Abstract
Background
Iron is an essential micronutrient with differing intake patterns and metabolism between men and women. Epidemiologic evidence on the association of dietary iron and its heme and non-heme components with colorectal cancer (CRC) development is inconclusive.
Methods
We examined baseline dietary questionnaire-assessed intakes of total, heme, and non-heme iron and CRC risk in the EPIC cohort. Sex-specific multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were computed using Cox regression. We modelled substitution of a 1 mg/day of heme iron intake with non-heme iron using the leave one-out method.
Results
Of 450,105 participants (318,680 women) followed for 14.2 ± 4.0 years, 6162 (3511 women) developed CRC. In men, total iron intake was not associated with CRC risk (highest vs. lowest quintile, HRQ5vs.Q1:0.88; 95%CI:0.73, 1.06). An inverse association was observed for non-heme iron (HRQ5vs.Q1:0.80, 95%CI:0.67, 0.96) whereas heme iron showed a non-significant association (HRQ5vs.Q1:1.10; 95%CI:0.96, 1.27). In women, CRC risk was not associated with intakes of total (HRQ5vs.Q1:1.11, 95%CI:0.94, 1.31), heme (HRQ5vs.Q1:0.95; 95%CI:0.84, 1.07) or non-heme iron (HRQ5vs.Q1:1.03, 95%CI:0.88, 1.20). Substitution of heme with non-heme iron demonstrated lower CRC risk in men (HR:0.94; 95%CI: 0.89, 0.99).
Conclusions
Our findings suggest potential sex-specific CRC risk associations for higher iron consumption that may differ by dietary sources.
Subject terms: Risk factors, Cancer
Introduction
According to the Global Cancer Observatory (GCO), over 1.9 million new cases of colorectal cancer (CRC) were diagnosed worldwide in 2020 [1]. CRC constitutes the third most common malignancy in men and the second in women [1]. CRC etiology is multifactorial and includes established lifestyle factors such as smoking, alcohol intake, physical inactivity, or being overweight or obese [2], but also dietary factors such as higher intake of red and processed meats [2, 3].
Red meat is an important source of heme iron. Around 15–40% of heme iron is absorbed in the duodenum, leaving the remainder to proceed toward the lower gastrointestinal tract and the colorectum [4, 5]. Chemically, heme iron is in the ferrous form (Fe2+), a potent pro-oxidative form which catalyzes the Fenton reaction to produce hydroxyl free radicals evidenced to cause direct damage to DNA [6]. Heme iron is also a nitrosating agent and could contribute indirectly to increased CRC risk by generating lipid peroxide radicals and increasing the production of carcinogenic nitrosamines in the colorectum [7, 8]. In contrast to heme iron, dietary non-heme iron originates mainly from plants and dairy products in the ferric form (Fe3+).
Despite experimental studies supporting heme iron as a possible mediating factor in the association between red and processed meats and CRC, there is limited evidence from cohort studies on the role of dietary iron, particularly comparing its heme and non-heme components. In particular, there is a need for additional evidence in men and women analyzed separately in consideration of sex-specific differences in iron intakes and metabolism across the life cycle [9]. It is known that men and women have divergent iron needs, absorption rates, turnover, and excretion in the body [10].
Eight prospective cohort studies have investigated the association between heme iron and CRC risk [11–18]. These studies, with the exception of the most recent [18], have been included in four meta-analyses, which all showed a modest positive association (e.g., 11% higher risk for each mg higher daily intake) for heme iron and CRC risk [2, 19–21]. Among these eight studies, two reported positive associations with CRC in women [13, 22], while two others reported no statistically significant associations [12, 14]. In men, a study found a positive cancer risk association restricted to the colon [12] whereas two others found no associations [16, 17].
We addressed these questions by investigating the association between dietary total, heme, and non-heme iron intake levels and CRC risk in men and women in the European Prospective Investigation into Cancer and Nutrition (EPIC), a large multinational prospective cohort with wide variations in intake of subtypes of iron and over 6000 cases of CRC ascertained during 14.2 years of mean follow-up.
Materials and methods
Study participants
From 1992 to 2000, 521,317 participants (approximately two-thirds were women) aged between 35 and 75 years were recruited from 23 centers located in 10 European countries (Denmark, France, Germany, Greece, Italy, the Netherlands, Norway, Spain, Sweden, and the United Kingdom) [23]. Socio-demographic information, anthropometric measures, and information on diet, and other lifestyle factors were collected at baseline for all EPIC participants. Participants diagnosed with cancer prior to recruitment (n = 25,184) were excluded from data analysis as were, those lacking follow-up information (n = 4148), lacking dietary data (n = 6259) or those with energy intake vs. energy requirement in the extreme highest or lowest 1% (n = 9573) (Supplementary Fig. 1). We also excluded data from participants from Greece (n = 26,048) due to restrictions on data use and access. Ethical approval was obtained from the IARC Ethical Committee and from local ethics committees pertaining to each EPIC center. All the participants provided written informed consent to enter the cohort.
Dietary assessment
Usual dietary intake was assessed at baseline for all the participants using country-specific validated dietary questionnaires. Dietary assessment instruments applied included self-administered quantitative food frequency questionnaires (FFQs, in the Netherlands, Germany, Greece and Italy except the center in Naples), semi-quantitative FFQs (Denmark, Norway, Naples, Umeå and, the UK), and a combination of methods such as quantitative FFQs and 1-week food records (Malmö) [24]. Food items consumed in EPIC were matched to the food composition database which was developed by the United States Department of Agriculture (USDA, Release 26 & 27) as part of the Nutrient Database for Standard Reference publications. The matching procedure has been previously described [25]. Briefly, for the estimation of dietary iron intake, the iron content of each food source, as compiled in the EPIC Nutrient Database (ENDB) matched to the USDA database, was multiplied with the individual daily intake of the food sources. To calculate heme iron intake, we applied specific coefficients based on the data from the literature: 0.65 for iron from beef, lamb and veal, 0.39 for iron from pork, 0.54 for iron from processed meats, 0.26 for iron from poultry, fish and shellfish, and 0.43 (a combined coefficient) for other meat sources of iron (including prepared foods with meats) [12, 26]. To calibrate our FFQ dietary iron data, a subsample of 5–12% of EPIC participants per center (in total, 36,000 participants) with additional baseline dietary data collected by 24 h dietary recall was used, as previously described [27]. The main dietary sources of heme iron are summarized in Supplementary Table 1. Non-heme iron intake was calculated for each participant as the difference between total iron and heme iron intake.
The Mediterranean dietary score was calculated for each participant based on the consumption of nine food items: vegetables, fruits and nuts, legumes, cereals, fish, monounsaturated fat: saturated fat ratio, dairy products, meat and poultry, and alcohol [28]. For vegetables, legumes, fruits and nuts, cereals, and fish, participants whose consumption were above the median were assigned 0, or 1 if they have intake values below the median. The score was reversed for the other food items, with the exception of ethanol consumption for which a value of 1 was assigned to men or women with consumption between 10 and 50 g/day or 5–25 g, respectively. The sum of the scores for all the nine food groups was calculated for each participant.
Anthropometry and lifestyle
Baseline weight and standing height were measured in all participants, except those recruited in Oxford, France, and Norway, where self-reported values were collected. Participants completed questionnaires on education, alcohol intake, smoking status, physical activity, reproductive health, and previous disease history.
Follow-up for cancer incidence and vital status
Vital status was collected by record linkage with mortality registries in all countries except for Germany, and the Italian center of Naples, where data were collected actively. Ascertainment of incident cancer cases was undertaken through record linkage with cancer registries or a combination of health data sources including health insurance records, pathology registries, or active follow-up of the participants and their relatives. We defined CRC based on the definitions by the International Classification of Diseases for Oncology (ICD-O; codes: colon cancer: C18, and rectal cancer: C19-C20). Colon cancer included proximal colon cancers (C18.0–C18.5: cecum, appendix, ascending colon, hepatic flexure, transverse colon, and splenic flexure), distal colon cancers (C18.6–C18.7: descending colon and sigmoid colon), overlapping and unspecified (C18.8 and C18.9). Rectal cancer included incident tumors from the rectosigmoid junction down to the rectum (C19: rectosigmoid junction, C20: rectum).
Statistical analyses
Frequencies for categorical variables and means and standard deviations for continuous variables of baseline characteristics were calculated. Cox proportional hazards regression models stratified by age at recruitment (1-year categories) were used to compute hazard ratios (HRs) and 95% confidence intervals (CI) separately for the associations between total iron, heme iron, and non-heme iron and CRC risk in men and women. Additional analyses combining men and women were also conducted. For the stratification of the models, we used the strata options in the Stcox program in STATA. This option uses the “no interaction” approach as defined by Kleinbaum and Klein [29], and computes distinct estimated hazard functions, but with similar HRs for each stratum. Time at study entry was age at recruitment, and exit time was considered as the age at whichever of the following occurred first: incident CRC diagnosis, emigration, death, or the last date at which the follow-up was considered complete (until December 2014 for the current analysis). The main analyses were run a priori separately in men and women. We ran three main models: model 1 (stratified by age at recruitment, unadjusted) and model 2 was model 1 additionally adjusted for established or putative lifestyle risk factors for CRC (excluding dietary factors): [2] body mass index (BMI, kg/m², continuous), height (cm, continuous), physical activity (inactive, moderately inactive, moderately active, active, missing), highest education level attained (none, primary, technical/professional, secondary, higher, missing), and smoking habits and intensity (never, 1–15 cigarettes/day, 16–25 cigarettes/day, 26 or more cigarettes/day, former smokers who quit <10 years, former smokers who quit 11–20 years, former smokers who quit >20 years, current pipe-cigar and occasional smokers, missing) and total energy minus energy from alcohol (kcal/day, continuous), and energy from alcohol intake (kcal/day, continuous). Model 3 was model 2 additionally adjusted for intakes of vitamin C (mg/day, continuous), calcium (g/day, continuous), tea (g/day, continuous), and coffee (g/day, continuous) as inhibitors/enhancers of iron absorption, and for menopause status (pre-, peri-, and post-menopause) and hormone use (never, ever, unknown/missing) in women. Adjustment variables in model 3 also included cereal and cereal products, legumes, and vegetables when analyzing heme iron; and red meat, processed meats, poultry, and fish and fish products when analyzing non-heme iron. We considered model 3 as our main analysis. We also ran four intermediate models: Model S1 was adjusted for all the variables in model 2 except energy intakes variables. Because several dietary factors such as red and processed meats, or dairy products, or fish which are associated with CRC are also main sources of dietary iron [2, 3], we also used a holistic approach by adjusting for the Mediterranean dietary score as a proxy of overall dietary patterns (Model S2). In model S3, we additionally adjusted for dietary intakes of calcium, vitamin C, tea, coffee, energy from alcohol, and total energy minus energy from alcohol, and menopause status and hormone use (in women), and in model S4, we mutually adjusted for iron type i.e., heme iron analysis adjusted for non-heme iron and vice versa. We divided intakes of total iron, heme iron and non-heme iron into country-specific quintiles. This decision was based on the observation of variation across the EPIC countries in level of iron intake (Supplementary Table 2). Trend analyses were run based on median values of each quintile. We also ran the analyses for continuous iron variables, per one standard deviation (SD) increment. In the continuous analyses, Cox regression models were additionally stratified by country in all models (including country as strata in the Cox models). No deviations from the proportional hazards assumption were observed after we assessed the proportionality using Schoenfeld residuals over time [30].
Non-linear associations were evaluated using restricted cubic splines with four knots [31]. To test the linearity of the associations, we performed likelihood ratio tests and compared for each outcome, the model with only the linear term to the model with both the linear and the cubic spline terms. We also assessed associations between the intake of types of iron and the risk by tumor anatomical subsite (colon vs. rectal cancers, and proximal vs. distal colon cancers). Differences in associations by tumor site were tested using competing risks analyses [32]. We ran Cox proportional hazards models to compute anatomical subsite-specific HRs using the duplication method [33] and further tested heterogeneity across sites (colon vs. rectal cancer and proximal colon vs. distal colon) using Wald tests. We conducted analyses stratified by menopause status (in women: premenopausal, postmenopausal, perimenopausal/unknown), and categories of age (<50, 50–<65, ≥65 years), and BMI (<25, 25–<30, ≥30 kg/m2) to investigate whether these factors which can regulate iron absorption and status may modify the risk of CRC.
Substitution analyses
We conducted substitution analyses to evaluate how the risk of CRC would change if replacing heme iron by non-heme iron, and vice versa [34]. We applied the leave one-out approach to calculate HRs and 95%CIs for the substitution analyses [35]. To evaluate CRC risk associated with 1 mg replacement of heme iron i.e., ~60 mg of cooked beef, with 1 mg of non-heme iron i.e., about two boiled eggs or 30 g of boiled beans, we included in multivariable-adjusted models total iron and non-heme iron, but not heme iron. Substitution models were stratified by age at recruitment and country, and adjusted for BMI, height, education, physical activity, smoking, and dietary intakes of calcium, tea, coffee, vitamin C, red meat, processed meats, poultry, and fish and fish products but not total energy intake [36]; and in women only, for menopause status and use of hormone replacement therapy. Substitution analyses assume that there is a linear association between the exposure variable and the disease risk [37], which was the case for dietary iron intake in both men and women, as observed by splines and likelihood ratio tests. Also, substitution analyses permitted us to evaluate whether the association observed could be attributed to higher/or lower intake of one specific group of iron (e.g., heme iron) while keeping tallied dietary iron from all sources unchanged [38].
Sensitivity analyses
To evaluate the possible impact of reverse causation, or effects of the exposure variables on tumor progression rather than initiation, all analyses were re-run excluding participants with <4 years of follow-up. We also conducted additional sensitivity analyses, by excluding participants from the UK (mostly vegetarians from the EPIC Oxford center), Italy and Spain (blood donor associations members) due to possible effects of their iron intake patterns and iron metabolism.
We considered two-sided P values < 0.05 as statistically significant. We corrected the P values obtained for multiple testing using the false discovery rate method by Benjamini-Hochberg [39]. All statistical analyses were carried out using Stata 15.1 (StataCorp, College Station, TX, USA). The figures were prepared using R software 3.5.1 (Foundation for Statistical Computing, Vienna, Austria).
Results
The final database used for statistical analyses included 450,105 participants among whom 318,680 were women (Supplementary Fig. 1). During a median follow-up of 15 years, 6162 incident CRC cases (3511 women) were identified. Table 1 summarizes the baseline characteristics of the cases and non-cases in men and women. In both men and women, cases tended to have slightly higher BMI and waist circumference; cases also had a lower education and consumed more alcohol and red and processed meats. Cases were less likely to be never smokers, consumed less calcium and were less physically active, compared to non-cases.
Table 1.
Selected characteristics of the study population, by sex, and colorectal cancer status, EPIC cohort study, 1992–2014.
| Men | Women | |||
|---|---|---|---|---|
| Cases (n = 2651) | Non-cases (n = 128,774) | Cases (n = 3511) | Non-cases (315,169) | |
| Age at recruitment, yrs | 56.0 ± 7.4 | 51.5 ± 9.9 | 56.4 ± 8.1 | 50.1 ± 9.7 |
| Anthropometry, mean ± SD | ||||
| BMI, kg/m² | 27.2 ± 3.8 | 26.4 ± 3.6 | 25.6 ± 4.4 | 24.8 ± 4.3 |
| Waist circumference, cm | 97.3 ± 10.2 | 94.3 ± 10.1 | 82.3 ± 11.5 | 79.6 ± 11.1 |
| Waist-to-hip ratio | 0.96 ± 0.06 | 0.94 ± 0.06 | 0.81 ± 0.07 | 0.79 ± 0.07 |
| Lifestyle variables, % | ||||
| Smoking status and intensity | ||||
| Never | 23.9 | 31.7 | 46.7 | 47.1 |
| Current | 18.5 | 20.7 | 19.5 | 18.7 |
| Current, 1–15 cig/day | 8.6 | 10.0 | 13.0 | 12.4 |
| Current, 16–25 cig/day | 7.4 | 7.9 | 5.4 | 5.4 |
| Current, 26+ cig/day | 2.45 | 2.8 | 1.1 | 0.9 |
| Former | 42.1 | 35.2 | 25.4 | 22.5 |
| Former, quit ≤ 10 years | 13.7 | 12.5 | 8.4 | 8.5 |
| Former, quit 11–20 years | 13.6 | 11.3 | 7.5 | 7.2 |
| Former, quit 20+ years | 14.8 | 11.4 | 9.5 | 6.8 |
| Current, pipe/cigar/occasional | 12.1 | 9.3 | 5.67 | 8.7 |
| Missing | 3.5 | 3.1 | 2.63 | 3.2 |
| Physical activity | ||||
| Inactive | 22.4 | 17.5 | 20.3 | 25.6 |
| Moderately inactive | 31.2 | 30.9 | 34.3 | 33.8 |
| Moderately active | 21.9 | 24.2 | 27.8 | 23.2 |
| Active | 22.7 | 25.1 | 15.7 | 15.6 |
| Missing | 1.8 | 2.3 | 1.8 | 1.8 |
| Highest education level attained | ||||
| None | 4.5 | 3.2 | 3.9 | 3.5 |
| Primary school completed | 34.1 | 28.6 | 29.4 | 23.0 |
| Technical/professional school | 25.1 | 24.9 | 25.5 | 22.3 |
| Secondary school | 11.4 | 13.3 | 18.8 | 24.1 |
| Higher education | 21.7 | 27.1 | 16.6 | 23.1 |
| Missing | 3.3 | 2.9 | 5.8 | 4.1 |
| Menopause | ||||
| Pre-menopause | 13.4 | 35.0 | ||
| Perimenopause | 15.5 | 19.8 | ||
| Post-menopause | 67.1 | 42.5 | ||
| Use of HRT | ||||
| Never | 62.9 | 68.1 | ||
| Ever | 28.9 | 25.2 | ||
| Missing/unknown | 8.3 | 6.8 | ||
| Dietary intake, mean ± SD | ||||
| Total energy, kcal/day | 2378 ± 631 | 2418 ± 662 | 1903 ± 514 | 1937 ± 541 |
| Alcohol, g/day | 24.2 ± 25.1 | 20.9 ± 23.1 | 8.7 ± 12.3 | 8.4 ± 12 |
| Red and processed meats, g/day | 105.0 ± 59 | 99.0 ± 62.2 | 67.6 ± 40.8 | 66.0 ± 43.0 |
| Poultry, g/day | 22.1 ± 22.3 | 20.9 ± 22.0 | 18.0 ± 18.8 | 18.1 ± 18.8 |
| Fish and shellfish, g/day | 41.5 ± 36.0 | 37.2 ± 35.2 | 37.8 ± 35.1 | 38.2 ± 36.8 |
| Fiber, g/day | 23.5 ± 8.1 | 24.3 ± 8.5 | 22.0 ± 7.3 | 22.2 ± 7.4 |
| Fat, g/day | 90.4 ± 30.9 | 92.5 ± 32.0 | 73.2 ± 25.7 | 75.3 ± 26.6 |
| Protein, g/day | 97.5 ± 29.1 | 97.6 ± 30.1 | 81.4 ± 23.4 | 82.9 ± 25.1 |
| Calcium, g/day | 1.00 ± 0.4 | 1.04 ± 0.4 | 0.97 ± 0.4 | 0.98 ± 0.4 |
| Tea, g/day | 213 ± 201 | 208 ± 195 | 233 ± 185 | 221 ± 172 |
| Coffee, g/day | 485 ± 444 | 464 ± 429 | 416 ± 369 | 367 ± 343 |
| Vitamin C, mg/day | 110 ± 60 | 114 ± 63 | 123 ± 63 | 125 ± 64 |
| Iron | ||||
| Total iron, mg/day | 14.5 ± 4.7 | 14.6 ± 4.7 | 11.8 ± 3.5 | 12.2 ± 3.8 |
| Heme iron, mg/day | 1.9 ± 1.2 | 1.8 ± 1.2 | 1.22 ± 0.8 | 1.22 ± 0.8 |
| Non heme iron, mg/day | 12.6 ± 4.1 | 12.8 ± 4.1 | 10.6 ± 3.2 | 11.0 ± 3.4 |
BMI body mass index, HRT hormone replacement therapy, SD standard deviation.
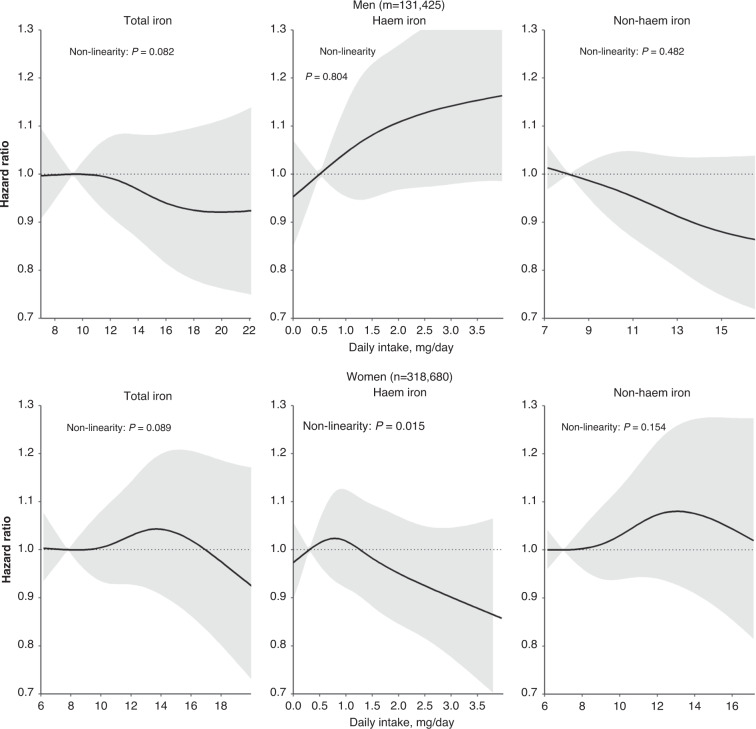
Total iron intake was not associated with CRC risk in men (Model 3: HR comparing the highest with the lowest quintile, HRQ5vs.Q1:0.88, 95%CI:0.73, 1.06) (Table 2). An inverse association was observed for non-heme iron intake (HRQ5vs.Q1:0.80, 95%CI:0.67, 0.96), whereas heme iron intake showed a statistically non-significant association with CRC risk (HRQ5vs.Q1:1.10, 95%CI:0.96, 1.27). In women, intakes of total iron (Model 3: HRQ5vs.Q1:1.11, 95%CI:0.94, 1.31), heme iron (HRQ5vs.Q1:0.95, 95%CI:0.84, 1.07) and non-heme iron (HRQ5vs.Q1:1.03, 95%CI:0.88, 1.20) were not associated with CRC risk. Illustrations by splines showed that CRC risk associated with total iron, heme iron, and non-heme iron were linear in men (Fig. 1). However, in women, heme iron (P for nonlinearity = 0.015), but not total iron (P for nonlinearity = 0.089), or non-heme iron (P for nonlinearity = 0.154) showed a non-linear association with CRC risk. After correcting for multiple testing, none of the associations remained statistically significant.
Table 2.
Hazard ratios and 95% confidence intervals for the risk of colorectal cancer associated with iron intakes, EPIC cohort study, 1992–2014.
| Quintiles of intakea | Ptrend | Continuous, per SD incrementb,c | |||||
|---|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | |||
| Men | |||||||
| Total iron | |||||||
| Median intake (IQR), mg/day | 9.3 (8.1; 10.2) | 12.0 (11.5; 12.6) | 14.1 (13.6; 14.6) | 16.4 (15.8; 17.2) | 20.5 (19.1; 23.0) | Per 4.7 mg | |
| n cases | 551 | 563 | 495 | 537 | 505 | 2651 | |
| Model 1 | 1.00 (Ref.) | 0.96 (0.85, 1.08) | 0.88 (0.78, 0.99) | 0.95 (0.85, 1.07) | 0.85 (0.75, 0.96) | 0.017 | 0.96 (0.92, 1.00) |
| Model 2 | 1.00 (Ref.) | 0.96 (0.85, 1.09) | 0.87 (0.77, 1.00) | 0.95 (0.82, 1.10) | 0.82 (0.69, 0.98) | 0.066 | 0.96 (0.90, 1.02) |
| Model 3 | 1.00 (Ref.) | 0.98 (0.87, 1.11) | 0.90 (0.79, 1.03) | 0.99 (0.86, 1.15) | 0.88 (0.73, 1.06) | 0.317 | 0.96 (0.90, 1.02) |
| Heme iron | |||||||
| Median intake (IQR), mg/day | 0.51 (0.2; 0.7) | 1.2 (1.0; 1.3) | 1.7 (1.6; 1.8) | 2.2 (2.1; 2.4) | 3.2 (2.9; 3.9) | Per 1.2 mg | |
| n cases | 435 | 488 | 575 | 582 | 571 | 2651 | |
| Model 1 | 1.00 (Ref.) | 1.09 (0.96, 1.23) | 1.12 (0.99, 1.26) | 1.09 (0.96, 1.24) | 1.16 (1.02, 1.31) | 0.040 | 1.05 (1.01, 1.09) |
| Model 2 | 1.00 (Ref.) | 1.07 (0.95, 1.22) | 1.10 (0.97, 1.25) | 1.07 (0.94, 1.22) | 1.13 (0.99, 1.29) | 0.135 | 1.04 (1.00, 1.09) |
| Model 3 | 1.00 (Ref.) | 1.07 (0.94, 1.21) | 1.09 (0.96, 1.23) | 1.05 (0.92, 1.20) | 1.10 (0.96, 1.27) | 0.270 | 1.03 (0.99, 1.08) |
| Non-heme iron | |||||||
| Median intake (IQR), mg/day | 8.1 (7.0; 8.8) | 10.5 (10.0; 11.0) | 12.4 (11.9; 12.8) | 14.4 (13.9; 15.1) | 18.1 (16.8; 20.2) | Per 4.1 mg | |
| n cases | 583 | 561 | 503 | 518 | 486 | 2651 | |
| Model 1 | 1.00 (Ref.) | 0.93 (0.82, 1.04) | 0.87 (0.78, 0.98) | 0.87 (0.78, 0.98) | 0.82 (0.72, 0.92) | 0.001 | 0.94 (0.91, 0.98) |
| Model 2 | 1.00 (Ref.) | 0.92 (0.82, 1.04) | 0.86 (0.76, 0.99) | 0.86 (0.74, 0.99) | 0.78 (0.65, 0.92) | 0.005 | 0.93 (0.88, 0.99) |
| Model 3 | 1.00 (Ref.) | 0.93 (0.82, 1.05) | 0.88 (0.77, 1.00) | 0.88 (0.76, 1.01) | 0.80 (0.67, 0.96) | 0.019 | 0.94 (0.89, 1.00) |
| Women | |||||||
| Total iron | |||||||
| Median intake (IQR), mg/day | 7.8 (6.9; 8.5) | 10.1 (9.6; 10.5) | 11.8 (11.4; 12.2) | 13.7 (13.2; 14.3) | 17.1 (15.9; 19.0) | Per 3.8 mg | |
| n cases | 758 | 726 | 762 | 704 | 561 | 3511 | |
| Model 1 | 1.00 (Ref.) | 1.00 (0.90, 1.11) | 1.03 (0.93, 1.14) | 1.10 (0.99, 1.22) | 0.95 (0.85, 1.06) | 0.919 | 0.99 (0.96, 1.03) |
| Model 2 | 1.00 (Ref.) | 1.02 (0.92, 1.14) | 1.07 (0.96, 1.20) | 1.17 (1.03, 1.32) | 1.03 (0.89, 1.20) | 0.156 | 1.02 (0.97, 1.07) |
| Model 3 | 1.00 (Ref.) | 1.04 (0.93, 1.16) | 1.11 (0.98, 1.25) | 1.22 (1.07, 1.40) | 1.11 (0.94, 1.31) | 0.025 | 1.02 (0.97, 1.07) |
| Heme iron | |||||||
| Median intake (IQR), mg/day | 0.3 (0.1; 0.4) | 0.75 (0.7; 0.8) | 1.1 (1.0; 1.2) | 1.5 (1.4; 1.6) | 2.2 (2.0; 2.7) | Per 0.8 mg | |
| n cases | 608 | 719 | 789 | 771 | 624 | 3511 | |
| Model 1 | 1.00 (Ref.) | 1.03 (0.92, 1.14) | 1.02 (0.92, 1.14) | 1.07 (0.97, 1.19) | 1.02 (0.92, 1.14) | 0.457 | 1.01 (0.97, 1.04) |
| Model 2 | 1.00 (Ref.) | 1.02 (0.92, 1.14) | 1.01 (0.90, 1.12) | 1.06 (0.95, 1.18) | 1.00 (0.89, 1.12) | 0.743 | 1.00 (0.96, 1.04) |
| Model 3 | 1.00 (Ref.) | 1.00 (0.90, 1.12) | 0.98 (0.88, 1.09) | 1.01 (0.91, 1.13) | 0.95 (0.84, 1.07) | 0.504 | 0.98 (0.95, 1.02) |
| Non heme iron | |||||||
| Median intake (IQR), mg/day | 7.0 (6.1; 7.6) | 9.0 (8.6; 9.4) | 10.6 (10.2; 11.0) | 12.4 (11.9; 12.9) | 15.4 (14.4; 17.1) | Per 3.4 mg | |
| n cases | 775 | 757 | 706 | 713 | 560 | 3511 | |
| Model 1 | 1.00 (Ref.) | 0.98 (0.89, 1.09) | 1.04 (0.94, 1.15) | 1.06 (0.96, 1.18) | 0.93 (0.84, 1.04) | 0.710 | 0.99 (0.96, 1.02) |
| Model 2 | 1.00 (Ref.) | 1.01 (0.90, 1.12) | 1.08 (0.96, 1.21) | 1.12 (0.99, 1.27) | 1.00 (0.86, 1.17) | 0.344 | 1.02 (0.97, 1.07) |
| Model 3 | 1.00 (Ref.) | 1.01 (0.91, 1.13) | 1.09 (0.97, 1.23) | 1.14 (1.01, 1.30) | 1.03 (0.88, 1.20) | 0.209 | 1.03 (0.98, 1.09) |
IQR interquartile range.
aThe analyses were run using country-specific quintiles.
bFor the continuous analysis, all the models were adjusted for the same variables as the in quintiles analysis, but with additional stratification by country.
cP values for heterogeneity by sex (using continuous values) for total iron, heme iron, and non-heme iron, respectively, were for Model 1, 0.124, 0.260 and 0.031; and Model 2, 0.528, 0.091, and 0.176; and Model 3, 0.802, 0.081, 0.308.
Model 1 was stratified by age at recruitment.
Model 2 was model 1 additionally adjusted for BMI, height, education, physical activity, smoking, energy intakes from alcohol, and total energy minus energy from alcohol.
Model 3 was model 2 additionally adjusted for intakes of vitamin C, calcium, tea, coffee, and in women specifically menopause status and hormone use. Model 3 for heme iron additionally included intakes of cereal, and cereal products, legumes, and vegetables whereas model 3 for non-heme iron included intakes of red meat, processed meats, poultry, and fish and fish products.
Fig. 1. Splines of the associations between dietary iron intake and colorectal risk in men and women.
Splines models were adjusted for body mass index, height, education, physical activity, smoking, and for energy intakes from alcohol, and total energy minus energy from alcohol, intakes of vitamin C, calcium, tea, coffee, and stratified by age at recruitment and country. In women specifically menopause status and hormone use were included in the model. For heme iron, additional confounders included intakes of cereal, and cereal products, legumes, and vegetables whereas for non-heme iron it included intakes of red meat, processed meats, poultry, and fish and fish products for non-heme iron. The median intake values of the lowest quintiles were used as the reference. Deviations from linearity were tested using likelihood ratio tests comparing the model with only the linear term to the model with both the linear and the cubic spline terms. The grayish area represents the 95% confidence intervals.
We assessed potential heterogeneity of the associations between men and women to verify whether the associations (for continuous variables) differed according to our primary hypothesis. There were no statistically significant differences between men and women in the associations between dietary heme iron (P for heterogeneity by sex = 0.081) or non-heme iron (P for heterogeneity by sex = 0.308) (Table 2).
The intermediate model (Model S1) and the model with adjustment for the Mediterranean diet (Model S2) showed similar associations to the Model 2 (Supplementary Table 3). In men, the inverse association observed for non-heme iron was stronger after adjustment for heme iron (Model S4: HRQ5vs.Q1 = 0.77, 95%CI:0.64–0.92).
Analyses by tumor anatomical location (colon vs. rectum) showed that the observed associations were consistent across sub-sites for all types of iron in men (P value for heterogeneity: 0.274, 0.814, 0.274 for total iron, heme iron, and non-heme iron, respectively) (Table 3). There was a positive significant association observed between intakes of heme iron and rectal cancer (HRQ5vs.Q1 = 1.29, 95%CI:1.03–1.61). When the associations were further examined by specific anatomical sub-divisions within the colon (proximal vs. distal), the results in men showed a statistically significant positive association in the proximal colon (HR per 1.2 mg:1.11, 95%CI:1.02, 1.20) and no association in the distal colon (HR per 1.2 mg:0.95, 95%CI:0.88, 1.04, P for heterogeneity = 0.008). In women, total iron, heme iron, and non-heme iron intakes showed no significant associations with CRC risk across tumor locations. Analyses combining men and women showed no significant associations between dietary total iron and non-heme iron and CRC risk (Supplementary Table 4). For both types of iron, no differences were observed in the associations by BMI categories for men and women, or by female menopausal status (Supplementary Table 5). However, we observed an inverse association between the intake of heme iron and the risk of CRC in women aged ≥65 years (P for heterogeneity = 0.046).
Table 3.
Hazard ratios and 95% confidence intervals for iron intake and the risk of colorectal cancer by anatomical subsites, EPIC cohort study, 1992–2014.
| Quintiles of intakea | Ptrend | Continuous, per SD incrementb,c,d | |||||
|---|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | |||
| Men | |||||||
| Total iron | |||||||
| Median intake (IQR), mg/day | 9.3 (8.1; 10.2) | 12.0 (11.5; 12.6) | 14.1 (13.6; 14.6) | 16.4 (15.8; 17.2) | 20.5 (19.1; 23.0) | Per 4.7 mg | |
| Colon cancer | |||||||
| n cases | 351 | 321 | 304 | 336 | 282 | 1594 | |
| HR (95%CI) | 1.00 (Ref.) | 0.91 (0.78, 1.06) | 0.83 (0.70, 0.99) | 0.92 (0.76, 1.11) | 0.75 (0.60, 0.95) | 0.059 | 0.95 (0.88, 1.03) |
| Proximal colon cancer | |||||||
| n cases | 137 | 128 | 125 | 147 | 122 | 659 | |
| HR (95%CI) | 1.00 (Ref.) | 0.94 (0.73, 1.20) | 0.91 (0.69, 1.19) | 1.08 (0.81, 1.45) | 0.92 (0.65, 1.31) | 0.922 | 1.04 (0.92, 1.17) |
| Distal colon cancer | |||||||
| n cases | 175 | 149 | 150 | 159 | 124 | 757 | |
| HR (95%CI) | 1.00 (Ref.) | 0.85 (0.68, 1.07) | 0.82 (0.64, 1.05) | 0.86 (0.66, 1.12) | 0.63 (0.45, 0.88) | 0.036 | 0.91 (0.81, 1.02) |
| Rectal cancer | |||||||
| n cases | 215 | 224 | 206 | 214 | 198 | 1057 | |
| HR (95%CI) | 1.00 (Ref.) | 1.06 (0.87, 1.28) | 0.95 (0.77, 1.18) | 1.01 (0.80, 1.27) | 0.94 (0.71, 1.25) | 0.634 | 0.97 (0.88, 1.07) |
| Heme iron | |||||||
| Median intake (IQR), mg/day | 0.51 (0.2; 0.7) | 1.2 (1.0; 1.3) | 1.7 (1.6; 1.8) | 2.2 (2.1; 2.4) | 3.2 (2.9; 3.9) | Per 1.2 mg | |
| Colon cancer | |||||||
| n cases | 286 | 323 | 346 | 319 | 320 | 1594 | |
| HR (95%CI) | 1.00 (Ref.) | 1.03 (0.87, 1.21) | 1.03 (0.87, 1.21) | 0.98 (0.83, 1.16) | 1.00 (0.83, 1.19) | 0.783 | 1.03 (0.97, 1.09) |
| Proximal colon cancer | |||||||
| n cases | 111 | 120 | 146 | 156 | 126 | ||
| HR (95%CI) | 1.00 (Ref.) | 0.97 (0.74, 1.25) | 1.09 (0.84, 1.40) | 1.22 (0.94, 1.57) | 1.00 (0.75, 1.33) | 0.414 | 1.11 (1.02, 1.20) |
| Distal colon cancer | |||||||
| n cases | 139 | 158 | 166 | 140 | 154 | 757 | |
| HR (95%CI) | 1.00 (Ref.) | 1.05 (0.83, 1.33) | 1.04 (0.82, 1.31) | 0.89 (0.70, 1.14) | 0.99 (0.77, 1.29) | 0.525 | 0.95 (0.88, 1.04) |
| Rectal cancer | |||||||
| n cases | 169 | 209 | 231 | 218 | 230 | 1057 | |
| HR (95%CI) | 1.00 (Ref.) | 1.13 (0.92, 1.39) | 1.20 (0.97, 1.47) | 1.18 (0.95, 1.45) | 1.29 (1.03, 1.61) | 0.036 | 1.05 (0.98, 1.12) |
| Non-heme iron | |||||||
| Median intake (IQR), mg/day | 8.1 (7.0; 8.8) | 10.5 (10.0; 11.0) | 12.4 (11.9; 12.8) | 14.4 (13.9; 15.1) | 18.1 (16.8; 20.2) | Per 4.1 mg | |
| Colon cancer | |||||||
| n cases | 366 | 316 | 309 | 319 | 284 | 1594 | |
| HR (95%CI) | 1.00 (Ref.) | 0.86 (0.74, 1.01) | 0.82 (0.69, 0.97) | 0.84 (0.69, 1.01) | 0.73 (0.58, 0.92) | 0.018 | 0.94 (0.86, 1.01) |
| Proximal colon cancer | |||||||
| n cases | 142 | 135 | 122 | 132 | 128 | 659 | |
| HR (95%CI) | 1.00 (Ref.) | 0.96 (0.75, 1.23) | 0.86 (0.65, 1.12) | 0.94 (0.70, 1.26) | 0.94 (0.66, 1.33) | 0.680 | 0.98 (0.87, 1.12) |
| Distal colon cancer | |||||||
| n cases | 182 | 145 | 153 | 151 | 126 | 757 | |
| HR (95%CI) | 1.00 (Ref.) | 0.80 (0.64, 1.01) | 0.81 (0.64, 1.04) | 0.79 (0.60, 1.03) | 0.62 (0.45, 0.87) | 0.021 | 0.92 (0.82, 1.04) |
| Rectal cancer | |||||||
| n cases | 218 | 226 | 214 | 203 | 196 | 1057 | |
| HR (95%CI) | 1.00 (Ref.) | 1.05 (0.86, 1.28) | 0.98 (0.80, 1.22) | 0.95 (0.75, 1.19) | 0.93 (0.70, 1.23) | 0.419 | 0.96 (0.87, 1.06) |
| Women | |||||||
| Total iron | |||||||
| Median intake (IQR), mg/day | 7.8 (6.9; 8.5) | 10.1 (9.6; 10.5) | 11.8 (11.4; 12.2) | 13.7 (13.2; 14.3) | 17.1 (15.9; 19.0) | Per 3.8 mg | |
| Colon cancer | |||||||
| n cases | 495 | 465 | 491 | 527 | 425 | 2403 | |
| HR (95%CI) | 1.00 (Ref.) | 0.97 (0.85, 1.11) | 1.06 (0.92, 1.22) | 1.19 (1.02, 1.39) | 1.03 (0.85, 1.24) | 0.138 | 1.02 (0.96, 1.09) |
| Proximal colon cancer | |||||||
| n cases | 244 | 251 | 237 | 258 | 207 | 1197 | |
| HR (95%CI) | 1.00 (Ref.) | 1.05 (0.87, 1.26) | 1.01 (0.83, 1.23) | 1.13 (0.91, 1.41) | 0.95 (0.73, 1.25) | 0.878 | 1.01 (0.92, 1.10) |
| Distal colon cancer | |||||||
| n cases | 209 | 169 | 210 | 214 | 167 | 969 | |
| HR (95%CI) | 1.00 (Ref.) | 0.85 (0.69, 1.05) | 1.11 (0.90, 1.38) | 1.20 (0.94, 1.52) | 1.02 (0.76, 1.37) | 0.166 | 1.05 (0.95, 1.16) |
| Rectal cancer | |||||||
| n cases | 192 | 226 | 211 | 214 | 203 | 1046 | |
| HR (95%CI) | 1.00 (Ref.) | 1.14 (0.93, 1.38) | 1.10 (0.89, 1.36) | 1.13 (0.90, 1.42) | 1.06 (0.80, 1.39) | 0.710 | 1.02 (0.93, 1.12) |
| Heme iron | |||||||
| Median intake (IQR), mg/day | 0.3 (0.1; 0.4) | 0.75 (0.7; 0.8) | 1.1 (1.0; 1.2) | 1.5 (1.4; 1.6) | 2.2 (2.0; 2.7) | Per 0.8 mg | |
| Colon cancer | |||||||
| n cases | 446 | 479 | 506 | 498 | 474 | 2403 | |
| HR (95%CI) | 1.00 (Ref.) | 1.05 (0.93, 1.20) | 1.02 (0.90, 1.17) | 1.03 (0.90, 1.17) | 0.99 (0.86, 1.14) | 0.774 | 0.99 (0.94, 1.03) |
| Proximal colon cancer | |||||||
| n cases | 232 | 242 | 249 | 245 | 229 | 1197 | |
| HR (95%CI) | 1.00 (Ref.) | 1.02 (0.85, 1.22) | 0.94 (0.78, 1.13) | 0.95 (0.78, 1.15) | 0.89 (0.73, 1.09) | 0.208 | 0.95 (0.89, 1.01) |
| Distal colon cancer | |||||||
| n cases | 174 | 196 | 210 | 197 | 192 | 969 | |
| HR (95%CI) | 1.00 (Ref.) | 1.13 (0.92, 1.38) | 1.14 (0.93, 1.40) | 1.10 (0.89, 1.36) | 1.10 (0.88, 1.39) | 0.515 | 1.02 (0.95, 1.09) |
| Rectal cancer | |||||||
| n cases | 202 | 194 | 209 | 233 | 208 | 1046 | |
| HR (95%CI) | 1.00 (Ref.) | 0.90 (0.74, 1.10) | 0.89 (0.73, 1.08) | 0.99 (0.81, 1.20) | 0.87 (0.71, 1.07) | 0.475 | 0.97 (0.91, 1.04) |
| Non-heme iron | |||||||
| Median intake (IQR), mg/day | 7.0 (6.1; 7.6) | 9.0 (8.6; 9.4) | 10.6 (10.2; 11.0) | 12.4 (11.9; 12.9) | 15.4 (14.4; 17.1) | Per 3.4 mg | |
| Colon cancer | |||||||
| n cases | 503 | 455 | 512 | 510 | 423 | 2403 | |
| HR (95%CI) | 1.00 (Ref.) | 0.95 (0.83, 1.08) | 1.10 (0.96, 1.26) | 1.14 (0.98, 1.33) | 1.01 (0.83, 1.22) | 0.226 | 1.03 (0.96, 1.10) |
| Proximal colon cancer | |||||||
| n cases | 241 | 245 | 257 | 243 | 211 | 1197 | |
| HR (95%CI) | 1.00 (Ref.) | 1.06 (0.88, 1.28) | 1.15 (0.94, 1.40) | 1.12 (0.90, 1.40) | 1.04 (0.79, 1.36) | 0.591 | 1.03 (0.94, 1.13) |
| Distal colon cancer | |||||||
| n cases | 213 | 170 | 207 | 214 | 165 | 969 | |
| HR (95%CI) | 1.00 (Ref.) | 0.84 (0.68, 1.04) | 1.06 (0.86, 1.32) | 1.16 (0.91, 1.47) | 0.97 (0.72, 1.31) | 0.308 | 1.05 (0.94, 1.16) |
| Rectal cancer | |||||||
| n cases | 208 | 239 | 220 | 231 | 210 | 1108 | |
| HR (95%CI) | 1.00 (Ref.) | 1.17 (0.96, 1.42) | 1.09 (0.88, 1.35) | 1.16 (0.93, 1.46) | 1.08 (0.82, 1.43) | 0.627 | 1.04 (0.94, 1.14) |
IQR interquartile range.
aThe analyses were run using country-specific quintiles.
bFor the continuous analysis, all the models were adjusted for the same variables as the in quintiles analysis, but with additional stratification by country.
cP values for heterogeneity by sex (using continuous values) for total iron, heme iron, and non-heme iron, respectively, were for all colon cancer, 0.994, 0.181, 0.573; proximal colon cancer 0.453, 0.004, 0.779; distal colon cancer, 0.505, 0.384, 0.665; rectal cancer, 0.691, 0.275, 0.352.
dIn men, P for heterogeneity colon vs. rectal cancer were 0.274, 0.814, 0.274 for total iron, heme iron, and non-heme iron, respectively; and P for heterogeneity proximal colon cancer vs. distal colon cancer were 0.129, 0.008, 0.448 for total iron, heme iron, and non-heme iron, respectively. In women, P for heterogeneity colon vs. rectal cancer were 0.999, 0.579, 0.999 for total iron, heme iron, and non-heme iron, respectively; and P for heterogeneity proximal colon cancer vs. distal colon cancer were 0.671, 0.162, 0.999 for total iron, heme iron, and non-heme iron, respectively.
There were 178 and 239 overlapping and non-specified colon or rectal cancer cases in men and women, respectively.
Models were adjusted for BMI, height, education, physical activity, smoking, energy intakes from alcohol, and total energy minus energy from alcohol, intakes of vitamin C, calcium, tea, coffee, and stratified by age at recruitment. In women specifically menopause status and hormone use were included in the model. For heme iron, additional confounders included intakes of cereal, and cereal products, legumes, and vegetables whereas for non-heme iron it included intakes of red meat, processed meats, poultry, and fish and fish products for non-heme iron.
Substitution analyses
Substitution analyses revealed that replacing 1 mg of heme iron with 1 mg of non-heme iron resulted in lower CRC risk in men (HR per 1 mg replacement:0.94, 95%CI:0.89, 0.99) (Table 4). The main benefit of the replacement was indicated by a 10% lower risk of proximal colon cancer (HR per 1 mg replacement:0.90, 95%CI:0.82, 0.99) whereas no benefit was shown for distal colon (HR per 1 mg replacement: 1.03, 95%CI: 0.93–1.14). Despite the non-linearity of the association with heme iron in women, we proceeded with the substitution analyses to provide comparability with results in men. Our analyses in women showed no apparent benefit of substituting heme iron with non-heme iron.
Table 4.
Hazard ratios and 95% confidence intervals for substituting dietary heme iron (1 mg) with non-heme iron.
| Men | Women | |
|---|---|---|
| Colorectal cancer | 0.94 (0.89, 0.99) | 1.01 (0.96, 1.07) |
| Colon cancer | 0.95 (0.89, 1.02) | 1.01 (0.94, 1.08) |
| Proximal colon cancer | 0.90 (0.82, 0.99) | 1.08 (0.98, 1.19) |
| Distal colon cancer | 1.03 (0.93, 1.14) | 0.98 (0.88, 1.09) |
| Rectal cancer | 0.92 (0.85, 1.00) | 1.01 (0.92, 1.12) |
Models were adjusted for BMI, height, education, physical activity, smoking, and dietary intakes of calcium, tea, coffee, vitamin C, red meat, processed meats, poultry, and fish and fish product and particularly in women for menopause status and use of hormone replacement therapy.
Sensitivity analyses
Analyses excluding participants with <4 years of follow-up did not materially change the risk associations (Supplementary Table 6). In addition, excluding participants from Italy and Spain (blood donors), or UK (health-conscious) from the regression models did not materially change the findings.
Discussion
In this large prospective cohort study, we found that higher intake of non-heme iron was inversely associated with CRC risk in men. Analyses by anatomical sub-site suggested a stronger positive association with heme iron in the proximal colon vs. the distal colon, while no difference in the risk association was observed between the colon and rectum. In women, higher consumption of total iron, heme iron, and non-heme iron was not associated with CRC risk. Substitution of heme with non-heme iron (per 1 mg replacement) was associated with a 6% lower risk in men and had no observable projected association in women.
Previous prospective studies have mostly examined CRC associations with iron intake in women [11–14, 17] and only a few studies presented data for both men and women [12, 16] or in sex-adjusted analyses of men and women combined [15, 18]. Similar to our results, most of these studies did not find an association between heme iron intake and CRC risk in women [12, 14, 16, 17]. Two studies found higher risk of CRC associated with heme iron intake in women [11, 13]. However, there are no previous prospective studies regarding the non-heme iron-CRC risk association. The only information to date derives from case-control studies, which show either an inverse [40] or null association [41]. In women, iron absorption may decrease after menopause due to natural amenorrhea [42]. Hypothetically, postmenopausal women may have higher levels of iron reaching the colon conferring an increased CRC risk, compared to pre-menopausal women. However, we did not observe a difference between pre- and post-menopausal women, although we observed that among women ≥65 years, higher heme iron intake was inversely associated with CRC risk.
The dissimilar associations observed between heme and non-heme iron in men could be due to multiple factors. First, the positive association between higher heme iron intake and the risk of proximal colon cancer observed in men could be explained by higher intakes and concentration of iron reaching the colon, considering the lower rate of absorption of iron in men compared to women [43]. Iron pool in the body is efficiently conserved and recycled in humans, as minor losses only occur with the death and removal of skin cells, and minor losses from the renewal of gastrointestinal tissues [44]. Non-heme iron is principally different from heme iron as it originates mainly from plant foods and does not promote the synthesis of nitrosamines. Non-heme iron is easily chelated by antinutrients and participates in fewer reactions within the gastrointestinal tract, compared to heme iron. Animal studies have shown that non-heme iron is absorbed throughout the gastrointestinal tract, mostly in the duodenum, but also in the colon [45, 46]. Sesink et al. [47] have reported that adding hemin, an iron-containing breakdown product of hemoglobin, to the diet of rats promotes colonic epithelial proliferation whereas non-heme iron did not. Nonetheless, there is a need for future studies to investigate the potential inverse association observed with non-heme iron in men. Interestingly, our substitution analyses found that replacing 1 mg of daily heme iron intake with an equivalent daily amount of non-heme iron was associated with 6% lower risk of CRC in men. Although a 6% reduction of CRC risk (10% for proximal colon cancer risk) associated with replacement of 1 mg of heme iron with 1 mg of non-heme iron may seem large, it is plausible and corresponds to the risk observed in low-meat eaters compared to regular meat eaters [48]. Thus, our findings provide additional evidence that it might be the amount of heme iron, not non-heme iron, in the diet that is associated with an increased CRC risk.
There is no clear explanation of the differences in the association of disease risk by tumor anatomical subsite, particularly in the colon. One plausible explanation may be the actions of the microbiome and their interactions with iron available in the colon. The microbiome is profoundly influenced by the availability of iron, and iron is important for the growth and functions of bacteria in the colon, but higher levels of heme essentially promote the growth of pathogenic bacteria [49]. Thus, heme iron-depleted conditions evaluated with a hemin-deficient culture medium limit the growth of pathogenic bacteria such as Salmonella typhimurium and Escherichia coli, while it has no effect on other bacteria such as Lactobacillus [49]. The mutagenic actions of deleterious bacteria is likely to occur in the proximal colon because its protective mucus barrier is thinner compared to the mucus barrier in the distal colon [50]. Nevertheless, we cannot rule out a possible chance finding as heme iron intake was associated with higher rectal cancer risk as well. It is highly likely that the production of nitrosamines is an important factor in CRC development. The center of the heme molecule, where the iron is located, can convert nitrite into nitric acid and higher intake of red meat has been shown to increase levels of O6-methyl-2-deoxyguanosine (O6MeG), a DNA adduct derived from nitrosamines in the distal colon [51]. Importantly O6MeG has been found in human rectal biopsies [52] as an endpoint to the cumulated production of nitrosamines throughout the colon. It is therefore possible that the mutagenic actions of nitrosamines may be enhanced in the rectum. This could explain the higher risk of rectal cancer observed with heme iron in our study. Similarly, it is intriguing that we observed an inverse association between dietary heme iron intake and CRC risk in women aged beyond 65 years. This could be the combined effects of lifestyle changes with age toward healthier behaviors such as leisure physical activity practice, smoking cessation, or reduction in drinking intensity. Another conceivable hypothesis may be the well-documented significant changes in the microbiome of older subjects, with abundance of short-chain fatty acids producing genera such as Collinsella [53]. There is a need to further investigate the potential effects and mechanisms of heme iron in the proximal colon vs. distal colon, and in elderly women.
Strengths of our study include its prospective study design and large population. Our study is mainly limited by the single collection of dietary intakes at baseline, and the absence of data on iron supplements intake in the population, although supplement intake has been reported to be recurrent and variable between countries [54]. Absence of data on gastrointestinal conditions such as inflammatory bowel diseases is another limitation in our study. Also, it is possible that some of our results may be due to residual confounding despite comprehensive adjustment for covariates and extended sensitivity analyses. Noteworthily, because our study was based on dietary iron, and did not consider iron absorption, our findings could not be interpreted in parallel with body iron status. In addition, our analyses did not adjust for family history of CRC, or for anti-nutrients such as phytic acid and oxalates which inhibit iron absorption, due to unavailability of these variables in our cohort. Other limitations include recall and measurements errors (both for lifestyle and dietary intake), and difficulty in comparing country-specific dietary questionnaires. Finally, our findings may not be generalizable to other settings where iron intakes are low, or where varying intakes of vitamin C (promotes non-heme iron absorption), dietary iron chelators (bind and inhibit iron action and absorption) or recurrent infections affect iron transit and absorption.
In conclusion, we found that dietary non-heme iron was inversely associated with the risk of CRC in men whereas heme iron intake showed a positive association restricted to the proximal colon. No significant associations were observed between intake of total or different types of iron and CRC risk in women. Our findings suggest that any CRC risk associations for higher dietary iron are likely to be sex-specific and may vary between its heme- and non-heme components. Additional research, looking at body iron status and metabolism may shed some more light on this important question. Moreover, evidence on the link between iron and CRC is required from other non-European populations with different intake levels, and behaviors that may promote or inhibit iron absorption and utilization.
Disclaimer
Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this paper and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer/World Health Organization.
Supplementary information
Supplementary Tables and Figures without track changes
Acknowledgements
The authors would like to thank the EPIC study participants and staff for their valuable contribution to this research. The coordination of EPIC is financially supported by International Agency for Research on Cancer (IARC) and also by the Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London which has additional infrastructure support provided by the NIHR Imperial Biomedical Research Centre (BRC). The national cohorts are supported by: Danish Cancer Society (Denmark); Ligue Contre le Cancer, Institut Gustave Roussy, Mutuelle Générale de l’Education Nationale, Institut National de la Santé et de la Recherche Médicale (INSERM) (France); German Cancer Aid, German Cancer Research Center (DKFZ), German Institute of Human Nutrition PotsdamRehbruecke (DIfE), Federal Ministry of Education and Research (BMBF) (Germany); Associazione Italiana per la Ricerca sul Cancro-AIRC-Italy, Compagnia di SanPaolo and National Research Council (Italy); Dutch Ministry of Public Health, Welfare and Sports (VWS), Netherlands Cancer Registry (NKR), LK Research Funds, Dutch Prevention Funds, Dutch ZON (Zorg Onderzoek Nederland), World Cancer Research Fund (WCRF), Statistics Netherlands (The Netherlands); Health Research Fund (FIS), Instituto de Salud Carlos III (ISCIII), Regional Governments of Andalucía, Asturias, Basque Country, Murcia and Navarra, and the Catalan Institute of Oncology, ICO (Spain); Swedish Cancer Society, Swedish Research Council, Region Skåne and Region Västerbotten (Sweden); Cancer Research UK (14136 to EPIC-Norfolk; C8221/A29017 to EPIC-Oxford), Medical Research Council (1000143 to EPIC-Norfolk; MR/M012190/1 to EPIC-Oxford). (United Kingdom). VF is supported by the Cancer Prevention and Research Institute of Texas (CPRIT) Rising Stars Award (Grant ID RR200056). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the paper.
Author contributions
Conception and design: MJ, EKA, Acquisition and interpretation of data: all authors; Performed analyses: EKA, MJ, Drafted the paper: EKA, AJC, ER, VF, PJ, HF, DJH; Critically revised paper: all authors; Approved of final submission: all authors.
Funding
Internal funds of the IARC were used to support these analyses.
Data availability
For information on how to submit an application for gaining access to EPIC data and/or biospecimens, please follow the instructions at http://epic.iarc.fr/access/index.php.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Ethical approval was obtained from the IARC Ethical Committee and from local ethics committees pertaining to each EPIC center. All the participants provided written informed consent to enter the cohort.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-023-02164-7.
References
- 1.Global Cancer Observatory-Cancer today [Internet]. IARC-WHO. 2020 [cited 22/12/2020]. https://gco.iarc.fr/today/home.
- 2.WCRF. Diet, Nutrition, Physical Activity and Colorectal Cancer. London, UK: World Cancer Research Fund; 2018.
- 3.Bouvard V, Loomis D, Guyton KZ, Grosse Y, Ghissassi FE, Benbrahim-Tallaa L, et al. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015;16:1599–600. doi: 10.1016/S1470-2045(15)00444-1. [DOI] [PubMed] [Google Scholar]
- 4.Hallberg L, Hultén L, Gramatkovski E. Iron absorption from the whole diet in men: how effective is the regulation of iron absorption? Am J Clin Nutr. 1997;66:347–56. doi: 10.1093/ajcn/66.2.347. [DOI] [PubMed] [Google Scholar]
- 5.Ishikawa S, Tamaki S, Ohata M, Arihara K, Itoh M. Heme induces DNA damage and hyperproliferation of colonic epithelial cells via hydrogen peroxide produced by heme oxygenase: a possible mechanism of heme-induced colon cancer. Mol Nutr food Res. 2010;54:1182–91. doi: 10.1002/mnfr.200900348. [DOI] [PubMed] [Google Scholar]
- 6.Glei M, Klenow S, Sauer J, Wegewitz U, Richter K, Pool-Zobel BL. Hemoglobin and hemin induce DNA damage in human colon tumor cells HT29 clone 19A and in primary human colonocytes. Mutat Res. 2006;594:162–71. doi: 10.1016/j.mrfmmm.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Lunn JC, Kuhnle G, Mai V, Frankenfeld C, Shuker DE, Glen RC, et al. The effect of haem in red and processed meat on the endogenous formation of N-nitroso compounds in the upper gastrointestinal tract. Carcinogenesis. 2007;28:685–90. doi: 10.1093/carcin/bgl192. [DOI] [PubMed] [Google Scholar]
- 8.Cross AJ, Pollock JRA, Bingham SA. Haem, not protein or inorganic iron, is responsible for endogenous intestinal N-nitrosation arising from red meat. Cancer Res. 2003;63:2358–60. [PubMed] [Google Scholar]
- 9.Abbaspour N, Hurrell R, Kelishadi R. Review on iron and its importance for human health. J Res Med Sci: Off J Isfahan Univ Med Sci. 2014;19:164–74. [PMC free article] [PubMed] [Google Scholar]
- 10.Hunt JR, Zito CA, Johnson LK. Body iron excretion by healthy men and women. Am J Clin Nutr. 2009;89:1792–8. doi: 10.3945/ajcn.2009.27439. [DOI] [PubMed] [Google Scholar]
- 11.Lee DH, Anderson KE, Folsom AR, Jacobs DR., Jr Heme iron, zinc and upper digestive tract cancer: the Iowa Women’s Health Study. Int J Cancer. 2005;117:643–7. doi: 10.1002/ijc.21215. [DOI] [PubMed] [Google Scholar]
- 12.Balder HF, Vogel J, Jansen MC, Weijenberg MP, van den Brandt PA, Westenbrink S, et al. Heme and chlorophyll intake and risk of colorectal cancer in the Netherlands cohort study. Cancer Epidemiol Biomark Prev: Publ Am Assoc Cancer Res Cosponsored Am Soc Preventive Oncol. 2006;15:717–25. doi: 10.1158/1055-9965.EPI-05-0772. [DOI] [PubMed] [Google Scholar]
- 13.Larsson SC, Adami H-O, Giovannucci E, Wolk A. Re: Heme Iron, Zinc, Alcohol Consumption, and Risk of Colon Cancer. J Natl Cancer Inst. 2005;97:232–3. doi: 10.1093/jnci/dji032. [DOI] [PubMed] [Google Scholar]
- 14.Kabat GC, Miller AB, Jain M, Rohan TE. A cohort study of dietary iron and heme iron intake and risk of colorectal cancer in women. Br J Cancer. 2007;97:118–22. doi: 10.1038/sj.bjc.6603837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cross AJ, Ferrucci LM, Risch A, Graubard BI, Ward MH, Park Y, et al. A large prospective study of meat consumption and colorectal cancer risk: an investigation of potential mechanisms underlying this association. Cancer Res. 2010;70:2406–14. doi: 10.1158/0008-5472.CAN-09-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X, Giovannucci EL, Smith-Warner SA, Wu K, Fuchs CS, Pollak M, et al. A prospective study of intakes of zinc and heme iron and colorectal cancer risk in men and women. Cancer Causes Control. 2011;22:1627–37. doi: 10.1007/s10552-011-9839-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hara A, Sasazuki S, Inoue M, Iwasaki M, Shimazu T, Sawada N, et al. Zinc and heme iron intakes and risk of colorectal cancer: a population-based prospective cohort study in Japan. Am J Clin Nutr. 2012;96:864–73. doi: 10.3945/ajcn.112.041202. [DOI] [PubMed] [Google Scholar]
- 18.Etemadi A, Abnet CC, Graubard BI, Beane-Freeman L, Freedman ND, Liao L, et al. Anatomical subsite can modify the association between meat and meat compounds and risk of colorectal adenocarcinoma: Findings from three large US cohorts. Int J Cancer. 2018;143:2261–70. doi: 10.1002/ijc.31612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fonseca-Nunes A, Jakszyn P, Agudo A. Iron and cancer risk—a systematic review and meta-analysis of the epidemiological evidence. Cancer Epidemiol Biomark Prev: Publ Am Assoc Cancer Res Cosponsored Am Soc Preventive Oncol. 2014;23:12–31. doi: 10.1158/1055-9965.EPI-13-0733. [DOI] [PubMed] [Google Scholar]
- 20.Qiao L, Feng Y. Intakes of heme iron and zinc and colorectal cancer incidence: a meta-analysis of prospective studies. Cancer Causes Control. 2013;24:1175–83. doi: 10.1007/s10552-013-0197-x. [DOI] [PubMed] [Google Scholar]
- 21.Bastide N, Pierre F, Corpet D. Heme Iron from Meat and Risk of Colorectal Cancer: A Meta-analysis and a Review of the Mechanisms Involved. Cancer Prev Res (Phila, Pa). 2011;4:177–84. doi: 10.1158/1940-6207.CAPR-10-0113. [DOI] [PubMed] [Google Scholar]
- 22.Lee DH, Anderson KE, Harnack LJ, Folsom AR, Jacobs DR., Jr Heme iron, zinc, alcohol consumption, and colon cancer: Iowa Women’s Health Study. J Natl Cancer Inst. 2004;96:403–7. doi: 10.1093/jnci/djh047. [DOI] [PubMed] [Google Scholar]
- 23.Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5:1113–24. doi: 10.1079/PHN2002394. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez CA. The European Prospective Investigation into Cancer and Nutrition (EPIC) Public Health Nutr. 2006;9:124–6. doi: 10.1079/PHN2005934. [DOI] [PubMed] [Google Scholar]
- 25.Van Puyvelde H, Perez-Cornago A, Casagrande C, Nicolas G, Versele V, Skeie G, et al. Comparing Calculated Nutrient Intakes Using Different Food Composition Databases: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC) Cohort. Nutrients. 2020;12:2906. doi: 10.3390/nu12102906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cross AJ, Harnly JM, Ferrucci LM, Risch A, Mayne ST, Sinha R. Developing a heme iron database for meats according to meat type, cooking method and doneness level. Food Nutr Sci. 2012;3:905–13. doi: 10.4236/fns.2012.37120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jakszyn P, Agudo A, Lujan-Barroso L, Bueno-de-Mesquita HB, Jenab M, Navarro C, et al. Dietary intake of heme iron and risk of gastric cancer in the European prospective investigation into cancer and nutrition study. Int J Cancer. 2012;130:2654–63. doi: 10.1002/ijc.26263. [DOI] [PubMed] [Google Scholar]
- 28.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599–608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 29.Kleinbaum D, Klein M. Survival Analysis: A Self-Learning Text. 1. NY, USA: Springer; 2005. p. 700.
- 30.Hess KR. Graphical methods for assessing violations of the proportional hazards assumption in cox regression. Stat Med. 1995;14:1707–23. doi: 10.1002/sim.4780141510. [DOI] [PubMed] [Google Scholar]
- 31.Harrell FEJ. [Internet]. Rms: Regression Modeling Strategies.R package version 6.3-0 [cited 31/12/2016]. https://CRAN.R-project.org/package=rms.
- 32.Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transpl. 2007;40:381–7. doi: 10.1038/sj.bmt.1705727. [DOI] [PubMed] [Google Scholar]
- 33.Wang M, Spiegelman D, Kuchiba A, Lochhead P, Kim S, Chan AT, et al. Statistical methods for studying disease subtype heterogeneity. Stat Med. 2016;35:782–800. doi: 10.1002/sim.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ibsen DB, Laursen ASD, Würtz AML, Dahm CC, Rimm EB, Parner ET, et al. Food substitution models for nutritional epidemiology. Am J Clin Nutr. 2021;113:294–303. doi: 10.1093/ajcn/nqaa315. [DOI] [PubMed] [Google Scholar]
- 35.Song M, Giovannucci E. Substitution analysis in nutritional epidemiology: proceed with caution. Eur J Epidemiol. 2018;33:137–40. doi: 10.1007/s10654-018-0371-2. [DOI] [PubMed] [Google Scholar]
- 36.Tomova GD, Gilthorpe MS, Tennant PWG. Theory and performance of substitution models for estimating relative causal effects in nutritional epidemiology. Am J Clin Nutr. 2022;116:1379–88. doi: 10.1093/ajcn/nqac188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van den Brandt PA. Red meat, processed meat, and other dietary protein sources and risk of overall and cause-specific mortality in The Netherlands Cohort Study. Eur J Epidemiol. 2019;34:351–69. doi: 10.1007/s10654-019-00483-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alferink LJ, Kiefte-de Jong JC, Erler NS, Veldt BJ, Schoufour JD, de Knegt RJ, et al. Association of dietary macronutrient composition and non-alcoholic fatty liver disease in an ageing population: the Rotterdam Study. Gut. 2019;68:1088–98. doi: 10.1136/gutjnl-2017-315940. [DOI] [PubMed] [Google Scholar]
- 39.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Ser B (Methodol) 1995;57:289–300. [Google Scholar]
- 40.Ronco A, Calderon J, Mendoza B, Espinosa E, Lasalvia-Galante E. Dietary Iron Sources and Colorectal Cancer Risk: A Role for Sex. Journal of Cancer Science and Treatment. 2019:93–110.
- 41.Luo H, Zhang NQ, Huang J, Zhang X, Feng XL, Pan ZZ, et al. Different forms and sources of iron in relation to colorectal cancer risk: a case-control study in China. Br J Nutr. 2019;121:735–47. doi: 10.1017/S0007114519000023. [DOI] [PubMed] [Google Scholar]
- 42.Fairweather-Tait SJ, Jennings A, Harvey LJ, Berry R, Walton J, Dainty JR. Modeling tool for calculating dietary iron bioavailability in iron-sufficient adults. Am J Clin Nutr. 2017;105:1408–14. doi: 10.3945/ajcn.116.147389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woodhead JC, Drulis JM, Nelson SE, Janghorbani M, Fomon SJ. Gender-Related Differences in Iron Absorption by Preadolescent Children. Pediatr Res. 1991;29:435–9. doi: 10.1203/00006450-199105010-00005. [DOI] [PubMed] [Google Scholar]
- 44.Aisen P, Enns C, Wessling-Resnick M. Chemistry and biology of eukaryotic iron metabolism. Int J Biochem Cell Biol. 2001;33:940–59. doi: 10.1016/S1357-2725(01)00063-2. [DOI] [PubMed] [Google Scholar]
- 45.Johnston KL, Johnson DM, Marks J, Srai SK, Debnam ES, Sharp PA. Non-haem iron transport in the rat proximal colon. Eur J Clin Investig. 2006;36:35–40. doi: 10.1111/j.1365-2362.2006.01585.x. [DOI] [PubMed] [Google Scholar]
- 46.Takeuchi K, Bjarnason I, Laftah AH, Latunde-Dada GO, Simpson RJ, McKie AT. Expression of iron absorption genes in mouse large intestine. Scand J Gastroenterol. 2005;40:169–77. doi: 10.1080/00365520510011489. [DOI] [PubMed] [Google Scholar]
- 47.Sesink AL, Termont DS, Kleibeuker JH, Van der Meer R. Red meat and colon cancer: the cytotoxic and hyperproliferative effects of dietary heme. Cancer Res. 1999;59:5704–9. [PubMed] [Google Scholar]
- 48.Watling CZ, Schmidt JA, Dunneram Y, Tong TYN, Kelly RK, Knuppel A, et al. Risk of cancer in regular and low meat-eaters, fish-eaters, and vegetarians: a prospective analysis of UK Biobank participants. BMC Med. 2022;20:73. doi: 10.1186/s12916-022-02256-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parmanand BA, Kellingray L, Le Gall G, Basit AW, Fairweather-Tait S, Narbad A. A decrease in iron availability to human gut microbiome reduces the growth of potentially pathogenic gut bacteria; an in vitro colonic fermentation study. J Nutr Biochem. 2019;67:20–7. doi: 10.1016/j.jnutbio.2019.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kamphuis JBJ, Mercier-Bonin M, Eutamène H, Theodorou V. Mucus organisation is shaped by colonic content; a new view. Sci Rep. 2017;7:8527. doi: 10.1038/s41598-017-08938-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winter J, Nyskohus L, Young GP, Hu Y, Conlon MA, Bird AR, et al. Inhibition by resistant starch of red meat-induced promutagenic adducts in mouse colon. Cancer Prev Res (Philos) 2011;4:1920–8. doi: 10.1158/1940-6207.CAPR-11-0176. [DOI] [PubMed] [Google Scholar]
- 52.Le Leu RK, Winter JM, Christophersen CT, Young GP, Humphreys KJ, Hu Y, et al. Butyrylated starch intake can prevent red meat-induced O6-methyl-2-deoxyguanosine adducts in human rectal tissue: a randomised clinical trial. Br J Nutr. 2015;114:220–30. doi: 10.1017/S0007114515001750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, et al. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS ONE. 2010;5:e10667. doi: 10.1371/journal.pone.0010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skeie G, Braaten T, Hjartåker A, Lentjes M, Amiano P, Jakszyn P, et al. Use of dietary supplements in the European Prospective Investigation into Cancer and Nutrition calibration study. Eur J Clin Nutr. 2009;63:S226–38. doi: 10.1038/ejcn.2009.83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables and Figures without track changes
Data Availability Statement
For information on how to submit an application for gaining access to EPIC data and/or biospecimens, please follow the instructions at http://epic.iarc.fr/access/index.php.