Abstract
Psychosocial support (PSS) to caregivers of HIV-infected infants on antiretroviral treatment (ART) is crucial to ensure ART adherence and sustained long-term viral suppression in children. A specific approach including tools to monitor and understand adherence behavior and risk factors that prevent optimal treatment compliance are urgently needed. This qualitative exploratory study, conducted in southern Mozambique, monitored the infants’ viral response trajectories during 18 months follow-up, as a measure of adherence, reviewed the caregiver’s PSS session notes and the answers to a study questionnaire, to analyze whether the standard PSS checklist applied to infants’ caregivers can identify barriers influencing their adherence. Only 9 of 31 infants had sustained virologic response. Reported factors affecting adherence were: difficulties in drugs administration, shared responsibility to administer treatment; disclosure of child’s HIV status to family members but lack of engagement; mother’s ART interruption and poor viral response. In conclusion, we found that the standard PSS approach alone, applied to caregivers, was lacking focus on many relevant matters that were identified by the study questionnaire. A comprehensive patient-centered PSS package of care, including an adherence risk factor monitoring tool, tailored to caregivers and their children must be developed.
Keywords: Antiretroviral therapy, adherence, psychosocial support, HIV-infected infants, viral suppression, Mozambique
Introduction
Starting antiretroviral treatment (ART) in the first months of life is crucial to reduce morbidity and mortality in perinatally HIV-infected infants (Violari et al., 2008), to achieve faster virologic suppression and obtain excellent immunological response (Chan et al., 2019; Chiappini et al., 2006; Rinaldi et al., 2018). Sustained virologic response and long-term benefits, including adequate child cognitive development are possible if optimal adherence to ART is ensured at all times (Chiappini et al., 2006; Goetghebuer et al., 2009; Struyf et al., 2020; Teasdale et al., 2018). In fact, suboptimal adherence was shown to be a major contributor to poor ART response in the pediatric population (Vreeman et al., 2008).
Providing adequate psychosocial support (PSS) to caregivers after their infant’s HIV diagnosis and during the first months on ART is crucial to prevent, identify and address barriers to optimal treatment adherence (Millar et al., 2020; Nasuuna et al., 2019).
Mozambique reported a vertical transmission rate of 13% and approximately 16,400 newly infected children started ART in 2020; the viral load suppression rate in the pediatric population was of 37% (MISAU, 2020). In 2007, following the WHO recommendation to decentralize service delivery and adopt standardized simplified care (Gilks et al., 2006), pediatric HIV care was decentralized to primary level and less specialized staff started to provide clinical care to children. Similarly, PSS care shifted from psychologists to lay counselors, peer educators, expert patients and mentor mothers who have been trained to offer a standard “PSS and positive prevention” package to support patients and caregivers to be adherent, reach and sustain viral suppression (MISAU, 2013; Teasdale & Besser, 2008).
Little is known about best practices of PSS tailored to infants and their caregivers, other than the family-centered approach (Leeper et al., 2010; Richter et al., 2009; Rochat et al., 2011) and different approaches are being studied to improve maternal adherence (Kim et al., 2020). In 2015, the HIV program in Mozambique launched the PSS and positive prevention national guidance, however, lacking specific PSS assessment and package of care for infants’ caregivers (MISAU, 2015b). Many studies described adherence and barriers among caregivers of older children (Campbell et al., 2012; Cruz et al., 2014; Davies et al., 2008; Fetzer et al., 2011; Mavhu et al., 2013; Naar-King et al., 2006; Nabukeera-Barungi et al., 2007) but limited evidence exists about ART adherence barriers among caregivers of youngest children.
Considering that infants depend on caregivers for taking the medication, it is critical to understand the nature of adherence behavior among caregivers and anticipate potential threats to optimal compliance. This exploratory study monitored the infant’s viral response trajectories after ART initiation to analyze whether the standard PSS package of care applied to infants’ caregivers can effectively identify barriers influencing their adherence behavior. To our best knowledge, no studies have assessed the PSS tools and model of care offered to caregivers of HIV-infected infants.
Methods
Study setting and population
This study was conducted at Matola Provincial Hospital, in Maputo Province, southern Mozambique, where the HIV prevalence was of 22.9% (MISAU et al., 2018). Patients included in the study were caregivers of HIV perinatally infected infants starting ART within two months of age and followed for 18 months, from Feb 2017 to August 2019. All caregivers had a PSS session with a trained psychologist after the first month of therapy, followed by monthly sessions and responded to a study questionnaire, administered by the study nurse, within the first three months after enrollment. The interview was conducted in Portuguese and in Changana, the local language.
This is an ancillary study of a cohort study started in 2017, called “Immunity and HIV persistence in perinatal HIV infection”, funded by the National Institute of Health – NIH (AI127347), developed in collaboration with Fundação Ariel Glaser contra o SIDA Pediátrico, the Instituto Nacional de Saúde, Mozambique, the University of Miami, U.S.A. and the Bambino Gesú Children’s Hospital, IRCCS, Rome, Italy.
Study design and data collection
This is an exploratory study consisting of a review of infants’ clinical file, where caregiver’s study questionnaire, visit notes from the first PSS session, and results of infant’s viral load measurements were reported. Collection of questionnaire answers and the first PSS visit notes was done using a systematic approach.
The study questionnaire explored the following topics: mother’s ART discontinuation in the past, mother’s education, number of people living in the same household, ART administration to infant and any assistance, difficulties encountered in child’s ingestion of drugs, times caregiver forgot giving drugs, attendance of traditional healer, mother’s and child serostatus disclosure within family or friends. The PSS notes were reported using the standardized Ministry of Health (MOH) PSS checklist (MISAU, 2015c) (Table 1).
Table 1.
The standardized Ministry of Health (MOH) PSS checklist.
| Themes explored in the PSS checklist | Specific questions |
|---|---|
|
| |
| HIV infection | HIV basic knowledge; prevention and disease progression; acceptance of HIV status; belief in ART efficacy; fear of adverse events and management; pill burden |
| Adherence to ART | Time in taking medication and dose, adherence plan |
| General health | Feeling sick, depression, anxiety, alcohol or drug abuse, sexual behavior and condoms use, sexual transmitted disease, family planning and safe pregnancy |
| Family and socioeconomic issues | Gender-based violence, support, disclosure to partner and family, food availability, transport issues, stigma and discrimination |
| Community issues | Traditional medicine, cultural factors, stigma and discrimination, community support and participation in support groups |
| Children care | Person in charge of administering the medication, disclosure, time and dosing of ART |
Data analysis
Qualitative data were analyzed using a content analysis approach. Two researchers (MGL and SC) agreed to use the MOH codes embedded in the PSS tool and jointly defined and added new codes. They jointly applied the codes to all collected data and agreed on any coding discrepancies across the entire data set. They jointly developed the higher order PSS themes to be reported. MGL and SC have not been involved in the collection of qualitative data, that were collected by a trained psychologist and nurse. Themes and factors related to caregiver’s adherence behavior were identified and considered in relation to infant’s viral response trends.
We assessed whether the MOH PSS checklist applied to caregivers, identified or missed factors influencing caregiver’s adherence behavior. Analysis of PSS baseline findings and infants’ viral load response was done to see if factors identified in the first visit corresponded with poor or good viral response and thus adherence.
Descriptive analysis was done considering two groups of infants, with good and poor virologic response VR. Good VR was defined in case of virologic suppression (i.e. plasma HIV-RNA <1000 copies/ml – MOH definition) (MISAU, 2015a) achieved and maintained in all the measurements. Poor VR was defined when the infant never achieved viral suppression, or when, after achieving viral suppression, had more than one measurement of HIV-RNA plasma above 1000 copies/ml in follow-up tests.
Ethical considerations
The study was approved by the National Bioethics Committee (IRB00002657, 102/CNBS/2016), the Mozambican MOH, the IRB of University of Miami and Bambino Gesú Children Hospital, IRCCS. Caregivers who agreed to participate in the study signed a written informed consent for themselves and for their infants, including consent to publish de-identified data.
Results
Characteristics of participants
A total of 31 infants (61% female) were followed for a median follow-up time of 18 months; one infant moved to another province, two died during follow up and three became orphans. Thirty-one caregivers received the PSS session and 26 the study questionnaire, five mothers were not interviewed (three died and two were not available). Sociodemographic characteristics of mothers are described in Table 2.
Table 2.
Mothers’ characteristics at enrollment.
| Mother’s characteristics | HIV-infected infants N = 31 (%) |
|---|---|
|
| |
| Mother’s age (years) | |
| Median | 28 [11] |
| Mother alive | |
| Yes | 28 (90) |
| No | 3 (10) |
| Marital status | |
| Single | 13 (42) |
| Married/living together | 17 (55) |
| Missing | 1 (3) |
| Disclosure of serostatus to family | |
| Yes | 28 (90) |
| No | 2 (7) |
| Missing | 1 (3) |
| Permanent job | |
| Yes | 4 (13) |
| No | 24 (77) |
| Missing | 3 (10) |
| Fixed income | |
| Yes | 17 (55) |
| No | 11 (35) |
| Missing | 3 (10) |
| Education | |
| No education | 2 (6) |
| Primary | 9 (29) |
| Secondary | 17 (55) |
| Missing | 3 (10) |
| Partner HIV-positive | |
| Yes | 12 (39) |
| No | 10 (32) |
| Don’t know | 6 (19) |
| Missing | 3 (10) |
Infants’ virologic response
Nine infants had good VR and 22 had poor VR. Among infants with poor VR: 12 never achieved viral suppression, and 10 reached viral suppression but had a rebound within six months after suppression. Of those who rebounded, six again achieved viral suppression (Figures 1 and 2).
Figure 1.
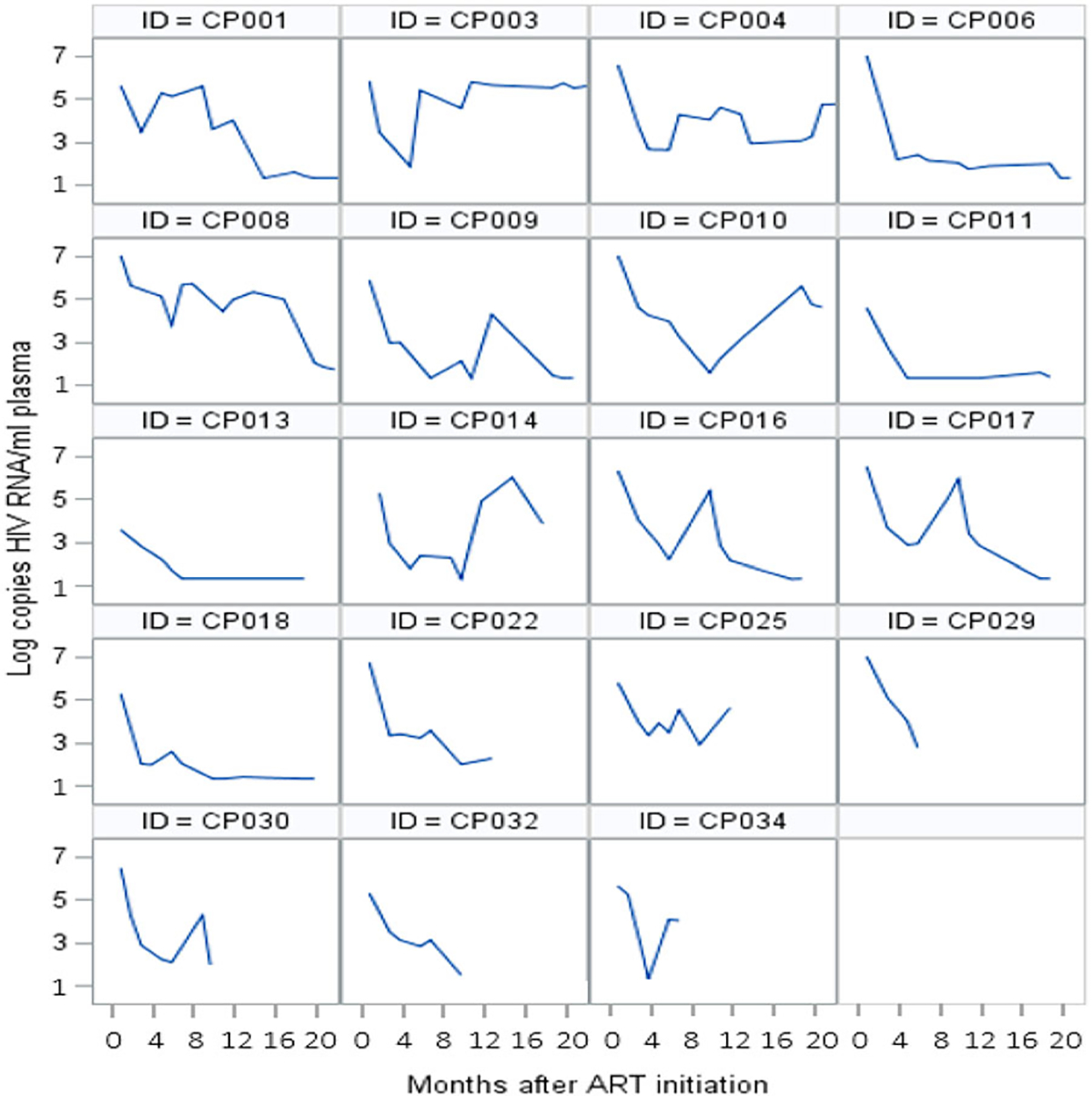
Individual trajectories of viral load of infants who reached viral suppression during follow-up.
Figure 2.
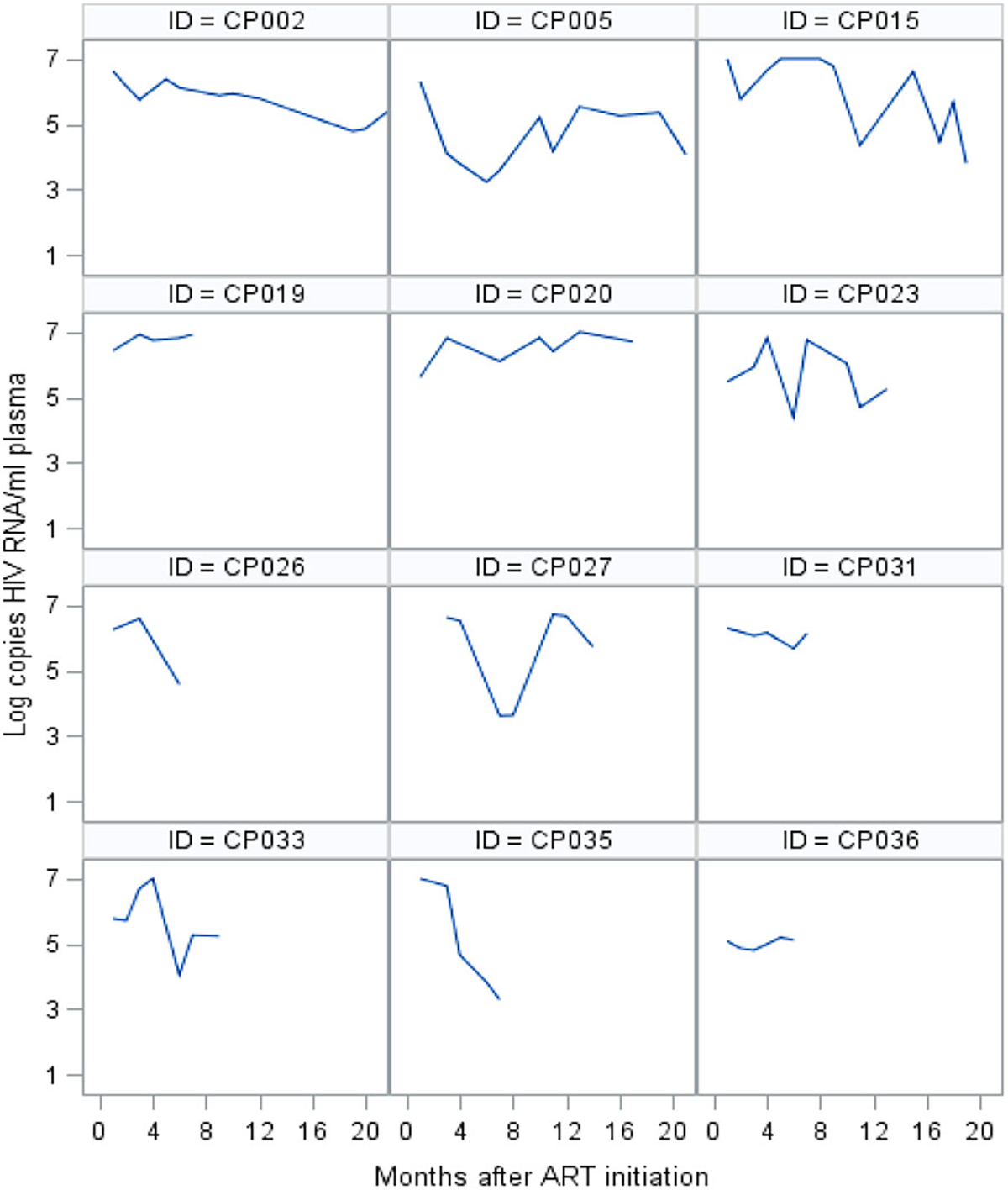
Individual trajectories of viral load of infants who never reached viral suppression during follow-up.
Psychosocial themes reported during the PSS visits and study questionnaire
A total of 31 first PSS visits reports and 26 study questionnaires were available and analyzed. All caregivers attended all the appointments: half of caregivers of infants with both good and poor VR returned on the scheduled day, half with a mean delay of three days.
Disclosure of infant HIV serostatus and family support
The majority of mothers (23 of 31), reported that they disclosed child status to family members or friends: 16/22 were in the group of poor VR and 7/9 in the other group (Table 3). Nevertheless, no records, in both groups, detailed whether any discussion happened about difficulties faced by the caregiver after disclosure. Irrespective of infant’s VR, few records indicated lack of family support (6/31) and very few reported any discrimination issues at home or within the community. A range of reports is shown below.
The husband’s family where mother and baby live is not aware of the infant’s and couple serostatus. (Poor VR)
The mother says she needs to disclose her serostatus to her partner; the mother-in-law, with whom she lives, knows she has anemia. (Good VR)
The infant’s father lives in South Africa, he is not aware of the child serostatus and he is seronegative. (Poor VR)
The father is HIV-positive and on ART since 2012 … but he didn’t disclose to the wife. However, he is supporting economically and also in giving drugs to the child. (Good VR)
Table 3.
Infant’s management at home.
| Good VR N = 9 |
Poor VR n = 22 |
|
|---|---|---|
|
| ||
| Problems in ART administration | ||
| Referred problems | 2 | 11 |
| Vomit | 1 | 9 |
| Delay | 0 | 4 |
| Wrong dose | 0 | 1 |
| Forgot | 1 | 2 |
| Baby refused | 0 | 1 |
| No problems referred | 6 | 8 |
| Not reported | 1 | 3 |
| Shared responsibility in ART administration | ||
| Yes | 5 | 12 |
| No | 2 | 7 |
| Not reported | 2 | 3 |
| Child’s HIV status disclosed to family members | ||
| Yes | 7 | 16 |
| No | 0 | 3 |
| Not reported | 2 | 3 |
VR = viral response; ART = antirretroviral therapy.
Administration of antiretroviral drugs and assessment of adherence
The majority of mothers (17 of 31), reported shared responsibility with other family member to administer ART to the infant: 12/22 of the group of infants with poor VR and 5/9 of the other group. Thirteen caregivers reported problems with administration of drugs, being multiple problems frequent in mothers of infants with poor VR (Table 3).
The adherence plan was documented in 16 infants, 10 of them who lately had poor VR and 6 who had good VR.
“The child lives with parents, mother HIV-positive and father HIV-negative. Father helps with the medication. Mother refers 10–15 min delay, in the evening dose” (Poor VR)
“The aunty refers difficulties in giving the correct dose at the scheduled time.” (Poor VR)
“Mother refer no delay nor skipping medication, just vomits sometimes” (Poor VR)
“Mother does not count on partner’s support in giving the medication to the baby” (Poor VR)
“Father is helping in giving the medication in the morning, at night he is at work” (Poor VR)
Traditional healer
The habit to visit traditional healer was reported in 42% (13/31) of cases, with similar frequency in both groups: 5/9 of good VR compared to 8/22 of poor VR, the rest of caregivers denied frequenting traditional healer.
“The mother refers she is giving the ‘moon remedy’ because the mother-in-law, is checking on her; she is not aware of mother and infant’s serostatus” (Poor VR)
Mother’s history of ART and viral response
Mothers of infants with poor VR were on ART for a shorter time compared to mothers of infants with good VR. More mothers in the group of infants with poor VR, reported to have interrupted ART for some time before delivery and had also poor VR during follow up compared to mother of infant with good VR (Table 4).
Table 4.
Mothers’ antiretroviral therapy and viral load profile per infants’ viral response.
| Infants with good VR n = 9 (%) |
Infants with poor VR n = 22 (%) |
|
|---|---|---|
|
| ||
| Time on ART before delivery | ||
| <3months | 1 (11%) | 3 (14%) |
| 3–9 months | 4 (44%) | 12 (55%) |
| >9 months | 4 (44%) | 5 (23%) |
| Missing | 0 | 1 |
| Self-report ART interruption | ||
| Yes | 4 (44%) | 13 (59%) |
| No | 4 (44%) | 9 (41%) |
| Missing | 1 (11%) | 0 |
| VL during follow-up | ||
| Undetectable | 5 (56%) | 10 (45%) |
| Detectable | 1 (11%) | 10 (45%) |
| Missing (mother died/left) | 3 (33%) | 2 (9%) |
ART = antiretroviral therapy; VL = viral load; VR = viral response; Good VR = infants who reached and maintained viral load suppression during follow up; Poor VR = infants who never reached viral load suppression or reached viral load suppression and had a subsequent rebound.
Mother mental status and stigma
With the exception of one mother of an infant with poor VR, who was diagnosed with depression, no information exploring mother’s mental health, situations of stigmatization, gender-based violence, substance abuse, nor linkage to support groups was reported in files.
HIV basic knowledge
No report in infants file was found about discussion of HIV basic knowledge.
Discussion
The evidence from our study is that the standard PSS checklist applied to infants’ caregiver poorly identified barriers to ART adherence, and unsuccessfully classified infants at risk of poor viral response.
To our best knowledge, there are no studies describing the optimal PSS approach for caregivers of infants starting ART in the first months of life, nor their adherence behavior, while most studies evaluated barriers among caregivers of older children (Campbell et al., 2012; Cruz et al., 2014; Davies et al., 2008; Fetzer et al., 2011; Mavhu et al., 2013; Naar-King et al., 2006; Nabukeera-Barungi et al., 2007; Tong et al., 2020; Verma et al., 2020) and among children or adolescents (Campbell et al., 2012; Fetzer et al., 2011; Gibb et al., 2003; Haberer et al., 2011; Haberer & Mellins, 2009; Ssanyu et al., 2020).
In our cohort, we found dynamic psychosocial factors related to the family dimension, such as poor family members and partner involvement, multiple caregivers, difficulties in drugs administration, which eventually negatively affected the adherence pattern even among those caregivers who initially were compliant and whose infants reached viral suppression.
Using the standard PSS checklist combined with the study questionnaire, it was possible to identify in the first session, more than half of the infants at risk of poor adherence by evaluating the infant’s acceptability of ART and administration pattern (Table 3). Similarly to another study in Brazil, we found administration difficulties and delegation of responsibility as characteristics associated to children with poor adherence (Santarem Ernesto et al., 2012).
From the questionnaire, we evinced that the mother was the primary caregiver for almost all children. Although the majority of them reported shared responsibility to administer the medication, counseling was not offered to other family members. In Uganda, having multiple caregivers and ignoring the baby’s status (Seth et al., 2014) were factors increasing the risk of non-adherence (Nabukeera-Barungi et al., 2007). Thereafter, promoting counseling session of other family members may be a key intervention to improve infant’s management at home (Amzel et al., 2013; Richter, 2010; Van Winghem et al., 2008).
Despite disclosure of mother’s and child’s HIV serostatus to family members and partners being reported in majority of mothers, it appears that disclosure alone had limited contribution to good adherence and achievement of infant’s viral suppression. No report on family dynamics were mentioned during the PSS session; importantly, fathers or relatives rarely attended the PSS session with the mother; the father of twin sisters, with good VR, was the only partner present at the clinic.
In our context, as in many other African countries, partner decision in mother’s and child’s health care issues often limits woman’s ability to decide and openly discuss health matters including HIV care (Bandali, 2011; Bwirire et al., 2008; Hodgson et al., 2014; O’Gorman et al., 2010; Ujiji et al., 2011). Implementing strategies to engage family members and males in the infant’s care requires a stronger program response (Lahai et al., 2020; MISAU, 2018).
No adherence plan was reported for half of the caregivers in our cohort, suggesting the need to improve support to staff offering PSS care. Inviting all infant’s caregivers and showing them how to prepare and give the drug to the baby during multiple PSS sessions, should be part of the standard of care.
One key element in evaluating infant’s adherence to ART not captured by the MOH standard PSS checklist, was the mother’s viral load test result. We found that almost all mothers with poor VR had infants with poor VR (Table 3). This correlation has been described in Kenya and shows that it is crucial to evaluate infant’s adherence and VR in light of mother’s viral load test result (Humphrey et al., 2019).
Another element poorly explored with the standard PSS checklist, but very important for the infant’s care, was the mother’s mental health status: only one mother appeared to be assessed for her mental health. This aspect needs to be better addressed as perinatal depression prevalence is of 18% in Sub-Saharan Africa (Myer et al., 2008; Sawyer et al., 2010; Stringer et al., 2014), affects also breastfeeding women living with HIV and has found associated with poor ART adherence among caregivers (Bennetts et al., 1999; Spielman et al., 2021; Stringer et al., 2014). Additional evidence has been published about the negative psychosocial impact on mothers of early HIV diagnosis in their infant and how proper counseling and support group participation was key to cope with the new condition (Varga et al., 2005). A study in Brazil found that mental health, cognitive status and quality of life were important predictors of adherence among caregivers although of older children than ours (Cruz et al., 2014). An easy tool to screen mother’s mental health should be developed and included in the mother’s PSS package of care in accordance to WHO recommendation (WHO, 2016).
In our study, adherence was self-reported and good in almost all the patients. Few mothers reported missing doses, and compliance to a scheduled visit and drug pick-ups were overall optimal. Our findings, once again, show that self-reported adherence is not a reliable indicator of good viral response, and retention into care does not necessarily reflect adherence to treatment (Bagenda et al., 2011; Cruz et al., 2014; Davies et al., 2008; Müller et al., 2008; Teasdale et al., 2013).
This finding also highlights how structural factors related to the health provider may influence mother’s disclosure of barriers she is facing and warns of the need to promote and build a safe and confidential environment between the patient and the health staff (Duff et al., 2010).
Viral load (VL) measurement is the test of choice to monitor response to ART and, indirectly, adherence (WHO, 2016). The frequent monitoring of VL in our population showed how variable and unpredictable the VR was, despite monthly APSS sessions. Few infants had a good sustained VR during the entire follow-up period, other infants had a rebound with further suppression, while some never controlled the virus again (Figures 1 and 2). Frequent rebound after viral suppression has been described in a South African cohort of infants (Teasdale et al., 2018) and reflects changes in caregiver’s adherence behavior.
The suboptimal viral control we observed during the first 18 months of life, when is crucial for an infant to control the virus for a healthy development (Violari et al., 2008), calls for innovative and efficient interventions. We suggest to include in the PSS approach the evaluation of VR trajectories, such as those we have presented (Figures 1 and 2). This measure can serve as a tool to assist less specialized staff to identify cases without good viral response, and indicate them for a more intense PSS response.
In case of poor VR or if risk factors for optimal adherence are identified, a more dynamic community support, complementing the PSS care offered at HF, can be proposed. More frequent home visits, such as weekly visits can be scheduled during one month, as reported in South Africa (Calmy et al., 2007) instead of monthly visits during three months, as recommended in Mozambique. A Directly Observed Therapy (DOT) for ART, proven effective in increasing adherence (Gigliotti et al., 2001), could be suggested for HIV programs that are implementing the mentors mothers (MM) strategy such as Mozambique (Teasdale & Besser, 2008). ART DOT at health facility followed by ART DOT at home, for one week under the supervision of a trained MM, should be considered for all newly infected infants or in case caregiver is reporting any difficulties in administering the medication.
The study has a few limitations. The qualitative approach did not allow us to generalize our findings, although we believe that it identifies specific areas for innovative PSS interventions tailored to caregivers of infants and children starting ART.
Moreover, reports from caregivers may present social desirability bias; depending on the relationship with the health provider, parents tend to respond in a favorable and expected way so as not to be judged, leading to over-report of good adherence and under reporting problematic situations or undesirable behaviors.
Another limitation, knowing that adherence factors change over time, is that our study analyzed the initial PSS session, when it was possible to identify risk factors present in the first period after ART initiation. However, we think that the first evaluation is crucial and needs to be as accurate and as comprehensive as possible in order to promptly identify potential risk factors and act on them quickly.
Our findings revealed that the standard PSS checklist alone applied to our population, was lacking focus on many relevant matters that affected caregivers’ adherence. A comprehensive patient-centered PSS approach for caregivers and their children needs to be designed considering the needs of infants early and evolving age in the context of family, extended-family and community, they are living in.
However, more robust evidence is needed on how factors related to adherence are changing over time and the way they can effectively be addressed.
Finally, a stronger program response is required to engage partners and family members in infant’s continuum health care.
Acknowledgements
The authors would like to thank all caregivers who participated in the study, the study staff at the Matola Provincial Hospital who took care of infants and caregivers; the Director of Matola Provincial Hospital and Maputo Province Provincial Health Directorate for the support in implementing the study.
Funding
This work was supported by the National Institute of Health (NIH) under the Grant number AI127347. The funders of this study did not play a role in design of the study, collection, analysis and interpretation of data or in writing the manuscript.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Amzel A, Toska E, Lovich R, Widyono M, Patel T, Foti C, Dziuban EJ, Phelps BR, Sugandhi N, Mark D, & Altschuler J (2013). Promoting a combination approach to paediatric HIV psychosocial support. AIDS (London, England), 27(2), S147–S157. 10.1097/QAD.0000000000000098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagenda A, Barlow-Mosha L, Bagenda D, Sakwa R, Fowler MG, & Musoke PM (2011). Adherence to tablet and liquid formulations of antiretroviral medication for paediatric HIV treatment at an urban clinic in Uganda. Annals of Tropical Paediatrics, 31(3), 235–245. 10.1179/1465328111Y.0000000025 [DOI] [PubMed] [Google Scholar]
- Bandali S (2011). Norms and practices within marriage which shape gender roles, HIV/AIDS risk and risk reduction strategies in Cabo Delgado, Mozambique. AIDS Care, 23(9), 1171–1176. 10.1080/09540121.2011.554529 [DOI] [PubMed] [Google Scholar]
- Bennetts A, Shaffer N, Manopaiboon C, Chaiyakul P, Siriwasin W, Mock P, Klumthanom K, Sorapipatana S, Yuvasevee C, Jalanchavanapate S, & Clark L (1999). Determinants of depression and HIV-related worry among HIV-positive women who have recently given birth, Bangkok, Thailand. Social Science & Medicine, 49(6), 737–749. 10.1016/S02779536(99)00108-2 [DOI] [PubMed] [Google Scholar]
- Bwirire LD, Fitzgerald M, Zachariah R, Chikafa V, Massaquoi M, Moens M, Kamoto K, & Schouten EJ (2008). Reasons for loss to follow-up among mothers registered in a prevention-of-mother-to-child transmission program in rural Malawi. Transactions of The Royal Society of Tropical Medicine and Hygiene, 102(12), 1195–1200. 10.1016/j.trstmh.2008.04.002 [DOI] [PubMed] [Google Scholar]
- Calmy A, Ford N, Hirschel B, Reynolds SJ, Lynen L, Goemaere E, de la Vega FG, Perrin L, & Rodriguez W (2007). HIV viral load monitoring in resource-limited regions: Optional or necessary? Clinical Infectious Diseases, 44(1), 128–134. 10.1086/510073 [DOI] [PubMed] [Google Scholar]
- Campbell C, Skovdal M, Mupambireyi Z, Madanhire C, Nyamukapa C, & Gregson S (2012). Building adherence-competent communities: Factors promoting children’s adherence to anti-retroviral HIV/AIDS treatment in rural Zimbabwe. Health & Place, 18(2), 123–131. 10.1016/j.healthplace.2011.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MK, Goodall R, Judd A, Klein N, Chiappini E, Klimkait T, Ngo-Giang-Huong N, Palma P, Rossi P, Thorne C, Turkova A, Zangari P, Fraaij PL, Pajkrt D, Marques L, Collins IJ, Gibb DM, Gonzalez-Tome MI, Navarro ML, … Babiker AGA (2019). Predictors of faster virological suppression in early treated infants with perinatal HIV from Europe and Thailand. AIDS 2019, 33(7), 1155–1165. 10.1097/QAD.0000000000002172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappini E, Galli L, Tovo P-A, Gabiano C, Gattinara GC, Guarino A, Baddato R, Giaquinto C, Lisi C, & de Martino M (2006). Virologic, immunologic, and clinical benefits from early combined antiretroviral therapy in infants with perinatal HIV-1 infection. AIDS, 20(2), 207–215. 10.1097/01.aids.0000200529.64113.3e [DOI] [PubMed] [Google Scholar]
- Cruz MLS, Cardoso CAA, Darmont MQ, Souza E, Andrade SD, D’Al Fabbro MM, Fonseca R, Bellido JG, Monteiro SS, & Bastos FI (2014). Viral suppression and adherence among HIV-infected children and adolescents on antiretroviral therapy: Results of a multicenter study. Jornal de Pediatria, 90(6), 563–571. 10.1016/j.jped.2014.04.007 [DOI] [PubMed] [Google Scholar]
- Davies M-A, Boulle A, Fakir T, Nuttall J, & Eley B (2008). Adherence to antiretroviral therapy in young children in Cape Town, South Africa, measured by medication return and caregiver self-report: A prospective cohort study. BMC Pediatrics, 8(1), 34. 10.1186/1471-2431-8-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff P, Kipp W, Wild TC, Rubaale T, & Okech-Ojony J (2010). Barriers to accessing highly active antiretroviral therapy by HIV-positive women attending an antenatal clinic in a regional hospital in western Uganda. Journal of the International AIDS Society, 13(1), 37. 10.1186/1758-2652-13-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetzer BC, Mupenda B, Lusiama J, Kitetele F, Golin C, & Behets F (2011). Barriers to and facilitators of adherence to pediatric antiretroviral therapy in a Sub-Saharan setting: Insights from a qualitative study. AIDS Patient Care and STDs, 25(10), 611–621. 10.1089/apc.2011.0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb DM, Goodall RL, Giacomet V, Mcgee L, Compagnucci A, Lyall H, & Paediatric European Network for Treatment of AIDS Steering Committee. (2003). Adherence to prescribed antiretroviral therapy in human immunodeficiency virus-infected children in the PENTA 5 trial. The Pediatric Infectious Disease Journal, 22(1),56. 10.1097/00006454-200301000-00015 [DOI] [PubMed] [Google Scholar]
- Gigliotti F, Murante BL, & Weinberg GA (2001). Short course directly observed therapy to monitor compliance with antiretroviral therapy in human immunodeficiency virus-infected children. The Pediatric Infectious Disease Journal, 20(7), 716. 10.1097/00006454-200107000-00017 [DOI] [PubMed] [Google Scholar]
- Gilks CF, Crowley S, Ekpini R, Gove S, Perriens J, Souteyrand Y, Sutherland D, Vitoria M, Guerma T, & De Cock K (2006). The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. The Lancet, 368(9534), 505–510. 10.1016/S0140-6736(06)69158-7 [DOI] [PubMed] [Google Scholar]
- Goetghebuer T, Haelterman E, Le Chenadec J, Dollfus C, Gibb D, Judd A, Green H, Galli L, Ramos JT, Giaquinto C, Warszawski J, Levy J, & Group, for the E. I. C. (2009). Effect of early antiretroviral therapy on the risk of AIDS/death in HIV-infected infants. AIDS, 23 (5), 597. 10.1097/QAD.0b013e328326ca37 [DOI] [PubMed] [Google Scholar]
- Haberer J, & Mellins C (2009). Pediatric adherence to HIV antiretroviral therapy. Current HIV/AIDS Reports, 6(4), 194–200. 10.1007/s11904-009-0026-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberer JE, Cook A, Walker AS, Ngambi M, Ferrier A, Mulenga V, Kityo C, Thomason M, Kabamba D, Chintu C, Gibb DM, & Bangsberg DR (2011). Excellent adherence to antiretrovirals in HIV+ Zambian children is compromised by Disrupted Routine, HIV non-disclosure, and paradoxical income effects. PLOS ONE, 6(4), e18505. 10.1371/journal.pone.0018505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson I, Plummer ML, Konopka SN, Colvin CJ, Jonas E, Albertini J, Amzel A, & Fogg KP (2014). A systematic review of individual and contextual factors affecting ART initiation, adherence, and retention for HIV-infected pregnant and postpartum women. PLOS ONE, 9(11), e111421. 10.1371/journal.pone.0111421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey JM, Genberg BL, Keter A, Musick B, Apondi E, Gardner A, Hogan JW, & Wools-Kaloustian K (2019). Viral suppression among children and their caregivers living with HIV in western Kenya. Journal of the International AIDS Society, 22(4), e25272. 10.1002/jia2.25272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MH, Tembo TA, Mazenga A, Yu X, Myer L, Sabelli R, Flick R, Hartig M, Wetzel E, Simon K, Ahmed S, Nyirenda R, Kazembe PN, Mphande M, Mkandawire A, Chitani MJ, Markham C, Ciaranello A, & Abrams EJ (2020). The video intervention to inspire treatment adherence for life (VITAL start): protocol for a multisite randomized controlled trial of a brief video-based intervention to improve antiretroviral adherence and retention among HIV-infected pregnant women in Malawi. Trials, 21(1), 207. 10.1186/s13063-020-4131-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahai M, James PB, Wannang NN, Wurie HR, Conteh S, Bah AJ, & Samai M (2020). A cross-sectional study on caregivers’ perspective of the quality of life and adherence of paediatric HIV patients to highly active anti-retroviral therapy. BMC Pediatrics, 20(1), 286. 10.1186/s12887-020-02194-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeper SC, Montague BT, Friedman JF, & Flanigan TP (2010). Lessons learned from family-centred models of treatment for children living with HIV: Current approaches and future directions. Journal of the International AIDS Society, 13(2), S3. 10.1186/1758-2652-13-S2-S3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavhu W, Berwick J, Chirawu P, Makamba M, Copas A, Dirawo J, Willis N, Araya R, Abas MA, Corbett EL, Mungofa S, Laver SM, & Cowan FM (2013). Enhancing psychosocial support for HIV positive adolescents in Harare, Zimbabwe. PLOS ONE, 8(7), e70254. 10.1371/journal.pone.0070254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar JR, Bengu N, Fillis R, Sprenger K, Ntlantsana V, Vieira VA, Khambati N, Archary M, Muenchhoff M, Groll A, Grayson N, Adamson J, Govender K, Dong K, Kiepiela P, Walker BD, Bonsall D, Connor T, Bull MJ, … Goulder P (2020). High-frequency failure of combination antiretroviral therapy in paediatric HIV infection is associated with unmet maternal needs causing maternal non-adherence. EClinicalMedicine, 22, 100344. 10.1016/j.eclinm.2020.100344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MISAU. (2013). Plano de Aceleração da resposta ao HIV SIDA em Moçambique 2013–2015 [Acceleration plan for HIV/AIDS response in Mozambique 2013–2015]. http://www.misau.gov.mz/index.php/planos-estrategicos-do-hiv
- MISAU. (2015a). Directriz, Implementação da carga viral de HIV em Moçambique [Guidelines for HIV viral load implementation in Mozambique]. http://www.misau.gov.mz/index.php/directrizes-nacionais
- MISAU. (2015b). Directriz Nacional de Apoio Psicossocial e Prevenção Positiva [National Guidelines on Psychosocial Support and Positive Prevention]. http://www.misau.gov.mz/index.php/guioes-de-cuidados-e-tratamento
- MISAU. (2015c). Guião de Actividades de Apoio Psicossocial e Prevenção Positiva [Manual for Psychosocial Support and Positive Prevention Activities]. http://www.misau.gov.mz/index.php/guioes-de-cuidados-e-tratamento
- MISAU. (2018). Directrizes para engajamento do homen nos cuidados de saúde [Guidelines for male engagement in health care]. http://www.misau.gov.mz/index.php/directrizes-nacionais
- MISAU. (2020). Relatório Anual das Actividades Relacionadas ao HIV/SIDA [Annual Report of HIV/AIDS activities]—2019. http://www.misau.gov.mz/index.php/relatorios-anuais#
- MISAU, INE, & ICF. (2018). Inquérito de Indicadores de Imunização, Malária e HIV/SIDA em Moçambique [Immunization, Malaria and HIV/AIDS Survey in Mozambique] (IMASIDA) 2015. https://dhsprogram.com/publications/publication-ais12-ais-final-reports.cfm
- Müller AD, Bode S, Myer L, Roux P, & von Steinbüchel N (2008). Electronic measurement of adherence to pediatric antiretroviral therapy in South Africa. The Pediatric Infectious Disease Journal, 27(3), 257–262. 10.1097/INF.0b013e31815b1ad4 [DOI] [PubMed] [Google Scholar]
- Myer L, Smit J, Roux LL, Parker S, Stein DJ, & Seedat S (2008). Common mental disorders among HIV-infected individuals in South Africa: Prevalence, predictors, and validation of brief psychiatric rating scales. AIDS Patient Care and STDs, 22(2), 147–158. 10.1089/apc.2007.0102 [DOI] [PubMed] [Google Scholar]
- Naar-King S, Arfken C, Frey M, Harris M, Secord E, & Ellis D (2006). Psychosocial factors and treatment adherence in paediatric HIV/AIDS. AIDS Care, 18(6), 621–628. 10.1080/09540120500471895 [DOI] [PubMed] [Google Scholar]
- Nabukeera-Barungi N, Kalyesubula I, Kekitiinwa A, Byakika-Tusiime J, & Musoke P (2007). Adherence to antiretroviral therapy in children attending Mulago Hospital, Kampala. Annals of Tropical Paediatrics, 27(2), 123–131. 10.1179/146532807X192499 [DOI] [PubMed] [Google Scholar]
- Nasuuna E, Kigozi J, Muwanguzi PA, Babirye J, Kiwala L, Muganzi A, Sewankambo N, & Nakanjako D (2019). Challenges faced by caregivers of virally non-suppressed children on the intensive adherence counselling program in Uganda: A qualitative study. BMC Health Services Research, 19(1), 150. 10.1186/s12913-019-3963-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Gorman DA, Nyirenda LJ, & Theobald SJ (2010). Prevention of mother-to-child transmission of HIV infection: Views and perceptions about swallowing nevirapine in rural Lilongwe, Malawi. BMC Public Health, 10(1), 354. 10.1186/1471-2458-10-354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter L (2010). An introduction to family-centred services for children affected by HIV and AIDS. Journal of the International AIDS Society, 13(2), S1. 10.1186/1758-2652-13-S2-S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter LM, Sherr L, Adato M, Belsey M, Chandan U, Desmond C, Drimie S, Haour-Knipe M, Hosegood V, Kimou J, Madhavan S, Mathambo V, & Wakhweya A (2009). Strengthening families to support children affected by HIV and AIDS. AIDS Care, 21(sup1), 3–12. 10.1080/09540120902923121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi S, Cotugno N, Pallikkuth S, Pahwa R, Palma P, & Pahwa S (2018). Time of ART initiation in perinatally HIV-infected children impacts on HIV-specific T cell functionality. The Journal of Immunology, 200(1 Supplement), 166.1–166.1. [Google Scholar]
- Rochat TJ, Bland R, Coovadia H, Stein A, & Newell M-L (2011). Towards a family-centered approach to HIV treatment and care for HIV-exposed children, their mothers and their families in poorly resourced settings. Future Virology, 6(6), 687–696. 10.2217/fvl.11.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarem Ernesto A, Muller Banzato Pinto de Lemos R, Huehara MI, Moreno Morcillo A, dos Santos Vilela MM, & Nolasco da Silva MT (2012). Usefulness of pharmacy dispensing records in the evaluation of adherence to antiretroviral therapy in Brazilian children and adolescents. The Brazilian Journal of Infectious Diseases, 16(4), 315–320. 10.1016/j.bjid.2012.06.006 [DOI] [PubMed] [Google Scholar]
- Sawyer A, Ayers S, & Smith H (2010). Pre- and postnatal psychological wellbeing in Africa: A systematic review. Journal of Affective Disorders, 123(1–3), 17–29. 10.1016/j.jad.2009.06.027 [DOI] [PubMed] [Google Scholar]
- Seth A, Gupta R, Chandra J, Maheshwari A, Kumar P, & Aneja S (2014). Adherence to antiretroviral therapy and its determinants in children with HIV infection – Experience from Paediatric Centre of Excellence in HIV Care in North India. AIDS Care, 26(7), 865–871. 10.1080/09540121.2013.859649 [DOI] [PubMed] [Google Scholar]
- Spielman KL, Soler-Hampejsek E, Muula AS, Tenthani L, & Hewett PC (2021). Depressive symptoms, HIV-related stigma and ART adherence among caregivers of children in vulnerable households in rural southern Malawi. PLOS ONE, 16(3), e0247974. 10.1371/journal.pone.0247974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ssanyu JN, Nakafeero M, & Nuwaha F (2020). Multi-measure assessment of adherence to antiretroviral therapy among children under five years living with HIV in Jinja, Uganda. BMC Public Health, 20(1), 1319. 10.1186/s12889-020-09430-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer EM, Meltzer-Brody S, Kasaro M, Stuebe AM, Wiegand S, Paul R, & Stringer JSA (2014). Depression, pregnancy, and HIV: The case to strengthen mental health services for pregnant and post-partum women in sub-Saharan Africa. The Lancet Psychiatry, 1(2), 159–162. 10.1016/S2215-0366(14)70273-1 [DOI] [PubMed] [Google Scholar]
- Struyf T, Dube Q, Cromwell EA, Sheahan AD, Heyderman RS, & Van Rie A (2020). The effect of HIV infection and exposure on cognitive development in the first two years of life in Malawi. European Journal of Paediatric Neurology, 25, 157–164. 10.1016/j.ejpn.2019.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale CA, Abrams EJ, Coovadia A, Strehlau R, Martens L, & Kuhn L (2013). Adherence and viral suppression among infants and young children initiating protease inhibitor-based antiretroviral therapy. The Pediatric Infectious Disease Journal, 32(5), 489–494. 10.1097/INF.0b013e31827e84ba [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale CA, & Besser MJ (2008). Enhancing PMTCT programmes through psychosocial support and empowerment of women: The mothers2mothers model of care. Southern African Journal of HIV Medicine, 9(1), 60–64. [Google Scholar]
- Teasdale CA, Sogaula N, Yuengling KA, Wang C, Mutiti A, Arpadi S, Nxele M, Pepeta L, Mogashoa M, Rivadeneira ED, & Abrams EJ (2018). HIV viral suppression and longevity among a cohort of children initiating antiretroviral therapy in Eastern Cape, South Africa. Journal of the International AIDS Society, 21(8), e25168. 10.1002/jia2.25168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong PD,Atuhairwe C,&Taremwa IM(2020).Differential self-reported determinants to antiretroviral therapy adherence: Findings from caregivers of children under five years living with human immunodeficiency virus infection attending Al-Sabah Hospital, South Sudan. HIV/AIDS, 12, 175–186. 10.2147/HIV.S248057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujiji OA, Ekström AM, Ilako F, Indalo D, Wamalwa D, & Rubenson B (2011). Reasoning and deciding PMTCT-adherence during pregnancy among women living with HIV in Kenya. Culture, Health & Sexuality, 13(7), 829–840. 10.1080/13691058.2011.583682 [DOI] [PubMed] [Google Scholar]
- Van Winghem J, Telfer B, Reid T, Ouko J, Mutunga A, Jama Z, & Vakil S (2008). Implementation of a comprehensive program including psycho-social and treatment literacy activities to improve adherence to HIV care and treatment for a pediatric population in Kenya. BMC Pediatrics, 8(1), 52. 10.1186/1471-2431-8-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga C, Sherman G, Maphosa J, & Jones S (2005). Psychosocial consequences of early diagnosis of HIV status in vertically exposed infants in Johannesburg, South Africa. Health Care for Women International, 26(5), 387–397. 10.1080/07399330590933935 [DOI] [PubMed] [Google Scholar]
- Verma D, Bachani D, Acharya AS, Seth A, & Hemal A (2020). Factors affecting adherence to treatment in children living with HIV. Indian Journal of Sexually Transmitted Diseases and AIDS, 41(2), 181–187. 10.4103/ijstd.IJSTD_43_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, Jean-Philippe P, & McIntyre JA (2008). Early antiretroviral therapy and mortality among HIV-infected infants. New England Journal of Medicine, 359(21), 2233–2244. 10.1056/NEJMoa0800971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreeman RC, Wiehe SE, Pearce EC, & Nyandiko WM (2008). A systematic review of pediatric adherence to antiretroviral therapy in low- and middle-income countries. The Pediatric Infectious Disease Journal, 27(8), 686. 10.1097/INF.0b013e31816dd325 [DOI] [PubMed] [Google Scholar]
- WHO. (2016). Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach. http://apps.who.int/iris/bitstream/10665/208825/1/9789241549684_eng.pdf [PubMed]


