Abstract
Prevention and control of foodborne pathogens are of vital public health importance, and poultry meat is recognized as a major source of Salmonella infection in humans. Therefore, it is necessary to reduce the presence of salmonella in poultry meat. This article provided a systematic review and modeling to assess the effect of various factors on bacteriophages' function on Salmonella spp. Reduction in poultry meat. Twenty-two studies were included based on the inclusion and exclusion criteria mentioned in the methodology. The results showed that each unit increase in bacterial dose, phage dose, and temperature increases the Salmonella reduction by about 7%, 20%, and 1%, respectively. In addition, wild-type phages were more efficient than commercial-type phages, and this result was statistically significant (β = 1.124; p-value <0.001). This multivariate analysis is a helpful tool to predict the role of various factors in the role of phage in reducing Salmonella in poultry meat.
Keywords: Bacteria, Phage, Meat, Biocontrol, Multivariate analysis
1. Introduction
Salmonella is one of the most important foodborne pathogens worldwide and the causative agent of the most common foodborne disease known as non-typhoidal salmonellosis. More than 2500 Salmonella serotypes have been described in the genus of Salmonella enterica. Salmonellosis symptoms are abdominal pain, vomiting, inflammatory diarrhea, headache, and nausea [[1], [2], [3]]. Salmonellosis, caused by non-typhoidal Salmonella spp, is currently the leading foodborne disease of bacterial etiology in the United States, causing approximately 1.35 million cases and 420 deaths annually. According to the Center for Disease Control and Prevention (CDC), Salmonella Enteritidis (S. Enteritidis) is the most commonly reported serotype. Between 2002 and 2017, there were 4265 food poisoning cases in Korea, 332 were related to Salmonella, and after enteropathogenic Escherichia coli and norovirus, Salmonella was the third most common food poisoning [4]. Salmonella is estimated to be responsible for around 85% of foodborne diseases worldwide. In 2007, the United States Department of Agricultural Economic Services (USDA) estimated that the United States had suffered 2.5 million $ in economic losses from 1.4 million cases of Salmonella [2]. In 2015 and 2008, 94,625 and 2551 cases of salmonellosis were reported in Japan and Europe, respectively [5]. In China, 70 salmonellosis outbreaks were reported from 2008 to 2012, resulting in 4151 hospitalizations and four deaths [3,6]. The above cases make clear the importance and impact it has worldwide.
Poultry meat is one of the essential sources of Salmonella infection in humans [4,5,7,8]. The animal intestine is an important reservoir for Salmonella, which can be transferred to meat during manufacturing and slaughtering procedures [4,9]. The spread of Salmonella in poultry meat can cause economic and health damage. With the increasing consumption of these products, there are concerns that Salmonella infectioncould pose a crucial public health risk [10,11]. Therefore, reducing Salmonella in poultry meat is necessary for human health, and prevention methods must be applied appropriately [5,12].
Conventional, physical, and chemical methods of reducing Salmonella can have various side effects [5,13]. Physical processes, such as washing with hot or cold water, freezing, cooling, and ionizing radiation can cause deterioration in the properties of raw meat and consumer satisfaction. Heat treatment changes the color, but radiation oxidizes the fats and changes the organoleptic properties of meat [10,14]. Chemical disinfectants, namely ascorbic acid and calcium carbonate have detrimental effects on texture, taste, and color. Besides, chemical preservatives, including sodium benzoate and benzoic acid can lead to complications, like asthma, hives, and seizures [3,15]. Conventional methods; therefore, do not meet the needs of the consumer, and new techniques have to be developed.
Bacteriophage is a virus with a bacterial host that injects its genetic material into the bacterial cell. After replication and rupture of the host cell, more phages are released [[16], [17], [18]]. The clinical use of phages to treat a wide range of infections began in the early 1920s [19]. Bacteriophages have drawn increased attention as a new approach to combat pathogens because of their advantages, like ubiquitous nature, easy extraction, cost-effectiveness, and safety [[20], [21], [22]]. However, phage cocktails are used to improve phage efficiency and performance, which several studies have shown to be effective [[23], [24], [25]]. Such strategies as combination therapies and genome engineering can further help prevent the spread of phage resistance in the future [19].
Recently, such phages as SalmoFresh® and Salmonelex™ have been identified as Generally Recognized as Safe (GRAS) by the Food and Drug Administration (FDA) and the United States Department of Agriculture (USDA) [26]. Many studies have examined the effects of phage in various foods that have shown phages are used as a biologically safe agent. The first factor in phage successfully is phage stability. Several external factors, namely temperature, pH, aw, and salt concentration can influence the lytic activity of phage [22].
According to various studies evaluating the effect of phage in reducing Salmonella in poultry meat, numerous factors can affect the effectiveness of phage, including temperature, time, phage inoculation method, bacterial dose, and phage dose. Sukumaran et al. (2015), for example, concluded that the dip treatment affect phage performance [8]. Thung et al. (2017) found that at 4 °C, the effect of phages increases slightly over time [27]. Duc et al. (2018) established that with an increase in temperature from 8 °C to 25 °C, phage efficincy increases [5]. Moon et al. stated that with an increase in phage dose, the effect of phages increases, and phages have a greater influence on single bacteria than a bacterial cocktail [11]. Adriana et al. performed a systematic review and meta-analysis on the effects of dietary additives, vaccinations, and processing aids as control measures for Salmonella spp. In chicken meat. They found that the interventions were effective in reducing Salmonella [28]. However, since there is no centralized data on the effect of different factors on phage function, this study aimed to systematically review previously published articles on bacteriophage-mediated Salmonella reduction and to model the effect of various factors on phages function Salmonella reduction in poultry meat.
2. Methods
2.1. Definition and literature search
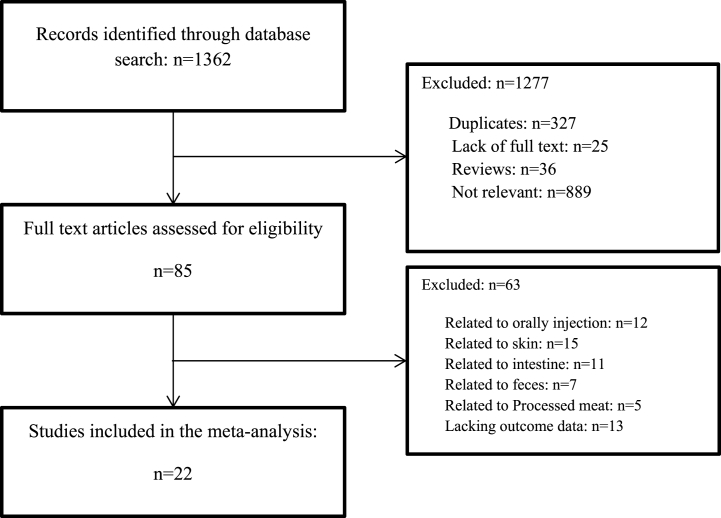
A comprehensive search for the effect of different phage factors on Salmonella reduction in poultry meat carried out using similar keywords in three major global electronic databases, including PubMed, Scopus, and Science Direct. The search was performed by systematic query in title, abstract, and keywords using key terms encompassing the following: Salmonella, phage, bacteriophage, poultry, and chicken. Articles based on their titles, abstracts, and full text included, and papers without the English language in the main text, review articles, and book chapters excluded. Studies on the reduction of Salmonella in poultry meat, articles with phage cocktails, and experimental studies were included; papers that examined the effects of phages with other substances were excluded. Following, full-text screening of the eligible studies was done from the databases. There were relevant articles in which the abstracts were the only text available and required additional efforts to access full text; so, they were excluded (Fig. 1).
Fig. 1.
Flow chart of the systematic literature research.
2.2. Data analysis
All data were entered into a file and analyzed with Stata software (version 13.0). The Salmonella reduction was presented descriptively. Univariate linear regression models were used to predict the regression coefficient for Salmonella reduction. In order to eliminate possible confounding factors, the variables were entered into the multivariate linear regression model with a significance level <0.2. The variance inflation factor (VIF) was used in the multivariate regression model to remove the linearity effect. Therefore, variables with a VIF greater than four were eliminated.
This equation () was used to predict Salmonella reduction. R2 was used as a criterion to select the best model. The significance level was 0.05 for two-sided tests.
3. Results
3.1. Eligible studies and characteristics
The initial search identified 1362 potentially relevant studies, of which 85 studies were further evaluated. Finally, 22 articles were included in this study. A total of 417 independent data were extracted from the 22 articles and entered into the regression analysis. The baseline characteristics of the included studies are shown in Table 1. The results showed that studies on the reduction of Salmonella bacteria were carried out between 2013 and 2021; the main topics examined are the effect of phage on the reduction of Salmonella in chicken meat, the main dose of phage is between 6 log10 PFU/mL and 9 log10 PFU/mL, the most used temperatures are 4 °C and 25 °C. The effect of the phages was mainly examined within 1–24 h. The results indicated that the phage could be effective at killing Salmonella and reducing it by up to 10 log10 CFU/g under some conditions.
Table 1.
Different Salmonella reduction factors investigated in poultry meat.
| Reference | Factors investigated | Result |
|---|---|---|
| [3] | Phage dose: 106 PFU/g and 107 PFU/g | These findings demonstrated that the phage cocktail described in this study can be potentially used as a biological control agent against Salmonella in food products. |
| Temperature: 4°C and 25 C | ||
| Time: 1, 3, 6, 12, 24, and 48 h | ||
| [33] | Temperature: 4°C and 25 C | A significant reduction in bacterial numbers (1.5–4 log CFU/sample,p < 0.05) was observed in all tested foods. |
| Time: 1, 2, 3, 4 and 5 h | ||
| [4] | Phage dose: 108, 109, and 1010 PFU/g | The potential efficacy of the bacteriophage cocktail as a biological agent against S. Enteritidis in raw chicken breast meat. |
| Time: 24, 48, 72, 92, 120, 144, and 168 h | ||
| [25] | Time: 24, 48, 72, and 96 h | A developed phage cocktail suggests a potential biocontrol against Salmonella in fresh foods. |
| [1] | Temperature: 4°C and −20 C | Significant reduction of SSL1-010 (0.4–1.0 log CFU/cm2,P < 0.05) throughout 72 h of storage at 4°C. At −20°C, phage treatment significantly decreased. The number of SSL1-010 by 0.4 log – 0.7 log CFU/cm2 (P < 0.05) during 0–24 h of storage. These findings demonstrated that phage cocktail can be used for controlling growth of SSL1-010 on chicken meat during storage at 4 C. Besides, phage cocktail can be employed for reduction of Salmonella load during the first hs of storage at −20 C. |
| Time: 3, 6, 24, 48, and 72 h | ||
| [50] | Time: 48, 120, and 168 h | The potential effectiveness of this bacteriophage cocktail as a biocontrol agent of Salmonella in several food matrices under conditions similar to those used in their production. |
| [27] | Time: 12, 24, and 48 h | Bacterial population was reduced by 2.0 log cycles on the bacteriophage treated chicken meat samples. |
| [8] | Phage dose: 108 PFU/g and 109 PFU/g | The surface applications of phage significantly reduced Salmonella counts on chicken breast fillets. |
| Time: 24 and 168 h | ||
| [51] | Time: 4, 8, 24, and 168 h | Lytic phage preparation was effective in reducing Salmonella on chicken breast fillets. |
| Temperature: 4°C and 25°C | ||
| [18] | Time: 0.5 and 8 h | Bacteriophage reduction was dependent on Salmonella's susceptibility to the bacteriophage, and treatment time. |
| [11] | Phage dose: 1.1 × 108 PFU/g, 1.1 × 109 PFU/g, and 108 × 2.2 PFU/g | The combined treatments resulted in significantly greater reduction of Salmonella than individual bacteriophage or essential oil treatments. |
| [10] | Bacterial dose: 3 and 6 log 10 CFU/g | SE-P3, P16, P37, and P47 phages have the potency to be used as a biocontrol strategy to control Salmonella in the poultry industry. |
| Time: 3, 24, 48, 96, 72, 120, and 192 h | ||
| Temperature: 4°C and 25 C | ||
| [5] | Temperature: 8°C and 25 C | Phages isolated from raw chicken meats are potential agents for controlling Salmonella in raw meats. |
| Time: 2, 4, 6, and 24 h | ||
| [2] | Temperature: 4°C and 25 C | A phage cocktail of SPHG1 and SPHG3 is considered as a promising candidate as a biocontrol agent against foodborne salmonellosis. |
| Time: 2, 6, 12, 16, 24, 36, and 48 h | ||
| [7] | Phage dose: 108 and 106 PFU/g | Two phages (vB_SalS_1–23 and vB_SalS_3–29) with lytic effects show a high potential to inhibit the growth of Salmonella contaminants and can be used as candidate biocontrol agents. |
| Time: 1, 2, 3, 4, 5, 6, and 7 h | ||
| [22] | Temperature: 4°C and 25 C | Thermostable phages could be applied as complementary tools to control post-contamination after thermal processing of food products. |
| Time: 3, 6, 9, 12, 24, and 48 h | ||
| [9] | Phage dose: 108 PFU/g and 107 PFU/g | Bacteriophage application during tumbling of red meat trim and poultry can provide additional Salmonella control in ground products. |
| Time: 0.5 and 6 h | ||
| [52] | Time: 2, 4, 6, 8, 10, 12, and 24 h | These findings highlighted phage vB_SalP_TR2 as a potential antibacterial agent for the control of Salmonella in food samples. |
| [53] | Time: 0.08, 0.25, 0.5, 1, 24, and 168 h | ST-W77 and SE-W109 are ideal phages for further development as Salmonella biocontrol agents for food production. |
| [54] | Time: 24 and 48 h | Phages may be useful in the control of food-borne pathogens. |
| [55] | Phage dose: 106 PFU/g and 107 PFU/g | Significant reductions of viable Salmonella were observed in diverse foods. |
| Temperature: 4°C and 25°C | ||
| Time: 1, 3, 6, and 12 h | ||
| [56] | Temperature: 4°C and 25 C | Phage LPST94 is a promising candidate for biological control agents against pathogenic Salmonella and has the potential to be applied across different food matrices. |
| Time: 1, 3, 6, 12, 24, and 48 h |
3.2. Univariate and multivariate analysis
Table 2 presents the regression coefficient as a measure of association for each exposure variable with the presence of Salmonella reduction. The Univariate regression coefficient (i.e., a separate logistic regression model for each exposure) was highly significant for all variates. The univariate analysis showed that with each unit of increase in the bacterial dose (i.e., from 106 CFU/mL to 107 CFU/mL) and phage dose (i.e., from 106 PFU/mL to 107 PFU/mL), the rate of Salmonella reduction increases by about 20% (p-value <0.001). In addition, commercial phage decreases Salmonella reduction by about 90%. The use of surface treatment increases Salmonella reduction by around 76% (p-value <0.001). They were significant for Salmonella serotypes, S. Enteritidis (β = 0.66, p-value <0.001), and other serotypes (β = 0.25, p-value = 0.025) showed a greater reduction in Salmonella.
Table 2.
Univariate and multivariate linear model for regression coefficient of Salmonella reduction in poultry meat.
| Univariate model |
Multivariate model |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | β | 95% Cl |
P value | β | 95% Cl |
P value | ||
| Lower | Upper | Lower | Upper | |||||
| Bacterial Dose | 0.197 | 0.142 | 0.252 | 0.000 | 0.071 | 0.010 | 0.132 | 0.022 |
| Phage Dose | 0.201 | 0.162 | 0.240 | 0.000 | 0.202 | 0.150 | 0.254 | <0.00 |
| Temperature | 0.011 | 0.002 | 0.020 | 0.014 | 0.015 | 0.007 | 0.022 | 0.000 |
| Time | 0.003 | 0.001 | 0.005 | 0.001 | −0.001 | −0.003 | −0.0003 | 0.108 |
| Method of inoculation | ||||||||
| Surface treatment | Ref | – | – | – | ||||
| Deep treatment | 0.764 | 0.470 | 1.058 | 0.000 | −0.151 | −0.451 | 0.149 | 0.391 |
| Phage type | ||||||||
| Wild phage | Ref | – | – | – | ||||
| Commercial phage | −0.900 | −1.240 | −0.554 | 0.000 | −1.124 | −1.445 | −0.803 | 0.000 |
| Type of Salmonella | ||||||||
| S. Typhimurium | Ref | – | – | – | ||||
| S. Enteritidis | 0.66 | 0.43 | 0.88 | 0.000 | 0.445 | 0.230 | 0.661 | 0.000 |
| Other | 0.25 | 0.032 | 0.48 | 0.025 | 0.356 | 0.133 | 0.579 | 0.002 |
Since all of the variables were highly correlated (colinear variables), the multivariable model was presented separately for each of these exposures. The results of the multivariate regression model are presented in Table 2. All of the variables were found to be highly significant with this outcome, except the method of inoculation (β = −0.151, p-value = 0.391). However, it was also shown that a one-unit increase in bacterial dose, phage dose, and temperature; increases the Salmonella reduction by about 7%, 20%, and 1.5%, respectively, and these results were statistically significant. Time, unlike the other factors, has a negative effect on the process, but it does affect the reduction of Salmonella; that is, each extra h in phage treatment is associated with a 0.1% decrease in Salmonella reduction (p-value = 0.108) … However, the imperative role of other factors and different test conditions should not be underestimated. The extraction environment had a higher effect on phage function, and commercial phage decreases Salmonella reduction by 1.12 units, and this result was statistically significant (p-value <0.001). Lastly, S. Enteritidis (β = 0.445, p-value <0.001) and other species (β = 0.356, p-value = 0.002) showed more reduction in Salmonella.
4. Discussion
4.1. Temperature
This is the first time that multivariate analysis has been applied to investigatethe effect of various factors on bacteriophage function on Salmonella spp. Reduction in poultry meat with such a broad set of data, revealing the external factors that can affect phage activity and their importance. Temperature is a vital factor in bacteriophage survival. It plays an essential role in adhesion, penetration, proliferation, and the length of the latent period. At lower than optimal temperatures, fewer phages’ genetic materials penetrate bacterial host cells; therefore, fewer of them can be involved in the proliferation phase. Higher temperatures can extend the duration of the latent period [29]; therefore, temperature is an important factor in phage function. This study indicated that an increase in temperature is positively correlated with Salmonella reduction; a 10-unit increase in temperature results in a 15% increase in Salmonella reduction. The positive relationship between temperature and Salmonella reduction observed in this study was similar to recent findings from China, Thailand, and Chile [[30], [31], [32]]. According to the present study, increasing the temperature from 4 °C to 25 °C can lead to rise in the rate of Salmonella reduction by about 32%. Guo et al. (2021) found that with an increase in temperature from 4 °C to 25 °C, bacterial reduction rises by approximately 30% [33]. In this regard, Greer (1988) argued that possibly low temperatures are responsible for the lysing of a very small proportion of the infected cells and blocking the lytic development of phage [34].
4.2. Time
It was shown that the potency of bacteriophage is significantly improved following the early times of inoculation [35]. According to Table 2, the negative effect of time is negligible so that after 24 h, bacterial reduction decreases by around 2.5%. These results are also supported by El-Dougdoug et al., Kim et al. and Li et al. [[36], [37], [38]]. In one study [39], the negative effect of time was considerably higher on the order of 50% per h. Jassim et al. (2012) indicated that longer exposure time do not remarkably increase the number of attacking phages [35]. However, it can result from various external factors anddisparate test conditions.
4.3. Phage dose
Phage dose has a notable effect on the reduction of Salmonella; a 5-unit increase in the phage dose entirely reduced the population of bacteria. The efficacy of phage therapy highly depends on phage dosage [40] and the positive relationship between phage dose and Salmonella reduction observed in other studies that have been done in Asia, Europe, and North America with similar trends [36,41,42]. Zhang et al. (2021) in their study found that a one-unit increase in phage dose rises Salmonella reduction by about 18%, which is similar to current results [43]. This may be the consequence of a positive correlation between the phage dose and the phage recovery [44].
4.4. Bacterial dose
The present study also showed that increasing the bacterial dose can have a positive impact on phage function. After increasing the bacterial dose by two units, there was a 15% reduction in Salmonella counts. Some researchers report that a 2-unit increase in the bacterial dose increases Salmonella reduction by around 12% [45]. In another study, Greer showed that phage load had no effect until the initial bacterial density reached a certain level, and at higher levels of bacterial contamination, fewer phages induced a significant increase [46].
4.5. Type of salmonella
Concerning the different serotypes, this study determined that phage biocontrol is more effective for S. Enteritidis than S. Typhimurium. After phage treatment of poultry meat contaminated with S. Enteritidis and other types, Salmonella was reduced by over 45% and 35%, respectively, compared to S. Typhimurium. These reductions are broadly consistent with those previously recorded [37,38,47] that have been performed on poultry skin, egg yolk, egg white, and liquid egg. Moreover, Vaz et al. found that S. Typhimurium had the highest phage resistance, and S. Heldiberg and S. Enteritidis was the most sensitive [40]. According to these studies [48,49], more decline in S. Enteritidis compared to other species could be due to the higher prevalence of the poultry products. However, if this study could be conducted under different conditions and more factors affecting the phage potency, the results might be equal for both strains of Salmonella.
4.6. Method of inoculation
Furthermore, one of the objectives of this study was to compare the effectiveness of dip treatment and surface bacteriophage application. Dip treatment with lytic bacteriophages is15% more effective against Salmonella thansurface treatment. Sukumaran et al. found that the bacterial reduction obtained after immersion treatment was 13% higher than surface treatment [8]. In the study by Jassim et al. at 103 PFU/mL, the sprayed mode showed a lower log reduction and was less effective than the immersion mode in reducing Salmonella overall. The immersion method may indeed provide better reductions in Salmonella. However, this effect declines rapidly with prolonged use because of contaminating the solution with various other foodborne bacteria. In addition, the optimal phage concentration for the spraying mode was higher than the immersion mode. The lower effectiveness could be due to the inability of the spray mode to cover samples with phage as efficiently as the immersion method [35]. It could also be due to the different phage doses used in the various studies.
4.7. Phage type
The current study also investigated the impact of phage type on the reduction rate of Salmonella in poultry meat, so employing wild type phages results in a two-fold reduction in the Salmonella population. Multiple passages of the commercial phages are responsible for reducing their efficiency compared to the wild-type. Vaz et al. (2020) proposed that long-term phage-therapy can lead to the evolution of host bacterial resistance, which negatively affects the efficacy of the treatment. The development of bacterial resistance is mainly due to the modification of the phage receptors on their surface, which prevents adsorption [40]. More utilization of commercial phages can be responsible for greater bacterial resistance.
Overall, this is the first study to globally examine the effect of the factors on phage performance through a systematic review and modeling. Nevertheless, there are obvious ways to survey the parameters, such as studying the time interval between the phage inoculation and bacterial reduction, which is not mentioned in all studies. A broader approach is currently being developed with the aim expanding the pool of data and enhancing the value of applicability and predictability.
5. Conclusion
It has been shown that the effectiveness of phage applications depends on several factors. The wild-type phages have the greatest potency and doubles phage performance. In addition, increasing the phage dose and temperature increases its effectiveness. Furthermore, S. Enteritidis has the lowest phage resistance; an immersive type of inoculation is more efficient than surface inoculation (e.g., spray). Ultimately, immersion treatment of wild-type phage with higher doses of bacteria and phage at elevated temperatures can be more effective, making phage a helpful tool for reducing Salmonella in poultry meat. However, further studies on the role of other factors, including MOI (multiplicity of infection), the exact number of phages, packaging systems, and combination use of phage with other antimicrobial agents, are advised.
Ethics approval and consent to participate
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Funding
This study was entirely financed by Shiraz University of Medical Sciences (SUMS), Shiraz, Iran with project number 24735.
Authorship contribution
Mohsen Shahdadi, Maryam Safarirad, Enayat Berizi, Seyed Mohammad Mazloomi, Saeid Hosseinzadeh, and Morteza Zare: conceived and designed the experiments; performed the experiments; analyzed and interpreted the data; contributed reagents, materials, analysis tools or data; wrote the paper.
Zahra Derakhshan and Saeed Rajabi: conceived and designed the experiments; analyzed and interpreted the data; contributed reagents, materials, analysis tools or data; wrote the paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Shiraz University of Medical Sciences for supporting us during the current study.
References
- 1.Abhisingha M., Dumnil J., Pitaksutheepong C. Efficiency of phage cocktail to reduce Salmonella Typhimurium on chicken meat during low temperature storage. LWT (Lebensm.-Wiss. & Technol.) 2020;129 doi: 10.1016/j.lwt.2020.109580. [DOI] [Google Scholar]
- 2.Esmael A., Azab E., Gobouri A.A., Nasr-Eldin M.A., Moustafa M.M., Mohamed S.A., et al. Isolation and characterization of two lytic bacteriophages infecting a multi-drug resistant Salmonella Typhimurium and their efficacy to combat salmonellosis in ready-to-use foods. Microorganisms. 2021;9(2):423. doi: 10.3390/microorganisms9020423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Islam M.S., Zhou Y., Liang L., Nime I., Liu K., Yan T., et al. Application of a phage cocktail for control of Salmonella in foods and reducing biofilms. Viruses. 2019;11(9):841. doi: 10.3390/v11090841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim J.H., Kim H.J., Jung S.J., Mizan M.F.R., Park S.H., Ha S.D. Characterization of Salmonella spp.‐specific bacteriophages and their biocontrol application in chicken breast meat. J. Food Sci. 2020;85(3):526–534. doi: 10.1111/1750-3841.15042. [DOI] [PubMed] [Google Scholar]
- 5.Duc H.M., Son H.M., Honjoh K-i, Miyamoto T. Isolation and application of bacteriophages to reduce Salmonella contamination in raw chicken meat. LWT (Lebensm.-Wiss. & Technol.) 2018;91:353–360. doi: 10.1016/j.lwt.2018.01.072. [DOI] [Google Scholar]
- 6.Ding Y., Zhang Y., Huang C., Wang J., Wang X. An endolysin LysSE24 by bacteriophage LPSE1 confers specific bactericidal activity against multidrug-resistant Salmonella strains. Microorganisms. 2020;8(5):737. doi: 10.3390/microorganisms8050737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cong C., Wei B., Cui H., Li X., Yuan Y., Wang L., et al. Isolation, characterization and comparison of lytic Epseptimavirus phages targeting Salmonella. Food Res. Int. 2021;147 doi: 10.1016/j.foodres.2021.110480. [DOI] [PubMed] [Google Scholar]
- 8.Sukumaran A.T., Nannapaneni R., Kiess A., Sharma C.S. Reduction of Salmonella on chicken meat and chicken skin by combined or sequential application of lytic bacteriophage with chemical antimicrobials. Int. J. Food Microbiol. 2015;207:8–15. doi: 10.1016/j.ijfoodmicro.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 9.Yeh Y., Purushothaman P., Gupta N., Ragnone M., Verma S., De Mello A. Bacteriophage application on red meats and poultry: effects on Salmonella population in final ground products. Meat Sci. 2017;127:30–34. doi: 10.1016/j.meatsci.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Demirarslan Ö.A., Alasalvar H., Yildirim Z. Biocontrol of Salmonella Enteritidis on chicken meat and skin using lytic SE-P3, P16, P37, and P47 bacteriophages. LWT (Lebensm.-Wiss. & Technol.) 2021;137 doi: 10.1016/j.lwt.2020.110469. [DOI] [Google Scholar]
- 11.Moon S.H., Waite-Cusic J., Huang E. Control of Salmonella in chicken meat using a combination of a commercial bacteriophage and plant-based essential oils. Food Control. 2020;110 doi: 10.1016/j.foodcont.2019.106984. [DOI] [Google Scholar]
- 12.Mead G., Lammerding A.M., Cox N., Doyle M.P., Humbert F., Kulikovskiy A., et al. Scientific and technical factors affecting the setting of Salmonella criteria for raw poultry: a global perspective. J. Food Protect. 2010;73(8):1566–1590. doi: 10.4315/0362-028x-73.8.1566. [DOI] [PubMed] [Google Scholar]
- 13.Hungaro H.M., Mendonça R.C.S., Gouvêa D.M., Vanetti M.C.D., de Oliveira Pinto C.L. Use of bacteriophages to reduce Salmonella in chicken skin in comparison with chemical agents. Food Res. Int. 2013;52(1):75–81. doi: 10.1016/j.foodres.2013.02.032. [DOI] [Google Scholar]
- 14.Mir N.A., Rafiq A., Kumar F., Singh V., Shukla V. Determinants of broiler chicken meat quality and factors affecting them: a review. J. Food Sci. Technol. 2017;54:2997–3009. doi: 10.1007/s13197-017-2789-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sohaib M., Anjum F.M., Arshad M.S., Rahman U.U. Postharvest intervention technologies for safety enhancement of meat and meat based products; a critical review. J. Food Sci. Technol. 2016;53:19–30. doi: 10.1007/s13197-015-1985-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Domingo-Calap P., Delgado-Martínez J. Bacteriophages: protagonists of a post-antibiotic era. Antibiotics. 2018;7(3):66. doi: 10.3390/antibiotics7030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jamal M., Bukhari S.M., Andleeb S., Ali M., Raza S., Nawaz M.A., et al. Bacteriophages: an overview of the control strategies against multiple bacterial infections in different fields. J. Basic Microbiol. 2019;59(2):123–133. doi: 10.1002/jobm.201800412. [DOI] [PubMed] [Google Scholar]
- 18.Parveen S., Schwarz J., Hashem F., Vimini B. Reduction of Salmonella in ground chicken using a bacteriophage. Poultry Sci. 2017;96(8):2845–2852. doi: 10.3382/ps/pex062. [DOI] [PubMed] [Google Scholar]
- 19.Pires D.P., Costa A.R., Pinto G., Meneses L., Azeredo J. Current challenges and future opportunities of phage therapy. FEMS Microbiol. Rev. 2020;44(6):684–700. doi: 10.1093/femsre/fuaa017. [DOI] [PubMed] [Google Scholar]
- 20.Ferriol-González C., Domingo-Calap P. Phages for biofilm removal. Antibiotics. 2020;9(5):268. doi: 10.3390/antibiotics9050268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ly-Chatain M.H. The factors affecting effectiveness of treatment in phages therapy. Front. Microbiol. 2014;5:51. doi: 10.3389/fmicb.2014.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park H., Kim J., Kim M., Park Y., Ryu S. Development of new strategy combining heat treatment and phage cocktail for post-contamination prevention. Food Res. Int. 2021;145 doi: 10.1016/j.foodres.2021.110415. [DOI] [PubMed] [Google Scholar]
- 23.Chen L., Yuan S., Liu Q., Mai G., Yang J., Deng D., et al. In vitro design and evaluation of phage cocktails against Aeromonas salmonicida. Front. Microbiol. 2018;9:1476. doi: 10.3389/fmicb.2018.01476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mateus L., Costa L., Silva Y., Pereira C., Cunha A., Almeida A. Efficiency of phage cocktails in the inactivation of Vibrio in aquaculture. Aquaculture. 2014;424:167–173. doi: 10.1016/j.aquaculture.2014.01.001. [DOI] [Google Scholar]
- 25.Petsong K., Benjakul S., Chaturongakul S., Switt A.I.M., Vongkamjan K. Lysis profiles of Salmonella phages on Salmonella isolates from various sources and efficiency of a phage cocktail against S. enteritidis and S. typhimurium. Microorganisms. 2019;7(4):100. doi: 10.3390/microorganisms7040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toprak Z.T., Şanlıbaba P. Application of phage for biocontrol of Salmonella species in food systems. Turkish Journal of Agriculture-Food Science and Technology. 2020;8(10):2214–2221. doi: 10.24925/turjaf.v8i10.2214-2221.3689. [DOI] [Google Scholar]
- 27.Thung T.Y., Premarathne J.M.K.J.K., San Chang W., Loo Y.Y., Chin Y.Z., Kuan C.H., et al. Use of a lytic bacteriophage to control Salmonella Enteritidis in retail food. LWT (Lebensm.-Wiss. & Technol.) 2017;78:222–225. doi: 10.1016/j.lwt.2016.12.044. [DOI] [Google Scholar]
- 28.Taboada A.C., Glass K., Chateau D., Pavic A. A systematic review and meta-analysis of the effect of dietary additives, vaccination and processing aids as control measures for Salmonella spp. in chicken meat. Applied Food Research. 2022 doi: 10.1016/j.afres.2022.100254. [DOI] [Google Scholar]
- 29.Jończyk E., Kłak M., Międzybrodzki R., Górski A. The influence of external factors on bacteriophages. Folia Microbiol. 2011;56:191–200. doi: 10.1007/s12223-011-0039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galarce N., Escobar B., Rojas V., Navarro C., Turra G., Robeson J., et al. Application of a virulent bacteriophage cocktail leads to reduction of Salmonella enterica serovar Enteritidis counts in processed meat products. Biocontrol Sci. Technol. 2016;26(4):462–475. doi: 10.1080/09583157.2015.1125447. [DOI] [Google Scholar]
- 31.Shan J., Korbsrisate S., Withatanung P., Adler N.L., Clokie M.R., Galyov E.E. Temperature dependent bacteriophages of a tropical bacterial pathogen. Front. Microbiol. 2014;5:599. doi: 10.3389/fmicb.2014.00599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taj M., Ling J., Bing L., Qi Z., Taj I., Hassani T., et al. Effect of dilution, temperature and pH on the lysis activity of T4 phage against E. coli bl21. J Anim Plant Sci. 2014;24(4):1252–1255. [Google Scholar]
- 33.Bao H., Zhang P., Zhang H., Zhou Y., Zhang L., Wang R. Bio-control of Salmonella Enteritidis in foods using bacteriophages. Viruses. 2015;7(8):4836–4853. doi: 10.3390/v7082847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bertani G., Nice S. Studies on LYSOGENESIS II. P1: the effect of temperature on the lysogenization of Shigella dysenteriae with phage. J. Bacteriol. 1954;67(2):202–209. doi: 10.1128/jb.67.2.202-209.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jassim S., Abdulamir A., Abu Bakar F. Novel phage-based bio-processing of pathogenic Escherichia coli and its biofilms. World J. Microbiol. Biotechnol. 2012;28:47–60. doi: 10.1007/s11274-011-0791-6. [DOI] [PubMed] [Google Scholar]
- 36.El-Dougdoug N., Cucic S., Abdelhamid A., Brovko L., Kropinski A., Griffiths M., et al. Control of Salmonella Newport on cherry tomato using a cocktail of lytic bacteriophages. Int. J. Food Microbiol. 2019;293:60–71. doi: 10.1016/j.ijfoodmicro.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Li Z., Ma W., Li W., Ding Y., Zhang Y., Yang Q., et al. A broad-spectrum phage controls multidrug-resistant Salmonella in liquid eggs. Food Res. Int. 2020;132 doi: 10.1016/j.foodres.2020.109011. [DOI] [PubMed] [Google Scholar]
- 38.Luo D., Li C., Wu Q., Ding Y., Yang M., Hu Y., et al. Isolation and characterization of new phage vB_CtuP_A24 and application to control Cronobacter spp. in infant milk formula and lettuce. Food Res. Int. 2021;141 doi: 10.1016/j.foodres.2021.110109. [DOI] [PubMed] [Google Scholar]
- 39.Kim H.-J., Kim Y.-T., Kim H.B., Choi S.H., Lee J.-H. Characterization of bacteriophage VVP001 and its application for the inhibition of Vibrio vulnificus causing seafood-borne diseases. Food Microbiol. 2021;94 doi: 10.1016/j.fm.2020.103630. [DOI] [PubMed] [Google Scholar]
- 40.Vaz C.S.L., Voss-Rech D., Alves L., Coldebella A., Brentano L., Trevisol I.M. Effect of time of therapy with wild-type lytic bacteriophages on the reduction of Salmonella Enteritidis in broiler chickens. Vet. Microbiol. 2020;240 doi: 10.1016/j.vetmic.2019.108527. [DOI] [PubMed] [Google Scholar]
- 41.Goode D., Allen V., Barrow P. Reduction of experimental Salmonella and Campylobacter contamination of chicken skin by application of lytic bacteriophages. Appl. Environ. Microbiol. 2003;69(8):5032–5036. doi: 10.1128/AEM.69.8.5032-5036.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan T., Liang L., Yin P., Zhou Y., Mahdy Sharoba A., Lu Q., et al. Application of a novel phage LPSEYT for biological control of Salmonella in foods. Microorganisms. 2020;8(3):400. doi: 10.3390/microorganisms8030400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y., Ding Y., Li W., Zhu W., Wang J., Wang X. Application of a novel lytic podoviridae phage Pu20 for biological control of drug-resistant Salmonella in liquid eggs. Pathogens. 2021;10(1):34. doi: 10.3390/pathogens10010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dąbrowska K. Phage therapy: what factors shape phage pharmacokinetics and bioavailability? Systematic and critical review. Med. Res. Rev. 2019;39(5):2000–2025. doi: 10.1002/med.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Çufaoğlu G., Onaran Acar B., Ayaz N., Göncüoğlu M., Ormanci F. Biocontrol of Escherichia coli O157: H7 in ready-to-eat salad using a lytic bacteriophage. Med. Weter. - Vet. Med. - Sci. Pract. 2017;73(7) doi: 10.21521/mw.5740. [DOI] [Google Scholar]
- 46.Greer G.G. Effects of phage concentration, bacterial density, and temperature on phage control of beef spoilage. J. Food Sci. 1988;53(4):1226–1227. doi: 10.1111/j.1365-2621.1988.tb13570.x. [DOI] [Google Scholar]
- 47.Ge H., Xu Y., Hu M., Zhang K., Zhang S., Jiao Xa, et al. Isolation, characterization, and application in poultry products of a salmonella-specific bacteriophage, S55. J. Food Protect. 2021;84(7):1202–1212. doi: 10.4315/JFP-20-438. [DOI] [PubMed] [Google Scholar]
- 48.Álvarez-Fernández E., Alonso-Calleja C., García-Fernández C., Capita R. Prevalence and antimicrobial resistance of Salmonella serotypes isolated from poultry in Spain: comparison between 1993 and 2006. Int. J. Food Microbiol. 2012;153(3):281–287. doi: 10.1016/j.ijfoodmicro.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 49.De Vylder J., Raspoet R., Dewulf J., Haesebrouck F., Ducatelle R., Van Immerseel F. Salmonella Enteritidis is superior in egg white survival compared with other Salmonella serotypes. Poultry Sci. 2013;92(3):842–845. doi: 10.3382/ps.2012-02668. [DOI] [PubMed] [Google Scholar]
- 50.Spricigo D.A., Bardina C., Cortés P., Llagostera M. Use of a bacteriophage cocktail to control Salmonella in food and the food industry. Int. J. Food Microbiol. 2013;165(2):169–174. doi: 10.1016/j.ijfoodmicro.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 51.Sukumaran A.T., Nannapaneni R., Kiess A., Sharma C.S. Reduction of Salmonella on chicken breast fillets stored under aerobic or modified atmosphere packaging by the application of lytic bacteriophage preparation SalmoFreshTM. Poultry Sci. 2016;95(3):668–675. doi: 10.3382/ps/pev332. [DOI] [PubMed] [Google Scholar]
- 52.Shang Y., Sun Q., Chen H., Wu Q., Chen M., Yang S., et al. Isolation and characterization of a novel Salmonella phage vB_SalP_TR2. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.664810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Phothaworn P., Supokaivanich R., Lim J., Klumpp J., Imam M., Kutter E., et al. Development of a broad-spectrum Salmonella phage cocktail containing Viunalike and Jerseylike viruses isolated from Thailand. Food Microbiol. 2020;92 doi: 10.1016/j.fm.2020.103586. [DOI] [PubMed] [Google Scholar]
- 54.Zinno P., Devirgiliis C., Ercolini D., Ongeng D., Mauriello G. Bacteriophage P22 to challenge Salmonella in foods. Int. J. Food Microbiol. 2014;191:69–74. doi: 10.1016/j.ijfoodmicro.2014.08.037. [DOI] [PubMed] [Google Scholar]
- 55.Guo Y., Li J., Islam M.S., Yan T., Zhou Y., Liang L., et al. Application of a novel phage vB_SalS-LPSTLL for the biological control of Salmonella in foods. Food Res. Int. 2021;147 doi: 10.1016/j.foodres.2021.110492. [DOI] [PubMed] [Google Scholar]
- 56.Islam M.S., Zhou Y., Liang L., Nime I., Yan T., Willias S.P., et al. Application of a broad range lytic phage LPST94 for biological control of Salmonella in foods. Microorganisms. 2020;8(2):247. doi: 10.3390/microorganisms8020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.