Abstract
Background
Endoscopic submucosal dissection (ESD) has become an established standard for endoscopic removal of large gastrointestinal (GI) lesions and early GI malignancies. However, ESD is technically challenging and requires significant health care infrastructure. As such, its adoption in Canada has been relatively slow. The practice of ESD across Canada remains unclear. Our study aimed to provide a descriptive overview of training pathways and practice trends of ESD in Canada.
Methods
Current ESD practitioners across Canada were identified and invited to participate in an anonymous cross-sectional survey.
Results
Twenty-seven ESD practitioners were identified; survey response rate was 74%. Respondents were from 15 different institutions. All practitioners underwent international ESD training of some type. Fifty per cent pursued long-term ESD training programs. Ninety-five per cent attended short-term training courses. Sixty per cent and 40% performed hands-on live human upper and lower GI ESD, respectively, before independent practice. In practice, 70% saw an increase per year in number of procedures performed from 2015 to 2019. Sixty per cent were dissatisfied with their institution’s health care infrastructure to support ESD. Thirty-five per cent perceived their institution as supportive of expanding the practice of ESD.
Conclusions
Several challenges exist to the adoption of ESD in Canada. Training pathways are variable, with no set standards. In practice, practitioners express dissatisfaction with access to necessary infrastructure and feel poorly supported in expanding the practice of ESD. As ESD is increasingly the accepted standard for the treatment of many neoplastic GI lesions, greater collaboration between practitioners and institutions is crucial to standardize training and ensure patient access.
Keywords: Endoscopic practice, Endoscopic submucosal dissection, Luminal endoscopy
BACKGROUND
Endoscopic submucosal dissection (ESD), first pioneered in Japan, has allowed for curative endoscopic en bloc resection of large superficial gastrointestinal (GI) lesions and early GI malignancies. ESD provides clear advantages for removing large or advanced GI lesions compared to other methods, with lower recurrence rates compared to endoscopic mucosal resection and lower cost, morbidity, and mortality compared to surgery (1,2). Despite this, the adoption of ESD has been limited in North America owing to various factors, including steep learning curves, limited expertise, and inadequate remuneration (3).
The indications for ESD are broadening to include dysplastic Barrett’s esophagus, superficial intramucosal esophageal adenocarcinoma, squamous cell carcinoma, early gastric cancer, and large colorectal neoplasms limited to the mucosa or superficial submucosa (3). ESD is increasingly regarded as a potential first-line option for the treatment of early malignant and large benign gastrointestinal lesions in place of surgery, with curative resection rates up to 89% (4,5). Although highly effective in expert hands, ESD is associated with significant risks of perforation and bleeding dependent on the lesion, patient, and endoscopist factors (2,6). Given differences between patient volumes, endoscopist training, and health care infrastructure available for the practice of ESD between Asian and Western countries, it is unclear whether previously reported outcomes for ESD from studies primarily conducted in Japan are consistent with its use in Canada.
In Japan, unlike in North America, there are many centers with sufficient expertise and volume to take trainees (7). Given these limitations, there remains no standardized pathway to gaining competency in North America. As such, only a handful of centers and endoscopists across Canada have enough experience to practice ESD. A prior study surveying endoscopists performing ESD in Canada gave a preliminary overview of barriers to uptake. However, their findings were limited by the survey distribution at the early stages of ESD adoption in Canada and the small number of active ESD practitioners, with only 11 respondents actively performing ESD (8). There has since been a significant increase in the number of ESD practitioners in Canada. With the growing recognition and acceptance of ESD, it is essential to evaluate its use in Canada thoroughly, particularly given our country’s unique multicultural population, differences in training opportunities, and health care model (6). Our study provides an updated comprehensive survey of endoscopists actively practicing ESD across Canada as of 2021 and an in-depth review of training pathways, growth trends, and practice patterns.
METHODS
Study Design
A cross-sectional survey of ESD training pathways, practice environment and trends was created and reviewed by the study team. The study team consisted of three therapeutic endoscopists (J.M., G.M., C.T.) actively performing ESD at the largest referral center for therapeutic endoscopy in Canada, where up to 100 ESD procedures are performed per year. The survey was divided into four general categories, evaluating (i) training history, (ii) ESD experience and practice environment, (iii) ESD technique/equipment, and (iv) health care infrastructure for ESD (Supplementary Appendix 1). The survey was created on the SurveyMonkey platform for distribution.
An up-to-date contact list of all endoscopists currently taking referrals for ESD at centers across Canada was generated by contacting known practitioners from internal networks and all tertiary Canadian gastroenterology departments for ESD practitioners at their respective sites. Identified endoscopists were contacted via e-mail for recruitment and provided a link to the survey on SurveyMonkey. Participants were made clear that the survey was anonymous and de-identified. Survey data collection was conducted from October 2020 to May 2021. Remuneration was not provided for survey completion. This study was approved by the Unity Health REB.
Analysis
Raw data were extracted from SurveyMonkey. Descriptive statistics and qualitative analysis were conducted via Microsoft Excel and SPSS v28.0 (IBM Corp).
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
RESULTS
The survey was distributed to 27 endoscopists identified as actively practicing ESD in Canada. One endoscopist indicated they were no longer performing ESD. Twenty endoscopists actively performing ESD completed the survey with a survey completion rate of 74% (n = 20/27).
Participant Demographics
Median age of respondents was 43 years (IQR 8.5). Ninety-five per cent (n = 19/20) of respondents were male. Ten respondents practiced in Ontario, five practiced in Quebec, one practiced in Manitoba, one practiced in Saskatchewan, one practiced in Alberta, and one practiced in British Columbia.
Median number of years in independent practice at the time of survey was 10.5 years (IQR 8.8). Median number of years of independent ESD practice at the time of survey was 3.5 years (IQR 2.8).
ESD Training History
Ninety per cent (n = 18/20) of respondents completed a traditional therapeutic endoscopy fellowship program (Table 1). During therapeutic endoscopy fellowship 94% (n = 17/18) received training in ERCP and/or EUS. Twenty-two per cent (n = 4/18) received training in ESD during therapeutic endoscopy fellowship. Fifty per cent (n = 10/20) underwent a formal ESD training program outside Canada. Median duration of training programs was 2 months (IQR 5.8). Ninety-five per cent of respondents (n = 19/20) attended short-term ESD training courses. Median number of courses attended was 2 (IQR 1.3). All respondents received international ESD training outside of Canada in some form. Ninety-five per cent (n = 19/20) received ESD training on animal models. Sixty-five per cent (n = 13/20) performed ESD on live human cases before independent practice; of which 54% (n = 7/13) received exposure on both upper and lower GI ESD, 38% (n = 5/13) on upper GI ESD only, and 8% (n = 1/13) on lower GI ESD only. As the primary operator in live human cases prior to independent ESD practice, 15% (n = 2/13) of respondents performed >20, 8% (n = 1/13) performed 11 to 20, 77% (n = 10/13) performed 1 to 10 cases.
Table 1.
Respondents training history
| Survey field | Participant responses |
|---|---|
| Underwent traditional advanced therapeutic endoscopy fellowship | 90% (n = 18/20) |
| Trained in ERCP + EUS | 61% (n = 11/18) |
| Trained in ERCP Only | 11% (n = 2/18) |
| Trained in EUS Only | 22% (n = 4/18) |
| Received ESD training during advanced fellowship | 22% (n = 4/18) |
| Attended short-term ESD training courses/workshops | 95% (n = 19/20) |
| Median number of attended courses/workshops | 2 (IQR 1.3) |
| Attended courses/workshops outside of Canada | 100% (18/18) |
| Received ESD training on animal models | 95% (n = 19/20) |
| Underwent long-term* ESD training program | 50% (n = 10/20) |
| Median duration of training program (Months) | 2 (IQR 5.8) |
| Received hands-on experience during program (versus observation only) | 80% (n = 8/10) |
| Performed ESD on human cases prior to independent practice | 65% (n = 13/20) |
| Both upper and lower GI ESD | 35% (n = 7/20) |
| Upper GI ESD only | 25% (n = 5/20) |
| Lower GI ESD only | 5% (n = 1/20) |
*Defined as >30 days
In general, 50% (n = 10/20) received ESD training via a combination of short-term courses and animal model training and 45% (n = 9/20) via short-term courses and a long-term formal ESD training program. One participant received training solely via a long-term formal ESD training program that lasted 14 months.
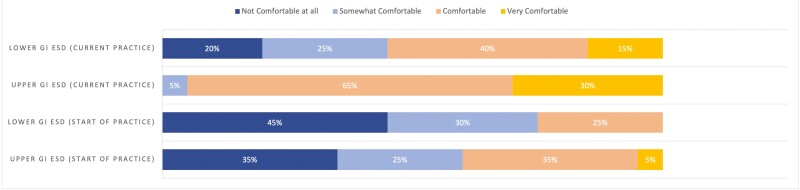
At the start of practice, 35% (n = 7/20) and 45% (n = 9/20) of respondents reported feeling ‘not comfortable at all’ with performing upper GI and lower GI ESD, respectively, independently. Only 35% (n = 7/20) and 25% (n = 5/20) reported feeling ‘comfortable’ with performing upper GI and lower GI ESD, respectively, independently at the start of practice. However, comfort level at the time of the survey was reported as ‘comfortable’ or ‘very comfortable’ by 95% (n = 19/20) of practitioners for upper GI ESD and 55% (n = 11/20) for lower GI ESD (Figure 1).
Figure 1.
Respondents comfort level with performing ESD.
Independent ESD Practice Environment
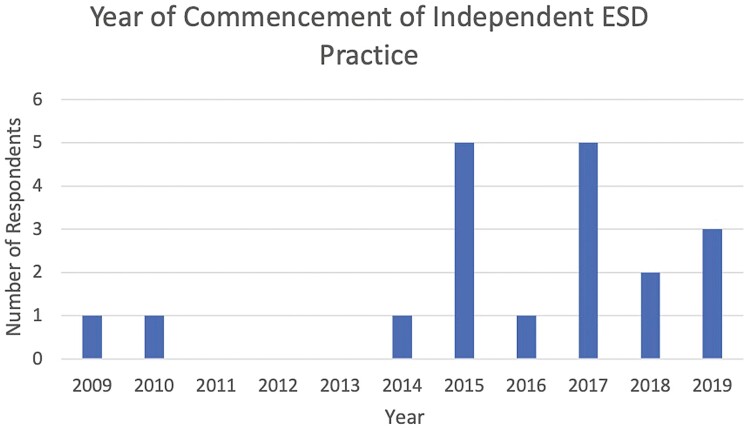
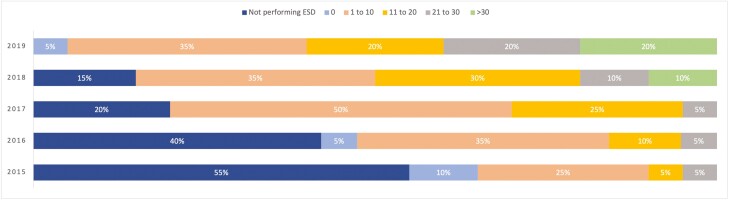
Eighty-five per cent (n = 17/20) of respondents began independent ESD practice after 2014 (Figure 2). The median number of practitioners offering ESD at each center was 1. As shown in Figure 3, the approximate number of ESD procedures performed by respondents each year starting from 2015 has sequentially increased, with only two respondents performing >10 cases per year in 2015 and twelve respondents performing >10 cases per year by 2019.
Figure 2.
Year of commencement of ESD practice.
Figure 3.
Approximate number of ESD procedures performed by respondents per year.
Fifty-eight per cent (n = 11/19) of respondents reported an approximate wait time for ESD of 1 to 3 months. The median duration of time usually booked for lower and upper GI ESD cases were 120 minutes (IQR 60) by respondents. Sixty per cent (n = 12/20) of respondents performed ESD in the endoscopy suite, and 35% (n = 7/20) performed ESD in an operating room. Seventy-five per cent (n = 15/20) of respondents performed ESD under monitored anesthesia care with propofol or general anesthesia. See Table 2 for further details.
Table 2.
ESD practice environment
| Survey field | Participant responses |
|---|---|
| Median number of ESD practitioners at center | 1 (range 1–3) |
| Approximate wait-time for ESD | |
| <2 weeks | 11% (n = 2/19) |
| 2–4 weeks | 26% (n = 5/19) |
| 1–3 months | 58% (n = 11/19) |
| 3–6 months | 5% (n = 1/19) |
| Median duration of time usually booked for ESD cases (minutes) | |
| Upper GI | 120 (IQR 60) |
| Lower GI | 120 (IQR 60) |
| Usual location for performing ESD | |
| Endoscopy Suite | 60% (n = 12/20) |
| Operating Room | 35% (n = 7/20) |
| Most common sedation used for ESD | |
| MAC with Propofol | 15% (n = 3/20) |
| General anesthesia | 60% (n = 12/20) |
| Conscious sedation (opioids/benzos) | 20% (n = 4/20) |
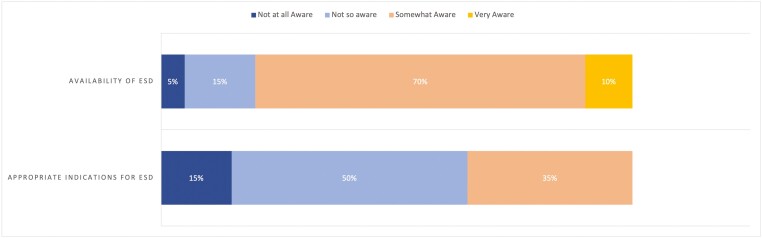
The most common sources of ESD referrals were from surgeons (95% [n = 19/20]) or gastroenterologists (90% [n = 18/20]). Only 10% (n = 2/20) of respondents reported referral providers were ‘very aware’ of the availability of ESD in their area and 65% (n = 13/20) were ‘not so aware’ or ‘not at all aware’ of the appropriate indications for ESD referral (Figure 4).
Figure 4.
Referral providers awareness of ESD availability and indications.
Health Care Infrastructure for ESD
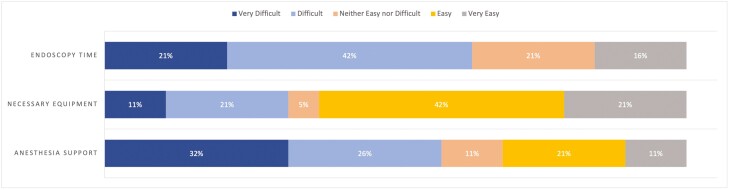
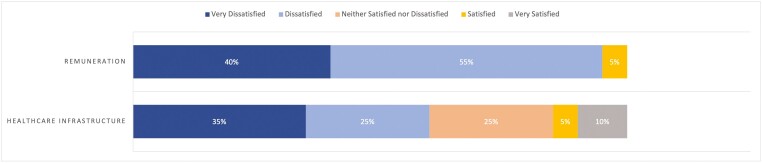
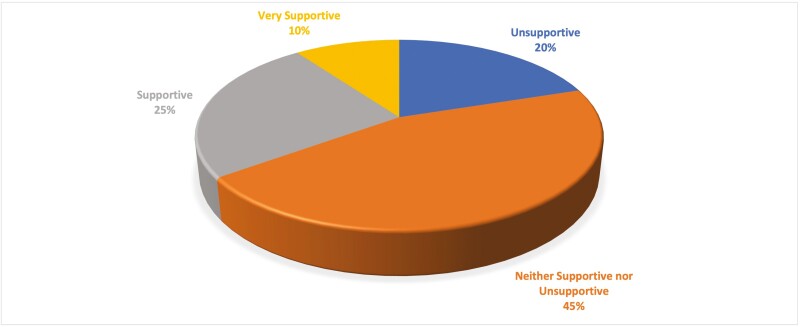
Most respondents reported difficulty in securing endoscopy time and anesthesia support for ESD (Figure 5). However, most respondents reported relative ease in securing necessary technical equipment (Figure 5). Ninety-five percent (n = 19/20) of respondents were either dissatisfied or very dissatisfied with the remuneration for ESD, and the majority (55% [n = 11/20]) were overall not satisfied with their center’s current health care infrastructure to support their ESD practice (Figure 6). Notably, only 35% (n = 7/20) felt their institution was either ‘supportive’ or ‘very supportive’ in expanding the practice of ESD (Figure 7).
Figure 5.
Ease of securing resources for ESD.
Figure 6.
Overall satisfaction with remuneration and infrastructure for ESD.
Figure 7.
Perceived supportiveness of institution in expanding practice of ESD.
DISCUSSION
The results of our study have provided an up-to-date overview of the evolution of the state of ESD practice in Canada. Our results demonstrate significant heterogeneity in ESD training pathways with no standard routes that are mainstream currently. We have also shown considerable growth in the number of ESD practitioners and the number of ESD cases being performed over the past few years. Despite this growth, however, there appears to be relative dissatisfaction among practitioners regarding the overall health infrastructure, remuneration and institutional support for ESD practice in Canada.
ESD training pathways remain variable, as evidenced by our results and remain a significant barrier to adopting ESD in North America, as supported by prior studies (9,10). Multiple reasons have been raised that precluded the development of standard training pathways in Western countries, including the availability of training/supervision, obtaining adequate case-loads, and obtaining the necessary equipment and infrastructure (10). As such, many endoscopists have traditionally looked toward visiting Japanese centers for training and/or attending international courses by expert ESD practitioners (10,11). In addition, our findings demonstrated that many practitioners felt uncomfortable performing ESD at the start of independent ESD practice, which may reflect the lack of structured training curriculums and guidance. However, as more western endoscopists have become proficient in ESD, there is now an opportunity to develop standardized curriculums in Western centers. To this end, the American Society of Gastrointestinal Endoscopy and the European Society of Gastrointestinal Endoscopy has recently published guidance statements for developing core curriculums for ESD training (9,12). In Canada, the availability of ESD training remains limited, with only six respondents currently providing hands-on ESD training based on our survey results. However, as ESD continues to grow, we expect the barriers to accessing ESD training in Canada will significantly decrease.
As speculated by Rai et al., there has been considerable growth in the number of ESD operators since their survey, with 20 respondents actively performing ESD responding to our study versus 11 respondents back in 2018 (8). Additionally, our survey results show that respondents progressively had increasing numbers of ESD procedures performed yearly. This likely reflects increasing recognition and referrals for ESD; however, as evidenced by our results, there remains relatively poor awareness of appropriate indications for ESD from referral providers. This is understandable given the complexities surrounding the selection of the best resection technique for the appropriate lesion (13). The American Gastroenterological Association and the Japanese Gastroenterological Endoscopy Society have previously published guidance regarding appropriate indications (3,14,15). However, with the nuances of lesion selection, some groups have proposed the development of regional multi-disciplinary teams (MDT) for decisions regarding lesion management (10). The development of MDTs may promote the growth of appropriate ESD referrals and increase patient access to this specialized service (16).
Lastly, our survey results show a perceived lack of institutional support and health care infrastructure for the practice and growth of ESD in Canada. This may have in part stemmed from earlier skepticism regarding the efficacy and safety of ESD in Western regions, particularly given most outcome studies were based in Japan (13,17). However, Draganov et al. recently published the largest prospective multicenter study on the outcomes of ESD in North American centers (including one Canadian center) of over 600 patients. Their results demonstrated the safety and efficacy of ESD in North America, with outcomes adhering to established consensus quality standards (17). The need for resources such as specialized equipment, endoscopy time and anesthesia support has also been a known barrier to the growth of ESD (18). This challenge in accessing resources remains evident in our survey results. However, several studies have now demonstrated the cost savings of ESD when performed on appropriate lesions as it allows the avoidance of surgical intervention (19–21). In public health care systems such as Canada, the proper implementation of ESD provides an opportunity to provide cost-effective care for the benefit of both patients and taxpayers and thus should be regarded as a priority for governments and health care systems.
CONCLUSION
There remain several challenges in adopting ESD in Canada, ranging from the lack of structured training pathways and perceived deficiencies in institutional support and health care infrastructure. However, there has been evident growth in the practice of ESD in Canada. This will undoubtedly continue to expand as ESD has become the standard of care for the resection of many neoplastic GI lesions. To ensure adequate patient access to care and the maintenance of high-quality practice standards, increased partnership with institutions and health care networks are crucial to facilitate the successful practice of ESD in Canada.
Supplementary Material
Contributor Information
Suqing Li, Division of Gastroenterology and Hepatology, Department of Medicine, University of Calgary, Calgary, Alberta, Canada; Division of Gastroenterology and Hepatology, Department of Medicine, The Center for Advanced Therapeutic Endoscopy and Endoscopic Oncology, St. Michael’s Hospital, Toronto, Ontario, Canada.
Jeffrey Mosko, Division of Gastroenterology and Hepatology, Department of Medicine, The Center for Advanced Therapeutic Endoscopy and Endoscopic Oncology, St. Michael’s Hospital, Toronto, Ontario, Canada.
Gary May, Division of Gastroenterology and Hepatology, Department of Medicine, The Center for Advanced Therapeutic Endoscopy and Endoscopic Oncology, St. Michael’s Hospital, Toronto, Ontario, Canada.
Christopher Teshima, Division of Gastroenterology and Hepatology, Department of Medicine, The Center for Advanced Therapeutic Endoscopy and Endoscopic Oncology, St. Michael’s Hospital, Toronto, Ontario, Canada.
REFERENCES
- 1. Daoud DC, Suter N, Durand M, Bouin M, Faulques B, von Renteln D.. Comparing outcomes for endoscopic submucosal dissection between Eastern and Western countries: A systematic review and meta-analysis. World J Gastroenterol 2018;24:2518–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang D, Aihara H, Perbtani YB, et al. Safety and efficacy of endoscopic submucosal dissection for rectal neoplasia: A multicenter North American experience. Endosc Int Open 2019;7:E1714–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Draganov PV, Wang AY, Othman MO, Fukami N.. AGA institute clinical practice update: Endoscopic submucosal dissection in the United States. Clin Gastroenterol Hepatol 2019;17:16–25.e1. [DOI] [PubMed] [Google Scholar]
- 4. Heitman SJ, Bourke MJ.. Endoscopic submucosal dissection and EMR for large colorectal polyps: ‘The perfect is the enemy of good’. Gastrointest Endosc 2017;86:87–89 [DOI] [PubMed] [Google Scholar]
- 5. Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc 2010;72:1217–25. [DOI] [PubMed] [Google Scholar]
- 6. Iacucci M, Eustace G, Uraoka T, et al. Endoscopic submucosal dissection in the colorectum: Feasibility in the Canadian setting. Can J Gastroenterol 2013;27:689–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Uraoka T, Parra-Blanco A, Yahagi N.. Colorectal endoscopic submucosal dissection: Is it suitable in western countries? J Gastroenterol Hepatol 2013;28:406–14. [DOI] [PubMed] [Google Scholar]
- 8. Rai M, Motomura D, Hookey L, Antillon MR, Bechara R.. The who, what, where, how, and why of endoscopic submucosal dissection in Canada: A survey among Canadian endoscopists. JGH Open 2021;5(734):734–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aihara H, Dacha S, Anand GS, et al. Core curriculum for endoscopic submucosal dissection (ESD). Gastrointest Endosc 2021;93:1215–21. [DOI] [PubMed] [Google Scholar]
- 10. Barbour JA, O’Toole P, Suzuki N, Dolwani S.. Learning endoscopic submucosal dissection in the UK: Barriers, solutions and pathways for training. Frontline Gastroenterol 2021;12:671–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schlachterman A, Yang D, Goddard A, Gotoda T, Draganov P.. v. Perspectives on endoscopic submucosal dissection training in the United States: A survey analysis. Endosc Int Open 2018;06:E399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pimentel-Nunes P, Pioche M, Albéniz E, et al. Curriculum for endoscopic submucosal dissection training in Europe: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2019;51:980–92. [DOI] [PubMed] [Google Scholar]
- 13. Emmanuel A, Gulati S, Burt M, Hayee B, Haji A.. Colorectal endoscopic submucosal dissection: Patient selection and special considerations. Clin Exp Gastroenterol 2017;10:121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tanaka S, Kashida H, Saito Y, et al. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Digestive Endoscopy 2015;27:417–34. [DOI] [PubMed] [Google Scholar]
- 15. Ono H, Yao K, Fujishiro M, et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer*. Digestive Endoscopy 2015. doi: 10.1111/den.12518. [DOI] [PubMed]
- 16. Weidner A, Wong H, Barbour J.. P281 Developing a safe and effective endoscopic submucosal dissection (ESD) service in the Northern Region. Gut 2022;71:A176–7. [Google Scholar]
- 17. Draganov P, Yao K, Fujishiro M, et al. Endoscopic submucosal dissection in north america: a large prospective multicenter study. Gastroenterology 2021;160:2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Friedel D, Stavropoulos SN.. Introduction of endoscopic submucosal dissection in the West. World J Gastrointest Endosc 2018;10:225–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bahin FF, Heitman SJ, Rasouli KN, et al. Wide-field endoscopic mucosal resection versus endoscopic submucosal dissection for laterally spreading colorectal lesions: A cost-effectiveness analysis. Gut 2018;67:1965–73. [DOI] [PubMed] [Google Scholar]
- 20. Sekiguchi M, Igarashi A, Mizuguchi Y, et al. Cost-effectiveness analysis of endoscopic resection for colorectal laterally spreading tumors: Endoscopic submucosal dissection versus piecemeal endoscopic mucosal resection. Digestive Endoscopy 2022;34:553–68. [DOI] [PubMed] [Google Scholar]
- 21. Dahan M, Pauliat E, Liva-Yonnet S, et al. What is the cost of endoscopic submucosal dissection (ESD)? A medico-economic study. United European Gastroenterol J 2019;7(138):138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.