Key Points
Question
Is maternal tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccination during pregnancy associated with a decrease in pertussis incidence among US infants younger than 2 months?
Findings
In this ecologic study of 57 460 pertussis cases reported in infants younger than 1 year between 2000 and 2019, pertussis incidence among infants younger than 2 months declined following maternal Tdap vaccination introduction; no similar decrease occurred among infants aged 6 months to less than 12 months. Overall, there was a significant difference between incidence rate differences between the pre–maternal and post–maternal Tdap vaccination periods.
Meaning
These findings suggest that maternal Tdap vaccination is associated with a reduction in pertussis among infants younger than 2 months, the strategy’s target age group.
Abstract
Importance
Infants younger than 1 year have the highest burden of pertussis morbidity and mortality. In 2011, the US introduced tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccination during pregnancy to protect infants before vaccinations begin.
Objective
To assess the association of maternal Tdap vaccination during pregnancy with the incidence of pertussis among infants in the US.
Design, Setting, and Participants
In this ecologic study, a time-trend analysis was performed of infant pertussis cases reported through the National Notifiable Diseases Surveillance System between January 1, 2000, and December 31, 2019, in the US. Statistical analysis was performed from April 1, 2020, to October 31, 2022.
Exposures
Maternal Tdap vaccination during pregnancy.
Main Outcomes and Measures
Pertussis incidence rates were calculated and compared between 2 periods—the pre–maternal Tdap vaccination period (2000-2010) and the post–maternal Tdap vaccination period (2012-2019)—for 2 age groups: infants younger than 2 months (target group of maternal vaccination) and infants aged 6 months to less than 12 months (comparison group). Incidence rate differences between the 2 age groups were modeled using weighted segmented linear regression. The slope difference between the 2 periods was estimated to assess the association of maternal Tdap vaccination with pertussis incidence among infants.
Results
A total of 57 460 pertussis cases were reported in infants younger than 1 year between 2000 and 2019; 19 322 cases (33.6%) were in infants younger than 2 months. During the pre–maternal Tdap vaccination period, annual pertussis incidence did not change among infants younger than 2 months (slope, 3.29 per 100 000 infants per year; P = .28) but increased slightly among infants aged 6 months to less than 12 months (slope, 2.10 per 100 000 infants per year; P = .01). There was no change in the difference in incidence between the 2 age groups (slope, 0.08 per 100 000 infants per year; P = .97) during the pre–maternal Tdap vaccination period overall. However, in the post–maternal Tdap vaccination period, incidence decreased among infants younger than 2 months (slope, −14.53 per 100 000 infants per year; P = .001) while remaining unchanged among infants aged 6 months to less than 12 months (slope, 1.42 per 100 000 infants per year; P = .29). The incidence rate difference between the 2 age groups significantly decreased during the post–maternal Tdap vaccination period (slope, −14.43 per 100 000 infants per year; P < .001). Pertussis incidence rate differences were significantly different between the pre–maternal and post–maternal Tdap vaccination periods (slope difference, −14.51 per 100 000 infants per year; P = .01).
Conclusions and Relevance
In this study, following maternal Tdap vaccine introduction, a sustained decrease in pertussis incidence was observed among infants younger than 2 months, narrowing the incidence gap with infants aged 6 months to less than 12 months. These findings suggest that maternal Tdap vaccination is associated with a reduction in pertussis burden in the target age group (<2 months) and that further increases in coverage may be associated with additional reductions in infant disease.
This ecologic study assesses the association of maternal tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccination during pregnancy with the incidence of pertussis among infants in the US from 2000 to 2019.
Introduction
Despite the introduction and widespread use of effective vaccines for infants, children, and adolescents, pertussis continues to cause substantial morbidity and mortality in the US. Pertussis can affect persons of all ages, but infants have the highest burden of disease and an elevated risk of severe pertussis-related morbidity and mortality. Although coverage of the 3-dose infant pertussis primary diphtheria, tetanus toxoid, and acellular pertussis (DTaP) vaccine series has consistently remained high in the US,1 vaccination is not recommended until 2 months of age, leaving infants susceptible to disease during the early months of life.2 In 2011, the US introduced tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccination during pregnancy to protect infants, specifically those who are too young for vaccination, against pertussis3; in October 2012, the recommendation was expanded to include a dose of Tdap vaccine during every pregnancy, preferably from 27 to 36 weeks’ gestation.4
Maternal vaccination during pregnancy is a safe and effective strategy for preventing pertussis among infants younger than 2 months.5 Postlicensure vaccine effectiveness studies have estimated the strategy to be 69% to 93% effective at preventing infant pertussis6,7,8,9,10,11; higher effectiveness has been observed against severe outcomes such as hospitalization.7,10,11 Tdap vaccine coverage among pregnant women has increased since introduction of the strategy, and as of 2019, 54.9% of US pregnant women had received a dose of Tdap vaccine during pregnancy.12 Although numerous studies have evaluated the effectiveness of maternal Tdap vaccination for preventing infant pertussis, less is known about the population-level association of this strategy with the reported pertussis burden in infants, especially in the US.13,14,15,16,17 We analyzed national surveillance data from 2000 to 2019 to assess the association of Tdap vaccination during pregnancy with infant pertussis trends in the US.
Methods
In this ecologic study, we conducted a time-series analysis by analyzing case data reported through the National Notifiable Diseases Surveillance System between January 1, 2000, and December 31, 2019. Cases were classified by reporting states according to the Council of State and Territorial Epidemiologists case definition.18 The pertussis clinical case definition for cases reported during the study period required cough of 2 weeks or longer duration with paroxysms, inspiratory whoop, or posttussive vomiting. A confirmed case was defined as acute cough illness of any duration with isolation of Bordetella pertussis from culture or a clinical case with either positive polymerase chain reaction test results or epidemiologic linkage to a laboratory-confirmed case. Clinical cases with no laboratory confirmation or no epidemiologic linkage to a laboratory-confirmed case were classified as probable. In 2014, apnea was added to the clinical case definition for infants younger than 1 year, and the infant probable category was expanded to include acute cough illness of any duration with 1 or more of the aforementioned clinical symptoms and either polymerase chain reaction test confirmation or epidemiologic linkage to a laboratory-confirmed case. We included cases classified as confirmed or probable or with an unknown status in our analysis. This study was reviewed by the Centers for Disease Control and Prevention (CDC) and was conducted consistent with applicable federal law and CDC policy (ie, 45 CFR part 46.102(1)(2), 21 CFR. part 56; 42 USC §241(d); 5 USC §552a; and 44 USC §3501 et seq). This study was determined to meet the requirements of public health surveillance. In addition, this study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Statistical Analysis
Statistical analysis was performed from April 1, 2020, to October 31, 2022. Incidence rates (cases per 100 000 population) in the pre–maternal Tdap vaccination period (2000-2010) and post–maternal Tdap vaccination period (2012-2019) were calculated for 2 age groups: the target group of maternal vaccination, defined as infants younger than 2 months, and a comparison group of infants aged 6 months to less than 12 months, a group for which disease incidence is not expected to be associated with maternal vaccination. Incidence rates were calculated using population estimates from the CDC National Center for Health Statistics as denominators (bridged-race, vintage 2019 estimates).19 To obtain denominators for each infant age group, estimates for infants younger than 1 year were divided by 12 to get monthly denominators and were multiplied by infant age in months.
Incidence rates for each age group for the 2 maternal vaccination periods were modeled using weighted, segmented linear regression assuming a Poisson distribution; the weights were the inverse of the variances of incidence. Piecewise regression lines were joined at the year when the maternal vaccination recommendation was made (2011). Pertussis incidence trends, as indicated by the slope of the regression line, were compared for each maternal vaccination period for both the target (<2 months) and the comparison (6 to <12 months) age groups.
We used a difference-in-differences approach to further assess the association of maternal Tdap vaccination with infant pertussis incidence while accounting for trends related to factors other than maternal vaccination. Trends in incidence rate differences between the target age group (<2 months) and the comparison age group (6 to <12 months) were calculated. The regression slope for each maternal Tdap vaccination period and the slope difference between the pre–maternal and post–maternal Tdap vaccination periods were estimated using weighted segmented linear regression. No significant difference would be expected in the absence of an association of maternal vaccination with infant pertussis incidence. The year 2011 was excluded from this analysis since the recommendation was made later in the year and to account for the gradual uptake of the recommendation. We used SAS, version 9.4 (SAS Institute Inc) to conduct the statistical analyses. All P values were from 2-sided tests and results were deemed statistically significant at P < .05.
To ensure the robustness of the data, 2 sensitivity analyses were conducted. In 1 analysis, infants aged 2 months to less than 12 months and children aged 1 to 6 years were used as comparison groups, and in the other analysis, the year 2011 was not excluded from the model.
Results
A total of 57 460 pertussis cases were reported in infants younger than 1 year between 2000 and 2019; 46 762 (81.4%) were classified as confirmed, 10 624 (18.5%) were classified as probable, and 74 (0.1%) were classified as unknown. A total of 19 322 cases (33.6%) occurred among infants younger than 2 months.
During the pre–maternal Tdap vaccination period, the mean annual pertussis incidence among infants younger than 2 months was 165.3 per 100 000 infants, with large fluctuations but no significant trend in annual incidence during this period (slope, 3.29 per 100 000 infants per year; P = .28). In the post–maternal Tdap vaccination period, incidence among infants younger than 2 months decreased, largely due to the steady decrease from 205.4 per 100 000 infants in 2012 to 75.4 per 100 000 infants in 2016. Incidence then stabilized at 80.9 per 100 000 infants during the latter part of the post–maternal Tdap vaccination period (2017-2019). Overall, the mean annual incidence of pertussis among infants younger than 2 months was 121.8 per 100 000 infants, and the annual pertussis incidence decreased significantly during the post–maternal Tdap vaccination period (slope, −14.53 per 100 000 infants per year; P = .001). The change in incidence trends was significant between the pre–maternal and post–maternal Tdap vaccination periods among infants younger than 2 months (slope difference, −17.82 per 100 000 infants per year; P = .01).
Among infants aged 6 months to less than 12 months, mean annual pertussis incidence was 19.7 per 100 000 infants and annual incidence fluctuated less compared with that among infants younger than 2 months during the pre–maternal Tdap vaccination period. Throughout the pre–maternal Tdap vaccination period, incidence among infants aged 6 months to less than 12 months remained 4 to 12 times lower in any given year than incidence among infants younger than 2 months (Figure 1),12,20,21,22 and annual incidence rates increased slightly among infants aged 6 months to less than 12 months (slope, 2.10 per 100 000 infants per year; P = .01). Annual incidence rates did not change significantly among infants aged 6 months to less than 12 months during the post–maternal Tdap vaccination period (slope, 1.42 per 100 000 infants per year; P = .29). There was no change in incidence trends between the pre–maternal and post–maternal Tdap vaccination periods among infants aged 6 months to less than 12 months (slope, −0.68 per 100 000 infants per year; P = .72).
Figure 1. Annual Incidence of Reported Pertussis Among Infants Younger Than 2 Months and Infants Aged 6 Months to Less Than 12 Months, 2000-2019.

Maternal tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccination during pregnancy was introduced in the US in 2011. National coverage estimates of maternal Tdap vaccination for available years (beginning in 2014) were obtained through the Centers for Disease Control and Prevention’s internet panel survey.12,20,21,22 Changes in the internet panel survey methods may limit the ability to compare estimates for 2017 to 2018 with estimates from previous seasons.
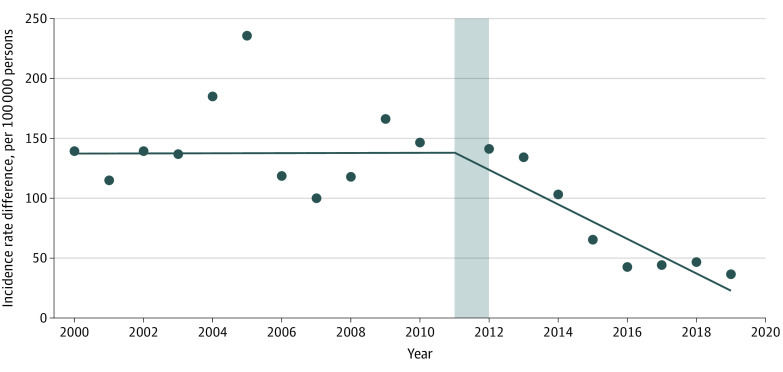
Between 2000 and 2010, there was no significant change in the difference in pertussis incidence between infants younger than 2 months and infants aged 6 months to less than 12 months (slope, 0.08 per 100 000 infants per year; P = .97); however, in the post–maternal Tdap vaccination period, the incidence rate difference between the 2 groups significantly decreased (slope, −14.43 per 100 000 infants per year; P < .001) (Figure 2). Overall, the difference between incidence rate differences between the pre–maternal and post–maternal Tdap vaccination periods was −14.51 per 100 000 infants per year (P = .01), suggesting an association of maternal vaccination with pertussis trends in infants younger than 2 months. Similar trends in incidence rate differences during both the pre–maternal and the post–maternal Tdap vaccination periods were obtained when using the comparison age groups of 2 to less than 12 months and 1 to 6 years and whether or not 2011 was included in the model.
Figure 2. Pertussis Incidence Difference Between Infants Younger Than 2 Months and Infants Aged 6 Months to Less Than 12 Months.
The gray area indicates the year of maternal tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccination introduction, which was excluded from the segmented regression analysis to account for the gradual uptake of the recommendation; the dots indicate observed differences in pertussis incidence between infants younger than 2 months and infants aged 6 months to less than 12 months; and the solid line indicates estimated differences in pertussis incidence between infants younger than 2 months and infants aged 6 months to less than 12 months.
Discussion
Given the documented high effectiveness of maternal vaccination for preventing infant pertussis that has been reported in the literature,5 coupled with the achievement of approximately 55% coverage among US pregnant women by 2019,12 our analysis of national surveillance data suggests that maternal Tdap vaccination is associated with a sustained decrease in pertussis incidence among infants too young to receive the primary childhood vaccination series. Prior to the introduction of maternal Tdap vaccination, the incidence of pertussis in infants younger than 2 months, the target age group of maternal vaccination, remained constant, but incidence then declined significantly in the post–maternal Tdap vaccination period. In addition, although pertussis incidence remained higher among infants younger than 2 months compared with infants aged 6 months to less than 12 months, the incidence difference between infants in these groups significantly decreased following the implementation of maternal Tdap vaccination during pregnancy. While changing DTaP coverage among young children may also contribute to fluctuations in pertussis incidence among infants aged 6 months to less than 12 months and may, therefore, have an association with the incidence difference between the 2 age groups, our study was conducted during a time of high and stable DTaP coverage among young children,1 further strengthening our findings.
Although uptake of maternal vaccination has progressed steadily in the US, there is early indication that coverage is beginning to plateau. In 2014, shortly after introduction of the strategy, coverage reached 27% of US pregnant women.20 By 2016, uptake of Tdap vaccination had almost doubled, reaching more than 48%.20 Although coverage increased to 54.9% by 2019, this represented only a 0.5% increase over the prior year.12,21 Our analysis found that, as Tdap vaccination coverage among pregnant women increased over time, the gap in pertussis incidence between infants younger than 2 months and infants aged 6 months to less than 12 months narrowed; further reductions in incidence might be achieved if a higher proportion of pregnant women are vaccinated.
Maternal vaccination during pregnancy is an effective strategy for preventing infant pertussis in the early months of life before infant DTaP vaccination begins6,7,8,9,10,11; however, studies have shown that passively transferred maternal antibodies from Tdap vaccination during pregnancy can result in blunting of an infant’s antibody response to the primary DTaP series.23,24,25,26,27,28,29,30,31,32,33,34,35 Importantly, the clinical relevance of antibody interference is yet to be determined. A slight increase in pertussis incidence was observed among infants aged 6 months to less than 12 months beginning in 2009, but this increase predated the introduction of maternal Tdap vaccination, and the incidence of pertussis among infants aged 6 months to less than 12 months decreased later in the post–maternal vaccination period even as coverage of maternal vaccination increased. In addition, we found that annual incidence rates did not change significantly across the entire post–maternal Tdap vaccination period. However, until more is understood about the clinical relevance of blunting, close monitoring of disease trends in older infant age groups will be important, especially as the coverage of maternal vaccination increases.
Limitations
This study has limitations. Maternal vaccination status was not available at the individual level in the surveillance data on reported infants with pertussis; however, our analysis used national notifiable disease data, the most representative source of pertussis disease trend data in the US, to assess the association of national maternal Tdap vaccination coverage during pregnancy with nationally reported infant pertussis trends. In addition, because vaccination history of the case patient is often incomplete or missing in the National Notifiable Diseases Surveillance System, we were unable to determine with certainty the proportion of infants younger than 2 months who received pertussis vaccines prior to 8 weeks of age. However, analysis of vaccine data that were available suggests that the estimated proportion was small overall (<0.2%) and did not cluster in any particular year of the study period.
Conclusions
In the context of an overall pertussis resurgence in the US and many countries worldwide during our study period,36,37,38,39 our data suggest that maternal Tdap vaccination is associated with a reduction in disease burden among the youngest and most vulnerable age group (<2 months). However, with only modest gains in Tdap vaccination coverage among pregnant women in recent years and no national program for maternal immunization, additional work is needed to ensure further reductions in infant disease. Efforts should continue to place emphasis on increasing Tdap vaccination uptake through education of prenatal care practitioners and other key practitioners who may routinely interact with pregnant women in their practices, such as pediatricians and family practice physicians.12,40 As a health care practitioner recommendation is a factor associated with vaccination during pregnancy,41,42 it is important for these practitioners to clearly state their recommendation for vaccination and to communicate the benefits and risks of vaccination as well as listen to and respond directly to pregnant patients’ questions. Additional increases in the uptake of Tdap vaccination during pregnancy may be associated with further decreases in the incidence of pertussis among infants younger than 2 months.
Data Sharing Statement
References
- 1.Hill HA, Yankey D, Elam-Evans LD, Singleton JA, Pingali SC, Santibanez TA. Vaccination coverage by age 24 months among children born in 2016 and 2017—National Immunization Survey–Child, United States, 2017–2019. MMWR Morb Mortal Wkly Rep. 2020;69(42):1505-1511. doi: 10.15585/mmwr.mm6942a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liang JL, Tiwari T, Moro P, et al. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2018;67(2):1-44. doi: 10.15585/mmwr.rr6702a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) . Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) in pregnant women and persons who have or anticipate having close contact with an infant aged <12 months—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60(41):1424-1426. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) . Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep. 2013;62(7):131-135. [PMC free article] [PubMed] [Google Scholar]
- 5.Vygen-Bonnet S, Hellenbrand W, Garbe E, et al. Safety and effectiveness of acellular pertussis vaccination during pregnancy: a systematic review. BMC Infect Dis. 2020;20(1):136. doi: 10.1186/s12879-020-4824-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amirthalingam G, Andrews N, Campbell H, et al. Effectiveness of maternal pertussis vaccination in England: an observational study. Lancet. 2014;384(9953):1521-1528. doi: 10.1016/S0140-6736(14)60686-3 [DOI] [PubMed] [Google Scholar]
- 7.Amirthalingam G, Campbell H, Ribeiro S, et al. Sustained effectiveness of the maternal pertussis immunization program in England 3 years following introduction. Clin Infect Dis. 2016;63(suppl 4):S236-S243. doi: 10.1093/cid/ciw559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baxter R, Bartlett J, Fireman B, Lewis E, Klein NP. Effectiveness of vaccination during pregnancy to prevent infant pertussis. Pediatrics. 2017;139(5):e20164091. doi: 10.1542/peds.2016-4091 [DOI] [PubMed] [Google Scholar]
- 9.Dabrera G, Amirthalingam G, Andrews N, et al. A case-control study to estimate the effectiveness of maternal pertussis vaccination in protecting newborn infants in England and Wales, 2012-2013. Clin Infect Dis. 2015;60(3):333-337. doi: 10.1093/cid/ciu821 [DOI] [PubMed] [Google Scholar]
- 10.Skoff TH, Blain AE, Watt J, et al. Impact of the US maternal tetanus, diphtheria, and acellular pertussis vaccination program on preventing pertussis in infants <2 months of age: a case-control evaluation. Clin Infect Dis. 2017;65(12):1977-1983. doi: 10.1093/cid/cix724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saul N, Wang K, Bag S, et al. Effectiveness of maternal pertussis vaccination in preventing infection and disease in infants: the NSW Public Health Network case-control study. Vaccine. 2018;36(14):1887-1892. doi: 10.1016/j.vaccine.2018.02.047 [DOI] [PubMed] [Google Scholar]
- 12.Lindley MC, Kahn KE, Bardenheier BH, et al. Vital signs: burden and prevention of influenza and pertussis among pregnant women and infants—United States. MMWR Morb Mortal Wkly Rep. 2019;68(40):885-892. doi: 10.15585/mmwr.mm6840e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedrich F, Valadão MC, Brum M, et al. Impact of maternal dTpa vaccination on the incidence of pertussis in young infants. PLoS One. 2020;15(1):e0228022. doi: 10.1371/journal.pone.0228022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langsam D, Anis E, Haas EJ, et al. Tdap vaccination during pregnancy interrupts a twenty-year increase in the incidence of pertussis. Vaccine. 2020;38(12):2700-2706. doi: 10.1016/j.vaccine.2020.01.095 [DOI] [PubMed] [Google Scholar]
- 15.Vizzotti C, Juarez MV, Bergel E, et al. Impact of a maternal immunization program against pertussis in a developing country. Vaccine. 2016;34(50):6223-6228. doi: 10.1016/j.vaccine.2016.10.081 [DOI] [PubMed] [Google Scholar]
- 16.Kim G, Berry JG, Janes JL, Perez A, Hall M. Association of maternal Tdap recommendations with pertussis hospitalizations of young infants. Hosp Pediatr. 2022;12(3):e106-e109. doi: 10.1542/hpeds.2021-006323 [DOI] [PubMed] [Google Scholar]
- 17.Boulet SL, Chamberlain AT, Biswas HH, Jamieson DJ. Trends in infant pertussis hospitalizations in the United States, 2009-2017. JAMA. 2019;322(21):2134-2136. doi: 10.1001/jama.2019.15577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention . Pertussis (whooping cough) (Bordetella pertussis) 2020. case definition. Accessed December 19, 2022. https://ndc.services.cdc.gov/case-definitions/pertussis-2020/
- 19.Centers for Disease Control and Prevention . CDC WONDER: bridged-race population estimates. Accessed December 28, 2022. https://wonder.cdc.gov/bridged-race-population.html
- 20.Centers for Disease Control and Prevention . Pregnant women and Tdap vaccination, internet panel survey, United States, April 2016. Accessed November 3, 2022. https://www.cdc.gov/vaccines/imz-managers/coverage/adultvaxview/pubs-resources/tdap-report-2016.html
- 21.Kahn KE, Black CL, Ding H, et al. Influenza and Tdap vaccination coverage among pregnant women—United States, April 2018. MMWR Morb Mortal Wkly Rep. 2018;67(38):1055-1059. doi: 10.15585/mmwr.mm6738a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention . Pregnant women and Tdap vaccination, internet panel survey, United States, April 2017. Accessed November 3, 2022. https://www.cdc.gov/vaccines/imz-managers/coverage/adultvaxview/pubs-resources/tdap-report-2017.html
- 23.Maertens K, Hoang TTH, Nguyen TD, et al. The effect of maternal pertussis immunization on infant vaccine responses to a booster pertussis-containing vaccine in Vietnam. Clin Infect Dis. 2016;63(suppl 4):S197-S204. doi: 10.1093/cid/ciw551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abu-Raya B, Maertens K, Munoz FM, et al. Factors affecting antibody responses to immunizations in infants born to women immunized against pertussis in pregnancy and unimmunized women: individual-participant data meta-analysis. Vaccine. 2021;39(44):6545-6552. doi: 10.1016/j.vaccine.2021.09.022 [DOI] [PubMed] [Google Scholar]
- 25.Hardy-Fairbanks AJ, Pan SJ, Decker MD, et al. Immune responses in infants whose mothers received Tdap vaccine during pregnancy. Pediatr Infect Dis J. 2013;32(11):1257-1260. doi: 10.1097/INF.0b013e3182a09b6a [DOI] [PubMed] [Google Scholar]
- 26.Munoz FM, Bond NH, Maccato M, et al. Safety and immunogenicity of tetanus diphtheria and acellular pertussis (Tdap) immunization during pregnancy in mothers and infants: a randomized clinical trial. JAMA. 2014;311(17):1760-1769. doi: 10.1001/jama.2014.3633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoang HTT, Leuridan E, Maertens K, et al. Pertussis vaccination during pregnancy in Vietnam: results of a randomized controlled trial pertussis vaccination during pregnancy. Vaccine. 2016;34(1):151-159. doi: 10.1016/j.vaccine.2015.10.098 [DOI] [PubMed] [Google Scholar]
- 28.Ladhani SN, Andrews NJ, Southern J, et al. Antibody responses after primary immunization in infants born to women receiving a pertussis-containing vaccine during pregnancy: single arm observational study with a historical comparator. Clin Infect Dis. 2015;61(11):1637-1644. doi: 10.1093/cid/civ695 [DOI] [PubMed] [Google Scholar]
- 29.Kent A, Ladhani SN, Andrews NJ, et al. ; PUNS study group . Pertussis antibody concentrations in infants born prematurely to mothers vaccinated in pregnancy. Pediatrics. 2016;138(1):e20153854. doi: 10.1542/peds.2015-3854 [DOI] [PubMed] [Google Scholar]
- 30.Maertens K, Caboré RN, Huygen K, et al. Pertussis vaccination during pregnancy in Belgium: follow-up of infants until 1 month after the fourth infant pertussis vaccination at 15 months of age. Vaccine. 2016;34(31):3613-3619. doi: 10.1016/j.vaccine.2016.04.066 [DOI] [PubMed] [Google Scholar]
- 31.Maertens K, Caboré RN, Huygen K, Hens N, Van Damme P, Leuridan E. Pertussis vaccination during pregnancy in Belgium: results of a prospective controlled cohort study. Vaccine. 2016;34(1):142-150. doi: 10.1016/j.vaccine.2015.10.100 [DOI] [PubMed] [Google Scholar]
- 32.Halperin SA, Langley JM, Ye L, et al. A randomized controlled trial of the safety and immunogenicity of tetanus, diphtheria, and acellular pertussis vaccine immunization during pregnancy and subsequent infant immune response. Clin Infect Dis. 2018;67(7):1063-1071. doi: 10.1093/cid/ciy244 [DOI] [PubMed] [Google Scholar]
- 33.Abu-Raya B, Maertens K, Munoz FM, et al. The effect of tetanus-diphtheria-acellular-pertussis immunization during pregnancy on infant antibody responses: individual-participant data meta-analysis. Front Immunol. 2021;12:689394. doi: 10.3389/fimmu.2021.689394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perrett KP, Halperin SA, Nolan T, et al. Impact of tetanus-diphtheria-acellular pertussis immunization during pregnancy on subsequent infant immunization seroresponses: follow-up from a large randomized placebo-controlled trial. Vaccine. 2020;38(8):2105-2114. doi: 10.1016/j.vaccine.2019.10.104 [DOI] [PubMed] [Google Scholar]
- 35.Barug D, Pronk I, van Houten MA, et al. Maternal pertussis vaccination and its effects on the immune response of infants aged up to 12 months in the Netherlands: an open-label, parallel, randomised controlled trial. Lancet Infect Dis. 2019;19(4):392-401. doi: 10.1016/S1473-3099(18)30717-5 [DOI] [PubMed] [Google Scholar]
- 36.Skoff TH, Hadler S, Hariri S. The epidemiology of nationally reported pertussis in the United States, 2000-2016. Clin Infect Dis. 2019;68(10):1634-1640. doi: 10.1093/cid/ciy757 [DOI] [PubMed] [Google Scholar]
- 37.Macina D, Evans KE. Bordetella pertussis in school-age children, adolescents and adults: a systematic review of epidemiology and mortality in Europe. Infect Dis Ther. 2021;10(4):2071-2118. doi: 10.1007/s40121-021-00520-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clark TA. Changing pertussis epidemiology: everything old is new again. J Infect Dis. 2014;209(7):978-981. doi: 10.1093/infdis/jiu001 [DOI] [PubMed] [Google Scholar]
- 39.Tan T, Dalby T, Forsyth K, et al. Pertussis across the globe: recent epidemiologic trends from 2000 to 2013. Pediatr Infect Dis J. 2015;34(9):e222-e232. doi: 10.1097/INF.0000000000000795 [DOI] [PubMed] [Google Scholar]
- 40.Razzaghi H, Kahn KE, Black CL, et al. Influenza and Tdap vaccination coverage among pregnant women—United States, April 2020. MMWR Morb Mortal Wkly Rep. 2020;69(39):1391-1397. doi: 10.15585/mmwr.mm6939a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Myers KL. Predictors of maternal vaccination in the United States: an integrative review of the literature. Vaccine. 2016;34(34):3942-3949. doi: 10.1016/j.vaccine.2016.06.042 [DOI] [PubMed] [Google Scholar]
- 42.Kilich E, Dada S, Francis MR, et al. Factors that influence vaccination decision-making among pregnant women: a systematic review and meta-analysis. PLoS One. 2020;15(7):e0234827. doi: 10.1371/journal.pone.0234827 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Sharing Statement