Highlights
-
•
Lp(a) testing occurred most commonly in patients with prevalent ASCVD and multiple prior CV events.
-
•
Elevated Lp(a) level was associated with greater odds of subsequent lipid lowering therapy initiation.
-
•
Elevated Lp(a) was associated with composite cardiovascular hospitalization.
-
•
Lp(a) testing occurs infrequently in clinical practice.
Keywords: Lipoprotein(a), Cardiovascular prevention
Abstract
Objective
Elevated lipoprotein(a) [Lp(a)] is associated with atherosclerotic cardiovascular disease, yet little is known about Lp(a) testing patterns in real-world practice. The objective of this analysis was to determine how Lp(a) testing is used in clinical practice in comparison with low density lipoprotein cholesterol (LDL-C) testing alone, and to determine whether elevated Lp(a) level is associated with subsequent initiation of lipid-lowering therapy (LLT) and incident cardiovascular (CV) events.
Methods
This is an observational cohort study, based on lab tests administered between Jan 1, 2015 and Dec 31, 2019. We used electronic health record (EHR) data from 11 United States health systems participating in the National Patient-Centered Clinical Research Network (PCORnet). We created two cohorts for comparison: 1) the Lp(a) cohort, of adults with an Lp(a) test and 2) the LDL-C cohort, of 4:1 date- and site-matched adults with an LDL-C test, but no Lp(a) test. The primary exposure was the presence of an Lp(a) or LDL-C test result. In the Lp(a) cohort, we used logistic regression to assess the relationship between Lp(a) results in mass units (< 50, 50-100, and > 100mg/dL) and molar units (<125, 125-250, > 250nmol/L) and initiation of LLT within 3 months. We used multivariable adjusted Cox proportional hazards regression to evaluate these Lp(a) levels and time to composite CV hospitalization, including hospitalization for myocardial infarction, revascularization and ischemic stroke.
Results
Overall, 20,551 patients had Lp(a) test results and 2,584,773 patients had LDL-C test results (82,204 included in the matched LDL-C cohort). Compared with the LDL-C cohort, the Lp(a) cohort more frequently had prevalent ASCVD (24.3% vs. 8.5%) and multiple prior CV events (8.6% vs. 2.6%). Elevated Lp(a) was associated with greater odds of subsequent LLT initiation. Elevated Lp(a) reported in mass units was also associated with subsequent composite CV hospitalization [aHR (95% CI): Lp(a) 50-100mg/dL 1.25 (1.02-1.53), p<0.03, Lp(a) > 100mg/dL 1.23 (1.08-1.40), p<0.01].
Conclusion
Lp(a) testing is relatively infrequent in health systems across the U.S. As new therapies for Lp(a) emerge, improved patient and provider education is needed to increase awareness of the utility of this risk marker.
1. Introduction
Lipoprotein(a) [Lp(a)] is a lipid molecule similar to low density lipoprotein cholesterol (LDL-C), with an apolipoprotein(a) [apo(a)] attached covalently to apolipoprotein B (apoB) [1]. Lp(a) is a known risk factor for atherosclerotic cardiovascular disease (ASCVD) and is thought to mediate atherosclerosis by promoting endothelial dysfunction, increasing inflammation, and inhibiting fibrinolysis [2]. Individual Lp(a) levels are 80-90% genetically determined via the LPA gene, which are inherited in an autosomal codominant fashion and remain relatively static throughout adulthood [3], with little potential for modification by therapeutic lifestyle changes or statin therapy.
Elevated Lp(a) has been associated with increased risk of coronary artery disease, peripheral artery disease and ischemic stroke in multiple epidemiologic, Mendelian randomization and genome-wide association studies [4], [5], [6]. The LPA gene locus has been identified as one of the strongest monogenetic risk factors for coronary artery disease, even more potent than low density lipoprotein and PCSK9-related genetic variants [7]. Circulating Lp(a) can be measured by mass, in milligrams per deciliter (mg/dL) or by particle number, in nanomoles per liter (nmol/L). Because apo(a) isoforms have variable size, Lp(a) mass assays must be carefully calibrated and are subject to measurement error [8]. The exact cutoff at which Lp(a) level confers increased ASCVD risk on a population level remains somewhat controversial and likely varies within racial groups [2,9]. Both the American College of Cardiology/American Heart Association and European Atherosclerosis Society consider Lp(a) to be elevated if greater than 50 mg/dL or > 125nmolL [10,11]. Observational and epidemiologic studies suggest that approximately 20% of the adult population meets this threshold [12].
Although the relationship between Lp(a) and ASCVD is known, Lp(a) testing patterns in real world practice have not been well described. Additionally, little is known about how Lp(a) levels affect subsequent treatment decisions (i.e., use of lipid lowering therapy) or outcomes (subsequent ASCVD events in clinical practice). Our goal was to describe the population who receives Lp(a) testing compared with those who undergo LDL-C testing alone, to characterize downstream changes in lipid lowering therapy (LLT) and subsequent cardiovascular (CV) outcomes.
2. Methods
Data was extracted retrospectively from 11 United States (U.S.) health systems participating in the National Patient-Centered Clinical Research Network (PCORnet®). PCORnet, developed with funding from the Patient-Centered Outcomes Research Institute (PCORI), is a distributed data network of Clinical Research Networks (CRNs) with data from health systems across the country. Data from individual electronic health records (EHR) within PCORnet have been standardized according to the PCORnet Common Data Model (https://pcornet.org). Data quality is maintained through quarterly data curation efforts and study-specific quality checks. Health systems were selected for inclusion in this analysis based on their Lp(a) lab test result availability and willingness to participate. This study received approval from the Duke Institutional Review Board. This work has been carried out in accordance with the Code of Ethics of the World Medical Association.
We created two cohorts based on available lab test results from January 1, 2015 to December 31, 2019: 1) the Lp(a) cohort, including patients who had at least one Lp(a) test result within the study period and 2) the LDL-C cohort, a control group with an LDL-C test but no Lp(a) test during the study period. The LDL-C cohort was matched 4:1 by date (quarter and year) and site to patients in the Lp(a) cohort. Patients included in both cohorts were ≥ 18 years of age at the time of testing and had at least one other encounter within the health system in the year prior to lab test order to ensure they received longitudinal care within that system. For the outcome analysis, patients from the Lp(a) cohort were included if their test result occurred between January 1, 2015 and December 31, 2018 to allow for adequate follow-up time.
The primary exposure was the presence of an Lp(a) or LDL-C test result. Index date was defined as the date of the first Lp(a) test result within the study period, or date-matched LDL-C test result. Lp(a) values were reported in either mass (mg/dL) or molar units (nmol/L) by sites. Given imprecision in the conversion between units due to the heterogeneity of apoB size, Lp(a) mass and molar results were analyzed separately [8,13,14]. LDL-C values were reported only in mass units (mg/dL).
Demographic data were abstracted from encounter information on the date of index lab test. Comorbid diagnoses, defined by International Classification of Disease (ICD) 9th and 10th revision codes, were recorded from within the 3 years prior to the index lab test. Vital signs were abstracted from encounters within ±90 days of index lab test. Baseline medication use and other laboratory data were recorded within 1 year prior to index lab test.
The primary endpoint was initiation of LLT within 3 months of the index lab test. This included initiation of statin, ezetimibe, proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i), or niacin in those not already on any of these therapies at index testing encounter. The secondary endpoint was time to a composite of CV hospitalization, including hospitalization for myocardial infarction, ischemic stroke and/or coronary revascularization. These encounters were defined by ICD 9 and 10 codes or procedure codes.
We summarized characteristics of both cohorts using descriptive statistics (median and 25th/75th percentiles for continuous variables, frequencies and percentages for categorical variables). Due to large sample sizes, we did not perform statistical hypothesis testing to compare patient characteristics between the Lp(a) and matched LDL-C cohorts. Notable differences are flagged based on clinical significance.
Due to the number of Lp(a) values reported as “less than” in the data (20%, with the majority of these reported as “< 6 mg/dL”), we assessed relationships between outcomes and Lp(a) level using pre-defined categories (Lp(a) < 50mg/dL, 50-100mg/dL, and > 100mg/dL for results in mass units and Lp(a) < 125nmol/L, 125-250nmol/L and > 250nmol/L) with Lp(a) < 50mg/dL and Lp(a) < 125nmol/L as reference, respectively. Lp(a) categories were based on American College of Cardiology (ACC)/American Heart Association (AHA) guideline cutoffs and population values over the 90th percentile [15,16].
A logistic regression model was used to assess the relationship between Lp(a) levels and initiation of LLT. A Cox proportional hazards model was used to evaluate the relationship between Lp(a) and time to composite CV hospitalization. All models considered clustering within sites using a robust sandwich covariance estimator and were adjusted for age, sex, race, prior ASCVD status, body mass index (BMI), history of hypertension, history of diabetes, total cholesterol, calculated low density LDL-C, high density lipoprotein cholesterol (HDL-C), and statin use. Continuous covariates were included as natural cubic splines to allow for flexible relationships. Missing data were excluded from denominators in descriptive analyses, with <10% missing data for all model covariates. Single imputation using the median was used for modeling purposes.
All analyses were conducted by the Duke Clinical Research institute (Durham, NC) using SAS version 9.4 (SAS Institute Inc, Cary NC) or R version 4.0 or higher (R Core Team).
3. Results
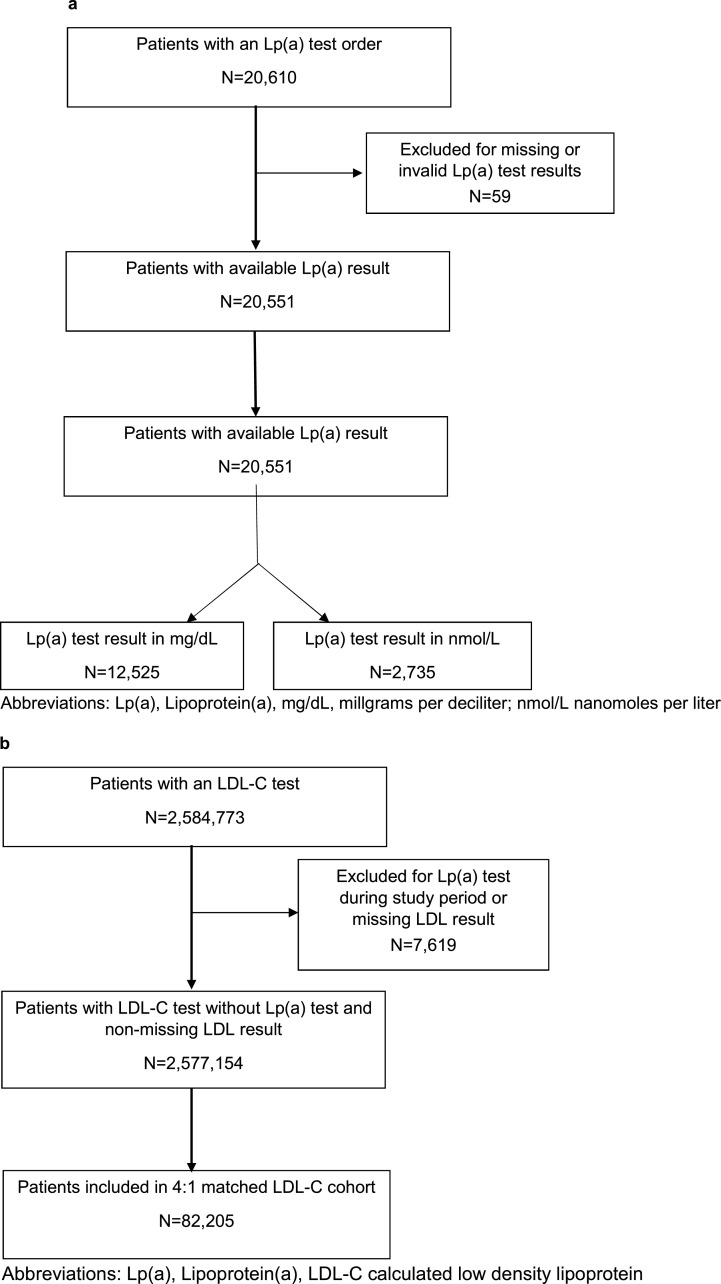
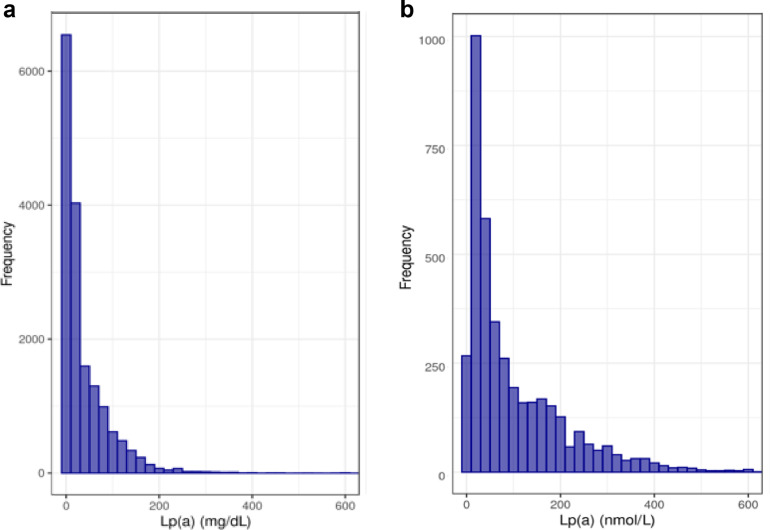
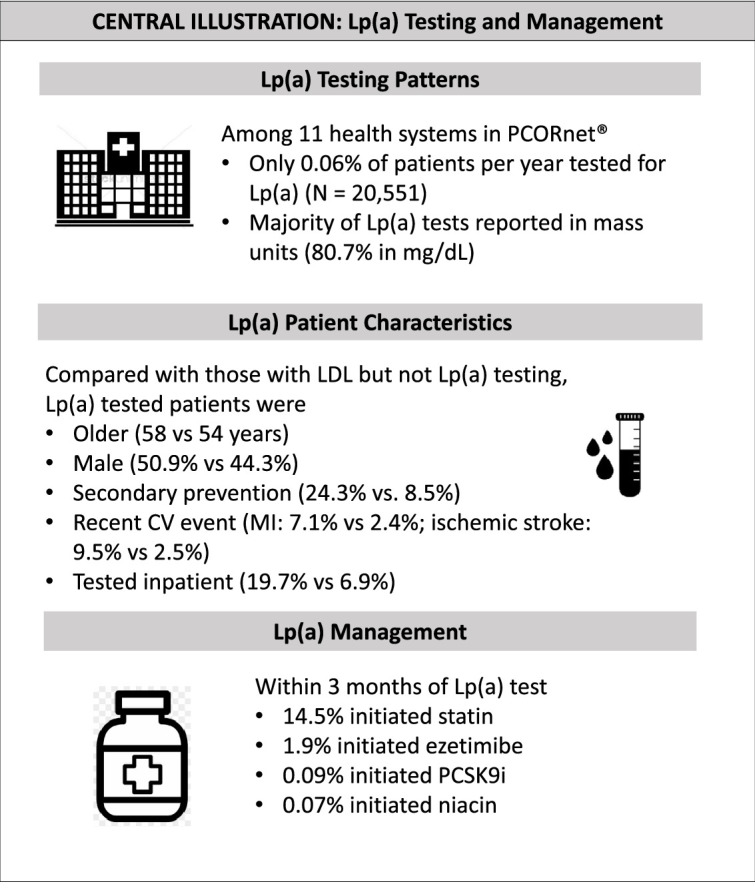
Overall, 20,551 patients had Lp(a) test results over the study period (Fig. 1a). This represents 0.06% of patients per year within the included health systems. There were 2,584,773 patients with LDL-C test results, of whom 82,204 were included in the matched LDL-C cohort. The majority of Lp(a) tests were reported in mass rather than molar units (80.7% in mg/dL vs. 19.3% in nmol/L). The median Lp(a) value was 16.0mg/dL (6.0, 55.0) for those results reported in mass units and 57.0nmol/L (23.0, 151.0) for those results reported in molar units (Fig. 2a, 2b). There were 473 Lp(a) tests in our dataset with very high Lp(a) vales (415 Lp(a) values > 175mg/dL and 58 Lp(a) test results > 425nmol/L).
Fig. 1.
a: Consort Diagram of Lp(a) Cohort, 1b: Consort Diagram of LDL-C Cohort.
Fig. 2.
a: Histogram of Lp(a) values in Lp(a) cohort with results in mass units. b: Histogram of Lp(a) values in Lp(a) cohort with results in molar units.
Baseline characteristics of the overall Lp(a) and LDL-C cohorts are presented in Table 1. Compared with the LDL-C cohort, the Lp(a) cohort was older (58 vs 54 years) and less frequently female (49.1% vs. 55.7%) (Central Illustration). Traditional cardiovascular risk factors were more common in the Lp(a) cohort (hypertension 45.5% vs 37.7%, diabetes 16.9% vs 14.6%, and hyperlipidemia 64.3 vs 33.6%), as was prior history of ASCVD (24.3% vs 8.5%). Individuals in the Lp(a) cohort more frequently had a cardiovascular (CV) event within 3 months of index lab test (myocardial infarction (7.1% vs 2.4%), coronary revascularization (3.2% vs 0.9%) or ischemic stroke (9.5% vs 2.5%)) as well as multiple prior CV events (8.6% vs 2.6%). Lp(a) testing was performed more often in the outpatient setting (79.6% outpatient vs 19.7% inpatient), although inpatient Lp(a) testing occurred more commonly than inpatient LDL-C testing (19.7% vs. 6.9%). Lp(a) testing increased over time (3295 Lp(a) tests in 2015 vs 5285 Lp(a) tests in 2019). Baseline characteristics of the Lp(a) outcomes cohort by those reported in mass and molar units are presented in Supplemental Table S2
Table 1.
Baseline characteristics of the Lp(a) and LDL-C cohorts.
| Lp(a) Cohort | LDL Cohort | |
|---|---|---|
| Characteristic | (N=20551) | (N=82204) |
| Demographics | ||
| Age (years) | 58 (47, 67) | 54 (41, 65) |
| Female | 10094 (49.1%) | 45750 (55.7%) |
| Race | ||
| Black or African American | 1498 (7.3%) | 7632 (9.3%) |
| White | 17357 (84.5%) | 67123 (81.7%) |
| Other | 1697 (8.3%) | 7449 (9.0%) |
| Hispanic Ethnicity | 577 (2.8%) | 2974 (3.6%) |
| Setting of Index Lab | ||
| Inpatient | 2145/10864 (19.7%) | 3213/46561 (6.9%) |
| Outpatient | 8647/10864 (79.6%) | 43140/46561 (92.7%) |
| Other/Unknown | 72/10864 (0.7%) | 208/46561 (0.4%) |
| Insurance | ||
| Public (Medicare/Medicaid) | 2546 (25.3%) | 8901 (20.7%) |
| Private Health Insurance | 3237 (32.2%) | 10303 (24.0%) |
| Self-Pay/No Payment | 647 (6.4%) | 2261 (5.3%) |
| Other/Unknown | 3626 (36.1%) | 21505 (50.0%) |
| Year of Index Test | ||
| 2015 | 3295 (16.0%) | 13180 (16.0%) |
| 2016 | 3703 (18.0%) | 14812 (18.0%) |
| 2017 | 3877 (18.9%) | 15508 (18.9%) |
| 2018 | 4391 (21.4%) | 17564 (21.4%) |
| 2019 | 5285 (25.7%) | 21140 (25.7%) |
| Site Region | ||
| Midwest | 12372 (60.2%) | 49488 (60.2%) |
| Northeast | 3570 (17.4%) | 14280 (17.4%) |
| South | 4609 (22.4%) | 18436 (22.4%) |
| Comorbidities | ||
| Hypertension | 9343 (45.5%) | 31025 (37.7%) |
| Hyperlipidemia | 13220 (64.3%) | 27587 (33.6%) |
| Diabetes | 3481 (16.9%) | 12004 (14.6%) |
| MI | 2066 (10.1%) | 3070 (3.7%) |
| MI within 3 Months of Index Lab | 1460 (7.1%) | 2010 (2.4%) |
| MI within 12 Months of Index Lab | 1797 (8.7%) | 2476 (3.0%) |
| Coronary Revascularization | 2478 (12.1%) | 3379 (4.1%) |
| Revascularization within 3 Months of Index Lab | 656 (3.2%) | 701 (0.9%) |
| Revascularization within 12 Months of Index Lab | 970 (4.7%) | 861 (1.0%) |
| Ischemic Stroke | 2237 (10.9%) | 2620 (3.2%) |
| Stroke within 3 Months of Index Lab | 1951 (9.5%) | 2041 (2.5%) |
| Stroke within 12 Months of Index Lab | 2076 (10.1%) | 2275 (2.8%) |
| Hemorrhagic Stroke | 281 (1.4%) | 467 (0.6%) |
| CAD | 6543 (31.8%) | 9915 (12.1%) |
| TIA | 716 (3.5%) | 1034 (1.3%) |
| PAD | 3018 (14.7%) | 6199 (7.5%) |
| Stage 3 and 4 CKD | 1123 (5.5%) | 4179 (5.1%) |
| Cancer | 419 (2.0%) | 1575 (1.9%) |
| Multiple CV Events | 1771 (8.6%) | 2122 (2.6%) |
| Labs and Vitals | ||
| Systolic Blood Pressure | 125 (114, 137) | 124 (114, 136) |
| Diastolic Blood Pressure | 76 (69, 82) | 76 (69, 82) |
| Body Mass Index | 28 (25, 32) | 29 (25, 34) |
| Current Smoker | 1492/16008 (9.3%) | 9035/65956 (13.7%) |
| eGFR ≤ 60 mL/min/1.73m2 | 8682 (47.4%) | 29874 (43.7%) |
| Total Cholesterol | 184 (150, 223) | 182 (155, 210) |
| High Density Lipoprotein | 50 (40, 64) | 52 (42, 66) |
| Low Density Lipoprotein | 103 (76, 136) | 102 (80, 127) |
| Triglycerides | 111 (78, 165) | 107 (76, 156) |
| Medication History | ||
| Statin Monotherapy | 9140 (44.5%) | 19129 (23.3%) |
| Statin + Ezetimibe Combination Therapy | 1047 (5.1%) | 503 (0.6%) |
| Ezetimibe Monotherapy | 261 (1.3%) | 235 (0.3%) |
| PCSK9i | 380 (1.8%) | 47 (0.1%) |
| Niacin | 712 (3.5%) | 1960 (2.4%) |
| Other Lipid Lowering Therapy | 1153 (5.6%) | 2299 (2.8%) |
| ACEi/ARB | 6331 (30.8%) | 19132 (23.3%) |
| Beta Blocker | 6153 (29.9%) | 16018 (19.5%) |
| Anticoagulant | 2996 (14.6%) | 3350 (4.1%) |
| Other Blood Pressure Medication | 6961 (33.9%) | 22597 (27.5%) |
| Aspirin | 7695 (37.4%) | 14745 (17.9%) |
| Any LLT | 10738 (52.3%) | 21104 (25.7%) |
Abbreviations: Angiotensin Converting Enzyme inhibitor, ACEi; Angiotensin Receptor Blocker, ARB; Coronary Artery Disease, CAD; Chronic Kidney Disease, CKD; Cardiovascular, CV; Estimated Glomerular Filtration Rate, eGFR; Lipid Lowering Therapy, LLT; Myocardial infarction, MI; Peripheral Artery Disease, PAD; Proprotein convertase subtilisin/kexin type 9 inhibitor, PCSK9i; Transient Ischemic Attack, TIA.
Lp(a) tests were more common among those with no prior history of ASCVD than among those with prior history of ASCVD (75.7% vs 24.3%). Among those with ASCVD (N=4988), Lp(a) testing was most common among individuals with a history of ischemic stroke (44.8%) or myocardial infarction (41.4%) compared to those with peripheral artery disease (27.3%).
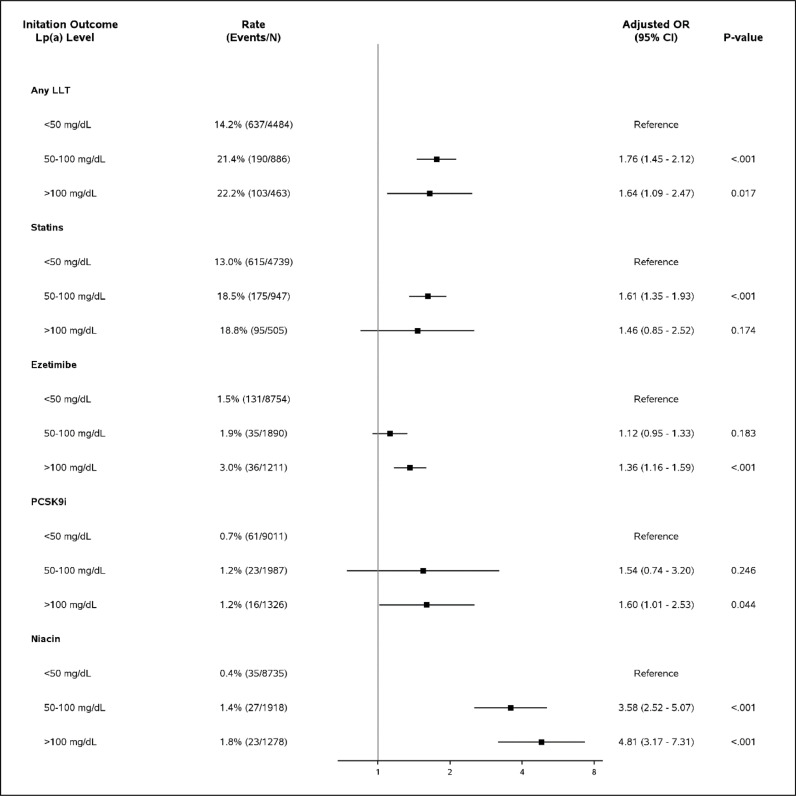
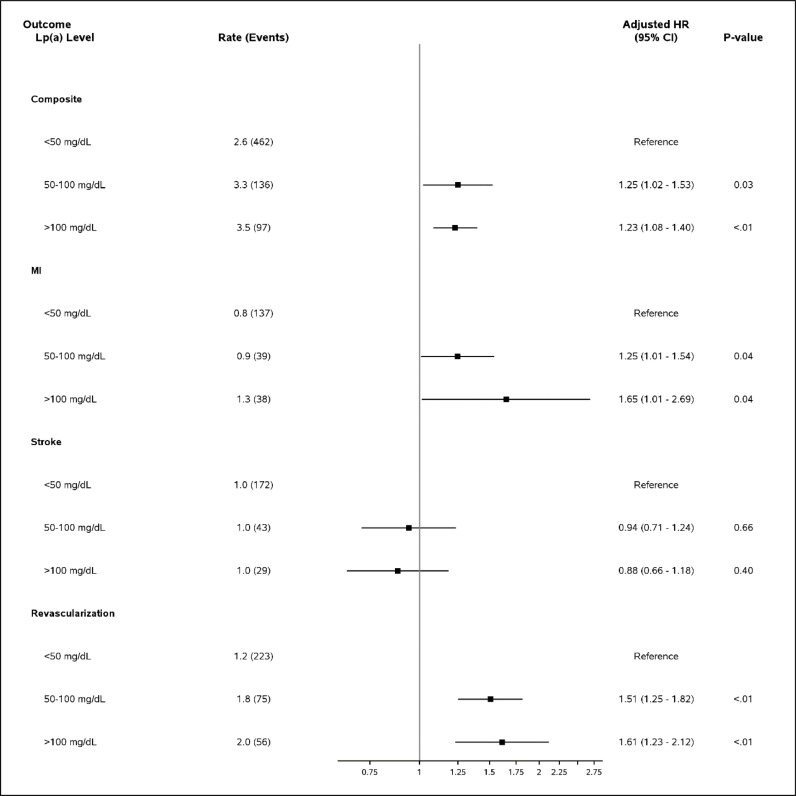
Elevated Lp(a) results reported in mass units of 50-100mg/dL and of > 100mg/dL were each associated with greater odds of LLT initiation in the 3 months following index lab test compared with Lp(a) < 50mg/dL [adjusted odds ratio (aOR) (95% CI): 50-100mg/dL:1.74 (1.45-2.12), p<0.001; >100mg/dL: 1.64 (1.09-2.47), p=0.017]. (Fig. 3). Markedly elevated Lp(a) > 100mg/dL was significantly associated with initiation of ezetimibe [aOR (95% CI): 1.36 (1.16-1.59)], PSCK9i [1.60 (1.01-2.53)], and niacin [4.81 (3.17-7.31)], but not statin [1.46 (0.85-2.52)], though prescription numbers were relatively lower in these subgroups. Elevated Lp(a) results in mass units were also significantly associated with composite CV hospitalization [Lp(a) 50-100mg/dL: 1.25 (1.02-1.53); Lp(a) > 100mg/dL: 1.23 (1.08-1.40)] compared to Lp(a) < 50mg/dL as reference (Fig. 4). A similar pattern was seen for Lp(a) results reported in molar units, with even stronger association with lipid lowering therapy initiation across subgroups (Supplemental Fig. 1, Supplemental Fig. 2).
Fig. 3.
Lipid lowering therapy initiation by Lp(a) level in Lp(a) Outcomes Cohort for Mass Units Results.
Fig. 4.
Lp(a) Level and CV hospitalization outcome in Lp(a) outcomes cohort for mass units results.
4. Discussion
In this large, contemporary analysis of 11 health systems across the U.S., we found that Lp(a) testing occurred rarely compared with LDL-C testing. When tested, elevated Lp(a) level was associated with initiation of LLT, though medication initiation rates were relatively low overall. Elevated Lp(a) was also associated with increased risk of CV hospitalization.
This analysis suggests Lp(a) testing occurs infrequently across multiple health systems in the U.S. Other, smaller studies have described similarly low rates of Lp(a) testing in both primary and secondary prevention populations [17], [18], [19], [20]. Lp(a) testing is indicated by the ACC/AHA guidelines in those with a family history of premature ASCVD [10]. The National Lipid Association (NLA) expands these recommendations to include those with personal history of premature ASCVD, those with primary severe hypercholesterolemia, and those at very high ASCVD risk [1]. The European Atherosclerosis society advocates for even broader testing – at least once in all adults [21]. Lp(a) testing was performed rarely in our cohort, even among individuals at increased risk with prior history of ASCVD and multiple prior CV events. Low overall rates of Lp(a) testing may be related to lack of knowledge, as providers may not recognize guideline indications for testing or may feel uncomfortable with interpretation of results.
The majority of Lp(a) test results in our analysis were reported in mass units (mg/dL) rather than molar units (nmol/L). Lp(a) isoforms can have different molecular weights, related to variability in size and composition of the attached apo(a) (2). Lp(a) measured in mass units assumes the lipid component of each Lp(a) particle is the same, and that apo(a) makes up a fixed percentage of the Lp(a) mass. Molar units instead, measure apo(a) concentration and as such, more accurately reflect the number of circulating Lp(a) particles [8]. Both the National Lipid Association and the International Federation of Clinical Chemistry and Laboratory Medicine have advocated for the exclusive use of molar units for Lp(a) testing, though our study indicates that this practice has not yet been widely adopted [[1], [22]].
Elevated Lp(a) was associated with LLT initiation in our analysis, though these results varied by medication class. ACC/AHA guidelines identify Lp(a) ≥ 50mg/dL as a risk-enhancing factor favoring statin initiation. As there is no randomized trial evidence yet to support Lp(a) as a target of therapy, treatment of elevated Lp(a) is aimed towards LDL-C lowering and other ASCVD-risk reduction measures [10]. Treatment with PSCK9i can lower Lp(a) levels, though the clinical implications of this are not fully known [1]. While niacin also lowers Lp(a), use of this medication has not been shown to reduce cardiovascular events and is therefore not generally recommended [1]. Although Lp(a) testing was associated with increased LLT initiation overall in our population, the absolute rates of these prescriptions were relatively low, even among those with Lp(a) > 100mg/dL. This prescribing practice may reflect a gap in care for these higher risk individuals. Improved patient and provider education on the implications of this risk marker is needed to optimize preventive treatment in this population. Several useful strategies have been proposed to mitigate this knowledge gap, including laboratory alerts when an elevated Lp(a) value is detected and early involvement of a lipid specialist when needed [21].
Elevated Lp(a) values were associated with increased risk of subsequent CV hospitalization among our cohort. Elevated Lp(a) has been associated with increased risk of CV events, including myocardial infarction and ischemic stroke in multiple prospective, population-based studies [23,24]. Although the exact risk estimate varies depending on the subgroup, in general those with the highest levels of Lp(a) have 3 to 4-fold increase risk of myocardial infarction [25,26] and 1.6-fold increased risk in ischemic stroke [27] compared to those with the lowest levels. This is consistent with the pattern seen in our cohort, as those with elevated Lp(a) values carried the highest risk even after adjustment for other factors. Enhanced risk in this population underscores the importance of aggressive risk factor management for these individuals.
These results must be interpreted with the following caveats. As with all EHR analyses, medication prescriptions or hospitalizations may have occurred outside of the designated health system and would not be captured in our study. We attempted to mitigate this risk by requiring patients to have at least one prior encounter within the health system to ensure a higher likelihood of longitudinal follow-up within a given health system. Our LLT initiation analyses were likewise limited to new medication initiation. There may have been LLT dose titration after laboratory testing not captured by our study. Associations derived from our outcome analyses may not be reflective of true, biological relationships, as our sample included only those who underwent testing and is thus subject to selection bias. Lastly, given the observational nature of our data, there may be other, unmeasured confounding variables not accounted for in our analysis.
5. Conclusion
Lp(a) testing remains relatively infrequent in health systems across the U.S. despite guidelines recommendations and increased understanding of its role in the pathogenesis of ASCVD. As new therapies for Lp(a) emerge, improved patient and provider education is needed to increase awareness of the utility of this risk marker and associated implications for ASCVD risk management.
Source of Funding
This study was supported by a grant from Amgen, Inc.
Disclosures
MDK is supported by a National Institute of Health (NIH) training grant (NIH 5T32HL069749-17. PHJ reports grant support from the Make Well Known Foundation through an unrestricted grant from Amgen; grant support from Novartis and Novo Nordisk. MFL has received research funding from the NIH (HL116263), Amgen, Regeneron, Ionis, Merck, REGENXBIO, Sanofi and Novartis and has served as a consultant for Esperion, Alexion Pharmaceuticals and REGENXBIO. EDP reports research support from: Amgen Inc., Janssen Pharmaceutical Products, Bristol Myers Squibb, Esperion; and consulting fees from: Jansen Pharmaceutical Products, Boehringer Ingelheim, Novartis, Cerner. NJP reports research grants from: Amgen, Inc.; AstraZeneca; Baseline Study LLC; Boehringer Ingleheim; Duke Clinical Research Institute; Eli Lilly & Company; Novartis Pharmaceuticals, Novo Nordisk Pharmaceutical Company; Regeneron Pharmaceuticals, Inc.; Sanofi-S.A.; Verily Sciences Research Company; Consulting fees from AstraZeneca; Boehringer Ingleheim; Esperion Therapeutics, Eli Lilly & Company, Novo Nordisk Pharmaceutical Company. The remaining authors have nothing to disclose.
Supplemental Fig. S1: Lipid lowering therapy initiation by Lp(a) level in Lp(a) Outcomes Cohort for Molar Units Results
Supplemental Fig. S2: Lp(a) Level and CV hospitalization outcome in Lp(a) outcomes cohort for molar units results
CRediT authorship contribution statement
Michelle D. Kelsey: Writing – original draft. Hillary Mulder: Writing – review & editing, Methodology, Formal analysis, Data curation. Karen Chiswell: Writing – review & editing, Methodology, Formal analysis. Zachary M. Lampron: Writing – review & editing, Project administration. Ester Nilles: Writing – review & editing, Data curation. Jacquelyn P. Kulinski: Writing – review & editing. Parag H. Joshi: Writing – review & editing. W. Schuyler Jones: Writing – review & editing. Alanna M. Chamberlain: Writing – review & editing. Thorsten M. Leucker: Writing – review & editing. Wenke Hwang: Writing – review & editing. M. Wesley Milks: Writing – review & editing. Anuradha Paranjape: Writing – review & editing. Jihad S. Obeid: Writing – review & editing. MacRae F. Linton: Writing – review & editing. Shia T. Kent: Writing – review & editing. Eric D. Peterson: Writing – review & editing. Emily C. O'Brien: Writing – review & editing. Neha J. Pagidipati: Conceptualization, Methodology, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Michelle D Kelsey reports financial support was provided by Amgen Inc. Disclosures: PHJ reports grant support from the Make Well Known Foundation through an unrestricted grant from Amgen; grant support from Novartis and Novo Nordisk. MFL has received research funding from the NIH (HL116263), Amgen, Regeneron, Ionis, Merck, REGENXBIO, Sanofi and Novartis and has served as a consultant for Esperion, Alexion Pharmaceuticals and REGENXBIO. EDP reports research support from: Amgen Inc., Janssen Pharmaceutical Products, Bristol Myers Squibb, Esperion; and consulting fees from: Jansen Pharmaceutical Products, Boehringer Ingelheim, Novartis, Cerner. NJP reports research grants from: Amgen, Inc.; AstraZeneca; Baseline Study LLC; Boehringer Ingleheim; Duke Clinical Research Institute; Eli Lilly & Company; Novartis Pharmaceuticals, Novo Nordisk Pharmaceutical Company; Regeneron Pharmaceuticals, Inc.; Sanofi-S.A.; Verily Sciences Research Company; Consulting fees from AstraZeneca; Boehringer Ingleheim; Esperion Therapeutics, Eli Lilly & Company, Novo Nordisk Pharmaceutical Company. The remaining authors have nothing to disclose.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajpc.2023.100478.
Appendix. Supplementary materials
References
- 1.Wilson DP, Jacobson TA, Jones PH, Koschinsky ML, McNeal CJ, Nordestgaard BG, et al. Use of Lipoprotein(a) in clinical practice: a biomarker whose time has come. A scientific statement from the National Lipid Association. J Clin Lipidol. 2019;13(3):374–392. doi: 10.1016/j.jacl.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Tsimikas S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. 2017;69(6):692–711. doi: 10.1016/j.jacc.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 3.Welsh P, Welsh C, Celis-Morales CA, Brown R, Ho FK, Ferguson LD, et al. Lipoprotein(a) and cardiovascular disease: prediction, attributable risk fraction, and estimating benefits from novel interventions. Eur J Prevent Cardiol. 2020 doi: 10.1093/eurjpc/zwaa063. [DOI] [PubMed] [Google Scholar]
- 4.Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, White IR, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302(4):412–423. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, Heath SC, et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361(26):2518–2528. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 6.Burgess S, Ference BA, Staley JR, Freitag DF, Mason AM, Nielsen SF, et al. Association of LPA variants with risk of coronary disease and the implications for lipoprotein(a)-lowering therapies: a mendelian randomization analysis. JAMA Cardiol. 2018;3(7):619–627. doi: 10.1001/jamacardio.2018.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, Thompson JR, et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. 2013;45(1):25–33. doi: 10.1038/ng.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcovina SM, Albers JJ. Lipoprotein(a) measurements for clinical application. J Lipid Res. 2016;57(4):526–537. doi: 10.1194/jlr.R061648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reyes-Soffer G, Ginsberg HN, Berglund L, Duell PB, Heffron SP, Kamstrup PR, et al. Lipoprotein(a): a genetically determined, causal, and prevalent risk factor for atherosclerotic cardiovascular disease: a scientific statement from the American heart association. Arterioscler Thromb Vasc Biol. 2022;42(1):e48–e60. doi: 10.1161/ATV.0000000000000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73(24):3168–3209. doi: 10.1016/j.jacc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Eur Heart J. 2019;41(1):111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 12.Varvel S, McConnell JP, Tsimikas S. Prevalence of Elevated Lp(a) Mass Levels and Patient Thresholds in 532 359 Patients in the United States. Arterioscler Thromb Vasc Biol. 2016;36(11):2239–2245. doi: 10.1161/ATVBAHA.116.308011. [DOI] [PubMed] [Google Scholar]
- 13.Brown WV, Ballantyne CM, Jones PH, Marcovina S. Management of Lp(a)†. J Clin Lipidol. 2010;4(4):240–247. doi: 10.1016/j.jacl.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Tsimikas S, Fazio S, Viney NJ, Xia S, Witztum JL, Marcovina SM. Relationship of lipoprotein(a) molar concentrations and mass according to lipoprotein(a) thresholds and apolipoprotein(a) isoform size. J Clin Lipidol. 2018;12(5):1313–1323. doi: 10.1016/j.jacl.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;140(11):e596–e646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gencer B, Kronenberg F, Stroes ES, Mach F. Lipoprotein(a): the revenant. Eur Heart J. 2017;38(20):1553–1560. doi: 10.1093/eurheartj/ehx033. [DOI] [PubMed] [Google Scholar]
- 17.Wilkinson M, Huynh A, AlGhawi R, Jin C, Ma G, Palakodeti S, et al. Trends in Testing for Lipoprotein(a) at an Academic Medical Center Over 13 Years. J Clin Lipidol. 2017;11(3):773. [Google Scholar]
- 18.Kelsey M, Page C, Alhanti B, Rhodes SL, Kent ST, Peterson E, et al. Lipoprotein(a) testing patterns in a large health system. Am J Cardiol. [DOI] [PMC free article] [PubMed]
- 19.Gautam R, Mehta R, Amari D, Heo J, Wang S, Sung JC, et al. A real-world assessment of patient characteristics and treatment patterns among patients with lipoprotein(a) measurement and receiving primary and secondary prevention for cardiovascular disease in the US. J Am Coll Cardiol. 2021;77(18_Supplement_1):1563. [Google Scholar]
- 20.Laguna A, Lahoz R, Fonseca AF, Amari DT, Ferber P, Heo JH, et al. Characteristics of patients with at least one lipoprotein(a) [Lp(a)] assessment and those with Lp(a) levels equal to or greater than 70 mg/dL: a real-world study in the US. Eur Heart J. 2020;41(Supplement_2) [Google Scholar]
- 21.Kronenberg F, Mora S, Stroes ESG, Ference BA, Arsenault BJ, Berglund L, et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: a European Atherosclerosis Society consensus statement. Eur Heart J. 2022;43(39):3925–3946. doi: 10.1093/eurheartj/ehac361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcovina SM, Albers JJ, Scanu AM, Kennedy H, Giaculli F, Berg K, et al. Use of a reference material proposed by the International Federation of Clinical Chemistry and Laboratory Medicine to evaluate analytical methods for the determination of plasma lipoprotein(a) Clin Chem. 2000;46(12):1956–1967. [PubMed] [Google Scholar]
- 23.Erqou S, Thompson A, Di Angelantonio E, Saleheen D, Kaptoge S, Marcovina S, et al. Apolipoprotein(a) isoforms and the risk of vascular disease: systematic review of 40 studies involving 58,000 participants. J Am Coll Cardiol. 2010;55(19):2160–2167. doi: 10.1016/j.jacc.2009.10.080. [DOI] [PubMed] [Google Scholar]
- 24.Willeit P, Ridker PM, Nestel PJ, Simes J, Tonkin AM, Pedersen TR, et al. Baseline and on-statin treatment lipoprotein(a) levels for prediction of cardiovascular events: individual patient-data meta-analysis of statin outcome trials. Lancet. 2018;392(10155):1311–1320. doi: 10.1016/S0140-6736(18)31652-0. [DOI] [PubMed] [Google Scholar]
- 25.Kamstrup PR, Benn M, Tybjærg-Hansen A, Nordestgaard BG. Extreme Lipoprotein(a) Levels and Risk of Myocardial Infarction in the General Population. Circulation. 2008;117(2):176–184. doi: 10.1161/CIRCULATIONAHA.107.715698. [DOI] [PubMed] [Google Scholar]
- 26.Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301(22):2331–2339. doi: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 27.Langsted A, Nordestgaard BG, Kamstrup PR. Elevated lipoprotein(a) and risk of ischemic stroke. J Am Coll Cardiol. 2019;74(1):54–66. doi: 10.1016/j.jacc.2019.03.524. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.