Abstract
Transthyretin related cardiac amyloidosis (TTR-CA) is an infiltrative cardiomyopathy that cause heart failure with preserved ejection fraction, mainly in aging people. Due to the introduction of a non invasive diagnostic algorithm, this disease, previously considered to be rare, is increasingly recognized. The natural history of TTR-CA includes two different stages: a presymptomatic and a symptomatic stage. Due to the availability of new disease-modifying therapies, the need to reach a diagnosis in the first stage has become impelling. While in variant TTR-CA an early identification of the disease may be obtained with a genetic screening in proband's relatives, in the wild-type form it represents a challenging issue. Once the diagnosis has been made, in order to identifying patients with a higher risk of cardiovascular events and death it is necessary to focus on risk stratification. Two prognostic scores have been proposed both based on biomarkers and laboratory findings. However, a multiparametric approach combining information from electrocardiogram, echocardiogram, cardiopulmonary exercise test and cardiac magnetic resonance may be warranted for a more comprehensive risk prediction. In this review, we aim at evaluating a step by step risk stratification, providing a clinical diagnostic and prognostic approach for the management of patients with TTR-CA.
Keywords: risk stratification, cardiac amyloidosis, natural history, transthyretin, heart failure, arrhythmias
Introduction
Transthyretin related cardiac amyloidosis (TTR-CA) is an infiltrative cardiomyopathy caused by extracellular deposition of transthyretin (TTR)-derived insoluble amyloid fibrils in the myocardium. TTR-CA is generally considered to be rare but in the last 20 years, due to advanced technology and improvement of diagnostic tools, it has been increasingly recognized (1). Two distinct types of the TTR protein (variant, vTTR, or wild type, wtTTR) become unstable, and misfolding forms aggregate resulting in amyloid fibrils.
The true prevalence of both forms of TTR-CA is hard to define, since the familial form present a highly uneven geographical distribution and the senile form is often underdiagnosed (2). The development of effective and specific drugs for TTR-CA marked the beginning of a new era for this disease once deemed incurable (3, 4).
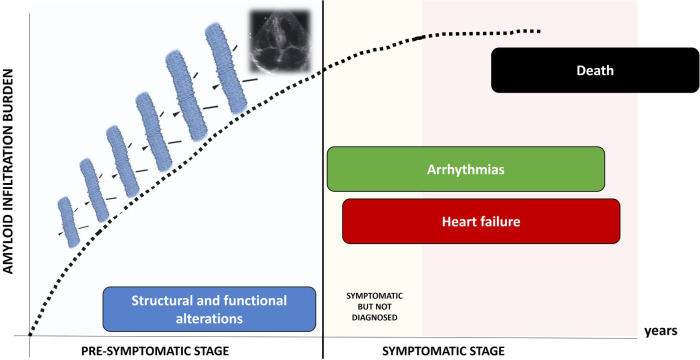
Clinical course of TTR-CA is characterized by two different stages (Figure 1): a pre-symptomatic stage, when fibrils progressively infiltrate the heart, causing initial and subclinical structural and functional alterations; in this stage, patients are usually asymptomatic and often elude the diagnosis; in a subsequent time, the symptomatic stage, the disease clinically manifests: patients suffer from progressive heart failure (HF), arrhythmias and conduction system disease, undergo clinical evaluation and are eventually diagnosed. Death occurs in a median time of 3–4 years (1).
Figure 1.
Natural history of TTR-CA: the pre-symptomatic stage and the symptomatic stage.
To improve care and risk stratification of TTR-CA patients, the identification of clinical and instrumental features associated with both the development and the progression of the disease is of paramount importance. Although the number of diagnoses of TTR-CA has increased markedly during the last 20 years (5), the main challenge in the management of this disease still remains its early recognition. Indeed, most of patients achieve a diagnosis when they have already reached hard endpoints, as arrhythmias or HF hospitalizations. Moreover, the specific TTR-CA therapies result more effective in patients with mild symptoms, further emphasizing the need to identify TTR-CA patients before clinical conditions worsen and HF develops and progresses.
Pre-symptomatic stage
Before the occurrence of clinical manifestations, clinical efforts need to be focused on recognizing those patients who can develop the disease and need to be screened.
In vTTR, more than 140 different mutations of the TTR gene have been described and specific variants often correlate with different clinical manifestations, ranging from a prevalent cardiac phenotype to mixed and prevalent neurological ones (6). The various mutations are associated with specific phenotypes and some of them are endemic in specific geographical areas (7). For example, the variant Val30Met has different manifestations and penetrance according to the geographical location, ranging from a fast-progressing disease dominated by neuropathy with early onset and high penetrance (Portuguese form), to a slowly progressing disease with late onset and low penetrance (Swedish form) (8, 9). Typical “cardiogenic” variants, like Val122Ile in North America and Ile68Leu in Italy, are characterized by clinical manifestations very similar to those of wtTTR, including a high prevalence of carpal tunnel syndrome (CTS), with a comparable age of onset (seventh-eight decade of life) (10, 11). These cardiac forms share with the wtTTR also the male prevalence, in contrast to mixed forms like those caused by Phe64Leu or Glu89Gln, in which the gender disparity is milder or absent (12, 13). In general, women who carry a pathogenic variant less likely have cardiac involvement and among asymptomatic carriers there is a relatively larger female presence (14), suggesting a lower penetrance in women. Furthermore, it has been reported that inheriting the pathogenetic TTR variant from the mother can cause an anticipation of disease onset and consequently a higher penetrance (15). In summary, carrying a pathogenic TTR mutation confers a variable risk of developing CA, which depends on the specific variant, the geographical area, gender and transmitting parent (father vs. mother). Genotype-positive phenotype-negative individuals should be periodically visited in order to detect the development of minor disease signs and in this way allow an early initiation of a specific therapy. It has been proposed that the clinical follow-up should start about 10 years before the predicted age of disease onset (PADO), estimated from the typical age of onset associated with the specific mutation, the age of onset of the affected relatives and the sex of the transmitting parent (16).
In wtTTR, extracardiac manifestations like CTS, which can represent an early sign of the systemic disease, are likely the only predictive factors of its development. CTS is a very frequent finding among TTR-CA patients, especially in the wild-type form, because of selective amyloid deposition in the transverse carpal ligament (17). This particular localisation may be explained by the presence of repetitive mechanical stimuli in carpal tunnel area, as well as in the heart, that facilitate TTR amyloidogenesis through the activation of plasminogen (18). Compared to the general population, the prevalence of CTS in TTR-CA is higher, ranging from 15% to 60%, especially in men in the seventh and eight decades (19, 20). It is well known that the diagnosis of CTS is often followed by the development of CA with a characteristic latency of 5–10 years (21). This interval is the most likely explanation for the low incidence of CA (2%) found by Sperry et al. (22) in patients undergoing carpal tunnel surgical release, despite the fact that amyloid deposits have been found in 10% patients. On the other hand, this approach offers the opportunity of a very early screening of patients at risk to develop TTR-CA in the following years. Indeed, a history of CTS has been associated with a 12 times higher risk of amyloidosis as compared to matched control subject without CTS; an odd that raises to 30 times in the case of bilateral CTS (23). A recent study (24) has shown that the prevalence of wtTTR, 5–15 years after surgery for bilateral CTS, reached 8.8% in men, getting closer to the aforementioned prevalence of TTR deposits in the carpal tunnel ligament (22), and suggesting that amyloid deposition in this specific site could predict future development of CA. Post hoc subgroup analysis has highlighted a prevalence of 25.7% in men >70 years old, after excluding patients with BMI > 30 and occupational risk factors for CTS. Moreover, this screening approach has allowed an early diagnosis, considering that almost all the TTR-CA patients identified had low disease severity scores (24). The presence of left ventricular (LV) hypertrophy or other red flags, especially NT-proBNP and a relative apical sparring pattern, may allow to increase the sensitivity of the screening method (25, 26).
Finally, some echocardiographic features may raise the suspicion of TTR-CA in the context of the LV hypertrophy. In a multicentric study, evaluating more than 1,000 patients with increased heart wall thickness, in which amyloidosis was suspected, relative wall thickness, evidence of diastolic dysfunction (E/e'), TAPSE and strain variables assessing the relative apical sparing had best diagnostic accuracy to individuate those with amyloid infiltration (27). Moreover, a simple score, obtained by the product of relative wall thickness and E/e' ratio, has been demonstrated to possibly have a role as an initial screening tool for patients with suspected TTR-CA (28). Recently, Merlo et al. in a multicentric Italian study enrolling 5,315 unselected consecutive patients undergoing echocardiogram for reasons other than known or suspected cardiac amyloidosis (CA), showed that 1.2% of them reached a diagnosis of TTR-CA. Echocardiographic findings as non-dilated, hypertrophic hearts with LV ejection fraction >50% in combination with apical sparing or at least two red flags (i.e., restrictive filling pattern, granular sparkling, pericardial effusion, interatrial septum thickness >5 mm, atrio-ventricular valve thickness >5 mm) provide a diagnostic accuracy >70% (29). An ECG discordance with echocardiographic findings of hypertrophied not dilated LV or a slightly increase of cardiac biomarkers further increase the suspicion of TTR-CA (1, 8).
Symptomatic stage
Risk factors for heart failure and death
HF is the main complication of TTR-CA, both in terms of number of hospitalizations and of mortality. In recent years, TTR-CA has been increasingly recognized as a cause of HF hospitalizations, exceeding the rate of 65 cases per 100,000 people/year in several regions of the United States (30). Current pharmacological management of HF is limited in patients with TTR-CA: drugs as beta blockers and renin angiotensin system inhibitors may be poorly tolerated (31). Moreover, the reduced size of LV cavity and the frequent involvement of right ventricle may hamper the use of long-term ventricular assist devices. Finally, heart transplantation may be an effective option, but only in carefully selected patients (32).
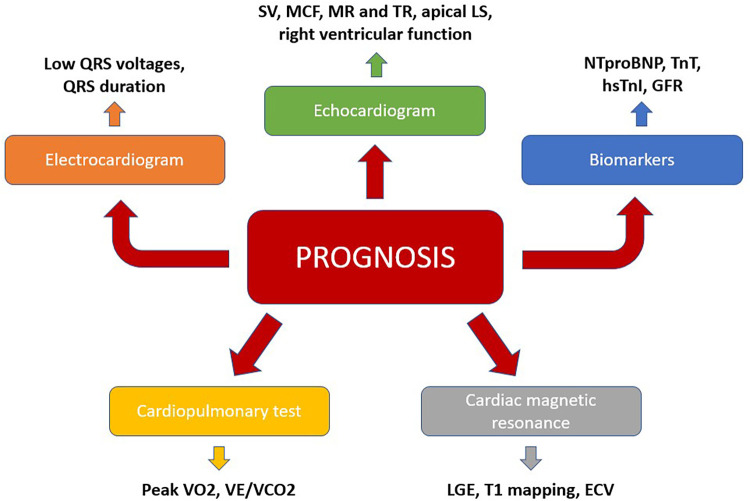
For these reasons, risk stratification of TTR-CA patients is imperative in order to identify patients with at risk of a faster disease progression towards HF, as this subset requires earlier and more aggressive therapies, as well as closer monitoring. In this regard, a multimodal approach, which integrates clinical, biomarkers and instrumental indicators need to be used (Figure 2).
Figure 2.
A multiparametric approach to predict prognosis in TTR-CA. ECV, extracellular volume; GLS, global longitudinal strain; GFR, glomerular filtration rate; LGE, late gadolinium enhancement; MR, mitral regurgitation; TR, tricuspid regurgitation; VCO2, carbon dioxide output; VE, minute ventilation; VO2, maximum oxygen consumption.
Risk scores
In 2016, Grogan et al. (33) proposed for wtTTR a three-group classification system (Mayo score) according to cut-offs of troponin T (50 ng/L) and NT-proBNP (3,000 ng/L). The 4-year survival was 57%, 42% and 18% for stage I (both values below the cut-off), stage II (one marker above the cut-off) and stage III (both markers above the cut-off), respectively. This staging system is not validated for vTTR. Moreover, the use of troponin T appeared to be overwhelmed by the current worldwide diffusion of newer high sensitivity troponin assays, which not only differ in sensitivity, but also give different numerical results as compared to older troponin assays. In 2018, Gillmore et al. (34) developed a three-stage grading system (NAC score) for vTTR and wtTTR amyloidosis using NT-proBNP (cut-off: 3,000 ng/L) and glomerular renal filtrate (cut-off: 45 ml/min/1.73 m2) with median survival of 69, 47 and 24 months in stage I, II and III, respectively. Since the number of early diagnosis has recently increased, a very early stage (Ia) defined by low NT-proBNP concentration (<500 pg/ml, < 1,000 pg/ml with atrial fibrillation) and need for low doses of loop diuretics (<0.75 mg/kg) has been proposed (35) to better risk stratify CA patients. Stage Ia patients had a longer median survival compared with stage Ib (>100 months vs. 75 months), comparable to the age- and gender-matched general population. Despite this, a considerable cardiovascular morbidity still characterizes this stage, getting worse during the follow-up period, even in the patients with primary non-cardiovascular clinical presentation (36). Besides the aforementioned inter-assay variability of troponin, the accuracy of the “Mayo” staging system (33) is limited by the incompleteness of data regarding some variables and by the lack of external validation. Compared with NAC staging system, externally validated in an unrelated French cohort (34), it provides less effective prognostic discrimination, especially between stage I and stage II (35).
Alongside these two scores, other clinical markers have been proposed in recent years to improve their accuracy. In 2020, Cheng et al. (37) demonstrated that diuretic dose and NYHA functional class were strong independent predictors of all-cause mortality and the composite outcome of all-cause mortality or cardiac transplantation. This study, including both vTTR e wtTTRpatients, reported the incremental value of these parameters added to the Mayo and NAC staging systems. According to a recent study by the University of Heidelberg (38), the risk score proposed by Gillmore et al. for TTR-CA may not be sufficient to predict outcomes leading to advanced HF. In this study, a simple risk stratification score (“HeiRisk” score) including clinical parameters and biomarkers was generated to identify patients with end-stage cardiac light-chain (AL) or TTR amyloidosis in order to facilitate clinical decisions, such as listing for heart transplantation. This study showed that only cardiac biomarkers - hsTnT (cut off: 55 pg/ml) and NT-proBNP (cut off: 6,330 ng/L) - and QRS duration (cut off: 104 ms), but not haemodynamic measures, were significant predictors in TTR-CA.
However, all these scores are binary systems with few variables and this, whilst ensures simplicity of use, may be a limitation for an accurate prediction of outcomes, essential to evaluate the effects of new therapies; for this purpose a multiparametric approach is probably required.
Electrocardiogram
The most striking electrocardiographic abnormality in patients with CA is the reduction of QRS voltages, particularly in the limb leads, and the disproportion between QRS voltages and LV thickness at echocardiography (39). Low QRS voltage is defined as a QRS amplitude <5 mm (0.5 mV) in all peripheral leads. This finding, considered pathognomonic of the disease, is present in 60% of AL and only in 20% of TTR-CA, and may reflect the burden of amyloid infiltration in the heart. In a recent study by Cipriani et al. (40), low QRS voltages paired with an advanced disease stage independently predicted cardiovascular death. Together with the NAC staging, low QRS voltages provided incremental prognostic value in TTR-CA.
Echocardiogram
Cardiac amyloid deposition usually causes HF with preserved ejection fraction. Therefore, different echocardiographic tools have been proved useful to define the prognosis of TTR-CA patients, beyond LV ejection fraction. A decreased SV index, which represents a marker of advanced disease, has shown to independently predict prognosis, even after adjustment for NYHA class and NAC staging system (41). Chacko et al. have demonstrated a progressive worsening of structural and functional echocardiographic parameters over time, although only worsening in the degree of mitral and tricuspid regurgitation at 12- and 24-month assessments associated with a worse prognosis (42). Moreover, myocardial contraction fraction (MCF), which is the ratio of LV systolic output to LV myocardial volume, has shown promising result to predict outcomes in CA patients. In the THAOS registry (43) the median survival of patients with MCF < 25% was less than 3 years compared with 6.8 years of patients with MCF ≥ 25%.
In recent years, assessment of LV global longitudinal strain (GLS) has proved to be of great diagnostic and prognostic significance. In patients with TTR-CA, GLS is reduced showing the characteristic apical sparing pattern with a “cherry on top” appearance at the bull's eye plot. Recently, a reduction in apical longitudinal strain (cut off: −14,5%) have shown to be an independent predictor of major cardiac adverse events (44). On the contrary, longitudinal strain of the basal and midcavity sections, where amyloid infiltration is more marked and early, has not been found to predict prognosis (44). These data suggest that a reduction of apical longitudinal strain, typical of the advanced stages of the disease with severe amyloid deposition, is uncommon and probably less helpful in the early course of CA.
Right ventricular dysfunction, assessed by TAPSE (cut off: 14 mm), has been associated with a higher rate of cardiovascular events (45, 46). A recent study (46) has also highlighted that right ventricular free wall strain (cut off: 16%) may have an independent prognostic role for all causes of death. A study by Bandera et al. (47) demonstrated that increased atrial stiffness, identified using echo speckle tracking and characterized by a reduction in the reservoir and contractile function of the atrium, remains independently associated with prognosis after adjusting for known predictors. Notably, the absence of atrial contraction,foundin 22% of patients in sinus rhythmis associated with a significantly poorer prognosis compared topatients who maintain an effective mechanical contraction,andsimilar tothose with atrial fibrillation (47).
Cardiopulmonary exercise test
The cardiopulmonary exercise test (CPET) is the gold standard test todetermine prognosis in chronic HF with reduced ejection fraction (48). CPET is performed to assess the cardiocirculatory exercise response, together with the ventilatory and peripheral muscular responses. All of these parameters can be altered in amyloidosis due to the restrictive cardiomyopathy, cardiac denervation and chronotropic insufficiency. The main CPET characteristics of CA patients include reduced peak VO2, increased VE-VCO2 slope and episodes of oscillatory ventilation (EOV) (49). Peak VO2 and circulatory power has been found to be strongly and independently predictive of death or HF (50, 51). The combination of peak VO2 (cut off: 13 ml/min/kg) and NT-proBNP was the best predictor of all-cause mortality and the composite of mortality or HF-related hospitalization (45). Furthermore, the increase in VE/VCO2 slope (cut off: 40), resulted from several factors like autonomic dysfunction, right ventricular dysfunction and the absence of tidal volume rise during exercise, was shown to be associated with clinical events in wtTTR (49, 52).
Cardiac magnetic resonance
Cardiac magnetic resonance (CMR)has the ability to provide unique information about myocardial tissue composition. Indeed, it can identify and quantify cardiac amyloid deposition, using late gadolinium enhancement (LGE) and T1 mapping with calculation of extracellular volume (ECV).
In CA, LGE shows a characteristic global subendocardial pattern, generally associated with abnormal myocardial and blood-pool gadolinium kinetics (53). Non-contrast T1-mapping has great diagnostic accuracy for CA, being more sensitive than LGE imaging for identifying early disease (54). Transmural LGE has been associated with higher mortality compared to subendocardial pattern, remaining an independent negative predictor of survival in multivariable Cox models, as well as NT-proBNP and stroke volume indexed (55). Both native T1 mapping and ECV correlate with mortality, but only ECV remains independently predictive of prognosis after adjustment for other prognostic factors, as evidence of its robustness as a marker of cardiac infiltration (56).
Risk factors for arrhythmias
Although the clinical course of TTR-CA is dominated by HF and its manifestations, arrhythmias and conduction system diseases are also very common (57). Sudden cardiac death has been reported to be one of the main causes of death (58), although often from pulseless electrical activity. Moreover, cardiac arrhythmias are associated with increased in-hospital mortality and acute HF exacerbations (59).
Atrial fibrillation is the most commonly observed heart rhythm disturbance in CA, especially in wtTTR, where it can be detected in up to 70% of patients (60, 61). The progressive diastolic dysfunction and the increase of filling pressures, together with the selective deposition of amyloid in the atria walls (62), lead to atrial structural and functional remodeling - also called atrial myopathy -, which accounts for the frequency of supraventricular arrhythmias. Age, HF, LV ejection fraction, left atrial size and right atrial pressure have shown to be independent predictors of developing atrial fibrillation (63). A history of atrial fibrillation is strongly associated with prevalent and incident HF (63); however, in contrast to other etiologies of HF, in TTR-CA atrial fibrillation doesn't seem to impact survival and all-cause mortality (60, 61, 63). Previous studies have emphasized the high prevalence of intracardiac thrombi in CA, in particular in patients with atrial fibrillation (64, 65). Restrictive filling pattern and low left atrial appendage emptying velocities at transesophageal echocardiogram have been shown to predict the presence of intracardiac thrombi (63). Furthermore, a significant proportion of arterial thromboembolic events occurred in patient in sinus rhythm or despite adequate anticoagulation therapy due to the amyloidosis related atrial myopathy, that causes a progressive decline of atrial function and, eventually, an electromechanical dissociation (66, 67). In view of this, in patients at high risk of thromboembolic events the execution of a transesophageal echocardiography should be considered before direct current cardioversion (68, 69).
High-grade atrioventricular (AV) blocks are present in 9.5% of TTR-CA patients at the time of diagnosis (70). Amyloid fibrils infiltrate the conduction system, making an increasing number of patients pacemaker (PMK)-dependent as the disease progresses (71). Several studies reported that device implantation is required in about 9%–11% of patients in the years following the diagnosis (70–72). PMK implantation impacts on outcomes, as right ventricular pacing may be associated with worsening HF symptoms, LV ejection fraction decline and mitral regurgitation severity (73). In a recent paper, it has been showed that history of atrial fibrillation, PR interval >200 ms and QRS duration predict future PMK implantation. The presence of these features should advice a close monitoring, while the absence of all these risk factors allow to exclude with great accuracy the need of PMK in the first 6 months after diagnosis (72).
Ventricular tachyarrhythmias, although frequent, have not been thought to contribute significantly to overall mortality in CA, especially TTR-CA (74). On the other hand, previous studies (75, 76) have reported a high rate of appropriate and successful implantable cardioverter defibrillator (ICD) therapies, even if involving mostly AL patients. A recent retrospective study cohort of 130 TTR-CA patients (77) have documented a high rate of ventricular arrhythmias and appropriate ICD therapies, in particular in those patients with systolic dysfunction. The evidence of non-sustained ventricular tachycardia (NSVT) and a history of unexplained syncope has been proposed as criteria for ICD implantation (78). In contrast, in a recent meta-analysis, the predictive value of NSVT has been debated and it has been shown that a NYHA class III-IV is associated with lower rate of appropriate ICD therapies. The physiopathological explanation of this result is that the focal amyloid deposits and associated fibrosis in the early stage of the disease can act as arrhythmogenic foci (79).
However, no studies have demonstrated a survival benefit related to ICD implantation, highlighting the need to better select patients at risk of lethal arrhythmic events. Furthermore, in an elegant study (80) CA was associated with a mortality rate of 26.9% at 1 year after ICD implantation compared with 11.3% among a propensity-matched cohort of patients with other non-ischemic cardiomyopathies; in this context the Authors found 5 predictors of mortality: a history of syncope, NSVT, diabetes mellitus, cerebrovascular disease and renal dysfunction. Therefore, it is clear that the risk of lethal arrhythmias should be balanced with the risk of other competitive causes of mortality. In this regard, ICD implantation should probably be considered in patients with lesser cardiac involvement and in the early stages of the disease (81).
Therapeutic implication of disease staging
The main goal of the emerging disease-modifying therapies - TTR gene silencers and TTR stabilizers - is to prevent further generation or deposition of amyloid fibrils. For this reason, an early diagnosis and a prompt start of this specific treatment allows to obtain a significant benefit in terms of survival and quality of life. On the other hand, patients with delayed diagnosis and advanced disease are unlikely going to benefit from these therapies (82). This is especially true in older patients with higher risk of competitive non-cardiovascular causes of mortality. Moreover, in the ATTR-ACT study (3) patients with NYHA class III disease at baseline had higher rates of cardiovascular-related hospitalizations, suggesting an unfavourable cost-benefit ratio of Tafamidis in this subgroup of patients. Risk scores have not been systematically used as criteria for inclusion or exclusion of patients in trials, neither as endpoints to determine drug efficacy. Nevertheless, it is reasonable to think that the use of these scores would be informative of the potential benefit of the treatment or of its futility.
Conclusion
TTR-CA is increasingly recognized, particularly in older patients. The advent of new disease-modifiers therapies highlights the importance of reaching the diagnosis early, ideally in the pre-symptomatic stage. A multiparametric approach, including not only biomarker scores, but also clinic, electrocardiographic and imaging data, is suggested for a careful risk stratification of mortality and HF-related events, in order to tailor CA management and therapy and to improve outcomes. Identifying reliable predictors of arrhythmic events is still an unmet need and the role of ICD in CA remains unclear. The improvement of survival hopefully related to new therapies will likely change this scenario.
Author contributions
RS, EC, LM, GT and BM: drafted the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The handling editor [JG] declared a past co-authorship with the authors [GT, RS].
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. (2019) 73(22):2872–91. 10.1016/j.jacc.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia-Pavia P, Rapezzi C, Adler Y, Arad M, Basso C, Brucato A, et al. Diagnosis and treatment of cardiac amyloidosis: a position statement of the ESC working group on myocardial and pericardial diseases. Eur Heart J. (2021) 42(16):1554–68. 10.1093/eurheartj/ehab072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. (2018) 379(11):1007–16. 10.1056/NEJMoa1805689 [DOI] [PubMed] [Google Scholar]
- 4.Minamisawa M, Claggett B, Adams D, Kristen AV, Merlini G, Slama MS, et al. Association of patisiran, an RNA interference therapeutic, with regional left ventricular myocardial strain in hereditary transthyretin amyloidosis: the APOLLO study. JAMA Cardiol. (2019) 4(5):466–72. 10.1001/jamacardio.2019.0849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ioannou A, Patel RK, Razvi Y, Porcari A, Sinagra G, Venneri L. Impact of earlier diagnosis in cardiac ATTR amyloidosis over the course of 20 years. Circulation. (2022) 146(22):1657–70. 10.1161/CIRCULATIONAHA.122.060852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maurer MS, Hanna M, Grogan M, Dispenzieri A, Witteles R, Drachman B, et al. Genotype and phenotype of transthyretin cardiac amyloidosis: THAOS (Transthyretin Amyloid Outcome Survey). J Am Coll Cardiol. (2016) 68(2):161–72. 10.1016/j.jacc.2016.03.596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scirpa R, Russo D, Tini G, Sclafani M, Tropea A, Cava F, et al. Clinical translation of genetic testing in TTR amyloidosis: genotype-phenotype correlations, management of asymptomatic carriers and familial screening. Vessel Plus. (2022) 6:52. 10.20517/2574-1209.2021.74 [DOI] [Google Scholar]
- 8.Rapezzi C, Quarta CC, Riva L, Longhi S, Gallelli I, Lorenzini M, et al. Transthyretin-related amyloidoses and the heart: a clinical overview. Nat Rev Cardiol. (2010) 7(7):398–408. 10.1038/nrcardio.2010.67 [DOI] [PubMed] [Google Scholar]
- 9.Conceição I, De Carvalho M. Clinical variability in type I familial amyloid polyneuropathy (Val30Met): comparison between late- and early-onset cases in Portugal. Muscle Nerve. (2007) 35(1):116–8. 10.1002/mus.20644 [DOI] [PubMed] [Google Scholar]
- 10.Gagliardi C, Perfetto F, Lorenzini M, Ferlini A, Salvi F, Milandri A, et al. Phenotypic profile of Ile68Leu transthyretin amyloidosis: an underdiagnosed cause of heart failure. Eur J Heart Fail. (2018) 20(10):1417–25. 10.1002/ejhf.1285 [DOI] [PubMed] [Google Scholar]
- 11.Quarta CC, Buxbaum JN, Shah AM, Falk RH, Claggett B, Kitzman DW, et al. The amyloidogenic V122I transthyretin variant in elderly black Americans. N Engl J Med. (2015) 372(1):21–9. 10.1056/NEJMoa1404852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gentile L, Tournev I, Amass L, Chapman D, Mazzeo A, THAOS investigators. Phenotypic differences of Glu89Gln genotype in ATTR amyloidosis from endemic loci: update from THAOS. Cardiol Ther. (2021) 10(2):481–90. 10.1007/s40119-021-00226-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damy T, Kristen AV, Suhr OB, Maurer MS, Planté-Bordeneuve V, Yu CR, et al. Transthyretin cardiac amyloidosis in continental Western Europe: an insight through the Transthyretin Amyloidosis Outcomes Survey (THAOS). Eur Heart J. (2019) 43(5):391–400. 10.1093/eurheartj/ehz173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caponetti AG, Rapezzi C, Gagliardi C, Milandri A, Dispenzieri A, Kristen AV, et al. Sex-related risk of cardiac involvement in hereditary transthyretin amyloidosis: insights from THAOS. JACC Heart Fail. (2021) 9(10):736–46. 10.1016/j.jchf.2021.05.005 [DOI] [PubMed] [Google Scholar]
- 15.Hellman U, Alarcon F, Lundgren HE, Suhr OB, Bonaiti-Pellié C, Planté-Bordeneuve V. Heterogeneity of penetrance in familial amyloid polyneuropathy, ATTR Val30Met, in the Swedish population. Amyloid. (2008) 15(3):181–6. 10.1080/13506120802193720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grandis M, Obici L, Luigetti M, Briani C, Benedicenti F, Bisogni G, et al. Recommendations for pre-symptomatic genetic testing for hereditary transthyretin amyloidosis in the era of effective therapy: a multicenter Italian consensus. Orphanet J Rare Dis. (2020) 15(1):348. 10.1186/s13023-020-01633-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sekijima Y, Uchiyama S, Tojo K, Sano K, Shimizu Y, Imaeda T, et al. High prevalence of wild-type transthyretin deposition in patients with idiopathic carpal tunnel syndrome: a common cause of carpal tunnel syndrome in the elderly. Hum Pathol. (2011) 42(11):1785–91. 10.1016/j.humpath.2011.03.004 [DOI] [PubMed] [Google Scholar]
- 18.Mangione PP, Verona G, Corazza A, Marcoux J, Canetti D, Giorgetti S, et al. Plasminogen activation triggers transthyretin amyloidogenesis in vitro. J Biol Chem. (2018) 293(37):14192–9. 10.1074/jbc.RA118.003990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milandri A, Farioli A, Gagliardi C, Longhi S, Salvi F, Curti S, et al. Carpal tunnel syndrome in cardiac amyloidosis: implications for early diagnosis and prognostic role across the spectrum of aetiologies. Eur J Heart Fail. (2020) 22(3):507–15. 10.1002/ejhf.1742 [DOI] [PubMed] [Google Scholar]
- 20.Aus dem Siepen F, Hein S, Prestel S, Baumgärtner C, Schönland S, Hegenbart U, et al. Carpal tunnel syndrome and spinal canal stenosis: harbingers of transthyretin amyloid cardiomyopathy? Clin Res Cardiol. (2019) 108(12):1324–30. 10.1007/s00392-019-01467-1 [DOI] [PubMed] [Google Scholar]
- 21.Nakagawa M, Sekijima Y, Yazaki M, Tojo K, Yoshinaga T, Doden T, et al. Carpal tunnel syndrome: a common initial symptom of systemic wild-type ATTR (ATTRwt) amyloidosis. Amyloid. (2016) 23(1):58–63. 10.3109/13506129.2015.1135792 [DOI] [PubMed] [Google Scholar]
- 22.Sperry BW, Reyes BA, Ikram A, Donnelly JP, Phelan D, Jaber WA, et al. Tenosynovial and cardiac amyloidosis in patients undergoing carpal tunnel release. J Am Coll Cardiol. (2018) 72(17):2040–50. 10.1016/j.jacc.2018.07.092 [DOI] [PubMed] [Google Scholar]
- 23.Fosbøl EL, Rørth R, Leicht BP, Schou M, Maurer MS, Kristensen SL, et al. Association of carpal tunnel syndrome with amyloidosis, heart failure, and adverse cardiovascular outcomes. J Am Coll Cardiol. (2019) 74(1):15–23. 10.1016/j.jacc.2019.04.054 [DOI] [PubMed] [Google Scholar]
- 24.Westin O, Fosbøl EL, Maurer MS, Leicht BP, Hasbak P, Mylin AK, et al. Screening for cardiac amyloidosis 5–15 years after surgery for bilateral carpal tunnel syndrome. J Am Coll Cardiol. (2022) 80(10):967–77. 10.1016/j.jacc.2022.06.026 [DOI] [PubMed] [Google Scholar]
- 25.Vianello PF, La Malfa G, Tini G, Mazzola V, Miceli A, Santolini E, et al. Prevalence of transthyretin amyloid cardiomyopathy in male patients who underwent bilateral carpal tunnel surgery: the ACTUAL study. Int J Cardiol. (2021) 329:144–7. 10.1016/j.ijcard.2020.12.044 [DOI] [PubMed] [Google Scholar]
- 26.Ladefoged B, Clemmensen T, Dybro A, Hartig-Andreasen C, Kirkeby L, Gormsen LC, et al. Identification of wild-type transthyretin cardiac amyloidosis in patients with carpal tunnel syndrome surgery (CACTuS) [published online ahead of print, 2022 Oct 3]. ESC Heart Fail. (2023) 10(1):234–44. 10.1002/ehf2.14173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boldrini M, Cappelli F, Chacko L, Restrepo-Cordoba MA, Lopez-Sainz A, Gian-noni A, et al. Multiparametric echocardiography scores for the diagnosis of cardiacamyloidosis. JACC Cardiovasc Imaging. (2020) 13:909–20. 10. 10.1016/j.jcmg.2019.10.011 [DOI] [PubMed] [Google Scholar]
- 28.Aimo A, Chubuchny V, Vergaro G, Barison A, Nicol M, Cohen-Solal A, et al. A simple echocardiographic score to rule out cardiac amyloidosis. Eur J Clin Invest. (2021) 51(5):e13449. 10.1111/eci.13449 [DOI] [PubMed] [Google Scholar]
- 29.Merlo M, Pagura L, Porcari A, Cameli M, Vergaro G, Musumeci B, et al. Unmasking the prevalence of amyloid cardiomyopathy in the real world: results from phase 2 of the AC-TIVE study, an Italian nationwide survey. Eur J Heart Fail. (2022) 24(8):1377–86. 10.1002/ejhf.2504 [DOI] [PubMed] [Google Scholar]
- 30.Gilstrap LG, Dominici F, Wang Y, El-Sady MS, Singh A, Di Carli MF, et al. Epidemiology of cardiac amyloidosis-associated heart failure hospitalizations among fee-for-service medicare beneficiaries in the United States. Circ Heart Fail. (2019) 12(6):e005407. 10.1161/CIRCHEARTFAILURE.118.005407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oghina S, Bougouin W, Bézard M, Kharoubi M, Komajda M, Cohen-Solal A, et al. The impact of patients with cardiac amyloidosis in HFpEF trials. JACC Heart Fail. (2021) 9(3):169–78. 10.1016/j.jchf.2020.12.005 [DOI] [PubMed] [Google Scholar]
- 32.Witteles RM. Cardiac transplantation and mechanical circulatory support in amyloidosis. JACC Cardio Oncol. (2021) 3(4):516–21. 10.1016/j.jaccao.2021.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grogan M, Scott CG, Kyle RA, Zeldenrust SR, Gertz MA, Lin G, et al. Natural history of wild-type transthyretin cardiac amyloidosis and risk stratification using a novel staging system [published correction appears in J Am Coll Cardiol. 2017 Jun 13;69(23):2882]. J Am Coll Cardiol. (2016) 68(10):1014–20. 10.1016/j.jacc.2016.06.033 [DOI] [PubMed] [Google Scholar]
- 34.Gillmore JD, Damy T, Fontana M, Hutchinson M, Lachmann HJ, Martinez-Naharro A, et al. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J. (2018) 39(30):2799–806. 10.1093/eurheartj/ehx589 [DOI] [PubMed] [Google Scholar]
- 35.Cappelli F, Martone R, Gabriele M, Taborchi G, Morini S, Vignini E, et al. Biomarkers and prediction of prognosis in transthyretin-related cardiac amyloidosis: direct comparison of two staging systems. Can J Cardiol. (2020) 36(3):424–31. 10.1016/j.cjca.2019.12.020 [DOI] [PubMed] [Google Scholar]
- 36.Law S, Bezard M, Petrie A, Chacko L, Cohen OC, Ravichandran S, et al. Characteristics and natural history of early-stage cardiac transthyretin amyloidosis. Eur Heart J. (2022) 43(27):2622–32. 10.1093/eurheartj/ehac259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng RK, Levy WC, Vasbinder A, Teruya S, De Los Santos J, Leedy D, et al. Diuretic dose and NYHA functional class are independent predictors of mortality in patients with transthyretin cardiac amyloidosis. JACC Cardio Oncol. (2020) 2(3):414–24. 10.1016/j.jaccao.2020.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kreusser MM, Volz MJ, Knop B, Ehlermann P, Schmack B, Ruhparwar A, et al. A novel risk score to predict survival in advanced heart failure due to cardiac amyloidosis. Clin Res Cardiol. (2020) 109(6):700–13. 10.1007/s00392-019-01559-y [DOI] [PubMed] [Google Scholar]
- 39.Cyrille NB, Goldsmith J, Alvarez J, Maurer MS. Prevalence and prognostic significance of low QRS voltage among the three main types of cardiac amyloidosis. Am J Cardiol. (2014) 114(7):1089–93. 10.1016/j.amjcard.2014.07.026 [DOI] [PubMed] [Google Scholar]
- 40.Cipriani A, De Michieli L, Porcari A, Licchelli L, Sinigiani G, Tini G, et al. Low QRS voltages in cardiac amyloidosis: clinical correlates and prognostic value. JACC Cardio Oncol. (2022) 4(4):458–70. 10.1016/j.jaccao.2022.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chacko L, Martone R, Bandera F, Lane T, Martinez-Naharro A, Boldrini M, et al. Echocardiographic phenotype and prognosis in transthyretin cardiac amyloidosis. Eur Heart J. (2020) 41:1439–47. 10.1093/eurheartj/ehz905 [DOI] [PubMed] [Google Scholar]
- 42.Chacko L, Karia N, Venneri L, Bandera F, Passo BD, Buonamici L, et al. Progression of echocardiographic parameters and prognosis in transthyretin cardiac amyloidosis. Eur J Heart Fail. (2022) 24(9):1700–12. 10.1002/ejhf.2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rubin J, Steidley DE, Carlsson M, Ong ML, Maurer MS. Myocardial contraction fraction by M-mode echocardiography is superior to ejection fraction in predicting mortality in transthyretin amyloidosis. J Card Fail. (2018) 24(8):504–11. 10.1016/j.cardfail.2018.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ternacle J, Bodez D, Guellich A, Audureau E, Rappeneau S, Lim P, et al. Causes and consequences of longitudinal LV dysfunction assessed by 2D strain echocardiography in cardiac amyloidosis. JACC Cardiovasc Imaging. (2016) 9(2):126–38. 10.1016/j.jcmg.2015.05.014 [DOI] [PubMed] [Google Scholar]
- 45.Bodez D, Ternacle J, Guellich A, Galat A, Lim P, Radu C, et al. Prognostic value of right ventricular systolic function in cardiac amyloidosis. Amyloid. (2016) 23(3):158–67. 10.1080/13506129.2016.1194264 [DOI] [PubMed] [Google Scholar]
- 46.Tjahjadi C, Fortuni F, Stassen J, Debonnaire P, Lustosa RP, Marsan NA, et al. Prognostic implications of right ventricular systolic dysfunction in cardiac amyloidosis. Am J Cardiol. (2022) 173:120–7. 10.1016/j.amjcard.2022.02.048 [DOI] [PubMed] [Google Scholar]
- 47.Bandera F, Martone R, Chacko L, Ganesananthan S, Gilbertson JA, Ponticos M, et al. Clinical importance of left atrial infiltration in cardiac transthyretin amyloidosis. JACC Cardiovasc Imaging. (2022) 15(1):17–29. 10.1016/j.jcmg.2021.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure [published correction appears in Eur Heart J. 2021 Oct 14;:]. Eur Heart J. (2021) 42(36):3599–726. 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 49.Banydeen R, Monfort A, Inamo J, Neviere R. Diagnostic and prognostic values of cardiopulmonary exercise testing in cardiac amyloidosis. Front Cardiovasc Med. (2022) 9:898033. 10.3389/fcvm.2022.898033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nicol M, Deney A, Lairez O, Vergaro G, Emdin M, Carecci A, et al. Prognostic value of cardiopulmonary exercise testing in cardiac amyloidosis. Eur J Heart Fail. (2021) 23(2):231–9. 10.1002/ejhf.2016 [DOI] [PubMed] [Google Scholar]
- 51.Hein S, Aus Dem Siepen F, Bauer R, Katus HA, Kristen AV. Peak VO2 is an independent predictor of survival in patients with cardiac amyloidosis. Amyloid. (2018) 25(3):167–73. 10.1080/13506129.2018.1496077 [DOI] [PubMed] [Google Scholar]
- 52.Yunis A, Doros G, Luptak I, Connors LH, Sam F. Use of ventilatory efficiency slope as a marker for increased mortality in wild-type transthyretin cardiac amyloidosis. Am J Cardiol. (2019) 124(1):122–30. 10.1016/j.amjcard.2019.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maceira AM, Joshi J, Prasad SK, Moon JC, Perugini E, Harding I, et al. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. (2005) 111(2):186–93. 10.1161/01.CIR.0000152819.97857.9D [DOI] [PubMed] [Google Scholar]
- 54.Karamitsos TD, Piechnik SK, Banypersad SM, Fontana M, Ntusi NB, Ferreira VM, et al. Noncontrast T1 mapping for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging. (2013) 6(4):488–97. 10.1016/j.jcmg.2012.11.013 [DOI] [PubMed] [Google Scholar]
- 55.Fontana M, Pica S, Reant P, Abdel-Gadir A, Treibel TA, Banypersad SM, et al. Prognostic value of late gadolinium enhancement cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. (2015) 132(16):1570–9. 10.1161/CIRCULATIONAHA.115.016567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martinez-Naharro A, Kotecha T, Norrington K, Boldrini M, Rezk T, Quarta C, et al. Native T1 and extracellular volume in transthyretin amyloidosis. JACC Cardiovasc Imaging. (2019) 12(5):810–9. 10.1016/j.jcmg.2018.02.006 [DOI] [PubMed] [Google Scholar]
- 57.Hartnett J, Jaber W, Maurer M, Sperry B, Hanna M, Collier P, et al. Electrophysiological manifestations of cardiac amyloidosis: JACC: cardioOncology state-of-the-art review. JACC Cardio Oncol. (2021) 3(4):506–15. 10.1016/j.jaccao.2021.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Falk RH, Rubinow A, Cohen AS. Cardiac arrhythmias in systemic amyloidosis: correlation with echocardiographic abnormalities. J Am Coll Cardiol. (1984) 3(1):107–13. 10.1016/s0735-1097(84)80436-2 [DOI] [PubMed] [Google Scholar]
- 59.Thakkar S, Patel HP, Chowdhury M, Patel K, Kumar A, Arora S, et al. Impact of arrhythmias on hospitalizations in patients with cardiac amyloidosis. Am J Cardiol. (2021) 143:125–30. 10.1016/j.amjcard.2020.12.024 [DOI] [PubMed] [Google Scholar]
- 60.Mints YY, Doros G, Berk JL, Connors LH, Ruberg FL. Features of atrial fibrillation in wild-type transthyretin cardiac amyloidosis: a systematic review and clinical experience. ESC Heart Fail. (2018) 5(5):772–9. 10.1002/ehf2.12308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Donnellan E, Wazni OM, Hanna M, Elshazly MB, Puri R, Saliba W, et al. Atrial fibrillation in transthyretin cardiac amyloidosis: predictors, prevalence, and efficacy of rhythm control strategies. JACC Clin Electrophysiol. (2020) 6(9):1118–27. 10.1016/j.jacep.2020.04.019 [DOI] [PubMed] [Google Scholar]
- 62.Cornwell GG, 3rd, Murdoch WL, Kyle RA, Westermark P, Pitkänen P. Frequency and distribution of senile cardiovascular amyloid. A clinicopathologiccorrelation. Am J Med. (1983) 75(4):618–23. 10.1016/0002-9343(83)90443-6 [DOI] [PubMed] [Google Scholar]
- 63.Longhi S, Quarta CC, Milandri A, Lorenzini M, Gagliardi C, Manuzzi L, et al. Atrial fibrillation in amyloidotic cardiomyopathy: prevalence, incidence, risk factors and prognostic role. Amyloid. (2015) 22(3):147–55. 10.3109/13506129.2015.1028616 [DOI] [PubMed] [Google Scholar]
- 64.Feng D, Edwards WD, Oh JK, Chandrasekaran K, Grogan M, Martinez MW, et al. Intracardiac thrombosis and embolism in patients with cardiac amyloidosis. Circulation. (2007) 116(21):2420–6. 10.1161/CIRCULATIONAHA.107.697763 [DOI] [PubMed] [Google Scholar]
- 65.Feng D, Syed IS, Martinez M, Oh JK, Jaffe AS, Grogan M, et al. Intracardiac thrombosis and anticoagulation therapy in cardiac amyloidosis. Circulation. (2009) 119(18):2490–7. 10.1161/CIRCULATIONAHA.108.785014 [DOI] [PubMed] [Google Scholar]
- 66.Cappelli F, Tini G, Russo D, Emdin M, Del Franco A, Vergaro G, et al. Arterial thrombo-embolic events in cardiac amyloidosis: a look beyond atrial fibrillation. Amyloid. (2021) 28(1):12–8. 10.1080/13506129.2020.1798922 [DOI] [PubMed] [Google Scholar]
- 67.Vilches S, Fontana M, Gonzalez-Lopez E, Mitrani L, Saturi G, Renju M, et al. Systemicembolism in amyloidtransthyretincardiomyopathy. Eur J Heart Fail. (2022) 24(8):1387–96. 10.1002/ejhf.2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Russo D, Limite LR, Arcari L, Autore C, Musumeci MB. Predicting the unpredictable: how to score the risk of stroke in cardiac amyloidosis? J Am Coll Cardiol. (2019) 73(22):2910–1. 10.1016/j.jacc.2019.02.078 [DOI] [PubMed] [Google Scholar]
- 69.El-Am EA, Dispenzieri A, Melduni RM, Ammash NM, White RD, Hodge DO, et al. Direct current cardioversion of atrial arrhythmias in adults with cardiac amyloidosis. J Am Coll Cardiol. (2019) 73(5):589–97. 10.1016/j.jacc.2018.10.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Donnellan E, Wazni OM, Saliba WI, Hanna M, Kanj M, Patel DR, et al. Prevalence, incidence, and impact on mortality of conduction system disease in transthyretin cardiac amyloidosis. Am J Cardiol. (2020) 128:140–6. 10.1016/j.amjcard.2020.05.021 [DOI] [PubMed] [Google Scholar]
- 71.Rehorn MR, Loungani RS, Black-Maier E, Coniglio AC, Karra R, Pokorney SD, et al. Cardiac implantable electronic devices: a window into the evolution of conduction disease in cardiac amyloidosis. JACC Clin Electrophysiol. (2020) 6(9):1144–54. 10.1016/j.jacep.2020.04.020 [DOI] [PubMed] [Google Scholar]
- 72.Porcari A, Rossi M, Cappelli F, Canepa M, Musumeci B, Cipriani A, et al. Incidence and risk factors for pacemaker implantation in light-chain and transthyretin cardiac amyloidosis. Eur J Heart Fail. (2022) 24(7):1227–36. 10.1002/ejhf.2533 [DOI] [PubMed] [Google Scholar]
- 73.Donnellan E, Wazni OM, Saliba WI, Baranowski B, Hanna M, Martyn M, et al. Cardiac devices in patients with transthyretin amyloidosis: impact on functional class, left ventricular function, mitral regurgitation, and mortality. J Cardiovasc Electrophysiol. (2019) 30(11):2427–32. 10.1111/jce.14180 [DOI] [PubMed] [Google Scholar]
- 74.Escher F, Senoner M, Doerler J, Zaruba MM, Messner M, Mussner-Seeber C, et al. When and how do patients with cardiac amyloidosis die? Clin Res Cardiol. (2020) 109(1):78–88. 10.1007/s00392-019-01490-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lin G, Dispenzieri A, Kyle R, Grogan M, Brady PA. Implantable cardioverter defibrillators in patients with cardiac amyloidosis. J Cardiovasc Electrophysiol. (2013) 24(7):793–8. 10.1111/jce.12123 [DOI] [PubMed] [Google Scholar]
- 76.Hamon D, Algalarrondo V, Gandjbakhch E, Extramiana F, Marijon E, Elbaz N, et al. Outcome and incidence of appropriate implantable cardioverter-defibrillator therapy in patients with cardiac amyloidosis. Int J Cardiol. (2016) 222:562–8. 10.1016/j.ijcard.2016.07.254 [DOI] [PubMed] [Google Scholar]
- 77.Brown MT, Yalamanchili S, Evans ST, Ram P, Blank EA, Lyle MA, et al. Ventricular arrhythmia burden and implantable cardioverter-defibrillator outcomes in transthyretin cardiac amyloidosis. Pacing Clin Electrophysiol. (2022) 45(4):443–51. 10.1111/pace.14458 [DOI] [PubMed] [Google Scholar]
- 78.Varr BC, Zarafshar S, Coakley T, Liedtke M, Lafayette RA, Arai S, et al. Implantable cardioverter-defibrillator placement in patients with cardiac amyloidosis. Heart Rhythm. (2014) 11(1):158–62. 10.1016/j.hrthm.2013.10.026 [DOI] [PubMed] [Google Scholar]
- 79.Halawa A, Woldu HG, Kacey KG, Alpert MA. Effect of ICD implantation on cardiovascular outcomes in patients with cardiac amyloidosis: a systematic review and meta-anaylsis. J Cardiovasc Electrophysiol. (2020) 31(7):1749–58. 10.1111/jce.14541 [DOI] [PubMed] [Google Scholar]
- 80.Higgins AY, Annapureddy AR, Wang Y, Minges KE, Lampert R, Rosenfeld LE, et al. Survival following implantable cardioverter-defibrillator implantation in patients with amyloid cardiomyopathy. J Am Heart Assoc. (2020) 9(18):e016038. 10.1161/JAHA.120.016038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liżewska-Springer A, Sławiński G, Lewicka E. Arrhythmic sudden cardiac death and the role of implantable cardioverter-defibrillator in patients with cardiac amyloidosis-a narrative literature review. J Clin Med. (2021) 10(9):1858. 10.3390/jcm10091858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Quarta CC, Fontana M, Damy T, Catini J, Simoneau D, Mercuri M, et al. Changing paradigm in the treatment of amyloidosis: from disease-modifying drugs to anti-fibril therapy. Front Cardiovasc Med. (2022) 9:1073503. 10.3389/fcvm.2022.1073503 [DOI] [PMC free article] [PubMed] [Google Scholar]