Abstract
Ectopic pregnancies are the leading cause of maternal mortality in the first trimester, with an incidence of 5%–10% of all pregnancy-related deaths. Diagnosis of ectopic pregnancies is difficult due to clinical mimics and non-specific symptoms of abdominal pain and vaginal bleeding. The current standard for ectopic pregnancy diagnosis includes ultrasound imaging and β-human chorionic gonadotropin (β-hCG) monitoring. In addition to β-hCG, serum markers are being explored as a potential for diagnosis, with activin-AB and pregnancy-associated plasma protein A specifically showing promise. Other diagnostic methods include endometrial sampling, with dilation and curettage showing the highest specificity; however, frozen section reduces the diagnostic timeline which may improve outcomes. Treatment options for confirmed ectopic pregnancies include medical, surgical, and expectant management. Chosen treatment methodology is based on β-hCG levels, hematologic stability, and risk of ectopic pregnancy rupture. Current innovations in ectopic pregnancy management aim to preserve fertility and include laparoscopic partial tubal resection with end-to-end anastomosis and uterine artery embolization with intrauterine infusion of methotrexate. Psychological interventions to improve patient mental health surrounding ectopic pregnancy diagnosis and treatment are also valuable innovations. This literature review aims to bring light to current ectopic pregnancy diagnostics, treatments, and future directions.
Keywords: ectopic pregnancy, ectopic pregnancy diagnosis, ectopic pregnancy innovation, ectopic pregnancy treatment, global women’s health, maternal health, public health, reproductive health, Roe v. Wade, women’s health
Introduction
Ectopic pregnancy (EP) ruptures are the leading cause of maternal mortality within the first trimester of pregnancy with a rate of 9%–14% and an incidence of 5%–10% of all pregnancy-related deaths.1 A gestational sac (GS) that implants in a location that is not the uterus is defined as an EP. Women with an EP may have nonspecific symptoms such as lower abdominal pain and vaginal bleeding, often presenting clinically similar to appendicitis, urinary calculi, early pregnancy loss, or trauma.2 Women with this presentation in the first trimester have an EP prevalence in emergency departments as high as 18%, which can be easily misdiagnosed as the previously described clinical mimics.3 Descriptions of EPs and their prevalence are found in Table 1.
Table 1.
Types of ectopic pregnancy (EP) and incidence.
| EP type | Description | Incidence | Characteristics |
|---|---|---|---|
| Tubal | Gestational sac (GS) implants in the fallopian tube | 95% | – |
| Interstitial | GS implants in interstitial portion of fallopian tube and transverses the myometrium in the uterine fundus | 2%–4% | May present later in pregnancy1 |
| Cesarean Scar (CSP) | GS implants into the anterior uterine wall of lower uterine segment where Cesarean scar resides | <1% | Treatment has a high success and high complication rate4 |
| Heterotopic | Concomitant intrauterine pregnancy (IUP) and EP | 1%–3% | Difficult to manage if desired IUP1 |
| Cervical | GS implants in the mucosa of the endocervical canal | <1% | Dilation and curettage in a previous pregnancy in 70% of cases5 |
| Ovarian | GS implantation in the ovaries | <3% | 81% associated with concomitant intrauterine devices1 |
| Abdominal | GS implants in the peritoneal cavity of the abdomen | ~1% | There are some reported cases of term deliveries of healthy babies1 |
Tubal EPs are the most common type and have high maternal morbidity and mortality when ruptured.1 The rate of ruptured EPs is approximately 15% in Western countries, with a retrospective study showing an increased rupture rate during the COVID-19 pandemic.6 Heterotopic EPs are particularly complex, and their incidence is increasing due to a correlation with assisted reproductive technologies (ART), with an incidence of 1/100 pregnancies with in vitro fertilization (IVF) and 1/7000 pregnancies from ART with ovulation induction.1 Increasing rates of IVF are correlated with rising reports of EPs among those individuals. The EP rate among IVF pregnancies is 2.1%–8.6% after embryo transfer, in comparison to 2% in natural conceptions.7 Furthermore, the World Health Organization (WHO) notes an increasing rate of cesarean sections, currently reported as 21% of childbirths globally, which may in turn increase the rate of cesarean scar EPs (CSPs) over time.8 The current standard for diagnostics includes ultrasound (US) imaging—transvaginal (TVUS) or transabdominal (TAUS)—and β-human chorionic gonadotropin (β-hCG) level monitoring. Earlier and more specific EP diagnosis can help reduce maternal mortality rates. Current experimental studies are identifying biomarkers and endometrial sampling techniques that may be useful for more effective diagnostics. Once an EP is diagnosed, treatment can consist of medical, surgical, or expectant management, with innovative emphasis on conservation of fertility.
Research methods
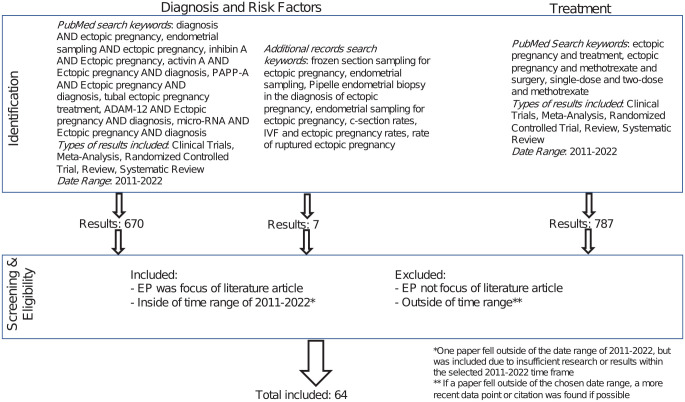
This analysis examines and reviews literature involving the diagnosis and treatment of EPs from 2011 to 2022. Using the online PubMed search engine and Google, this review compiles 64 literature articles. While compiling literature for this review, several methodologies were followed as outlined in Figure 1.
Figure 1.
Research methods.
Research was reviewed and screened based on relevance to EP diagnosis, risk factors, treatment, and clinical trials involving EPs. PubMed search results were filtered by article type to examine meta-analysis, reviews, systematic reviews, reviews, clinical trials, and randomized control trials. Literature was included based on direct relevance to EP and was excluded if not relevant. As an example, search results that included the words “ectopic pregnancy” and “diagnosis,” but did not have the diagnosis of EP as the primary focus were excluded. Books and documents were also excluded article types, as review of up-to-date literature was the goal of the study. One paper out of the 64 included fell outside of the date range of 2011–2022 due to insufficient research or results within the selected time frame.9
In addition to the methodology above, researchers conducted direct Google searches on topics relating to EP with key words as seen in Figure 1. The process of filtering, inclusion, and exclusion followed the outlined methodologies above.
PubMed was the primary search engine for our research and allowed for a comprehensive review of current medical knowledge of EPs. Literature not found in PubMed may not have been included and is one source of bias and limitation to this study. Minimization of search criteria allowed for inclusion of all relevant papers.
At risk populations
Half of patients diagnosed with an EP have no known risk factors.2 Risk factors include prior EP, damage to fallopian tubes, prior pelvic surgery, complications from ascending pelvic infection, prior fallopian tube surgery or pathology, infertility, smoking, age greater than 35 years old, pelvic inflammatory disease, endometriosis, variant reproductive system anatomy, pregnancy that occurs with an intrauterine device (IUD) in place, or use of ART.2,3,10,11
Individuals with IUDs are at lower risk for EP than individuals who do not use contraception; however, 53% of pregnancies that occur in patients with IUDs are ectopic.3 Patients with a history of one prior EP have a 10% risk of subsequent EP recurrence while those with a history of two or more prior EPS have a risk greater than 25%.3 Specific risk factors associated with ART include increased number of embryos transferred, fresh instead of cryo-thawed embryo transfers, and cleavage-stage (Day 3) instead of blastocyst (Day 5) embryo transfers.11
Oral contraceptive use, prior termination of pregnancy, emergency contraception failure, cesarean delivery, and loss of pregnancy have not been found to have any significant association with increased risk of EP.3
Diagnostic methods
Current diagnostic methods for EP rely on serum β-hCG levels in correlation with TVUS or TAUS findings. TVUS has been shown to be more accurate and sensitive compared to TAUS in the diagnosis of early EP.12 Specifically, three-dimensional TVUS combined with color Doppler US was shown to be more effective than conventional 3D-US for the diagnosis of early CSP.13 Imaging mimics make EPs difficult to diagnose; however, awareness of differentiating features on US allows for more effective diagnosis, as summarized in Table 2.
Table 2.
Ultrasound (US) diagnostics of ectopic pregnancies (EPs) and clinical mimics.
| Type | Incidencea | US visualization | Clinical mimics | Features |
| Tubal | ~95% | • Extraovarian mass containing yolk sac and/or fetal pole
with/without cardiac motion • “blob” or “bagel” sign |
• Hemorrhagic cyst • Acute appendicitis |
• 70% occur at ampullary segment |
| Interstitial | 2%–4% | • “interstitial line sign” • “bulging sign” • “myometrial mantle sign” |
• Angular pregnancy • Fundal fibroid |
• 15× higher mortality rate |
| Cesarean Scar (CSP) | <1% | • Sagittal plane eccentrically embedded within anterior lower uterine segment with thinning/non-existent myometrium anteriorly | • Low-lying intrauterine pregnancy
(IUP) • Hematoma/abscess • Pedunculated fibroid |
• If growth extends into the uterine cavity normal pregnancy is possible |
| Heterotopic | 1%–3% | • IUP in conjunction with para-ovarian adnexal mass, “tubal ring,” or adnexal gestational sac (GS) | • Hyper-stimulated ovaries may obscure presence of adnexal EP | • Incidence rising • 1/100 in vitro fertilization (IVF) pregnancies • 1/7000 assisted reproductive technologies (ART) with ovulation induction pregnancies |
| Cervical (CEP) | <1% | • Eccentrically located round GS within cervical wall below the
cervical os • Cervical ballooning • Negative “sliding sign” |
• Abortion in progress • Nabothian cyst |
• Massive hemorrhage risk • Recent dilation and curettage reported in 70% of CEPs • 10× more likely in patients with ART |
| Ovarian | <3% | • GS containing either yolk sac or fetal pole inseparable from
the ovary • Thick echogenic trophoblastic rim • Hyper-vascular and “ring of fire” Doppler |
• Tubal EP in infundibulum • Corpus luteum cyst • Involuting follicle |
• 81% associated with intrauterine devices |
| Abdominal | 0.9%–1.4% | • Intraperitoneal GS with echogenic trophoblastic tissue, located in peritoneum | • Large unruptured tubal EP | • 7.7× risk of organ perforation and catastrophic
hemorrhage • Maternal mortality upward of 10% |
Source: Table modified and summarized from Houser et al.1
US: ultrasound; CSP: cesarean scar; IUP: intrauterine pregnancy; GS: gestational sac; EP: ectopic pregnancy; IVF: in vitro fertilization; ART: assisted reproductive technology; CEP: cervical ectopic pregnancy.
Incidence as a measure of the % of all EPs.
β-hCG trends are used in conjunction with US to determine EP diagnosis. A patient with a β-hCG level > 2000 mIU/mL with no sign of intrauterine pregnancy (IUP) is highly suspicious of EP.1 β-hCG level is monitored to determine a miscarriage or fetal development pattern. Viable IUPs are 99% likely to have a 49% increase in β-hCG levels over 48 h when initial levels were < 1500 mIU/mL.2 Decreasing levels or a slower rate is suggestive of miscarriage or EP, with a decrease of 21% or greater most likely a failed IUP.2 Overall, the complexity of diagnosis depends on the type of EP.
Experimental markers
In patients with a pregnancy of unknown location (PUL), 50%–70% are found to have either an EP or miscarriage, while the remaining 30% may have a normal IUP.14 Serial β-hCG levels are monitored to determine pregnancy location and prognosis. A rise less than 35% in 2 days suggests EP with an accuracy of 80.2%.14
Outside of β-hCG, experimental markers are being researched for potential use in diagnostics; however, they are not traditionally used in clinical settings. Such markers include inhibin A, activins, pregnancy-associated plasma protein A (PAPP-A), A disintegrin and metalloprotease-12 (ADAM-12), vascular endothelial growth factor (VEGF), and messenger and micro-RNA. Table 3 summarizes the efficacy of markers in various studies. Activin-AB was found to have a strong EP diagnostic correlation.15 ADAM-12 as a biomarker for PUL was shown to be promising for EP diagnostics in a study performed by Rausch et al.,16 however Horne et al.17 was unable to replicate these findings. Micro-RNA as a diagnostic tool for EP has been promising in recent years. Specifically, micro-RNAs are linked to placental pathologies and their relationship with EPs is being studied.18 Sun et al.19 found miR-378d in serum exosomes promising in EP diagnosis, with even higher significance when miR-100-5p and miR-215-5P are used in conjunction with a panel of β-hCG and progesterone. PAPP-A expression was found to be significantly lower in patients with EP, suggesting diagnostic and therapeutic value.20 Serum progesterone is higher in IUP compared to failing pregnancies and EPs.14 EPs have a serum progesterone cutoff of 10 ng/mL in most cases or 30 ng/mL 28–49 days post-menstruation in patients receiving clomiphene citrate fertility treatments.14 Serum progesterone is non-specific for EP or miscarriages and has been shown to misclassify normal IUPs, making it a less desirable standard for diagnosis.14
Table 3.
Sensitivity and specificity of promising biomarkers for ectopic pregnancy diagnosis.
| Biomarker | Reference | Sample size | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
|---|---|---|---|---|---|---|
| Activin-AB | Refaat and Bahathiq15 | 120 | 92.5 | 85 | 75.5 | 95.8 |
| A disintegrin and metalloprotease-12 (ADAM-12) a | Rausch et al.16 | 199 | 70 | 84 | – | – |
| β-human chorionic gonadotropin | Refaat and Bahathiq15 | 120 | 67.5 | 51.2 | 40.9 | 75.9 |
| Micro-RNA miR-378d | Sun et al.19 | 36 | 89.1 | 64 | – | – |
| Pregnancy-associated plasma protein A | Zhang and Wang20 | 134 | 92.13 | 78.33 | – | – |
| Progesterone | Refaat and Bahathiq15 | 120 | 27.5 | 50 | 21.5 | 58 |
There is limited literature on the efficacy of the biomarkers described above. For example, ADAM-12 was shown to have conflicting value in diagnostics.16,17 As such, further studies should be conducted to confirm diagnostic value.
Hematological assessment of complete blood count (CBC) samples has also been investigated as a diagnostic tool for EPs. Retrospective reviews have shown that white blood cell (WBC) levels, specifically monocyte counts, are higher in patients with tubal EPs.21 When assessing platelet characteristics, platelet distribution width may also indicate the presence of an EP, but the exact trend has been debated.21,22 Creatinine phosphokinase (CPK) may also be used in the early detection of EP, although further validation of this measurement is required.23
Exploratory diagnostics
In addition to experimental markers, endometrial sampling is being explored as a new format for EP diagnosis. Endometrial sampling allows for differentiation of failed IUPs from EPs, thus allowing patients to avoid unnecessary methotrexate (MTX) treatment.11 EP diagnostic assumption without dilation and curettage (D&C) endometrial sampling resulted in up to 40% of patients being treated for falsely diagnosed EPs.9 Failed IUPs are confirmed by presence of villi on endometrial sampling and/or a 15%–20% decline of β-hCG the day following the procedure.14 Endometrial sampling can be completed using endometrial biopsy pipelles, D&C, Karman cannula aspiration, or frozen sections. Table 4 summarizes the sensitivity and specificity of exploratory diagnostic tools as described below.
Table 4.
Sensitivity and specificity of endometrial sampling methods.
| Method | Reference | Sample size | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
|---|---|---|---|---|---|---|
| Dilation and curettage | Batig et al.24 | 31 | 88.9 | 100 | 100 | 57.1 |
| Frozen section | Odeh et al.25 | 106 | 72.7 | 95.9 | 88.9 | 88.6 |
| Karman aspiration | Brady et al.26 | 45 | 67.7 | 100 | – | – |
| Pipelle sampling | Batig et al.24 | 31 | 70.1 | 100 | 100 | 33.3 |
D&C is found to have higher sensitivity rates for EP diagnosis in comparison to endometrial biopsy pipelles; however, both procedures are limited in accuracy and further studies are needed to confirm diagnostic value.24 Frozen section technique is performed on endometrial material shortly after curettage and decreases the time needed to disprove EP diagnosis.25 Of 106 women who underwent frozen section technique, nine patients with IUP were falsely started on MTX therapy and three patients with EPs were incorrectly diagnosed with IUP and discharged.25 Concurrent methods for diagnosis are necessary to avoid unwanted pregnancy termination and missed EPs. Karman cannula aspiration of endometrium allowed 2/3 of women to avoid EP treatment and showed faster recovery times of 12.6 days for IUP when compared to 26.3 days for patients treated with MTX.27 Pipelle sampling was found to have higher sensitivity in patients with β-hCG ⩽ 2000 mIU/mL, suggesting selective diagnostic potential.24
Review of Table 4 indicates that all methods of endometrial sampling have > 95% specificity for diagnosis; however, D&C demonstrates the highest sensitivity, thus confirming it as the most effective protocol.
EP diagnostics are complex and difficult to determine early in the pregnancy. Current methods of US imaging alongside β-hCG are effective in diagnosis, however serum biomarkers and endometrial sampling show promise as future diagnostic methods. Further studies and investigations can help to confirm their value in early EP diagnostics, in hopes of diminishing the maternal mortality rate.
Treatment
Once diagnosis of EP is confirmed, treatment can take a conservative or aggressive approach depending on EP location, pregnancy timeline, and GS size. There are three different approaches to the treatment of EPs—medical, surgical, and expectant management—which are based on the type of EP, as seen in Table 5.
Table 5.
Summary of treatment recommendations for various types of ectopic pregnancies (EP).
| Type | Medical management | Surgical management | Expectant management |
|---|---|---|---|
| Tubal | Intramuscular (IM) methotrexate (MTX) may be considered
for: • Clinically stable patients with unruptured EP |
Recommended for: • Hemodynamically unstable patients May be considered for: • Clinically stable patients with unruptured EP Options include minimally invasive laparoscopy |
— |
| Interstitial | IM MTX may be considered for: • Hemodynamically stable patients |
Hysterectomy recommended for: • Patients who experience life-threatening hemorrhage • Patients who do not wish to maintain fertility Laparoscopic guided cornual resection recommended for: • Patients who wish to maintain fertility |
— |
| Cesarean Scar (CSP)a | • Intra-gestational MTX is the treatment of choice for medical
management • Systemic IM MTX on its own is not recommended |
Common approach: • Surgical resection with transvaginal or laparoscopic approach Alternative approaches: • Ultrasound (US)-guided vacuum aspiration • Hysterectomy (if future fertility is not desired) Sharp curettage alone should be avoided, due to risk of uterine rupture |
• Recommended against by Society for Maternal Fetal Medicine |
| Heterotopica | • IM MTX is contraindicated given simultaneous presence of an intrauterine pregnancy (IUP) | Approaches: • Salpingectomy/Salpingostomy • US-guided ablation • Laparoscopic removal of the EP Note: Surgical management has the worst outcomes for IUP |
• Greatest maternal mortality |
| Cervical | MTX can be administered in one of the following
ways: • Systemically via IM injection • Directly into the amniotic cavity Optional: • Supplementation with intraamniotic potassium chloride injection to increase efficacy |
Fertility preserving techniques that allow for bleeding
control: • Uterine artery embolization • Balloon tamponade • Endocervical curettage Cerclage can augment balloon tamponade in patients with severe hemorrhage |
– |
| Ovarian | • Not a common approach | • Gold standard for treatment • All attempts are made to preserve as much ovarian reserve as possible |
|
| Abdominal | – | • Standard treatment | • Recommended against due to risk of catastrophic intra-abdominal hemorrhage |
Source: Table modified and summarized from Houser et al.1
EP: ectopic pregnancy; IM: intramuscular; MTX: methotrexate; CSP: Cesarean scar pregnancy; US: ultrasound; IUP: intrauterine pregnancy.
There is no standardized treatment or algorithm for management, but common approaches are described.
Medical management
Intramuscular (IM) MTX injection is the current standard for medical management of EPs. MTX, a folate antagonist, inhibits rapid cell division, consequently resulting in EP termination.2 Contraindications to medical management include hemodynamic instability, anemia, leukopenia, thrombocytopenia, pelvic pain or hemoperitoneum indicative of EP rupture, renal or hepatic insufficiency, pulmonary disease, active peptic ulcer disease, coinciding IUP, breast feeding, fetal cardiac activity, serum β-hCG levels > 5000 mIU/mL, or EP > 4 cm in diameter.11 Surgical management is indicated in patients exhibiting MTX contraindications.11 However, most EPs that are diagnosed early are clinically stable, allowing patients to pursue nonsurgical options.28 Healthcare accessibility and patient compliance should be considered for medical management, as inability to follow-up may lead to higher risk of complications and treatment failure, thus making surgical management the safer treatment option.2,11
MTX is administered in single, double, or multi-dose regimens.11 The name of each regimen indicates the number of planned doses. The actual number of doses may vary depending on patient β-hCG trends.3 Patients with higher β-hCG levels may benefit from double-dose MTX therapy.29 Multi-dose regimens differ in dosing and include coadministration of leucovorin (folinic acid). Leucovorin reduces the adverse effects of MTX, but also reduces treatment efficacy.30 A breakdown of treatment protocols is described in Table 6.
Table 6.
Methotrexate (MTX) treatment protocols.
| Day | Single-dose regimen | Two-dose regimen | Fixed multi-dose regimen |
|---|---|---|---|
| 1 | Measure β-human chorionic gonadotropin (β-hCG) level and administer single dose of MTX (50 mg/m2) intramuscularly (IM) | Measure β-hCG level and administer single dose of MTX (50 mg/m2) IM | Measure β-hCG level and administer MTX (1 mg/kg) IM |
| 2 | – | – | Administer leucovorin (0.1 mg/kg) IM |
| 3 | – | – | Measure β-hCG level • If β-hCG level decreases > 15% between Days 1–3, measure β-hCG levels weekly until returned to nonpregnant levels (skip remaining steps)a • If β-hCG level decreases < 15% between Days 1–3, readminister single dose of MTX (1 mg/kg IM) IM |
| 4 | Measure β-hCG level (levels commonly increase between Days 1–4)2 |
Measure β-hCG level and administer single dose of MTX (50 mg/m2) IM | Administer leucovorin (0.1 mg/kg) IM |
| 5 | – | – | Measure β-hCG level • If β-hCG level decreases > 15% between Days 3–5, measure β-hCG levels weekly until returned to nonpregnant levels (skip remaining steps)a • If β-hCG level decreases < 15% between Days 3–5, readminister single dose of MTX (1 mg/kg) IM |
| 6 | – | – | Administer leucovorin (0.1 mg/kg) IM |
| 7 | Measure β-hCG level • If β-hCG level decreases > 15% between Days 4–7, measure β-hCG levels weekly until returned to nonpregnant levelsa • If β-hCG level decreases < 15% between Days 4–7 (20% of patients), readminister single dose of MTX (50 mg/m2) IM and repeat protocol ◦ Refer for surgical management if β-hCG does not decline after 2 doses (< 1% of patients) Statistics from Brady11 |
Measure β-hCG level • If β-hCG level decreases > 15% between Days 4–7, measure β-hCG levels weekly until returned to nonpregnant levels (skip remaining steps)a • If β-hCG level decreases < 15% between Days 4–7, readminister single dose of MTX (50 mg/m2) IM |
Measure β-hCG level • If β-hCG level decreases > 15% between Days 5–7, measure β-hCG levels weekly until returned to nonpregnant levels (skip remaining steps)a • If β-hCG level decreases < 15% between Days 5–7, readminister single dose of MTX (1 mg/kg) IM |
| 8 | – | – | Administer leucovorin (0.1 mg/kg) IM |
| 9 | – | – | Measure β-hCG level • If β-hCG level decreases > 15% between Days 7–9, measure β-hCG levels weekly until returned to nonpregnant levelsa • If β-hCG level decreases < 15% between Days 7–9, refer for surgical management |
| 11 | – | Measure β-hCG level • If β-hCG level decreases > 15% between Days 7–11, measure β-hCG levels weekly until returned to nonpregnant levels (skip remaining steps)a • If β-hCG level decreases < 15% between Days 7–11, readminister single dose of MTX (50 mg/m2) IM |
– |
| 14 | - | Measure β-hCG level • If β-hCG level decreases > 15% between Days 11–14, measure β-hCG levels weekly until returned to nonpregnant levels a • If β-hCG level decreases < 15% between Days 11–14, refer for surgical management |
- |
| Follow-up | Consider administering MTX treatment for persistent EP if β-hCG levels plateau or increase | ||
Common side effects of MTX treatment include vaginal spotting and gastrointestinal issues such as nausea, diarrhea, and vomiting.3 Some women may exhibit abdominal pain 2–3 days after treatment which can be managed expectantly in the absence of symptoms indicative of EP tubal rupture.3 Patients undergoing treatment should avoid taking folic acid supplements or non-steroidal anti-inflammatory drugs which can decrease the effectiveness of MTX, refrain from using substances such as opioids, alcohol, or other analgesics that can mask symptoms of EP rupture, and abstain from activities that increase EP rupture risk such as vaginal intercourse.2 As MTX is a potent teratogen, it is suggested that patients use contraception for 3 months following treatment, although there is limited evidence to support this recommendation.11
Current literature estimates the percent resolution of EPs via MTX treatment without need for surgical intervention to be 70%–95%, with lower success rates in patients with higher initial β-hCG levels.2,3 However, recent meta-analyses display conflicting results regarding success and risks of adverse effects with different treatment regimens.28–32 As such, there is a need for further investigation into this area of research.
Additional therapeutic agents administered in conjunction with MTX have been studied. Seven days of oral gefitinib in addition to single dose IM MTX effectively treated patients with stable tubal EPs and eliminated the need for surgical intervention.33 This treatment regimen must be validated by a randomized control trial, but may present as another option with minimal adverse effects.
Independent of the treatment regimen, β-hCG levels that continue to rise correlate with increased risk of treatment failure, which in turn can lead to tubal rupture, abdominal hemorrhage, future infertility, and death.11,32 If a patient develops significant pain or exhibits hemodynamic instability at any time throughout treatment, surgical management should be pursued.11
In addition to declining β-hCG levels, other markers are being studied to determine MTX success.34 Studies have proposed that women successfully treated for EP will have a significantly higher serum CPK.35,36 Red cell distribution width, mean platelet volume, and neutrophil-lymphocyte ratio may also inform efficacy of EP MTX treatment.37,38
Surgical management
Salpingostomy and salpingectomy are the two common approaches for surgical management of EPs. Salpingostomy consists of removing solely the EP via an incision in the fallopian tube, whereas salpingectomy includes removal of part or all the fallopian tube along with the EP.2 Salpingectomy is advised for patients with EPs ⩾ 5 cm in diameter, significant tubal damage, tubal rupture, bleeding, or previous tubal ligation.11 However, patients who undergo salpingectomy and have absent/obstructed contralateral fallopian tubes will be unable to procreate without ART, making salpingostomy preferred by patients who wish to retain fertility.11 In patients with normal contralateral fallopian tubes, salpingostomy and salpingectomy are shown to have equivalent future pregnancy outcomes, as supported by the ESEP study.11,39 Following salpingectomy, pathologic confirmation of EP in the removed fallopian tube is sufficient to confirm success of the procedure.11 Contrarily, salpingostomy requires subsequent β-hCG measurements to ensure absence of residual trophoblastic tissue (~20% of patients), which generally requires additional MTX treatment.11 A retrospective clinical trial discovered that early post-linear salpingostomy β-hCG values are predictive of persistent EP before Day 5, with a positive predictive value of 88% and negative predictive value of 99%.40
Overall, surgical management has been shown to have a higher rate of success in terminating EPs than medical management and is indicated in patients who exhibit signs of EP rupture (e.g. hemodynamic instability), have contraindications to medical management, or express personal preference to pursue surgical treatment.11 Current literature suggests there is no difference between medical and surgical management regarding their effect on subsequent fertility, with limited exceptions as mentioned above.2 Disadvantages of surgical management include anesthesia complications, secondary injuries, and blood loss.28
Expectant management
Expectant management is the most conservative approach for the treatment of EPs. This method can be considered for patients with decreasing or plateaued β-hCG levels.3 EPs presenting with β-hCG levels < 200 mIU/mL will spontaneously resolve in 88% of cases; however, the rate of spontaneous resolution declines as β-hCG levels exceed this threshold.3 Patients who choose to pursue expectant management must have β-hCG tested every 48 h and should consider other options if levels do not decline.2 Risks of expectant management include tubal rupture, hemorrhage, and emergency surgery.3 The relative efficacy and safety of expectant management is an area of ongoing research, with medical and surgical management remaining the primary approaches to EP treatment.11
Evidence in literature suggests initial β-hCG levels greater or less than 1500 mIU/mL may provide a method to inform expectant management versus MTX treatment for specific types of EP.41,42 Research has shown that IM MTX injections for patients with confirmed tubal EPs did not result in significantly different outcomes when compared to placebo, especially for clinically stable women with β-hCG < 1500 mIU/mL.43 Patients with β-hCG > 1500 mIU/mL, however, did have statistically significant changes and negative pregnancy tests after MTX injection compared to placebo.43 More research is necessary to determine effectiveness of MTX in patients with a confirmed tubal EP and β-hCG > 1500 mIU/mL. Serum markers other than β-hCG may also inform treatment success. Memtsa et al. found progesterone and β-hCG to be significantly different in both successful and failed expectant management of tubal EPs, whereas inhibin A, activin A, and high sensitivity C-reactive protein were unlikely to improve selection for conservative management.41
Innovations/other
Innovations in surgical management of EPs may provide better immediate and future fertility outcomes. Studies such as the DEMETER trial have shown that fertility rates between medical treatment and conservative surgery are not significantly different.42,44 A recent trial exploring the effectiveness of laparoscopic partial tubal resection with end-to-end anastomosis found significantly higher postoperative fallopian tube patency compared to controls.45 There was no significant difference between ovarian function, operation time, intra blood β-hCG recovery time, and hospital time compared with the control group.45 This method, however, may be an effective measure to preserve fertility. Uterine artery embolization (UAE) with intrauterine infusion of MTX alone was shown to effectively manage EP and preserve fertility.46 UAE with local infusion of MTX and 5-fluorouracil (5-FU) has also been studied and shown to be effective, but the addition of 5-FU is linked to more adverse effects.46 UAE before or after uterine curettage for the treatment of CSP was effective at preventing the need for hysterectomy in 11 of 12 patients.47 One of the 12 patients in this study required hysterectomy due to continued hemorrhage after uterine curettage followed by emergent UAE and further curettage.47 High-intensity focused US (HIFU) followed by D&C or UAE are additional methods to treat CSP that have been shown to preserve fertility.4,48 Nonsurgical approaches being studied, including HIFU, may offer additional benefits for future fertility.
Utility of UAE for successful treatment of EPs and bleeding due to EP resolution has also been studied in recent years. UAE in conjunction with MTX was shown to effectively resolve and control bleeding in the treatment of tubal EPs, CSP, cervical EPs, and abdominal EPs.47,49–51 Use of UAE in an emergent setting has also been shown to resolve EPs and control bleeding after a misdiagnosis of EP.47 For patients with persistently high β-hCG levels and vaginal bleeding after systemic MTX treatment, UAE has been shown to be a safe and effective option.52
Hysteroscopy also shows promise for the treatment of CSP.53 Hysteroscopy allows for direct visualization of the uterine cavity and a CSP, and thus may be a promising surgical option.54,55 A recent systematic review revealed that hysteroscopic treatment after HIFU or UAE resulted in 91% resolution of CSP.53 Larger studies are required to further assess the safety of hysteroscopic treatment.
Noninvasive treatments are a continued area of study for patients with EPs, especially CSPs. US-guided HIFU has been shown to effectively resolve CSP.56 HIFU in this study was notably conducted as an outpatient procedure 2–5 times, with patients experiencing minimal side effects.56 In a 2019 meta-analysis comparing UAE and HIFU, early management of CSP with HIFU resulted in better outcomes, including decreased blood loss, shorter hospital durations, and less adverse events; however, β-hCG levels took longer to normalize.57
Psychological interventions are also important to consider in the management of EPs. Counseling and patient education throughout EP intervention has been shown to improve mental health and self-esteem in one randomized controlled clinical trial.58 Methods in this trial included education on EP medical intervention, the physical and psychological complications of interventions, and the sadness, self-esteem, and mental health changes that can be seen after an EP. Another randomized control study found that patient education, attentive and enhanced perioperative care, including heated blankets, and postoperative and discharge education can lower anxiety and depression after laparoscopic management of EP.59 Muscle relaxation training after MTX administration has also been shown to reduce anxiety in patients with EPs.60 Overall, psychological management and patient education play crucial roles in the quality-of-life following an EP.
Implications for practice and/or policy
The reversal of Roe v. Wade offers additional challenges in the treatment of EPs. Mifepristone and misoprostol are medications that can be given to treat a PUL. These medications are associated with more rapid exclusion of EP when administered before a definitive diagnosis is made and traditional treatment of MTX is started.61 With the overturn of Roe v. Wade, some government officials have hinted medications traditionally associated with abortion, including mifepristone and misoprostol, may no longer be readily available. Leading healthcare organizations like the American Medical Association and American College of Obstetricians and Gynecologists consider all forms of healthcare a human right, including abortion and contraception.62 They also state and believe the government should not be involved in the patient-physician relationship.63,64 Current legal proceedings and changes to state legislature may affect EP treatment and should be considered in the future management of EPs.
Study limitations
PubMed was the primary search engine for research in this publication and allowed for a comprehensive review of current medical knowledge of EPs. Literature not found in PubMed may not have been included and is one source of bias and limitation to this study. Minimization of search criteria allowed for inclusion of all relevant papers. Overall, there is limited innovation reported in EP diagnosis and treatment, thus opening the opportunity for more research to be conducted.
Conclusion
This review consolidates current diagnostic and treatment strategies for EP. Many tools and markers for EP diagnosis along with treatment strategies have been studied and shown to be effective; however, the amount of large multicenter trials within the last 5 years is sparse.
The current diagnostic standard for EP is a combination of US imaging and serum levels of β-hCG. Imaging mimics make EP difficult to diagnose; however, awareness of differentiating features allows for better diagnosis. Additional serum markers outside of β-hCG are being investigated to confirm diagnosis when US results are inconclusive. While not widely used in clinical practice, these experimental markers, specifically activin-AB and PAPP-A, show promise for EP diagnosis. Utilization of these markers may improve outcomes by allowing for earlier diagnosis. In addition, endometrial sampling is promising for effective EP diagnosis. Review of Table 4 indicates that the majority of methods of endometrial sampling have 100% specificity for diagnosis; however, D&C continues to demonstrate the highest sensitivity for diagnostics, thus confirming it as the most effective protocol to be used.
Medical, surgical, and expectant management are the three main treatment options for the management of EPs. MTX is the most common medication given for the treatment of EP and is administered using single or multi-dose regimens. However, recent meta-analyses have offered conflicting findings regarding success rates and risk of adverse effects between the different treatment regimens.28,30–32 As such, there is a need for further investigation into this area of research. Dosage of MTX is often governed by serum levels of β-hCG, with additional doses given if β-hCG levels do not decline after MTX administration. Medications given in conjunction with MTX, such as 5-FU, and procedures, such as UAE, HIFU, and D&C, have shown to be effective at resolving extrauterine pregnancy.
When medical management alone is contraindicated or does not resolve EP, surgical interventions including salpingostomy or salpingectomy are often performed. UAE and hysteroscopy are additional surgical procedures being studied, especially for the removal of CSPs. Expectant management via monitoring of β-hCG levels can be used in place of medical management for patients with decreasing or plateaued β-hCG levels; however, more studies are required to assess the safety of this form of treatment.3,43
Future fertility is an important factor to consider during the treatment of EP. Studies, such as the DEMETER trial, have shown no significant difference in fertility rates following medical treatment and conservative surgery.42,44 The ESEP study also showed that salpingotomy and salpingectomy do not significantly affect future pregnancy outcomes.39 Nonsurgical approaches being studied, including HIFU, may offer additional benefits for future fertility. Increasing the number of multicenter trials is necessary to demonstrate the efficacy of treatments that improve surgical outcomes and future fertility after an EP.
Finally, psychological management is a crucial factor in successful outcomes following EP diagnosis and treatment. Trials have shown that attention to patient mental health, comfort, and education following diagnosis have resulted in lower anxiety and depression.58–60 Consideration of these factors can improve patient quality-of-life.
Acknowledgments
Not Applicable.
Footnotes
ORCID iDs: Kellie Mullany  https://orcid.org/0000-0002-6806-1032
https://orcid.org/0000-0002-6806-1032
Madeline Minneci  https://orcid.org/0000-0003-0983-1022
https://orcid.org/0000-0003-0983-1022
Ryan Monjazeb  https://orcid.org/0000-0001-9558-1909
https://orcid.org/0000-0001-9558-1909
Olivia C. Coiado  https://orcid.org/0000-0003-1322-5877
https://orcid.org/0000-0003-1322-5877
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contribution(s): Kellie Mullany: Conceptualization; Investigation; Project administration; Writing—original draft; Writing—review & editing.
Madeline Minneci: Conceptualization; Investigation; Writing—original draft; Writing—review & editing.
Ryan Monjazeb: Conceptualization; Investigation; Writing—original draft; Writing—review & editing.
Olivia C. Coiado: Conceptualization; Investigation; Supervision; Writing—review & editing.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: Not applicable.
References
- 1. Houser M, Kandalaft N, Khati NJ. Ectopic pregnancy: a resident’s guide to imaging findings and diagnostic pitfalls. Emerg Radiol 2022; 29(1): 161–172. [DOI] [PubMed] [Google Scholar]
- 2. Hendriks E, Rosenberg R. Ectopic pregnancy: diagnosis and management—American Family Physician. Am Fam Physician 2020; 101: 599–606. [PubMed] [Google Scholar]
- 3. ACOG. ACOG practice bulletin no. 193: tubal ectopic pregnancy. Obstetrics and Gynecology 2018; 131: e91–e103. [DOI] [PubMed] [Google Scholar]
- 4. Zhang C, Zhang Y, He J, et al. Outcomes of subsequent pregnancies in patients following treatment of cesarean scar pregnancy with high intensity focused ultrasound followed by ultrasound-guided dilation and curettage. Int J Hyperthermia 2019; 36(1): 926–931. [DOI] [PubMed] [Google Scholar]
- 5. Stabile G, Mangino FP, Romano F, et al. Ectopic cervical pregnancy: treatment route. Medicina 2020; 56: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dvash S, Cuckle H, Smorgick N, et al. Increase rate of ruptured tubal ectopic pregnancy during the COVID-19 pandemic. Eur J Obstet Gynecol Reprod Biol 2021; 259: 95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng LY, Lin PY, Huang FJ, et al. Ectopic pregnancy following in vitro fertilization with embryo transfer: a single-center experience during 15 years. Taiwan J Obstet Gynecol 2015; 54(5): 541–545. [DOI] [PubMed] [Google Scholar]
- 8. WHO. Caesarean section rates continue to rise, amid growing inequalities in access, https://www.who.int/news/item/16-06-2021-caesarean-section-rates-continue-to-rise-amid-growing-inequalities-in-access (2021, accessed 3 December 2022).
- 9. Barnhart KT, Gracia CR, Reindl B, et al. Usefulness of Pipelle endometrial biopsy in the diagnosis of women at risk for ectopic pregnancy. Am J Obstet Gynecol 2003; 188(4): 906–909. [DOI] [PubMed] [Google Scholar]
- 10. Ucisik-Keser FE, Matta EJ, Fabrega MG, et al. The many faces of ectopic pregnancies: demystifying the common and less common entities. Abdom Radiol 2021; 46(3): 1104–1114. [DOI] [PubMed] [Google Scholar]
- 11. Brady PC. New evidence to guide ectopic pregnancy diagnosis and management. Obstet Gynecol Surv 2017; 72(10): 618–625. [DOI] [PubMed] [Google Scholar]
- 12. Mathlouthi N, Slimani O, Ferchichi A, et al. [Medical treatment of ectopic pregnancy]. Tunis Med 2013; 91: 49–53. [PubMed] [Google Scholar]
- 13. Shi L, Huang L, Liu L, et al. Diagnostic value of transvaginal three-dimensional ultrasound combined with color Doppler ultrasound for early cesarean scar pregnancy. Ann Palliat Med 2021; 10(10): 10486–10494. [DOI] [PubMed] [Google Scholar]
- 14. Panelli DM, Phillips CH, Brady PC. Incidence, diagnosis and management of tubal and nontubal ectopic pregnancies: a review. Fertil Res Pract 2015; 1: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Refaat B, Bahathiq AO. The performances of serum activins and follistatin in the diagnosis of ectopic pregnancy: a prospective case-control study. Clin Chim Acta 2019; 500: 69–74. [DOI] [PubMed] [Google Scholar]
- 16. Rausch ME, Beer L, Sammel MD, et al. ADAM-12 as a novel marker for the diagnosis of ectopic pregnancy. Fertil Steril 2011; 95: 1373–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Horne AW, Brown JK, Tong S, et al. Evaluation of ADAM-12 as a diagnostic biomarker of ectopic pregnancy in women with a pregnancy of unknown location. PLoS ONE 2012; 7(8): e0041442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kontomanolis EN, Kalagasidou S, Fasoulakis Z. MicroRNAs as potential serum biomarkers for early detection of ectopic pregnancy. Cureus 2018; 10: e2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sun J, Deng G, Ruan X, et al. Exosomal microRNAs in serum as potential biomarkers for ectopic pregnancy. Biomed Res Int 2020; 2020: 3521859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang X, Wang C. Predictive value of PAPP-A for ectopic pregnancy and analysis of related factors. Exp Ther Med 2021; 22(2): 801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eskicioğlu F, Özdemir AT, Turan GA, et al. The efficacy of complete blood count parameters in the diagnosis of tubal ectopic pregnancy. Ginekol Pol 2014; 85(11): 823–827. [DOI] [PubMed] [Google Scholar]
- 22. Eskicioglu F, Turan GA, Gur EB. The efficacy of platelet activation indicators for the diagnosis of tubal ectopic pregnancy. Pak J Med Sci 2015; 31(3): 745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Colombo GE, Leonardi M, Armour M, et al. Efficacy and safety of expectant management in the treatment of tubal ectopic pregnancy: a systematic review and meta-analysis. Hum Reprod Open 2020; 2020(4): hoaa044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Batig AL, Elliott D, Rumjahn H, et al. Pipelle endometrial sampling in the evaluation of abnormal first trimester pregnancies. Mil Med 2014; 179(9): 1030–1035. [DOI] [PubMed] [Google Scholar]
- 25. Odeh M, Qasoum A, Tendler R, et al. Pregnancy of unknown location: the value of frozen section analysis and its relation to beta-hCG levels and endometrial thickness. Rev Bras Ginecol Obstet 2019; 41(3): 142–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brady P, Imudia AN, Awonuga AO, et al. Pregnancies of unknown location after in vitro fertilization: minimally invasive management with Karman cannula aspiration. Fertil Steril 2014; 101(2): 420–426. [DOI] [PubMed] [Google Scholar]
- 27. Insogna IG, Farland LV, Missmer SA, et al. Outpatient endometrial aspiration: an alternative to methotrexate for pregnancy of unknown location. Am J Obstet Gynecol 2017; 217(2): 185.e1–185.e9. [DOI] [PubMed] [Google Scholar]
- 28. Xiao C, Shi Q, Cheng Q, et al. Non-surgical management of tubal ectopic pregnancy: a systematic review and meta-analysis. Medicine 2021; 100: E27851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hamed HO, Ahmed SR, Alghasham AA. Comparison of double- and single-dose methotrexate protocols for treatment of ectopic pregnancy. Int J Gynaecol Obstet 2012; 116(1): 67–71. [DOI] [PubMed] [Google Scholar]
- 30. Yuk JS, Lee JH, Park WI, et al. Systematic review and meta-analysis of single-dose and non-single-dose methotrexate protocols in the treatment of ectopic pregnancy. Int J Gynaecol Obstet 2018; 141(3): 295–303. [DOI] [PubMed] [Google Scholar]
- 31. Alur-Gupta S, Cooney LG, Senapati S, et al. Two-dose versus single-dose methotrexate for treatment of ectopic pregnancy: a meta-analysis. Am J Obstet Gynecol 2019; 221(2): 95–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang C, Cai J, Geng Y, et al. Multiple-dose and double-dose versus single-dose administration of methotrexate for the treatment of ectopic pregnancy: a systematic review and meta-analysis. Reprod Biomed Online 2017; 34(4): 383–391. [DOI] [PubMed] [Google Scholar]
- 33. Skubisz MM, Tong S, Doust A, et al. Gefitinib and methotrexate to treat ectopic pregnancies with a pre-treatment serum hCG 1000–10,000 IU/L: phase II open label, single arm multi-centre trial. Ebiomedicine 2018; 33: 276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Orozco EM, Sánchez-Durán MA, Bello-Muñoz JC, et al. ß-hCG and prediction of therapeutic success in ectopic pregnancies treated with methotrexate, results from a prospective observational study. J Matern Fetal Neonatal Med 2015; 28(6): 695–699. [DOI] [PubMed] [Google Scholar]
- 35. Davari-Tanha F, Ghazi M, Mohseni M, et al. The role of plasma creatine phosphokinase (CPK) level in prediction of response to methotrexate for ectopic pregnancy. J Family Reprod Health 2016; 10(2): 59–63. [PMC free article] [PubMed] [Google Scholar]
- 36. Gnisci A, Rua S, Courbiere B, et al. Plasma creatine phosphokinase level may predict successful treatment after a single injection of methotrexate for ectopic pregnancy. Fertil Steril 2011; 95(6): 2131–2133. [DOI] [PubMed] [Google Scholar]
- 37. Kanmaz AG, Inan AH, Beyan E, et al. Role of various complete blood count parameters in predicting the success of single-dose Methotrexate in treating ectopic pregnancy. Pak J Med Sci 2018; 34(5): 1132–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Akkaya H, Uysal G. Can hematologic parameters predict treatment of ectopic pregnancy. Pak J Med Sci 2017; 33(4): 937–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mol F, van Mello NM, Strandell A, et al. Salpingotomy versus salpingectomy in women with tubal pregnancy (ESEP study): an open-label, multicentre, randomised controlled trial. Lancet 2014; 383: 1483–1489. [DOI] [PubMed] [Google Scholar]
- 40. Morse AN, Si W, Qin S, et al. Optimal use of peri-operative human chorionic gonadotrophin concentrations to identify persistent ectopic pregnancy after laparoscopic salpingostomy: a retrospective cohort study. Reprod Biomed Online 2018; 36(3): 361–368. [DOI] [PubMed] [Google Scholar]
- 41. Memtsa M, Jauniaux E, Gulbis B, et al. Evaluation of maternal serum biomarkers in predicting outcome of successful expectant management of tubal ectopic pregnancies. Eur J Obstet Gynecol Reprod Biol 2020; 250: 61–65. [DOI] [PubMed] [Google Scholar]
- 42. Düz SA. Fertility outcomes after medical and surgical management of tubal ectopic pregnancy. Acta Clin Croat 2022; 60(3): 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jurkovic D, Memtsa M, Sawyer E, et al. Single-dose systemic methotrexate vs expectant management for treatment of tubal ectopic pregnancy: a placebo-controlled randomized trial. Ultrasound Obstet Gynecol 2017; 49(2): 171–176. [DOI] [PubMed] [Google Scholar]
- 44. Fernandez H, Capmas P, Lucot JP, et al. Fertility after ectopic pregnancy: the DEMETER randomized trial. Hum Reprod 2013; 28(5): 1247–1253. [DOI] [PubMed] [Google Scholar]
- 45. Cheng P, Yang XH. Preservation of the fallopian tube in ectopic tubal pregnancy: an analysis of the outcome of two laparoscopic surgical approaches. Ann Ital Chir 2022; 93: 241–247. [PubMed] [Google Scholar]
- 46. Gao J, Li X, Chen J, et al. Uterine artery embolization combined with local infusion of methotrexate and 5- fluorouracil in treating ectopic pregnancy: a CONSORT-compliant article. Medicine 2018; 97(5): e9722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang B, Jiang ZB, Huang MS, et al. Uterine artery embolization combined with methotrexate in the treatment of cesarean scar pregnancy: results of a case series and review of the literature. J Vasc Interv Radiol 2012; 23(12): 1582–1588. [DOI] [PubMed] [Google Scholar]
- 48. Chen L, Xiao S, Zhu X, et al. Analysis of the reproductive outcome of patients with cesarean scar pregnancy treated by high-intensity focused ultrasound and uterine artery embolization: a retrospective cohort study. J Minim Invasive Gynecol 2019; 26(5): 883–890. [DOI] [PubMed] [Google Scholar]
- 49. Elmokadem AH, Abdel-Wahab RM, El-Zayadi AA, et al. Uterine artery embolization and methotrexate infusion as sole management for caesarean scar and cervical ectopic pregnancies: a single-center experience and literature review. Can Assoc Radiol J 2019; 70(3): 307–316. [DOI] [PubMed] [Google Scholar]
- 50. Li Z, Xu W, Hu B, et al. Uterine artery embolization in association with methotrexate infusion for the treatment of tubal ectopic pregnancy. J Interven Med 2019; 1: 182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ozen M, Birmingham E, Hoffman M, et al. Non-surgical management of abdominal ectopic pregnancy with uterine artery embolization. Radiol Case Rep 2022; 17(5): 1631–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kwon JH, Kim GM, Han K, et al. Safety and efficacy of uterine artery embolization in ectopic pregnancies refractory to systemic methotrexate treatment: a single-center study. Cardiovasc Intervent Radiol 2017; 40(9): 1351–1357. [DOI] [PubMed] [Google Scholar]
- 53. Diakosavvas M, Kathopoulis N, Angelou K, et al. Hysteroscopic treatment of cesarean scar pregnancy: a systematic review. Eur J Obstet Gynecol Reprod Biol 2022; 270: 42–49. [DOI] [PubMed] [Google Scholar]
- 54. Chueh HY, Pai AHY, Su YY, et al. Hysteroscopic removal, with or without laparoscopic assistance, of first-trimester cesarean scar pregnancy. Fertil Steril 2022; 117: 643–645. [DOI] [PubMed] [Google Scholar]
- 55. Zhang X, Pang Y, Ma Y, et al. A comparison between laparoscopy and hysteroscopy approach in treatment of cesarean scar pregnancy. Medicine 2020; 99: e22845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xiao J, Zhang S, Wang F, et al. Cesarean scar pregnancy: noninvasive and effective treatment with high-intensity focused ultrasound. Am J Obstet Gynecol 2014; 211(4): 356e1–7. [DOI] [PubMed] [Google Scholar]
- 57. Xiao X, Feng Z, Li T, et al. Comparing the efficacy and safety of high-intensity focused ultrasound and uterine artery embolization in caesarean scar pregnancy: a meta-analysis. Adv Ther 2019; 36(6): 1314–1325. [DOI] [PubMed] [Google Scholar]
- 58. Hasani S, Mirghafourvand M, Esmaeilpour K, et al. The effect of counseling based on health promotion awareness on mental health and self-esteem in women with ectopic pregnancy: a randomized controlled clinical trial. J Matern Fetal Neonatal Med 2021; 34(11): 1687–1694. [DOI] [PubMed] [Google Scholar]
- 59. Zhong L, Zhao Y, Zhu H. Randomized trial of the application value of comprehensive nursing intervention in the perioperative period of ruptured bleeding of ectopic pregnancy. Ann Palliat Med 2021; 10(4): 4593–4600. [DOI] [PubMed] [Google Scholar]
- 60. Pan L, Zhang J, Li L. Effects of progressive muscle relaxation training on anxiety and quality of life of inpatients with ectopic pregnancy receiving methotrexate treatment. Res Nurs Health 2012; 35(4): 376–382. [DOI] [PubMed] [Google Scholar]
- 61. Goldberg AB, Fulcher IR, Fortin J, et al. Mifepristone and misoprostol for undesired pregnancy of unknown location. Obst Gynecol 2022; 139: 771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. ACOG Board of Directors. Abortion policy. ACOG, https://www.acog.org/clinical-information/policy-and-position-statements/statements-of-policy/2022/abortion-policy (2022, accessed 11 July 2022). [Google Scholar]
- 63. O’Reilly KB. Highlights from the 2022 AMA annual meeting. American Medical Association, https://www.ama-assn.org/house-delegates/annual-meeting/highlights-2022-ama-annual-meeting (2022, accessed 11 July 2022).
- 64. O’Reilly KB. With abortion under attack, doctors push back on criminalizing care. American Medical Association, https://www.ama-assn.org/delivering-care/population-care/abortion-under-attack-doctors-push-back-criminalizing-care (2022, accessed 11 July 2022).