Abstract
Background
We investigated the utility of a molecular classifier tool and genetic alterations for predicting prognosis in Japanese patients with endometrial cancer.
Methods
A total of 1029 patients with endometrial cancer from two independent cohorts were classified into four molecular subtype groups. The primary and secondary endpoints were relapse-free survival (RFS) and overall survival (OS), respectively.
Results
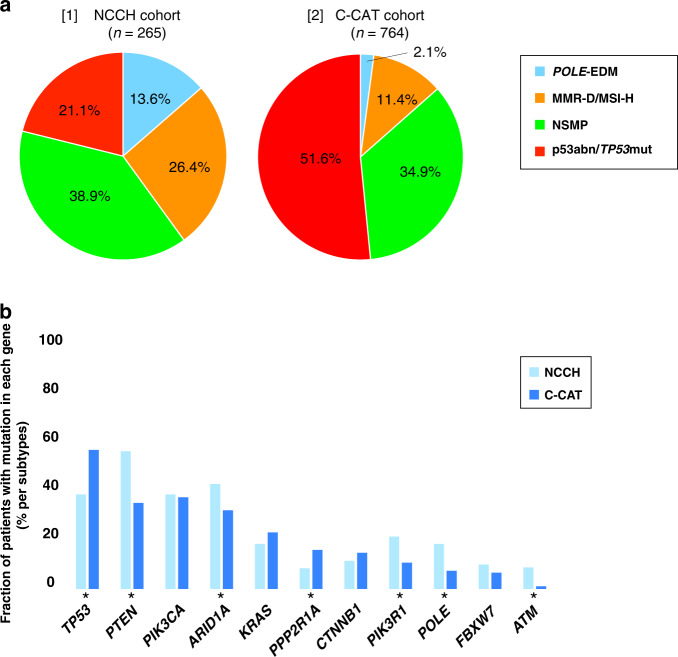
Among the 265 patients who underwent initial surgery, classified according to immunohistochemistry, patients with DNA polymerase epsilon exonuclease domain mutation had an excellent prognosis (RFS and OS), patients with no specific molecular profile (NSMP) and mismatch repair protein deficiency had an intermediate prognosis, and those with protein 53 abnormal expression (p53abn) had the worst prognosis (P < 0.001). In the NSMP group, mutant KRAS and wild-type ARID1A were associated with significantly poorer 5-year RFS (41.2%) than other genomic characteristics (P < 0.001). The distribution of the subtypes differed significantly between patients with recurrence/progression and classified by sequencing (n = 764) and patients who underwent initial surgery (P < 0.001). Among patients with recurrence/progression, 51.4% had the opportunity to receive molecular targeted therapy.
Conclusions
A molecular classifier is a useful tool for determining prognosis and eligibility for molecularly targeted therapy in patients with endometrial cancer.
Subject terms: Endometrial cancer, Tumour biomarkers
Background
Endometrial cancer, the fifth most common cancer in women, accounts for an estimated 382,000 new cancer cases and 90,000 deaths annually worldwide [1]. In Japan, during 2018, 17,089 patients were newly diagnosed with endometrial cancer, and 2597 patients with endometrial cancer died [2]. The overall prognosis in patients with endometrial cancer is generally considered favourable, with a 5-year overall survival (OS) of 80%; nevertheless, 15–20% of patients experience recurrence [3]. Outcomes in patients with endometrial cancer and systemic recurrence are poor, with a median survival hardly exceeding 12 months [4]. A risk stratification system based on clinicopathological factors, such as stage, histopathologic type, grade, myometrial invasion, and lymphovascular space invasion, has been used to identify patients with endometrial cancer who are at risk of a poor prognosis [5, 6]. Patients with endometrial cancer are categorised into risk groups to identify those requiring adjuvant treatment [7, 8]; however, risk stratification using clinical factors evaluated after surgery cannot inform decision-making concerning surgical procedures. Given these deficiencies, the development of an accurate diagnosis and risk stratification system for endometrial cancer is required.
In 2013, The Cancer Genome Atlas Endometrial Collaborative Project proposed four different prognostic subtypes based on genomic abnormalities that reflected endometrial cancer tumour biology [9]. Subsequently, a clinically applicable molecular classification system based on immunohistochemistry (IHC) was developed [10–13]. The molecular classification includes four subtypes: (i) DNA polymerase epsilon mutant (POLE-mut), (ii) mismatch repair protein deficiency (MMR-D), (iii) protein 53 abnormal expression (p53abn), and (iv) no specific molecular profile (NSMP) [14]. Molecular subtype assignment is highly reproducible and can be performed on diagnostic endometrial biopsy or curettage. Moreover, molecular subtyping is highly concordant with classification based on subsequent hysterectomy specimens [15, 16]. Recently, a molecular classifier has been reported to be useful in distinguishing prognosis in the Caucasian population with endometrial cancer [13, 15, 17]. The 2020 European Society of Gynaecological Oncology, European Society for Radiotherapy and Oncology, and European Society of Pathology guidelines assign risk groups and make treatment decisions according to these molecular subtypes [5].
Endometrial cancer, similar to colorectal cancer and non-small cell lung cancer [18, 19], has a different prognosis depending on ethnicity [20, 21]. Mahdi et al. reported that Asians had a favourable prognosis despite a higher tumour grade and more advanced stage of illness compared to American Indians/Alaska Natives and non-Hispanic White patients with endometrial cancer [22]. Clinicopathological prognostic factors are defined by stage, histology, grade, myometrial invasion, and lymphovascular space invasion, according to the National Comprehensive Cancer Network and European Society for Medical Oncology regardless of ethnicity, although the genetic alterations in endometrial cancer may differ depending on ethnicity. Therefore, molecular classification should consider the biological differences in endometrial cancer according to ethnicity to be broadly applicable to patients with endometrial cancer. However, few studies have described the prognostic value of a molecular classifier in non-Caucasian patients with endometrial cancer.
Furthermore, few studies have focused on the genomic profiles of patients with advanced endometrial cancer. The majority of patients with endometrial cancer are diagnosed at an early stage, and most patients are cured using surgery alone. However, patients with advanced or recurrent endometrial cancer who do not respond to localised therapy, such as surgery or radiotherapy, have a poor prognosis [23, 24]. Treatment options for advanced endometrial cancer have not changed in the last decade. There are limited options for cytoreductive therapy after initial treatment, and no standard options are available for subsequent recurrence and progression. Therefore, evaluating genomic profiles, including the frequency of actionable mutations in advanced endometrial cancer stages, is important to increase options for molecular targeted therapies after relapse to further improve OS in patients with advanced endometrial cancer.
In this study, we investigated the impact of molecular classification on prognosis in >1000 Asian patients with endometrial cancer, including those who underwent initial surgical treatment, and those who recurred or progressed. Furthermore, we aimed to identify novel molecular prognostic factors and indications for molecularly targeted agents by comprehensively decoding genomic changes in patients with endometrial cancer using targeted sequences.
Materials and methods
Study design
The study consisted of two independent retrospective cohorts of 1029 Japanese patients with primary endometrial cancer.
The National Cancer Center Hospital (NCCH) cohort included 265 patients, all of whom underwent initial surgery, 99 (37.4%) received adjuvant therapy, and 73 (27.6%) experienced recurrence or progression after the initial surgery.
The Center for Cancer Genomics and Advanced Therapeutic (C-CAT) cohort included 764 patients, all of whom were cases of recurrence or progression; 553 (72.4%) underwent initial surgery, although data were not available for the initial treatment regimen used in 211 patients. In the 705 patients for whom therapeutic records were available, the median number of administered chemotherapy regimens was 2 (range: 1–9 regimens). One patient who overlapped across both cohorts was excluded. The patient characteristics for each cohort are summarised in Table 1.
Table 1.
Characteristics of Japanese patients with endometrial cancer.
| Characteristics | NCCH cohort | C-CAT cohort | P value | ||
|---|---|---|---|---|---|
| (n = 265) | (%) | (n = 764) | (%) | ||
| Clinicopathological parameters | |||||
| Age [year] (median, range) | 57 | (28–89) | 63 | (25–85) | <0.001* |
| Histological types | |||||
| Endometrioid | |||||
| Grade 1 | 92 | (34.7%) | 27 | (3.5%) | |
| Grade 2 | 30 | (11.3%) | 31 | (4.1%) | |
| Grade 3 | 76 | (28.7%) | 43 | (5.6%) | |
| Unknown grade | 0 | (0.0%) | 255 | (33.4%) | |
| Carcinosarcoma | 23 | (8.7%) | 130 | (17.0%) | |
| Serous | 18 | (6.8%) | 132 | (17.3%) | |
| Clear | 12 | (4.4%) | 25 | (3.3%) | |
| Mixed | 11 | (4.2%) | 26 | (3.4%) | |
| Un/de-differentiated | 1 | (0.4%) | 18 | (2.4%) | |
| Neuroendocrine | 0 | (0.0%) | 7 | (0.9%) | |
| Poorly differentiated carcinomaa | 1 | (0.4%) | 14 | (1.8%) | |
| Carcinoma | 1 | (0.4%) | 46 | (6.0%) | |
| Others | 0 | (0.0%) | 10 | (1.3%) | |
| Histological gradeb | <0.001** | ||||
| Low | 122 | (46.0%) | 58 | (7.6%) | |
| High | 143 | (54.0%) | 405 | (53.0%) | |
| Unclassifiable | 0 | (0.0%) | 301 | (39.4%) | |
| FIGO (2008) stage | – | ||||
| IA | 99 | (37.4%) | NA | (–) | |
| lB | 41 | (15.5%) | NA | (−) | |
| ll | 22 | (8.3%) | NA | (–) | |
| lllA | 16 | (6.0%) | NA | (–) | |
| lllB | 4 | (1.5%) | NA | (–) | |
| lllC | 58 | (21.9%) | NA | (–) | |
| lVB | 25 | (9.4%) | NA | (–) | |
| Specimens for targeted gene panel | – | ||||
| Initial surgery | 265 | (100.0%) | 553 | (72.4%) | |
| Recurrent tumour | 0 | (0.0%) | 209 | (27.3%) | |
| Unknown | 0 | (0.0%) | 2 | (0.3%) | |
| Type of targeted gene panel | – | ||||
| Ion Ampliseq Hotspot and a custom panel | 265 | (100.0%) | 0 | (0.0%) | |
| FoundationOne CDx | 0 | (0.0%) | 749 | (98.0%) | |
| NCC Oncopanel | 0 | (0.0%) | 15 | (2.0%) | |
| Adjuvant therapy | – | ||||
| None | 166 | (62.6%) | NA | (–) | |
| Chemotherapy | 97 | (36.6%) | NA | (–) | |
| Radiotherapy | 2 | (0.8%) | NA | (–) | |
| Number of chemotherapy lines given (median, range) | 1 | (0–3) | 2 | (1–9) | <0.001* |
| Recurrence or progression | – | ||||
| None | 192 | (72.4%) | NA | (–) | |
| Recurrence | 67 | (25.3%) | NA | (–) | |
| Progression | 6 | (2.3%) | NA | (–) | |
| Outcome | 0.833*** | ||||
| Alive | 217 | (81.9%) | 630 | (82.5%) | |
| Death | 48 | (18.1%) | 134 | (17.5%) | |
| Follow-up period [month] (median, range) | 61 | (3–149) | 28 | (1–270) | <0.001* |
NCCH National Cancer Center Hospital, C-CAT Center for Cancer Genomics and Advanced Therapeutics, FIGO International Federation of Gynecology and Obstetrics, NA not available.
*Mann–Whitney’s U test; **low grade vs high grade, Chi-squared test; ***Chi-squared test.
aThe detailed histological subtype or grade according to the 2020 WHO classification was not provided for this case.
bLow: endometrioid carcinoma Grade 1 or 2, high: endometrioid carcinoma Grade 3, carcinosarcoma, serous, clear, mixed, and others.
National Cancer Center Hospital cohort
Characteristics
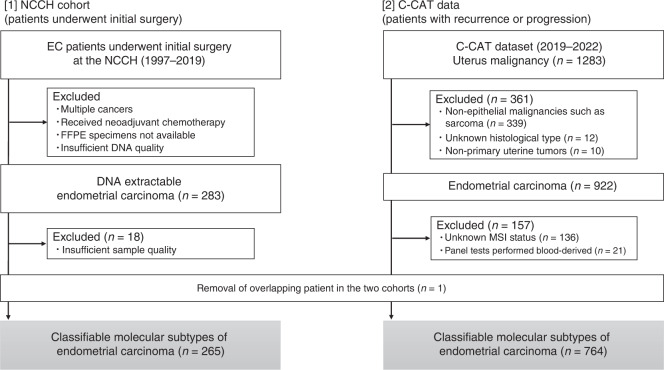
Of all the patients who underwent initial surgery at the NCCH between 1997 and 2019 and had a pathological diagnosis of endometrial cancer; 265 were included in the study. Patients who received neoadjuvant chemotherapy were excluded (Fig. 1 [1]). All the cases were reviewed by at least two gynaecological pathologists, and the pathological diagnoses were confirmed according to the 2020 World Health Organization tumour classification. Clinicopathological data, including age and stage (defined by the International Federation of Gynecology and Obstetrics [FIGO] in 2008) were retrospectively obtained for each patient.
Fig. 1. Consort diagram of two cohorts.
[1] Two hundred and sixty-five patients with endometrial cancer who underwent initial surgical treatment at our hospital. [2] Seven hundred and sixty-four patients with recurrence or progression from the C-CAT dataset.
DNA preparation and next-generation sequencing
Genomic DNA was extracted from 265 hysterectomy formalin-fixed paraffin-embedded (FFPE) endometrial tumour tissue samples using the QIAamp DNA FFPE tissue kit according to the manufacturer’s instructions (Qiagen, Hilden, Germany). DNA obtained from tumour tissues (50 ng) was used for library construction using the Ion AmpliSeqTM Cancer Hotspot Panel v2 and Ion AmpliSeqTM Custom Panel (Thermo Fisher Scientific, Waltham, MA, USA). Experimental details are provided in the Supplementary Methods. Pathological variants in the 50 cancer-related genes were defined using previously reported criteria [25–28] and “high-impact variants,” such as frameshift, stop-gain, stop-loss, and start-loss were defined using SnpEff v4.3 [29] for all exon sequences in the Ion AmpliSeqTM Custom Panel, in addition to pathogenic/oncogenic variants in the ClinVar [30] and OncoKB [31].
Classification of molecular subtypes using IHC
Using a molecular classifier, patients with endometrial cancer were divided into four groups: (i) POLE exonuclease domain mutation (POLE-EDM), (ii) MMR-D, (iii) p53abn and (iv) NSMP. MMR-D and p53abn have been defined in the Supplementary Methods. In this study, all exons of POLE were sequenced using the Ion AmpliSeqTM Custom Panel, and we defined POLE-EDM as oncogenic/pathogenic, nonsense, and in-frame deletion variants detected within the exonuclease domain of POLE that were reported as somatic hotspots [32]. Oncogenic/pathogenic mutations, nonsense mutations, and in-frame deletion mutations that did not contain an exonuclease domain were defined as non-POLE-EDM. The molecular classification was conducted as follows: first, tumours were assessed for POLE-EDM; next, the presence/deficiency of MMR proteins was assessed using IHC; and finally, tumours were assessed for p53 aberrations using IHC, yielding four subgroups: POLE-EDM, MMR-D, p53abn, and NSMP. In this study, double/dual classifier endometrial cancer was classified into upstream branching groups as previously reported [14]. Due to low DNA quality from the FFPE sample, we analysed frozen tumour tissue specimens that were different from the tumour section for MMR-D and p53 IHC in 18 of 265 cases.
Center for Cancer Genomics and Advanced Therapeutic cohort
Characteristics
In June 2019, insurance coverage for the Comprehensive Genome Profiling (CGP) test was introduced in Japan for patients with solid tumours for whom no standard treatment was available or was expected to be completed [33]. The C-CAT was established under the national health insurance system to consolidate, store, and utilise the mutation data and medical information of patients who underwent CGP tests, such as FoundationOne CDx and NCC Oncopanel (https://for-patients.c-cat.ncc.go.jp/). By the end of April 2022, the data of >32,000 patients with advanced cancer had been collected. The definitions of somatic mutations in the C-CAT cohort are provided in the Supplementary Methods.
Classification of molecular subtypes using next-generation sequencing
In April 24, 2022, we accessed the C-CAT database (ver. 20220406) and obtained clinical and genomic data for 764 patients with clearly defined endometrial carcinoma, excluding patients with sarcoma (Fig. 1 [2]). C-CAT cases of endometrial cancer were divided into four different molecular subtypes based on genomic abnormalities as follows: (i) POLE-EDM, (ii) microsatellite instability high (MSI-H), (iii) TP53 oncogenic mutation (TP53mut), and (iv) NSMP. In endometrial cancer, p53 evaluation using IHC is an excellent surrogate marker for TP53 mutation status determined by sequencing and has comparable performance and excellent reproducibility among pathologists [34, 35]. Double/dual classification endometrial cancer was classified into upstream branch groups similar to the NCCH cohort [14]. The molecular classification methods by cohort are summarised in the Supplementary Methods.
Clinical outcomes
In the NCCH cohort, the primary endpoint was relapse-free survival (RFS), which was measured from the date of random assignment to the date of documented relapse or death, whatever the cause. Patients who were alive and relapse-free at the last follow-up were censored. The secondary endpoint was OS, which was measured from the date of random assignment to the date of death, regardless of the cause. Six patients whose tumours could not be completely resected in the initial surgery were excluded from the survival analysis. Patients alive at the time of the analysis were censored at the date of the last follow-up.
In the C-CAT cohort, the primary endpoint was OS. Forty-six patients whose survival period was unknown because the date of diagnosis or last follow-up was not known were excluded from the survival analysis.
Statistical analysis
Statistical analysis was performed using R software ver. 4.1.2 (R Foundation, Vienna, Austria) and JMP version 16.0.0 software (SAS Institute, New York, USA). Variables that achieved statistical significance in the univariate analysis were subsequently included in the multivariate analysis. The level of statistical significance was set at P < 0.05. Cumulative survival was estimated using the Kaplan–Meier method, and differences in survival between two groups was analysed using the log-rank test. The effect of variables on OS or RFS was determined via univariate and multivariate analyses using the Cox proportional hazard model with JMP software.
Results
NCCH cohort
Characteristics of the patients by molecular subtype
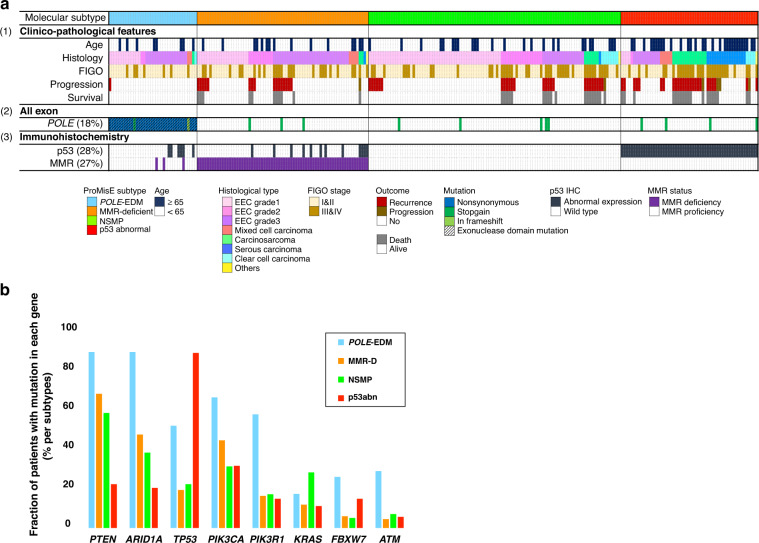
The clinical characteristics and pathological data of the 265 patients from this cohort are summarised in Table 1. Thirty-six patients were assigned to the POLE-EDM group, 70 to the MMR-D group, 103 to the NSMP group, and 56 to the p53abn group (Fig. 2a). The age of the patients in the p53abn group was significantly higher than that of patients in the other molecular subtype groups (P < 0.001) (Supplementary Table S1). The distribution of histological types depended on the molecular subtypes, with Grade 3 endometrioid endometrial carcinoma (EEC) prevalent in the POLE-EDM and MMR-D groups, low-grade (Grade 1 and 2) EEC in the NSMP group, and serous carcinoma in the p53abn group (P < 0.001). The number of progression events was significantly lower in the POLE-EDM group and significantly higher in the p53abn group (P < 0.001). There was no association between the FIGO stage and molecular subtype. The median follow-up period for all patients was 61 months (range: 3–149 months).
Fig. 2. Genetic alteration spectrum by molecular subtype in the NCCH cohort.
a Clinicopathological factors and molecular subtype in the NCCH cohort. The 265 patients were classified according to (1) histological type and clinicopathological features, (2) somatic mutations used for molecular classification and (3) IHC used for molecular classification. b Differences among the four subgroups of recurrently mutant genes. Shown are the mutation frequencies of all genes that were significantly mutated in at least one of the four subgroups.
Distribution of somatic mutations by molecular subtype
The most frequently mutated gene in patients included in the NCCH cohort was PTEN, which was detected in 147/265 (55.5%) patients, followed by ARID1A, TP53, PIK3CA and PIK3R1, detected in 112/265 (43.2%), 101/265 (38.1%), 101/265 (38.1%)c and 56/265 (21.1%) patients, respectively. The pattern of somatic mutations varied among different molecular subtypes (Fig. 2b). PTEN and ARID1A mutations were significantly less frequent in the p53abn group than in the other groups (P < 0.001). KRAS mutations were found more frequently in patients with endometrial carcinoma and NSMP than in those with other subtypes. There were no typical mesonephric-like adenocarcinomas in 48 patients with KRAS mutations. TP53 mutations were significantly more common in the p53abn group than in the other three groups. This distribution of somatic mutations by molecular subtype was similar to that of the histological type. TP53mut/p53 wild-type (wt) was more common in endometrioid carcinoma, while TP53wt/p53abn was more common in non-endometrioid carcinoma, and the histological types differed significantly between the two groups (P < 0.001) (Supplementary Table S2).
Correlation between molecular subtype and clinical outcomes
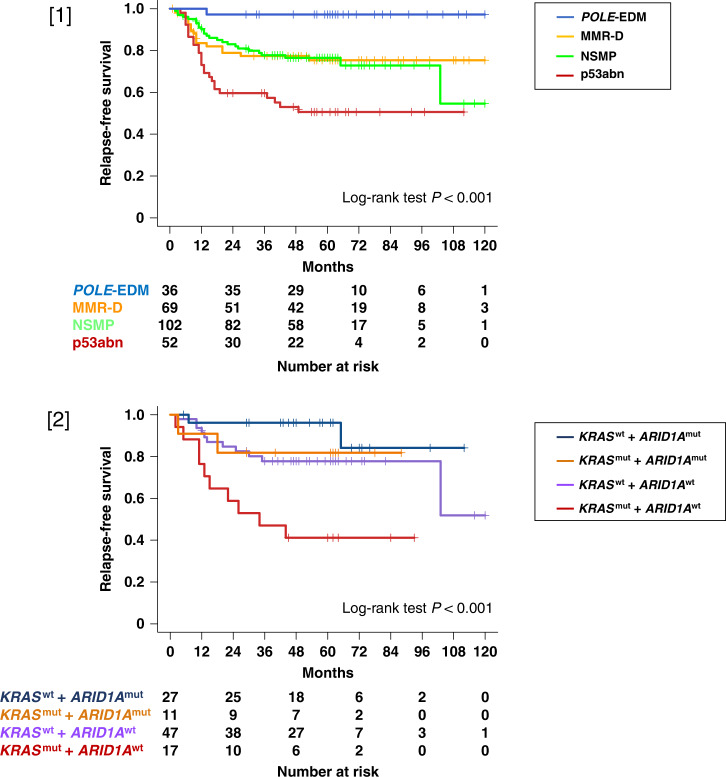
The survival probability for the entire NCCH cohort of endometrial cancer patients is shown in Supplementary Fig. S1. Patients with endometrial cancer with POLE-EDM had the best prognosis in terms of RFS and OS; those with MMR-D and NSMP exhibited intermediate prognosis, with no significant difference between the two groups; and those with p53abn had the worst prognosis (Fig. 3 [1]) and Supplementary Fig. S2). In the multivariate analysis performed using a Cox proportional hazards model, patients with endometrial cancer and p53abn were independently found to have a worse RFS than those with POLE-EDM (hazard ratio [HR] = 16.2, 95% confidence interval [CI] = 2.16–121.0, P = 0.007) (Supplementary Table S3). Patients with endometrial cancer with POLE-EDM had a favourable prognosis, with a 100% 5-year RFS rate and 5-year OS rate despite the advanced FIGO Stage (III–IV) (Supplementary Fig. S3 [1], [2]). In the early stage, patients with POLE-EDM endometrial cancer had a favourable prognosis, those with MMR-D and NSMP had an intermediate prognosis, and those with p53abn had an unfavourable prognosis, although the differences were not significant (Supplementary Fig. S3 [3], [4]). In the multivariate analysis performed using a Cox proportional hazards model, patients with endometrial cancer with mutant PTEN and ARID1A had a better RFS than those with wild-type PTEN and ARID1A (HR = 0.42, 95% CI = 0.25–0.69, P < 0.001; HR = 0.58, 95% CI = 0.35–0.97, P = 0.040, respectively) (Supplementary Table S3). In 198 patients with EEC, those with PTEN mutations had better RFS than those without mutations, with no significant difference in OS (Supplementary Fig. S4).
Fig. 3. Kaplan–Meier survival curves in the NCCH cohort.
[1] RFS according to molecular subtype in all stages of POLE-EDM (blue line), MMR-D (orange line), NSMP (green line) and p53abn (red line). [2] RFS according to KRAS and ARID1A status in patients with endometrial cancer with no specific molecular profile (NSMP). KRAS wt and ARID1A mut (navy blue line), KRAS mut and ARID1A mut (dark orange line), KRAS wt and ARID1A wt (purple line) and KRAS mut and ARID1A wt (dark red line). mut mutant, wt wild type.
Forty-eight of the 265 patients had POLE mutations, 36 with mutations in the exonuclease domain (P286R/C, 18 patients; V411L, 7 patients; A456P, 5 patients; S297F/Y, 2 patients; S459F/del, 2 patients; F367C, 1 patient; W369*, 1 patient) and 12 with mutations in the non-exonuclease domain (Supplementary Fig. S5A). Patients with exonuclease domain mutations in POLE had significantly better RFS and OS than those with non-exonuclease domain mutations in POLE (P < 0.001) (Supplementary Fig. S5B).
Somatic mutations as prognostic factors in patients with no significant molecular profile
Among the 103 patients with NSMP, 17 with KRAS mutation and without ARID1A mutation had unfavourable RFS (log-rank test; P < 0.001) and OS (log-rank test; P = 0.002) (Fig. 3 [2] and Supplementary Fig. S6). In the Cox proportional hazard model analysis, patients with mutant KRAS and wt ARID1A had the worst RFS when wt KRAS and mutant ARID1A were used as the reference (HR = 6.98, 95% CI = 1.47–33.2, P = 0.015) (Supplementary Table S4). The clinical characteristics of the 17 patients with KRAS mutation and without ARID1A mutation are summarised in Supplementary Table S5.
C-CAT cohort
Patient characteristics
The clinical characteristics and pathological data of the 764 patients with recurrence or progression of endometrial cancer are summarised in Table 1. Sixteen patients were assigned to the POLE-EDM group, 87 to the MSI-H group, 267 to the NSMP group, and 394 to the TP53mut group (Supplementary Table S6). The C-CAT data showed a high frequency of low-grade endometrial cancer in the NSMP group and high-grade endometrial cancer in the TP53mut group, although the histological grades were not available for many patients (Supplementary Table S6). The genetic alterations by molecular subtype for the C-CAT cohort are shown in Supplementary Fig. S7A. In the C-CAT cohort, similar to the NCCH cohort, the distribution of many genetic alterations differed among the four subtypes (Supplementary Fig. S7B). PTEN, ARID1A and PIK3CA mutations were more common in the POLE-EDM and MSI-H groups, KRAS mutations were more common in the NSMP group, and PPP2R1A mutations were more common in the TP53mut group (P < 0.001). There was no significant association between molecular subtype and histological grade in the 463 patients for whom histological grades were available in the C-CAT cohort (P = 0.394).
Correlation between molecular subtype and clinical outcome and potential therapeutic targets
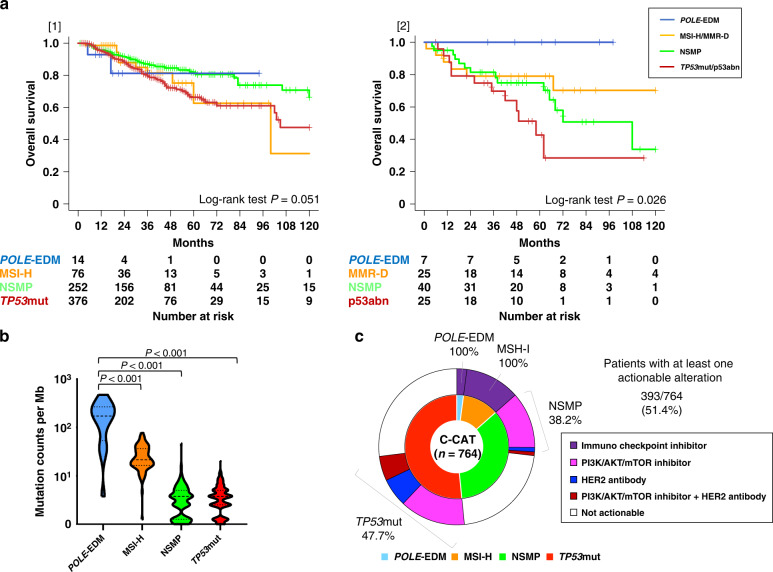
The survival probabilities for the entire C-CAT cohort of endometrial cancer patients are shown in Supplementary Fig. S1. In patients with endometrial cancer who had recurrence or progression, OS showed no significant difference among the four subtypes (Fig. 4a [1]). Among patients with advanced disease (FIGO Stage III–IV) who underwent initial surgery, OS was classified into four subtypes (Fig. 4a [2]). The tumour mutation burden (TMB), obtained from C-CAT data, was significantly higher in the POLE-EDM group than in the other three groups (Fig. 4b). Among the 764 patients in the C-CAT cohort, there were 393 patients (51.4%) with at least one actionable alteration (Fig. 4c).
Fig. 4. Patient outcomes and therapeutic targets in the C-CAT cohort.
a Kaplan–Meier survival curves. [1] OS according to molecular subtype in the C-CAT cohort of POLE-EDM (blue line), MSI-H (orange line), NSMP (green line), and TP53mut (red line). [2] OS according to molecular subtype in advanced Stages (III–IV) among the NCCH cohort of POLE-EDM (blue line), MMR-D (orange line), NSMP (green line), and p53abn (red line). b Distribution of the tumour mutation burden by four subtypes in the C-CAT cohort. c Frequency of actionable mutations by subtypes in the C-CAT cohort.
Comparison of molecular subtypes and mutation patterns between patients who underwent initial surgery and patients with recurrence or progression
In patients with recurrence or progression, the frequency of occurrence of POLE-EDM in the C-CAT cohort was significantly lower than that in the NCCH cohort, in contrast to the frequency of TP53mut, which was significantly higher (2.1% vs. 13.6% and 51.2% vs. 21.1%, respectively; P < 0.001; Fig. 5a). The patients with recurrence or progression had a significantly lower frequency of PTEN and ARID1A mutations and higher frequency of TP53mut than patients who underwent initial surgery (Fig. 5b). Conversely, the frequency of PIK3CA, KRAS and CTNNB1 mutations was not different between the two cohorts.
Fig. 5. Differences in molecular subtypes and gene frequencies between the NCCH and C-CAT cohorts.
a Frequency of molecular subtypes in the NCCH cohort (initial surgery patients) vs. the C-CAT (recurrence or progression patients). b Differences between the two cohorts of recurrent mutant genes. Asterisks indicate genes with significant differences between the two groups.
The genetic alterations by cohort in Japanese patients with POLE-EDM endometrial cancer are shown in Supplementary Fig. S8A. The frequency of gene mutations did not differ between the two cohorts (Supplementary Fig. S8B). Fifty-six of the 764 patients from the C-CAT cohort had POLE mutations, including 16 with mutations in the exonuclease domain (P286R, 6 patients; V411L, 6 patients; S297F, 1 patient; P436R, 1 patient; A456P, 1 patient; S459F, 1 patient), and 40 with mutations in the non-exonuclease domain (Supplementary Fig. S9A). In the C-CAT cohort, there was no significant difference in OS between patients with exonuclease mutations in POLE and those with non-exonuclease mutations in POLE (P = 0.733) (Supplementary Fig. S9B). OS rates according to molecular subtype for all patients by each cohort are shown in Supplementary Fig. S10. The analysis of the correlation between molecular subtype and outcome in both cohorts, excluding histologic types for which the molecular classification algorithm is not valid (carcinosarcoma, undifferentiated/differentiated carcinoma, neuroendocrine carcinoma, and carcinomas of unknown histology), showed that endometrial carcinoma with abnormal p53 expression/TP53 mutation exhibited poor prognostic behaviour (Supplementary Fig. S11).
Discussion
We demonstrated that molecular classification can be useful for determining prognosis in patients with endometrial cancer who underwent initial surgery, regardless of ethnicity. As previously reported, endometrial cancer with p53abn was associated with an unfavourable prognosis, and since the p53abn was a poor prognostic factor independent of FIGO stage and histological grade, the molecular classifier system included prognostic factors independent of the stages and histological types. Patients with NSMP (as identified by an IHC-based on molecular classifier) endometrial cancer with mutant KRAS and wt ARID1A showed poor RFS compared with those with other KRAS/ARID1A statuses. Conversely, there were significantly fewer patients classified as POLE-EDM and significantly more patients classified as TP53mut among patients with recurrence or progression than among those who underwent initial surgery, and the frequency of molecular subtypes was clearly different between the two groups. Furthermore, in patients with endometrial cancer with recurrence or progression, the molecular subclassification based on comprehensive genomic analysis in the C-CAT database could enable appropriate treatment of recurrence, and ~50% of patients may be eligible for molecularly targeted therapies such as immune checkpoint inhibitors.
Somatic alterations in the exonuclease domain of POLE occur in a subgroup of endometrial cancers with ultra-mutation (frequently ≥100 mutations/Mb) [9] and excellent clinical outcome [9, 11–13]; the former was replicated in the C-CAT cohort and the latter in the NCCH cohort. The high mutation burden results in an enriched antigenic neoepitope and enhanced anti-tumour immune response, suggesting a favourable prognosis for this subtype [36, 37]. We first reported that the frequency of POLE-EDM was significantly lower in the recurrence or progression group than in the initial surgery group, suggesting that there may not be a good prognosis after recurrence. Since only 2.1% of patients with endometrial cancer with recurrence or progression presented with POLE-EDM tumour characteristic behaviour, these findings support the omission of adjuvant treatment and a decrease in surveillance for Asian patients with POLE-EDM endometrial cancer. In addition, patients with POLE-EDM endometrial cancer have significantly higher TMB than those with other subtypes; therefore, appropriate treatment such as immune checkpoint inhibitor therapy may improve prognosis [38].
Patients with MMR-D endometrial cancer had an intermediate prognosis in the NCCH cohort. Both POLE, which controls base incorporation and proofreading, and the MMR system, which monitors post-replication, play central roles in facilitating accurate DNA replication [39]; therefore, somatic mutations in POLE- or MMR-related genes (MLH1, MSH2, MSH6 and PMS2) result in DNA replication repair deficiency. However, endometrial cancers with MMR-D were reported to have lower TMB and neoantigen loads than those with POLE mutations [36], which might explain the difference in prognosis between the two groups. Pembrolizumab, an anti-PD-1 monoclonal antibody, demonstrated robust and durable anti-tumour activity with manageable toxicity and promising survival in patients with advanced MSI-H/MMR-D endometrial cancer [40].
Among the four groups in the NCCH cohort, the highest number of patients were classified in the NSMP group, with no molecular characteristics for molecular classification. In the NCCH cohort, we identified mutant KRAS and wt ARID1A as novel biomarkers associated with poor prognosis in the NSMP group. Mutant KRAS and wt ARID1A may identify high-grade or advanced-stage disease characterised by aggressive behaviour in the NSMP population. KRAS mutations are found in 10–30% of well-differentiated endometrial cancers and are known for their role as an early checkpoint in the transition from hyperplasia to cancer [41, 42] and as markers of invasive potential in well-differentiated tumours [42, 43]. However, there is no consensus on how KRAS mutation affects the prognosis of endometrial cancers, with only a few reports associating it with poor prognosis [44] and aggressive clinical behaviour [45]. ARID1A mutations are frequently detected in patients with endometrial cancer, and cell line assays have indicated increased tumorigenicity when ARID1A mutations are combined with other genomic abnormalities [46, 47]. However, a study that used The Cancer Genome Atlas dataset reported that ARID1A mutation alone was associated with better prognosis in patients with endometrial cancer compared to wt ARIDIA [47]. These mechanisms need to be elucidated in the future. A total of 38.2% of patients with recurrence or progression classified as NSMP had PIK3CA oncogenic mutations or ERBB2 alterations, suggesting that they may be candidates for molecular targeted therapies such as PI3K/AKT/mTOR inhibitors and HER2 antibody drugs.
Patients with p53abn endometrial cancer had significantly worse clinical outcomes than those with other molecular subtypes in the NCCH cohort. However, patients with endometrial cancer with p53abn with POLE-EDM or MMR-D have been reported to have a favourable prognosis [14]. TP53mut is associated with more aggressive tumours and poor overall outcome in various cancer types compared to wt TP53 [48], including endometrial cancers, and ~27.8% of patients with endometrial cancer have been reported to have TP53mut regardless of histological type [49]. In a comparison between the C-CAT and NCCH cohorts, the percentage of TP53 abnormalities was higher in patients with metastatic progression or recurrence (51.6%) than in those who underwent initial surgery (21.1%). Patients with TP53mut exhibited a trend toward a worse prognosis than those with other subtypes in the recurrence or progression patient cohort. This suggests that patients with TP53 abnormalities should be carefully surveilled, considering their susceptibility to recurrence.
In the C-CAT cohort, approximately half of the patients with TP53mut (47.7%) had at least one genetic alteration that could be a potential therapeutic target. Although there have been few studies on molecular targeted therapy for patients carrying TP53mut, a therapy for targeting a neoantigen derived from a common TP53mut has been reported [50]. Recently, Leslie et al. found that bevacizumab plus chemotherapy more significantly improved OS in patients with advanced endometrial cancer with TP53mut compared with those without TP53mut [51]. These results indicate that precision medicine for uterine cancer is expected to advance in the future.
The sensitivity, specificity, and diagnostic odds ratio for detecting abnormal TP53 by IHC in this study were 0.60 (95% CI 0.54–0.65), 0.92 (95% CI 0.87–0.95) and 16.2 (95% CI 8.05–32.5), respectively. Compared to the results of previous studies [34], the sensitivity of p53 IHC appears to be low, and a combination of factors may explain this finding. We analysed frozen tumour tissue specimens that were different from the tumour section used for p53 IHC in 18 of 265 cases because of low-quality DNA from the FFPE sample. In these cases, the tumour section used for IHC was different from the tumour tissue used for TP53 sequencing; therefore, intratumoral heterogeneity of TP53 mutations may result in a discrepancy between p53 status determined by IHC and the TP53 sequencing results. The small number of subclones with TP53 mutations, the small number of cases with TP53 mutations showing wild-type staining patterns in IHC, and technical issues with IHC may also explain this discrepancy.
We consider that the inability of the molecular classification of the C-CAT cohort to predict prognosis is explained by the fact that patients with Stage I–II endometrioid endometrial carcinoma do not qualify for sequencing by the C-CAT programme because of lack of recurrence or progression of the disease. The prognostic impact of molecular classification of recurrent/advanced endometrial cancer requires further investigation. Moreover, it is important to note that POLE-EDM tumour is not a favourable prognostic factor in advanced stage and metastatic disease. In aggressive tumours, we speculate that as the tumour progresses, the clones survive by acquiring favourable features for survival, such as tumour immune escape mechanisms; however, further studies are required to determine the reason for this finding.
In conclusion, endometrial cancer molecular subtypes represent a useful classification system that evaluates tumour characteristics regardless of ethnicity, with the potential to predict the prognosis of patients who underwent initial surgery and determine the usefulness of appropriate molecular targeted therapy for patients with recurrence or progression. Furthermore, patients with NSMP endometrial cancer and combined mutant KRAS and wt ARID1A who underwent initial surgery have a poor prognosis. These results indicate that molecular classification can distinguish patients with similar histological features but different prognoses, as well as guide therapeutic strategies and appropriate surveillance.
Supplementary information
Acknowledgements
The authors are grateful to Hitoshi Ichikawa, Sachiyo Mitani, and to other physicians and staff at the National Cancer Center and other hospitals for their help and support. We would like to thank Editage (https://www.editage.jp) for English language editing.
Author contributions
YA, KS, TK and HY designed the study. YA wrote the initial draft of the manuscript. YA, KS, MM, YS and HY contributed to the analysis and interpretation of the data and assisted in manuscript preparation. All the other authors contributed to the data collection, interpretation and critical review of the manuscript. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the study. We will ensure that all questions related to the accuracy or integrity of any part of the study are appropriately investigated and resolved.
Funding
This work was supported by a Grant-in-Aid for Young Scientists (B) Number 20K18207 and 19K16572, Grant-in-Aid for Scientific Research (C) Number 20K09636, and National Cancer Center Research and Development Fund (2020-J-2, NCC Biobank and NCC Core Facility).
Data availability
The data generated in this study are available upon reasonable request from the corresponding authors.
Ethics approval and consent to participate
Institutional Review Board Statement: The study protocol was approved by the Institutional Review Board (IRB) of the National Cancer Center (NCC) (approval number 2017-331), and the study was conducted in accordance with the ethical guidelines of the Helsinki Declaration. C-CAT data were accessed through the C-CAT Research-Use Portal site after obtaining approval from the NCC-IRB (approval number 2020-067) and the C-CAT Data Utilization Review Board (approval number CDU2021-001N).
Consent to publish
Informed consent was given by patients diagnosed with endometrial carcinoma between 2000 and 2018, for the use of their samples in research, at their first visit to our hospital. Information derived from our research, using the samples collected after informed consent was obtained, has been summarised on our hospital website. Patients were free to revoke their consent at any time point. In this study, only samples from patients who did not revoke their consent were used. We also informed patients treated before the year 2000 that the information summary of the study is published on the official hospital website. Patients who refused to provide consent for the use of their residual samples were excluded from this study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kouya Shiraishi, Email: kshirais@ncc.go.jp.
Hiroshi Yoshida, Email: hiroyosh@ncc.go.jp.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-023-02203-3.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Katanoda K, Hori M, Saito E, Shibata A, Ito Y, Minami T, et al. Updated trends in cancer in Japan: incidence in 1985-2015 and mortality in 1958-2018-A sign of decrease in cancer incidence. J Epidemiol. 2021;31:426–50. doi: 10.2188/jea.JE20200416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005;366:491–505. doi: 10.1016/S0140-6736(05)67063-8. [DOI] [PubMed] [Google Scholar]
- 4.Odagiri T, Watari H, Hosaka M, Mitamura T, Konno Y, Kato T, et al. Multivariate survival analysis of the patients with recurrent endometrial cancer. J Gynecol Oncol. 2011;22:3–8. doi: 10.3802/jgo.2011.22.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Concin N, Matias-Guiu X, Vergote I, Cibula D, Mirza MR, Marnitz S, et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer. 2021;31:12–39. doi: 10.1136/ijgc-2020-002230. [DOI] [PubMed] [Google Scholar]
- 6.Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, et al. Uterine neoplasms, version 1.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:170–99.. doi: 10.6004/jnccn.2018.0006. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton CA, Pothuri B, Arend RC, Backes FJ, Gehrig PA, Soliman PT, et al. Endometrial cancer: a society of gynecologic oncology evidence-based review and recommendations. Gynecol Oncol. 2021;160:817–26.. doi: 10.1016/j.ygyno.2020.12.021. [DOI] [PubMed] [Google Scholar]
- 8.Hamilton CA, Pothuri B, Arend RC, Backes FJ, Gehrig PA, Soliman PT, et al. Endometrial cancer: a society of gynecologic oncology evidence-based review and recommendations, part II. Gynecol Oncol. 2021;160:827–34. doi: 10.1016/j.ygyno.2020.12.024. [DOI] [PubMed] [Google Scholar]
- 9.Cancer Genome Atlas Research N, Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stelloo E, Bosse T, Nout RA, MacKay HJ, Church DN, Nijman HW, et al. Refining prognosis and identifying targetable pathways for high-risk endometrial cancer; a TransPORTEC initiative. Mod Pathol. 2015;28:836–44. doi: 10.1038/modpathol.2015.43. [DOI] [PubMed] [Google Scholar]
- 11.Stelloo E, Nout RA, Osse EM, Jurgenliemk-Schulz IJ, Jobsen JJ, Lutgens LC, et al. Improved risk assessment by integrating molecular and clinicopathological factors in early-stage endometrial cancer-combined analysis of the PORTEC cohorts. Clin Cancer Res. 2016;22:4215–24. doi: 10.1158/1078-0432.CCR-15-2878. [DOI] [PubMed] [Google Scholar]
- 12.Talhouk A, McConechy MK, Leung S, Li-Chang HH, Kwon JS, Melnyk N, et al. A clinically applicable molecular-based classification for endometrial cancers. Br J Cancer. 2015;113:299–310. doi: 10.1038/bjc.2015.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talhouk A, McConechy MK, Leung S, Yang W, Lum A, Senz J, et al. Confirmation of ProMisE: a simple, genomics-based clinical classifier for endometrial cancer. Cancer. 2017;123:802–13. doi: 10.1002/cncr.30496. [DOI] [PubMed] [Google Scholar]
- 14.Leon-Castillo A, Gilvazquez E, Nout R, Smit VT, McAlpine JN, McConechy M, et al. Clinicopathological and molecular characterisation of ‘multiple-classifier’ endometrial carcinomas. J Pathol. 2020;250:312–22. doi: 10.1002/path.5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kommoss S, McConechy MK, Kommoss F, Leung S, Bunz A, Magrill J, et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Ann Oncol. 2018;29:1180–8. doi: 10.1093/annonc/mdy058. [DOI] [PubMed] [Google Scholar]
- 16.Abdulfatah E, Wakeling E, Sakr S, Al-Obaidy K, Bandyopadhyay S, Morris R, et al. Molecular classification of endometrial carcinoma applied to endometrial biopsy specimens: towards early personalized patient management. Gynecol Oncol. 2019;154:467–74. doi: 10.1016/j.ygyno.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Britton H, Huang L, Lum A, Leung S, Shum K, Kale M, et al. Molecular classification defines outcomes and opportunities in young women with endometrial carcinoma. Gynecol Oncol. 2019;153:487–95. doi: 10.1016/j.ygyno.2019.03.098. [DOI] [PubMed] [Google Scholar]
- 18.Le H, Ziogas A, Taylor TH, Lipkin SM, Zell JA. Survival of distinct Asian groups among colorectal cancer cases in California. Cancer. 2009;115:259–70. doi: 10.1002/cncr.24034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soo RA, Loh M, Mok TS, Ou SH, Cho BC, Yeo WL, et al. Ethnic differences in survival outcome in patients with advanced stage non-small cell lung cancer: results of a meta-analysis of randomized controlled trials. J Thorac Oncol. 2011;6:1030–8. doi: 10.1097/JTO.0b013e3182199c03. [DOI] [PubMed] [Google Scholar]
- 20.Plaxe SC, Saltzstein SL. Impact of ethnicity on the incidence of high-risk endometrial carcinoma. Gynecol Oncol. 1997;65:8–12. doi: 10.1006/gyno.1996.4594. [DOI] [PubMed] [Google Scholar]
- 21.Connell PP, Rotmensch J, Waggoner SE, Mundt AJ. Race and clinical outcome in endometrial carcinoma. Obstet Gynecol. 1999;94:713–20. doi: 10.1016/s0029-7844(99)00381-6. [DOI] [PubMed] [Google Scholar]
- 22.Mahdi H, Schlick CJ, Kowk LL, Moslemi-Kebria M, Michener C. Endometrial cancer in Asian and American Indian/Alaskan Native women: tumor characteristics, treatment and outcome compared to non-Hispanic white women. Gynecol Oncol. 2014;132:443–9. doi: 10.1016/j.ygyno.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 23.Francis SR, Ager BJ, Do OA, Huang YJ, Soisson AP, Dodson MK, et al. Recurrent early stage endometrial cancer: patterns of recurrence and results of salvage therapy. Gynecol Oncol. 2019;154:38–44. doi: 10.1016/j.ygyno.2019.04.676. [DOI] [PubMed] [Google Scholar]
- 24.Wylie J, Irwin C, Pintilie M, Levin W, Manchul L, Milosevic M, et al. Results of radical radiotherapy for recurrent endometrial cancer. Gynecol Oncol. 2000;77:66–72. doi: 10.1006/gyno.2000.5727. [DOI] [PubMed] [Google Scholar]
- 25.Hirose S, Murakami N, Takahashi K, Kuno I, Takayanagi D, Asami Y, et al. Genomic alterations in STK11 can predict clinical outcomes in cervical cancer patients. Gynecol Oncol. 2020;156:203–10. doi: 10.1016/j.ygyno.2019.10.022. [DOI] [PubMed] [Google Scholar]
- 26.Kuno I, Takayanagi D, Asami Y, Murakami N, Matsuda M, Shimada Y, et al. TP53 mutants and non-HPV16/18 genotypes are poor prognostic factors for concurrent chemoradiotherapy in locally advanced cervical cancer. Sci Rep. 2021;11:19261. doi: 10.1038/s41598-021-98527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murakami N, Asami Y, Yoshida H, Takayanagi D, Hirose S, Kuno I, et al. Distribution of genetic alterations in high-risk early-stage cervical cancer patients treated with postoperative radiation therapy. Sci Rep. 2021;11:10567. doi: 10.1038/s41598-021-90139-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takayanagi D, Hirose S, Kuno I, Asami Y, Murakami N, Matsuda M, et al. Comparative analysis of genetic alterations, HPV-status, and PD-L1 expression in neuroendocrine carcinomas of the cervix. Cancers. 2021;13:1215. doi: 10.3390/cancers13061215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cingolani P, Platts A, Wang le L, Coon M, Nguyen T, Wang L, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly. 2012;6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landrum MJ, Lee JM, Benson M, Brown GR, Chao C, Chitipiralla S, et al. ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2018;46:D1062–D7.. doi: 10.1093/nar/gkx1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chakravarty D, Gao J, Phillips SM, Kundra R, Zhang H, Wang J, et al. OncoKB: a precision oncology knowledge base. JCO Precis Oncol. 2017;1:1–16. [DOI] [PMC free article] [PubMed]
- 32.Rayner E, van Gool IC, Palles C, Kearsey SE, Bosse T, Tomlinson I, et al. A panoply of errors: polymerase proofreading domain mutations in cancer. Nat Rev Cancer. 2016;16:71–81. doi: 10.1038/nrc.2015.12. [DOI] [PubMed] [Google Scholar]
- 33.Kohno T, Kato M, Kohsaka S, Sudo T, Tamai I, Shiraishi Y, et al. C-CAT: the national datacenter for cancer genomic medicine in Japan. Cancer Discov. 2022;12:2509–15. doi: 10.1158/2159-8290.CD-22-0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh N, Piskorz AM, Bosse T, Jimenez-Linan M, Rous B, Brenton JD, et al. p53 immunohistochemistry is an accurate surrogate for TP53 mutational analysis in endometrial carcinoma biopsies. J Pathol. 2020;250:336–45. doi: 10.1002/path.5375. [DOI] [PubMed] [Google Scholar]
- 35.Raffone A, Travaglino A, Cerbone M, De Luca C, Russo D, Di Maio A, et al. Diagnostic accuracy of p53 immunohistochemistry as surrogate of TP53 sequencing in endometrial cancer. Pathol Res Pr. 2020;216:153025. doi: 10.1016/j.prp.2020.153025. [DOI] [PubMed] [Google Scholar]
- 36.Howitt BE, Shukla SA, Sholl LM, Ritterhouse LL, Watkins JC, Rodig S, et al. Association of polymerase e-mutated and microsatellite-instable endometrial cancers with neoantigen load, number of tumor-infiltrating lymphocytes, and expression of PD-1 and PD-L1. JAMA Oncol. 2015;1:1319–23. doi: 10.1001/jamaoncol.2015.2151. [DOI] [PubMed] [Google Scholar]
- 37.van Gool IC, Eggink FA, Freeman-Mills L, Stelloo E, Marchi E, de Bruyn M, et al. POLE proofreading mutations elicit an antitumor immune response in endometrial cancer. Clin Cancer Res. 2015;21:3347–55. doi: 10.1158/1078-0432.CCR-15-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Das A, Sudhaman S, Morgenstern D, Coblentz A, Chung J, Stone SC, et al. Genomic predictors of response to PD-1 inhibition in children with germline DNA replication repair deficiency. Nat Med. 2022;28:125–35. doi: 10.1038/s41591-021-01581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cortez D. Replication-coupled DNA repair. Mol Cell. 2019;74:866–76.. doi: 10.1016/j.molcel.2019.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Malley DM, Bariani GM, Cassier PA, Marabelle A, Hansen AR, De Jesus Acosta A, et al. Pembrolizumab in patients with microsatellite instability-high advanced endometrial cancer: results from the KEYNOTE-158 study. J Clin Oncol. 2022;40:752–61. doi: 10.1200/JCO.21.01874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banno K, Yanokura M, Iida M, Masuda K, Aoki D. Carcinogenic mechanisms of endometrial cancer: involvement of genetics and epigenetics. J Obstet Gynaecol Res. 2014;40:1957–67. doi: 10.1111/jog.12442. [DOI] [PubMed] [Google Scholar]
- 42.Sideris M, Emin EI, Abdullah Z, Hanrahan J, Stefatou KM, Sevas V, et al. The role of KRAS in endometrial cancer: a mini-review. Anticancer Res. 2019;39:533–9. doi: 10.21873/anticanres.13145. [DOI] [PubMed] [Google Scholar]
- 43.Tsuda H, Jiko K, Yajima M, Yamada T, Tanemura K, Tsunematsu R, et al. Frequent occurrence of c-Ki-ras gene mutations in well differentiated endometrial adenocarcinoma showing infiltrative local growth with fibrosing stromal response. Int J Gynecol Pathol. 1995;14:255–9. doi: 10.1097/00004347-199507000-00010. [DOI] [PubMed] [Google Scholar]
- 44.Mizuuchi H, Nasim S, Kudo R, Silverberg SG, Greenhouse S, Garrett CT. Clinical implications of K-ras mutations in malignant epithelial tumors of the endometrium. Cancer Res. 1992;52:2777–81. [PubMed] [Google Scholar]
- 45.Ito K, Watanabe K, Nasim S, Sasano H, Sato S, Yajima A, et al. K-ras point mutations in endometrial carcinoma: effect on outcome is dependent on age of patient. Gynecol Oncol. 1996;63:238–46. doi: 10.1006/gyno.1996.0313. [DOI] [PubMed] [Google Scholar]
- 46.Reske JJ, Wilson MR, Holladay J, Siwicki RA, Skalski H, Harkins S, et al. Co-existing TP53 and ARID1A mutations promote aggressive endometrial tumorigenesis. PLoS Genet. 2021;17:e1009986. doi: 10.1371/journal.pgen.1009986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen J, Ju Z, Zhao W, Wang L, Peng Y, Ge Z, et al. ARID1A deficiency promotes mutability and potentiates therapeutic antitumor immunity unleashed by immune checkpoint blockade. Nat Med. 2018;24:556–62. doi: 10.1038/s41591-018-0012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333–9. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Momeni-Boroujeni A, Dahoud W, Vanderbilt CM, Chiang S, Murali R, Rios-Doria EV, et al. Clinicopathologic and genomic analysis of TP53-mutated endometrial carcinomas. Clin Cancer Res. 2021;27:2613–23. doi: 10.1158/1078-0432.CCR-20-4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hsiue EH, Wright KM, Douglass J, Hwang MS, Mog BJ, Pearlman AH, et al. Targeting a neoantigen derived from a common TP53 mutation. Science. 2021;371:eabc8697. doi: 10.1126/science.abc8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leslie KK, Filiaci VL, Mallen AR, Thiel KW, Devor EJ, Moxley K, et al. Mutated p53 portends improvement in outcomes when bevacizumab is combined with chemotherapy in advanced/recurrent endometrial cancer: an NRG Oncology study. Gynecol Oncol. 2021;161:113–21.. doi: 10.1016/j.ygyno.2021.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are available upon reasonable request from the corresponding authors.