Abstract
Background:
Cisternostomy is a surgical technique thought of and developed as an option for severe brain trauma treatment. It demands a particular knowledge and skill to microsurgically approach basal cisterns and effectively manipulate their contents. To perform this procedure safely, the anatomy and pathophysiology must be clearly understood.
Methods:
Detailed microscopic dissection and anatomical review were done, after a detailed reading of facts and recent publications about cisternostomy. Cisternal pathways and landmark planning are described and augmented using a new method to show de arachnoid borders. Finally, a brief discussion is written as a synopsis.
Results:
Cisternostomy requires thorough microscopic knowledge and microsurgical skills. This paper intends to provide information to understand better the anatomy related, thus, easing the learning curve. The technique used to show arachnoid borders, complementing cadaveric and surgical images, was useful for this purpose.
Conclusion:
To perform this procedure safely, it is mandatory to handle microscopic details of cistern anatomy. Reaching a core cistern is necessary to assure effectiveness. This procedure needs, as well, surgical step-by-step landmark planning and performing. Cisternostomy could be a life-saving procedure and a new powerful tool for severe brain trauma treatment. Evidence is being collected to support its indications.
Keywords: Basal cisterns anatomy, Cisternostomy, Microsurgical technique, Severe brain trauma
INTRODUCTION
Since 1699, the basal cisterns were defined as particular spaces in the cranial cavity, limited by meninx and formed as an enlargement of subarachnoid space.[13] These spaces divide and take names according to their anatomical position. Those names were adapted, transformed, and classified differently by many authors, but mostly following the first complete description made by professor Yasargil et al. in the mid-1970s.[39]
Many attempts to homolog both names and classifications, do not reach a unanimous response even now. Many of them still use clinical eponyms or other terms outside the Federative International Programme for Anatomical Terminology (FIPAT), a program of the International Federation of Associations of Anatomists (IFAA) chapter for neuroscience.[14] To simplify this reading, we followed FIPAT/IFAA rules (n. 285–316), offering an anatomical review, which follows recent papers and most of the classical descriptions.[4,33,36,38]
Cisternostomy is a microsurgical procedure that approaches basal cisterns intimately. It was thought of as an option for severe and diffuse brain trauma treatment, following a new perspective on pathophysiology and pathology.[9,23] Nowadays, this project is collecting evidence from randomized controlled trials.[7]
Herein, we intend to report a detailed description of cisterns anatomy, with the purpose of illustrating the procedure of cisternostomy. Being the main surgical purpose to reach the pre-pontine cistern, running through a safe anatomical pathway is mandatory.
MATERIALS AND METHODS
Methods for anatomical description
Multiserver research about classical cisternal anatomy descriptions and recent surgical publications was done (PubMed®, NEJM®, Scielo®). This was followed by the production of images to illustrate the most important concepts.
For the image production, two objectives were aimed: first to build a solid spatial concept; second to show, in parallel, the real-surgical view. To achieve those goals, three kinds of images will be presented: (i) surgical interventions video captures where the different cisterns are approached; (ii) cadaveric dissection images where the different elements could be better described; and (iii) microanatomy laboratory images produced to show the arachnoid limits and membranes insertions, using a new technique developed for this intention.
Cisterns overview
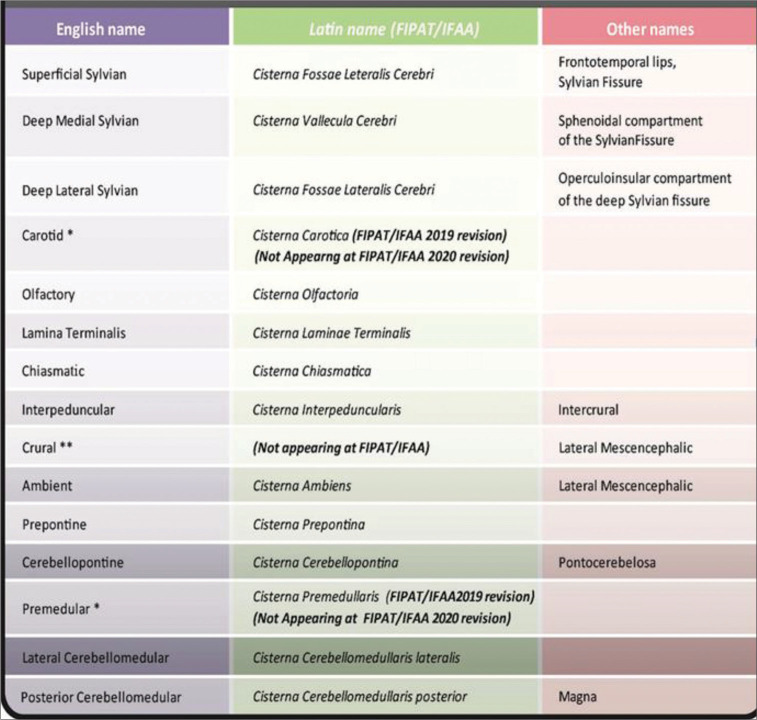
A cranial cistern is a space between the two known layers of soft meninx (arachnoid and pia mater, which limits a big space named the subarachnoid space). The external layer, the arachnoid, covers the whole surface of the brain without getting into deep landmarks, keeping faithful to the dura surface and bridging big spaces. The internal layer, pia mater, otherwise, gets into every single fissure, following vessels just up to microcirculation levels, marking a three-dimensional tube around them, which is named perivascular space of Virchow-Robin.[16,23] This Virchow-Robin space plays the main role in severe brain trauma pathophysiology. Filling spaces in the brain and skull base cisterns adopt anatomical neighbor’s names, such as “carotid,” “supra-cerebellar” or “chiasmatic.”[33,39] A list of cranial cisterns is offered as a general synopsis in the Figures 1 and 2.
Figure 1:
List of cranial cisterns.
Figure 2:
List of cranial cisterns.
The cisternal spaces are filled with cerebrospinal fluid (CSF) and are transited by structural elements (i.e., a nerve or a vessel). Taken as a group, cisterns CSF acts as a reservoir, playing a role as a part of the total intracranial volume. Approximately 125 mL corresponds to cisternal CSF (75% spinal/25% cranial, approximately) and just 25 mL to ventricular CSF.[3] Both, cisternal and ventricular, are only connected through the lateral aperture of the fourth ventricle (foramen of Luschka) to the Cerebellopontine Cistern, and the median aperture of the fourth ventricle (foramen of Magendie) to the Posterior cerebellomedullary cistern or Cisterna Magna.
Once in the cisternal space, CSF can move from one compartment to another. Divisions between them are formed by an incomplete porous wall with various-sized openings.[24,39] Trabecular adhesions between its walls confer this space the universal term of “subarachnoid” space. The latter is not a small detail; these adhesions turn into the main challenge to dissecting a cistern, manipulating its contents, or opening a surgical pathway between them.
CSF physiopathology
CSF has a regular way to be produced, flowing, and to be reabsorbed by intracranial structures. Particularly, cerebral cisterns act as a pathway and a reservoir.[19]
Once the CSF is produced by the choroidal plexus, it starts an intraventricular route going to the IVth ventricle to finally reach the subarachnoid space. Once here, it flows filling every cistern and space in the cranial cavity and the spinal compartment. The CSF has continuity between intraventricular space and the central canal (Canalis centralis) at spinal levels. The same continuity is present for subarachnoid space, through the posterior cerebromedullary cistern (Cisterna magna) and the premedullary cistern to the perimedullary space.
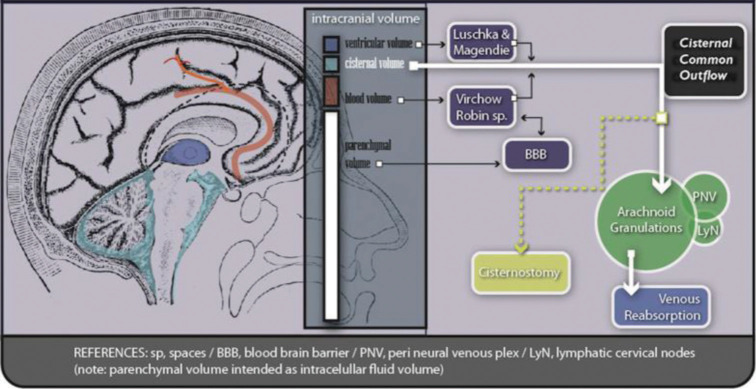
The subarachnoid space offers certain places for CSF to be reabsorbed.[4,19,35] Arachnoid villi (mostly at the superior sagittal sinus), rich venous plexus near cranial and spinal nerves, and the submucosal plate at the lamina cribosa. All of them have the internal jugular vein as the main exit route, but also to the cervicofacial lymphatic net. The latter presents a particular linking to the perivascular space of Virchow Robin, through perineural veins, but a definitive route for them remains poorly elucidated.[35] A general description of this CSF flow and anatomy is shown in Figure 3.
Figure 3:
Intracranial fluids circulation. This figure represents a schematic drawing showing the implication of cisternostomy (light green box) in the common outflow of all brain fluids.
Little volume, in normal conditions, is added to the choroidal plexus production, generally coming from neuronal and neuroglia metabolism from the brain parenchyma. It is collected through the Virchow Robin spaces and regulated by the blood-brain barrier (BBB).[19,23,35] This little amount of volume may vary when a pathological situation arrives, for example, trauma.
After trauma the situation changes drastically: BBB turns much more permeable and neuroglial metabolism tends to water accumulation both cellular and extracellularly.[19,23,35] This situation, in general terms known as diffuse cerebral edema, initiates the main problem of severe brain trauma: Rising intracranial pressure (ICP), which untreated, leads to a certain and definitive disability and/or dead.
Following many experiences and expert reviews, diffuse edema and rising ICP treatment, may include decompression of the cranial cavity with the intention of minimizing effects over de brain parenchyma and perfusion.[6,20]
According to a new approach-analysis of the same problem (having in mind the previously mentioned pathophysiology mechanisms), cisternostomy comes to the scene. Opening basal cisterns and communicating them with a catheter to a controlled extracranial environment (a normal pressurized reservoir), allow maintaining the exceeding edema fluid out of the cranial cavity, thus, preventing ICP from rising[7,9,23] [Figure 3].
The pivotal concepts of intracranial fluid circulation are as follows: (a) CSF is produced in the ventricles; (b) CSF communicates with the subarachnoid space (cisterns) through Lushka and Magendie foramina to the Cisterna magna; (c) once in the cisternal compartment, CSF flows in many directions filling every space; and (d) parenchymal fluids are added to the cisternal volume through Virchow Robin spaces (Virchow Robin spaces represent anatomical direct communication between parenchymal interstitium and basal cisterns). This last component generates, by inflammatory mechanisms, a big increase in cerebral volume and pressure during the active phase of trauma and other pathologies.[16,23,32]
It has to be remembered that the diameter and structure of the foramina at the IVth Ventricle act as a valve, thus CSF and cerebral fluids cannot return to the ventricular compartment efficiently, being unable to offer a pathway out to edema-related fluids.
Although this physiopathology, medical and surgical issues are thrilling, their details exceed this anatomical paper.
RESULTS
Microscopic anatomy review
Cerebral cisterns can be described following many authors, routes, and classifications. In this paper, surgical-based anatomy will be taken as a guide to thoroughly descript the cisternostomy procedure.
The surgical goal is to set an external catheter tip at the iterpeduncular/prepontine cistern to keep the increased edema fluid (coming from parenchyma to the basal cisterns, via the Virchow Robin spaces) out of the cranial vault. This cistern represents a core cisternal communication, both lateral to medial and infra to supratentorial.
The first anatomical obstacle is the skin, soft tissues, and the bonny cranial vault. Several approaches are described in this respect. Among them, the one used in previous randomized trials for cisternostomy, is the frontotemporal approach or modification of the pterional approach.[2,17,25,26,30,34]
The main target for this approach is the wide exposure of the superficial sylvian fissure, to allow proper manipulation in a swollen brain and then sstart a cisternal pathway and dissection [Figures 4A-C]. Special care must be taken to remove bone from the sphenoidal ridge to have a good visualization at deeper levels.[34]
Figure 4:
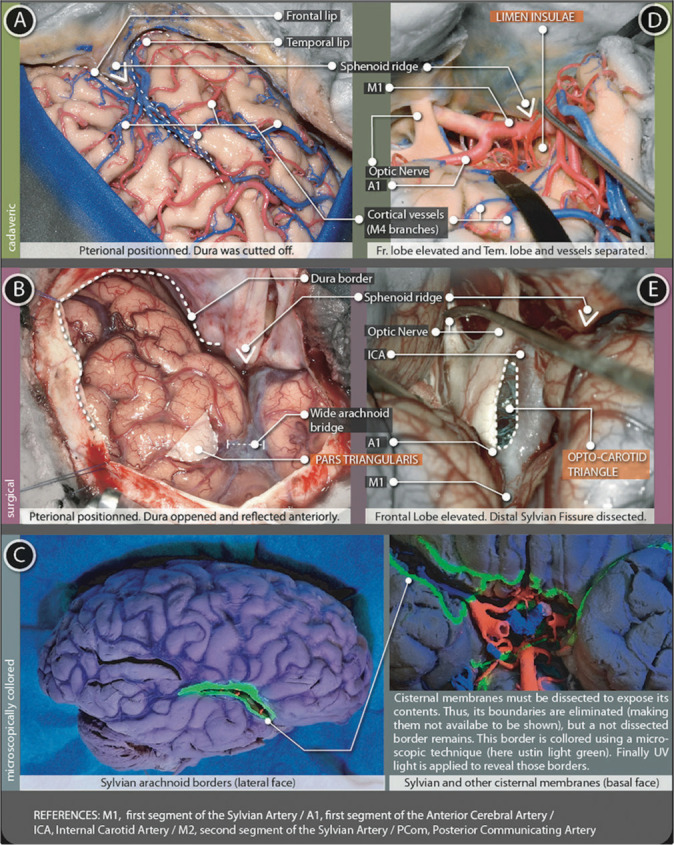
Sylvian cistern figure. The superficial part (A and B) is represented using (A) cadaveric and (B) surgical pictures. The deep part (D-E) is represented with (D) cadaveric and (E) surgical pictures (C) microscopically colored images demark the arachnoidal borders in the lateral and basal surfaces of the brain.
Once the cisternal space is entered, it is possible to define each boundary, connections with other cisterns, elements contained, and specific relationships with the surrounding parenchyma.
Cistern of lateral cerebral fossa (sylvian cistern)
The most remarkable and constant landmark in the lateral surface are the Lateral sulcus (Sylvian Fissure). This fissure has a superficial part and a deep part, only visible after surgical opening.
Superficial part
It has a linear shape and extends from the anterior clinoid process, following the junction of the frontal with the temporal lobes (before the dura is opened, this same point is marked by a dense tract named orbito-meningeal band, which hides the anterior clinoid process and must be dissected to enter the subfrontal-extradural pathway). After the dura is opened, the superficial sylvian cistern continues without stop up to the supra marginalis gyrus. At simple sight, it presents three extensions: anterior-horizontal, anterior-vertical, and posterior. The former two, confine a particular triangle-shaped portion of the inferior frontal gyrus, the pars triangularis [Figure 4A], which is the first surgical landmark - LANDMARK 1. Once identified, it becomes the opening point to reach deeper levels. The arachnoid tissue bridging frontal and temporal lips separates one from the other widely at this point.[33]
Deep Part (opercuralis + sphenoidal)
It has a complex volumetrical presentation divided into two segments: the Sphenoidal segment (located medially and deeper at the anterior cranial base) and the operculo-insular segment (located laterally and more superficial, directly under the superficial part of the sylvian fissure and only visible after the surgical separation of frontal lip and temporal lip). sphenoidal and operculo-insular segments can be divided by the presence of a particular landmark, a dense tract of white matter: the limen insulae.
Contents
Superficial branches of the Middle cerebral artery or sylvian artery can be seen at the brain surface (M4).[17] Once the superficial part of the fissure is dissected, these artery trajectories can be followed and guide a proper dissection. Deep in the operculo-insular part of the sylvian fissure, main elements are:
Middle cerebral artery: M4 arterial branches can be followed up to M1 segment: M4 are cortical vessels at the surface up to the Sylvian frontotemporal lips; M3 from here to the Circularis Gyrus Insulae; M2 from here and up to the Limen Insulae - LANDMARK 2. At this point, there are two M1 (post bifurcation, medial to the limen insulae). Those two arteries come from a single trunk: the M1 pre-bifurcation segment
Lenticulostriate arteries: Perforating branches mostly for the Anterior perforated substance, named as early branches when from M1 pre-bifurcation
Polar temporal artery (temporopolar artery): Perforating branch to the Temporal pole (A2 branch, after the Orbitofontal artery and the Long striate artery or Heubner’s Recurrent Artery)
Superficial sylvian vein: receiving cortical veins, taking the Sylvian trajectory and finally draining to venous sinus at the sphenoid ridge (sphenopetrosal sinus)
Deep sylvian vein: receives deep insular veins and drains into the sphenopetrosal Sinus or directly into the Cavernous Sinus.
Relationships
At the cerebral surface, the Lateral sulcus (Sylvian fissure) represents the intimate contact of the Inferior Frontal Gyrus (superior lip) and the Superior Temporal Gyrus (inferior lip). After gentle dissection and retraction of the arachnoid bridging those two lips, Lobus Insularis appears. Deeper in the Sylvian Valley, the Limen Insulae marks the start of the Sphenoideal segment of the Deep Sylvian Fissure. The Anterior Perforated Substance and the medial part of the Pars Orbitalis conform to the roof in an anatomic position (in a surgical position it rests anteriorly). The floor is the Planum Polare. The posterior wall corresponds to a part of the Uncus Hippocampus. The medial wall correlates to the internal carotid artery (ICA) (Supra-clinoid segment and its surrounding arachnoidal membrane, limited to the Carotid Cistern) Optic Tract, Optic Chiasma, and Optic nerve. The anterior wall is incompletely marked by a thickening of the arachnoid layer just over the Anterior and Medial Olfactory Stria. At this point, the Sylvian Fissure also connects with the Olfactory Cistern.
Once the bifurcation of M1 is noticed, the medial boundary of the deep Laeral sulcus (Sylvian Fissure) is almost achieved. About 8–15 millimeters further, over the M1 trajectory, an arachnoid membrane steps just in front of the Supra-clinoid Carotid Segment. Some of the most important points are illustrated in the Figure 4D.
Olfactory cistern
The olfactory cistern starts in front of the Deep Portion of the Sylvian Fissure (Sphenoidal compartment). At this point, a frontobasal sulcus can be found between the Straight (Rectus) and Orbital (Orbitalis) giri. The arachnoid membrane bridging over them conforms to the floor of the cistern (over the Cribiform Plate, bone surface of the Frontoethmoidal region). From here, an ascending reflection runs deep in the sulcus and blends with the rest of the arachnoid tissue.
Relationships
This cistern has no posterior wall, thus, a direct connection to the Sylvian, Carotid, Chiasmatic, Lamina terminalis (LT), and Pericallosal cisterns can be easily found.
Contents
The Olfactory Bulb represents the main content. It runs anteriorly with an A2 first branch: the Orbitofrontal artery.
Carotid cistern
Contiguous laterally to the Lateral cerebral fossa (Sylvian Cistern), a new rhomboidal space surrounding the supraclinoid ICA can be noticed.
Following a Lateral sulcus (Sylvian fissure) route, after the visualization of previous landmarks, the carotid artery should be observed underneath a thin arachnoid layer - LANDMARK 3. This is the lateral carotid sheet, just proximal to the A1-M1 confluence.
Lateral carotid sheet
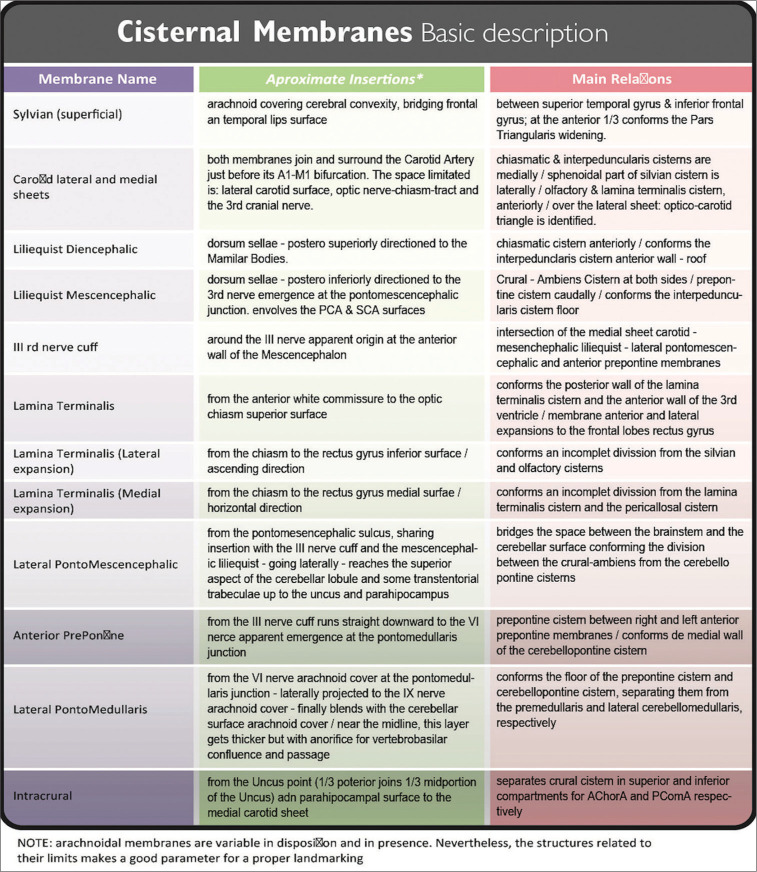
Starting at the lateral carotid face, it goes forward (reaching the optical nerve-chiasm junction) and downward (up to the III cranial nerve or oculomotor nerve). This point is important to understand the basal cisterns membranes arrangement. It is named III cranial nerve cuff.[33] This arachnoid structure represents the confluence of various membranes. It must be remembered that some described membranes may not be present in part or at all because they have a wide number of inter-patient variations.[22,38,39]
Medial carotid sheet
This layer begins once the lateral layer reaches the optic nerve. Here, with no disruption, it starts a backward pathway along the optic tract and all way down to the III cranial nerve. Thus, this membrane covers the ICA and the space between the ICA and the Optic nerve (space known as the Opto-Carotid Triangle - LANDMARK 4) and covers the proximal posterior communicating artery (PComA). This triangle serves as a window for the entrance to midline cisterns, particularly to the Interpeduncular Cistern[2,33] [Figure 4E].
Contents
Once the lateral carotid sheet is opened, Carotid Cistern is reached, and its contents exposed:
ICA: In its supraclinoid segment. It describes an anteromedial to the posterolateral trajectory, starting at the distal dural ring
PComA: emerging from de posterolateral face of the artery just next to the III cranial nerve
Anterior choroidal artery: emerging from the last carotid segment before it bifurcates into the Anterior and Middle Cerebral Artery
Ophthalmic artery: it can be seen accompanying the Optic Nerve, near the Carotid emergence from the distal dural ring, beneath the medial carotid sheet.
Relationships
As it was previously said, a rhomboid space should be described. The posterior angle is represented by the III nerve cuff. The III nerve then runs in a separated cover until it enters the cavernous sinus. The anterior angle is at the optic nerve-chiasm junction, where medial and lateral sheets join. Both lateral-sided segments of the rhomboid represent the shared limit with the Sphenoidal deep part of the lateral cerebral fossa (Sylvian Cistern). Medial-sided segments of the rhomboid are contoured by the optic nerve-chiasm-tract. They connect the Carotid Cistern with the Cistern of the LT (superiorly), the Interpeduncular Cistern (posteriorly), and the Chiasmatic Cistern (medially).[2,33]
Chiasmatic cistern
Located in the midline, the Chiasmatic Cistern represents a crossroads for cisternal anatomy. It connects superiorly with the Cistern of the LT; laterally with both Carotid Cisterns (separated by its medial sheet); posteriorly with the Interpeduncular Cistern (separated by the diencephalic membrane of Liliequist).
Relationships
The Optic Chiasm is the main anatomic element. This structure has an anterior-superior border which represents the site of insertion for the LT. Starting here, the LT runs up and posteriorly directly to the Anterior White Commissure, building the posterior wall of the Cistern of the LT and the anterior wall of the Third Ventricle. Just posterior to the Optic Chiasm the Pituitary Stalk appears, coming from diencephalic structures and going to the sellar region (pia mater and arachnoid tissue build a cilindric-shaped passage through the Chiasmatic Cistern, with a prior space named Infundibular Recess). Posterior to the Pituitary Stalk, a thin portion of grey matter constitutes the Tuber Cinereum, which is the roof of the Chiasmatic Cistern and part of the floor of the 3rd Ventricle (anterior to the Mamillar Bodies and posterior to the Infundibular Recess). The posterior-superior corners of the Chiasmatic Cistern are related directly to the PComA and the III Cranial Nerve as they enter the Interpeduncular Cistern after they went through the Carotid Cistern. The posterior wall is represented by the Diencephalic Membrane of Liliequist.
Contents
Optic Nerves and Chiasm, Hypophyseal and Infundibular arteries, Pituitary Stalk, venous plexus from Optic Chiasm and Pituitary Stalk.
LT cistern
The LT membrane extends from the lower border of the Anterior White Commissure downward to the Optic Chiasm.[22,37] This membrane has also two expansions:
Lateral LT membrane expansion: From the Optic Chiasm to the Straight gyrus (Gyrus Rectus) posterior part. It also has an ascending direction, contacting the Olfactory Cistern
Medial LT membrane expansion: It departs from the medial part of the Straight gyrus (Gyrus Rectus) and goes directly to the Inter Frontalis Space. It has also a brief anterior direction with no complete fixation in its rear part. This space is used by the anterior arterial complex to pass through.
Most of these membranes (as well as some others) have the main difficulty to be described: to explore them microscopically, it is necessary to dissect (mostly eliminate) their boundaries. In attention to this issue, endoscopic approaches have been made, allowing cisterns to be described from a different point of view.[22]
Relationships
The Cistern of the LT is located as the entrance to the Pericallosal Cistern (separated from the latter by the cisternal roof, the medial expansion of LT membrane). On both sides, it is separated from the Carotid Cistern by the lateral expansion of the LT membrane (this membrane is perforated by the A1 segment). The floor of this cistern is represented by the lower part of the LT membrane itself and the upper surface of the Optic Chiasm.
Contents
Optic Nerves and Chiasm; A1 segment (perforating the lateral expansion membrane) and A2 segment (going through the incomplete posterior border of the medial expansion); the long striate artery or Recurrent Artery of Heubner is frequently an A1 branch; Anterior Communicating Artery; and Frontopolar and Superior Hypophyseal branches.
Interpeduncular cistern
Once LANDMARK 4 was identified, it must be dissected and trespassed to reach a midline cistern (cisterns can be classified as “paired/lateral” or “unpaired/midline”).[4] If PComA trajectory LANDMARK 5 is followed, Interpeduncular Cistern is reached.
As it is known, perimesencephalic structures correspond to the Tentorial notch (Tentorial Incisura) level. This space is the theoretical limit and real pathway of communication between supra and infratentorial spaces. Thus, reaching this cistern is not only access to the midline but also finding a connection to infratentorial levels.
The tentorial notch (Incisura) is classically divided into anterior, middle, and posterior parts.[33,36,39] Each one has a particular cistern associated: Interpeduncular Cistern (anterior part), Cistern Ambiens (middle part), and Quadrigeminal Cistern (posterior part). The anterior part has an important element, which constitutes both upper and lower limits of the interpeduncular cistern: the Liliequist’s Membrane.
Liliequist’s membrane
First covering the Dorsum Sellae, this arachnoid layer then extends laterally to both Posterior Clinoidal Processes, and finally runs posteriorly in the search of the brainstem. As soon as this membrane leaves the Posterior Clinoidal Process (with its posteriorly oriented trajectory), it spreads into two membranes:
Diencephalic membrane: Continuing a slightly posterior-superior direction (with intimate adhesion to the III nerve cuff at both sides) this membrane finally arrives at brainstem levels. Here, it attaches to Mamillary Bodies. This membrane is thick, regular, and uniform along its surface, forming the roof of the Interpeduncular Cistern. When this membrane is surgically perforated (i.e. while making a Third Ventriculocisternostomy, after the Tuber Cinereum was traversed) the Third Ventricle and basal cisterns are then communicated. After this perforation, the Basilar Artery should be easily seen among arachnoid trabeculae and beneath the mesencephalic membrane.[1,11,15]
Mesencephalic membrane: From the same anterior insertion as the diencephalic membrane, this one instead, runs in an oblique manner (with a posteroinferior direction). Once at the brainstem level, it attaches to the Pontomesencephalic Sulcus, just above the Basilar Artery tip and bifurcation. PComA end should be encountered here, dividing P1 from P2 segment of the posterior cerebral artery (PCA). Laterally, the Medial Carotid Sheet, descending up to the oculomotor nerve cuff, is also seen and joins this Mesencephalic Membrane of Liliequist. They continue as one membrane over the Pontomescencephalic Sulcus (forming the Lateral Pontomesencephalic Membrane). The Liliequist mesencephalic membrane is very thin and mostly incomplete, but it has several attachments to the oculomotor nerve and PComA sheath.
Contents
Basilar tip and bifurcation LANDMARK 6: This arterial complex is exactly situated where the mesencephalic membrane starts its insertion. Just a few millimeters downward, dissecting arachnoid space, the Prepontine Cistern is reached
PComA: This artery can be seen coming from the Carotid Cistern (serving as a guide for dissection). It arrives and ends at the P1-P2 level of the PCA [Figures 5Aand B]
PCA and superior cerebellar artery (SCA): Mesencephalic membrane insertion involves both arteries, side to side of the midbrain anterior surface, marked laterally by the apparent origin of the oculomotor nerve
Oculomotor nerve: This nerve can be seen up to its apparent origin at the brainstem surface. It passes between PCA and SCA
Venous complex: It includes drainage veins from posterior communicating, pontomesencephalic, and thalamoperforating veins.
Figure 5:
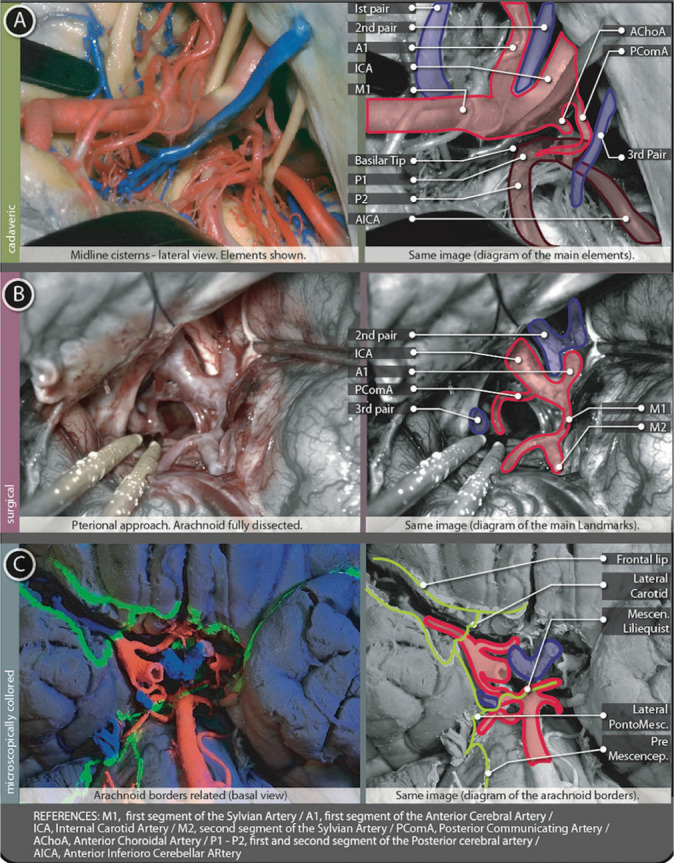
Tentorial cisterns figure. (A) Shows a cadaveric preparation with cisternal contents from a lateral view and a schematic explanation of them. (B) Surgical image from a pterional left-sided approach after full arachnoid dissection. Opto-carotid triangle, posterior communicating artery and the Third cranial nerve are shown. A schematic explanation of this image is placed just next to the surgical picture. (C) Basal view of a microscopically colored preparation where the same contents can be founded in relation to arachnoidal borders.
Relationships
The roof of this cistern is composed of the Mamillary Bodies and the diencephalic membrane of Lieliquist. This roof is directly situated behind the Chiasmatic Cistern. Only a thin layer of grey matter (the Tuber Cinereum, part of the floor of the third ventricle, point of perforation for the previously named Third Ventriculo-cisternostomy) is situated between this cistern and the third ventricle above. On both sides, the Cistern Ambiens occupy the middle part of the Tentorial Incisura. The Posterior Perforated Substance (perforated by small branches of the Basilar and P1 arteries) is at the back of the Interpeduncular cistern. Downward, beyond the incomplete mesencephalic membrane of Liliequist, the cisternal space is continued by the Prepontine Cistern LANDMARK 7.
Crural cistern
Classical anatomic references describe this Cistern as located in front of the Ambient Cistern and just lateral to the Interpeduncularis Cistern. Nevertheless, the FIPAT/IFA nomenclature does not name it particularly. Crural Cistern is a paired cistern at the tentorial/mesencephalic level, midway between Interpenduncularis and Ambient ones. The Anterior Choroidal Artery (as an anterolateral limit with the Carotid Cistern) and the PComA (as the posteromedial limit with the Interpeduncularis Cistern) demarcates the Cural Cistern location.[22,38,39] Crural and Cistern Ambiens can be denominated as one as “Lateral Mesenchepalic Cistern.”23. The Crural Membrane 8 (separating Ambiens from Crural Cistern) must be differentiated from the Intracrual Membrane 23 (separating crural cistern in superior and inferior compartments), both appearing as incomplete and not always present membranes.[22,24,38,39]
Relationships
The anterior and mid part of the Uncus forms its lateral boundary (the division between the mid and posterior portion of the Uncus) are marked by an arachnoid layer running from lateral to medial, the Lateral Pontomesencephalic Membrane. This membrane continues up to the III nerve cuff; once here, it runs down as the Anterior Pontine Membrane. This same membrane, once it gets near the VI cranial nerve, at the Ponto-Medullaris junction, starts a lateral trajectory as the Lateral Ponto-Medullaris Membrane, just up to the IX cranial nerve. Here, this membrane blends with the exterior cerebellar arachnoid membrane [Figure 5C].
From this same posterior uncus point, another thin membrane (the Intracrural Membrane) runs anteromedially dividing Crural Cistern in a superior and inferior compartment (Anterior Choroidal Artery and PComA belong to each one of them in that order). The medial wall of the cistern is the lateral surface of the Mesencephalon.
Contents
Anterior Choroidal Artery (Carotid last branch previous to bifurcation), Posterior Choroidal Artery (PCA branch), P2a segment of the PCA, and proximal part of the Basal Vein of Rosenthal.
Pre-pontine cistern
The midline Cisterns (LANDMARK 6), from the tentorium down, are as follows: Interpeduncularis, Pre-Pontine, and Pre-Medullary cisterns.
Previously described membranes situate properly the PrePontine Cistern between the right and left Anterior Pontine Membrane. Superiorly, relates to the Interpeduncularis Cistern. Inferiorly, continues to the Pre-Medullary Cistern.
Relationships
The anterior surface of this cistern is related to the Clivus. Posteriorly, the Pons and its centrally located element, the Basilar Artery in its sulcus. The Mesencephalic membrane of Liliequist represents the roof [Figure 5C]. On the floor of this cistern, a thickened membrane (where the Vertebral Arteries join at the Basilar point, approximately at the Pontomedullaris junction) separates this Cistern from the Premedullaris one. Laterally on both sides, the Cerebellopontine Cisterns are present.
Contents
The main structure located as a central axis is the Basilar Artery. The VI Cranial Nerve (Abducens) and all its trajectory looking forward to the Abducens nerve canal or Dorello’s Canal. The Anterior Inferior Cerebellar Artery (AICA) starts here in Figure 5A.
Cistern ambiens
The space between the mesial surface of the Temporal Lobe (Uncus posterior part, Parahypocampus, Dentate Gyrus, and Fornix Fimbria) and the lateral surface of the Mesencephalon (from the lateral end of the Quadrigeminal Plate) takes the name of Cistern Ambiens. Anteriorly its limits the Crural Cistern. Posteriorly, it is limited to the Quadrigeminal Cistern.
Cerebello-pontine cistern
The Cerebello-pontine cistern is related superiorly to the Lateral Mesencephalic Cistern (Crural + Ambiens). This paired cistern occupies both sides of the Prepontine Cistern. Superior limit, the Ponto-Mesencephalic junction. Inferior limit, the Ponto-Medularis junction.
Peri-callosal cistern
Between medial surfaces of both right and left frontal lobes, the Anterior Interhemisferic Fissure houses the Pericallosal Cistern. Where the Splenium part of the Corpus Callosum ends, the Pericallosal Cistern connects with the Quadrigeminal Cistern at the level of the pineal gland and the mesencephalon dorsum.
Quadrigeminal cistern
Continuing the Pericallosal cistern posterior and caudally, the Pineal Gland and Quadrigeminal Plate are reached just behind-below the Splenium of the Corpus Callosum. At this level, the subarachnoid space conforms to a wide cistern fully occupied by multiple venous confluences and arterial branches.
Cistern of transverse fissure (Cistern of velum interpositum)
With no separation from the anterior part of the Quadrigeminal Cistern, this space runs anteriorly beneath the Fornix and above the roof of the IIIrd Ventricle. The anteroposterior extension goes from the Habenular Commissure to the Interventricular foramen (foramen of Monro).
Superior-cerebellar cistern
Mostly without precise lateral limits, and continuing the Quadrigeminal Cistern posteriorly, the Superior-cerebellar Cistern follows the Straight Sinus up to the confluence of the sinus (Torcula of Herophilus). From here, it continues with the Posterior cerebellomedullary cistern (Cisterna Magna) after many arachnoid adhesions have been dissected.
This cistern contains median and paramedian branches from the SCA. The Superior Vermian Vein is formed by its feeders, here.
Pre-medullary cistern
Cranial cisternal space ends caudally at the margins of the Foramen Magnum, building a ring around the inferior portion of the brainstem (the Medulla). This cisternal space corresponds anteriorly to the Premedullaris Cistern, laterally to the Lateral Cerebellomedullary Cistern, and posteriorly to the Posterior Cerebellomedullary Cistern (namely the Cisterna Magna).
Lateral cerebello-medullary cistern
Situated laterally to the Premedullaris cistern on both sides, this paired Cistern is also situated caudally to the CerebelloPontine Cistern (separated from the latter by the Lateral Ponto-Medullary Membrane).
Posterior cerebellomedullary cistern (cisterna magna)
It is easy to locate this cistern just above de Foramen Magnum in its posterior margin, and posterior to the Lateral Cerebellomedullary Cistern, recently described. Nevertheless, describing its anatomic particularities is not a simple task. Due to Cerebellar anatomy, the presence or absence of Falx Cerebelli and the bony compliance of the Posterior Fossa, the Cisterna Magna description may vary largely (escaping to this paper’s objective). These anatomic relationships can be better understood by taking into count the central importance and location of the Fourth Ventricle.[31]
DISCUSSION
Cisternostomy procedure and landmark
Literature evidence reported cisternostomy as a new therapeutic option for Brain Trauma treatment.[7-9] For decades before, decompressive craniectomy (DC) was the only tool for trauma neurosurgeons, being mostly palliative, as it just allows the swelling of an injured brain to expand with no big changes in the physiopathology happening under the skull. DC was studied by many trials, mostly with similar conclusions: less mortality but high rates of vegetative status and disabilities.[10,12,21]
The surgical purpose of the cisternostomy is to act actively in the physiopathology process, allowing the exceeding amount of water, proceeding from the swelling of an injured brain, to be derived out of the cranial vault. Thus, the intracranial volume will not increase and so it happens with the ICP.
Remembering that intracellular and paracellular/interstitial edema follows the Virchow Robin pathway to Basal Cisterns), the goal to be achieved is the surgical connection from within the cisterns to out of the Cranial Vault, should be created.[16,23,32] This is performed, by using an intra-cisternal catheter, placed in the core Prepontine cistern which receives all added volume in an external reservoir.
The frontotemporal approach is useful.[29] It is used for many pathologies, so it is well known by neurosurgeons in general. The whole procedure can be done in a proper manner after the skull base is reached from this particular position and vision.
Working on an injured and swelling brain is not the perfect situation for any microsurgical intervention, so it is mandatory to make a thorough plan and approach to minimize operation time and parenchyma manipulation. A detailed landmark progression is the best way for this purpose.
Basal cisterns could be reached using both extradural and intradural modalities.[8]
Therefore, the landmarks collected after the Lateral cerebral fossa (Sylvian fissure) approached are as follows:
Landmark 1 - Pars triangularis should be found after a frontotemporal craniotomy to start dissecting the Sylvian fissure.
Landmark 2 - The main curvature of M1-M2 over the limen insulae is found after separating the frontal and temporal lips and Sylvian vessels followed.
Landmark 3 - Carotid artery encountered after dissecting a thin membrane covering it, just a few millimeters beyond the M1-A1 bifurcation.
Landmark 4 - Optico-Carotid triangle appears once the optic nerve is visualized just next to the supraclinoidal Carotid. After dissection of the membrane covering the triangle, the Interpeduncular Cistern is reached, and its contents can be visualized.
Landmark 5 - PComA appears after the Interpeduncular Cistern is opened, coming from the laterally situated Crural Cistern.
Landmark 6 - Basilar tip is identified in the proximities, after following the PComA up to the PCA.
Landmark 7 - Prepontine cistern position is confirmed by the visualization of the Basilar Artery beneath the Mesencephalic membrane of Liliequist.
Deep knowledge of the anatomical structures is paramount to set properly the catheter tip.
There may appear difficulties with some anatomical variants such as big posterior clinoidal processes or bulging parenchyma in the Optico-Carotid window. In those rare cases, alternative routes could be taken to achieve the same goal.[8]
PRELIMINARY RESULTS, FORECAST, AND PITFALLS
After several case reports and a first randomized control trial, cisternostomy appears as a promising intervention. 11 Mortality rates improved, but the most important change was achieving significantly better outcomes.
Another positive aspect of cisternostomy is that there is no need for re-intervention after decompression. Cisternostomy uses a small craniotomy approach instead of a big craniectomy (i.e., used in DC).
As was expected, there are negative aspects to this procedure too. More operation time is needed as well as more infrastructures in the operation room (microscope, microscopic instruments, and skilled surgical team). Parenchymal retraction is necessary. All of those negative aspects seem to be affordable and even necessary to reach good therapeutic levels.
The DC has been the gold standard for several decades and its indication is part of a routine at almost all places in the world where neurotrauma patients arrive. Some inertia is expected against proposing cisternostomy or any other changes to this routine. However, evidence-based medicine will finally enlighten this situation in favor of the patient. It is easy to imagine that any options using novel advanced technologies, bearing in mind microscopical anatomy and physiopathology, should be considered.[5,8,9,16,18,22,24,27,28,32,33,35,39]
CONCLUSION
Deep knowledge of neuroanatomy, microscopic details of cisterns, and careful surgical step-by-step landmark planning are mandatory to perform a safe and effective cisternostomy. Cisternostomy may be a life-saving strategy and an innovative therapeutic tool in case of severe brain trauma.
There is still a need of collecting more information and evidence about this procedure. Multicenter randomized controlled trials should be planned and programmed to acquire quality evidence.
Institutional review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board.
Footnotes
How to cite this article: Villanueva P, Baldoncini M, Forlizzi V, Campero A, Rangel CC, Granja JO, et al. Microneurosurgical anatomy of the basal cisterns: A brief review for cisternostomy. Surg Neurol Int 2023;14:97.
Contributor Information
Pablo Villanueva, Email: pvillanuevach@gmail.com.
Matías Baldoncini, Email: drbaldoncinimatias@gmail.com.
Valeria Forlizzi, Email: vforlizzi@gmail.com.
Alvaro Campero, Email: alvarocampero@yahoo.com.ar.
Carlos Castillo Rangel, Email: neuro_cast27@yahoo.com.
Jaime Ordóñez Granja, Email: ordoncx@gmail.com.
Albert Sufianov, Email: sufianov@gmail.com.
Alice Giotta Lucifero, Email: alicelucifero@gmail.com.
Sabino Luzzi, Email: sabino.luzzi@unipv.it.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
REFERENCES
- 1.Abdalá-Vargas NJ, Cifuentes-Lobelo HA, Ordóñez-Rubiano EG, Patiño-Gómez JG, Villalonga JF, Lucifero AG, et al. Anatomic variations of the floor of the third ventricle: Surgical implications for endoscopic third ventriculostomy. Surg Neurol Int. 2022;13:218. doi: 10.25259/SNI_404_2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adib SD, Herlan S, Ebner FH, Hirt B, Tatagiba M, Honegger J. Interoptic, trans-lamina terminalis, opticocarotid triangle, and caroticosylvian windows from mini-supraorbital, frontomedial, and pterional perspectives: A comparative cadaver study with artificial lesions. Front Surg. 2019;6:40. doi: 10.3389/fsurg.2019.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alperin N, Bagci AM, Lee SH, Lam BL. Automated quantitation of spinal CSF volume and measurement of craniospinal CSF redistribution following Lumbar withdrawal in idiopathic intracranial hypertension. AJNR Am J Neuroradiol. 2016;37:1957–63. doi: 10.3174/ajnr.A4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altafulla J, Bordes S, Jenkins S, Litvack Z, Iwanaga J, Loukas M, et al. The basal subarachnoid cisterns: Surgical and anatomical considerations. World Neurosurg. 2019;129:190–9. doi: 10.1016/j.wneu.2019.05.087. [DOI] [PubMed] [Google Scholar]
- 5.Bellantoni G, Guerrini F, Del Maestro M, Galzio R, Luzzi S. Simple schwannomatosis or an incomplete Coffin-Siris? Report of a particular case. eNeurologicalSci. 2019;14:31–3. doi: 10.1016/j.ensci.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GW, Bell MJ, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2017;80:6–15. doi: 10.1227/NEU.0000000000001432. [DOI] [PubMed] [Google Scholar]
- 7.Chandra VR, Prasad BC, Banavath HN, Reddy KC. Cisternostomy versus Decompressive craniectomy for the management of traumatic brain injury: A randomized controlled trial. World Neurosurg. 2022;162:e58–64. doi: 10.1016/j.wneu.2022.02.067. [DOI] [PubMed] [Google Scholar]
- 8.Cherian I, Grasso G, Bernardo A, Munakomi S. Anatomy and physiology of cisternostomy. Chin J Traumatol. 2016;19:7–10. doi: 10.1016/j.cjtee.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherian I, Yi G, Munakomi S. Cisternostomy: Replacing the age old decompressive hemicraniectomy? Asian J Neurosurg. 2013;8:132–8. doi: 10.4103/1793-5482.121684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D’Urso P, et al. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011;364:1493–502. doi: 10.1056/NEJMoa1102077. [DOI] [PubMed] [Google Scholar]
- 11.Dezena RA. Endoscopic Third Ventriculostomy: Classic Concepts and a State-of-the-Art Guide. Cham: Springer International Publishing; 2020. Surgical Technique of Endoscopic Third Ventriculostomy (ETV) pp. 81–91. [Google Scholar]
- 12.Elsawaf Y, Anetsberger S, Luzzi S, Elbabaa SK. Early decompressive craniectomy as management for severe traumatic brain injury in the pediatric population: A comprehensive literature review. World Neurosurg. 2020;138:9–18. doi: 10.1016/j.wneu.2020.02.065. [DOI] [PubMed] [Google Scholar]
- 13.Ettmüller ME, Ruysch F. De Cerebri Corticali substantia, &c. Karnataka: Clear Prints; 1699. Epistola Anatomica, Problematica duodecima, Authore Mich, Ernesto Ettmullero, M. D. &c. Ad Virum Clarissimum Fredericum Ruyschium Med. Doct. Anatomiae ac Botanices Professorem. [Google Scholar]
- 14.Federative International Programme for Anatomical Terminology . Terminologia Anatomica. 2nd ed. Rio de Janeiro: Federative International Programme for Anatomical Terminology; 2019. Terminology. [Google Scholar]
- 15.Fernandes-Silva J, Silva SM, Alves H, Andrade JP, Arantes M. Neurosurgical anatomy of the floor of the third ventricle and related vascular structures. Surg Radiol Anat. 2021;43:1915–25. doi: 10.1007/s00276-021-02785-8. [DOI] [PubMed] [Google Scholar]
- 16.Gess B, Niederstadt TU, Ringelstein EB, Schäbitz WR. Clinical relevance of normal and enlarged Virchow-Robin spaces. Nervenarzt. 2010;81:727–33. doi: 10.1007/s00115-010-2983-y. [DOI] [PubMed] [Google Scholar]
- 17.Giotta Lucifero A, Fernandez-Miranda JC, Nunez M, Bruno N, Tartaglia N, Ambrosi A, et al. The modular concept in skull base surgery: Anatomical basis of the median, paramedian and lateral corridors. Acta Biomed. 2021;92:e2021411. doi: 10.23750/abm.v92iS4.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giotta Lucifero A, Luzzi S, Brambilla I, Trabatti C, Mosconi M, Savasta S, et al. Innovative therapies for malignant brain tumors: The road to a tailored cure. Acta Biomed. 2020;91:5–17. doi: 10.23750/abm.v91i7-S.9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall JE. Netherlands: Elsevier Health Sciences; 2015. Guyton and Hall Textbook of Medical Physiology. [Google Scholar]
- 20.Hawryluk GW, Rubiano AM, Totten AM, O’Reilly C, Ullman JS, Bratton SL, et al. Guidelines for the management of severe traumatic brain injury: 2020 Update of the decompressive craniectomy recommendations. Neurosurgery. 2020;87:427–34. doi: 10.1093/neuros/nyaa278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutchinson PJ, Kolias AG, Timofeev IS, Corteen EA, Czosnyka M, Timothy J, et al. Trial of decompressive craniectomy for traumatic intracranial hypertension. N Engl J Med. 2016;375:1119–30. doi: 10.1056/NEJMoa1605215. [DOI] [PubMed] [Google Scholar]
- 22.Inoue K, Seker A, Osawa S, Alencastro LF, Matsushima T, Rhoton AL., Jr Microsurgical and endoscopic anatomy of the supratentorial arachnoidal membranes and cisterns. Neurosurgery. 2009;65:644–4. doi: 10.1227/01.NEU.0000351774.81674.32. discussion 665. [DOI] [PubMed] [Google Scholar]
- 23.Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The glymphatic system: A beginner’s guide. Neurochem Res. 2015;40:2583–99. doi: 10.1007/s11064-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lü J, Zhu XL. Characteristics of distribution and configuration of intracranial arachnoid membranes. Surg Radiol Anat. 2005;27:472–81. doi: 10.1007/s00276-005-0025-4. [DOI] [PubMed] [Google Scholar]
- 25.Luzzi S, Lucifero AG, Bruno N, Baldoncini M, Campero A, Galzio R. Cranio-orbito-zygomatic approach. Acta Biomed. 2022;92:e2021350. doi: 10.23750/abm.v92iS4.12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luzzi S, Lucifero AG, Bruno N, Baldoncini M, Campero A, Galzio R. Pterional approach. Acta Biomed. 2022;92:e2021346. doi: 10.23750/abm.v92iS4.12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luzzi S, Lucifero AG, Del Maestro M, Marfia G, Navone SE, Baldoncini M, et al. Anterolateral approach for retrostyloid superior parapharyngeal space schwannomas involving the jugular foramen area: A 20-year experience. World Neurosurg. 2019;132:e40–52. doi: 10.1016/j.wneu.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Luzzi S, Lucifero AG, Martinelli A, Maestro MD, Savioli G, Simoncelli A, et al. Supratentorial high-grade gliomas: maximal safe anatomical resection guided by augmented reality high-definition fiber tractography and fluorescein. Neurosurg Focus. 2021;51:E5. doi: 10.3171/2021.5.FOCUS21185. [DOI] [PubMed] [Google Scholar]
- 29.Luzzi S, Lucifero AG, Spina A, Baldoncini M, Campero A, Elbabaa SK, et al. Cranio-orbito-zygomatic approach: Core techniques for tailoring target exposure and surgical freedom. Brain Sci. 2022;12:405. doi: 10.3390/brainsci12030405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luzzi S, Maestro MD, Elia A, Vincitorio F, Perna GD, Zenga F, et al. Morphometric and radiomorphometric study of the correlation between the foramen magnum region and the anterior and posterolateral approaches to ventral intradural lesions. Turk Neurosurg. 2019;29:875–86. doi: 10.5137/1019-5149.JTN.26052-19.2. [DOI] [PubMed] [Google Scholar]
- 31.Matsushima T, Rhoton AL, Jr, Lenkey C. Microsurgery of the fourth ventricle: Part 1. Microsurgical anatomy. Neurosurgery. 1982;11:631–67. doi: 10.1227/00006123-198211000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Proulx ST. Cerebrospinal fluid outflow: A review of the historical and contemporary evidence for arachnoid villi, perineural routes, and dural lymphatics. Cell Mol Life Sci. 2021;78:2429–57. doi: 10.1007/s00018-020-03706-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rhoton AL. Rhoton’s Cranial Anatomy and Surgical Approaches. Oxford: Oxford University Press; 2019. Surgeons CoN. [Google Scholar]
- 34.Rubio RR, Chae R, Vigo V, Abla AA, McDermott M. Immersive surgical anatomy of the pterional approach. Cureus. 2019;11:e5216. doi: 10.7759/cureus.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakka L, Coll G, Chazal J. Anatomy and physiology of cerebrospinal fluid. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128:309–16. doi: 10.1016/j.anorl.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Testut L, Latarjet A, Latarjet M, Devy G, Dupret S. 1949. Tratado de Anatomia Humana: Salvat Editores, S.A. [Google Scholar]
- 37.Tubbs RS, Nguyen HS, Loukas M, Cohen-Gadol AA. Anatomic study of the lamina terminalis: Neurosurgical relevance in approaching lesions within and around the third ventricle. Childs Nerv Syst. 2012;28:1149–56. doi: 10.1007/s00381-012-1831-8. [DOI] [PubMed] [Google Scholar]
- 38.Yasargil MG. General Operative Techniques and Pathological Considerations of the Intracranial Aneurysms. Vol. 1. United States: Thieme; 1984. Microneurosurgery, Microsurgical Anatomy of the Basal Cisterns and Vessels of the Brain, Diagnostic Studies. [Google Scholar]
- 39.Yasargil MG, Kasdaglis K, Jain KK, Weber HP. Anatomical observations of the subarachnoid cisterns of the brain during surgery. J Neurosurg. 1976;44:298–302. doi: 10.3171/jns.1976.44.3.0298. [DOI] [PubMed] [Google Scholar]