Abstract
Articular cartilage (AC), a bone-to-bone protective device made of up to 80% water and populated by only one cell type (i.e. chondrocyte), has limited capacity for regeneration and self-repair after being damaged because of its low cell density, alymphatic and avascular nature. Resulting repair of cartilage defects, such as osteoarthritis (OA), is highly challenging in clinical treatment. Fortunately, the development of tissue engineering provides a promising method for growing cells in cartilage regeneration and repair by using hydrogels or the porous scaffolds. In this paper, we review the therapeutic strategies for AC defects, including current treatment methods, engineering/regenerative strategies, recent advances in biomaterials, and present emphasize on the perspectives of gene regulation and therapy of noncoding RNAs (ncRNAs), such as circular RNA (circRNA) and microRNA (miRNA).
Keywords: Articular cartilage, tissue engineering, therapeutic strategies, osteoarthritis, ncRNAs
Introduction
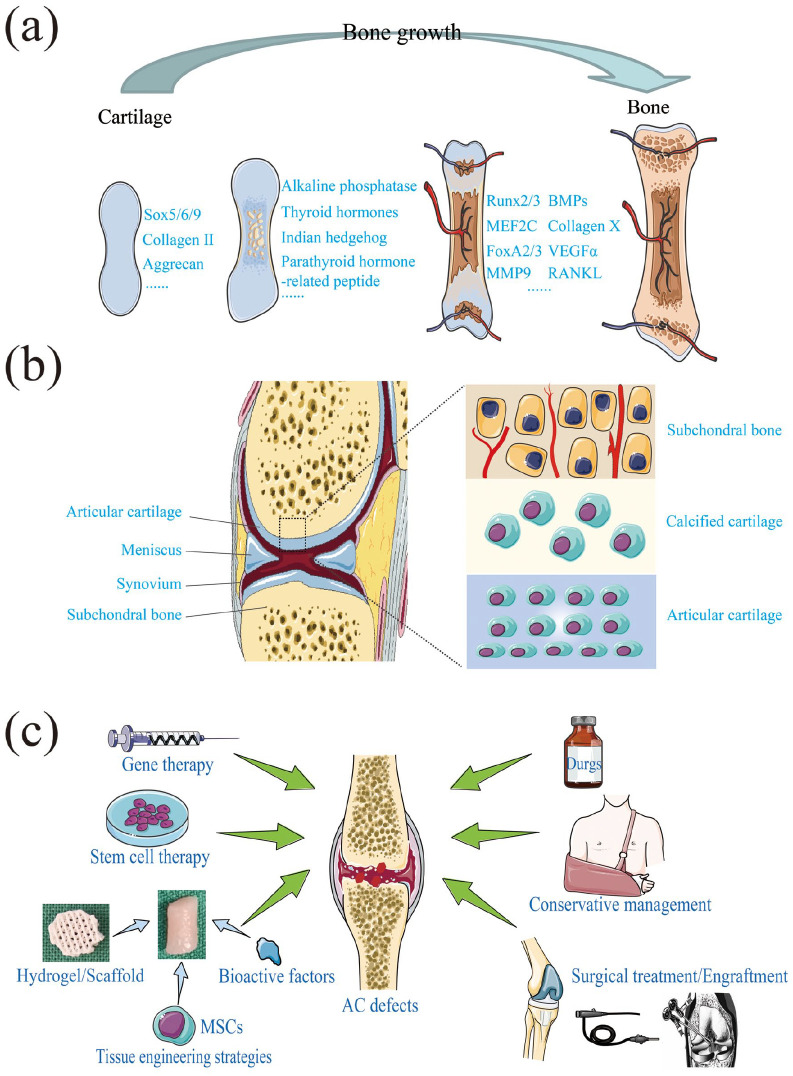
Cartilage is a kind of dense, supportive, resilient connective tissue, consisting of numerous of intercellular substances and chondrocytes with cysts. In an embryo, a great mass of bones is cartilaginous. However, most of them are ossified and replaced by bone tissue as the age rising (Figure 1(a)).1,2 In adults, only a few bones are cartilaginous, for example, the outer ear, the tip of the nose, the tip of the ribs, and so on. Cartilage is divided into hyaline cartilage, elastic cartilage and fibrous cartilage according to the difference of intercellular substance. Among them, hyaline cartilage is mainly distributed in the sternal end of the rib and the surfaces of the bone and joints, also known as articular cartilage (AC).
Figure 1.
(a) Bone growth process, (b) the joint and AC anatomy, and (c) AC defects therapeutic strategies.
Unlike other tissues, its avascular and no lymphatics nature resulting nonnutritive for regeneration, AC has limited capacity for self-restoration after being damaged; thus, its repair and regenerate hold highly challenging.3 AC injuries leads to an imbalance in tissue homeostasis, and the affected tissue often exhibits OA changes due to the poor self-healing capacity of cartilage, resulting produce proinflammatory cytokines (IL-1) and tissue destructive enzymes (such as MMPs and ADAMTs), and loss its normal phenotype and microenvironment synchronously (Figure 1(b)).4,5 After years of development, the treatment methods of AC injuries, such as traditional therapy (palliative or surgical treatment), biological therapy (stem cell or chondrocyte treatment, cell-free fat extract (CEFFE) and platelet-rich plasma (PRP) treatment); and regenerative therapy by using scaffolds, seed cells, and bioactive factors, have become abundant (Figure 1(c)).
Noncoding RNAs (ncRNAs), such as microRNA (miRNA), circular RNA (circRNA), and long non-coding RNA (lncRNA), have also been found to play an important regulatory role in cartilage injuries and chondrocyte proliferation.6 –9 Particularly, circRNA, which has a stable structure, has the ability to resist RNA enzymes and sequence conserved characteristics; its regulatory role on cartilage injury and regeneration is increasingly favored,10 –14 thus laying a foundation for its future clinical application. This work reviewed the therapeutic strategies and biomaterials of AC regeneration, and made a prospect.
Articulate cartilage defects therapeutic strategies
Palliative management
Palliative management, such as hyaluronic acid (HA), analgesics therapy, corticosteroid injection, and hormones, is used for early mild cartilage injuries (grades Ⅰ-Ⅱ according to the International Cartilage Repair Society (ICRS) and Kellgren & Lawrence (K-L) cartilage injury classification). Among them, drugs and corticosteroids have been used in clinic/clinical trials or emerging research to alleviate the inflamed joint environment.15 For example, kartogenin (KGN) was confirmed as an emerging stable nonprotein compound that can promote the differentiation of bone marrow mesenchymal stem cells (BMSCs) and synovial mesenchymal stem cells (SMSCs) into chondrocytes both in vitro and in vivo.16,17 Besides, nonsteroidal anti-inflammatory drugs (NSAIDs), such as Halofuginone, Etoricoxib, Ibuprofen, and Diclofenac, have shown some benefits for AC regeneration and OA treatment.18,19 Natural products, including terpenoids, polysaccharides, polyphenols, flavonoids, alkaloids, and saponins, are advantageous as their low toxicity, low costs, and act on multiple targets, are gradually being applied in the treatment of OA.20 But, the function of AC tissue is largely conferred by its compartmentalized zonal microstructure and composition.
Unfortunately, clinical studies have shown that current treatments such as palliative/conservative management of cartilage defects just relieve the symptoms, and often fails to regenerate new tissue that recapitulates this zonal structure and patients with these lesions may need surgical intervention.21 For example, palliative management are recommended by at least 75% of the knee OA treatment guidelines in USA, but the effect size of acetaminophen on pain is only 0.13, and the pain meliorative effect of NSAIDs has no statistical difference detected between taking NSAIDs versus placebo, which implies a trivial clinical effect.22,23 Furthermore, a clinical research showed that HA injection for knee OA was no evidence of benefit on account of it just effect on pain, function, and stiffness in a short-term (1–4 weeks).24
Surgical intervention
Surgeons try to promote a natural fibrocartilaginous response by using marrow stimulating techniques, aiming to reduce swelling and pain and improving the joint function of the patients.25 Numerous surgical techniques have been developed to address focal cartilage defects. These surgical treatment strategies are characterized as palliation (e.g. fixation, metallic spacing devices, chondroplasty, and debridement), repair (e.g. drilling and microfracture (MFX)), or restoration (e.g. osteochondral autograft or allograft transplantation, autologous/allogeneic chondrocyte implantation (ACI), even osteotomies).26 –30 Among them, the transplanted products are difficult to commercialize in osteochondral transplantation surgery due to the constraints of the transplanted cartilage source and the timeliness of transplantation.
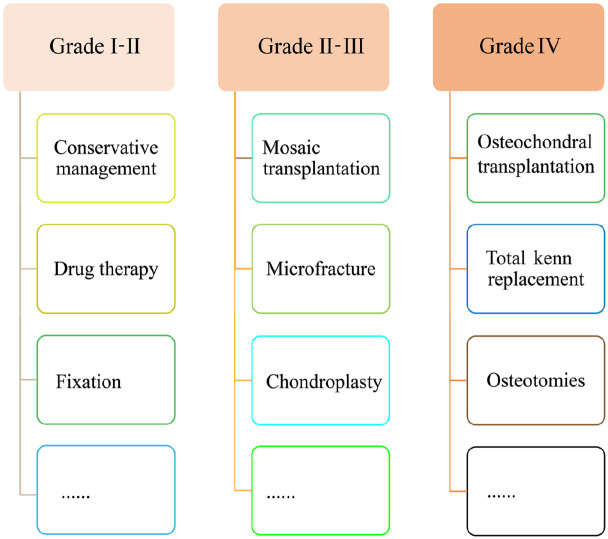
Surgical treatment, such as MFX and mosaic transplantation, is performed for midterm cartilage injuries (grades Ⅱ and Ⅲ). Furthermore, artificial joint replacement, even osteotomies, is performed for advanced severe cartilage injuries (grade Ⅳ) (Figure 2). However, current surgical management provides short-term symptom relief and fails to restore the articular surface or regenerate new tissue that recapitulates the focal structure; thus, it faces the problem of poor prognosis, complications, secondary injury, insufficient donors, incomplete chondrogenesis or rapid degradation and fibrosis of the repair tissue, almost always progressing to further deterioration in the long term.15 For example, the average secondary injury incidences at the donor site of osteochondral autografts in the knee and ankle were 5.9% and 19.6%, respectively.31 Although the autologous cartilage particles product CAIS from Depuy company and the allogeneic juvenile cartilage particles DeNovo NT were commercialized. Both of them are just suitable for the small size cartilage defect repair (<3.5 cm2), and the safety and repair effectiveness lack long-term follow-up data, which need more clinical data to be verified.32 Fortunately, advancement in molecular biology, biological treatment and regenerative treatment has revealed the potential of growth factors, differentiation factors, and cytokines in directing cellular differentiation, metabolic activity, chondrogenesis, and promoting the formation of functionally acceptable cartilage-like tissue.33
Figure 2.
Palliative or surgical therapeutic strategies for different degrees of AC defects.
Gene regulation
The transfer of specific target gene-encoding proteins with therapeutic or regenerative properties into target cells or tissues in the suffering environment makes continuous production and specific release at relevant sites possible; thus it provides long-term treatment for cartilage repair and is a good strategy for the treatment of AC injuries.34 In preclinical studies, gene regulation has been successfully used to treat cartilaginous, bone, and skeletal muscle injuries by balancing the management of temporary joint mechanical incompetence with altered metabolic and inflammatory homeostasis.35,36 For example, anabolic factors, such as CCN family protein 2,37 insulin-like growth factor-1 (IGF-1),38 fibroblast growth factor (FGF),39 and transforming growth factor β (TGF-β) superfamily,40,41 have proven their potential to stimulate chondrogenesis and synthesis of cartilage-specific matrix components, allowing the formation of a hyaline cartilage-like tissue repair in experimental studies. Moreover, anticatabolic or anti-inflammatory factors, such as interleukin 4/10 (IL-4/10), interleukin 1 receptor a (IL-1Ra), and tumor necrosis factors receptor, could inhibit excessive cartilage degradation and destruction.33,42
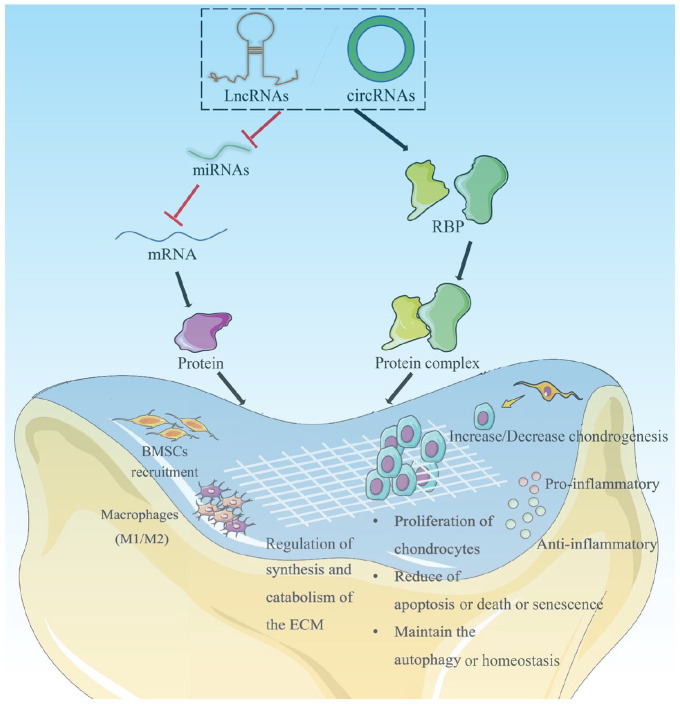
Furthermore, multifarious ncRNAs have been proven to regulate cell proliferation, apoptosis, death and senescence, inflammation, cartilage homeostasis, extracellular matrix (ECM) maintenance, and BMSCs recruitment, thus playing a crucial role in promoting the regeneration of AC, which shows strong feasibility in gene therapy of AC defects.43 For example, circRSU1 regulated oxidative stress-triggered inflammation and ECM maintenance in human chondrocyte by modulating the MEK/ERK1/2 and NF-κB cascades via circRSU1/miR-93-5p/MAP3K8 axis.14 In addition, others ncRNAs, such as circCDK14/miR-125a-5p,11 circFOXO3,44 circVMA21/miR-495-3p,45 circHIPK3/miR-30a-3p,46 lncRNA PILA,47 lncRNA NEAT1/miR-122-5p,48 miR-29b-5p,49 miR-17,50 etc., were reported could serve as a potential target for gene therapy of AC defects via competitive endogenous RNA network or scaffolding protein, including OA, as shown in Figure 3.
Figure 3.
Gene regulatory mechanisms of circRNA/lncRNA/miRNA in AC defects. Reproduced from Xiang et al.51
Notwithstanding, the widespread application of gene therapy requires the development of safe and efficient gene delivery vectors and supportive gene-activated matrices; thus, polymeric biomaterials are particularly attractive due to their tunable physiochemical properties (the sustained and tunable release of gene therapies in a spatiotemporally explicit manner).34,52 In addition, gene therapy offers a cracking tool to stimulate chondrogenic profit from the effective, safe, and durable delivery of candidate sequences with chondroprotective and/or chondroregenerative properties.53 For instance, hydrogel-guided, a recombinant adeno-associated virus vector coding for human IGF-1, resulted in IGF-1 overexpression, enabling long-term AC repair, and protection against perifocal OA in minipigs’ full-thickness chondral defect model.38 Gene therapy with lentiviral vectors encoding CLOCK, a core component of the molecular circadian clock machinery, in counteracting human mesenchymal stem cells (MSCs) decay, promoted cartilage regeneration, and attenuated age-related articular degeneration in mice.54 In addition, exosomes were used in gene therapy of AC defects as vectors with good absorbability.55
Tissue engineering strategies
The rapid development of cartilage tissue engineering technology (TET) provides a new and highly viable mentality for AC defect repair, including OA, which has been developing since the 1980s and with the process of combining the obtained seed cells with biomaterials and bioactive factors to induce stem cell differentiation and proliferation to cultivate a biomimetic functional tissue, and then transplanting it into the defected section to perform its repair function.56,57 It was first applied to AC construction, which is easy to study without interference benefit by its simple structure.58 –60
The landmark study of cartilage TET was that of Vacanti et al.61 who successfully constructed hyaluronic tissues with well properties in nude mice by using bovine articular chondrocytes (ACs) and biodegradable macromolecular scaffold in 1991; their work provided a new approach for tissue creation using synthetic biocompatible and biodegradable polymers as templates onto which cells are seeded is presented. And then in 1997, Cao et al.62 seed cow chondrocytes into a biodegradable human ear-shaped scaffold and then implanted into subcutaneous pockets on the dorsa of athymic mice to generate new cartilage. Afterward, various cartilage TET parameters were preliminarily explored and a relatively mature culture system was established via experiments on small animals. The subsequent experiment on large animals provided a solid foundation for cartilage TET from laboratory to clinic because of the complete immunogenicity and high similarity in anatomical structure with human.63,64 Matrix-assisted autologous chondrocyte implantation (MACI), which based on ACI technology, has been commercialized for several years in the clinic, and showed positive repair effects, such as MACI® from Vericel, CaReS® from Arthro Kinetics Biotechnology GmbH, Chondro-Gide® from Geistlich Biomaterials Company, and BioSeed® from BioTissue etc.65 –68 Furthermore, MaioRegen scaffold was consisting of collagen I and hydroxyapatite. By indirectly loading endogenous stem cells, it significantly improved knee symptoms of the patients with full layer knee AC defects, and averted twice surgeries and chondrocytes in vitro expansion, which increases the complexity of surgeries and prolongs the recovery time.69
Although the preclinical trial is currently underway, this focuses on exploring the key technologies for clinical transformation of cartilage TET. It also faces some ethical, policy and technical difficulties. For example, AC defects, including OA, often damages not only the AC layer, but also involves the calcified cartilage and subchondral bone. Therefore, just repairing the AC layer is often difficult to achieve complete treatment. The preparation of a zonal microstructured tissue engineered scaffold materials to mimic the structure of AC and osteochondral (Figure 4), particularly the integrated living joint, might provide better treatment for serious AC defects. Hence, the subsequent research needs to ensure its safety, quality, and efficacy of cartilage TET products before going public, including materials, biocompatibility, clinical application, risk assessment management, and difficulty level of clinical evaluation with medical devices.
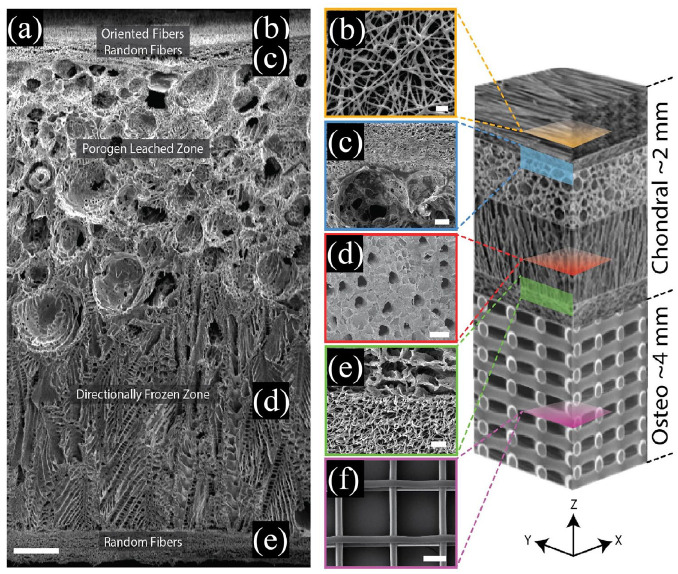
Figure 4.
A zonal microstructured scaffold mimics the structure of AC and subchondral bone to repairing osteochondral defects: (a) cross-section of the complete scaffold showing each unique zone, (b) partially fused poly-ε-caprolactone (PCL) fibers used to adhere the electrospun mat to the underlying foam using residual solvent (top-down image), (c) a cross-sectional view of the porogen-electrospun interface, (d) vertical channels through the directionally frozen foam (top-down image), (e) a cross-sectional view of the directionally frozen-electrospun interface, and (f) melt-electrowritten osteo component (top-down image). The osteo component consisted of 20 μm diameter fibers stacked at 200 μm intervals in a 90-degree lay-down pattern. Figure at right is a conceptual schematic of the zonal microstructured osteochondral scaffold, features are not proportionally represented. Scale bars for images a, b, c, d, e, and f are 250, 10, 50, 100, 25, and 100 μm, respectively. Reproduced from Steele et al.70
Stem cell
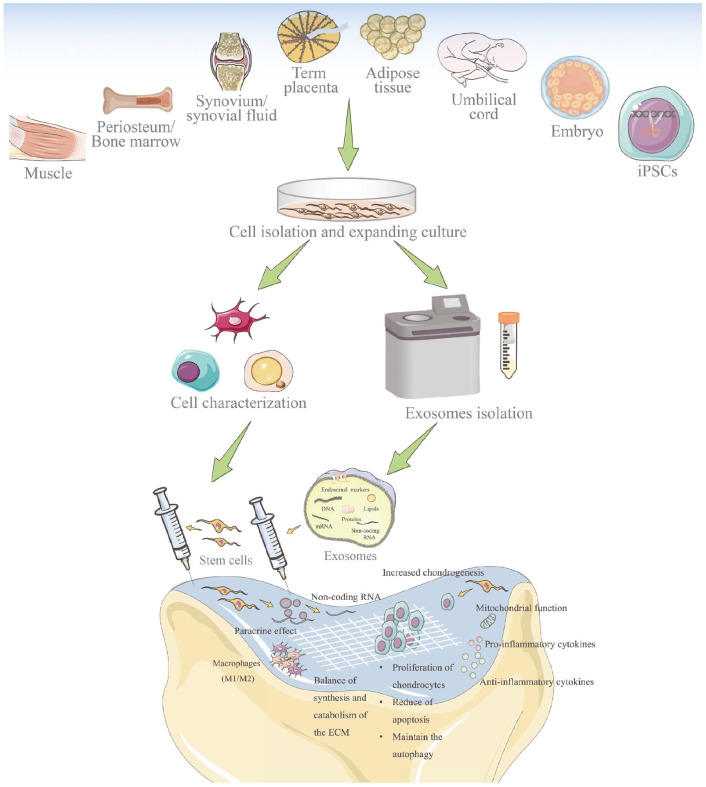
Chondrocytes are a great option for the seed cells in cartilage TET due to their ECM and collagen II productive capability. However, they can lose their chondrogenic phenotype during cultivation in vitro. Moreover, the instability source, and the chondrocyte from older patient become smaller and less uniform aggrecans greatly limited their further regeneration function.3 Luckily, stem cells, including MSCs, embryonic stem cells, and induced pluripotent stem cells (iPSCs) are increasingly favored by researchers for AC defect therapy, such as focal chondral lesions, due to its accessibility and low immunogenicity characteristic (Figure 5).71 –73 Some evidence suggests that the tissue type from which MSCs are harvested play a role in determining their ability to regenerate cartilage.74 Among them, BMSCs remain the gold standard for AC and bone TET or regenerative medicine. However, adipose-derived mesenchymal stem cells (ADSCs) have similar properties and some advantages and thus are considered a good alternative to BMSCs.75 Unfortunately, their chondrogenesis potential is inferior.76 Umbilical cord-derived mesenchymal stem cells (UCMSCs) show a higher proliferative potential than BMSCs and are capable of osteogenic, chondrogenic, and adipogenic differentiation. Interestingly, they show comparable transfection efficiency as BMSCs using a nucleofection method but are more amenable to transfection with liposomal methods.77 Studies have also reported successful cartilage repair with SMSCs transplantation and held promise as a treatment option for focal cartilage defects.74,78 In comparison, SMSCs and BMSCs, especially SMSCs that have greater proliferative rate and chondrogenic potential than ADSCs, periosteum-MSCs, and muscle-MSCs, may be the main driver of cartilage repair. Interestingly, an ADSCs-derived scaffold-free tissue-engineered construct was developed as a novel cell therapy system for intervertebral disks degeneration using a rat tail total nucleotomy model, which performed promising AC regenerative potential (Table 1).79
Figure 5.
Stem cells and their derived exosomes used in AC defects repair. Stem cells can protect cartilage by differentiation into chondrocyte lineages, affecting the chondrocytes, mediating mitochondrial function, regulating cytokines, balancing the synthesis and catabolism of ECM, modifying immune reactions, and paracrine activity that might be involved with the secreted exosomes. Exosomes are small extracellular vesicles that include lipids, nucleic acids, and proteins. Reproduced from Xiang et al.51
Table 1.
MSCs used in AC defects repair.
| MSCs | Source | Autologous/Allogeneic | Available volume | Chondrogenesis potential |
|---|---|---|---|---|
| BMSCs | Bone marrow | Autologous | ++ | ++ |
| ADSCs | Adipose | Autologous | +++ | ++ |
| UCMSCs | Umbilical cord | Almost allogeneic | +++ | +++ |
| SMSCs | Synovium | Autologous | + | +++ |
| PMSCs | Periosteum | Autologous | + | + |
| MMSCs | Muscle | Autologous | + | + |
However, MSCs therapy has numerous limitations: only suitable for early/middle mild injury treatment, poor cell adhesion and retention, phenotypic alteration of cells, regulation of mechanical properties, heterogeneity, and lower engraftment rates after implantation.80,81 In addition, the efficient recruitment of MSCs to defective or damaged tissues in vivo has been a difficult hurdle. Current studies aim to construct biomaterials that could effectively recruit BMSCs to facilitate the repair of pathological tissues and promote MSCs proliferation, viability, adhesion and chondrogenic differentiation.82 For example, an injectable thermosensitive sodium alginate (SA)/agarose (AG) composite hydrogel with coculture of BMSCs and ACs could promote the chondrocyte differentiation of BMSCs, resulting in its application to AC regeneration.83 In addition, targeted cell delivery by porous magnetic nanoparticles was a promising technique to enhance the low targeting efficiency of MSCs in tissue regeneration. A 3D porous microbead that was formed as a MSCs cargo consisting of poly(lactic-co-glycolic acid) (PLGA) and coated with amine-functionalized magnetic nanoparticles showed 2D/3D targeting of multiple microbeads loaded with MSCs; the microbead performed targeted MSCs delivery, proliferation, viability, and chondrogenic differentiation for cartilage repair.84 In addition, a human ADSCs-based medical microrobot system, which consists of a microrobot body capable of supporting MSCs, an electromagnetic actuation system for microrobot 3D targeting, and a magnet for the fixation of a microrobot to the damaged cartilage, was proposed; its efficacy was verified in a cartilage defect model in vivo trial for AC regeneration.85 In addition, MSCs-binding peptide E7 was fused with the exosomal membrane protein Lamp 2b to yield exosomes with E7 peptide displayed on the surface; the exosomes had SMSCs targeting capability, could deliver KGN enter SMSCs efficiently, and induce a higher degree of cartilage differentiation, resulting in an advanced stem cell therapy for cartilage defect.17
Moreover, generating phenotypic chondrocytes from pluripotent stem cells is of great interest in AC regeneration. Subcutaneous implantation of assembled iPSC-derived cartilage microtissues resulted in a homogenous cartilaginous tissue positive for collagen II but negative for osteocalcin. Human iPSC-derived neural crest cells (iPSC-NCCs) with high potential to undergo chondrogenesis through MSCs differentiation were used to fabricate cartilage.86 In addition, human iPSC-derived isogenic mesodermal cells (iPSC-MCs) and iPSC-NCCs expressed hyaline cartilage-associated markers and could generate hyaline cartilage-like tissue ectopically and at joint defects. By comparison, iPSC-NCCs revealed closer morphological and transcriptional similarities to native ACs than iPSC-MCs, and its implants induced by growth factor mixture demonstrated increased matrix production and stiffness in comparison with iPSC-MCs implants.87 Moreover, a baculovirus system was used to confer prolonged and robust TGF-β3/bone morphogenetic protein 6 (BMP-6) expression in genetically engineered rabbit ADSCs (rADSCs), which cultured in porous scaffolds critically augmented rADSCs chondrogenesis and suppressed osteogenesis/hypertrophy, leading to the formation of cartilaginous constructs with improved maturity and mechanical properties in hyaline cartilage regeneration.76 Happily, clinical studies have shown that CARTISTEM®, a combination of human UCMSCs and sodium hyaluronate for cartilage repair, got into the market in Korea in 2012. Short-term and long-term studies have verified its safety, functions and advantages on AC repair and regeneration.88,89 However, a standard and therapeutic mechanism for MSCs-based therapy in AC defects is required in the future research, including cell selection, characterization and quality inspection (such as phenotypic and multipotent differentiation potential analysis), isolation, culture and expansion methods, dosages, and rehabilitation program. Finally, how to get it through supervision department and into clinical application also needs to be overcome.
Materials of scaffolds
Hydrogels
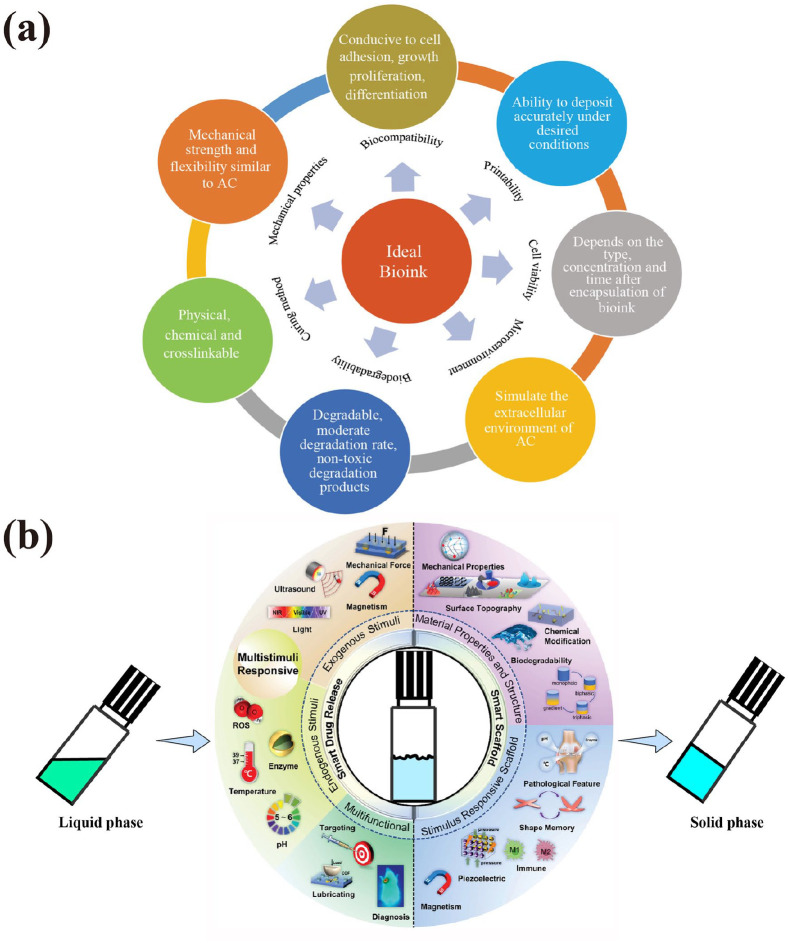
Hydrogels, which are elastic and display smooth surfaces while exhibiting high water content, are promising candidates for cartilage regeneration. In recent years, injectable and highly tunable composite hydrogels for cartilage TET, drug or cell delivery (e.g. KGN, stem cells, and chondrocytes) have been introduced as a desirable alternative to invasive treatments. However, given its multilayer composite tissue, the single-layer hydrogel or bionic scaffold is not enough to accomplish joint injury repair; thus, bilayered or even multilayered hydrogels must be developed and used. Although polyethylene glycol (PEG), gelatin, HA, methacrylic anhydride (MA), glycosaminoglycan (GAG), methacrylated pullulan, and silk fibroin (SF) are often used to prepare hydrogels, hydrogels with different components still have certain limitations for cartilage TET. For example, these hydrogels do not meet the requirements of AC repair because of their fast degradation rate and poor mechanical strength. With the continuous discovery of new members, hydrogel preparation materials are becoming increasingly abundant; whether as 3D printing ideal bioink or scaffold materials, hydrogel or its composite materials are optimizing its biocompatibility, printability, cell viability, microenvironment, biodegradability, curing method, and mechanical properties, making its application increasingly extensive (Figure 6(a)).90,91
Figure 6.
(a) Design strategy of ideal bioink. Reproduced from Weng et al.92 and (b) smart stimuli-responsive hydrogels for articular cartilage repair and regeneration. Reproduced from Gu et al.93
Improving the properties of single hydrogels by adding components with different biological properties to form composite hydrogels might solve the above problems. For example, pullulan could be modified with MA, lithium phenyl (2,4,6-trimethylbenzoyl) phosphinate (LAP), and PEG diacrylate (PEGDA) to form a hybrid hydrogel system, which has good mechanical properties and slow degradation rate for cartilage repair and regeneration.94 In addition, gelatin, HA and MA are used together in a composite hydrogel to make scaffolds, which regenerated mature cartilage with typical lacunae structure and cartilage-specific ECM successfully when combined with chondrocytes; the hydrogel provided a novel natural 3D scaffold with satisfactory outer shape, pore structure, mechanical strength, degradation rate, and weak immunogenicity for cartilage regeneration.95
With the increasing application, some hydrogels need special processing to adapt to the corresponding application conditions due to the different microenvironments of body parts. Complex hydrogels composed of natural or synthetic polymers can exhibit high mechanical strength and good reproducibility but may be problematic due to biocompatibility issues, which are insufficiently investigated.96 Some natural or synthetic polymer-based stimuli-responsive (endogenous or exogenous stimuli) smart hydrogels with specific material properties and structure, and multifunctions, such as photo-crosslinkable hydrogels, thermogel, mechanical force-responsive hydrogels, pH-responsive hydrogels, electricresponsive hydrogels, redox-responsive hydrogels, enzyme-responsive hydrogels, magnet-responsive hydrogels, ultrasound-responsive hydrogels, and multiresponsive hydrogel, have appeared successively, and provided new possibilities for various advanced technologies, great research and application potentials in regenerative medicine, including cartilage TET (Figure 6(b)).93,97,98 Among them, gelatin methacrylate (GelMA), gelatin norbornene, and methacrylatedHA (HAMA) were used as gradient biomimetic visible light photo-polymerized hydrogel 99 –101; LAP and sericin methacryloyl were used as UVphotoinitiator 94,102; PLGA, poly(N-isopropylacrylamide), poly(N-vinylcaprolactam), poloxamer 407, and chitosan were used as thermoresponsive polymer according to their special characteristics (Table 2).103 –106
Table 2.
Advantages and limitations of different biomaterials for hydrogels used in AC TET.
| No. | Name (Abbreviation) | Advantages | Limitations | Refs. |
|---|---|---|---|---|
| 1 | Polyethylene glycol (PEG) | 1. Biocompatible | Biologically inert | Wei et al.96 |
| 2. Easy to be functionalized | Roberts and Bryant107 | |||
| 2 | Poly (2-methacryloyloxyethyl phosphorylcholine) (PMPC) | 1. Monomer and polymer biocompatible | NO cell adhesion molecules | Wei et al.96 |
| 2. Lubricant | ||||
| 3. Mechanical strength | ||||
| 3 | Polyvinyl alcohol (PVA) | 1. MSCs chondrogenic differentiation | 1. Mechanical strength | Wei et al.96 |
| 2. Biologically inert | Shi et al.108 | |||
| 3. Low cell adhesion | Maher et al.109 | |||
| 4. Low bioactivity | Bichara et al.110 | |||
| 5. Non-degradable | ||||
| 6. Low equilibrium water content | ||||
| 4 | Poly(N-vinylcaprolactam) (PVCL) | 1. Thermosensitive | 1. Wettability | Wei et al.96 |
| 2. Biocompatibility | 2. Mechanical properties | Whittaker et al.111 | ||
| 3. Water uptake capacity | 3. Antibiofouling | |||
| 5 | Poly(N-isopropylacrylamide) (PNIPAm) | 1. Thermosensitive | 1. Low viscosity | Lima et al.112 |
| 2. Biocompatibility | 2. Fast adhesive polymerized properties | Guo et al.113 | ||
| 3. Mechanical properties | ||||
| 4. Reversible cell adhesion | ||||
| 6 | Polyacrylic acid (PAA) | pH-responsive | 1. Ionized at high pH | Jiang et al.97 |
| 2. Lower coefficient of friction | Bichara et al.110 | |||
| 7 | Polyaniline | 1. Electric-responsive | Limited hydrophilicity | Jiang et al.97 |
| 2. Rigid backbone | ||||
| 8 | Poly(N,Ndiethylaminoethyl meth acrylate) (PDEAEM) | pH-responsive | Ionized at low pH | Jiang et al.97 |
| 9 | Poly(lactic-co-glycolic acid) (PLGA) | 1. Thermosensitive | 1. Mechanical strength | Sadat Tabatabaei Mirakabad et al.114 |
| 2. Approved by the US FDA and European Medicine Agency to use in drug delivery systems | 2. Poor loading | Qu et al.115 | ||
| 3. Biodegradation | 3. High burst release | |||
| 4. Improved cartilage regeneration | 4. Production of acids | |||
| 5. Biocompatibility | ||||
| 10 | Poloxamer 407 (PX) | 1. Thermosensitive | Induced hyperlipidemia | Goo et al.116 |
| 2. Mechanical properties | Wang et al.117 | |||
| 3. Supersaturating | ||||
| 11 | Azobenzene and its derivatives | 1. Thermosensitive | 1. Stability of the switch in vivo | Jiang et al.97 |
| 2. Light-responsive | 2. Rapid spatiotemporal control | |||
| 3. Hypoxia-response | 3. Absorption wavelengths compatible with biological tissue optical windows | |||
| 4. UV phototoxic damage | ||||
| 12 | Lithium phenyl (2,4,6-trimethylbenzoyl) phosphinate (LAP) | 1. UV photoinitiator | 1. Cytotoxic | Qin et al.94 |
| 2. Water-soluble | 2. Mutagenic | |||
| 13 | Cellulose | 1. Can be sulfated | 1. Mechanical properties | Wei et al.96 |
| 2. Nanofibrils similar to collagen fibrils of tissue ECM | 2. Limited 3D nano-scale pore structure | Li et al.118 | ||
| 3. Lack of biological function | ||||
| 14 | Chondroitin sulfate (CS) | 1. Component of natural cartilage | Rapid degradation | Wei et al.96 |
| 2. Regulate hypertrophy during MSCs chondrogenesis | Chen et al.119 | |||
| 3. Promote cartilage ECM production | ||||
| 4. Inhibiting inflammation | ||||
| 15 | Hyaluronic acid (HA) | 1. pH-responsive | NO cell adhesion molecules | Wei et al.96 |
| 2. Component of natural cartilage | Jiang et al.97 | |||
| 3. Increase the synthesis of ECM and chondrogenesis | ||||
| 4. Easy to be functionalized | ||||
| 16 | Chitosan, chitin and their derivatives | 1. Thermosensitive | 1. Low solubility | Wei et al.96 |
| 2. Ions-responsive | 2. High viscosity | Jiang et al.97 | ||
| 3. Electric-responsive | ||||
| 4. pH-responsive | ||||
| 5. Drug delivery | ||||
| 6. Easy to be functionalized | ||||
| 17 | Alginate | 1. Thermosensitive | 1. Lack of biodegradability | Wei et al.96 |
| 2. pH-responsive | 2. Immunological responses for its endotoxin contents | Liu et al.120 | ||
| 3. 3D bioprinting | 3. The rapid gelling process | |||
| 4. Mechanical strength | 4. Limited capacity to retain GAGs | |||
| 5. Gene carriers | 5. Insufficiently cell adhesion | |||
| 6. Biocompatible component with a high water content, good porosity and tunable viscosity | ||||
| 7. Powerful capacity to retain collagen | ||||
| 18 | Collagen | 1. Natural ECM protein | 1. Limited number of functional groups for crosslinking | Wei et al.96 |
| 2. Immunomodulation | 2. Fast degradation | Long et al.121 | ||
| 3. Poor mechanical properties | ||||
| 4. Excessive cell-mediated shrinkage | ||||
| 19 | Gelatin | 1. Promote cell adhesion | 1. Weakly mechanical properties and brittleness | Shi et al.122 |
| 2. UV photoinitiator | 2. Low thermal stability | Vassallo et al.123 | ||
| 3. 3D bioprinting | 3. High sensitivity to enzymatic degradation | |||
| 4. Highly biocompatible, biodegradable, and cost-effective | ||||
| 20 | Silk fibroin (SF) | 1. Mimicking the collagen structure of native cartilage with biocompatibility, biodegradability, high tensile strength, and various cells adherence | 1. Limited options for anchoring growth factor | Cheng et al.124 |
| 2. Immunocompatible | 2. Large brittleness | Qi et al.125 | ||
| 3. The diverse ability to crosslink or solution-to-gel | 3. Low stability in aqueous solution | |||
| 4. Natural photoluminescence | 4. Difficult to be crosslinked | |||
| 21 | Fibrin | Easy to be functionalized | No chondro-permissive | Thorpe et al.126 |
| 22 | Agarose (AG) | 1. Thermosensitive | 1. Low mechanical properties | Bahcecioglu et al.127 |
| 2. Biocompatibility | 2. Low cell adhesion | |||
| 3. Promote expression of GAGs | ||||
| 4. Non-immunogenic properties | ||||
| 23 | Methacrylic anhydride (MA) | Photo-crosslinkable | Low mechanical properties | Xia et al.95 |
| 24 | Peptides | Promote MSCs chondrogenesis | Need proper peptide design, synthesis, and purification | Jiang et al.97 |
Collagen-based scaffolds
Cartilage tissue is rich in collagen, such as collagen type Ⅰ, II, III, V, VI, IX, XI, and XIV, which are an important natural component of ECM as a bioactive protein to provide high elasticity for cartilage tissue.128 –130 Increased knowledge about the organization, structure and properties of collagen has inspired us to design and develop innovative collagen-based biomaterials and tissue engineered products.131 Multiple collagen-based scaffolds have been proposed for TET to promote a biological response and to work as artificial biomimetic ECM to guide the regeneration of different tissues/organs, such as AC,132 bone,133 blood vessel,134 and periodontal ligament,135 etc.
For TET in cartilage regeneration, collagen is often used to assemble hydrogels or scaffolds as cell, drug, or bioactive molecular loaders in cell proliferation, adhesion, and chondrogenic differentiation of stem cells for cartilage repair and regeneration,136 –138 which is due to its natural cartilage component, excellent biocompatibility and a wide range of sources, including animal skin, hooves, cartilage, and some marine products. Among them, collagen-based scaffolds are frequently used to deliver and retain cells at the site of cartilage damage.139 For example, a BMSCs/porous tantalum associated with chondrocytes/collagen membranes demonstrated remarkably therapeutic efficacy in goat models of large osteochondral defect.132 Collagen/oligomeric proanthocyanidin/oxidized HA composite scaffolds that show high biocompatibility and excellent mechanical properties were prepared, and were found to be favorable surfaces for the deposition of apatite and can be used as potential candidates for AC repair.140
Moreover, different types of collagen have various properties that apply to different tissues. Type I collagen is sufficient to support cellular activities of ACs and MSCs; however, it shows limited chondrogenic performance compared with type II collagen. Nonetheless, type I collagen is the clinically feasible option because type II collagen shows arthritogenic potency.141 The effects of native collagen and denatured collagen scaffolds on rabbit chondrocytes’ proliferation, adhesion, differentiation and interaction with matrix were investigated, and the results suggested that the native collagen scaffolds may contribute to cartilage repair more than denatured collagen scaffolds.137 Overall, native collagen is an ideal tissue engineering scaffold material, especially for cartilage, because it can provide good biological compatibility and flexibility and can serve as a carrier for cell, drug, or bioactive molecules to promote chondrocyte proliferation and adhesion while accelerating stem cell differentiation into chondrocytes; thus, stimulating the repair of cartilage injury. So eventually, numerous collagen-based products have got into the market or in clinical research for cartilage repair, such as Neocart, CaReS, and Novocart 3D. But, almost of current collagen-based scaffolds on market or in the clinic are solidity, which increases the operating difficulty. The injectable collagen-based hydrogels, which is injectable before transplantation but solid after implantation, may be a good candidate in the near future for AC repairs to reduce the trauma and improve the filling of materials to the defect area.
ECM scaffolds
Joint biomechanical and tribological functions rely on the integrity of cartilage ECM. ECM, generally refers to decellularized ECM, which is among the most promising scaffold for cartilage repair due to its natural cartilage components (Figure 7). However, ECM sourced from different developmental stages, such as neonatal, childhood, and adolescent cartilage tissues, shows diversified temporal dependency on cellular chondrogenesis because of its components and discrepant growth factors. Among them, childhood cartilage is considered the optimal ECM source for the further development of ECM-based tissue engineering scaffolds in AC repair.142 In addition, ECM from different cartilage types has specific functions in inducing stem cells to osteogenesis or chondrogenic differentiation due to their different bioactive factors. Mass spectrometry revealed that the growth plate and AC ECM contain a unique array of regulatory proteins. Moreover, porous bilayered scaffolds composed of growth plate ECM overlaid by AC ECM were fabricated; the growth plate layer supported the formation of endochondral bone, whereas the AC layer stimulated the formation of the covering hyaline cartilage through the collagen fiber architecture to improve the recapitulation of the native tissue.143
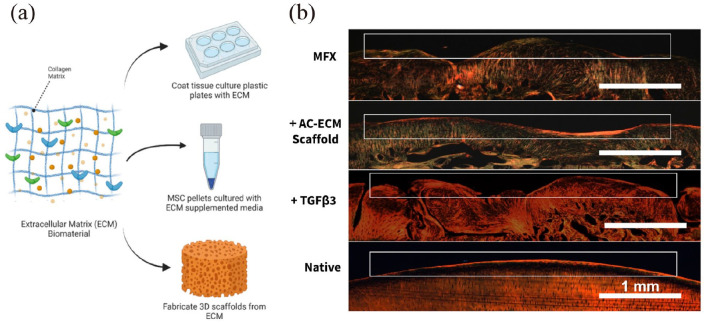
Figure 7.
Scaffolds derived from AC ECM can be used alone or in combination with exogenous growth factors for the repair of chondral lesions: (a) ECM biomaterial used in MSCs chondrogenic differentiation and 3D scaffolds fabrication, (b) AC ECM scaffolds used alone or in combination with TGF-β3 promotes the recapitulation of native collagen fiber alignment in the superficial zone, and shows better repair effect compared to traditional MFX therapy. Histological sections were stained with picrosirius red and then imaged using polarized light microscopy. Reproduced from Weng et al.144
ECM scaffolds, which are cell-free biomimetic scaffolds with cartilage ECM-like architectures, are often fabricated as polymer nanocomposite scaffolds that mimic the structural design and mechanical characteristics of native AC and are widely used in cartilage regeneration. This kind of scaffolds guide the morphology, orientation and phenotypic state of chondrocytes in a spatially controlled manner, support the growth of tissue with features that are reminiscent of the natural analog; and promote localized hydroxyapatite formation to permit integration with the subchondral bone.145 However, endogenous cells hardly migrate into ECM derived from native cartilage due to its nonporous and dense structure, especially for severe grade Ⅳ AC injuries involving subchondral bones. To solve this problem, multilayered scaffolds with a compact interfacial layer, hierarchical organization and heterogeneous composition were developed to mimic the stratified structure and complex components of natural osteochondral tissues to enhance its defect repair. For example, a trilayer scaffold comprising an oriented AC ECM-derived cartilage layer, a porous three-dimensional (3D) printing PLGA/β-tricalcium phosphate (PLGA/β-TCP) bone layer, and an intermediate PLGA/β-TCP compact interfacial layer was designed to evaluate the ability of repairing osteochondral defect in a goat model; this scaffold enhanced the biomechanical and biochemical properties of the neo-osteochondral tissue.146 Another multilayer scaffold was built; the oriented cartilage layer was fabricated with cartilage ECM-chitosan materials, and a 3D-printed core-sheath-structured bone layer was fabricated with PLGA/β-TCP phosphate-collagen materials and a compact interfacial layer, which was more effective in repairing osteochondral defect.147 Moreover, a spatially patterned, gradient, macroporous, trilayer microribbon (microRB) scaffold, which led to minimal cartilage deposition and weak mechanical properties, took only 21 days for MSCs-seeded scaffolds to reach cartilage-mimicking compressive moduli without requiring high cell seeding density.148 In addition, different decellularization approaches generate different structures and composition of ECM scaffold. Scaffolds decellularized with 0.5%−1% SDS, followed by 1% Triton X-100 and DNase solution, could ensure the integrity and bioactivity of ECM scaffolds; thus, this type of scaffold is favorable to BMSCs recruitment and osteochondral regeneration and conducive to AC repair.149,150
The injury repair performance of ECM scaffolds can also be optimized via combination with other biological materials. For example, ECM-mimetic SF scaffolds with horizontally aligned, vertically aligned, and random pore architectures of joint were synthesized and showed a good effect in repairing the rabbit osteochondral defect.151 Hierarchical macro-microporous waterborne polyurethane-ECM scaffolds were constructed by water-based 3D printing and were found to be suitable for cell distribution, adhesion, and proliferation, thus promoting in situ AC regeneration in rabbits. Exhilaratingly, the repaired cartilage showed a similar histological structure and mechanical performance to that of normal cartilage.152 Loaded with BMSCs, a graphene oxide-modified (2 mg/mL) 3D ECM scaffold, in which GO was crosslinked with the ECM by 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride and N-hydroxy succinimide, completely bridged the AC defect in rabbit knee with hyaline cartilage.153
Nanofibrous/cellulose scaffolds
SF and cellulose have been reported as advanced natural materials with safety and excellent biocompatibility, extensive source (silkworm cocoon, plants and bacteria etc.) and low cost features, and they are well used as structural constituent materials of injectable hydrogels or TET scaffolds because of their remarkable mechanical properties, long-lasting in vivo stability, hypoimmunity and nontoxicity, and can be used efficiently in crosslinking applications.154 –156 For example, a porous SF scaffolds derived from horseradish peroxidase (HRP)-mediated crosslinking by salt-leaching and freeze-drying methodologies were developed for AC TET; these scaffolds presented high porosity, wide pore distribution, high interconnectivity, large swelling capacity, and favorable degradation rate, which made it promising candidates as 3D matrices for cartilage regeneration.157 For cellulose, bacterial cellulose in general, was found to enhance the mechanical properties of hydrogel and thus was applied for osteochondral defect repair.158
Furthermore, SF and cellulose are often used in combining with other materials to modify their properties and hence become more conducive to cartilage regeneration. For example, an injectable hydrogel was engineered using SF, carboxymethyl cellulose, and gelatin, in which the ectopically regenerated cartilage was mature and closely resembled native AC.155 3D-printed PCL/porous SF scaffolds were proposed for meniscus TET and had more favorable microstructure compared with PCL scaffolds. The capture of porous SF in the PCL cage reduced the high compressive modulus in the wet favorable material due to its higher expansion properties in comparison with the PCL cage.159 A biomimetic and composite scaffold consisting of PCL/SF/SMSCs-specific affinity peptide (LTHPRWP, L7) with extraordinary biomechanical properties and biocompatibility was used to develop a 3D-printed, tissue-engineered meniscus, which showed improved cartilage protection and could greatly strengthen meniscus regeneration and chondroprotection.160 Analogously, SF greatly balanced the mechanical properties and degradation rate to match the newly formed cartilage by integrating gelatin with BMSCs-specific-affinity peptide.161 Through 3D printing, a PCL/fiber network was also used to reinforce interpenetrating network hydrogels consisting of alginate and GelMA mechanically. The network supported robust chondrogenesis when laden with a coculture of BMSCs and chondrocytes.162
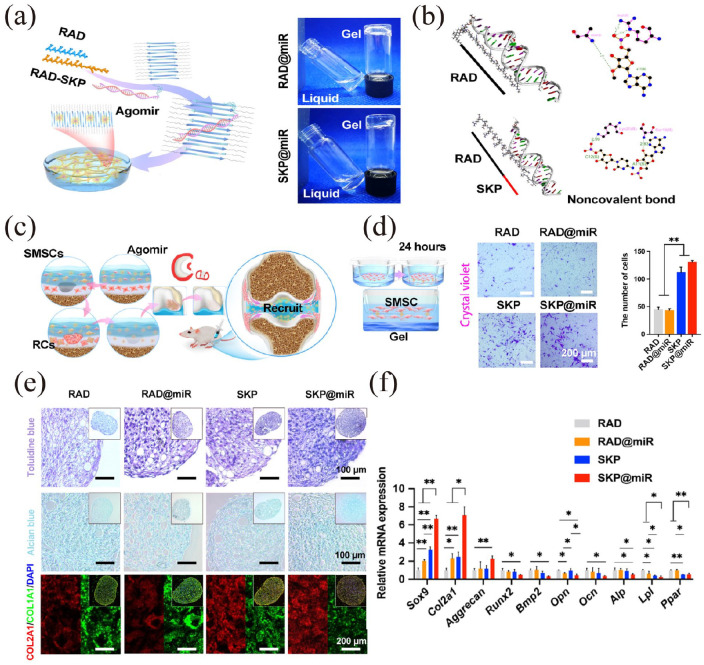
The molecular and supramolecular design of bioactive materials might have significant implications for regenerative medicine. An ideal regenerative therapy should be minimally invasive; thus, the concept of self-assembling biomaterials programed to transform from injected fluids into solid bioactive structures in tissues is attractive for clinical translation.163 Therefore, the design and optimization of natural bionic nanofibrous or nanocellulose microcarriers for the delivery of cells, molecules/macromolecules to promote osteochondral repair and regeneration is of great clinical value.164 –167 For instance, cellulose nanocrystals have been reported to reinforce the self-healing and stress-relaxation properties of collagen-based nanocomposite hydrogels for cell delivery.168 An injectable bioactive self-assembling stem cell-homing peptide nanofiber hydrogel-based aging-related miRNA delivery to rejuvenate impaired AC was prepared and validated (Figure 8).49 Combined with SA, nanocellulose was used to 3D print hydrogels for AC engineering and showed good integration, promising cartilage regeneration and mechanical stability in mice.166 Therefore, cellulose nanofibrils are promising nanomaterial candidates due to their high mechanical strength and excellent biocompatibility.169
Figure 8.
An injectable nanofiber hydrogel to deliver an aging-related miRNA to rejuvenate impaired AC: (a) RAD (RADA) and SKP (SKPPGTSS) peptides self-assemble to form a stable nanofiber hydrogel with aging-related agomir-29b-5p distributed inside by adjusting the pH value to neutral, (b) molecular docking analysis of RAD or SKP peptide interacted with agomir-29b-5p, (c) the recruitment process pattern of SMSCs during AC repair, (d) images and cell quantification of Transwell bottom membrane stained with crystal violet after 24 h to monitor SMSCs recruitment in vitro, (e) Toluidine blue, Alcian blue, and immunofluorescence staining of COL2A1 and COL1A1 in SMSCs pellets after 14-day culture on RAD, RAD@miR, SKP, and SKP@miR, (f) qRT-PCR analysis of gene expression of chondrogenesis-related and osteogenesis-related genes in SMSCs pellets. Values were normalized to β-actin levels, and RAD group was used as the control group. Statistical analysis was performed using one-way ANOVA. *p < 0.05 and **p < 0.01. Reproduced from Zhu et al.49
Other biomaterials
As mentioned above, biomaterials play a key role in cartilage TET and regeneration through different combinations and treatments, where cells must migrate through the scaffold, fill the defect, and then proliferate and differentiate to facilitate tissue remodeling. For example, a mixture of gelatin and PCL with 70:30 ratio could be used for 3D printing of electrospun membranes.170 Gelatin and PLA cross-linked with HA could be used to construct superabsorbent 3D scaffold based on electrospun nanofibers for AC TET.171 Eletrospun PCL nanofibers were surface-modified with poly(glycidyl methacrylate) and subsequently reacted with ECM and self-assembled with ADSCs; the modified nanofibers exhibited differentiation hallmarks of chondrogenesis without additional biological additives.167
In addition, metal ions play a crucial role in AC regeneration. For example, strontium (Sr) ions could simultaneously promote BMSCs proliferation, upregulate cartilage-related gene expression, and improve GAG secretion; consequently, injectable hydrogels crosslinked by Sr-doped bioglass (BG) could modulate both BMSCs differentiation and inflammatory response.172 Tannic acid/Sr(2+)-coated silk/graphene oxide-based meniscus scaffold could perform anti-inflammatory and anti-ROS functions in cartilage protection and OA delay.173 In addition, molybdenum (Mo)-doped BG scaffolds were prepared, and results revealed that MoO42− ions elicited anabolic responses by activating the HIF-1α signaling pathway, inhibiting catabolic responses, and protecting the cartilage matrix from degradation.174 A copper sulfate and hydroxylysine or Cu-alginate hydrogel treatment regimen enhanced collagen cross-linking and biomechanical properties in antimicrobial wound dressings, TET scaffolds or AC implants.175,176 A composite scaffold, which was composed of poly (l-lactide) (PLLA) and nano-hydroxyapatite (10 wt%), was functionalized with 3 mol% europium (Eu3+) and promoted the osteogenesis and chondrogenesis of ADSCs.177 Furthermore, other metal ions, such as magnesium (Mg)178 and cobalt (Co),179 and nonmetal ions, such as boron,180 have been confirmed to promote AC regeneration.
Research and achievement on different build approaches, such as enzymatically cross-linked hydrogels/scaffolds, are increasing. The HRP-mediated crosslinking of SF and tyramine-substituted gelatin was conducted to fabricate a macroporous hydrogel scaffold, which could be used as a cell seeding strategy for cartilage regeneration.181 Microbial transglutaminase was used to cross-link gelatin hydrogel enriched with an AC ECM for hyaline cartilage regeneration in rabbits.182 Furthermore, biofors, pH, electric, redox, and magnet-responsive hydrogels/scaffolds were used in AC regeneration.96 For osteochondral regeneration, constructing multilayered bionic scaffolds with different properties remains the gold standard to imitate different tissue types at the injured site of joints so as to promote injury repair. For example, an HRP-SF cartilage-like layer fully integrated into an HRP-SF/ZnSr/β-TCP subchondral bone-like layer was proposed as a biofunctional hierarchical scaffold via enzymatic cross-linking.183 A bilayer scaffold, in which gellan gum and SA act as the chondral layer and gellan gum and hydroxyapatite act as the subchondral layer, could seed with MSCs for effective osteochondral repair.184 A composite porous support obtained by cryopolymerization of PEG dimethacrylate in the presence of PLGA, β-TCP, and strontium folate (SrFO), with HAMA-based hydrogel containing zinc folic acid derivative (ZnFO), was reported to promote the regeneration of cartilage-like tissue over the scaffold and neoformation of osteochondral tissue.185
Bioactive factors
Several bioactive factors, such as growth factors, mineral ions and anabolic factors, have been used to alleviate the inflamed joint microenvironment or promote new cartilage tissue formation.15 For example, BMP-2 is efficient at stimulating bone or cartilage regeneration in tracheal grafts.186 Stromal cell-derived factor 1 (SDF-1) and platelet-derived growth factor-BB could enhance endogenous cell recruitment and cartilage matrix formation.187 Recombinant human transglutaminase-4 enhances rabbit cartilage regeneration of the SMSCs encapsulated in HA/collagen/fibrinogen composite hydrogel.80 Cell-permeable, superpositively charged SOX9 is able to promote hyaline-like cartilage regeneration by inducing chondrogenic differentiation of BMSCs.188 And the relatively high concentration (2–10 μM) of Mg ions facilitate chondrogenesis and osteogenesis instead of adipogenesis of BMSCs and tendon-derived MSCs under induction conditions respectively ex vivo.189
However, these therapies remain limited in terms of duration and effectiveness. Consequently, current efforts are focused on the potential ways for improving future long-term clinical outcomes. For instance, HA and fucoidan was blended with gelatin and then further cross-linked with genipin to prepare injectable hydrogels, which facilitated the sustained release of PRP growth factors and promoted cartilage regeneration in rabbits.190 Implantation of the scaffolds containing biodegradable polyurethane, 200 ng/mL SDF-1, and 22 wt% Y27632-encapsulated microspheres (55 µg/mL Y27632 in microspheres) in rabbit AC defects, which allowed each of SDF-1 and Y27632 to be released sequentially, supported the potential of the scaffolds to promote cartilage regeneration.191
Moreover, some acellular bioactive factor aggregates from autologous tissues have been shown to play a key role in promoting cartilage regeneration, and they are often derived from unimportant or waste tissues, regenerative tissues, or from stem cell secretome. For example, CEFFE attenuates osteoarthritis via chondrocyte regeneration and macrophage immunomodulation.192 In addition, PRP may promote AC lubrication and regeneration, and retards the progression of OA by stimulating cell migration, proliferation, differentiation of progenitor/MSCs, joint homeostasis, and joint lubrication.193,194 The function of BMSCs extracted exosomes was verified in vitro and in OA model in vivo.195
Perspectives
AC acts as a low-friction cushion in joints and is essential for skeletal movements in mammals. Given its avascular nature and low cell density, AC has a limited regenerative ability, and damage caused by wear, traumas, inflammatory conditions, degenerative diseases and biomechanic alterations often needs conservative or surgical treatment. AC defects, such as OA, affect millions of people worldwide and place a considerable socioeconomic burden on the society. But, conservative treatment often fails and almost always progresses to further deterioration. What’s worse, some people may have anaphylactic reactions to some drugs because of their special corporeity. Due to the drug treatment is not anaphylactic and short-acting, long-term medication will also produce greater damage to the digestive system. Although surgeons try to promote a natural fibrocartilaginous response by using marrow stimulating techniques, aiming to reduce swelling and pain, and improve the joint function of patients.25 But, the therapeutic effect, especially the long-term effect, is often not ideal. In addition, surgical treatment effect may vary from person to person. for example, the injury of the donor site and non-union with the recipient site caused by autologous cartilage transplantation often occur clinically, while the risk of rejection, disease transmission and other complications may arise from allograft osteochondral transplantation.
Genes that promote chondrogenesis, chondrogenic differentiation of stem cells, and chondrocyte proliferation have been widely reported to regulate AC defects repair. However, the premise is that it requires the effective, safe and durable gene delivery vectors and supportive gene-activated matrices. Adenovirus (viral vectors) and plasmids (nonviral expression vectors) were the most used vectors in preclinical studies. However, their toxicity, immunogenicity and the risk of foreign gene insertion limit their clinical application prospects. Cell-mediated transfer of the respective genes may ideally combine the supply of a chondrogenic cell population with the production of bioactive factors directly at the site of the lesion.42 Controlled delivery of gene vectors using biocompatible materials is emerging as a novel strategy for the sustained and tunable release of gene therapies in a spatiotemporally precise manner, thereby reducing intra-articular vector spread and possible loss of therapeutic gene product.52 In addition, ncRNAs, such as miRNA,49 lncRNA,196 and circRNA,197 have been proven to promote chondrocyte differentiation, proliferation, and retention; moreover, they regulate the development of OA and recruit BMSCs through competitive endogenous RNA networks in regulatory upstream, thus playing a crucial role in promoting the regeneration of AC (Table 3). Particularly, circRNA is more advanced in gene therapy due to its RNase resistance and higher stability than linear RNA. After the later basic research has proven its regulatory mechanism of circRNA, it can be used as a bioactive factor in the repair of AC injury and TET by connecting and integrating the effective fragment to the vector or engineered MSCs to improve its transfection and working efficiency. Consequently, with the availability of optimized gene transfer systems, circRNA gene therapy offers powerful tools to stimulate the chondrogenic process in MSCs via the effective, safe, and durable delivery of candidate sequences with chondroprotective and/or chondroregenerative properties.
Table 3.
LncRNA, circRNA, and miRNA used for AC homeostasis.
| Gene ID | Species | Effects | References | Merits | Demerits |
|---|---|---|---|---|---|
| Circ0001236 | Human | Enhances MSCs chondrogenesis, maintains chondrocytes function | Mao et al.197 | Sequence conservation; RNA enzyme resistant, may has a long half-life | Requires the effective, safe and durable gene delivery vectors and supportive gene-activated matrices |
| Circ.33186 | Mouse | Knockdown of circRNA.33186 promotes proliferation and inhibited apoptosis | Zhou et al.198 | ||
| CircSERPINE2 | Human | Regulates apoptosis and ECM metabolism | Shen et al.12 | ||
| CircZSWIM6 | Human | Dysregulation of ECM and energy homeostasis | Gong et al.199 | ||
| CircSLC7A2 | Human | Regulates ECM homeostasis and inflammation, prevents apoptosis | Ni et al.200 | ||
| CircATRNL1 | Human | Enhances BMSCs proliferation and chondrogenic differentiation | Zheng et al.201 | ||
| CircVMA21 | Human and rat | Alleviates nucleus pulposus cell apoptosis and ECM homeostasis | Cheng et al.202 | ||
| CircCDK14 | Human | Regulates metabolism, inhibites apoptosis, promotes proliferation | Shen et al.11 | ||
| Circ0083429 | Human | Regulates ECM homeostasis, alleviates OA | Yao et al.203 | ||
| CircPDE4B | Human and mouse | Prevents AC degeneration and promotes its repair | Shen et al.13 | ||
| Wu et al.204 | |||||
| CircRNA3503 | Human | Alleviates inflammation-induced apoptosis and ECM homeostasis | Tao et al.205 | ||
| CircRSU1 | Human | Regulates oxidative stress-triggered inflammation and ECM maintenance | Yang et al.14 | ||
| MiR-23b | Human | Induces MSCs chondrogenic differentiation | Ham et al.206 | Short sequence and easy transfection | Low stability means difficult store and transport |
| MiR-92a | Human | Promotes chondroprogenitors proliferation and matrix synthesis | Ning et al.207 | ||
| MiR-145 | Murine | Promotes MSCs chondrogenesis | Yang et al.208 | ||
| MiR-140-5p | Human | Regulates MSCs chondrogenesis and the metabolism | Meng et al.209 | ||
| MiR-200a | Human | Inhibits chondrocytes differentiation and promotes proliferation | Umeda et al.210 | ||
| MiR-9 | Human and rabbit | Regulates chondroblasts survival and maintains cartilage homeostasis | Song et al.211 | ||
| MiR-142-3p | Mouse | Inhibits chondrocytes apoptosis and inflammation | Wang et al.212 | ||
| MiR-204 and MiR-211 | Mouse | Synergistically maintains joint homeostasis to suppress OA pathogenesis | Huang et al.213 | ||
| MiR-455 | Human and mouse | Protects against surgery-induced cartilage degradation | Ito et al.214 | ||
| MiR-17 | Mouse | Maintains ECM homeostasis | Zhang et al.50 | ||
| LncRNA MM2P | Mouse | Maintains chondrocytes phenotype | Bai et al.215 | Long sequence general means more potential functional areas | Low stability means difficult store and transport; requires the effective, safe and durable gene delivery vectors and supportive gene-activated matrices |
| LncRNA MALAT-1 | Human | Promotes chondrocytes proliferation and migration, suppresses apoptosis, inflammation and cartilage degradation | Pan et al.216 | ||
| LncRNA THUMPD3-AS1 | Human | Enhances chondrocytes proliferation and inflammation | Wang et al.217 | ||
| LncRNA CRNDE | Rat | Promotes BMSCs chondrogenic differentiation | Shi et al.218 | ||
| LncRNA NEAT1 | Human | Inhibits chondrocytes proliferation, promote apoptosis | Xiao et al.219 | ||
| LncRNA XIST | Rat | Promotes OA progression | Liu et al.220 | ||
| LINC00671 | Mouse | Inhibites chondrocytes proliferation, enhances apoptosis and ECM degradation | Chen and Xu221 | ||
| LncRNA OIP5-AS1 | Human and mouse | Facilitates CHON-001 and ATDC5 cells proliferation and migration, ameliorates apoptosis and inflammation | Zhi et al.222 | ||
| LncRNA H19 | Rat | Promotes chondrocytes migration, matrix secretion, suppresses apoptosis and senescence | Yan et al.9 | ||
| LncRNA DNM3OS | Rat | Suppresses early MSCs chondrogenic differentiation under hypoxic conditions | Zhou et al.223 |
MSCs have a reliable potential for chondrogenic differentiation, which is a promising approach to treat AC injury (focal defects and OA) and enhance the self-repair capabilities of AC. However, the need for improved designs remains critical because the reproduction of a native structural and functional unit in sites of cartilage damage is formidable occurring upon implantation of MSCs. Moreover, the retention time of MSCs in vivo is relatively short, and the current exploratory research on MSCs therapy for AC defects still needs multiple injections to achieve better therapeutic effect, which also puts forward higher requirements on the source and quality of MSCs. In addition, exosomes derived from MSCs are rich in therapeutic cargos, such as miRNA, lncRNA, small interfering RNA, DNA, protein, and bioactive lipids.224 Spontaneously, they are thought to function primarily as communication vehicles to transfer bioactive factors between cells to evoke biological responses in recipient cells. For MSCs exosomes, many of these biological responses translated to a therapeutic outcome in injured or diseased cells.55
Although AC is a successful pioneering area of regenerative medicine, restoring the complex architecture, biomechanical properties, and biological function of the native tissue remains challenging and thus is a common problem in orthopedic practice.145,225 Especially, repairing osteochondral defects is a considerable challenge due to it involves the breakdown of AC and underlying bone. Traditional hydrogels with a homogenized single-layer structure cannot fully restore the function of osteochondral cartilage tissue.226 In a certain degree, utilizing 3D printing technologies to prepare biodegradable natural composite scaffolds could replace chemically synthesized polymers with more natural polymers or low-toxicity crosslinkers by using the ideal bioink/hydrogels.92,140 Furthermore, nanotechnology has been proven to be a powerful engineering strategy in the past few decades. However, it is critical that the degradation rate of the scaffolds used for tissue engineering AC at the injury site can match the formation rate of neo-tissues. For example, studies have shown that defects treated with slow-dissolving scaffolds (HYAFF® and PLLA) presented more cartilage than fast-dissolving scaffolds (ACP™ and PLGA) 12 weeks after surgery. Among them, ACP™ sponges (a hyaluronan-based auto cross-linked polysaccharide polymer from Fidia Advanced Biopolymers srl (Abano Terme, Italy)) dissolve in vitro in 2 weeks when placed in daily changes of PBS at 37°C. But HYAFF®-11 sponges (another hyaluronan-based polymer from Fidia Advanced Biopolymers srl) dissolve in 10 weeks. Based on GPC results (molecular weight of polymer), the half-life of PLGA foams with similar physical characteristics to those used in the study was ∼3 weeks in vitro and ∼2 weeks in vivo. Based on weight, the time required to lose approximately half of the dry weight was ∼12 weeks in vitro. But the half-life of PLLA foams was respectively ∼20 and ∼40 weeks in vitro.227 Fortunately, the living tissue-engineered cartilage, which can perfectly fused with surrounding tissue, is a promising therapeutic strategy for resolving cartilage defects once and for all. In particular, the integrated living biological joints provide the possibility for the treatment of extensive osteochondral defects at joint sites, such as grade IV injuries.
But, as shown in Table 4, current different therapeutic strategy has different hurdles need to battle. For instance, in consideration of the limitation of AC in self-healing and the complexity of osteochondral tissue, osteochondral defects are needed for new therapeutic strategies urgently.228 And, being the hallmark of OA, ECM destruction and abnormal homeostasis are gaining increasing attention as a therapeutic target in cartilage regeneration.229 In preclinical settings, high-quality cartilage tissue has been produced using combination strategies involving stem/progenitor cells or chondrocytes, biomaterials, and bioactive factors to generate a construct for implantation at the lesion site.230 Successful cartilage TET seeks to repair or regenerate neo-tissues via intricately coordinated interactions between biomaterial scaffolds, cells, biological factors, and physical/mechanical factors, but still faces a multitude of challenges.231,232 For regenerative medicine products, including tissue engineered cartilage, it is the ultimate goal to transform it into clinical application through certain steps to solving AC defects more perfectly (Figure 9). Therefore, the approval policies of regulatory authorities should also be fully considered. For cartilage TET, which is mainly regulated by a combination of medical device and drug (biological products) regulatory regulations,3 different kinds of materials that will be used need meet the different test standard. For instance, in general, biomaterials should be followed ISO 13485 and ISO 14971 to ensure the quality, its biocompatibility should be followed ISO 10993 to guarantee the biosafety, and for clinical trials, ISO 14155 is not a bed way for the clinical evaluation before clinical transformation and application.
Table 4.
The current hurdles in AC repair.
| Treatment strategy | Hurdles |
|---|---|
| Palliative management | 1. Short-term curative effect, long-term drug damage other organs |
| 2. Monosymptomatic resolution, often needs a combined drugs | |
| 3. No effect on injury development and fails to regenerate new AC tissue | |
| 4. Cannot solve the larger damage | |
| Surgical intervention | 1. Robbing Peter to pay Paul |
| 2. Short-term symptom relief and poor prognosis | |
| 3. Complications | |
| 4. Insufficient donors | |
| 5. Incomplete chondrogenesis or rapid degradation and fibrosis of the repaired tissue | |
| 6. The transplant tissue is difficult to preserve and integrate with the surrounding cartilage tissue | |
| 7. Risk of disease transmission | |
| 8. Long recovery time | |
| 9. High costs | |
| 10. Ineffective in repairing large cartilage defects | |
| Gene regulation/ therapy | 1. Requires the effective, safe and durable gene delivery vectors and supportive gene-activated matrices |
| 2. Short duration | |
| 3. Low stability resulting difficult to store and transport | |
| 4. Potential immunogenicity | |
| Stem cell therapy | 1. The stricter regulations on policy and ethics |
| 2. A long culture time and a complex culturing procedure | |
| 3. Cell viability and differentiation capacity were greatly affected by age | |
| 4. Poor cell adhesion and retention | |
| 5. Phenotypic alteration | |
| 6. Heterogeneity | |
| 7. Allograft rejection | |
| 8. High costs | |
| 9. Potential tumorigenicity and immunogenicity | |
| Materials scaffolds | 1. Might intercept cell-cell signaling and exogenous stimulus signals |
| 2. The degradation rate does not match the regeneration rate | |
| 3. Difficult to fully mimic the natural microenvironment resulting poor integration with surrounding cartilage tissue | |
| 4. Complicated preparation process | |
| 5. Solid material leads to inconvenient surgical implantation |
Figure 9.
Steps involved in the cartilage tissue engineering. Reproduced from Chinta et al.232
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors acknowledge funding from the open project program of the Third Affiliated Hospital of Xinxiang Medical University (Nos. KFKTYB202119, KFKTZD202105, KFKTYB202107), the Key Research and Development Program of Henan province (No. 221111310100), the Key Research and Development and Promotion Special (Science and Technology) Project of Henan Province (No. 232102310331), and the Major Science and Technology Projects of Xinxiang City (No. 21ZD006).
ORCID iD: Xueqiang Guo  https://orcid.org/0000-0003-2318-8678
https://orcid.org/0000-0003-2318-8678
References
- 1. Romeo SG, Alawi KM, Rodrigues J, et al. Endothelial proteolytic activity and interaction with non-resorbing osteoclasts mediate bone elongation. Nat Cell Biol 2019; 21(4): 430–441. [DOI] [PubMed] [Google Scholar]
- 2. Prein C, Beier F. ECM signaling in cartilage development and endochondral ossification. Curr Top Dev Biol 2019; 133: 25–47. [DOI] [PubMed] [Google Scholar]
- 3. Guo X, Ma Y, Min Y, et al. Progress and prospect of technical and regulatory challenges on tissue-engineered cartilage as therapeutic combination product. Bioact Mater 2023; 20: 501–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ziadlou R, Rotman S, Teuschl A, et al. Optimization of hyaluronic acid-tyramine/silk-fibroin composite hydrogels for cartilage tissue engineering and delivery of anti-inflammatory and anabolic drugs. Mater Sci Eng C Mater Biol Appl 2021; 120: 111701. [DOI] [PubMed] [Google Scholar]
- 5. Lories RJ, Luyten FP. The bone-cartilage unit in osteoarthritis. Nat Rev Rheumatol 2011; 7(1): 43–49. [DOI] [PubMed] [Google Scholar]
- 6. Chen J, Huang T, Liu R, et al. Congenital microtia patients: the genetically engineered exosomes released from porous gelatin methacryloyl hydrogel for downstream small RNA profiling, functional modulation of microtia chondrocytes and tissue-engineered ear cartilage regeneration. J Nanobiotechnol 2022; 20(1): 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shen K, Duan A, Cheng J, et al. Exosomes derived from hypoxia preconditioned mesenchymal stem cells laden in a silk hydrogel promote cartilage regeneration via the miR-205–5p/PTEN/AKT pathway. Acta Biomater 2022; 143: 173–188. [DOI] [PubMed] [Google Scholar]
- 8. Jiang Y, Zhang C, Long L, et al. A comprehensive analysis of SE-lncRNA/mRNA differential expression profiles during chondrogenic differentiation of human bone marrow mesenchymal stem cells. Front Cell Dev Biol 2021; 9: 721205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yan L, Liu G, Wu X. The umbilical cord mesenchymal stem cell-derived exosomal lncRNA H19 improves osteochondral activity through miR-29b-3p/FoxO3 axis. Clin Transl Med 2021; 11(1): e255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duan L, Liang Y, Xu X, et al. Noncoding RNAs in subchondral bone osteoclast function and their therapeutic potential for osteoarthritis. Arthritis Res Ther 2020; 22(1): 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shen P, Yang Y, Liu G, et al. CircCDK14 protects against osteoarthritis by sponging miR-125a-5p and promoting the expression of Smad2. Theranostics 2020; 10(20): 9113–9131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shen S, Wu Y, Chen J, et al. CircSERPINE2 protects against osteoarthritis by targeting miR-1271 and ETS-related gene. Ann Rheum Dis 2019; 78(6): 826–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shen S, Yang Y, Shen P, et al. circPDE4B prevents articular cartilage degeneration and promotes repair by acting as a scaffold for RIC8A and MID1. Ann Rheum Dis 2021; 80(9): 1209–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang Y, Shen P, Yao T, et al. Novel role of circRSU1 in the progression of osteoarthritis by adjusting oxidative stress. Theranostics 2021; 11(4): 1877–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patel JM, Saleh KS, Burdick JA, et al. Bioactive factors for cartilage repair and regeneration: improving delivery, retention, and activity. Acta Biomater 2019; 93: 222–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang W, Zhu P, Huang H, et al. Functionalization of novel theranostic hydrogels with kartogenin-grafted USPIO nanoparticles to enhance cartilage regeneration. ACS Appl Mater Interfaces 2019; 11(38): 34744–34754. [DOI] [PubMed] [Google Scholar]
- 17. Xu X, Liang Y, Li X, et al. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials 2021; 269: 120539. [DOI] [PubMed] [Google Scholar]
- 18. da Costa BR, Pereira TV, Saadat P, et al. Effectiveness and safety of non-steroidal anti-inflammatory drugs and opioid treatment for knee and hip osteoarthritis: network meta-analysis. BMJ 2021; 375: n2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cui Z, Crane J, Xie H, et al. Halofuginone attenuates osteoarthritis by inhibition of TGF-β activity and H-type vessel formation in subchondral bone. Ann Rheum Dis 2016; 75(9): 1714–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gao M, Chen C, Zhang Q, et al. Research progress on the antiosteoarthritic mechanism of action of natural products. Evid Based Complement Alternat Med 2021; 2021: 7714533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Idaszek J, Costantini M, Karlsen TA, et al. 3D bioprinting of hydrogel constructs with cell and material gradients for the regeneration of full-thickness chondral defect using a microfluidic printing head. Biofabrication 2019; 11(4): 044101. [DOI] [PubMed] [Google Scholar]
- 22. Dillon CF, Rasch EK, Gu Q, et al. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991-94. J Rheumatol 2006; 33(11): 2271–2279. [PubMed] [Google Scholar]
- 23. Scott DL, Berry H, Capell H, et al. The long-term effects of non-steroidal anti-inflammatory drugs in osteoarthritis of the knee: a randomized placebo-controlled trial. Rheumatology 2000; 39(10): 1095–1101. [DOI] [PubMed] [Google Scholar]
- 24. Bellamy N, Campbell J, Robinson V, et al. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev 2005; 2: Cd005321. [DOI] [PubMed] [Google Scholar]
- 25. Longo UG, Petrillo S, Franceschetti E, et al. Stem cells and gene therapy for cartilage repair. Stem Cells Int 2012; 2012: 168385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Richter DL, Schenck RC, Jr, Wascher DC, et al. Knee articular cartilage repair and restoration techniques: a review of the literature. Sports Health 2016; 8(2): 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Montgomery SR, Foster BD, Ngo SS, et al. Trends in the surgical treatment of articular cartilage defects of the knee in the United States. Knee Surg Sports Traumatol Arthrosc 2014; 22(9): 2070–2075. [DOI] [PubMed] [Google Scholar]
- 28. McCormick F, Harris JD, Abrams GD, et al. Trends in the surgical treatment of articular cartilage lesions in the United States: an analysis of a large private-payer database over a period of 8 years. Arthroscopy 2014; 30(2): 222–226. [DOI] [PubMed] [Google Scholar]
- 29. Kellett CF, Boscainos PJ, Gross AE. Surgical options for articular defects of the knee. Expert Rev Med Devices 2006; 3(5): 585–593. [DOI] [PubMed] [Google Scholar]
- 30. Wasiak J, Villanueva E. Autologous cartilage implantation for full thickness articular cartilage defects of the knee. Cochrane Database Syst Rev 2002; 4: Cd003323. [DOI] [PubMed] [Google Scholar]
- 31. Andrade R, Vasta S, Pereira R, et al. Knee donor-site morbidity after mosaicplasty - a systematic review. J Exp Orthop 2016; 3(1): 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tompkins M, Hamann JC, Diduch DR, et al. Preliminary results of a novel single-stage cartilage restoration technique: particulated juvenile articular cartilage allograft for chondral defects of the patella. Arthroscopy 2013; 29(10): 1661–1670. [DOI] [PubMed] [Google Scholar]
- 33. Gelse K, von der Mark K, Schneider H. Cartilage regeneration by gene therapy. Curr Gene Ther 2003; 3(4): 305–317. [DOI] [PubMed] [Google Scholar]
- 34. Yang R, Chen F, Guo J, et al. Recent advances in polymeric biomaterials-based gene delivery for cartilage repair. Bioact Mater 2020; 5(4): 990–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bougioukli S, Evans CH, Alluri RK, et al. Gene therapy to enhance bone and cartilage repair in orthopaedic surgery. Curr Gene Ther 2018; 18(3): 154–170. [DOI] [PubMed] [Google Scholar]
- 36. Bellavia D, Veronesi F, Carina V, et al. Gene therapy for chondral and osteochondral regeneration: is the future now? Cell Mol Life Sci 2018; 75(4): 649–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nishida T, Kubota S, Aoyama E, et al. Low-intensity pulsed ultrasound (LIPUS) treatment of cultured chondrocytes stimulates production of CCN family protein 2 (CCN2), a protein involved in the regeneration of articular cartilage: mechanism underlying this stimulation. Osteoarthr Cartil 2017; 25(5): 759–769. [DOI] [PubMed] [Google Scholar]
- 38. Maihöfer J, Madry H, Rey-Rico A, et al. Hydrogel-guided, rAAV-mediated IGF-I overexpression enables long-term cartilage repair and protection against perifocal osteoarthritis in a large-animal full-thickness chondral defect model at one year in vivo. Adv Mater 2021; 33(16): e2008451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li Q, Liu T, Zhang L, et al. The role of bFGF in down-regulating α-SMA expression of chondrogenically induced BMSCs and preventing the shrinkage of BMSC engineered cartilage. Biomaterials 2011; 32(21): 4773–4781. [DOI] [PubMed] [Google Scholar]
- 40. Bian L, Zhai DY, Tous E, et al. Enhanced MSC chondrogenesis following delivery of TGF-β3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials 2011; 32(27): 6425–6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim SH, Kim SH, Jung Y. TGF-β 3 encapsulated PLCL scaffold by a supercritical CO 2 –HFIP co-solvent system for cartilage tissue engineering. J Control Release 2015; 206: 101–107. [DOI] [PubMed] [Google Scholar]
- 42. Gelse K, Schneider H. Ex vivo gene therapy approaches to cartilage repair. Adv Drug Deliv Rev 2006; 58(2): 259–284. [DOI] [PubMed] [Google Scholar]
- 43. Ali SA, Peffers MJ, Ormseth MJ, et al. The non-coding RNA interactome in joint health and disease. Nat Rev Rheumatol 2021; 17(11): 692–705. [DOI] [PubMed] [Google Scholar]
- 44. Zhao C, Li X, Sun G, et al. CircFOXO3 protects against osteoarthritis by targeting its parental gene FOXO3 and activating PI3K/AKT-mediated autophagy. Cell Death Dis 2022; 13(11): 932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li Z, Meng D, Liu Y, et al. Circular RNA VMA21 ameliorates IL-1β-engendered chondrocyte injury through the miR-495-3p/FBWX7 signaling axis. Clin Immunol 2022; 238: 108995. [DOI] [PubMed] [Google Scholar]
- 46. Shang J, Li H, Wu B, et al. CircHIPK3 prevents chondrocyte apoptosis and cartilage degradation by sponging miR-30a-3p and promoting PON2. Cell Prolif 2022; 55(9): e13285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tang S, Cao Y, Cai Z, et al. The lncRNA Pila promotes NF-κB signaling in osteoarthritis by stimulating the activity of the protein arginine methyltransferase prmt1. Sci Signal 2022; 15(735): eabm6265. [DOI] [PubMed] [Google Scholar]
- 48. Zhang S, Jin Z. Bone mesenchymal stem cell-derived extracellular vesicles containing long noncoding RNA NEAT1 relieve osteoarthritis. Oxid Med Cell Longev 2022; 2022: 5517648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhu J, Yang S, Qi Y, et al. Stem cell–homing hydrogel-based miR-29b-5p delivery promotes cartilage regeneration by suppressing senescence in an osteoarthritis rat model. Sci Adv 2022; 8(13): eabk0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang Y, Li S, Jin P, et al. Dual functions of microRNA-17 in maintaining cartilage homeostasis and protection against osteoarthritis. Nat Commun 2022; 13(1): 2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xiang XN, Zhu SY, He HC, et al. Mesenchymal stromal cell-based therapy for cartilage regeneration in knee osteoarthritis. Stem Cell Res Ther 2022; 13(1): 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cucchiarini M, Madry H. Biomaterial-guided delivery of gene vectors for targeted articular cartilage repair. Nat Rev Rheumatol 2019; 15(1): 18–29. [DOI] [PubMed] [Google Scholar]
- 53. Frisch J, Venkatesan JK, Rey-Rico A, et al. Current progress in stem cell-based gene therapy for articular cartilage repair. Curr Stem Cell Res Ther 2015; 10(2): 121–131. [DOI] [PubMed] [Google Scholar]
- 54. Liang C, Liu Z, Song M, et al. Stabilization of heterochromatin by CLOCK promotes stem cell rejuvenation and cartilage regeneration. Cell Res 2021; 31(2): 187–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Toh WS, Lai RC, Hui JHP, et al. MSC exosome as a cell-free MSC therapy for cartilage regeneration: Implications for osteoarthritis treatment. Semin Cell Dev Biol 2017; 67: 56–64. [DOI] [PubMed] [Google Scholar]
- 56. Gaut C, Sugaya K. Critical review on the physical and mechanical factors involved in tissue engineering of cartilage. Regen Med 2015; 10(5): 665–679. [DOI] [PubMed] [Google Scholar]
- 57. Cao L, Tong Y, Wang X, et al. Effect of amniotic membrane/collagen-based scaffolds on the chondrogenic differentiation of adipose-derived stem cells and cartilage repair. Front Cell Dev Biol 2021; 9: 647166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhao Z, Fan C, Chen F, et al. Progress in articular cartilage tissue engineering: a review on therapeutic cells and macromolecular scaffolds. Macromol Biosci 2020; 20(2): e1900278. [DOI] [PubMed] [Google Scholar]
- 59. Keeney M, Lai JH, Yang F. Recent progress in cartilage tissue engineering. Curr Opin Biotechnol 2011; 22(5): 734–740. [DOI] [PubMed] [Google Scholar]
- 60. Langer R, Vacanti JP. Tissue engineering. Science 1993; 260(5110): 920–926. [DOI] [PubMed] [Google Scholar]
- 61. Vacanti CA, Langer R, Schloo B, et al. Synthetic polymers seeded with chondrocytes provide a template for new cartilage formation. Plast Reconstr Surg 1991; 88(5): 753–759. [DOI] [PubMed] [Google Scholar]
- 62. Cao Y, Vacanti JP, Paige KT, et al. Transplantation of chondrocytes utilizing a polymer-cell construct to produce tissue-engineered cartilage in the shape of a human ear. Plast Reconstr Surg 1997; 100(2): 297–302; discussion 303–304. [DOI] [PubMed] [Google Scholar]
- 63. Khademhosseini A, Vacanti JP, Langer R. Progress in tissue engineering. Sci Am 2009; 300(5): 64–71. [DOI] [PubMed] [Google Scholar]
- 64. Iwasa J, Engebretsen L, Shima Y, et al. Clinical application of scaffolds for cartilage tissue engineering. Knee Surg Sports Traumatol Arthrosc 2009; 17(6): 561–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. McCarthy HS, Roberts S. A histological comparison of the repair tissue formed when using either chondrogide(®) or periosteum during autologous chondrocyte implantation. Osteoarthr Cartil 2013; 21(12): 2048–2057. [DOI] [PubMed] [Google Scholar]
- 66. Carey JL, Remmers AE, Flanigan DC. Use of MACI (autologous cultured chondrocytes on porcine collagen membrane) in the United States: preliminary experience. Orthop J Sports Med 2020; 8(8): 2325967120941816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Huang BJ, Hu JC, Athanasiou KA. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials 2016; 98: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schneider U, Rackwitz L, Andereya S, et al. A prospective multicenter study on the outcome of type I collagen hydrogel-based autologous chondrocyte implantation (CaReS) for the repair of articular cartilage defects in the knee. Am J Sports Med 2011; 39(12): 2558–2565. [DOI] [PubMed] [Google Scholar]
- 69. Park IS, Jin RL, Oh HJ, et al. Sizable scaffold-free tissue-engineered articular cartilage construct for cartilage defect repair. Artif Organs 2019; 43(3): 278–287. [DOI] [PubMed] [Google Scholar]
- 70. Steele JAM, Moore AC, St-Pierre JP, et al. In vitro and in vivo investigation of a zonal microstructured scaffold for osteochondral defect repair. Biomaterials 2022; 286: 121548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Harrell CR, Markovic BS, Fellabaum C, et al. Mesenchymal stem cell-based therapy of osteoarthritis: Current knowledge and future perspectives. Biomed Pharmacother 2019; 109: 2318–2326. [DOI] [PubMed] [Google Scholar]
- 72. De Bari C, Roelofs AJ. Stem cell-based therapeutic strategies for cartilage defects and osteoarthritis. Curr Opin Pharmacol 2018; 40: 74–80. [DOI] [PubMed] [Google Scholar]
- 73. Johnson K, Zhu S, Tremblay MS, et al. A stem cell-based approach to cartilage repair. Science 2012; 336(6082): 717–721. [DOI] [PubMed] [Google Scholar]
- 74. To K, Zhang B, Romain K, et al. Synovium-derived mesenchymal stem cell transplantation in cartilage regeneration: a PRISMA review of in vivo studies. Front Bioeng Biotechnol 2019; 7: 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bionaz M, Monaco E, Wheeler MB. Transcription adaptation during in vitro adipogenesis and osteogenesis of porcine mesenchymal stem cells: dynamics of pathways, biological processes, up-stream regulators, and gene networks. PLoS One 2015; 10(9): e0137644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lu CH, Yeh TS, Yeh CL, et al. Regenerating cartilages by engineered ASCs: prolonged TGF-β3/BMP-6 expression improved articular cartilage formation and restored zonal structure. Mol Ther 2014; 22(1): 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells 2007; 25(6): 1384–1392. [DOI] [PubMed] [Google Scholar]
- 78. Jones BA, Pei M. Synovium-derived stem cells: a tissue-specific stem cell for cartilage engineering and regeneration. Tissue Eng Part B Rev 2012; 18(4): 301–311. [DOI] [PubMed] [Google Scholar]
- 79. Ishiguro H, Kaito T, Yarimitsu S, et al. Intervertebral disc regeneration with an adipose mesenchymal stem cell-derived tissue-engineered construct in a rat nucleotomy model. Acta Biomater 2019; 87: 118–129. [DOI] [PubMed] [Google Scholar]
- 80. Kim JK, Bae HC, Ro DH, et al. Enhancement of cartilage regeneration of synovial stem cells/hydrogel by using transglutaminase-4. Tissue Eng Part A 2021; 27(11–12): 761–770. [DOI] [PubMed] [Google Scholar]
- 81. Zha K, Li X, Yang Z, et al. Heterogeneity of mesenchymal stem cells in cartilage regeneration: from characterization to application. NPJ Regen Med 2021; 6(1): 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wang G, Man Z, Xin H, et al. Enhanced adhesion and proliferation of bone marrow mesenchymal stem cells on β-tricalcium phosphate modified by an affinity peptide. Mol Med Rep 2019; 19(1): 375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhu Y, Kong L, Farhadi F, et al. An injectable continuous stratified structurally and functionally biomimetic construct for enhancing osteochondral regeneration. Biomaterials 2019; 192: 149–158. [DOI] [PubMed] [Google Scholar]
- 84. Go G, Han J, Zhen J, et al. A magnetically actuated microscaffold containing mesenchymal stem cells for articular cartilage repair. Adv Healthc Mater 2017; 6(13): 6. [DOI] [PubMed] [Google Scholar]
- 85. Go G, Jeong SG, Yoo A, et al. Human adipose-derived mesenchymal stem cell-based medical microrobot system for knee cartilage regeneration in vivo. Sci Robot 2020; 5(38): eaay6626. [DOI] [PubMed] [Google Scholar]
- 86. Nakamura A, Murata D, Fujimoto R, et al. Bio-3D printing iPSC-derived human chondrocytes for articular cartilage regeneration. Biofabrication 2021; 13(4): 13. [DOI] [PubMed] [Google Scholar]
- 87. Lee MS, Stebbins MJ, Jiao H, et al. Comparative evaluation of isogenic mesodermal and ectomesodermal chondrocytes from human iPSCs for cartilage regeneration. Sci Adv 2021; 7(21): eabf0907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lim HC, Park YB, Ha CW, et al. Allogeneic umbilical cord blood-derived mesenchymal stem cell implantation versus microfracture for large, full-thickness cartilage defects in older patients: a multicenter randomized clinical trial and extended 5-year clinical follow-up. Orthop J Sports Med 2021; 9(1): 2325967120973052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Park YB, Ha CW, Lee CH, et al. Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up. Stem Cells Transl Med 2017; 6(2): 613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sun JY, Zhao X, Illeperuma WR, et al. Highly stretchable and tough hydrogels. Nature 2012; 489(7414): 133–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Klotz BJ, Gawlitta D, Rosenberg AJWP, et al. Gelatin-methacryloyl hydrogels: towards biofabrication-based tissue repair. Trends Biotechnol 2016; 34(5): 394–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Weng T, Zhang W, Xia Y, et al. 3D bioprinting for skin tissue engineering: current status and perspectives. J Tissue Eng 2021; 12: 20417314211028574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Gu Z, Wang J, Fu Y, et al. Smart biomaterials for articular cartilage repair and regeneration. Adv Funct Mater 2023; 33(10): 2212561. [Google Scholar]
- 94. Qin X, He R, Chen H, et al. Methacrylated pullulan/polyethylene (glycol) diacrylate composite hydrogel for cartilage tissue engineering. J Biomater Sci Polym Ed 2021; 32(8): 1057–1071. [DOI] [PubMed] [Google Scholar]
- 95. Xia H, Zhao D, Zhu H, et al. Lyophilized scaffolds fabricated from 3D-printed photocurable natural hydrogel for cartilage regeneration. ACS Appl Mater Interfaces 2018; 10(37): 31704–31715. [DOI] [PubMed] [Google Scholar]
- 96. Wei W, Ma Y, Yao X, et al. Advanced hydrogels for the repair of cartilage defects and regeneration. Bioact Mater 2021; 6(4): 998–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Jiang Y, Wang Y, Li Q, et al. Natural polymer-based stimuli-responsive hydrogels. Curr Med Chem 2020; 27(16): 2631–2657. [DOI] [PubMed] [Google Scholar]
- 98. Sood N, Bhardwaj A, Mehta S, et al. Stimuli-responsive hydrogels in drug delivery and tissue engineering. Drug Deliv 2016; 23(3): 758–780. [DOI] [PubMed] [Google Scholar]
- 99. Li F, Truong VX, Thissen H, et al. Microfluidic encapsulation of human mesenchymal stem cells for articular cartilage tissue regeneration. ACS Appl Mater Interfaces 2017; 9(10): 8589–8601. [DOI] [PubMed] [Google Scholar]
- 100. Jiang G, Li S, Yu K, et al. A 3D-printed PRP-GelMA hydrogel promotes osteochondral regeneration through M2 macrophage polarization in a rabbit model. Acta Biomater 2021; 128: 150–162. [DOI] [PubMed] [Google Scholar]
- 101. Lee C, O'Connell CD, Onofrillo C, et al. Human articular cartilage repair: sources and detection of cytotoxicity and genotoxicity in photo-crosslinkable hydrogel bioscaffolds. Stem Cells Transl Med 2020; 9(3): 302–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Qi C, Deng Y, Xu L, et al. A sericin/graphene oxide composite scaffold as a biomimetic extracellular matrix for structural and functional repair of calvarial bone. Theranostics 2020; 10(2): 741–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kesti M, Müller M, Becher J, et al. A versatile bioink for three-dimensional printing of cellular scaffolds based on thermally and photo-triggered tandem gelation. Acta Biomater 2015; 11: 162–172. [DOI] [PubMed] [Google Scholar]
- 104. Wang C, Feng N, Chang F, et al. Injectable cholesterol-enhanced stereocomplex polylactide thermogel loading chondrocytes for optimized cartilage regeneration. Adv Healthc Mater 2019; 8(14): e1900312. [DOI] [PubMed] [Google Scholar]
- 105. Dehghan-Baniani D, Mehrjou B, Chu PK, et al. A biomimetic nano-engineered platform for functional tissue engineering of cartilage superficial zone. Adv Healthc Mater 2021; 10(4): e2001018. [DOI] [PubMed] [Google Scholar]
- 106. Hanafy AS, El-Ganainy SO. Thermoresponsive hyalomer intra-articular hydrogels improve monoiodoacetate-induced osteoarthritis in rats. Int J Pharm 2020; 573: 118859. [DOI] [PubMed] [Google Scholar]
- 107. Roberts JJ, Bryant SJ. Comparison of photopolymerizable thiol-ene PEG and acrylate-based PEG hydrogels for cartilage development. Biomaterials 2013; 34(38): 9969–9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Shi W, Fang F, Kong Y, et al. Dynamic hyaluronic acid hydrogel with covalent linked gelatin as an anti-oxidative bioink for cartilage tissue engineering. Biofabrication 2021; 14(1): 014107. [DOI] [PubMed] [Google Scholar]
- 109. Maher SA, Doty SB, Torzilli PA, et al. Nondegradable hydrogels for the treatment of focal cartilage defects. J Biomed Mater Res A 2007; 83(1): 145–155. [DOI] [PubMed] [Google Scholar]
- 110. Bichara DA, Bodugoz-Sentruk H, Ling D, et al. Osteochondral defect repair using a polyvinyl alcohol-polyacrylic acid (PVA-paac) hydrogel. Biomed Mater 2014; 9(4): 045012. [DOI] [PubMed] [Google Scholar]
- 111. Whittaker JL, Dutta NK, Zannettino A A, et al. Engineering DN hydrogels from regenerated silk fibroin and poly(N-vinylcaprolactam). J Mater Chem B 2016; 4(33): 5519–5533. [DOI] [PubMed] [Google Scholar]
- 112. Lima LH, Morales Y, Cabral T. Poly-N-isopropylacrylamide (pnipam): a reversible bioadhesive for sclerotomy closure. Int J Retina Vitreous 2016; 2: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Guo W, Douma L, Hu MH, et al. Hyaluronic acid-based interpenetrating network hydrogel as a cell carrier for nucleus pulposus repair. Carbohydr Polym 2022; 277: 118828. [DOI] [PubMed] [Google Scholar]
- 114. Sadat Tabatabaei Mirakabad F, Nejati-Koshki K, Akbarzadeh A, et al. PLGA-based nanoparticles as cancer drug delivery systems. Asian Pac J Cancer Prev 2014; 15(2): 517–535. [DOI] [PubMed] [Google Scholar]
- 115. Qu M, Liao X, Jiang N, et al. Injectable open-porous PLGA microspheres as cell carriers for cartilage regeneration. J Biomed Mater Res A 2021; 109(11): 2091–2100. [DOI] [PubMed] [Google Scholar]
- 116. Goo YT, Sa CK, Choi JY, et al. Development of a solid supersaturable micelle of revaprazan for improved dissolution and oral bioavailability using box-behnken design. Int J Nanomedicine 2021; 16: 1245–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wang Y, Li L, Ma Y, et al. Multifunctional supramolecular hydrogel for prevention of epidural adhesion after laminectomy. ACS Nano 2020; 14(7): 8202–8219. [DOI] [PubMed] [Google Scholar]
- 118. Li Y, Xun X, Xu Y, et al. Hierarchical porous bacterial cellulose scaffolds with natural biomimetic nanofibrous structure and a cartilage tissue-specific microenvironment for cartilage regeneration and repair. Carbohydr Polym 2022; 276: 118790. [DOI] [PubMed] [Google Scholar]
- 119. Chen S, Chen W, Chen Y, et al. Chondroitin sulfate modified 3D porous electrospun nanofiber scaffolds promote cartilage regeneration. Mater Sci Eng C Mater Biol Appl 2021; 118: 111312. [DOI] [PubMed] [Google Scholar]
- 120. Liu W, Madry H, Cucchiarini M. Application of alginate hydrogels for next-generation articular cartilage regeneration. Int J Mol Sci 2022; 23(3): 1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Long S, Huang D, Ma Z, et al. A sonication-induced silk-collagen hydrogel for functional cartilage regeneration. J Mater Chem B 2022; 10(26): 5045–5057. [DOI] [PubMed] [Google Scholar]
- 122. Shi C, Yao Y, Wang L, et al. Human salivary histatin-1-functionalized gelatin methacrylate hydrogels promote the regeneration of cartilage and subchondral bone in temporomandibular joints. Pharmaceuticals 2021; 14(5): 484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Vassallo V, Tsianaka A, Alessio N, et al. Evaluation of novel biomaterials for cartilage regeneration based on gelatin methacryloyl interpenetrated with extractive chondroitin sulfate or unsulfated biotechnological chondroitin. J Biomed Mater Res A 2022; 110(6): 1210–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Cheng G, Davoudi Z, Xing X, et al. Advanced silk fibroin biomaterials for cartilage regeneration. ACS Biomater Sci Eng 2018; 4(8): 2704–2715. [DOI] [PubMed] [Google Scholar]
- 125. Qi C, Liu J, Jin Y, et al. Photo-crosslinkable, injectable sericin hydrogel as 3D biomimetic extracellular matrix for minimally invasive repairing cartilage. Biomaterials 2018; 163: 89–104. [DOI] [PubMed] [Google Scholar]
- 126. Thorpe SD, Buckley CT, Steward AJ, et al. European Society of Biomechanics S.M. Perren Award 2012: the external mechanical environment can override the influence of local substrate in determining stem cell fate. J Biomech 2012; 45(15): 2483–2492. [DOI] [PubMed] [Google Scholar]
- 127. Bahcecioglu G, Hasirci N, Bilgen B, et al. Hydrogels of agarose, and methacrylated gelatin and hyaluronic acid are more supportive for in vitro meniscus regeneration than three dimensional printed polycaprolactone scaffolds. Int J Biol Macromol 2019; 122: 1152–1162. [DOI] [PubMed] [Google Scholar]
- 128. Bielajew BJ, Donahue RP, Lamkin EK, et al. Proteomic, mechanical, and biochemical characterization of cartilage development. Acta Biomater 2022; 143: 52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Altman RD, Kates J, Chun LE, et al. Preliminary observations of chondral abrasion in a canine model. Ann Rheum Dis 1992; 51(9): 1056–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Lo S, Fauzi MB. Current update of collagen nanomaterials-fabrication, characterisation and its applications: a review. Pharmaceutics 2021; 13(3): 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Ferreira AM, Gentile P, Chiono V, et al. Collagen for bone tissue regeneration. Acta Biomater 2012; 8(9): 3191–3200. [DOI] [PubMed] [Google Scholar]
- 132. Wei X, Liu B, Liu G, et al. Mesenchymal stem cell-loaded porous tantalum integrated with biomimetic 3D collagen-based scaffold to repair large osteochondral defects in goats. Stem Cell Res Ther 2019; 10(1): 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Sheehy EJ, Miller GJ, Amado I, et al. Mechanobiology-informed regenerative medicine: dose-controlled release of placental growth factor from a functionalized collagen-based scaffold promotes angiogenesis and accelerates bone defect healing. J Control Release 2021; 334: 96–105. [DOI] [PubMed] [Google Scholar]
- 134. Copes F, Pien N, Van Vlierberghe S, et al. Collagen-based tissue engineering strategies for vascular medicine. Front Bioeng Biotechnol 2019; 7: 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Lin HH, Chao PG, Tai WC, et al. 3D-printed collagen-based waveform microfibrous scaffold for periodontal ligament reconstruction. Int J Mol Sci 2021; 22(14): 7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Jiang X, Huang X, Jiang T, et al. Correction: the role of sox9 in collagen hydrogel-mediated chondrogenic differentiation of adult mesenchymal stem cells (MSCs). Biomater Sci 2019; 7(9): 3926–1568. [DOI] [PubMed] [Google Scholar]
- 137. Jiang LB, Su DH, Liu P, et al. Shape-memory collagen scaffold for enhanced cartilage regeneration: native collagen versus denatured collagen. Osteoarthr Cartil 2018; 26(10): 1389–1399. [DOI] [PubMed] [Google Scholar]
- 138. Matsiko A, Levingstone TJ, Gleeson JP, et al. Incorporation of TGF-beta 3 within collagen-hyaluronic acid scaffolds improves their chondrogenic potential. Adv Healthc Mater 2015; 4(8): 1175–1179. [DOI] [PubMed] [Google Scholar]
- 139. Sevastianov VI, Basok YB, Kirsanova LA, et al. A comparison of the capacity of mesenchymal stromal cells for cartilage regeneration depending on collagen-based injectable biomimetic scaffold type. Life 2021; 11(8): 756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Lee CF, Hsu YH, Lin YC, et al. 3D printing of collagen/oligomeric proanthocyanidin/oxidized hyaluronic acid composite scaffolds for articular cartilage repair. Polymers 2021; 13(18): 3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Irawan V, Sung TC, Higuchi A, et al. Collagen scaffolds in cartilage tissue engineering and relevant approaches for future development. Tissue Eng Regen Med 2018; 15(6): 673–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Cao H, Wang X, Chen M, et al. Childhood cartilage ecm enhances the chondrogenesis of endogenous cells and subchondral bone repair of the unidirectional collagen-dECM scaffolds in combination with microfracture. ACS Appl Mater Interfaces 2021; 13(48): 57043–57057. [DOI] [PubMed] [Google Scholar]
- 143. Cunniffe GM, Díaz-Payno PJ, Sheehy EJ, et al. Tissue-specific extracellular matrix scaffolds for the regeneration of spatially complex musculoskeletal tissues. Biomaterials 2019; 188: 63–73. [DOI] [PubMed] [Google Scholar]
- 144. Browe DC, Burdis R, Díaz-Payno PJ, et al. Promoting endogenous articular cartilage regeneration using extracellular matrix scaffolds. Mater Today Bio 2022; 16: 100343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Camarero-Espinosa S, Rothen-Rutishauser B, Weder C, et al. Directed cell growth in multi-zonal scaffolds for cartilage tissue engineering. Biomaterials 2016; 74: 42–52. [DOI] [PubMed] [Google Scholar]
- 146. Jia S, Wang J, Zhang T, et al. Multilayered scaffold with a compact interfacial layer enhances osteochondral defect repair. ACS Appl Mater Interfaces 2018; 10(24): 20296–20305. [DOI] [PubMed] [Google Scholar]
- 147. Zhang T, Zhang H, Zhang L, et al. Biomimetic design and fabrication of multilayered osteochondral scaffolds by low-temperature deposition manufacturing and thermal-induced phase-separation techniques. Biofabrication 2017; 9(2): 025021. [DOI] [PubMed] [Google Scholar]
- 148. Gegg C, Yang F. Spatially patterned microribbon-based hydrogels induce zonally-organized cartilage regeneration by stem cells in 3D. Acta Biomater 2020; 101: 196–205. [DOI] [PubMed] [Google Scholar]
- 149. Wang Z, Li Z, Li Z, et al. Cartilaginous extracellular matrix derived from decellularized chondrocyte sheets for the reconstruction of osteochondral defects in rabbits. Acta Biomater 2018; 81: 129–145. [DOI] [PubMed] [Google Scholar]
- 150. Wang Z, Han L, Sun T, et al. Extracellular matrix derived from allogenic decellularized bone marrow mesenchymal stem cell sheets for the reconstruction of osteochondral defects in rabbits. Acta Biomater 2020; 118: 54–68. [DOI] [PubMed] [Google Scholar]
- 151. Zhang W, Ling C, Li X, et al. Cell-free biomimetic scaffold with cartilage extracellular matrix-like architectures for in situ inductive regeneration of osteochondral defects. ACS Biomater Sci Eng 2020; 6(12): 6917–6925. [DOI] [PubMed] [Google Scholar]
- 152. Chen M, Li Y, Liu S, et al. Hierarchical macro-microporous WPU-ECM scaffolds combined with microfracture promote in situ articular cartilage regeneration in rabbits. Bioact Mater 2021; 6(7): 1932–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Gong M, Sun J, Liu G, et al. Graphene oxide-modified 3D acellular cartilage extracellular matrix scaffold for cartilage regeneration. Mater Sci Eng C Mater Biol Appl 2021; 119: 111603. [DOI] [PubMed] [Google Scholar]
- 154. Zhou F, Zhang X, Cai D, et al. Silk fibroin-chondroitin sulfate scaffold with immuno-inhibition property for articular cartilage repair. Acta Biomater 2017; 63: 64–75. [DOI] [PubMed] [Google Scholar]
- 155. Mahajan A, Singh A, Datta D, et al. Bioinspired injectable hydrogels dynamically stiffen and contract to promote mechanosensing-mediated chondrogenic commitment of stem cells. ACS Appl Mater Interfaces 2022; 14(6): 7531–7550. [DOI] [PubMed] [Google Scholar]
- 156. Yuan T, Li Z, Zhang Y, et al. Injectable ultrasonication-induced silk fibroin hydrogel for cartilage repair and regeneration. Tissue Eng Part A 2021; 27(17–18): 1213–1224. [DOI] [PubMed] [Google Scholar]
- 157. Ribeiro VP, da Silva Morais A, Maia FR, et al. Combinatory approach for developing silk fibroin scaffolds for cartilage regeneration. Acta Biomater 2018; 72: 167–181. [DOI] [PubMed] [Google Scholar]
- 158. Zhu X, Chen T, Feng B, et al. Biomimetic bacterial cellulose-enhanced double-network hydrogel with excellent mechanical properties applied for the osteochondral defect repair. ACS Biomater Sci Eng 2018; 4(10): 3534–3544. [DOI] [PubMed] [Google Scholar]
- 159. Cengiz IF, Maia FR, da Silva Morais A, et al. Entrapped in cage (EiC) scaffolds of 3D-printed polycaprolactone and porous silk fibroin for meniscus tissue engineering. Biofabrication 2020; 12(2): 025028. [DOI] [PubMed] [Google Scholar]
- 160. Li Z, Wu N, Cheng J, et al. Biomechanically, structurally and functionally meticulously tailored polycaprolactone/silk fibroin scaffold for meniscus regeneration. Theranostics 2020; 10(11): 5090–5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Shi W, Sun M, Hu X, et al. Structurally and functionally optimized silk-fibroin-gelatin scaffold using 3D printing to repair cartilage injury in vitro and in vivo. Adv Mater 2017; 29(29): 1701089. [DOI] [PubMed] [Google Scholar]
- 162. Schipani R, Scheurer S, Florentin R, et al. Reinforcing interpenetrating network hydrogels with 3D printed polymer networks to engineer cartilage mimetic composites. Biofabrication 2020; 12(3): 035011. [DOI] [PubMed] [Google Scholar]
- 163. Shah RN, Shah NA, Del Rosario Lim MM, et al. Supramolecular design of self-assembling nanofibers for cartilage regeneration. Proc Natl Acad Sci USA 2010; 107(8): 3293–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Liu Q, Wang J, Chen Y, et al. Suppressing mesenchymal stem cell hypertrophy and endochondral ossification in 3D cartilage regeneration with nanofibrous poly(l-lactic acid) scaffold and matrilin-3. Acta Biomater 2018; 76: 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Wang Y, Yuan X, Yu K, et al. Fabrication of nanofibrous microcarriers mimicking extracellular matrix for functional microtissue formation and cartilage regeneration. Biomaterials 2018; 171: 118–132. [DOI] [PubMed] [Google Scholar]
- 166. Al-Sabah A, Burnell SEA, Simoes IN, et al. Structural and mechanical characterization of crosslinked and sterilised nanocellulose-based hydrogels for cartilage tissue engineering. Carbohydr Polym 2019; 212: 242–251. [DOI] [PubMed] [Google Scholar]
- 167. Kim HS, Mandakhbayar N, Kim HW, et al. Protein-reactive nanofibrils decorated with cartilage-derived decellularized extracellular matrix for osteochondral defects. Biomaterials 2021; 269: 120214. [DOI] [PubMed] [Google Scholar]
- 168. Zhang S, Huang D, Lin H, et al. Cellulose nanocrystal reinforced collagen-based nanocomposite hydrogel with self-healing and stress-relaxation properties for cell delivery. Biomacromolecules 2020; 21(6): 2400–2408. [DOI] [PubMed] [Google Scholar]
- 169. Zhao H, Zhang Y, Liu Y, et al. In situ forming cellulose nanofibril-reinforced hyaluronic acid hydrogel for cartilage regeneration. Biomacromolecules 2021; 22(12): 5097–5107. [DOI] [PubMed] [Google Scholar]
- 170. Zheng R, Duan H, Xue J, et al. The influence of gelatin/PCL ratio and 3-D construct shape of electrospun membranes on cartilage regeneration. Biomaterials 2014; 35(1): 152–164. [DOI] [PubMed] [Google Scholar]
- 171. Chen W, Chen S, Morsi Y, et al. Superabsorbent 3D scaffold based on electrospun nanofibers for cartilage tissue engineering. ACS Appl Mater Interfaces 2016; 8(37): 24415–24425. [DOI] [PubMed] [Google Scholar]
- 172. Cai Z, Li Y, Song W, et al. Anti-inflammatory and prochondrogenic in situ-formed injectable hydrogel crosslinked by strontium-doped bioglass for cartilage regeneration. ACS Appl Mater Interfaces 2021; 13(50): 59772–59786. [DOI] [PubMed] [Google Scholar]
- 173. Li Y, Chen M, Yan J, et al. Tannic acid/Sr(2+)-coated silk/graphene oxide-based meniscus scaffold with anti-inflammatory and anti-ROS functions for cartilage protection and delaying osteoarthritis. Acta Biomater 2021; 126: 119–131. [DOI] [PubMed] [Google Scholar]
- 174. Dang W, Wang X, Li J, et al. 3D printing of Mo-containing scaffolds with activated anabolic responses and bi-lineage bioactivities. Theranostics 2018; 8(16): 4372–4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Madzovska-Malagurski I, Vukasinovic-Sekulic M, Kostic D, et al. Towards antimicrobial yet bioactive Cu-alginate hydrogels. Biomed Mater 2016; 11(3): 035015. [DOI] [PubMed] [Google Scholar]
- 176. Makris EA, MacBarb RF, Responte DJ, et al. A copper sulfate and hydroxylysine treatment regimen for enhancing collagen cross-linking and biomechanical properties in engineered neocartilage. FASEB J 2013; 27(6): 2421–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Marycz K, Smieszek A, Targonska S, et al. Three dimensional (3D) printed polylactic acid with nano-hydroxyapatite doped with europium(III) ions (nhap/PLLA@eu(3+)) composite for osteochondral defect regeneration and theranostics. Mater Sci Eng C Mater Biol Appl 2020; 110: 110634. [DOI] [PubMed] [Google Scholar]
- 178. Yuan Z, Lyu Z, Liu X, et al. Mg-bgns/DCECM composite scaffold for cartilage regeneration: a preliminary in vitro study. Pharmaceutics 2021; 13(10): 1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Focaroli S, Teti G, Salvatore V, et al. Calcium/cobalt alginate beads as functional scaffolds for cartilage tissue engineering. Stem Cells Int 2016; 2016: 2030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Liu S, Pu Y, Yang R, et al. Boron-assisted dual-crosslinked poly (γ-glutamic acid) hydrogels with high toughness for cartilage regeneration. Int J Biol Macromol 2020; 153: 158–168. [DOI] [PubMed] [Google Scholar]
- 181. Li Q, Xu S, Feng Q, et al. 3D printed silk-gelatin hydrogel scaffold with different porous structure and cell seeding strategy for cartilage regeneration. Bioact Mater 2021; 6(10): 3396–3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Tsai CC, Kuo SH, Lu TY, et al. Enzyme-cross-linked gelatin hydrogel enriched with an articular cartilage extracellular matrix and human adipose-derived stem cells for hyaline cartilage regeneration of rabbits. ACS Biomater Sci Eng 2020; 6(9): 5110–5119. [DOI] [PubMed] [Google Scholar]
- 183. Ribeiro VP, Pina S, Costa JB, et al. Enzymatically cross-linked silk fibroin-based hierarchical scaffolds for osteochondral regeneration. ACS Appl Mater Interfaces 2019; 11(4): 3781–3799. [DOI] [PubMed] [Google Scholar]
- 184. Xing J, Peng X, Li A, et al. Gellan gum/alginate-based Ca-enriched acellular bilayer hydrogel with robust interface bonding for effective osteochondral repair. Carbohydr Polym 2021; 270: 118382. [DOI] [PubMed] [Google Scholar]
- 185. Asensio G, Benito-Garzón L, Ramírez-Jiménez RA, et al. Biomimetic gradient scaffolds containing hyaluronic acid and Sr/Zn folates for osteochondral tissue engineering. Polymers 2021; 14(1): 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Li X, Wang J, Ni Y, et al. Bone morphogenetic protein-2 stimulation of cartilage regeneration in canine tracheal graft. J Heart Lung Transplant 2009; 28(3): 285–289. [DOI] [PubMed] [Google Scholar]
- 187. Vainieri ML, Lolli A, Kops N, et al. Evaluation of biomimetic hyaluronic-based hydrogels with enhanced endogenous cell recruitment and cartilage matrix formation. Acta Biomater 2020; 101: 293–303. [DOI] [PubMed] [Google Scholar]
- 188. Zhang X, Wu S, Zhu Y, et al. Exploiting joint-resident stem cells by exogenous SOX9 for cartilage regeneration for therapy of osteoarthritis. Front Med 2021; 8: 622609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Lim MH, Jeun JH, Kim DH, et al. Evaluation of collagen gel-associated human nasal septum-derived chondrocytes as a clinically applicable injectable therapeutic agent for cartilage repair. Tissue Eng Regen Med 2020; 17(3): 387–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Lu HT, Chang WT, Tsai ML, et al. Development of injectable fucoidan and biological macromolecules hybrid hydrogels for intra-articular delivery of platelet-rich plasma. Mar Drugs 2019; 17(4): 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Wen YT, Dai NT, Hsu SH. Biodegradable water-based polyurethane scaffolds with a sequential release function for cell-free cartilage tissue engineering. Acta Biomater 2019; 88: 301–313. [DOI] [PubMed] [Google Scholar]
- 192. Jia Z, Kang B, Cai Y, et al. Cell-free fat extract attenuates osteoarthritis via chondrocytes regeneration and macrophages immunomodulation. Stem Cell Res Ther 2022; 13(1): 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Özdemir E, Emet A, Hashemihesar R, et al. Articular cartilage regeneration utilizing decellularized human placental scaffold, mesenchymal stem cells and platelet rich plasma. Tissue Eng Regen Med 2020; 17(6): 901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Sakata R, Reddi AH. Platelet-rich plasma modulates actions on articular cartilage lubrication and regeneration. Tissue Eng Part B Rev 2016; 22(5): 408–419. [DOI] [PubMed] [Google Scholar]
- 195. He L, He T, Xing J, et al. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res Ther 2020; 11(1): 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Yoon DS, Kim EJ, Cho S, et al. RUNX2 stabilization by long non-coding RNAs contributes to hypertrophic changes in human chondrocytes. Int J Biol Sci 2023; 19(1): 13–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Mao G, Xu Y, Long D, et al. Exosome-transported circRNA_0001236 enhances chondrogenesis and suppress cartilage degradation via the miR-3677-3p/Sox9 axis. Stem Cell Res Ther 2021; 12(1): 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Zhou ZB, Huang GX, Fu Q, et al. circRNA.33186 contributes to the pathogenesis of osteoarthritis by sponging miR-127-5p. Mol Ther 2019; 27(3): 531–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Gong Z, Wang K, Chen J, et al. CircZSWIM6 mediates dysregulation of ECM and energy homeostasis in ageing chondrocytes through RPS14 post-translational modification. Clin Transl Med 2023; 13(1): e1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Ni W, Jiang C, Wu Y, et al. CircSLC7A2 protects against osteoarthritis through inhibition of the miR-4498/TIMP3 axis. Cell Prolif 2021; 54(6): e13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Zheng J, Lin Y, Tang F, et al. Promotive role of circATRNL1 on chondrogenic differentiation of BMSCs mediated by miR-338-3p. Arch Med Res 2021; 52(5): 514–522. [DOI] [PubMed] [Google Scholar]
- 202. Cheng X, Zhang L, Zhang K, et al. Circular RNA VMA21 protects against intervertebral disc degeneration through targeting miR-200c and X linked inhibitor-of-apoptosis protein. Ann Rheum Dis 2018; 77(5): 770–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Yao T, Yang Y, Xie Z, et al. Circ0083429 regulates osteoarthritis progression via the mir-346/SMAD3 axis. Front Cell Dev Biol 2020; 8: 579945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Wu Y, Hong Z, Xu W, et al. Circular RNA circPDE4D protects against osteoarthritis by binding to miR-103a-3p and regulating FGF18. Mol Ther 2021; 29(1): 308–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Tao SC, Huang JY, Gao Y, et al. Small extracellular vesicles in combination with sleep-related circRNA3503: a targeted therapeutic agent with injectable thermosensitive hydrogel to prevent osteoarthritis. Bioact Mater 2021; 6(12): 4455–4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Ham O, Song BW, Lee SY, et al. The role of microRNA-23b in the differentiation of MSC into chondrocyte by targeting protein kinase A signaling. Biomaterials 2012; 33(18): 4500–4507. [DOI] [PubMed] [Google Scholar]
- 207. Ning G, Liu X, Dai M, et al. MicroRNA-92a upholds bmp signaling by targeting noggin3 during pharyngeal cartilage formation. Dev Cell 2013; 24(3): 283–295. [DOI] [PubMed] [Google Scholar]
- 208. Yang B, Guo H, Zhang Y, et al. MicroRNA-145 regulates chondrogenic differentiation of mesenchymal stem cells by targeting sox9. PLoS One 2011; 6(7): e21679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Meng F, Li Z, Zhang Z, et al. MicroRNA-193b-3p regulates chondrogenesis and chondrocyte metabolism by targeting HDAC3. Theranostics 2018; 8(10): 2862–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Umeda M, Terao F, Miyazaki K, et al. MicroRNA-200a regulates the development of mandibular condylar cartilage. J Dent Res 2015; 94(6): 795–802. [DOI] [PubMed] [Google Scholar]
- 211. Song J, Kim D, Chun CH, et al. MicroRNA-9 regulates survival of chondroblasts and cartilage integrity by targeting protogenin. Cell Commun Signal 2013; 11: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Wang X, Guo Y, Wang C, et al. MicroRNA-142-3p inhibits chondrocyte apoptosis and inflammation in osteoarthritis by targeting HMGB1. Inflammation 2016; 39(5): 1718–1728. [DOI] [PubMed] [Google Scholar]
- 213. Huang J, Zhao L, Fan Y, et al. The microRNAs miR-204 and miR-211 maintain joint homeostasis and protect against osteoarthritis progression. Nat Commun 2019; 10(1): 2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Ito Y, Matsuzaki T, Ayabe F, et al. Both microRNA-455-5p and -3p repress hypoxia-inducible factor-2α expression and coordinately regulate cartilage homeostasis. Nat Commun 2021; 12(1): 4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Bai J, Zhang Y, Zheng X, et al. LncRNA MM2P-induced, exosome-mediated transfer of sox9 from monocyte-derived cells modulates primary chondrocytes. Cell Death Dis 2020; 11(9): 763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Pan C, Huang W, Chen Q, et al. LncRNA Malat-1 from MSCs-derived extracellular vesicles suppresses inflammation and cartilage degradation in osteoarthritis. Front Bioeng Biotechnol 2021; 9: 772002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Wang Y, Li T, Yang Q, et al. LncRNA THUMPD3-AS1 enhances the proliferation and inflammatory response of chondrocytes in osteoarthritis. Int Immunopharmacol 2021; 100: 108138. [DOI] [PubMed] [Google Scholar]
- 218. Shi C, Zheng W, Wang J. lncRNA-CRNDE regulates BMSC chondrogenic differentiation and promotes cartilage repair in osteoarthritis through SIRT1/SOX9. Mol Cell Biochem 2021; 476(4): 1881–1890. [DOI] [PubMed] [Google Scholar]
- 219. Xiao P, Zhu X, Sun J, et al. LncRNA NEAT1 regulates chondrocyte proliferation and apoptosis via targeting miR-543/PLA2G4A axis. Hum Cell 2021; 34(1): 60–75. [DOI] [PubMed] [Google Scholar]
- 220. Liu Y, Liu K, Tang C, et al. Long non-coding RNA XIST contributes to osteoarthritis progression via miR-149-5p/DNMT3A axis. Biomed Pharmacother 2020; 128: 110349. [DOI] [PubMed] [Google Scholar]
- 221. Chen C, Xu Y. Long noncoding RNA LINC00671 exacerbates osteoarthritis by promoting ONECUT2-mediated smurf2 expression and extracellular matrix degradation. Int Immunopharmacol 2021; 90: 106846. [DOI] [PubMed] [Google Scholar]
- 222. Zhi L, Zhao J, Zhao H, et al. Downregulation of lncRNA OIP5-AS1 induced by IL-1β aggravates osteoarthritis via regulating mir-29b-3p/pgrn.undefined. Cartilage 2021; 13(2_suppl): 1345S–1355S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Zhou X, Xu W, Wang Y, et al. LncRNA DNM3OS regulates GREM2 via miR-127-5p to suppress early chondrogenic differentiation of rat mesenchymal stem cells under hypoxic conditions. Cell Mol Biol Lett 2021; 26(1): 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Foo JB, Looi QH, How CW, et al. Mesenchymal stem cell-derived exosomes and microRNAs in cartilage regeneration: biogenesis, efficacy, miRNA enrichment and delivery. Pharmaceuticals 2021; 14(11): 1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Richter W. Mesenchymal stem cells and cartilage in situ regeneration. J Intern Med 2009; 266(4): 390–405. [DOI] [PubMed] [Google Scholar]
- 226. Gan D, Wang Z, Xie C, et al. Mussel-inspired tough hydrogel with in situ nanohydroxyapatite mineralization for osteochondral defect repair. Adv Healthc Mater 2019; 8(22): e1901103. [DOI] [PubMed] [Google Scholar]
- 227. Solchaga LA, Temenoff JS, Gao J, et al. Repair of osteochondral defects with hyaluronan- and polyester-based scaffolds. Osteoarthr Cartil 2005; 13(4): 297–309. [DOI] [PubMed] [Google Scholar]
- 228. Deng C, Xu C, Zhou Q, et al. Advances of nanotechnology in osteochondral regeneration. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2019; 11(6): e1576. [DOI] [PubMed] [Google Scholar]
- 229. Peng Z, Sun H, Bunpetch V, et al. The regulation of cartilage extracellular matrix homeostasis in joint cartilage degeneration and regeneration. Biomaterials 2021; 268: 120555. [DOI] [PubMed] [Google Scholar]
- 230. Madeira C, Santhagunam A, Salgueiro JB, et al. Advanced cell therapies for articular cartilage regeneration. Trends Biotechnol 2015; 33(1): 35–42. [DOI] [PubMed] [Google Scholar]
- 231. Li KC, Hu YC. Cartilage tissue engineering: recent advances and perspectives from gene regulation/therapy. Adv Healthc Mater 2015; 4(7): 948–968. [DOI] [PubMed] [Google Scholar]
- 232. Chinta ML, Velidandi A, Pabbathi NPP, et al. Assessment of properties, applications and limitations of scaffolds based on cellulose and its derivatives for cartilage tissue engineering: a review. Int J Biol Macromol 2021; 175: 495–515. [DOI] [PubMed] [Google Scholar]