
| n (%) | All patients (N = 5578) |
|---|---|
| Any AEs | 4662 (83.6) |
| Any ADRs | 3767 (67.5) |
| Serious AEs | 2246 (40.3) |
| Serious ADRs | 597 (10.7) |
| AEs leading to dose reduction of nintedanib | 1242 (22.3) |
| ADRs leading to dose reduction of nintedanib | 1196 (21.4) |
| AEs leading to discontinuation of nintedanib | 2276 (40.8) |
| ADRs leading to discontinuation of nintedanib | 1356 (24.3) |
| AEs leading to death | 1107 (19.8) |
| ADRs leading to death | 140 (2.5) |
AE adverse event, ADR adverse drug reaction
The most common AEs that occurred in ≥ 4% of patients in either subgroups were diarrhoea, hepatic function abnormal, liver disorder, decreased appetite, IPF (including disease progression), nausea and cough (Table 4). Similarly, the most common ADRs that occurred in ≥ 2% of patients in either subgroup were diarrhoea, hepatic function abnormal, liver disorder, decreased appetite, nausea, vomiting and IPF (Table 4). Decreased appetite and IPF were more frequent AEs and ADRs in the ‘discontinued’ than in the ‘continued’ subgroup. The frequency of common ADRs, including diarrhoea and hepatic function abnormal, decreased after a nintedanib dose reduction (Table 5).
Table 4.
Major adverse events in the ‘continued’ and ‘discontinued’ subgroups
| MedDRA PT, n (%) | ‘Continued’ subgroup (n = 2509) | ‘Discontinued’ subgroup (n = 2795) |
|---|---|---|
| Major AEs occurring in ≥ 4% of patients | ||
| Diarrhoea | 1051 (41.9) | 659 (23.6) |
| Hepatic function abnormal | 374 (14.9) | 420 (15.0) |
| Liver disorder | 192 (7.7) | 232 (8.3) |
| Decreased appetite | 170 (6.8) | 354 (12.7) |
| IPF | 151 (6.0) | 601 (21.5) |
| Nausea | 149 (5.9) | 164 (5.9) |
| Cough | 117 (4.7) | 59 (2.1) |
| Major ADRs occurring in ≥ 2% of patients | ||
| Diarrhoea | 1038 (41.4) | 648 (23.2) |
| Hepatic function abnormal | 351 (14.0) | 400 (14.3) |
| Liver disorder | 182 (7.3) | 228 (8.2) |
| Decreased appetite | 150 (6.0) | 327 (11.7) |
| Nausea | 138 (5.5) | 156 (5.6) |
| Vomiting | 39 (1.6) | 58 (2.1) |
| IPF | 29 (1.2) | 93 (3.3) |
AE adverse event, ADR adverse drug reaction, IPF idiopathic pulmonary fibrosis, MedDRA Medical Dictionary for Regulatory Activities, PT preferred term
Table 5.
Incidence of adverse drug reactions before and after nintedanib dose reduction in the safety analysis set (N = 1595)
| MedDRA PT | ADR severity | ADR incidence, n (%) | p-valuea | |
|---|---|---|---|---|
| Pre-dose reduction | Post-dose reduction | |||
| Diarrhoea | Total | 592 (37.1) | 301 (18.9) | < 0.001 |
| Mild | 401 (25.1) | 219 (13.7) | < 0.001 | |
| Moderate | 214 (13.4) | 89 (5.6) | < 0.001 | |
| Severe | 8 (0.5) | 6 (0.4) | 0.774 | |
| Missing | 5 (0.3) | 1 (0.1) | 0.125 | |
| Hepatic function abnormal | Total | 363 (22.8) | 119 (7.5) | < 0.001 |
| Mild | 287 (18.0) | 96 (6.0) | < 0.001 | |
| Moderate | 73 (4.6) | 21 (1.3) | < 0.001 | |
| Severe | 5 (0.3) | 2 (0.1) | 0.453 | |
| Missing | 1 (0.1) | 0 | – | |
| Liver disorder | Total | 203 (12.7) | 71 (4.5) | < 0.001 |
| Mild | 160 (10.0) | 61 (3.8) | < 0.001 | |
| Moderate | 43 (2.7) | 11 (0.7) | < 0.001 | |
| Severe | 1 (0.1) | 0 | – | |
| Missing | 0 | 0 | – | |
| Decreased appetite | Total | 170 (10.7) | 104 (6.5) | < 0.001 |
| Mild | 90 (5.6) | 51 (3.2) | < 0.001 | |
| Moderate | 74 (4.6) | 51 (3.2) | 0.035 | |
| Severe | 5 (0.3) | 4 (0.3) | 1.000 | |
| Missing | 4 (0.3) | 0 | – | |
| Nausea | Total | 119 (7.5) | 54 (3.4) | < 0.001 |
| Mild | 90 (5.6) | 38 (2.4) | < 0.001 | |
| Moderate | 28 (1.8) | 17 (1.1) | 0.117 | |
| Severe | 1 (0.1) | 0 | – | |
| Missing | 4 (0.3) | 0 | – | |
| Vomiting | Total | 36 (2.3) | 20 (1.3) | 0.026 |
| Mild | 26 (1.6) | 11 (0.7) | 0.011 | |
| Moderate | 9 (0.6) | 8 (0.5) | 1.000 | |
| Severe | 3 (0.2) | 2 (0.1) | 1.000 | |
| Missing | 0 | 0 | – | |
| IPF | Total | 20 (1.3) | 14 (0.9) | 0.377 |
| Mild | 0 | 0 | – | |
| Moderate | 1 (0.1) | 3 (0.2) | 0.500 | |
| Severe | 2 (0.1) | 9 (0.6) | 0.065 | |
| Missing | 17 (1.1) | 2 (0.1) | < 0.001 | |
ADR adverse drug reaction, IPF idiopathic pulmonary fibrosis, MedDRA Medical Dictionary for Regulatory Activities, PT preferred term
aMcNemar's test for incidence pre- vs. post-dose reduction
There was no apparent increase in the frequency of any AE, diarrhoea or hepatic function disorder in patients who took corticosteroids ± immunosuppressants at baseline, although these patients had a small but statistically significant increase in infections and infestations (corticosteroids, odds ratio 1.28; 95% CI 1.06–1.54; corticosteroids + immunosuppressants, odds ratio 1.53; 95% CI 1.09–2.14; see Figure S1 in the electronic supplementary material). However, the risk of acute exacerbations (recorded as an AE or ADR) was significantly increased in patients who were taking corticosteroids at baseline (see Table S1 in the electronic supplementary material). In these patients (n = 1329), acute exacerbations were reported in 14.7% of patients (n = 196) as an AE and in 1.7% (n = 23) as an ADR. In contrast, in patients not taking corticosteroids at baseline (n = 3845), acute exacerbations were reported in 4.8% of patients (n = 185) as an AE and in 0.6% (n = 24) as an ADR.
Discontinuation
Overall, 1292 patients (23.2%) and 2795 patients (50.1%) discontinued nintedanib within 3 and 12 months of initiation, respectively (Table 6). Among patients who discontinued nintedanib within 12 months, 448 (16.0%) and 1442 (51.6%) discontinued because of an AE and ADR, respectively, and 221 (7.9%) because of insufficient effectiveness (Table 6).
Table 6.
Reasons for, and timing of, early nintedanib discontinuation
| Baseline total (N = 5578) | Patient status at each time point, n (%) | |
|---|---|---|
| 3 months | 12 months | |
| Treatment continuation | ||
| Yes | 4286 (76.8) | 2783 (49.9) |
| No | 1292 (23.2) | 2795 (50.1) |
| Reason for discontinuation | (n = 1292) | (n = 2795) |
| ADR | 730 (56.5) | 1442 (51.6) |
| AE | 206 (15.9) | 448 (16.0) |
| Insufficient effectiveness | 82 (6.3) | 221 (7.9) |
| Lost to follow-up | 68 (5.3) | 210(7.5) |
| Clinical improvement | 3 (0.2) | 14 (0.5) |
| Other | 198(15.3) | 450 (16.1) |
| Data missing | 5 (0.4) | 10 (0.4) |
| ADRs as reasons for discontinuation (≥ 2%, MedDRA PT) | (n = 730) | (n = 1442) |
| Hepatic function abnormal | 137 (18.8) | 177 (12.3) |
| Decreased appetite | 75 (10.3) | 141 (9.8) |
| Diarrhoea | 76 (10.4) | 190(13.2) |
| Liver disorder | 65 (8.9) | 84 (5.8) |
| Nausea | 49 (6.7) | 63 (4.4) |
| IPF | 27 (3.7) | 38 (2.6) |
| Vomiting | 22 (3.0) | 28 (1.9) |
AE adverse event, ADR adverse drug reaction, IPF idiopathic pulmonary fibrosis, MedDRA Medical Dictionary for Regulatory Activities, PT preferred term
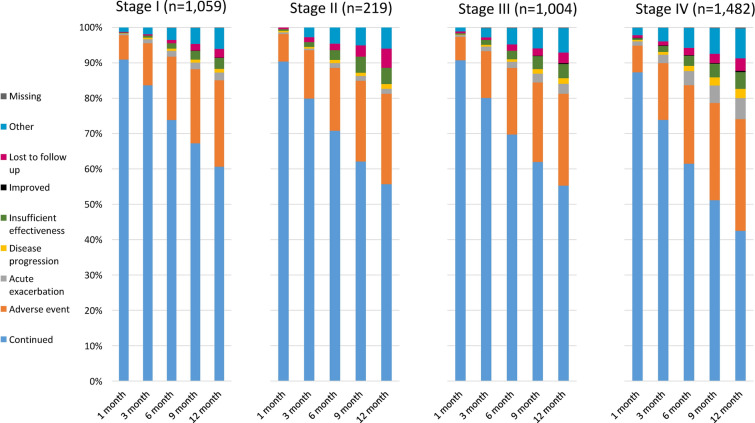
The reasons for discontinuation of nintedanib treatment were generally similar across different IPF severity stages (Fig. 1). The proportions of patients who discontinued nintedanib because of an AE or an acute exacerbation tended to be higher among patients with stage IV IPF than those with stage I–III disease.
Fig. 1.
Treatment continuation and discontinuations rates over time, according to idiopathic pulmonary fibrosis severity stage
The ADRs most commonly causing nintedanib treatment discontinuation within 3 months (≥ 2% by MedDRA PT) were hepatic function abnormal (18.8%), decreased appetite (10.3%), diarrhoea (10.4%), liver disorder (8.9%), nausea (6.7%), IPF (3.7%) and vomiting (3.0%) (Table 6). The ADRs most commonly causing treatment discontinuation within 12 months (≥ 2% by MedDRA PT) were diarrhoea (13.2%), hepatic function abnormal (12.3%), decreased appetite (9.8%), liver disorder (5.8%), nausea (4.4%) and IPF, i.e. worsening of primary disease (2.6%; Table 6).
Liver dysfunction, including two MedDRA PTs (hepatic function abnormal and liver disorders), was the most common type of ADR causing discontinuation within the first 3 months of nintedanib treatment, with 137/730 patients discontinuing within 3 months because of hepatic function abnormal and 65/730 because of liver disorders. Most patients with diarrhoea were able to continue taking nintedanib; diarrhoea was the cause of 13.2% of ADR-related discontinuations at 12 months.
Hepatic function disorder developed in 635/2147 (29.6%) patients with BSA < 1.58 m2 and 742/3114 (23.8%) with BSA ≥ 1.58 m2; the risk of developing hepatic function disorder was significantly higher in patients with lower BSA (odds ratio for < 1.58 m2 vs. ≥ 1.58: 1.34; 95% CI 1.19–1.52).
According to the multivariate analysis, patients aged ≥ 75 years, males, those with BSA of < 1.58 m2, those with stage III/IV IPF and those with FVC < 70% predicted at baseline were more likely to discontinue nintedanib treatment within 3 and 12 months (Table 7). Patients who developed hepatic function disorder after starting nintedanib were more likely to discontinue treatment within 3 months than those without hepatic function disorder, while patients who developed diarrhoea were less likely to discontinue treatment within 12 months than those without diarrhoea (Table 7).
Table 7.
Multivariate analysis of factors associated with nintedanib discontinuation within 3 and 12 months
| Discontinuation within 3 months | Discontinuation within 12 months | |||
|---|---|---|---|---|
| OR (95% CI) | p-valuea | OR (95% CI) | p-valuea | |
| Age, ≥ 75 vs. < 75 years | 1.38 (1.15–1.66) | < 0.001 | 1.29 (1.10–1.52) | 0.002 |
| Sex, female vs. male | 0.72 (0.57–0.92) | 0.010 | 0.69 (0.56–0.86) | 0.001 |
| BSA, < 1.58 vs. ≥ 1.58 m2 | 1.78 (1.45–2.20) | < 0.001 | 2.01 (1.67–2.43) | < 0.001 |
| IPF severity stage, III + IV vs. I + II | 1.29 (1.06–1.57) | 0.012 | 1.32 (1.12–1.55) | < 0.001 |
| FVC % predicted, < 70 vs. ≥ 70% | 1.75 (1.44–2.12) | < 0.001 | 2.06 (1.76–2.41) | < 0.001 |
| Occurrence of ADR (diarrhoea), yes vs. no | 0.53 (0.41–0.70) | < 0.001 | 0.43 (0.36–0.50) | < 0.001 |
| Occurrence of ADR (hepatic function disorder), yes vs. no | 1.65 (1.36–2.01) | < 0.001 | 1.09 (0.92–1.29) | 0.313 |
ADR adverse drug reaction, BSA body surface area, CI confidence interval, FVC forced vital capacity, IPF idiopathic pulmonary fibrosis, OR odds ratio
aChi-squared test
Effectiveness
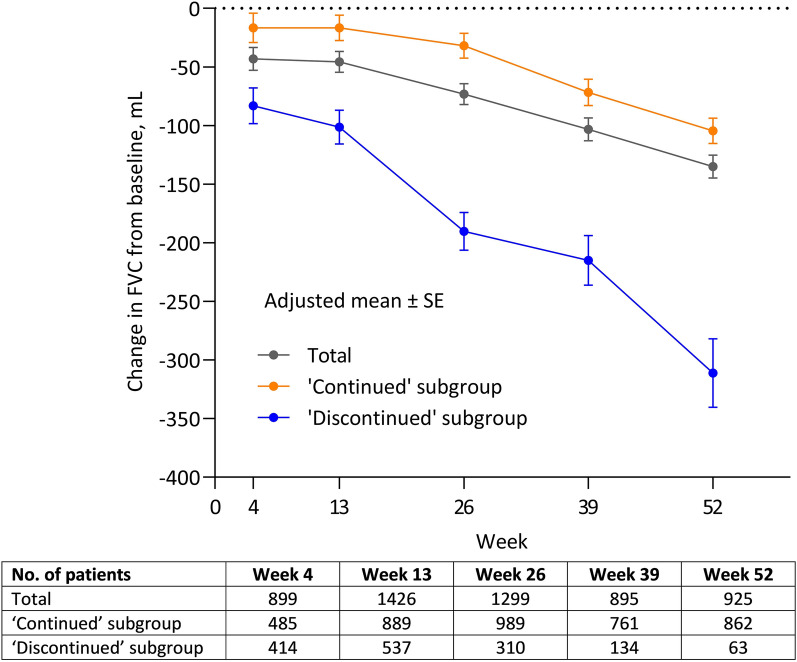
At 12 months, the adjusted mean ± SE change in FVC from baseline was – 135.0 ± 9.7 ml or − 7.2 ± 4.9% predicted. The adjusted mean ± SE change in FVC from baseline was – 311.2 ± 29.2 ml and – 104.4 ± 10.9 ml in the ‘discontinued’ and ‘continued’ subgroups, respectively (Fig. 2).
Fig. 2.
Change from baseline in forced vital capacity (FVC) during nintedanib treatment in the ‘continued’ and ‘discontinued’ subgroups. SE, standard error
Discussion
This prospective analysis of the largest patient population to date shows that approximately 50% of Japanese patients with IPF discontinue nintedanib treatment within 12 months and that patients with stage III or IV IPF and FVC < 70% predicted at baseline are at increased risk of early treatment discontinuation.
These results confirm the findings of previous smaller studies in Japanese patients, which reported nintedanib discontinuation rates of 47–51% [15, 16]. In one of these previous studies, the only factor significantly associated with early discontinuation of nintedanib was poor performance status at baseline [15], whereas we found that older age, male sex, lower BSA and worse lung function (severe IPF and lower percentage predicted FVC) were associated with an increased risk of treatment discontinuation. A previous retrospective study that included patients aged ≥ 75 years also suggested that low BMI and FVC were risk factors for early discontinuation [21].
Based on the current study, as well as previous real-world studies, it appears that Asian patients (including those in Japan or Korea) have higher rates of nintedanib discontinuation (47–53%) [15–17] than patients in the USA or Europe (11–26%) [10–14]. The reason for this difference is not clear, but previous data indicate that patients with a smaller BSA are at higher risk of hepatotoxicity, dose reduction or discontinuation [20, 22], suggesting that the smaller physique of Asian individuals places them at higher risk of developing AEs. Indeed, our study found that the rate of nintedanib discontinuation within 12 months was higher in the patients with lower BSA (< 1.58 m2) than in the patients with higher BSA (≥ 1.58 m2); thus, we consider that careful attention should be paid to high nintedanib exposure in patients with a smaller physique.
While we did not make a direct comparison of discontinuation rates by starting dose in our study, we noted there was no difference in the nintedanib starting dose between patients who discontinued treatment within 12 months and those who continued treatment beyond 12 months. It should be noted that, regardless of the starting dose, as mentioned above, the discontinuation rates were higher among patients with a BSA of < 1.58 m2 than among patients with a BSA ≥ 1.58 m2, again suggesting that if an AE is observed in patients with a low BSA, in addition to symptomatic treatment, the nintedanib dose should be reduced or interrupted until the patient recovers to a treatable condition. Our findings differ from those of a previous study, which reported that using a low starting dose of nintedanib may reduce the incidence of severe AEs and limit the rate of discontinuation [23].
Similar to previous studies [8, 15], the most common ADRs leading to treatment discontinuation within 12 months in our cohort of Japanese patients receiving nintedanib included diarrhoea and abnormal hepatic function. Diarrhoea was the most common reason for discontinuation of nintedanib within 12 months (13.2%), while liver dysfunction (hepatic function abnormal and hepatic disorder) was the most common reason for early discontinuation of nintedanib (i.e. within 3 months), with 18.8% and 8.9% of patients discontinuing nintedanib within 3 months because of hepatic function abnormal and liver disorders, respectively. Interestingly, diarrhoea appeared to have a lower impact on nintedanib treatment discontinuations in our study than hepatic function disorder, as patients who developed hepatic function disorder were more likely to discontinue treatment within 3 months than those without hepatic function disorder, while patients with diarrhoea were less likely to discontinue treatment within 12 months than those without diarrhoea. This may be because treatment discontinuation for hepatic function disorder occurred before the onset of severe diarrhoea in many patients. In addition, many patients with diarrhoea may have had dose reduction or temporary treatment interruption rather than discontinuation, thereby reducing the odds of treatment discontinuation in these patients.
These findings are consistent with data from the INPULSIS-ON open-label extension study [24]. In the Asian subgroup of that study, diarrhoea was the most common AE in patients who continued nintedanib treatment, but few patients who continued to take nintedanib during the open-label extension subsequently discontinued therapy because of diarrhoea [24]. Post-marketing data from the US reported a diarrhoea incidence rate of 1053 events per 1000 patient-year during nintedanib treatment for IPF, lower than the rate reported in INPULSIS (1331 per 1000 patient-year) and similar to the rate in the general US population (980 per 1000 patient-year) [25].
The risk of liver enzyme elevations is positively correlated with plasma concentrations of nintedanib in patients with IPF and those with other fibrosing interstitial lung diseases [26]. Therefore, reducing drug exposure through dose reduction or treatment interruption is recommended in the prescribing information for nintedanib [27]. Indeed, we found that nintedanib dose was a better predictor of liver dysfunction than diarrhoea, with the incidence of hepatic function abnormal decreasing by 67% (from 22.8 to 7.5%) and liver disorder decreasing by 65% (from 12.7 to 4.5%) after nintedanib dose reduction. In fact, with the exception of disease progression, the frequency and severity of ADRs decreased markedly once the dose of nintedanib was reduced, supporting current recommendations for the management of AEs with this drug. Overall, the results of the exposure–efficacy and exposure–safety analyses support a nintedanib starting dose of 150 mg bid in patients with fibrotic interstitial lung diseases [26, 28] and indicate consideration of dose reduction in the event of liver dysfunction.
Since patients who discontinue nintedanib treatment experience a greater decline in clinical status and lung function compared with those who continue treatment [15], all steps should be taken to manage AEs through dose adjustment or treatment interruption rather than permanent discontinuation. Moreover, advanced IPF disease stage and low baseline FVC were identified as risk factors for early discontinuation, which indicates that nintedanib treatment should be initiated early in the disease course to maximise clinical benefits and treatment persistence.
The combination of corticosteroids with nintedanib has been reported to reduce the risk of diarrhoea but increase the risk of infections and infestations, which may lead to an acute exacerbation [29]. In our study, we confirmed that the baseline use of corticosteroids and/or immunosuppressants did not increase the risk of any AE, diarrhoea or hepatic function disorder with nintedanib. However, the baseline use of immunosuppressants (with or without corticosteroids) was associated with a small increase in the risk of infections and infestations, and baseline corticosteroid therapy was associated with an increased risk of acute exacerbation of IPF compared with no corticosteroid therapy.
In this study, patients who continued nintedanib for 12 months had a significantly smaller mean decline in FVC compared with those who discontinued nintedanib. While this result may be attributed in part to the higher baseline FVC among the patients in the ‘continued’ subgroup (compared with both the ‘discontinued’ subgroup and the overall cohort), the same trend showing less marked FVC decline in patients who continued versus discontinued nintedanib was also reported in a previous Japanese real-world study, in which baseline FVC was similar between the two patient groups [15]. However, in the Japanese study, the size of the decline in FVC in continued versus discontinued groups (median change: – 10 vs. − 165 ml/year, respectively) [15] was smaller than observed in the present study (mean change: – 104.4 vs. – 311.2 ml/year, respectively).
One of the major strengths of our study is that it includes the largest patient cohort to date—we were able to identify only one other observational study with nintedanib that included > 1000 patients, which was conducted in the US [25]. Other strengths include the prospective collection of data, the external validity and the overall generalisability of the findings from this real-world population of patients.
The limitations of the study include the lack of a control group and incomplete data collection for many patients. In addition, since the data of AEs are reported at the discretion of the researcher, we assessed only those AEs that were recorded by the physician and therefore may have failed to collect some AE data.
Conclusions
Our study demonstrates that approximately 50% of Japanese patients receiving nintedanib for IPF discontinue treatment during the first year of treatment, with > 50% of discontinuations caused by ADRs, including liver dysfunction and diarrhoea. Most ADRs respond to dose reduction, which should be attempted first before discontinuing nintedanib to prevent deterioration of clinical status and lung function. Because patients with worse lung function at baseline are at increased risk of early treatment discontinuation, nintedanib treatment should be initiated early in the IPF disease course.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
Sponsorship for this study, the journal Rapid Service Fee and the Open Access Fee were funded by Boehringer Ingelheim.
Medical Writing, Editorial and Other Assistance
The authors thank Catherine Rees on behalf of Springer Healthcare Communications, who wrote the outline of this article and Sarah Greig, PhD, of Springer Healthcare Communications, who wrote the first draft. Support for this assistance was funded by Boehringer Ingelheim.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Author Contributions
All authors contributed to the study design, data interpretation, and manuscript preparation. Kaori Ochiai analysed the data. All authors read and approved the final manuscript as submitted, and agree to be accountable for all aspects of the work.
Disclosures
Takashi Ogura reports consulting fees from Boehringer Ingelheim, Bristol-Myers Squibb and Taiho Pharmaceutical Co.; and lecture fees or honoraria from Boehringer Ingelheim, Eisai Inc., Kyorin Pharmaceutical Co., Ltd., Shionogi and Co., Ltd., and Teijin Pharma. Yoshikazu Inoue reports grants from the Japanese Ministry of Health, Labour, and Welfare, and from the Japan Agency for Medical Research and Development; lecture fees and consulting fees from Boehringer Ingelheim; consulting fees from Taiho Pharmaceutical Co. and Roche; and lecture fees from Shionogi, Kyorin and GSK. Arata Azuma reports consulting fees from Toray Co., Ltd.; lecture fees and participation on an advisory board from Boehringer Ingelheim and Taiho Pharmaceutical Co.; and support for travel from Boehringer Ingelheim. Sakae Homma reports consulting fees from Boehringer Ingelheim. Yasuhiro Kondoh reports consulting fees from Boehringer Ingelheim, Chugai Pharmaceutical Co., Ltd., Healios K.K., Janssen Pharmaceutical K.K., Shionogi and Co., Ltd. and Taiho Pharmaceutical Co.; payments or honoraria from Boehringer Ingelheim, Bristol-Myers Squibb, Eisai Inc., Janssen Pharmaceutical K.K., Kyorin Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma, Novartis Pharma K.K., Shionogi and Co., Ltd. and Teijin Pharma. Katsumi Tanaka is an employee of Nippon Boehringer Ingelheim Co., Ltd. Kaori Ochiai is an employee of EPS Corporation and is in charge of analysis work on behalf of Nippon Boehringer Ingelheim Co., Ltd. Yukihiko Sugiyama reports participation on a data safety advisory board from Boehringer Ingelheim. Toshihiro Nukiwa reports consulting fees from Boehringer Ingelheim and payments or honoraria from Grifols.
Compliance with Ethics Guidelines
This post-marketing surveillance study was conducted under the direction of the Ministry of Health, Labour, and Welfare (MHLW) and the protocol was approved by the MHLW before study initiation. Participating institutes were contracted by Boehringer Ingelheim. As per the Japanese Pharmaceutical and Medical Device Act, the study was conducted in accordance with Good Post-marketing Study Practice (GPSP) guidance. Further, as per GPSP guidance, the study was exempt from protocol review and approval by ethics committees of the participating institutions and exempt from the requirement for the collection of informed consent from patients, unless such procedures were specifically required by the participating institutions.
Data Availability
To ensure independent interpretation of clinical study results and enable authors to fulfil their role and obligations under the ICMJE criteria, Boehringer Ingelheim grants all external authors access to relevant clinical study data. In adherence with the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data, scientific and medical researchers can request access to clinical study data after publication of the primary manuscript in a peer-reviewed journal, regulatory activities are complete and other criteria are met. Researchers should use the https://vivli.org/ link to request access to study data and visit https://www.mystudywindow.com/msw/datasharing for further information.
Contributor Information
Takashi Ogura, Email: takaoguogu@gmail.com.
Yoshikazu Inoue, Email: giichiyi@me.com.
Arata Azuma, Email: azuma_arata@yahoo.co.jp.
Sakae Homma, Email: sahomma@med.toho-u.ac.jp.
Yasuhiro Kondoh, Email: konyasu2003@yahoo.co.jp.
Katsumi Tanaka, Email: katsumi.tanaka@boehringer-ingelheim.com.
Kaori Ochiai, Email: kaori.ochiai.ext@boehringer-ingelheim.com.
Yukihiko Sugiyama, Email: sugiyuki14@gmail.com.
Toshihiro Nukiwa, Email: toshinkw47@gmail.com.
References
- 1.Kondoh Y, Suda T, Hongo Y, et al. Prevalence of idiopathic pulmonary fibrosis in Japan based on a claims database analysis. Respir Res. 2022;23:24. doi: 10.1186/s12931-022-01938-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Natsuizaka M, Chiba H, Kuronuma K, et al. Epidemiologic survey of Japanese patients with idiopathic pulmonary fibrosis and investigation of ethnic differences. Am J Respir Crit Care Med. 2014;190:773–779. doi: 10.1164/rccm.201403-0566OC. [DOI] [PubMed] [Google Scholar]
- 3.Homma S, Bando M, Azuma A, et al. Japanese guideline for the treatment of idiopathic pulmonary fibrosis. Respir Investig. 2018;56:268–291. doi: 10.1016/j.resinv.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198:e44–e68. [DOI] [PubMed]
- 5.Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 6.Richeldi L, Cottin V, du Bois RM, et al. Nintedanib in patients with idiopathic pulmonary fibrosis: combined evidence from the TOMORROW and INPULSIS® trials. Respir Med. 2016;113:74–79. doi: 10.1016/j.rmed.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Crestani B, Huggins JT, Kaye M, et al. Long-term safety and tolerability of nintedanib in patients with idiopathic pulmonary fibrosis: results from the open-label extension study, INPULSIS-ON. Lancet Respir Med. 2019;7:60–68. doi: 10.1016/S2213-2600(18)30339-4. [DOI] [PubMed] [Google Scholar]
- 8.Azuma A, Taniguchi H, Inoue Y, et al. Nintedanib in Japanese patients with idiopathic pulmonary fibrosis: a subgroup analysis of the INPULSIS® randomized trials. Respirology. 2017;22:750–757. doi: 10.1111/resp.12960. [DOI] [PubMed] [Google Scholar]
- 9.Deaton A, Cartwright N. Understanding and misunderstanding randomized controlled trials. Soc Sci Med. 2018;210:2–21. doi: 10.1016/j.socscimed.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonella F, Kreuter M, Hagmeyer L, et al. Insights from the German compassionate use program of nintedanib for the treatment of idiopathic pulmonary fibrosis. Respiration. 2016;92:98–106. doi: 10.1159/000448288. [DOI] [PubMed] [Google Scholar]
- 11.Galli JA, Pandya A, Vega-Olivo M, Dass C, Zhao H, Criner GJ. Pirfenidone and nintedanib for pulmonary fibrosis in clinical practice: tolerability and adverse drug reactions. Respirology. 2017;22:1171–1178. doi: 10.1111/resp.13024. [DOI] [PubMed] [Google Scholar]
- 12.Toellner H, Hughes G, Beswick W, et al. Early clinical experiences with nintedanib in three UK tertiary interstitial lung disease centres. Clin Transl Med. 2017;6:41. doi: 10.1186/s40169-017-0172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tzouvelekis A, Karampitsakos T, Kontou M, et al. Safety and efficacy of nintedanib in idiopathic pulmonary fibrosis: a real-life observational study in Greece. Pulm Pharmacol Ther. 2018;49:61–66. doi: 10.1016/j.pupt.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Antoniou K, Markopoulou K, Tzouvelekis A, et al. Efficacy and safety of nintedanib in a Greek multicentre idiopathic pulmonary fibrosis registry: a retrospective, observational, cohort study. ERJ Open Res. 2020;6:00172–2019. doi: 10.1183/23120541.00172-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato M, Sasaki S, Tateyama M, et al. Clinical significance of continuable treatment with nintedanib over 12 months for idiopathic pulmonary fibrosis in a real-world setting. Drug Des Devel Ther. 2021;15:223–230. doi: 10.2147/DDDT.S284819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobashi M, Tanaka H, Taima K, et al. The efficacy of nintedanib in 158 patients with idiopathic pulmonary fibrosis in real-world settings: a multicenter retrospective study. SAGE Open Med. 2021;9:20503121211023357. doi: 10.1177/20503121211023357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon HY, Park S, Kim DS, Song JW. Efficacy and safety of nintedanib in advanced idiopathic pulmonary fibrosis. Respir Res. 2018;19:203. doi: 10.1186/s12931-018-0907-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Homma S, Sugino K, Sakamoto S. Usefulness of a disease severity staging classification system for IPF in Japan: 20 years of experience from empirical evidence to randomized control trial enrollment. Respir Investig. 2015;53:7–12. doi: 10.1016/j.resinv.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Lancaster L, Crestani B, Hernandez P, et al. Safety and survival data in patients with idiopathic pulmonary fibrosis treated with nintedanib: pooled data from six clinical trials. BMJ Open Respir Res. 2019;6:e000397. doi: 10.1136/bmjresp-2018-000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikeda S, Sekine A, Baba T, et al. Low body surface area predicts hepatotoxicity of nintedanib in patients with idiopathic pulmonary fibrosis. Sci Rep. 2017;7:10811. doi: 10.1038/s41598-017-11321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uchida Y, Ikeda S, Sekine A, et al. Tolerability and safety of nintedanib in elderly patients with idiopathic pulmonary fibrosis. Respir Investig. 2021;59:99–105. doi: 10.1016/j.resinv.2020.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Toi Y, Kimura Y, Domeki Y, et al. Association of low body surface area with dose reduction and/or discontinuation of nintedanib in patients with idiopathic pulmonary fibrosis: a pilot study. Sarcoidosis Vasc Diffuse Lung Dis. 2019;36:74–78. doi: 10.36141/svdld.v36i1.7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikeda S, Sekine A, Baba T, et al. Low starting-dosage of nintedanib for the reduction of early termination. Respir Investig. 2019;57:282–285. doi: 10.1016/j.resinv.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Song JW, Ogura T, Inoue Y, et al. Long-term treatment with nintedanib in Asian patients with idiopathic pulmonary fibrosis: results from INPULSIS®-ON. Respirology. 2020;25:410–416. doi: 10.1111/resp.13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noth I, Oelberg D, Kaul M, Conoscenti CS, Raghu G. Safety and tolerability of nintedanib in patients with idiopathic pulmonary fibrosis in the USA. Eur Respir J. 2018;52:1702106. doi: 10.1183/13993003.02106-2017. [DOI] [PubMed] [Google Scholar]
- 26.Schmid U, Weber B, Sarr C, Freiwald M. Exposure-safety analyses of nintedanib in patients with chronic fibrosing interstitial lung disease. BMC Pulm Med. 2021;21:244. doi: 10.1186/s12890-021-01598-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bendstrup E, Wuyts W, Alfaro T, et al. Nintedanib in idiopathic pulmonary fibrosis: practical management recommendations for potential adverse events. Respiration. 2019;97:173–184. doi: 10.1159/000495046. [DOI] [PubMed] [Google Scholar]
- 28.Schmid U, Weber B, Magnusson MO, Freiwald M. Exposure-efficacy analyses of nintedanib in patients with chronic fibrosing interstitial lung disease. Respir Med. 2021;180:106369. doi: 10.1016/j.rmed.2021.106369. [DOI] [PubMed] [Google Scholar]
- 29.Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. An International Working Group report. Am J Respir Crit Care Med. 2016;194:265–75. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
To ensure independent interpretation of clinical study results and enable authors to fulfil their role and obligations under the ICMJE criteria, Boehringer Ingelheim grants all external authors access to relevant clinical study data. In adherence with the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data, scientific and medical researchers can request access to clinical study data after publication of the primary manuscript in a peer-reviewed journal, regulatory activities are complete and other criteria are met. Researchers should use the https://vivli.org/ link to request access to study data and visit https://www.mystudywindow.com/msw/datasharing for further information.