Abstract
Microglia are the only resident innate immune cells derived from the mesoderm in the nerve tissue. They play a role in the development and maturation of the central nervous system (CNS). Microglia mediate the repair of CNS injury and participate in endogenous immune response induced by various diseases by exerting neuroprotective or neurotoxic effects. Traditionally, microglia are considered to be in a resting state, the M0 type, under physiological conditions. In this state, they perform immune surveillance by constantly monitoring pathological responses in the CNS. In the pathological state, microglia undergo a series of morphological and functional changes from the M0 state and eventually polarize into classically activated microglia (M1) and alternatively activated microglia (M2). M1 microglia release inflammatory factors and toxic substances to inhibit pathogens, while M2 microglia exert neuroprotective effects by promoting nerve repair and regeneration. However, in recent years, the view regarding M1/M2 polarization of microglia has gradually changed. According to some researchers, the phenomenon of microglia polarization is not yet confirmed. The M1/M2 polarization term is used for a simplified description of its phenotype and function. Other researchers believe that the microglia polarization process is rich and diverse, and consequently, the classification method of M1/M2 has limitations. This conflict hinders the academic community from establishing more meaningful microglia polarization pathways and terms, and therefore, a careful revision of the concept of microglia polarization is required. The present article briefly reviews the current consensus and controversy regarding microglial polarization typing to provide supporting materials for a more objective understanding of the functional phenotype of microglia.
Keywords: Microglia, Polarization, M1/M2 subtype, Multiple subtypes
Highlights
-
•
Microglia polarization towards M1/M2 type.
-
•
The idea that microglia polarize into two M1/M2 phenotypes is questioned.
-
•
Several possible subtypes of microglia.
1. Introduction
In the early 19th century, central nervous system (CNS) anatomists used different stains to reveal the morphology of neurons to gain insights into the anatomy of the brain at a time when glial cells were yet to be discovered. In the mid-19th century, German pathologist Rudolf Virchow used the term “glue” (the word “glue” originated from ancient Greek) to describe the entire non-neuronal compartment of the CNS [1,2]. Virchow believed that glial cells are a type of connective tissue that connects and supports various neurons; he also found that this tissue contains various cells. However, the concept of microglia was still not proposed at that time. In the early 20th century, microglia were discovered by the Spanish neuroscientist Río Hortega as a distinct cell type. Hortega [3] showed that microglia are mainly derived from primordial macrophages produced in the yolk sac and migrate from the external environment of the brain to the brain parenchyma in the early stage of brain development (approximately 10.5 days of embryonic development). Before the early 21st century, researchers did not recognize the critical role of microglia in the CNS. In subsequent studies, scientists found that microglia in the CNS can interact with neurons, astrocytes, and oligodendrocytes and play a persistent role in immune surveillance [[4], [5], [6]]. The phenotype of microglia has been a key issue while investigating its origin and function. In particular, with the advancement in sequencing technology, microglia have been found to occur from the periphery to the center of the CNS. This not only enabled researchers to achieve great milestones in the study of microglia in the past 20 years, but it also made them aware of the current challenges in this field. According to some researchers, the classification of microglia based on its polarization, that is, the strict division of microglia based on its M1/M2 polarization is a too simplified theory, and they believe that the phenotype and function of microglia are diverse. Presently, the review of microglial cell typing is mostly focused on M1/M2 phenotypes. This article initially presents the view that microglial polarization is based on M1/M2 phenotypes. In the context of the current challenges for this consideration and the several newly discovered microglial subtypes, the present article will provide support for a more comprehensive and objective understanding of microglial polarization typing and then provide novel ideas for developing new brain neural research and related treatment methods based on microglia.
2. M1/M2 polarization of microglia
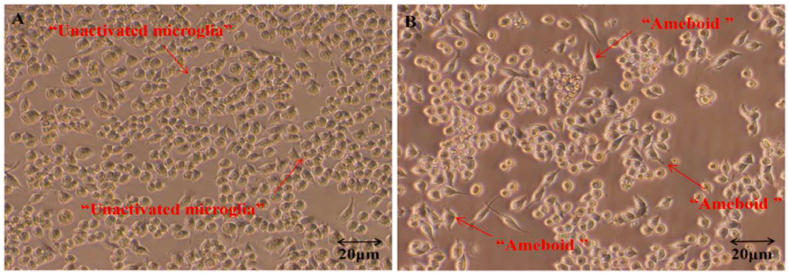
As resident immune cells in the brain, microglia are involved in the development of brain tissue structure, maintenance of the physiological functions of the CNS, and restoration of impaired immune responses and subsequent repair. Under physiological conditions, microglia maintain a relatively quiescent neuron-specific monitoring phenotype (M0 phenotype) and play an “immune surveillance and defense” role in the microenvironment of nerve cells [[7], [8], [9]]. Microscopic dynamic real-time observations revealed that under physiological conditions, microglia appear to be in a “quiescent” state; however, they actually extend out of the cell body, constantly monitoring for any potential damage in the brain. Thus, microglia perform a complete assessment of the brain for any damage every few hours. To maintain this immune surveillance state, microglia need to receive a continuous “all is well” signal from the surrounding cells. The lack of this signal will immediately prompt microglia to conduct an immune status investigation [[10], [11], [12]]. In the state of pathological injury, microglial cells are rapidly activated and polarized, accompanied by a series of phenomena such as proliferation, chemotaxis, phagocytosis, migration, and cytokine secretion; these cells then rapidly generate complex responses depending on the degree of injury. If the injury is mild, the cells at the injury site send a “find me” signal; subsequently, microglial cells are rapidly activated to the M2 state, which is characterized by distal branches, small cell body-like changes, and secretion of anti-inflammatory factors. The M2 microglial cells can promote cell tissue repair and regeneration, enhance phagocytic activity, and play a neuroprotective role. If severe or persistent tissue damage occurs, the cells at the injury site send an “eat me” signal. Subsequently, microglial cells are completely activated into the pro-inflammatory or toxic M1 state, which is characterized by amoeboid changes with large and round cell bodies and thick protrusions [[13], [14], [15]]. These cells mainly exert cytotoxic effects and can quickly remove infected cells and debris of dying cells. Fig. 1 shows the morphological changes of microglia before and after polarization. Excessive activation of M1 microglia, however, can cause loss, damage, and degeneration of neuronal function, and M1 microglia have been shown to play an important role in cerebrovascular diseases and neurodegenerative diseases [8,14]. In summary, several studies have shown that M1 and M2 microglia perform different functions at different stages of inflammation and disease; in other words, M1 and M2 microglia switch between functional phenotypes according to different environmental stimuli.
Fig. 1.
Morphological changes in microglia before and after polarization. A: Microglia in the unactivated state of growth in good condition, with a clear background. The cell body is small and occasionally observed between cell synaptic connections. The microglial cell has a full three-dimensional shape with fewer branches. B: After activation, microglia cell debris increased. The background is not clear; with an increase in the size of the cell body, the emergence of “amoeba-like cells,” increased synaptic connections between the cell bodies, and increased branches.
2.1. M1 polarization of microglia (classically activated)
M1 microglia are considered to be pro-inflammatory cells. Following appropriate stimuli, M1 microglia serve as the first line of defense of the neuroimmune system, and they usually appear within a few hours of stimulation [[16], [17], [18]]. Microglia use a series of immune receptors to recognize harmful stimuli, such as Toll-like receptors, nucleotide oligomerization domain receptors, and scavenger receptors. Activated microglia release high levels of pro-inflammatory cytokines such as interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), and interleukin-1β (IL-1β), and a sustained inflammatory response continues to aggravate brain damage. In addition to pro-inflammatory cytokines, M1 microglia secrete a large amount of reactive oxygen species (ROS); proteolytic enzymes, including heme oxygenase 1 (HO-1), matrix metalloproteinases (MMPs), and inducible nitric oxide synthase (iNOS); and interleukin 12 (IL-12). This process causes T cells to trigger an adaptive immune response and remove foreign pathogens [[19], [20], [21], [22]].
In many model studies, the level of M1 microglia was found to increase when bacterial components such as lipopolysaccharide (LPS) or infection-related signals such as IFN-γ increase [[23], [24], [25]]. When the central nervous system experience a sterile inflammatory response caused by trauma or ischemic reperfusion, microglia polarize into the M1 type and secrete pro-inflammatory cytokines, including interleukin-1α (IL-1α), interleukin-6 (IL-6), IL-12, chemokines, reduced nicotinamide adenine dinucleotide phosphate (NADPH), surface antigen CD40, etc. [[26], [27], [28]]. Microglia models in rodents have shown that polarized M1 microglia induced by LPS and IFN-γ inhibit phagocytosis. The outcome of M1 microglia polarization depends on many aspects, including the production of iNOS, ROS, or NLRP3 inflammasome.
In conclusion, classically activated M1 microglia mainly secrete several pro-inflammatory factors that are involved in the process of pathogen clearance and alleviation of inflammatory injury of diseases. Microglia with M1 phenotype can inhibit phagocytosis and promote inflammation which can produce several inflammatory mediators that cause neuronal damage and further activate the release of pro-inflammatory factors, leading to a neuroinflammatory cascade. Moreover, following the overactivation of microglia with M1 phenotype, the inflammatory response of the CNS is further amplified and sustained, thereby interfering with nerve repair.
2.2. M2 polarization of microglia (alternative activation)
It is generally believed that M2 microglia are protective cells that secrete anti-inflammatory factors and upregulate neuroprotective factors. They play an active role in immune defense by providing protection against organisms, fighting infections, acting as antigen-presenting cells, killing tumor cells, performing tissue repair, providing chemotaxis orientation, and eliminating invading bacteria.
Under various microenvironment stimuli, M2 microglia can further differentiate into three subtypes: M2a, M2b, and M2c [2,29,30]. M2a and M2b play an immunoregulatory role or promote M2 immune response, while M2c inhibits immune response and tissue remodeling. M2a is activated by interleukin-4 (IL-4) or interleukin-13 (IL-13), which can promote fibrosis and repair tissues [31]. M2b type is jointly induced by Toll-like receptors or IL-1 receptors combined with immune complexes [31]. M2c type is stimulated by interleukin-10 (IL-10), transforming growth factor-β (TGF-β), and glucocorticoid release in response to specific anti-inflammatory effects [31]. Other interleukin cytokines such as interleukin-3 (IL-3), interleukin-21 (IL-21), and interleukin-33 (IL-33) and chemokines CCL2 and CXCL4 can also induce microglia to undergo M2 polarization [23,32,33]. The M2 polarization of microglia is identical to the polarization process of peripheral macrophages. This association was confirmed in in vitro experiments. However, in the absence of IL-4 or IL-13, microglia also polarize to M2 in aseptic wounds. This process suggests the presence of an alternative stimulus. In this process, M2 microglia are derived from M1 microglia, and they are transformed from pro-inflammatory microglia in the circulatory system to mature microglia with the ability to repair [[34], [35], [36]]. Therefore, the intrinsic phenotype of cells may vary due to different sources and environments.
M2 microglia promote inflammation resolution and restore homeostasis through anti-inflammatory factors such as IL-10, IL-13, TGF-β, and arginase 1 (Arg1). This includes reducing the levels of inflammatory factors by producing IL-10 and increasing the phagocytosis of apoptotic cells through IL-10-stimulated cells. In contrast, in the absence of IL-10 overexpression, the phagocytosis of macrophages is not enhanced [[37], [38], [39]]. M2 microglia polarization is characterized by the activation of peroxisome proliferator-activated receptors, following which M2 microglia express chemokines (CCL17, CCL22, and CCL24) [[40], [41], [42]]. Researchers believe that gladetomil acetate, a drug used to treat multiple sclerosis, induces the expression of insulin-like growth factor 1 (IGF-1) in microglia to exert neuroprotective effects. In IL-4-deficient model mice, the clinical symptoms of multiple sclerosis were observed to be aggravated, thus, suggesting that IL-4 deficiency is associated with reduced M2 microglial phenotype [33,43,44]. Microglial cells induced by LPS and IFN-γ damage neurons, whereas those induced by IL-4 stimulate neuronal synaptic growth. This series of studies have established a relationship between the M2 microglial phenotype and neuronal repair and damage reduction [45,46]. A schematic diagram of microglial polarization is shown in Fig. 2.
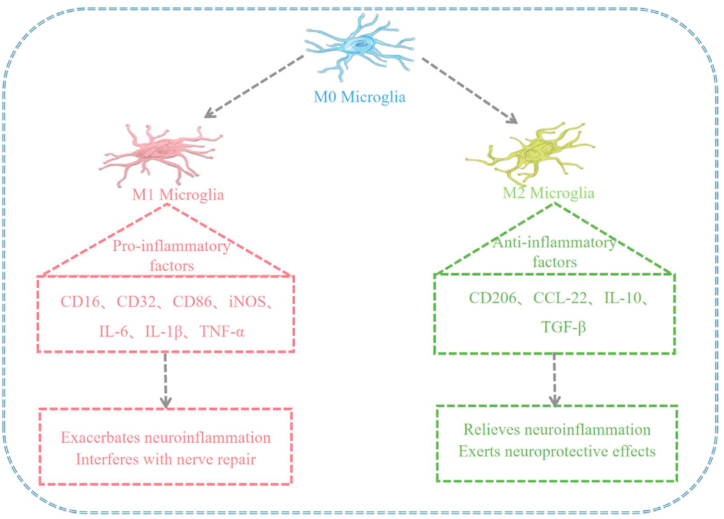
Fig. 2.
Microglia polarization diagram: M1 microglia release pro-inflammatory factors and toxic substances to kill pathogens, while M2 microglia exert neuroprotective effects by promoting tissue repair and regeneration. Overactivated M1 microglia can cause neuronal disability, damage, and degeneration and play an important role in cerebrovascular diseases, neurodegenerative diseases, neurodevelopmental disorders, and mental disorders.
In summary, the current belief is that M2 microglia are the key cells involved in repair after CNS injury, and they possess neuroprotective effects. Compared to the pro-inflammatory effects of M1 microglia, M2 microglia promote the release of anti-inflammatory factors, thus showing an anti-inflammatory effect and inhibiting immune response. M2 microglia can also reduce local inflammation by engulfing cell debris at the injury site, thereby promoting the recovery of brain tissue and participating in damaged tissue remodeling. M2 microglia can be further divided into M2a, M2b, and M2c subtypes. Microglia can be categorized into different subtypes according to different functions, thus, suggesting the diversity of microglia phenotypes. A schematic diagram of Microglia activation and inactivation diagram is shown in Fig. 3.
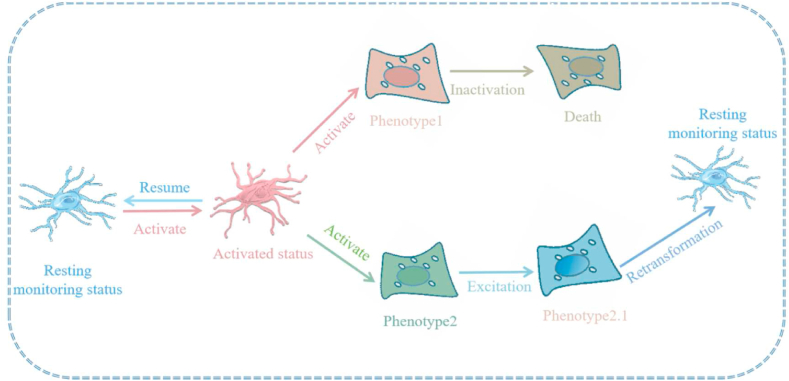
Fig. 3.
Microglia activation and inactivation diagram: Resting microglia are constantly in an “immune surveillance” state. After stimulation, microglia convert to an activated state and then return again to the resting state. Following stimulation, microglial cells change their morphological appearance and become “amoeba-like” glial cells, which respond to external stimuli, play an immunological role, and then return to the resting state. Some microglial cells are inactivated and die during the immune response.
3. Questioning the view that microglial polarization is an M1/M2 phenotype process
The microglial subtype was originally proposed by the Spanish neurologist Río Hortega in the early 20th century; however, it was not until 2016 that the idea was re-proposed. Following the emergence of photon imaging, genome-wide transcription analysis, and epigenetic analysis, the research on microglia entered a white-hot stage. An increasing number of researchers believe that the polarization pattern of M1 and M2 microglia is a simplified form to interpret data without the determination of the individual source and function of microglia. The description of the M1/M2 phenotype hinders the establishment of more meaningful microglia polarization pathways and terms in academic research and should therefore be abandoned [47].
3.1. Naming convention used for peripheral macrophages is not suitable for microglia
The M1/M2 polarization pattern of microglia is identical to that of macrophages; however, several lines of evidence show a large difference between macrophages and microglia in peripheral blood [34,[48], [49], [50]]. For example, the transcriptomes of microglia and macrophages from blood and tissues are significantly different; moreover, microglia and mononuclear macrophages show significant differences in expression profile, function, and structural characteristics. These studies showed that activated microglia and macrophages derived from monocytes are completely different cells. Infiltrating macrophages after tissue damage or infection do not follow the M1/M2 polarization pattern. If the nomenclature of peripheral macrophage typing is applied to microglia, we will have an insufficient understanding of the concept of microglial typing. Furthermore, peripheral macrophages themselves include dozens of different types of cells. The nomenclature of M1/M2 is only a macroscopic and broad concept [[51], [52], [53]]. Considering the irreplaceable role of microglia in the CNS, this nomenclature may not be appropriate for microglia.
Researchers also consider that if this model is still used, it will seriously hinder the progress of research on microglia. The specific reasons include the following aspects [54]. First, the classification of M1/M2 is made to enable in vitro culture of microglia by using a single stimulation mode, which is inconsistent with the highly complex environment of the human CNS [55]. Second, according to recent studies, the phenomenon that microglia polarize only to the M1 or M2 phenotype does not occur exclusively in the body [56]. Finally, the M1/M2 polarization of macrophages is an inflammatory response that occurs when infiltrating monocytes or bone marrow-derived macrophages encounter pathogens, dead cells, bacterial debris, or tumor cells in diseased tissues. In contrast, microglia are resident macrophages in the body that are different from the macrophages occurring in the hematopoietic circulatory system [57,58]. On the basis of these reasons, the simple division of microglia into the M1/M2 phenotype may not be applicable.
3.2. Microglia subtypes should not be classified solely on the basis of molecular characteristics and markers
Vassilis Stratoulias et al. believed that [59] microglia may constitute a cell community in which different subtypes exhibit varying properties, perform different physiological functions, and respond differently to stimuli. Stratoulias suggested that microglia subtypes should be classified according to function rather than molecular characteristics and markers. There is also new evidence that different phenotypes of microglia exhibit varying functions, which are determined not only by their environment but also by their intrinsic characteristics. That is, such cells are a highly plastic single-cell phenotype with various different functional phenotypes, thus exerting different physiological and pathological effects. This study also recognized that the multiple activation states of microglia are not entirely consistent with the M1/M2 classification currently used at this stage. Microglia perform multiple functions through various phenotypes, each of which corresponds to a different phenotype.
Some evidence also shows that the different phenotypes of microglia exhibit different intrinsic characteristics acquired during the development and maturation of the CNS. Under physiological conditions, these subtypes coexist in the CNS. Furthermore, under pathological conditions, these cell subtypes undergo different phenotypic transformations according to different stimuli to perform varying physiological functions. Therefore, researchers suggest that microglia subtypes should be classified according to the function of microglia rather than according to their molecular characteristics [[60], [61], [62]].
4. Possible microglial subtypes
Researchers believe that microglia exhibit varying levels of response to different stimuli. Although microglia are widely distributed in the CNS, their distribution varies in different regions. In an unbiased single-cell RNA sequencing study [56], 47 different cell subtypes were identified by analyzing the brain and hippocampus of unstimulated adult mice, and two of these cell subtypes belonged to microglia. Microglia may constitute a cell community in which different subtypes exhibit different properties, perform varying physiological functions, and respond differently to stimuli.
4.1. “Satellite” microglia
In 1919, Rio-Hortega found that the cell body of a type of microglia was associated and interacted with the initial segment of a neuronal axon. The researcher named this type of microglia as “satellite” microglia, which was located on the axon side of the neuron cell body and overlapped with the initial segment of the neuronal axon (i.e., the part of the neuron potential initiation). “Satellite” microglia are found in the development stage of mice and during the adult stage, and they also occur in the cerebral cortex of primates; thus, they are closely related to neuronal excitation [63,64]. Researchers believe that “satellite” microglia may be a transitional state in which microglia exert immune surveillance [65]. Because no markers of “satellite” microglia have been found, it is not yet possible to determine the intrinsic differences between them and microglia. Wogram et al., however, confirmed that “satellite” microglia mainly exist in the cerebral cortex, thalamus, and hippocampus [66]. Stowell et al. observed the dynamic migration process of “satellite” microglia through two-photon in vivo imaging and observed their interaction with the proximal dendrites of neurons [67,68]. These microglia also show differences in self-renewal and transformation under normal physiological conditions and LPS stimulation. Presently, there are a limited number of studies on the function of “satellite” microglia; thus, resulting in an insufficient understanding of many aspects of these microglia. We expect that further exploration of these cells will enable us to elucidate more new functions of microglia.
4.2. KSPG microglia
Keratan sulfate proteoglycan (KSPG) is an extracellular matrix molecule that forms boundaries for axon growth in the developing brain and spinal cord. KSPG microglia, located on the extracellular matrix and cell surface, are keratin sulfate-positive microglia detected around motor neurons in amyotrophic lateral sclerosis [69,70]. Tuszynsk et al. [71] used 5D4 monoclonal antibodies in the brain of healthy adult rats for in situ observation and found that KSPG microglia showed heterogeneity, which contributed to axonal growth and cell adhesion. In particular, 5D4-KSPG microglia are expressed by branch microglia subsets, which completely contrasts with amoeboid microglia. The expression of 5D4-KSPG microglia significantly varied in different varieties of inbred rats. A subset of microglia expressing 5D4-KSPG was also detected in the mammalian spinal cord [72]. Although 5D4-KSPG microglia are abundantly found in the hippocampus and brainstem, only a few of them are detected in the cerebellum and cerebral cortex. Previous studies have shown that 5D4-KSPG microglia and 5D4-KSPG-negative microglia coexist in the same region of the CNS; however, these studies suggest the existence of two different subtypes of KSPG microglia. On the basis of the reactivity of KSPG microglia, a comprehensive and systematic approach is needed.
4.3. Hoxb8 microglia
Compared to typical microglial subtypes, Hoxb8 microglial cells differ in molecular characteristics and spatial distribution. Mice carrying the Hoxb8-Cre and ROSA26-YFP genes were hybridized to track the expression of YFP-Hoxb8 [73]. In the adult brain, microglial cells were the lone cells that showed the YFP signal, i.e., the localization of the YFP signal revealed the presence of microglial cells throughout the brain, especially in the cerebral cortex. Transcriptional analysis of Hoxb8-positive and Hoxb8-negative microglia revealed significant differences in the expression of only 21 genes [74]. Compared to non-Hoxb8 microglia, Hoxb8 microglia express microglia-specific genes such as Tmem119, Sall3, and Ms4a7 and also express hematopoietic-related characteristic genes such as Clel12a, Klra2, and Lilra5. In Hoxb8 mutant mice, selective inactivation of Hoxb8 is sufficient to cause pathological phenomena in the hematopoietic system [75]. Previous studies have also shown that the loss of Hoxb8 function in microglia affects corticostriatal neuronal circuits, leading to anxiety and impaired social behavior [[76], [77], [78]]. The emergence of Hoxb8 microglia reveals that microglia play an important role in the hematopoietic system and behavior, thereby further enriching the function of microglia.
4.4. CD11c microglia
In 2017, Kohno et al. reported another subtype of microglia, CD11c, in the brain of neonatal mice, which showed a positive result for CD11c expression [79]. The number of CD11c microglia first increased to 20% of the total number of microglia after birth and then decreased to 3% of the total microglia number. The reasons for this alteration in the microglial population need to be determined, which may be related to cell death, cell migration, or differentiation of microglia subsets [80]. CD11c-positive microglia are unevenly distributed during brain development, and they are concentrated in the corpus callosum and cerebellar white matter. CD11c-positive microglia are also the main source of IGF1, and this microglia subtype selectively consumes IGF1 and affects the development of myelin [79,81]. Therefore, CD11c-positive microglia in the brain of neonatal mice play an important role in myelin development and neurogenesis. Kamphuis et al. suggested that the number of CD11c microglia is positively correlated with age and is also associated with Aβ protein deposition in different Alzheimer’s disease models [81]. This clarifies that the close correlation between the subtype and age should not be ignored when focusing on the number and function of microglia.
Two specific subtypes of microglia exist, namely “microglial cells supporting neurogenesis,” which are critical for the survival and migration of nerve cells [82], and “dark microglia,” which interact with blood vessels and synapses and are characterized by a dark color in electron microscopy [[83], [84], [85]]. “Microglia supporting neurogenesis” are critical for neuroblast survival and migration. On the other hand, dark microglial cells are rarely observed in healthy adult mice; however, they are commonly detected in aged mice, CX3CR1 knockout mice, and Alzheimer’s disease model mice. These findings suggest that dark microglial cells may represent a subtype of microglia and play a special role in development, stress, and disease [83].
The abovementioned studies confirmed the presence of several different subtypes of microglia that can be differentiated on the basis of their function. It should be noted that in most cases, microglial subtypes and typical microglial cells exist simultaneously in the same environment, and this state will change according to the internal and external environment; this aspect should be investigated in detail in further studies. Single-cell RNA sequencing technology also supports the existence of various subtypes of microglia. For example, Hammond et al. [86] reported the presence of eight transcriptomes of microglial clusters in the mouse brain, some of which are involved in cellular immune function, some microglial clusters decrease dramatically during postnatal development, and some microglial clusters show gender differences. RNA sequencing revealed that different subtypes of microglial cells correspond to varying functions, thus, providing strong evidence for the presence of microglial subtypes. Many scientists also classify microglia on the basis of their functions and propose the concept of microglial diversity, as shown in Table 1.
Table 1.
Different types of glial cells.
| Number | Types of microglia | Concept | Function | Reference |
|---|---|---|---|---|
| 1 | “Satellite” microglia | “Satellite” microglia are located on the axon side of the neuronal cell body and overlap with the site where the neuronal potential starts, mainly in the cerebral cortex, thalamus, and hippocampus | “Satellite” glial cells are the most abundant in sensory ganglia and play a major role in sensory function; they are closely related to neuronal excitation | [15,23,44,45,62] |
| 2 | KSPG microglia | KSPG microglia are located in the extracellular matrix and cell surface; they are positive for keratin sulfate | Help cell axon growth and adhesion, establish boundaries for axonal growth in the developing brain and spinal cord and are a potential inhibitor of axon growth | [30,31,33,34] |
| 3 | Hoxb8 microglia | Hoxb8 glial cells are derived from the Hoxb8 lineage in the second wave (E8.5) hematopoiesis of the yolk sac | Loss of Hoxb8 glial function causes behaviors such as chronic anxiety and obsessive-compulsive disorder | [10,17,29,53,69,73] |
| 4 | CD11c microglia | CD11c expression is positive in these glial cells | Play an important role in myelin development and neurogenesis and are associated with Aβ protein deposition | [36,42,52] |
| 5 | Support neurons to form microglia | Glial cells associated with neurogenesis contribute to embryonic neurogenesis and neuronal differentiation and development | Critical for adult neurogenesis, removal of apoptotic neurons, learning-dependent synapse formation, synaptic and spinal remodeling, axon guidance, and survival and migration of neuroblasts | [32] |
| 6 | “Dark” microglia | Immunohistochemically appear as “dark” glial cells in transmission electron microscopy, which are often in contact with the synaptic cleft while extensively surrounding axon terminals | Interactions with blood vessels and synapses; they may be linked with the pathological remodeling in neural circuits that can impair learning, memory, and other basic cognitive functions | [4,63,64] |
5. Summary
Significant advancements have been made in the study of microglia polarization typing. RNA sequencing expression profiles, complementary techniques, and epigenomic analysis provide broad prospects for studying the activity of microglia in the healthy and pathological CNS and for developing appropriate therapeutic strategies for these cells. However, regarding the use of the nomenclature of peripheral macrophages to study microglia, our understanding of the concept of microglia typing is insufficient, and it is difficult to continue to conduct more systematic and in-depth research without an appropriate approach for microglia typing. Researchers have also found that microglia have richer neurobiological functions during development, including regulating overactive neurons, pruning synapses, and promoting neuronal survival. M1/M2 microglia typing does not show the complete biological functions of microglia. Therefore, on the basis of the source, we need a new nomenclature system to redefine microglia and their subtypes. Similarly, if the M1/M2 microglia polarization typing method is no longer used, it also implies that researchers cannot determine the activation status of microglia based only on the typical markers of M1/M2 (iNOS, Arg1, CD206, TGF-β, TNF-α, and CD11b). These components have been widely used to identify the activation status of microglia. Thus, the complete expression of reactive microglia can be determined by monitoring one or several gene expression markers. Reactive microglia express only two polarization products and are thus not comprehensive.
Summarizing previous studies, RNA transcriptomics, proteomics, and other computational biology and transcriptomics techniques should be used to investigate the CNS from the embryonic development stage to the aging stage and to reclassify the subtypes of microglia in different pathological states such as trauma, infection, ischemia, inflammation, and neurodegeneration. The repeated use of the M1/M2 typing method cannot positively promote the transcriptional expression profile analysis of microglia. Existing studies have revealed that the subtypes of microglia may coexist in the CNS and that microglia exhibit varying characteristics in different environments. Therefore, researchers should use different methods to recognize microglia and classify their subtypes in detail. In summary, a complete microglial subtype structure should be established that could help understand the biological functions of microglia through whole-genome transcriptomics, epigenomics, and proteomics. Considering that different microglia play various functions under normal physiological condition, we can better understand the diversity of microglia according to their regional distribution and gene expression. The classification of microglia should not be limited to its division into M1/M2 phenotypes based on cell surface markers. Although sufficient progress has been made in the study of microglia phenotypic polarization, the complete picture remains to be further discovered.
5. Outlook
The development and evolution of microglia in biology have undergone a revolution, resulting in significant progress in the understanding of their roles and types. However, many questions about microglia and their role in human diseases remain unanswered, including the different functions of the various subtypes of microglia in the CNS. Microglia are generally considered to be associated with the entire process of development, aging, disease, trauma, and infection of the CNS. Substantial progress has been made in understanding the phenotype and function of microglia by using their whole genome expression profile. However, in recent years, there has been no consensus on the topic of the polarization of microglia, and many obstacles and unsolved problems remain in the study of microglia phenotype. In the context of these controversies, future research needs to use more highly sensitive methods for evaluation, especially with advances in epigenetics, single-cell sequencing techniques, and imaging techniques in recent years. Significant advances have been reported in the extraction of whole-genome expression profiles of microglia. In future studies, other techniques such as multi-omics methods at the single-cell level, new animal models, patient-derived microglia, mass spectrometry cell analysis, immunohistochemical methods with high-resolution laser ablation, and other innovative techniques should be combined to help us clarify the unresolved issues in microglial phenotypes. This will enable a more in-depth understanding of microglia.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article. </p>
Funding statement
Junong Zhang Junlong Zhang was supported by National Natural Science Foundation of China [81874420], Shanxi Provincial Key Research and Development Project [No. 201903D421018]. Wenbin He was supported by National Natural Science Foundation of China [U21A20410], Shanxi Provincial Key Research and Development Project [201803D421006]. This work was supported by National Natural Science Foundation of China [82004236].
Data availability statement
No data was used for the research described in the article.
6. Declaration of interest's statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article..
Contributor Information
Wenbin He, Email: hewb@sxtcm.edu.cn.
Junlong Zhang, Email: zhangjl@sxtcm.edu.com.
The authors declare no competing interests.References
- 1.Deckert-Schlüter M. [Rudolf-Virchow Prize 1998. Award lecture. Toxoplasmosis: a model infection for studying systemic and intracerebral immune reactions] Verh. Dtsch. Ges. Pathol. 1998;82:9–22. [PubMed] [Google Scholar]
- 2.Kober C., Rohn S., Weibel S., Geissinger U., Chen N.G., Szalay A.A. Microglia and astrocytes attenuate the replication of the oncolytic vaccinia virus LIVP 1.1.1 in murine GL261 gliomas by acting as vaccinia virus traps. J. Transl. Med. 2015;13:216. doi: 10.1186/s12967-015-0586-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sierra A., de Castro F., Del Río-Hortega J., Rafael Iglesias-Rozas J., Garrosa M., Kettenmann H. The “Big-Bang” for modern glial biology: translation and comments on Pío del Río-Hortega 1919 series of papers on microglia. Glia. 2016;64:1801–1840. doi: 10.1002/glia.23046. [DOI] [PubMed] [Google Scholar]
- 4.Borst K., Dumas A.A., Prinz M. Microglia: immune and non-immune functions. Immunity. 2021;54:2194–2208. doi: 10.1016/j.immuni.2021.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Colonna M., Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Annu. Rev. Immunol. 2017;35:441–468. doi: 10.1146/annurev-immunol-051116-052358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masuda T., Sankowski R., Staszewski O., Prinz M. Microglia heterogeneity in the single-cell era. Cell Rep. 2020;30:1271–1281. doi: 10.1016/j.celrep.2020.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Spiteri A.G., Wishart C.L., Pamphlett R., Locatelli G., King N.J.C. Microglia and monocytes in inflammatory CNS disease: integrating phenotype and function. Acta Neuropathol. 2022;143:179–224. doi: 10.1007/s00401-021-02384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang Y., Le W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol. Neurobiol. 2016;53:1181–1194. doi: 10.1007/s12035-014-9070-5. [DOI] [PubMed] [Google Scholar]
- 9.Wolf S.A., Boddeke H.W.G.M., Kettenmann H. Microglia in physiology and disease. Annu. Rev. Physiol. 2017;79:619–643. doi: 10.1146/annurev-physiol-022516-034406. [DOI] [PubMed] [Google Scholar]
- 10.Borst K., Dumas A.A., Prinz M. Microglia: immune and non-immune functions. Immunity. 2021;54:2194–2208. doi: 10.1016/j.immuni.2021.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Cowan M., Petri W.A. Microglia: immune regulators of neurodevelopment. Front. Immunol. 2018;9:2576. doi: 10.3389/fimmu.2018.02576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harry G.J. Microglia during development and aging. Pharmacol. Ther. 2013;139:313–326. doi: 10.1016/j.pharmthera.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Umpierre A.D., Wu L.-J. How microglia sense and regulate neuronal activity. Glia. 2021;69:1637–1653. doi: 10.1002/glia.23961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiong X.-Y., Liu L., Yang Q.-W. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog. Neurobiol. 2016;142:23–44. doi: 10.1016/j.pneurobio.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Young K., Morrison H. Quantifying microglia morphology from photomicrographs of immunohistochemistry prepared tissue using ImageJ. J. Vis. Exp. 2018 doi: 10.3791/57648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bai Q., Xue M., Yong V.W. Microglia and macrophage phenotypes in intracerebral haemorrhage injury: therapeutic opportunities. Brain. 2020;143:1297–1314. doi: 10.1093/brain/awz393. [DOI] [PubMed] [Google Scholar]
- 17.Böttcher C., Schlickeiser S., Sneeboer M.A.M., Kunkel D., Knop A., Paza E., Fidzinski P., Kraus L., Snijders G.J.L., Kahn R.S., Schulz A.R., Mei H.E., NBB-Psy, Hol E.M., Siegmund B., Glauben R., Spruth E.J., de Witte L.D., Priller J. Human microglia regional heterogeneity and phenotypes determined by multiplexed single-cell mass cytometry. Nat. Neurosci. 2019;22:78–90. doi: 10.1038/s41593-018-0290-2. [DOI] [PubMed] [Google Scholar]
- 18.Troutman T.D., Kofman E., Glass C.K. Exploiting dynamic enhancer landscapes to decode macrophage and microglia phenotypes in health and disease. Mol. Cell. 2021;81:3888–3903. doi: 10.1016/j.molcel.2021.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colonna M., Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Annu. Rev. Immunol. 2017;35:441–468. doi: 10.1146/annurev-immunol-051116-052358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwon H.S., Koh S.-H. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl. Neurodegener. 2020;9:42. doi: 10.1186/s40035-020-00221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voet S., Prinz M., van Loo G. Microglia in central nervous system inflammation and multiple sclerosis pathology. Trends Mol. Med. 2019;25:112–123. doi: 10.1016/j.molmed.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Xu L., He D., Bai Y. Microglia-Mediated inflammation and neurodegenerative disease. Mol. Neurobiol. 2016;53:6709–6715. doi: 10.1007/s12035-015-9593-4. [DOI] [PubMed] [Google Scholar]
- 23.Arioz B.I., Tastan B., Tarakcioglu E., Tufekci K.U., Olcum M., Ersoy N., Bagriyanik A., Genc K., Genc S. Melatonin attenuates LPS-induced acute depressive-like behaviors and microglial NLRP3 inflammasome activation through the SIRT1/nrf2 pathway. Front. Immunol. 2019;10:1511. doi: 10.3389/fimmu.2019.01511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fourrier C., Remus-Borel J., Greenhalgh A.D., Guichardant M., Bernoud-Hubac N., Lagarde M., Joffre C., Layé S. Docosahexaenoic acid-containing choline phospholipid modulates LPS-induced neuroinflammation in vivo and in microglia in vitro. J. Neuroinflammation. 2017;14:170. doi: 10.1186/s12974-017-0939-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu W., Taso O., Wang R., Bayram S., Graham A.C., Garcia-Reitboeck P., Mallach A., Andrews W.D., Piers T.M., Botia J.A., Pocock J.M., Cummings D.M., Hardy J., Edwards F.A., Salih D.A. Trem2 promotes anti-inflammatory responses in microglia and is suppressed under pro-inflammatory conditions. Hum. Mol. Genet. 2020;29:3224–3248. doi: 10.1093/hmg/ddaa209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bettegazzi B., Bellani S., Cattaneo S., Codazzi F., Grohovaz F., Zacchetti D. Gα13 contributes to LPS-induced morphological alterations and affects migration of microglia. Mol. Neurobiol. 2021;58:6397–6414. doi: 10.1007/s12035-021-02553-0. [DOI] [PubMed] [Google Scholar]
- 27.Dos-Santos-Pereira M., Guimarães F.S., Del-Bel E., Raisman-Vozari R., Michel P.P. Cannabidiol prevents LPS-induced microglial inflammation by inhibiting ROS/NF-κB-dependent signaling and glucose consumption. Glia. 2020;68:561–573. doi: 10.1002/glia.23738. [DOI] [PubMed] [Google Scholar]
- 28.Liu H., Bian W., Yang D., Yang M., Luo H. Inhibiting the Piezo1 channel protects microglia from acute hyperglycaemia damage through the JNK1 and mTOR signalling pathways. Life Sci. 2021;264 doi: 10.1016/j.lfs.2020.118667. [DOI] [PubMed] [Google Scholar]
- 29.Kalkman H.O., Feuerbach D. Microglia M2A polarization as potential link between food allergy and autism spectrum disorders. Pharmaceuticals. 2017;10:95. doi: 10.3390/ph10040095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kisucká A., Bimbová K., Bačová M., Gálik J., Lukáčová N. Activation of neuroprotective microglia and astrocytes at the lesion site and in the adjacent segments is crucial for spontaneous locomotor recovery after spinal cord injury. Cells. 2021;10:1943. doi: 10.3390/cells10081943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kisucká A., Bimbová K., Bačová M., Gálik J., Lukáčová N. Activation of neuroprotective microglia and astrocytes at the lesion site and in the adjacent segments is crucial for spontaneous locomotor recovery after spinal cord injury. Cells. 2021;10:1943. doi: 10.3390/cells10081943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chu X., Cao L., Yu Z., Xin D., Li T., Ma W., Zhou X., Chen W., Liu D., Wang Z. Hydrogen-rich saline promotes microglia M2 polarization and complement-mediated synapse loss to restore behavioral deficits following hypoxia-ischemic in neonatal mice via AMPK activation. J. Neuroinflammation. 2019;16:104. doi: 10.1186/s12974-019-1488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou D., Ji L., Chen Y. TSPO modulates IL-4-induced microglia/macrophage M2 polarization via PPAR-γ pathway. J. Mol. Neurosci. 2020;70:542–549. doi: 10.1007/s12031-019-01454-1. [DOI] [PubMed] [Google Scholar]
- 34.Hardeland R. Melatonin and microglia. Int. J. Mol. Sci. 2021;22:8296. doi: 10.3390/ijms22158296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim S., Son Y. Astrocytes stimulate microglial proliferation and M2 polarization in vitro through crosstalk between astrocytes and microglia. Int. J. Mol. Sci. 2021;22:8800. doi: 10.3390/ijms22168800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsai C.-F., Chen G.-W., Chen Y.-C., Shen C.-K., Lu D.-Y., Yang L.-Y., Chen J.-H., Yeh W.-L. Regulatory effects of quercetin on M1/M2 macrophage polarization and oxidative/antioxidative balance. Nutrients. 2021;14:67. doi: 10.3390/nu14010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shemer A., Scheyltjens I., Frumer G.R., Kim J.-S., Grozovski J., Ayanaw S., Dassa B., Van Hove H., Chappell-Maor L., Boura-Halfon S., Leshkowitz D., Mueller W., Maggio N., Movahedi K., Jung S. Interleukin-10 prevents pathological microglia hyperactivation following peripheral endotoxin challenge. Immunity. 2020;53:1033–1049.e7. doi: 10.1016/j.immuni.2020.09.018. [DOI] [PubMed] [Google Scholar]
- 38.Shen H., Xu B., Yang C., Xue W., You Z., Wu X., Ma D., Shao D., Leong K., Dai J. A DAMP-scavenging, IL-10-releasing hydrogel promotes neural regeneration and motor function recovery after spinal cord injury. Biomaterials. 2022;280 doi: 10.1016/j.biomaterials.2021.121279. [DOI] [PubMed] [Google Scholar]
- 39.Yang L., Liu C., Li W., Ma Y., Huo S., Ozathaley A., Ren J., Yuan W., Ni H., Li D., Zhang J., Liu Z. Depression-like behavior associated with E/I imbalance of mPFC and amygdala without TRPC channels in mice of knockout IL-10 from microglia. Brain Behav. Immun. 2021;97:68–78. doi: 10.1016/j.bbi.2021.06.015. [DOI] [PubMed] [Google Scholar]
- 40.Ellwanger D.C., Wang S., Brioschi S., Shao Z., Green L., Case R., Yoo D., Weishuhn D., Rathanaswami P., Bradley J., Rao S., Cha D., Luan P., Sambashivan S., Gilfillan S., Hasson S.A., Foltz I.N., van Lookeren Campagne M., Colonna M. Prior activation state shapes the microglia response to antihuman TREM2 in a mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2017742118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kinuthia U.M., Wolf A., Langmann T. Microglia and inflammatory responses in diabetic retinopathy. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.564077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Louboutin J.-P., Strayer D.S. Relationship between the chemokine receptor CCR5 and microglia in neurological disorders: consequences of targeting CCR5 on neuroinflammation, neuronal death and regeneration in a model of epilepsy. CNS Neurol. Disord.: Drug Targets. 2013;12:815–829. doi: 10.2174/18715273113126660173. [DOI] [PubMed] [Google Scholar]
- 43.Liu X., Liu J., Zhao S., Zhang H., Cai W., Cai M., Ji X., Leak R.K., Gao Y., Chen J., Hu X. Interleukin-4 is essential for microglia/macrophage M2 polarization and long-term recovery after cerebral ischemia. Stroke. 2016;47:498–504. doi: 10.1161/STROKEAHA.115.012079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu J., Chen Z., Yu F., Liu H., Ma C., Xie D., Hu X., Leak R.K., Chou S.H.Y., Stetler R.A., Shi Y., Chen J., Bennett M.V.L., Chen G. IL-4/STAT6 signaling facilitates innate hematoma resolution and neurological recovery after hemorrhagic stroke in mice. Proc. Natl. Acad. Sci. U. S. A. 2020;117:32679–32690. doi: 10.1073/pnas.2018497117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Subedi L., Lee J.H., Yumnam S., Ji E., Kim S.Y. Anti-inflammatory effect of sulforaphane on LPS-activated microglia potentially through JNK/AP-1/NF-κB inhibition and Nrf2/HO-1 activation. Cells. 2019;8:194. doi: 10.3390/cells8020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang J., Rong P., Zhang L., He H., Zhou T., Fan Y., Mo L., Zhao Q., Han Y., Li S., Wang Y., Yan W., Chen H., You Z. IL4-driven microglia modulate stress resilience through BDNF-dependent neurogenesis. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abb9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ransohoff R.M. A polarizing question: do M1 and M2 microglia exist? Nat. Neurosci. 2016;19:987–991. doi: 10.1038/nn.4338. [DOI] [PubMed] [Google Scholar]
- 48.Cai W., Dai X., Chen J., Zhao J., Xu M., Zhang L., Yang B., Zhang W., Rocha M., Nakao T., Kofler J., Shi Y., Stetler R.A., Hu X., Chen J. STAT6/Arg1 promotes microglia/macrophage efferocytosis and inflammation resolution in stroke mice. JCI Insight. 2019;4 doi: 10.1172/jci.insight.131355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He Y., Gao Y., Zhang Q., Zhou G., Cao F., Yao S. IL-4 switches microglia/macrophage M1/M2 polarization and alleviates neurological damage by modulating the JAK1/STAT6 pathway following ICH. Neuroscience. 2020;437:161–171. doi: 10.1016/j.neuroscience.2020.03.008. [DOI] [PubMed] [Google Scholar]
- 50.Zeiner P.S., Preusse C., Golebiewska A., Zinke J., Iriondo A., Muller A., Kaoma T., Filipski K., Müller-Eschner M., Bernatz S., Blank A.-E., Baumgarten P., Ilina E., Grote A., Hansmann M.L., Verhoff M.A., Franz K., Feuerhake F., Steinbach J.P., Wischhusen J., Stenzel W., Niclou S.P., Harter P.N., Mittelbronn M. Distribution and prognostic impact of microglia/macrophage subpopulations in gliomas. Brain Pathol. 2019;29:513–529. doi: 10.1111/bpa.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Locati M., Curtale G., Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu. Rev. Pathol. 2020;15:123–147. doi: 10.1146/annurev-pathmechdis-012418-012718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shapouri-Moghaddam A., Mohammadian S., Vazini H., Taghadosi M., Esmaeili S.-A., Mardani F., Seifi B., Mohammadi A., Afshari J.T., Sahebkar A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018;233:6425–6440. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 53.Yunna C., Mengru H., Lei W., Weidong C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020;877 doi: 10.1016/j.ejphar.2020.173090. [DOI] [PubMed] [Google Scholar]
- 54.Butovsky O., Jedrychowski M.P., Moore C.S., Cialic R., Lanser A.J., Gabriely G., Koeglsperger T., Dake B., Wu P.M., Doykan C.E., Fanek Z., Liu L., Chen Z., Rothstein J.D., Ransohoff R.M., Gygi S.P., Antel J.P., Weiner H.L. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat. Neurosci. 2014;17:131–143. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hickman S.E., Kingery N.D., Ohsumi T.K., Borowsky M.L., Wang L., Means T.K., El Khoury J. The microglial sensome revealed by direct RNA sequencing. Nat. Neurosci. 2013;16:1896–1905. doi: 10.1038/nn.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y., Chen K., Sloan S.A., Bennett M.L., Scholze A.R., O'Keeffe S., Phatnani H.P., Guarnieri P., Caneda C., Ruderisch N., Deng S., Liddelow S.A., Zhang C., Daneman R., Maniatis T., Barres B.A., Wu J.Q. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu M.-Y., Lin Y.-Y., Zhang B.-J., Lu D.-L., Lu Z.-Q., Cai W. Update of inflammasome activation in microglia/macrophage in aging and aging-related disease. CNS Neurosci. Ther. 2019;25:1299–1307. doi: 10.1111/cns.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prineas J.W., Parratt J.D.E. Multiple sclerosis: microglia, monocytes, and macrophage-mediated demyelination. J. Neuropathol. Exp. Neurol. 2021;80:975–996. doi: 10.1093/jnen/nlab083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stratoulias V., Venero J.L., Tremblay M.-È., Joseph B. Microglial subtypes: diversity within the microglial community. EMBO J. 2019;38 doi: 10.15252/embj.2019101997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheray M., Stratoulias V., Joseph B., Grabert K. The rules of engagement: do microglia seal the fate in the inverse relation of glioma and Alzheimer's disease? Front. Cell. Neurosci. 2019;13:522. doi: 10.3389/fncel.2019.00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saidi D., Cheray M., Osman A.M., Stratoulias V., Lindberg O.R., Shen X., Blomgren K., Joseph B. Glioma-induced SIRT1-dependent activation of hMOF histone H4 lysine 16 acetyltransferase in microglia promotes a tumor supporting phenotype. OncoImmunology. 2018;7 doi: 10.1080/2162402X.2017.1382790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stratoulias V., Heino T.I. MANF silencing, immunity induction or autophagy trigger an unusual cell type in metamorphosing Drosophila brain. Cell. Mol. Life Sci. 2015;72:1989–2004. doi: 10.1007/s00018-014-1789-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hanani M., Verkhratsky A. Satellite glial cells and astrocytes, a comparative review. Neurochem. Res. 2021;46:2525–2537. doi: 10.1007/s11064-021-03255-8. [DOI] [PubMed] [Google Scholar]
- 64.Lee J.H., Kim W. The role of satellite glial cells, astrocytes, and microglia in oxaliplatin-induced neuropathic pain. Biomedicines. 2020;8:324. doi: 10.3390/biomedicines8090324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lim H., Lee H., Noh K., Lee S.J. IKK/NF-κB-dependent satellite glia activation induces spinal cord microglia activation and neuropathic pain after nerve injury. Pain. 2017;158:1666–1677. doi: 10.1097/j.pain.0000000000000959. [DOI] [PubMed] [Google Scholar]
- 66.Wogram E., Wendt S., Matyash M., Pivneva T., Draguhn A., Kettenmann H. Satellite microglia show spontaneous electrical activity that is uncorrelated with activity of the attached neuron. Eur. J. Neurosci. 2016;43:1523–1534. doi: 10.1111/ejn.13256. [DOI] [PubMed] [Google Scholar]
- 67.Coomey R., Stowell R., Majewska A., Tropea D. The role of microglia in neurodevelopmental disorders and their therapeutics. Curr. Top. Med. Chem. 2020;20:272–276. doi: 10.2174/1568026620666200221172619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stowell R.D., Wong E.L., Batchelor H.N., Mendes M.S., Lamantia C.E., Whitelaw B.S., Majewska A.K. Cerebellar microglia are dynamically unique and survey Purkinje neurons in vivo. Dev Neurobiol. 2018;78:627–644. doi: 10.1002/dneu.22572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jander S., Stoll G. Strain-specific expression of microglial keratan sulfate proteoglycans in the normal rat central nervous system: inverse correlation with constitutive expression of major histocompatibility complex class II antigens. Glia. 1996;18:255–260. doi: 10.1002/(SICI)1098-1136(199611)18:3<255::AID-GLIA9>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 70.Jones L.L., Tuszynski M.H. Spinal cord injury elicits expression of keratan sulfate proteoglycans by macrophages, reactive microglia, and oligodendrocyte progenitors. J. Neurosci. 2002;22:4611–4624. doi: 10.1523/JNEUROSCI.22-11-04611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jones L.L., Tuszynski M.H. Spinal cord injury elicits expression of keratan sulfate proteoglycans by macrophages, reactive microglia, and oligodendrocyte progenitors. J. Neurosci. 2002;22:4611–4624. doi: 10.1523/JNEUROSCI.22-11-04611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jander S., Stoll G. Downregulation of microglial keratan sulfate proteoglycans coincident with lymphomonocytic infiltration of the rat central nervous system. Am. J. Pathol. 1996;148:71–78. [PMC free article] [PubMed] [Google Scholar]
- 73.Tränkner D., Boulet A., Peden E., Focht R., Van Deren D., Capecchi M. A microglia sublineage protects from sex-linked anxiety symptoms and obsessive compulsion. Cell Rep. 2019;29:791–799.e3. doi: 10.1016/j.celrep.2019.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nagarajan N., Jones B.W., West P.J., Marc R.E., Capecchi M.R. Corticostriatal circuit defects in Hoxb8 mutant mice. Mol. Psychiatr. 2018;23:1868–1877. doi: 10.1038/mp.2017.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van Deren D.A., De S., Xu B., Eschenbacher K.M., Zhang S., Capecchi M.R. Defining the Hoxb8 cell lineage during murine definitive hematopoiesis. Development. 2022;149 doi: 10.1242/dev.200200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen S.-K., Tvrdik P., Peden E., Cho S., Wu S., Spangrude G., Capecchi M.R. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell. 2010;141:775–785. doi: 10.1016/j.cell.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.De S., Van Deren D., Peden E., Hockin M., Boulet A., Titen S., Capecchi M.R. Two distinct ontogenies confer heterogeneity to mouse brain microglia. Development. 2018;145:dev152306. doi: 10.1242/dev.152306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hyman S.E. A bone to pick with compulsive behavior. Cell. 2010;141:752–754. doi: 10.1016/j.cell.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 79.Kohno K., Shirasaka R., Yoshihara K., Mikuriya S., Tanaka K., Takanami K., Inoue K., Sakamoto H., Ohkawa Y., Masuda T., Tsuda M. A spinal microglia population involved in remitting and relapsing neuropathic pain. Science. 2022;376:86–90. doi: 10.1126/science.abf6805. [DOI] [PubMed] [Google Scholar]
- 80.Mayrhofer F., Dariychuk Z., Zhen A., Daugherty D.J., Bannerman P., Hanson A.M., Pleasure D., Soulika A., Deng W., Chechneva O.V. Reduction in CD11c+ microglia correlates with clinical progression in chronic experimental autoimmune demyelination. Neurobiol. Dis. 2021;161 doi: 10.1016/j.nbd.2021.105556. [DOI] [PubMed] [Google Scholar]
- 81.Kamphuis W., Kooijman L., Schetters S., Orre M., Hol E.M. Transcriptional profiling of CD11c-positive microglia accumulating around amyloid plaques in a mouse model for Alzheimer's disease. Biochim. Biophys. Acta. 2016;1862:1847–1860. doi: 10.1016/j.bbadis.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 82.Johnson E.C.B., Dammer E.B., Duong D.M., Ping L., Zhou M., Yin L., Higginbotham L.A., Guajardo A., White B., Troncoso J.C., Thambisetty M., Montine T.J., Lee E.B., Trojanowski J.Q., Beach T.G., Reiman E.M., Haroutunian V., Wang M., Schadt E., Zhang B., Dickson D.W., Ertekin-Taner N., Golde T.E., Petyuk V.A., De Jager P.L., Bennett D.A., Wingo T.S., Rangaraju S., Hajjar I., Shulman J.M., Lah J.J., Levey A.I., Seyfried N.T. Large-scale proteomic analysis of Alzheimer's disease brain and cerebrospinal fluid reveals early changes in energy metabolism associated with microglia and astrocyte activation. Nat. Med. 2020;26:769–780. doi: 10.1038/s41591-020-0815-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bisht K., Sharma K., Lacoste B., Tremblay M.-È. Dark microglia: why are they dark? Commun. Integr. Biol. 2016;9 doi: 10.1080/19420889.2016.1230575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.St-Pierre M.-K., Bordeleau M., Tremblay M.-È. Visualizing dark microglia. Methods Mol. Biol. 2019;2034:97–110. doi: 10.1007/978-1-4939-9658-2_8. [DOI] [PubMed] [Google Scholar]
- 85.St-Pierre M.-K., Šimončičová E., Bögi E., Tremblay M.-È. Shedding light on the dark side of the microglia. ASN Neuro. 2020;12 doi: 10.1177/1759091420925335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hammond T.R., Dufort C., Dissing-Olesen L., Giera S., Young A., Wysoker A., Walker A.J., Gergits F., Segel M., Nemesh J., Marsh S.E., Saunders A., Macosko E., Ginhoux F., Chen J., Franklin R.J.M., Piao X., McCarroll S.A., Stevens B. Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity. 2019;50:253–271.e6. doi: 10.1016/j.immuni.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.