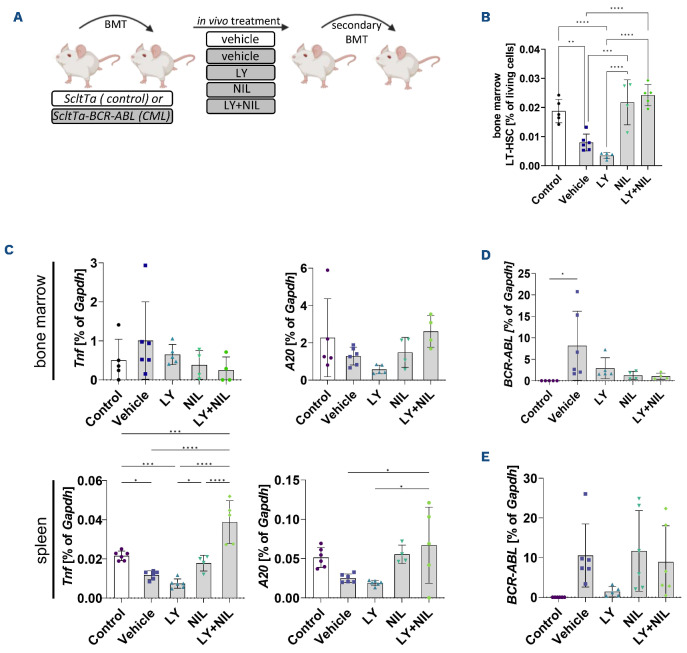
Figure 2.
IKK2 inhibition reduces long-term hematopoietic stem cells and inhibits disease re-initiation in vivo. (A) Schematic overview of the experimental setup: 2x106 bone marrow (BM) cells of either FVB/N Scl-tTa or Scl-tTa-BCR-ABL mice were transplanted into irradiated wild-type FVB/N recipients. Expression of BCR-ABL was induced 1 week after transplantation and treatment was started at 2 weeks after transplant and continued for 3 weeks. During that time, LY2409881 (LY) was administered via intraperitoneal injection, 3 times per week (50 mg/kg body weight, dissolved in 5% dextrose) and/or nilotinib (NIL) treatment was performed via oral gavage, daily (50 mg/kg body weight, dissolved in 10% N-methyl-2-pyrrolidon and 90% polyethylene glycol) and/or the corresponding vehicle control for 3 weeks daily. Following treatment groups were evaluated: ScltTa (vehicle, designated as control) or Scl-tTa-BCR-ABL (vehicle, LY, NIL, and LY + NIL). After sacrifice, 2x106 BM cells of treated animals were transplanted into secondary irradiated recipients. These mice were sacrificed for analyses 6 weeks after transplantation. (B) BM-derived long-term hematopoietic stem cells (LT-HSC) (lin-,Sca1+,ckit+,CD48low,CD150+) of treated recipients, shown as % of living cells. (C) Quantitative real time polymerase chain reaction (qRT-PCR) analyses of Tnf and A20 mRNA expression in BM and spleen of treated primary recipients is shown relative to Gapdh (%). (D) BCR-ABL mRNA expression in BM of primary and secondary (E) recipients. Significance was calculated using one or two-way ANOVA; mean ± standard deviation; *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.