Abstract
Background
The role of inflammation and cytokines in the pathophysiology of primary headache disorders is uncertain. We performed a systematic review and meta-analysis to synthesise the results of studies comparing peripheral blood cytokine levels between patients with migraine, tension-type headache, cluster headache, or new daily persistent headache (NDPH), and healthy controls; and in migraine between the ictal and interictal stages.
Methods
We searched PubMed/Medline and Embase from inception until July 2022. We included original research studies which measured unstimulated levels of any cytokines in peripheral blood using enzyme-linked immunosorbent assay or similar assay. We assessed risk of bias using the Newcastle–Ottawa Quality Assessment Scale. We used random effects meta-analysis with inverse variance weighted average to calculate standardised mean difference (SMD), 95% confidence intervals, and heterogeneity for each comparison. This study is registered with PROSPERO (registration number CRD42023393363). No funding was received for this study.
Results
Thirty-eight studies, including 1335 patients with migraine (32 studies), 302 with tension-type headache (nine studies), 42 with cluster headache (two studies), and 1225 healthy controls met inclusion criteria.
Meta-analysis showed significantly higher interleukin (IL)-6 (SMD 1.07, 95% CI 0.40–1.73, p = 0.002), tumour necrosis factor (TNF)-α (SMD 0.61, 95% CI 0.14–1.09, p = 0.01), and IL-8 (SMD 1.56, 95% CI 0.03–3.09, p = 0.04), in patients with migraine compared to healthy controls, and significantly higher interleukin-1β (IL-1β) (SMD 0.34, 95% CI 0.06–0.62, p = 0.02) during the ictal phase of migraine compared to the interictal phase. Transforming growth factor (TGF)-β (SMD 0.52, 95% CI 0.18–0.86, p = 0.003) and TNF-α (SMD 0.64, 95% CI 0.33–0.96, p = 0.0001) were both higher in patients with tension-type headache than controls.
Conclusions
The higher levels of the proinflammatory cytokines IL-6, IL-8 and TNF-α in migraine compared to controls, and IL-1β during the ictal stage, suggest a role for inflammation in the pathophysiology of migraine, however prospective studies are required to confirm causality and investigate the mechanisms for the increase in cytokine levels identified. Cytokines may also have a role in tension-type headache. Due a lack of data, no conclusions can be made regarding cluster headache or NDPH.
Keywords: Migraine disorders, Tension-type headache, Cytokine, Neurogenic inflammation, Immunology
Background
The pathophysiology of the primary headache disorders tension-type headache, migraine, cluster headache, and new daily persistent headache (NDPH), and the degree of overlap in pathophysiology between the disorders, is incompletely understood [1–4].
Cytokines are small proteins important in cell signaling, particularly in regulation of the immune system. They have complex mechanisms of action but are broadly classified as pro-inflammatory, such as interleukin (IL)-6 and tumour necrosis factor alpha (TNF-α); or anti-inflammatory, such as IL-4 and IL-10 [5, 6].
Several observations suggest that the neuroinflammation and pro-inflammatory cytokines, may be involved in the pathophysiology of episodic headache attacks. These observations include the high frequency of acute headache as a symptom of systemic infection such as influenza and Covid-19 which may be mediated by cytokines [7–9], the high frequency of headache as an adverse effect of the therapeutic administration of the cytokines TNF-α or beta interferon for cancer or multiple sclerosis [10, 11], and the efficacy of non-steroidal anti-inflammatory drugs in acute treatment of headache attacks [12].
Cytokines and neurogenic inflammation are also hypothesised to be involved in the process of transformation from an episodic headache disorder to chronic daily headache, and/or in the development of de-novo chronic daily headache in post-infectious NDPH [9, 13, 14].
Several studies have measured serum levels of cytokines in patients with primary headache disorders, either making a comparison between patients with primary headache disorders and healthy controls, or between the ictal and interictal periods of migraine. We sought to synthesise the results of these studies to better determine the significance of cytokines in primary headache disorders.
Objectives
To perform a systematic review and meta-analysis of studies measuring peripheral blood cytokine levels in primary headache disorders. Specifically, to determine whether there are differences in cytokine levels between the following groups:
Patients with migraine compared to healthy controls
During the ictal phase of migraine compared to the interictal phase
Patients with tension-type headache compared to healthy controls
Patients with cluster headache compared to healthy controls
Patients with new daily persistent headache compared to healthy controls
Methods
This systematic review and meta-analysis was conducted and reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement [15]. It is registered with PROSPERO (registration number CRD42023393363). The protocol was not pre-published. No funding was received for this review.
Search strategy
Two reviewers (AKM and SC) independently searched the databases PubMed and Embase, with no start date and last search date on 7th July 2022. Search terms were or “migraine” or “tension-type headache” or “cluster headache” or “trigeminal autonomic cephalalgia(s)” or “new daily persistent headache” or “primary headache” and “cytokine(s)”. The result was limited to humans as an automated filter. Duplicates and non-English language articles were removed before screening.
Selection criteria
Titles and abstracts were screened for relevance by a single author (AKM), and full text was reviewed if the abstract indicated that cytokines were measured in any of the primary headaches under investigation. Full texts were then screened against the inclusion criteria. We included original research studies which included patients with migraine, tension-type headache, cluster headache, and/or new daily persistent headache according to International Headache Society (IHS) criteria; and which measured levels of any cytokines in peripheral blood using enzyme-linked immunosorbent assay (ELISA) or similar assay. We excluded any study where cytokine production was stimulated, any study where headache attacks were pharmacologically provoked, or interventional studies where baseline cytokine levels were not reported prior to intervention. In addition, we excluded any study where mean/standard deviation or median/IQR could not be extracted from the text, tables, or graphs.
Data extraction
Data were extracted manually from included studies by a single author (AKM) into a Microsoft Excel spreadsheet. Data in this paper are presented as means and standard deviations. If only standard error of the mean (SEM) was reported, this was converted to standard deviation (SD). If only median and interquartile range (IQR) were reported, the median was assumed mean and the IQR converted to SD.
Risk of bias assessment
Risk of bias was assessed by a single author (AKM) using the Newcastle–Ottawa Quality Assessment Scale [16].
Statistical analysis
Data on demographics and study types were analysed using descriptive statistics in Microsoft Excel. Meta-analysis was performed where at least two studies had examined a particular cytokine for each of the group comparisons of interest. We used random effects meta-analysis with inverse variance weighted average, using the software RevMan 5 (Cochrane Collaboration). As all studies did not use the same ELISA-based methods for measurement of cytokines or report the same measurement units, standardised mean difference (SMD) was calculated for comparison of group differences and calculation of 95% confidence intervals. The I2 measure of heterogeneity was calculated for each meta-analysis. Funnel plots were used to assess for publication bias for those meta-analyses which included at least ten studies.
Results
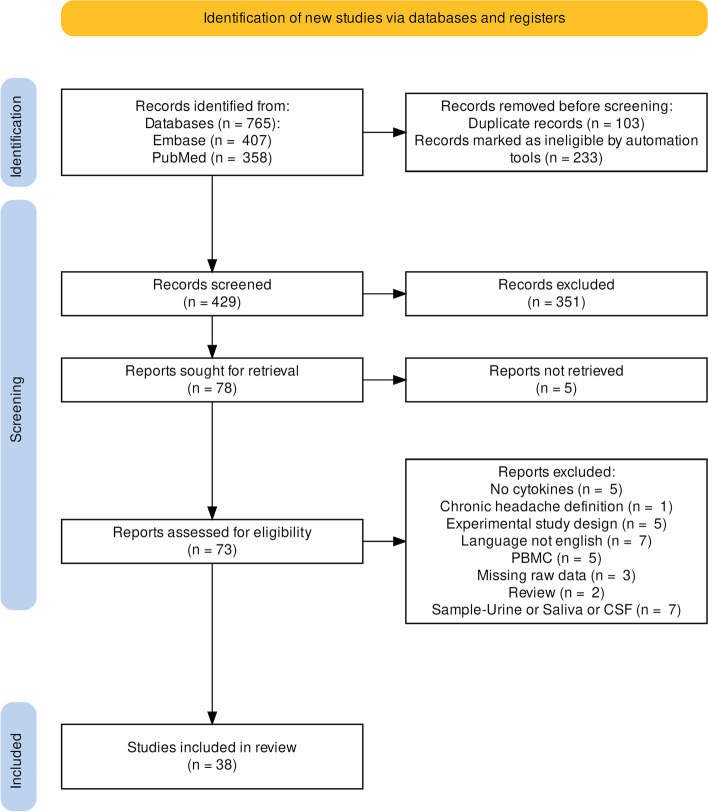
The search resulted in 765 records, of which 38 met inclusion criteria (see Fig. 1). The studies included a total of 2904 participants, of whom 1335 had migraine, 302 had tension-type headache, 42 had cluster headache, and 1225 were healthy controls. No studies including patients with NDPH met inclusion criteria.
Fig. 1.
PRISMA flow diagram of study selection. Abbreviations: PMBC, studies measuring cytokine production by peripheral blood mononuclear cells in vitro, rather than circulating serum cytokines
Of the 38 studies, 32 included patients with migraine, nine with tension-type headache, two with cluster headache, and none with new daily persistent headache. All studies used IHS criteria, most commonly ICHD-2 (17 studies) and ICHD-3 (11 studies) [17, 18]. Most (26/38) studies were conducted in outpatients. The most commonly measured cytokines were IL-6 (21 studies), TNF-α (20 studies), IL-10 (12 studies), IL-1β (11 studies), and IL-8 (10 studies). Study quality was rated as good (7 or 8 on Newcastle–Ottawa scale) in 31/38 studies. Details of individual studies are shown in Table 1.
Table 1.
Summary of individual studies and quality assessment scores
| Author | Year | Country | Condition(s) studied | ICHD criteria | Setting | Cytokines studied | NOS |
|---|---|---|---|---|---|---|---|
| Covelli et al. [19] | 1991 | Italy | M & T | 1 | NR | TNF-α | 4 |
| Shimomura et al. [20] | 1991 | Japan | M & T | I | OP | IL-2 | 7 |
| Martelletti et al. [21] | 1993a | Italy | C | I | OP | IL-1β | 8 |
| Martelletti et al. [22] | 1993b | Italy | M | I | IP | IL-4, IL-6 IFN-γ | 8 |
| Munno et al. [23] | 1998 | Italy | M | I | OP | IL-4, IL-8, IFN-γ | 8 |
| Martelletti et al. [24] | 2001 | Italy | M | I | NR | IL-2, IL-4, IFN-γ | 7 |
| Empl et al. [25] | 2003a | Germany | C | I | OP | IL-1β, IL-6 | 7 |
| Empl et al. [26] | 2003b | Germany | M | I | OP | IL-6, TNF-α | 7 |
| Sarchielli et al. [27] | 2004 | Italy | M | II | IP | IL-8 | 8 |
| Perini et al. [28] | 2005 | Italy | M | I | OP | IL-1β, IL-2, IL-4, IL-6, IL-10, TNF-α | 8 |
| Ishizaki et al. [29] | 2005 | Japan | M & T | I | OP | TGF-β | 8 |
| Sarchielli et al. [30] | 2006 | Italy | M | II | IP | IL-1β, IL-4, IL-6, TNF-α | 8 |
| Fidan et al. [31] | 2006 | Turkey | M | II | IP | IL-6, IL-10 | 7 |
| Koçer et al. [32] | 2009 | Turkey | M | II | OP | IL-6 | 6 |
| Bockowski et al. [33] | 2009 | Poland | M & T | II | IP | IL-1α, TNF-α | 8 |
| Koçer et al. [34] | 2010 | Turkey | T | II | OP | IL-8 | 8 |
| Bockowski et al. [35] | 2010 | Poland | M & T | II | IP | IL-4, IL-10, IL-13 | 5 |
| Uzar et al. [36] | 2011 | Turkey | M | II | IP | IL-1β, IL-2, IL-6, IL-10, TNF-α | 7 |
| Güzel et al. [37] | 2013 | Turkey | M | II | OP | TGF-β | 6 |
| Della Vidova et al. [38] | 2013 | Australia | T | II | OP | IL-2, IL-5, IL-10, IL-13, I IFN-γ, TGF-β, TNF-α | 8 |
| Wang et al. [39] | 2015 | China | M | II | OP | IL-6 | 8 |
| Lee et al. [40] | 2015 | Taiwan | M | III | OP | IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10 | 8 |
| Duarte et al. [41] | 2015 | Brazil | M | II | OP | IL-8 | 8 |
| Domingues et al. [42] | 2015 | Brazil | T | II | OP | IL-8 | 6 |
| Deitos et al. [43] | 2015 | Brazil | T | II | OP | IL-8, IL-12, TNF-α | 5 |
| Aydin et al. [44] | 2015 | Turkey | M | III | NR | IL-4, IL-5, IL-6, IL-10, IFN-γ, TNF-α | 7 |
| Yucel et al. [45] | 2016 | Turkey | M | II | IP | IL-1β, IL-6, TNF-α | 7 |
| Oliveira et al. [46] | 2017 | Brazil | M | II | OP | IL-6, IL-8, IL-10, TNF-α | 8 |
| Michalak et al. [47] | 2017 | Poland | M | II | OP | TNF-α | 8 |
| Martami et al. [48] | 2018 | Iran | M | III | OP | IL-6, TNF-α | 7 |
| Dominguez et al. [49] | 2018 | Spain | M | III | OP | IL-6, IL-10, TNF-α | 8 |
| Han et al. [50] | 2019 | China | M | III | OP | IL-1β, IL-2, IL-6, IL-10, TNF-α | 7 |
| Flook et al. [51] | 2019 | Spain | M | III | OP | IL-1α, IL-1β, IL-4, IL-6, IL-8, IFN-γ | 3 |
| Chaudhry et al. [52] | 2019 | Germany | M | III | NR | IL-1β, IL-6, IL-10, TNF-α | 8 |
| Togha et al. [53] | 2020 | Iran | M | III | OP | IL-6, TNF-α | 8 |
| Karaaslan et al. [54] | 2020 | Turkey | M | III | OP | IL-1β, IL-6, TNF-α | 7 |
| Dönder et al. [55] | 2021 | Turkey | M | III | OP | IL-18 | 8 |
| Cowan et al. [56] | 2021 | USA | M | III | OP | IL-6, IL-8, IL-10, IFN-γ, TNF-α | 6 |
IL-1α, IL-5, IL-12 and IL-13 were not used for meta-analysis as they were only reported in single studies for any of the comparisons of interest
Abbreviations: C Cluster headache, ICHD International Classification of Headache Disorders, IP Inpatient, M Migraine, NOS Newcastle–Ottawa score, NR Not recorded, OP Outpatient, T Tension-type headache
Migraine versus healthy controls
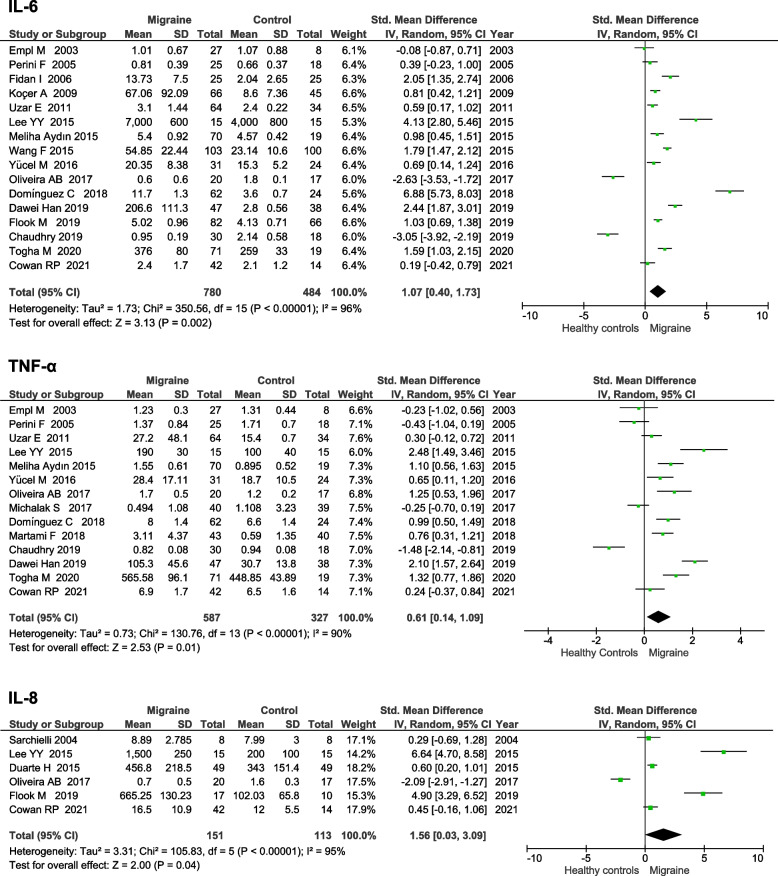
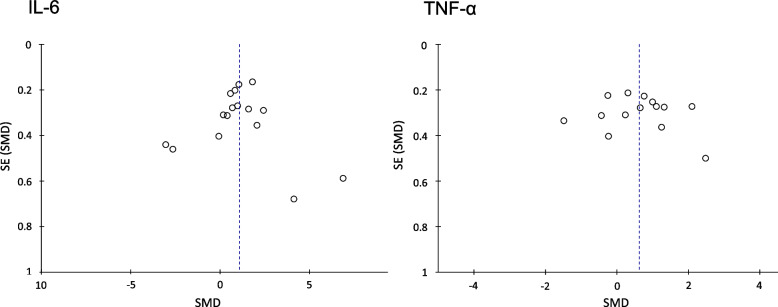
Nine cytokines were assessed in at least two studies comparing migraine to healthy controls (see Table 2). The two most commonly measured cytokines (IL-6 and TNF-α) were both higher in migraine than controls. IL-6 had a SMD of 1.07 (95% CI 0.40–1.73, p = 0.002) and TNF-α had a SMD of 0.61 (95% CI 0.14–1.09, p = 0.01). IL-8 was also higher in migraine than healthy controls (SMD 1.56 95% CI 0.03–3.09, p = 0.04). Forest plots for IL-6, TNF-α, and IL-8 are displayed in Fig. 2. Funnel plots for IL-6 and TNF-α did not show evidence of publication bias (See Fig. 3), funnel plot for IL-8 was not generated as there were fewer than ten studies included. There were no significant differences in the other cytokines measured (see Table 2). Heterogeneity levels were high for all cytokines in the comparison of migraine and healthy controls.
Table 2.
Differences in circulating cytokines levels between patients with migraine and healthy controls
| Cytokine | N of studies | Total participants | Std. Mean Difference (95% CI) Effect Estimate | P Value | I2 |
|---|---|---|---|---|---|
| IL-1β | 7 | 502 | 0.50 (-0.54, 1.54) | 0.34 | 96% |
| IL-2 | 6 | 348 | 0.58 (-0.37, 1.54) | 0.23 | 92% |
| IL-4 | 5 | 346 | 0.88 (-1.65, 3.41) | 0.49 | 98% |
| IL-6 | 16 | 264 | 1.07 (0.40, 1.73) | 0.002 | 96% |
| IL-8 | 6 | 163 | 1.56 (0.03, 3.09) | 0.04 | 95% |
| IL-10 | 12 | 706 | 0.10 (-0.74, 0.94) | 0.41 | 95% |
| TGF-β | 2 | 219 | 2.05 (-0.29, 4.40) | 0.09 | 98% |
| TNF-α | 14 | 914 | 0.61 (0.14, 1.09) | 0.01 | 90% |
| IFN-γ | 6 | 301 | 1.23 (-0.03, 2.49) | 0.06 | 93% |
Abbreviations: CI Confidence interval, I2 Measure of heterogeneity, IL-1β Interleukin-1β, IL-, Interleukin-2, IL-6 Interleukin-6, IL-4 Interleukin-4, IL-8 Interleukin-8, IL-10 Interleukin-10, TGF-β Transforming growth factor β, TNF-α Tumor necrosis factor alpha, IFN-γ Interferon gamma
Fig. 2.
Forest plots of IL-6, TNF-α, and IL-8 levels in migraine compared to healthy controls. Abbreviations: IL, interleukin; IV, inverse variance; I2, measure of heterogeneity; SD, standard deviation; TNF-α, tumour necrosis factor alpha
Fig. 3.
Funnel plots for studies of IL-6 and TNFα in migraine compared to healthy controls. Abbreviations: IL, interleukin; TNF-α, tumour necrosis factor alpha; SD, standard deviation; SEM, standard error of the mean
Migraine ictal phase versus interictal phase
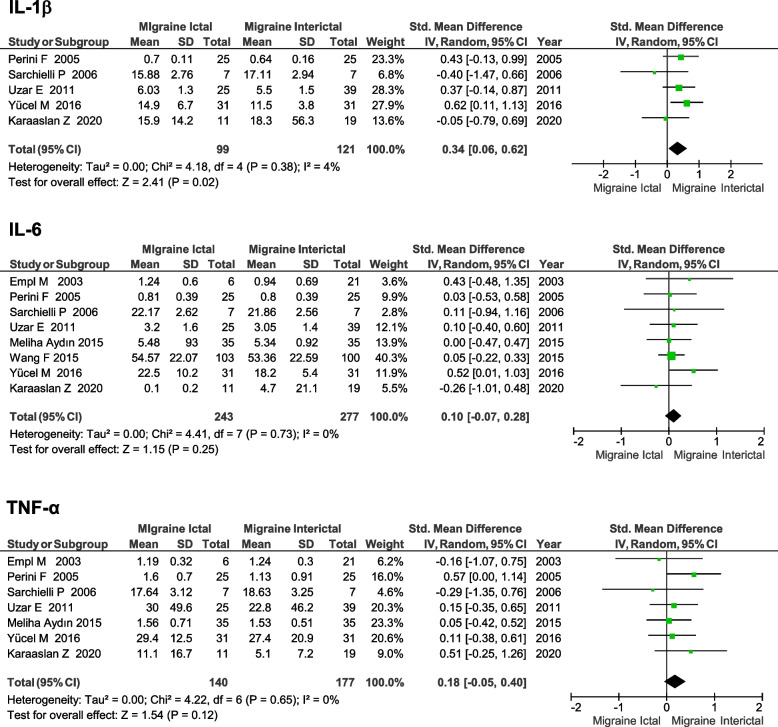
Six cytokines were assessed in at least two studies comparing the ictal phase of migraine to the interictal stage (see Table 3). IL-1β was higher in the ictal stage (SMD 0.34, 95% CI 0.06–0.62, p = 0.02), based on five studies with a low degree of heterogeneity (I2 = 4%) (see Fig. 4). There were no significant differences in the other cytokines measured (see Table 3).
Table 3.
Differences in circulating cytokines levels in the ictal stage of migraine compared to the inter-ictal stage
| Cytokine | N of studies | Total participants | Std. Mean Difference (95% CI) Effect Estimate | P Value | I2 |
|---|---|---|---|---|---|
| IL-1β | 5 | 220 | 0.34 (0.06, 0.62) | 0.02 | 4% |
| IL-2 | 2 | 114 | -0.09 (-0.89, 0.70) | 0.82 | 77% |
| IL-4 | 4 | 174 | -0.24 (-0.90, 0.42) | 0.48 | 76% |
| IL-6 | 8 | 520 | 0.10 (-0.07, 0.28) | 0.25 | 0% |
| IL-10 | 3 | 184 | 0.23 (-0.23, 0.68) | 0.33 | 57% |
| TNF-α | 7 | 317 | 0.18 (-0.05, 0.40) | 0.12 | 0% |
Abbreviations: CI Confidence interval, I2 Measure of heterogeneity, IL-2 Interleukin-2, IL-8 Interleukin-8, TGF-β Transforming growth factor β, TNF-α tumor necrosis factor alpha
Fig. 4.
Forest plot of IL-1β, IL-6 and TNF-α levels in patients with migraine in the ictal stage compared to interictally. Abbreviations: IL, interleukin; IV, inverse variance; I2, measure of heterogeneity; SD, standard deviation
Tension-type headache
Four cytokines were assessed in at least two studies comparing tension-type headache and healthy controls (see Table 4). There were significantly higher levels of both Transforming growth factor (TGF)-β (SMD 0.52, 95% CI 0.18–0.86, p = 0.003) and TNF-α (SMD 0.64, 95% CI 0.33, 0.96, p = 0.0001) in patients with tension-type headache. Both were only in two studies, but with similar results and low heterogeneity (I2 = 0%). There was no significant difference in IL-2 or IL-8 (see Table 4).
Table 4.
Differences in circulating cytokines levels between patients with tension-type headache and healthy controls
| Cytokine | N of studies | Total participants | Std. Mean Difference (95% CI) Effect Estimate | P Value | I2 |
|---|---|---|---|---|---|
| IL-2 | 2 | 187 | -5.48 (-14.13, 3.17) | 0.21 | 99% |
| IL-8 | 3 | 242 | 1.08 (-1.02, 3.18) | 0.31 | 98% |
| TGF-β | 2 | 168 | 0.52 (0.18, 0.86) | 0.003 | 0% |
| TNF-α | 2 | 165 | 0.64 (0.33, 0.96) | 0.0001 | 0% |
Abbreviations: CI Confidence interval, I2 Measure of heterogeneity, IL-2 Interleukin-2, IL-8 Interleukin-8, TGF-β Transforming growth factor β, TNF-α Tumor necrosis factor alpha
Cluster headache
Only a single cytokine (IL-1β) was compared between cluster headache and healthy controls in at least two studies, which was not significantly different (SMD 3.36, 95% CI -1.96–8.68, p = 0.22).
New daily persistent headache
No studies were identified which compared cytokine levels with healthy controls or provided raw data on blood cytokine levels in NDPH.
Discussion
We have identified higher levels of IL-6, IL-8 and TNF-α in patients with migraine compared to healthy controls, higher levels of TGF-β and TNF-α in tension-type headache compared to healthy controls, and higher levels of IL-1β during attacks of migraine compared to the interictal period. This corroborates the findings of previous narrative reviews in this area that pro-inflammatory cytokines are typically raised in migraine suggesting the presence of neuroinflammation [57–60].
A meta-analyses of cytokine levels in migraine has recently been published but included far fewer studies than the current study [61]. It found that IL-1β, IL-6, and TNF-α were all higher in patients with migraine than controls. The discrepancy between the results for IL-1β in that study and the current study is likely because it included only two studies measuring IL-1β, both of which had positive results, whereas we have included seven studies. All studies from this meta-analysis were also included in the current study.
Another systematic review has compiled the results of studies comparing cytokine levels in migraine between the ictal and interictal periods [62]. The authors did not conduct a meta-analysis, but they did observe a lack of a consistent relationship between cytokine levels during the ictal and interictal states. They did find a trend for the pro-inflammatory cytokines TNF-α and IL-6 to be higher, and the anti-inflammatory cytokine IL-10 to be decreased, in the interictal period in migraine compared to healthy controls. A study in experimentally induced migraine found that the anti-inflammatory cytokine IL-4 was downregulated during attacks, along with intercellular adhesion molecule 1, suggesting that these proteins are involved in the pathway of nitric oxide stimulated (and potentially spontaneous) migraine attacks [63, 64].
To the best of our knowledge, a systematic review or meta-analysis has not previously been used to assess cytokines in tension-type headache, cluster headache, or new daily persistent headache. Our finding of increased TNF-α in tension-type headache, similarly to migraine, may suggest either an overlap in pathophysiology between migraine and tension-type headache, or it may be a non-specific finding secondary to the chronic daily headache disorder. TGF-β, which we also found to be raised in tension-type headache is usually considered an anti-inflammatory cytokine and was the only anti-inflammatory cytokine we found to be raised in any primary headache disorder. This result should be interpreted with caution as the analysis only included two studies, however it is possible that TGF-β is elevated as a response to pain, or as a compensatory response to the elevation of one or more of the proinflammatory cytokines. Cytokines have only been compared to healthy controls in a few small studies of cluster headache. Neuroimmunological mechanisms of cluster headache have been previously proposed but require further supporting evidence [65]. Cytokines have not been compared to healthy controls in any studies of NDPH. NDPH is a primary headache disorder which often has a post-infectious onset and since its first description has been hypothesised to have an immune basis [14]. Studies are required to identify whether serum cytokine levels are altered in NDPH (especially those with a post-infectious onset) in comparison to controls and patients with chronic migraine.
Migraine is not thought to be a classical inflammatory disease, and classical clinical symptoms of inflammation or blood or cerebrospinal fluid inflammatory markers are not found. However, the trend for pro-inflammatory cytokines to be higher in patients with migraine suggests that neuroinflammation mediated by cytokines could be involved in its pathophysiology. There is human and animal evidence that the pro-inflammatory cytokines IL-1β, IL-6, and TNF-α are involved in both the initiation and persistence of pain by their direct effects on nociceptive sensory neurons, and central sensitisation [5]. The presence of neuroinflammation in migraine is supported by a neuroimaging study using PET/MRI imaging with [11C]PBR28 ligand (a marker of glial activation) in patients with migraine and showed increased tracer uptake in the thalamus, primary/secondary somatosensory cortices, and insular cortices compared to controls [66]. A second study using similar methodology found increased tracer uptake in the meninges and occipital parameningeal tissues in patients’ migraine with visual aura, compared to both healthy controls and those with lower back pain [67]. The authors hypothesized that meningeal inflammation may be related to cortical spreading depression which initiates migraine with aura.
A pathway linking cortical spreading depression with trigeminovascular system activation has been identified via the neuronal channel Panx1, the activation of which stimulates the production of IL-1β [58, 68]. This corresponds with our finding that IL-1β was the cytokine which was consistently raised during the ictal period of migraine. Calcitonin gene-related peptide (CGRP) is present in both peripheral trigeminal neurons and central neurons, is released upon activation of the trigeminovascular system, and CGRP blocking drugs are effective in the treatment of migraine. A COX-2 dependent pathway has been identified whereby IL-1β can induce CGRP release in trigeminal ganglia neurons, which can be blocked by indomethacin [69]. CGRP is thought to induce sterile “neurogenic inflammation” in migraine, which could further induce neuroinflammation via production of inflammatory cytokines [13, 70]. Pre-clinical studies have shown that CGRP triggers the release of cytokines from T cells [71, 72]. In patients with migraine CGRP levels have been shown to highly correlate (r = 0.94) with IL-6 levels [50].
It is important to recognise that none of the studies which have measured cytokines in primary headache disorders have been longitudinal, and they did not recruit patients prior to the onset of the headache disorder. Therefore, it is possible that the higher levels of cytokines found may be secondary to chronic pain, rather than part of the biology of the headache disorder itself. There is a large literature on the possible role of cytokines in pain disorders such as fibromyalgia [73], and psychiatric disorders, particularly depression [74], where cytokine profiles appear similar to what has been found in the headache literature, suggesting they could be a non-specific biomarkers of chronic pain or chronic stress. Alternatively, they could help explain the known association of primary headache disorders with depression and other chronic pain conditions such as fibromyalgia [75, 76].
Generally, studies of cytokines in primary headache disorder have matched patient groups by age and sex, but they have not been matched by headache frequency, severity, duration, or disability levels; or used these factors as covariates when the results are analysed. A few studies have compared cytokine levels between patients with chronic (headache on at least 15 days per month) and episodic (headache on fewer than 15 days per month) headache disorders. These have found higher IL-6 levels in chronic than episodic tension-type headache [34], higher TNF-α in chronic than episodic migraine [48, 53], and higher IL-6 and CGRP in chronic than episodic migraine [53]. These studies suggest a correlation between headache frequency and higher proinflammatory cytokine levels.
A limitation of all studies measuring peripheral cytokine levels is that the activity of cytokines is predominantly paracrine (local) rather than endocrine, therefore it is possible that measuring peripherally circulating cytokines may not be reflective of their likely site of action in headache disorders either in the brain or trigeminal afferents. For this reason, one study has measured cytokine levels in jugular venous blood during migraine attacks. This study did find similar results to those studies which have investigated peripheral cytokines—an increase in proinflammatory cytokines including IL-6 and TNF-α during the attack compared to baseline [30]. Cytokine levels have also been measured in the cerebrospinal fluid (CSF) of patients with primary headache disorders, but in too few studies to include in the current systematic review and meta-analysis. A small study of patients with NDPH and chronic migraine found that TNF-α levels in the CSF were above the normal range in the majority of patients with both NDPH and chronic migraine, but were in the normal range in most patients in the serum [77]. A CSF study in patients with migraine and tension-type headache found that IL-1ra, Monocyte Chemoattractant Protein-1 (MCP-1), and TGF-β1 were higher in the CSF of patients with both episodic tension-type headache and migraine without aura, compared to controls [78].
A limitation of all cytokine studies is that there are a multitude of factors which can affect cytokine levels, including time of day the blood is taken, site blood is taken from, speed of analysis, presence of comorbidities, medications being taken, and nutritional status of the patient. This means that cytokines are unlikely to be helpful as diagnostic biomarkers, however they may still prove helpful in determining prognosis, or influencing response to treatment. Cytokine antagonists and monoclonal antibodies against cytokines or their receptors are in clinical use for autoimmune disorders. To the best of our knowledge there are no case reports or anecdotal evidence of monoclonal antibodies targeting TNF-a, IL-6, or IL-8 improving primary headache disorders, but they have not been trialled specifically for this purpose.
Conclusions
The proinflammatory cytokines IL-6, TNF-α, and IL-8 are higher in patients with migraine than healthy controls, and IL-1β levels are raised during migraine attacks. This suggests that they may be involved in the pathophysiology of migraine. Prospective studies are required to determine causality, and to determine whether cytokines are useful biomarkers in differentiating different subtypes of headache disorders, determining prognosis, or influencing treatment response.
Abbreviations
- CSF
Cerebrospinal fluid
- ICHD-3
International Classification of Headache Disorders, 3rd Edition
- IHS
International Headache Society
- IFN-γ
Interferon gamma
- IL
Interleukin
- IQR
Interquartile range
- NDPH
New daily persistent headache
- SD
Standard deviation
- SMD
Standardised mean difference
- TGF-β
Transforming growth factor beta
- TNF-α
Tumour necrosis factor alpha
Authors’ contributions
AM: design of study, acquisition, analysis and interpretation of data, drafting and revising the manuscript. SC: conception and design of study, analysis and interpretation of data, drafting and revising the manuscript. JR: interpretation of data, revising the manuscript. EH: interpretation of data, revising the manuscript. MM: conception and design of study, interpretation of data, revising the manuscript. All authors approved the final version of the manuscript.
Funding
No funding was received for this project.
Availability of data and materials
The extracted data used for the analyses is available upon reasonable request to the corresponding author.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
AKM has no conflicts of interest.
SC has received a research fellowship sponsored by Abbott.
JR has served on advisory boards for Viatris, Lilly and Pfizer and has received compensation for educational presentations supported by Allergan, Novartis and Viatris.
EH has served on advisory boards for Sanofi-Genzyme, Novartis, Teva, Eli Lilly, Allergan and Lundbeck, been involved in clinical trials sponsored by Novartis,Teva, Xalud, Cerecin, AEON Biopharma and Abbvie and has received payment for educational presentations from Allergan, Teva, Eli Lilly and Novartis.
MM is chair of the medical advisory board of the CSF Leak Association; has served on advisory boards for Allergan, Autonomic Technologies Inc, Eli Lilly, Novartis, Pfizer, Salvia and TEVA; has received payment for educational presentations from Allergan, electroCore, Eli Lilly, Novartis and TEVA; has received grants from Abbott, Medtronic and electroCore; and has a patent on system and method for diagnosing and treating headaches (WO2018051103A1, issued).
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abdu Kisekka Musubire and Sanjay Cheema contributed equally as first authors.
References
- 1.Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol Rev. 2017;97(2):553–622. doi: 10.1152/physrev.00034.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei DY, Goadsby PJ. Cluster headache pathophysiology - insights from current and emerging treatments. Nat Rev Neurol. 2021;17(5):308–324. doi: 10.1038/s41582-021-00477-w. [DOI] [PubMed] [Google Scholar]
- 3.Ashina S, Bendtsen L, Ashina M. Pathophysiology of tension-type headache. Curr Pain Headache Rep. 2005;9(6):415–422. doi: 10.1007/s11916-005-0021-8. [DOI] [PubMed] [Google Scholar]
- 4.Peng KP, Rozen TD. Update in the understanding of new daily persistent headache. Cephalalgia. 2023;43(2):3331024221146314. doi: 10.1177/03331024221146314. [DOI] [PubMed] [Google Scholar]
- 5.Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. 2007;45(2):27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu C, Chu D, Kalantar-Zadeh K, George J, Young HA, Liu G. Cytokines: from clinical significance to quantification. Adv Sci (Weinh) 2021;8(15):e2004433. doi: 10.1002/advs.202004433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5(11):718–725. doi: 10.1016/S1473-3099(05)70270-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith RS. The cytokine theory of headache. Med Hypotheses. 1992;39(2):168–174. doi: 10.1016/0306-9877(92)90181-b. [DOI] [PubMed] [Google Scholar]
- 9.Caronna E, Pozo-Rosich P. Headache as a symptom of COVID-19: narrative review of 1-year research. Curr Pain Headache Rep. 2021;25(11):73. doi: 10.1007/s11916-021-00987-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapman PB, Lester TJ, Casper ES, Gabrilove JL, Wong GY, Kempin SJ, et al. Clinical pharmacology of recombinant human tumor necrosis factor in patients with advanced cancer. J Clin Oncol. 1987;5(12):1942–1951. doi: 10.1200/JCO.1987.5.12.1942. [DOI] [PubMed] [Google Scholar]
- 11.Elmazny A, Hamdy SM, Abdel-Naseer M, Shalaby NM, Shehata HS, Kishk NA, et al. Interferon-beta-induced headache in patients with multiple sclerosis: frequency and characterization. J Pain Res. 2020;13:537–545. doi: 10.2147/JPR.S230680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pardutz A, Schoenen J. NSAIDs in the acute treatment of migraine: a review of clinical and experimental data. Pharmaceuticals (Basel) 2010;3(6):1966–1987. doi: 10.3390/ph3061966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edvinsson L, Haanes KA, Warfvinge K. Does inflammation have a role in migraine? Nat Rev Neurol. 2019;15(8):483–490. doi: 10.1038/s41582-019-0216-y. [DOI] [PubMed] [Google Scholar]
- 14.Vanast WJD-M, F.; Tyrrell, D. L. J. Hypothesis: chronic benign daily headache is an immune disorder with a viral trigger. Headache. 1987;27(3):138–42. [DOI] [PubMed]
- 15.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wells GA, Shea B, D. OC, J. P, Welch V, Losos M, et al.: The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (2000). Accessed 1 Feb 2023.
- 17.Headache Classification Subcommittee of the International Headache Society The international classification of headache disorders: 2nd edition. Cephalalgia. 2004;24:9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 18.Headache Classification Committee of the International Headache Society The international classification of headache disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211. doi: 10.1177/0333102417738202. [DOI] [PubMed] [Google Scholar]
- 19.Covelli V, Massari F, Fallacara C, Munno I, Pellegrino NM, Jirillo E, et al. Increased spontaneous release of tumor necrosis factor-alpha/cachectin in headache patients. A possible correlation with plasma endotoxin and hypothalamic-pituitary-adrenal axis. Int J Neurosci. 1991;61(1–2):53–60. doi: 10.3109/00207459108986270. [DOI] [PubMed] [Google Scholar]
- 20.Shimomura T, Araga S, Esumi E, Takahashi K. Decreased serum interleukin-2 level in patients with chronic headache. Headache. 1991;31(5):310–313. doi: 10.1111/j.1526-4610.1991.hed3105310.x. [DOI] [PubMed] [Google Scholar]
- 21.Martelletti P, Granata M, Giacovazzo M. Serum interleukin-1 beta is increased in cluster headache. Cephalalgia. 1993;13(5):343–5. doi: 10.1046/j.1468-2982.1993.1305343.x. [DOI] [PubMed] [Google Scholar]
- 22.Martelletti P, Stirparo G, Rinaldi C, Frati L, Giacovazzo M. Disruption of the immunopeptidergic network in dietary migraine. Headache. 1993;33(10):524–527. doi: 10.1111/j.1526-4610.1993.hed3310524.x. [DOI] [PubMed] [Google Scholar]
- 23.Munno I, Centonze V, Marinaro M, Bassi A, Lacedra G, Causarano V, et al. Cytokines and migraine: increase of IL-5 and IL-4 plasma levels. Headache. 1998;38(6):465–467. doi: 10.1046/j.1526-4610.1998.3806465.x. [DOI] [PubMed] [Google Scholar]
- 24.Martelletti P, Zicari A, Realacci M, Fiore G, De Filippis S, Stirparo G, et al. Expression of NOS-2, COX-2 and Th1/Th2 cytokines in migraine. J Headache Pain. 2001;2(SUPPL. 1):S51–S56. doi: 10.1007/s101940170010. [DOI] [Google Scholar]
- 25.Empl M, Forderreuther S, Schwarz M, Muller N, Straube A. Soluble interleukin-2 receptors increase during the active periods in cluster headache. Headache. 2003;43(1):63–68. doi: 10.1046/j.1526-4610.2003.03011.x. [DOI] [PubMed] [Google Scholar]
- 26.Empl M, Sostak P, Riedel M, Schwarz M, Muller N, Forderreuther S, et al. Decreased sTNF-RI in migraine patients? Cephalalgia. 2003;23(1):55–58. doi: 10.1046/j.1468-2982.2003.00453.x. [DOI] [PubMed] [Google Scholar]
- 27.Sarchielli P, Alberti A, Vaianella L, Pierguidi L, Floridi A, Mazzotta G, et al. Chemokine levels in the jugular venous blood of migraine without aura patients during attacks. Headache. 2004;44(10):961–968. doi: 10.1111/j.1526-4610.2004.04189.x. [DOI] [PubMed] [Google Scholar]
- 28.Perini F, D'Andrea G, Galloni E, Pignatelli F, Billo G, Alba S, et al. Plasma cytokine levels in migraineurs and controls. Headache. 2005;45(7):926–931. doi: 10.1111/j.1526-4610.2005.05135.x. [DOI] [PubMed] [Google Scholar]
- 29.Ishizaki K, Takeshima T, Fukuhara Y, Araki H, Nakaso K, Kusumi M, et al. Increased plasma transforming growth factor-beta1 in migraine. Headache. 2005;45(9):1224–1228. doi: 10.1111/j.1526-4610.2005.00246.x. [DOI] [PubMed] [Google Scholar]
- 30.Sarchielli P, Alberti A, Baldi A, Coppola F, Rossi C, Pierguidi L, et al. Proinflammatory cytokines, adhesion molecules, and lymphocyte integrin expression in the internal jugular blood of migraine patients without aura assessed ictally. Headache. 2006;46(2):200–207. doi: 10.1111/j.1526-4610.2006.00337.x. [DOI] [PubMed] [Google Scholar]
- 31.Fidan I, Yuksel S, Ymir T, Irkec C, Aksakal FN. The importance of cytokines, chemokines and nitric oxide in pathophysiology of migraine. J Neuroimmunol. 2006;171(1–2):184–188. doi: 10.1016/j.jneuroim.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Kocer A, Memisogullari R, Domac FM, Ilhan A, Kocer E, Okuyucu S, et al. IL-6 levels in migraine patients receiving topiramate. Pain Pract. 2009;9(5):375–379. doi: 10.1111/j.1533-2500.2009.00301.x. [DOI] [PubMed] [Google Scholar]
- 33.Bockowski L, Sobaniec W, Zelazowska-Rutkowska B. Proinflammatory plasma cytokines in children with migraine. Pediatr Neurol. 2009;41(1):17–21. doi: 10.1016/j.pediatrneurol.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Kocer A, Kocer E, Memisogullari R, Domac FM, Yuksel H. Interleukin-6 levels in tension headache patients. Clin J Pain. 2010;26(8):690–693. doi: 10.1097/AJP.0b013e3181e8d9b6. [DOI] [PubMed] [Google Scholar]
- 35.Bockowski L, Smigielska-Kuzia J, Sobaniec W, Zelazowska-Rutkowska B, Kulak W, Sendrowski K. Anti-inflammatory plasma cytokines in children and adolescents with migraine headaches. Pharmacol Rep. 2010;62(2):287–291. doi: 10.1016/S1734-1140(10)70268-1. [DOI] [PubMed] [Google Scholar]
- 36.Uzar E, Evliyaoglu O, Yucel Y, UgurCevik M, Acar A, Guzel I, et al. Serum cytokine and pro-brain natriuretic peptide (BNP) levels in patients with migraine. Eur Rev Med Pharmacol Sci. 2011;15(10):1111–1116. [PubMed] [Google Scholar]
- 37.Güzel I, Taşdemir N, Celik Y. Evaluation of serum transforming growth factor β1 and C-reactive protein levels in migraine patients. Neurol Neurochir Pol. 2013;47(4):357–362. doi: 10.5114/ninp.2013.36760. [DOI] [PubMed] [Google Scholar]
- 38.Della Vedova C, Cathcart S, Dohnalek A, Lee V, Hutchinson MR, Immink MA, et al. Peripheral interleukin-1beta levels are elevated in chronic tension-type headache patients. Pain Res Manage. 2013;18(6):301–306. doi: 10.1155/2013/796161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang F, He Q, Ren Z, Li F, Chen W, Lin X, et al. Association of serum levels of intercellular adhesion molecule-1 and interleukin-6 with migraine. Neurol Sci. 2015;36(4):535–540. doi: 10.1007/s10072-014-2010-3. [DOI] [PubMed] [Google Scholar]
- 40.Lee YY, Yang YP, Huang PI, Li WC, Huang MC, Kao CL, et al. Exercise suppresses COX-2 pro-inflammatory pathway in vestibular migraine. Brain Res Bull. 2015;116:98–105. doi: 10.1016/j.brainresbull.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 41.Duarte H, Teixeira AL, Rocha NP, Domingues RB. Increased interictal serum levels of CXCL8/IL-8 and CCL3/MIP-1α in migraine. Neurol Sci. 2015;36(2):203–208. doi: 10.1007/s10072-014-1931-1. [DOI] [PubMed] [Google Scholar]
- 42.Domingues RB, Duarte H, Rocha NP, Teixeira AL. Increased serum levels of interleukin-8 in patients with tension-type headache. Cephalalgia. 2015;35(9):801–806. doi: 10.1177/0333102414559734. [DOI] [PubMed] [Google Scholar]
- 43.Deitos A, Dussán-Sarria JA, Souza A, Medeiros L, TarragôMda G, Sehn F, et al. Clinical value of serum neuroplasticity mediators in identifying the central sensitivity syndrome in patients with chronic pain with and without structural pathology. Clin J Pain. 2015;31(11):959–967. doi: 10.1097/ajp.0000000000000194. [DOI] [PubMed] [Google Scholar]
- 44.Aydin M, Demir CF, Arikanoglu A, Bulut S, Ilhan N. Plasma cytokine levels in migraineurs during and outside of attacks. Eur J Gen Med. 2015;12(4):307–12. doi: 10.15197/ejgm.01255. [DOI] [Google Scholar]
- 45.Yucel M, Kotan D, Ciftci GG, Ciftci IH, Cikriklar HI. Serum levels of endocan, claudin-5 and cytokines in migraine. Eur Rev Med Pharmacol Sci. 2016;20(5):930–936. [PubMed] [Google Scholar]
- 46.Oliveira AB, Bachi ALL, Ribeiro RT, Mello MT, Tufik S, Peres MFP. Unbalanced plasma TNF-alpha and IL-12/IL-10 profile in women with migraine is associated with psychological and physiological outcomes. J Neuroimmunol. 2017;313:138–144. doi: 10.1016/j.jneuroim.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 47.Michalak S, Kalinowska-Lyszczarz A, Wegrzyn D, Niezgoda A, Losy J, Osztynowicz K, et al. Increased Serum CD14 Level Is Associated with Depletion of TNF-α in Monocytes in Migraine Patients during Interictal Period. Int J Mol Sci. 2017;18(2). 10.3390/ijms18020398. [DOI] [PMC free article] [PubMed]
- 48.Martami F, RazeghiJahromi S, Togha M, Ghorbani Z, Seifishahpar M, Saidpour A. The serum level of inflammatory markers in chronic and episodic migraine: a case-control study. Neurol Sci. 2018;39(10):1741–1749. doi: 10.1007/s10072-018-3493-0. [DOI] [PubMed] [Google Scholar]
- 49.Domínguez C, Vieites-Prado A, Pérez-Mato M, Sobrino T, Rodríguez-Osorio X, López A, et al. CGRP and PTX3 as predictors of efficacy of Onabotulinumtoxin type A in chronic migraine: an observational study. Headache. 2018;58(1):78–87. doi: 10.1111/head.13211. [DOI] [PubMed] [Google Scholar]
- 50.Han D. Association of Serum Levels of Calcitonin Gene-related Peptide and Cytokines during Migraine Attacks. Ann Indian Acad Neurol. 2019;22(3):277–281. doi: 10.4103/aian.AIAN_371_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flook M, Frejo L, Gallego-Martinez A, Martin-Sanz E, Rossi-Izquierdo M, Amor-Dorado JC, et al. Differential proinflammatory signature in vestibular migraine and meniere disease. Front Immunol. 2019;10(JUN) (no pagination)(1229). 10.3389/fimmu.2019.01229. [DOI] [PMC free article] [PubMed]
- 52.Chaudhry SR, Lendvai IS, Muhammad S, Westhofen P, Kruppenbacher J, Scheef L, et al. Inter-ictal assay of peripheral circulating inflammatory mediators in migraine patients under adjunctive cervical non-invasive vagus nerve stimulation (nVNS): a proof-of-concept study. Brain Stimul. 2019;12(3):643–651. doi: 10.1016/j.brs.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 53.Togha M, RazeghiJahromi S, Ghorbani Z, Ghaemi A, Rafiee P. Evaluation of inflammatory state in Migraineurs: a case-control study. Iran J Allergy Asthma Immunol. 2020;19(S1):83–90. doi: 10.18502/ijaai.v19i(s1.r1).2864. [DOI] [PubMed] [Google Scholar]
- 54.Karaaslan Z, Ozcelik P, Ulukan C, Ulusoy C, Orhan KS, Orhan EK, et al. Plasma levels of inflammatory mediators in vestibular migraine. Int J Neurosci. 2020;130(4):330–335. doi: 10.1080/00207454.2019.1681994. [DOI] [PubMed] [Google Scholar]
- 55.Dönder A, Cafer V, Yilmaz A, Aslanhan H, Arikanoğlu A. Investigation of serum vaspin, visfatin, chemerin and IL-18 levels in migraine patients. Arq Neuropsiquiatr. 2021;79(9):789–794. doi: 10.1590/0004-282x-anp-2020-0425. [DOI] [PubMed] [Google Scholar]
- 56.Cowan RP, Gross NB, Sweeney MD, Sagare AP, Montagne A, Arakaki X, et al. Evidence that blood-CSF barrier transport, but not inflammatory biomarkers, change in migraine, while CSF sVCAM1 associates with migraine frequency and CSF fibrinogen. Headache. 2021;61(3):536–545. doi: 10.1111/head.14088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Biscetti L, De Vanna G, Cresta E, Bellotti A, Corbelli I, Letizia Cupini M, et al. Immunological findings in patients with migraine and other primary headaches: a narrative review. Clin Exp Immunol. 2022;207(1):11–26. doi: 10.1093/cei/uxab025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kursun O, Yemisci M, van den Maagdenberg A, Karatas H. Migraine and neuroinflammation: the inflammasome perspective. J Headache Pain. 2021;22(1):55. doi: 10.1186/s10194-021-01271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spekker E, Tanaka M, Szabo A, Vecsei L. Neurogenic Inflammation: The Participant in Migraine and Recent Advancements in Translational Research. Biomedicines. 2021;10(1). 10.3390/biomedicines10010076. [DOI] [PMC free article] [PubMed]
- 60.Kemper RH, Meijler WJ, Korf J, Ter Horst GJ. Migraine and function of the immune system: a meta-analysis of clinical literature published between 1966 and 1999. Cephalalgia. 2001;21(5):549–557. doi: 10.1046/j.1468-2982.2001.00196.x. [DOI] [PubMed] [Google Scholar]
- 61.Geng C, Yang Z, Xu P, Zhang H. Aberrations in peripheral inflammatory cytokine levels in migraine: a systematic review and meta-analysis. J Clin Neurosci. 2022;98:213–218. doi: 10.1016/j.jocn.2022.02.026. [DOI] [PubMed] [Google Scholar]
- 62.Thuraiaiyah J, Erritzoe-Jervild M, Al-Khazali HM, Schytz HW, Younis S. The role of cytokines in migraine: a systematic review. Cephalalgia. 2022;42(14):1565–1588. doi: 10.1177/03331024221118924. [DOI] [PubMed] [Google Scholar]
- 63.Martelletti P, Stirparo G, Morrone S, Rinaldi C, Giacovazzo M. Inhibition of intercellular adhesion molecule-1 (ICAM-1), soluble ICAM-1 and interleukin-4 by nitric oxide expression in migraine patients. J Mol Med (Berl) 1997;75(6):448–453. doi: 10.1007/s001090050130. [DOI] [PubMed] [Google Scholar]
- 64.Martelletti P, Morrone S. The role of adhesion molecules in migraine: a debate. Cephalalgia. 2000;20(2):136. doi: 10.1046/j.1468-2982.2000.00027.x. [DOI] [PubMed] [Google Scholar]
- 65.Martelletti P, Giacovazzo M. Putative neuroimmunological mechanisms in cluster headache. An integrated hypothesis. Headache. 1996;36(5):312–315. doi: 10.1046/j.1526-4610.1996.3605312.x. [DOI] [PubMed] [Google Scholar]
- 66.Albrecht DS, Mainero C, Ichijo E, Ward N, Granziera C, Zurcher NR, et al. Imaging of neuroinflammation in migraine with aura: A [(11)C]PBR28 PET/MRI study. Neurology. 2019;92(17):e2038–e2050. doi: 10.1212/WNL.0000000000007371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hadjikhani N, Albrecht DS, Mainero C, Ichijo E, Ward N, Granziera C, et al. Extra-Axial Inflammatory Signal in Parameninges in Migraine with Visual Aura. Ann Neurol. 2020;87(6):939–949. doi: 10.1002/ana.25731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Karatas H, Erdener SE, Gursoy-Ozdemir Y, Lule S, Eren-Kocak E, Sen ZD, et al. Spreading depression triggers headache by activating neuronal Panx1 channels. Science. 2013;339(6123):1092–1095. doi: 10.1126/science.1231897. [DOI] [PubMed] [Google Scholar]
- 69.Neeb L, Hellen P, Boehnke C, Hoffmann J, Schuh-Hofer S, Dirnagl U, et al. IL-1beta stimulates COX-2 dependent PGE(2) synthesis and CGRP release in rat trigeminal ganglia cells. PLoS One. 2011;6(3):e17360. doi: 10.1371/journal.pone.0017360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ramachandran R. Neurogenic inflammation and its role in migraine. Semin Immunopathol. 2018;40(3):301–314. doi: 10.1007/s00281-018-0676-y. [DOI] [PubMed] [Google Scholar]
- 71.Levite M. Neuropeptides, by direct interaction with T cells, induce cytokine secretion and break the commitment to a distinct T helper phenotype. Proc Natl Acad Sci U S A. 1998;95(21):12544–12549. doi: 10.1073/pnas.95.21.12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cuesta MC, Quintero L, Pons H, Suarez-Roca H. Substance P and calcitonin gene-related peptide increase IL-1 beta, IL-6 and TNF alpha secretion from human peripheral blood mononuclear cells. Neurochem Int. 2002;40(4):301–306. doi: 10.1016/s0197-0186(01)00094-8. [DOI] [PubMed] [Google Scholar]
- 73.O'Mahony LF, Srivastava A, Mehta P, Ciurtin C. Is fibromyalgia associated with a unique cytokine profile? A systematic review and meta-analysis. Rheumatology (Oxford) 2021;60(6):2602–2614. doi: 10.1093/rheumatology/keab146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229. doi: 10.1016/j.neuroscience.2013.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lampl C, Thomas H, Tassorelli C, Katsarava Z, Lainez JM, Lanteri-Minet M, et al. Headache, depression and anxiety: associations in the Eurolight project. J Headache Pain. 2016;17:59. doi: 10.1186/s10194-016-0649-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Penn IW, Chuang E, Chuang TY, Lin CL, Kao CH. Bidirectional association between migraine and fibromyalgia: retrospective cohort analyses of two populations. BMJ Open. 2019;9(4):e026581. doi: 10.1136/bmjopen-2018-026581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rozen T, Swidan SZ. Elevation of CSF tumor necrosis factor alpha levels in new daily persistent headache and treatment refractory chronic migraine. Headache. 2007;47(7):1050–1055. doi: 10.1111/j.1526-4610.2006.00722.x. [DOI] [PubMed] [Google Scholar]
- 78.Bo SH, Davidsen EM, Gulbrandsen P, Dietrichs E, Bovim G, Stovner LJ, et al. Cerebrospinal fluid cytokine levels in migraine, tension-type headache and cervicogenic headache. Cephalalgia. 2009;29(3):365–372. doi: 10.1111/j.1468-2982.2008.01727.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The extracted data used for the analyses is available upon reasonable request to the corresponding author.