Abstract
Among sleep-related disordered breathing events, hypopneas are the most frequent. Like obstructive and central apneas, hypopneas may be obstructive or central (reduced drive) in origin. Nevertheless, unlike apneas, categorizing hypopneas as either “obstructive” or “central” is often difficult or ambiguous. It has been suggested that hypopneas could be categorized as obstructive when associated with snoring, inspiratory flow limitation, or paradoxical thoraco-abdominal excursions. This approach, however, has not been extensively tested and misclassification of hypopneas is unavoidable. Yet, much rides on the accurate distinction of these events to guide therapy with medical devices or pharmacological therapy in each patient. Additionally, accurate hypopnea classification is critical for design of clinical trials, because therapeutic responses differ depending on the subtype of hypopnea. Correctly classifying hypopneas can also allay concerns about obtaining coverage for therapies that specifically target either central or obstructive sleep-disordered breathing events. The present paper expands on the current criteria for differentiating obstructive from central hypopneas and provides illustrative tracings that can help classify these events.
Citation:
Javaheri S, Rapoport DM, Schwartz AR. Distinguishing central from obstructive hypopneas on a clinical polysomnogram. J Clin Sleep Med. 2023;19(4):823–834.
Keywords: obstructive sleep apnea, central sleep apnea, polysomnography
INTRODUCTION
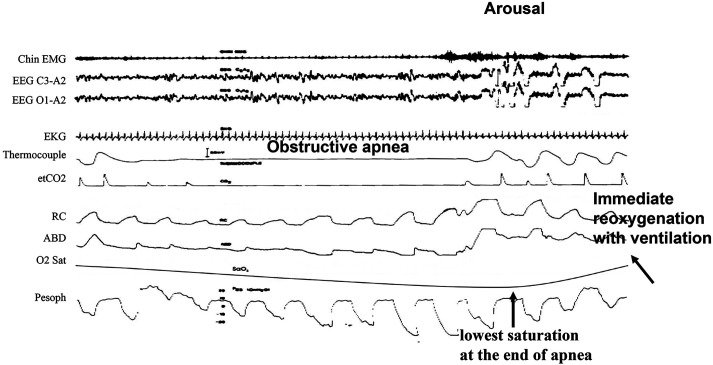
Classifying respiratory events as due either to obstruction or reduced central drive may be conceptually simple but can be challenging in practice; physiologically, schemes for defining obstruction are based on detecting either a reduced flow for a given effort or an increased effort for a given flow, suggesting airway obstruction. For apneas, obstructive can be distinguished from central events with reasonable accuracy based on the rule that in the absence of any flow whatsoever, obstruction is defined by any level of inspiratory effort. Figure 1 depicts an example of obstructive apnea.1 Please note the presence of paradoxical thoracoabdominal excursions associated with apnea indicating upper airway obstruction confirmed by the presence of progressively increasing negative pressure swings during the apnea. This leaves no doubt as to the nature of the event. An arousal is associated with termination of apnea and resumption of ventilation.
Figure 1. Example of an obstructive apnea from polysomnography.
Tracings are from top to bottom: Chin EMG = electromyogram, EEGs = electroencephalograms, EKG = electrocardiogram, Thermocouple = measured airflow, etCO2 = carbon dioxide, RC = rib cage and ABD = abdominal excursions, O2 Sat = oxygen saturation measured by figure pulse oximetry, Pesoph = esophageal pressure. Airflow is absent in the face of effort observed on rib cage, abdominal and esophageal pressure tracings. Breathing resumes with the onset of arousal (increase in chin EMG and EEG α waves) and opening of upper airway. Please note the nadir of saturation is at the end of apnea just before resumption of breathing, followed by quick reoxygenation associated with arousal-terminating apnea. Contrast with Figure 2. (Modified from Javaheri et al. Sleep apnea types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol. 2017;69:841–858.)
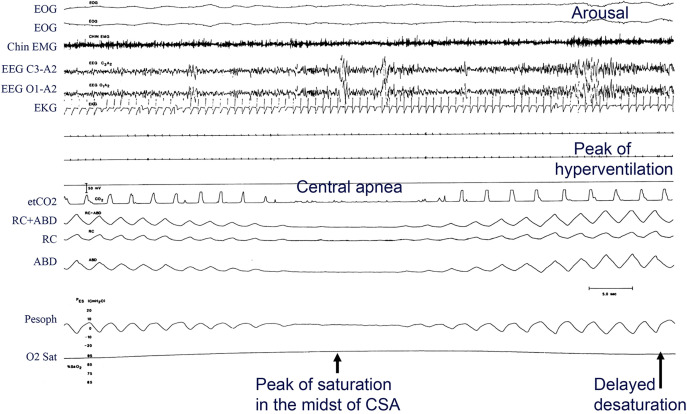
In contrast to obstructive apnea, central apnea (Figure 2) is defined by the absence of both airflow and effort, revealed by the absence of thoracoabdominal excursion (and in this case lack of esophageal pressure swings, leaving no doubt as to the subtype of apnea).2 These principles regarding airflow and thoracoabdominal excursions (effort) are the basis of the rules put forth in the American Academy of Sleep Medicine (AASM) scoring manual for distinguishing subtypes of apneas.3
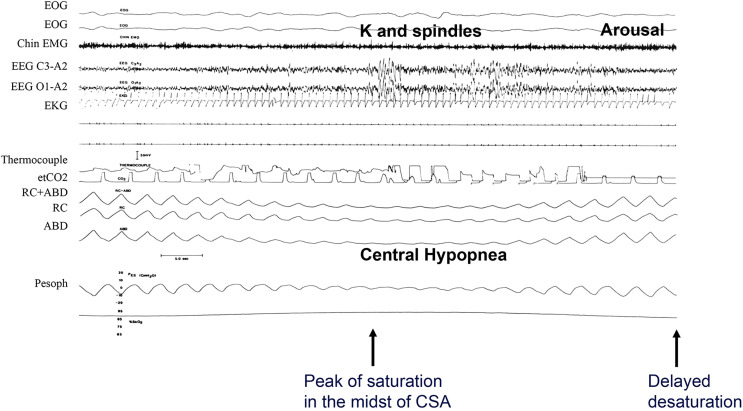
Figure 2. Example of a central apnea from polysomnography.
This epoch shows breath-by-breath physiology of central apnea observed in a patient with heart failure and reduced ejection fraction. Note breath-by-breath smooth and gradual changes in the thoracoabdominal excursions and esophageal pressure in the crescendo and decrescendo arms of the cycle. The arousal occurs at the peak of hyperventilation. Due to long circulation time, the peak of saturation occurs in the midst of apnea, and there is delayed reoxygenation. Tracings are: EOG = electro-oculograms (first and second), Chin EMG = electromyogram, EEGs = electroencephalograms, EKG = electrocardiogram, etCO2 = measured carbon dioxide, RC+ABD = combined rib cage and abdominal electrocardiogram, RC and ABD = excursions, Pesoph = esophageal pressure, O2 Sat = oxygen saturation. Airflow is absent in the effort channels observed on rib cage, abdominal, and esophageal pressure tracings. (Modified from Javaheri et al. Sleep apnea types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol. 2017;69:841–858.)
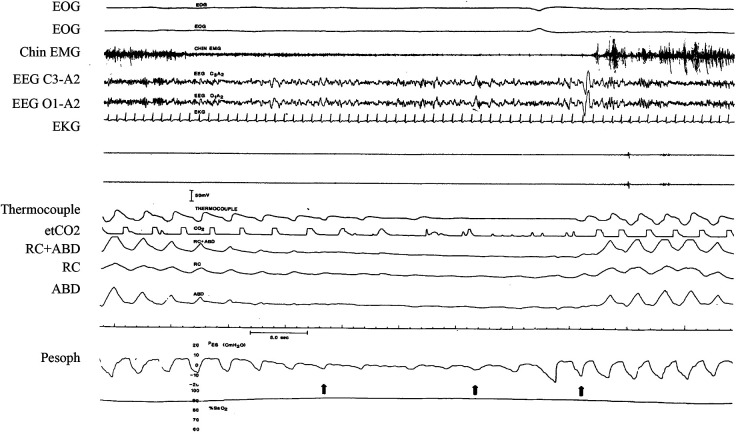
Difficulty arises with accurately differentiating subtypes of hypopneas; however, these events contribute equal weight to the index (apnea-hypopnea index [AHI]) and are more common than apneas on sleep recordings.4 To date, it has been conventional for hypopneas to be scored by default as obstructive in nature, because distinguishing obstructive from central hypopneas is difficult. This difficulty arises for several reasons that include: (1) ambiguity in the tracings, (2) practical difficulty in implementing existing rules, and (3) true physiological ambiguity, ie, when a single hypopnea appears to contain both obstructive and central features or when it evolves within the event (Figure 3).
Figure 3. Example of ambiguous respiratory event.
In this epoch, there is ambiguity as to the nature of the respiratory event. However, the balloon in the esophagus show activation of pump muscles, an indication of upper airway obstruction and that the event is obstructive in nature. See Figure 1 and Figure 2 legends for key to abbreviations and identification of traces.
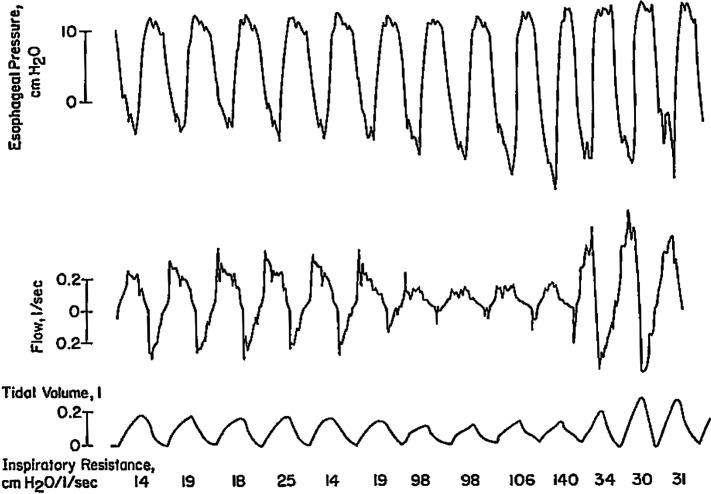
These sources of ambiguity can often be resolved by using a chest wall electromyography (EMG)5 or esophageal pressure2,6 signals to detect respiratory effort. While some researchers, such as Berry and colleagues,5 have successfully implemented chest wall or diaphragm EMG, this has not been widely adopted, especially in ongoing large epidemiological studies, and this signal is rarely available in clinical studies. In this context, Berry and colleagues5 have shown that a chest wall electromyogram provides a useful complementary signal for hypopnea classification. However, up to 50% of Berry’s studies showed inadequate EMG signals. Javaheri et al have used esophageal pressure for research studies,2 but a number of patients refused swallowing the esophageal balloon. Figure 4 and Figure 5 depict examples of obstructive and central hypopneas with esophageal pressure swings. In the case of obstructive hypopnea (Figure 4), both airflow and tidal volume decreased, despite increasing effort depicted in swings in esophageal pressure revealing increased upper airway resistance.6
Figure 4. Physiology of an obstructive hypopnea in non-REM sleep.
This figure shows breath-by-breath physiology of an obstructive hypopnea in non-REM sleep by simultaneously measuring airflow, tidal volume, airway resistance, along with esophageal pressure. Airway resistance for each breath was calculated at peak inspiratory esophageal pressure ie, increase in resistive pressure (after subtracting the pressure required for volume change)/flow rate. Note breath-by-breath progressively increasing (negative) esophageal pressure during obstructive hypopnea, with increase resistance and decreasing airflow tidal volume. Also note flattening of inspiratory airflow. (Modified from: Lung: Scientific Foundations, second edition, 1997. Eds R.G. Crystal et al. Effects on Breathing and Breathing Stability. J Dempsey, C Harms, B Morgan and S Badr. Courtesy of Dr Dempsey.)
Figure 5. Example of a central hypopnea in a patient with heart failure.
Please note crescendo-decrescendo changes in thoracoabdominal excursions commensurate with parallel changes in the esophageal pressure. Similar to findings in central apnea (Figure 2), there are time-dependent changes in saturation and in arousal occurring at the peak of hyperventilation. The channels are the same as in Figure 2. See Figure 1 and Figure 2 legends for key to abbreviations and identification of traces.
Figure 5 depicts an example of a central hypopnea in a patient with heart failure and reduced ejection fraction.2 In contrast to obstructive hypopnea (Figure 4), in a central hypopnea7 esophageal pressure swings are commensurate with thoracoabdominal efforts on a breath-to-breath basis, showing a parallel gradual reduction followed by an increase in efforts and airflow, consistent with waxing and waning ventilatory drive.
As noted earlier, the sine qua non for obstructive events is either reduced airflow for a given effort or an increased effort for a given flow. None of these criteria is present in Figure 2 (central sleep apnea [CSA]) and Figure 5 (central hypopnea), confirming their designations as central rather than obstructive events.
Distinguishing obstructive from central hypopnea has important therapeutic implications for the individual patient and in the design of clinical trials.7–9 Whereas continuous positive airway pressure (CPAP) devices remain the therapy of choice for obstructive sleep apnea/hypopnea, studies have shown that both CPAP7,10 and adaptive servoventilation8 may not effectively treat central events and can be associated with excess cardiovascular mortality. Nonetheless, central sleep apnea/hypopnea (CSAH) can respond to oxygen,11 theophylline,12 acetazolamide,13 phrenic nerve stimulation,14 and adaptive servoventilation, as, for example, in opioid-induced CSA15
In this manuscript, we expand on the previous criteria set in The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications3 and the excellent articles by Randerath et al16 and Dupuy-McCauley et al17 and discuss signal characteristics (Table 1) on readily available signals and provide illustrative examples that may help distinguish central from obstructive hypopneas.
Table 1.
Features distinguishing central from obstructive hypopneas.
| Signal | Obstructive Hypopnea | Central Hypopnea |
|---|---|---|
| Overall characteristics | ||
| AHI worse in REM sleep | ✓ | — |
| Frequent central apneas present | — | ✓ |
| Positional change | ± | ± |
| Sleep-disordered breathing event characteristics | ||
| Snoring during hypopnea | ✓ | — |
| Thoracoabdominal paradox during hypopnea | ✓ | — |
| Abrupt resumption of ventilation at arousal | ✓ | — |
| Crescendo-decrescendo breathing with: | — | ✓ |
| Gradual event termination | — | ✓ |
| Delayed arousal occurring at peak of ventilation | — | ✓ |
| SpO2 nadir delayed (due to prolonged circulation time) | — | ✓ |
| SpO2 zenith in the midst of hypopnea | — | ✓ |
| Breath characteristics | ||
| Inspiratory airflow limitation during hypopnea | ✓ | — |
| Prolonged inspiratory duty cycle | ✓ | — |
AHI = apnea-hypopnea index, REM = rapid eye movement sleep.
DISTINGUISHING RESPIRATORY EVENTS
Breath-wise flow signal characteristics that differentiate obstructive from central hypopneas
Scoring definitions
The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.6 (2020),3 defines the requirements for scoring a hypopneas based on using a nasal pressure probe (or other flow device) during a diagnostic study as follows:
Peak signal excursions must drop by at least 30% of the pre-event baseline for at least 10 seconds or more.
For the hypopnea to count toward the AHI (Medicare criteria, referred to as the AHI4 in the present paper), there must be at least a 4% drop in oxygen saturation by pulse oximetry associated with the event marked by the drop in flow.
The AASM manual2 further states that one can classify an hypopnea as obstructive if any of the following criteria are met:
there is snoring during the event,
there is increased inspiratory flattening of nasal pressure (inspiratory flow limitation, IFL), and
there is associated thoracoabdominal paradoxing that occurs during the event.
Conversely, these rules suggest that hypopneas should be classified as central if none of the criteria are met. While these rules provide some guidance, they are difficult to apply consistently and do not apply to all situations encountered. Thus, hypopnea classification often remains ambiguous, leading to an arbitrary default designation as an obstructive event.
Inspiratory airflow limitation—a cardinal feature of partial upper airway obstruction (hypopnea)
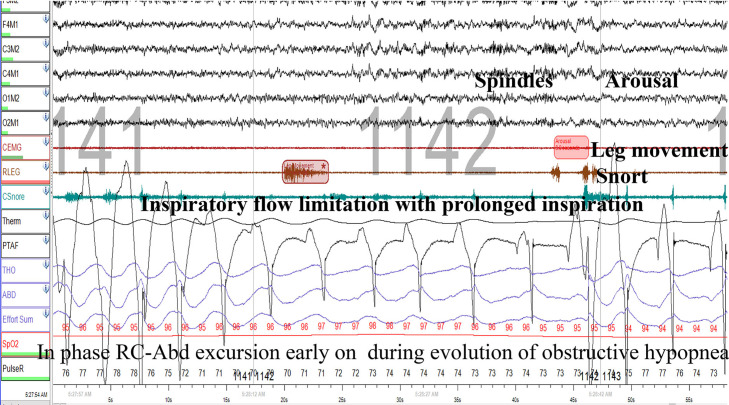
Presence of inspiratory flow limitation (IFL) is a noninvasive reflection of residual upper airway resistance.18 As depicted in Figure 6 and Figure 7, during inspiration airflow plateaus (flattens) while effort increases, indicating dynamic upper airway collapse or inspiratory flow limitation. In addition, there is a dissociation between inspiratory airflow and respiratory effort within successive breaths. IFL is often associated with paradoxical thoracoabdominal excursions like that seen in obstructive apnea (Figure 1). The mechanism of this finding is that diaphragmatic effort exerted against a partially or completely obstructed upper airway generates a negative intrathoracic pressure that pulls the rib cage inward, while the positive abdominal pressure increases abdominal girth. We emphasize that both the flattening of the inspiratory airflow signal and paradoxical excursions are most relevant when they occur throughout the obstructive hypopnea but are not present before and after the event, as these features should be absent when the upper airway is widely patent. An example of this is shown in Figure 6: as hypopnea is evolving, thoracoabdominal excursions are in phase, only to become out of phase later in the event, and before and after the event, excursions are in phase. Paradoxical thoracoabdominal excursions, however, may be absent in some patients with a stiff rib cage that resists inward excursion, as depicted in Figure 7.
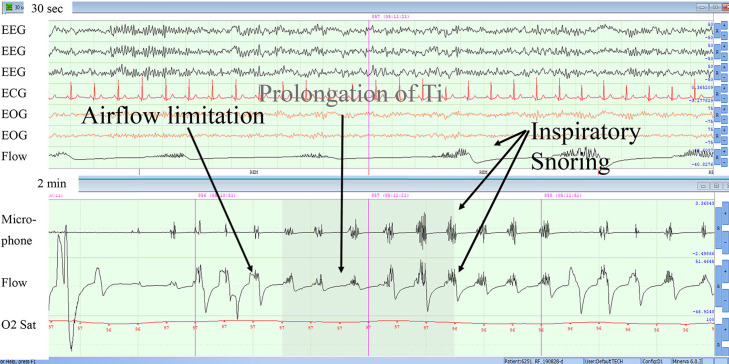
Figure 6. Example of obstructive hypopnea in non-REM sleep (stage N2).
The figure shows multiple facets of an obstructive hypopnea. However, please note that as hypopnea is evolving, early on thoracoabdominal excursions are in phase for 3 breaths but become out of phase as the event progresses. Also, thoracoabdominal excursions are in phase with recovery simultaneous with an arousal and a leg movement. ECG = electrocardiogram, EEG = electroencephalogram, EOG = electro-oculogram, Microphone = , Flow = , O2 Sat = oxygen saturation, Ti = inspiratory time.
Figure 7. Obstructive hypopnea with snoring.
The epoch is an obstructive hypopnea showing increasing intensity of snoring as hypopnea evolves and snoring goes away with arousal. This pattern of snoring within the events and its absence with resolution strongly indicates progressively increasing upper airway resistance (in contrast to decreasing effort as measured by esophageal pressure in Figure 5) and upper airway collapse, with recovery. Note that either a direct recording of snoring, or a vibration artifact on an unfiltered flow signal corresponds to the inspiratory flow period and is loudest during the hypopnea. Snoring may also be expiratory, but this is less frequent.
It should be noted that IFL and thoracoabdominal paradoxical motion can occur in a few breaths at the end of a central event (as during Hunter-Cheyne-Stokes breathing), when the upper airway narrows or completely occludes before the resumption of normal breathing. This event is usually referred to as a mixed apnea.2 Under these circumstances, IFL or complete upper airway closure occurs in the context of primarily central events, even though a few partially or completely obstructed breathings can occur. This pattern stands in contrast to a progressive series of sustained obstructed breaths that predominate from mid- to end-event in events that should be considered obstructive hypopneas or apneas.
Finally, not all respiratory inductance plethysmography (“RIP”) devices used to detect thoracoabdominal movement are identical. True inductance plethysmography can be calibrated and give a “sum” signal and, together with the paradox, show an estimate of total volume and/or flow. This may be more accurate in assessing “effort” than the simple effort bands that rely on stretch of a piezoelectric band.
In summary, paradoxical excursions during the event are relatively specific, but are not necessarily a sensitive sign of obstructive hypopneas.
Prolongation of inspiration or the inspiratory duty cycle
The inspiratory duty cycle (IDC) is defined by the ratio of inspiratory time (TI) over the total breath time (TTOT). When the upper airway obstructs and inspiratory airflow becomes limited, brainstem mechanisms prolong inspiration in order to preserve tidal volume and minute ventilation.19
Characteristically in obstructive hypopnea, the inspiratory time and the IDC increase progressively, either in absolute (TI) or relative (TI/TTOT) terms, and especially when the values of TI and TI/TTOT are compared to values from the larger adjacent breaths surrounding the event. This prolongation of inspiration (Figure 6) in absolute or relative terms results largely from a reflex that serves to maintain ventilation in the face of upper airway obstruction. Determining the IDC is time-consuming (unless an automated algorithm is deployed), but the prolongation of the IDC is nonetheless easily recognized visually over the course of an obstructive hypopnea.
One issue with both IFL and IDC is that they cannot be assessed if the nasal flow signal is flat and only the thermistor picks up flow (making the event a hypopnea, not an apnea). Due to its physical characteristics, the nasal pressure signal is least sensitive in the very low flow situation, and thus may look flat whenever an “hypopnea” has severely reduced flow or when the signal is poor due to displacement of the cannula. In either case, other ways of separating obstructive from central events must be used, but the default of assuming this is to be judged like an apnea (ie, “any effort makes it obstructive”) may be inadequate, and caution in classifying events is advised.
Snoring
Another characteristic of obstructive hypopnea is the presence of snoring, which when present during an event suggests some degree of partial upper airway obstruction. Inspiratory snoring can be detected as vibrations in the inspiratory airflow (Figure 6 and Figure 7) or tracheal sound signals.
High frequency noise in these signals can denote the start of an obstructive hypopnea, and often increases progressively during the event. To be specific for obstructive hypopnea, snoring should be absent before the event, present during the event, sometimes increasing in intensity, and usually abating at event termination after a resuscitative snort as airway patency is restored and normal breathing resumes (Figure 7).
It is also worth noting that the upper airway can narrow during central hypopneas and may even close completely during central apneas. Such narrowing has been observed endoscopically.20 Monitoring can further identify central and obstructive features within the same event (ie, mixed apnea).2 Under these circumstances, the initial central component has been attributed to hypocapnia that results from the hyperventilatory phase of the preceding sleep-disordered breathing event. As CO2 accumulates over the course of the event, however, respiratory drive returns and unmasks upper airway obstruction as an obstructive component ensues. Despite dynamic narrowing of the upper airway during central events, snoring and inspiratory airflow limitation often remain absent. In contrast to obstructive events, snoring is absent during the central hypopnea but usually occurs at the zenith of the hyperventilation phase (Figure 8) rather than progressively increasing during the event itself until it terminates abruptly at the arousal.
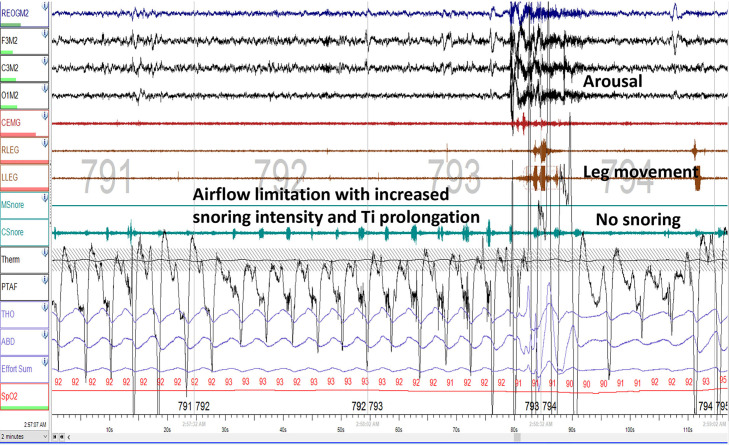
Figure 8. Obstructive hypopnea without paradoxical thoracoabdominal excursions.
With arousal, there is a leg movement and snoring dissipating.
Lack of snoring during central hypopnea is best explained by proportional reduction in ventilatory drive to both upper airway and pump muscles and consequently the absence of inspiratory flow limitation or sufficient flow to cause vibration. The upper airway can only flow-limit when inspiratory drive increases to the point that the pharynx collapses. Snoring specifically denotes the presence of inspiratory flow limitation caused by dynamic collapse of the airway and partial flow obstruction.
Breathing patterns and temporal relationship to oxygen saturation and arousal
Despite overlap between central and obstructive components of sleep-disordered breathing episodes, distinct differences in the overall breathing patterns can be discerned between those with a predominance of obstructive compared to central sleep-disordered breathing events. These patterns often differ as a function of (1) sleep stage, (2) ventilatory profile at event termination, (3) SpO2 contour among groups of sleep-disordered breathing episodes, and (4) timing of the arousal with respect to event termination. We first describe these features in patients with a common form of central sleep apnea, viz, Hunter-Cheyne-Stokes breathing (HCSB), and then consider variant CSA patterns that often occur with opiates and at high altitude.
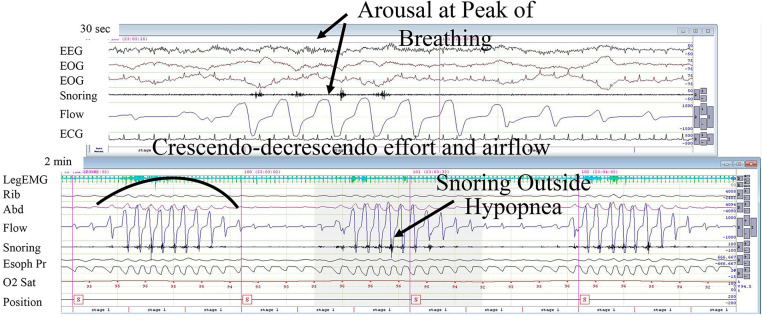
In HCSB, central apneas and hypopneas occur against the backdrop of left ventricular dysfunction, atrial fibrillation, cardiac chamber enlargement, and volume overload. Left ventricular systolic dysfunction remains the most common cause.21 In these cardiac disorders, sleep-disordered breathing patterns are often characterized by a crescendo-decrescendo breathing pattern with progressive, gradual increases and decreases in airflow, respiratory (thoracoabdominal) effort, as well as esophageal pressure (Figure 1 and Figure 3). At the nadirs of these signals, reductions in airflow meet criteria for central hypopnea (Figure 9) or apnea (Figure 2).
Figure 9. Hunter-Cheyne-Stokes breathing with hypopnea.
Note waxing and waning of flow signal followed by apnea or hypopnea. The arousal, if any, occurs at the peak of breathing, rather than at its resumption. Please note absence of snoring during central hypopnea but its appearance with hyperventilation, during the peak of breathing. There is no paradox in the rib and abdomen signals. If recorded, the esophageal pressure is lowest during the hypopnea (noted in Figure 4). Top panel shows 30-second epoch, lower panel shows 2-minute epoch. EEG = electroencephalogram (central derivations), EOG = electrooculogram (left and right), ECG = electrocardiogram, Rib and Abd = inductance plethysmography, Flow = nasal flow estimated from pressure in nasal cannula, Snoring = from filtered flow signal, O2 Sat = oxygen saturation, Position = , Esoph Pr = esophageal catheter pressure.
HCSB was first described by Hunter almost 4 decades before it was reported by Cheyne.21 As in obstructive hypopneas, the airflow could decrease progressively in decrescendo fashion but generally rises more gradually during recovery in central than obstructive hypopneas and apneas (compare Figure 1, Figure 2, Figure 4, Figure 5, and Figure 9). Similarly, ventilation rises abruptly in obstructive events with the first couple of breaths being the largest in amplitude (Figure 1 and Figure 4). The sudden rise in ventilation at event terminations results because the airway reopens promptly at the moment the patient is aroused from sleep. At this point in time, the asphyxic stimulus to ventilatory drive (from accumulated alterations in blood gases) during the preceding apnea or hypopnea has peaked. In other words, obstructive hypopneas terminate with arousals that reopen the upper airway, unmasking a progressive rise in ventilatory drive during the event while signaling ventilatory demand with a huge deep breath or large inspiratory effort and an accompanying large increase in airflow. This pattern differs markedly from the temporal relationship between arousal and ventilation seen in HCSB (Figure 5 and Figure 9).
In contrast to obstructive events, central hypopneas and apneas are often characterized by an arousal that is delayed well past the restart of ventilation, and usually occurs at the apex of respiratory effort and ventilation (Figure 5).
Differential impact of sleep stage on central sleep-disordered breathing episodes
In non–rapid eye movement (REM) sleep, normal breathing is metabolically controlled by the level of the partial pressure of carbon dioxide (PaCO2). As PaCO2 approaches the apneic threshold, hypopnea ensues, and gives way to apnea when PaCO2 drops below the apneic threshold.22 The impact of the apnea threshold on ventilation is particularly pronounced in non-REM sleep when central events typically occur, because ventilation is largely driven by PaCO2. REM sleep, however, overrides metabolic control of ventilation. Thus, differences in the distribution of sleep-disordered breathing episodes between non-REM and REM sleep can help distinguish predominantly central from obstructive patterns. Central sleep apnea is usually characterized by a greater propensity for events in non-REM rather than REM sleep, as often occurs in heart failure,21,23 at high altitude.24 and in opioid users.15,25,26 In contrast, obstructive events frequently increase in number and duration during REM sleep, and the obstructive hypopneas seen during non-REM sleep can give way to complete obstructive apneas during REM sleep due to pronounced reductions in upper airway muscle tone.
Positional responses common to central and obstructive sleep-disordered breathing episodes
It is well known that obstructive events worsen in the supine position due to gravitational effects, favoring prolapse of the tongue and other soft tissues into the pharynx. Less well understood is that, like obstructive sleep apnea, central sleep apnea can also worsen in the supine position27 and improve in the lateral position.28 Therefore, worsening of sleep-disordered breathing in supine position and improvement in nonsupine position is not specific to obstructive events and should not be used determine whether an event is obstructive or central in nature.
Relationship between event termination, arousal, and ventilation
In central hypopneas and apneas, arousal usually appears in the middle of the hyperpneic phase and often coincides with its apex (Figure 2 and Figure 5). In contrast, obstructive events are usually terminated by arousal and a large increase in ventilation followed by a progressive decrease in ventilation thereafter. Thus, increases in ventilation coincide with the arousal, which occurs at event termination in obstructive apneas and hypopneas, but ventilation and arousal are both delayed well past event termination in central episodes.
Symmetry in the shape of the oxyhemoglobin saturation profile is a hallmark finding in some central sleep-disordered breathing events (Figure 2, Figure 5, and Figure 9), especially with HCSB. In central events, the saturation profile parallels the breathing pattern, which often exhibits a gradual crescendo-decrescendo pattern in HCSB. In contrast, obstructive events are characterized by an asymmetric oxygen saturation profile with a slow desaturation and a relatively rapid resaturation upstroke at event termination (Figure 1). The abrupt rise in SpO2 (reoxygenation) is dictated by the sudden resumption of ventilation in the first couple of large breaths when arousals terminate these events.
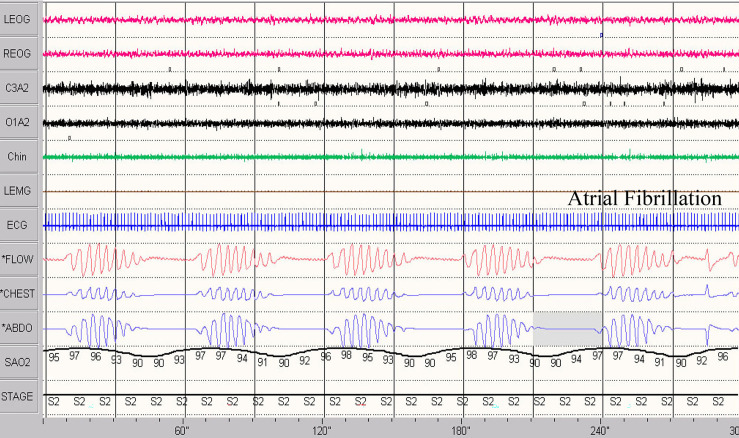
Timing differences in the patterns of desaturation-resaturation can also distinguish central HCSB from obstructive events. In patients with cardiac disease (heart failure, atrial fibrillation, chamber enlargement) or volume overload, the transit time for reoxygenated blood to pass from the lungs through the congested pulmonary veins and left heart to the ventilatory control centers in the brainstem is often prolonged. We can approximate this delay by measuring the circulation time: the time delay between the onset or resumption of ventilation at event termination and the onset of the upslope in the SpO2 signal. The normal circulation time from lung to finger (oximeter) is 15–17 seconds.29 Patients with underlying cardiac disease and HSCB will often demonstrate a prolongation in circulation time (circulatory delay) that may reach 40 seconds or more.30 We note that, circulatory delay calculated from lung to ear31 or lung to tongue32 is shorter than lung to finger (where the pulse oximeters are currently placed to measure saturation), but still much prolonged in those with CSA due to heart failure. In heart failure, prolonged circulatory delay is multifactorial and could be related to reduced cardiac output and left ventricular ejection fraction,33 increased blood volume in the left heart,34 and pulmonary vascular congestion.35 This delay is also observed in heart failure with OSA compared to OSA without heart failure.35 In the absence of increased blood volume contained within the central circulation (left ventricle, left atrium, and pulmonary veins and capillaries), and with normal cardiac output, circulatory delay is normal and much shorter in those with CSA without heart failure.31
Prolongation of the circulation time can play a significant role in the pathogenesis of HCSB. During apneas and hypopneas, a rise in CO2 in the central circulation (eg, lung and pulmonary capillaries) will not reach brainstem CO2-sensing regions promptly, thereby delaying the resumption of ventilation. The ensuing rise in CO2 is consequently increased, leading to a marked overshoot in ventilation with a consequent precipitous fall in CO2. As CO2 approaches or falls below the apnea threshold, central hypopneas and apneas ensue. Thus, a prolonged circulation time features prominently in the pathogenesis of ventilatory over- and undershooting, converting a negative feedback system to a positive one in HCSB. This delay is also recognized when the apex in SpO2 is either in the middle of apnea (Figure 1 and Figure 2) or overlaps the subsequent sleep-disordered breathing event (Figure 9). This contrasts with changes in obstructive events (Figure 1).
Central sleep apnea/hypopnea variants
Ataxic breathing
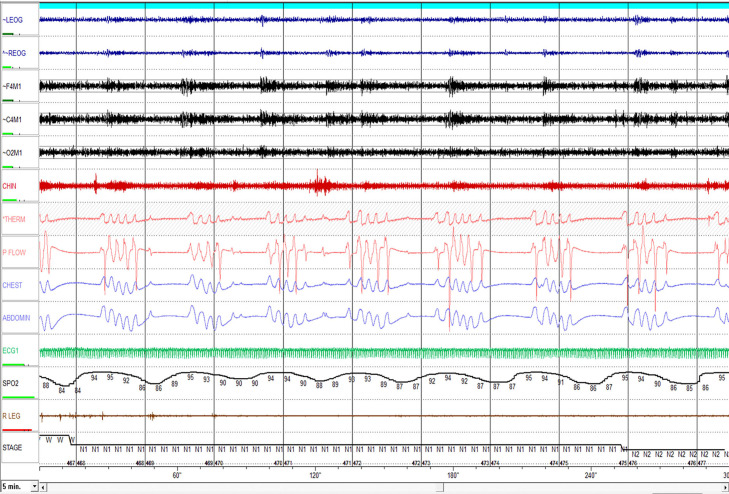
Despite the aforementioned indicators of central hypopneas, accurately differentiating obstructive from central hypopneas can still be difficult, especially when it is not HCSB and particularly when opiates underlie the central sleep-disordered breathing diathesis (Figure 10). This pattern is quite ataxic and sleep-disordered breathing events with both obstructive and central etiology can co-occur or be quite difficult to accurately score (Figure 11). Opiates can blunt neuromotor tone to both the respiratory pump and upper airway muscles, which can trigger central and obstructive episodes, respectively. Opioids act on pre-Bötzinger complex and hypoglossal motor neurons in the brainstem, potentially contributing to a complex breathing pattern of central, mixed, and obstructive events. A similar pattern can also be observed in normal subjects at high altitude, patients with brainstem or neurodegenerative disorders in whom pre-Bötzinger complex is involved. If a dramatic reduction in sleep-disordered breathing ensues on CPAP, it suggests that an obstructive pattern predominated, whereas central events are seldom completely suppressed by CPAP in non-HSCB.15,25,26
Figure 10. Hunter-Cheyne-Stokes breathing with central apnea.
EEG = electroencephalogram (central derivations), EOG = electrooculogram (left and right), ECG = electrocardiogram, flow = nasal flow estimated from pressure in nasal cannula, rib/abd = inductance plethysmography, SAO2 = O2 saturation.
Figure 11. Opioid-associated sleep-disordered breathing.
This 10-minute epoch shows different breathing disorders associated with opioids. In regard to the pattern of breathing, it is quite ataxic; central apneas are of variable duration and breaths out of apnea could be of variable tidal volume and airflow. This pattern is quite distinct and contrasts with that of Hunter-Cheyne-Stokes breathing (Figures 9 and 10).
Central apneas and hypopneas at high altitude
Central apneas and hypopneas occur frequently in normal people at high altitude,24 mediated by hypoxic stimulation of the carotid bodies. Furthermore, the phenotype of sleep-disordered breathing changes such that obstructive events present in lowlanders convert to central events with sojourn to high altitude.24 Importantly, the aforementioned criteria that differentiate central from obstructive events also apply to scoring at high altitude.
Cardiogenic oscillation
The presence or absence of cardiogenic oscillation in the airflow signal or the expired carbon dioxide during an apnea has been suggested to indicate airway patency, allowing the event to be labeled central apnea.36 It has been hypothesized that during the airway-open central apnea, heart beats change intrathoracic pressure, which produces visible jets of airflow coinciding with systole. This hypothesis was tested in a meticulously executed physiologic study37 in which fiberoptic endoscopy was used to visualize the pharyngeal lumen in people and animals with both spontaneous and induced obstructive and central sleep apnea. During full night polysomnography, airflow was measured by a pneumotachometer attached to a nasal mask. The pharyngeal lumen was visualized using a pediatric fiberoptic scope positioned at the level of velopharynx or oropharynx. In addition, inspiratory muscle EMG was obtained. The authors observed cardiogenic oscillation in both central and obstructive apneas. Based on human and animal studies, Morrell and colleagues37 concluded that cardiogenic oscillation cannot reliably differentiate obstructive from central apneas. They suggested an alternative mechanism to account for cardiogenic oscillations in flow caused by arterial pulsations in upper airway structures. Notably, however, the reappearance of cardiogenic oscillations once airway obstruction is relieved by CPAP can strongly suggest that any residual apneas on CPAP are of central origin.
SUMMARY
Hypopneas are the most prevalent sleep-related disordered breathing events and contribute importantly to the AHI. However, unlike apnea, categorization of hypopnea as either obstructive or central is often difficult or ambiguous, and the conventional default is that hypopneas are assumed to be obstructive in nature; this bias is in part because obstructive events are the most common form of sleep apnea hypopnea in general population. In certain clinical populations, however, central apneas and hypopneas can predominate. The distinction is key since treatment of central sleep apnea and hypopnea differs considerably from that of obstructive sleep apnea. We have expanded on the criteria put forward by the AASM, emphasizing distinct differences in respiratory signals and overall breathing patterns. Distinguishing breath-wise features includes identifying the presence of inspiratory flow limitation and associated increases in inspiratory duty cycle and snoring temporally related to the event. Central hypopneas and apneas can also be recognized by their predominance in non-REM sleep, and their most common form, HCSB, by symmetric oximetry and ventilation profiles, delayed timing of the arousal, snoring during the crescendo rather than decrescendo phase of ventilation, and a frequent prolongation in circulation time. These characteristics can help determine whether hypopneas and apneas are due to primary reductions in ventilatory drive and can be recognized from simple, readily available signals collected during routine clinical sleep studies, with automation as a logical next step.38 In this article, we have expanded further on the current guidelines put forth by the American Academy of Sleep Medicine to facilitate this distinction. These characteristics can help determine whether hypopneas and apneas are due to primary reductions in ventilatory drive and can be recognized from simple, readily available signals collected during routine clinical sleep studies.
DISCLOSURE STATEMENT
All authors have seen and approved the final manuscript. S.J. has received grant money from the National Institutes of Health for a trial on low-flow nocturnal oxygen therapy, is a consultant to Zoll Respicardia, and has received money for advising JAZZ and Harmony Pharmaceuticals. He is in the speaker bureau for Idorsia. D.R. receives royalty payments on licensed patents for modifications to CPAP from Fisher and Paykel Healthcare (through NYU) and does consulting on respiratory issues for Fisher and Paykel Healthcare. He is on a clinical advisory board for SomnoMed advising on the use of oral appliances, and has grant support from ProSomnus given to Icahn School of Medicine at Mt. Sinai on use of oral appliances in moderate-severe OSA. A.R.S. reports sponsored research from NIH, Apnimed, Periodic Breathing LLC, Respimetrix, and Signifier Medical, and paid consulting from AE Mann Foundation, Apnimed, Deerfield Catalyst, Itamar Medical/Zoll, Invicta Medical, LivaNova, Nyxoah, Periodic Breathing LLC, Philip Respironics, ResMed, Respicardia/Zoll, and Respimetrix. He also reports equity in Dayzz and Respimetrix.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- CPAP
continuous positive airway pressure
- CSA
central sleep apnea
- CSAH
central sleep apnea/hypopnea
- EMG
electromyography
- HCSB
Hunter-Cheyne-Stokes breathing
- IDC
inspiratory duty cycle
- IFL
inspiratory flow limitation
- PaCO2
partial pressure of carbon dioxide
- REM
rapid eye movement
- TI
inspiratory time
REFERENCES
- 1. Javaheri S , Barbe F , Campos-Rodriguez F , et al . Sleep apnea: types, mechanisms, and clinical cardiovascular consequences . J Am Coll Cardiol. 2017. ; 69 ( 7 ): 841 – 858 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dowdell WT , Javaheri S , McGinnis W . Cheyne-Stokes respiration presenting as sleep apnea syndrome. Clinical and polysomnographic features . Am Rev Respir Dis. 1990. ; 141 ( 4 ): 871 – 879 . [DOI] [PubMed] [Google Scholar]
- 3. Berry RB , Quan SF , Abreu AR , et al. for the American Academy of Sleep Medicine . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.6. Darien, IL: : American Academy of Sleep Medicine; ; 2020. . [Google Scholar]
- 4. Redline S , Kapur VK , Sanders MH , et al . Effects of varying approaches for identifying respiratory disturbances on sleep apnea assessment . Am J Respir Crit Care Med. 2000. ; 161 ( 2 ): 369 – 374 . [DOI] [PubMed] [Google Scholar]
- 5. Berry RB , Ryals S , Wagner MH . Use of chest wall EMG to classify hypopneas as obstructive or central . J Clin Sleep Med. 2018. ; 14 ( 5 ): 725 – 733 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dempsey J , Harms C , Morgan B , Badr S . Effects on Breathing and Breathing Stability . In: Crystal RG , West JB , eds. The Lung: Scientific Foundations. 2nd ed . Philadelphia: : Lippincott Williams & Wilkins; ; 1997. . [Google Scholar]
- 7. Bradley TD , Logan AG , Kimoff RJ , et al. CANPAP Investigators . Continuous positive airway pressure for central sleep apnea and heart failure . N Engl J Med. 2005. ; 353 ( 19 ): 2025 – 2033 . [DOI] [PubMed] [Google Scholar]
- 8. Cowie MR , Woehrle H , Wegscheider K , et al . Adaptive servo-ventilation for central sleep apnea in systolic heart failure . N Engl J Med. 2015. ; 373 ( 12 ): 1095 – 1105 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. National Institutes of Health Clinical Center . The Impact of Low-Flow Oxygen Therapy on Hospital Admissions and Mortality in Patients with Heart Failure and Central Sleep Apnea (LOFT-HF). NCT03745898. ClinicalTrials.gov . Bethesda, MD: National Institutes of Health; 2019. . https://clinicaltrials.gov/ct2/show/NCT03745898 . Accessed January 26, 2020.
- 10. Javaheri S . Effects of continuous positive airway pressure on sleep apnea and ventricular irritability in patients with heart failure . Circulation. 2000. ; 101 ( 4 ): 392 – 397 . [DOI] [PubMed] [Google Scholar]
- 11. Javaheri S , Ahmed M , Parker TJ , Brown CR . Effects of nasal O2 on sleep-related disordered breathing in ambulatory patients with stable heart failure . Sleep. 1999. ; 22 ( 8 ): 1101 – 1106 . [DOI] [PubMed] [Google Scholar]
- 12. Javaheri S , Parker TJ , Wexler L , Liming JD , Lindower P , Roselle GA . Effects of theophylline on sleep disordered breathing in stable heart failure: a prospective, double-blind, placebo controlled, and crossover study . N Engl J Med. 1996. ; 335 : 562 – 567 . [DOI] [PubMed] [Google Scholar]
- 13. Javaheri S . Acetazolamide improves central sleep apnea in heart failure: a double-blind, prospective study . Am J Respir Crit Care Med. 2006. ; 173 ( 2 ): 234 – 237 . [DOI] [PubMed] [Google Scholar]
- 14. Costanzo MR , Ponikowski P , Javaheri S , et al. remedé System Pivotal Trial Study Group . Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial . Lancet. 2016. ; 338 ( 10048 ): 974 – 982 . [DOI] [PubMed] [Google Scholar]
- 15. Javaheri S , Malik A , Smith J , Chung E . Adaptive pressure support servoventilation: a novel treatment for sleep apnea associated with use of opioids . J Clin Sleep Med. 2008. ; 4 ( 4 ): 305 – 310 . [PMC free article] [PubMed] [Google Scholar]
- 16. Randerath WJ , Treml M , Priegnitz C , Stieglitz S , Hagmeyer L , Morgenstern C . Evaluation of a noninvasive algorithm for differentiation of obstructive and central hypopneas . Sleep. 2013. ; 36 ( 3 ): 363 – 368 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dupuy-McCauley KL , Mudrakola HV , Colaco B , Arunthari V , Slota KA , Morgenthaler TI . A comparison of 2 visual methods for classifying obstructive vs central hypopneas . J Clin Sleep Med. 2021. ; 17 ( 6 ): 1157 – 1165 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Condos R , Norman RG , Krishnasamy I , Peduzzi N , Goldring RM , Rapoport DM . Flow limitation as a noninvasive assessment of residual upper-airway resistance during continuous positive airway pressure therapy of obstructive sleep apnea . Am J Respir Crit Care Med. 1994. ; 150 ( 2 ): 475 – 480 . [DOI] [PubMed] [Google Scholar]
- 19. Chin CH , Kirkness JP , Patil SP , et al . Compensatory responses to upper airway obstruction in obese apneic men and women . J Appl Physiol (1985). 2012. ; 112 ( 3 ): 403 – 410 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Badr MS , Toiber F , Skatrud JB , Dempsey J . Pharyngeal narrowing/occlusion during central sleep apnea . J Appl Physiol 1985. 1995. ; 78 ( 5 ): 1806 – 1815 . [DOI] [PubMed] [Google Scholar]
- 21. Javaheri S . Heart Failure . In: Kryger MH , Roth T , Goldstein C , eds. Principles and Practices of Sleep Medicine . 7th ed . Philadelphia: : WB Saunders; ; 2022. : 1462 – 1476 . [Google Scholar]
- 22. Javaheri S , Dempsey JA . Central sleep apnea . Compr Physiol. 2013. ; 3 ( 1 ): 141 – 163 . [DOI] [PubMed] [Google Scholar]
- 23. Javaheri S , Parker TJ , Liming JD , et al . Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations . Circulation. 1998. ; 97 ( 21 ): 2154 – 2159 . [DOI] [PubMed] [Google Scholar]
- 24. Manning E , Bloch KE , Dempsey JA , Javaheri S , Mohsenin V . Sleep and Breathing at High Altitude . In: Kryger MH , Roth T , Goldstein C , eds. Principles and Practices of Sleep Medicine . 7th ed . Philadelphia: : WB Saunders; ; 2022. : 1389 – 1400 . [Google Scholar]
- 25. Javaheri S , Harris N , Howard J , Chung E . Adaptive servo-ventilation for treatment of opioids-associated central sleep apnea . J Clin Sleep Med. 2014. ; 10 ( 6 ): 637 – 643 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Javaheri S , Patel S . Opioids cause central and complex sleep apnea in humans and reversal with discontinuation: a plea for detoxification . J Clin Sleep Med. 2017. ; 13 ( 6 ): 829 – 833 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sahlin C , Svanborg E , Stenlund H , Franklin KA . Cheyne-Stokes respiration and supine dependency . Eur Respir J. 2005. ; 25 ( 5 ): 829 – 833 . [DOI] [PubMed] [Google Scholar]
- 28. Szollosi I , Roebuck T , Thompson B , Naughton MT . Lateral sleeping position reduces severity of central sleep apnea/Cheyne-Stokes respiration . Sleep. 2006. ; 29 ( 8 ): 1045 – 1051 . [DOI] [PubMed] [Google Scholar]
- 29. Xu WD , Fang JC . Back to the future: circulation time revisited . J Card Fail. 2016. ; 22 ( 11 ): 928 – 929 . [DOI] [PubMed] [Google Scholar]
- 30. American Academy of Sleep Medicine . International Classification of Sleep Disorders. 3rd ed . Darien, IL: : American Academy of Sleep Medicine; ; 2014. . [Google Scholar]
- 31. Hall MJ , Xie A , Rutherford R , Ando S , Floras JS , Bradley TD . Cycle length of periodic breathing in patients with and without heart failure . Am J Respir Crit Care Med. 1996. ; 154 ( 2 ): 376 – 381 . [DOI] [PubMed] [Google Scholar]
- 32. Rubin AE , Gottlieb SH , Gold AR , Schwartz AR , Smith PL . Elimination of central sleep apnoea by mitral valvuloplasty: the role of feedback delay in periodic breathing . Thorax. 2004. ; 59 ( 2 ): 174 – 176 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oldenburg O , Bitter T , Wiemer M , Langer C , Horstkotte D , Piper C . Pulmonary capillary wedge pressure and pulmonary arterial pressure in heart failure patients with sleep-disordered breathing . Sleep Med. 2009. ; 10 ( 7 ): 726 – 730 . [DOI] [PubMed] [Google Scholar]
- 34. Tkacova R , Hall MJ , Liu PP , Fitzgerald FS , Bradley TD . Left ventricular volume in patients with heart failure and Cheyne-Stokes respiration during sleep . Am J Respir Crit Care Med. 1997. ; 156 ( 5 ): 1549 – 1555 . [DOI] [PubMed] [Google Scholar]
- 35. Efken C , Bitter T , Prib N , Horstkotte D , Oldenburg O . Obstructive sleep apnoea: longer respiratory event lengths in patients with heart failure . Eur Respir J. 2013. ; 41 : 1340 – 1346 . [DOI] [PubMed] [Google Scholar]
- 36. Ayappa I , Norman RG , Rapoport DM . Cardiogenic oscillations on the airflow signal during continuous positive airway pressure as a marker of central apnea . Chest. 1999. ; 116 : 660 – 666 . [DOI] [PubMed] [Google Scholar]
- 37. Morrell MJ , Badr SM , Harms CA , Dempsey JA . The assessment of upper airwway patency during apnea using cardiogenic oscillations in the airflow signal . Sleep. 1995. ; 18 ( 8 ): 651 – 658 . [DOI] [PubMed] [Google Scholar]
- 38. Parekh A , Tolbert TM , Mooney AM , et al . Endotyping sleep apnea one breath at a time: an automated approach for separating obstructive from central sleep-disordered breathing . Am J Respir Crit Care Med. 2021. ; 204 ( 12 ): 1452 – 1462 . [DOI] [PMC free article] [PubMed] [Google Scholar]