Abstract
Objectives:
This study aimed to evaluate functional abdominal pain disorders and functional constipation prevalence in the central region of Saudi Arabia, and compare it to that of the western region.
Methods:
This was a cross-sectional study using online questionnaires targeting the general population of Riyadh region of Saudi Arabia. Subjects were randomly selected by sharing links on social media groups. Any parent with a 3–18-year-old child was included, and children with chronic medical illnesses or symptoms of organic GI disorders were excluded.
Results:
Three hundred nineteen subjects were included in the final analysis; the prevalence of functional abdominal pain disorders overall was 6.2% and the prevalence of functional constipation was 8.1%.
Conclusions:
Functional constipation diagnosis seems to be affected by life stressors or a previous viral illness. Seasonal variations had minimal effect on functional abdominal pain disorder and functional constipation symptom frequency and severity.
Keywords: Functional gastrointestinal disorders, COVID-19, Saudi Arabia, irritable bowel syndrome, pediatric
Introduction
Functional abdominal pain disorders (FAPDs) and functional constipation (FC) belong to a large group of gastrointestinal (GI) disorders that are traditionally characterized by the lack of any significant histological, anatomical, or biochemical abnormalities, but rather involves various afferent (pain) and efferent (motility and secretion) functions—hence the name—of the enteric nervous system and its interactions with the central nervous system.1 The exact pathophysiology of those disorders is not fully elucidated.1 Many theories such as the polyvagal theory revolve around the dysfunction of the gut–brain axis in response to an early life event, in genetically susceptible individuals, leading to a state of autonomic nervous system imbalance.2
The diagnosis of functional GI disorders (FGIDs) relies on the presence of a combination of symptoms that cannot be explained by another organic GI disorder without the need for extensive testing.3 Several expert groups proposed different diagnostic criteria to serve that purpose, with the most commonly used criteria in current clinical practice being Rome IV criteria.4
The prevalence of FGIDs varies from one region in the world to another and ranges from 9.9% to 29%.5 Adult irritable bowel syndrome (IBS) and functional dyspepsia (FD) have a prevalence of 30.5% and 18% respectively in Saudi Arabia.6,7 The diagnostic criteria used in the majority of those studies were Rome III and Apley’s for IBS and recurrent abdominal pain respectively.6–8 We previously reported a prevalence of 3.1% and 4.7% for FAPDs and FC, respectively in our western region of Saudi Arabia.9 Since those numbers were considerably lower than that of adults or other pediatric studies from the same region albeit different criteria being used, we hypothesized that this difference could be explained by regional or seasonal variations within the same population. We aimed to assess the prevalence of those two disorders in the central (Riyadh) region to examine whether regional variations on the same population do exist and whether seasons can also contribute to such difference given our western region is characterized by a very mild winter in contrast to the central region that typically experiences a significantly colder winter. And similar to our previous study, we aimed to assess the impact of socio-economic and COVID-19 related factors (e.g. distant learning) on the prevalence of FAPDs and FC.
Methods
The study is cross-sectional, targeting the central (Riyadh) region population of Saudi Arabia. Questionnaires were designed by google forms and links were shared via Whatsapp®, the most used social media app in Saudi Arabia based on a 2021 global media insights report of 28.24 (80.50%) million users.
Questions included in the questionnaire were adapted from Rome IV criteria.4 Subjects can meet criteria for more than one FAPD subtype or an FAPD and FC simultaneously. The only exception is IBS-C and FC since they are mutually exclusive by definition, and thus subjects meeting criteria for both IBS-C and FC were classified as IBS-C only.
Subject selection was done by randomly sharing the questionnaire link with random Whatsapp groups. Sample collection took place from August 2021 to November 2021. All participants that live in the central (Riyadh) region and have at least one child between the ages 3–18 were included. Children with any established chronic organic GI disorder, special needs, chronic medical conditions (e.g. diabetes) or taking medications on daily basis were excluded. In addition, participants who answered yes to the chronic abdominal pain question and had two or more organic GI symptoms or one organic GI symptom and a positive family history of organic GI disorders were also excluded. Those symptoms include blood in stools, mouth ulcers, joint pains or swellings, visual symptoms (uveitis), and weight loss.
Data that was collected included location, child’s age, gender, parental age, living and marital status, occupation, income, history of COVID-19 infection and COVID-related death in the family, and perception of distant learning as well as seasonal variations on symptoms.
Sample size was determined using OpenEpi® version 3.0 software that is representative of Riyadh region population of 8 million. For a confidence level of 99%, and an expected prevalence between 5% and 10%; the sample size required was 127–239. We aimed to obtain at least 500 participants to compensate for potential exclusions.
Statistical analysis
After coding and proper translation, data obtained were entered into the statistical package for social science software (SPSS®) for analysis. Using complex operators and logical expressions in the case selection command, cases that do meet Rome IV criteria for FAPD and its subcategories (FD, abdominal migraine, IBS, and abdominal pain not otherwise specified) as well as FC were identified. Total cases were expressed in numbers (n) and percentages (%) of the entire sample. A χ2 (or Fisher’s exact when applicable) test was used to measure any association between selected cases and different categorical variables (e.g. marital status). A multinomial logistic regression model was used to assess the effect of combining all variables on selected cases. For seasonal variation and online learning parental perception on FAPD and FC symptom frequency, cases that did meet Rome IV criteria were compared to cases that had abdominal pain, and constipation respectively but did not meet Rome IV criteria, using χ2 (or Fisher’s exact when applicable) tests. p Values < 0.05 were considered significant.
Results
Five hundred sixty-four responded to the questionnaire, and 245 were excluded (review exclusions) or removed because they were duplicates. The demographic characteristics of the remaining 319 are summarized in Table 1.
Table 1.
Baseline characteristics overall and baseline characteristics of subjects with FAPD and FC.
| Variable | Overall N = 319 (%) | FAPD N = 20 (%) | p-Value | FC N = 26 (%) | p-Value |
|---|---|---|---|---|---|
| Living with both parents | 258 (81) | 17 (85) | 0.7 | 25 (96) | 0.03 |
| Parent age: | 0.4 | 0.6 | |||
| >40 | 83 (26) | 6 (30) | 5 (19) | ||
| 31–40 | 152 (47) | 10 (50) | 14 (54) | ||
| 20–30 | 84 (26) | 4 (20) | 7 (27) | ||
| Married | 268 (84) | 18 (90) | 0.4 | 26 (100) | 0.06 |
| Divorced | 40 (12) | 1 (5) | |||
| Widowed | 1 (5) | ||||
| Educational level: | 0.4 | 0.36 | |||
| Undergraduate or higher | 166 (52) | 8 (40) | 13 (50) | ||
| High school | 127 (40) | 11 (55) | 9 (35) | ||
| Occupation: | 0.06 | 0.06 | |||
| Fieldwork | 81 (25) | 5 (25) | 2 (8) | ||
| Business | 83 (26) | 9 (45) | 6 (23) | ||
| Student/unemployed | 109 (34) | 5(25) | 11 (42) | ||
| Healthcare | 46 (14) | 1 (5) | 7 (27) | ||
| Monthly income: | 0.6 | 0.8 | |||
| <5000 SAR | 34 (10) | 3 (15) | 3 (11) | ||
| 5000–10,000 SAR | 110 (34) | 5 (25) | 10 (38) | ||
| >10,000 SAR | 66 (20) | 2 (10) | 6 (23) | ||
| Unanswered | 109 (34) | 10 (50) | 7 (27) | ||
| Child’s age: | 0.7 | 0.1 | |||
| <5 | 111 (35) | 4 (20) | 11 (42) | ||
| 5–12 | 152 (47) | 13 (65) | 14 (54) | ||
| 13–18 | 56 (17) | 3 (15) | 1 (4) | ||
| Nationality: | 0.5 | 0.1 | |||
| Saudi | 296 (93) | 19 (95) | 22 (85) | ||
| Child’s gender: | 0.3 | 0.5 | |||
| Male | 162 (51) | 8 (40) | 15 (58) | ||
| Learning issues: | 1 | 0.7 | |||
| Yes | 29 (9) | 3 (15) | 3 (11) | ||
| COVID in the family: | 0.6 | 0.6 | |||
| Yes | 159 (50) | 9 (45) | 14 (54) | ||
| COVID death in the family: | 0.1 | 0.2 | |||
| Yes | 77 (24) | 3 (15) | 9 (35) | ||
| COVID diagnosis in child: | 0.7 | 0.01 | |||
| Yes | 112 (35) | 10 (50) | 15 (58) |
p Values shown are for Fisher’s exact tests for the respective variables in association with FAPD cases versus non-selected cases, and with FC cases versus non-selected cases. FAPD: functional abdominal pain disorder; FC: functional constipation; SAR: Saudi Arabian Riyal*.
Statistically signifcant values are shown in bold.
Functional abdominal pain disorders
One hundred twenty-nine (40.4%) participants reported the presence of chronic abdominal pain, of whom 18 met the criteria for IBS, and 3 of those had the IBS-C subtype. Fifteen cases out of the 18 also met the criteria for FD. Seventeen cases met the criteria for FD, 15 out of whom also met the criteria for IBS. Figure 1 illustrates this overlap of FAPD subtypes. No cases met the criteria for either abdominal migraine or “functional abdominal pain not otherwise specified” subtypes in our study. Collectively, there were 20/319 (6.2%) cases that did meet criteria for at least one of the FAPD subcategories. Their demographic characteristics are summarized in Table 1.
Figure 1.
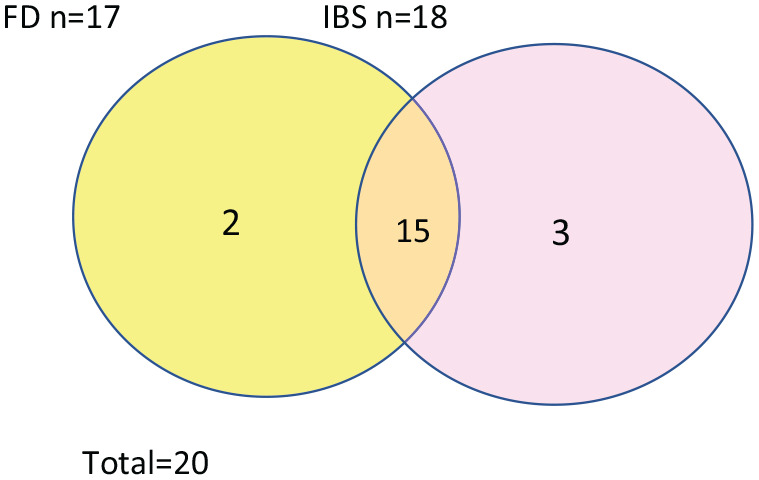
Diagrammatic representation of the subcategories of FAPDs. The overlap area represents cases that met criteria for both subcategories.
FAPD: functional abdominal pain disorder; FD: functional dyspepsia; IBS: irritable bowel syndrome.
There was no statistically significant association between cases with FAPDs and any independent variable including child’s age and gender, parental age, parental educational level, parental occupation, monthly income, marital status, learning issues, COVID-19 diagnosis, and COVID-19-related death. χ2 or Fisher’s exact test p values for each variable are summarized in Table 1.
Combining all independent variables in a multinomial logistic regression model showed no impact on the diagnosis of FAPD (final model fitting χ2 = 30.8, p = 0.07).
Distant learning (before and after COVID-19 pandemic) doesn’t seem to affect FAPDs’ symptoms severity or frequency based on parental perception (χ2 = 6.5, p = 0.08). Similarly, seasonal variations don’t seem to affect FAPDs’ symptoms severity or frequency based on parental perception (χ2 = 3.6, p = 0.4).
Functional constipation
One hundred seven (33.5%) participants reported the presence of chronic constipation. Twenty-nine cases did meet the criteria for FC but three were excluded because they also met the criteria for IBS. Thus, the total number of FC cases was 26/319 (8.1%). FC was significantly associated with living with both parents and COVID-19 diagnosis in the child (p = 0.03 and 0.01, respectively). Otherwise, there was no statistically significant association between cases with FC and any other independent variable including child’s age and gender, learning issues, parental age, parental educational level, parental occupation, monthly income, and marital status. Fisher’s exact test p values for each variable are summarized in Table 1.
Combining all independent variables in a multinomial logistic regression model showed a significant impact (final model fitting 182, χ2 = 34, p = 0.029), with FC cases being 9.5 times more likely to have a parent with an educational level less than high school (p = 0.01) in the same model.
FC symptoms severity (symptoms before and after COVID-19 pandemic) didn’t seem to be affected by distant learning based on parental perception (χ2 = 4.2, p = 0.2). Similarly, seasonal variations don’t seem to affect FC symptoms severity or frequency based on parental perception (χ2 = 7.6, p = 0.1).
In summary: The prevalence of cases with any FAPD was 6.2%, and the prevalence of FC was 8.1%.
Discussion
The pooled prevalence of all FAPDs is 13.5% based on a large meta-analysis.10 The prevalence varies from one region to another and ranges from as low as 1.6% to as high as 41.2%.10 Rome III criteria tended to give a relatively higher prevalence potentially due to less stringent criteria in comparison with Rome IV.10,11 Our Prevalence from the central region is slightly higher than the prevalence we previously reported from the western region despite using the same criteria in both studies, which redemonstrates what has been observed with worldwide regional variations in FAPDs prevalence.9 Regional variation in the incidence and prevalence of organic GI disorders such as inflammatory bowel disease (IBD) is well documented in the literature.12,13 This variation can be explained by genetic or more importantly, environmental factors such as diet, climate, and infectious agents that are more prevalent in certain regions and seasons.14–16 Seasonal variation itself had been similarly hypothesized to affect the prevalence of multiple organic GI disorders and autoimmune conditions.16–18 The hypothesis revolves around the interactions of climate and human biologic functions such as vitamin D activity, and melatonin secretion and their effects on the immune system.16 While FGIDs are classically defined by the absence of any alterations in histological or biochemical norms, more recent evidence points toward a role of the gut microbiome in the pathogenesis with the newly established microbiome-gut-brain axis concept.19–21 Interestingly, gut microbiota populations vary by host geographic location based on a multi-center study in China.22 This hypothesis, similar to the many observations from organic GI disorders (e.g. IBD) studies, could be the bridge to understanding the regional and seasonal differences in FGIDs’ prevalence.
The pooled prevalence of FC from a large meta-analysis is 9.5%.23 Similar to FAPDs, FC prevalence seems to be affected by different geographic locations.23 This is demonstrated in the present study, where our prevalence in the central region was higher than what we previously reported from the western region.9 This could—at least in part—be attributed to environmental factors. After all, the pathophysiology of all FGIDs indeed seems to involve an environmental insult in genetically susceptible individuals.19,20 This was also observed in the present study, where FC cases were more likely to have a history of COVID-19 diagnosis. Viral illness—as discussed in the microbiome-gut-brain axis concept—could be the inciting event that alters the gut microbiome or transiently slows down colonic motility, resulting in the classic vicious cycle of rectal distension, and desensitization, and eventually sustained constipation with the hallmark symptoms usually seen in FC but not habitual or intermittent constipation.20,24,25 Studies reporting on the prevalence of constipation as an isolated symptom usually show a much higher prevalence.26 Furthermore, the relationship between dietary fiber intake and FC is controversial compared to habitual or intermittent constipation.27–29 These observations along with the altered microbiome-gut-brain axis and colonic motility theorized with viral illnesses can give us clues as to why FC and habitual/intermittent constipation prevalence differ significantly with the former being far less common. It can be argued that FC is a more chronic disorder with a mechanism involving rectal desensitization to distension, rather than a direct and intermittent response to dietary fiber intake or lifestyle habits seen in habitual/intermittent constipation.4,20,25,30,31 A recent study on constipation prevalence during COVID-19 lockdown again showed a relatively higher prevalence of “new-onset” constipation at 25% and linked it to reduced physical activity and water intake as a result of the lockdown. That study however did not use validated criteria to distinguish habitual/intermittent constipation from chronic FC which reiterates the importance of making that distinction when comparing prevalence.32
Patients with FAPDs and FGIDs frequently exhibit features of more than one disorder simultaneously since criteria for FAPD subcategories do tend to overlap.4,33,34 We redemonstrated in the present study that the majority of our FAPDs cases did meet criteria for at least two disorders. Rome IV expert committee classified FAPDs into four subclasses mainly for research and clinical purposes, treatment approach, and long-term outcomes are generally quite similar however.4,35
FGIDs are commonly associated with psychiatric conditions such as depression and anxiety disorders.36,37 It had been postulated that FGIDs are direct manifestations of somatization disorders or brain dysfunction seen in some of those psychiatric disorders; however, other studies have demonstrated that GI symptoms arise well in advance of psychiatric diagnoses in a significant number of patients.1 Thus, the role of psychiatric illnesses in FGIDs pathogenesis remains unclear.1 It is indeed possible that a common inciting factor or an early life stressor could potentially trigger both conditions (FGIDs and psychiatric illnesses) in a genetically predisposed individual.1,34,38 We aimed to assess the effect of potential life stressors such as sociodemographic factors, on the prevalence of FAPDs and FC in the present study. Interestingly we found that living status (both parents versus divorced and widowed parents) was significantly associated with FC but not FAPD. Additionally, FC cases were 9.5 times more likely to have a parent with an educational level less than high school. The educational level of the parent can be considered a direct life stressor when it affects the parents’ ability to cope with a chronic condition or a child with social issues such as stool withholding or soiling which are hallmark features of FC.39 Such association was not observed with FAPD cases in this study.
Strengths of the present study include decent sample size, and the use of Rome IV criteria to diagnose cases of FAPD and FC as well as FAPD subtypes. The study is limited by the inherent risk of selection, recall, interviewer, and response bias, and being online, effectively limiting the ability to physically evaluate and examine cases to accurately exclude organic GI disorders. The exclusion of patients with established organic GI disorders or other chronic illnesses might have underestimated FAPD and FC prevalence since FGIDs can certainly coexist with organic GI disorders. 4
Conclusions
The prevalence of FAPDs and FC seems to be affected by regional variations even at a smaller scale within the same population. FC but not FAPD prevalence again seems to be affected by stressors such as living situation, parental educational level, and COVID-19 diagnosis. The prevalence of FAPDs or FC was not affected by seasonal variation and distant/online learning.
Supplemental Material
Supplemental material, sj-docx-2-smo-10.1177_20503121231163519 for Regional and seasonal variations in functional abdominal pain and functional constipation prevalence among Saudi children by Ammar Khayat, Sarah Salem Aldharman, Njoud Naif Alharbi, Abdulaziz Saad Alayyaf, Jannat Abdullah abdulmuttalib and Elaf Rudda Altalhi in SAGE Open Medicine
Supplemental material, sj-pdf-1-smo-10.1177_20503121231163519 for Regional and seasonal variations in functional abdominal pain and functional constipation prevalence among Saudi children by Ammar Khayat, Sarah Salem Aldharman, Njoud Naif Alharbi, Abdulaziz Saad Alayyaf, Jannat Abdullah abdulmuttalib and Elaf Rudda Altalhi in SAGE Open Medicine
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: Ethical approval for this study was obtained from Umm Al Qura University’s Institutional Review Board (approval number/ID HAPO-02-K-012-2021-09-743).
Informed consent: Written consent was obtained from each participant before participation (please review supplemental material).
ORCID iDs: Ammar Khayat  https://orcid.org/0000-0002-4819-9552
https://orcid.org/0000-0002-4819-9552
Sarah Salem Aldharman  https://orcid.org/0000-0002-6714-9964
https://orcid.org/0000-0002-6714-9964
Supplemental material: Supplemental material for this article is available online.
References
- 1. Holtmann G, Shah A, Morrison M. Pathophysiology of functional gastrointestinal disorders: a holistic overview. Dig Dis 2017; 35(Suppl 1): 5–13. [DOI] [PubMed] [Google Scholar]
- 2. Kolacz J, Kovacic KK, Porges SW. Traumatic stress and the autonomic brain-gut connection in development: Polyvagal Theory as an integrative framework for psychosocial and gastrointestinal pathology. Dev Psychobiol 2019; 61: 796–809. [DOI] [PubMed] [Google Scholar]
- 3. Nurko S, Di Lorenzo C. Functional abdominal pain: time to get together and move forward. J Pediatr Gastroenterol Nutr 2008; 47: 679–680. [DOI] [PubMed] [Google Scholar]
- 4. Hyams JS, Di Lorenzo C, Saps M, et al. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology 2016; 150: 1456–1468.e1452. [Google Scholar]
- 5. Boronat AC, Ferreira-Maia AP, Matijasevich A, et al. Epidemiology of functional gastrointestinal disorders in children and adolescents: a systematic review. World J Gastroenterol 2017; 23: 3915–3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zacharakis G, Al-Ghamdi S, AlZahrani J, et al. Effects of the Rome IV Criteria to functional dyspepsia symptoms in Saudi Arabia: epidemiology and clinical practice. Korean J Gastroenterol 2020; 76: 304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aljammaz KI, Alrashed AA, Alzwaid AA. Irritable bowel syndrome: epidemiology and risk factors in the adult Saudi population of the central region. Niger J Clin Pract 2020; 23: 1414–1418. [DOI] [PubMed] [Google Scholar]
- 8. Telmesani AM. Helicobacter pylori: prevalence and relationship with abdominal pain in school children in Makkah City, western Saudi Arabia. Saudi J Gastroenterol 2009; 15: 100–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khayat A, Algethami G, Baik S, et al. The effect of using Rome IV Criteria on the prevalence of functional abdominal pain disorders and functional constipation among children of the western region of Saudi Arabia. Glob Pediatr Health 2021; 8: 2333794x211022265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Korterink JJ, Diederen K, Benninga MA, et al. Epidemiology of pediatric functional abdominal pain disorders: a meta-analysis. PloS One 2015; 10: e0126982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saps M, Velasco-Benitez CA, Langshaw AH, et al. Prevalence of functional gastrointestinal disorders in children and adolescents: comparison between Rome III and Rome IV Criteria. J Pediatr 2018; 199: 212–216. [DOI] [PubMed] [Google Scholar]
- 12. El Mouzan MI, AlEdreesi MH, Hasosah MY, et al. Regional variation of pediatric inflammatory bowel disease in Saudi Arabia: results from a multicenter study. World J Gastroenterol 2020; 26: 416–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2020; 5: 17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Owczarek D, Rodacki T, Domagała-Rodacka R, et al. Diet and nutritional factors in inflammatory bowel diseases. World J Gastroenterol 2016; 22: 895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tarris G, de Rougemont A, Charkaoui M, et al. Enteric viruses and inflammatory bowel disease. Viruses 2021; 13(1). DOI: 10.3390/v13010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Watad A, Azrielant S, Bragazzi NL, et al. Seasonality and autoimmune diseases: The contribution of the four seasons to the mosaic of autoimmunity. J Autoimmun 2017; 82: 13–30. [DOI] [PubMed] [Google Scholar]
- 17. Yoon JY, Cha JM, Kim HI, et al. Seasonal variation of peptic ulcer disease, peptic ulcer bleeding, and acute pancreatitis: a nationwide population-based study using a common data model. Medicine 2021; 100: e25820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fares A. Global patterns of seasonal variation in gastrointestinal diseases. J Postgrad Med 2013; 59: 203–207. [DOI] [PubMed] [Google Scholar]
- 19. Osadchiy V, Martin CR, Mayer EA. The gut-brain axis and the microbiome: mechanisms and clinical implications. Clin Gastroenterol Hepatol 2019; 17: 322–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Margolis KG, Cryan JF, Mayer EA. The microbiota-gut-brain axis: from motility to mood. Gastroenterology 2021; 160: 1486–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mars RAT, Frith M, Kashyap PC. Functional gastrointestinal disorders and the microbiome-what is the best strategy for moving microbiome-based therapies for functional gastrointestinal disorders into the clinic? Gastroenterology 2021; 160: 538–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. He Y, Wu W, Zheng HM, et al. Regional variation limits applications of healthy gut microbiome reference ranges and disease models. Nat Med 2018; 24: 1532–1535. [DOI] [PubMed] [Google Scholar]
- 23. Koppen IJN, Vriesman MH, Saps M, et al. Prevalence of functional defecation disorders in children: a systematic review and meta-analysis. J Pediatr 2018; 198: 121–130.e126. [DOI] [PubMed] [Google Scholar]
- 24. Vassallo M, Camilleri M, Caron BL, et al. Gastrointestinal motor dysfunction in acquired selective cholinergic dysautonomia associated with infectious mononucleosis. Gastroenterology 1991; 100: 252–258. [DOI] [PubMed] [Google Scholar]
- 25. Berumen A, Edwinson AL, Grover M. Post-infection irritable bowel syndrome. Gastroenterol Clin North Am 2021; 50: 445–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zubaidi AM, Al-Saud NH, Al-Qahtani XA, et al. Bowel function and its associated variables in Saudi adults: a population-based study. Saudi Med J 2012; 33: 627–633. [PubMed] [Google Scholar]
- 27. Yang J, Wang HP, Zhou L, et al. Effect of dietary fiber on constipation: a meta analysis. World J Gastroenterol 2012; 18: 7378–7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Axelrod CH, Saps M. The role of fiber in the treatment of functional gastrointestinal disorders in children. Nutrients 2018; 10(11). DOI: 10.3390/nu10111650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Piccoli de, Mello P, Eifer DA, Daniel de, Mello E. Use of fibers in childhood constipation treatment: systematic review with meta-analysis. J Pediatr 2018; 94: 460–470. [DOI] [PubMed] [Google Scholar]
- 30. Mugie SM, Benninga MA, Di Lorenzo C. Epidemiology of constipation in children and adults: a systematic review. Best Pract Res Clin Gastroenterol 2011; 25: 3–18. [DOI] [PubMed] [Google Scholar]
- 31. Prat D, Jacobs F, Hamzaoui O, et al. Impact of delayed transit in severe COVID 19 critical care patients: a retrospective analysis. Clin Res Hepatol Gastroenterol 2021; 45: 101676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Remes-Troche JM, Coss-Adame E, Amieva-Balmori M, et al. Incidence of ‘new-onset’ constipation and associated factors during lockdown due to the COVID-19 pandemic. BMJ Open Gastroenterol 2021; 8: e000729. DOI: 10.1136/bmjgast-2021-000729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Velasco-Benítez CA, Ramírez-Hernández CR, Moreno-Gómez JE, et al. Overlapping of functional gastrointestinal disorders in latinamerican schoolchildren and adolescents. Revista Chilena de Pediatria 2018; 89: 726–731. [DOI] [PubMed] [Google Scholar]
- 34. Lewis ML, Palsson OS, Whitehead WE, et al. Prevalence of functional gastrointestinal disorders in children and adolescents. J Pediatr 2016; 177: 39–43.e33. [DOI] [PubMed] [Google Scholar]
- 35. Horst S, Shelby G, Anderson J, et al. Predicting persistence of functional abdominal pain from childhood into young adulthood. Clin Gastroenterol Hepatol 2014; 12: 2026–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stasi C, Nisita C, Cortopassi S, et al. Subthreshold psychiatric psychopathology in functional gastrointestinal disorders: can it be the bridge between gastroenterology and psychiatry? Gastroenterol Res Pract 2017; 2017: 1953435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Van Oudenhove L, Vandenberghe J, Demyttenaere K, et al. Psychosocial factors, psychiatric illness and functional gastrointestinal disorders: a historical perspective. Digestion 2010; 82: 201–210. [DOI] [PubMed] [Google Scholar]
- 38. O’Mahony SM, Clarke G, Dinan TG, et al. Irritable bowel syndrome and stress-related psychiatric co-morbidities: focus on early life stress. Handb Exp Pharmacol 2017; 239: 219–246. [DOI] [PubMed] [Google Scholar]
- 39. Makhoul Khoury S, Ben-Zur H, Ben-Arush M. Mastery and social support moderate the effects of educational level on adjustment of Arab mothers of children diagnosed with cancer. Eur J Cancer Care 2018; 27: e12906. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-2-smo-10.1177_20503121231163519 for Regional and seasonal variations in functional abdominal pain and functional constipation prevalence among Saudi children by Ammar Khayat, Sarah Salem Aldharman, Njoud Naif Alharbi, Abdulaziz Saad Alayyaf, Jannat Abdullah abdulmuttalib and Elaf Rudda Altalhi in SAGE Open Medicine
Supplemental material, sj-pdf-1-smo-10.1177_20503121231163519 for Regional and seasonal variations in functional abdominal pain and functional constipation prevalence among Saudi children by Ammar Khayat, Sarah Salem Aldharman, Njoud Naif Alharbi, Abdulaziz Saad Alayyaf, Jannat Abdullah abdulmuttalib and Elaf Rudda Altalhi in SAGE Open Medicine


