Abstract
Black pepper essential oil has the same disadvantages as other plant essential oils, such as volatilization, high sensitivity to light and heat and poor water solubility, which leads to great limitations in application. This study improved the stability and antibacterial properties of black pepper essential oil (BPEO) based on a nano-emulsification process. Tween 80 was selected as the emulsifier to prepare the BPEO nanoemulsion. Gas chromatograph - mass spectrometer (GC-MS) was used to analyze the composition of BPEO, of which d-limonene was the main component (37.41%). After emulsification, black pepper nanoemulsion was obtained (droplet size was 11.8 nm). The water solubility and stability of the emulsions at 25 °C were also improved with decreasing particle size. Antimicrobial properties of plant pathogens (Colletotrichum gloeosporioides, Botryodiplodia theobromae) and foodborne pathogens (Staphylococcus aureus, Escherichia coli) were evaluated by disk diffusion and other techniques for determining minimum inhibitory concentration (MIC) and minimal bactericidal concentration (MBC). With 12.5 mg mL−1 MIC and 25 mg mL−1 MBC, BPEO inhibited the growth of two tested plant pathogens and two foodborne pathogens. Essential oils (EO) were encapsulated in a nanoemulsion system to enhance the bacteriostatic effect of essential oils and reduce MIC and MBC concentrations. After emulsification, the biological activity (antimicrobial and antioxidant) of the BPEO nanoemulsion was considerably improved, nano-emulsification had certain significance for the study of EOs.
Keywords: Black pepper essential oil, Nanoemulsion, Stability, Antimicrobial activity, Antioxidant activity
1. Introduction
Black pepper (Piper nigrum L.) is native to Southeast Asia, and Hainan Province is the main producing area and accounting for more than 90% of the total pepper production in China [1,2]. Black pepper can treat colds, fevers, and many inflammatory diseases. Black pepper essential oil (BPEO) is extracted from black pepper and is widely used in food and medicine. BPEO is rich in monoterpenes and sesquiterpenes and has strong antioxidant, anti-inflammatory and antibacterial properties, making it a suitable alternative to synthetic antioxidants, antibiotics and antibacterial agents [3]. However, due to its low solubility, the dispersibility of BPEO in food and its absorption in the human body have been greatly restricted [4]. Therefore, suitable processing methods were needed to improve the utilization of BPEO without affecting its biological activity.
Nanoemulsification technology has achieved remarkable results in solving the problems of water solubility and the stability of essential oils (EOs). The nanoemulsion prepared by nanoemulsification technology is relatively small in size and has high dynamic stability, low viscosity, and optical transparency, which has attracted the interest of the food industry [5,6]. The EO nanoemulsion system can minimize the effect of adding EOs on the organoleptic properties of food and enhance the bioactive properties of EOs, including their antibacterial properties. Compared with naked EOs, EO nanoemulsions show higher biological activity. The Litsea cubeba EO nanoemulsion prepared by Ref. [7] not only showed good inhibition on the food-borne pathogenic bacteria Listeria monocytogenes (L. monocytogenes) and the spoilage bacteria Shewanella baltica (S. baltica), but also showed that nanoemulsion has a stronger antibacterial effect than pure EO [8]. studied the effect of BPEO on the storage quality of fresh pork, and the results showed that gram-negative bacteria are more sensitive to BPEO than gram-positive bacteria.
When conditions were right, harmful microorganisms can grow and multiply, breaking down nutrients in food and causing food to spoil and go rancid, making it unsuitable for human consumption [9,10]. Currently, the use of physical sterilization in food processing may reduce nutritional value, and chemical antimicrobial agents may be potentially harmful to human health [11,12]. Unlike chemical preservatives, EOs are natural food preservatives derived from plant material. In the food industry, the use of EO can prevent the infection and reproduction of spoilage bacteria, so that the food has a longer shelf life [13]. The effectiveness and specificity of EO against various microorganisms is derived from the action of the diverse antimicrobial compounds it contains (20–60 different biologically active compounds) [1,14]. EOs contained in the emulsion system have low reactivity and hydrophobicity, which is not conducive to their application in the food industry [15,16].
However, the development of BPEO has been greatly limited by its shortcomings, such as volatility, easy oxidation, poor stability, and short preservation time [16,17]. In addition, there are few reports on the antibacterial properties of BPEO against common plant pathogens. Therefore, improving the volatility, stability, and durable availability of BPEO and expanding the development of BPEO and high value-added products are important. In this study, based on ultrasonic nanoemulsion technology to improve the physical properties of black pepper, the antioxidant activity of black pepper nanoemulsion and the determination of activity of this nanoemulsion on spoilage microorganisms and pathogenic bacteria were evaluated.
2. Materials and methods
2.1. Materials
BPEO (98%, India), Tween 80, NaCl, HCl, NaOH, Bromocresol violet, Ethanol, FeSO4, H2O2, Potassium persulfate, ABTS, DPPH purchased from Hainan Haidaosen Technology Co., LTD, all reagents are analytically pure. Two mango plant pathogens (Colletotrichum gloeosporioides, Botryodiplodia theobromae) and two foodborne pathogens (Staphylococcus aureus, Escherichia coli) were obtained from China General Microbiological Culture Collection Center (the strain activity was qualified by PCR test).
2.2. Analysis of composition of BPEO
GC-MS analysis was used to explore the volatile components in BPEO, as the following gas chromatography conditions: SLB-5MS column (30 m*0.25 mm I.D. *0.25 μm); shunt ratio, 40:1; and injection volume, 1.00 mL. The initial temperature of the column was 60 °C (maintained for 2 min), and then the temperature was raised to 260 °C at 10 °C·min−1 (maintained for 5 min). The chemicals were identified using the NIST-MS and WILEY-MS libraries [[18], [19], [20]].
2.3. Preparation and physical properties of BPEO nanoemulsion
BPEO, Tween 80, and water were mixed in a ratio of 10:1:89 (W/W) for the preparation of BPEO nanoemulsions. The emulsion (HLB = 15) was formed using the SC-1500F ultrasonic homogenizer under the following homogenizing conditions: duration, 15 min; power, 500 W; and ultrasonic frequency, 20 kHz; emulsifier 4.23% black pepper essential oil 5% [10,14].
The average particle size and polydispersity index of the emulsion were measured by Zetasizer Nano (MAL1077738, Malvern Instrument, England). The BPEO nanoemulsion was diluted 100-fold with deionize ed water and stirred sufficiently to avoid multiple scattering effects. The temperature was 25 °C, the detection angle was 90°, and this measurement was repeated three times. The thermodynamic stability of the emulsions was analyzed according to the method of [21,22].
2.4. Microstructure of BPEO nanoemulsion
Transmission electron microscopy (TEM) was used to analyze the microstructure of the BPEO nanoemulsion. The nanoemulsion was dropped on the copper mesh with the film, followed by 2% phosphotungstic acid dye for 30 min. Excess dye was absorbed using filter paper, and the samples were subsequently observed with TEM at an accelerating voltage of 80 kV [18].
2.5. Storage stability of BPEO nanoemulsions
The stability of the nanoemulsion within 30 d can be determined by droplet size and size distribution [14,16,23]. The freshly prepared BPEO nanoemulsion samples were stored at a temperature of 25 °C ± 5 °C and a relative humidity of 65% ± 5% for 30 d. The average particle size and zeta potential of the emulsion were measured with particle size analyzer (Malvern Instruments, UK) each 5 d. Different concentrations of NaCl solutions (0.1, 0.2, 0.3, 0.4, 0.5 g L−1) was prepared. The BPEO nanoemulsion was diluted 1 time with NaCl solutions, and after standing for 2 h, the average particle size and zeta potential of the emulsion were measured. A volume of 10 mL of BPEO nanoemulsion was placed in a test tube, and heated it in a water bath at 40, 50, 60, 70 and 80 °C for 60 min. After standing overnight at room temperature, the particle size and zeta potential of the Nano emulsion were measured. The pH value of the BPEO nanoemulsion was adjusted to 4.0, 6.0, 8.0, 10.0, 12.0 with 0.1 mol L−1 HCl and 0.1 mol L−1 NaOH. After standing for 2 h, the average particle size and zeta potential were measured.
2.6. In vitro antioxidant activity of BPEO nanoemulsion
2.6.1. Analysis of the total antioxidant capacity
FRAP method was used to test the total antioxidant capacity of BPEO nanoemulsion [14]. Dissolved 50 mg of bromocresol violet in 95% ethanol solution and make up to 100 mL; 273.6 mg of 1.8 mol L−1 FeSO4 was joined in evaporate water, constant into 100 mL volumetric flask; brought 6 mL of 30% H2O2 to 100 mL with heavy steam water. 0.4 mL of 0.05% bromocresol purple solution (diluted into 95% ethanol), 0.5 mL of 0.1 mol L−1 HCl, and 0.5 mL of 1.8 mmol mL−1 FeSO4 were placed in separate test tubes, diluted with heavy steam water, and then reacted with water for 8 min at 30 °C. Absorbance (A0) was measured at 420 nm. Then, 0.5 mL of 0.1 mol L−1 H2O2 was added into 1 mmol L−1 FeSO4 solution, and the absorbance (A) was measured. The result value is the average of three determinations. Low absorbance value was calculated as follow (Equation (1)):
| ΔA = A0 − A. | (1) |
2.6.2. Measurement of the DPPH free radical scavenging rate
Method was used to measure the DPPH free radical scavenging rate [15,16]. 0.2 mL of the sample, 4 mL of 0.1 mmol L−1 DPPH ethanol solution was added and then added distilled water up to 8 mL. After 30 min, the OD value (Ax) was measured at 517 nm 4 mL of distilled water with 4 mL of DPPH solution were mixed, and the OD value (Ao) was measured. A mixture of 4 mL ethanol and 4 mL distilled water was set as control group. The calculation method is as follow (Equation (2)):
| the free radical scavenging rate (%) = (1-Ax/A0) × 100. | (2) |
2.6.3. The ABTS free radical scavenging rate
Method was used to measure the ABTS free radical scavenging rate [15,16]. 5 mL of 7 mmol L−1 ABTS solution and 88 μL of 140 mmol L−1 potassium persulfate solution was mixed, and kept for 15 h at room temperature in the dark. The solution was diluted with ultrapure water to an absorbance of about 0.7 at 734 nm, and the resulting solution was the ABTS+ solution. 0.2 mL sample and 2 mL ABTS solution were mixed uniformly, and after 10 min of reaction in the dark, the absorbance A1 was measured at 734 nm. The control group replaced the sample with 0.2 mL ethanol solution, and the obtained absorbance was A2. The calculation method is as follow (Equation (3)):
| the free radical scavenging rate (%) = (1-Ax/A2) × 100. | (3) |
2.7. Analysis of antimicrobial properties
The determination of the minimum bacteriostatic concentration (MIC) and the minimal bactericide concentration (MBC) of BPEO nanoemulsion was also slightly modified with reference to previous studies [24]. The concentration of microorganisms was diluted to approximately 105 CFU mL−1 for stock, and 0.5 mL of each bacterial inoculum was inoculated into 2.5 mL of nutrient broth. Then, different volume fractions of essential oils (Dissolved in 10% ethanol solution) or nanomoemulsions were added to the tube and stirred in a whirl for 30 s. When the mixture was incubated at 37 °C for 24 h. The minimum concentration of invisible turbidity (OD600 change ≤0.05) is 1 MIC of the EO and its nanoemulsion. A 100 μL aliquot of the test microorganism culture medium showing no turbidity at OD600 was transferred to plate colony count (PCA) nutrient agar and cultured at 37 °C for 24 h. The corresponding concentration of non-growing microorganisms was taken as the minimal bactericide concentration (MBC). After 48 h of culture, without bacterial growth and the lowest concentration of EO and its nanoemulsion, which can be defined as 1 MBC of BPEO nanoemulsion on the bacteria. 30 μg tetracycline, 10 μg streptomycin and 5 μg neomycin were the three positive controls, and Tween 80 was the negative control. Approximately 50 μL of BPEO and its nanoemulsion were added and cultured in a Petri dish at 37 °C for 24 h. The average of the three measurements represented the results [9,12,16].
2.8. Statistical analysis
The statistical software is SPSS 23. Final data were expressed as the mean ± standard deviation ( ± s). ANOVA was employed to assess the differences among groups. Significant differences were denoted by P < 0.05.
3. Results and discussion
3.1. Volatile composition of BPEO nanoemulsion
To make clear the volatile composition of BPEO, GC-MS method was conducted and the main composition of BPEO was showed in Table 1. In total, 25 compounds were found in BPEO. d-Limonene is the dominant terpene, comprising 37.41% of the total identified compounds. The levels determined were similar to those reported in other studies [25]. Other compounds present in quantities exceeding 5% included 3 - carene (13.77%), Cyclohexane, 1 - methylene - 4 - (1 - methylethenyl) (9.45%), Bicyclo [5.2.0] nonane, 2 - methylene - 4, 8, 8 - trimethyl - 4 - vinyl (7.96%) and Bicyclo [3.1.0] hex - 2 - ene, 4 - methyl - 1 - (1 - methylethyl) (5.20%).
Table 1.
Determination on the composition of BPEO.
| Compound | Molecular | Percentage (%) |
|---|---|---|
| d-Limonene | C10H16 | 37.41 |
| 3-Carene | C10H16 | 13.77 |
| Cyclohexane, 1-methylene-4-(1-methylethenyl) | C10H16 | 9.45 |
| Bicyclo [5.2.0]nonane, 2-methylene-4,8,8-trimethyl-4-vinyl | C15H24 | 7.96 |
| Bicyclo [3.1.0]hex-2-ene, 4-methyl-1-(1-methylethyl) | C10H16 | 5.20 |
| Ylangene | C15H24 | 4.01 |
| Cyclohexene, 4-ethenyl-4-methyl-3-(1-methylethenyl)-1-(1-methylethyl)-, (3 R-trans) | C15H24 | 3.73 |
| o-Cymene | C10H14 | 2.73 |
| Naphthalene, decahydro-4a-methyl-1-methylene-7-(1-methylethenyl)-, [4aR-(4a. Alpha.,7. Alpha.,8a. beta.)]- | C15H24 | 1.98 |
| 1,4,7, -Cycloundecatriene, 1,5,9,9-tetramethyl-, Z, Z, Z- | C15H24 | 1.93 |
| 1,5-dimethyl-8-(1-methylethenyl)-, [S- (Z, E)]- | C15H24 | 1.61 |
| 2-Isopropenyl-4a,8-dimethyl-1,2,3,4,4a,5,6,8a-octahydronaphthalene | C15H24 | 1.35 |
| Cyclohexene, 4-(1,5-dimethyl-1,4-hexadienyl)-1-methyl- | C15H24 | 1.34 |
| Naphthalene, 1,2,3,5,6,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl)-, (1 S-cis)- | C15H24 | 1.16 |
| α-Phellandrene | C10H16 | 0.99 |
| Elemene isomer | C15H24 | 0.57 |
3.2. Physical properties
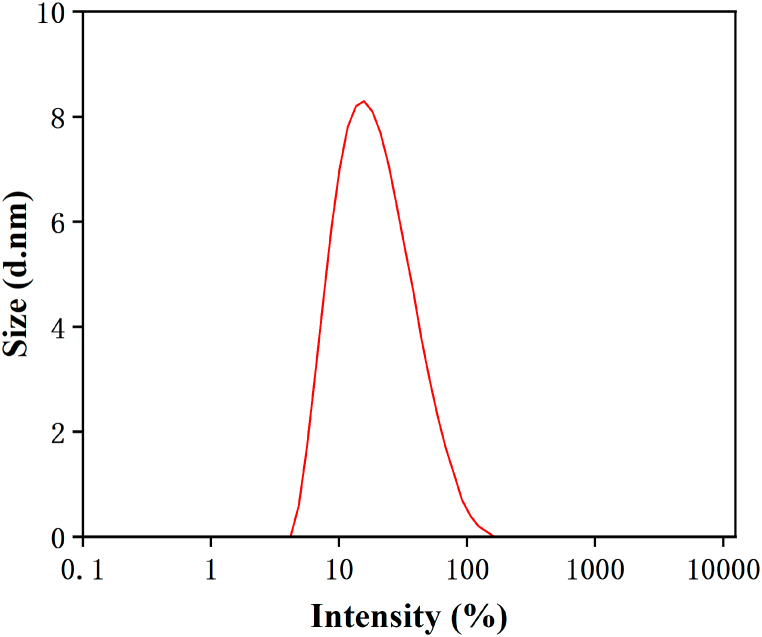
This appropriate nanoemulsion was selected for subsequent studies. The average droplet size and PDI of black pepper nanoemulsion were 15.69 ± 0.017 nm and 0.251 ± 0.004 (Fig. 1). In orthogonal experiments, the ratio of BPEO to oil carrier appears to be the key factor affecting the formation, stability, and performance of nanoemulsions [26]. When the ratio of essential oil to oil carrier is appropriate, nanoemulsions with smaller droplet sizes can be formed, conversely, too much oil carrier can lead to coalescence, so that nanoemulsions have better gravitational separation and aggregation than traditional emulsions stability. Compared with some previous studies [27,28], this study successfully reduced the particle size of essential oil-based emulsions to nanoscale (11.8 nm) (Fig. 1), which indicated that the functionality of the LECO-based nanoemulsion was improved.
Fig. 1.
The particle size distribution of BPEO nanoemulsion.
3.3. TEM observations of BPEO nanoemulsion
From the TEM image, it can be intuitively observed that the nanoemulsion droplets were spherical, and the emulsion droplets were distributed uniformly, with good formability, and a large number of small droplets (Fig. 2).
Fig. 2.
The particle size and morphology of nanoemulsions. The observation magnification of TEM was 60.000 × .
3.4. Long-term stability of BPEO nanoemulsion
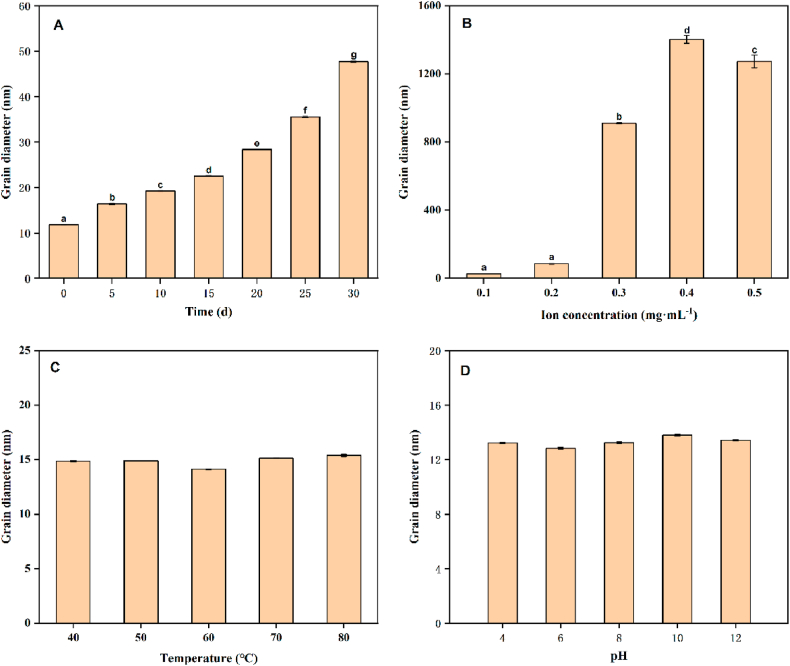
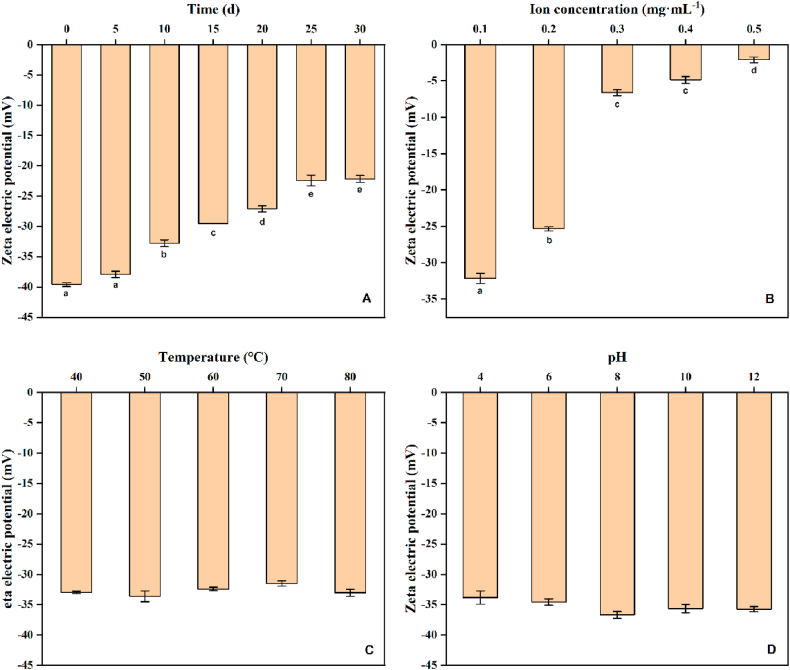
The size of the particle size is one of the standards to measure the stability of the nanoemulsion [9,10]. When the temperature was 25 °C ± 5 °C and the relative humidity was 65% ± 5%, the appearance of the BPEO nanoemulsion did not change significantly even after airtight storage for one months, showed no stratification or precipitation, and remained milky white. At Days 0, 5, 10, and 15, the particle size and zeta potential were basically unchanged (Fig. 3, Fig. 4A), indicating that the BPEO nanoemulsion exhibited good stability. As the storage time increased, the particle size of the BPEO nanoemulsion gradually increased. This is likely because the aggregation phenomenon of the nanoemulsion occurred with the increase of time, which reduced the stability of the emulsion. When stored for 30 d, the particle size of the nanoemulsion was 4 times the particle size on the 0 d. Although the stability decreased during storage, the particle size still did not exceed 50 nm after storage for 30 d, indicating that the nanoemulsion has good stability.
Fig. 3.
The effect of different storage time (A), ion concentration (B), temperature (C), and PH (D) on the stability of BPEO nanoemulsion. Different letters indicated significant differences and denoted by P < 0.05.
Fig. 4.
The effect of different storage time (A), ion concentration (B), temperature (C), and PH (D) on the zeta potential of BPEO nanoemulsion. Different letters indicated significant differences and denoted by P < 0.05.
It can be seen from Fig. 3, Fig. 4B, when the NaCl concentration increased from 0.1 mg mL−1 to 0.2 mg mL−1, the particle size and zeta potential of the BPEO nanoemulsion increased slightly, and the change was not significant. When the ion concentration increased to 0.3 mg mL−1 and 0.4 mg mL−1, the particle size increased significantly, about 900 nm and 1400 nm, respectively. However, when the NaCl concentration was 0.5 mg mL−1, the particle size dropped to about 1100 nm. This indicated that the stability of the BPEO nanoemulsion will decrease with the increase of NaCl concentration, and the BPEO nanoemulsion had better stability under the condition of low NaCl concentration.
When the temperature was lower than 60 °C, there was no significant difference in the particle size and zeta potential of the BPEO nanoemulsion changes. When the temperature was 80 °C, the particle size of the nanoemulsion increased, but the particle size only changes by 1.3 nm. Therefore, the BPEO nanoemulsion prepared in this study had good temperature stability (Fig. 3, Fig. 4C).
When the pH was 4, 6, 8, 10, 12, there was no significant difference in the particle size of the BPEO nanoemulsion. When the pH was 6, the average particle size was the smallest (∼13 nm), and the zeta potential was −34 mV. By contrast, when the pH was 10, the average particle size was the largest (∼14 nm), and the zeta potential was −37 mV. But the difference in particle size between the two was only 1 nm. Therefore, the BPEO nanoemulsion had good acid-base resistance, and under the condition of pH 4–12, the nanoemulsion showed good physical stability (Fig. 3, Fig. 4D).
3.5. Antioxidant activity of BPEO nanoemulsions
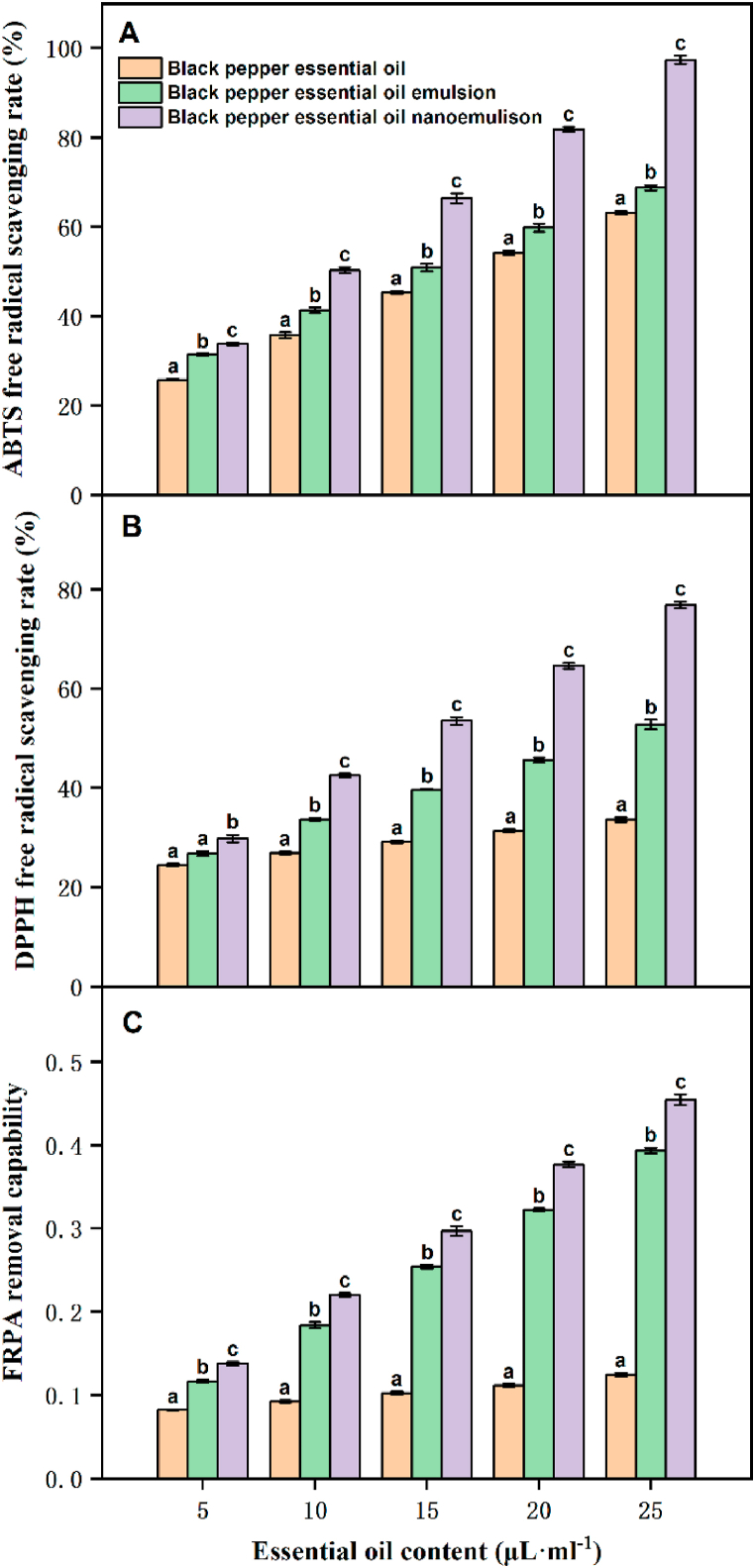
One of the urgent problems to be solved in the food industry is the loss of food nutrition caused by the oxidative deterioration of oil. Antioxidants are added to prevent oxidative spoilage and prolong the shelf life of food products. These antioxidants can scavenge free radicals and form stable products [29,30]. EOs have drawn significant attention as natural antioxidants and are used as free radical scavengers to prevent oxidative spoilage in oxidable foods [31,32]. FRAP, DPPH and ABTS methods are rapid, simple, sensitive, and reproducible [30,33]. Therefore, this experiment comprehensively evaluated the changes of the antioxidant activity for black pepper EO with or without nanoemulsion, results were shown in Fig. 2. We found that the BPEO had a certain antioxidant effect, and the free radical scavenging rates of ABTS (Fig. 5A), DPPH (Fig. 5B) and FRAP (Fig. 5C) by nanoemulsions were significantly increased relative to that of BPEO (P < 0.05). Under the same concentration of essential oil, the scavenging ability of the sample on ABTS, DPPH and FRTA free radicals is BPEO < BPEO emulsion < BPEO nanoemulsion. This difference may be attributed to the emulsion system improved the water solubility of BPEO, thereby increased its antioxidant activity in water-soluble systems. BPEO has high volatility, resulting in shorter reaction times and higher contents of active ingredients than that in nanoessential oil [9,10]. In addition, the free radical scavenging ability of the sample increased with the increase of essential oil content. This showed that the free radical scavenging ability of BPEO was improved after nanoemulsions treatment, and the construction of nanoemulsion does not affect the antioxidant ability of BPEO. Moreover, the smaller the nanoemulsion droplet size, the more comprehensive the contact with free radicals [12,13]. The free radical removal rate of the BPEO nanoemulsion was higher than that of BPEO. A similar observation has previously been reported [2]. Therefore, nanoemulsification was beneficial to the antioxidant capacity of BPEO. These results suggest that BPEO nanoemulsion was a natural antioxidant.
Fig. 5.
The scavenging effects of nanoemulsion, emulsion and pure essential oils on ABTS (A), DPPH free radicals (B) and FRAP (C). Different letters indicated significant differences and denoted by P < 0.05.
3.6. Antimicrobial activity of the BPEO nanoemulsions
The results of the diffusion of antibacterial activity by paper plate are listed in Table 2. Tween-80 acted as a negative control and did not inhibit the tested bacteria. BPEO nanoemulsion showed antifungal effect against C. gloeosporioides, B. theobromae and a bacteriostatic effect against S. aureus, E. coli (Table 3). The bacteriostatic effect of BPEO nonamulsion against all tested strains can be observed. Although Gram-positive bacteria are generally more susceptible to plant essential oils, this trend was not observed in this study, possibly due to their less complex cell wall structures [19]. MIC and MBC analysis showed that the antibacterial effect of the BPEO nanoemulsion on some bacteria was improved, but for the bacteria, E. Coli and S. Aureus the MIC obtained is the same for EO and emulsion/nanoemulsion. Some physical and chemical properties of nanoemulsions may affect their antibacterial properties. These factors include the droplet size, electrical properties, location, and concentration of antibacterial compounds [23,34]. In addition, the type of emulsifier or other additives can also have an impact on the effectiveness of the nanoemulsion [2,17]. The antibacterial properties of low molecular weight emulsifiers such as Tween 80 also depend on the type of strain, which will transfer the antibacterial properties to the various bacteria observed when using Tween 80 [35,36].
Table 2.
Antimicrobial activity of BPEO and its nanoemulsion against foodborne pathogens.
| Pathogens | BPEO Zone Diameter (mm) | BPEO Nanoemulsion Zone Diameter (mm) | Streptomycin | Neomycin | Tetracycline |
|---|---|---|---|---|---|
| Colletotrichum gloeosporioides | 5.00 ± 0.16b | 13.36 ± 0.58a | 20 ± 0.27b | 22.67 ± 0.22b | 17.66 ± 0.36d |
| Botryodiplodia theobromae | 3.64 ± 0.46c | 11.16 ± 0.26b | 23.38 ± 0.34a | 21.87 ± 0.49bc | 18.19 ± 0.63cd |
| Staphylococcus aureus | 6.8 ± 0.45a | 9.17 ± 0.48c | 15.15 ± 0.24c | 19.94 ± 0.57c | 21.05 ± 0.35b |
| Escherichia coli | 6.18 ± 26a | 8.69 ± 0.46d | 10.6 ± 0.38d | 24.62 ± 0.36a | 29.26 ± 0.42a |
Table 3.
MIC and MBC determination of BPEO and its nanoemulsion on foodborne pathogens.
| Pathogen | BPEO |
BPEO Nanoemulsion |
Tween 80 |
|||
|---|---|---|---|---|---|---|
| MIC (mg·mL−1) | MBC (mg·mL−1) | MIC (mg·mL−1) | MBC (mg·mL−1) | MIC (mg·mL−1) | MBC (mg·mL−1) | |
| Colletotrichum gloeosporioides | 6.25 | 12.5 | 3.125 | 6.25 | >25 | >25 |
| Botryodiplodia theobromae | 6.25 | 12.5 | 3.125 | 6.25 | >25 | >25 |
| Staphylococcus aureus | 12.5 | 25 | 12.5 | 25 | >25 | >25 |
| Escherichia coli | 12.5 | 25 | 12.5 | 25 | >25 | >25 |
Changing the method of preparing nanoemulsions may also improve antibacterial performance. In this study, the nanoemulsion had a small average droplet size (11.8 nm). The size of nanoemulsion droplets was closely related to their antibacterial properties; the smaller the droplet, the easier it is for the active substance to be transported through the bacterial membrane [37]. In contrast, shortening the ultrasonic time can enhance the antibacterial performance of the EO nanoemulsion despite the larger droplet size [12]. In addition, the nanoemulsions obtained by microfluidization exhibited significantly superior antibacterial performance against E. coli. This improvement may be attributed to high temperatures and the degradation of volatile substances in the presence of EO as longer ultrasonic time leads to an increase in the exit temperature of the system [10]. BPEO nanoemulsion has a good inhibitory effect on mango postharvest pathogens and can be used for mango postharvest preservation.
4. Conclusions
25 compounds in BPEO were identified by the GC-MS method, and a stable BPEO nanoemulsion was successfully prepared using an ultrasonic homogenizer, which improved the solubility and utilization of BPEO. The test results showed that BPEO nanoemulsion has good storage stability, temperature stability, and exhibits good physical stability at low NaCl concentration and a wide pH range (4–12). In this study, the research results also showed When the concentration reached 12.5 mg mL−1, BPEO exerted a bacteriostatic effect on all tested strains, and the antimicrobial properties of BPEO nanoemulsion were improved to a certain extent compared with the non-emulsified essential oils. In addition, BPEO nanoemulsion showed effective bacteriostasis against Colletotrichum gloeosporioides and Botryodiplodia theobromae and had moderate bacteriostasis against Staphylococcus aureus and Escherichia coli. Therefore, BPEO nanoemulsions can be used for the low-temperature, pH and NaCl concentration preservation of food and control of bacterial growth. The results of this study had important implications for the future development of natural antibacterial agents and their application in postharvest mango insurance.
Author contribution statement
Yudong Nie: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Contributed reagents, materials, analysis tools or data.
Yonggui Pan: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Yue Jiang, Rong Yuan, Yi Zhu: Performed the experiments; Analyzed and interpreted the data.
Dandan Xu, Zhengke Zhang: Analyzed and interpreted the data; Wrote the paper.
Funding statement
Dr. Yonggui Pan was supported by the National Spark Program——Demonstration and promotion of logistics distribution and preservation technology of main tropical fruits in Hainan [Grant No. 2014GA800001].
Data availability statement
Data will be made available on request.
Declaration of interest's statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Kristiniak S., Harpel J., Breckenridge D.M., Buckle J. Black pepper essential oil to enhance intravenous catheter insertion in patients with poor vein visibility: a controlled study. J. Alternative Compl. Med. 2012;18:1003–1007. doi: 10.1089/acm.2012.0106. [DOI] [PubMed] [Google Scholar]
- 2.Truong Dam Thai V., Ly Thi Minh H., Dong Thi Anh D. Formulation of black pepper (Piper nigrum L.) essential oil nano-emulsion via phase inversion temperature method. Food Sci. Nutr. 2020;8:1741–1752. doi: 10.1002/fsn3.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amalraj A., Haponiuk J.T., Thomas S., Gopi S. Preparation, characterization and antimicrobial activity of polyvinyl alcohol/gum Arabic/chitosan composite films incorporated with black pepper essential oil and ginger essential oil. Int. J. Biol. Macromol. 2020;151:366–375. doi: 10.1016/j.ijbiomac.2020.02.176. [DOI] [PubMed] [Google Scholar]
- 4.Shetta A., Kegere J., Mamdouh W. Comparative study of encapsulated peppermint and green tea essential oils in chitosan nanoparticles: encapsulation, thermal stability, in-vitro release, antioxidant and antibacterial activities. Int. J. Biol. Macromol. 2019;126:731–742. doi: 10.1016/j.ijbiomac.2018.12.161. [DOI] [PubMed] [Google Scholar]
- 5.da Silva B.D., do Rosário D.K.A., Weitz D.A., Conte-Junior C.A. Essential oil nanoemulsions: properties, development, and application in meat and meat products. Trends Food Sci. Technol. 2022;121:1–13. doi: 10.1016/j.tifs.2022.01.026. [DOI] [Google Scholar]
- 6.Kreutz T., Carneiro S.B., Soares K.D., Limberger R.P., Apel M.A., Veiga-Junior V.F., Koester L.S. Aniba canelilla (Kunth) Mez essential oil-loaded nanoemulsion: improved stability of the main constituents and in vitro antichemotactic activity. Ind. Crop. Prod. 2021;171 doi: 10.1016/j.indcrop.2021.113949. [DOI] [Google Scholar]
- 7.Wang Y., Cen C., Chen J., Zhou C., Fu L. Nano-emulsification improves physical properties and bioactivities of litsea cubeba essential oil. LWT--Food Sci. Technol. 2021;137 doi: 10.1016/j.lwt.2020.110361. [DOI] [Google Scholar]
- 8.Zhang J., Wang Y., Pan D.D., Cao J.X., Shao X.F., Chen Y.J., Sun Y.Y., Ou C.R. Effect of black pepper essential oil on the quality of fresh pork during storage. Meat Sci. 2016;117:130–136. doi: 10.1016/j.meatsci.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Chandran J., Nayana N., Roshini N., Nisha P. Oxidative stability, thermal stability and acceptability of coconut oil flavored with essential oils from black pepper and ginger. Journal of Food Science and Technology-Mysore. 2017;54:144–152. doi: 10.1007/s13197-016-2446-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eren H., Turkmen A.S., Aslan A. Explore; New York, N.Y.: 2021. Effect of Topical Application of Black Pepper Essential Oil on Peripheral Intravenous Catheter Insertion: A Randomized Controlled Study. [DOI] [PubMed] [Google Scholar]
- 11.da Silva J.K.R., Silva J.R.A., Nascimento S.B., da Luz S.F.M., Meireles E.N., Alves C.N., Ramos A.R., Maia J.G.S. Antifungal activity and computational study of constituents from piper divaricatum essential oil against Fusarium infection in black pepper. Molecules. 2014;19(11):17926–17942. doi: 10.3390/molecules191117926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han X., Beaumont C., Rodriguez D., Bahr T. Black pepper (Piper nigrum) essential oil demonstrates tissue remodeling and metabolism modulating potential in human cells. Phytother Res. 2018;32:1848–1852. doi: 10.1002/ptr.6110. [DOI] [PubMed] [Google Scholar]
- 13.Heckert Bastos L.P., Vicente J., Correa dos Santos C.H., de Carvalho M.G., Garcia-Rojas E.E. Encapsulation of black pepper (Piper nigrum L.) essential oil with gelatin and sodium alginate by complex coacervation. Food Hydrocolloids. 2020;102 doi: 10.1016/j.foodhyd.2019.105605. [DOI] [PubMed] [Google Scholar]
- 14.Kapoor I.P.S., Singh B., Singh S., Singh G. Essential oil and oleoresins of black pepper as natural food preservatives for orange juice. J. Food Process. Preserv. 2014;38:146–152. doi: 10.1111/j.1745-4549.2012.00756.x. [DOI] [Google Scholar]
- 15.Kumoro A.C., Hasan M., Singh H. The Pacific zonal asymmetry and its influence on Southern Hemisphere sea ice variability. Arabian J. Sci. Eng. 2010;35:7–16. doi: 10.1017/S0954102010000283. [DOI] [Google Scholar]
- 16.Misharina T.A. Antiradical properties of essential oils and extracts from coriander, cardamom, white, red, and black peppers. Appl. Biochem. Microbiol. 2016;52:79–86. doi: 10.1134/S0003683816010087. [DOI] [Google Scholar]
- 17.Nikolic M., Stojkovic D., Glamoclija J., Ciric A., Markovic T., Smiljkovic M., Sokovic M. Could essential oils of green and black pepper be used as food preservatives? Journal of Food Science and Technology-Mysore. 2015;52:6565–6573. doi: 10.1007/s13197-015-1792-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y., Wang L., Tan J., Li R., Jiang Z.-T., Tang S.-H. Comparative analysis of intracellular and in vitro antioxidant activities of essential oil from white and black pepper (piper nigrum L.) Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.680754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu G.-W., Cheng Q., Nie J., Wang P., Wang X.-J., Li Z.-G., Lee M.-R. DES-based microwave hydrodistillation coupled with GC-MS for analysis of essential oil from black pepper (Piper nigrum) and white pepper. Anal. Methods. 2017;9:6777–6784. doi: 10.1039/C7AY02072D. [DOI] [Google Scholar]
- 20.Wang Y., Jiang Z.-T., Li R. Composition comparison of essential oils extracted by hydrodistillation and microwave-assisted hydrodistillation from black pepper (piper nigrum L.) grown in China. Journal of Essential Oil Bearing Plants. 2009;12:374–380. doi: 10.1080/0972060X.2009.10643734. [DOI] [Google Scholar]
- 21.Shafiq S., Shakeel F., Talegaonkar S., Ahmad F.J., Khar R.K., Ali M. Development and bioavailability assessment of ramipril nanoemulsion formulation. J. European Journal of Pharmaceutics and Biopharmaceutics. 2007;66(2):227–243. doi: 10.1016/j.ejpb.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Hien L.T.M., Dao D.T.A. Black pepper essential oil nanoemulsions formulation using EPI and PIT methods. J. Food Process. Preserv. 2021;45:3. doi: 10.1111/jfpp.15216. [DOI] [Google Scholar]
- 23.Zhang Y., Bai C., Shi W., Alvarez-Manzo H., Zhang Y. Identification of essential oils including garlic oil and black pepper oil with high activity against babesia duncani. Pathogens. 2020;9:446. doi: 10.3390/pathogens9060466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ying Z., Ruiqi S., Wenting Z., Guang-Long Y., Jian C. Antibacterial activity of tea saponin from Camellia oleifera shell by novel extraction method. Ind. Crop. Prod. 2020;153(1) doi: 10.1016/j.indcrop.2020.112604. [DOI] [Google Scholar]
- 25.Augustine Amalraj, Józef T., Haponiuk, Thomas Sabu, Sreeraj Gopi. Preparation, characterization and antimicrobial activity of polyvinyl alcohol/gum Arabic/chitosan composite films incorporated with black pepper essential oil and ginger essential oil. Int. J. Biol. Macromol. 2020;151(15):366–375. doi: 10.1016/j.ijbiomac.2020.02.176. [DOI] [PubMed] [Google Scholar]
- 26.McClements D.J., Rao J. Food-grade nanoemulsions: formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit. Rev. Food Sci. Nutr. 2011;51:285–330. doi: 10.1080/10408398.2011.559558. [DOI] [PubMed] [Google Scholar]
- 27.Li P.-H., Chiang B.-H. Process optimization and stability of D-limonene-in-water nanoemulsions prepared by ultrasonic emulsification using response surface methodology. Ultrason. Sonochem. 2012;19:192–197. doi: 10.1016/j.ultsonch.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 28.Li Y., Zhang Z., Yuan Q., Liang H., Vriesekoop F. Process optimization and stability of D-limonene nanoemulsions prepared by catastrophic phase inversion method. J. Food Eng. 2013;119:419–424. doi: 10.1016/j.jfoodeng.2013.06.001. [DOI] [Google Scholar]
- 29.Dinh P.N., Cam H., Quoc T.P. Comparison of essential oil extracted from black pepper by using various distillation methods in laboratory scale. IOP Conf. Ser. Mater. Sci. Eng. 2020;991 doi: 10.1088/1757-899X/991/1/012050. [DOI] [Google Scholar]
- 30.Thien Hien T., Le Ke H., Duy Chinh N., Tan Phat D., Le Thi Hong N., Dai Hai N., Trinh Duy N., Vo D.-V.N., Quoc Toan T., Long Giang B. The study on extraction process and analysis of components in essential oils of black pepper (piper nigrum L.) seeds harvested in gia lai Province, vietnam. Processes. 2019;7:56. doi: 10.3390/pr7020056. [DOI] [Google Scholar]
- 31.Ghosh S., Kumar A., Sachan N., Chandra P. Anxiolytic and antidepressant-like effects of essential oil from the fruits of Piper nigrum Linn. (Black pepper) in mice: involvement of serotonergic but not GABAergic transmission system. Heliyon. 2021;7 doi: 10.1016/j.heliyon.2021.e06884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heckert Bastos L.P., Costa B.d.S., Siqueira R.P., Garcia-Rojas E.E. Complex coacervates of beta-lactoglobulin/sodium alginate for the microencapsulation of black pepper (Piper nigrum L.) essential oil: simulated gastrointestinal conditions and modeling release kinetics. Int. J. Biol. Macromol. 2020;160:861–870. doi: 10.1016/j.ijbiomac.2020.05.265. [DOI] [PubMed] [Google Scholar]
- 33.Tomas N., Myszka K., Wolko L., Nuc K., Szwengiel A., Grygier A., Majcher M. Effect of black pepper essential oil on quorum sensing and efflux pump systems in the fish-borne spoiler Pseudomonas psychrophila KM02 identified by RNA-seq, RT-qPCR and molecular docking analyses. Food Control. 2021;130 doi: 10.1016/j.foodcont.2021.108284. [DOI] [Google Scholar]
- 34.Zhang Jing, Ye Ke-Ping, Zhang Xin, Pan Dao-Dong, Sun Yang-Ying, Jin-Xuan Cao. Antibacterial activity and mechanism of action of black pepper essential oil on meat-borne Escherichia coli. Front. Microbiol. 2017;7:2094. doi: 10.3389/fmicb.2016.02094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeena K., Liju V.B., Umadevi N.P., Kuttan R. Antioxidant, anti-inflammatory and antinociceptive properties of black pepper essential oil (piper nigrum linn) Journal of Essential Oil Bearing Plants. 2014;17(1):1–12. doi: 10.1016/j.foodhyd.2019.105605. [DOI] [Google Scholar]
- 36.Morsy N.F.S., Abd El-Salam E.A. Antimicrobial and antiproliferative activities of black pepper (piper nigrum L.) essential oil and oleoresin. Journal of Essential Oil Bearing Plants. 2017;20:779–790. doi: 10.1080/0972060X.2017.1341342. [DOI] [Google Scholar]
- 37.Zhang C., Zhao J., Famous E., Pan S., Peng X., Tian J. Antioxidant, hepatoprotective and antifungal activities of black pepper (Piper nigrum L.) essential oil. Food Chem. 2021;346 doi: 10.1016/j.foodchem.2020.128845. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.