Abstract
Background:
The ophthalmic segment of the internal carotid artery (ICA) represents a common site for cerebral aneurysms. However, aneurysms of the ophthalmic artery (OphA) itself represent rare lesions and have been associated with trauma and flow-related lesions such as arteriovenous fistulas or malformations. Here, we explore clinical and radiological features of four patients managed for five proper ophthalmic artery aneurysms (POAAs).
Methods:
Patients undergoing diagnostic cerebral angiogram (DCA) between January 2018 and November 2021 with newly or previously identified POAA were retrospectively reviewed. Clinical and radiological data were analyzed to identify common and unique features.
Results:
Four patients with identification of five POAA were identified. Three patients suffered traumatic brain injury with subsequent identification of POAA on DCA. Patient 1 presented with a traumatic carotid-cavernous-sinus fistula requiring transvenous coil embolization and second stage flow diversion of the ICA. Patient 2 suffered a gunshot wound with ICA compromise, ethmoidal dural arteriovenous fistula (dAVF) development with rapid growth of two POAAs eventually requiring Onyx embolization. Patient 3 was assaulted and DCA showed a POAA without any other cerebrovascular pathology. Patient 4 had undergone N-butyl cyanoacrylate embolization of an ethmoidal dAVF 13 years ago with the feeding OphA carrying a large POAA. Re-DCADCA was performed for a newly developed and unrelated transverse-sigmoid-sinus dAVF.
Conclusion:
Management of POAAs poses a challenge to neurovascular surgeons since POAAs inherit a risk for visual deterioration or hemorrhage. DCA facilitates identification of coexisting cerebrovascular pathology. If clinically silent and not accompanied by cerebrovascular disease, observation appears reasonable.
Keywords: Aneurysm, Dural arteriovenous fistula, Ophthalmic artery

INTRODUCTION
The term ophthalmic artery (OphA) aneurysm is commonly used to delineate ophthalmic segment internal carotid artery (ICA) aneurysms. Terminology for aneurysms of the OphA itself is diverse. Terms such as proper, true, itself, and stem have been used. However, those terms often do not appropriately display anatomical or histological features. Qiao et al. utilized the term peripheral OphA aneurysm. In their report, intracranial, intracanalicular, intraorbital, and terminal aneurysms of the proper OphA have been reviewed.[18] Proper OphA aneurysms (POAAs) have been commonly associated with blunt or penetrating cranial trauma or cerebrovascular diseases that change cerebrovascular hemodynamics such as dural arteriovenous fistulas (dAVF).[2,11] The majority of reported POAAs are unruptured, while in few cases, SAH or complications from an intraorbital rupture have been reported.[16,18] Although several case reports of POAAs have been published, they still do represent rare lesions. Here, the authors seek to review similarities and differences in the presentation, neuroimaging, and management of four patients with five POAAs encountered at their institution.
MATERIALS AND METHODS
Exploring the institutional prospectively maintained case log, a total of four cases with newly or previously identified POAAs between January 2018 and June 2021 were found. In the study period, 3542 diagnostic cerebral angiograms (DCAs) in 2704 patients were performed. Local institutional review board (IRB) approval was obtained for retrospective analysis (IRB #2021-0901), patient consent was waived. The herein presented study is in accordance with ethical standards of the Declaration of Helsinki. Clinical and radiological data from the electronic medical records were reviewed.
RESULTS
Patient 1
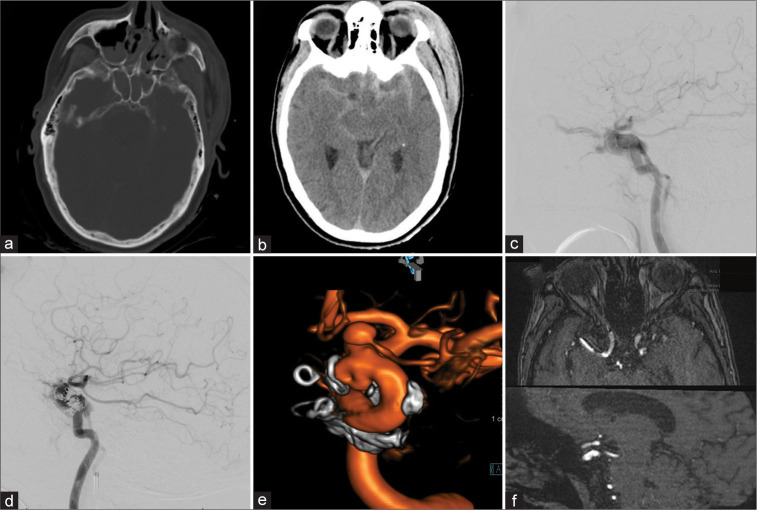
A 55-year-old female presented after a motor bicycle accident with subarachnoid hemorrhage, bilateral subdural hematoma pronounced around the tentorial leaflets, bilateral sphenoid, nasal septal, and left zygomaticomaxillary complex fractures with an associated pansinus hemorrhage and a large left scalp hematoma [Figures 1a and b]. The patient was noted to have left eye exophthalmos, chemosis, and decreased visual acuity – only able to see light and shapes. A DCA showed a left Type A carotid-cavernous-sinus-fistula (CCF) [Figure 1c] which was subsequently treated with transvenous coil embolization [Figure 1d]. A follow-up DCA 8 months later showed no residual arteriovenous shunting but did show a focal outpouching along the dorsomedial aspect of the supraclinoid left ICA likely representing the traumatic site of the ICA injury. Furthermore, a 4 mm broad-based aneurysm of the proximal segment of the left OphA was evident [Figure 1e]. The patient underwent flow diversion using pipeline embolization device. The patient refused to undergo follow-up DCA at 6 months. However, magnetic resonance angiography (MRA) demonstrated disappearance of the outpouching [Figure 1f]. The visual status remained unchanged.
Figure 1:
Patient 1, 55 F, traumatic brain injury. Admission computed tomography-head (a and b). Type A carotid-cavernous fistula in lateral diagnostic cerebral angiogram (DCA)-projection, the retrograde filling of the left superior ophthalmic vein is evident (c). Transvenous coil-embolization was performed in the acute setting (d). Follow-up DCA after 8 months showed a dorsal-medial focal outpouching of the internal carotid artery (ICA) as well as a prominent intracranial proper ophthalmic artery aneurysm (e). Flow diversion using Pipeline embolization device was performed and 6-month follow-up magnetic resonance angiography shows resolution of the ICA defect (f).
Patient 2
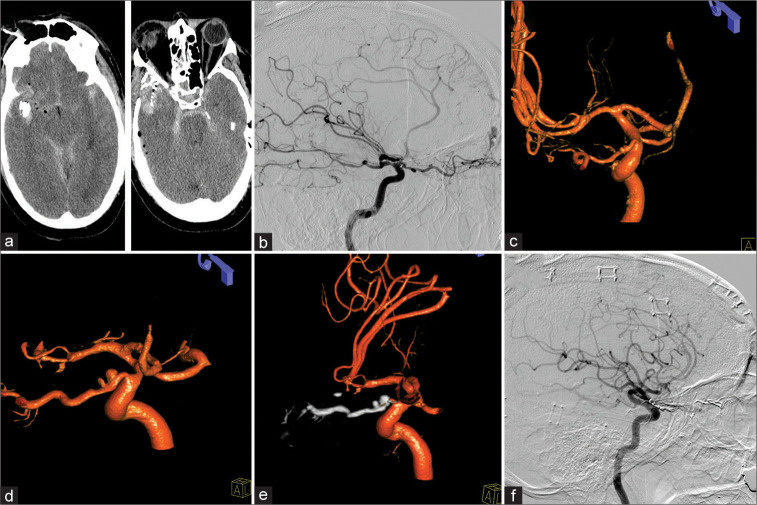
A 58-year-old male suffered a right-sided temporal gunshot wound. The projectile tract had a downward trajectory extending through the right posterior orbit, nasal septum, and left maxillary sinus with sparing of the left orbit [Figure 2a]. Bullet and bone fragments within the right temporal lobe with surrounding intraparenchymal hematoma required emergent hemicraniectomy, wound debridement, and partial temporal lobectomy. The patient’s demonstrated a right sided fixed and dilated pupil on admission with a Glasgow coma scale of 7. Computed tomography-angiography (CTA) did not show evidence for vascular injury but interpretation was limited due to bullet fragment artifacts. DCA eventually revealed a right-sided ethmoidal dAVF [Figure 2b] and two superiorly directed minimal outpouchings of the dAVF feeding OphA [Figure 2c]. Due to severe right-sided ICA, anterior cerebral artery, and middle cerebral artery vasospasm, the patient underwent repeated intra-arterial vasodilation treatment with nicardipine, nitroglycerine, and verapamil. Ophthalmologic evaluation confirmed right eye vision loss. Two weeks out, the patient developed a right frontotemporal abscess requiring surgical evacuation. Another week later (3 weeks in total), both minor outpouchings had progressed to two distinct POAAs, one at the intracranial and one at the intracanalicular segment of OphA [Figure 2d]. Due to loss of vision on admission and the OphA feeding the dAVF, decision was made to embolize the feeding artery and the POAAs using Onyx [Figure 2e]. Six-week follow-up DCA demonstrated obliteration of the OphA, the POAAs, and the dAVF. However, left ICA injection demonstrated dAVF recurrence fed by the left OphA. The patient underwent open surgical treatment for the fistula and 6 months follow-up eventually demonstrated no recurrence [Figure 2f]. Cranioplasty was performed 3 months later and at 8 months after the trauma, the patient remained with right amaurosis but otherwise recovered without other significant disabilities.
Figure 2:
Patient 2, 58 M, right temporal gunshot wound. Admission computed tomography-head (a). A traumatic right-sided ethmoidal dural arteriovenous fistula (dAVF) was evident on lateral diagnostic cerebral angiogram (b). Two minimal outpouchings of the intracranial and intracanalicular segment of the ophthalmic artery were identified as well (c). Three weeks after a complicated course, both proper ophthalmic artery aneurysm (POAAs) grew (d). Treatment of dAVF as well as POAAs was performed through Onyx embolization (e) with successful resolution of the POAAs as well as dAVF (f).
Patient 3
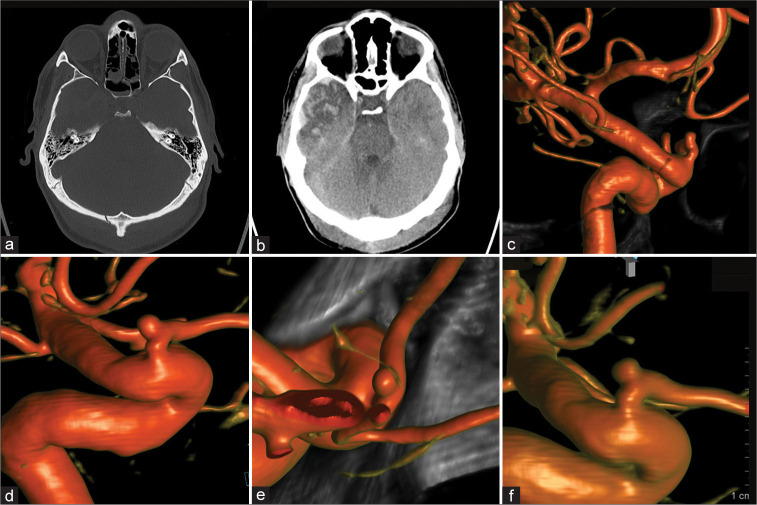
A 31-year-old male was assaulted with a metallic objected and suffered a strike against the head. He was admitted to an outside hospital and a non contrast head CT imaging showed an occipital skull fracture, small falcine SDH, and a right temporal contusion [Figures 3a and b]. Neurological decline with increased intracranial pressures (ICPs) required invasive ICP monitoring but no further neurosurgical intervention. The patient eventually recovered with residual tinnitus. The initially performed CTA did not reveal vascular injuries. Follow-up with DCA was recommended and performed at our institution 4 months after trauma. It demonstrated a small left POAA [Figure 3c] without any other cerebrovascular pathologies. Observation was recommended due to the asymptomatic POAA. A follow-up DCA 4 months, nine, and finally 21 months later demonstrated stable size and morphology [Figures 3d-f, respectively]. The patient remained asymptomatic.
Figure 3:
Patient 3, 31 M, traumatic brain injury. Admission computed tomography-head (a and b). Four-month follow-up diagnostic cerebral angiogram (DCA) for potential vascular compromise was performed revealing a small intracranial proper ophthalmic artery aneurysm (c). Subsequent 4, 9, and 21 months DCA follow-ups demonstrated stable aneurysm size and morphology (d-f, respectively).
Patient 4
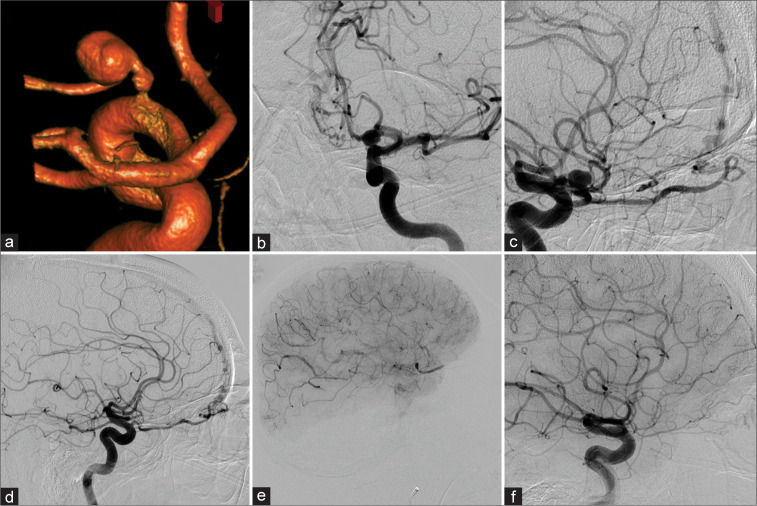
In 2008, a 68-year-old male patient complaining of retro-orbital pain was found to have a left POAA on a CT scan of the orbits. A magnetic resonance imaging and MRA were completed that redemonstrated the aneurysm. The subsequent DCA showed the aneurysm arising from the left OphA [Figures 4a and b] and bilateral ethmoidal artery dAVFs [Figures 4c and d]. Based on the treating physician’s preference, the right AVF was treated with N-butyl cyanoacrylate (NBCA) and the patient’s vision remained intact. Two weeks later, the left AVF was treated with NBCA. Despite embolization distally in the OphA at the fistulous point, the intraoperative angiography had shown contrast stasis within the intraorbital OphA segment. Stasis was also observed in the POAA [Figure 4e]. The patient suffered visual loss with subsequent decreased vision in the left eye. Five months later, DCA demonstrated that the aneurysm had resolved completely and the minute reconstitution of the OphA including a choroidal blush [Figure 4f]. The patient represented 12 years later with double vision on upgaze, still with only light-dark perception in the left eye. CTA imaging was inconclusive. A DCA was completed and did not show any cerebrovascular pathology in the anterior circulation. The OphA caliber was stable without evidence of the previous POAA. Bilateral posterior meningeal arteries feeding a dAVF draining into the straight sinus were identified. The AVF was embolized with Onyx through the right side with complete obliteration.
Figure 4:
Patient 4 was diagnosed with a left-sided proper ophthalmic artery aneurysm (POAA) (a-c) in a setting of a left (b and c) and right (d) sided ethomoidal dural arterio-venous fistula. After right-sided N-butyl cyanoacrylate (NBCA) embolization, the left side was treated with NBCA as well. However, the ophthalmic artery (OphA) demonstrated contrast stasis in the intraorbital segment – as well as in the POAA – in the postembolization run (e). Follow-up diagnostic cerebral angiogram (DCA) 5 months later revealed reconstitution of a small calibered OphA. However, vision did not recover beyond light-dark perception. Twelve years later, the DCA demonstrated equal findings (f).
DISCUSSION
To date, several case reports of POAAs have been published. Still, POAAs remain poorly understood and thus pose a challenge if identified on cerebrovascular imaging. POAAs have been identified arising from all OphA segments with the intracanalicular location representing the least common site, while the intracranial and the intraorbital locations appear to be the most prominent.[2,13,17,18,21] Clinically, visual deterioration and visual loss are the predominant symptoms associated with POAAs attributable to the proximity of the vasculature and optic nerve.[8-10] Intraorbital POAAs have been reported to also present with exophthalmos, extraocular muscle palsies and pain. Only a subset of reported POAAs had ruptured causing aneurysmal subarachnoid, intracerebral, intraorbital, or subconjunctival hemorrhage.[16,18,21] Etiology of POAAs is not yet established and a genetic component has also not yet been identified.[6] However, POAAs have commonly been described in the setting of traumatic brain injury with vascular comprise. Hence, pseudoaneurysm formation or development of dissecting/fusiform aneurysms prone to rapid growth are likely.[3,23] The rapid growth of both POAAs of Patient 3 likely was subject to this etiology. The high-impact traumatic brain injury followed by a prolonged course of severe vasospasm as well as adjacent parenchymal abscess formation might have rendered aneurysm stabilization unlikely facilitating rapid progression. Presence and formation of POAAs in context with cerebrovascular pathologies that alter intracranial flow hemodynamics have also been established. Flow-related aneurysms are frequently encountered with anatomic variations of the cerebral arterial vasculature,[12] brain arteriovenous malformations[14,19] and brain dAVF.[1,7] In the presented case series, three patients harbored arteriovenous fistulas involving the ophthalmic segment of the ICA (two ethmoidal dAVFs and one Type A CCF). In contrast to trauma, altered hemodynamics is more likely to cause true (saccular) aneurysm formation. Subsequently, treatment of the underlying cause such as dAVF embolization or surgical obliteration represents a curative approach that also affect flow-related aneurysms. This has been observed in Patient 4 and also observed by others.[13] In distinct cases, endovascular proximal OphA sacrifice[17] or OphA flow diversion has been successful.[21] Besides endovascular treatment, microsurgical options include clip obliteration specifically for intracranial segment POAAs. Depending on lesion location and aneurysm size, anterior clinoidectomy and optic canal decompression (unroofing) are required.[11,20,22] Finally, several case reports recommended watchful waiting for asymptomatic and incidental POAAs.[4,15] Predictive aneurysm rupture score such as PHASES is likely not applicable to POAAs due to most ruptured aneurysms presenting with low PHASES scores, the potential different etiology of POAAs opposed to true saccular aneurysms as well as the original studies not including POAAs.[5] Repeated DCAs in the asymptomatic Patient 3 from the present series demonstrated stable POAA size and morphology over a 2-year course. Although the lesion was identified after cranial trauma, etiology cannot be determined. However, the stable clinical and radiological course underline that conservative management is a valuable option for a certain subset of POAAs.
Limitation
The small series of POAAs bases on imaging reports during the study period. However, the herein identified patient count with POAAs per patient-angiograms in the study period cannot reliably be used to estimate a prevalence of POAAs. Not every patient underwent 3D-rotational angiography and small POAAs might easily be overlooked without proper angiographic assessment of the OphA.
CONCLUSION
POAAs represent a rare entity that may go unnoticed in patients suffering from traumatic brain injury or cerebrovascular pathologies. Still, they do pose a risk for visual deterioration or even hemorrhage. This report adds to the scant literature on POAAs and may help informing clinical decision-making.
Ethical statement
Ethical approval: Approval was obtained by the local IRB #2021-0901
Informed consent: waived by IRB for retrospective analysis.
Footnotes
How to cite this article: Hendrix P, Bohan C, Dalal SS, Weiner GM, Kanmounye US, Schirmer CM, et al. Proper ophthalmic artery aneurysms. Surg Neurol Int 2023;14:105.
Contributor Information
Philipp Hendrix, Email: phendrix@geisinger.edu.
Christian Bohan, Email: cobohan1@geisinger.edu.
Shamsher Singh Dalal, Email: ssdalal@geisinger.edu.
Gregory M. Weiner, Email: gweiner@geisinger.edu.
Ulrick S. Kanmounye, Email: ukanmounyekouokam@geisinger.edu.
Clemens M. Schirmer, Email: cmschirmer@geisinger.edu.
Oded Goren, Email: ogoren@geisinger.edu.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
REFERENCES
- 1.Cagnazzo F, Peluso A, Vannozzi R, Brinjikji W, Lanzino G, Perrini P. Arterial aneurysms associated with intracranial dural arteriovenous fistulas: Epidemiology, natural history, and management. A systematic review. Neurosurg Rev. 2019;42:277–85. doi: 10.1007/s10143-017-0929-6. [DOI] [PubMed] [Google Scholar]
- 2.Choi BK, Lee TH, Choi CH, Lee SW. Fusiform intracanalicular ophthalmic artery aneurysm; Case report and review of literature. J Korean Neurosurg Soc. 2008;44:43–6. doi: 10.3340/jkns.2008.44.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chun HJ, Yi HJ. Traumatic extracranial pseudoaneurysm on the peripheral ophthalmic artery presenting as delayed intraparenchymal hematoma: Case report. Surg Neurol. 2009;71:701–4. doi: 10.1016/j.surneu.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Dehdashti AR, Safran AB, Martin JB, Rüfenacht DA, de Tribolet N. Intraorbital ophthalmic artery aneurysm associated with basilar tip saccular aneurysm. Neuroradiology. 2002;44:600–3. doi: 10.1007/s00234-002-0786-y. [DOI] [PubMed] [Google Scholar]
- 5.Foreman PM, Hendrix P, Harrigan MR, Fisher WS, 3rd, Vyas NA, Lipsky RH, et al. PHASES score applied to a prospective cohort of aneurysmal subarachnoid hemorrhage patients. J Clin Neurosci. 2018;53:69–73. doi: 10.1016/j.jocn.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Griessenauer CJ, Farrell S, Sarkar A, Zand R, Abedi V, Holland N, et al. Genetic susceptibility to cerebrovascular disease: A systematic review. J Cereb Blood Flow Metab. 2018;38:1853–71. doi: 10.1177/0271678X18797958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gross BA, Ropper AE, Du R. Cerebral dural arteriovenous fistulas and aneurysms. Neurosurg Focus. 2012;32:E2. doi: 10.3171/2011.12.FOCUS11336. [DOI] [PubMed] [Google Scholar]
- 8.Hendrix P, Griessenauer CJ, Foreman P, Loukas M, Fisher WS, 3rd, Rizk E, et al. Arterial supply of the lower cranial nerves: A comprehensive review. Clin Anat. 2014;27:108–17. doi: 10.1002/ca.22318. [DOI] [PubMed] [Google Scholar]
- 9.Hendrix P, Griessenauer CJ, Foreman P, Shoja MM, Loukas M, Tubbs RS. Arterial supply of the upper cranial nerves: A comprehensive review. Clin Anat. 2014;27:1159–66. doi: 10.1002/ca.22415. [DOI] [PubMed] [Google Scholar]
- 10.Hendrix P, Griessenauer CJ, Foreman P, Shoja MM, Tubbs RS. Nerves and Nerve Injuries. Netherlands: Elsevier Science; 2015. Blood supply of the cranial nerves; pp. 427–38. [Google Scholar]
- 11.Kawaguchi S, Sakaki T, Okuno S, Uchiyama Y, Nishioka T. Peripheral ophthalmic artery aneurysm. Report of two cases. J Neurosurg. 2001;94:822–5. doi: 10.3171/jns.2001.94.5.0822. [DOI] [PubMed] [Google Scholar]
- 12.Kayembe KN, Sasahara M, Hazama F. Cerebral aneurysms and variations in the circle of Willis. Stroke. 1984;15:846–50. doi: 10.1161/01.str.15.5.846. [DOI] [PubMed] [Google Scholar]
- 13.Martínez-Pérez R, Tsimpas A, Ruiz Á, Montivero A, Mura J. Spontaneous regression of a true intracanalicular fusiform ophthalmic artery aneurysm after endovascular treatment of an associated dural arteriovenous fistula. World Neurosurg. 2018;119:362–5. doi: 10.1016/j.wneu.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 14.Meisel HJ, Mansmann U, Alvarez H, Rodesch G, Brock M, Lasjaunias P. Cerebral arteriovenous malformations and associated aneurysms: Analysis of 305 cases from a series of 662 patients. Neurosurgery. 2000;46:793–800. doi: 10.1097/00006123-200004000-00004. discussion 800-2. [DOI] [PubMed] [Google Scholar]
- 15.Pandey P, Rayes M, Guthikonda M, Xavier A. Peripheral ophthalmic artery aneurysm associated with multiple intracranial aneurysms: A case report. J Neurointerv Surg. 2010;2:211–2. doi: 10.1136/jnis.2009.001107. [DOI] [PubMed] [Google Scholar]
- 16.Peschillo S, Biraschi F, Diana F, Colonnese C, Marenco M, Delfini R. Aneurysms of the intracranial segment of the ophthalmic artery trunk: Case report and systematic literature review. J Neurol Surg A Cent Eur Neurosurg. 2018;79:257–61. doi: 10.1055/s-0037-1604268. [DOI] [PubMed] [Google Scholar]
- 17.Piché SL, Haw CS, Redekop GJ, Heran MK. Rare intracanalicular ophthalmic aneurysm: Endovascular treatment and review of the literature. AJNR Am J Neuroradiol. 2005;26:1929–31. [PMC free article] [PubMed] [Google Scholar]
- 18.Qiao L, Wang H, Mao L, Chen S, Xie W, Wu Q. Peripheral ophthalmic artery aneurysm. Neurosurg Rev. 2011;34:29–38. doi: 10.1007/s10143-010-0290-5. [DOI] [PubMed] [Google Scholar]
- 19.Redekop G, TerBrugge K, Montanera W, Willinsky R. Arterial aneurysms associated with cerebral arteriovenous malformations: Classification, incidence, and risk of hemorrhage. J Neurosurg. 1998;89:539–46. doi: 10.3171/jns.1998.89.4.0539. [DOI] [PubMed] [Google Scholar]
- 20.Sato S, Suga S, Ohira T, Takayama H, Kawase T. Aneurysm of the ophthalmic artery trunk. Acta Neurochir (Wien) 1999;141:321–2. doi: 10.1007/s007010050304. [DOI] [PubMed] [Google Scholar]
- 21.Sirakov S, Sirakov A, Tsonev H, Hristov H. Ruptured intracanalicular ophthalmic artery aneurysm treated with low profile flow diverter device: Case report. Clin Neuroradiol. 2020;30:177–80. doi: 10.1007/s00062-019-00792-2. [DOI] [PubMed] [Google Scholar]
- 22.Yanaka K, Matsumaru Y, Kamezaki T, Nose T. Ruptured aneurysm of the ophthalmic artery trunk demonstrated by three-dimensional rotational angiography: Case report. Neurosurgery. 2002;51:1066–9. doi: 10.1097/00006123-200210000-00038. discussion 1069-70. [DOI] [PubMed] [Google Scholar]
- 23.Zheng Y, Lu Z, Shen J, Xu F. Intracranial pseudoaneurysms: Evaluation and management. Front Neurol. 2020;11:582. doi: 10.3389/fneur.2020.00582. [DOI] [PMC free article] [PubMed] [Google Scholar]