Abstract
Anxiety is being increasingly diagnosed in the elderly population. In this sense, epidemiologic data have linked late-life anxiety disorders to increased cognitive decline, morbidity, and even mortality. In addition, studies have already reported the influence of the environment on the association between aging and anxiety. Therefore, the present study aimed to conduct a comparative analysis between Elevated Plus Maze (EPM) and Open Field (OF) tests as methods for evaluating mice's anxiety-like behavior, considering environmental and age variables. For this, eighty Female albino Swiss mice aged 6, 12, and 18 months were housed in an impoverished environment (IE) and enriched environment (EE). Following this, the animals were tested in EPM and OF tests. The environment and age affect the anxiety-like behavior of the mice in the OF, with a difference between the animals of 6 and 18 months, only in the EE (p < 0.021). However, in the EPM, it does not occur. Despite that, the environment affected the distance traveled by the mice in the EPM, where the IE animals showed greater exploratory activity than the EE, only in the 18-month group (p < 0.001). No environmental influences were detected in the OF. Concerning age, in the EPM, animals in the 18-month-old group traveled shorter distances compared to the 6-month group (p < 0.001) and the 12-month group (p < 0.001), only in EE. In turn, in the OF there was a decrease in the distance traveled in the 18-month group compared to the 6-month group (p = 0.012), only in the IE. Thus, the divergences between the results of EPM and OF instigate a better evaluation of the parameters analyzed in each test.
Keywords: Elevated plus maze, Open field, Aging, Enriched environment
Highlights
-
•
Elevated Plus Maze and Open Field tests are recommended to assess anxiety-like behavior in murine experimental models.
-
•
However, the assessment of anxiety-like behavior seems to depend on what type of variable is acting on anxiogenic behavior.
-
•
Divergences between the results of the Open Field and Elevated Plus Maze tests instigate a better evaluation of the parameters analyzed in each test.
1. Introduction
Anxiety disorders are baring increasingly diagnosed in the population, especially in elderly people [1]. This disorder is defined as the anticipation of a future threat and can be considered a habitual and adaptive emotion of everyday life, as it incites a survival instinct that keeps an individual away from risky situations [2]. In this sense, epidemiologic data have linked anxiety disorders in late life to increased cognitive decline, morbidity, and even mortality [2].
Preclinical studies have been performed to elucidate the influence of environmental in anxiety-like behavior [3]. In this sense, the type of environment for housing animals in laboratory research, especially mice, is also discussed [4]. Evidence shows that an impoverished environment (IE), poor in stimuli, limits the inherent natural behaviors of species, leading to stress, preferring an enriched environment (EE). EE consists of a type of manipulated environment that aims to include environmental modifications to improve an animal's psychological well-being, going beyond its basic needs. It can be achieved through larger and more complex domestic cages, with objects to promote play, such as running wheels or climbing apparatus [5]. Such modifications induce sensory and motor stimulation that leads manifestation of natural behaviors of species, promoting a sense of control over the environment and a better ability to deal with stress [5,6].
Morphologically, EE induces changes in the nervous system, including increased brain volume, reduced apoptosis of nerve cells, increased neurogenesis, and neuronal plasticity [7]. These findings seem to have positive effects on learning and memory, in addition to decreasing anxiety-like levels and contributing to greater exploratory activity [[8], [9], [10]].
Another important point is the increasing prevalence of the diagnosis of anxiety in elderly individuals and the possible consequences of this disorder on the quality of life of this population [11]. Clinical and preclinical studies point to a greater cognitive deficit in anxious elderly, demonstrating that anxiety in the elderly contributes to hyper agitation, dementia, and neurodegenerative diseases [12].
Therefore, the age variable is also relevant to be studied in cases of anxiety. Elevated Plus Maze (EPM) and Open Field (OF) tests are among animal models commonly used to assess anxiety-like behavior and exploratory activity [13]. They are based on the natural aversion of rodents to exposed environments, where an unprotected or completely open elevated area is an anxiogenic challenge [14,15].
Thus, as both tests are considered the most used to assess anxiety-like behavior, the present study, through an experimental murine model, aims to investigate whether they are equally sensitive to the effects of age and environment variables, translating results similar or not.
2. Methods
2.1. Experimental groups
Eighty Female albino Swiss mice were housed under different conditions represented in Fig. 1. (A-C), part in an impoverished environment (IE), Fig. 1 (B), and part in an enriched environment (EE), Fig. 1 (C). The IE consisted of plastic cages of standard dimensions (32 × 39 × 16.5 cm), lined with rice straw, and covered with metal grids. The EE consisted of two-level wire cages (50 × 50 × 50 cm), equipped with tunnels, bridges, sliding wheels, and toys, which were changed periodically, keeping the animals exposed to new stimuli.
Fig. 1.
Impoverished environment and enriched environment
Note. (A–B) Impoverished environment: (A) Plastic cages, covered with a metal grid, allowing free access to water and feed. (B) Mice in a housing containing minimal stimuli, lined with rice straw. (C) Enriched environment: two-level housing containing ropes, tunnels, bridges, rods, racing wheels, and toys. The objects were changed or replaced weekly, and the animals had free access to water and food, arranged on different floors.
Considering that the life span of a mouse is 24 months [16], animals from each environment were divided by age, where the 6-month-old group represented young animals (n= 16 for IE and EE), the 12-month-old group was middle-aged (n= 16 for IE and EE) and, finally, the 18-month-old group a senile animal (n= 7 for IE and n= 9 for EE), with different animals for each age.
The temperature was controlled (23 ± 1 °C) and the light-dark cycles lasted 12 h, with the light period from 6:00 p.m. to 6:00 a.m. and the dark period from 6:00 a.m. to 6:00 p.m. The animals provided by Instituto Evandro Chagas were handled according to the "Principles of Laboratory Animal Care” (National Institutes of Health), at Laboratory for Research in Neurodegeneration and Infection, University Hospital João de Barros Barreto, of the Federal University of Pará, Brazil. The experimental protocol was tested and approved before the study began by the Ethics Committee on Experimental Animal Research (from the Institute of Biological Sciences, Federal University of Pará, Brazil, CEPAE-UFPA: 223-14).
2.2. Behavioral testing of mice
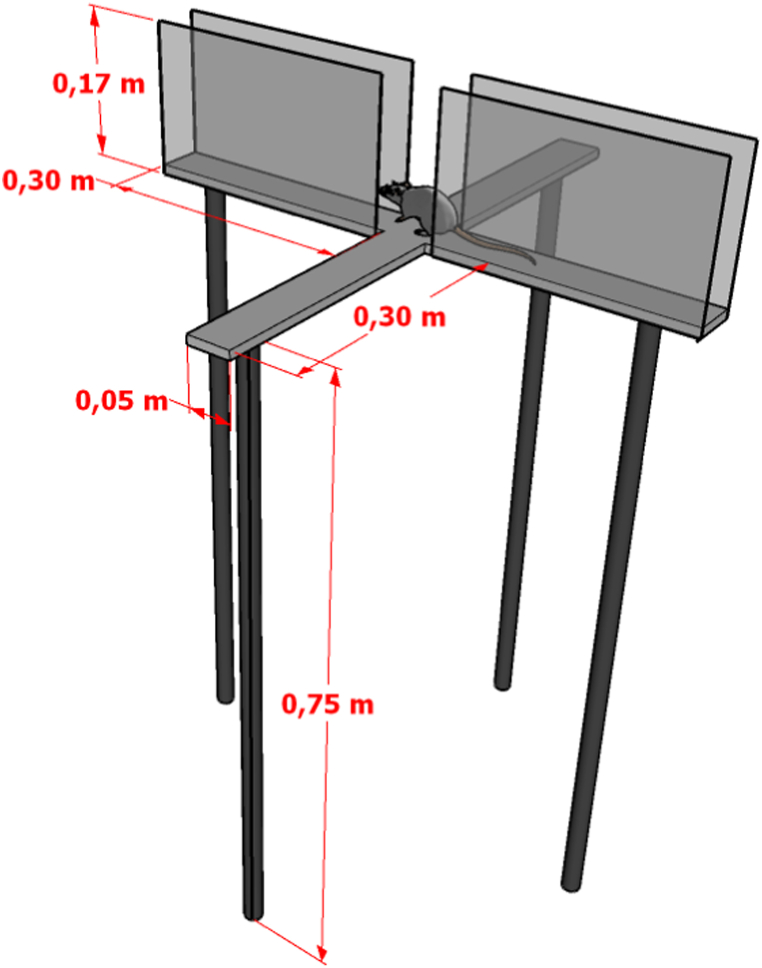
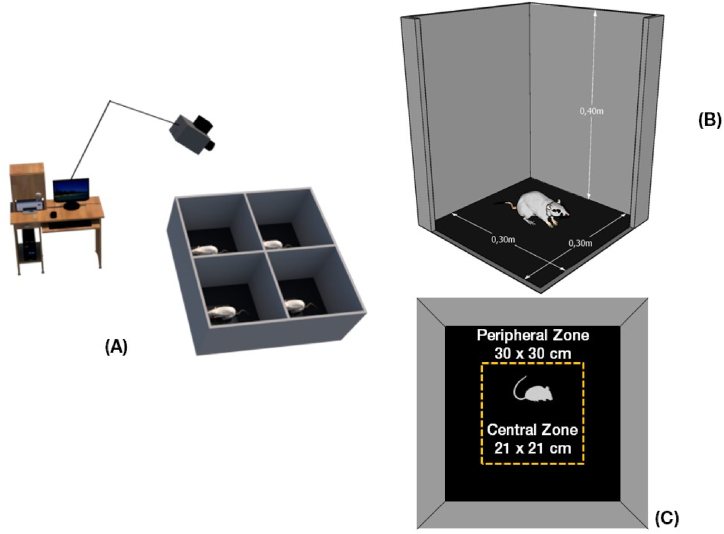
The animals were evaluated for anxiety-like behavior through the EPM and OF tests, in each aging group. Then, each animal was kindly introduced into the central platform of the EPM apparatus and later into the center of box for 5 min. EPM apparatus consisted of two arms, one open and one closed of 30 × 5 cm each, in the opposite positions and joined at a 5 × 5 cm central platform. The closed arms (CA) are surrounded by 17 cm high walls and open arms (OA) do not have walls. The entire apparatus, which is represented in Fig. 2, is wood-made and painted with black paint suspended 75 cm from the floor. OF apparatus consisted of an open box with 30 × 30 × 40 cm, covered in a gray casing and a black background, which can be seen in Fig. 3 (A-B). After each session, devices were sanitized with 70% ethanol solution to remove olfactory clues, as well as organic waste from the animal.
Fig. 2.
Illustration of the Elevated Plus Maze apparatus with its dimensions.
Note. The apparatus is suspended 0.75 m from the ground and consists of two opposing arms, one open and one closed of 0.30 × 0.05 m each. The closed arms are surrounded by 0.17 m high walls and the open arms have no walls.
Fig. 3.
Illustration of the Open Field apparatus.
Note. (A) Representation of the test scenario, with a monitoring system for video capture and apparatus used in the test, being possible to test 4 animals concurrently, but without communication between them or behavior interference. (B) Dimensions of the box, 0.3 × 0.3 × 0.4 m. (C) Virtual division of the Open Field apparatus floor into central and peripheral zones, with approximately equal areas.
Tests were recorded using a video camera (Philips® SPC 611NC) installed on the ceiling of the behavioral room and connected to a computer to record images. To process and analyze behavioral parameters detected in videos, the computer software ANY-maze Video Tracking System (StoeltingCo©) was used. The tests were performed with the same protocol in handling procedures, with the same levels of luminosity measured using a photometer (4–5 cd/m2) and at the same time of day (dark cycle) for all groups.
In the EPM, one of the parameters used to assess anxiety-like behavior is the time spent in each arm (open arms, closed arms, and central platform), expecting a spontaneous tendency to avoid unprotected OA, concentrating permanence in CA. The total distance traveled, and the distance traveled minute by minute were also used.
In the OF, the computer program virtually divided the box floor into two different square zones of approximately equal sizes: central zone (CZ) and peripheral zone (PZ) (Fig. 3C). This division allowed the analysis of time spent in each zone expressed in seconds, in addition to the distance traveled in each zone. In the same way, a spontaneous tendency of the animal is expected to avoid the unprotected central zone and to concentrate its permanence in the peripheral zone, thus allowing the evaluation of anxiety-like behavior. In addition, it was evaluated the total distance traveled and the distance traveled minute by minute.
Statistical analysis was based on the video records of each session, extracted by ANY-maze Video Tracking System (Stoelting©). The normality of quantitative data was tested with the Shapiro-Wilk test. Regarding the behavioral parameters, significant differences between the groups were evaluated with two-way and one-way Analysis of Variance (ANOVA) tests, depending on the analyzed parameter, and Tukey's post hoc test, assuming an interval of 95% confidence (p < 0.05). The influences of age and environment were investigated, and analyses were performed with the Jamovi® software.
3. Results
3.1. Time in the closed arm and peripheral zone
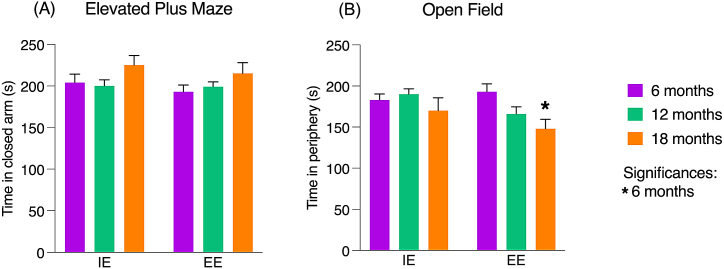
To evaluate the anxiety-like behavior, the parameter used was the time spent by the animals in each arm (OA and CA) in EPM and in each zone (CZ and PZ) in OF. In EPM, no significant differences were identified when evaluating the influence of age and environment on anxiety-like behavior, independent of the arm. In OF, as CZ and PZ are complementary zones, a significant difference was found related to time spent in CZ and PZ between the 6 and 18-month-old animals, only in EE (F [2.74] = 3.97, p < 0.021). Fig. 4 (A-B) shows the graphical representation of data obtained in the areas where animal is expected to concentrate its permanence, CA of the EPM, Fig. 4 (A), and PZ of the OF, Fig. 4 (B).
Fig. 4.
Anxiety-like behavior of young, middle-aged, and senile albino Swiss mice submitted to an impoverished and enriched environment.
Note. (A) Time (s) in the closed arm of the Elevated Plus Maze and (B) time (s) in the peripheral zone in the Open Field test. Both tests had a total duration of 300 s. Values are expressed as mean ± standard error, highlighting the significant differences (p < 0.05): (*) significant differences about 6 months. Significance values were obtained through Analysis of Variance (ANOVA) 2 criteria (age and environment) and post Tukey test. IE – impoverished environment; EE – enriched environment.
3.2. Total distance traveled
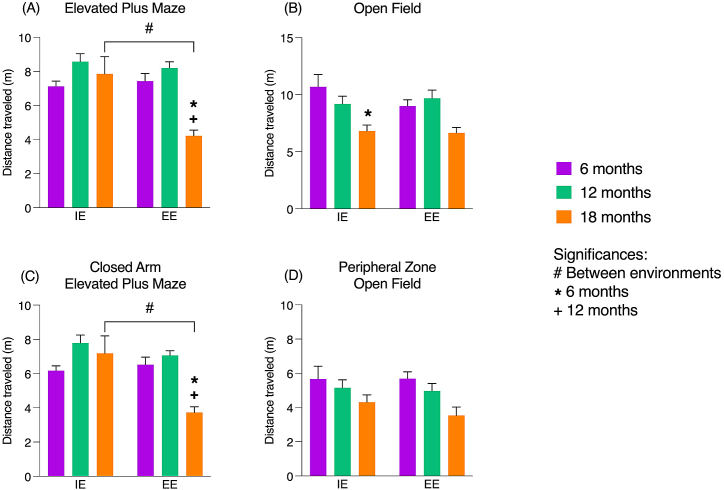
According to the results presented so far, few differences were observed between the EPM and OF tests, or even those induced by age and environment. In this sense, we performed a complementary analysis based on the total distance traveled, expressed in meters. EPM showed significant differences in the total distance traveled between the groups, where the interaction of variables age and environment was observed (F [2.74] = 7.52, p = 0.001). In the EPM, at an 18-month-old, there were significant differences in the total distance traveled when comparing animals from IE and EE (F [1.74] = 9.45, p < 0.001), which was not observed at 6- and 12-month-old. 18-month-old animals in IE showed greater distance traveled than those in the EE. Regarding age, in EE, a decrease was detected in the distance traveled at 18 months old with significant differences when compared to 6-month-old animals (F [2.74] = 10.54, p < 0.001) and to 12-month-old. (F [2.74] = 10.54 p < 0.001).
The OF revealed a significant difference in distance traveled between 6- and 18-month-old in IE (F [2.73] = 6.92, p = 0.012), with no interaction between age and environment. There was a decrease in the distance traveled in 18-month-old group compared to 6-month-old group.
An analysis of the distance traveled in the closed arm and the peripheral zone was also performed. The results obtained in the closed arm were like those found when considering the entire apparatus, indicating a predilection for this area. In the OF test, no significant differences were identified unlike when the entire apparatus was considered. The graphical representation of the data obtained is shown in Fig. 5 (A-D).
Fig. 5.
Distance traveled by young, middle-aged, and senile albino Swiss mice, submitted to impoverished and enriched environments.
Note. (A–B) Total distance traveled (m): (A) in the Elevated Plus Maze and (B) in the Open Field test. (C) Distance traveled (m) in the closed arm of the Elevated Plus Maze and (D) distance traveled (m) in the peripheral zone of the Open Field test. Values are expressed as mean ± standard error, highlighting the significant differences (p < 0.05): (#) significant differences between environments; (*) significant differences to 6 months and (+) about 12 months. Significance values were obtained through Analysis of Variance (ANOVA) 2 criteria (age and environment) and post Tukey test. IE – impoverished environment; EE – enriched environment.
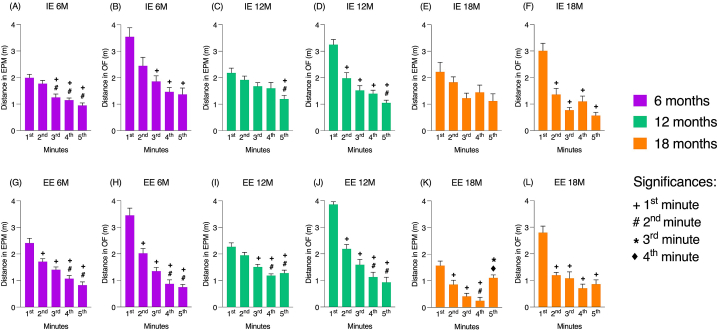
3.3. Distance traveled minute by minute
Exploratory activity was also evaluated through minute-by-minute distance traveled in each of the tests. In EPM and OF, for both environments and at 6 and 12-month-old, a higher pattern of exploratory activity was observed in the 1st minute decreasing in subsequent minutes. The 18-month-old animals in the EPM did not follow this pattern, as there was no significant reduction in the distance covered at the first minute of the test. The graphical representation of the data is shown in Fig. 6 (A-L).
Fig. 6.
Albino Swiss mice exploratory activity in each group formed.
Note. (A–F) Distance traveled minute by minute (m) of the animals in an impoverished environment, being (A, C, and E) in the Elevated Plus Maze and (B, D, F) in the Open Field test, where (A and B) represent 6 months, (C and D) 12 months and (E and F) 18 months. (G–L) Distance traveled minute by minute (m) of the animals in an enriched environment, being (G, I and K) in the Elevated Plus Maze and (H, J, L) in the Open Field test, where (G and H) represent 6 months, (I and J) 12 months and (K and L) 18 months. Values are expressed as mean ± standard error, highlighting the significant differences (p < 0.05): (+) significant differences about the 1st minute, (#) to the 2 nd min, (*) to the 3rd minute, and (♦) at the 4th minute. Significance values were obtained through Analysis of Variance (ANOVA) and after Tukey's test. EPM – Elevated Plus Maze; IE – impoverished environment; EE – enriched environment; 6 M–6 months; 12 M–12 months; 18 M–18 months.
4. Discussion
This study aimed to investigate whether EPM and OF are equally sensitive to the effects of age and environmental variables, translating similar results or not, in an experimental murine model.
The present study identified different influences of the environment on anxiety-like behavior in the OF, but not in the EPM. At this point, Sparling et al. [5] considered that there is no unanimity in the literature about the impact of environmental enrichment on anxiety-like behavior in studies with both tests.
In the OF, 18-month-old animals raised in the EE spent less time in the peripheral zone when compared to the 6-month group. In a study carried out by Shoji and Miyakawa [17], mice of different ages (2, 11, 17, and 23 months) did not show a predilection for a certain zone in the first 5 min of the test. However, this same study extended the OF to 120 min and, only then, identified a shorter time spent in the peripheral zone for senile animals showing a decrease in anxiety-like behavior. It is worth noting that, in the present study, the duration of the OF was 5 min, as recommended by other studies [18,19].
On the other hand, Nolte et al. [20] reported results that contradict our findings. In the study, 24-month-old mice submitted to 30 min in the OF remained longer in the peripheral zone compared to 3 and 12-month mice. Thus, given the divergences in the findings on the anxiety-like behavior when using the OF, there is a need for better standardization regarding the methodology used, especially regarding the duration of the test.
In the EPM, no significant differences were observed in the time spent in the CA and OA of EPM when comparing ages, which agrees with the findings of Shoji and Miyakawa [17] but differs from those of Gokdemir et al. [21].
In the analysis of distance traveled, the EPM showed an interaction between age and environmental factors influencing the exploratory activity. The 18-month-old animals kept in IE traveled a greater distance during the test than the EE animals at the same age. This can be explained by the fact that, since these animals were kept in a poor stimuli environment, exposure to EPM was realized as a novelty, leading to a greater exploratory activity different from those from EE. On the other hand, the findings by Tarasova et al. demonstrate that animals kept in EE had a greater exploratory activity [9]. No environmental influences were detected in the OF.
In the EPM, the aging animals in EE traveled a shorter distance when compared to 6- and 12-month-olds revealing the influence of aging on exploratory activity. These findings agree with other studies where it was observed that older animals traveled shorter distances compared to younger ones in several behavioral tests, including EPM [17,22].
The OF showed a significant decrease in the distance traveled only between the 18-month-old animals compared to the 6-month-old in IE. In this way, environmental enrichment seems to attenuate the effects of aging in exploratory activity. The differences in terms of age agree with the findings of Shoji and Miyakawa [17], that reported a decreased exploratory activity in older mice compared to younger ones. However, another study using OF demonstrated that mice maintained similar patterns of exploratory activity at all ages [20], while an increase in exploratory activity in senile animals has also been reported [21].
The present study included an analysis of the distance traveled minute by minute in EPM and OF tests, considering the apparatus as a whole. There was a decrease in the distance traveled over the minutes of both tests showing significant reductions in exploratory activity, which has already been reported by de Siqueira Mendes et al. [23]. Such decrease occurred abruptly in OF, while in EPM it occurred gradually. Since OF apparatus consists of an open area without so many possibilities or alternatives, the animal tends to quickly explore the environment, a fact represented by the high distances traveled in the first minute. However, the significant differences from the second minute onwards about the first show an adaptation to the test proposal quickly losing interest in exploring the apparatus. The same is not observed in EPM, which can be explained by the conflict experienced by the animal between the motivation to explore a new environment and the desire to avoid OA of apparatus [24]. In addition, the decrease in exploratory activity was slightly altered in the EPM for 18-month-old animals raised in IE, with no significant difference in the distance covered in the apparatus between the test minutes.
About the experimental animal models, it is known that EPM and OF tests are widely used to assess anxiety-like behavior in addition to being able to measure parameters related to exploratory activity [14,15]. They are based on the natural aversion of rodents to exposed environments, where unprotected or completely open elevated areas are considered anxiogenic agents. The time spent in each of the zones, as well as the distance traveled during the tests, are some aspects to be estimated to assess anxiety-like behavior and exploratory activity [25,26].
However, when it comes to anxiety-like behavior, the findings obtained by EPM and OF were contradictory. As already mentioned, our results showed that the EPM was not able to identify significant differences in the time spent in OA and CA between animals of different ages and kept in different environments, while the OF showed a significant difference between 6-month and 18-month-old animals kept in EE, where senile animals spent less time in the peripheral zone.
For a possible explanation, it must first be considered that anxiety can be classified as an anxiety state or an anxiety trait, based on its duration and consistency. The anxious state reflects situations that create anxiety at a given moment when facing a threat. On the other hand, the anxious trait characterizes stable anxiety over time, which reflects a threat perceived by an individual but not by others. Thus, an anxious individual will show a greater anxiety trait than a healthy individual [27,28].
Therefore, EPM and OF tests are recommended to assess the state of anxiety-like behavior, since they create an anxiogenic environment for the animal. However, EPM seems to be more effective in this regard since the test apparatus can cause more anxiety than OF [29]. The animal in EPM is suspended, without protection, when exploring the OA, which is very different from what happens in OF. It is also worth noting that, primarily, EPM is commonly applied for pharmacological studies, such as for the evaluation of anxiolytic drugs. Thus, given everything that has already been mentioned and considering again that the present study did not identify changes in the anxiety-like behavior through EPM and, in OF, it identified differences, finally some hypotheses can be raised.
First, it is questioned whether EPM is adequately sensitive to assess the influence of age and environment on anxiety-like behavior, knowing that the animals were within their natural conditions, or if its application would be more opportune in situations with variables that act directly on anxiogenic behavior, such as the use of anxiolytic drugs. In addition, as it is a test that assesses the anxious state resulting from a threat that it momentarily imposes, a lasting anxious trait resulting from aging may not be identified. In agreement, studies mention that the only test proposed as an animal model to assess anxiety traits is the free-exploratory paradigm [10,27,30]. Regarding OF, it is contested how much the apparatus used can induce an anxiogenic behavior, which can be identified statistically, suggesting its effectiveness is more evident in the evaluations of exploratory activity since it does not impose the same threat level as the EPM.
A final point to be highlighted is the use of only female species in our study. Although gender is discussed in the literature, recent studies suggest that there are no qualitative differences between males and females when it comes to anxiogenic analysis [31]. On the other hand, the choice to use female animals is also a matter of convenience for our laboratory, since there are reports in the literature of increased aggression, particularly among male mice, in the enriched environment [4]. In addition, although there is a difference between the animals due to age and estrous cycle, it is assumed that this influence may be equally distributed between the tests since they are the same animals. Therefore, any difference observed leads us to believe that it is due to the sensitivity of the tests to these variables.
Finally, given the reported findings, the differences in the results found between the EPM and OF tests further instigate the discussion about the use of such tests to evaluate similar parameters related to anxiety-like behavior and exploratory activity.
Production notes
Author contribution statement
Mariah Cerqueira; Micaele Lopes Castro: Analyzed and interpreted the data; Wrote the paper.
Amanda Almeida Vieira; Juliana Ayumi Azevedo Kurosawa; Fabio Leite do Amaral Junior: Performed the experiments; Wrote the paper.
Fabíola de Carvalho Chaves de Siqueira Mendes: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Marcia Consentino Kronka Sosthenes: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Marcia Consentino Kronka Sosthenes was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico [475677/2008-0 and 304411/2022-1], Fundação Amazônia Paraense de Amparo à Pesquisa [136/08].
Fabíola de Carvalho Chaves de Siqueira Mendes was supported by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior).
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest’s statement
The authors declare no competing interests.
References
- 1.Lucas S.J., et al. 2015. High-Intensity Interval Exercise And Cerebrovascular Health: Curiosity, Cause, And Consequence; pp. 902–911. 35(6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crocq, M.-A.J.D.i.c.n . 2022. A History of Anxiety: from Hippocrates to DSM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gong X., et al. 2018. Environmental Enrichment Reduces Adolescent Anxiety-And Depression-Like Behaviors Of Rats Subjected To Infant Nerve Injury; pp. 1–13. 15(1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oatess T.L., et al. 2021. Effects Of Acrylic Tunnel Enrichment On Anxiety-Like Behavior, Neurogenesis, And Physiology Of C57BL/6J Mice; pp. 44–53. 60(1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sparling J.E., et al. vol. 197. 2020. (Environmental Enrichment and its Influence on Rodent Offspring and Maternal Behaviours, a Scoping Style Review of Indices of Depression and Anxiety). [DOI] [PubMed] [Google Scholar]
- 6.André V., et al. 2018. Laboratory Mouse Housing Conditions Can Be Improved Using Common Environmental Enrichment Without Compromising Data; p. e2005019. 16(4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H., et al. 2020. Enriched Environment And Social Isolation Affect Cognition Ability Via Altering Excitatory And Inhibitory Synaptic Density In Mice Hippocampus; pp. 2417–2432. 45(10) [DOI] [PubMed] [Google Scholar]
- 8.Bayne K.J.A.m., medicine e. 2018. Environmental Enrichment And Mouse Models: Current Perspectives; pp. 82–90. 1(2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tarasova A.Y., et al. 2018. Influence of" Enriched Environment" on Behavior and Neurogenesis in Mice Selected by Cognitive Trait; pp. 583–587. 164(5) [DOI] [PubMed] [Google Scholar]
- 10.Goes T.C., Antunes F.D., Teixeira-Silva F.J.N.l. vol. 584. 2015. pp. 93–96. (Environmental Enrichment for Adult Rats: Effects on Trait and State Anxiety). [DOI] [PubMed] [Google Scholar]
- 11.Kim Y.-K. vol. 1191. Springer Nature; 2020. (Anxiety Disorders: Rethinking and Understanding Recent Discoveries). [Google Scholar]
- 12.Santabarbara J., et al. Clinically relevant anxiety and risk of Alzheimer's disease in an elderly community sample: 4.5 years of follow-up. J. Affect. Disord. 2019;250:16–20. doi: 10.1016/j.jad.2019.02.050. [DOI] [PubMed] [Google Scholar]
- 13.Knight P., et al. vol. 204. 2021. (Sex Differences in the Elevated Plus-Maze Test and Large Open Field Test in Adult Wistar Rats). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kraeuter A.-K., Guest P.C., Sarnyai Z. Pre-clinical Models. Springer; 2019. The elevated plus maze test for measuring anxiety-like behavior in rodents; pp. 69–74. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka S., et al. 2012. Four Factors Underlying Mouse Behavior In An Open Field; pp. 55–61. 233(1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown-Borg H.M., et al. Dwarf mice and the ageing process. Nature. 1996;384(6604):33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- 17.Shoji H., Miyakawa T.J.N.r. 2019. Age‐Related Behavioral Changes From Young To Old Age In Male Mice Of A C57 BL/6J Strain Maintained Under A Genetic Stability Program; pp. 100–118. 39(2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carola V., et al. 2002. Evaluation Of The Elevated Plus-Maze And Open-Field Tests For The Assessment Of Anxiety-Related Behaviour In Inbred Mice; pp. 49–57. 134(1–2) [DOI] [PubMed] [Google Scholar]
- 19.Kumar V., et al. 2013. Animal Models Of Anxiety: A Comprehensive Review; pp. 175–183. 68(2) [DOI] [PubMed] [Google Scholar]
- 20.Nolte E.D., et al. 2019. Anxiety And Task Performance Changes In An Aging Mouse Model; pp. 246–251. 514(1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gokdemir O., et al. 2020. The Effect Of Exercise On Anxiety-And Depression-Like Behavior Of Aged Rats; pp. 8–17. 95(1) [DOI] [PubMed] [Google Scholar]
- 22.Shoji H., et al. 2016. Age-Related Changes In Behavior In C57BL/6J Mice From Young Adulthood To Middle Age; pp. 1–18. 9(1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Siqueira Mendes F.d.C.C., et al. Environmental impoverishment, aging, and reduction in mastication affect mouse innate repertoire to explore novel environments and to assess risk. Front. Neurosci. 2019;13:107. doi: 10.3389/fnins.2019.00107. 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahn S.-H., et al. 2013. Basal Anxiety During An Open Field Test Is Correlated With Individual Differences In Contextually Conditioned Fear In Mice; pp. 154–159. 17(3) [Google Scholar]
- 25.Seibenhener M.L., Wooten M.C.J.J. 2015. Use Of The Open Field Maze To Measure Locomotor And Anxiety-Like Behavior In Mice. (96) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Komada M., Takao K., Miyakawa T.J.J. 2008. Elevated Plus Maze For Mice; p. e1088. (22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goes T.C., et al. 2018. Excitotoxic Lesion Of The Medial Prefrontal Cortex In Wistar Rats: Effects On Trait And State Anxiety; pp. 313–319. 142. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy B.L., et al. 2001. Assessment Of State And Trait Anxiety In Subjects With Anxiety And Depressive Disorders; pp. 263–276. 72(3) [DOI] [PubMed] [Google Scholar]
- 29.Erdoğan F., et al. 2005. Effects Of Pentylenetetrazole-Induced Status Epilepticus On Behavior, Emotional Memory And Learning In Immature Rats; pp. 537–542. 6(4) [DOI] [PubMed] [Google Scholar]
- 30.Goes T.C., Antunes F.D., Teixeira-Silva F.J.N.l. 2009. Trait And State Anxiety In Animal Models: Is There Correlation? pp. 266–269. 450(3) [DOI] [PubMed] [Google Scholar]
- 31.Lovick T.A., Zangrossi H.J.F.i.P., Jr. 2021. Effect Of Estrous Cycle On Behavior Of Females In Rodent Tests Of Anxiety; p. 1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.