Abstract
Background
In rodents, glomerular expression of insulin receptor substrate 1 (IRS1) is decreased in diabetic kidney disease (DKD) and reduced associated functioning is involved in the development and progression of DKD. This study aimed to evaluate the significance of glomerular IRS1 expression in DKD patients, and investigated whether glomerular IRS1 expression can reflect renal pathology and predict renal outcomes.
Methods
This study included 10 patients who underwent renal biopsy and were diagnosed with DKD or minor glomerular abnormality (MGA). IRS1-positive cells were determined based on renal biopsy and immunostaining, and the associations of the number of these cells with baseline and prognostic parameters were analyzed.
Results
IRS1-positive cells were significantly decreased in DKD than in MGA. IRS1 positivity tended to be negatively correlated with global glomerulosclerosis and tubulointerstitial fibrosis. The rate of change in estimated glomerular filtration rate before and 12 months after renal biopsy was positively correlated to the number of IRS1-positive cells. Furthermore, a tendency towards negative correlation was observed between the number of glomerular IRS1-positive cells and the proteinuria.
Conclusions
This study shows the glomerular IRS1-positive cell count was significantly decreased in DKD, and that the degree IRS1 positivity was partially correlated with renal pathology and function.
Keywords: Diabetic kidney disease, IRS1, Proteinuria, Kidney biopsy, Estimated glomerular filtration rate
1. Introduction
Diabetic kidney disease (DKD) is most common cause of chronic kidney disease and end-stage renal disease (ESRD), which are associated with an increased risk of mortality [1,2]. The prevalence of dialysis due to ESRD caused by DKD has exceeded that of glomerulonephritis-induced ESRD in the U.S [3].
Insulin/insulin receptor substrate-1 (IRS1) signaling can increase nitric oxide (NO) production via the phosphatidylinositol-3 (PI3K)/Akt pathway, thereby increasing its anti-inflammatory effects [4,5]. In contrast, in diabetic and insulin-resistant states, while the IRS1/Akt/endothelial NO synthase (eNOS) pathway is inhibited selectively, another major pathway of insulin signaling, p38 mitogen-activated protein kinase (MAPK), is not inhibited [6,7]. We have previously shown that the glomerulus is the site of the insulin-resistant state in diabetes and obesity [4]. Furthermore, our previous results suggest that increasing IRS1 levels or inhibiting the action of protein kinase C-β (PKCβ) may improve renal function in patients with diabetes and insulin-resistance [4]. Linagliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor, shows renoprotective effects by activation of IRS1 signaling, which is suppressed in the diabetic state, thereby reducing podocyte apoptosis [8]. However, there have been no reports investigating glomerular expression of IRS1 in patients with type 2 diabetes and its association with renal pathology and function.
The aim of this study was to determine glomerular expression of IRS1 in the kidneys of patients with DKD or minor glomerular abnormality (MGA) diagnosed by kidney biopsy, and to evaluate whether glomerular IRS1 expression can reflect renal pathology and predict renal outcomes.
2. Material and methods
2.1. Patient groups
All procedures involving human participants in this study were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The ethics committee of Osaka Medical and Pharmaceutical University approved this study (approval number: 2020-095). Written informed consent was obtained from all participants at the time the renal biopsy performed. This study included a retrospective cohort of 10 patients who underwent kidney biopsy at Osaka Medical and Pharmaceutical University Hospital between April 2015 and July 2020. Data were collected and analyzed retrospectively by using electronic medical records maintained by the Department of Nephrology at Osaka Medical and Pharmaceutical University Hospital. Blood and urine biochemical analyses were performed on a Hitachi labospect 008 (Hitachi, Tokyo, Japan) autoanalyzer. Data on the levels of serum creatinine (Cr), blood urea nitrogen (BUN), total protein, albumin, total cholesterol, high density lipoprotein (HDL) cholesterol, low density lipoprotein (LDL) cholesterol, triglyceride, fasting blood glucose, C-reactive protein, urinary N-acetyl-β-D-glucosaminidase, and urinary β2 microglobulin, along with estimated glomerular filtration rate (eGFR), urine protein-to-creatinine ratio, and patient characteristics (e.g., underlying diseases, age, sex, blood pressure, and body mass index), were obtained from the electronic medical records and kidney biopsy database [9]. The definition of DKD includes four criteria: [I] a duration of more than 5 years after the onset of diabetes, [II] the presence of micro- or macro-albuminuria, [III] histopathological changes in line with DKD, such as glomerular basement membrane thickening, mesangial expansion, nodular sclerosis, and/or arteriolar hyalinosis, and [IV] the exclusion of other possible causes for renal disorders [10]. The exclusion criteria were history of cancer, other nephritis complications, and autoimmune disease complications including collagen disease.
2.2. Kidney biopsy
Kidney specimens were obtained using a 16-gauge biopsy needle (Bard, New Providence, NJ). Specimens were fixed in 10% formalin, and the prepared sections were stained with hematoxylin-eosin, Masson's trichrome, or periodic acid-Schiff stains (PAS). Immunohistochemical staining was performed with antibodies specific to IRS1 (Abcam, Cambridge, UK) using an established avidin-biotin detection method [11,12] (Roche, Indianapolis, IN). Glomerular morphometry and interstitial fibrosis were evaluated in PAS-stained and Masson's trichrome-stained tissues, respectively [13]. At least three pathologists evaluated the specimens [9].
2.3. Calculation of eGFR
Levels of serum creatinine were measured in all samples using an enzymatic laboratory method [14], and the values are represented using two decimal places. The eGFR of each patient was calculated using the following formula: 194 × serum creatinine−1.094 × age−0.287 × [0.739 (if female)]. The rate of change in eGFR was used to evaluate renal function at each time point and was calculated using the following formula: [(eGFR 12 months after kidney biopsy – eGFR before kidney biopsy)/eGFR before kidney biopsy] × 100; this calculation is a modification of the previously reported method [9,15].
2.4. Statistical analyses
The data are expressed as mean values ± SD. Between-group comparisons were made using the Mann–Whitney U test; categorical variables are presented as numbers (percentage) and were compared using Fisher's exact test, as appropriate. Spearman's correlation coefficients were estimated to determine associations between two variables. The log-transformed values of IRS1 signals were used. All analyses were performed using StatView (SAS Institute, Cary, CA, USA) and Excel software (Microsoft, Redmond, WA, USA).
3. Results
Patient's baseline characteristics and concomitant medications are presented in Table 1. A total of 40% and 50% of the patients were treated using renin-angiotensin-aldosterone system (RAAS) inhibitors or DPP-4 inhibitors, respectively. For DKD, the patients had a history of type 2 diabetes and presented with overt proteinuria and reduced eGFR. The median duration of DKD diagnosis was 13 ± 8.4 years. All cases were in stage 4 of DKD. In contrast, for MGA, we confirmed the absence of proteinuria and no reduction in eGFR (Table 1). Kidney biopsies were performed, and the pathology was analyzed by at least three pathologists, who confirmed the absence of pathological features in MGA, while for DKD, diabetic glomerular sclerotic lesions were recognized on PAS saining.
Table 1.
Baseline clinical characteristics of patients at renal biopsy who were analyzed for glomerular IRS1 protein expression by immunohistochemistry.
| MGA (n = 4) | DN (n = 6) | P value | |
|---|---|---|---|
| Sex (male/female) | 2/2 | 4/2 | P = 0.598 |
| Age (years) | 48.8 ± 25.2 | 59.7 ± 13.0 | P = 0.124 |
| Diabetes duration (years) | N/A | 12.8 ± 8.4 | N/A |
| RAAS inhibitor (yes/no) | 0/0 | 4/2 | N/A |
| DPP-4 inhibitor (yes/no) | 0/0 | 5/1 | N/A |
| Metformin (yes/no) | 0/0 | 1/5 | N/A |
| Glinide (yes/no) | 0/0 | 1/5 | N/A |
| α-glucosidase inhibitor (yes/no) | 0/0 | 1/5 | N/A |
| Sulfonylureas (yes/no) | 0/0 | 1/5 | N/A |
| Insulin (yes/no) | 0/0 | 1/5 | N/A |
| BMI (kg/m2) | 22.8 ± 5.4 | 24.7 ± 3.0 | P = 0.4892 |
| Fasting blood glucose | 106.3 ± 5.5 | 106.3 ± 36.9 | N/A |
| Systolic blood pressure (mmHg) | 111.5 ± 14.8 | 160.0 ± 22.2 | P = 0.005 |
| Diastolic blood pressure (mmHg) | 65.0 ± 3.7 | 87.8 ± 19.9 | P = 0.057 |
| Urinary protein (g/g creatinine) | 0.29 ± 0.25 | 4.5 ± 2.2 | P = 0.0056 |
| Creatinine (mg/dl) | 0.84 ± 0.13 | 1.53 ± 0.97 | P = 0.2066 |
| eGFR(ml/min/1.73m2) | 70.9 ± 19.4 | 43.2 ± 16.7 | P = 0.0421 |
| BUN (mg/dl) | 13.8 ± 4.3 | 26.5 ± 14.9 | P = 0.1399 |
| Total protein (g/dl) | 6.6 ± 0.5 | 5.9 ± 0.7 | P = 0.1795 |
| Albumin (g/dl) | 3.8 ± 0.1 | 3.1 ± 0.4 | P = 0.0145 |
| Total cholesterol (mg/dl) | 181.3 ± 22.5 | 198.0 ± 42.1 | P = 0.4919 |
| Triglyceride (mg/dl) | 129.5 ± 53.7 | 152.5 ± 59.4 | P = 0.5516 |
| HDL cholesterol (mg/dl) | 58.8 ± 14.3 | 57.8 ± 17.9 | P = 0.9342 |
| LDL cholesterol (mg/dl) | 96.5 ± 26.0 | 112.6 ± 32.6 | P = 0.5022 |
| CRP (mg/dl) | 0.28 ± 0.20 | 0.11 ± 0.10 | P = 0.1145 |
N/A; not applicable, MGA; minor glomerular abnormality, DKD; diabetic kidney disease; RAAS; renin-angiotensin-aldosterone system, DPP-4; dipeptidyl peptidase-4, BMI; body mass index, eGFR; estimated glomerular filtration rate, BUN; blood urea nitrogen, HDL; high density lipoprotein, LDL; low density lipoprotein, CRP; C-reactive protein.
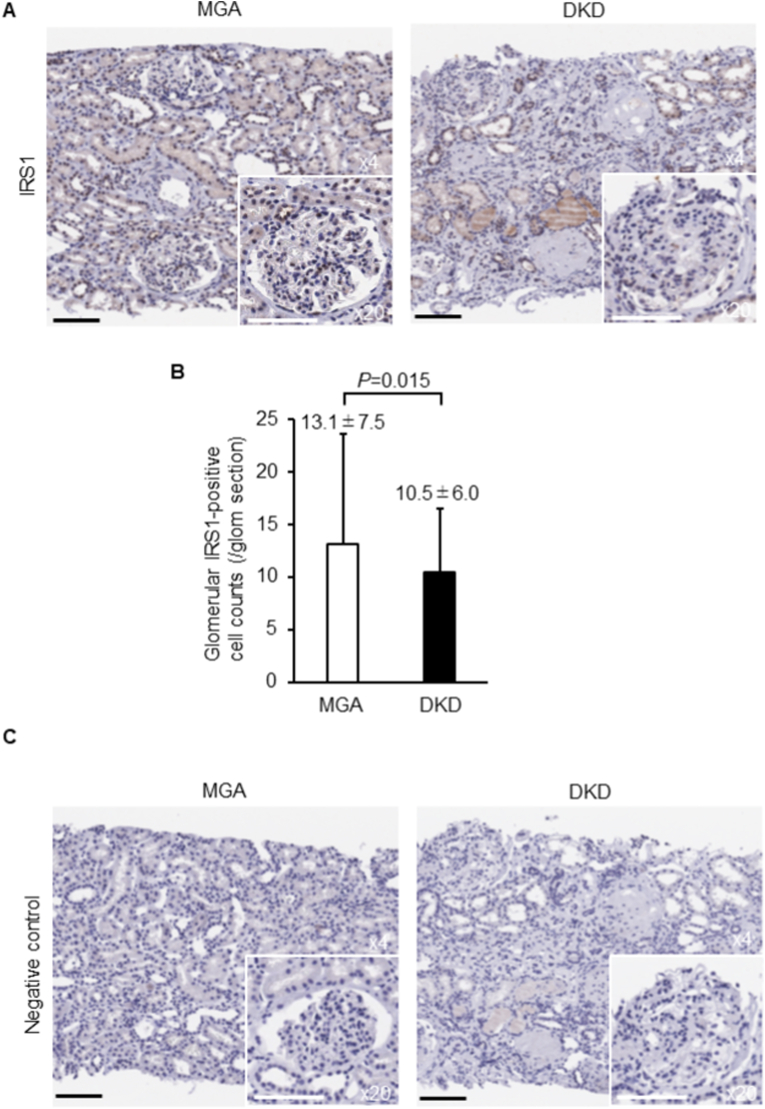
The glomerular IRS1 expression levels were compared between DKD and MGA. Immunohistochemical analysis revealed that the IRS1-positive cell count in DKD was significantly decreased compared to that in MGA (average number of IRS1-positive cells per glomerulus; 10.5 ± 6.0 in DKD and 13.1 ± 7.5 in MGA; P = 0.015; Fig. 1A and B). Negative control for primary antibody immunohistochemical staining data is shown in Fig. 1c.
Fig. 1.
Immunohistochemical analysis for IRS1 in kidney biopsy samples.
(A) Representative immunohistochemistry of IRS1 in MGA and DKD. (B) Quantification of glomerular IRS1-positive cell count. (C) Representative negative control for primary antibody immunohistochemical staining. Bar = 100 μm. IRS1: insulin receptor substrate 1, MGA: minor glomerular abnormality, DKD: diabetic kidney disease.
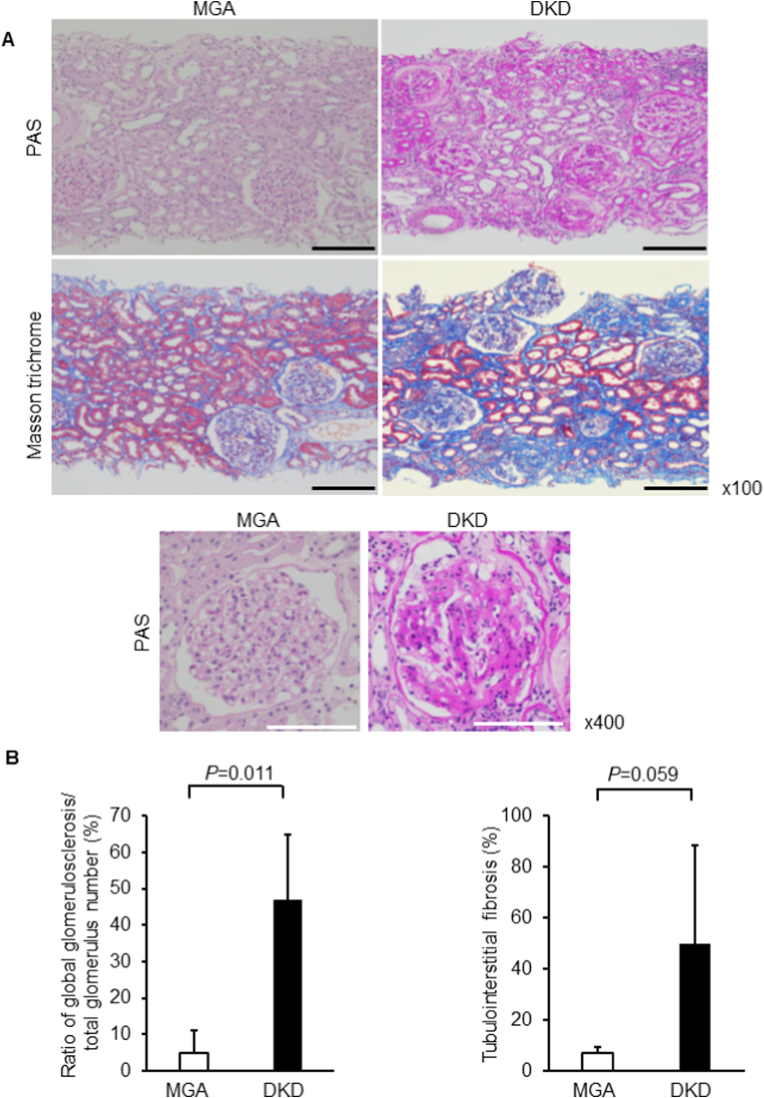
As shown in the representative images, the degree of global glomerulosclerosis was significantly increased in DKD than in MGA (46.9 ± 17.9 in DKD and 4.8 ± 6.3% in MGA, P = 0.011, respectively, Fig. 2A and B, Supplementary Figs. 1 and 2). While the area of tubulointerstitial fibrosis was larger in DKD than in MGA, the difference was not statistically significant (50.0 ± 38.3% in DKD and 6.9 ± 2.4% in MGA, P = 0.059; Fig. 2A and B, Supplementary Figs. 1 and 2). In terms of renal function, eGFR was decreased and urinary protein level was increased in DKD compared to those in MGA (eGFR: 43.2 ± 16.7 in DKD and 70.9 ± 19.4 ml/min/1.73 m2 in MGA, P = 0.0421; urinary protein: 4.5 ± 2.2 in DKD and 0.29 ± 0.25 g/g creatinine in MGA, P = 0.0056; Table 1).
Fig. 2.
Renal morphology in the experimental groups.
(A) Representative light microscopic appearance (periodic acid-Schiff [PAS], Masson's trichrome). (B) Morphometric analysis of the ratio of global glomerulosclerosis per total number of glomeruli and tubulointerstitial fibrosis area. Bar = 100 μm. MGA: minor glomerular abnormality, DKD: diabetic kidney disease.
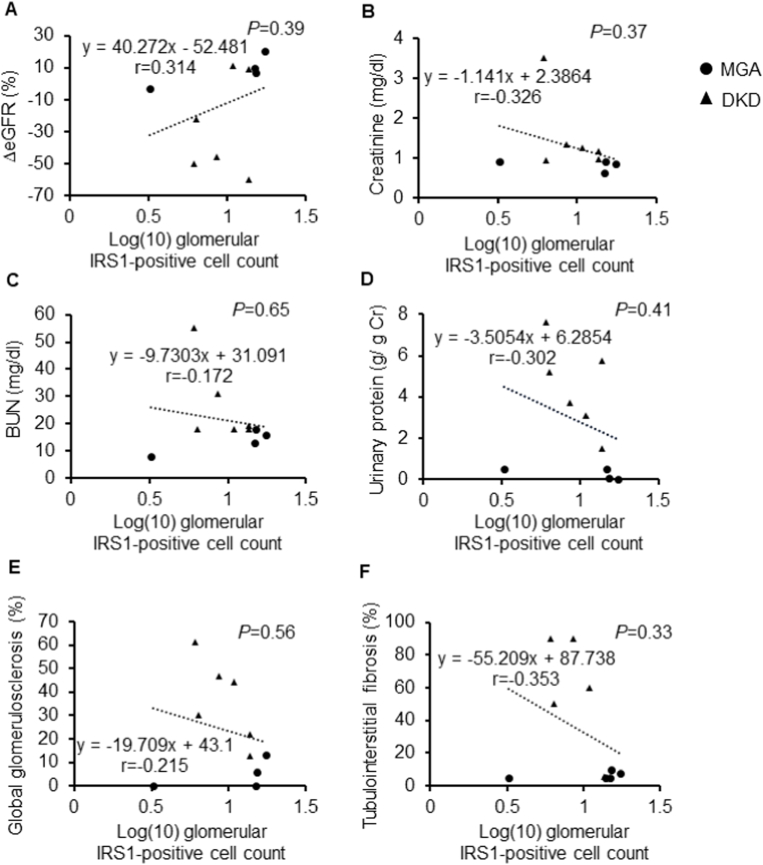
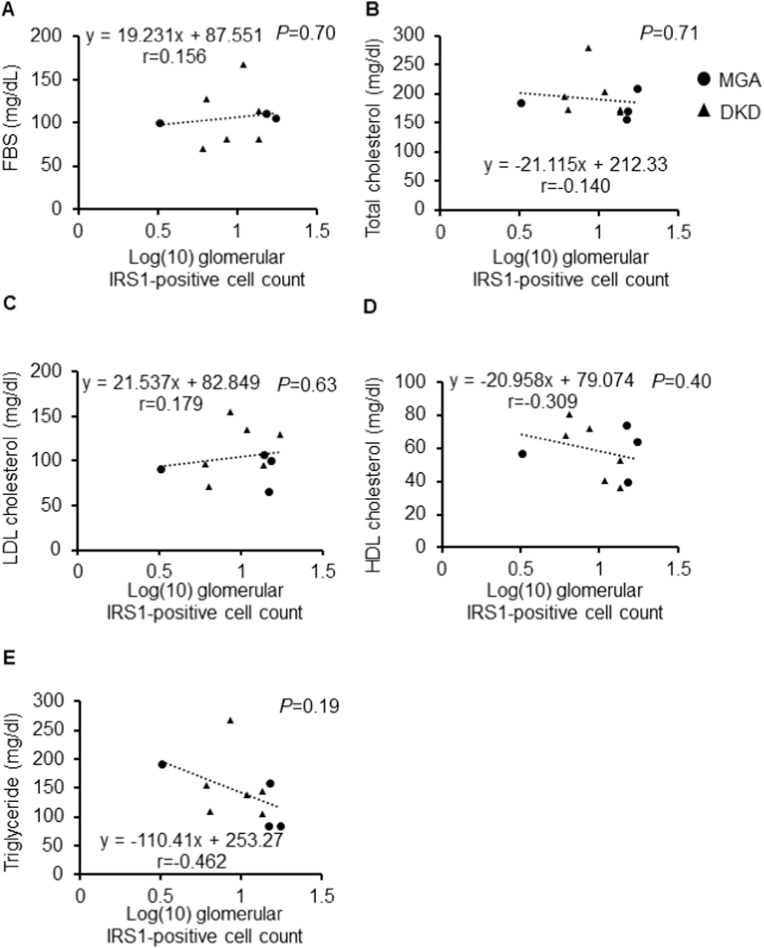
Serum albumin level was decreased in DKD, reflecting increased urinary protein levels (3.1 ± 0.4 in DKD and 3.8 ± 0.1 g/dl in MGA, P = 0.0145; Table 1). Changes in eGFR tended to increase with an increase in the number of glomerular IRS1-positive cells, though the differences were not significant (r = 0.314, P = 0.39, Fig. 3A). Similar to the relation of changes in eGFR with IRSI positivity, the higher the number of glomerular IRS1-positive cells, the lower the serum creatinine, BUN, and urinary protein levels tended to be (serum creatinine: r = −0.326, P = 0.37; BUN: r = −0.172, P = 0.65; urinary protein: r = −0.302, P = 0.41; Fig. 3B–D). Regarding renal pathology, a higher number of glomerular IRS1-positive cells were related with a greater and lower degree of global glomerulosclerosis and interstitial fibrosis, respectively (global glomerular sclerosis: r = −0.215, P = 0.56; tubulointerstitial fibrosis: r = −0.353, P = 0.33; Fig. 3E and F). No association was found between fasting blood sugar levels or other blood biochemical data and glomerular IRS1 positivity (fasting blood sugar: r = 0.156, P = 0.70: total cholesterol: r = −0.140, P = 0.71; LDL cholesterol: r = 0.179, P = 0.63: HDL cholesterol: r = −0.309, P = 0.40; Fig. 4A–D). Although not statistically significant, higher triglyceride levels tended to be correlated with fewer glomerular IRS1-positive cells (r = −0.462, P = 0.19; Fig. 4E).
Fig. 3.
Association of the IRS1-positive cell count with renal function markers and renal morphology.
Correlation between the IRS1-positive cell count and estimated glomerular filtration rate (A), serum creatinine (B), blood urea nitrogen (BUN) (C), urine protein-creatinine ratio (D), global glomerulosclerosis (E), and tubulointerstitial fibrosis (F). The log-transformed values of IRS1 signals were used. IRS1: insulin receptor substrate 1.
Fig. 4.
Correlation between the IRS1-positive cell count and clinical parameters. Correlation between the IRS1-positive cell count and fasting blood sugar (FBS) (A), total cholesterol (B), low density lipoprotein (LDL) cholesterol (C), high density lipoprotein (HDL) cholesterol (D), and triglyceride (E). The log-transformed values of IRS1 signals were used. IRS1: insulin receptor substrate 1.
4. Discussion
The present study demonstrated that IRS1 is abundantly expressed in the glomeruli in MGA and significantly decreased in DKD. Furthermore, glomerular IRS1 positivity tended to be negatively correlated with global glomerulosclerosis and tubulointerstitial fibrosis; however, the differences were not statistically significant.
In diabetes and insulin-resistant states, while the IRS1/Akt/eNOS pathway is selectively inhibited, the MAPK remains uninhibited [4,16]. Furthermore, our previous data indicated that diabetes inhibited the phosphorylation of Akt, eNOS, and glycogen synthase kinase (GSK) 3α, decreased the protein levels of IRS1, and had an increased association with ubiquitin degradation in glomerular endothelial cells [4]. Studies using mice overexpressing PKCβ2 in endothelial cells (EC-PKCβ2) showed impaired insulin/IRS1 signaling in the renal cortex, with diabetic EC-PKCβ2 showing increased albuminuria and glomerulosclerosis [17,18]. In contrast, overexpression of IRS1 or the chemical PKCβ inhibitor ruboxistaurin reversed the inhibitory effect of high glucose [4]. These data suggest that activation of IRS1 in glomerular endothelial cells could be a novel treatment approach for DKD.
A higher number of glomerular IRS1-positive cells tended to be associated with improved renal function prognosis and proteinuria. However, the lack of statistically significant differences in this study may be due to the similar levels of glycemic control in the DKD and MGA groups. Furthermore, administration of DPP-4 inhibitors in almost all patients with DKD may have also contributed to the lack of statistical differences from the MGA groups; DPP-4 inhibitors may induce renoprotective effects via their pleiotropic actions [[19], [20], [21]]. Interestingly, linagliptin, a DPP-4 inhibitor, improved renal pathology and function by restoring IRS1/Akt signaling, which were inhibited in DKD [8].
Similar to DPP-4 inhibitor administration in this study, RAAS inhibitors were administered in most patients with DKD. RAAS plays an important role in the pathogenesis of DKD [22,23]. Angiotensin-converting enzyme converts angiotensin I to angiotensin II (Ang II), increasing the aldosterone secretion, antidiuretic hormone secretion, microvascular constriction, sympathetic nerve activity, inflammation, and the quantity of the extracellular matrix [7,17,24]. We have previously shown that the Ang II-activated transforming growth factor-β (TGF-β)/Smad1 pathway induces mesangial expansion [25]. Furthermore, Ang II inhibits vascular endothelial growth factor-A action in glomerular endothelial cells [26]. RAAS inhibitors directly inhibit Smad1 expression, thereby decreasing mesangial expansion in DKD [25]. Glucagon-like peptide-1 (GLP-1), the levels of which are elevated by DPP-4 inhibitors, suppresses inflammation caused by Ang II signaling [17]. Thus, the fact that patients in this study were adequately treated for DKD using RAAS and DPP-4 inhibitors may be why no statistically significant differences were observed in the relationship between glomerular IRS1-positive cells and various parameters in MGA and DKD.
The number of glomerular IRS1-positive cells did not correlate with the levels of total cholesterol, LDL cholesterol, or HDL cholesterol, while a higher serum triglyceride level tended to lower the number of glomerular IRS1-positive cells. A recent study has shown that IRS1 gene polymorphism may influence insulin resistance and is associated with non-alcoholic fatty liver disease [27]. However, further investigation is required to elucidate the mechanisms underlying the association between triglyceride and IRS1 expression.
In obesity, free fatty acid is known to be increased, and it may activate the PKC pathway [28]. We have reported that abnormal metabolic factors, such as hyperglycemia and free fatty acids, can induce inflammation and selective insulin resistance, leading to IRS1 dysfunction in the glomerulus [4,29,30].
Resistance to insulin signaling and its action in the glomerulus are selective when the IRS1/PI3K/Akt pathway is enhanced; contrastingly, activation of the extracellular signal-regulated kinase (Erk)/MAPK pathway by insulin remains fully active [4]. This type of selective loss of insulin signaling in insulin resistant and diabetic states has been reported in many vascular beds, including the glomerulus, aorta, and microvessels in adipose tissues [16,31,32].
This study has several limitations. Sample size in this study was small. Glomerular protein expression of IRS1 was studied only by immunostaining, not by western blotting assay. This was a retrospective analysis of a cohort from a single center. As we exclusively examined patients who had undergone kidney biopsy, the patient cohort investigated here might not be a true representation of the overall population of individuals with MGA or DKD. Examining glomerular IRS1-positive cells through kidney biopsy can help in comprehending the pathophysiology and prognosis of DKD. Nonetheless, the limitation of this approach is its impracticality for frequent and regular employment in outpatient settings.
In summary, the present study suggests that the protein expression of glomerular IRS1 reflects the renal pathology and may predict renal outcomes in patients with MGA and DKD. Further large-scale investigations regarding glomerular IRS1 levels among patients with MGA or DKD may validate the study results.
Funding
This work was supported by JSPS KAKENHI, Japan Grant Number 22K08368 (to A. Mima).
Data availability statement
The data that support the findings of this study are available in the figshare at https://figshare.com/articles/dataset/IRS1_DKD/20484177, but restrictions apply to the availability of these data, and so are not publicity available. Data are however available from the author upon reasonable request and with permission of figshare.
CRediT authorship contribution statement
Akira Mima: Conceptualization, Methodology, Software, Data curation, Writing – original draft, preparation, Writing – review & editing. Ami Murakami: Visualization, Investigation, Writing – review & editing. Rina Lee: Visualization, Investigation, Software, Validation. Shinji Lee: Supervision.
Declaration of competing interest
A. Mima received a speaker's honorarium from Novartis, Kyowa Kirin, Mitsubishi Tanabe, Torii, Kowa, Bayer, Eli Lilly, Mochida, Astellas, and Boehringer Ingelheim. A. Mima received research grants from Kyowa Kirin, Sumitomo Pharma, Otsuka, Torii, Kowa, and Boehringer Ingelheim.
Acknowledgments
The authors would like to thank Dr. Tatsuhiko Mori and Dr. Yoshinobu Hirose from Osaka Medical and Pharmaceutical University for their valuable discussions on renal pathology. The authors would also like to thank Ms. Ayumi Noguchi for her contribution in preparing the renal pathology data and for her insights on statistical analysis. In addition, we extend our appreciation to Genetic Labs Co., Ltd. for providing IRS1 staining and to Honyaku Center Inc. for their assistance with English language editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.metop.2023.100240.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
Fig. S1.
Fig. S2.
References
- 1.Diabetes C., Complications Trial Research G., Nathan D.M., Genuth S., Lachin J., Cleary P., et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 3.Saran R., Robinson B., Abbott K.C., Bragg-Gresham J., Chen X., Gipson D., et al. US renal data system 2019 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2020;75(1 Suppl 1):A6–A7. doi: 10.1053/j.ajkd.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Mima A., Ohshiro Y., Kitada M., Matsumoto M., Geraldes P., Li C., et al. Glomerular-specific protein kinase C-beta-induced insulin receptor substrate-1 dysfunction and insulin resistance in rat models of diabetes and obesity. Kidney Int. 2011;79(8):883–896. doi: 10.1038/ki.2010.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mima A., Qi W., King G.L. Implications of treatment that target protective mechanisms against diabetic nephropathy. Semin Nephrol. 2012;32(5):471–478. doi: 10.1016/j.semnephrol.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mima A. Diabetic nephropathy: protective factors and a new therapeutic paradigm. J Diabet Complicat. 2013;27(5):526–530. doi: 10.1016/j.jdiacomp.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Mima A. Inflammation and oxidative stress in diabetic nephropathy: new insights on its inhibition as new therapeutic targets. J Diabetes Res. 2013;2013 doi: 10.1155/2013/248563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mima A., Yasuzawa T., Nakamura T., Ueshima S. Linagliptin affects IRS1/Akt signaling and prevents high glucose-induced apoptosis in podocytes. Sci Rep. 2020;10(1):5775. doi: 10.1038/s41598-020-62579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mima A. Prediction of decreased estimated glomerular filtration rate using liver fibrosis markers: a renal biopsy-based study. Sci Rep. 2022;12(1) doi: 10.1038/s41598-022-22636-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gross J.L., de Azevedo M.J., Silveiro S.P., Canani L.H., Caramori M.L., Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28(1):164–176. doi: 10.2337/diacare.28.1.164. [DOI] [PubMed] [Google Scholar]
- 11.Hsu S.M. Immunohistochemistry. Methods Enzymol. 1990;184:357–363. doi: 10.1016/0076-6879(90)84294-q. [DOI] [PubMed] [Google Scholar]
- 12.Bratthauer G.L. The avidin-biotin complex (ABC) method and other avidin-biotin binding methods. Methods Mol Biol. 2010;588:257–270. doi: 10.1007/978-1-59745-324-0_26. [DOI] [PubMed] [Google Scholar]
- 13.Fern R.J., Yesko C.M., Thornhill B.A., Kim H.S., Smithies O., Chevalier R.L. Reduced angiotensinogen expression attenuates renal interstitial fibrosis in obstructive nephropathy in mice. J Clin Invest. 1999;103(1):39–46. doi: 10.1172/JCI4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peake M., Whiting M. Measurement of serum creatinine--current status and future goals. Clin Biochem Rev. 2006;27(4):173–184. [PMC free article] [PubMed] [Google Scholar]
- 15.Oshima M., Toyama T., Haneda M., Furuichi K., Babazono T., Yokoyama H., et al. Estimated glomerular filtration rate decline and risk of end-stage renal disease in type 2 diabetes. PLoS One. 2018;13(8) doi: 10.1371/journal.pone.0201535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang Z.Y., Lin Y.W., Clemont A., Feener E.P., Hein K.D., Igarashi M., et al. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J Clin Invest. 1999;104(4):447–457. doi: 10.1172/JCI5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mima A., Hiraoka-Yamomoto J., Li Q., Kitada M., Li C., Geraldes P., et al. Protective effects of GLP-1 on glomerular endothelium and its inhibition by PKCbeta activation in diabetes. Diabetes. 2012;61(11):2967–2979. doi: 10.2337/db11-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mima A. Incretin-based therapy for prevention of diabetic vascular complications. J Diabetes Res. 2016;2016 doi: 10.1155/2016/1379274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanasaki K., Shi S., Kanasaki M., He J., Nagai T., Nakamura Y., et al. Linagliptin-mediated DPP-4 inhibition ameliorates kidney fibrosis in streptozotocin-induced diabetic mice by inhibiting endothelial-to-mesenchymal transition in a therapeutic regimen. Diabetes. 2014;63(6):2120–2131. doi: 10.2337/db13-1029. [DOI] [PubMed] [Google Scholar]
- 20.Mima A. A narrative review of diabetic kidney disease: previous and current evidence-based therapeutic approaches. Adv Ther. 2022;39(8):3488–3500. doi: 10.1007/s12325-022-02223-0. [DOI] [PubMed] [Google Scholar]
- 21.Mima A. Mitochondria-targeted drugs for diabetic kidney disease. Heliyon. 2022;8(2) doi: 10.1016/j.heliyon.2022.e08878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qian Y., Feldman E., Pennathur S., Kretzler M., Brosius F.C., 3rd From fibrosis to sclerosis: mechanisms of glomerulosclerosis in diabetic nephropathy. Diabetes. 2008;57(6):1439–1445. doi: 10.2337/db08-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mima A., Arai H., Matsubara T., Abe H., Nagai K., Tamura Y., et al. Urinary Smad1 is a novel marker to predict later onset of mesangial matrix expansion in diabetic nephropathy. Diabetes. 2008;57(6):1712–1722. doi: 10.2337/db07-1726. [DOI] [PubMed] [Google Scholar]
- 24.Culver S., Li C., Siragy H.M. Intrarenal angiotensin-converting enzyme: the old and the new. Curr Hypertens Rep. 2017;19(10):80. doi: 10.1007/s11906-017-0778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mima A., Matsubara T., Arai H., Abe H., Nagai K., Kanamori H., et al. Angiotensin II-dependent Src and Smad1 signaling pathway is crucial for the development of diabetic nephropathy. Lab Invest. 2006;86(9):927–939. doi: 10.1038/labinvest.3700445. [DOI] [PubMed] [Google Scholar]
- 26.Mima A., Kitada M., Geraldes P., Li Q., Matsumoto M., Mizutani K., et al. Glomerular VEGF resistance induced by PKCdelta/SHP-1 activation and contribution to diabetic nephropathy. Faseb J. 2012;26(7):2963–2974. doi: 10.1096/fj.11-202994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhatt S.P., Guleria R. Association of IRS1 (Gly972Arg) and IRS2 (Gly1057Asp) genes polymorphisms with OSA and NAFLD in asian Indians. PLoS One. 2021;16(8) doi: 10.1371/journal.pone.0245408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inoguchi T., Li P., Umeda F., Yu H.Y., Kakimoto M., Imamura M., et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49(11):1939–1945. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- 29.Mima A., Qi W., Hiraoka-Yamomoto J., Park K., Matsumoto M., Kitada M., et al. Retinal not systemic oxidative and inflammatory stress correlated with VEGF expression in rodent models of insulin resistance and diabetes. Invest Ophthalmol Vis Sci. 2012;53(13):8424–8432. doi: 10.1167/iovs.12-10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mima A., Yasuzawa T., King G.L., Ueshima S. Obesity-associated glomerular inflammation increases albuminuria without renal histological changes. FEBS Open Bio. 2018;8(4):664–670. doi: 10.1002/2211-5463.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuboki K., Jiang Z.Y., Takahara N., Ha S.W., Igarashi M., Yamauchi T., et al. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo : a specific vascular action of insulin. Circulation. 2000;101(6):676–681. doi: 10.1161/01.cir.101.6.676. [DOI] [PubMed] [Google Scholar]
- 32.Li Q., Park K., Li C., Rask-Madsen C., Mima A., Qi W., et al. Induction of vascular insulin resistance and endothelin-1 expression and acceleration of atherosclerosis by the overexpression of protein kinase C-beta isoform in the endothelium. Circ Res. 2013;113(4):418–427. doi: 10.1161/CIRCRESAHA.113.301074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available in the figshare at https://figshare.com/articles/dataset/IRS1_DKD/20484177, but restrictions apply to the availability of these data, and so are not publicity available. Data are however available from the author upon reasonable request and with permission of figshare.