Abstract
Extracellular vesicles (EVs)-based cell-free strategy has shown therapeutic potential in tissue regeneration. Due to their important roles in intercellular communications and their natural ability to shield cargos from degradation, EVs are also emerged as novel delivery vehicles for various bioactive molecules and drugs. Accumulating studies have revealed that EVs can be modified to enhance their efficacy and specificity for the treatment of many diseases. Engineered EVs are poised as the next generation of targeted delivery platform in the field of precision therapy. In this review, the unique properties of EVs are overviewed in terms of their biogenesis, contents, surface features and biological functions, and the recent advances in the strategies of engineered EVs construction are summarized. Additionally, we also discuss the potential applications of engineered EVs in targeted therapy of cancer and damaged tissues, and evaluate the opportunities and challenges for translating them into clinical practice.
Keywords: Extracellular vesicles, Engineering, Targeted delivery
Introduction
As heterogeneous groups of membranous vesicles secreted by living cells, extracellular vesicles (EVs) consist of exosomes, microvesicles and apoptotic bodies [1, 2]. EVs contain various bioactive molecules including proteins, nucleic acids and lipids, and transport them from donor cells to recipient cells mainly through receptor-ligand interaction, direct membrane fusion and endocytosis [3, 4]. Accumulating studies reveal that EVs represent a novel pattern of intercellular communication and play important roles in numerous physiological and pathological processes [5]. Various specific cells-derived EVs exert therapeutic roles in tissue regeneration [6, 7]. EVs secretion is recognized as the crucial mechanisms underlying cell transplantation-based disease therapeutics [8]. Moreover, the ability to transfer cargos between cells positions EVs as potential delivery vehicles in nanomedicine. Compared with synthetic biomaterials, EVs exhibit advantages including low immunogenicity, non-toxicity, excellent biocompatibility and flexible drug loading ability, which make them serve as an emerging delivery platform in vivo [9]. The stable plasma membrane structure endows EVs with the intrinsic property to protect their contents from degradation. As a result of the nanoscale size, EVs can circulate through physiological barriers [10]. Thus, these unique characteristics make EVs show great promise as an endogenous delivery system.
Previous studies have demonstrated that a variety of natural EVs alleviate tissue degeneration and create beneficial microenvironments in many refractory diseases including diabetes, lupus erythematosus and Alzheimer's disease [11–13]. However, the application of natural EVs-based cell-free therapy still has several significant limitations. For instance, the heterogeneity of EVs derived from the same cell source leads to the poor reproducibility of therapeutic effects [14]. Moreover, the complex contents may weaken EVs-mediated protective efficiency and even induce safety issues [15]. Accurate quantification of therapeutic molecules in EVs also needs further investigations. Therefore, engineered EVs construction opens a new avenue for safer and more effective cell-free therapy. The introduction of exogenous ingredients into EVs has achieved encouraging progress. Currently, there are two major approaches to load cargos into EVs. One approach involves the integration of cargos into the donor cells, followed by the release and isolation of the cargos-loaded EVs [16]. The other is to adopt advanced biotechniques to directly introduce cargos into purified EVs [17]. In addition, successful EVs-mediated delivery of bioactive molecules requires the precise docking onto the target cells. The enrichment of surface proteins including tetraspanins, integrin and membrane fusion proteins offers opportunities for EVs to combine with targeting components [18]. Combination of cargo loading and surface modification advances engineered EVs as a novel delivery platform for precision therapy.
Herein, we systematically summarize the main characteristics of EVs, with a focus on the modification approaches to endow EVs with enhanced protective efficiency and targeting potential. We also highlight the recent advances in the application of engineered EVs as nanovehicles for targeted delivery of therapeutic agents in tissue regeneration, which will contribute to understand future opportunities and challenges of this field.
Biogenesis, components and characteristics of EVs
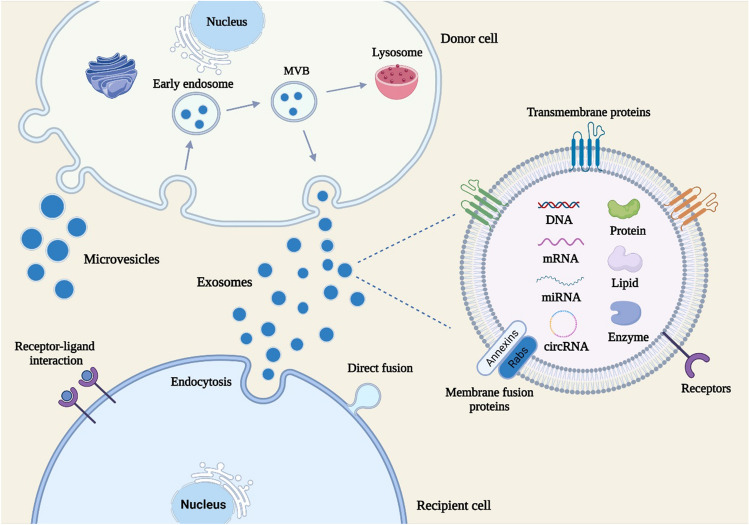
EVs are natural nanosized membrane vesicles derived from living cells and exist in body fluids such as blood, urine, saliva and cerebrospinal fluids [19]. According to the differences in size, biogenesis and components, EVs are mainly divided into three categories: exosomes, microvesicles and apoptotic bodies [20]. Exosomes, with the size ranging from 30 to 150 nm, are generated as a result of the cargo sorting into endosomes to form multivesicular bodies (MVBs) [21]. After the fusion of MVBs with plasma membranes, exosomes are released into the extracellular environment (Fig. 1). Microvesicles arise by the direct outward budding of plasma membranes, with the diameter up to 1000 nm [22]. The asymmetric distribution of phospholipids in the plasma membrane bilayer is associated with the generation of microvesicles. The outer membrane is mainly composed of phosphatidylcholine and sphingomyelin, while the intima is dominated by phosphatidylserine and phosphatidylethanolamine [23]. Due to the cytosolic calcium influx-induced the activation of phospholipid crawling enzyme, the destroyed phospholipid asymmetry initiates the process of cell budding [24]. Calcium-dependent proteolysis also contributes to the release of microvesicles by reorganizing the cytoskeleton [25]. Apoptotic bodies, with the diameter above 1000 nm, are the largest subtype of EVs and usually released by cells undergoing apoptosis [26]. Among those vesicles, the roles of exosomes and microvesicles in tissue regeneration have attracted wide attention. However, it remains a critical challenge to distinguish exosomes and microvisicles due to their overlapping sizes, characteristics and cargos. In this review, an umbrella term “EVs” is used to refer to those vesicles.
Fig. 1.
The biogenesis, contents and uptake of EVs. Exosomes are generated by the fusion of MVBs with plasma membranes and released to the extracellular space. Microvesicles are derived from the direct outward budding of plasma membranes. EVs can transport cargos including proteins, nucleic acids and lipids to recipient cells through endocytosis, direct fusion and receptor-ligand interaction
Initially EVs were considered one way to transport metabolic waste produced by cells. Emerging studies have shown that EVs carry various bioactive substances including proteins, nucleic acids and lipids [27]. The biological roles of EVs in physiological and pathological conditions have been widely revealed. As a novel mode of intercellular communication, EVs can transport cargos from donor cells to recipient cells, resulting in the exchange of genetic information and reprogramming of recipient cells [28]. The components of EVs are closely associated with the pathophysiological status of source cells [29]. Increasing evidence suggests that EVs are involved in the pathogenesis of many diseases such as tumor, inflammation, infection and nerve injury by shuttling pathogenic molecules [30–33]. The differential expression of contents in EVs during the disease progress endows them the potential to serve as promising biomarkers for early diagnosis and prognosis [34]. As a result of antigen-presenting function, EVs are recognized to exert critical roles in immune response [35]. In addition, EVs also display the therapeutic value to promote tissue regeneration. The beneficial effects of cell transplantation are mainly mediated by the paracrine pathway [36]. EVs not only inherit the ability of source cells to repair injured tissues, but also avoid limitations of cell therapy including cell senescence, tumorigenicity and low cell survival [37].
Currently, several possible mechanisms underlying the cargo sorting into EVs have been proposed. For instance, it is widely recognized that endosomal sorting complexes required for the transport (ESCRT)-dependent pathway is responsible for the loading of protein molecules into MVBs [38]. The ESCRT system includes 4 core components, named ESCRT 0, I, II and III respectively. ESCRT-0-induced the recognition and retention of the ubiquitinated proteins initiate this progress. ESCRT- I/II take charge of inward budding and ESCRT-III causes the formation of spiral-shaped structure and the shrinkage of neck bud, followed by the ATPase vacuolar protein sorting-4-induced the membrane scission. Moreover, syntenin-mediated ESCRT-independent pathway account for the sorting of several specific cargos such as latent membrane protein 1 and lysyl-tRNA synthetase [39, 40]. Tetraspanins on the surface of EVs also contribute to the introduction of proteins into EVs [41]. In addition to proteins, EVs transport various nucleic acids to exert functional roles, including mRNA, miRNA, long noncoding RNA (lncRNA), circular RNA (circRNA), genomic DNA and mitochondrial DNA [42]. Although the enrichment of DNAs in EVs has been clarified, the mechanisms by which DNAs can be packaged into EVs require further explorations. Recent studies reveal that the introduction of RNAs into EVs involves the help of complicated RNA sorting system. RNA binding proteins (RBPs) are key factors to recognize RNAs with the unique features including specific sequences, structures and modifications, and then load them into EVs [43]. For example, heterogeneous nuclear ribonucleoprotein A2B1 (hnRNPA2B1) exhibits the potential to sort numerous miRNAs and lncRNAs into EVs relying on its RNA-binding domains [44]. In addition, adenylation and urylation at the 3′ end of miRNAs, human antigen R (HuR) and argonaute 2 (Ago2) are also reported to exert critical roles in the loading of RNAs into EVs [45–47]. Lipids are another major components of EVs and participate in the regulation of various biological processes such as metabolic balance, immune surveillance and inflammatory response [48]. The lipid bilayer membrane structure also endows EVs the ability to protect internal cargos from degradation and transport them to recipient cells, thus promoting the idea to serve EVs as effective delivery vehicles [49]. Furthermore, various isolation and purification methods have been established based on the physical and chemical features of EVs, including differential ultracentrifugation [50], density gradient centrifugation [51], size exclusion chromatography [52], ultrafiltration [53], immune-affinity capture [54] and polymer precipitation [55]. Researchers can choose appropriate extraction method according to their own experimental requirements (Table 1).
Table 1.
Evaluation of isolation methods of EVs
| Method | Principles | Advantages | Limitations | References |
|---|---|---|---|---|
| Differential ultracentrifugation | Size | Low cost, convenience | Time consuming, low yield, poor integrity | [50] |
| Density gradient centrifugation | Size and density | High purity, suitable for cargo analysis | Time consuming, distinguish proteins with similar density to EVs hardly | [51] |
| Size exclusion chromatography | Size | High yield, high purity, good integrity | Special equipment required | [52] |
| Ultrafiltration | Size and shape | High efficiency, convenience | Low purity | [53] |
| Immune-affinity capture | Antigen–antibody response | High specificity, high purity | High cost, low yield | [54] |
| Polymer precipitation | Surface charge and solubility | High efficiency, simple, high yield | Low purity, high cost | [55] |
The unique features qualify EVs as a novel cell-free therapy strategy and effective delivery platform. During the shutting process, EVs can be absorbed by adjacent or distant recipient cells mainly through endocytosis, direct fusion with the plasma membrane and receptor-ligand interaction, followed by the release of bioactive molecules [56]. The enrichment of CD47 on the surface of EVs allows them to escape the clearance of mononuclear phagocytic system, resulting in the inherent stability of EVs in circulation [57]. Moreover, EVs exhibit excellent biocompatibility and can be rapidly metabolized after intravenous injection [58]. Compared with traditional carriers, EVs possess the ability to cross biological barriers such as blood–brain barrier (BBB), blood-retinal barrier and placental barrier due to their nanoscale size, opening encouraging avenues to use EVs as a delivery system for various refractory diseases [59]. Increasing studies show that specific donor cells-derived EVs also display natural targeting potential. For instance, EVs derived from mesenchymal stem cells (MSCs–EVs) inherit the tumor-homing ability of parent cells and exert therapeutic effects [60]. Importantly, the negatively charged surface and membrane proteins of EVs provide possibilities to construct engineered EVs for further enhancing their tissue targeting [61].
Engineering strategies of EVs
In order to use EVs as carriers for drugs and bioactive molecules, developing effective loading strategies are required. Moreover, surface modification of EVs can also enhance their targeting potential. Many attempts have been made for engineered EVs construction. It is worth noting that precision therapy usually requires the simultaneous application of diverse strategies.
Cargo loading methods
Currently, the cargo loading methods of EVs are mainly divided into two categories: endogenous strategies and exogenous strategies. The endogenous strategies mainly rely on the engineering of parent cells to release cargos-loaded EVs. The exogenous strategies refer to the direct introduction of cargos into isolated EVs through advanced techniques. Next, these strategies are introduced in detail (Table 2).
Table 2.
Evaluation of strategies for cargo loading into EVs
| Strategies | Methods | Principles | Advantages | Limitations | References |
|---|---|---|---|---|---|
| Endogenous strategy | Gene transfection | Liposomes-mediated cargos delivery | Simple, no damage to membrane integrity | Cytotoxicity, low loading efficiency | [63, 64] |
| Co-incubation | Penetration of cargos into cells | Simple, no damage to membrane integrity | Low specificity, low loading efficiency | [65, 66] | |
| Exogenous strategy | Incubation | Interaction between cargos and EV membrane | Simple and feasible | Low loading efficiency, toxicity | [68–70] |
| Electroporation | Transient voltage-induced the generation of pores on EV membrane | Simple, high loading efficiency | EV aggregation, nucleic acids precipitation | [71–73] | |
| Sonication | Deformation of EV membrane | High loading efficiency | EV aggregation | [74–77] | |
| Extrusion | Mechanical force-induced the temporarily destroyed membrane of EVs | Efficient packaging | May compromise the membrane structure of EVs | [78–80] | |
| Freeze–thaw cycles | Ice crystals-induced changes in EV membrane | High loading efficiency | EV aggregation, may change the membrane structure of EVs | [81–83] |
Endogenous cargo loading strategies
Endogenous strategies involve the modification of donor cells, followed by the release of engineered EVs based on the aforementioned sorting mechanisms. Gene transfection and co-incubation are two major approaches for the introduction of cargos into cells [62]. For gene transfection, plasmids and viral vectors are common tools to induce the overexpression of nucleic acids or proteins in cells [63, 64]. Through natural biogenesis processes, these components can be then encapsulated into EVs. In general, gene transfection represents one simple and feasible method to obtain EVs expressing specific genes with the preservation of membrane structure. However, some disadvantages including low loading efficiency and genetic instability limit its extensive application. Co-incubation of small molecule drugs and donor cells is considered one practicable strategy for their introduction into EVs [65]. For example, Kalimuthu et al. demonstrated that paclitaxel can be encapsulated into EVs after the mixing with MSCs [66]. Co-incubation exhibits the superiorities of convenience, and maintains the structure of EVs, whereas the loading efficiency is closely associated with drug properties and incubation conditions. There is an urgent need to customize the co-incubation schemes for specific drugs.
Exogenous cargo loading strategies
Different from the endogenous cargo loading strategies, exogenous cargo loading strategies are usually adopted after the purification of EVs. The commonly used approaches consist of incubation, electroporation, sonication, extrusion, and freeze–thaw cycles [67]. Simple incubation provides possibilities for several hydrophobic or small-molecule drugs to enter EVs. The principles of other physical methods are to form temporary pores on the membrane of EVs for cargo loading.
Various hydrophobic drugs such as paclitaxel, doxorubicin and curcumin can be directly encapsulated into EVs by simple mixing at different conditions, owing to the interaction of hydrophobic molecules and hydrophobic membrane structure of EVs [68, 69]. Incubation exhibits the protection of the properties of drugs and EVs, whereas the loading efficiency is associated with the nature of drugs. Increasing studies show that some compounds can enhance the loading efficiency. For instance, saponin-mediated surface penetration is used to load proteins and drugs into EVs through removing cholesterol on the membrane of EVs [70]. However, inefficient packaging of hydrophilic drugs limits the application of this method.
Electroporation is a common method for the introduction of drugs and nucleic acids into EVs. Due to the stimulation of electric field, transit pores are created on the membrane of EVs to enable cargos packaging [71, 72]. Although there are studies reporting that the membrane integrity of EVs can be restored after electroporation, the aggregation of EVs and the precipitation of nucleic acids may result in the poor loading efficiency, thus impairing the therapeutic effects [73].
Sonication is a simple and effective strategy to envelop exogenous drugs or small molecules with EVs. Sonication-induced shear forces lead to the deformation of EV membrane, thereby allowing the entry of cargos into EVs [74, 75]. The setting of sonication parameters determines the loading efficiency and the EV integrity [76]. Violent sonication treatment may cause irreversible damage to EVs. Accumulating evidence reveals that the combination with microfluidics technologies can optimize the cargo loading strategy based on traditional sonication method [77].
EVs and cargos are mixed and continuously extruded in a mini-extruder with polycarbonate films [78]. During the extrusion process, the membrane of EVs is destroyed to achieve the cargo loading [79]. Recent studies suggest that the packaging efficiency of extrusion is more excellent compared with incubation and electroporation [80]. However, repeated extrusion can compromise the membrane structure of EVs and cause adverse reactions.
The freeze–thaw method relies on the repeated cycles of freezing and thawing to form ice crystals, thus changing the EV membrane structure and function [81, 82]. The loading efficiency of freeze–thaw cycles is reported to be higher than incubation, but lower than sonication and extrusion [83]. Moreover, this method may induce the aggregation of EVs, resulting in the increased size of engineered EVs (Fig. 2).
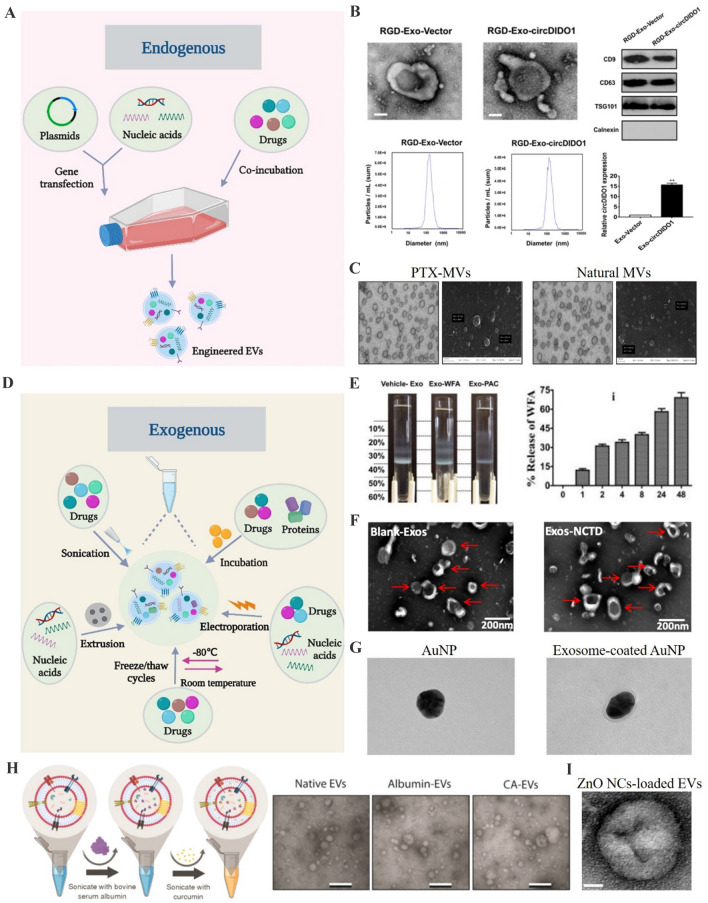
Fig. 2.
Strategies for cargo loading into EVs. A Schemes for the modification of donor cells to release cargos-loaded EVs. B Characterization of exosomes derived from circDIDO1 plasmids-transfected gastric cancer cells.
(Adapted from Guo et al. J Transl Med. 2022;20:326, with permission from Springer Nature [64]) C Transmission electron microscope and scanning electron microscopy for the microvesicles derived from SR4987 cells co-incubated with PTX. (Adapted from Pascucci et al. J Control Release. 2014;192:262–70, with permission from Elsevier [65]) D Schemes for the direct cargo loading into EVs. E Separation of vehicle- and WFA-loaded milk exosomes by Opti-prep density gradient and the release study for Exo-WFA. (Adapted from Munagala et al. Cancer Lett. 2016;371:48–61, with permission from Elsevier [69]) F Transmission electron microscope for drug-loaded exosomes after electroporation. (Adapted from Liang et al. Mol Pharm. 2021;18:1003–13, with permission from American Chemical Society [72]) G Transmission electron microscope for exosome-coated AuNPs after extrusion. (Adapted from Khongkow et al. Sci Rep. 2019;9:8278, with permission from Springer Nature [79]) H Schematic showing the mild sonication procedure and transmission electron microscopy for EVs. (Adapted from Yerneni et al. Acta Biomater. 2022;149:198–212, with permission from Elsevier [75]) I Transmission electron microscopy for ZnO NCs-loaded EVs. (Adapted from Dumontel et al. Cell Biosci. 2022;12:61, with permission from Springer Nature [82])
Membrane modification methods
Proper modification of cargos-loaded EVs endows them with enhanced targeting potential, improved biological distribution and increased cellular uptake. Due to the enrichment of bioactive molecules and unique structure in EV membrane, current studies focus on the surface modification for further modifying engineered EVs. Based on the principles, membrane modification approaches can be divided into gene engineering, covalent modification and noncovalent modification (Fig. 3).
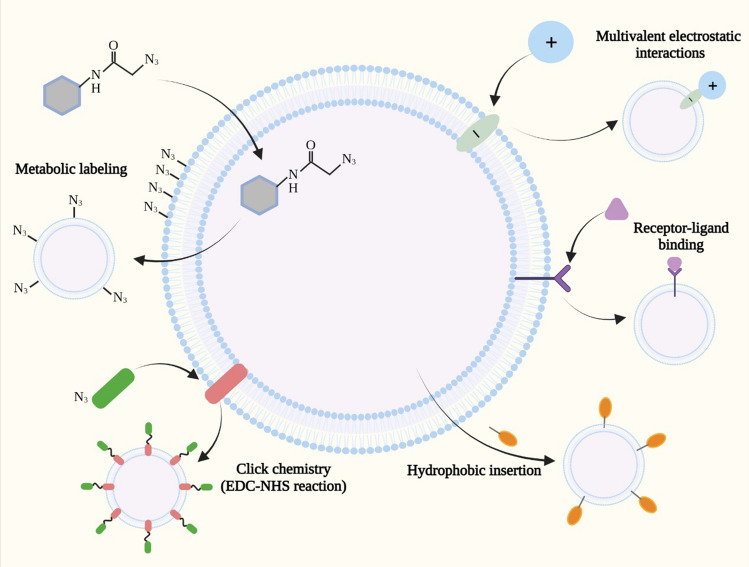
Fig. 3.
Membrane modification strategies for EV functionalization. Metabolic labeling, click chemistry, receptor-ligand binding, hydrophobic insertion and multivalent electrostatic interactions are used to modify EVs for enhancing their targeting
Gene engineering
Gene engineering is a widely used cell manipulation approach to produce modified EVs for tissue regeneration. Transmembrane proteins such as Lamp, CD9, CD63 and CD81 expressed on the surface of EVs provide opportunities for the fusion of targeting ligands to enhance their specific accumulation [84]. Therefore, donor cells-based genetic engineering involving the transfection with the plasmid encoding the fusion gene of the guidance peptide and transmembrane proteins is considered a feasible strategy to display targeting proteins on EV membrane. Zhao et al. have prepared RGD-modified EVs to target tumor vessels by transfecting 293 T cells with RGD-Lamp2b plasmids [85]. Wang et al. have transfected MSCs with the plasmids encoding the cTnI short peptide to construct cTnI-targeted EVs, which show the accumulation at the infarct area and promote cardiomyocyte proliferation [86].
Covalent modification
Compared with cells, the reagents and reaction conditions applied to the direct modification of EVs are more extensive. However, the osmotic pressure changes caused by inappropriate temperature, pressure and salt concentration can still lead to the destruction and aggregation of EVs [87]. Additionally, the stability of membrane modification is closely associated with the strength of the binding between functional groups and EVs. Covalent bonds are generally stable and less likely to dissociate due to the change of ambient conditions [88]. Click chemistry and some bioconjugation methods have emerged as promising candidates to form chemical bonds under environment conditions for the covalent modification of EVs. Click chemistry attaches the alkyne group to EV surface through 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide-N-hydroxysuccinimide (EDC-NHS) condensation reaction, followed by the conjugation to the azido group of targeting aptamers [89]. Accumulating evidence suggests that click chemistry offers advantages including high efficiency and maintaining the membrane integrity of EVs. For instance, Hao et al. have combined SILY with the surface of MSCs–EVs by click chemistry to construct SILY-EVs, which display longer tissue retention and enhance therapeutic effects to promote muscle regeneration and vascularization [90]. Moreover, neutrophil-derived EVs functionalized with sub-5 nm ultrasmall Prussian blue nanoparticles via click chemistry are reported to target inflammatory synovitis, inhibit inflammatory reaction and alleviate rheumatoid arthritis progress [91]. However, whether this modification reduces the activity of membrane proteins of EVs remains to be clarified. Moreover, bioconjugation methods also provide possibilities for exogenous functional ligands to form chemical bonds with EVs, resulting in the preparation of EVs with improved capabilities. Shamili et al. have prepared engineered EVs by conjugating LJM-3064 aptamer to the surface of EVs to further ameliorate multiple sclerosis [92]. Importantly, bioconjugation is a relatively mild covalent modification method compared with click chemistry.
Noncovalent modification
In addition to covalent modification strategies, several noncovalent methods, such as hydrophobic insertion, receptor-ligand binding and multivalent electrostatic interactions, can also be applied to provide effective modification of EVs to improve their targeting [93]. The enrichment of cholesterol, sphingomyelin and glycolipids in EV membrane creates a stable structure to prevent the facile insertion of exogenous materials [94]. However, the lipophilic substance can be spontaneously embedded in the surface of EVs under the hydrophobic interaction. Various functional molecules or biomaterials connected with lipophilic components in advance achieve the combination with EVs through simple co-incubation [95]. Emerging studies show that DSPE-PEG, a commercial amphiphilic molecule with the ability of self-assembly into EV membrane, exhibits the property to conjugate exogenous ligands for EV modification [96].
The principle of multivalent electrostatic strategies involves the multiple charge interactions. Due to the negative charged groups present on EV membrane, cationic species can bind to the surface of EVs through electrostatic interaction. Furthermore, this method also results in the formation of EVs with the positive charged surface, which contributes to their internalization into recipient cells [97]. For instance, Tumara et al. have prepared engineered EVs by surface modification with cationized pullulan to target the injured liver and achieve enhanced therapeutic effects [98]. However, some cationic nanomaterials-induced cytotoxicity is still a thorny issue [99].
As a result of the natural receptors enriched on EV membrane, targeting ligands can specifically attach to the surface of EVs. Moreover, non-native binding groups can also be introduced into EVs for the connection with functional molecules. Qi et al. reported that the binding of transferrin-conjugated superparamagnetic nanoparticles endows blood reticulocytes-derived EVs with targeting potential via the interaction with the transferrin receptors [100]. This method is a promising strategy for the specific modification of EVs to enhance their diagnosis and treatment value in some diseases, whereas the synthetic challenge and safety evaluation of exogenous modify groups still need further investigations.
Engineered EVs for precision therapy
Early studies mainly focus on the repairing potential of natural EVs in various diseases. However, there are many limitations in their application for tissue regeneration including insufficient targeting, large dose and high frequency [101]. The intrinsic property of EVs to transport cargos promotes the construction of engineered EVs. Compared with natural EVs, engineered EVs exhibit increased accumulation in injured tissues and exert enhanced therapeutic effects. Proper modification of EVs as a targeted delivery platform holds promising clinical perspective. Next, the utilization of engineered EVs to transport bioactive molecules and drugs for the precision therapy of several diseases is introduced.
Breast cancer
Breast cancer is one of the most common cancers in women worldwide [102]. Current treatment strategies such as chemotherapy and surgical intervention have several limitations including poor targeting, easy removal and high toxicity [103]. Recently, EVs-mediated therapeutic agents delivery has attracted wide attention. Many attempts have been made to design EVs for tumor targeting. Increasing studies report that several specific cells-derived EVs inherit the tumor-tropism ability of donor cells. For instance, the natural tumor-homing capacity of adult MSCs highlights the potential of MSCs–EVs as delivery nanovehicles of drugs and bioactive molecules for cancer therapy [104]. O'Brien et al. have obtained EVs enriched with miR-379 by utilizing lentiviral transduction to induce the overexpression of miR-379 in bone marrow MSCs. Systemic administration of these EVs shows therapeutic functions in metastatic breast cancer [105]. Moreover, EVs from myeloid-derived suppressor cells which are transfected with Timp3 and Rarres2 expression plasmids exhibit the abilities to accumulate in cancerous tissues and inhibit the invasion and proliferation of cancer cells in the mouse model of breast cancer [106]. However, the targeting potential of EVs relied on the characteristics of source cells still cannot meet the requirement of precision medicine. Accumulating researches have suggested that the surface modification of EVs represents a promising strategy to enhance their specificity in cancer therapy. For example, it is recognized that the progress of breast cancer is closely related to the high expression of human epidermal growth factor receptor 2 (HER2) gene. Results of Limoni et al. show that HEK293T cells after lentiviral vector transfection produce EVs expressing DARPin G3 on the surface. These engineered EVs can bind specifically to HER2 gene and transport TPD52 siRNA to cancer cells, providing considerable promise for breast cancer therapy [107]. Shi et al. have developed an EVs-based platform displaying the monoclonal antibodies of CD3 and HER2 on the surface to dually target T cell and breast cancer, resulting in the activation of cytotoxic T cells to attack HER2-expressing breast cancer cells [108]. HER2 dual-targeting EVs carrying HER2 ligand and miRNA are also reported to adhere to HER2 gene in breast cancer cells and block HER2 synthesis, thus exerting antitumor effects [109]. In addition, immune cells represent an ideal source for generating EVs to target cancer cells. Li et al. have engineered macrophage-derived EVs with a peptide targeting c-Met to become refined vehicles for the delivery of doxorubicin [110]. Nucleolin-targeting aptamer AS1411-displayed, paclitaxel loaded EVs derived from dendritic cells show targeting affinity for breast cancer, reduce tumor volume and weight, and inhibit tumor proliferation [111]. Emerging evidence has suggested that sonodynamic therapy and photothermal therapy are promising candidates for cancer treatment. Cao et al. have encapsulated the sonosensitizer indocyanine green into HEK-293 T cells-derived EVs followed by the attachment of folic acid to their surface for sonodynamic breast cancer therapy [112]. RGD-modified, vanadium carbide quantum dots-loaded EVs exhibit increased tumor accumulation and achieve low-temperature photothermal therapy with excellent tumor destruction efficiency (Fig. 4) [113].
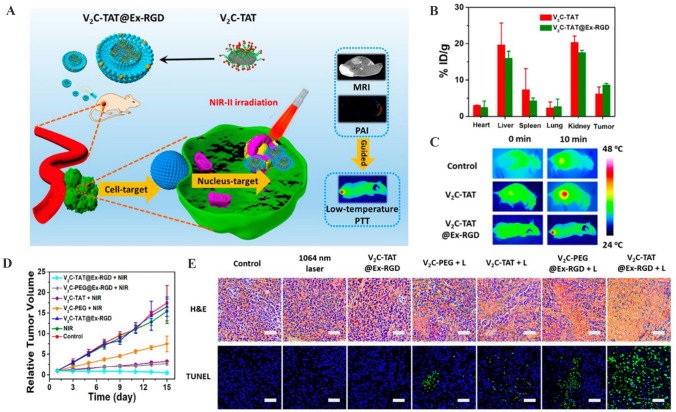
Fig. 4.
V2C-TAT@Ex-RGD for low-temperature nucleus-targeted PTT in the near-infrared-II region. A Schematic diagram showing the preparation and dual targeting ability of V2C-TAT@Ex-RGD. B Biodistribution of V in main tissues and tumor 24 h in MCF-7 tumor-bearing mice after intravenous injection. C Infrared thermal images at the tumor sites of the MCF-7-tumor-bearing mice under laser irradiation after different treatments. D Relative tumor growth curves of the MCF-7 tumor- bearing mice in different groups. E H&E and TUNEL staining of pathological changes in tumor tissues from different groups.
(Adapted from Cao et al. ACS Nano. 2019;13:1499–510, with permission from American Chemical Society [113])
Prostate cancer
The treatment for prostate cancer patients is still a major challenge in the clinic and requires further explorations of novel targets and strategies [114]. Recently, EVs-mediated targeted delivery of therapeutic agents has achieved encouraging progress for prostate cancer therapy. Due to the unique integrin expression pattern, prostate cancer cells-derived EVs display the preferential targeting to their original tumor tissues. Pan et al. have encapsulated PMA/Fe-HSA@DOX nanoparticles into urinary EVs from prostate cancer patients to realize the prostatic cancer targeting, inhibit tumor volume and weight, promote tumor apoptosis, and prolong the survival time of mice [115]. Considering that E3 aptamer is only recognized and absorbed by prostate cancer cells, Han et al. have designed aptamer-modified EVs loaded with small interfering RNA to specifically silence SIRT6 expression in prostate cancer cells and inhibit tumor growth and metastasis (Fig. 5) [116, 117]. Moreover, as a result of the increased expression of PSMA in prostate cancer cells, U937 cells-derived EVs are decorated with anti-PSMA peptide. These engineered EVs exhibit enhanced targeting potential for prostate cancer cells, representing a smart delivery system for prostate cancer therapy [118].
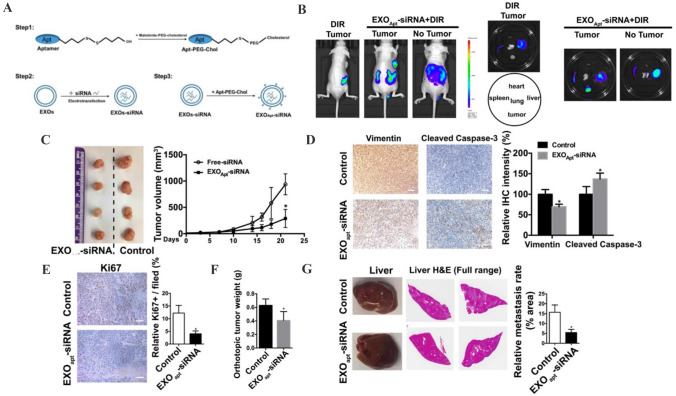
Fig. 5.
Aptamer-modified siRNA-loaded exosomes treatment suppresses prostate tumor proliferation and metastasis. A Schematic illustration of the procedure for the E3 aptamer-modified siRNA loaded exosomes. B Fluorescence images of tumor- bearing (subcutaneous implantation of PC3 cells) or no tumor-bearing mice after the injection of aptamer-modified, DIR-labeled exosomes. C Representative image of the subcutaneous tumors in mice after treatment with aptamer-modified siRNA-loaded exosomes compared with the control group. D, E Immunohistochemistry staining of Vimentin, Cleaved Caspase-3 and Ki67 in tumor tissues treated with aptamer-modified siRNA-loaded exosomes. F The weight of tumor in orthotopically implanted mouse model after treatment with aptamer-modified siRNA-loaded exosomes compared with the control group. G Full-scale H&E staining of livers from the orthotopic mouse model. Metastatic nodules in livers were dissected counted.
(Adapted from Han et al. Theranostics. 2021;11:6526–41, with permission from Ivyspring International Publisher [116])
Brain diseases
The lack of safe and effective BBB delivery vehicles significantly limits the treatment of brain diseases [119]. The superiorities of EVs including strong cargo loading capacity and BBB penetration bring new hope for brain diseases therapy. However, the targeting property of natural EVs remains unsatisfactory. To this end, the potential application of engineered EVs after cargo loading and membrane modification for targeted delivery in injured brain area has been widely investigated. Tian et al. demonstrated that ReN cells-derived EVs loaded with small interfering RNA against programmed cell death ligand-1 and decorated with a brain tumor-targeting cyclic RGDyK peptide possess excellent glioblastoma-targeting ability and prevent tumor growth [120]. Jia et al. have loaded SPIONs and curcumin into EVs and then connected the membrane of EVs with a neuropilin-1-targeted peptide. Engineered EVs are found to abundantly accumulate in glioma and induce potent antitumor effects [121]. Furthermore, treatment with methotrexate-loaded and low-density lipoprotein-attached EVs results in the increased distribution in the glioma site and prolonged survival period of mice (Fig. 6) [122]. In addition, due to the difficulty of transporting drugs to the ischemic area, ischemic stroke is recognized the main cause of acquired disability worldwide [123]. Currently, engineered EVs-mediated drug delivery for ischemic stroke therapy has been assessed in many preclinical studies and achieved inspired progress. Guo et al. have developed an effective delivery platform based on the engineered EVs conjugated with a monoclonal antibody against GAP43 to enhance the accumulation of quercetin in ischemic neurons [124]. Tian et al. revealed that MSCs–EVs decorated with c(RGDyK) peptide show the high efficiency to deliver curcumin to ischemic brain, leading to the inhibition of cellular inflammation and apoptosis in the lesion region [125]. Using the similar strategy, Wu et al. have isolated PDGFα-modified EVs from lentivirus transfected-neural stem cells, followed by the introduction of Bryostatin-1. Compared with native Bryostatin-1 treatment, engineered EVs display central nervous system targeting property and further alleviate the infiltration of pro-inflammatory cells [126]. Increasing studies have suggested that rabies virus glycoprotein (RVG) can bind to nicotinic acetylcholine receptor, therefore selectively targeting neuronal cells and brain endothelial cells. The engineering strategy based on RVG modified-EVs is a novel method for brain targeted delivery. For instance, EVs engineered to express RVG on the membrane show effective targeting to neuronal cells and transport gold nanoparticles to cross BBB [79]. Yu et al. demonstrated that the neprilysin variant after the encapsulation into RVG displayed-EVs exhibits deep penetration into the hippocampus region of the brain to reduce the release of inflammatory cytokines [127]. Similarly, Zhang et al. have generated RVG-modified, ZIKV-specific siRNA-loaded EVs to achieve the neuro-specific targeting and alleviate the neurological damage caused by ZIKV in the fetal mouse model [128]. In major depressive disorder mouse model, administration of EVs engineered with RVG and circDYM significantly ameliorate depressive-like behaviours and inhibit BBB leakage [129]. These findings suggest that engineered EVs represent an efficient and ideal delivery system for brain diseases therapy.
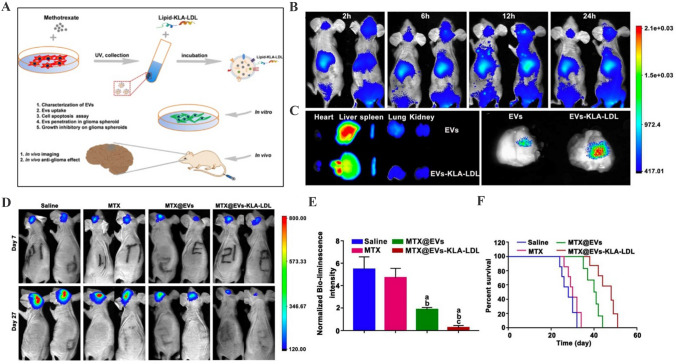
Fig. 6.
Targeted therapeutic efficacy of MTX@EVs-KLA-LDL in glioma. A Schematic illustration of the design of EVs-KLA-LDL. B The fluorescent image of the experimental animals at the different time points after treatment in vivo. C Fluorescent imaging of the brains harvested at 24 h after treatment and other major organs including heart, liver, spleen, lung and kidney. D Representative fluorescent images of U87-luci-bearing mice with treatments of MTX, MTX@EVs and EVs-KLA-LDL on days 7 and 27. E Normalized bioluminescence intensity of U87MG-luci glioma at days 27 against that of days 7 for different groups. F Survival curves of glioblastoma multiforme-bearing mice after different treatment.
(Adapted from Ye et al. ACS Appl Mater Interfaces. 2018;10:12,341–50, with permission from American Chemical Society [122])
Bone diseases
The ability of engineered EVs to transport bioactive molecules and drugs for bone diseases therapy has been evaluated in many preclinical studies. Currently, delivery of drugs into chondrocytes across the nonvascular extracellular matrix is considered an important challenge for osteoarthritis therapy [130]. The strategy based on engineered EVs as delivery nanovehicles suggests a promising approach to enhance the accumulation of therapeutic agents in cartilage area. Liang et al. have fused a chondrocyte-affinity peptide on the surface of EVs, followed by the loading of miR-140 [131]. These engineered EVs are retained in deep cartilage regions and inhibit cartilage-degrading proteases. Recent evidence reveals that transplantation of synovial fluid-derived MSCs represents a feasible approach for cartilage degeneration, whereas the differentiation ability of transplanted MSCs in the joints is usually low. The results of Xu et al. have demonstrated that E7 peptide-decorated EVs effectively deliver Kartogenin to synovial fluid-derived MSCs and induce high degree of cartilage differentiation [132]. In addition, MSCs–EVs conjugated with dextran sulfate show the accumulation in the inflamed joints and release therapeutic miRNAs to induce macrophage phenotype regulation, leading to the inhibition of inflammatory reactions in rheumatoid arthritis mice [133]. Moreover, the ability of EVs to transport bioactive molecules for the precision therapy of osteosarcoma has also been reported. Huang et al. have used c(RGDyK)-modified EVs which encapsulate lncRNA MEG3 to target osteosarcoma cells and facilitate the anti-osteosarcoma effects (Fig. 7) [134]. Taken together, engineered EVs after proper modification show great potential to be developed as a novel delivery platform for bone diseases therapy.
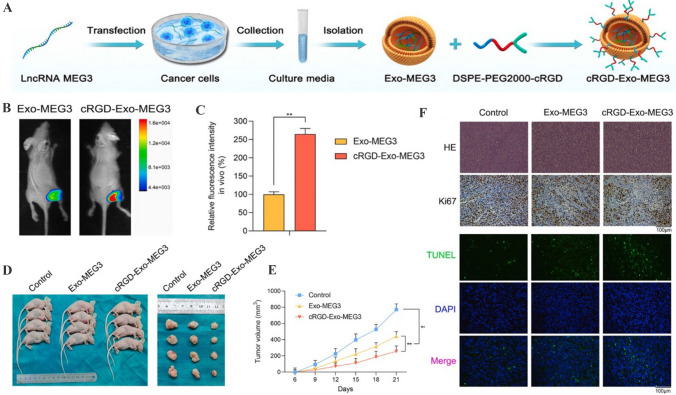
Fig. 7.
Targeted anti-osteosarcoma therapy of cRGD-Exo-MEG3 in vivo. A Schematic illustration showing the synthesis of cRGD-Exo-MEG3. B, C The tumor accumulation ability of cRGD-Exo-MEG3 was detected using xenograft tumor model in vivo. D, E Nude mice were divided into three groups and the volume of subcutaneously transplanted tumors of these three groups were recorded. F H&E staining, Ki67 immunohistochemistry and TUNEL staining for the proliferation and apoptotic rates of cells in osteosarcoma tissues.
(Adapted from Huang et al. J Control Release. 2022;343:107–17, with permission from Elsevier [134])
Heart diseases
Previous studies have demonstrated that EVs derived from several donor cells especially MSCs exert therapeutic functions in various heart diseases such as myocarditis, myocardial infarction and heart failure [135–137]. However, the low tissue specificity and limited therapeutic contents hinder the wide application of natural EVs [138]. The introduction of bioactive molecules and drugs into EVs provides considerable promise to enhance their repairing value. Moreover, attaching targeting moieties including antibodies, homing peptides and other biological ligands to the surface of EVs is recognized a practicable strategy to enhance their myocardial tissue targeting [139]. To potentiate the accumulation in injured cardiomyocyte and enhance the protective efficiency, Wang et al. have constructed engineered MSCs–EVs that are modified with ischemic myocardium-targeting peptide [140]. Compared with natural EVs, engineered EVs display increased distribution in ischemic heart area and further attenuate myocardial fibrosis. Li et al. have fused the membranes of MSCs–EVs with platelet membranes by extrusion to prepare platelet-mimetic EVs, which inherit the targeting potential of platelets to damaged cardiac endothelial cells and promote angiogenesis in the mouse model of myocardial ischemia reperfusion [141]. In addition, angiogenin-1 hydrogel modification further boosts the retention of EVs in myocardial infarction sites and strengthens the islet-1-induced angiogenic and anti-apoptosis effects to promote myocardial infarction recovery (Fig. 8) [142]. Thus, the use of engineered EVs to deliver therapeutic molecules further expands the applicability of EVs in heart diseases therapy.
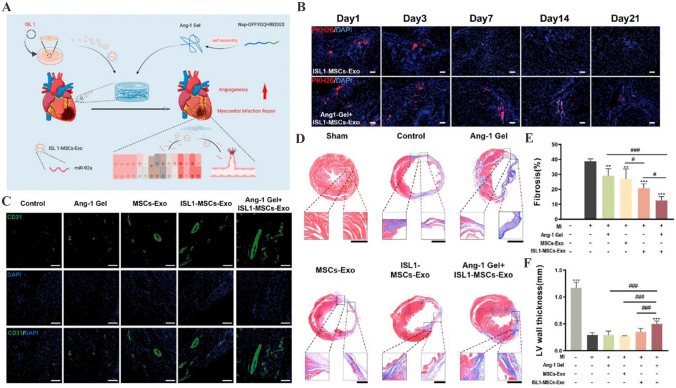
Fig. 8.
Ang-1 Gel-modified ISL1-MSCs-Exo ameliorate cardiac fibrosis after myocardial infarction. A Schematic illustration showing the preparation of Ang-1 Gel and ISL1-MSCs-Exo. B Ang-1 gel increased the retention rate of ISL1-MSCs-Exo in the border area of myocardial infarction. C Angiogenesis in the border zone of ischemic myocardium at day 28 after myocardial infarction was detected by CD31 immunofluorescence staining. D Masson’s trichrome staining showing the degree of cardiac fibrosis in different treatment groups at day 28 after myocardial infarction operation. E, F Fibrosis areas and infarct wall thickness analysis.
(Adapted from Hu et al. ACS Appl Mater Interfaces. 2022;14:36,289–303, with permission from American Chemical Society [142])
Metabolic diseases
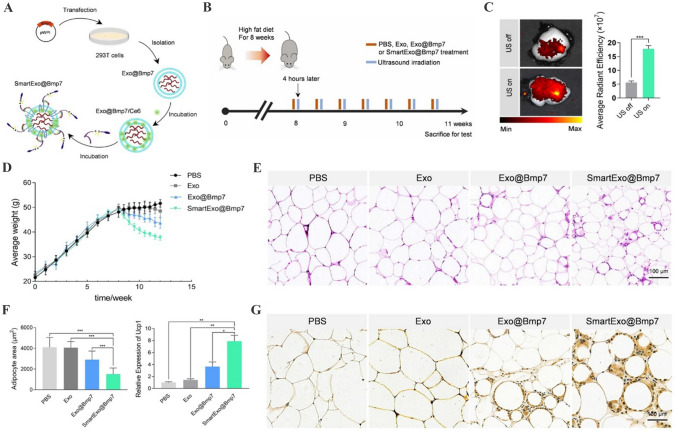
Emerging studies have reported that engineered EVs also play a targeted therapeutic role in many metabolic diseases. Komuro et al. [143] demonstrated that 293 T cells-derived EVs decorated with peptide p88 can specifically bind to pancreatic β-cells for transporting encapsulated plasmid DNA with no observed side effects in mouse model. Tang et al. [144] have utilized Kim-1-binding LTH peptide to engineer red blood cell-derived EVs, which are found to be accumulated at the injured tubules. SiRNAs targeting P65 and Snai1 after the introduction into engineered EVs effectively alleviate tubulointerstitial inflammation and fibrosis and restore renal metabolic function. Guo et al. [145] have developed omental adipose tissue-targeting EVs by displaying peptide CP05 on the membrane. Engineered EVs act as an effective delivery system to shuttle bone morphogenetic protein 7 mRNA to induce omental adipose tissue browning, representing a promising strategy for anti-obesity therapy (Fig. 9). Current researches focus on the targeting ligand to modify EVs for the precision therapy of metabolic diseases. The engineering strategies of EVs and their roles in other metabolic diseases still need further investigations.
Fig. 9.
Smart exosome-based system applied in Bmp7 targeting delivery for omental adipose tissue browning. A Schematic illustration of exosome-based Bmp7 delivery system SmartExo@Bmp7 construction. B Schematic diagram of the experimental procedure. C Representative confocal images of DiI-labeled exosomes (red) in abdominal adipose tissue of mice with or without ultrasound irradiation. D Average weight of each group recorded starting from the high fat diet to the end of treatment period. E Representative images of H&E staining of omental adipose tissue. F Relative area of lipid droplets for HE staining images and relative expression level of Ucp1 in omental adipose tissue. G Representative images of Ucp1 staining of omental adipose tissue.
(Adapted from Guo et al. J Nanobiotechnology. 2021;19:402, with permission from Springer Nature [145])
Conclusions and future perspectives
EVs are emerged as a novel delivery nanoplatform owing to their excellent biocompatibility and stability. Great progress has been achieved to the studies of EV purification, identification, biogenesis and biological features. It is widely recognized that EVs-mediated cargos delivery shows protective functions in various diseases. Recently, increasing studies have reported that proper modification can improve the targeting potential and therapeutic ability of EVs. Engineered EVs-based cell-free strategy represents a promising candidate for precision therapy with great application value.
Although many studies have revealed that engineered EVs can significantly repair tissue damage, there still exists several challenges for their clinical transformation. Firstly, the mass production of EVs is a major challenge [146, 147]. The traditional purification methods including ultracentrifugation and polymer precipitation have many limitations such as low yield, poor purity and complex and time-consuming procedures [148]. There is an urgent need to develop advanced purification approaches with high efficiency. It is reported that the use of bioreactor to enhance the production of EVs and subsequent microfluidics-based isolation technologies may achieve large-scale acquisition of high-quality EVs [149]. Moreover, the accurate identification of EVs to assess their heterogeneity, contents and functions is critical to ensure quality control. There is still a lack of standardization of EVs from different sources or batches [150]. The question of which type of producer cells and culture conditions is optimal for the generation of homogeneous EVs is required further investigations. Secondly, the cargo loading efficiency of engineered EVs needs further improvement. The development of optimized method to achieve the maximum packaging efficiency can reduce the use dose and administration frequency of engineered EVs, which may provide an important foundation for this novel cell-free therapeutic strategy [151]. Thirdly, considering that the contents and surface compositions of EVs may change significantly after modification procedures, the use of engineered EVs may induce safety issues [152]. Therefore, the application of engineered EVs needs comprehensive preclinical evaluations to avoid potential side effects.
Overall, engineered EVs construction provides new insights into the efficient and targeted delivery of therapeutic agents for refractory diseases. The potential of engineered EVs in precision therapy still has great exploration space. With concerted efforts from researchers, the utilization of engineered EVs as a targeted delivery platform for future nanomedicine is attractive and promising.
Acknowledgement
This study was supported by the National Natural Science Foundation of China (81971757).
Author contribution
YS and FS drafted the manuscript and drew the figures. WX and HQ revised the manuscript. All authors read and approved the final manuscript.
Declarations
Conflict of interest
The authors declare they have no conflict of interest.
Ethical statement
There are no animal experiments carried out for this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuntong Sun and Fengtian Sun contributed equally to this work.
References
- 1.Grange C, Bussolati B. Extracellular vesicles in kidney disease. Nat Rev Nephrol. 2022;18:499–513. doi: 10.1038/s41581-022-00586-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang X, Zhang H, Gu J, Zhang J, Shi H, Qian H, et al. Engineered extracellular vesicles for cancer therapy. Adv Mater. 2021;33:e2005709. doi: 10.1002/adma.202005709. [DOI] [PubMed] [Google Scholar]
- 3.Kim HY, Kwon S, Um W, Shin S, Kim CH, Park JH, et al. Functional extracellular vesicles for regenerative medicine. Small. 2022;2022:e2106569. doi: 10.1002/smll.202106569. [DOI] [PubMed] [Google Scholar]
- 4.Yu D, Li Y, Wang M, Gu J, Xu W, Cai H, et al. Exosomes as a new frontier of cancer liquid biopsy. Mol Cancer. 2022;21:56. doi: 10.1186/s12943-022-01509-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Niel G, Carter DRF, Clayton A, Lambert DW, Raposo G, Vader P. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat Rev Mol Cell Biol. 2022;23:369–382. doi: 10.1038/s41580-022-00460-3. [DOI] [PubMed] [Google Scholar]
- 6.Cheng L, Hill AF. Therapeutically harnessing extracellular vesicles. Nat Rev Drug Discov. 2022;21:379–399. doi: 10.1038/s41573-022-00410-w. [DOI] [PubMed] [Google Scholar]
- 7.de Abreu RC, Fernandes H, da Costa Martins PA, Sahoo S, Emanueli C, Ferreira L. Native and bioengineered extracellular vesicles for cardiovascular therapeutics. Nat Rev Cardiol. 2020;17:685–697. doi: 10.1038/s41569-020-0389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed L, Al-Massri K. New approaches for enhancement of the efficacy of mesenchymal stem cell-derived exosomes in cardiovascular diseases. Tissue Eng Regen Med. 2022;19:1129-46. [DOI] [PMC free article] [PubMed]
- 9.Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 2021;16:748–759. doi: 10.1038/s41565-021-00931-2. [DOI] [PubMed] [Google Scholar]
- 10.Fan B, Gu J, Wu J, Sun Y, Huang R, Shen H, et al. Circulating abnormal extracellular vesicles: their mechanism for crossing blood-brain barrier, effects on central nervous system and detection methods. J Biomed Nanotechnol. 2022;18:640–659. doi: 10.1166/jbn.2022.3293. [DOI] [PubMed] [Google Scholar]
- 11.Sun Y, Shi H, Yin S, Ji C, Zhang X, Zhang B, et al. Human mesenchymal stem cell derived exosomes alleviate type 2 diabetes mellitus by reversing peripheral insulin resistance and relieving β-cell destruction. ACS Nano. 2018;12:7613–7628. doi: 10.1021/acsnano.7b07643. [DOI] [PubMed] [Google Scholar]
- 12.Yang C, Sun J, Tian Y, Li H, Zhang L, Yang J, et al. Immunomodulatory effect of MSCs and MSCs-derived extracellular vesicles in systemic lupus erythematosus. Front Immunol. 2021;12:714832. doi: 10.3389/fimmu.2021.714832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo M, Yin Z, Chen F, Lei P. Mesenchymal stem cell-derived exosome: a promising alternative in the therapy of Alzheimer's disease. Alzheimers Res Ther. 2020;12:109. doi: 10.1186/s13195-020-00670-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Psaraki A, Ntari L, Karakostas C, Korrou-Karava D, Roubelakis MG. Extracellular vesicles derived from mesenchymal stem/stromal cells: the regenerative impact in liver diseases. Hepatology. 2022;75:1590–1603. doi: 10.1002/hep.32129. [DOI] [PubMed] [Google Scholar]
- 15.Bister N, Pistono C, Huremagic B, Jolkkonen J, Giugno R, Malm T. Hypoxia and extracellular vesicles: a review on methods, vesicular cargo and functions. J Extracell Vesicles. 2020;10:e12002. doi: 10.1002/jev2.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim H, Kim D, Nam H, Moon S, Kwon YJ, Lee JB. Engineered extracellular vesicles and their mimetics for clinical translation. Methods. 2020;177:80–94. doi: 10.1016/j.ymeth.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Chen S, Sun F, Qian H, Xu W, Jiang J. Preconditioning and engineering strategies for improving the efficacy of mesenchymal stem cell-derived exosomes in cell-free therapy. Stem Cells Int. 2022;2022:1779346. doi: 10.1155/2022/1779346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruan S, Greenberg Z, Pan X, Zhuang P, Erwin N, He M. Extracellular vesicles as an advanced delivery biomaterial for precision cancer immunotherapy. Adv Healthc Mater. 2022;11:e2100650. doi: 10.1002/adhm.202100650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanayama R. Emerging roles of extracellular vesicles in physiology and disease. J Biochem. 2021;169:135–138. doi: 10.1093/jb/mvaa138. [DOI] [PubMed] [Google Scholar]
- 20.Aires ID, Ribeiro-Rodrigues T, Boia R, Ferreira-Rodrigues M, Girão H, Ambrósio AF, et al. Microglial extracellular vesicles as vehicles for neurodegeneration spreading. Biomolecules. 2021;11:770. doi: 10.3390/biom11060770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou B, Xu K, Zheng X, Chen T, Wang J, Song Y, et al. Application of exosomes as liquid biopsy in clinical diagnosis. Signal Transduct Target Ther. 2020;5:144. doi: 10.1038/s41392-020-00258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menck K, Sivaloganathan S, Bleckmann A, Binder C. Microvesicles in cancer: small size, large potential. Int J Mol Sci. 2020;21:5373. doi: 10.3390/ijms21155373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.You B, Yang Y, Zhou Z, Yan Y, Zhang L, Jin J, et al. Extracellular vesicles: a new frontier for cardiac repair. Pharmaceutics. 2022;14:1848. doi: 10.3390/pharmaceutics14091848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shkair L, Garanina EE, Stott RJ, Foster TL, Rizvanov AA, Khaiboullina SF. Membrane microvesicles as potential vaccine candidates. Int J Mol Sci. 2021;22:1142. doi: 10.3390/ijms22031142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laberge A, Arif S, Moulin VJ. Microvesicles: Intercellular messengers in cutaneous wound healing. J Cell Physiol. 2018;233:5550–5563. doi: 10.1002/jcp.26426. [DOI] [PubMed] [Google Scholar]
- 26.van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 27.Maacha S, Bhat AA, Jimenez L, Raza A, Haris M, Uddin S, et al. Extracellular vesicles-mediated intercellular communication: roles in the tumor microenvironment and anti-cancer drug resistance. Mol Cancer. 2019;18:55. doi: 10.1186/s12943-019-0965-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo L, Wu Z, Wang Y, Li H. Regulating the production and biological function of small extracellular vesicles: current strategies, applications and prospects. J Nanobiotechnolgy. 2021;19:422. doi: 10.1186/s12951-021-01171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohan A, Agarwal S, Clauss M, Britt NS, Dhillon NK. Extracellular vesicles: novel communicators in lung diseases. Respir Res. 2020;21:175. doi: 10.1186/s12931-020-01423-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marar C, Starich B, Wirtz D. Extracellular vesicles in immunomodulation and tumor progression. Nat Immunol. 2021;22:560–570. doi: 10.1038/s41590-021-00899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang F, Chen Q, Wang W, Ling Y, Yan Y, Xia P. Hepatocyte-derived extracellular vesicles promote endothelial inflammation and atherogenesis via microRNA-1. J Hepatol. 2020;72:156–166. doi: 10.1016/j.jhep.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 32.McNamara RP, Dittmer DP. Extracellular vesicles in virus infection and pathogenesis. Curr Opin Virol. 2020;44:129–138. doi: 10.1016/j.coviro.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo Y, Gil Z. The role of extracellular vesicles in cancer-nerve crosstalk of the peripheral nervous system. Cells. 2022;11:1294. doi: 10.3390/cells11081294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saleem T, Sumrin A, Bilal M, Bashir H, Khawar MB. Tumor-derived extracellular vesicles: potential tool for cancer diagnosis, prognosis, and therapy. Saudi J Biol Sci. 2022;29:2063–2071. doi: 10.1016/j.sjbs.2022.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shao J, Li S, Liu Y, Zheng M. Extracellular vesicles participate in macrophage-involved immune responses under liver diseases. Life Sci. 2020;240:117094. doi: 10.1016/j.lfs.2019.117094. [DOI] [PubMed] [Google Scholar]
- 36.Weng Z, Zhang B, Wu C, Yu F, Han B, Li B, et al. Therapeutic roles of mesenchymal stem cell-derived extracellular vesicles in cancer. J Hematol Oncol. 2021;14:136. doi: 10.1186/s13045-021-01141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keshtkar S, Azarpira N, Ghahremani MH. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther. 2018;9:63. doi: 10.1186/s13287-018-0791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Juan T, Fürthauer M. Biogenesis and function of ESCRT-dependent extracellular vesicles. Semin Cell Dev Biol. 2018;74:66–77. doi: 10.1016/j.semcdb.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 39.Nkosi D, Sun L, Duke LC, Patel N, Surapaneni SK, Singh M, et al. Epstein-barr virus LMP1 promotes syntenin-1- and hrs-induced extracellular vesicle formation for its own secretion to increase cell proliferation and migration. MBio. 2020;11:00589–e620. doi: 10.1128/mBio.00589-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim SB, Kim HR, Park MC, Cho S, Goughnour PC, Han D, et al. Caspase-8 controls the secretion of inflammatory lysyl-tRNA synthetase in exosomes from cancer cells. J Cell Biol. 2017;216:2201–2216. doi: 10.1083/jcb.201605118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ding H, Li LX, Harris PC, Yang J, Li X. Extracellular vesicles and exosomes generated from cystic renal epithelial cells promote cyst growth in autosomal dominant polycystic kidney disease. Nat Commun. 2021;12:4548. doi: 10.1038/s41467-021-24799-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y, Zhao J, Yu S, Wang Z, He X, Su Y, et al. Extracellular vesicles long RNA sequencing reveals abundant mRNA, circRNA, and lncRNA in human blood as potential biomarkers for cancer diagnosis. Clin Chem. 2019;65:798–808. doi: 10.1373/clinchem.2018.301291. [DOI] [PubMed] [Google Scholar]
- 43.Fabbiano F, Corsi J, Gurrieri E, Trevisan C, Notarangelo M, D'Agostino VG. RNA packaging into extracellular vesicles: an orchestra of RNA-binding proteins? J Extracell Vesicles. 2020;10:e12043. doi: 10.1002/jev2.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Groot M, Lee H. Sorting mechanisms for microRNAs into extracellular vesicles and their associated diseases. Cells. 2020;9:1044. doi: 10.3390/cells9041044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goswami A, Mukherjee K, Mazumder A, Ganguly S, Mukherjee I, Chakrabarti S, et al. MicroRNA exporter HuR clears the internalized pathogens by promoting pro-inflammatory response in infected macrophages. EMBO Mol Med. 2020;12:e11011. doi: 10.15252/emmm.201911011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koppers-Lalic D, Hackenberg M, Bijnsdorp IV, van Eijndhoven MAJ, Sadek P, Sie D, et al. Nontemplated nucleotide additions distinguish the small RNA composition in cells from exosomes. Cell Rep. 2014;8:1649–1658. doi: 10.1016/j.celrep.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 47.Weaver AM, Patton JG. Argonautes in extracellular vesicles: artifact or selected cargo? Cancer Res. 2020;80:379–381. doi: 10.1158/0008-5472.CAN-19-2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vanherle S, Haidar M, Irobi J, Bogie JFJ, Hendriks JJA. Extracellular vesicle-associated lipids in central nervous system disorders. Adv Drug Deliv Rev. 2020;159:322–331. doi: 10.1016/j.addr.2020.04.011. [DOI] [PubMed] [Google Scholar]
- 49.Yong T, Wei Z, Gan L, Yang X. Extracellular vesicle-based drug delivery systems for enhanced anti-tumor therapies through modulating cancer-immunity cycle. Adv Mater. 2022;34:e2201054. [DOI] [PubMed]
- 50.Tian Y, Gong M, Hu Y, Liu H, Zhang W, Zhang M, et al. Quality and efficiency assessment of six extracellular vesicle isolation methods by nano-flow cytometry. J Extracell Vesicles. 2020;9:1697028. doi: 10.1080/20013078.2019.1697028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coumans FAW, Brisson AR, Buzas EI, Dignat-George F, Drees EEE, El-Andaloussi S, et al. Methodological guidelines to study extracellular vesicles. Circ Res. 2017;120:1632–1648. doi: 10.1161/CIRCRESAHA.117.309417. [DOI] [PubMed] [Google Scholar]
- 52.Liu DSK, Upton FM, Rees E, Limb C, Jiao LR, Krell J, et al. Size-exclusion chromatography as a technique for the investigation of novel extracellular vesicles in cancer. Cancers (Basel) 2020;12:3156. doi: 10.3390/cancers12113156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oeyen E, Van Mol K, Baggerman G, Willems H, Boonen K, Rolfo C, et al. Ultrafiltration and size exclusion chromatography combined with asymmetrical-flow field-flow fractionation for the isolation and characterisation of extracellular vesicles from urine. J Extracell Vesicles. 2018;7:1490143. doi: 10.1080/20013078.2018.1490143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Popovic M, Mazzega E, Toffoletto B, de Marco A. Isolation of anti-extra-cellular vesicle single-domain antibodies by direct panning on vesicle-enriched fractions. Microb Cell Fact. 2018;17:6. doi: 10.1186/s12934-017-0856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cai S, Luo B, Jiang P, Zhou X, Lan F, Yi Q, et al. Immuno-modified superparamagnetic nanoparticles via host-guest interactions for high-purity capture and mild release of exosomes. Nanoscale. 2018;10:14280–14289. doi: 10.1039/C8NR02871K. [DOI] [PubMed] [Google Scholar]
- 56.Ocansey DKW, Zhang L, Wang Y, Yan Y, Qian H, Zhang X, et al. Exosome-mediated effects and applications in inflammatory bowel disease. Biol Rev Camb Philos Soc. 2020;95:1287–1307. doi: 10.1111/brv.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shimizu A, Sawada K, Kobayashi M, Yamamoto M, Yagi T, Kinose Y, et al. Exosomal CD47 plays an essential role in immune evasion in ovarian cancer. Mol Cancer Res. 2021;19:1583–1595. doi: 10.1158/1541-7786.MCR-20-0956. [DOI] [PubMed] [Google Scholar]
- 58.Hutcheson JD, Aikawa E. Extracellular vesicles in cardiovascular homeostasis and disease. Curr Opin Cardiol. 2018;33:290–297. doi: 10.1097/HCO.0000000000000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Claridge B, Lozano J, Poh QH, Greening DW. Development of extracellular vesicle therapeutics: challenges, considerations, and opportunities. Front Cell Dev Biol. 2021;9:734720. doi: 10.3389/fcell.2021.734720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li X, Liu LL, Yao JL, Wang K, Ai H. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles inhibit endometrial cancer cell proliferation and migration through delivery of exogenous miR-302a. Stem Cells Int. 2019;2019:8108576. doi: 10.1155/2019/8108576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jia X, Tang J, Yao C, Yang D. Recent progress of extracellular vesicle engineering. ACS Biomater Sci Eng. 2021;7:4430–4438. doi: 10.1021/acsbiomaterials.1c00868. [DOI] [PubMed] [Google Scholar]
- 62.Zhang X, Xu Q, Zi Z, Liu Z, Wan C, Crisman L, et al. Programmable extracellular vesicles for macromolecule delivery and genome modifications. Dev Cell. 2020;55:784–801. doi: 10.1016/j.devcel.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang H, Wang Y, Bai M, Wang J, Zhu K, Liu R, et al. Exosomes serve as nanoparticles to suppress tumor growth and angiogenesis in gastric cancer by delivering hepatocyte growth factor siRNA. Cancer Sci. 2018;109:629–641. doi: 10.1111/cas.13488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guo Z, Zhang Y, Xu W, Zhang X, Jiang J. Engineered exosome-mediated delivery of circDIDO1 inhibits gastric cancer progression via regulation of MiR-1307-3p/SOCS2 axis. J Transl Med. 2022;20:326. doi: 10.1186/s12967-022-03527-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pascucci L, Coccè V, Bonomi A, Ami D, Ceccarelli P, Ciusani E, et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J Control Release. 2014;192:262–270. doi: 10.1016/j.jconrel.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 66.Kalimuthu S, Gangadaran P, Rajendran RL, Zhu L, Oh JM, Lee HW, et al. A new approach for loading anticancer drugs into mesenchymal stem cell-derived exosome mimetics for cancer therapy. Front Pharmacol. 2018;9:1116. doi: 10.3389/fphar.2018.01116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ou YH, Liang J, Czarny B, Wacker MG, Yu V, Wang JW, et al. Extracellular vesicle (EV) biohybrid systems for cancer therapy: recent advances and future perspectives. Semin Cancer Biol. 2021;74:45–61. doi: 10.1016/j.semcancer.2021.02.006. [DOI] [PubMed] [Google Scholar]
- 68.Wu P, Zhang B, Ocansey DKW, Xu W, Qian H. Extracellular vesicles: a bright star of nanomedicine. Biomaterials. 2021;269:120467. doi: 10.1016/j.biomaterials.2020.120467. [DOI] [PubMed] [Google Scholar]
- 69.Munagala R, Aqil F, Jeyabalan J, Gupta RC. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016;371:48–61. doi: 10.1016/j.canlet.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fuhrmann G, Chandrawati R, Parmar PA, Keane TJ, Maynard SA, Bertazzo S, et al. Engineering extracellular vesicles with the tools of enzyme prodrug therapy. Adv Mater. 2018;30:e1706616. doi: 10.1002/adma.201706616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lennaárd AJ, Mamand DR, Wiklander RJ, El Andaloussi S, Wiklander OPB. Optimised electroporation for loading of extracellular vesicles with doxorubicin. Pharmaceutics. 2021;14:38. doi: 10.3390/pharmaceutics14010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liang L, Zhao L, Wang Y, Wang Y. Treatment for hepatocellular carcinoma is enhanced when norcantharidin is encapsulated in exosomes derived from bone marrow mesenchymal stem cells. Mol Pharm. 2021;18:1003–1013. doi: 10.1021/acs.molpharmaceut.0c00976. [DOI] [PubMed] [Google Scholar]
- 73.Liang G, Zhu Y, Ali DJ, Tian T, Xu H, Si K, et al. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J Nanobiotechnology. 2020;18:10. doi: 10.1186/s12951-019-0563-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lamichhane TN, Jeyaram A, Patel DB, Parajuli B, Livingston NK, Arumugasaamy N, et al. Oncogene knockdown via active loading of small RNAs into extracellular vesicles by sonication. Cell Mol Bioeng. 2016;9:315–324. doi: 10.1007/s12195-016-0457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yerneni SS, Yalcintas EP, Smith JD, Averick S, Campbell PG, Ozdoganlar OB. Skin-targeted delivery of extracellular vesicle-encapsulated curcumin using dissolvable microneedle arrays. Acta Biomater. 2022;149:198–212. doi: 10.1016/j.actbio.2022.06.046. [DOI] [PubMed] [Google Scholar]
- 76.Sun F, Xu W, Qian H. The emerging role of extracellular vesicles in retinal diseases. Am J Transl Res. 2021;13:13227–13245. [PMC free article] [PubMed] [Google Scholar]
- 77.Liu C, Zhang W, Li Y, Chang J, Tian F, Zhao F, et al. Microfluidic sonication to assemble exosome membrane-coated nanoparticles for immune evasion-mediated targeting. Nano Lett. 2019;19:7836–7844. doi: 10.1021/acs.nanolett.9b02841. [DOI] [PubMed] [Google Scholar]
- 78.Rayamajhi S, Aryal S. Surface functionalization strategies of extracellular vesicles. J Mater Chem B. 2020;8:4552–4569. doi: 10.1039/D0TB00744G. [DOI] [PubMed] [Google Scholar]
- 79.Khongkow M, Yata T, Boonrungsiman S, Ruktanonchai UR, Graham D, Namdee K. Surface modification of gold nanoparticles with neuron-targeted exosome for enhanced blood-brain barrier penetration. Sci Rep. 2019;9:8278. doi: 10.1038/s41598-019-44569-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tran PHL, Xiang D, Tran TTD, Yin W, Zhang Y, Kong L, et al. Exosomes and nanoengineering: a match made for precision therapeutics. Adv Mater. 2020;32:e1904040. doi: 10.1002/adma.201904040. [DOI] [PubMed] [Google Scholar]
- 81.Xi XM, Xia SJ, Lu R. Drug loading techniques for exosome-based drug delivery systems. Pharmazie. 2021;76:61–67. doi: 10.1691/ph.2021.0128. [DOI] [PubMed] [Google Scholar]
- 82.Dumontel B, Susa F, Limongi T, Vighetto V, Debellis D, Canta M, et al. Nanotechnological engineering of extracellular vesicles for the development of actively targeted hybrid nanodevices. Cell Biosci. 2022;12:61. doi: 10.1186/s13578-022-00784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Raimondo S, Giavaresi G, Lorico A, Alessandro R. Extracellular vesicles as biological shuttles for targeted therapies. Int J Mol Sci. 2019;20:1848. doi: 10.3390/ijms20081848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11:3183–3195. doi: 10.7150/thno.52570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao Z, Shuang T, Gao Y, Lu F, Zhang J, He W, et al. Targeted delivery of exosomal miR-484 reprograms tumor vasculature for chemotherapy sensitization. Cancer Lett. 2022;530:45–58. doi: 10.1016/j.canlet.2022.01.011. [DOI] [PubMed] [Google Scholar]
- 86.Wang Y, Ding N, Guan G, Liu G, Huo D, Li Y, et al. Rapid delivery of hsa-miR-590-3p using targeted exosomes to treat acute myocardial infarction through regulation of the cell cycle. J Biomed Nanotechnol. 2018;14:968–977. doi: 10.1166/jbn.2018.2493. [DOI] [PubMed] [Google Scholar]
- 87.Zhu M, Li S, Li S, Wang H, Xu J, Wang Y, et al. Strategies for engineering exosomes and their applications in drug delivery. J Biomed Nanotechnol. 2021;17:2271–2297. doi: 10.1166/jbn.2021.3196. [DOI] [PubMed] [Google Scholar]
- 88.Dong J, Zhang RY, Sun N, Hu J, Smalley MD, Zhou A, et al. Coupling nanostructured microchips with covalent chemistry enables purification of sarcoma-derived extracellular vesicles for downstream functional studies. Adv Funct Mater. 2020;30:2003237. doi: 10.1002/adfm.202003237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Y, Yao J, Cai L, Liu T, Wang X, Zhang Y, et al. Bone-targeted extracellular vesicles from mesenchymal stem cells for osteoporosis therapy. Int J Nanomedicine. 2020;15:7967–7977. doi: 10.2147/IJN.S263756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hao D, Lu L, Song H, Duan Y, Chen J, Carney R, et al. Engineered extracellular vesicles with high collagen-binding affinity present superior in situ retention and therapeutic efficacy in tissue repair. Theranostics. 2022;12:6021–6037. doi: 10.7150/thno.70448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang L, Qin Z, Sun H, Chen X, Dong J, Shen S, et al. Nanoenzyme engineered neutrophil-derived exosomes attenuate joint injury in advanced rheumatoid arthritis via regulating inflammatory environment. Bioact Mater. 2022;18:1–14. doi: 10.1016/j.bioactmat.2022.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hosseini Shamili F, Alibolandi M, Rafatpanah H, Abnous K, Mahmoudi M, Kalantari M, et al. Immunomodulatory properties of MSC-derived exosomes armed with high affinity aptamer toward mylein as a platform for reducing multiple sclerosis clinical score. J Control Release. 2019;299:149–164. doi: 10.1016/j.jconrel.2019.02.032. [DOI] [PubMed] [Google Scholar]
- 93.Hernandez-Oller L, Seras-Franzoso J, Andrade F, Rafael D, Abasolo I, Gener P, et al. Extracellular vesicles as drug delivery systems in cancer. Pharmaceutics. 2020;12:1146. doi: 10.3390/pharmaceutics12121146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ke W, Afonin KA. Exosomes as natural delivery carriers for programmable therapeutic nucleic acid nanoparticles (NANPs) Adv Drug Deliv Rev. 2021;176:113835. doi: 10.1016/j.addr.2021.113835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee ES, Sul JH, Shin JM, Shin S, Lee JA, Kim HK, et al. Reactive oxygen species-responsive dendritic cell-derived exosomes for rheumatoid arthritis. Acta Biomater. 2021;128:462–473. doi: 10.1016/j.actbio.2021.04.026. [DOI] [PubMed] [Google Scholar]
- 96.Wei Z, Zhao Y, Hsu P, Guo S, Zhang C, Zhong B. Exosomes for gene therapy effectively inhibit the endothelial-mesenchymal transition in mouse aortic endothelial cells. BMC Musculoskelet Disord. 2021;22:1000. doi: 10.1186/s12891-021-04896-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Salunkhe S, Basak M, Chitkara D, Mittal A. Surface functionalization of exosomes for target-specific delivery and in vivo imaging & tracking strategies and significance. J Control Release. 2020;326:599–614. doi: 10.1016/j.jconrel.2020.07.042. [DOI] [PubMed] [Google Scholar]
- 98.Tamura R, Uemoto S, Tabata Y. Augmented liver targeting of exosomes by surface modification with cationized pullulan. Acta Biomater. 2017;57:274–284. doi: 10.1016/j.actbio.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 99.Armstrong JPK, Holme MN, Stevens MM. Re-engineering extracellular vesicles as smart nanoscale therapeutics. ACS Nano. 2017;11:69–83. doi: 10.1021/acsnano.6b07607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Qi H, Liu C, Long L, Ren Y, Zhang S, Chang X, et al. Blood exosomes endowed with magnetic and targeting properties for cancer therapy. ACS Nano. 2016;10:3323–3333. doi: 10.1021/acsnano.5b06939. [DOI] [PubMed] [Google Scholar]
- 101.Barile L, Vassalli G. Exosomes: therapy delivery tools and biomarkers of diseases. Pharmacol Ther. 2017;174:63–78. doi: 10.1016/j.pharmthera.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 102.McDonald ES, Clark AS, Tchou J, Zhang P, Freedman GM. Clinical diagnosis and management of breast cancer. J Nucl Med. 2016;57:9s–16. doi: 10.2967/jnumed.115.157834. [DOI] [PubMed] [Google Scholar]
- 103.Pondé NF, Zardavas D, Piccart M. Progress in adjuvant systemic therapy for breast cancer. Nat Rev Clin Oncol. 2019;16:27–44. doi: 10.1038/s41571-018-0089-9. [DOI] [PubMed] [Google Scholar]
- 104.Lan T, Luo M, Wei X. Mesenchymal stem/stromal cells in cancer therapy. J Hematol Oncol. 2021;14:195. doi: 10.1186/s13045-021-01208-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.O'Brien KP, Khan S, Gilligan KE, Zafar H, Lalor P, Glynn C, et al. Employing mesenchymal stem cells to support tumor-targeted delivery of extracellular vesicle (EV)-encapsulated microRNA-379. Oncogene. 2018;37:2137–2149. doi: 10.1038/s41388-017-0116-9. [DOI] [PubMed] [Google Scholar]
- 106.Duarte-Sanmiguel S, Panic A, Dodd DJ, Salazar-Puerta A, Moore JT, Lawrence WR, et al. In situ deployment of engineered extracellular vesicles into the tumor niche via myeloid-derived suppressor cells. Adv Healthc Mater. 2022;11:e2101619. doi: 10.1002/adhm.202101619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Limoni SK, Moghadam MF, Moazzeni SM, Gomari H, Salimi F. Engineered exosomes for targeted transfer of siRNA to HER2 positive breast cancer cells. Appl Biochem Biotechnol. 2019;187:352–364. doi: 10.1007/s12010-018-2813-4. [DOI] [PubMed] [Google Scholar]
- 108.Shi X, Cheng Q, Hou T, Han M, Smbatyan G, Lang JE, et al. Genetically engineered cell-derived nanoparticles for targeted breast cancer immunotherapy. Mol Ther. 2020;28:536–547. doi: 10.1016/j.ymthe.2019.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang L, Zhou X, Zou W, Wu Y, Zhao J, Chen X, et al. Exosomes containing miRNAs targeting HER2 synthesis and engineered to adhere to HER2 on tumor cells surface exhibit enhanced antitumor activity. J Nanobiotechnology. 2020;18:153. doi: 10.1186/s12951-020-00711-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li S, Wu Y, Ding F, Yang J, Li J, Gao X, et al. Engineering macrophage-derived exosomes for targeted chemotherapy of triple-negative breast cancer. Nanoscale. 2020;12:10854–10862. doi: 10.1039/D0NR00523A. [DOI] [PubMed] [Google Scholar]
- 111.Wan Y, Wang L, Zhu C, Zheng Q, Wang G, Tong J, et al. Aptamer-conjugated extracellular nanovesicles for targeted drug delivery. Cancer Res. 2018;78:798–808. doi: 10.1158/0008-5472.CAN-17-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nguyen Cao TG, Kang JH, You JY, Kang HC, Rhee WJ, Ko YT, et al. Safe and targeted sonodynamic cancer therapy using biocompatible exosome-based nanosonosensitizers. ACS Appl Mater Interfaces. 2021;13:25575–25588. doi: 10.1021/acsami.0c22883. [DOI] [PubMed] [Google Scholar]
- 113.Cao Y, Wu T, Zhang K, Meng X, Dai W, Wang D, et al. Engineered exosome-mediated near-infrared-II region V2C quantum dot delivery for nucleus-target low-temperature photothermal therapy. ACS Nano. 2019;13:1499–1510. doi: 10.1021/acsnano.8b07224. [DOI] [PubMed] [Google Scholar]
- 114.Teo MY, Rathkopf DE, Kantoff P. Treatment of advanced prostate cancer. Annu Rev Med. 2019;70:479–499. doi: 10.1146/annurev-med-051517-011947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pan S, Zhang Y, Huang M, Deng Z, Zhang A, Pei L, et al. Urinary exosomes-based engineered nanovectors for homologously targeted chemo-chemodynamic prostate cancer therapy via abrogating EGFR/AKT/NF-kB/IkB signaling. Biomaterials. 2021;275:120946. doi: 10.1016/j.biomaterials.2021.120946. [DOI] [PubMed] [Google Scholar]
- 116.Han Q, Xie QR, Li F, Cheng Y, Wu T, Zhang Y, et al. Targeted inhibition of SIRT6 via engineered exosomes impairs tumorigenesis and metastasis in prostate cancer. Theranostics. 2021;11:6526–6541. doi: 10.7150/thno.53886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Powell Gray B, Kelly L, Ahrens DP, Barry AP, Kratschmer C, Levy M, et al. Tunable cytotoxic aptamer-drug conjugates for the treatment of prostate cancer. Proc Natl Acad Sci U S A. 2018;115:4761–4766. doi: 10.1073/pnas.1717705115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Severic M, Ma G, Pereira SGT, Ruiz A, Cheung CCL, Al-Jamal WT. Genetically-engineered anti-PSMA exosome mimetics targeting advanced prostate cancer in vitro and in vivo. J Control Release. 2021;330:101–110. doi: 10.1016/j.jconrel.2020.12.017. [DOI] [PubMed] [Google Scholar]
- 119.Gao X, Xu J, Yao T, Liu X, Zhang H, Zhan C. Peptide-decorated nanocarriers penetrating the blood-brain barrier for imaging and therapy of brain diseases. Adv Drug Deliv Rev. 2022;187:114362. doi: 10.1016/j.addr.2022.114362. [DOI] [PubMed] [Google Scholar]
- 120.Tian T, Liang R, Erel-Akbaba G, Saad L, Obeid PJ, Gao J, et al. Immune checkpoint inhibition in GBM primed with radiation by engineered extracellular vesicles. ACS Nano. 2022;16:1940–1953. doi: 10.1021/acsnano.1c05505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jia G, Han Y, An Y, Ding Y, He C, Wang X, et al. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials. 2018;178:302–316. doi: 10.1016/j.biomaterials.2018.06.029. [DOI] [PubMed] [Google Scholar]
- 122.Ye Z, Zhang T, He W, Jin H, Liu C, Yang Z, et al. Methotrexate-loaded extracellular vesicles functionalized with therapeutic and targeted peptides for the treatment of glioblastoma multiforme. ACS Appl Mater Interfaces. 2018;10:12341–12350. doi: 10.1021/acsami.7b18135. [DOI] [PubMed] [Google Scholar]
- 123.Uniken Venema SM, Dankbaar JW, van der Lugt A, Dippel DWJ, van der Worp HB. Cerebral collateral circulation in the era of reperfusion therapies for acute ischemic stroke. Stroke. 2022;53:3222–3234. doi: 10.1161/STROKEAHA.121.037869. [DOI] [PubMed] [Google Scholar]
- 124.Guo L, Huang Z, Huang L, Liang J, Wang P, Zhao L, et al. Surface-modified engineered exosomes attenuated cerebral ischemia/reperfusion injury by targeting the delivery of quercetin towards impaired neurons. J Nanobiotechnol. 2021;19:141. doi: 10.1186/s12951-021-00879-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tian T, Zhang HX, He CP, Fan S, Zhu YL, Qi C, et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137–149. doi: 10.1016/j.biomaterials.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 126.Wu WC, Tian J, Xiao D, Guo YX, Xiao Y, Wu XY, et al. Engineered extracellular vesicles encapsulated Bryostatin-1 as therapy for neuroinflammation. Nanoscale. 2022;14:2393–2410. doi: 10.1039/D1NR05517H. [DOI] [PubMed] [Google Scholar]
- 127.Yu Y, Li W, Mao L, Peng W, Long D, Li D, et al. Genetically engineered exosomes display RVG peptide and selectively enrich a neprilysin variant: a potential formulation for the treatment of Alzheimer's disease. J Drug Target. 2021;29:1128–1138. doi: 10.1080/1061186X.2021.1929257. [DOI] [PubMed] [Google Scholar]
- 128.Zhang R, Fu Y, Cheng M, Ma W, Zheng N, Wang Y, et al. sEVs(RVG) selectively delivers antiviral siRNA to fetus brain, inhibits ZIKV infection and mitigates ZIKV-induced microcephaly in mouse model. Mol Ther. 2022;30:2078–2091. doi: 10.1016/j.ymthe.2021.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yu X, Bai Y, Han B, Ju M, Tang T, Shen L, et al. Extracellular vesicle-mediated delivery of circDYM alleviates CUS-induced depressive-like behaviours. J Extracell Vesicles. 2022;11:e12185. doi: 10.1002/jev2.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liang Y, Xu X, Xu L, Prasadam I, Duan L, Xiao Y, et al. Non-surgical osteoarthritis therapy, intra-articular drug delivery towards clinical applications. J Drug Target. 2021;29:609–616. doi: 10.1080/1061186X.2020.1870231. [DOI] [PubMed] [Google Scholar]
- 131.Liang Y, Xu X, Li X, Xiong J, Li B, Duan L, et al. Chondrocyte-targeted microRNA delivery by engineered exosomes toward a cell-free osteoarthritis therapy. ACS Appl Mater Interfaces. 2020;12:36938–36947. doi: 10.1021/acsami.0c10458. [DOI] [PubMed] [Google Scholar]
- 132.Xu X, Liang Y, Li X, Ouyang K, Wang M, Cao T, et al. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials. 2021;269:120539. doi: 10.1016/j.biomaterials.2020.120539. [DOI] [PubMed] [Google Scholar]
- 133.You DG, Lim GT, Kwon S, Um W, Oh BH, Song SH, et al. Metabolically engineered stem cell-derived exosomes to regulate macrophage heterogeneity in rheumatoid arthritis. Sci Adv. 2021;7:eabe0083. doi: 10.1126/sciadv.abe0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Huang X, Wu W, Jing D, Yang L, Guo H, Wang L, et al. Engineered exosome as targeted lncRNA MEG3 delivery vehicles for osteosarcoma therapy. J Control Release. 2022;343:107–117. doi: 10.1016/j.jconrel.2022.01.026. [DOI] [PubMed] [Google Scholar]
- 135.Pan L, Yan B, Zhang J, Zhao P, Jing Y, Yu J, et al. Mesenchymal stem cells-derived extracellular vesicles-shuttled microRNA-223-3p suppress lipopolysaccharide-induced cardiac inflammation, pyroptosis, and dysfunction. Int Immunopharmacol. 2022;110:108910. doi: 10.1016/j.intimp.2022.108910. [DOI] [PubMed] [Google Scholar]
- 136.Zhang J, Lu Y, Mao Y, Yu Y, Wu T, Zhao W, et al. IFN-γ enhances the efficacy of mesenchymal stromal cell-derived exosomes via miR-21 in myocardial infarction rats. Stem Cell Res Ther. 2022;13:333. doi: 10.1186/s13287-022-02984-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yan F, Cui W, Chen Z. Mesenchymal stem cell-derived exosome-loaded microRNA-129-5p inhibits TRAF3 expression to alleviate apoptosis and oxidative stress in heart failure. Cardiovasc Toxicol. 2022;22:631–645. doi: 10.1007/s12012-022-09743-9. [DOI] [PubMed] [Google Scholar]
- 138.Chen P, Wang L, Fan X, Ning X, Yu B, Ou C, et al. Targeted delivery of extracellular vesicles in heart injury. Theranostics. 2021;11:2263–2277. doi: 10.7150/thno.51571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Antes TJ, Middleton RC, Luther KM, Ijichi T, Peck KA, Liu WJ, et al. Targeting extracellular vesicles to injured tissue using membrane cloaking and surface display. J Nanobiotechnology. 2018;16:61. doi: 10.1186/s12951-018-0388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang X, Chen Y, Zhao Z, Meng Q, Yu Y, Sun J, et al. Engineered exosomes with ischemic myocardium-targeting peptide for targeted therapy in myocardial infarction. J Am Heart Assoc. 2018;7:e008737. doi: 10.1161/JAHA.118.008737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li Q, Song Y, Wang Q, Chen J, Gao J, Tan H, et al. Engineering extracellular vesicles with platelet membranes fusion enhanced targeted therapeutic angiogenesis in a mouse model of myocardial ischemia reperfusion. Theranostics. 2021;11:3916–3931. doi: 10.7150/thno.52496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hu X, Ning X, Zhao Q, Zhang Z, Zhang C, Xie M, et al. Islet-1 mesenchymal stem cells-derived exosome-incorporated angiogenin-1 hydrogel for enhanced acute myocardial infarction therapy. ACS Appl Mater Interfaces. 2022;14:36289–36303. doi: 10.1021/acsami.2c04686. [DOI] [PubMed] [Google Scholar]
- 143.Komuro H, Kawai-Harada Y, Aminova S, Pascual N, Malik A, Contag CH, et al. Engineering extracellular vesicles to target pancreatic tissue in vivo. Nanotheranostics. 2021;5:378–390. doi: 10.7150/ntno.54879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tang TT, Wang B, Li ZL, Wen Y, Feng ST, Wu M, et al. Kim-1 targeted extracellular vesicles: a new therapeutic platform for RNAi to treat AKI. J Am Soc Nephrol. 2021;32:2467–2483. doi: 10.1681/ASN.2020111561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Guo Y, Wan Z, Zhao P, Wei M, Liu Y, Bu T, et al. Ultrasound triggered topical delivery of Bmp7 mRNA for white fat browning induction via engineered smart exosomes. J Nanobiotechnol. 2021;19:402. doi: 10.1186/s12951-021-01145-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lee JH, Yoon JY, Lee JH, Lee HH, Knowles JC, Kim HW. Emerging biogenesis technologies of extracellular vesicles for tissue regenerative therapeutics. J Tissue Eng. 2021;12:20417314211019015. doi: 10.1177/20417314211019015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Grangier A, Branchu J, Volatron J, Piffoux M, Gazeau F, Wilhelm C, et al. Technological advances towards extracellular vesicles mass production. Adv Drug Deliv Rev. 2021;176:113843. doi: 10.1016/j.addr.2021.113843. [DOI] [PubMed] [Google Scholar]
- 148.Liangsupree T, Multia E, Riekkola ML. Modern isolation and separation techniques for extracellular vesicles. J Chromatogr A. 2021;1636:461773. doi: 10.1016/j.chroma.2020.461773. [DOI] [PubMed] [Google Scholar]
- 149.Corrêa RR, Juncosa EM, Masereeuw R, Lindoso RS. Extracellular vesicles as a therapeutic tool for kidney disease: current advances and perspectives. Int J Mol Sci. 2021;22:5787. doi: 10.3390/ijms22115787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Meng W, He C, Hao Y, Wang L, Li L, Zhu G. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Deliv. 2020;27:585–598. doi: 10.1080/10717544.2020.1748758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen C, Sun M, Wang J, Su L, Lin J, Yan X. Active cargo loading into extracellular vesicles: highlights the heterogeneous encapsulation behaviour. J Extracell Vesicles. 2021;10:e12163. doi: 10.1002/jev2.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li S, Xu J, Qian J, Gao X. Engineering extracellular vesicles for cancer therapy: recent advances and challenges in clinical translation. Biomater Sci. 2020;8:6978–6991. doi: 10.1039/D0BM01385D. [DOI] [PubMed] [Google Scholar]