Abstract
Background
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are a relatively new class of antidiabetic drugs with demonstrated renal and cardiovascular disease benefit. This study evaluates the role of SGLT2 inhibitors on the survival of non-small cell lung cancer (NSCLC) patients.
Methods
We used National Surveillance, Epidemiology and End Results (SEER)-Medicare linked data. Twenty four thousand nine hundred fifteen NSCLC patients newly diagnosed between 2014 and 2017 with pre-exiting diabetes and aged 66 years or older were included and followed to the end of 2019. Information on SGLT2 inhibitors use was extracted from the Medicare Part D file.
Results
SGLT2 inhibitor use was associated with significantly reduced mortality risk after adjusting for potential confounders (HR = 0.68, 95% CI = 0.60–0.77) with stronger association for longer duration of use (HR = 0.54, 85% CI = 0.44–0.68). Further, we found that SGLT2 inhibitor use was associated with a significant reduced risk of mortality regardless of patients’ demographic, tumour characteristics and cancer treatments.
Conclusion
Our large SEER-Medicare linked data study indicates that SGLT2 inhibitors use was associated with improved overall survival of NSCLC patients with pre-existing diabetes. Further studies are needed to confirm our findings and elucidate the possible mechanisms behind the association.
Subject terms: Epidemiology, Outcomes research
Background
Lung cancer is the second most common cancer and the leading cancer death in both men and women in the US. It is estimated that 135,720 Americans died from lung cancer in 2020, accounting for more than 22 percent of all cancer deaths, more than breast, prostate and colon cancers combined [1]. Lung cancer has a poor survival due to late diagnosis and limited treatment interventions; over half of people diagnosed with lung cancer die within one year of diagnosis and the 5-year survival is about 19% [2]. Therefore, novel therapy is urgently needed to improve lung cancer survival.
Diabetes is a common comorbidity among lung cancer patients. Based on a SEER-Medicare 2010 linked analysis, 28% of patients age 66 years and over with non-small cell lung cancer (NSCLC) had a diagnosis of diabetes identified from 12 months preceding the lung cancer diagnosis [3]. Diabetes is an independent unfavourable prognosis factor for patients with lung cancer [4–6]. Although lifestyle interventions such as diet, exercise and weight control remain the foundation for managing type 2 diabetes, most patients will eventually require pharmacotherapy for glycemic control [7].
Sodium-glucose cotransporter (SGLT2) inhibitors (or gliflozins) is a relatively new class of oral prescription medications for patients with type 2 diabetes. Four SGLT2 inhibitors, canagliflozin, dapagliflozin, empagliflozin and ertugliflozin, have been approved by the Food and Drug Administration (FDA), beginning with canagliflozin in March 2013 [8, 9]. Although less widely used than metformin, over 3 million canagliflozin prescriptions for diabetes treatment were written in the US in 2019 [10]. Canagliflozin may be used as a stand-alone adjunct to diet and exercise, or may be combined with metformin, insulin or a sulfonylurea in cases where patients with type 2 diabetes have not responded adequately to other medications [11].
SGLT2 inhibitors lower blood glucose concentrations by blocking sodium-glucose cotransporter 2, thereby promoting urinary glucose excretion [12, 13]. In addition to its benefits in treatment of diabetes, gliflozins are considered to have additional renal and cardiovascular risk reduction benefits [14–16]. SGLT2 inhibitors are among the first-choice drugs after metformin failure, because of their favourable effects on cardiovascular risk and renal disease progression [17].
Recently, several laboratory studies have suggested intriguing cancer benefits related to gliflozins. Kaji et al. [18]. conducted a xenograft study of human liver cells and concluded that canagliflozin attenuated liver cancer growth by inhibiting glucose uptake. Similarly, Scafoglio et al. [19]. showed in mouse models and xenografts that SGLT2 inhibitors reduced tumour growth of early-stage lung adenocarcinoma and prolonged survival. Other laboratory studies have demonstrated that canagliflozin (but not dapagliflozin) inhibited proliferation and survival of lung cancer cells [20], or showed that canagliflozin induced apoptosis in vitro in resistant non-small cell lung cancer cell lines [21]. Early clinical evidence also indicates gliflozins may have anti-cancer benefits for gastrointestinal, lung, pancreas, prostate and liver cancers [22, 23].
These findings from laboratory and early clinical studies highlight the need to investigate associations between SGLT2 inhibitors and cancer survival in human populations. However, population level epidemiological studies to investigate this exciting possibility have not been undertaken. If evidence is found in a large, heterogeneous, clinical population that SGLT2 inhibitors generally, or canagliflozin specifically, promote cancer survival, the impact is potentially profound, especially for lung cancer patients with limited treatment options and very poor survival. The current study uses national Surveillance, Epidemiology and End Results (SEER)-Medicare data to identify a population of adults aged 66 and over with lung cancer who also have pre-existing diabetes and tests the emerging hypothesis that SGLT2 inhibitors, including canagliflozin for diabetes will be associated with better lung cancer survival. We focused on NSCLC, the most common type of lung cancer, accounting for more than 85% of all lung cancers [24].
Methods
SEER-Medicare data
The SEER-Medicare linked database was used for this study. The SEER programme is population-based tumour registries that routinely collect information on all newly diagnosed cancer cases that occur in persons residing in SEER areas covering ~48.0% of the US population [25]. The Medicare programme, federally funded and administered by the Center for Medicare and Medicaid Services (CMS), provides health insurance for people aged 65 years and older, people under age 65 with certain disabilities, and people of all ages with End-Stage Renal Disease (permanent kidney failure requiring dialysis or a kidney transplant) [26]. The linkage of these two data sources provides detailed information about Medicare beneficiaries with cancer and offers a unique population-based source of information that can be used for an array of epidemiological and health serves research [27]. The linkage was first completed in 1991 and is updated in 1995, 1999, and biennially in recent years. For each of the linkages, 95% of persons age 65 and older in the SEER files were matched to the Medicare enrolment file [28]. As of 2021, the data include all Medicare eligible persons who were diagnosed with cancer through 2017, and their Medicare claims through 2019 [27].
Study population
From the latest SEER-Medicare dataset, we considered all lung cancer as a primary cancer between 2014 and 2017. 2014 was chosen because canagliflozin was approved in 2013 and only a small number of patients used it in 2013. This study population was also restricted to those with continuous Medicare Part A and Part B insurance coverage and no HMO coverage for at least 12 months prior to lung cancer diagnosis. The reason for this inclusion criterion is to entail 12 months of coverage for assessment of diabetes status and other comorbidities. The study was also restricted to those with continuous Medicare Part A, Part B and Part D (prescription drug) coverage, and no HMO coverage for at least 3 months after a cancer diagnosis or until death, for tracking of Medicare treatment choices and diabetes treatment. A study population flow chart is shown in Fig. 1. An initial sample of 107,762 NSCLC patients were identified. Of these, 77,771 (72.17%) met inclusion criteria for continuous Medicare Part A and Part B insurance coverage, and no HMO coverage for at least 12 months prior to cancer diagnosis and three months after cancer diagnosis. There were 24,915 (32.04%) of these patients with pre-existing diabetes that formed the final study sample. The diabetes status in CMS data was determined on the basis of either a single inpatient claim or at least two outpatient claim ICD-9 or ICD-10 diagnoses separated by at least one day during the interval beginning 2 years before lung cancer diagnosis.
Fig. 1.
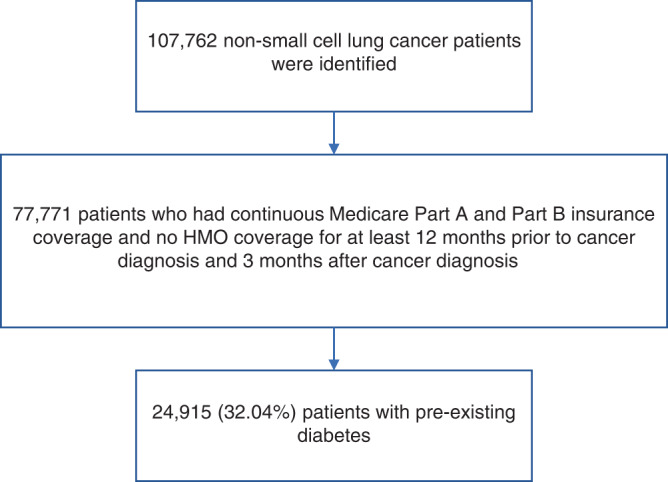
Study population flow chart.
Exposures
Information on SGLT2 inhibitors use was extracted from the Medicare Part D event file. Any SGLT2 inhibitors use (including canagliflozin, dapagliflozin, empagliflozin and ertugliflozin), and one specific SGLT2 inhibitor—canagliflozin use, were the primary exposures of interest. To avoid confounding by metformin use, we also extracted information on metformin use from the same Part D event file.
Outcomes
All-cause mortality was our primary outcome. The mortality data were extracted from claims data as of 2019. Mortality month was included to assess survival time.
Covariates
Cancer stage was extracted from the SEER cancer file. Cancer stage was categorised as: localised (confined to primary site); regional (spread to regional lymph nodes); and distant (cancer has metastasised). We further categorised our cases into squamous cell carcinoma, adenocarcinoma and other specific histological types.
Cancer treatment information was determined using both the cancer file and CMS data, including cancer-directed surgery, chemotherapy, radiation, immunotherapy/checkpoint inhibitor use and epidermal growth factor receptor (EGFR) antagonists. Although the SEER programme routinely collects information regarding certain anti-cancer therapies (i.e. surgery, radiation therapy) occurring within 4 months of diagnosis, SEER does not report information pertaining to chemotherapy administration. Also, based on an article comparing SEER treatment data with Medicare claims [29], the overall sensitivity of using SEER data only to identify these treatments is only moderate. Thus, we used claims data to supplement the SEER information to identify cancer treatment and flagged a patient to have a specific type of treatment if either database indicated yes to the type of treatments.
Comorbidities
Chronic conditions, including diabetes, chronic kidney disease, hypertension and cardiovascular disease were based on the chronic condition flags file from the claims data.
Other covariates included patient’s demographic characteristics (age in years, sex, race/ethnicity (including non-Hispanic White, non-Hispanic Black or African American, non-White Hispanic or Asian/Pacific Islander), and marital status (married or not)) from SEER.
Statistical analysis
Distribution of patients’ characteristics, tumour characteristics and treatment were compared first between patients with and without SGLT2 inhibitor use. Chi-square tests were used to evaluate differences for categorical covariates, and t-tests were used for continuous variables.
Second, multivariate Cox proportional hazards regression models were used to estimate adjusted relative hazard ratios for mortality in relation to SGLT2 inhibitor use, duration of its use adjusting for potential confounders including age, gender, race/ethnicity, comorbidities, tumour stage and cancer treatment. The survival time was measured as months from date of cancer diagnosis to death or to the end of 2019 if the patient survived, whichever comes first.
Third, similar methods were used to estimate adjusted relative hazard ratios for mortality in relation to a specific SGLT2 inhibitor (canagliflozin use). More detailed diabetes treatment, including duration of SGLT2 inhibitor use, and combination of metformin and SGLT2 inhibitor use (neither, metformin alone, SGLT alone or both) were examined adjusting for potential confounders. Finally, we performed subgroup analyses according to patients’ demographic characteristics (age, sex, race/ethnicity), tumour characteristics (stage and histological types) and tumour treatment. In addition, cause of death information was not provided in the Medicare claims data but was available for a shorter follow-up time through 2017 in SEER among a subset of individuals (about 70% of study population) (N = 17,547). We conducted a sensitivity analysis for lung cancer specific mortality as the outcome among this subset of the population.
Results
Of 24,915 NSCLC patients with pre-existing diabetes (average diabetes duration was 7.8 years), 531 (2.13%) patients used SGLT2 inhibitors and 324 (1.30%) used canagliflozin specifically. Of the 531 patients who used SGLT2 inhibitors, 402 (75.7%) patients used a combination of SGLT2 inhibitors and metformin. Of those who received SGLT2 inhibitors, the average length of this medication treatment was 15.2 months and was 13.7 months for canagliflozin specifically. Over an average of 21.2 months of follow-up, there were 18,181 patients who died in total. 260 patients died out of 531 who used SGLT2 inhibitors (crude mortality = 48.0%) while 17921 died out of 24384 patients who did not use SGLT2 inhibitors (crude mortality = 73.5%).
Table 1 provides a summary of sample characteristics according to SGLT2 inhibitor status. Persons who used SGLT2 inhibitors, compared to non-users, on average were younger, more likely to be Asian, Pacific Islander or Hispanic, more likely to have chronic kidney disease but less likely to have cardiovascular disease, more likely to have local or regional cancer stage, be squamous cell carcinoma, and more likely to receive surgery but less likely to receive radiation treatment.
Table 1.
Patients’ characteristics by sodium-glucose cotransporter 2 (SGLT2) inhibitor use status among 24,915 non-small cell lung cancer patientsa.
| Total (24,915) | Use SGLT2 inhibitors | |||
|---|---|---|---|---|
| No (24,384) | Yes (531) | P-value | ||
| Age at diagnosis (years) mean (std) | 76.9 (6.73) | 77.0 (6.73) | 73.5 (5.45) | <0.0001 |
| Diabetes duration (years) | 7.8 (4.71) | 7.8 (4.71) | 7.9 (4.63) | 0.70 |
| Sex | 0.12 | |||
| Male | 50.90 | 50.82 | 54.24 | |
| Female | 49.10 | 49.49 | 45.76 | |
| Race/ethnicity (%) | <0.0001 | |||
| Non-Hispanic White | 74.80 | 74.88 | 71.37 | |
| Non-Hispanic Black | 10.99 | 11.06 | 7.53 | |
| Asian or Pacific Islander | 6.473 | 6.64 | 11.11 | |
| Hispanic/Latino | 6.90 | 6.84 | 9.79 | |
| Others | 0.58 | — | — | |
| Marital status (%) | 0.30 | |||
| No | 33.65 | 33.72 | 30.51 | |
| Yes | (34.04 | 34.01 | 35.59 | |
| Missing | 32.31 | 32.28 | 33.90 | |
| Chronic conditions | ||||
| Chronic kidney disease (yes, %) | 42.17 | 42.04 | 51.98 | 0.006 |
| Hypertension (yes, %) | 94.29 | 94.25 | 95.48 | 0.23 |
| Cardiovascular disease (yes, %) | 76.14 | 76.25 | 71.19 | 0.007 |
| Cancer stage (%) | <0.0001 | |||
| Localised | 28.31 | 28.05 | 35.97 | |
| Regional | 24.64 | 24.53 | 29.00 | |
| Distant | 42.44 | 42.67 | 32.02 | |
| Missing | 4.72 | 4.75 | 3.01 | |
| Cancer histological types (%) | 0.0008 | |||
| Squamous cell carcinoma | 45.8 | 45.64 | 52.54 | |
| Adenocarcinoma | 28.98 | 28.99 | 28.63 | |
| Other types | 25.23 | 25.37 | 18.83 | |
| Cancer treatment | ||||
| Surgery (yes, %) | 23.30 | 22.89 | 42.18 | <0.0001 |
| Chemotherapy (yes, %) | 37.70 | 37.67 | 39.17 | 0.48 |
| Radiation (yes, %) | 45.04 | 45.20 | 37.85 | 0.0008 |
| Immunotherapy (yes, %) | 4.3 | 4.3 | 4.0 | 0.73 |
| EGFR antagonist (yes, %) | 3.9 | 3.9 | 5.46 | 0.07 |
aNumbers have been marked because of small sample size.
Table 2 summarises the results of the Cox proportional hazards models to test associations between SGLT2 inhibitor use and duration and NSCLC survival. Associations are shown for dichotomised SGLT2 inhibitor and canagliflozin use (yes/no) as well as duration of use divided at less than 12 months versus 12 months or more with no use as the referent. Adjusting for covariates, SGLT2 inhibitor use was associated with significantly reduced mortality risk (HR = 0.68, 95% CI = 0.60–0.77). Compared to no use, both a shorter and a longer duration of SGLT2 inhibitor use was associated with significantly lower mortality risk, with stronger effects associated with longer duration of use (HR = 0.54, 85% CI = 0.44–0.68). The most commonly used SGLT2 inhibitor, canagliflozin, showed a marginally significant association with lower mortality risk (HR = 0.88, 95% CI = 0.76–1.01). Compared to no use, the use of canagliflozin for 12 or more months was associated with lower mortality risk (HR = 0.74, 95% CI = 0.58–0.96) whereas shorter duration of use was not significantly associated with mortality risk.
Table 2.
Associations between sodium-glucose cotransporter 2 (SGLT2) inhibitor use and mortality among 24,915 non-small cell lung cancer patients.
| Number of deaths /number of patients |
HR (95% CI)a | |
|---|---|---|
| SGLT2 inhibitor use | ||
| No | 17,921/24,384 | Reference |
| Yes | 260/531 | 0.68 (0.60–0.77) |
| Duration of use | ||
| <12 months | 179/312 | 0.76 (0.66–0.89) |
| ≥12 months | 81/219 | 0.54 (0.44–0.68) |
| P for trend | 0.0001 | |
| Canagliflozin use | ||
| No | 17,993/24,591 | Reference |
| Yes | 188/324 | 0.88 (0.76–1.01) |
| Duration of use | ||
| <12 months | 128/197 | 0.96 (0.80–1.14) |
| ≥12 months | 60/127 | 0.74 (0.58–0.96) |
| P for trends | 0.03 | |
| Combination of metformin and SGLT2 inhibitors use | ||
| Neither | 12,519/16,688 | Reference |
| Metformin only | 5402/7696 | 0.93 (0.90–0.96) |
| SGLT2 only | 70/129 | 0.71 (0.56–0.89) |
| Both | 190/402 | 0.62 (0.54–0.72) |
aModels were adjusted for age at diagnosis, sex, race/ethnicity, marital status, chronic conditions (chronic kidney disease, hypertension, cardiovascular disease), cancer stage, cancer histological types (squamous cell carcinoma, adenocarcinoma, other types), cancer treatment (cancer-directed surgery, chemotherapy, radiation, immunotherapy and EGFR antagonist) and diabetes duration. All models were also adjusted for metformin use except for the analysis of combination of metformin and SGLT2 inhibitors use.
We further examined the associations between combinations of SGLT2 inhibitors and metformin use (Table 2). Compared to neither use of metformin or SGLT2 inhibitors, all other categories (including metformin use alone, SGLT2 inhibitors use alone, or use both drugs) were significantly associated with lower risk of NSCLC mortality, with the strongest association for use both of metformin and SGLT2 inhibitors (HR = 0.62, 95% CI: 0.54–0.72).
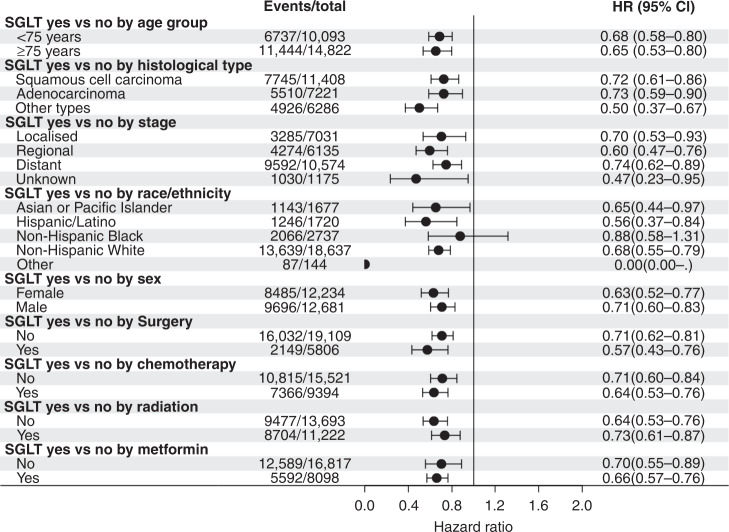
In addition, we examined the covariate-adjusted association between SGLT2 inhibitor use and risk of mortality stratified by different covariates and found that SGLT2 inhibitor use was associated with a significant reduced risk of mortality regardless of patients’ demographic, tumour characteristics and cancer treatments (Fig. 2). For example, SGLT2 inhibitor use was associated with significantly reduced risk of mortality across all cancer stages adjusted for all the covariates. Finally, the sensitivity analysis using lung cancer specific morality as an outcome among a subset of the cohort confirmed the findings that SGLT2 inhibitor use was associated with reduced risk of lung cancer mortality (HR = 0.69, 95% CI = 0.58–0.83).
Fig. 2. Forest plot of association between sodium-glucose cotransporter 2 (SGLT2) inhibitor use and mortality by covariates.
All the results were adjusted for the same covariates except for the stratified variable.
Discussion
The results indicate that SEER-Medicare NSCLC patients with pre-existing diabetes had improved survival when prescribed SGLT2 inhibitors compared to patients with diabetes who did not use SGLT2 inhibitors. A longer duration of SGLT2 inhibitor use in general, and canagliflozin specifically, was associated with better survival. Models controlled for metformin use, cancer stage, histological types, cancer treatment and other covariates. We further found a reduced mortality risk associated with SGLT2 inhibitor use across demographic, cancer treatment and tumour characteristics. Finally, we observed a similar reduced lung cancer mortality associated with SGLT2 inhibitors based on a sensitivity analysis.
This study is among the first epidemiological evaluation of the association between SGLT2 inhibitors and cancer survival using large nationally representative datasets. Early clinical evidence indicates SGLT2 inhibitors may have anti-cancer benefits for gastrointestinal, lung, pancreas, prostate and liver cancers [22, 23]. A meta-analysis of 46 randomised clinical trials by Tang et al. [23]. showed no benefit of SGLT2 inhibitors on reducing overall cancer incidence but did show a protective effect of canagliflozin specifically (OR 0.15, CI 0.04–0.60) for gastrointestinal cancer. Major limitations to these studies include the short-term length of the trials and the small numbers of cancers that were observed. Previous clinical studies also focused on cancer incidence rather than cancer survival. A number of laboratory studies have suggested intriguing lung cancer benefits related to SGLT2 inhibitors. These observed benefits from SGLT2 inhibitors include reduced tumour growth of early-stage lung adenocarcinoma and prolong survival in a mouse model [19], inhibited proliferation and survival of lung cancer cells [20, 30], or induced apoptosis in vitro in resistant non-small cell lung cancer cell lines [21].
The mechanism by which SGLT2 inhibitors may contribute to better survival of NSCLC remains to be elucidated. Potential mechanisms may include both glycemic-dependent or glycemic-independent pathways. Glucose is required for cancer cell survival and growth [31]. Lung cancer cells show increased glucose uptake and utilisation compared to normal lung cells [32, 33]. Inhibition of glucose uptake and glucose deprivation have been shown to induce apoptosis [34, 35]. It may be possible to induce apoptosis of tumour cells by inhibiting glucose uptake. The blocking of cellular glucose uptake by SGLT2 inhibitors as a means to impede tumour growth is consistent with the well-documented Warburg Effect, the observation that tumour cells demonstrate a preference for energy production through increased glucose uptake and fermentation of glucose to lactate even in the presence of oxygen [36]. These findings suggest that SGLT2 inhibitors may play a role in inhibiting glucose uptake in lung cancer metastases.
Among the many putative glycemic-independent mechanisms, there is a speculative link through oxidative stress and chronic inflammation following SGLT2 treatment [37], which are important contributors to the formation and progression of cancer [38, 39]. Recent data suggest that SGLT2 inhibitors have antioxidant properties that may be key to the reduction in cardiovascular death found in clinical trials [37]. Studies have also demonstrated that SGLT2 inhibitors have properties to reduce glucose, increase antioxidative capacity and lower free-radical production [37, 40–42]. These potent antioxidant properties may lead to favourable outcomes for cancer patients, since oxidative stress and chronic inflammation are important contributors to the formation and progression of cancer [38, 39]. Also, research has shown that SGLT2 but not SGLT1 mRNA expression was significantly higher in the metastatic lesions and lymph nodes compared with primary lung cancer tissue [43]. These findings suggest that SGLT2 inhibitors may play a role in lung cancer progression.
The strengths of our study include using a large, population-based, nationally representative database with a rich source for comorbidity data, tumour characteristics, and cancer treatments that are important for studying survival. However, there are several limitations that deserve to be mentioned. First, analysis of drug exposure is complicated by changes in therapy. Diabetes treatment regimens may change over time because of diabetes progression or evolving concepts regarding optimal management, making it difficult to disentangle the effects of variable lengths of exposure to different drug combinations. Second, there are concerns about confounding by indication because patients treated with SGLT2 inhibitors may have different clinical characteristics than patients with other diabetes-related treatment, such as those treated with metformin. However, SGLT2 inhibitors may be more likely to be prescribed for advanced diabetes after metformin failure. Third, although the linked SEER-Medicare dataset is rich, which allows us to adjust for most major confounders, including patients’ demographic factors, tumour characteristics and cancer treatments, there is lack of health behaviour data, such as smoking, physical activity or BMI available. Thus, we were not able to account for these potential confounders in our data analysis. For example, smoking is not only the predominant cause of lung cancer but also a major determinant of worse survival in lung cancer. Although it is not clear whether smoking was associated with SGLT2 inhibitor use in the data, future studies are needed to assess the potentially complex role that cigarette smoking may have on this association. Furthermore, based on our data, the patients who were prescribed SGLT2 inhibitors were likely to be younger and have early-stage disease, and less likely to be Black American people. Although we adjusted for these variables, residual confounding may still be present. Fourth, because Medicare claims data did not include cause of death, lung cancer specific mortality analysis was limited to a subset of data and shorter follow-up time from SEER in a sensitivity analysis. Finally, all of the findings presented here are from patients ≥66 years of age. Future studies that include younger patients will be needed to address this gap.
In conclusion, our large retrospective cohort analysis of the linked SEER-Medicare database indicated that SGLT2 inhibitors use appeared to be beneficial for prolonged NSCLC survivorship among people with type 2 diabetes. Additional research is warranted to confirm the findings and examine potential mechanisms that drive the association. Our findings may be helpful to inform the design of future clinical trials of SGLT2 inhibitors for lung cancer treatment.
Author contributions
JL: Conceptualisation, methodology, formal analysis, writing–original draft and writing-review and editing. MH: Conceptualisation, methodology and writing-review and editing. YD: Conceptualisation and writing-review and editing.
Funding
This study is supported by the National Institutes of Health (R03CA256238).
Data availability
Data were available from the SEER-Medicare website (https://healthcaredelivery.cancer.gov/seermedicare/obtain/) on reasonable request.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was reviewed and approved by the Human Research Protection Program (HRPP) Office of Research Compliance—Indiana University, Protocol #: 2012984604.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu, M et al. SEER Cancer Statistics Review, 1975-2016. 2019. https://seer.cancer.gov/csr/1975_2016/. Accessed 15 Oct 2015.
- 3.Lin CC, Virgo KS, Robbins AS, Jemal A, Ward EM. Comparison of comorbid medical conditions in the national cancer database and the SEER-medicare database. Ann Surg Oncol. 2016;23:4139–48. doi: 10.1245/s10434-016-5508-5. [DOI] [PubMed] [Google Scholar]
- 4.Deng HY, Zheng X, Zha P, Peng L, Huang KL, Qiu XM. Diabetes mellitus and survival of non-small cell lung cancer patients after surgery: a comprehensive systematic review and meta-analysis. Thorac Cancer. 2019;10:571–8. doi: 10.1111/1759-7714.12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu L, Cao H, Zhang T, Shen H, Dong W, Wang L, et al. The effect of diabetes mellitus on lung cancer prognosis: a PRISMA-compliant meta-analysis of cohort studies. Medicine (Baltim) 2016;95:e3528. doi: 10.1097/MD.0000000000003528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bi, G, Yao G, Bian Y, Xue L, Zhang Y, Lu T, et al. The effect of diabetes mellitus on prognosis of patients with non-small-cell lung cancer: a systematic review and meta-analysis. Ann Thorac Cardiovasc Surg. 2019. 10.5761/atcs.ra.19-00170. [DOI] [PMC free article] [PubMed]
- 7.Blonde L. Current antihyperglycemic treatment guidelines and algorithms for patients with type 2 diabetes mellitus. Am J Med. 2010;123:S12–18. doi: 10.1016/j.amjmed.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Tentolouris A, Vlachakis P, Tzeravini E, Eleftheriadou I, Tentolouris N SGLT2 inhibitors: a review of their antidiabetic and cardioprotective effects. Int J Environ Res Public Health. 2019;16:2965. [DOI] [PMC free article] [PubMed]
- 9.Nainggolan L FDA approves Canagliflozin, a first-in-class diabetes drug. 2013. https://www.medscape.com/viewarticle/781709 Accessed 30 Sept 2019.
- 10.ClinCalc. The top 200 of 2021. Provided by the ClinCalc Drugstats database. 2021. https://clincalc.com/DrugStats/Top200Drugs.aspx Accessed 11 Jan 2021.
- 11.CADTH. Canagliflozin (Invokana) for Type 2 Diabetes Mellitus [Internet]. Ottawa: Canadian Agency for Drugs and Technology in Health; 2015.
- 12.Zhang XL, Zhu QQ, Chen YH Li, XL Chen F, Huang JA, et al. Cardiovascular safety, long-term noncardiovascular safety, and efficacy of sodium-glucose cotransporter 2 inhibitors in patients with type 2 diabetes mellitus: a systemic review and meta-analysis with trial sequential analysis. J Am Heart Assoc. 2018;7:e007165. [DOI] [PMC free article] [PubMed]
- 13.Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 2011;91:733–94. doi: 10.1152/physrev.00055.2009. [DOI] [PubMed] [Google Scholar]
- 14.Rosenwasser RF, Sultan S, Sutton D, Choksi R, Epstein BJ. SGLT-2 inhibitors and their potential in the treatment of diabetes. Diabetes Metab Syndr Obes. 2013;6:453–67. doi: 10.2147/DMSO.S34416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallwitz B. The cardiovascular benefits associated with the use of sodium-glucose cotransporter 2 inhibitors—real-world data. Eur Endocrinol. 2018;14:17–23. doi: 10.17925/EE.2018.14.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cavaiola TS, Pettus J. Cardiovascular effects of sodium glucose cotransporter 2 inhibitors. Diabetes Metab Syndr Obes. 2018;11:133–48. doi: 10.2147/DMSO.S154602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2018;41:2669–701. doi: 10.2337/dci18-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaji K, Nishimura N, Seki K, Sato S, Saikawa S, Nakanishi K, et al. Sodium glucose cotransporter 2 inhibitor canagliflozin attenuates liver cancer cell growth and angiogenic activity by inhibiting glucose uptake. Int J Cancer. 2018;142:1712–22. doi: 10.1002/ijc.31193. [DOI] [PubMed] [Google Scholar]
- 19.Scafoglio CR, Villegas B, Abdelhady G, Bailey ST, Liu J, Shirali AS, et al. Sodium-glucose transporter 2 is a diagnostic and therapeutic target for early-stage lung adenocarcinoma. Sci Transl Med. 2018;10:eaat5933. [DOI] [PMC free article] [PubMed]
- 20.Villani LA, Smith BK, Marcinko K, Ford RJ, Broadfield LA, Green AE, et al. The diabetes medication Canagliflozin reduces cancer cell proliferation by inhibiting mitochondrial complex-I supported respiration. Mol Metab. 2016;5:1048–56. doi: 10.1016/j.molmet.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H, Tong CW, Leung Y, Wong MH, To KK, Leung KS. Identification of clinically approved drugs indacaterol and canagliflozin for repurposing to treat epidermal growth factor tyrosine kinase inhibitor-resistant lung cancer. Front Oncol. 2017;7:288. doi: 10.3389/fonc.2017.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin HW, Tseng CH. A review on the relationship between SGLT2 inhibitors and cancer. Int J Endocrinol. 2014;2014:719578. doi: 10.1155/2014/719578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang H, Dai Q, Shi W, Zhai S, Song Y, Han J. SGLT2 inhibitors and risk of cancer in type 2 diabetes: a systematic review and meta-analysis of randomised controlled trials. Diabetologia. 2017;60:1862–72. doi: 10.1007/s00125-017-4370-8. [DOI] [PubMed] [Google Scholar]
- 24.Molina JR, Yang PG, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–94. doi: 10.1016/S0025-6196(11)60735-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.SEER. Overview of the SEER Program. https://seer.cancer.gov/about/overview.html. Accessed 2 Nov 2021.
- 26.CMS. Medicare Program - General Information. http://www.cms.gov/MedicareGenInfo/. Accessed 24 Nov 2021.
- 27.SEER-Medicare:: Brief Description of the SEER-Medicare Database. https://healthcaredelivery.cancer.gov/seermedicare/overview/. Accessed 29 Oct 2021.
- 28.Engels EA, Pfeiffer RM, Ricker W, Wheeler W, Parsons R, Warren JL. Use of Surveillance, Epidemiology, and End Results-Medicare Data to Conduct Case-Control Studies of Cancer Among the US Elderly. Am J Epidemiol. 2011;174:860–70. doi: 10.1093/aje/kwr146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noone AM, Lund JL, Mariotto A, Cronin K, McNeel T, Deapen D, et al. Comparison of SEER treatment data with medicare claims. Med Care. 2016;54:e55–64. doi: 10.1097/MLR.0000000000000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto L, Yamashita S, Nomiyama T, Kawanami T, Hamaguchi Y, Shigeoka T, et al. Sodium-glucose cotransporter 2 inhibitor canagliflozin attenuates lung cancer cell proliferation in vitro. Diabetol Int. 2021;12:389–98. doi: 10.1007/s13340-021-00494-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scafoglio C, Hirayama BA, Kepe V, Liu J, Ghezzi C, Satyamurthy N, et al. Functional expression of sodium-glucose transporters in cancer. Proc Natl Acad Sci USA. 2015;112:E4111–4119. doi: 10.1073/pnas.1511698112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blair SL, Heerdt P, Sachar S, Abolhoda A, Hochwald S, Cheng H, et al. Glutathione metabolism in patients with non-small cell lung cancers. Cancer Res. 1997;57:152–5. [PubMed] [Google Scholar]
- 33.Hochwald SN, Harrison LE, Rose DM, Anderson M, Burt ME. gamma-Glutamyl transpeptidase mediation of tumor glutathione utilization in vivo. J Natl Cancer Inst. 1996;88:193–7. doi: 10.1093/jnci/88.3-4.193. [DOI] [PubMed] [Google Scholar]
- 34.Kan O, Baldwin SA, Whetton AD. Apoptosis is regulated by the rate of glucose-transport in an interleukin-3 dependent cell-line. J Exp Med. 1994;180:917–23. doi: 10.1084/jem.180.3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shim H, Chun YS, Lewis BC, Dang CV. A unique glucose-dependent apoptotic pathway induced by c-Myc. Proc Natl Acad Sci USA. 1998;95:1511–6. doi: 10.1073/pnas.95.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yaribeygi H, Atkin SL, Butler AE, Sahebkar A. Sodium-glucose cotransporter inhibitors and oxidative stress: an update. J Cell Physiol. 2019;234:3231–7. doi: 10.1002/jcp.26760. [DOI] [PubMed] [Google Scholar]
- 38.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603–16. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Multhoff G, Molls M, Radons J. Chronic inflammation in cancer development. Front Immunol. 2011;2:98. doi: 10.3389/fimmu.2011.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sugizaki T, Zhu S, Guo G, Matsumoto A, Zhao J, Endo M, et al. Treatment of diabetic mice with the SGLT2 inhibitor TA-1887 antagonizes diabetic cachexia and decreases mortality. npj Aging Mech Dis. 2017;3:12. doi: 10.1038/s41514-017-0012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishibashi Y, Matsui T, Yamagishi S. Tofogliflozin, a highly selective inhibitor of SGLT2 blocks proinflammatory and proapoptotic effects of glucose overload on proximal tubular cells partly by suppressing oxidative stress generation. Horm Metab Res. 2016;48:191–5. doi: 10.1055/s-0035-1555791. [DOI] [PubMed] [Google Scholar]
- 42.Osorio H, Coronel I, Arellano A, Pacheco U, Bautista R, Franco M, et al. Sodium-glucose cotransporter inhibition prevents oxidative stress in the kidney of diabetic rats. Oxid Med Cell Longev. 2012;2012:542042. [DOI] [PMC free article] [PubMed]
- 43.Ishikawa N, Oguri T, Isobe T, Fujitaka K, Kohno N. SGLT gene expression in primary lung cancers and their metastatic lesions. Jpn J Cancer Res. 2001;92:874–9. doi: 10.1111/j.1349-7006.2001.tb01175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data were available from the SEER-Medicare website (https://healthcaredelivery.cancer.gov/seermedicare/obtain/) on reasonable request.