Letter
Drug discovery and development is a multi-phase process that takes long years (10–15) of experimenting and optimization with roughly $1–2 billion for every new drug to be approved by Food and Drug Administration (FDA) (Hinkson et al., 2020). Target validation, compound screening, and lead optimization are the first three in silico and in vitro experimentation processes that precede in vivo investigation. Once successfully passed, the drug candidates go into preclinical tests performed on lab animals. This is followed by entry to the three-consecutive clinical trials (phases I–III) which decide the major outcome of the drug in hand. Nevertheless, from drug approval to launch of marketing and distribution, it takes about 1.5 years on average (Dowden and Munro, 2019). For any pharma company or academic institution, the advancement to phase I is an outstanding achievement bypassing rigorous drug optimization stages and preclinical evaluation. Notably, about 90% of all drugs approval failures are upon phase I advance (Takebe et al., 2018). Such high failure rate is traced to 4 main reasons: (i) lack/poor clinical efficacy (accounting for 40–50%), (ii) unbearable toxicity (30%), (iii) weak pharmacokinetic and druglikeness properties (10–15%) and (iv) poor financial and management strategy (10%) (Sun et al., 2022).
In 2022, FDA has listed new 37 approvals for new drugs (https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2022, accessed on January 18). The list can be grouped into 10 groups based on the disease they treat as illustrated in Fig. 1. The majority of approved drugs fall in cancer treatment (31%) which targets many types and states of cancer. This involves non-Hodgkin lymphoma, non-small cell lung cancer, relapsed or refractory acute myeloid leukemia, recurrent ovarian cancer that is resistant to platinum therapy and unresectable hepatocellular carcinoma, among others. Given that cancer therapeutics are indispensable, chemotherapy FDA-approvals gradually replace the first-line care products. Indeed, between 2016, and 2021, there were 207 FDA drug approvals in oncology and malignant hematology. Among those, 28 drugs (14%) were standard-care displacing therapeutics (Benjamin et al., 2022). The second most targeted disease was neuropathies (14%) such as amyotrophic lateral sclerosis and polyneuropathy of hereditary transthyretin-mediated amyloidosis. Besides, the list also involved gadopiclenol and hyperpolarized Xe-129 designed for CNS and pulmonary diagnostic purposes. Surprisingly, the list also included enzyme replacement therapy of sphingomyelinase as well as pyruvate kinase deficiencies.
Fig. 1.
Disease categories targeted by the newly approved drugs.
With respect to the drug entity, the newly approved drugs can be grouped into 7 groups as elucidated in Fig. 2. Approximately 53% (19 out of 37) of which is small molecules followed by monoclonal antibody (mAb, 25%) and 6% enzymes. This is no surprise as it is evident that 35 years on from the FDA’s approval of the first mAb, these biologics represent nearly a fifth of the agency’s new drug approvals each year (Mullard, 2021). Notably, this year also witnessed the introduction of short interfering RNA (siRNA) approval to treat polyneuropathy of hereditary transthyretin-mediated amyloidosis. After a decade from the first siRNA approval, only few siRNAs got approved by FDA. Although the demonstrated potency in vitro, its clinical in vivo translation forms an obstacle due to some issues concerning mainly target selection and sequence design, delivery to the intended site besides the negative side effects (Friedrich and Aigner, 2022). The number of approvals is decreasing year after year given that there were 53, 50 and 37 approvals in the last three years (Kinch et al., 2022). It is worth noting that no vaccine nor small molecules targeting coronavirus disease-19 (COVID-19) were listed despite the ongoing infection waves and the emergence of novel variants.
Fig. 2.
Drug structure type of the newly approved drugs.
Since the small molecule category was the category having the largest drug entities (53%), the individual drug entities were given in some details in Table 1.
Table 1.
List of drug entities of small molecule cateogry along with the corresponding structure and indication.
| Category | Molecule | Structure | Indication |
|---|---|---|---|
| Infectious disease | Lenacapavir |  |
Anti-HIV |
| Oteseconazole | 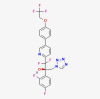 |
Vulvovaginal candidiasis | |
| Cancer | Adagrasib |  |
Non-small cell lung cancer |
| Olutasidenib |  |
Acute myeloid leukemia | |
| Futibatinib | 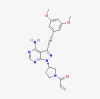 |
Intrahepatic cholangiocarcinoma | |
| Eflapegrastim | 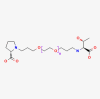 |
Decrease the incidence of infection in patients with non-myeloid malignancies receiving myelosuppressive anti-cancer drugs | |
| Lutetium (177Lu) vipivotide tetraxetan | 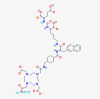 |
Prostate cancer | |
| Pacritinib | 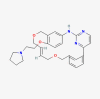 |
Myelofibrosis | |
| Neuropathy | Sodium phenylbutyrate/ | 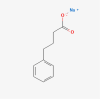 |
Amyotrophic lateral sclerosis |
| taurursodiol | 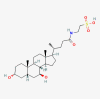 |
||
| Daridorexant | 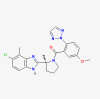 |
Insomnia | |
| Ganaxolone | 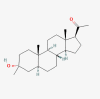 |
Seizures in cyclin-dependent kinase-like 5 deficiency | |
| Omidenepag isopropyl |  |
Glaucoma | |
| Gadopiclenol |  |
CNS imaging | |
| Kidney disease | Terlipressin |  |
Improves kidney function in hepatorenal syndrome |
| Bone disease | Deucravacitinib | 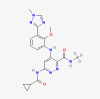 |
Plaque psoriasis |
| Tapinarof |  |
Plaque psoriasis | |
| Diabetes | Tirzepatide |  |
Diabetes |
| Cardiovascular disease | Mavacamten |  |
Obstructive hypertrophic cardiomyopathy |
| Skin care products | Abrocitinib | 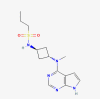 |
Atopic dermatitis |
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Benjamin D.J., Xu A., Lythgoe M.P., Prasad V. Cancer Drug Approvals That Displaced Existing Standard-of-Care Therapies, 2016–2021. JAMA Netw. Open. 2022;5:e222265. doi: 10.1001/jamanetworkopen.2022.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowden H., Munro J. Trends in clinical success rates and therapeutic focus. Nat. Rev. Drug Discov. 2019;18:495–496. doi: 10.1038/d41573-019-00074-z. [DOI] [PubMed] [Google Scholar]
- Friedrich M., Aigner A. Therapeutic siRNA: State-of-the-Art and Future Perspectives. BioDrugs. 2022;36:549–571. doi: 10.1007/s40259-022-00549-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkson I.V., Madej B., Stahlberg E.A. Accelerating therapeutics for opportunities in medicine: a paradigm shift in drug discovery. Front. Pharmacol. 2020;11:770. doi: 10.3389/fphar.2020.00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinch M.S., Kraft Z., Schwartz T. 2021 in review: FDA approvals of new medicines. Drug Discov. Today. 2022;27:2057–2064. doi: 10.1016/j.drudis.2022.04.010. [DOI] [PubMed] [Google Scholar]
- Mullard A. FDA approves 100th monoclonal antibody product. Nat. Rev. Drug Discov. 2021;20:491–495. doi: 10.1038/d41573-021-00079-7. [DOI] [PubMed] [Google Scholar]
- Sun D., Gao W., Hu H., Zhou S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B. 2022 doi: 10.1016/j.apsb.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebe T., Imai R., Ono S. The current status of drug discovery and development as originated in United States academia: the influence of industrial and academic collaboration on drug discovery and development. Clin. Transl. Sci. 2018;11:597–606. doi: 10.1111/cts.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]




