Abstract
Abstract
Outcomes of neonatal encephalopathy (NE) have improved since the widespread implementation of therapeutic hypothermia (TH) in high-resource settings. While TH for NE in term and near-term infants has proven beneficial, 30–50% of infants with moderate-to-severe NE treated with TH still suffer death or significant impairments. There is therefore a critical need to find additional pharmacological and non-pharmacological interventions that improve the outcomes for these children. There are many potential candidates; however, it is unclear whether these interventions have additional benefits when used with TH. Although primary and delayed (secondary) brain injury starting in the latent phase after HI are major contributors to neurodisability, the very late evolving effects of tertiary brain injury likely require different interventions targeting neurorestoration. Clinical trials of seizure management and neuroprotection bundles are needed, in addition to current trials combining erythropoietin, stem cells, and melatonin with TH.
Impact
The widespread use of therapeutic hypothermia (TH) in the treatment of neonatal encephalopathy (NE) has reduced the associated morbidity and mortality. However, 30–50% of infants with moderate-to-severe NE treated with TH still suffer death or significant impairments.
This review details the pathophysiology of NE along with the evidence for the use of TH and other beneficial neuroprotective strategies used in term infants.
We also discuss treatment strategies undergoing evaluation at present as potential adjuvant treatments to TH in NE.
Introduction
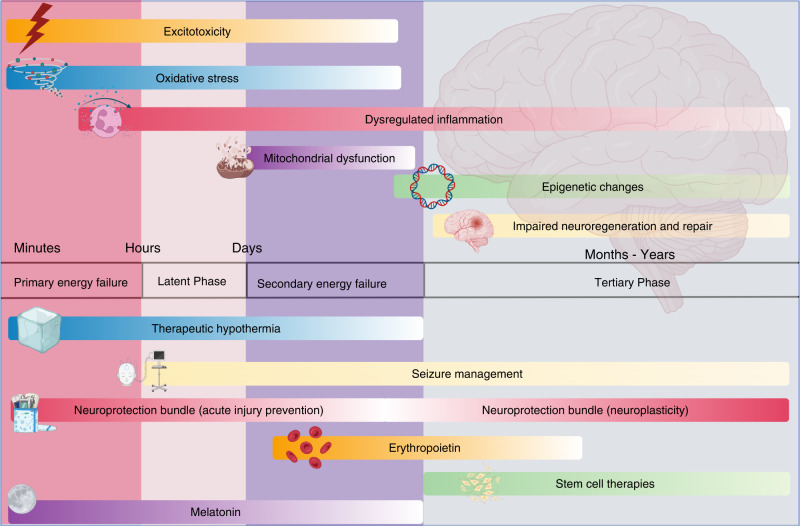
Improvements in antenatal care and advances in neonatal intensive care have reduced neonatal morbidity and mortality. However, adverse neurodevelopmental outcomes remain significant for many children. A variety of complications, such as cerebral congenital malformations, genetic anomalies, congenital epilepsies, and congenital cardiac defects, may also contribute to long-term neurological disability. Neonatal encephalopathy (NE) is a heterogeneous problem that contributes about 700,000 deaths per year worldwide in term and near-term infants1 and affects 1–4 per 1000 births in high-resource settings. The development and successful translation of therapeutic hypothermia (TH) has confirmed the fundamental principle that it is possible to reduce the risk of disability after acute hypoxia–ischemia (HI) (Fig. 1).
Fig. 1. Pathophysiology, phases of injury, and therapeutic windows for present and future neuroprotective interventions in term newborns.
Earliest phases of injury are targeted by interventions, including therapeutic hypothermia, acute neuroprotective bundles, and melatonin. Neuroplasticity bundles, erythropoietin, and stem cell therapies aim to reduce injury during the later phases. Improved seizure management offers neuroprotection throughout all stages of injury.
The seminal finding that underpinned development and translation of TH is that perinatal brain damage after HI evolves over time, with initial transient recovery of oxidative metabolism followed by progressive activation of cell death pathways, leading to secondary deterioration after approximately 4–8 h, with failure of oxidative metabolism, delayed seizures, and ultimately cell death. This delay provides a window of time after HI during which it is possible to intervene with TH. In addition, tertiary mechanisms of brain injury may continue for weeks, months, or years,2 involving dysregulated immune responses and loss of trophic support, which may be amenable to novel therapies after the end of hypothermia treatment.3
Many different treatment strategies have been postulated as adjunctive treatment strategies to TH to further decrease morbidity. These include allopurinol, azithromycin, ascorbic acid, ibuprofen, magnesium sulfate, xenon gas treatment, and sildenafil. This paper will concentrate on the discussion of melatonin (MT), erythropoietin, and mesenchymal stromal cells as these therapies are undergoing evaluation in human clinical trials at present.
Therapeutic hypothermia
TH is now routine care for infants with moderate-to-severe NE.4 It was first been recommended as standard treatment by the International Liaison Committee on Resuscitation (ILCOR) in 2010, based on compelling evidence from randomized controlled trials (RCTs) that TH, and improvements in supportive neonatal intensive care unit (NICU) care during treatment, reduces brain injury detected by modern imaging,5 and improves survival and neurological outcomes into middle childhood.6,7 The parameters for optimal neuroprotection are now well understood.8 Brain temperature needs to be reduced by ~3.5 °C, starting as soon as possible in the first 6 h after HI and then continued for ~72 h. Shorter or longer cooling than 72 h, or deeper cooling (by >5 °C) reduces neuroprotection both in preclinical studies9–11 and in a large randomized clinical trial.12 Thus, current clinical protocols are close to optimal.
In large randomized trials, hypothermic neuroprotection was incomplete, reducing the combined risk of death and severe disabilities at 18 months of age by ~12%, from 58 to 46%.13 Thus, many infants still die or survive with major debilitating handicaps, despite TH intervention. There is evidence that the risk of adverse outcome despite TH has fallen from about 45% in the original trials to about 29% in a recent RCT.12 The recent trial of late cooling found an overall risk of adverse outcome of just 26%.14 This improvement mainly reflects a reduction in mortality, from 25% in the original trials to 10%, with little change in the rate of disability after NE.
The challenge now is twofold: first, to find ways to improve the outcomes for infants with NE who have been treated with TH, and second, to improve treatment strategies in settings in which TH is not beneficial or is contraindicated, such as low–middle-income countries.15 Broadly, the key mechanisms of TH are to attenuate evolving programmed cell death and inflammation, raising the possibility of overlap with the mechanisms of potential adjunct treatment.16
Neuroprotection bundles in term and near-term neonates in NeuroNICU
Neonatal brain injury is a complex, multifactorial process.17 Genetic, epigenetic, metabolic, prenatal, perinatal, and postnatal factors interact to protect, cause, or exaggerate neonatal brain injury.18–22 This complexity makes developing a monotherapy challenging since it is improbable that any one intervention will be applicable in all settings.23 There is an increasing interest in a multi-intervention bundled approach using quality improvement methodology to alleviate neonatal brain injury.24 Neuroprotection bundles can be divided into (1) acute brain injury prevention and (2) neuroplasticity bundles.
The key concepts in the acute brain injury prevention bundles are early identification and referral, preventing fluctuation in physiologic parameters (such as carbon dioxide, glucose, blood pressure, temperature), minimal handling and pain management, seizure diagnosis and management, early nutrition, and fluid and electrolyte balance.25–30 Implementation of neuroprotection bundles targeting those key elements through outreach and Neonatal Neuro-Critical Care programs have proven to be effective in improving NE identification and preventing short term morbidities such as rate of brain injury on magnetic resonance imaging (MRI), antiseizure medication (ASM) doses and timing of treatment, use of boluses and inotropes, temperature fluctuation, and overall hospital length of stay.31–35 Evidence for the long-term impact of such programs is still lacking and is required for any of these approaches to become standard care.
Neuroplasticity bundles target potential brain injury and growth well beyond the first few days of birth and after discharge.36 Key elements in such bundles (evaluated in preterm and/or term infants) have variably included (1) empowering families through Family Integrated Care (FICARE);37 (2) optimizing nutrition;38 (3) developmental care;39 (4) skin-to-skin care and massage therapy;40,41 (5) positive stimulating sounds such as music therapy,42 reading programs,43 parental voice,44 minimizing disturbing noises,45 and enhancing physiologic sleep–wake cycles;46 and (6) encouraging positive social interaction.47 Although there is limited evidence that neuroplasticity interventions can improve long-term cognitive and motor outcomes, well-powered studies are still lacking.40,41,43
Seizure management
One potential neuroprotective strategy is improved treatment of seizures associated with acute neonatal brain injury, i.e., seizures related to hypoxic–ischemic encephalopathy (HIE), stroke, and intracranial hemorrhage (ICH). These three disorders underlie ~75% of neonatal seizures.48–50 With increasing recognition of the association between neonatal seizures and adverse outcomes, there has been increased attention and research effort on the improved detection and management of acute symptomatic neonatal seizures using gold standard continuous, conventional video electroencephalograph (EEG) monitoring.51–53 Similarly, there has been increased interest in developing and testing more effective and safer treatments for neonatal seizures.54 The direction of causality is still not clear.
TH reduces seizure burden substantially in cohort studies of infants with moderate-to-severe HIE compared to normothermia.55–57 Interestingly, some studies found this only after moderate HIE, whereas others report an improvement with severe HIE as well.55 The overall incidence of seizures is reported not to be affected by TH, suggesting that the duration of individual seizures and the total time of seizures is lower in infants who do seize,57 consistent with animal studies.58
Experimental models have shown that intense neonatal seizures by themselves can lead to decreased neurogenesis, synaptic reorganization, dendritic spine loss, and other effects on the developing brain that correlate well with later cognitive deficits, such as memory impairments.59 However, it is challenging to disentangle the impact of seizures from brain injury in experimental models of HI injury without seizures. For example, injection of the excitotoxin kainic acid in normoxic P10 rat induced clinical and electrographic seizures lasting a mean of 282 min, but notably did not cause brain injury after either 3 or 20 days recovery.60 The authors then tested the effect of seizures after a model of HI for 30 min that induced moderate neuropathological injury, but no electrographic seizures. Kainic acid injection after this period of HI induced superimposed seizures and increased neuronal loss in the hippocampus. Critically, a subsequent study found that the kainite-induced seizures were associated with a small increase in brain temperature—and that preventing hyperthermia abolished the increase in neuronal necrosis up to 20 days of recovery.61 Thus, spontaneous seizures may not exacerbate injury after HI, and at least part of their injurious effects may be mediated by hyperthermia, consistent with association of pyrexia in multiple preclinical studies and clinical trials of infants with neonatal encephalopathy.62,63 Conversely, in near-term fetal sheep receiving cerebral ischemia that led to status epilepticus and severe watershed brain injury, treatment with the potent anti-excitotoxic agent dizocilpine starting at 6 h, before the onset of seizures, completely abolished seizures but had only a modest effect to reduce injury in mildly affected regions and no effect on parasagittal cortical infarction.64
Determining the direction of causality in humans is challenging, as it is impossible to determine whether more severe brain injury begets more severe seizures, or the reverse, or a combination of both. There are data showing that higher seizure burden is associated with worse short- and long-term outcomes,50,65 although the higher seizure burden may reflect greater injury. One small study suggested that seizure severity was associated with outcome independently of severity of HI injury by MRI.66 Two small, randomized trials of treatment of clinical vs EEG-proven seizures also suggested that higher seizure burden is associated with worse brain injury and neurodevelopmental outcome.67,68 Although these trials were small, treatment of EEG-proven seizures resulted in reduced seizure burden compared with treatment of only clinical seizures, showing that EEG-guided treatment is more effective in reducing seizure burden than treatment of only clinically suspected seizures. Notably, both trials found that the reduced seizure burden was associated with less brain injury by brain MRI and improved neurologic outcome. Although these data suggest that higher seizure burden is harmful, the clinical impact of mild-to-moderate seizure burden is unclear. An expert consensus recommended a threshold of 30 s/h of seizure activity for randomization in a clinic trial,69 but the threshold that should prompt treatment in routine clinical care is unknown, and requires further research.
Importantly, the ASMs used to treat neonatal seizures are often ineffective, as ~40–60% of neonates will have persistent seizures after an initial loading dose of an ASM.50,70 Moreover, there is limited evidence for the efficacy and safety of ASMs for neonatal seizures,71 as there have been only three randomized trials of ASMs to treat neonatal seizures,70,72,73 two of which used a crossover design,70,73 in addition to open-label, uncontrolled studies.74–77 Phenobarbital and phenytoin had efficacy in the first RCT in which EEG monitoring was not continuous.70 More recently, levetiracetam 40–60 mg/kg was shown to be much less effective than phenobarbital 20–40 mg/kg, albeit with a marginally better adverse effect profile.73 Add-on treatment with bumetanide enhanced seizure reduction in a randomized, double-blind controlled trial, particularly with higher bumetanide exposure, compared with standard therapy alone (phenobarbital).72 This promising result will need to be tested in a larger trial to determine ideal dose, efficacy, and safety before it is incorporated into clinical care, as this class of drugs can be ototoxic. Other ASMs such as lidocaine and midazolam have been studied only in small cohort and/or retrospective studies and had low reported efficacy.74,76 Some of the ASMs used widely in older children, such as topiramate, which could have an additional neuroprotective effect, have been rarely used or studied.78,79 In a randomized trial of 110 infants with HIE, add-on therapy with topiramate (by nasogastric tube) vs placebo with TH (HYPOTOP), topiramate reduced seizures in the subset who reached therapeutic levels in the first 24 h but had no significant effect on mortality or neurodevelopmental outcomes at 2 years.80 The lack of adequate efficacy and safety data for currently used ASMs speaks of the compelling need to develop novel ASMs targeted to the specific pathophysiology of neonatal seizures and to test their efficacy and safety in rigorously designed RCTs that balance important covariates such as severity of both neonatal encephalopathy and seizures.69
Animal models have raised concerns about the potential effects of frequently used ASMs especially in the area of brain development and neurodevelopmental outcome. Phenobarbital was seen to induce apoptosis in rodent neurons in the cortex, hypothalamus, thalamus, basal ganglia, and the developing white matter, however, at a higher dose than typically used clinically.81–83 In rats, a threshold dose of 40 mg/kg was associated with apoptosis. Importantly, when phenobarbital was combined with diazepam, even lower doses were associated with apoptosis.83 Phenobarbital and phenytoin have been shown to disrupt the maturation of synapses in the developing brain of the neonatal rate and thus impair behavior.84 In comparison, although levetiracetam seems to be a less effective anticonvulsant it has a superior safety profile,85 and there is some evidence from rat models that it may reduce apoptosis after HI.86,87
Erythropoietin in term infants
Erythropoietin (Epo) is a 30.4-kDa glycoprotein primarily produced in the liver in the fetus and in the kidney and liver after the neonatal period. Epo and its receptor (Epo-R) are expressed by many cell types in the brain. In animal models, Epo can modulate inflammation, angiogenesis, and neurogenesis and promotes white matter development.88,89 The response to injury is mediated via hypoxia-inducible factor-1-mediated increase in Epo expression, signaling protein Janus kinase 2, and downstream phosphatidylinositol 3-kinase/Akt, Stat5, and the extracellular signal-regulated kinase.
Serum Epo levels are significantly elevated in both term and preterm infants with adverse neurodevelopmental outcomes and remain dysregulated in later childhood post-NE.3,90–93 Recombinant Epo may upregulate Epo receptors in animal models of neonatal HI and Epo levels for tissue protection may be up to 1000-fold higher than required for erythropoiesis.94
Numerous studies of Epo neuroprotection performed in rodents, sheep, and nonhuman primates have provided consistent evidence that exogenous Epo results in both histologic and functional benefit, with most benefit seen in multiple, high-dose treatment regimens.95 There is a U-shaped dose–response curve, with extremely high doses resulting in a loss of neuroprotection or even increased vulnerability.96,97 Less than 1% of circulating Epo crosses the intact blood–brain barrier (BBB), most likely via passive diffusion.98 In contrast, higher doses of Epo have been shown in rats, primates, and humans to achieve significant elevations in CSF Epo concentrations, particularly after HI when permeability of the BBB is increased.99 Of concern, recent large animal studies found that combined therapy using continuous Epo infusion with TH after HI does not seem to be additive.100,101
In humans, Epo monotherapy for neonatal encephalopathy has been tested in small clinical trials in settings where TH was not available.102,103 These studies suggest short-term neurodevelopmental benefit after high-dose Epo over the first 5 days of life or three times per week for 2–4 weeks, or long-term benefit with every other day dosing for 2 weeks. No safety concerns have been reported, but their small sample sizes limit extrapolation of results for clinical use of Epo for presumed NE.
In hospitals where TH is standard of care, Epo must be studied in this context to examine both safety and long-term efficacy. Phase I and phase II trials of combination Epo and hypothermia therapy have demonstrated safety of high-dose Epo, with a dose of 1000 U/kg intravenously (IV) achieving serum concentrations that most closely approximated optimal neuroprotective levels in preclinical models.96 The phase II NEATO trial EPO boluses at 1, 2, 3, 5, and 7 days of age may provide additional benefit in MRI injury scores and motor outcomes at 12 months of age.104 However, two children in the standard care group had confounding conditions; if these infants are excluded, there was no significant difference between groups. Combination therapy was also found to reduce serum tau protein but did not affect neurodevelopmental outcome at 9 months of age.105
Unfortunately, consistent with this interpretation, the phase III High-Dose Erythropoietin for Asphyxia and Encephalopathy Trial (HEAL, NCT# 02811263) that randomized infants to either 1000 U/kg of Epo (n = 257) or saline placebo (n = 243) given IV within 26 h after birth, and then at 2, 3, 4, and 7 days,106 found no effect on the risk of death or neurodevelopmental impairment at 22–36 months of age (52.5% after Epo vs 49.5% after placebo). Moreover, Epo was associated with a higher rate of serious adverse events. The similar, phase III, Preventing Adverse Outcomes of Neonatal Hypoxic Ischemic Encephalopathy with Erythropoietin (PAEAN) Trial (NCT# 03079167) is in progress.107
Darbepoetin, a long-acting erythropoiesis-stimulating agent that may provide similar neuroprotective benefit as Epo with a more practical dosing schedule, is also currently being investigated in cooled neonates with NE (DANCE trial: NCT01471015)108 and as monotherapy for milder NE (MEND Trial: NCT03071861).109 No trials have directly compared these agents.
Stem cell therapies
Volume and red blood cell reduced human umbilical cord blood mononuclear cells (hUCB cells), collected and processed with established procedures, have been used for allogeneic transplants, for hematopoietic disorders, as well as inherited metabolic disease110 (https://www.fda.gov/home). Mesenchymal stromal cells (MSCs), which have been found in multiple tissues and have been phenotypically defined in a standardized way, have been tested in hundreds of clinical trials, including studies that enrolled hundreds of children testing MSCs as potential therapy for graft vs host disease.111–115 Meta-analysis of 46 trials of MSCs in a wide variety of species, including humans, rats, and mice, and in adult animals with stroke indicate improved outcome with MSC treatment compared with placebo.116 There was no apparent effect of the origin of the MSCs or the target species, administration route, timing, immunogenicity, or dose.
In neonatal animal studies, both hUCB cells and MSCs have shown promise for neuroprotection after HI.117–122 In small and large animal studies, treatment after HI with hUCB cells increases neurotrophic and angiogenic factors, decreases inflammation and microglial activation, and modifies T lymphocyte migration into injured areas of the brain. MSCs work mainly via paracrine secretion of multiple cytokines, morphogens, small molecules, and exosomes, which carry a variety of substances, which can affect the biology of adjacent and distant responder cells and tissue.112 Recent in vitro studies describe the formation of membranous channels between MSCs and injured cells (tunneling nanotubules (TNT)); MSCs are thought to inhibit apoptosis and restore cellular bioenergetics by transferring healthy mitochondria to injured cells through TNT.123 Exosomes from MSCs may also promote regenerative responses from the neurogenic stem cell niche.124 In addition to decreasing markers of inflammation, administration of MSCs in a neonatal brain injury model was associated with increased differentiation towards neurons and oligodendrocytes and decreased proliferating inflammatory cells post-injury. Repeat dosing, several days after injury, seemed to enhance cell differentiation and functional outcome.122 Although preclinical trials where MSCs were administered before and immediately after TH were associated with improved anatomic and functional outcomes, one study in P9 mice showed that administering MSCs 3 days after TH for 4 h might be deleterious.125 In neonatal piglets, cooling from 1 to 13 h after HI plus intranasally administered MSCs at 24 and 48 h was associated with (i) faster aEEG recovery after injury; (ii) improved brain energy metabolism based on phosphorus-31 magnetic resonance spectroscopy (MRS) but not the Lac/NAA ratio; (iii) reduced total number of TUNEL-positive cells and increased oligodendrocytes in the white matter compared to cooling alone,126 but had no effect on gray matter. It is unknown whether these limited benefits would be achieved after a full clinical protocol of TH. In addition to inherent properties of MSCs and their exosomes, further benefits may be achieved by modifications to enhance production of certain neurotrophic factors.115 One key issue is that the immunomodulatory effects of MSCs appear to be determined by local inflammatory conditions in the host, with polarization of MSCs to pro-inflammatory or anti-inflammatory phenotype depending on the initial inflammatory milieu.127 Therefore, the timing of MSCs administration may be critical in determining the therapeutic response.
Human trials for the use of hUCB cells and MSCs are still at an extremely early stage. Two studies in human infants with moderate-to-severe NE have been published, demonstrating safety and feasibility of collection and preparation of the nucleated cord blood cells.128,129 In addition, a small phase I/II, open-label, single-arm study, which evaluated the safety and tolerability of intranasally administered MSC for perinatal arterial ischemic stroke (Perinatal Arterial Stroke Treated With Stromal Cells Intranasally, PASSIoN), has just completed enrolment (NCT03356821: https://clinicaltrials.gov/ct2/show/NCT03356821). The most promising study was a phase II multi-site double-blinded RCT, which aimed at assessing the neuroprotective efficacy of autologous hUCB cells in neonates with moderate-to-severe NE. That study was stopped prematurely after randomization of 35 out of the planned 160 infants due to slow enrolment and funding difficulties (NCT02612155: https://clinicaltrials.gov/ct2/show/NCT02612155). While short-term safety signals have been reassuring, much work is needed to establish the safety and efficacy of cell therapy for brain injury in newborn infants.
Melatonin
MT is an endogenous hormone released by the pineal gland. Its release is inhibited by light stimulation so there is significant 24-h variation in MT levels, with higher nighttime and lower daytime physiological concentration.130 MT easily crosses the BBB131 and MT receptors are widely distributed in different brain regions132 and among a wide variety of immune cells, including neutrophils, monocytes, and microglia.133 MT has anti-inflammatory properties primarily through prevention of inflammasome activation134 and inhibition of pro-inflammatory cytokines production. It also has antioxidant properties as a direct free radical scavenger and by upregulating antioxidant enzymes through activation of MT receptors MT1 and MT2,135 and anti-apoptotic properties by preventing mitochondrial release of cytochrome C and apoptosis-inducing factor.136 MT is a chronobiotic agent that regulates other circadian rhythms including the expression of circadian rhythm genes,137 which exert a major influence on inflammatory responses and immune function.138
Evidence from several animal studies including piglets, lambs, sheep, and rats have demonstrated compelling neuroprotective benefits of MT as a single therapy and as an adjunct therapy with TH. In a piglet model of perinatal asphyxia, piglets that received TH plus 30 mg/kg MT within 10 min of HI had improved markers of neuronal viability on MRS and reduced markers of neuronal cell death compared to those that received TH alone.139 Further studies in piglets and fetal sheep suggest that the benefit of MT appears to be time critical, dependent on therapeutic levels (15–30 mg/L) achieved within 3–4 h after HI, and that formulations with ethanol excipient are most effective.140–143
More recently, small pilot studies in human neonates suggest possible neuroprotective benefits of MT as an adjunctive therapy to TH. The first small RCT of 25 infants with NE to examine the long-term effects of MT (5 mg/kg IV) as an adjunct to TH found that patients receiving MT treatment had better cognitive ability on Bayley-III at 18 months of age compared to those receiving placebo. There was no difference in survival or incidence of cerebral palsy between groups. However, the trial was not powered to detect a difference.144 An earlier small trial also found improved survival with reduced neurodevelopmental abnormalities at 6 months of age in patients who received MT (5 daily enteral doses of 10 mg/kg) and TH compared to TH alone.145 Two further studies compared MT monotherapy orally to placebo and reported reduced mortality,146,147 using eight 10 mg/kg doses every 2 h and a one-off dose of 10 mg/kg, respectively. A recent systematic review and meta-analysis described the paucity of high quality RCTs of MT as an adjunct to TH in NE due to inadequate sample size, subtherapeutic levels with uncertain oral bioavailability in sick neonates, no pharmacokinetic studies and no consistent validated outcome measure.148 Large clinical trials of MT are needed.
The Use of Melatonin for Neuroprotection in Asphyxiated Newborns (MELPRO) study, the first phase III placebo-controlled trial of enteral MT as an adjunctive therapy to TH, is currently recruiting and will report on the primary outcome of Bayley scale III neurodevelopmental outcome at 12 months and secondary outcomes of neurodevelopmental outcomes at 24 months, MRI, and aEEG results (NCT03806816). One hundred neonates with moderate-to-severe NE will be randomized to TH or TH with 5 daily enteral doses of MT 10 mg/kg. The bioavailability of enteral MT may be variable in sick neonates undergoing TH; however, serum MT and autophagy levels will be measured at enrolment, daily during TH, at days 5 and 7.
Other proposed interventions include the nonpsychotropic cannabinoid, cannabidiol (CBD), and allopurinol. CBD has been safely used in the treatment of seizures resistant to other ASMs in the pediatric population.149 CBD has shown mixed results after HI, with some short-term evidence of benefit after immediate IV infusion after HI in piglets during normothermia or TH.150,151 However, other studies found no evidence of neuroprotection, and hypotension developed during higher-dose therapy.152,153 Thus, further preclinical studies are needed resolve its potential value, and practical constraints before it can be considered for translation. Allopurinol, a xanthine-oxidase inhibitor, is currently being assessed in a multicenter RCT in 13 European centers as an adjunctive treatment to TH.154
Conclusions
Following TH, a significant proportion of neonates with NE still develop long-term neurodisability. Therefore, optimizing and further improving neonatal intensive care and neonatal neurocritical care is vital. Additional neuroprotective interventions such as erythropoietin, MT, and stem cells are currently being tested in clinical trials. Follow-up from hospital discharge through childhood to optimize systemic and neurodevelopmental outcomes will be valuable as they may be opportunities for further neuroprotective therapies to prevent tertiary brain injury. The recently established Newborn Brain Society (newbornbrainsociety.org) will have an important role in setting clinical practice guidelines for caring of these infants, facilitating international registries, and organizing/coordinating multicenter research activities to advance this important field.
Data sharing statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author contributions
All authors were involved in drafting the article and revising it critically for important intellectual content. Each author has approved the final version for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Eleanor J. Molloy, Email: Eleanor.molloy@tcd.ie
On behalf of the Newborn Brain Society Guidelines and Publications Committee:
Sonia Bonifacio, Pia Wintermark, Hany Aly, Taeun Chang, Vann Chau, Hannah Glass, Monica Lemmon, An Massaro, Courtney Wusthoff, Gabrielle deVeber, Andrea Pardo, and Melisa Carrasco McCaul
References
- 1.Liu L, et al. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the sustainable development goals. Lancet. 2016;388:3027–3035. doi: 10.1016/S0140-6736(16)31593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleiss B, Gressens P. Tertiary mechanisms of brain damage: a new hope for treatment of cerebral palsy? Lancet Neurol. 2012;11:556–566. doi: 10.1016/S1474-4422(12)70058-3. [DOI] [PubMed] [Google Scholar]
- 3.Zareen Z, et al. Cytokine dysregulation persists in childhood post neonatal encephalopathy. BMC Neurol. 2020;20:115. doi: 10.1186/s12883-020-01656-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azzopardi D, et al. Implementation and conduct of therapeutic hypothermia for perinatal asphyxial encephalopathy in the UK–analysis of national data. PLoS ONE. 2012;7:e38504. doi: 10.1371/journal.pone.0038504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shankaran S, et al. Neonatal magnetic resonance imaging pattern of brain injury as a biomarker of childhood outcomes following a trial of hypothermia for neonatal hypoxic-ischemic encephalopathy. J. Pediatr. 2015;167:987.e3–993.e3. doi: 10.1016/j.jpeds.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobs SE, et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst. Rev. 2013;2013:Cd003311. doi: 10.1002/14651858.CD003311.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Natarajan G, Pappas A, Shankaran S. Outcomes in childhood following therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy (HIE) Semin. Perinatol. 2016;40:549–555. doi: 10.1053/j.semperi.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wassink G, Gunn ER, Drury PP, Bennet L, Gunn AJ. The mechanisms and treatment of asphyxial encephalopathy. Front. Neurosci. 2014;8:40. doi: 10.3389/fnins.2014.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidson JO, et al. How long is sufficient for optimal neuroprotection with cerebral cooling after ischemia in fetal sheep? J. Cereb. Blood Flow Metab. 2018;38:1047–1059. doi: 10.1177/0271678X17707671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alonso-Alconada D, et al. Brain cell death is reduced with cooling by 3.5°C to 5°C but increased with cooling by 8.5°C in a piglet asphyxia model. Stroke. 2015;46:275–278. doi: 10.1161/STROKEAHA.114.007330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davidson JO, et al. How long is too long for cerebral cooling after ischemia in fetal sheep? J. Cereb. Blood Flow Metab. 2015;35:751–758. doi: 10.1038/jcbfm.2014.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shankaran S, et al. Effect of depth and duration of cooling on death or disability at age 18 months among neonates with hypoxic-ischemic encephalopathy: a randomized clinical trial. JAMA. 2017;318:57–67. doi: 10.1001/jama.2017.7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards AD, et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ. 2010;340:c363. doi: 10.1136/bmj.c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laptook AR, et al. Effect of therapeutic hypothermia initiated after 6 h of age on death or disability among newborns with hypoxic-ischemic encephalopathy: a randomized clinical trial. JAMA. 2017;318:1550–1560. doi: 10.1001/jama.2017.14972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thayyil S, et al. Hypothermia for moderate or severe neonatal encephalopathy in low-income and middle-income countries (HELIX): a randomised controlled trial in India, Sri Lanka, and Bangladesh. Lancet Glob. Health. 2021;9:e1273–e1285. doi: 10.1016/S2214-109X(21)00264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wassink G, et al. A working model for hypothermic neuroprotection. J. Physiol. 2018;596:5641–5654. doi: 10.1113/JP274928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aslam S, Strickland T, Molloy EJ. Neonatal encephalopathy: need for recognition of multiple etiologies for optimal management. Front. Pediatr. 2019;7:142. doi: 10.3389/fped.2019.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calkavur S, et al. Genetic factors that influence short-term neurodevelopmental outcome in term hypoxic-ischaemic encephalopathic neonates. J. Int. Med. Res. 2011;39:1744–1756. doi: 10.1177/147323001103900517. [DOI] [PubMed] [Google Scholar]
- 19.Tan ES. Inborn errors of metabolism presenting as neonatal encephalopathy: practical tips for clinicians. Ann. Acad. Med. Singap. 2008;37:94–93. [PubMed] [Google Scholar]
- 20.Wood S, Crawford S, Hicks M, Mohammad K. Hospital-related, maternal, and fetal risk factors for neonatal asphyxia and moderate or severe hypoxic-ischemic encephalopathy: a retrospective cohort study. J. Matern. Fetal Neonatal Med. 2021;34:1448–1453. doi: 10.1080/14767058.2019.1638901. [DOI] [PubMed] [Google Scholar]
- 21.Tann CJ, et al. Perinatal risk factors for neonatal encephalopathy: an unmatched case-control study. Arch. Dis. Child. Fetal Neonatal Ed. 2018;103:F250–f256. doi: 10.1136/archdischild-2017-312744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossi AC, Prefumo F. Antepartum and intrapartum risk factors for neonatal hypoxic-ischemic encephalopathy: a systematic review with meta-analysis. Curr. Opin. Obstet. Gynecol. 2019;31:410–417. doi: 10.1097/GCO.0000000000000581. [DOI] [PubMed] [Google Scholar]
- 23.Oliveira V, et al. Hypothermia for encephalopathy in low-income and middle-income countries: feasibility of whole-body cooling using a low-cost servo-controlled device. BMJ Paediatr. Open. 2018;2:e000245. doi: 10.1136/bmjpo-2017-000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kofke WA. Incrementally applied multifaceted therapeutic bundles in neuroprotection clinical trials…time for change. Neurocrit Care. 2010;12:438–444. doi: 10.1007/s12028-010-9332-7. [DOI] [PubMed] [Google Scholar]
- 25.Williams KP, Galerneau F. Intrapartum fetal heart rate patterns in the prediction of neonatal acidemia. Am. J. Obstet. Gynecol. 2003;188:820–823. doi: 10.1067/mob.2003.183. [DOI] [PubMed] [Google Scholar]
- 26.Lingappan K, Kaiser JR, Srinivasan C, Gunn AJ. Relationship between PCO2 and unfavorable outcome in infants with moderate-to-severe hypoxic ischemic encephalopathy. Pediatr. Res. 2016;80:204–208. doi: 10.1038/pr.2016.62. [DOI] [PubMed] [Google Scholar]
- 27.Ivy AS, Clark CL, Bahm SM, Meurs KP, Wusthoff CJ. Improving the identification of neonatal encephalopathy: utility of a web-based video tool. Am. J. Perinatol. 2017;34:520–522. doi: 10.1055/s-0036-1593846. [DOI] [PubMed] [Google Scholar]
- 28.Mohammad K, et al. Hemodynamic instability associated with increased risk of death or brain injury in neonates with hypoxic ischemic encephalopathy. J. Neonatal Perinat. Med. 2017;10:363–370. doi: 10.3233/NPM-1816162. [DOI] [PubMed] [Google Scholar]
- 29.Ilves P, Kiisk M, Soopõld T, Talvik T. Serum total magnesium and ionized calcium concentrations in asphyxiated term newborn infants with hypoxic-ischaemic encephalopathy. Acta Paediatr. 2000;89:680–685. doi: 10.1111/j.1651-2227.2000.tb00364.x. [DOI] [PubMed] [Google Scholar]
- 30.Wintermark P, Mohammad K, Bonifacio SL. Proposing a care practice bundle for neonatal encephalopathy during therapeutic hypothermia. Semin. Fetal Neonatal Med. 2021;26:101303. doi: 10.1016/j.siny.2021.101303. [DOI] [PubMed] [Google Scholar]
- 31.Roychoudhury S, et al. Implementation of neonatal neurocritical care program improved short-term outcomes in neonates with moderate-to-severe hypoxic ischemic encephalopathy. Pediatr. Neurol. 2019;101:64–70. doi: 10.1016/j.pediatrneurol.2019.02.023. [DOI] [PubMed] [Google Scholar]
- 32.Bashir RA, et al. Implementation of a neurocritical care program: improved seizure detection and decreased antiseizure medication at discharge in neonates with hypoxic-ischemic encephalopathy. Pediatr. Neurol. 2016;64:38–43. doi: 10.1016/j.pediatrneurol.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Goswami I, et al. Introduction of continuous video EEG monitoring into 2 different NICU models by training neonatal nurses. Adv. Neonatal Care. 2018;18:250–259. doi: 10.1097/ANC.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 34.Mohammad K, et al. Impact of outreach education program on outcomes of neonates with hypoxic ischemic encephalopathy. Paediatr. Child Health. 2020;26:e215–e221. doi: 10.1093/pch/pxaa075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arriagada S, Huang H, Fletcher K, Giannone P. Prevention of excessive hypothermia in infants with hypoxic ischemic encephalopathy prior to admission to a quaternary care center: a Neonatal Outreach Educational Project. J. Perinatol. 2019;39:1417–1427. doi: 10.1038/s41372-019-0391-0. [DOI] [PubMed] [Google Scholar]
- 36.Cramer SC, et al. Harnessing neuroplasticity for clinical applications. Brain. 2011;134:1591–1609. doi: 10.1093/brain/awr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Brien K, et al. Effectiveness of family integrated care in neonatal intensive care units on infant and parent outcomes: a multicentre, multinational, cluster-randomised controlled trial. Lancet Child Adolesc. Health. 2018;2:245–254. doi: 10.1016/S2352-4642(18)30039-7. [DOI] [PubMed] [Google Scholar]
- 38.DeMaster D, et al. Nurturing the preterm infant brain: leveraging neuroplasticity to improve neurobehavioral outcomes. Pediatr. Res. 2019;85:166–175. doi: 10.1038/s41390-018-0203-9. [DOI] [PubMed] [Google Scholar]
- 39.Maguire CM, et al. Effects of individualized developmental care in a randomized trial of preterm infants <32 weeks. Pediatrics. 2009;124:1021–1030. doi: 10.1542/peds.2008-1881. [DOI] [PubMed] [Google Scholar]
- 40.Procianoy RS, Mendes EW, Silveira RC. Massage therapy improves neurodevelopment outcome at two years corrected age for very low birth weight infants. Early Hum. Dev. 2010;86:7–11. doi: 10.1016/j.earlhumdev.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Feldman R, Rosenthal Z, Eidelman AI. Maternal-preterm skin-to-skin contact enhances child physiologic organization and cognitive control across the first 10 years of life. Biol. Psychiatry. 2014;75:56–64. doi: 10.1016/j.biopsych.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 42.Chorna O, et al. Neuroprocessing mechanisms of music during fetal and neonatal development: a role in neuroplasticity and neurodevelopment. Neural Plast. 2019;2019:3972918. doi: 10.1155/2019/3972918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Braid S, Bernstein J. Improved cognitive development in preterm infants with shared book reading. Neonatal Netw. 2015;34:10–17. doi: 10.1891/0730-0832.34.1.10. [DOI] [PubMed] [Google Scholar]
- 44.Shellhaas, R. A., Burns, J. W., Barks, J. D. E., Hassan, F. & Chervin, R. D. Maternal voice and infant sleep in the neonatal intensive care unit. Pediatrics144, e20190288 (2019). [DOI] [PMC free article] [PubMed]
- 45.Smith SW, Ortmann AJ, Clark WW. Noise in the neonatal intensive care unit: a new approach to examining acoustic events. Noise Health. 2018;20:121–130. doi: 10.4103/nah.NAH_53_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shellhaas, R. A. et al. Neonatal sleep-wake analyses predict 18-month neurodevelopmental outcomes. Sleep40, zsx144 (2017). [DOI] [PMC free article] [PubMed]
- 47.Forcada-Guex M, Pierrehumbert B, Borghini A, Moessinger A, Muller-Nix C. Early dyadic patterns of mother-infant interactions and outcomes of prematurity at 18 months. Pediatrics. 2006;118:e107–e114. doi: 10.1542/peds.2005-1145. [DOI] [PubMed] [Google Scholar]
- 48.Osmond E, et al. Neonatal seizures: magnetic resonance imaging adds value in the diagnosis and prediction of neurodisability. Acta Paediatr. 2014;103:820–826. doi: 10.1111/apa.12583. [DOI] [PubMed] [Google Scholar]
- 49.Soul JS. Acute symptomatic seizures in term neonates: etiologies and treatments. Semin. Fetal Neonatal Med. 2018;23:183–190. doi: 10.1016/j.siny.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glass HC, et al. Contemporary profile of seizures in neonates: a prospective cohort study. J. Pediatr. 2016;174:98–103.e101. doi: 10.1016/j.jpeds.2016.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shellhaas RA, et al. The American Clinical Neurophysiology Society’s Guideline on continuous electroencephalography monitoring in neonates. J. Clin. Neurophysiol. 2011;28:611–617. doi: 10.1097/WNP.0b013e31823e96d7. [DOI] [PubMed] [Google Scholar]
- 52.Massey SL, Jensen FE, Abend NS. Electroencephalographic monitoring for seizure identification and prognosis in term neonates. Semin. Fetal Neonatal Med. 2018;23:168–174. doi: 10.1016/j.siny.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 53.Pavel AM, et al. A machine-learning algorithm for neonatal seizure recognition: a multicentre, randomised, controlled trial. Lancet Child Adolesc. Health. 2020;4:740–749. doi: 10.1016/S2352-4642(20)30239-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hellström-Westas L, Boylan G, Ågren J. Systematic review of neonatal seizure management strategies provides guidance on anti-epileptic treatment. Acta Paediatr. 2015;104:123–129. doi: 10.1111/apa.12812. [DOI] [PubMed] [Google Scholar]
- 55.Guidotti I, et al. Hypothermia reduces seizure burden and improves neurological outcome in severe hypoxic-ischemic encephalopathy: an observational study. Dev. Med. Child Neurol. 2016;58:1235–1241. doi: 10.1111/dmcn.13195. [DOI] [PubMed] [Google Scholar]
- 56.Srinivasakumar P, et al. Therapeutic hypothermia in neonatal hypoxic ischemic encephalopathy: electrographic seizures and magnetic resonance imaging evidence of injury. J. Pediatrics. 2013;163:465–470. doi: 10.1016/j.jpeds.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 57.Low E, et al. Cooling and seizure burden in term neonates: an observational study. Arch. Dis. Child. Fetal Neonatal Ed. 2012;97:F267. doi: 10.1136/archdischild-2011-300716. [DOI] [PubMed] [Google Scholar]
- 58.Davidson JO, et al. Non-additive effects of delayed connexin hemichannel blockade and hypothermia after cerebral ischemia in near-term fetal sheep. J. Cereb. Blood Flow Metab. 2015;35:2052–2061. doi: 10.1038/jcbfm.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holmes GL. The long-term effects of neonatal seizures. Clin. Perinatol. 2009;36:901–914. doi: 10.1016/j.clp.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 60.Wirrell EC, Armstrong EA, Osman LD, Yager JY. Prolonged seizures exacerbate perinatal hypoxic-ischemic brain damage. Pediatr. Res. 2001;50:445–454. doi: 10.1203/00006450-200110000-00005. [DOI] [PubMed] [Google Scholar]
- 61.Yager JY, Armstrong EA, Jaharus C, Saucier DM, Wirrell EC. Preventing hyperthermia decreases brain damage following neonatal hypoxic-ischemic seizures. Brain Res. 2004;1011:48–57. doi: 10.1016/j.brainres.2004.02.070. [DOI] [PubMed] [Google Scholar]
- 62.Wyatt JS, et al. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics. 2007;119:912–921. doi: 10.1542/peds.2006-2839. [DOI] [PubMed] [Google Scholar]
- 63.Laptook A, et al. Elevated temperature after hypoxic-ischemic encephalopathy: risk factor for adverse outcomes. Pediatrics. 2008;122:491–499. doi: 10.1542/peds.2007-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tan WK, Williams CE, Gunn AJ, Mallard CE, Gluckman PD. Suppression of postischemic epileptiform activity with MK-801 improves neural outcome in fetal sheep. Ann. Neurol. 1992;32:677–682. doi: 10.1002/ana.410320511. [DOI] [PubMed] [Google Scholar]
- 65.Kharoshankaya L, et al. Seizure burden and neurodevelopmental outcome in neonates with hypoxic-ischemic encephalopathy. Dev. Med. Child Neurol. 2016;58:1242–1248. doi: 10.1111/dmcn.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Glass HC, et al. Clinical neonatal seizures are independently associated with outcome in infants at risk for hypoxic-ischemic brain injury. J. Pediatr. 2009;155:318–323. doi: 10.1016/j.jpeds.2009.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Srinivasakumar P, et al. Treating EEG seizures in hypoxic ischemic encephalopathy: a randomized controlled trial. Pediatrics. 2015;136:e1302–e1309. doi: 10.1542/peds.2014-3777. [DOI] [PubMed] [Google Scholar]
- 68.van Rooij LG, et al. Effect of treatment of subclinical neonatal seizures detected with aEEG: randomized, controlled trial. Pediatrics. 2010;125:e358–e366. doi: 10.1542/peds.2009-0136. [DOI] [PubMed] [Google Scholar]
- 69.Soul JS, et al. Recommendations for the design of therapeutic trials for neonatal seizures. Pediatr. Res. 2019;85:943–954. doi: 10.1038/s41390-018-0242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Painter MJ, et al. Phenobarbital compared with phenytoin for the treatment of neonatal seizures. N. Engl. J. Med. 1999;341:485–489. doi: 10.1056/NEJM199908123410704. [DOI] [PubMed] [Google Scholar]
- 71.El-Dib M, Soul JS. The use of phenobarbital and other anti-seizure drugs in newborns. Semin. Fetal Neonatal Med. 2017;22:321–327. doi: 10.1016/j.siny.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 72.Soul JS, et al. A pilot randomized, controlled, double-blind trial of bumetanide to treat neonatal seizures. Ann. Neurol. 2021;89:327–340. doi: 10.1002/ana.25959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sharpe, C. et al. Levetiracetam versus phenobarbital for neonatal seizures: a randomized controlled trial. Pediatrics145, e20193182 (2020). [DOI] [PMC free article] [PubMed]
- 74.Boylan GB, et al. Second-line anticonvulsant treatment of neonatal seizures: a video-EEG monitoring study. Neurology. 2004;62:486–488. doi: 10.1212/01.WNL.0000106944.59990.E6. [DOI] [PubMed] [Google Scholar]
- 75.Castro Conde JR, Hernández Borges AA, Doménech Martínez E, González Campo C, Perera Soler R. Midazolam in neonatal seizures with no response to phenobarbital. Neurology. 2005;64:876–879. doi: 10.1212/01.WNL.0000152891.58694.71. [DOI] [PubMed] [Google Scholar]
- 76.Weeke LC, et al. Lidocaine response rate in aeeg-confirmed neonatal seizures: retrospective study of 413 full-term and preterm infants. Epilepsia. 2016;57:233–242. doi: 10.1111/epi.13286. [DOI] [PubMed] [Google Scholar]
- 77.Pressler RM, et al. Bumetanide for the treatment of seizures in newborn babies with hypoxic ischaemic encephalopathy (NEMO): an open-label, dose finding, and feasibility phase 1/2 trial. Lancet Neurol. 2015;14:469–477. doi: 10.1016/S1474-4422(14)70303-5. [DOI] [PubMed] [Google Scholar]
- 78.Koh S, Tibayan FD, Simpson JN, Jensen FE. Nbqx or topiramate treatment after perinatal hypoxia-induced seizures prevents later increases in seizure-induced neuronal injury. Epilepsia. 2004;45:569–575. doi: 10.1111/j.0013-9580.2004.69103.x. [DOI] [PubMed] [Google Scholar]
- 79.Glass HC, Poulin C, Shevell MI. Topiramate for the treatment of neonatal seizures. Pediatr. Neurol. 2011;44:439–442. doi: 10.1016/j.pediatrneurol.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nuñez-Ramiro A, et al. Topiramate plus cooling for hypoxic-ischemic encephalopathy: a randomized, controlled, multicenter, double-blinded trial. Neonatology. 2019;116:76–84. doi: 10.1159/000499084. [DOI] [PubMed] [Google Scholar]
- 81.van den Broek MP, et al. Pharmacokinetics and clinical efficacy of phenobarbital in asphyxiated newborns treated with hypothermia: a thermopharmacological approach. Clin. Pharmacokinet. 2012;51:671–679. doi: 10.1007/s40262-012-0004-y. [DOI] [PubMed] [Google Scholar]
- 82.Shellhaas RA, Ng CM, Dillon CH, Barks JD, Bhatt-Mehta V. Population pharmacokinetics of phenobarbital in infants with neonatal encephalopathy treated with therapeutic hypothermia. Pediatr. Crit. Care Med. 2013;14:194–202. doi: 10.1097/PCC.0b013e31825bbbc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bittigau P, et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc. Natl Acad. Sci. USA. 2002;99:15089–15094. doi: 10.1073/pnas.222550499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Forcelli PA, Kim J, Kondratyev A, Gale K. Pattern of antiepileptic drug-induced cell death in limbic regions of the neonatal rat brain. Epilepsia. 2011;52:e207–e211. doi: 10.1111/j.1528-1167.2011.03297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou, K. Q. et al. Treating seizures after hypoxic-ischemic encephalopathy-current controversies and future directions. Int. J. Mol. Sci.22, 7121 (2021). [DOI] [PMC free article] [PubMed]
- 86.Kilicdag H, et al. The effect of levetiracetam on neuronal apoptosis in neonatal rat model of hypoxic ischemic brain injury. Early Hum. Dev. 2013;89:355–360. doi: 10.1016/j.earlhumdev.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 87.Komur M, et al. Neuroprotective effect of levetiracetam on hypoxic ischemic brain injury in neonatal rats. Childs Nerv. Syst. 2014;30:1001–1009. doi: 10.1007/s00381-014-2375-x. [DOI] [PubMed] [Google Scholar]
- 88.Gonzalez FF, et al. Erythropoietin increases neurogenesis and oligodendrogliosis of subventricular zone precursor cells after neonatal stroke. Stroke. 2013;44:753–758. doi: 10.1161/STROKEAHA.111.000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shingo T, Sorokan ST, Shimazaki T, Weiss S. Erythropoietin regulates the in vitro and in vivo production of neuronal progenitors by mammalian forebrain neural stem cells. J. Neurosci. 2001;21:9733–9743. doi: 10.1523/JNEUROSCI.21-24-09733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.O’Dea MI, et al. Altered cytokine endotoxin responses in neonatal encephalopathy predict MRI outcomes. Front Pediatr. 2021;9:734540. doi: 10.3389/fped.2021.734540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.O’Hare FM, et al. Serial cytokine alterations and abnormal neuroimaging in newborn infants with encephalopathy. Acta Paediatr. 2017;106:561–567. doi: 10.1111/apa.13745. [DOI] [PubMed] [Google Scholar]
- 92.Ruth V, Widness JA, Clemons G, Raivio KO. Postnatal changes in serum immunoreactive erythropoietin in relation to hypoxia before and after birth. J. Pediatr. 1990;116:950–954. doi: 10.1016/S0022-3476(05)80659-6. [DOI] [PubMed] [Google Scholar]
- 93.Hagag AA, El Frargy MS, Abd El-Latif AE. Study of cord blood erythropoietin, leptin and adiponectin levels in neonates with hypoxic ischemic encephalopathy. Endocr. Metab. Immune Disord. Drug Targets. 2020;20:213–220. doi: 10.2174/1871530319666190725110619. [DOI] [PubMed] [Google Scholar]
- 94.Teramo KA, Klemetti MM, Widness JA. Robust increases in erythropoietin production by the hypoxic fetus is a response to protect the brain and other vital organs. Pediatr. Res. 2018;84:807–812. doi: 10.1038/s41390-018-0054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.van der Kooij MA, Groenendaal F, Kavelaars A, Heijnen CJ, van Bel F. Neuroprotective properties and mechanisms of erythropoietin in in vitro and in vivo experimental models for hypoxia/ischemia. Brain Res. Rev. 2008;59:22–33. doi: 10.1016/j.brainresrev.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 96.Kellert BA, McPherson RJ, Juul SE. A comparison of high-dose recombinant erythropoietin treatment regimens in brain-injured neonatal rats. Pediatr. Res. 2007;61:451–455. doi: 10.1203/pdr.0b013e3180332cec. [DOI] [PubMed] [Google Scholar]
- 97.Weber A, et al. Neuronal damage after moderate hypoxia and erythropoietin. Neurobiol. Dis. 2005;20:594–600. doi: 10.1016/j.nbd.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 98.Juul SE, et al. Erytropoietin concentrations in cerebrospinal fluid of nonhuman primates and fetal sheep following high-dose recombinant erythropoietin. Biol. Neonate. 2004;85:138–144. doi: 10.1159/000074970. [DOI] [PubMed] [Google Scholar]
- 99.Statler PA, McPherson RJ, Bauer LA, Kellert BA, Juul SE. Pharmacokinetics of high-dose recombinant erythropoietin in plasma and brain of neonatal rats. Pediatr. Res. 2007;61:671–675. doi: 10.1203/pdr.0b013e31805341dc. [DOI] [PubMed] [Google Scholar]
- 100.Wassink G, et al. Recombinant erythropoietin does not augment hypothermic white matter protection after global cerebral ischaemia in near-term fetal sheep. Brain Commun. 2021;3:fcab172. doi: 10.1093/braincomms/fcab172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wassink G, et al. Non-additive effects of adjunct erythropoietin therapy with therapeutic hypothermia after global cerebral ischaemia in near-term fetal sheep. J. Physiol. 2020;598:999–1015. doi: 10.1113/JP279131. [DOI] [PubMed] [Google Scholar]
- 102.Malla RR, Asimi R, Teli MA, Shaheen F, Bhat MA. Erythropoietin monotherapy in perinatal asphyxia with moderate to severe encephalopathy: a randomized placebo-controlled trial. J. Perinatol. 2017;37:596–601. doi: 10.1038/jp.2017.17. [DOI] [PubMed] [Google Scholar]
- 103.Zhu C, et al. Erythropoietin improved neurologic outcomes in newborns with hypoxic-ischemic encephalopathy. Pediatrics. 2009;124:e218–e226. doi: 10.1542/peds.2008-3553. [DOI] [PubMed] [Google Scholar]
- 104.Wu, Y. W. et al. High-dose erythropoietin and hypothermia for hypoxic-ischemic encephalopathy: a phase II trial. Pediatrics137, e20160191 (2016). [DOI] [PubMed]
- 105.Massaro AN, et al. Plasma biomarkers of brain injury in neonatal hypoxic-ischemic encephalopathy. J. Pediatr. 2018;194:67.e61–75.e61. doi: 10.1016/j.jpeds.2017.10.060. [DOI] [PubMed] [Google Scholar]
- 106.Wu YW, et al. Trial of erythropoietin for hypoxic-ischemic encephalopathy in newborns. N. Engl. J. Med. 2022;387:148–159. doi: 10.1056/NEJMoa2119660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.University of S. & National H., Medical Research Council A. PAEAN - Erythropoietin for Hypoxic Ischaemic Encephalopathy in Newborns. https://clinicaltrials.gov/ct2/show/NCT03079167 (2016).
- 108.University of Utah, Darbe Administration in Newborns Undergoing Cooling for Encephalopathy. https://clinicaltrials.gov/ct2/show/NCT01471015 (2015).
- 109.University of New M. & University of U. Mild Encephalopathy in the Newborn Treated With Darbepoetin. https://clinicaltrials.gov/ct2/show/NCT03071861 (2021).
- 110.Kurtzberg J. A history of cord blood banking and transplantation. Stem Cells Transl. Med. 2017;6:1309–1311. doi: 10.1002/sctm.17-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang X, et al. Effect of umbilical cord mesenchymal stromal cells on motor functions of identical twins with cerebral palsy: pilot study on the correlation of efficacy and hereditary factors. Cytotherapy. 2015;17:224–231. doi: 10.1016/j.jcyt.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 112.Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat. Immunol. 2014;15:1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 113.Cunningham CJ, Redondo-Castro E, Allan SM. The therapeutic potential of the mesenchymal stem cell secretome in ischaemic stroke. J. Cereb. Blood Flow Metab. 2018;38:1276–1292. doi: 10.1177/0271678X18776802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fisher SA, et al. Mesenchymal stromal cells as treatment or prophylaxis for acute or chronic graft-versus-host disease in haematopoietic stem cell transplant (HSCT) recipients with a haematological condition. Cochrane Database Syst. Rev. 2019;1:Cd009768. doi: 10.1002/14651858.CD009768.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vaes JEG, et al. The potential of stem cell therapy to repair white matter injury in preterm infants: lessons learned from experimental models. Front. Physiol. 2019;10:540. doi: 10.3389/fphys.2019.00540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vu Q, Xie K, Eckert M, Zhao W, Cramer SC. Meta-analysis of preclinical studies of mesenchymal stromal cells for ischemic stroke. Neurology. 2014;82:1277–1286. doi: 10.1212/WNL.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Meier C, et al. Spastic paresis after perinatal brain damage in rats is reduced by human cord blood mononuclear cells. Pediatr. Res. 2006;59:244–249. doi: 10.1203/01.pdr.0000197309.08852.f5. [DOI] [PubMed] [Google Scholar]
- 118.Rosenkranz K, Meier C. Umbilical cord blood cell transplantation after brain ischemia–from recovery of function to cellular mechanisms. Ann. Anat. 2011;193:371–379. doi: 10.1016/j.aanat.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 119.Rosenkranz K, et al. Transplantation of human umbilical cord blood cells mediated beneficial effects on apoptosis, angiogenesis and neuronal survival after hypoxic-ischemic brain injury in rats. Cell Tissue Res. 2012;348:429–438. doi: 10.1007/s00441-012-1401-0. [DOI] [PubMed] [Google Scholar]
- 120.McDonald CA, et al. Effects of umbilical cord blood cells, and subtypes, to reduce neuroinflammation following perinatal hypoxic-ischemic brain injury. J. Neuroinflammation. 2018;15:47. doi: 10.1186/s12974-018-1089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ. Mesenchymal stem cell treatment after neonatal hypoxic-ischemic brain injury improves behavioral outcome and induces neuronal and oligodendrocyte regeneration. Brain Behav. Immun. 2010;24:387–393. doi: 10.1016/j.bbi.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 122.van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ. Repeated mesenchymal stem cell treatment after neonatal hypoxia-ischemia has distinct effects on formation and maturation of new neurons and oligodendrocytes leading to restoration of damage, corticospinal motor tract activity, and sensorimotor function. J. Neurosci. 2010;30:9603–9611. doi: 10.1523/JNEUROSCI.1835-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nair S, et al. Neuroprotection offered by mesenchymal stem cells in perinatal brain injury: role of mitochondria, inflammation, and reactive oxygen species. J. Neurochem. 2021;158:59–73. doi: 10.1111/jnc.15267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kaminski N, et al. Mesenchymal stromal cell-derived extracellular vesicles reduce neuroinflammation, promote neural cell proliferation and improve oligodendrocyte maturation in neonatal hypoxic-ischemic brain injury. Front. Cell Neurosci. 2020;14:601176. doi: 10.3389/fncel.2020.601176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Herz J, et al. Interaction between hypothermia and delayed mesenchymal stem cell therapy in neonatal hypoxic-ischemic brain injury. Brain Behav. Immun. 2018;70:118–130. doi: 10.1016/j.bbi.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 126.Robertson NJ, et al. Human umbilical cord mesenchymal stromal cells as an adjunct therapy with therapeutic hypothermia in a piglet model of perinatal asphyxia. Cytotherapy. 2021;23:521–535. doi: 10.1016/j.jcyt.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bernardo ME, Fibbe WE. Mesenchymal stromal cells and hematopoietic stem cell transplantation. Immunol. Lett. 2015;168:215–221. doi: 10.1016/j.imlet.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 128.Cotten CM, et al. Feasibility of autologous cord blood cells for infants with hypoxic-ischemic encephalopathy. J. Pediatr. 2014;164:973.e1–979.e1. doi: 10.1016/j.jpeds.2013.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tsuji M, et al. Autologous cord blood cell therapy for neonatal hypoxic-ischaemic encephalopathy: a pilot study for feasibility and safety. Sci. Rep. 2020;10:4603. doi: 10.1038/s41598-020-61311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Reiter RJ. Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 1991;12:151–180. doi: 10.1210/edrv-12-2-151. [DOI] [PubMed] [Google Scholar]
- 131.Tarocco A, et al. Melatonin as a master regulator of cell death and inflammation: molecular mechanisms and clinical implications for newborn care. Cell Death Dis. 2019;10:317. doi: 10.1038/s41419-019-1556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ng KY, Leong MK, Liang H, Paxinos G. Melatonin receptors: distribution in mammalian brain and their respective putative functions. Brain Struct. Funct. 2017;222:2921–2939. doi: 10.1007/s00429-017-1439-6. [DOI] [PubMed] [Google Scholar]
- 133.Calvo JR, González-Yanes C, Maldonado MD. The role of melatonin in the cells of the innate immunity: a review. J. Pineal Res. 2013;55:103–120. doi: 10.1111/jpi.12075. [DOI] [PubMed] [Google Scholar]
- 134.Hardeland R. Melatonin and inflammation-story of a double-edged blade. J. Pineal Res. 2018;65:e12525. doi: 10.1111/jpi.12525. [DOI] [PubMed] [Google Scholar]
- 135.Reiter RJ, et al. Melatonin as an antioxidant: under promises but over delivers. J. Pineal Res. 2016;61:253–278. doi: 10.1111/jpi.12360. [DOI] [PubMed] [Google Scholar]
- 136.Sun FY, et al. Neuroprotection by melatonin against ischemic neuronal injury associated with modulation of DNA damage and repair in the rat following a transient cerebral ischemia. J. Pineal Res. 2002;33:48–56. doi: 10.1034/j.1600-079X.2002.01891.x. [DOI] [PubMed] [Google Scholar]
- 137.Vriend J, Reiter RJ. Melatonin feedback on clock genes: a theory involving the proteasome. J. Pineal Res. 2015;58:1–11. doi: 10.1111/jpi.12189. [DOI] [PubMed] [Google Scholar]
- 138.Curtis AM, Bellet MM, Sassone-Corsi P, O’Neill LA. Circadian clock proteins and immunity. Immunity. 2014;40:178–186. doi: 10.1016/j.immuni.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 139.Robertson NJ, et al. Melatonin augments hypothermic neuroprotection in a perinatal asphyxia model. Brain. 2013;136:90–105. doi: 10.1093/brain/aws285. [DOI] [PubMed] [Google Scholar]
- 140.Aridas JD, et al. Melatonin augments the neuroprotective effects of hypothermia in lambs following perinatal asphyxia. J. Pineal Res. 2021;71:e12744. doi: 10.1111/jpi.12744. [DOI] [PubMed] [Google Scholar]
- 141.Pang R, et al. Melatonin and/or erythropoietin combined with hypothermia in a piglet model of perinatal asphyxia. Brain Commun. 2020;3:fcaa211. doi: 10.1093/braincomms/fcaa211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Robertson NJ, et al. Melatonin as an adjunct to therapeutic hypothermia in a piglet model of neonatal encephalopathy: a translational study. Neurobiol. Dis. 2019;121:240–251. doi: 10.1016/j.nbd.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 143.Robertson NJ, et al. High-dose melatonin and ethanol excipient combined with therapeutic hypothermia in a newborn piglet asphyxia model. Sci. Rep. 2020;10:3898. doi: 10.1038/s41598-020-60858-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jerez-Calero A, et al. Hypothermia plus melatonin in asphyctic newborns: a randomized-controlled pilot study. Pediatr. Crit. Care Med. 2020;21:647–655. doi: 10.1097/PCC.0000000000002346. [DOI] [PubMed] [Google Scholar]
- 145.Aly H, et al. Melatonin use for neuroprotection in perinatal asphyxia: a randomized controlled pilot study. J. Perinatol. 2015;35:186–191. doi: 10.1038/jp.2014.186. [DOI] [PubMed] [Google Scholar]
- 146.Fulia F, et al. Increased levels of malondialdehyde and nitrite/nitrate in the blood of asphyxiated newborns: reduction by melatonin. J. Pineal Res. 2001;31:343–349. doi: 10.1034/j.1600-079X.2001.310409.x. [DOI] [PubMed] [Google Scholar]
- 147.Ahmad QM, Chishti AL, Waseem N. Role of melatonin in management of hypoxic ischaemic encephalopathy in newborns: a randomized control trial. J. Pak. Med. Assoc. 2018;68:1233–1237. [PubMed] [Google Scholar]
- 148.Ahmed J, Pullattayil SA, Robertson NJ, More K. Melatonin for neuroprotection in neonatal encephalopathy: a systematic review & meta-analysis of clinical trials. Eur. J. Paediatr. Neurol. 2021;31:38–45. doi: 10.1016/j.ejpn.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 149.Moreira GA, Moraes Neto R, Ribeiro RG, Crippa ACS. Cannabidiol for the treatment of refractory epilepsy in children: a critical review of the literature. Rev. Paul. Pediatr. 2022;41:e2021197. doi: 10.1590/1984-0462/2023/41/2021197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lafuente H, et al. Effects of cannabidiol and hypothermia on short-term brain damage in new-born piglets after acute hypoxia-ischemia. Front. Neurosci. 2016;10:323. doi: 10.3389/fnins.2016.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Barata L, et al. Neuroprotection by cannabidiol and hypothermia in a piglet model of newborn hypoxic-ischemic brain damage. Neuropharmacology. 2019;146:1–11. doi: 10.1016/j.neuropharm.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 152.Garberg HT, et al. Short-term effects of cannabidiol after global hypoxia-ischemia in newborn piglets. Pediatr. Res. 2016;80:710–718. doi: 10.1038/pr.2016.149. [DOI] [PubMed] [Google Scholar]
- 153.Garberg HT, et al. High-dose cannabidiol induced hypotension after global hypoxia-ischemia in piglets. Neonatology. 2017;112:143–149. doi: 10.1159/000471786. [DOI] [PubMed] [Google Scholar]
- 154.Maiwald CA, et al. Effect of allopurinol in addition to hypothermia treatment in neonates for hypoxic-ischemic brain injury on neurocognitive outcome (Albino): study protocol of a blinded randomized placebo-controlled parallel group multicenter trial for superiority (phase III) BMC Pediatr. 2019;19:210. doi: 10.1186/s12887-019-1566-8. [DOI] [PMC free article] [PubMed] [Google Scholar]