Abstract
The cost-effectiveness of conventional population-based breast cancer screening strategies (e.g. mammography) has been found controversial, while evidence shows that genetic testing for early detection of pathogenic variants is cost-effective. We aimed to review the economic evaluations of breast cancer screening in China to provide an information summary for future research on this topic. We searched the literature to identify the economic evaluations that examined breast cancer screening and testing in China, supplemented by hand-searching the reference lists of the included studies. We finally included five studies satisfying our inclusion criteria. Four articles examined mammography while the rest investigated multigene testing. The existing breast cancer screening programmes were found to be cost-effective among urban Chinese women, but one study concluded that they might cause harm to women in rural areas. Contextual factors, such as data absence, urban–rural disparity, willingness-to-pay threshold, and model design, imposed barriers to cost-effectiveness analysis. Multigene testing was found to be cost-effective and has a promising population impact among all women with breast cancer in China. Future research should investigate the cost-effectiveness of screening and identifying breast cancer through precision medicine technologies, including genetic testing, genome sequencing, cascade testing, and the return of secondary findings.
Keywords: Economic evaluation, Breast cancer, Screening, Cost-effectiveness analysis, China, Precision medicine
Key Summary Points
| Why carry out this study? |
| The cost-effectiveness of conventional population-wide breast cancer screening programmes (e.g. mammography) is controversial, while that of genetic testing to identify pathogenic variants may improve the cost-effectiveness. We aimed to present an evidence summary to inform policymakers in low- or middle-income countries such as China on the cost-effectiveness of different breast cancer screening strategies. |
| What was learned from the study? |
| Through a review of economic evaluations of breast cancer screening strategies in China, we found that precision medicine technologies may potentially improve the cost-effectiveness of screening breast cancer among Chinese women. |
| Future research may investigate screening strategies through precision medicine technologies in China, including genetic testing, genome sequencing, cascade testing, and the return of secondary genomic findings. |
Introduction
In 2020, the estimated number of new breast cancer cases and deaths increased respectively to 2.3 million and 685,000 globally, ranking as the most diagnosed cancer (11.7% of all cancer cases) and the fifth leading cause of death (6.9% of all cancer deaths) among women in the world [1, 2]. The incidence and deaths are rising rapidly and disproportionately in low- and middle-income countries (LMICs), among which China ranked first in new cases and deaths worldwide, accounting for approximately 18.4% of total new cases and 17.1% of total deaths globally in 2020 [1, 3]. The high incidence and mortality rate have imposed a substantial burden on healthcare systems of countries with low resources [4].
Population-based breast cancer screening for early diagnosis and prognosis, such as mammography, is widely argued to reduce mortality and enhance patients’ health outcomes [5, 6]. Although such a screening programme has been broadly implemented in high-income countries and proved cost-effective [7], it was found to be not economically attractive in China [8–10] and some other LMICs such as India [11], Ghana [12], and Egypt [13]. Screening tools such as clinical breast examination and ultrasound are less costly, yet the evidence of their feasibility and economic impact in LMICs remains controversial [14]. An alternative innovative approach is screening through genetic testing. Genetic testing is an established diagnostic tool that identifies inherited variants associated with breast cancer among family members, which can facilitate family-focused management and support physicians in making accurate clinical suggestions [15, 16]. Emerging evidence indicates that genetic testing for identifying pathogenic variants is cost-effective compared to routine screening strategies in some high-income countries and has been recommended in their national guidelines for breast cancer management [17–19]. However, gene-based testing services and the health economics evidence on the associated health benefits given its high cost are lacking in low-resource settings [20, 21].
With growing incidence and considerable disease and economic burden, it becomes urgent to assess current breast cancer screening strategies regarding both costs and health outcomes. Evidence from China could representatively reflect the cost-effectiveness of breast cancer screening strategies in low-resource countries considering the highest incidence and mortality rate. Systematic reviews of such evidence may inform future economic evaluations on breast cancer screening, and therefore inform policymakers on screening strategies for breast cancer control and management in LMICs such as China. Therefore, we aim to conduct a review to provide an up-to-date description of the current literature on the cost-effectiveness of breast cancer screening strategies in China and the methodological approaches’ characteristics. We aim to address the following research questions: (1) What is known from the literature about the cost-effectiveness of breast cancer screening strategies in China and the key drivers of cost-effectiveness outcomes? (2) What are the methodological challenges to the evaluation of screening strategies? (3) What methodological considerations can we make for future economic evaluations of breast cancer screening strategies in China.
Methods
Search Strategy
We searched PubMed, MEDLINE, EMBASE, CNKI, and WanFang for economic evaluations that examined breast cancer screening interventions in China up to 10 July 2022. The search strategy combined the following terms: (1) “breast cancer”, “screening”, “prevention”, “preventive”, “strategy”, “testing”, “genetic”; (2) “economic evaluation”, “cost-effectiveness analysis”, “cost–benefit analysis”, “cost–utility analysis”, “health technology assessment”, “modelling” or “economic models”; and (3) “China” or “Chinese”. The search was supplemented by screening the reference lists of included articles for the identification of additional studies. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Eligibility Criteria
Studies eligible for inclusion were economic evaluations that assessed breast cancer screening strategies, including ultrasound screening, mammography screening, clinical breast examination, and genetic testing, compared with alternative strategies including the assessed interventions, no screening, clinical diagnosis on presentation of symptoms, or family history-based testing for adults with or without risk of developing breast cancer in China. We included studies that measured the monetary value and health outcomes of the assessed interventions and comparators from all stakeholder perspectives, including healthcare, societal, payer, and third-party perspectives. Studies that were not full-length articles (e.g. conference abstracts) or did not focus on the Chinese context were ineligible. Studies that did not report the details of the model structure and parameters and the cost-effectiveness outcomes were also excluded. As a result of the different healthcare systems in Hong Kong, articles that evaluated breast cancer screening among women in Hong Kong were not considered. We also screened the studies by their reporting quality according to the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist (2022 version) and excluded the studies with low quality [22]. The selection process included two rounds, first by titles and abstracts of each document and then by full text, cross-validated by two reviewers independently (JJ and SJ). A PRISMA flow diagram was created to illustrate the screening process. Patients or the public were not involved in the design, conduct, reporting, or dissemination plans of our research.
Data Extraction
Data extraction of included studies was performed by two reviewers (JJ and SJ) using a bespoke form developed in Microsoft Excel. The collected data included the year of publication, study aim, studied population and the number of participants, assessed intervention and comparator(s), study design, model structure and software used, perspective and time horizon, discount rate, outcome measure, limitations, and main findings. With a particular focus on characteristics of cost components, we extracted cost data, including inputs of direct medical costs; direct non-medical costs; indirect costs, cost data source, unit cost, total costs of baseline strategy, and incremental costs. The modelling approach adopted and cost estimation in each study were then analyzed to present the methodological challenges of economically assessing breast cancer screening in China.
Quality of Reporting
Quality assessment was performed by two authors (JJ and JS), with disagreements addressed by discussions with senior authors (LY and YG). In line with our goal of compiling the existing literature and bridging research gaps, a formal quality appraisal was not conducted. Instead, each included study was assessed for completeness of reporting using the CHEERS checklist [22]. There was no minimum threshold set for inclusion. As no scoring mechanism was outlined by CHEERS, we therefore used different colours representing “reported”, “partially reported”, and “not reported” by green, light green, and red, respectively.
Results
Search Results
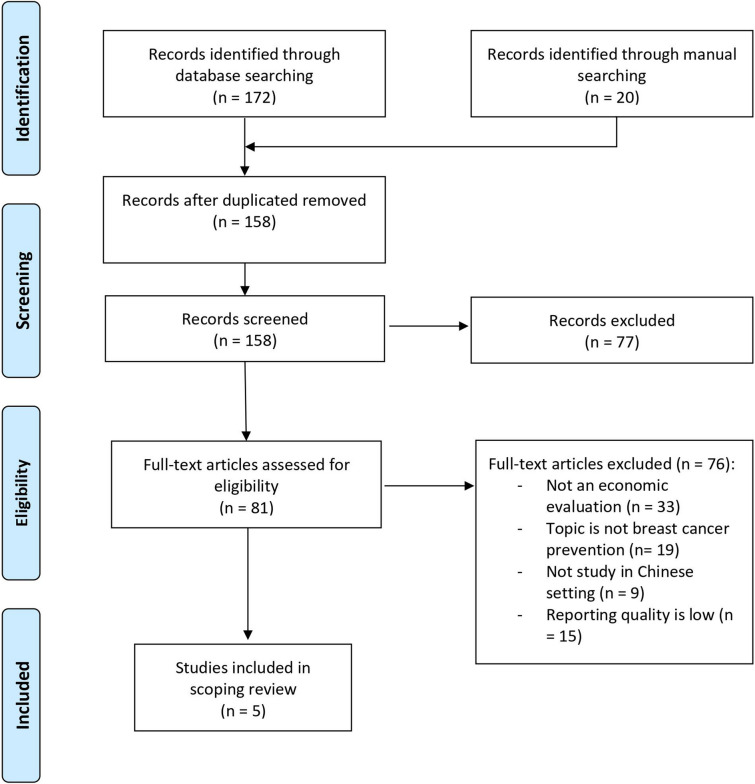
The process of article selection is illustrated in Fig. 1. The database search yielded 158 articles after the removal of duplicates. Eighty-one studies proceeded to full-text screening. Thirty-three studies were not economic evaluations. Nineteen articles were irrelevant to breast cancer screening interventions, nine studies were outside of the Chinese context, and 15 articles were excluded due to low quality of reporting. As such, we included five articles in our review.
Fig. 1.
Process of selection of eligible studies
Breast Cancer Screening Strategies and Cost-effectiveness
The major model characteristics, key study outcomes, and conclusions of the five included studies are presented in Tables 1 and 2 [23–27]. All eligible studies were published in recent years, between 2018 and 2022 [23–27]. Four articles assessed the cost-effectiveness of population-based breast cancer screening strategies, and the screening interval varied between 1 and 3 years [23, 24, 26, 27]. All articles focused on women aged between 35 and 70 years. Three studies [23, 26, 27] targeted urban settings and one study [24] focused on rural areas. Only one of five identified articles analyzed the cost-effectiveness of unselected multigene testing strategies (BRCA1/BRCA2/PALB2 testing, family history/clinical criteria-based BRCA testing) for all Chinese patients with breast cancer [25]. The main comparator in all studies was non-screening. Four studies incorporated additional comparators of varied screening intervals or different target populations [23, 25–27]. The sample size varied between 26,244 and 1,252,074, wherein two studies used 100,000 hypothetical cohorts in their model [26, 27].
Table 1.
Characteristics of included studies
| Study (year) | Study aim | Population | Strategies | Study design | Model structure and software | Perspective and time horizon | Discount rate | Outcome measure |
|---|---|---|---|---|---|---|---|---|
| Sun et al. (2018) [23] | To model the cost-effectiveness of a risk-based breast cancer screening programme in urban China, launched in 2012 | Urban Chinese women aged 40–69 years with a risk of developing breast cancer |
(1) Annual ultrasound screening for high-risk women aged 40–44 years, with further mammography screening for women with suspected results; annual ultrasound and mammography screening for high-risk women aged 45–69 years (2) No screening for women with low risk |
CUA | Natural history Markov model (TreeAge software) | Societal perspective; lifetime | 3% for both cost and outcome | Cost per QALY gained |
| Sun et al. (2019) [24] | To compare for the first time the cost-effectiveness of breast cancer screening using clinical breast examination coupled with ultrasound as a primary screening test compared to no screening in rural China | Rural Chinese women aged 35–64 years from rural areas in 31 provinces with no history of breast cancer in China |
(1) Clinical breast examination coupled with ultrasound, followed by undergoing mammography or clinical judgement if required every 3 years (2) No screening |
CUA | Natural history Markov model (TreeAge software) | Societal perspective; lifetime | 3% for both cost and outcome | Cost per QALY gained |
| Sun et al. (2022) [25] | To estimate cost-effectiveness and population impact of multigene testing for all Chinese patients with breast cancer | Patients with breast cancer in China |
(1) BRCA1/BRCA2/PALB2 testing for all patients with breast cancer (2) BRCA1/BRCA2 testing for patients with breast cancer fulfilling family history/clinical criteria (3) No genetic testing |
CUA | Patient level microsimulation model (TreeAge-Pro 2018) | Societal and payer perspectives; lifetime | 3% for both cost and outcome | Cost per QALY gained; cost per life year gained |
| Wang et al. (2021) [26] | To assess the cost-effectiveness of implementing a biennial mammography screening programme for Chinese women | Urban Chinese women aged 45–70 years |
(1) Mammography screening every 2 years (2) No screening (3) Alternative Scenarios including varying the screening interval (2 or 3 years), screening start age (from age of 40, 45 or 50 years) and stop age (65 or 70 years) |
CEA |
The Simulation Model on radiation Risk and breast cancer Screening (SiMRiSc) model (C++) |
Societal perspective; lifetime | 5% for both cost and outcome | Average cost-effectiveness ratio (ACER); cost per life year gained |
| Yang et al. (2018) [27] | To predict the feasibility of a community-based breast cancer screening strategy in China | Women aged 35–69 years in China |
(1) Annual community- based breast cancer screening (first tested by clinical breast examination, then advised to undergo breast ultrasonography and/or mammography) (2) Biennial community- based breast cancer screening (3) Triennial community- based breast cancer screening (4) No screening |
CUA | State-transition Markov model | Societal perspective; 50 years | 3% for both cost and outcome | Cost per QALY gained |
CUA cost–utility analysis, CEA cost-effectiveness analysis, QALY quality-adjusted life year, LYG life year gained
Table 2.
Key outcomes and study conclusions
| Study (year) | Incremental costs | Incremental QALYs/LYG | Main conclusion |
|---|---|---|---|
| Sun et al. (2018) [23] | $235.76 (annual screening vs no screening) | 0.0286 QALY (annual screening vs no screening) | The probability of the risk-based breast cancer screening programme in urban China being cost-effective is nearly 100% at the threshold of US $23,050/QALY, with an ICER of $8253/QALY |
| Sun et al. (2019) [24] | $186.7 (screening every 3 years vs no screening) | − 0.20 QALY (screening every 3 years vs no screening) | Clinical breast examination and ultrasound as the primary tool in rural China leads to higher costs and poorer health with a discounted ICER of − $916/QALY |
| Sun et al. (2022) [25] |
$132 (multigene testing vs no testing; payer perspective) $82 (societal perspective) |
0.018 QALY (multigen testing vs no testing) | Family history/clinical-criteria-based BRCA testing was dominated. Unselected multigene testing had an ICER of $4506/QALY (societal perspective) and $7266/QALY (payer perspective), which were well below the threshold of $10,260/QALY and significantly cost-effective |
| Wang et al. (2021) [26] | Not reported | Not reported | At a threshold of $30,785/LYG, biennial mammography screening was cost-effective in urban China with a discounted ACER of $17,309/LYG. It was also the optimal scenario with a discounted ICER of $25,261/LYG compared to other scenarios |
| Yang et al. (2018) [27] |
Per 100,000 simulated cohort (1) $12.19 million (annual screening vs no screening) (2) $6.2 million (biennial screening vs no screening) (3) $4.15 million (triennial screening vs no screening) |
Per 100,000 simulated cohort (1) 1583 QALYs (annual screening vs no screening) (2) 839 QALYs (biennial screening vs no screening) (3) 1587 QALYs (triennial screening vs no screening) |
Annual community-based breast cancer screening and screening every 3 years were 100% cost-effective for a WTP threshold of $20,272/QALY |
ACER average cost-effectiveness ratio, ICER incremental cost-effectiveness ratio, LYG life year gained, QALY quality-adjusted life year, WTP willingness-to-pay
Clinical Screening Strategies
Of the four studies focused on breast cancer screening strategies, three conducted a cost–utility analysis (CUA), while one was a cost-effectiveness analysis (CEA) which measured outcome as cost per life year gained. The evaluated screening strategies were ongoing cancer screening programmes in China, including breast cancer screening.
Yang et al. investigated the cost-effectiveness of a community breast cancer screening programme that has been implemented in Tianjin, China since 2009 [27]. They performed a decision-analytic Markov model to compare annual community-based clinical breast examination (CBE) coupled with further diagnosis undertaking ultrasound and/or mammography if CBE result yielded breast cancer suspicion, with no screening, biennial, and triennial screening strategy. The model considered eight health states: healthy women could transit to ductal carcinoma in situ (DCIS) or stage I or remain healthy. Then patients could progress from stage I to stage II, stage III, and stage IV sequentially. All modelled women could die from non-cancer causes, but only stage IV patients could die from breast cancer. The study simulated a hypothetical cohort of 100,000 healthy women aged between 35 and 69 years. A 50-year time horizon was applied, and the analysis was conducted from a societal perspective. The model outcome was the cost per quality-adjusted life year (QALY) gained. Several clinical outcomes were also reported, including deaths from breast cancer and other causes, invasive breast cancer deaths from other causes, and the number of DCIS cases that died from other causes. The model excluded the biennial screening strategy based on the principle of extended dominance.
The Chinese government launched a cancer screening programme, including breast cancer screening in 14 cities in 2012. Sun and colleagues developed a natural history Markov model to compare the lifetime costs and effects between breast cancer screening strategies and no screening for Chinese women aged 40–69 years [23]. The same health states reported by Yang et al. were included in the model. The programme participants were first categorized by risk of developing breast cancer using a risk assessment questionnaire as high-risk and low-risk groups. Then age-specific assumptions were included in the screening strategies for the high-risk group. High-risk women aged 40–44 years were given an annual ultrasound followed by mammography if suspicious results appeared. Additionally, it was assumed that high-risk women aged 45–69 years underwent both ultrasound and mammography screening every year. Women with low risk were not screened but only diagnosed on symptoms presented. A total of 198,097 women participated in the risk assessment questionnaire between 2012 and 2013, and 17,104 were identified as high risk. A societal perspective was adopted. The health outcome was measured as QALYs, and the incremental cost-effectiveness ratio (ICER) was reported as a model outcome.
The “two-cancer screening” (i.e. breast and cervical cancer) programme for rural women in 31 Chinese provinces took place in 2009 [28]. Sun and colleagues adopted the same Markov model structure to examine a breast cancer screening strategy’s lifetime costs, health effects, and cost-effectiveness compared with no screening for women aged 35–64 years [24]. Participants underwent a CBE and ultrasound in the screening programme, followed by mammography screening if the primary result was suspicious or unclear. The study obtained data from 26,224 participants. Both life years and QALYs were measured as health outcomes, and the model outcome was presented as cost per QALY gained at a discount rate of 3% for both costs and QALYs. A societal perspective was adopted. The ICER threshold was three times China’s gross domestic product (GDP) in 2014.
Wang et al. conducted an economic evaluation of the most recent breast cancer screening strategy which was introduced in 2019—a biennial mammography screening strategy for urban Chinese women aged 45–70 years [26]. They adopted a micro-simulation model (Simulation Model on radiation Risk and breast cancer Screening, SiMRiSc) to estimate the lifetime costs and benefits of the screening strategy compared with no screening using patient-level data. The model incorporated a series of risk factors, including life expectancy, tumour growth, tumour self-detection probability depending on tumour size and mammographic density, survival probability from breast cancer, the introduction of false positives associated with mammography specificity, and the probability of tumour induction due to ionizing radiation from mammography. The study modelled a hypothetical cohort of 100,000 women. Health outcomes from simulation estimates, including averted tumour deaths, screening-detected tumours, interval cancers, and life years gained, were reported. Both average cost-effectiveness ratios (ACERs), defined as the ratio of the cost to benefit of an intervention without reference to a comparator [29], when comparing the programme screening with no screening and ICERs when comparing alternative strategies were determined for model outcomes. The analysis was performed from a societal perspective. A discount rate of 5% for costs and health benefits was used.
Genetic Testing Strategies
Genetic testing for breast cancer diagnosis is an effective technology for the early detection of heritable variants and primary screening. Sun et al. and colleagues were interested in BRCA1/BRCA2/PALB2 genetic testing for breast cancer and developed a microsimulation model to assess the lifetime costs and QALYs of multigene testing for Chinese women with breast cancer [25]. The study compared strategy (1) BRCA1/BRCA2/PALB2 genetic testing in all women with breast cancer with strategy (2) BRCA genetic testing only for women with breast cancer family history clinical criteria and strategy (3) no screening. The team employed individual-level data from 8085 unselected patients with breast cancer to estimate the age distribution of patients with breast cancer and the total number of breast cancer cases by age group in the Chinese population. Concerning those fulfilling clinical criteria, first-degree relatives were tested for variants in BRCA1/BRCA2/PALB2 genes and then second-degree relatives underwent the same testing if the variants were identified in the first-degree relatives, known as cascade testing. The researchers also considered variants of uncertain significance (VUS), whereby relatives with a probability of 8.7% carrying VUS were offered cascade testing. Following the testing, unaffected and affected BRCA1/BRCA2/PALB2 path vars and breast cancer path vars carriers opted to minimize their breast cancer and ovarian cancer (OC) risk through risk-reducing interventions, e.g. risk-reducing mastectomy and risk-reducing salpingo-oophorectomy. The analysis was performed from both payer and societal perspectives and used a 3% discount rate. A more conservative willingness-to-pay (WTP) threshold of one GDP (10,260 USD per QALY in 2019) was adopted compared to the other four studies. Health outcomes, such as breast cancer incidence, OC incidence, mortality, and life years gained were also reported.
Cost-effectiveness Results
Yang et al. reported that compared to no screening, the deterministic ICER of the annual, biennial, and triennial community-based screening strategy was 7701.68 USD per QALY, 7392 USD per QALY, and 7075.77 USD per QALY, respectively. The probabilistic sensitivity analysis (PSA) observed that 100% of the iterations of screening every year and every 3 years were cost-effective at a cost-effectiveness threshold (CET), defined as three times the annual Chinese gross domestic product (GDP), and the annual screening strategy remained acceptable at a lower threshold value of two times the GDP [27]. The results of the scenario analysis showed that the probability of the annual screening strategy being cost-effective was over 95% when the attendance rate was greater than 50%. If 10% of stage I tumours could be detected by screening, the probability of the annual screening strategy being cost-effective could reach 95% at a greater CET (more than three times the GDP). When the compliance rate for receiving diagnostic tests was over 80%, the probability of the annual screening strategy being cost-effective would be up to 95% at a CET of two times the GDP.
Sun et al. estimated an ICER of 8253 USD per QALY gained, resulting in nearly 100% cost-effectiveness of the annual risk-based screening strategy at the threshold of three times the GDP [23]. The result was robust in the sensitivity analysis. Multiple scenario analyses were conducted and indicated that screening every 3 years was the most cost-effective strategy, which yielded an ICER of 6671 USD per QALY. The cost-effectiveness of the annual screening strategy was observed to decrease if not all the diagnosed women received treatment. Further, compared to mammography alone, the cost-effectiveness of both women aged 45–69 years receiving ultrasound and mammography remained uncertain.
Sun and colleagues predicted that the CBE and ultrasound screening strategy led to no benefit but harm to women’s health in rural China, yielding a negative ICER of − 916 USD per QALY gained [24]. The result was robust to one-way sensitivity analyses and scenarios analysis, wherein varied screening intervals, compliance rate, age-specific incidence, and utility loss due to false positives were explored.
Wang et al. reported that compared to no screening, the biennial mammography screening strategies with a 100% participation rate were cost-effective with a discounted ACER of 17,305 USD per life year gained at the WTP of 30,785 USD per life year gained (three times the GDP in 2019) [26]. The researchers subsequently explored seven alternative strategies by varying the screening interval and age span in scenario analyses concluding that the base case was the most cost-effective and robust to sensitivity analysis. On top of the CEA, a budget impact analysis was performed using a 10-year time horizon and showed that the screening programme would yield a cost of USD 38.1 million for a medium city with one million citizens.
In the multigene testing study, the strategy based on family history and clinical criteria was ruled out. It was revealed that multigene testing was cost-effective compared with no screening, yielding an ICER of 4506 USD per QALY gained from the societal perspective and an ICER of 7266 USD per QALY gained from the payer perspective; 94.2% and 86.6% of PSA iterations were cost-effective from the societal and payer perspective, respectively [25]. The results were reported to be robust to multiple scenario analysis. Concerning population impact, the study estimated that 7868 cases and 5164 deaths by both breast cancer and OC could be averted annually in China.
Study Assessment
Variations in Interventions and Comparators
Most studies compared different breast cancer screening strategies by varying intervention intervals, age range, or a combination of them [23, 24, 26, 27]. All included studies compared the assessed breast cancer screening strategy or strategies with “no screening” [23–27]. Of the five included studies, four studies compared different breast cancer screening strategies (such as ultrasound, CBE, and mammography) [23, 24, 26, 27] and one study compared multigene testing strategies with “no screening” [25]. In addition to “no screening” as the primary comparator, different breast cancer screening strategies with varied age ranges were compared in two studies [23, 26], and strategies with varied screening intervals were compared in two studies [26, 27]. Sun et al. compared multigene testing in all patients with breast cancer with no genetic testing and multigene testing in patients fulfilling family history or clinical criteria [25].
Variations in Perspectives and Time Horizon
The costs and study outcomes are determined by the perspective and time horizon applied. All studies adopted a societal perspective, although not all included indirect costs. Four of the five studies [23–26] used a lifetime time horizon, while a 50-year time horizon was applied in the fifth study [27]. The latter was justified considering that 50 years with a starting age of 35 years old was long enough to capture the differences in costs and outcomes between the intervention and comparators, as survival probability was less than 10% for Chinese women at 85 years old.
Variations in Model Assumptions
All five studies were model-based economic evaluations [23–27]. Three articles incorporated the same Markov model structure consisting of eight health states as described above [23, 24, 27]. Two studies [25, 26] adopted a patient-level model to predict the expected costs and outcomes, of which one study [26] evaluated a breast cancer screening strategy and another study [25] evaluated genetic testing. Screening effectiveness and attendance rate are important indicators to reflect the screening programme performance. Of the screening-focused studies, all four studies [23, 24, 26, 27] considered screening sensitivity and specificity in their model, whereas the attendance rate was explored in two studies [26, 27]. Three studies included DCIS in the model to assess screening efficiency, which ultimately could affect the cost-effectiveness results [23, 24, 27]. However, Wang et al. excluded DCIS from their model because of the harm associated with the overdiagnosis and overtreatment of DCIS and its extra costs [26]. Disutility was considered in three studies, with two breast cancer screening-focused studies [23, 24] incorporating disutility of false positive results and the multigene testing study incorporating disutility of risk-reducing surgery and development of heart disease [25]. In the microsimulation model of the multigene testing study, family members and associated cascade testing were considered to extend the benefits of genetic testing to the population.
Variations in Cost Estimation
To better understand the cost discrepancy among breast cancer screening strategies, we summarised the key characteristics of cost estimation for the five included studies (Table 3). The inclusion of cost categories varied across studies. All five studies included direct medical costs. Direct non-medical costs were included but not specified in three studies [23, 24, 27], described in one study [26], and not included in one study [25]. Three of the five articles included productivity loss as an indirect cost [23–25], and two studies did not include indirect cost [26, 27]. Two studies reported all three cost categories [23, 24]. Medical cost inputs were similar across the three screening-focused studies that adopted the same Markov model structure, including the cost of screening, diagnosis, treatment of breast cancer in four stages, and DCIS [23, 24, 27]. On the other hand, Wang et al. [26] considered treatment costs depending on the tumour size and excluded DCIS in their microsimulation model. The genetic testing study considered a wide range of medical costs, including genetic testing, prevention to minimize breast cancer and OC risk, diagnosis, and treatment for both breast cancer and OC, as well as palliative care [25]. It is worth noting that the costs were estimated in different years, which could affect the costs comparison between studies regarding price change of the intervention as well as treatments and inflation [23–26]. The most commonly used data sources were the corresponding programme-related publication and literature.
Table 3.
Characteristics of cost estimation
| Study (year) | Direct costs | Indirect costs | Cost data source | Intervention cost (unit) | Total cost of baseline strategy | |
|---|---|---|---|---|---|---|
| Medical costs | Non-medical costs | |||||
| Sun et al. (2018) [23] |
(1) Questionnaire (2) Screening (3) Biopsy for diagnosis (4) Treatment DCIS Stage I–IV |
Not specified | Productivity loss |
Cancer Screening Programme in Urban China; literature |
Screening: $85.5 | $335.43 |
| Sun et al. (2019) [24] |
(1) Screening (2) Biopsy for diagnosis (3) Treatment DCIS Stage I–IV |
Not specified | Productivity loss | Rural breast cancer screening programme; literature | Screening: $22.7 | $230 |
| Sun et al. (2022) [25] |
(1) Genetic testing (2) Minimizing breast cancer and OC risk RRSO (and HRT and osteoporosis prevention) RRM (and reconstruction and complications) CPM (and reconstruction and complications) Breast cancer screening for non-carriers Breast cancer screening for mutation carriers Chemoprevention (4) Diagnosis and treatment Ovarian cancer diagnosis and initial and follow-up treatment Breast cancer diagnosis and initial and follow-up treatment (5) Terminal care Ovarian cancer Breast cancer with fatal CHD Breast cancer with excess CHD |
Not included | Productivity loss (temporary and permanent disability, premature mortality) | Urban Basic Medical Insurance Database in China; Word Bank; literature | Genetic testing: $367 |
$4686 (payer perspective) $6808 (societal perspective) |
| Wang et al. (2021) [26] |
(1) Screening (2) Biopsy for diagnosis (3) Medical treatment during 2 months before and 10 months after diagnosis |
(1) Additional meals and nutrition (2) Transportation (3) Accommodation (4) Informal nursing (5) Other out-of-pocket costs |
Not included | Tianjin Development and Reform Commission; literature | Mammography: $34 |
$79.1 million per 100,000 simulated cohort |
| Yang et al. (2018) [27] |
(1) Clinical breast examination (2) Community health service (3) Evaluating abnormal results(ultrasound and mammography) (4) Biopsy of diagnosis (5) Primary treatment (inpatient and outpatient cost of treating invasive cancer and DCIS) (6) Follow up treatment (outpatient cost of follow-up screening test, radiotherapy, chemotherapy and targeted therapy) |
Not specified | Not included | Project management of rural women "Two Cancers" in 2010; literature |
CBE: $4.3 Mammography: $29.0 Ultrasound: $10.2 |
Per 100,000 simulated cohort $108.27 million (annual screening vs no screening) $102.28 million (biennial screening vs no screening) $100.23 million (triennial screening vs no screening) |
DCIS ductal carcinoma in situ, CPM contralateral prophylactic mastectomy, RRSO risk-reducing salpingo-oophorectomy, RRM risk-reducing mastectomy, CHD coronary heart disease
The unit cost of the screening interventions varied greatly in the five studies. The unit cost of genetic testing is significantly higher than breast cancer screening in China, costing over ten times more than the cost of mammography per screening. CBE was the cheapest option, with a unit cost of $4.3 [27]. The annual multigene testing strategy showed the lowest incremental cost compared to no screening among included studies [25]. Wang et al. did not report incremental costs [26]. However, it should be noted that a higher discount rate (5%) was adopted by Wang et al., which could result in a relatively lower incremental cost.
Variation in Outcome Measurement
Most of the included studies used QALYs gained as the main outcome measure [23–25, 27]. Two studies reported outcomes as life years gained [25, 26]. Sun et al. (2018) [23] obtained the utility scores for patients at stages I–IV from a cross-sectional survey performed using the EuroQol five-dimension (EQ5D) questionnaire that was conducted alongside the programme. Sun et al. (2019) adopted the same utility values [24]. Sun et al. (2022) and Yang et al. (2018) sourced the utility scores from literature [25, 27].
Some studies also reported clinical outcomes. For example, Yang et al. reported the number of deaths from breast cancer and other causes, invasive breast cancer deaths from other causes, and the number of DCIS cases that died from other causes [27]. Wang et al. reported averted tumour deaths, screening-detected tumours, interval cancers, and life years gained [26]. Sun et al. reported the incidence of breast cancer and OC and related cases and deaths prevented [25].
Variation in Conclusions
Four studies concluded the assessed breast cancer screening strategies to be cost-effective, with three breast cancer screening-focused studies [23, 26, 27] reporting that the strategies were cost-effective at a threshold of three times GDP per capita. Sun et al. concluded that multigene testing was significantly cost-effective at a one GDP per capita threshold [25]. Sun et al. reported that clinical breast examination and ultrasound led to higher costs and fewer benefits for rural Chinese women [24].
Key Drivers for Cost-effectiveness
The factors influencing the cost-effectiveness of the current breast cancer screening interventions were seen distinctively due to the different study designs and model structures. The key influential parameters include the cost of breast cancer screening/genetic testing, screening accuracy, compliance rate, and participation rate.
Cost of Breast Cancer Intervention
Intervention cost was underlined as one of the most influential factors on the ICER among the included studies. Sun et al. noted that varying screening costs drove the ICER to fluctuate the most [23]. Wang et al. predicted that ACER and the budget impact analysis were most sensitive to the cost of mammography screening [26]. The unit cost of multigene testing was estimated to be one of the most influential parameters to the ICER from both payer and societal perspectives [25]. Nevertheless, it is important to note that the cost of breast cancer screening and intervention continues to decrease, directly affecting the medical costs and leading to a greater population impact. Although the costs varied considerably among included studies, none of them identified that the cost-effectiveness conclusion was averted as a result of the variation in costs.
Compliance Rate for Treatment
Some studies highlighted that the treatment compliance rate influenced the examined strategies’ cost-effectiveness. Sun et al. estimated that the annual ultrasound followed by a mammography programme among high-risk women was less cost-effective if not all diagnosed cases received treatment [23]. Sun et al. underlined that rural patients were less likely to seek treatment because of the high out-of-pocket costs compared to urban patients [24]. This could result in poorer prognosis and higher costs, which may reduce the cost-effectiveness of the screening programme. Therefore, the researchers suggested further examining the cost-effectiveness result if sufficient data on treatment costs of rural patients was available. Additionally, the compliance rate for receiving a diagnostic test was also identified as having a certain impact on the study result. Yang et al. found that a compliance rate of at least 50% was required to maintain the 95% probability of the community-based screening programme being cost-effective [27]. Wang et al. validated their model using data from Japan and revealed that a lower cancer detection rate was associated with a decreased compliance rate [26].
Accuracy of Screening
The accuracy of screening was another important factor that impacted the cost-effectiveness. Yang et al. predicted that the community-based screening programme remained highly cost-effective at a lower WTP threshold (two times the GDG) if 20% of early-stage tumours were detected [27]. Another study noted that a lower mammographic density and a higher specificity could result in a more favourable ICER [26].
Attendance Rate
The proportion of participants undertaking the breast cancer screening was recognized as having influenced the ICER. Yang et al. estimated that the community-based screening programme was not cost-effective with a 30% attendance rate but became highly effective when the attendance rate increased to 50% [27]. Conversely, Wang et al. predicted that a decreased attendance rate could lead to a favourable ACER of the biennial mammography screening programme [26]. However, this was at the cost of a substantial reduction in averted deaths, screening-detected cancers, and life years gained.
Other Factors
There were additional factors that affected the cost-effectiveness outcome. For example, the community-based screening programme was predicted to be more cost-effective with a later starting age of 40 rather than 35 [27]. Compared to ultrasound plus mammography, mammography alone was found to be more cost-effective for high-risk Chinese women aged 45–69 years [23]. Sun et al. (2019) [24] predicted that low breast cancer incidence among rural Chinese women might lead to a less favourable cost-effectiveness outcome of the assessed screening programme.
Reporting
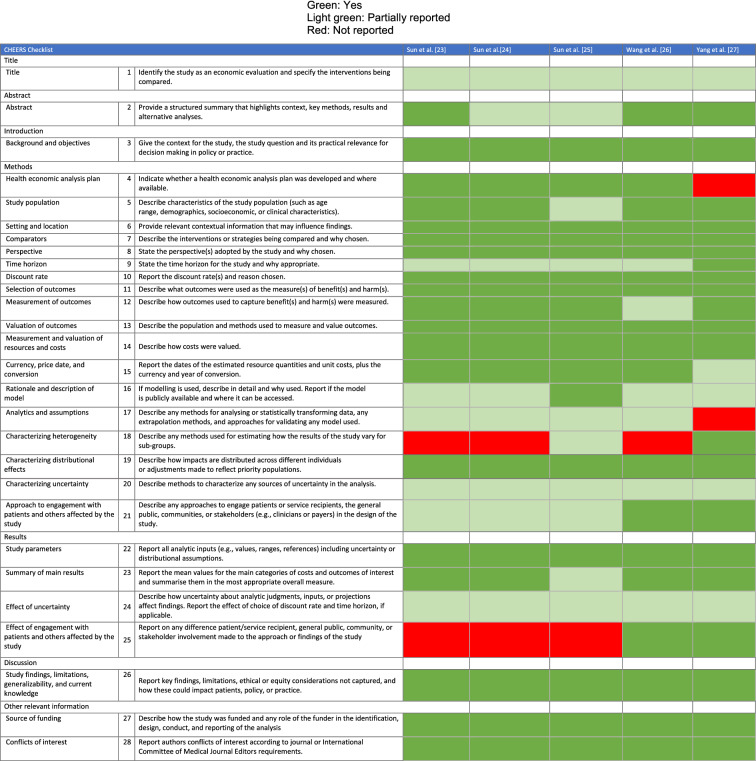
A summary of the reporting quality assessment for the included articles is presented in Table 4. All studies had an acceptable reporting quality, though some studies did not clearly specify the target population [30], or the model assumptions [18, 26].
Table 4.
CHEERS quality results
Discussion
This review outlined the economic evaluations of the current practice of breast cancer screening in China. A small number of studies were identified as eligible. There were two main study categories: four articles examined the cost-effectiveness of breast cancer screening strategies for high-risk women aged 35–70 years, including ultrasound, mammography, and CBE [23, 24, 26, 27]; and one article performed an economic evaluation of genetic testing [25]. A Markov model was used in three studies [23, 24, 27], and the other two developed a microsimulation model [25, 26]. Lifetime time horizon and societal perspective were commonly adopted to weigh up the long-term costs and effects. Three of the studies evaluating breast cancer screening programmes deemed the intervention to be cost-effective but only for women living in urban areas [23, 26, 27]. Conversely, the CBE coupled with the ultrasound programme resulted in decreased QALYs with additional costs compared to no screening in women residing in rural China, indicating apparent harm to rural women [24]. Despite the high cost, multigene testing was estimated to have promising health benefits for all Chinese women [25]. All studies were of high reporting quality, yet most failed to provide disaggregated costing details.
Challenges
The evidence on assessing the current breast cancer screening and intervention on an economic basis remains limited. This is mainly restricted because of several key challenges.
Data Absence
Data scarcity and accessibility have been the key challenges in economic evaluations in LMICs [31]. Indeed, data absence for model inputs and validation was recognized as the major challenge for the included studies in this review. Consequently, main model inputs had to be obtained from other countries or determined upon assumptions. For example, in the absence of the attendance rate, two studies assumed that all programme participants received breast cancer screening in their baseline analysis but failed to give the assumption’s rationale nor explored its uncertainty [23, 24]. Wang et al. [26] justified an attendance rate of 60–80% among urban Chinese women on the basis of an estimation of a higher than 70% attendance rate in Asian countries [32]. However, much lower attendance rates (21.7–52.1%) have been reported in other studies in some Chinese cities [33, 34]. From the programme perspective, although varied attendance rates may not change the cost-effectiveness, it could influence policy recommendations on such interventions as a key indicator of screening performance.
Similar to the attendance rate, an assumption of a 70–100% uptake rate of treatment was adopted in the reviewed studies without a reference. Additionally, most studies did not specify what type of treatment was provided to patients at each disease stage. The lack of data on treatment, including treatment uptake rate and treatment options, increased the uncertainty of the overall cost-effectiveness outcomes as receiving treatment accounted for substantial costs. Although the robustness of the model results with varied treatment costs was confirmed, it should be noted that the treatment pathway and costs vary considerably between urban and rural areas.
Challenges were observed in incorporating productivity loss in the CEA, mostly due to a lack of methodological guidance and appropriate data on productivity loss in the Chinese context [35, 36]. Three reviewed studies considered productivity loss, but no detailed process description was presented [18, 23, 25]. Including productivity loss as the main part of indirect costs is recommended in China’s current national guideline for health economic evaluation considering its effect on total costs that may ultimately influence the ICER from a societal perspective [35, 37, 38]. However, further research on this topic is warranted to address this challenge.
Other commonly observed challenges include the paucity of data on transition probabilities, utility values, risk index, long-term follow-up data, and ancillary costs (i.e. administration, training, or other clinical support). This was partly explained by population-based breast cancer screening programmes that were only implemented in China in the recent decade, and long-term data is still lacking. In practice, some essential programme data were not well documented and reported to ensure data consistency and transparency [23–25]. To address this challenge, some instruments, such as the Client Services Receipt Inventory, can be adopted and redesigned alongside the programme to plan and collect data on healthcare resource use that can particularly serve subsequent economic evaluations [39].
Urban–Rural Discrepancies
Urban–rural discrepancies in women with breast cancer and the associated resource use have been reported. Rural women generally have lower breast cancer incidence, poorer treatment adherence, fewer treatment choices due to scarce healthcare resources, and higher indirect medical costs (i.e. potentially higher travelling costs for rural patients) compared to urban women [40, 41]. Sun et al. (2019) [18] concluded that breast cancer screening programmes cause harm to rural Chinese women but also pointed out the need to review the results when more data is available for rural women. The urban–rural discrepancy also complicates the economic modelling process by impacting the generalizability of the cost-effectiveness outcomes at the whole country level [42].
Willingness-to-Pay Threshold
A clearly defined threshold is not available in China currently, though some health economists have made empirical estimations [43, 44]. Four of the five included studies adopted three times the GDP per capita as the WTP threshold. This was based on the suggestion by the World Health Organization on using disability-adjusted life year (DALY) as an outcome index, indicating that interventions with an ICER lower than three times GDP per capita per DALY averted were considered cost-effective and lower than one GDP per capita per DALY averted was considered very cost-effective [37]. Recently, a study estimated a threshold of 63% (5540, 2017 USD) GDP per capita per DALY averted, reflecting health opportunity costs when judging cost-effectiveness in China [45]. However, the GDP-based threshold has received extensive criticism and the World Health Organization has alerted its limited use in decision-making, which means that adopting three times the GDP could be problematic regarding cost-effectiveness evidence that informs policymakers on the value of money [46]. Some countries have published explicit CET ranges, but the values vary considerably from country to country [47]. To promote the health technology assessment on healthcare interventions, the Netherlands considers disease severity when setting a WTP threshold, indicating the threshold ranges from €10,000 per QALY for diseases with a severity of 0.10 to €80,000 per QALY for diseases with a severity of 1.0 [48]. This approach resulted in much higher WTP thresholds for life-threatening diseases, with breast cancer at an estimated severity of 0.86 [48]. In this review, Yang et al. [27] addressed this challenge by investigating the cost-effectiveness outcome and uncertainty around some key parameters, such as cancer incidence, attendance rate, the compliance rate for diagnosis, and detection of the tumour at the early stage through threshold analysis, and Sun et al. (2022) adopted a one GDP threshold in their baseline analysis to mitigate the uncertainty around the WTP threshold [25]. To which level the WTP threshold should be determined in general or for different diseases in China is a topic that remains to be addressed. Other than the WTP thresholds, it is equally important to consider local policies and stakeholders that determine the programme implementation plan and budget deployment, which may influence the intervention effects at the population level.
Model Design
Another common challenge in CEA is to choose a decision-analytic framework that would most accurately reflect the expected health costs and consequences associated with clinical care pathways of the interventions and alternative options [36]. Three studies developed a Markov model to weigh the long-term costs and benefits of breast cancer screening programmes [23, 24, 27]. However, Markov models fail to track patients’ relevant history to reflect the benefits of screening programmes over no screening or alternative strategies. To mitigate this issue, the other two studies adopted a patient-level simulation model that overcame this issue by incorporating a range of risk factors and tracking the clinical events of each patient with breast cancer as well as applied health services [25, 26]. This raises another challenge: microsimulation demands high quantities of data, which is mostly unavailable in China, leading to studies relying on international data [36]. It is important to note that risk factors of breast cancer in other countries could lead to substantial bias as disparities between countries exist regarding the characteristics of the breast cancer population. For example, compared to women of European descent, Chinese women are often diagnosed at a younger age (around 10 years earlier) and advanced stage and have less possibility of strong breast cancer family history [49–51]. Of particular concern is that individuals may follow different clinical pathways based on age, medical conditions, and socioeconomic characteristics, which raises challenges in identifying and incorporating all potential factors into the simulation. Numerous risk factors for breast cancer (e.g. polygenic risk score, hormonal factors, other family history factors) could be considered in future models to increase the precision of CEA.
Suggestions for Future Economic Evaluation
Overall, the cost-effectiveness of current breast cancer screening presented uncertainty among women across China. Comparably, genetic testing has shown significant cost-effectiveness among all Chinese women and promising health benefits in averting breast cancer cases and deaths. Genetic testing promotes the early detection process by identifying genetic variants associated with a high risk of breast cancer in patients and family members who usually are missed by using age and clinical-based screening approaches. For example, women with disease-causing variants receive more frequent surveillance than those without variants. This allows early diagnosis and timely treatment for the variant carriers and, from a societal perspective, could reduce the disease burden of society. Those favourable outcomes need further research to be confirmed.
Sun et al. (2021) only focused on the most common variants (i.e. BRCA1/BRCA2/PALB2) associated with high breast cancer risk. To maximize breast cancer detection, it is suggested that future CEA should also consider other genes associated with a high or moderate risk of developing breast cancer, such as TP53, PTEN, CDH1, and STK11 [52–55]. If established multigene testing could precisely identify those with disease-causing variants, policymakers may consider a tailored surveillance approach for women with high/moderate/low level of cancer risk. That implies a screening strategy with risk-adapted starting age for women with different levels of risk [56]. The risk-adapted starting age of screening could ensure the fairness and effectiveness of breast cancer screening.
Cascade testing extends genetic testing among family members after identifying the first person in the family with particular genetic variants. This aids in the surveillance and care management of family members. Evidence supported that covering family members with cascade testing improved the cost-effectiveness of genetic testing in women with breast cancer [57]. Sun et al. (2021) considered cascade testing among the relatives of patients with breast cancer. However, an elaboration of the assumptions upon cascade testing, including but not limited to the costs of testing, its sensitivity and specificity, relatives’ age, the respective uptake rate among the first- and second-degree relatives, and downstream interventions and health consequences, should be considered in future research.
Moreover, it would further increase the value of economic evaluations to consider secondary findings (SFs) in future models [58]. This refers to genetic testing results irrelevant to the primary indication for genome sequencing but that may have potential clinical importance. There has been an intense debate on returning SFs to patients [58, 59]. The American College of Medical Genetics and Genomics (ACMG) developed a list of SFs considered medically actionable and having high penetrance. They recommended that these SFs be returned to patients receiving genomic sequencing based on their consent [60]. By allowing the disclosure of SFs to patients, the ACMG is expecting to maximize the screening effects in the population. However, some scientists have concerns about its potentially increased downstream healthcare costs relevant to diagnosis and prophylactic treatments, as well as associated information overload and psychological distress [61–63]. Nevertheless, Bennette and colleagues reported that returning SFs was considered cost-effective for certain patient populations, such as patients with cardiomyopathy, patients with colorectal cancer, or healthy individuals [64]. Internationally, some studies examined the cost-effectiveness of genetic testing with extensive cascade testing covering family members for breast cancer and OC, but SFs have not been considered in their model [19, 65–67]. Further search is warranted to fill in this gap in cost-effectiveness evidence on cascade testing and SFs, which is needed to support developing national guidelines for advanced breast cancer screening and management in China.
Strengths and Limitations
This is the first review summarizing the up-to-date evidence on economic evaluations of breast cancer screening strategies in China. We also assessed the analytic approaches and outlined the factors that influenced the cost-effectiveness outcomes and potential methodological challenges to identify research gaps and provide suggestions for future work. This review is also subject to certain limitations. The exclusion of non-English studies may have introduced language bias. However, this may be mitigated by the small number of non-English studies excluded. Since grey literature searching was not conducted, some eligible studies could be missed out.
Conclusion
This review described the current literature on the cost-effectiveness of breast cancer screening strategies in China as representative of low-resource settings. The included studies examined the current breast cancer screening strategies including genetic testing. The conventional strategies were cost-effective among urban Chinese women but not among rural women. The genetic testing strategy was found to be economically attractive among Chinese women, indicating a potentially promising direction. Context-specific and methodological challenges impose barriers to evaluating the cost-effectiveness of breast cancer screening. Given that genetic testing may improve the cost-effectiveness of screening, we suggest that future research explores the feasibility of genetic testing as a screening approach for breast cancer and its value for money, which may mitigate the disease and financial burden to society.
Acknowledgements
Funding
Dr. Li Yang was funded by the China Medical Board (Grant No.19-336), the National Natural Science Foundation of China (Grant No. 71911530221 and 72174010) and the National Key Research and Development Program of China (Grant No. 2021YFC2500405). No funding or sponsorship was received for the publication of this article. The journal’s Open Access fee was funded by the authors.
Author Contributions
Conceptualization: Shan Jiang; Methodology: Jingjing Jiang, Shan Jiang, and Antonio Ahumada-Canale; Literature search: Jingjing Jiang and Shan Jiang; Screening and data extraction: Jingjing Jiang and Shan Jiang; Reporting quality assessment: Jingjing Jiang and Shan Jiang; Formal analysis and investigation: Jingjing Jiang and Shan Jiang; Writing—original draft preparation: Jingjing Jiang; Writing—review and editing: Shan Jiang, Antonio Ahumada-Canale, Zhuo Chen, Lei Si, Yawen Jiang, Li Yang, and Yuanyuan Gu; Funding acquisition: Li Yang; Resources: Zhuo Chen, Lei Si, Yawen Jiang, Li Yang, and Yuanyuan Gu; Supervision: Li Yang and Yuanyuan Gu.
Disclosures
Jingjing Jiang, Shan Jiang, Antonio Ahumada-Canale, Zhuo Chen, Lei Si, Yawen Jiang, Li Yang, and Yuanyuan Gu have nothing to declare.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
Footnotes
Jingjing Jiang and Shan Jiang contributed equally.
Contributor Information
Li Yang, Email: lyang@bjmu.edu.cn.
Yuanyuan Gu, Email: yuanyuan.gu@mq.edu.au.
References
- 1.Lei S, Zheng R, Zhang S, et al. Global patterns of breast cancer incidence and mortality: a population-based cancer registry data analysis from 2000 to 2020. Cancer Commun (Lond) 2021;41(11):1183–1194. doi: 10.1002/cac2.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.DeSantis CE, Bray F, Ferlay J, et al. International variation in female breast cancer incidence and mortality rates. Cancer Epidemiol Biomark Prev. 2015;24(10):1495–1506. doi: 10.1158/1055-9965.Epi-15-0535. [DOI] [PubMed] [Google Scholar]
- 4.El Saghir NS, Adebamowo CA, Anderson BO, et al. Breast cancer management in low resource countries (LRCs): consensus statement from the Breast Health Global Initiative. Breast. 2011;20(Suppl 2):S3–11. doi: 10.1016/j.breast.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Merino Bonilla JA, Torres Tabanera M, Ros Mendoza LH. Breast cancer in the 21st century: from early detection to new therapies. Radiologia. 2017;59(5):368–379. doi: 10.1016/j.rx.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Myers ER, Moorman P, Gierisch JM, et al. Benefits and harms of breast cancer screening: a systematic review. JAMA. 2015;314(15):1615–1634. doi: 10.1001/jama.2015.13183. [DOI] [PubMed] [Google Scholar]
- 7.Rashidian A, Barfar E, Hosseini H, et al. Cost effectiveness of breast cancer screening using mammography; a systematic review. Iran J Public Health. 2013;42(4):347–357. [PMC free article] [PubMed] [Google Scholar]
- 8.Wong IO, Kuntz KM, Cowling BJ, et al. Cost effectiveness of mammography screening for Chinese women. Cancer. 2007;110(4):885–895. doi: 10.1002/cncr.22848. [DOI] [PubMed] [Google Scholar]
- 9.Wong IO, Kuntz KM, Cowling BJ, et al. Cost-effectiveness analysis of mammography screening in Hong Kong Chinese using state-transition Markov modelling. Hong Kong Med J. 2010;16(Suppl 3):38–41. [PubMed] [Google Scholar]
- 10.Wong IO, Tsang JW, Cowling BJ, et al. Optimizing resource allocation for breast cancer prevention and care among Hong Kong Chinese women. Cancer. 2012;118(18):4394–4403. doi: 10.1002/cncr.27448. [DOI] [PubMed] [Google Scholar]
- 11.Okonkwo QL, Draisma G, der Kinderen A, et al. Breast cancer screening policies in developing countries: a cost-effectiveness analysis for India. J Natl Cancer Inst. 2008;100(18):1290–1300. doi: 10.1093/jnci/djn292. [DOI] [PubMed] [Google Scholar]
- 12.Zelle SG, Nyarko KM, Bosu WK, et al. Costs, effects and cost-effectiveness of breast cancer control in Ghana. Trop Med Int Health. 2012;17(8):1031–1043. doi: 10.1111/j.1365-3156.2012.03021.x. [DOI] [PubMed] [Google Scholar]
- 13.Denewer A, Hussein O, Farouk O, et al. Cost-effectiveness of clinical breast assessment-based screening in rural Egypt. World J Surg. 2010;34(9):2204–2210. doi: 10.1007/s00268-010-0620-3. [DOI] [PubMed] [Google Scholar]
- 14.Zelle SG, Baltussen RM. Economic analyses of breast cancer control in low-and middle-income countries: a systematic review. Syst Rev. 2013;2(1):1–14. doi: 10.1186/2046-4053-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Copur MS. Universal genetic testing for all breast cancer patients. Oncology (Williston Park). 2019;33(8):683731. [PubMed]
- 16.Valencia OM, Samuel SE, Viscusi RK, et al. The role of genetic testing in patients with breast cancer: a review. JAMA Surg. 2017;152(6):589–594. doi: 10.1001/jamasurg.2017.0552. [DOI] [PubMed] [Google Scholar]
- 17.Koldehoff A, Danner M, Civello D, et al. Cost-effectiveness of targeted genetic testing for breast and ovarian cancer: a systematic review. Value Health. 2021;24(2):303–312. doi: 10.1016/j.jval.2020.09.016. [DOI] [PubMed] [Google Scholar]
- 18.Sun L, Brentnall A, Patel S, et al. A cost-effectiveness analysis of multigene testing for all patients with breast cancer. JAMA Oncol. 2019;5(12):1718–1730. doi: 10.1001/jamaoncol.2019.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asphaug L, Melberg HO. The cost-effectiveness of multigene panel testing for hereditary breast and ovarian cancer in Norway. MDM Policy Pract. 2019;4(1):2381468318821103. doi: 10.1177/2381468318821103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yip CH, Evans DG, Agarwal G, et al. global disparities in breast cancer genetics testing, counselling and management. World J Surg. 2019;43(5):1264–1270. doi: 10.1007/s00268-018-04897-6. [DOI] [PubMed] [Google Scholar]
- 21.Manchanda R, Sun L, Patel S, et al. Economic evaluation of population-based BRCA1/BRCA2 mutation testing across multiple countries and health systems. Cancers. 2020;12(7):1929. doi: 10.3390/cancers12071929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Husereau D, Drummond M, Augustovski F, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BMJ. 2022;376:e067975. doi: 10.1136/bmj-2021-067975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun L, Legood R, Sadique Z, et al. Cost-effectiveness of risk-based breast cancer screening programme. China Bull World Health Organ. 2018;96(8):568–577. doi: 10.2471/blt.18.207944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun L, Sadique Z, Dos-Santos-Silva I, et al. Cost-effectiveness of breast cancer screening programme for women in rural China. Int J Cancer. 2019;144(10):2596–2604. doi: 10.1002/ijc.31956. [DOI] [PubMed] [Google Scholar]
- 25.Sun L, Cui B, Wei X, et al. Cost-effectiveness of genetic testing for all women diagnosed with breast cancer in China. Cancers (Basel) 2022 doi: 10.3390/cancers14071839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Greuter MJW, Zheng S, et al. Assessment of the benefits and cost-effectiveness of population-based breast cancer screening in urban China: a model-based analysis. Int J Health Policy Manag. 2021 doi: 10.34172/ijhpm.2021.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang L, Wang J, Cheng J, et al. Quality assurance target for community-based breast cancer screening in China: a model simulation. BMC Cancer. 2018;18(1):261. doi: 10.1186/s12885-018-4168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Health Commission of the People's Republic of China. "Two cancers" screening for rural women project management plan (2015 version). 2015. http://www.nhc.gov.cn/jkfpwlz/zcwj1ge/201902/602bbe4b1de24410ad355f38d9b4bfe4.shtml.
- 29.Bang H, Zhao H. Average cost-effectiveness ratio with censored data. J Biopharm Stat. 2012;22(2):401–415. doi: 10.1080/10543406.2010.544437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun L, Cui B, Wei X, et al. Cost-effectiveness of genetic testing for all women diagnosed with breast cancer in China. Cancers. 2022;14(7):1839. doi: 10.3390/cancers14071839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pitt C, Vassall A, Teerawattananon Y, et al. Foreword: health economic evaluations in low- and middle-income countries: methodological issues and challenges for priority setting. Health Econ. 2016;25 Suppl 1(Suppl 1):1–5. doi: 10.1002/hec.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen TH-H, Yen AMF, Fann JC-Y, et al. Clarifying the debate on population-based screening for breast cancer with mammography: a systematic review of randomized controlled trials on mammography with Bayesian meta-analysis and causal model. Medicine. 2017;96(3):e5684. doi: 10.1097/md.0000000000005684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang B, He M, Wang L, et al. Breast cancer screening among adult women in China, 2010. Prev Chronic Dis. 2013;10:E183. doi: 10.5888/pcd10.130136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang Y, Zhou K, Li H, et al. Knowledge, attitudes, and behaviour regarding breast cancer screening among women from different socio-economic regions in southwest China: a cross-sectional study. Asian Pac J Cancer Prev. 2011;12(1):203–209. [PubMed] [Google Scholar]
- 35.Jiang S, Wang Y, Si L, et al. Incorporating productivity loss in health economic evaluations: a review of guidelines and practices worldwide for research agenda in China. Bmj Global Heal. 2022;7(8):e009777. doi: 10.1136/bmjgh-2022-009777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang S, Chen Z, Wu J, et al. Addressing methodological and ethical issues in practicing health economic evaluation in China. J Glob Health. 2020 doi: 10.7189/jogh.10.020322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.China Society for Pharmacoeconomics and Outcomes Research. China Guidelines for Pharmacoeconomic Evaluations 2020 Edition. 2020. https://www.ispor.org/heor-resources/more-heor-resources/pharmacoeconomic-guidelines/pe-guideline-detail/china-mainland.
- 38.Chen Z, Jiang S, Wang Y, et al. Pharmacoeconomics of obesity in China: a scoping review. Expert Rev Pharm Out. 2021 doi: 10.1080/14737167.2021.1882306. [DOI] [PubMed] [Google Scholar]
- 39.Beecham J, Knapp M. Costing psychiatric interventions. Meas Mental Health Needs. 2001;2:200–224. [Google Scholar]
- 40.Xuan Q, Gao K, Song Y, et al. Adherence to needed adjuvant therapy could decrease recurrence rates for rural patients with early breast cancer. Clin Breast Cancer. 2016;16(6):e165–e173. doi: 10.1016/j.clbc.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 41.Wen D, Wen X, Yang Y, et al. Urban rural disparity in female breast cancer incidence rate in China and the increasing trend in parallel with socioeconomic development and urbanization in a rural setting. Thorac Cancer. 2018;9(2):262–272. doi: 10.1111/1759-7714.12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang F, Jiang S, He X-n, et al. Do rural residents in China understand EQ-5D-5L as intended? Evidence from a qualitative study. Pharmacoecon Open. 2020 doi: 10.1007/s41669-020-00212-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cai D, Shi S, Jiang S, et al. Estimation of the cost-effective threshold of a quality-adjusted life year in China based on the value of statistical life. Eur J Health Econom. 2021 doi: 10.1007/s10198-021-01384-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ochalek J, Wang H, Gu Y, et al. Informing a cost-effectiveness threshold for health technology assessment in China: a marginal productivity approach. Pharmacoeconomics. 2020 doi: 10.1007/s40273-020-00954-y. [DOI] [PubMed] [Google Scholar]
- 45.Ochalek J, Wang H, Gu Y, et al. Informing a cost-effectiveness threshold for health technology assessment in China: a marginal productivity approach. Pharmacoeconomics. 2020;38(12):1319–1331. doi: 10.1007/s40273-020-00954-y. [DOI] [PubMed] [Google Scholar]
- 46.Bertram MY, Lauer JA, De Joncheere K, et al. Cost-effectiveness thresholds: pros and cons. Bull World Health Organ. 2016;94(12):925–930. doi: 10.2471/blt.15.164418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McDougall JA, Furnback WE, Wang BCM, et al. Understanding the global measurement of willingness to pay in health. J Mark Access Health Policy. 2020;8(1):1717030. doi: 10.1080/20016689.2020.1717030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schurer M, Matthijsse SM, Vossen CY, et al. Varying willingness to pay based on severity of illness: impact on health technology assessment outcomes of inpatient and outpatient drug therapies in the Netherlands. Value Health. 2022;25(1):91–103. doi: 10.1016/j.jval.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 49.Chen C, Sun S, Yuan JP, et al. Characteristics of breast cancer in Central China, literature review and comparison with USA. Breast. 2016;30:208–213. doi: 10.1016/j.breast.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 50.Song QK, Li J, Huang R, et al. Age of diagnosis of breast cancer in china: almost 10 years earlier than in the United States and the European Union. Asian Pac J Cancer Prev. 2014;15(22):10021–10025. doi: 10.7314/apjcp.2014.15.22.10021. [DOI] [PubMed] [Google Scholar]
- 51.Sun J, Meng H, Yao L, et al. Germline mutations in cancer susceptibility genes in a large series of unselected breast cancer patients. Clin Cancer Res. 2017;23(20):6113–6119. doi: 10.1158/1078-0432.Ccr-16-3227. [DOI] [PubMed] [Google Scholar]
- 52.Nieuwenhuis MH, Kets CM, Murphy-Ryan M, et al. Cancer risk and genotype-phenotype correlations in PTEN hamartoma tumor syndrome. Fam Cancer. 2014;13(1):57–63. doi: 10.1007/s10689-013-9674-3. [DOI] [PubMed] [Google Scholar]
- 53.Heymann S, Delaloge S, Rahal A, et al. Radio-induced malignancies after breast cancer postoperative radiotherapy in patients with Li-Fraumeni syndrome. Radiat Oncol. 2010;5:104. doi: 10.1186/1748-717x-5-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cobain EF, Milliron KJ, Merajver SD. Updates on breast cancer genetics: clinical implications of detecting syndromes of inherited increased susceptibility to breast cancer. Semin Oncol. 2016;43(5):528–535. doi: 10.1053/j.seminoncol.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 55.Pederson HJ, Padia SA, May M, et al. Managing patients at genetic risk of breast cancer. Cleve Clin J Med. 2016;83(3):199–206. doi: 10.3949/ccjm.83a.14057. [DOI] [PubMed] [Google Scholar]
- 56.Zheng Y, Dong X, Li J, et al. Use of breast cancer risk factors to identify risk-adapted starting age of screening in China. Jama Netw Open. 2022;5(11):e2241441. doi: 10.1001/jamanetworkopen.2022.41441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tuffaha HW, Mitchell A, Ward RL, et al. Cost-effectiveness analysis of germ-line BRCA testing in women with breast cancer and cascade testing in family members of mutation carriers. Genet Med. 2018;20(9):985–994. doi: 10.1038/gim.2017.231. [DOI] [PubMed] [Google Scholar]
- 58.Jiang S, Anis AH, Cromwell I, et al. Health-care practitioners’ preferences for the return of secondary findings from next-generation sequencing: a discrete choice experiment. Genet Med. 2020 doi: 10.1038/s41436-020-0927-x. [DOI] [PubMed] [Google Scholar]
- 59.Jiang S. A scoping review of global guidelines for the disclosure of secondary genomic findings to inform the establishment of guidelines in China. China CDC Wkly. 2022;4(32):697–705. doi: 10.46234/ccdcw2022.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Green RC, Berg JS, Grody WW, et al. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med. 2013;15(7):565–574. doi: 10.1038/gim.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vayena E, Tasioulas J. Genetic incidental findings: autonomy regained? Genet Med. 2013;15(11):868–870. doi: 10.1038/gim.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Downing NR, Williams JK, Daack-Hirsch S, et al. Genetics specialists’ perspectives on disclosure of genomic incidental findings in the clinical setting. Patient Educ Couns. 2013;90(1):133–138. doi: 10.1016/j.pec.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gray SW, Hicks-Courant K, Lathan CS, et al. Attitudes of patients with cancer about personalized medicine and somatic genetic testing. J Oncol Pract. 2012;8(6):329–335. doi: 10.1200/jop.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bennette CS, Gallego CJ, Burke W, et al. The cost-effectiveness of returning incidental findings from next-generation genomic sequencing. Genet Med. 2015;17(7):587–595. doi: 10.1038/gim.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guzauskas GF, Garbett S, Zhou Z, et al. Cost-effectiveness of population-wide genomic screening for hereditary breast and ovarian cancer in the United States. JAMA Netw Open. 2020;3(10):e2022874. doi: 10.1001/jamanetworkopen.2020.22874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Müller D, Danner M, Rhiem K, et al. Cost-effectiveness of different strategies to prevent breast and ovarian cancer in German women with a BRCA 1 or 2 mutation. Eur J Health Econ. 2018;19(3):341–353. doi: 10.1007/s10198-017-0887-5. [DOI] [PubMed] [Google Scholar]
- 67.Mavaddat N, Peock S, Frost D, et al. Cancer risks for BRCA1 and BRCA2 mutation carriers: results from prospective analysis of EMBRACE. J Natl Cancer Inst. 2013;105(11):812–822. doi: 10.1093/jnci/djt095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.