Abstract
Objectives
To elucidate antibody responses after the second and third dose of COVID-19 vaccine in patients with inflammatory rheumatic diseases (IRD) treated with biologic/targeted disease modifying anti-rheumatic drugs (b/ts DMARDs).
Methods
Antibody levels to antigens representing spike full length protein and spike S1 were measured before vaccination, 2–12 weeks after the second dose, before and after the third dose using multiplex bead-based serology assay. Positive antibody response was defined as antibody levels over cut off (seropositivity) in seronegative individuals or ≥ 4-fold increase in antibodies in individuals seropositive for both spike proteins.
Results
Patients (n = 414) receiving b/ts DMARDs (283 had arthritis, 75 systemic vasculitis and 56 other autoimmune diseases) and controls (n = 61) from five Swedish regions participated. Treatments groups were: rituximab (n = 145); abatacept (n = 22); Interleukin 6 receptor inhibitors [IL6i (n = 79)]; JAnus Kinase Inhibitors [JAKi (n = 58)], Tumour Necrosis Factor inhibitor [TNFi (n = 68)] and Interleukin12/23/17 inhibitors [IL12/23/17i (n = 42)]. Percentage of patients with positive antibody response after two doses was significantly lower in rituximab (33,8%) and abatacept (40,9%) (p < 0,001) but not in IL12/23/17i, TNFi or JAKi groups compared to controls (80,3%). Higher age, rituximab treatment and shorter time between last rituximab course and vaccination predicted impaired antibody response. Antibody levels collected 21–40 weeks after second dose decreased significantly (IL6i: p = 0,02; other groups: p < 0,001) compared to levels at 2–12 week but most participants remained seropositive. Proportion of patients with positive antibody response increased after third dose but was still significantly lower in rituximab (p < 0,001).
Conclusions
Older individuals and patients on maintenance rituximab have an impaired response after two doses of COVID-19 vaccine which improves if the time between last rituximab course and vaccination extends and also after an additional vaccine dose. Rituximab patients should be prioritized for booster vaccine doses. TNFi, JAKi and IL12/23/17i does not diminished humoral response to primary and an additional vaccination.
Keywords: COVID-19 vaccine, Antibody response, Immunogenicity, Inflammatory rheumatic disease, Rituximab, Abatacept, Methotrexate
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic has caused catastrophic effects worldwide and causes great challenges to healthcare. Patients with inflammatory rheumatic diseases (IRD) show an aberrant immune response, and increased occurrence of infections and many of them are receiving drugs interfering with the immune system [[1], [2], [3]]. Early during the pandemic, concerns were raised regarding increased susceptibility to SARS-CoV-2 disease called COVID-19 and presumptive harmful effects on underlying chronic conditions [[4], [5]]. Having an IRD has been shown to be associated with increased mortality and morbidity after COVID-19 infections at least in the Swedish setting [[6], [7]], whereas different effects have been reported from use of immunosuppressive drugs on morbidity and mortality in COVID-19 infections. Thus, Bower at al reported no effect on morbidity and mortality or increased hospitalisation from use of different anti-rheumatic drugs [[6], [7]] whereas other studies have reported more serious disease, and deaths among IRD patients treated with steroids (prednisone) in doses > 10 mg daily and with B cells depleting therapy (rituximab) [[8], [9]]. There is consequently a special need to diminish risk for severe COVID-19 infections in IRD patients by vaccination and other measures irrespective of actual treatment.
Vaccination is an effective measure to prevent serious infections. After the licensure of the first vaccine against COVID-19 in late 2020, three vaccines using two different platforms (two mRNA vaccine and adenovirus vector vaccines) became available in Sweden during 2021 [[10], [11], [12]]. The safety and immunogenicity of these vaccines with development of antibodies against SARS-CoV-2 virus and protective immunity has been demonstrated in numerous studies [[10], [11], [12]]. However, immunosuppressed patients known to have a diminished antibody response to some vaccines and thereby potentially insufficient protection against infection, were not included in these pre-licensure studies [[10], [11], [12]]. Our group and others previously reported that disease modifying anti-rheumatic drugs (DMARDs) such as methotrexate (MTX), B cells depleting therapy (rituximab) and T cells co-stimulation inhibition (abatacept) are associated with impaired antibody response following common vaccinations (such as against pneumococci or influenza) while other biological treatments interleukin-6 receptor inhibitors (IL6i) or interleukin IL17/23-ihibitors (IL17/23i) did not exerted a profound negative effect on the vaccine response [[13], [14], [15]]. Consequently, the impact of different immunomodulating anti-rheumatic drugs on the immunogenicity of each vaccine needs to be investigated. Recent studies and a meta-analysis concluded that seroconversion rates after vaccination against COVID-19 were lowered in patients with immune mediated inflammatory disease and response rates were attenuated further in the patients treated with rituximab or abatacept [[16], [17], [18], [19]].
Our aims hereby were to investigate: 1) if any of biologic or targeted synthetic DMARD (b/tsDMARD) treatment given in monotherapy or in combination with conventional synthetic DMARDs (csDMARDs) impairs antibody response to two doses of COVID-19 vaccine in patients with IRD compared to healthy controls without immunosuppressive treatment 2) possible predictors of impaired antibody response 3) the persistence of antibody response up to 6 months after two vaccine doses 4) the immunogenicity of the third (booster) vaccine dose in these patients.
2. Methods
The present study was conducted at five University Rheumatology departments in five different regions (Region Västerbotten, Region Stockholm, Region Östergötland, Västra Götalandsregionen and Region Skåne) across Sweden.
2.1. Patient population
Patients with IRD with regular follow up at the 5 rheumatology departments in Sweden who were treated with predefined b/tsDMARDs participated. Patients were consecutively offered to participate in the study at their regular follow up visit at out-patient clinics. Very few patients decline participation (n = 10), mainly those living far away from the department or patients with a need of assistance due to impaired physical mobility. Patients with following b/tsDMARDs therapies were included: B cells depleting therapy (rituximab), JAnus Kinase inhibitors (JAKi: tofacitinib, baricitinib, upadacitinib), tumour necrosis factor inhibitors (TNFi: infliximab, adalimumab, etanercept including the biosimilars of these, certolizumab pegol, golimumab), IL6i (tocilizumab, sarilumab), T cells co-stimulation inhibitor (abatacept), IL12/23i (ustekinumab) and IL17i (secukinumab, ixekizumab). These treatments were administrated as monotherapy or in combination with other csDMARDs (MTX, sulfasalazine, hydroxychloroquine, azathioprine, leflunomide or mycophenolate mofetil) or oral prednisolone. Following rheumatic diseases were represented: rheumatoid arthritis (RA), spondylarthritis including psoriatic arthritis, juvenile arthritis (JIA) and other arthritides, patients with systemic vasculitis including anti-neutrophil cytoplasmic autoantibody (ANCA) associated systemic vasculitis and giant cells arteritis as well as patients with other inflammatory diseases (such as systemic lupus erythematosus (SLE), myositis, mixed connective tissue disease) (Table 1 ). Controls included adults without any rheumatic disease and who were not treated with immunosuppressing drugs for any other condition. The control group consisted mainly of the employees at the rheumatology departments, their acquaints and friends and patientś acquaints and relatives who chose to participate in the study.
Table 1.
The disease and treatment characteristics before the first dose of COVID-19 vaccine.
|
Patients n = 414 |
Controls (n = 61) | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatment groups | Patients with IRD (n = 414) |
Rituximab (n = 145) |
TNFi (n = 68) | IL6i (n = 79) | Abatacept (n = 22) |
IL12/23/17i (n = 42) |
JAKi (n = 58) | Controls |
| Age (median, range years) | 57 (21–85) | 65 (31–85) | 44 (21–65) | 63 (24–82) | 63 (22–82) | 52 (22–81) | 53 (23–77) | 49 (26–74) * |
| Females (n, %) | 284(68.5) | 94 (64.8) | 44 (65.7) | 58 (72.5) | 18 (78.3) | 24 (57.1) | 46 (80.7) | 45 (73.8) * |
| Ever smokers (former, current) (n, %) | 214 (51.7) | 87 (60.0) | 18 (26.9) | 45 (56.3) | 13 (56.5) | 17 (40.5) | 34 (59.6) | 13 (21.3) * |
| Disease duration (median, range years) | 16.8 (1–60) | 14.9 (1–49) | 14.1 (1–36) | 15.9 (1–60) | 24.6 (9–43) | 16.4 (1–42) | 15.4 (1–42) | – |
| Any comorbidity (n, %) | 318 (76.8) | 129 (89) | 33 (49.3) | 64 (80.0) | 19 (82.6) | 35 (83.3) | 38 (66.7) | 21 (34.4) * |
| Diabetes mellitus (n, %) | 34 (8.2) | 19 (13.1) | 2 (3.0) | 5 (6.3) | 1 (4.3) | 4 (9.5) | 3 (5.3) | 1 (1.6) |
| Hypertension (n, %) | 103 (24.9) | 44 (30.3) | 8 (11.9) | 24 (30.0) | 6 (26.1) | 10 (23.8) | 11 (19.3) | 6 (9.8) |
| Chronic obstructive lung disease (n, %) | 38 (9.2) | 15 (10.3) | 6 (9.0) | 5 (6.3) | 4 (17.4) | 2 (4.8) | 6 (10.5) | 3 (4.9) |
| Glucocorticoids (n, %) | 144 (34.8) | 71 (49.0) | 7 (10.4) | 31 (38.8) | 12 (52.2) | 2 (4.8) | 21 (36.8) | – |
| Prednisolone > 7.5 mg/d (n, %) | 20(4.8) | 13 (9.0) | 1 (1.5) | 3 (3.8) | 1 (4.3) | 0 | 2 (3.5) | – |
| csDMARD, any (n, %) | 181 (43.7) | 64 (44.1) | 34 (50.7) | 35 (43.8) | 14 (60.9) | 11 (26.2) | 23 (40.4) | – |
| Methotrexate (n, %) | 136 (32.9) | 46 (31.7) | 25 (37.3) | 28 (35.0) | 11 (47.8) | 8 (19.0) | 18 (31.6) | – |
| Sulfasalazin(n, %) | 16 (3.9) | 2 (1.4) | 6 (9.0) | 3 (3.8) | 1 (4.3) | 1 (2.4) | 3 (5.3) | – |
| Hydroxychloroquine (n, %) | 22 (5.3) | 12 (8.3) | 3 (4.8) | 3 (3.8) | 1 (4.3) | 2 (4.8) | 1 (1.8) | – |
| Other (n, %) | 16 (3.9) | 11 (7.6) | 2 (3.0) | 2 (2.5) | 1 (4.3) | 0 | 1 (1.8) | – |
| Type of vaccine | ||||||||
| mRNA both doses (n, %) | 371 (89.6) | 126 (82.3) | 67 (100) | 65 (81.3) | 19 (82.6) | 40 (95.2) | 54 (94.7) | 49 (80.3) |
| Adenovector virus vaccine two doses (n, %) | 39 (9.4) | 17 (11.7) | 0 | 14 (17.5) | 4 (17.4) | 2 (4.8) | 2 (3.5) | 5 (8.2) |
| Combination of a mRNA and a vector vaccine dose (n, %) | 4 (1.0) | 2 (1.4) | 0 | 1 (1.3) | 0 | 0 | 1 (1.8) | 7 (11.5) * |
| COVID-19 infection before vaccination#(yes, %) | 32 (7.7) | 4 (2.8) | 4 (6.0) | 4 (5.0) | 2 (8.7) | 9 (21.4) | 9 (15.8) | 14 (23.0) |
TNFi = tumour necrosis factor inhibitors, csDMARD = conventional synthetic anti-rheumatic drugs, IL6i = interleukin 6 receptor inhibitors; IL12/23/17i = interleukin 12/23 inhibitors and IL17 inhibitors; JAKi = Janus Kinase inhibitors.
Significant difference between patients with IRD and controls.
Symptoms and positive SARS-CoV-2 RNA in nasopharynx secrete.
2.2. Vaccination
Vaccination against COVID-19 was performed according to the Swedish national vaccination program during 2021. Three different COVID-19 vaccines were used (one adenovirus vector vaccine (ChAdOx1 nCoV-19, AstraZeneca) and two mRNA vaccines (BNT162b2, Pfizer-BioNtech and CX-024414, Moderna). The second vaccination was performed using either the same vaccine type at the first vaccine dose or a combination of the two vaccine types according to recommendations from the Swedish authorities [20]. The second vaccine dose was administrated approximately 12 weeks after the first dose of the adenovirus vector vaccine and after 3 weeks after a dose of mRNA vaccine. Only mRNA vaccines were used as a third vaccine dose [20].
2.3. Data collection
At inclusion i.e. before the first vaccine dose, demographic data, diagnose, time for disease onset, previous and current anti-rheumatic treatments, smoking status, co-morbidities, physiciańs assessment of activity scores using 28- tender and swollen joint count and ESR (DAS28) (in patients with arthritis) or patient́s own assessment of the activity in the underlying rheumatic disease and physical function measured by health assessment questionnaire (HAQ) was collected. Patients were instructed to take a notice of possible side-effects or other unexpected reactions after each vaccine dose as well as the possible effects of vaccination on their rheumatic disease. Patient reported outcomes (PRO) such as pain and patient assessment of diseases activity was also collected in the Swedish Rheumatology Registry (SRQ) [21] which is a part of the routine clinical praxis in Sweden. At the same time point routine blood samples [blood cell count, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), creatinine] were analysed. Patients were scheduled for a second visit, 2–12 weeks after vaccination when postvaccination blood samples were taken, the changes in current anti-rheumatic treatments, data on the date and type of vaccine as well as the possible side effects of the received vaccines were registered. The Swedish authorities recommended the third (booster) vaccine dose to all adults, administrated at least 6 months after the second one. All participants in the study were offered an optional follow up visit scheduled before the third dose. The final blood samples were collected up to 12 weeks after the third (booster) vaccine dose.
2.4. Analysis of antibody response
IgG antibody levels to two spike antigens (spike full length protein and spike S1) and a nucleocapsid C-terminal fragment (used to detect previously COVID-19 infected individuals) were measured in pre- and postvaccination sera using multiplex bead-based serology assay. The panel of 3 antigens was selected after evaluating more than 100 SARS-CoV-2 antigen representations (i.e. whole proteins, protein domains and peptides) (assay performance of 99.7 % sensitivity and 100 % specificity) [22]. Briefly, the full-length Spike, S1 and the nucleocapsid C-terminal fragment were coupled to color coded magnetic beads (MagPlex, Luminex Corp., Austin, TX, USA), each to a different color-code, and the beads mixed to generate a multiplex suspension bead array. Samples were diluted 1:50 in assay buffer containing 3 % (w/v) bovine serum albumin (BSA), 5 % (w/v) skim milk powder in PBS-T (0.05 % Tween20) and incubated with the array. IgG binding to the spike full length, S1, and nucleocapsid were detected by using a phycoerythrin-conjugated anti-human IgG antibody (H10104, Invitrogen, Waltham, MA, USA). The assay readout was performed by using the FLEXMAP 3D® instrument (Luminex Corp., Austin, TX, USA). An antigen and assay specific cut-off was calculated including in each assay run the same set of 12 pre-pandemic samples selected as the best combination to represent the background distribution among more than 2000 pre-pandemic samples tested during assay development phase [22]. The cut-off for spike full length and S1 was calculated as the mean of the signal intensity of the 12 pre-pandemic samples + 6SD, while for nucleocapsid was calculated as the mean of the signal intensity of the 12 controls + 12SD. Samples showing signals passing the cut-off were defined as seropositive for antibodies towards the specific antigen. Samples showing signals above the cut-off for both spike full length and S1 were defined as seropositivity in individuals with antibody levels below cut off before the first vaccine dose. Positive antibody response was defined as postvaccination antibody levels over cut off (seropositivity) for both spike protein antigens in previously seronegative individuals or ≥ 4-fold increase in prevaccination antibody levels in individuals already seropositive for both spike protein antigens (seroconversion).
2.5. Statistics
Based on available information on the response to various (non-COVID-19) vaccines in individuals with IRD we estimate the percentage of individuals reaching seroconversion for COVID-19 vaccines to be approximately 75 %, compared to the estimated 95 % among immunocompetent controls. To detect a 20 %-unit difference between any of treatment group and controls with 80 % statistical power and a 0.05 significance, 62 participants/treatment group needed to be included.
Percentage (%) of individuals with positive antibody response (responders) was calculated in each treatment group and in controls. In addition, percentage responders among individuals vaccinated with different vaccinations strategies (2 doses of mRNA vaccines vs 2 doses of vector vaccines vs a combination of two vaccine types) irrespective of anti-rheumatic treatment were calculated. For the purpose of comparing patients and controls overall, Mann-Whitney tests were used for numeric and chi-square (Chi2) tests for categorical variables. Predictors of antibody response among all patients were determined using binary logistic regression analysis. All tests were two-sided and statistical significance set at 0,05.
Data analyses were performed using Graph pad prism 9 and SPSS 28.
3. Results
In total, 414 patients and 61 controls participated in the study. Out of these, 283 had RA/JIA/psoriatic arthritis/axial spondylarthritis, 75 had systemic vasculitis and 56 had other autoimmune diseases. Patients with IRD receiving the following treatments were studied: rituximab (n = 145), abatacept (n = 22), IL6i (n = 79), JAKi (n = 58), TNFi (n = 68), IL12/23/IL17i (n = 42) and controls (n = 61). All together 166 (40,1%) patients received b/tsDMARD in combination with csDMARDs. Methotrexate was the most common csDMARD, used by 121 (29,2%) patients followed by hydroxychloroquine being used by 22 (5,3%), sulfasalazine 16 (3,9%) and other cs DMARDs (azathioprine, leflunomide or mycophenolate mofetil) were used in 16 (3,9%) of patients. Concomitant prednisolone was used in 144 (34,8%) patients. Mean prednisolone dose/day was 5,5 mg (range 0–25 mg) (Table 1). In total 19 (3,9%) patients were treated with prednisolone in dose ≥ 10 mg/day.
Briefly, compared to controls, IRD patients were significantly older (mean age 57 vs 49 years), had lower proportion females (69 % vs 74 %), had higher proportion ever smokers (52 % vs 21 %), and had more comorbidities at inclusion (77 % vs 34 %).
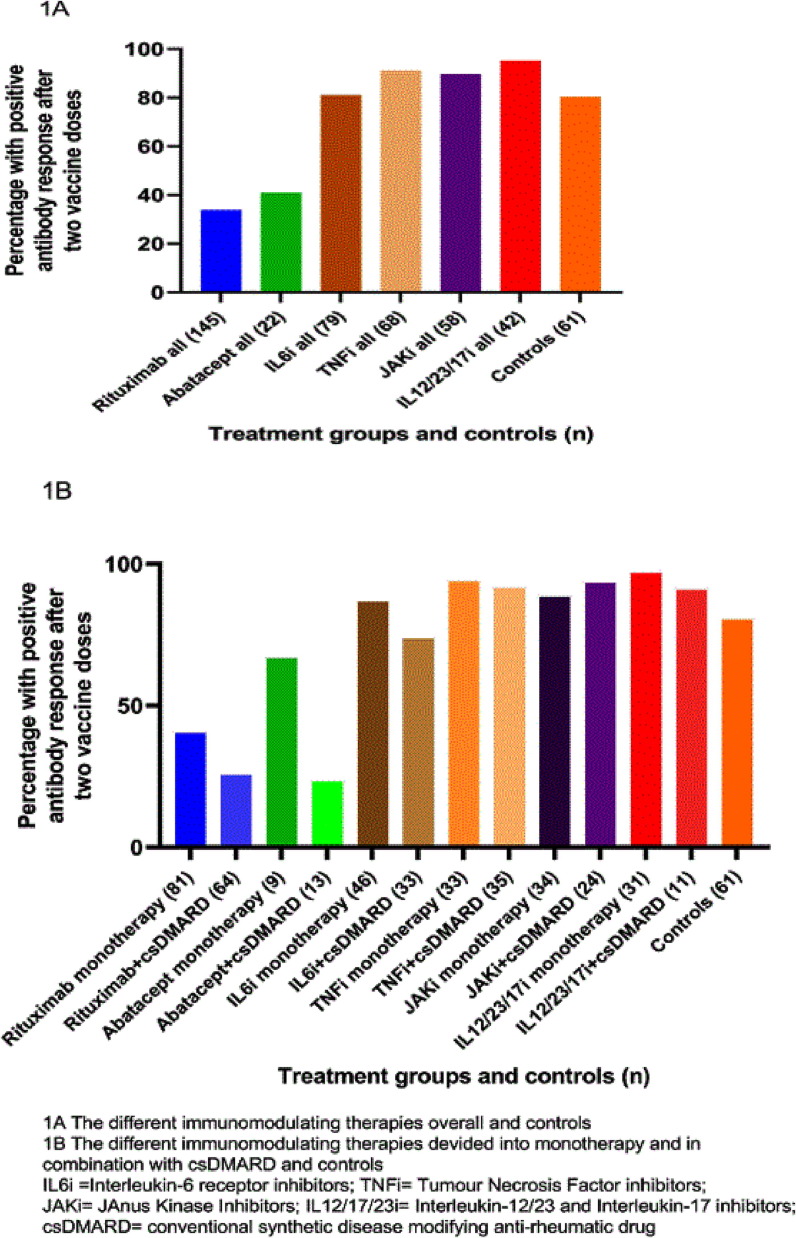
In total 14 (4 %) patients and 28 controls (46 %) had antibody levels over the cut off (were seropositive) for at least 2 antigens tested before vaccination. Percentage of patients with positive antibody response after two doses was significantly lower in rituximab (33,8%) and abatacept (40,9%) (p < 0,001) but not in IL12/23/17i, TNFi or JAKi groups compared to controls (80,3%). Fig. 1 shows the proportion of participants with positive antibody response defined as postvaccination antibody levels over the cut-off (seropositivity) in seronegative patients or ≥ 4-fold increase in antibody levels in patients with seropositivity for both spike antigens in each treatment group and controls (1A =b/tsDMARD overall and controls; 1B=b/tsDMARD monotherapy or b/tsDMARD+sDMARDs and controls).
Fig. 1.
A and B. Proportion of participants with positive antibody response defined as postvaccination antibody levels over the cut-off (seropositivity) in seronegative patients or ≥ 4-fold increase in antibody levels in patients with seropositivity for both spike antigens in each treatment group (biologic/ts DMARD as monotherapy or biologic/ts DMARD + csDMARD; B Biologic/ts DMARDs all) and controls.
Similar proportion of patients and controls received 2 doses of mRNA vaccines (89 % vs 80 %), 2 doses of adenovirus vector vaccine (10 % vs 8 %) but combination of both vaccines were more often given to controls (1 % and 11 %, respectively). A higher antibody response rate was seen for individuals receiving mRNA vaccines in comparison with the vector vaccine and the combination of vaccine types although the individuals in these groups were fewer, especially in the group immunized with a combination of both vaccine types (p < 0,0001).
3.1. Predictor analysis of positive antibody response after two vaccine doses
After 2 vaccine doses and compared to controls, patients with IRD as a group had an impaired antibody response [p = 0,034; Odds ratio (OR) 0,49 (95 % CI 0,25–0,95; unadjusted logistic regression analysis, Table 2 )]. Compared to controls, rituximab treatment, abatacept and concomitant csDMARDs, concomitant MTX, higher age, concomitant prednisolone, higher daily prednisolone dose, current or previous smoking, systemic vasculitis diagnosis, having any comorbidity at vaccination, lower HAQ, but not IL6r, TNFi or JAKi were each associated with the impaired antibody response in the unadjusted logistic regression analysis (Table 2).
Table 2.
Predictors of antibody response after two doses of COVID-19 vaccine, defined as postvaccination antibody levels over the cut-off (seropositivity) in seronegative patients or ≥ 4-fold increase in antibody levels in patients with seropositivity for both spike protein antigens. All 414 patients with inflammatory rheumatic diseases and 61 controls are included in the analysis.
|
A. Crude (unadjusted) logistic regression analysis (patients vs controls) | ||||
|---|---|---|---|---|
| B | p-value | Odds Ration (OR) | 95 % CI | |
| Patients (yes) /controls (no) | −0.72 | 0.034 | 0.49 | 0.25–0.95 |
|
Treatment groups vs controls: Rituximab Abatacept IL6i JAKi TNFi IL12/23/17i |
−2.08 −1.78 0.04 0.75 0.92 1.59 |
<0.001 <0.001 <0.001 0.919 0.162 0.089 0.045 |
0.13 0.17 1.04 2.12 2.49 4.90 |
0.06–0.26 0.06–0.49 0.45–2.43 0.74–6.09 0.87–7.12 1.04–2.17 |
| Age (years) | −0.06 | <0.001 | 0.95 | 0.93–0.96 |
| Sex (female/male) | 0.31 | 0.147 | 1.36 | 0.90–2.10 |
| Comorbidity (yes/no) | −1.33 | <0.001 | 0.26 | 0.16–0.45 |
| Smoking (ever/never) | −0.51 | <0.015 | 0.60 | 0.40–0.91 |
| Methotrexate at vaccination (yes/no) | −0.76 | 0.001 | 0.48 | 0.32–0.74 |
| csDMARD at vaccination (yes/no) | −0.72 | <0.001 | 0.49 | 0.33–0.73 |
| Prednisolone mg/day | −0.11 | <0.001 | 0.90 | 0.85–0.95 |
| Disease activity score (DAS28) 0–10 | −0.03 | 0.903 | 0.97 | 0.60–1.57 |
| Health assessment questionnaire (HAQ) 0–3 | −0.41 | 0.015 | 0.66 | 0.48–0.92 |
| B. Adjusted binary logistic regression analysis (patients vs controls)* | ||||
| Treatment groups vs controls: | <0.001 | |||
| Rituximab | −1.61 | 0.033 | 0.20 | 0.05–0.88 |
| Abatacept | −1.24 | 0.171 | 0.29 | 0.05–1.70 |
| IL6i | 0.64 | 0.433 | 1.90 | 0.38–9.46 |
| JAKi | 0.67 | 0.457 | 1.96 | 0.33–11.44 |
| TNFi | 1.17 | 0.254 | 3.23 | 0.43–24.21 |
| IL12/23/17i | 1.45 | 0.164 | 4.26 | 0.55–32.77 |
| Age (years) | −0.04 | 0.001 | 0.96 | 0.94–0.98 |
| Sex (female/male) | −0.10 | 0.750 | 0.90 | 0.48–1.71 |
| csDMARD (yes/no) | −0.74 | 0.016 | 0.48 | 0.26–0.87 |
| Prednisolone (mg/day) | −0.04 | 0.263 | 0.96 | 0.88–1.04 |
| Smoking (ever/never) | 0.08 | 0.805 | 1.08 | 0.59–1.97 |
| Comorbidity (yes/no) | −0.37 | 0.427 | 0.69 | 0.28–1.72 |
| Disease duration (years) | 0.00 | 0.99 | 1.0 | 0.97–1.28 |
| HAQ (0–3) | 0.09 | 0.73 | 1.10 | 0.65–1.85 |
IL6i = Interleukin 6 receptor inhibitors; TNFi = Tumour Necrosis Factor inhibitors; JAKi = JAnus Kinase Inhibitors; IL12/23/17 = Interleukin12/23 and Interleukin 17 inhibitors; csDMARD = conventional synthetic disease modifying anti-rheumatic drugs; HAQ = Health assessment questionnaire.
Binary logistic regression analysis adjusted for age, sex, cs DMARD or methotrexate (two separate regression models), prednisolone dose, smoking status, comorbidity, disease duration and health assessment questionnaire (HAQ) before vaccination.
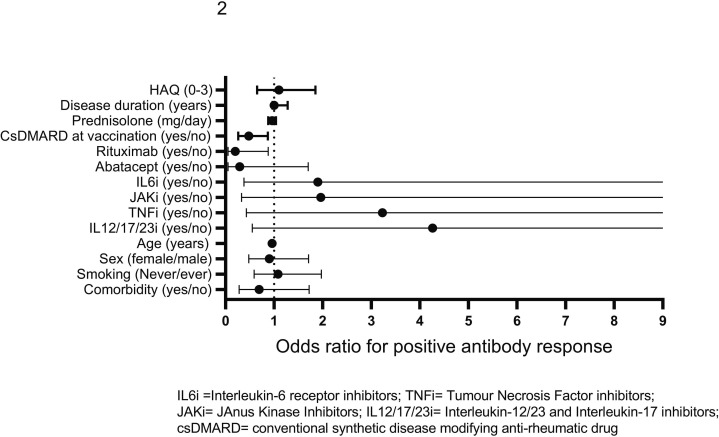
Higher age, rituximab and concomitant csDMARDs treatment remained predictors of impaired antibody response after adjustment in the logistic regression model (Table 2, Fig. 2 ).
Fig. 2.
Predictors of antibody response to two doses of COVID-19 vaccine, defined as postvaccination antibody levels over the cut-off (seropositivity) in seronegative patients or ≥ 4-fold increase in antibody levels in patients with seropositivity for both spike antigens. All 414 patients with inflammatory rheumatic diseases and 61 controls are included in the analysis. Unadjusted and adjusted odds ratios (OR) and 95 % CI are given.
3.1.1. Persistence of antibody response after two vaccine doses
In total, blood samples were collected from 131 individuals who volunteered in the optional visit before the third (booster) vaccination. Patients with following treatment participated: rituximab = 43, TNF-inhibitors = 6, IL6r-inhibitors = 21, abatacept = 4, IL-12/23/IL-17 inhibitors = 3, JAK- inhibitors = 23 and controls = 31. Serum samples were collected after mean (SD) 184 [26] days following the second vaccine among these participants.
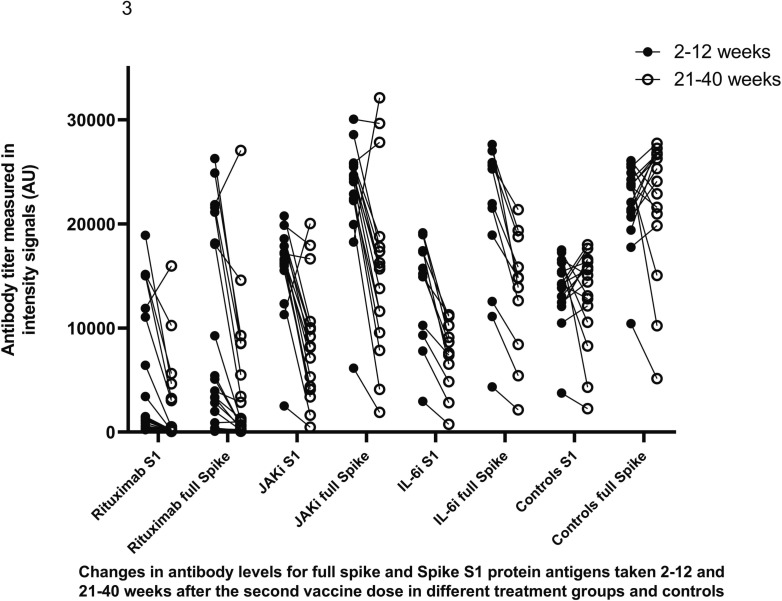
Compared to antibody levels in samples taken 2–12 week after the second vaccine those taken after 21–40 weeks decreased significantly in in groups IL6i and JAKi (p = 0,02). Corresponding comparisons were not performed among other treatment groups due to limited number of patients. However, most participants remained still seropositive (Fig. 3 ).
Fig. 3.
Antibody levels to full spike protein antigen and S1 spike protein in samples collected 2–12 and 21–40 weeks after the second vaccine dose.
In the rituximab group, 15 patients (34.9 %) were seropositive at 2–12 weeks and 14 patients (32.6 %) remained seropositive 21–40 weeks after second vaccine dose. In the IL6i group, 18 patients (85.7 %) were positive at 2–12 weeks and 21 patients (100 %) at 21–40 weeks after the second vaccine dose. All patients with JAKi were seropositive at both time points. One control seroconverted to positive during follow-up.
3.1.2. Antibody response after the third (booster) vaccine dose
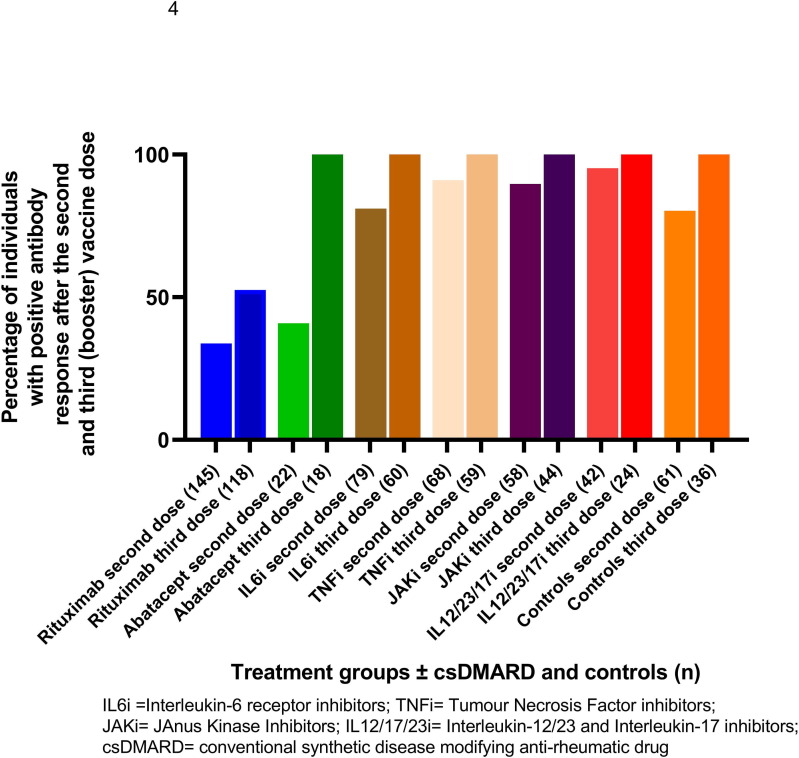
Blood samples were collected in 323 of 414 patients and 36 controls who received three vaccine doses. Patients receiving the following b/tsDMARDs participated: rituximab (n = 118; 68 % female; mean age 67 years), abatacept (n = 18; 72 % female; mean age 64 years), IL6i (n = 60; 73 % female; mean age 64 years), JAKi (n = 44; 80 % female, mean age 52 years), TNFi (n = 59; 70 % female; mean age 47 years;), IL12/23/17i (n = 24; 46 % female; mean age 54 years). Controls (n = 36) were 75 % female, mean age 51 years. All biological/tsDMARDs were administrated as monotherapy or in combination with cs DMARDs, MTX being the most frequently used csDMARD (32.5 %). Compared to results in samples 2–12 weeks after two vaccine doses, proportion (%) of seropositivity after three vaccine doses increased significantly in groups: rituximab monotherapy and rituximab + csDMARD (p = 0,004 and p = 0,003; respectively), IL6i + DMARD (p = 0,02), and abatacept + DMARD (p = 0,01). However, the proportion of seropositivity after three vaccine doses was still significantly lower in rituximab treated patients (52 %) compared to other treatment groups or controls (p < 0,001) (Fig. 4 ).
Fig. 4.
Proportion (%) of patients in different treatment groups and controls with positive antibody response after the second and third vaccine dose defined as postvaccination antibody levels over the cut-off (seropositivity) in seronegative patients or ≥ 4-fold increase in antibody levels in patients with seropositivity for both spike antigens.
3.1.3. Rituximab treatment
In total, 145 patients were treated with rituximab and of these 82 received rituximab as monotherapy (67 % women; mean age was 66 years and mean disease duration was 13 years). Rituximab monotherapy was more common in patients with systemic vasculitis (60 %) than in patients with RA/JIA (30 %). The large predominance of patients with rituximab treatment in this study may not be representative for the distribution of biological treatment in patients with IRD in Sweden.
Rituximab was given in combination with at least one csDMARD to 63 patients (62 % women; mean age was 66 years and mean disease duration was 17 years. Rituximab in combination with csDMARD was mostly used in RA/JIA (76 %) and only in 9.5 % of systemic vasculitis patients. MTX was the most used csDMARD also among these patients. The last rituximab treatment was administrated intravenously within a mean (range) 193 (23–501) days before the first vaccine dose.
Possible predictors of antibody response among patients treated with rituximab were studied applying binary logistic regression model adjusted for time between last rituximab course and vaccination (days), age, sex, csDMARDs or MTX, prednisolone dose, smoking status, systemic vasculitis diagnosis and rituximab dose (Table 3A ). Only shorter time between the last rituximab treatment and vaccination and higher age remained significant predictors of an impaired antibody response (adjusted logistic regression analysis). When time between the last rituximab treatment and vaccination was divided in 4 categories (≤6 months, 6 to ≤ 9, 9 to ≤ 12 and > 12 months) OR of the positive antibody response increased with the time after the last rituximab treatment and vaccination (Table 3B ).
Table3A.
Predictors of positive antibody response after two doses of COVID-19 vaccine in patients with inflammatory rheumatic diseases treated with rituximab- in relation to time between the last rituximab course and vaccination.
| B | p-value | OR | 95 % CI | |
|---|---|---|---|---|
| Time between last rituximab treatment and vaccination (months) | 0.24 | 0.003 | 1.28 | 1.09–1.50 |
| Age at vaccination (years) | −0.04 | 0.023 | 0.96 | 0.93–0.99 |
| Sex (female/male) | 0.63 | 0.159 | 1.87 | 0.78–4.47 |
| Methotrexate at vaccination (yes/no)* | −0.69 | 0.143 | 0.50 | 0.20–1.26 |
| Rituximab dos (1000 mg vs 500 mg) | −0.42 | 0.337 | 0.65 | 0.28–1.56 |
| Smoking (never/ever) | 0.01 | 0.823 | 1.10 | 0.47–2.57 |
| Diagnosis at vaccination (systemic vasculitis vs others) | −0.22 | 0.659 | 0.81 | 0.31–2.10 |
| Comorbidity (yes/no) | −0.68 | 0.347 | 0.50 | 0.12–2.10 |
Binary logistic regression analysis adjusted for age, sex, methotrexate, prednisolone dose, smoking status, diagnosis (systemic vasculitis), rituximab dose, comorbidity at vaccination.
Concomitant csDMARD (conventional synthetic anti-rheumatic drugs) and methotrexate were tested in two different models.
Table3B.
Predictors of positive antibody response after two doses of COVID-19 vaccine in patients with inflammatory rheumatic diseases treated with rituximab -in relation to time between the last rituximab treatment and vaccination using different time intervals as categories.
| B | p-value | OR | 95 % CI | |
|---|---|---|---|---|
|
Time between last rituximab treatment and vaccination 0–6 months 6–9 months 9–12 months >12 months |
1.40 1.49 2.11 |
0.006 0.002 0.028 0.034 |
4.04 4.42 8.26 |
1.66–9.81 1.18–16.60 1.18–58.10 |
| Age at vaccination (years) | −0.40 | 0.023 | 0.96 | 0.93–0.99 |
| Sex (female/male) | 0.55 | 0.223 | 1.74 | 0.71–4.22 |
| Methotrexate at vaccination (yes/no)* | −0.77 | 0.110 | 0.46 | 0.18–1.19 |
| Prednisolone (mg daily) | −0.10 | 0.119 | 0.90 | 0.79–1.03 |
| Rituximab dos (1000 mg vs 500 mg) | −0.45 | 0.327 | 0.64 | 0.26–1.57 |
| Smoking (never/ever) | 0.14 | 0.755 | 1.15 | 0.49–2.71 |
| Diagnosis at vaccination (systemic vasculitis vs others) | −0.14 | 0.779 | 0.87 | 0.33–2.55 |
| Comorbidity at vaccination(yes/no) | −0.54 | 0.473 | 0.58 | 0.13–2.55 |
Binary logistic regression analysis adjusted for age, sex, methotrexate, prednisolone dose, smoking status, diagnosis (systemic vasculitis), rituximab dose and comorbidity at vaccination.
Concomitant csDMARD (conventional synthetic anti-rheumatic drugs) and methotrexate were tested in two different models.
3.1.4. Vaccination schedules and tolerability of the vaccines
The mean (SD) time interval between the first two doses was 47 [25] days in patients and 65 (73) days in controls. All three vaccines were well tolerated with mostly mild or moderate side-effects corresponding to those reported from the manufacturer or other studies. No cases of vaccine-induced immune thrombotic thrombocytopenia (VITT) or other unexpected reactions were reported. The most prevalent side-effects reported were tenderness at the injection site, fatigue, headache, muscle pain or increased body temperature for a few days.
We did not notice any increased disease activity after vaccination; only fourteen (3.4 %) patients reported an increased activity in their IRD which is likely within the normal variation of disease activity over time in these patients.
4. Discussion
In this large, national study, conducted in five regions across Sweden, we report that ongoing treatment with rituximab significantly impairs the immunogenicity of COVID-19 vaccine in patients with IRD. These findings confirm and extend data from several previous studies and a meta-analysis [[16], [17], [18], [19]].
Results from the adjusted logistic regression analysis shows the odds ratio of satisfactory antibody response increases almost 30 % for each month after the last rituximab treatment. This is in line with results from some other studies, one showing a 20 % response rate six months after rituximab, raising to 60 % after 1 year [[23], [24], [25], [26], [27]].
Interestingly, a large study of MS patients treated with rituximab before being infected with COVID-19 or, in a small group, before vaccination, showed a significant reduction of anti-virus antibody titers [28]. Assessing vaccine response for patients with rituximab may thus be considered together with recommendations of vaccine booster-doses.
Another finding is that treatment with abatacept also decreases antibody response but to a lower extent compared to rituximab which is consistent with results published by Furer et al and Jena et al [[18], [19]]. However, this effect did not persist after the adjustment in the regression analysis indicating that the other patient or treatment characteristics play a more important role in the vaccine response than the mode of action of the drug.
Importantly, we observed a good antibody response in patients using TNFi, IL12/23/IL17i and JAKi.This was in parity with other studies [[19], [29]].
An attenuation in the antibody response was seen for all treatments investigated herein when combined with a csDMARD or prednisolone except for TNF inhibitors, proportion of patients with a positive antibody response rose to 94 %. Although concomitant prednisolone was associated with diminished antibody response in the unadjusted regression analysis, neither the usage of prednisolone nor the daily prednisolone dose predicted antibody response after adjustment in the regressions analysis. Of importance, the patients on prednisolone were mostly on a low prednisolone dose which may explain the diverging results compared to other reports [[17], [18], [19]].
Higher age was a significant negative predictor of vaccine response both in patients (regardless the immunosuppressive treatment) and controls. As for the other vaccines, these finding may be explained by the “immunosenesence” characterised by the weak and less effective immune response in the elderly [30]. As higher age is by far the strongest risk factor for a more severe COVID-19 infection, encouraging vaccination in older patients is extremely important.
A two-dose regime of mRNA COVID-19 vaccines has conferred over 94 % and the adenovirus vaccine 70 % efficacy against COVID-19 infection with a good tolerability in volunteers [[10], [11], [12]]. The mean time interval between the two doses was shorter in patients than in and controls in our study (47 and 65 days, respectively). This difference could be explained by the fact that “risk groups” were prioritized for vaccination in the beginning of the vaccination program in Sweden. For patients with IRD concerns against potential antigenic cross-reactivity between SARS-CoV-2 and human tissue causing autoimmunity or exacerbating already existing diseases have been raised [31]. In this study, for the majority of patients the disease activity remained stable postvaccination, which is in agreement with other studies although long-term follow up data is yet lacking [[14], [15], [16], [17]]. In agreement with former studies the patients herein reported few adverse advents, mostly pain at the injection site, headache, fatigue and chills for less than 3 days [[10], [11], [12], [13]]. No side-effects of special interest for patients with IRD such as herpes zoster, uveitis, pericarditis or VITT occurred.
A lower antibody response rate was seen for patients with systemic vasculitis, which however did not remain after adjustment in the regression analysis. Several studies demonstrated similar rates of seroconversion and antibody titres across autoimmune diseases suggesting that immunosuppressive treatment rather than the autoimmune disease itself, is influencing the immunogenicity to the vaccines [[16], [17], [18], [19]].
This study supports earlier findings that most DMARDs can be continued in relation to the administration of COVID-19 vaccination, except for rituximab where timing of treatment is of high importance to receive as good immunogenicity.
A waning antibody response after 2 vaccine doses has been observed when antibody levels were measured in samples collected approximately 6 months after the second COVID-19 vaccine [[32], [33], [34]]. Since most patients with exception for rituximab and controls maintained positive antibody response for 6 months in our study, the currently recommended 6 months interval between the second and third dose seems reasonable [20]. In addition, we here could confirm that additional (booster) vaccine dose resulted in more patients achieving positive antibody response including the rituximab treated patients. Still, the seropositivity of rituximab treated patients was significantly lower than in other treatment groups and in controls where vast majority of participants achieved sufficient antibody responses. This is in accordance with results from others [[33], [34], [35]].
A strength of this study is the heterogenous patient population with a broad range of autoimmune rheumatic diseases, the prospective design and in most cases sufficiently large treatment with a sufficient statistical power for the comparisons with a control group. The results from analysis of several different treatments in a real-life setting enable generalisability of the results and differentiates our results from many previous studies which only included one diagnosis and one, or few anti-rheumatic treatments.
One disadvantage of the study is that patients and controls were not age and sex matched. To account for this, we performed a logistic regression analysis adjusted for age, sex, comorbidity and smoking status. The analysis confirmed that rituximab, concomitant csDMARDs and higher age were predictors of an impaired antibody response.
Another obvious limitation of the present study is the lack of the data on the T cell response. T cells play an important role in the eradication of the virus and robust CD4 + T response to spike-proteins has been demonstrated in otherwise healthy individuals after vaccination against COVID-19 [35]. In addition, in healthy individuals, these CD4 + T cells were shown to correlate with anti-SARS-CoV-2 IgG antibodies [[36], [37]]. Notably, the large study of effects of COVID-19 infection on T cell responses to SARS-Cov2 spike and nucleocapsid peptides/protein in MS patients treated with rituximab showed a robust T cell response even in the absence of antibody titers over cut-offs which is in line also with another study on sufficient T cell response despite severely impaired antibody responses [[16], [25]]. It will thus be of large interest to investigate T cell response to the virus also in patients with rheumatic disease treated with anti-rheumatic therapies. The final proof of which immune responses on the T and B cell side that provide good protection against infection and severe COVID-19 disease will require further analysis of infection and morbidity rates in relation the outcome of the immunological studies. If a satisfactory T cell response provides a sufficient protection against infection in absence of antibody response remains to be determined. Furthermore, antibody and T cells response are still a surrogate measure for the vaccine response and studies showing the lower prevalence of infections among vaccinated patients compared to non-vaccinated ones are needed.
5. Conclusion
Older individuals and patients with inflammatory rheumatic diseases on maintenance rituximab as monotherapy or in combination with csDMARDs had an impaired responses to two doses of COVID-19 vaccine which improves moderately after an additional vaccine dose and when the time between last rituximab course and vaccination extends as long as possible. Rituximab patients should be encouraged to get the booster vaccine doses. Patients treated TNFi, JAKi or IL12/23/IL17i have a satisfactory humoral response to a primary and an additional vaccine dose.
Declarations
Ethics approval and consent to participate
Ethical approval was obtained from the Swedish national Ethical Review Board nr 2021–00636. The study was registered at EudraCT (European Union Drug Regulating Authorities Clinical Trials Database) with number 2021–000880-63. Oral and written consent was obtained from all patients and controls before inclusion in the study.
Availability of data and material
All data are available from the corresponding author on request.
Funding
This study was supported by grants from the Swedish Rheumatism Association, the Region Östergötland (ALF grants RÖ-940181), Anna-Greta Crafoord foundation, unrestricted research grants from Roche, Wilhelm and Martina Lundgrens science foundation (2021-3774), the Swedish state under the agreement between the Swedish Government and the county councils, the ALF-agreement (ALFGBG-966169).
The funders of the present study had no role in designing or conducting the study, inclusion, or selection of patients. Analysis, and interpretation of the data was performed by the authors as well as preparation of the paper.
Authors' contributions
MCK and LK designed the study. Inclusion of patient and conduction of the study was performed by MF, KC, EK, AS, MCK and SO. MH coordinated the processing of serum samples. EP performed the serological analysis. All authors have contributed to interpretation of the results. MF and MCK wrote the draft of the manuscript and all authors have contributed to writing the final version of the manuscript. The final submitted version was approved by all authors.
Competing interests
MF, PN, LK, AS, EK, AB, MH, EP declare no competing interests related to the present study. MK has received unrestricted research grants from Roche. KC: consultancy fees and speaker’s honoraria from Eli Lilly, Abbvie, Janssen and Pfizer.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Aikaterina Chatzidionysiou reports a relationship with Eli Lilly, Abbvie, Janssen and Pfizer that includes: consulting or advisory and speaking and lecture fees. Meliha Kapetanovic reports a relationship with Roche that includes: funding grants.
Acknowledgments
We thank Marianne Petersson, Anna-Lena Åblad and Lina Wirestam for laboratory work and biobank administration at the Rheumatology unit at Linköping University hospital; research nurse Anita Nihlberg at Skåne University Hospital in Lund; Louise Lundén, research nurse at Karolinska University Hospital in Stockholm; research nurse Åsa Loman at Sahlgrenska University Hospital in Gothenburg; Viktoria von Zweigbergk, research nurse at University Hospital Umeå for recruiting the patients, blood samples drawing and taking care of all administrative tasks in the study. We also thank the Autoimmunity and Serology Profiling Unit at Scilifelab for the serology data.
Data availability
Data will be made available on request.
References
- 1.Gron K.L., Arkema E.V., Glintborg B., Mehnert F., Ostergaard M., Dreyer L., et al. Risk of serious infections in patients with rheumatoid arthritis treated in routine care with abatacept. rituximab and tocilizumab in Denmark and Sweden. Ann Rheum Dis. 2019;78(3):320–327. doi: 10.1136/annrheumdis-2018-214326. [DOI] [PubMed] [Google Scholar]
- 2.Furer V., Rondaan C., van Heijstek M., Assen S., et al. Incidence and prevalence of vaccine preventable infections in adult patients with autoimmune inflammatory rheumatic diseases (AIIRD) RMD Open. 2019;5(2):e001041. doi: 10.1136/rmdopen-2019-001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Aalst M., Lotsch F., Spijker R., van der Meer J.T.M., Langendam M.W., Goorhuis A., et al. Incidence of invasive pneumococcal disease in immunocompromised patients: A systematic review and meta-analysis. Travel Med Infect Dis. 2018;24:89–100. doi: 10.1016/j.tmaid.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 4.Sjowall J. Azharuddin M. Frodlund M. Zhang Y. Sandner L. Dahle C. et al. SARS-CoV-2 Antibody Isotypes in Systemic Lupus Erythematosus Patients Prior to Vaccination: Associations With Disease Activity. Antinuclear Antibodies. and Immunomodulatory Drugs During the First Year of the Pandemic. Front Immunol 2021; 12: 724047. [DOI] [PMC free article] [PubMed]
- 5.Strangfeld A., Schafer M., Gianfrancesco M.A., Lawson-Tovey S., Liew J.W., Ljung L., et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2021;80(7):930–942. doi: 10.1136/annrheumdis-2020-219498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bower H, Frisell T, Di Giuseppe D, Delcoigne B, Ahlenius GM, Baecklund E, et al. Impact of the COVID-19 pandemic on morbidity and mortality in patients with inflammatory joint diseases and in the general population: a nationwide Swedish cohort study. Ann Rheum Dis 2021; 80(8): 1086–93. doi: 10.1136/annrheumdis-2021-219845. [DOI] [PMC free article] [PubMed]
- 7.Bower H, Frisell T, di Giuseppe D, Delcoigne B, Ahlenius GM, Baecklund E, et al. Effects of the COVID-19 pandemic on patients with inflammatory joint diseases in Sweden: from infection severity to impact on care provision. RMD Open 2021; 7(3): e00198. doi: 10.1136/rmdopen-2021-00198Erratum in: RMD Open. 2022 Jan;8(1). [DOI] [PMC free article] [PubMed]
- 8.Akiyama S. Hamdeh S. Micic D. Sakuraba A. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann Rheum Dis. 2020: annrheumdis-2020-218946. doi: 10.1136/annrheumdis-2020-218946. [DOI] [PubMed]
- 9.Grainger R. Kim AHJ. Conway R. Yazdany J. Robinson PC. COVID-19 in people with rheumatic diseases: risks, outcomes, treatment considerations. Nat Rev Rheumatol 2022: 1–14. doi: 10.1038/s41584-022-00755-x. Epub ahead of print. PMID: 35217850; PMCID: PMC8874732. [DOI] [PMC free article] [PubMed]
- 10.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rondaan C., Furer V., Heijstek M.W., Agmon-Levin N., Bijl M., Breedveld F.C., et al. Efficacy. immunogenicity and safety of vaccination in adult patients with autoimmune inflammatory rheumatic diseases: a systematic literature review for the 2019 update of EULAR recommendations. RMD Open. 2019;5(2):e001035. doi: 10.1136/rmdopen-2019-001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hua C., Barnetche T., Combe B., Morel J. Effect of methotrexate. anti-tumor necrosis factor alpha. and rituximab on the immune response to influenza and pneumococcal vaccines in patients with rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2014;66(7):1016–1026. doi: 10.1002/acr.22246. [DOI] [PubMed] [Google Scholar]
- 15.Kapetanovic M.C., Saxne T., Sjoholm A., Truedsson L., Jonsson G., Geborek P. Influence of methotrexate. TNF blockers and prednisolone on antibody responses to pneumococcal polysaccharide vaccine in patients with rheumatoid arthritis. Rheumatology (Oxford) 2006;45(1):106–111. doi: 10.1093/rheumatology/kei193. [DOI] [PubMed] [Google Scholar]
- 16.Prendecki M., Clarke C., Edwards H., McIntyre S., Mortimer P., Gleeson S., et al. Humoral and T-cell responses to SARS-CoV-2 vaccination in patients receiving immunosuppression. Ann Rheum Dis. 2021;80(10):1322–1329. doi: 10.1136/annrheumdis-2021-220626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boekel L., Steenhuis M., Hooijberg F., Besten Y.R., van Kempen Z.L.E., Kummer L.Y., et al. Antibody development after COVID-19 vaccination in patients with autoimmune diseases in the Netherlands: a substudy of data from two prospective cohort studies. Lancet Rheumatol. 2021;3(11):e778–e788. doi: 10.1016/S2665-9913(21)00222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furer V., Eviatar T., Zisman D., Peleg H., Paran D., Levartovsky D., et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80(10):1330–1338. doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]
- 19.Jena A., Mishra S., Deepak P., Kumar M.P., Sharma A., Patel Y.I., et al. Response to SARS-CoV-2 vaccination in immune mediated inflammatory diseases: Systematic review and meta-analysis. Autoimmun Rev. 2021 doi: 10.1016/j.autrev.2021.102927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Public Health Agency Sweden. Folkhälsomyndigheten. Covid-19 — Folkhälsomyndigheten (folkhalsomyndigheten.se). Website visited January 28, 2023.
- 21.The Swedish Rheumatology Quality Register (SRQ): Svensk Reumatologis Kvalitetsregister. https://srq.nu. Website visited January 28, 2023.
- 22.Hober S., Hellström C., Olofsson J., Andersson E., Bergström S., Jernbom Falk A., et al. Systematic evaluation of SARS-CoV-2 antigens enables a highly specific and sensitive multiplex serological COVID-19 assay. Clin Transl Immunology. 2021;10(7):e1312. doi: 10.1002/cti2.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konig M., Lorentzen A.R., Torgauten H.M., Tran T.T., Schikora-Rustad S., Vaage E.B., et al. Humoral immunity to SARS-CoV-2 mRNA vaccination in multiple sclerosis: the relevance of time since last rituximab infusion and first experience from sporadic revaccinations. J Neurol Neurosurg Psychiatry. 2021 doi: 10.1136/jnnp-2021-327612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Troldborg A., Thomsen M.K., Bartels L.E., Andersen J.B., Vils S.R., Mistegaard C.E., et al. Time since rituximab treatment is essential for developing a humoral response to COVID-19 mRNA vaccines in patients with rheumatic diseases. J Rheumatol. 2022 doi: 10.3899/jrheum.211152. jrheum .211152. [DOI] [PubMed] [Google Scholar]
- 25.van der Togt CJT, Ten Cate DF, van den Bemt BJF, Rahamat-Langendoen J, den Broeder N, den Broeder AA. Seroconversion after a third COVID-19 vaccine is affected by rituximab dose but persistence is not in patients with rheumatoid arthritis. Rheumatology (Oxford, England). 2022 Aug 24: keac486. doi: 10.1093/rheumatology/keac486. [DOI] [PMC free article] [PubMed]
- 26.Furer V., Eviatar T., Zisman D., Peleg H., Paran D., Levartovsky D., et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80(10):1330–1338. doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]
- 27.Frey S., Chiang T.P., Connolly C.M., Teles M., Alejo J.L., Boyarsky B.J., et al. Antibody durability 6 months after two doses of SARS-CoV-2 mRNA vaccines in patients with rheumatic and musculoskeletal disease. Lancet Rheumatol. 2022;4(4):e241–e243. doi: 10.1016/S2665-9913(21)00417-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asplund Högelin K, Ruffin N, Pin E, Månberg A, Hober S, Gafvelin G, et al. Development of humoral and cellular immunological memory against SARS-CoV-2 despite B cell depleting treatment in multiple sclerosis. iScience 2021 Sep 24; 24(9): 103078. [DOI] [PMC free article] [PubMed]
- 29.Seror R., Camus M., Salmon J.H., Roux C., Dernis E., Basch A., et al. Do JAK inhibitors affect immune response to COVID-19 vaccination? Data from the MAJIK-SFR Registry. Lancet Rheumatol. 2022;4(1):e8–e11. doi: 10.1016/S2665-9913(21)00314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lynch S.M., Guo G., Gibson D.S., Bjourson A.J., Rai T.S. Role of Senescence and Aging in SARS-CoV-2 Infection and COVID-19 Disease. Cells. 2021;10(12):3367. doi: 10.3390/cells10123367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vojdani A., Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217 doi: 10.1016/j.clim.2020.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dayam R.M., Law J.C., Goetgebuer R.L., Chao G.Y., Abe K.T., Sutton M., et al. Accelerated waning of immunity to SARS-CoV-2 mRNA vaccines in patients with immune mediated inflammatory diseases. JCI Insight. 2022;7(11):e159721. doi: 10.1172/jci.insight.159721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jyssum I., Kared H., Tran T.T., Tveter A.T., Provan S.A., Sexton J., et al. Humoral and cellular immune responses to two and three doses of SARS-CoV-2 vaccines in rituximab-treated patients with rheumatoid arthritis: a prospective, cohort study. Lancet Rheumatol. 2022;4(3):e177–e187. doi: 10.1016/S2665-9913(21)00394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benucci M., Damiani A., Gobbi F.L., Lari B., Grossi V., Infantino M., et al. Role of booster with BNT162b2 mRNA in SARS-CoV-2 vaccination in patients with rheumatoid arthritis. Immunol Res. 2022;70(4):493–500. doi: 10.1007/s12026-022-09283-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schietzel S., Anderegg M., Limacher A., et al. Humoral and cellular immune responses on SARS-CoV-2 vaccines in patients with antiCD20 therapies: a systematic review and meta-analysis of 1342 patients. RMD Open. 2022;8:e002036. doi: 10.1136/rmdopen-2021-002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan A.T., Linster M., Tan C.W., Le Bert N., Chia W.N., Kunasegaran K., et al. Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell Rep. 2021;34(6) doi: 10.1016/j.celrep.2021.108728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell. 2020;181(7):1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available from the corresponding author on request.
Data will be made available on request.