Abstract
The consumption of edible insects can be anadvantageous alternative to the conventional food supply chain, which involves global water waste, land deficit, undernutrition, and starvation. Besides the nutritional aspects, insect proteins have demonstrated a wide range of functional properties such as foamability, emulsifying and gelling abilities. The protein content and amino acid profile of some insects have revealed a good nutritional value and interesting functional properties. However, it is crucial to comprehend how the protein quality is affected by insect feeding, drying, and defatting. There is a knowledge gap about the impact of industrial treatment, such as pH, ionic strength, and heat treatment, on insect proteins' functional properties. In this review, we have aimed to highlight the potential application of insect proteins as a nutritional source and their promising technological applications. The study reported the principal insect protein characterization methodologies that have been investigated in the literature aiming to correlate the physicochemical parameters to possible protein functionalities. The research on the functional properties of insect proteins is at the exploratory level. Further detailed studies are needed to clarify the structure-function relation of insect proteins and how these functionalities and insect processing can increase consumer acceptance.
Keywords: Edible insect, Insect protein, Techno-functionality, Protein extraction, Sustainability
Graphical abstract
Highlights
-
•
SC-CO2 and HHP are promising defatting methods compared to solvent extraction.
-
•
Insect protein extracts showed greater WHC/OHC than insect flour.
-
•
Insect proteins show promising gelling capacity and strength at neutral and alkaline pH.
-
•
There is an improvement in colloidal properties when protein extract is used instead of flour.
1. Introduction
The world's population counts more than 7.8 billion people, and in 2050 this number is expected to reach around the 10 billion [1]. The increased population and the higher food demand inevitably impact agricultural and livestock growth, which elevates deforestation, water consumption, and greenhouse gas emission [2]. Even with an inefficient feed conversion rate the FAO (2011) projects that the consumption of livestock meat is expected to increase around 173% until 2050 [3]. The reported numbers highlight the importance of adopting a more balanced and sustainable food source, focusing on biodiversity, food safety, and more effective distribution of high-quality proteins for the world's population [4,5].
Some examples of emergent and sustainable food sources include vegetables (e.g. wheat, chia, moringa, beans, lentils), microorganisms (e.g., fungi and microalgae), and insects (Hermetia illucens, Tenebrio molitor, etc.). In recent years, plant-based products have gained great popularity mainly due to consumers’ growing interest in a more ecofriendly diet [6]. Soy proteins are an excellent example of plant-based products that have been produced for the fabrication of simulated meats and tofu [7,8].
Nonetheless, proteins from plants have been found to lack certain essential amino acids, mainly leucine, methionine, and lysine [9] and to show less digestibility, compared to animal-based proteins, which makes it necessary to combine various plant-based sources to obtain a complete and nutritious diet [9].
Insects are invertebrate animals that belong to the Phylum Arthropoda. Their biomass represents 95% of the animal kingdom with enormous biodiversity; however, only a few studies have reported their application in food systems for nutritional and technological aspects. Insects are a promising source of proteins with nutritional quality comparable to that offered by cattle [10]. In addition, insect farming presents a substantially smaller impact on the environment in terms of land, water usage, deforestation, and greenhouse gas production [11]. Part of this advantage comes from the efficient feed-to-meat conversion ratio of insects which, in the case of crickets, only 2.2 kg of feed is required to produce 1 kg of edible insect [12,13].
Some organizations have been evaluating the prospect of using insects as food sources and feed. The Food and Agricultural Organization of the United Nations (FAO) has extensively studied the advantages of consuming insects, considering the cultural, economic, safety, production, and nutritional aspects. In general, insect proteins meet all the requirements of the WHO for amino acid composition showing high values for phenylalanine, tyrosine, tryptophane, lysine, and threonine [13]. The insect order Orthoptera is one of the most valuable alternative protein sources. Most edible insects in this order fulfill the required essential amino acids for human consumption [14,15].
Although insects as alternative human food can bring all the reported advantages, consumers’ acceptance remains the most significant barrier to be overcome since entomophagy is constantly associated with disgusting feelings and diseases [54,83]. Therefore, an excellent way to improve insect acceptance by consumers would be to process the whole insect as insect flour or protein concentrate powder to be further implemented as a food ingredient.
The presence of legislation that supports the production and marketing of insects as human food is another fact that can affect consumers' acceptance. Recently, Tenebrio molitor, a beetle of the Tenebrionidae family, has been the first authorized insect to be used as a novel food for human consumption. This fact can be considered an advantageous breakthrough made by the Commission Implementing Regulation 2021/882, which opens new market opportunities and can increase people's interest in insects as food ingredients [16].
Beyond nutritional aspects, insect species can provide proteins to be further explored for their functionalities. For that, it is crucial to understand that the physicochemical properties of these proteins and their conformation will play a fundamental role in their functionality and that those features can be modified by the processing parameters used before and after the protein extraction [11,17].
Recent studies have unveiled the technological potential of insect proteins by exploiting their solubility, gelling ability, foamability, and emulsifying properties. Complex studies on protein characterization and their physicochemical properties have been evaluated to address these colloidal properties. The main goal is to determine how conventional and new technologies can affect the main functionalities of insect proteins to be further implemented in food [15,[17], [18], [19], [20], [21], [22]].
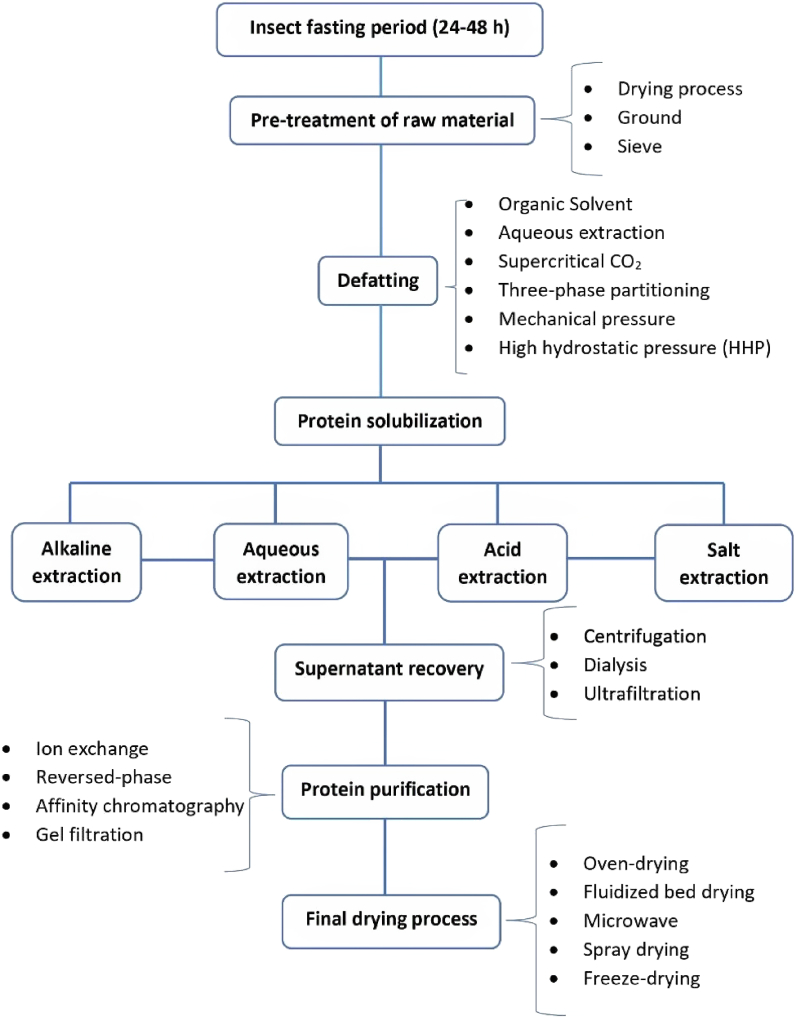
Gravel & Doyen [7] have described a standard 5-step food protein processing starting with pre-treatment, defatting, protein solubilization/recovery, protein purification, and finally, the drying process as the fifth step. An extra additional step can be added at the beginning, the insect farming, feeding, and fasting, as they might affect the future explored functionalities and insect protein content (Fig. 1). It is important to notice that all reported steps are optional and will depend on the product's starting material and final purpose.
Fig. 1.
Flowchart of insect processing for protein extraction.
To support the studies, this review aimed to highlight that insects may be an interesting protein-enriched source with good technological attributes. Moreover, the present work discussed the importance of different methods applied for protein extraction and their effects on protein quality and yield of extraction. The focus was also to discuss how some pre-treatments such as salt addition, pH alteration, and heat, when applied to protein matrix, can impact the protein extraction efficiency and their colloidal properties to be used in food.
2. Insect processing methods applied for protein extraction
2.1. Insect feed and fasting time
Black soldier fly larvae (BSFL) are considered the insect converter of organic waste into edible biomass, and the composition of such material relies on the substrate. Spranghers et al. [23] have grown BSFL on four substrates: chicken feed, vegetable waste, biogas digestate, and restaurant waste. The authors reported that the total biomass of the harvested prepupae differed substantially among the four substrates, where the total prepupal biomass was highest for chicken feed and the lowest for biogas digested. In addition, the slow development of larvae feeding on restaurant waste was observed. Protein content varied from 399 to 431 g/kg of dry matter among the four feeds. Differences between amino acid composition were not significant but, ether extract and ash content significantly changed.
A research performed by Fuso et al. [24] evaluated the nutritional composition of BSF reared on vegetable mixture. 49 different substrates were divided into three groups according to their seasonal availability. Protein content of BSF prepupae ranged between 35 and 49% of dry matter, where the lowest content was detected for insects fed with fruit by-products and the highest when autumnal leftovers were used.
Considerable effort has been placed on the development of an optimized diet for insects that would provide a maximized biomass with highly nutritious larvae. A study performed by Rumbos et al. [25] evaluated the usage of various commodities for the development and nutritional quality of Tenebrio molitor. The work stated that protein content of T. molitor fed on the different substrates tested ranged between 41 and 68% and the lipid content also from 14 to 38%. Interestingly the study showed through linear regression analysis that the protein content in the tested substrates did not show a strong correlation with the protein content of the larvae.
Insect fasting time is another parameter to be considered before handling insects for processing. This process is important as the number of contaminants can be reduced to a slight trace and then not interfere with further analysis and protein composition [22]. To avoid this contaminant, [21]; reared in a controlled environment adult grasshopper (S. gregaria), the larvae, and honeybee pupae (Apis mellifera). Before the protein extraction, the insects were fasted for at least 24 h. A similar procedure was performed by Ref. [26] when larvae of Protaetia brevitarsis were fasted for 24 h. The time duration of 24 h fastening has been reported as the most efficient to obtain the insect gut clear of gastrointestinal feces and any other residues that can compromise the nutritional composition.
2.2. Pre-treatment
Before the protein extraction step, the raw material needs to be pre-treated in order to reduce moisture and produce a finer powder. These initial steps typically improve the efficiency of the next applied processing. For instance, in legumes, this initial step can increase protein content as most fiber-rich parts are removed [7]. As insects are also full of fibers, they can be directly compared to legumes. Drying can increase the shelf-life of foods by reducing water content and, consequently, their availability for degradative reactions [27].
Therefore, in the first step, insects are usually dried, ground, and finally sieved to produce a fine powder that provides a larger surface area of contact with the solvents [7,22,28,29]. The drying step can occur either in the beginning or at the last stage of the protein isolation/purification process. A review performed by Melgar-Lalanne et al. [27] reported the application of a range of drying processing methods on different insects as raw materials, including conventional and modern methods such as solar drying and freeze-drying, respectively. The Freeze-drying treatment is a common method applied for insect drying in lab scales. Through this process, frozen specimens are dehydrated by sublimation and vacuum, with no significant changes in color, flavor, smells, and physical form [30]. These drying methods are constantly evaluated in order to verify their impact on the insect nutritional, chemical and functional characteristics.
Different drying technologies were applied to mealworm (Tenebrio molitor) in order to study their influence on the stability of nutrients and the possible lipid oxidation. The study reported that the methods could potentially alter nutritional components, and protein solubility was reduced when microwave, fluidized bed, and drying with vacuum were used. Lipid oxidation was also higher, considering the high production of 4-hydroxy-2-nonenal (4-HNE), for freeze-dried samples. The authors pointed out microwave and vacuum oven as advantageous alternatives compared to conventional methods for mealworm [31]. Fuso et al. [32] evaluated the nutritional composition and physicochemical parameters of Hermetia illucens dried by conventional and microwave drying methods. The authors stated that microwave drying influenced the amino acid composition and had a more compact product with larger particle size. Samples dried by microwave also had the lowest digestibility and digestible indispensable amino acid score (DIAAS).
Finally, spray drying has been largely used for protein solutions or suspensions, basically, the technique requires proteins to be atomized into a small droplet and pass through a hot air fed that dries the samples. Only a few studies have documented this technique as insect drying pre-treatment [33]. In another study, spray-dried mealworm powder was produced, the data showed some physical efficiency for particle formation, as it allowed the formation of a rounder shape powder with high homogeneity of particle size [34].
2.3. Insect powder defatting step
Defatting is typically considered a pre-step for protein extraction, as it cleans the flour, optimizing the yield of protein extraction. Different methods of defatting can yield protein extracts with different techno-functional properties. Hexane has been a commonly used solvent in defatting; however, it has become undesirable due to its negative environmental impact, safety issues, and effects on protein functionality [35]. A work by Kim et al. [36] evaluated the impact of aqueous fat removal and defatting by organic solvents, such as hexane, methanol, and ethanol, on Protaetia brevitarsis samples. The experiment intended to understand how the two approaches may affect insect proteins’ nutritious, color, and techno-functional elements. The amino acid composition, protein solubility, and functional properties (foamability, and emulsifying capacity) had the most interesting result when proteins were defatted with hexane. Conversely [37], studied Acheta domesticus. They stated that the aqueous extraction was more interesting when the techno-functionality was aimed. Hexanic extraction showed to be more promising considering the nutritional aspects. In addition, the efficiency of defatting with aqueous extraction was compared to a range of organic solvent-based extraction methods for different insects. The authors stated that aqueous extraction gave the lowest oil yield of all reported techniques [38].
Supercritical CO2 (SC–CO2) extraction has been used as an alternative defatting method for silkworm chrysalis [39]. According to Ref. [40] SC-CO2 method showed promising results giving 95% oil recovery for T. molitor. When compared to conventional solvent extraction, SC-CO2 showed some advantages such as reduced sample oxidation, the possibility to extract thermolabile metabolites and obtention of solvent-free residues [40,41].
Moreover, the high hydrostatic pressure (HHP) has been documented in the literature for the same purpose; the method was recently applied for insect defatting for Acheta domesticus and T. molitor [42]. This method was previously documented for metabolites extraction from plants, which causes air in the plant cell vacuoles to leak and damage the cell membrane. This fact increases the efficiency of solvent extraction (Sadus, 1992). The method was compared to traditional hexane extraction, and the results showed the influence of HHP process over the techno-functional properties of mealworm and cricket powders. The study concluded that the application of high hydrostatic pressure (500 MPa), for 15 min resulted in changes in the functional parameters of proteins and that HHP can be used for defatting purposes to implement insect powders as a food additive.
Nongonierma & FitzGerald [43] reported that the organic extraction of lipids might imply protein losses, as some proteins that have more affinity to solvent can be partitioned to the solvent phase being further removed. However, it is not commonly reported in the literature. Therefore, to avoid this solvent impact on samples and to use a more sustainable extraction method, SC-CO2 seems to be the most recommended technique, using low temperatures and preserving the native protein structure.
2.4. Supernatant recovery and extraction
Protein extraction is influenced by previous processing and can be carried out through numerous methods. The extraction rate efficiency also depends on the raw material characteristics, which in the case of insects, the insect species, life stage, and insect feeding can play an important role. Moreover, sample concentration, pH, salt addition, temperature, and extraction time are crucial parameters to be considered to optimize protein extraction.
Proteins are commonly separated from other components by varying the pH and ionic strength of the media to increase protein solubility. As the isoelectric point (pI) of most food matrices, including insects, are between pH 4 and 5, at high values of pH, proteins surfaces are mostly charged. This promotes electrostatic repulsion between proteins and increases protein-water interaction, resulting in higher solubility [44,45]. To extract a different group of proteins, the specific pI needs to be reached Hall et al. [19]. Some different pH range has been used for protein precipitation, i.e., around pH 4.0–5.0 for yellow mealworm proteins [29,46] pH 5.0 for silkworm chrysalis [39]. The pI can be determined for the whole group of proteins or even for specific protein fractions, as it was selected for B. mori proteins at pH 2.5 (albumins), pH 2.7 (globulins), pH 4.0 (glutelins), and pH 4.5 (prolamins) [47].
Ionic strength is another parameter that might be modified to change protein solubility during the extraction. At low concentrations of salt (NaCl), ionic strength of 0.5–1 M, salt ions will act as a “shield” on the protein surface, promoting higher protein-water interaction and water solubility. This method is also called “salting-in” [48]. As the charge and electrical repulsion favors protein solubility, salt addition and the change in pH can be a good strategy for protein extraction [46]. Studying the influence of NaCl concentration upon T. molitor protein extraction [46], found that at pH 8.0 the proportion of water-soluble proteins raised from 59.2% to 94.7% (w/w) with 0.1 and 1.0 M NaCl, respectively. The non-water-soluble proteins were found concentrated in the pellets [49]. have shown by LC-MS/MS, that pellets from aqueous extraction mainly contained muscle proteins, i.e., actin, myosin, tropomyosin and troponin.
The most common method for insect protein solubilization is based on alkaline addition followed by acid precipitation of the proteins at their isoelectric point, as stated by Ref. [50]. The author sought to establish an optimized procedure for protein extraction from microwave-dried larvae of Hermetia illucens (HIL), using ethanol as a defatting agent. The authors explored three extracting parameters: temperature, the alkaline solution to sample ratio, and time. An optimal condition using a mathematical polynomial model was used to illustrate the influence of extraction parameters over protein yield. The extraction condition was studied at 52.23 , and 59.43 min, protein 63.12 ± 1.05%. Experimentally, the protein extraction using the optimized protocol was compared with a modified protocol (60 min, 15:1 alkaline solution to sample ratio, and 40 ). Protein yield for the optimized protocol was 80.42 ± 0.90% and 76.91 ± 0.91% for the modified protocol. The study was essential to show how mathematical models can be successfully applied to investigate the optimum parameters for protein extraction and how the results might change under practical conditions.
A similar alkaline process has been explored by other authors using black cricket [51]. Following a similar method but intending to provide chitin separation [52], mixed a defatted migratory locust coarse meal with deionized water and adjusted the pH to 9.0 (NaOH 4 M). The slurry was filtered through a fine-mesh cheese filtering cloth. Zhao et al. [29] also reported a protein extraction method for mealworm larvae but based on four different factors: NaOH concentration, NaOH to defatted larvae ratio using ethanol, temperature, and time. The results showed that under optimized extraction conditions, 0.25 M NaOH, a 15:1 mL: g NaOH: fat-free larvae ratio, 40 for 1 h of extraction, a powder with 70% of protein content was obtained
Yi et al. [15] reported an eco-friendlier extraction system by adding demineralized water and ascorbic acid to an N2-frozen insect. Three fractions were obtained: the residue, the pellet, and the supernatant. The pellet fraction ranged from 65% to 75%, which had the highest protein content compared to other fractions. All chitin-bound nitrogen was present only in the pellet fraction since it is not soluble in the aqueous solvents.
An alternative and specific protein extraction method was carried by Okagu et al. [53] in order to obtain non-cuticular yellow mealworm proteins. The samples were washed and frozen in liquid nitrogen, blended with 1.6% w/v sulfite, frozen and lyophilized. The grounded powder was defatted using hexane 1:5 (w/v), and the non-cuticular protein was extracted using potassium tetraborate decahydrate (1%) at pH 9.1, the supernatant was removed from the residue containing the cuticular protein after centrifugation. The sample was lyophilized, and then a dialysis system took place for salt and other non-protein components removal.
Intending to improve protein extraction, additional steps might be included during alkaline extraction. Ultrasonic technique has been successfully applied by Yang et al. (2013). The method was implemented by replacing the shaker after adding the alkaline solution. Mishyna et al. [21] have compared the sonication extraction to conventional defatting and alkaline ones. Data showed that the highest protein extraction rate was obtained after the sonication method. The highest values of grasshopper powder's foaming and emulsifying capacity were also found when the ultrasound method was applied. Conversely [54], have reported the usage of alternative treatments such as ohmic heating and ultrasound to improve protein extraction and further emulsifying property. Samples treated with alternative methods did not significantly differ for protein content when compared to the conventional alkaline method. However, the authors mentioned that different ohmic heating parameters can affect the extraction and can be further explored in order to increase protein solubility.
In order to avoid protein denaturation, the supernatant can be freeze-dried or further purified in a membrane filtration system. Ultrafiltration (UF) can reduce the occurrence of protein denaturation, and the method can produce protein concentrates with great content (65%) or even protein isolate with 90% purity if coupled with diafiltration [7,33]. However, further studies are necessary on insect protein size and properties as these methods were not yet deeply reported for insect proteins in the literature.
3. Insect protein properties under processing
3.1. Processing parameters
Each insect species has its own protein profile, even insects in the same species but at different life stages can produce a particular group of proteins and then show a contrasting behavior over the explored techno-functionalities [11,55]. Herein, we understand the importance of having greater knowledge about physicochemical characteristics of insect proteins and how the variation of heat, pH and NaCl concentration can impact their intrinsic properties. Including solubilization, ζ-potential, hydrodynamic diameter, hydrophobic surface, free sulfhydryl group, water holding capacity, oil holding capacity, gelling ability, emulsifying, foamability, rheological and texture ability (Table 1 [15,15,15,15,15,[17], [18], [19],21,21,22,22,22,29,36,37,51,52,52,56,56,57,[57], [57], [58], [59], [60], [61], [62],81]).
Table 1.
Insect characterization and functionalities over NaCl, pH and Heat treatment.
| Source | Treatment |
Characterization Colloidal properties |
Reference |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NaCl | pH | Heat | sol | ζ | Size | Hyd | SH | WHC | OHC | R&T | Emul | Foam | Gel | ||
| Acheta domesticus | X | X | X | [15] | |||||||||||
| Acheta domesticus | X | X | X | X | X | [37] | |||||||||
| Allomyrina dichotoma | X | X | X | [56] | |||||||||||
| Alphitobius diaperinus | X | X | X | [15] | |||||||||||
| Anacridium melanorhodon | X | X | X | X | X | El Hassan., 2008 | |||||||||
| Apis mellifera | X | X | X | X | X | X | X | X | X | [21] | |||||
| [17] | |||||||||||||||
| Blaptica dubia | X | X | X | [15] | |||||||||||
| Bombyx mori | X | X | [81] | ||||||||||||
| Bombyx mori | X | X | X | X | Omotoso, 2015 | ||||||||||
| Chondracris roseapbrunner | X | X | X | X | X | X | [57] | ||||||||
| Cirina forda | X | X | X | X | X | [58] | |||||||||
| Cirina forda | X | X | X | X | X | X | X | Omotoso, 2006 | |||||||
| Gryllus assimilis | X | X | X | X | X | X | X | X | [51] | ||||||
| Gryllodes sigillatus | X | X | X | X | X | [22] | |||||||||
| Gryllodes sigillatus | X | X | X | [19] | |||||||||||
| Hermetica illucens | X | X | X | X | X | X | X | Buβler et al., 2018 | |||||||
| Hermetica illucens | X | X | X | X | Queiroz et al., 2021 | ||||||||||
| Imbrasia oyemensis | X | X | X | X | X | X | [59] | ||||||||
| Locusta migratoria | X | X | X | X | X | X | [52] | ||||||||
| Locusta migratoria | X | X | X | X | X | X | X | X | [52] | ||||||
| Oryctes owariensi | X | X | X | X | [60] | ||||||||||
| Patanga succincta | X | X | X | X | X | [57] | |||||||||
| Protaetia brevitarsis | X | X | X | X | X | [36] | |||||||||
| Rhynchophorus phoenicis | X | X | X | Koffi et al., 2017 | |||||||||||
| Schistocerca gregaria | X | X | X | X | X | Zielinska et al., 2018 [21] | |||||||||
| Schistocerca gregaria | X | X | X | X | X | [22] | |||||||||
| Sphenarium purpurascens | X | X | X | X | X | X | X | Torruco-Uco et al., 2018 | |||||||
| Tenebrio molitor | X | X | X | X | X | X | X | Buβler et al., 2018 | |||||||
| Tenebrio molitor | X | X | X | [56] | |||||||||||
| Tenebrio molitor | X | X | X | X | X | X | X | X | [61] | ||||||
| Tenebrio molitor | X | X | X | X | X | X | X | X | [62] | ||||||
| Tenebrio molitor | X | X | X | X | X | X | X | [29] | |||||||
| Tenebrio molitor | X | X | X | X | X | [18] | |||||||||
| Tenebrio molitor | X | X | X | X | X | [22] | |||||||||
| Tenebrio molitor | X | X | X | [15] | |||||||||||
| Zophobas morio | X | X | X | [15] | |||||||||||
Sol = Solubility; ζ = Zeta potential; Hyd = Hydrophobic surface; SH = sulfhydryl group; WHC= Water holding capacity; OHC = Oil holding capacity; Gel = gelling ability; Emul = Emulsifying; Foam = foamability; R&T = rheological and texture ability.
A common practice in industrial food processing is using salt, pH alteration, and heat variation over food matrixes [63]. Sodium chloride (NaCl) is largely applied as a preservative, spice, color maintenance, texture agent, and flavor agent. In the same way, variation in pH can impact different aspects of food such as flavor, consistency, and shelf-life. As pH is a logarithmic measurement, even small changes in values mean significant alterations [64]. Heat variation, another commonly applied process, can be used to control microorganisms’ growth and to improve product shelf life. Thermal techniques such as bleaching, pasteurization, and ultra-high temperature (UHT) are generally applied during food processing and aim to enhance color, texture, food quality, stability, and safety [65]. Intending to preserve and study the matrix behavior all reported processing parameters can be extended to alternative food sources such as insects. Some studies have demonstrated how these processes can alter protein functionality and cause structural changes Bubler et al., 2016 [29,51].
3.2. 3.2 Protein solubility
Protein solubility, an important functional property, directly depends on amino acid composition (hydration and surface hydrophobicity), protein size, and structural conformation, and can be affected by extrinsic factors, including ionic strength, pH changes, and temperature [66]. Most of the techno functionalities mentioned beforehand depend on how the protein interacts with the solvent and its solubility [22].
Buβler et al. (2016) investigated the influence of pH, NaCl, and temperature changes over techno-functionalities of T. molitor and H. illucens protein fractions. Protein solubility of these insects (pI around 4) was highly dependent on pH, showing higher solubility in the alkaline region, ph 10 for T. molitor and ph 12 for H. illucens and at extreme acid conditions (pH 2). Through the measurements of exposed hydrophobic tryptophan residues under extreme pH conditions (acidic or alkaline), the authors reported that protein might unfold and then expose the hydrophobic groups. Buβler et al. (2016) also verified the protein profile under the influence of pH by SDS-PAGE analysis. At pH 7 for protein extraction, the result showed that H. illucens had only two major protein bands, in the molecular weight region of 14.3 KDa and 80.5 kDa; the protein extract was composed of 75.9% of high molecular weight fraction and 24.1% of low molecular weight fraction. However, when taking extreme pHs (2 and 12) an expressive increase in low molecular weight protein was observed. The data shows that extreme pH conditions led to great proteolysis of the 80.5 kDa band to 14.3 kDa or lower.
Increasing NaCl molarity also improved protein solubility for both insects. Increasing ionic strength from 0 to 0.4 M reduced the solubility; however, NaCl molarity of 3 and 4 maximized protein solubility to 55% (T. molitor) and 70% (H. illucens), the results are expressed for T. molitor protein fraction, the increase in ionic strength decreased protein solubility again. Altering the extraction temperature from 20 to 60 increased protein yields by 20% and 10% for T. molitor and H. illucens, respectively (Bußler et al., 2016) [67]. also evaluated Locusta migratoria L. protein extract solubility upon pH change. Protein solubility showed low results, ranging from 10 to 22%, with minimum and maximum at pH 5 and pH 9, respectively. The protein pI was registered at pH 4. In the literature, pH alteration is one of the most common parameters for studying insect protein solubility [22,68].
High hydrostatic pressure (HHP) was firstly reported in the literature as a defatting process for later comprehension of how the process can affect the functionalities of insect protein extract [42]. The author compared the untreated group with HHP group for two species of insects T. molitor and A. domesticus. The pressure had no effect on primary or secondary structures, but it is known that tertiary and quaternary structure are susceptible to HHP, which can cause changes in solubility. Pressure by itself was unable to cause changes in cricket protein solubility but in T. Molitor. The results were confirmed based on protein surface hydrophobicity.
Commonly methods applied to conventional food processing have been switched to understand insect protein behavior. Therefore, other treatments, such as ultrasound, ohmic heating, and UHT aligned with pH and NaCl alterations might be further explored for the same purpose.
3.3. Surface hydrophobicity and –SH groups
Physico-chemical parameters of proteins can be individually evaluated in order to understand their role in protein functionalities. The presence of free sulfhydryl groups (SH) is one of them. Proteins with cysteine and cystine groups can suffer polymerization during heat, causing a thiol-disulfide exchange reaction and forming a strong polymer structure. During denaturation, proteins can unfold and reveal the thiol groups which will be available for intermolecular interactions with other proteins [64,69].
Generally, in globular tertiary protein structures, hydrophilic amino acid side groups are predominantly at the surface, and hydrophobic ones stay in the structure's core (Leu, Ile, Val, Phe, Trp). Due to food processing, most hydrophobic residues become exposed upon unfolding, which usually leads to reduced solubility as the nonpolar groups will form intermolecular hydrophobic bonds and cause a further increase in protein aggregation [50,70].
Santiago et al. [51] evaluated the effect of heat treatment and NaCl concentration on the secondary structure of black crickets proteins. The study investigated the hydrophobic surface exposure and the presence of sulfhydryl groups. The authors noticed that when the heat raise up to 85 for 15 min, there was a progressive increase in surface hydrophobicity. This can be explained by the unfolding process and consequent exposure of hydrophobic patches. Similar data was also observed by Ref. [17] when testing proteins from honeybee brood. Complementary [51], reported that hydrophobicity decreased under temperatures above 90 for 15 min and decreased even more after NaCl addition, indicating that heat and increasing NaCl concentration favored the thermo-induced aggregation of black cricket proteins.
Jiang et al. [61] tested the physicochemical and techno functionality of T. molitor protein extract under different conditions of heat and salt addition (salting-in/out). The presence of SH was evaluated under salting-in treatment and compared to the untreated group; no significant differences were found at levels of exposed SH patches. However, under salting-out treatment, protein samples showed higher disulfide bond content than untreated and the salting-in group. It is known that SS bonding can stabilize the protein folded structures and then decrease the conformational entropy, which improves thermal stability.
Wang et al. [47] noticed how surface hydrophobicity vary according to the protein extraction protocol, besides the intrinsic factors. The data was compared with a previous paper by Azagoh et al. [71]. Chatsuwan et al. [57] also evaluated the total sulfhydryl content and the free sulfhydryl content for protein extracts from Chondracris roseapbrunner and Patanga succincta. The results showed a similar content for both insect samples, which was expected based on the amount of sulfur-containing amino acids in the samples.
3.4. Water holding capacity/oil holding capacity
Another important functional property of a protein matrix is retaining water against gravity, the water holding capacity (WHC). It also includes bound, hydrodynamic, capillary, and entrapped water. The amount of water linked to a protein is directly affected by the amino acid profile, the number of charged residues, protein conformation, hydrophobicity, and extrinsic factors such as pH, temperature, and ionic strength. The water holding capacity positively affects the texture and moisture of food and is highly connected to gelation properties.
Oil-holding capacity (OHC) is crucial for understanding the techno-functionality of samples and their possible application in colloidal systems. Generally, small and low-density proteins tend to absorb more oil than big and high-density ones [7,72]. The presence of hydrophobic amino acids in the structure and the food processing method can affect the OHC of the protein matrix. This property is explored mainly in food systems to improve texture and flavor, making food more palatable [72,73]. OHC of proteins tends to be an interesting property to enhance insect supplementation in food formulations and, therefore, provides greater consumer acceptability.
Tenebrio molitor is one of the most explored insects for its protein's functional property. The WHC of T. molitor protein fractions, when compared to T. molitor flour, decreased by 0.41 g/g for high protein fractions and 0.37 g/g for low protein fractions. In addition, the OHC of protein extract compared to the flour decreased by 0.21 g/g for the low protein fraction, whereas it significantly increased by 0.26 g/g when the high protein fraction was tested (Buβler et al., 2016) (Buβler et al., 2018). In contrast to this study, Zielinska et al. (2018) have shown that protein preparation had a higher WHC (3.95 g/g) and OHC (2.74 g/g) than the whole insect flour (1.29 g/g and 1.71 g/g, respectively). Further studies are necessary to comprehend better how the different protein extraction and flour production methods might affect the WHC and OHC properties. However, it is known that the presence of fat in the flour and the protein size and conformation, which might be different on each fraction, will act on the functional property of those proteins.
Besides T. molitor, Zielinska et al.(2018) have also reported the WHC and OHC for two other insect species, Gryllodes sigillatus and Schistocerca gregaria, and the protein preparation for all insects showed higher WHC and OHC when compared to the insect flour. Lee et al. [62] have also tested the OHC of T. molitor protein preparation under heating treatment (55 , 75 and 95 ) for different heating times (20, 40, and 60 min). The result showed that the OHC was dependent on the temperature variation. The OHC decreased in all tested temperatures getting lower as the time increased, a similar result was found for Sphenarium purpurascens Ch. (grasshopper) [74]. The authors explained that the temperature treatment collapses the protein network, which might decrease the OHC. In addition, the longer heating time promotes protein aggregation due to conformational changes leading to a decline in the protein-oil holding capacity.
4. Colloidal properties
According to the states of matter, colloidal systems in food are classified as multicomponent systems such as sols, gels, emulsions, and foam. Food colloids are responsible for the structure, texture, and mouth-feel characteristics of many different food products and, therefore, will affect the final technological aspects and consumer acceptance [64,75]. Some authors have correlated the protein structural changes with a range of colloidal properties. Surface hydrophobicity, free sulfhydryl groups, zeta potential, and molecular protein weight were measured. These correlations are herein reported in Table 2 [21,51,57,61],. It is worth mentioning that to compare the protein functionalities of different insects, it is crucial to consider the processing and the extraction method applied in each study.
Table 2.
Insect protein characterization and functionalities under different treatments.
| Insect specie | Extract | Particle charge mmole(charge)/liter | ζ- potential (mV) | Surface hydrophobicity | Free Sulfhydryl (μmol/g) | Most abundant Molecular weight (kDa) | EC | FC (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Schistocerca gregaria | Raw | 0.8 | – | 180.5 | – | <20 | 40%a | 40-60a | [21] |
| Defatted | 0.8 | – | 207.8 | – | <20 | 45%a | 80-100a | ||
| Alkaline | 1.2 | – | 213.7 | – | <20 | 100%a | 80-100a | ||
| sonicated | 1.3 | – | 183.3 | – | <20 | 100%a | 40-60a | ||
| Apis mellifera | Raw | 0.6 | – | 159.8 | – | 75–200 | 20%a | 0-20a | |
| Defatted | 0.7 | – | 188.7 | – | 75–200 | 20–35%a | 20-40a | ||
| Alkaline | 0.3 | – | 237.3 | – | <20 | 100%a | 40-60a | ||
| sonicated | 0.3 | – | 212.2 | – | <20 | 100%a | 40-60a | ||
| Patanga succincta | aqueous extract | – | – | 15.9 | 0.48 | 20–250 | 29.23 m2/g | 8.57 | [57] |
| Chondracris roseapbrunner | Aqueous extract | – | – | 22.6 | 0.48 | 20–250 | 36.96 m2/g | 25.71 | |
| Tenebrio molitor | Alkaline (AEAP) | – | −14.40 | 1146.57 | 7.21 | – | 21.99 m2/g | 107.5 | [61] |
| Salting-in-AEAP | – | −15.85 | 1116.68 | 7.46 | – | 20–30 m2/ga | 110 | ||
| AEAP-salting-out | – | −23.10 | 683.7 | 5-6a | – | 40–50 m2/ga | 155 | ||
| Salting-in-AEAP-out | – | −26.20 | 564.86 | 5-6a | – | 55.50 m2/g | 205 | ||
| Gryllus assimilis | Untreated | – | - 44.0 | 8000–10000 | Near to zero | 10-15 (94.3% of abundance) | – | 190 | [51] |
| Heat 95 °C/15min | – | - 44.2 | 12000–14000 | Near to zero | 10-15 (34.1% abundance) | – | 1070 | ||
| 0.3 M NaCl 95 °C/15min | – | – | 6000–7000 | Near to zero | No distinguishable band | – | 1150 | ||
| Clanis Bilineata Tingtauica Mell | untreated | – | – | 200-250a | 5.6 | 30–130 | 2.5–5.0 m2/g | Wang et al. | |
| Sonic treated 200 W | – | – | 250-300a | 9.0a | 30–130 | 20.0–25.0 m2/g | |||
| Sonic treated 400 W | – | – | 376.4 | 10.0 | 30–130 | 27.5–30.0 m2/g | |||
| Sonic treated 600 W | – | – | 250-300a | 10.0 | 30–130 | 25.0–27.5 m2/g | |||
| Sonic treated 800 W | – | – | 200-250a | 8.0a | 30–130 | 25.0–27.5 m2/g |
All the values herein stated are in a range or is an approximation of what is reported in the graphics of each reference.
4.1. Gelling properties
Macroscopically, a gel is a material that behaves as an elastic solid, suffering partially reversible deformation under certainly applied tension, and simultaneously as a viscous liquid that can partially drain. Microscopically, a gel is a colloidal dispersion in which the solid phase (the network of a polymer chain) forms a structured three-dimensional matrix in the interstices of which are lodged with molecules of the liquid phase, water in case of hydrogels [76]. The gelling capacity is a protein functionality of great importance, used in food formulations such as jellies, desserts, yogurts, meat products, etc. The gelling agents, such as proteins and other polymers, are food additives employed as thickening and stabilizer agents that provide food texture through gel formation. Several proteins have been employed as a gelling agent, including zein, from corn, soy proteins, and egg and whey protein [77].
The least gelation concentration measures the gelling capacity or the critical minimum concentration specific for each hydrocolloid. Thus, if the concentration is in the range of the critical minimum concentration, the higher the molar mass, the faster the gelation process (Walstra, 2002.). Even though the gelling property of animal and plant proteins has been well documented in the literature, there have been only a few papers investigating the gelling agents from edible insects and their application in food formulations. Yi et al. [15] have studied the gelling property of protein fraction of five insect species, Tenebrio molitor, Zophobas morio, Alphitobius diaperinus, Acheta domesticus, and Blaptica dubia. In this study, the insect supernatant solutions were heated, and the concentrations from 3 to 30% (w/v) were evaluated at pH 3, 5, 7, and 10. The evolution of storage modulus (G′) and loss modulus (G”) were studied during temperature ramp using a rheometer, where G′ kept increasing for the first and second phases when the temperature was constant at 90 . During the cooling phase, G’ and G” sharply increased, which characterize the presence of hydrogens bonds between structural elements in the gel. The authors reported the gelation temperature for all five insects, T. molitor 61.7 , A. diaperinus 58.2 , Z. morio 51.2 , A. domesticus 56.2 , B. dubia 63.2 . The authors reported that 30% (w/v) protein samples of all insects formed a gel at pH 7 and pH 10. House cricket fraction (Acheta domesticus) was the only one to form a gel at 3% (w/v) and pH 7. In addition, the adult stage showed stronger gels than the larvae ones, implying that the insect life stage influences the protein profile.
Mishyna et al. [17] have reported the least gelation concentration of raw powder from honeybee brood; the study reported results at a lower protein concentration of 5% (w/v) at pH 7 and at a higher concentration of 11% (w/v) at pH 3. Honeybee brood gel hardness increased as a function of pH, showing maximum results at pH 7 and 9. The microstructure analysis by scanning microscopy corroborates with the above data as an alkaline pH environment caused the formation of gels with more regular, continuous, and smaller pores. The authors concluded that protein solubility at different pH directly affects the gel formation and textural properties, and soluble proteins from honeybee brood demonstrated great similarities to conventional proteins, such as whey [21].
In another study, protein concentrates from Locusta migratoria showed gel formation at a pH of 7.9 and a concentration of 20% or 16.5% (w/v) [67]. The least gelation concentration of Sphenarium purpurascens Ch., grasshopper, was evaluated under a range of temperatures. The concentration of 14% was identified as the minimum to start forming the gel structure in the tube [74].
Black cricket (Gryllus assimilis) protein isolate (BCPI) was tested for its critical gelling concentration (CGC) at a fixed pH 7. The protein isolate was heated at 80,85 and 90 for 15 min [51]. Whey protein isolate (WPI) was used as a comparative parameter. The critical gelling concentration was determined as 6.5% w/w at 90 . The value was interesting when compared to WPI, a conventional gelling agent with a minimum gelling concentration of 6%. In addition, data was compared to other insect-based protein-rich ingredients, for example, Osasona & Olaofe [58] reported a higher value of 14% w/w for Cirina forda larvae.
Santiago et al. [51] also evaluated the influence of NaCl addition over the gelation capacity of BCPI. It was observed that NaCl concentration between 0.3 and 0.5 M impaired the gelation. Lee et al. [62] evaluated the gel strength of protein extract of yellow mealworm under different heat treatments (55, 75, and 95 ) and exposure time (20, 40, and 60 min). The gel strength was obtained at the maximum time, 60 min, and at a higher temperature of 95 . Temperature acted over the protein unfolding process that will expose the hydrophobic groups and possibly facilitate the net formation between proteins. The ability of a protein to form gels is useful in developing new formulations as this property can retain water, flavor, sugar, and food ingredients in the structural matrix [74].
A recent study on the gelling properties of black soldier fly larvae proteins showed how ultrasound treatment can impact the gel-like structure and affect protein physicochemical/functional properties of proteins. After 15 min of ultrasound treatment, at fixed pH 7, and under heat (until 85 ) BSFL proteins showed the highest gel strength. The confocal microscope evaluated gel structure pre-treated with ultrasound for 15 min. Samples showed the most compact microstructure, containing the smallest pore size [78].
In general, the gelling capacity of insect proteins revealed promising results even when compared to WPI. It was stated that beyond intrinsic parameters, the presence of NaCl, and pH alterations in the medium influence gelation, which implies that electrostatic interaction directly affects the formation of the gel network of insect proteins. Protein concentration and temperature are other important parameters to consider, as they can accelerate the three-dimensional structure formation and the sol-gel transition.
The studies intend to demonstrate how extrinsic parameters, including ultrasound treatment, may impact on protein unfolding, sol-gel transition, and gel strength. The life stage also affects gel strength, whereas the adult stage showed the most interesting for gelling properties. Therefore, a more detailed study is necessary to understand how extrinsic parameters can optimize insect protein gelling abilities and how alternative methods of treatment such as ultrasound can be applied to improve protein functionality. The data also revealed that different insect species demonstrated a contrasting gelling capacity and gel strength under the same external temperature and pH conditions. This fact concluded that each insect presented a non-identical protein profile, and the difference will impact in the protein techno-functionality.
4.2. Foaming properties
Food foams are composed of a continuous aqueous phase that surrounds a gaseous (air) dispersed phase that can be stabilized by a viscoelastic film of proteins or other compounds [79]. In foam formulations, proteins spontaneously migrate from a bulk liquid to an air/water interface which implies that the free energy of proteins is lower at the interface compared to the bulk aqueous area [64]. Due to the interfacial properties of proteins, these amphiphilic molecules are the main surface-active agents that help with bubble formation and stabilization. Foam-type foods have been well explored on the market, such as whipped cream, meringue, marshmallow, ice cream, bread, and many other formulations [64,72]. The foaming property will rely on proteins’ ability to form a thin stable film at gas-liquid interfaces. Thus, it will depend on intrinsic parameters such as protein profile, the number of hydrophobic and hydrophilic residues over protein surface, structural form as globular or non-globular protein, size, steric effect, flexibility, charge density and distribution, and amino acid composition [72,80,81]. In addition, extrinsic parameters might also affect the foaming property, such as changes in ionic strength, heating, the addition of salt, sugars, and lipids, and protein concentration. In short, for a protein to perform a good foaming agent, it must follow three basic requirements: (i) it needs to quickly adsorb to the air-water interface, (ii) it must rapidly unfold and rearrange at the interface and, (iii) it needs to form a viscous cohesive film through intermolecular interaction [60,72].
Regarding foam structure, two parameters are generally evaluated, foamability and foam stability. Protein's foamability or foam capacity is related to the amount of interfacial area a protein can create. Complementary foam stability (FS) expresses the capacity of the protein to stabilize foam against the opposite force as the gravitational and mechanical ones [22,57].
Kim et al. [36] have reported different defatting processes using methanol, ethanol, or n-hexane of proteins extract from Protaetia brevitarsis larvae and the effect of organic solvents on the functional properties. The study revealed that aqueous extract showed the lowest foam capacity (20.0 ± 7.1%) while methanol, ethanol, and hexane extract showed higher foam capacity, 72.2 ± 7.9; 56.3 ± 8.8; and 88.9 ± 15.7%, respectively. The foam capacity and foam stability for P. brevitarsis larvae protein extracts showed a positive correlation with surface hydrophobicity and protein solubility, as was also stated by Ref. [82]. Besides, the high-fat content in the aqueous extract may hamper foam formation [83].
Torruco-Uco et al. [74] studied the foam capacity of grasshopper meals. To obtain the meal Grasshoppers (S. purpurascens Ch.) insects were dried at 55 and then ground in a coffee mill to reach a uniform particle size of 0.420 mm. Foam capacity was equal to 7.17%, and the author compared with A. domesticus (1.42%) [37]. However, it is important to note that [74] studied the whole ground insect meal, and [37] reported the results for an aqueous extracted residue, which are different materials and therefore, making the studies difficult to compare.
The foamability and foam stability of black cricket protein isolate (BCPI) were reported by Santiago et al. [51] under different treatments. The foamability increased as the temperature increased, reaching 1070% at 95 Untreated BCPI showed foamability of only 190% and foam stability (the remaining foam) between 50 and 70%. The group with the higher NaCl concentration (0.5 M) and heat treatment of 95 showed 35% of foam remaining after 30 min (the results were compared to WPI). The greater foaming property upon NaCl addition can be explained by the capacity of the proteins to form a more elastic and cohesive film in the air bubbles [51].
Other studies performed by Hall et al. [19] have also reported the foam properties of another cricket species, Gryllodes sigillatus, which showed lower foamability (100–155%), but better foam stability up to 90 min of standing. However, different devices were used to prepare the foam, and other insect processing methods were applied. Water-soluble protein extract from Patanga succincta (WSPP) and Chondracris roseapbrunner (WSPC) was tested for its functional properties, including FC and FS [57]. The results were compared to Bovine serum albumin (BSA) (values of BSA for FC and FS were 81.23% and 67.41%, respectively) WSPP, and WSPC exhibited lower FC values when compared to BSA, 8.57 and 25.71%, respectively. However, the FS of both insects was significantly higher than BSA, 98.72 and 86.41% for WSPP and WSPC, respectively. The results indicated the promising capacity of both water-soluble protein extract to stabilize foam. It is important to mention that the devices used for foam production and the methods applied for insect processing and foam formation will strongly impact the final result.
A recent study performed by Rumbos et al. [84] evaluated the FS and FC of black soldier fly larvae protein extract after heating treatment of 15 min. The data showed that FC was similar even after 85 /15 min compared to the untreated sample, but the FS at treatment 85 /15 min showed more promising results with significant difference during 20 min of storage. The study emphasizes the possibility of applying BSFL proteins as stabilizer agents and the optimization of proteins functionality after simple heating treatment.
The literature reports show that foamability is generally improved when the defatting step is included in the protein extraction method. In addition, proteins obtained through aqueous extraction have shown lower foam capacity. Lipids can normally compete with proteins during the stabilization of air bubbles and hamper foam capacity and stability. Extrinsic treatment such as heat might cause protein unfolding, which might cause fast protein adsorption at the air/water interface. Temperature combined with NaCl addition also revealed to be interesting for foam formation as they prevented protein drainage and/or formed a more elastic and cohesive film. Intrinsic parameters such as amino acid composition and their position in the protein structure will also affect the reported functionalities.
All reported data reveals the potential of insect protein functionalities which can further allow edible insect powders to be used in suitable food applications. The studies also demonstrated how insect proteins are affected either by intrinsic or extrinsic parameters. Moreover, food processing parameters are essential for protein foamability and require more profound research.
4.3. Emulsification properties
Emulsions are a homogeneous mix of at least two immiscible liquids (colloidal dispersion), which can be characterized according to the relative spatial distribution of both liquids [80]. The presence of oil droplets in a continuous phase of water is called an oil-in-water (O/W) emulsion, and a water droplet in the continuous oil phase is referred to as a water-in-oil (W/O) emulsion [64]. As the energy associated with the interface is proportional to the interfacial area (Δinterf G = γΔinterf A), during emulsion formation, there is an increase in energy due to the bigger interface system. Even though, smaller droplets can assume more positions in the system, which generates a higher configurational entropy (ΔconfigS).
The energy associated with emulsion formation is much higher than the configurational entropy variation, Δinterf G > ΔconfigS. The higher energy associated makes the whole system thermodynamically unstable. The amphiphilic character of proteins allows the formation and stabilization of emulsions by reducing surface tension at the oil-water interface. Proteins can create a film that prevents coalescence, flocculation, creaming, sedimentation, and Ostwald ripening [7]. Therefore, proteins play an important role as an emulsifier and surfactant in many natural and processed foods, such as egg yolk, soy milk, butter, and mayonnaise. In homogenized milk, for example, casein micelles and whey proteins replace the lipoprotein membrane with a stronger protein film, which is more stable against creaming than natural milk [18,22].
As foamability and other proteins functionalities, the emulsifying property is also influenced by intrinsic (hydrophobic groups) and extrinsic (pH, ionic strength) factors [36]. Protein size directly affects emulsion formation and stability as small proteins enhance diffusion and generally form a better emulsion. Larger proteins tend to have lower diffusion but greater emulsion stability once the layer surrounding the oil-water interface has formed [18,22]. Some edible insects' emulsion properties have been explored in the literature. Different materials were tested for emulsion capacity and stability, such as insect flour and protein extract obtained through different solvents extraction, all under various extrinsic factors.
Emulsion capacity (EC) is a measure of the number of grams of oil per gram of protein that can be emulsified, and emulsion stability (ES) measures the resistance of the emulsion over a specific time [85]. Protein concentrate of edible crickets (Acheta domesticus) was evaluated for its emulsifying capacity and emulsion stability using corn oil and water to form oil-in-water (O/W) emulsion. Three different materials were evaluated, hexane extracted proteins (HE), aqueous extracted precipitate (AE precipitate), and aqueous extracted residue (AE residue). The aqueous extraction revealed a high emulsion capacity and stability, and AE precipitate recorded the highest value, 41.7% (v/v) and 33.6% (v/v) for EC and ES, respectively [37]. EC and ES of T. molitor (TM), Allomyrina dichotoma (AD), Protaetia brevitarsis seulensis (PB) were explored under three different processes, ground (G), defatted (D), and extraction (E). Only EPB had a significantly higher EC, around 100%, compared to all other insects and treatments, which showed a similar result for all insects and parameters, approximately 70%. The study reported that following the grounding, defatting, and extraction steps, the emulsion stability decreased, except for PB [56]. Previous studies have also reported that edible insects' emulsion capacity and stability might reduce after the defatting step as the process might increase surface hydrophobicity and further protein aggregation [59,86,87]. However [56], concluded that the emulsion stability of PB (oil-in-water emulsion) did not reduce like the other insects, which makes PB a promising functional protein source.
A recent study on the emulsifying property of BSFL and its physical and oxidative stability reported the advantageous usage of sustainable treatments such as ultrasound and ohmic-heating to optimize protein functionality [84]. The study reported that over seven days of storage, samples treated with ohmic heating showed the most interesting stability compared to the untreated and ultrasound-treated. The interfacial tension (IFT) analysis by pendant drop corroborates with the storage test as the ohmic-heated sample showed the lowest IFT. It was the first time that ohmic heating was applied for the optimization of BSFL protein functionalities. The data stated the importance of using alternative strategies to improve protein functionalities and how BSFL proteins show advantageous potential to be applied in food systems.
Mishyna et al. [21] tested the emulsion capacity and stability of different fractions from S. gregaria and A. mellifera (raw powder, defatted powder, the powder obtained by alkaline extraction, and the powder obtained by alkaline and sonication extractions). The study showed that both insects' emulsions stabilized by raw and defatted fractions showed significantly lower values. The highest emulsion stability was detected for protein fraction from S. gregaria, and more than 80% of the emulsified layer remained stable after 120 min when the sonication was applied. The higher ES values from S. gregaria alkaline and sonication fraction can be due to the high surface charge of proteins from these fractions, preventing droplets approaching and aggregation, as [47] have reported similar results for barley protein extract. The EC of protein fractions (alkaline and sonication fractions) showed the highest values (100%). Raw and defatted powder of S. gregaria showed EC (oil-in-water emulsion) values of 39.5 and 45.0%, respectively. The higher values of EC in the protein fractions might be due to the greater protein content, improved solubility, and the presence of hydrophobic groups in protein-enriched fractions of insects. Damodaran [88] have concluded that low molecular weight surfactants might easier adsorb and reduce the interfacial tension, directly improving emulsion formation. This statement correlates with higher ES of S. gregaria, as the protein extract was characterized by low molecular weight proteins when compared to A. mellifera. Gould and Wolf [18] have also reported a synergistic enhancement of surfactant efficiency by the presence of small proteins. In addition, emulsifying capacity and stability were improved when hydrolysates from Gryllodes sigillatus were used [19]. It is also important to consider the protein composition based on hydrophobic amino acids' presence and position on protein structure, these intrinsic parameters can directly contribute to the emulsion stabilization, and it will vary according to each protein profile.
In conclusion, insect products such as protein concentrate, or insect flour, showed promising features to be used in food formulation as an emulsifying agent. Emulsion capacity and emulsion stability of insect proteins have been recently more explored in the literature, and it was stated that the defatting process, grounding, and extraction method, directly modify protein functionality beyond its intrinsic aspects [50,51].The aqueous extraction revealed greater emulsion property and the defatting process reduced this property, which might be explained by the protein/solvent interaction during the defatting step, causing protein surface hydrophobicity increase and possible protein aggregation. The result reinforces how the physicochemical characteristic of proteins and their interaction with added ingredients should be completely understood, as it can be considered a key step for developing insect food formulation and improving consumer acceptance.
5. Conclusion
Edible insects represent a promising alternative protein source for the world's growing population, and they fulfill all requirements to achieve the main Sustainable Development Goals (SDG) of WHO. Insect cultivation and its inclusion in the mainstream food culture represent a more sustainable and efficient food system. Besides the nutritional factor, insect proteins have revealed great technological applications. To optimize protein extraction with great functional and nutritional quality, insects need to be thoroughly studied from their early life stage, providing an appropriate feed and fasting treatment before processing. All other parameters, such as the defatting process, drying step, and extraction method, proved to affect protein functionality and, therefore, need to be completely comprehended. Besides, each insect life stage might represent a completely different protein profile and then show a different result regarding colloidal properties. Further studies should consider alternative techniques to defatting insects by avoiding organic solvents. Similarly, sonication, ohmic heating, and other non-thermal treatments can be applied to improve protein extraction with a more efficient and sustainable performance. The techno functionalities of insect proteins showed to be promising and a possible alternative to the conventional source. Exploring these colloidal properties can help increase consumer acceptance as the proteins allow the structuration of a range of formulations in food systems. In addition, further research should shed light on consumer willingness to pay for products from animals fed with insects and products made from insects.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) (88887.585466/2020-00), CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and (FAPEMIG (Minas Gerais State Agency for Research and Development).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interest's statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
This article is a part of the "Unconventional sources of food and food ingredients".
Contributor Information
Lucas Sales Queiroz, Email: lusaqu@food.dtu.dk.
Federico Casanova, Email: feca@food.dtu.dk.
References
- 1.Worldometers, 2017. From 1950 to Current Year: Elaboration of Data by United Nations, Department of Economic and Social Affairs, population division. World population prospects: The 2017 revision (Medium-fertility variant).
- 2.FAO . 2017. The Future of Food and Agriculture. Trends and Challenges. [Google Scholar]
- 3.Alexander P., Brown C., Arneth A., Finnigan J., Rounsevell M.D.A. Human appropriation of land for food: the role of diet. Global Environ. Change. 2016;41:88–98. [Google Scholar]
- 4.Aiking H., de Boer J. The next protein transition. Trends Food Sci. Technol. 2020;105:515–522. doi: 10.1016/j.tifs.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henchion M., Hayes M., Mullen A., Fenelon M., Tiwari B. Future protein supply and demand: strategies and factors influencing a sustainable equilibrium. Foods. 2017;6:53. doi: 10.3390/foods6070053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aschemann-Witzel J., Gantriis R.F., Fraga P., Perez-Cueto F.J.A. Plant-based food and protein trend from a business perspective: markets, consumers, and the challenges and opportunities in the future. Crit. Rev. Food Sci. Nutr. 2021;61:3119–3128. doi: 10.1080/10408398.2020.1793730. [DOI] [PubMed] [Google Scholar]
- 7.Gravel A., Doyen A. The use of edible insect proteins in food: challenges and issues related to their functional properties. Innovat. Food Sci. Emerg. Technol. 2020;59 [Google Scholar]
- 8.Singh P., Kumar R., Sabapathy S.N., Bawa A.S. Functional and edible uses of soy protein products. Compr. Rev. Food Sci. Food Saf. 2008;7:14–28. [Google Scholar]
- 9.Gorissen S.H.M., Crombag J.J.R., Senden J.M.G., Waterval W.A.H., Bierau J., Verdijk L.B., van Loon L.J.C. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids. 2018;50:1685–1695. doi: 10.1007/s00726-018-2640-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Huis A. Potential of insects as food and feed in assuring food security. Annu. Rev. Entomol. 2013;58:563–583. doi: 10.1146/annurev-ento-120811-153704. [DOI] [PubMed] [Google Scholar]
- 11.Halloran A., Roos N., Eilenberg J., Cerutti A., Bruun S. Life cycle assessment of edible insects for food protein: a review. Agron. Sustain. Dev. 2016;36:57. doi: 10.1007/s13593-016-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poma G., Cuykx M., Amato E., Calaprice C., Focant J.F., Covaci A. Evaluation of hazardous chemicals in edible insects and insect-based food intended for human consumption. Food Chem. Toxicol. 2017;100:70–79. doi: 10.1016/j.fct.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 13.van Huis A. Edible insects are the future? Proc. Nutr. Soc. 2016;75:294–305. doi: 10.1017/S0029665116000069. [DOI] [PubMed] [Google Scholar]
- 14.Rumpold B.A., Schluter O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013;57:802–823. doi: 10.1002/mnfr.201200735. [DOI] [PubMed] [Google Scholar]
- 15.Yi L., Lakemond C.M.M., Sagis L.M.C., Eisner-Schadler V., van Huis A., van Boekel M.A.J.S. Extraction and characterisation of protein fractions from five insect species. Food Chem. 2013;141:3341–3348. doi: 10.1016/j.foodchem.2013.05.115. [DOI] [PubMed] [Google Scholar]
- 16.Errico S., Spagnoletta A., Verardi A., Moliterni S., Dimatteo S., Sangiorgio P. Tenebrio molitor as a source of interesting natural compounds, their recovery processes, biological effects, and safety aspects. Compr. Rev. Food Sci. Food Saf. 2022;21:148–197. doi: 10.1111/1541-4337.12863. [DOI] [PubMed] [Google Scholar]
- 17.Mishyna M., Martinez J.-J.I., Chen J., Davidovich-Pinhas M., Benjamin O. Heat-induced aggregation and gelation of proteins from edible honey bee brood (Apis mellifera) as a function of temperature and pH. Food Hydrocolloids. 2019;91:117–126. [Google Scholar]
- 18.Gould J., Wolf B. Interfacial and emulsifying properties of mealworm protein at the oil/water interface. Food Hydrocolloids. 2018;77:57–65. [Google Scholar]
- 19.Hall F.G., Jones O.G., Ohaire M.E., Liceaga A.M. Functional properties of tropical banded cricket (Gryllodes sigillatus) protein hydrolysates. Food Chem. 2017;224:414–422. doi: 10.1016/j.foodchem.2016.11.138. [DOI] [PubMed] [Google Scholar]
- 20.Jantzen da Silva Lucas A., Menegon de Oliveira L., da Rocha M., Prentice C. Edible insects: an alternative of nutritional, functional and bioactive compounds. Food Chem. 2020;311 doi: 10.1016/j.foodchem.2019.126022. [DOI] [PubMed] [Google Scholar]
- 21.Mishyna M., Martinez J.-J.I., Chen J., Benjamin O. Extraction, characterization and functional properties of soluble proteins from edible grasshopper (Schistocerca gregaria) and honey bee (Apis mellifera) Food Res. Int. 2019;116:697–706. doi: 10.1016/j.foodres.2018.08.098. [DOI] [PubMed] [Google Scholar]
- 22.Zielinska E., Karas M., Baraniak B. Comparison of functional properties of edible insects and protein preparations thereof. Lebensm. Wiss. Technol. 2018;91:168–174. [Google Scholar]
- 23.Spranghers T., Ottoboni M., Klootwijk C., Ovyn A., Deboosere S., De Meulenaer B., Michiels J., Eeckhout M., De Clercq P., De Smet S. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2017;97:2594–2600. doi: 10.1002/jsfa.8081. [DOI] [PubMed] [Google Scholar]
- 24.Fuso A., Barbi S., Macavei L.I., Luparelli A.V., Maistrello L., Montorsi M., Sforza S., Caligiani A. Effect of the rearing substrate on total protein and amino acid composition in black soldier fly. Foods. 2021;10:1773. doi: 10.3390/foods10081773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rumbos C.I., Karapanagiotidis I.T., Mente E., Psofakis P., Athanassiou C.G. Evaluation of various commodities for the development of the yellow mealworm, Tenebrio molitor. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-67363-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khampakool A., Soisungwan S., You S., Park S.H. Infrared assisted freeze-drying (IRAFD) to produce shelf-stable insect food from protaetia brevitarsis (white-spotted flower chafer) larva. Food Sci. Anim. Resour. 2020;40:813–830. doi: 10.5851/kosfa.2020.e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melgar‐Lalanne G., Hernandez‐Alvarez A., Salinas‐Castro A. Edible insects processing: traditional and innovative technologies. Compr. Rev. Food Sci. Food Saf. 2019;18:1166–1191. doi: 10.1111/1541-4337.12463. [DOI] [PubMed] [Google Scholar]
- 28.BuBler S., Rumpold B.A., Jander E., Rawel H.M., Schluter O.K. Recovery and techno-functionality of flours and proteins from two edible insect species: meal worm (Tenebrio molitor) and black soldier fly (Hermetia illucens) larvae. Heliyon. 2016;2 doi: 10.1016/j.heliyon.2016.e00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao X., Vazquez-Gutierrez J.L., Johansson D.P., Landberg R., Langton M. Yellow mealworm protein for food purposes - extraction and functional properties. PLoS One. 2016;11 doi: 10.1371/journal.pone.0147791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shadung Kagiso G. Influence of drying method and location on proximate chemical composition of African metallic wood boring beetle, Sternocera Orissa (Coleoptera: buprestidae) in Republic of South Africa. Afr. J. Food Sci. 2012;6 [Google Scholar]
- 31.Kroncke N., Boschen V., Woyzichovski J., Demtroder S., Benning R. Comparison of suitable drying processes for mealworms (Tenebrio molitor) Innovat. Food Sci. Emerg. Technol. 2018;50:20–25. [Google Scholar]
- 32.Huang C., Feng W., Xiong J., Wang T., Wang W., Wang C., Yang F. Impact of drying method on the nutritional value of the edible insect protein from black soldier fly (Hermetia illucens L.) larvae: amino acid composition, nutritional value evaluation, in vitro digestibility, and thermal properties. Eur. Food Res. Technol. 2019;245:11–21. [Google Scholar]
- 33.Boye J.I., Barbana C. Food and Industrial Bioproducts and Bioprocessing. Wiley-Blackwell; Oxford, UK: 2012. Protein processing in food and bioproduct manufacturing and techniques for analysis; pp. 85–113. [Google Scholar]
- 34.Son Y.-J., Lee J.-C., Hwang I.-K., Nho C.W., Kim S.-H. Physicochemical properties of mealworm (Tenebrio molitor) powders manufactured by different industrial processes. LWT. 2019;116 [Google Scholar]
- 35.Lhocine L., Boye J.I., Arcand Y. Composition and functional properties of soy protein isolates prepared using alternative defatting and extraction procedures. J. Food Sci. 2006;71:C137–C145. [Google Scholar]
- 36.Kim T.-K., Yong H.I., Kim Y.-B., Jung S., Kim H.-W., Choi Y.-S. Effects of organic solvent on functional properties of defatted proteins extracted from Protaetia brevitarsis larvae. Food Chem. 2021;336 doi: 10.1016/j.foodchem.2020.127679. [DOI] [PubMed] [Google Scholar]
- 37.Ndiritu A.K., Kinyuru J.N., Kenji G.M., Gichuhi P.N. Extraction technique influences the physico-chemical characteristics and functional properties of edible crickets (Acheta domesticus) protein concentrate. J. Food Meas. Char. 2017;11:2013–2021. [Google Scholar]
- 38.Tzompa-Sosa D.A., Yi L., van Valenberg H.J.F., van Boekel M.A.J.S., Lakemond C.M.M. Insect lipid profile: aqueous versus organic solvent-based extraction methods. Food Res. Int. 2014;62:1087–1094. [Google Scholar]
- 39.Yang, R., Zhao, X., Kuang, Z., Ye, M., Luo, G., Xiao, G., & Xiong, Z., n.d. Optimization of antioxidant peptide production in the hydrolysis of silkworm (Bombyx mori L.) pupa protein using response surface methodology. J. Food Agric. Environ. 11, 952–956.
- 40.Purschke B., Stegmann T., Schreiner M., Jager H. Pilot-scale supercritical CO2 extraction of edible insect oil from Tenebrio molitor L. larvae—influence of extraction conditions on kinetics, defatting performance and compositional properties. Eur. J. Lipid Sci. Technol. 2017;119(2) [Google Scholar]
- 41.Mariod A.A., Abdelwahab S.I., Gedi M.A., Solati Z. Supercritical carbon dioxide extraction of sorghum bug (agonoscelis pubescens) oil using response surface methodology. J. Am. Oil Chem. Soc. 2010;87:849–856. [Google Scholar]
- 42.Bolat B., Ugur A.E., Oztop M.H., Alpas H. Effects of High Hydrostatic Pressure assisted degreasing on the technological properties of insect powders obtained from Acheta domesticus & Tenebrio molitor. J. Food Eng. 2021;292 [Google Scholar]
- 43.Nongonierma A.B., FitzGerald R.J. Unlocking the biological potential of proteins from edible insects through enzymatic hydrolysis: a review. Innovat. Food Sci. Emerg. Technol. 2017;43:239–252. [Google Scholar]
- 44.Lam A.C.Y., Can Karaca A., Tyler R.T., Nickerson M.T. Pea protein isolates: structure, extraction, and functionality. Food Rev. Int. 2018;34:126–147. [Google Scholar]
- 45.Singhal A., Karaca A.C., Tyler R., Nickerson M. Grain Legumes. InTech; 2016. Pulse proteins: from processing to structure-function relationships. [Google Scholar]
- 46.Yi L., Van Boekel M.A.J.S., Lakemond C.M.M. Extracting Tenebrio molitor protein while preventing browning: effect of pH and NaCl on protein yield. J. Insects as Food Feed. 2017;3:21–31. [Google Scholar]
- 47.Wang W., Wang N., Zhou Y., Zhang Y., Xu L., Xu J., Feng F., He G. Isolation of a novel peptide from silkworm pupae protein components and interaction characteristics to angiotensin I-converting enzyme. Eur. Food Res. Technol. 2011;232:29–38. [Google Scholar]
- 48.Duong-Ly K.C., Gabelli S.B. 2014. Salting Out of Proteins Using Ammonium Sulfate Precipitation; pp. 85–94. [DOI] [PubMed] [Google Scholar]
- 49.Yi L., Van Boekel M.A.J.S., Boeren S., Lakemond C.M.M. Protein identification and in vitro digestion of fractions from Tenebrio molitor. Eur. Food Res. Technol. 2016;242:1285–1297. [Google Scholar]
- 50.Mintah Benjamin K., He R., Agyekum A.A., Dabbour M., Golly M.K., Ma H. Edible insect protein for food applications: extraction, composition, and functional properties. J. Food Process. Eng. 2020;43 [Google Scholar]
- 51.Santiago L.A., Fadel O.M., Tavares G.M. How does the thermal-aggregation behavior of black cricket protein isolate affect its foaming and gelling properties? Food Hydrocolloids. 2021;110 [Google Scholar]
- 52.Purschke B., Meinlschmidt P., Horn C., Rieder O., Jager H. Improvement of techno-functional properties of edible insect protein from migratory locust by enzymatic hydrolysis. Eur. Food Res. Technol. 2018;244:999–1013. [Google Scholar]
- 53.Okagu O.D., Verma O., McClements D.J., Udenigwe C.C. Utilization of insect proteins to formulate nutraceutical delivery systems: encapsulation and release of curcumin using mealworm protein-chitosan nano-complexes. Int. J. Biol. Macromol. 2020;151:333–343. doi: 10.1016/j.ijbiomac.2020.02.198. [DOI] [PubMed] [Google Scholar]
- 54.Queiroz L.S., Casanova F., Feyissa A.H., Jessen F., Ajalloueian F., Perrone I.T., de Carvalho A.F., Mohammadifar M.A., Jacobsen C., Yesiltas B. Physical and oxidative stability of low-fat fish oil-in-water emulsions stabilized with black soldier fly (Hermetia illucens) larvae protein concentrate. Foods. 2021;10:2977. doi: 10.3390/foods10122977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Castro R.J.S., Ohara A., Aguilar J.G.D.S., Domingues M.A.F. Nutritional, functional and biological properties of insect proteins: processes for obtaining, consumption and future challenges. Trends Food Sci. Technol. 2018;76:82–89. [Google Scholar]
- 56.Kim T.-K., Yong H.I., Chun H.H., Lee M.-A., Kim Y.-B., Choi Y.-S. Changes of amino acid composition and protein technical functionality of edible insects by extracting steps. J. Asia Pac. Entomol. 2020;23:298–305. [Google Scholar]
- 57.Chatsuwan N., Nalinanon S., Puechkamut Y., Lamsal B.P., Pinsirodom P. Characteristics, functional properties, and antioxidant activities of water-soluble proteins extracted from grasshoppers, Patanga succincta and Chondracris roseapbrunner. J. Chem. 2018:1–11. [Google Scholar]
- 58.Osasona A.I., Olaofe O. Nutritional and functional properties of Cirina forda larva from Ado-Ekiti, Nigeria. Afr. J. Food Sci. 2010;4(12):775–777. [Google Scholar]
- 59.Akpossan R., Digbeu Y., Koffi M., Kouadio J., Dué E., Kouamé P. Protein fractions and functional properties of dried imbrasia oyemensis larvae full-fat and defatted flours. Int. J. Biochem. Res. Rev. 2015;5:116–126. [Google Scholar]
- 60.Assielou B., Due E., Koffi M., Dabonne S., Kouame P. Oryctes owariensis larvae as good alternative protein source: nutritional and functional properties. Annu. Res. Rev. Biol. 2015;8:1–9. [Google Scholar]
- 61.Jiang Y., Zhu Y., Zheng Y., Liu Z., Zhong Y., Deng Y., Zhao Y. Effects of salting-in/out-assisted extractions on structural, physicochemical and functional properties of Tenebrio molitor larvae protein isolates. Food Chem. 2021;338 doi: 10.1016/j.foodchem.2020.128158. [DOI] [PubMed] [Google Scholar]
- 62.Lee H., Kim J., Ji D., Lee C. Effects of heating time and temperature on functional properties of proteins of yellow mealworm larvae (Tenebrio molitor L.) Food Sci. Anim. Resour. 2019;39:296–308. doi: 10.5851/kosfa.2019.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lassé M., Deb-Choudhury S., Haines S., Larsen N., Gerrard J.A., Dyer J.M. The impact of pH, salt concentration and heat on digestibility and amino acid modification in egg white protein. J. Food Compos. Anal. 2015;38:42–48. [Google Scholar]
- 64.Fennema O. third ed. Marcel Dekker, Inc; 1996. Food Chemistry. [Google Scholar]
- 65.Jo Y., Benoist D.M., Barbano D.M., Drake M.A. Flavor and flavor chemistry differences among milks processed by high-temperature, short-time pasteurization or ultra-pasteurization. J. Dairy Sci. 2018;101:3812–3828. doi: 10.3168/jds.2017-14071. [DOI] [PubMed] [Google Scholar]
- 66.Sathe S.K., Zaffran V.D., Gupta S., Li T. Protein solubilization. J. Am. Oil Chem. Soc. 2018;95:883–901. [Google Scholar]
- 67.Purschke B., Tanzmeister H., Meinlschmidt P., Baumgartner S., Lauter K., Jager H. Recovery of soluble proteins from migratory locust (Locusta migratoria) and characterisation of their compositional and techno-functional properties. Food Res. Int. 2018;106:271–279. doi: 10.1016/j.foodres.2017.12.067. [DOI] [PubMed] [Google Scholar]
- 68.Ravi H.K., Degrou A., Costil J., Trespeuch C., Chemat F., Vian M.A. Effect of devitalization techniques on the lipid, protein, antioxidant, and chitin fractions of black soldier fly (Hermetia illucens) larvae. Eur. Food Res. Technol. 2020;246:2549–2568. [Google Scholar]
- 69.Plancken Iesel Van der Loey, Ann Van.Hendrickx M.E.G. Changes in sulfhydryl content of egg white proteins due to heat and pressure treatment. J. Agric. Food Chem. 2005;53(14):5726–5733. doi: 10.1021/jf050289+. [DOI] [PubMed] [Google Scholar]
- 70.Trevino S.R., Scholtz J.M., Pace C.N. Amino acid contribution to protein solubility: asp, glu, and ser contribute more favorably than the other hydrophilic amino acids in RNase sa. J. Mol. Biol. 2007;366:449–460. doi: 10.1016/j.jmb.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Azagoh C., Ducept F., Garcia R., Rakotozafy L., Cuvelier M.-E., Keller S., Lewandowski R., Mezdour S. Extraction and physicochemical characterization of Tenebrio molitor proteins. Food Res. Int. 2016;88:24–31. doi: 10.1016/j.foodres.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 72.Aryee A.N.A., Agyei D., Udenigwe C.C. Proteins in Food Processing. Elsevier; 2018. Impact of processing on the chemistry and functionality of food proteins; pp. 27–45. [Google Scholar]
- 73.Aremu M.O., Olaofe O., Akintayo E. Functional properties of some Nigerian varieties of legume seed flour concentration effect on foaming and gelation properties. J. Food Technol. 2007;5(2):109–115. [Google Scholar]
- 74.Torruco-Uco J.G., Hernandez-Santos B., Herman-Lara E., Martinez-Sanchez C.E., Juarez-Barrientos J.M., Rodriguez-Miranda J. Chemical, functional and thermal characterization, and fatty acid profile of the edible grasshopper (Sphenarium purpurascens Ch.) Eur. Food Res. Technol. 2019;245:285–292. [Google Scholar]
- 75.Milani M., Ni J., Golkar A. Some New Aspects of Colloidal Systems in Foods. IntechOpen; 2019. Introductory chapter: some new aspects of colloidal systems in foods. [Google Scholar]
- 76.Wu H., Morbidelli M. A model relating structure of colloidal gels to their elastic properties. Langmuir. 2001;17:1030–1036. [Google Scholar]
- 77.Banerjee S., Bhattacharya S. Food gels: gelling process and new applications. Crit. Rev. Food Sci. Nutr. 2012;52:334–346. doi: 10.1080/10408398.2010.500234. [DOI] [PubMed] [Google Scholar]
- 78.Kumar S., Queiroz L.S., Marie R., Nascimento L.G.L., Mohammadifar M.A., de Carvalho A.F., Brouzes C.M.C., Fallquist H., Fraihi W., Casanova F. Gelling properties of black soldier fly (Hermetia illucens) larvae protein after ultrasound treatment. Food Chem. 2022;386 doi: 10.1016/j.foodchem.2022.132826. [DOI] [PubMed] [Google Scholar]
- 79.Murray B.S., Ettelaie R. Foam stability: proteins and nanoparticles. Curr. Opin. Colloid Interface Sci. 2004;9:314–320. [Google Scholar]
- 80.Dickinson E. Structuring of colloidal particles at interfaces and the relationship to food emulsion and foam stability. J. Colloid Interface Sci. 2015;449:38–45. doi: 10.1016/j.jcis.2014.09.080. [DOI] [PubMed] [Google Scholar]
- 81.Dickinson E. Food emulsions and foams: stabilization by particles. Curr. Opin. Colloid Interface Sci. 2010;15:40–49. [Google Scholar]
- 82.Medrano A., Abirached C., Araujo A., Panizzolo L., Moyna P., Anon M. Correlation of average hydrophobicity, water/air interface surface rheological properties and foaming properties of proteins. Food Sci. Technol. Int. 2012;18:187–193. doi: 10.1177/1082013211415137. [DOI] [PubMed] [Google Scholar]
- 83.Russin T.A., Boye J.I., Arcand Y., Rajamohamed S.H. Alternative techniques for defatting soy: a practical review. Food Bioprocess Technol. 2011;4:200–223. [Google Scholar]
- 84.Queiroz L.S., Regnard M., Jessen F., Mohammadifar M.A., Sloth J.J., Petersen H.O., Ajalloueian F., Brouzes C.M.C., Fraihi W., Fallquist H., de Carvalho A.F., Casanova F. Physico-chemical and colloidal properties of protein extracted from black soldier fly (Hermetia illucens) larvae. Int. J. Biol. Macromol. 2021;186:714–723. doi: 10.1016/j.ijbiomac.2021.07.081. [DOI] [PubMed] [Google Scholar]
- 85.Lam R.S.H., Nickerson M.T. Food proteins: a review on their emulsifying properties using a structure–function approach. Food Chem. 2013;141:975–984. doi: 10.1016/j.foodchem.2013.04.038. [DOI] [PubMed] [Google Scholar]
- 86.Li F., Wang B., Kong B., Shi S., Xia X. Decreased gelling properties of protein in mirror carp (Cyprinus carpio) are due to protein aggregation and structure deterioration when subjected to freeze-thaw cycles. Food Hydrocolloids. 2019;97 [Google Scholar]
- 87.OSullivan J., Murray B., Flynn C., Norton I. The effect of ultrasound treatment on the structural, physical and emulsifying properties of animal and vegetable proteins. Food Hydrocolloids. 2016;53:141–154. [Google Scholar]
- 88.Damodaran S. Protein stabilization of emulsions and foams. J. Food Sci. 2006;70:R54–R66. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.