Abstract
Background
Rural dwellers with inflammatory bowel disease (IBD) face barriers to accessing specialized health services. We aimed to contrast health care utilization between rural and urban residents diagnosed with IBD in Saskatchewan, Canada.
Methods
We completed a population-based retrospective study from 1998/1999 to 2017/2018 using administrative health databases. A validated algorithm was used to identify incident IBD cases aged 18+. Rural/urban residence was assigned at IBD diagnosis. Outpatient (gastroenterology visits, lower endoscopies, and IBD medications claims) and inpatient (IBD-specific and IBD-related hospitalizations, and surgeries for IBD) outcomes were measured after IBD diagnosis. Cox proportional hazard, negative binomial, and logistic models were used to evaluate associations adjusting by sex, age, neighbourhood income quintile, and disease type. Hazard ratios (HR), incidence rate ratios (IRR), odds ratios (OR), and 95% confidence intervals (95% CI) were reported.
Results
From 5,173 incident IBD cases, 1,544 (29.8%) were living in rural Saskatchewan at IBD diagnosis. Compared to urban dwellers, rural residents had fewer gastroenterology visits (HR = 0.82, 95% CI: 0.77–0.88), were less likely to have a gastroenterologist as primary IBD care provider (OR = 0.60, 95% CI: 0.51–0.70), and had lower endoscopies rates (IRR = 0.92, 95% CI: 0.87–0.98) and more 5-aminosalicylic acid claims (HR = 1.10, 95% CI: 1.02–1.18). Rural residents had a higher risk and rates of IBD-specific (HR = 1.23, 95% CI: 1.13–1.34; IRR = 1.22, 95% CI: 1.09–1.37) and IBD-related (HR = 1.20, 95% CI: 1.11–1.31; IRR = 1.23, 95% CI: 1.10–1.37) hospitalizations than their urban counterparts.
Conclusion
We identified rural-urban disparities in IBD health care utilization that reflect rural-urban inequities in the access to IBD care. These inequities require attention to promote health care innovation and equitable management of patients with IBD living in rural areas.
Keywords: Crohn’s disease, Inflammatory bowel disease, Health services, Health care inequities, Rural health, Ulcerative colitis
What is already known on this topic?
Individuals living with inflammatory bowel disease (IBD) require access to specialized care and integrated models of care.
Individuals living in rural locations face additional challenges in accessing health care.
Fewer outpatient gastroenterology visits and higher hospitalizations among rural patients with IBD than their urban counterparts have been reported.
Studies in Canada evaluating differences in medication claims between rural and urban patients with IBD are needed.
Despite that a third of individuals with IBD in Saskatchewan live in rural areas, there are no studies from this province contrasting IBD health care utilization between rural and urban dwellers.
What this study adds?
Individuals diagnosed with IBD residing in rural Saskatchewan have 18% fewer gastroenterology visits than urban dwellers.
Compared to urban Saskatchewan dwellers, individuals diagnosed with IBD living in rural locations were 40% less likely to have a gastroenterologist as their primary IBD care provider.
Individuals diagnosed with IBD living in rural Saskatchewan have lower rates of lower endoscopies than their urban counterparts.
Rural residents in Saskatchewan with the diagnosis of IBD have higher 5-aminosalicylic acid medication claims than urban residents.
Individuals diagnosed with IBD in rural Saskatchewan have higher risk and rates of IBD-specific and IBD-related hospitalizations than their urban counterparts.
Introduction
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), is a lifelong chronic illness that causes inflammation and ulceration within the gastrointestinal system. IBD can be diagnosed in any age group but is most often diagnosed in early adulthood (1, 2). Canada has one of the highest rates of IBD in the world, with over 270,000 diagnoses of the illness in 2018 (2, 3). The number of IBD cases has been steadily increasing in Canada over time with an expected continued increase in the next years (3).
Individuals living with IBD experience periods of exacerbation, when the disease is active, and remission, when the disease is inactive (4). Health outcomes of patients with IBD could be affected if they do not receive the appropriate care (5, 6). Patients with IBD require access to specialized IBD care and integrated models of care (7–9). However, not all individuals living with IBD have access to these services (8, 10). Equitable health care is a significant social determinant of health (11) and is understood as patients’ ability to obtain suitable health care services based on their needs (12). Despite having lower incidence rates of IBD reported among rural dwellers in comparison to urban ones (1), patients with IBD living in rural and remote Canadian locations face challenges in accessing specialized health care (10, 13). As such, the lack of patient access to specialist care and greater geographical distance away from specialist care for chronic diseases, such as IBD, can lead to gaps in care and increased risk for IBD-related complications (9, 10, 14).
Research evaluating the provision of services to rural patients with IBD in Canada is crucial to advocate for health care equity and improve the quality of care (13). There are limited rural-urban comparisons among patients with IBD. One study examined disparities in the care of rural and urban Canadians with IBD in three provinces (10). This study used data from Alberta, Manitoba, and Ontario and identified health services utilization differences between rural and urban patients with IBD, specifically fewer outpatient gastroenterology visits and higher hospitalizations among rural patients with IBD in comparison to urban ones (10). No Canadian studies have evaluated differences in medication claims between rural and urban patients with IBD in Canada.
In this study, we aimed to contrast health care utilization (i.e., outpatient gastroenterology visits, lower endoscopies, claims for IBD medications, IBD-specific and IBD-related hospitalizations, and surgeries for IBD) between rural and urban residents diagnosed with IBD in Saskatchewan, Canada.
METHODS
This study is part of a patient-oriented research initiative that utilizes a mixed-methods approach. While the qualitative component examined health care use and access to care by individuals diagnosed with IBD in rural Saskatchewan (13), this article reports the findings of the quantitative portion of the study. Ethics approval was obtained from the University of Saskatchewan Ethics Board (Beh-REB 954).
Setting, Data Source, and Study Population
We completed a population-based retrospective study using Saskatchewan administrative health databases. Saskatchewan’s landscape covers an area of 588,244 square kilometers and has a population of around 1.1 million (15). More than a quarter (35.6%) of the Saskatchewan population resides in rural and remote locations (15). Saskatchewan residents (~99%) benefit from publicly funded provincial health coverage (16–18).
The province has records of provided health care services and health coverage in administrative databases, including the hospital discharge abstracts data (DAD), Medical Services Branch (MSB), Prescription drug plan (PDP), and Person Health Registration System (PHRS) datasets (16–19). The DAD has acute patient hospital discharge information such as type of diagnosis, performed procedures, admissions, and discharge date. Information on outpatient physician services is contained in the MSB data. Data on outpatient medications claims are available in the PDP, with details on dispensation date and drug identification numbers (DINs), among others.
The PDP data have information on every dispensed outpatient prescription medication regardless of coverage or how the claim was paid (either by the federal or provincial government, patients, or private insurance companies) (18). Over the counter and medications administered in the hospital setting are not captured by this database. Demographic information including place of residence, date of birth, sex, and health care is contained in the PHRS. We linked these administrative health databases deterministically using the encrypted unique identifiers.
To identify diagnosed IBD cases, the International Classification of Disease (ICD) codes of UC (i.e., ICD-9 556.x/ICD-10-CA K51.xx) and CD (i.e., ICD-9 555.x/ICD-10-CA K50.xx) were used and a previously validated administrative algorithm for IBD was applied (20). This algorithm required at least 5 health care encounters with the diagnosis of IBD within 2 years of continuous health coverage or 3 or more health care encounters within less than 2 years of health coverage (20). Each case was classified as CD or UC case based on the most frequent diagnosis across health care contacts (8). Incident IBD cases were selected, which required 8 years of continuous health care coverage with no records of health care contacts with the diagnosis of CD or UC before the date of diagnosis, specifically the first eligible IBD contact (17).
Subsequently, incident IBD cases aged ≥18 years old residing in Saskatchewan between 1998/1999 and 2017/208 fiscal years were included in this study (i.e., between April 1, 1998, and March 31, 2018). Data between 1990/1991 and 1997/1998 were considered to assess the 8 years of continuous health care coverage without IBD contacts and differentiate incident from prevalent cases.
The rural or urban location of residence was assigned at the date of IBD diagnosis based on postal codes and was considered dichotomous. Incident IBD cases with postal codes within census agglomeration or metropolitan areas with a population size of ≥15,000 were classified as urban and those outside as rural (17).
Study Outcomes
In this study, we evaluated outpatient (i.e., gastroenterology visits, lower endoscopies, and claims for IBD medications) and inpatient (i.e., IBD-specific and IBD-related hospitalizations, and surgeries for IBD) health care utilization outcomes.
Outpatient Outcomes
Gastroenterology visits
—Outpatient visits with gastroenterologists were measured after the date of IBD diagnosis. We used the main specialty of claiming physician variable in the Saskatchewan MSB database to identify visits with gastroenterologists. We classified IBD cases who had and did not have outpatient visits with a gastroenterologist (ever having seen a gastroenterologist, dichotomous) and measured the number of these visits from diagnosis date until the end of the study period (continuous). The time from the date of IBD diagnosis to the first outpatient visit with a gastroenterologist was also measured. Gastroenterology visits were classified in IBD-specific (i.e., those with the diagnosis code of UC [ICD-9 556.x/ICD-10-CA K51.xx] or CD [ICD-9 555.x/ICD-10-CA K50.xx]) and IBD-related contacts (i.e., those with IBD-specific diagnoses or codes of signs and symptoms of IBD (see Supplementary Table 1 for details) (10). In addition, data from the MSB were used to determine the primary IBD care providers within the 6 months after the IBD diagnosis (gastroenterologist or non-gastroenterologist, dichotomous). Specifically, IBD cases who had more than 50% of the outpatient IBD visits with physicians who had gastroenterology as the main specialty was classified as primary IBD care provider “gastroenterologist,” otherwise “non-gastroenterologist.”
Lower endoscopies
—We used the Canadian Classification of Diagnostic, Therapeutic, and Surgical Procedures (CCP) and the Canadian Classification of Health Interventions (CCI) codes in the DAD to determine IBD cases who had lower endoscopies (i.e., colonoscopies, sigmoidoscopies, rectoscopies, and anoscopies) between the diagnosis date until the end of the study period (ever having a lower endoscopy, dichotomous). Supplementary Table 2 lists the procedure codes used to identify lower endoscopies. The time from IBD diagnosis to the first lower endoscopy was measured. In addition, we measured the number of lower endoscopies from diagnosis date until the end of the study period (continuous).
Medication claims for IBD
—We accessed outpatient medication claims information from the PDP data. Medications claims for IBD were identified using the DINs. The medications for IBD were classified into three groups, immunomodulators (e.g., azathioprine, mercaptopurine, and methotrexate), biologics (e.g., infliximab, adalimumab, golimumab, certolizumab, vedolizumab, and ustekinumab), and 5-aminosalicylic acid (5-ASA, e.g., mesalamine, sulfasalazine, and olsalazine sodium). The list of medications and corresponding DINs are shown in Supplementary Table 3. We evaluated medication claims for immunomodulator, biologic, and 5-ASA therapies from the date of IBD diagnosis to the end of the study period or end of the coverage. Each case was classified as ever having a medication claim for IBD (dichotomous each immunomodulator, biologic, and 5-ASA medications). The time from the diagnosis date to the first medication claim was measured for each category. In addition, corticosteroid dependency (CsDep) was measured 6 months after the date of diagnosis and was defined as having two or more prescriptions of oral corticosteroids within 180 days (8, 21). The DINs of corticosteroid medications are listed in Supplementary Table 3.
Inpatient Outcomes
IBD-specific and -related hospitalizations—
The DAD was used to identify hospitalizations after the date of diagnosis. IBD-specific hospitalizations were those with two or more days of inpatient care that had as the most responsible diagnosis UC (i.e., ICD-9 556.x/ICD-10-CA K51.xx) or CD (i.e., ICD-9 555.x/ICD-10-CA K50.xx). IBD-related hospitalizations were those with two or more days of hospital care related to IBD-specific diagnoses (i.e., UC or CD) and signs or symptoms associated with IBD (Supplementary Table 1) (10). Each case was classified as having an IBD-specific and -related hospitalization (dichotomous each) and the number of hospitalizations were measured from diagnosis date until the end of the study period or end of coverage (continuous). In addition, the time from the diagnosis date to the first IBD-specific and -related hospitalizations were measured.
Surgeries for IBD
—The DAD was also used to identify surgeries for IBD from the date of diagnosis until the end of the study period (having surgery for IBD, dichotomous). A previously developed and tested list of surgical procedures for CD and UC was used (Supplementary Table 4) (10, 22). The time from the IBD diagnosis to the first surgery for IBD was measured as well.
Statistical Analysis
Multivariable regression models were run to test the association between rural/urban location of residence at the time of IBD diagnosis and the study outcomes, specifically gastroenterology visits, lower endoscopies, prescription medication claims (i.e., immunomodulator, biologic, and 5-ASA claims), IBD-specific and IBD-related hospitalizations, and surgeries for IBD.
To contrast risk differences, we used Cox proportional hazard regression models and estimated hazard ratios (HRs) and 95% confidence intervals (95% CIs). The length of follow-up (i.e., in person-months of health coverage from the IBD diagnosis to either the date of the study outcome or censoring) was the offset term for the models, accounting for varying lengths of the observation time for each IBD case.
To determine differences in the rate of outpatient gastroenterology visits, lower endoscopies, and hospitalization, negative binomial regression models were used, and incidence rate ratios (IRRs) and their 95% CIs were estimated. The negative binomial distribution of the generalized linear model was used because it provided the best fit for the data. We used the ratio of the deviance to the model degrees of freedom to evaluate the fitness of the model. A well-fitting model will generate a ratio value close to 1 (23). Model comparisons revealed that the Poisson distribution (suitable for modeling rates) was over-dispersed for our data (24).
Logistic regression models were used to evaluate the association between rural/urban residence and the odds of having a gastroenterologist as primary IBD care provider and corticosteroid dependency (CsDep) within the 6 months after the date of IBD diagnosis. Odds ratios (ORs) and 95% CIs were reported for these model results. This data analysis was restricted to incident IBD cases with 6 months of continuous health coverage after the date of diagnosis.
The model covariates included rural/urban residence, sex (male or female), age at diagnosis (continuous), disease type (UC or CD), and mean neighborhood income quintile (categorized from lowest to highest income). Stratified analyses by disease type were run considering sex, age at diagnosis, and neighborhood income quintile as covariates. In addition, a potential confounding effect of having a gastroenterologist as the primary IBD care provider was assessed. We also stratified the analyses by age group (i.e., 18–39, 40–59, ≥60 years old) at IBD diagnosis to explore if rural and urban disparities were more pronounced in certain age groups.
SAS software version 9.4 (SAS Institute Inc., Cary NC) was used to carry out all the analysis and an α = 0.05 was adopted as the significance level.
RESULTS
In total, 5,173 (CD 2,796 and UC 2,377) diagnosed IBD incidence cases from April 1, 1999, to March 31, 2018, were included in the study (Table 1). The mean age of IBD diagnosis was 42.46 years (SD = 17.57) and females accounted for 53.0% of the study. About a third of the IBD cases (29.8%) resided in rural locations at the date of diagnosis. We explored if rural residence changed 6 and 12 months after the date of IBD diagnosis, observing negligible variations on this variable. Compared with urban patients with IBD, rural patients with IBD were older (43.85 ± 18.09 vs. 41.86 ± 17.32, P = 0.0002), were more likely to be male (49.7% vs. 45.9%, P < 0.001), and less likely to have CD (50.8% vs. 55.4%, P < 0.0001). Statistically significant differences between rural and urban residents were observed by neighbourhood income quintiles (Table 1).
Table 1.
Descriptive characteristics of rural and urban patients diagnosed with IBD in Saskatchewan between 1998/1999 and 2017/2018 fiscal years
| Characteristics | Full cohort (n = 5173) | Rural (n = 1544) | Urban (n = 3629) | P-value* |
|---|---|---|---|---|
| Age at diagnosis (years), mean±SD | 42.46 ± 17.57 | 43.85 ± 18.09 | 41.86 ± 17.32 | 0.0002 |
| Sex, n (%) | ||||
| Female | 2742 (53.0) | 777 (50.3) | 1965 (54.1) | <0.0001 |
| Male | 2431 (47.0) | 767 (49.7) | 1664 (45.9) | |
| Length of follow-up (years), mean±SD | 10.42 ± 5.47 | 10.60 ± 5.52 | 10.35 ± 5.45 | 0.13 |
| Diagnosis, n (%) | ||||
| CD | 2796 (54.0) | 785 (50.8) | 2011 (55.4) | <0.0001 |
| UC | 2377 (46.0) | 759 (49.2) | 1618 (44.6) | |
| Mean neighbourhood income quintile, n (%) | ||||
| 1 (lowest) | 695 (13.4) | 173 (11.2) | 522 (14.4) | <0.0001 |
| 2 | 992 (19.2) | 347 (22.5) | 645 (17.8) | |
| 3 | 973 (18.8) | 260 (16.8) | 713 (19.6) | |
| 4 | 1171(22.6) | 365 (23.6) | 806 (22.2) | |
| 5 (highest) | 1032 (19.9) | 247 (16.0) | 785 (21.6) | |
| Unknown | 310 (6.0) | 152 (9.8) | 158 (4.4) | |
*t-test and chi-square tests.
Variations in the frequencies of the study outcomes were observed between individuals living in rural and urban locations at the date of diagnosis, particularly among gastroenterology visits, prescription medication claims, and IBD-specific and IBD-related hospitalizations (Table 2).
Table 2.
Study outcomes observed among rural and urban IBD cases between 1998/1999 and 2017/2018 fiscal years, n = yes (%)
| Full cohort | Crohn’s disease | Ulcerative colitis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Study outcomes | Total (n = 5173) | Rural (n = 1544) | Urban (n = 3629) | Total (n = 2796) | Rural (n = 785) | Urban (n = 2011) | Total (n = 2377) | Rural (n = 759) | Urban (n = 1618) |
| Outpatient | |||||||||
| Gastroenterology visit | 4338 (83.9) | 1250 (81.0) | 3088 (85.1) | 2369 (84.7) | 654 (83.3) | 1715 (85.3) | 1969 (82.8) | 596 (78.5) | 1373 (84.9) |
| Lower endoscopy | 4509 (87.2) | 1356 (87.8) | 3153 (86.9) | 2255 (80.7) | 642 (81.8) | 1613 (802) | 2254 (94.8) | 714 (94.1) | 1540 (95.2) |
| Prescription claim of 5-ASA | 4121 (79.7) | 1282 (83.0) | 2839 (78.2) | 1912 (68.4) | 570 (72.6) | 1342 (66.7) | 2209 (92.9) | 712 (93.8) | 1497 (92.5) |
| Prescription claim of IMs | 1971 (38.1) | 554 (35.9) | 1417 (39.0) | 1336 (47.8) | 356 (45.4) | 980 (48.7) | 635 (26.7) | 198 (26.1) | 437 (27.0) |
| Prescription claim of biologics | 1252 (24.2) | 339 (22.0) | 913 (25.2) | 859 (30.7) | 219 (27.9) | 640 (31.8) | 393 (16.5) | 120 (15.8) | 273 (16.9) |
| Corticosteroid dependency | 216 (4.2) | 62 (4.0) | 154 (4.2) | 116 (4.1) | 33 (4.2) | 83 (4.1) | 100 (4.2) | 29 (3.8) | 71 (4.4) |
| Inpatient | |||||||||
| IBD-specific hospitalization | 2545 (49.2) | 839 (54.3) | 1706 (47.0) | 1498 (53.6) | 468 (59.6) | 1030 (51.2) | 1047 (44.0) | 371 (48.9) | 676 (41.8) |
| IBD-related hospitalization | 2761 (53.4) | 897 (58.1) | 1864 (51.7) | 1605 (57.4) | 491 (62.5) | 1114 (55.4) | 1156 (48.6) | 406 (53.5) | 750 (46.4) |
| Surgery for IBD | 1744 (33.7) | 526 (34.1) | 1218 (33.6) | 1016 (36.3) | 277 (35.3) | 739 (36.7) | 728 (30.6) | 249 (32.8) | 479 (29.6) |
5-ASA, 5-aminosalicylic acid; IM, immune modulators.
Adjusted associations are presented in Table 3 and outlined in the sections below. We evaluated if having a gastroenterologist as the primary IBD care provider confounded any of the associations and observed that none of the regression estimates changed in more than 10%; consequently, having a gastroenterologist as the primary IBD care provider was not included in the final models.
Table 3.
Adjusted measures of association between rural residence at the date of diagnosis and the study outcomes
| Study outcomes | Full cohort (n = 5173) | Crohn’s disease (n = 2796) | Ulcerative colitis (n = 2377) | |
|---|---|---|---|---|
| Adjusted* HR (95% CI) |
Adjusted** HR(95% CI) | Adjusted* HR (95% CI) |
Adjusted* HR (95% CI) |
|
| Outpatient | ||||
| Gastroenterology visit | 0.82 (0.77 to 0.88) | 0.82 (0.77 to 0.88) | 0.85 (0.77 to 0.93) | 0.80 (0.72 to 0.88) |
| Lower endoscopy | 0.98 (0.92 to 1.05) | 0.94(0.87 to 1.00) | 1.04 (0.95 to 1.15) | 0.85 (0.77 to 0.93) |
| Prescription claim of 5-ASA | 1.13 (1.05 to 1.21) | 1.10 (1.02 to 1.18) | 1.13 (1.02 to 1.26) | 1.06 (0.97 to 1.16) |
| Prescription claim of IMs | 0.90 (0.81 to 1.00) | 0.93 (0.84 to 1.03) | 0.91 (0.81 to 1.04) | 0.94 (0.79 to 1.13) |
| Prescription claim of biologics | 0.86 (0.76 to 0.99) | 0.89 (0.78 to 1.01) | 0.87 (0.74 to 1.02) | 0.93 (0.74 to 1.17) |
| Corticosteroid dependency† | 1.01 (0.74 to 1.38) | 0.94 (0.79 to 1.12) | 0.91 (0.60 to 1.39) | 1.14 (0.73 to 1.79) |
| Inpatient | ||||
| IBD-specific hospitalization | 1.21 (1.11 to 1.32) | 1.23 (1.13 to 1.34) | 1.25 (1.11 to 1.40) | 1.21 (1.06 to 1.39) |
| IBD-related hospitalization | 1.19 (1.09 to 1.29) | 1.20 (1.11 to 1.31) | 1.21 (1.09 to 1.36) | 1.19 (1.05 to 1.35) |
| Surgery for IBD | 0.97 (0.87 to 1.08) | 0.98 (0.88 to 1.09) | 0.95 (0.82 to 1.10) | 1.01 (0.86 to 1.19) |
HR, hazard ratio; 95% CI, 95% confidence interval; 5-ASA, 5-aminosalicylic acid; IMs, immune modulators; Bold values denote statistically significant results.
*Adjusted by age, sex, and mean neighborhood income quintile.
**Adjusted by age, sex, mean neighborhood income quintile, and type of disease (i.e., Crohn's disease, ulcerative colitis).
†Odds ratios and 95% CIs; logistic regression models restricted to individuals with 6-months follow-up after the date of diagnosis.
Outpatient Outcomes
Gastroenterology visits
Rural Saskatchewan dwellers with the diagnosis of IBD had fewer gastroenterology visits (HR = 0.82, 95% CI: 0.77–0.88) compared to urban residents. This difference was significant among both CD (HR = 0.85, 95% CI: 0.77–0.93) and UC (HR = 0.80, 95% CI: 0.72–0.88) cases (Table 3). The stratified analysis by age groups showed lower gastroenterology visits limited to the youngest age group (HR = 0.76, 95% CI: 0.68–0.84; IRR), Supplementary Table 5.
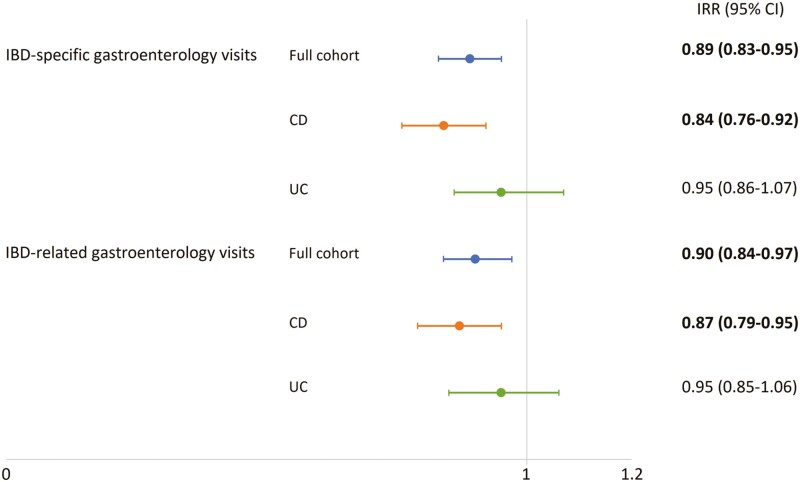
Among individuals diagnosed with IBD who had outpatient gastroenterology visits, IBD cases living in rural Saskatchewan had lower rates of gastroenterology visits than their urban counterparts (IBD-specific visits: IRR = 0.89, 95% CI: 0.83–0.95; IBD-related visits: IRR = 0.90, 95% CI: 0.84–0.97). These disparities in the number of IBD-specific and IBD-related outpatients gastroenterology visits were also observed among those with CD; however, no significant differences were observed among patients with UC (Figure 1).
Figure 1.
Association between the location of residence at IBD diagnosis and rates of outpatient gastroenterology visits. The figure shows adjusted incidence rate ratios (IRRs) and 95% confidence intervals (95% CIs) for rural residents. Adjusted IRRs are presented for IBD-specific and -related visits in the full cohort analysis (blue lines), as well as in the Crohn’s disease (CD, orange lines) and ulcerative colitis (UC, green lines) groups. Bold values denote statistically significant results.
Six months after the IBD diagnosis, 30.6% (n = 1350) of individuals had a gastroenterologist as the primary IBD care provider (other providers 69.4%, n = 3065). We observed that rural dwellers were less likely to have a gastroenterologist as their primary IBD care provider within the 6 months after IBD diagnosis (rural = 23.0%, urban = 77.0%; adjusted OR = 0.60, 95% CI: 0.51–0.70) compared to their urban counterparts. This difference was significant for both CD (rural = 24.0%, urban = 34.7%; adjusted OR = 0.61, 95% CI: 0.49–0.75) and UC (rural = 21.9%, urban = 32.7%; adjusted OR = 0.59, 95% CI: 0.46–0.74) cases.
Lower endoscopies
As presented in Table 3, we did not observe a statistically significant difference in the HR of lower endoscopies between IBD cases living in rural and urban areas (HR = 0.94; 95% CI: 0.87–1.00). However, fewer lower endoscopies were observed among patients with UC living in rural Saskatchewan (HR = 0.85, 95% CI: 0.77–0.93) than those in urban dwellers. No significant results were observed among CD cases (HR = 1.04, 95% CI: 0.95–1.15).
In addition, we observed that individuals diagnosed with IBD living in rural Saskatchewan had lower rates of lower endoscopies after the date of diagnosis than their urban counterparts (IRR = 0.92, 95% CI: 0.87–0.98). This disparity in the number of lower endoscopies was significant among individuals with UC (IRR = 0.89, 95% CI: 0.82–0.95), but not significant in the CD group (IRR = 0.96, 95% CI: 0.88–1.05). Furthermore, this difference in the rates of lower endoscopies was only significant in the youngest age group (i.e., 18–39 years; IRR = 0.88, 95% CI: 0.81–0.96), Supplementary Table 5.
Prescription medication claims
Compared to urban residents, rural Saskatchewan dwellers had higher prescription claims of 5-ASA (HR = 1.10, 95% CI: 1.02–1.18). This difference was significant (HR = 1.13, 95% CI: 1.02–1.26) among diagnosed patients with CD. We did not observe significant rural-urban differences in biologic and immune modulator claims. Similarly, there were no significant results in CsDep between the two groups (Table 3).
Inpatient Outcomes
Hospitalizations
The risk of hospitalizations was significantly higher among rural than urban dwellers, both IBD-specific (HR = 1.23, 95% CI: 1.13–1.34) and IBD-related (HR = 1.20, 95% CI: 1.11–1.31) hospitalizations. In the stratified analysis by disease type, there were higher risks of hospitalizations for CD (specific HR = 1.25, 95% CI: 1.11–1.40; related HR = 1.21, 95% CI: 1.09–1.36) and UC (specific HR = 1.21, 95% CI: 1.06–1.39; related HR = 1.19, 95% CI: 1.05–1.35) cases living in rural settings than their urban counterparts (Table 3). The stratified analysis by age groups showed that the differences in hospitalization between rural and urban patients were present across all age groups; however, slightly higher HRs of both IBD-specific (HR = 1.43, 95% CI: 1.17–1.74) and IBD-related (HR = 1.37, 95% CI: 1.15–1.65) hospitalizations were observed among those ≥60 years old (Supplementary Table 5).
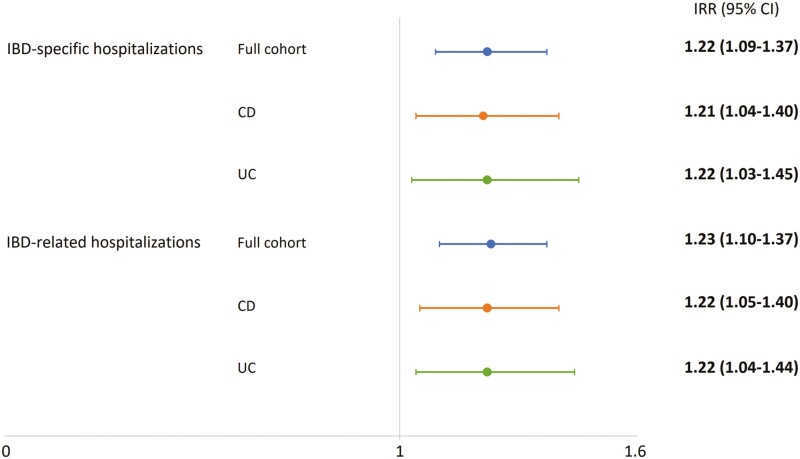
Furthermore, higher IBD hospitalization rates were identified in rural Saskatchewan patients (IBD-specific IRR = 1.22, 95% CI: 1.09–1.37; IBD-related IRR = 1.23, 95% CI: 1.10–1.37) compared to those residing in urban locations. Similarly, differences in the rates of hospitalizations were observed among patients with CD and UC (Figure 2).
Figure 2.
Association between the location of residence at IBD diagnosis and rates of hospitalizations. The figure shows adjusted incidence rate ratios (IRRs) and 95% confidence intervals (95% CIs) for rural residents. Adjusted IRRs are presented for IBD-specific and -related hospitalizations in the full cohort analysis (blue lines), as well as in the Crohn’s disease (CD, orange lines) and ulcerative colitis (UC, green lines) groups. Bold values denote statistically significant results.
Surgeries for IBD
In the full group and stratified analyses, as well as in the stratified analysis, we did not identify significant differences in the risks of surgeries for IBD between rural and urban dwellers (Table 3).
DISCUSSION
In this population-based study of patients with IBD in Saskatchewan, we identified disparities in IBD health care utilization that reflect rural-urban inequities in the access to IBD care. Compared to urban residents, rural dwellers had fewer gastroenterology outpatient visits, and rural residents had 40% lower odds of having a gastroenterologist as their primary IBD care provider. These findings are aligned with other studies in Canada and worldwide. Benchimol et al (10) identified fewer IBD-specific gastroenterologist visits for Canadian rural patients. Internationally, researchers have also reported that rural residents are less likely to be treated by a gastroenterologist for their gastrointestinal diseases (25, 26), have reduced digestive disease-related office visits (27), and decreased surveillance colonoscopies (25). In our study, we observed lower rates of lower endoscopies among rural Saskatchewan dwellers with the diagnosis of IBD in comparison to their urban counterparts. Particularly, individuals with UC living in rural areas had evidence of both fewer endoscopies and lower endoscopy rates than UC urban dwellers. Difficulty accessing specialist services for rural residents in Saskatchewan is not new, or specific for individuals living with IBD. In a province-wide survey, Karunanayake et al. (28) reported that 23% of individuals residing in rural Saskatchewan experienced difficulties in accessing specialist care, and the greater the distance from specialist centers, the greater the difficulties in accessing care. By disease type (Figure 1), we observed a statistically significant difference in the rates of outpatient gastroenterology visits between rural and urban individuals with the diagnosis of CD. However, these differences were not significant for individuals diagnosed with UC. A potential explanation of this difference could be the sample size; these differences might be confirmed with larger cohorts. Interestingly, Benchimol et al. (10) observed significant differences in the rates of outpatient visits between rural and urban individuals in Ontario, the largest cohort included in the study, but not significant in the Alberta and Manitoba IBD cohorts.
Limited studies have assessed differences in medication claims between rural and urban patients with IBD. We identified differences in this regard, with prescriptions claims of 5-ASA 10% higher among rural dwellers. This difference was significant for CD, but not UC. We did not observe significant rural-urban differences in other IBD medication outcomes including claims for biologics and immune modulators, or CsDep. An important limitation of using administrative health data is the lack of measurement of disease severity. Previous studies have described a lower incidence of IBD in rural residents (1), but there are no data to suggest any differences in IBD phenotype or severity between rural and urban patients. The finding of increased use of 5-ASA medications in rural patients with CD is therefore concerning, as 5-ASA medications have a limited role in the management of this disease with guidelines suggesting against the use of 5-ASA in patients with CD of any severity (29, 30). The only other study assessing differences in medication use between areas with different population densities is a German study by Lange et al (25). They observed that individuals with IBD in urban areas were more likely to receive steroids or immunosuppressive therapies than those individuals in rural areas (25). Lack of access to gastroenterology care could also affect 5-ASA prescription medication claims among individuals with IBD living in rural areas. However, we did not find evidence that having gastroenterology as the primary IBD care provider confounded the association between 5-ASA claims and rural residence.
The risk of IBD-specific and IBD-related hospitalizations was higher among rural Saskatchewan patients, consistent with findings in Benchimol et al.’s (10) and Xu et al.’s (27) studies. In addition, as reported by Benchimol et al. (10), we observed higher risks of IBD-specific and IBD-related hospitalizations in the oldest age group. This finding requires particular attention when planning and delivering of IBD care to promote integrative and tailored models of care for this specific age group and advocate for their wellbeing. Benchimol et al. (10) also reported increased rates of emergency department visits from rural patients with IBD. However, we did not examine differences in emergency department visits due to limited data in Saskatchewan. Although Benchimol et al. (10) and Borren et al. (14) identified rurality as a risk factor for IBD-related surgeries, we did not find similar disparities in our population in Saskatchewan.
Our study findings highlight the lack of access to specialized IBD care for individuals living in rural Saskatchewan and the associated increased risk of negative disease outcomes, IBD-specific and IBD-related hospitalizations. The lack of access to specialized IBD care can result in increased direct costs and health services utilization for individuals who reside in rural areas. Hospitalizations contribute to increased direct costs of care (31). If measures can be established to increase access to gastroenterologist care for rural residents of the province living with IBD, direct costs to the health system could potentially be diminished (31). Better clinical outcomes such as remission and decreased hospitalizations and surgeries are seen when individuals with IBD are cared for by gastroenterologists (5, 31). In addition, access to gastroenterologists in hospitalized patients with UC reduces in-hospital mortality (32).
Strengths of this study include its population-based nature with a large and representative sample size of the rural and urban population over 18 years, and that it is the first study in Canada to demonstrate differences in medication claims between rural and urban patients with IBD. Notwithstanding, we acknowledge the limitations of using administrative health data, including the presence of potential misclassification bias in assessing diseases surveillance for individuals with chronic diseases (33). We applied a validated case definition that required multiple IBD health care contacts to overcome potential misclassification bias. The applied case definition has been also recognized as one of the most accurate algorithms to identify adults with the diagnosis of IBD (34). An additional concern is that of differential accuracy of these algorithms in the identification of patients in rural or urban settings. However, the algorithm used has been validated in a variety of populations (20, 34, 35), including in settings with large rural populations (20). As in-hospital medications are not captured in the PDP, individuals who initiate biologic therapy in hospital but who are not continued this therapy upon discharge would not be captured. However, this is likely a very small percentage of patients and it is unlikely to affect our results. Given that in Canada infliximab and adalimumab were authorized for IBD in the 2000s (22), another limitation that needs to be acknowledged is that individuals diagnosed before 1998 and with biologic prescription claims were not considered due to the study period and use of an incident IBD cohort. In addition, we evaluated prescription medication claims for IBD as a dichotomous outcome. This approach does not consider continuation, duration, or rates of medication claims. Further studies could continue assessing rural-urban inequities in prescription medication claims considering prevalent and multiprovince IBD cohorts, as well as the measurement and assessment of episodes of medication therapies for IBD. Detailed approaches to assess medication claims could make evident further rural and urban inequities in the management of IBD.
Finding creative solutions to enhance access to gastroenterologist care for individuals with IBD living in rural areas of the province is critical. A study of patients with IBD living in rural areas of Saskatchewan identified that patients were often required to travel to urban centres to receive gastroenterologist care (13). Participants in this same study highly recommended the use of virtual care technologies (i.e., telephone services, video-conferencing, and telehealth) to access gastroenterologist care. Virtual IBD care can reduce barriers to accessing specialist care, provide an opportunity to reduce out-of-pocket expenses for patients, increase the quality of life, and provide optimal IBD care (13, 36). Virtual IBD care has been suggested as comparable to in-person care (36).
In conclusion, we identified that Saskatchewan rural residents diagnosed with IBD had fewer gastroenterology outpatient visits, lower odds of having a gastroenterologist as their primary IBD care provider, lower rates of lower endoscopies, more 5-ASA prescription claims, and higher risk and rates of hospitalizations due to IBD than their urban counterparts. These disparities in IBD health care utilization reflect rural-urban inequities in the access to IBD care. These rural-urban inequities require the attention of health care providers, decision-makers, and patients living with IBD to promote health care innovation and equitable management of patients with IBD living in rural areas.
Supplementary Material
Funding
This study received funding from the Saskatchewan Health Research Foundation (SHRF) and Saskatchewan Centre for Patient-Oriented Research (SCPOR), Sprout Grant award (ID #4756).
Acknowledgments
We would like to thank the staff of Saskatchewan Health Quality Council and SCPOR Data Services Platform for their support in the development of this project. This study is based in part on de-identified data provided by the Saskatchewan Ministry of Health and eHealth Saskatchewan. The interpretation and conclusions contained herein do not necessarily represent those of the Government of Saskatchewan, the Saskatchewan Ministry of Health, or eHealth Saskatchewan.
Contributor Information
Juan Nicolás Peña-Sánchez, Department of Community Health and Epidemiology, College of Medicine, University of Saskatchewan, Canada.
Jessica Amankwah Osei, Department of Community Health and Epidemiology, College of Medicine, University of Saskatchewan, Canada.
Noelle Rohatinsky, College of Nursing, University of Saskatchewan, Canada.
Xinya Lu, Health Quality Council, Saskatchewan, Canada.
Tracie Risling, College of Nursing, University of Calgary, Canada.
Ian Boyd, Kinistino, Saskatchewan, Canada.
Kendall Wicks, Cabri, Saskatchewan, Canada.
Mike Wicks,, Cabri, Saskatchewan, Canada.
Carol-Lynne Quintin, Crohn’s and Colitis Canada, Saskatchewan Chapter, Canada.
Alyssa Dickson, Saskatchewan Health Authority, Saskatchewan, Canada.
Sharyle A Fowler, Department of Medicine, College of Medicine, University Saskatchewan, Canada.
Authors’ Contributions
J.N.P.S provided substantial contributions to the study design and methods and led the data extraction and analysis. N.R and S.F provided substantial contributions to the conception and design of the study. X.L revised the study protocol and analyzed the data. J.N.P.S, J.O, N.R, and S.F contributed to the data interpretation and drafted the manuscript. All the authors contributed to the study conception and data interpretation, revised the manuscript critically for important intellectual content, approved the final version for publication, and agreed to be accountable for all aspects of the work.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1. Benchimol EI, Kaplan GG, Otley AR, et al. Rural and urban residence during early life is associated with risk of inflammatory bowel disease: A population-based inception and birth cohort study. Am J Gastroenterol. 2017;112:1412–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kaplan GG, Bernstein CN, Coward S, et al. The impact of inflammatory bowel disease in Canada 2018: Epidemiology. J Can Assoc Gastroenterol. 2019;2:S6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coward S, Clement F, Benchimol EI, et al. Past and future burden of inflammatory bowel diseases based on modeling of population-based data. Gastroenterology. 2019;156:1345–53.e4. [DOI] [PubMed] [Google Scholar]
- 4. Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:s1–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Law CC, Sasidharan S, Rodrigues R, et al. Impact of specialized inpatient IBD care on outcomes of IBD hospitalizations: A cohort study. Inflamm Bowel Dis. 2016;22:2149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Taxonera Samso C. Specialist care in the management of inflammatory bowel disease. Rev Esp Enferm Dig. 2016;108:607–8. [DOI] [PubMed] [Google Scholar]
- 7. Mikocka-Walus AA, Andrews JM, Bernstein CN, et al. Integrated models of care in managing inflammatory bowel disease: A discussion. Inflamm Bowel Dis. 2012;18:1582–7. [DOI] [PubMed] [Google Scholar]
- 8. Peña-Sánchez JN, Lix LM, Teare GF, Li W, Fowler SA, Jones JL.. Impact of an integrated model of care on outcomes of patients with inflammatory bowel diseases: Evidence from a population-based study. J Crohns Colitis. 2017;11:1471–9. [DOI] [PubMed] [Google Scholar]
- 9. Mawdsley JE, Irving PM, Makins RJ, Rampton DS.. Optimizing quality of outpatient care for patients with inflammatory bowel disease: The importance of specialist clinics. Eur J Gastroenterol Hepatol. 2006;18:249–53. [DOI] [PubMed] [Google Scholar]
- 10. Benchimol EI, Kuenzig ME, Bernstein CN, et al. Rural and urban disparities in the care of Canadian patients with inflammatory bowel disease: A population-based study. Clin Epidemiol. 2018;10:1613–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meyer SB, Luong TC, Mamerow L, Ward PR.. Inequities in access to healthcare: Analysis of national survey data across six Asia-Pacific countries. BMC Health Serv Res. 2013;13:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Allin S. Does equity in healthcare use vary across Canadian provinces? Healthc Policy. 2008;3:83–99. [PMC free article] [PubMed] [Google Scholar]
- 13. Rohatinsky N, Boyd I, Dickson A, et al. Perspectives of health care use and access to care for individuals living with inflammatory bowel disease in rural Canada. Rural Remote Health. 2021;21:6358. [DOI] [PubMed] [Google Scholar]
- 14. Borren NZ, Conway G, Tan W, et al. Distance to specialist care and disease outcomes in inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:1234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. StatisticsCanada. Focus on Geography Series, 2016 Census. Statistics Canada Catalogue no. 98-404-X2016001, 2021. https://www12.statcan.gc.ca/census-recensement/2016/as-sa/fogs-spg/Facts-PR-Eng.cfm?TOPIC=1&LANG=Eng&GK=PR&GC=47 (Accessed November 12, 2021).
- 16. Anderson M, Revie CW, Quail JM, et al. The effect of socio-demographic factors on mental health and addiction high-cost use: A retrospective, population-based study in Saskatchewan. Can J Public Health. 2018;109:810–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Osei JA, Peña-Sánchez JN, Fowler SA, Muhajarine N, Kaplan GG, Lix LM.. Population-based evidence from a Western Canadian province of the decreasing incidence rates and trends of inflammatory bowel disease among adults. J Can Assoc Gastroenterol. 2020. doi: 10.1093/jcag/gwaa028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Osei JA, Peña-Sánchez JN, Fowler SA, Muhajarine N, Kaplan GG, Lix LM.. Increasing prevalence and direct health care cost of inflammatory bowel disease among adults: A population-based study from a Western Canadian Province. J Can Assoc Gastroenterol. 2021. doi: 10.1093/jcag/gwab003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sari N, Osman M.. The effects of patient education programs on medication use among asthma and COPD patients: A propensity score matching with a difference-in-difference regression approach. BMC Health Serv Res. 2015;15:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bernstein CN, Blanchard JF, Rawsthorne P, Wajda A.. Epidemiology of Crohn’s disease and ulcerative colitis in a central Canadian province: A population-based study. Am J Epidemiol. 1999;149:916–24. [DOI] [PubMed] [Google Scholar]
- 21. Munkholm P, Langholz E, Davidsen M, Binder V.. Frequency of glucocorticoid resistance and dependency in Crohn’s disease. Gut. 1994;35:360–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Murthy SK, Begum J, Benchimol EI, et al. Introduction of anti-TNF therapy has not yielded expected declines in hospitalisation and intestinal resection rates in inflammatory bowel diseases: A population-based interrupted time series study. Gut. 2020;69:274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCullagh P. Generalized Linear Models. 2nd ed. London; New York: Chapman and Hall, 1989. [Google Scholar]
- 24. Frome EL, Checkoway H.. Epidemiologic programs for computers and calculators. Use of Poisson regression models in estimating incidence rates and ratios. Am J Epidemiol. 1985;121:309–23. [DOI] [PubMed] [Google Scholar]
- 25. Lange A, Prenzler A, Bachmann O, et al. Regional differences in health care of patients with inflammatory bowel disease in Germany. Health Econ Rev. 2015;5:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin YH, Tseng YH, Chen YC, et al. The rural–urban divide in ambulatory care of gastrointestinal diseases in Taiwan. BMC Int Health Hum Rights. 2013;13:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu F, Carlson SA, Liu Y, Greenlund KJ.. Urban-rural differences in health care utilization for inflammatory bowel disease in the USA, 2017. Dig Dis Sci. 2021. doi: 10.1007/s10620-021-07264-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Karunanayake CP, Rennie DC, Hagel L, et al. Access to specialist care in rural Saskatchewan: The Saskatchewan Rural Health Study. Healthcare. 2015;3:84–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Panaccione R, Steinhart AH, Bressler B, et al. Canadian Association of Gastroenterology Clinical Practice guideline for the management of luminal Crohn’s disease. Clin Gastroenterol Hepatol. 2019;17:1680–713. [DOI] [PubMed] [Google Scholar]
- 30. Panaccione R, Steinhart AH, Bressler B, et al. Canadian Association of Gastroenterology Clinical Practice guideline for the management of luminal Crohn’s disease. J Can Assoc Gastroenterol 2019;2:e1–e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kuenzig ME, Benchimol EI, Lee L, et al. The impact of inflammatory bowel disease in Canada 2018: Direct costs and health services utilization. J Can Assoc Gastroenterol. 2019;2:S17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Murthy SK, Steinhart AH, Tinmouth J, Austin PC, Nguyen GC.. Impact of gastroenterologist care on health outcomes of hospitalised ulcerative colitis patients. Gut. 2012;61:1410–6. [DOI] [PubMed] [Google Scholar]
- 33. Manuel DG, Rosella LC, Stukel TA.. Importance of accurately identifying disease in studies using electronic health records. BMJ. 2010;341:c4226. [DOI] [PubMed] [Google Scholar]
- 34. Benchimol EI, Guttmann A, Mack DR, et al. Validation of international algorithms to identify adults with inflammatory bowel disease in health administrative data from Ontario, Canada. J Clin Epidemiol. 2014;67:887–96. [DOI] [PubMed] [Google Scholar]
- 35. Bernstein CN, Wajda A, Svenson LW, et al. The epidemiology of inflammatory bowel disease in Canada: A population-based study. Am J Gastroenterol. 2006;101:1559–68. [DOI] [PubMed] [Google Scholar]
- 36. Painchaud M, Singh S, Penner RM.. A86 Inflammatory bowel disease patients’ satisfaction with telehealth: During the Covid-19 pandemic. J Can Assoc Gastroenterol. 2021;4(Supplement_1):56–7. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.