Abstract
Study Objectives:
The development of restless legs syndrome (RLS) has been rarely reported during and following opioid withdrawal. We aimed to determine the presence and severity of RLS symptoms during and after supervised opioid tapering.
Methods:
Ninety-seven adults enrolled in the Mayo Clinic Pain Rehabilitation Center who underwent supervised prescription opioid tapering were prospectively recruited. RLS presence and severity was assessed with the Cambridge-Hopkins Questionnaire 13 and International Restless Legs Syndrome Study Group Rating Severity Scale at admission, midpoint, and dismissal from the program as well as 2 weeks, 4 weeks, and 3 months after completion. Frequency and severity of RLS symptoms were compared between admission and each time point.
Results:
Average age of the cohort was 52.6 ± 13.3 years with a morphine milligram equivalent dose for the cohort of 45.6 ± 48.3 mg. Frequency of RLS symptoms increased from 28% at admission to peak frequency of 41% at 2 weeks following discharge from the Mayo Pain Rehabilitation Clinic (P = .01), returning to near baseline frequency 3 months after opioid discontinuation. International Restless Legs Syndrome Study Group Rating Severity Scale increased from baseline and then remained relatively stable at each time point following admission. Thirty-five (36.1%) participants developed de novo symptoms of RLS during their opioid taper, with those being exposed to higher morphine milligram equivalent doses having higher risk of developing RLS.
Conclusions:
Moderately severe symptoms of RLS, as assessed by survey, occur commonly in individuals undergoing opioid tapering, particularly if exposed to higher doses. In many cases, symptoms appear to be self-limited, although a minority develop persistent symptoms. Our results may have implications for successful opioid tapering, but future confirmatory studies with structured clinician interview are needed to establish that these symptoms truly represent restless legs syndrome given the potential for RLS-mimicking symptoms in individuals with chronic pain syndromes.
Citation:
McCarter SJ, Labott JR, Mazumder MK, et al. Emergence of restless legs syndrome during opioid discontinuation. J Clin Sleep Med. 2023;19(4):741–748.
Keywords: restless legs syndrome, opioids, withdrawal, chronic pain, taper
BRIEF SUMMARY
Current Knowledge/Study Rationale: Evaluation of the emergence of restless legs syndrome (RLS) in individuals undergoing opioid withdrawal has been limited to case reports and a few small studies. We aimed to determine the frequency of the development of RLS in patients undergoing supervised opioid tapering.
Study Impact: RLS symptoms emerge commonly in individuals undergoing supervised opioid taper, with the majority being transient and maximal in the first month of opioid discontinuation, although rarely symptoms may persist for months after opioid discontinuation. These data suggest practitioners supervising individuals undergoing opioid withdrawal should be aware of the potential development of RLS in these patients, although the impact of RLS on successful opioid withdrawal and quality of life requires further study.
INTRODUCTION
Restless legs syndrome (RLS) is a sensorimotor disorder characterized by an irresistible urge to move the legs, triggered by rest and relieved with movement. The disease has a nocturnal predilection which can lead to significant sleep disruption and distress.1 Dopaminergic dysfunction is thought to play a role in the pathogenesis of RLS. Opioid medications are effective treatment options in the management of chronic refractory RLS, potentially through modulation of dopaminergic neurotransmission.2 In 2018, worldwide estimates of patients taking prescribed opiates (for all indications) numbered 28 million.3 The risk of opioid use disorder, overdose, and withdrawal are among factors influencing reduction and discontinuation in a medication class with utility in appropriate patients. Classic symptoms of opioid withdrawal include nonspecific restlessness that may be confused with symptoms for RLS. However, evolution of de novo RLS in the setting of opioid withdrawal may occur and persist potentially complicating successful abstinence.4,5 Reports of the emergence of RLS symptoms during or following opioid withdrawal are primarily limited to case reports and a few studies, with prevalence of RLS symptoms ranging from 13% to 52% depending on the study.4–7 However, prior reports of RLS development were limited to patients withdrawing from illicit opioids or transitioning from opioids to buprenorphine.5,7
Utilizing a standardized and validated survey for RLS,8 we aimed to determine the prevalence of the development of de novo RLS symptoms as well as the frequency and severity of RLS symptoms in a sample of patients undergoing supervised opioid taper.
METHODS
One-hundred fifteen consecutive patients taking chronic daily opioids enrolled in the Mayo Clinic Pain Rehabilitation Center (PRC) from October 2017 through September 2021 were enrolled in this questionnaire-based study. Of the patients admitted into the program, 14 (11.2%) did not complete the program and were excluded from the final analyses. In addition, 2 patients did not complete their opioid taper by the time of the PRC program completion and 2 participants were started on buprenorphine/naloxone maintenance while in PRC treatment thus excluded from analysis. Our final sample consisted of 97 patients who successfully completed opioid taper while in the PRC. Details of the PRC inclusion/exclusion criteria, structure, and medication tapering process have been described elsewhere.9–11 In brief, the Mayo Clinic PRC is a 15-day (8 hours a day of intervention) intensive, outpatient, interdisciplinary pain rehabilitation program focusing on functional restoration. Patients receive an empirically supported protocol of cognitive behavioral therapy for chronic pain as well as daily physical and occupational therapy over the course of the program. Physician- and pharmacist-supervised opioid tapering is performed for patients entering treatment taking prescribed opioids for pain and is individualized for each patient. Oral morphine milligram equivalents (MME), based on Center for Disease Control conversions, are calculated on admission through a medication history reconciliation with the patient as well as a thorough assessment of the electronic health record, review of the original prescription bottles, and the state prescription drug monitoring program where access is available.12 Opioid tapering begins shortly after patients are admitted to the PRC and completed prior to discharge. Time for tapering includes the 15-day program plus weekends so may be as long as 21 days; however, most patients are tapered over approximately 10 days.11 Severity of opioid withdrawal symptoms are monitored utilizing the Clinical Opioid Withdrawal Scale which also assesses nonspecific restlessness related to opioid withdrawal although does not evaluate whether symptoms are isolated to the legs nor improve with movement.13
Assessment of RLS symptoms
The presence of RLS symptoms was assessed through the Cambridge Hopkins questionnaire for RLS symptoms while severity of RLS was assessed by the International Restless Legs Study Group RLS severity scale (IRLSS).8,14 Participants were considered to have RLS if they met “definitive criteria” for RLS, which has been previously shown to be 87.2% sensitive and 94.4% specific for RLS in the general population.8 Definitive criteria for RLS were defined by Allen et al8 and indicate positive responses to questions regarding an uncomfortable urge to move, present at rest and relieved with movement, worst at night, and not associated with leg cramps. Participants were considered to develop “de novo” RLS symptoms during their taper if they did not meet definitive criteria for RLS on admission but met definitive criteria for RLS at any point during or after their taper. IRLSS scores of 1–10 indicate mild symptoms, 11–20 moderate symptoms, 21–30 severe symptoms, and 31–40 very severe RLS symptoms. To assess for potential for RLS symptoms contributing to sleep disruption, participants completed the insomnia severity index (ISI) with scores of 8–14 indicating subthreshold insomnia, 15–21 moderate insomnia, and 22–28 severe insomnia.15 Participants filled out all 3 questionnaires at admission, midpoint of the program, day of discharge, as well as 2 weeks, 4 weeks, and 3 months after discharge from the PRC. Of note, time to discharge from opioid discontinuation varied by patient based on individualized tapering regimens.
Statistical analysis
Statistical analysis was performed using BlueSky Statistics software v. 7.10 (BlueSky Statistics LLC, Chicago, IL). Continuous variables are presented as means and standard deviations while categorical variables are presented as frequencies and percentages. Categorical variables between admission and subsequent time points were compared using McNemar’s tests, while continuous variables were compared with paired t tests. Individuals with missing survey data from a specific time-point were excluded from analysis for that specific time-point but included for time points with completed survey data either before or after the missing data. Frequency of RLS symptoms at each time point were reported as percentages of participants with completed surveys for that time point. Multivariable regression models were fit to evaluate predictors of developing de novo RLS symptoms. Exploratory subgroup analyses comparing individuals taking low dose (< 20 MME) vs doses ≥ 20 MME opioids and individuals who developed de novo RLS symptoms during their opioid taper were performed using Chi square or Fisher exact tests for categorical data and analysis of variance or Kruskal-Wallis tests for continuous data depending on the distribution of data by visual inspection. A cutoff of 20 MME was chosen, as an MME of 23 mg was the best combined cutoff for predicting de novo RLS (80% sensitive and 51% specific, area under the curve 0.65) and 20 MME is an easy to use and remember cutoff value for practical clinical purposes. P-values < 0.05 were considered statistically significant. This study was approved by the Mayo Clinic Institutional Review Board.
RESULTS
Of the 97 participants on daily opioids, 60 (62%) were female with an average age of 52.6 ± 13.3 years (Table 1). Fibromyalgia (37%) was the most common diagnosis followed by back pain (33%) and extremity pain (12.4%). Only 5 (5.2%) patients had a clinical diagnosis of RLS at admission and all were taking dopamine agonists. Mean MME for the cohort was 45.6 ± 48.3 mg (range 5–270 mg) while median MME was 30 mg with 96% of patients utilizing synthetic opioids and 4% using nonsynthetic opioids. Average duration of daily opioid use was 87.3 ± 96.3 months. Fifty (51.5%) participants were taking alpha-2-delta ligand medications during their PRC stay, 13 of whom were taking pregabalin (mean dose 326.9 mg, median dose 400 mg) and 37 taking gabapentin (mean dose 1,735 mg, median dose 1,600 mg). Most participants (63%) completed their opioid taper shortly after the midpoint of the PRC program while 32% completed their taper just prior to dismissal from the PRC. Opioid withdrawal symptoms were overall mild in the cohort with an average peak Clinical Opiate Withdrawal Scale score of 4.1 ± 3.1.
Table 1.
Cohort demographics (n = 97).
| Age at PRC admission (years) | 52.56 (13.30) |
| Sex | |
| Female | 60 (61.9%) |
| Male | 37 (38.1%) |
| Clinical diagnosis of RLS | |
| No | 92 (94.8%) |
| Yes | 5 (5.2%) |
| Duration opioid use (months) | 87.31 (96.27) |
| Opioid type | |
| Synthetic | 93 (96%) |
| Nonsynthetic | 4 (4%) |
| MME | 45.59 (48.26) |
| MME > 20 | |
| No | 36 (37.1%) |
| Yes | 61 (62.9%) |
| Alpha-2-delta ligand use | |
| No | 47 (48.5%) |
| Yes | 50 (51.5%) |
| Dopamine agonist use | |
| No | 92 (94.8%) |
| Yes | 5 (5.2%) |
| Timepoint taper completed | |
| Admission | 5 (5.2%) |
| Midpoint | 61 (62.9%) |
| Dismissal | 31 (32.0%) |
MME = morphine milligram equivalents, RLS = restless legs syndrome, PRC = Mayo Clinic Pain Rehabilitation Center.
RLS symptoms
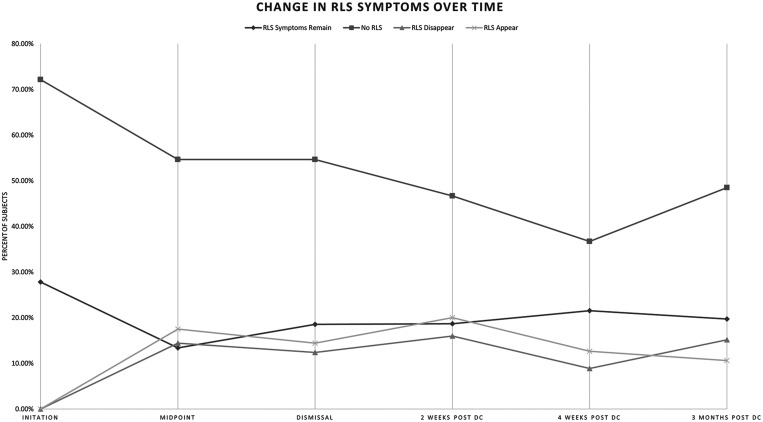
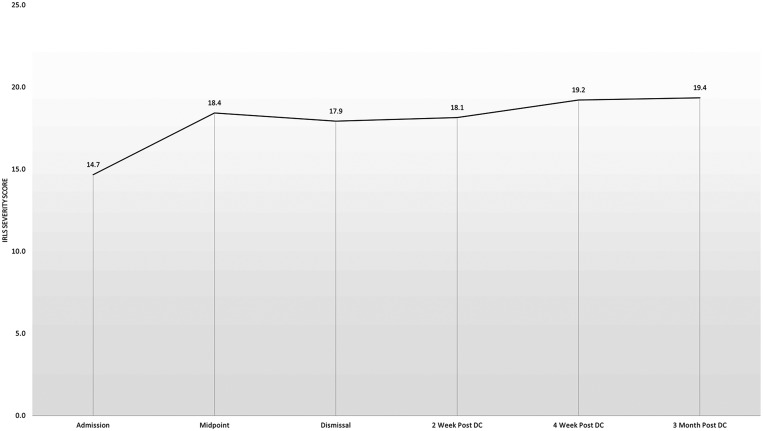
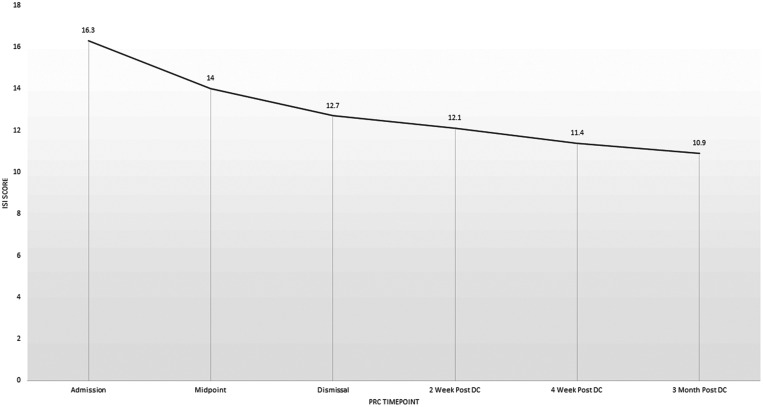
Twenty-seven (27.8%) participants met survey criteria for definitive RLS at admission to the PRC. Some participants continued to meet survey criteria for RLS at multiple time points during the study, while RLS symptom presence seemed to fluctuate in other patients during the course of the study (Figure 1). However, the overall frequency of participants meeting definitive RLS criteria increased significantly during and following their opioid taper, peaking at 40.8% 2 weeks after completion of the PRC. The frequency of RLS symptoms returned to near baseline levels of 30.3% endorsing RLS symptoms 3 months after completion of the PRC. Despite the increased frequency of RLS symptoms during the study, only the difference between baseline and 2 weeks following completion of the PRC program was significant (P = .01). In those meeting definitive RLS criteria at each time point, mean IRLSS increased from baseline 14.7 ± 10.3 to 18.4 ± 8.6 at midpoint of the PRC program and remained stably elevated between 17.9 ± 7.9 and 19.3 ± 5.9 at each timepoint, although there were no statistically significant differences in IRLSS between baseline and any timepoints (Figure 2). ISI score progressively decreased from 16.3 ± 7.9 at admission to 10.9 ± 7.9 three months after discharge from PRC, with each time point significantly lower compared with admission (all P < .001, Figure 3).
Figure 1. Frequency of definitive RLS by Cambridge Hopkins Questionnaire during and after opioid withdrawal.
DC = discharge, No RLS = participants with negative response on Cambridge Hopkins compared with prior timepoint, PRC = pain rehabilitation clinic, RLS = restless legs syndrome, RLS appear = participants who had a negative response on Cambridge Hopkins at prior timepoint but screened positive at the next timepoint, RLS disappear = participants who had positive response on Cambridge Hopkins at prior timepoint but no longer screen positive at the next timepoint, RLS symptoms remain = participants with positive response on Cambridge Hopkins compared with prior timepoint.
Figure 2. Severity of RLS symptoms as measured by the International Restless Legs Study Group Severity Scale in participants meeting criteria for definite RLS by Cambridge Hopkins Questionnaire during and after opioid withdrawal.
DC = discharge, PRC = Mayo Clinic Pain Rehabilitation Center, RLS = restless legs syndrome.
Figure 3. Insomnia Severity Index score during and after opioid withdrawal.
DC = discharge, ISI = insomnia severity index, PRC = Mayo Clinic Pain Rehabilitation Center.
We subsequently compared those who had RLS symptoms at baseline (n = 27, 28%), participants who developed de novo RLS symptoms during opioid taper (n = 35, 36%), and those who remained without RLS symptoms throughout the duration of the study (n = 35, 36%) (Table 2). Participants with RLS symptoms at baseline were younger than those with de novo RLS (48 vs 57 years, P = .04) and those who never developed RLS symptoms (48 vs 56 years, P = .02), and those with baseline RLS were more likely to be female (82% vs 54% and 54%, respectively, P = .025 and P = .025, respectively) than those with de novo RLS and those who never developed RLS. The median morphine equivalent dose was higher in those who developed de novo RLS symptoms compared with those who did not develop RLS symptoms (38 mg vs 22.8 mg, P = .03), while median morphine dose in the group with RLS symptoms at baseline was no different compared with other groups. There was no difference in timepoint the opioid taper was completed, duration of opioid exposure, alpha-2 delta-ligand exposure, or peak COWS between groups.
Table 2.
Group comparisons between those who had RLS at baseline, developed de novo RLS, and never developed RLS.
| Baseline RLS (n = 27) [A] | De Novo RLS (n = 35) [B] | Never Developed RLS (n = 35) [C] | Significance* | |
|---|---|---|---|---|
| Age at PRC admission (years) | 46.630 (12.555) | 54.857 (12.486) | 54.829 (13.587) | B,C>A |
| MME (mean) | 53.900 (61.407) | 50.686 (48.034) | 34.235 (35.080) | B>C |
| MME (median) | 22.500 | 38.000 | 22.750 | B>C |
| Duration opioid use (months) | 90.000 (101.032) | 51.478 (53.819) | 116.444 (113.498) | NS |
| Sex | A>B,C | |||
| Female | 22 (81.5%) | 19 (54.3%) | 19 (54.3%) | |
| Male | 5 (18.5%) | 16 (45.7%) | 16 (45.7%) | |
| MME > 20 | B>A,C | |||
| No | 14 (51.9%) | 6 (17.1%) | 16 (45.7%) | |
| Yes | 13 (48.1%) | 29 (82.9%) | 19 (54.3%) | |
| Alpha-2-delta ligand use | NS | |||
| No | 11 (40.7%) | 18 (51.4%) | 18 (51.4%) | |
| Yes | 16 (59.3%) | 17 (48.6%) | 17 (48.6%) | |
| MedWorsenRLS | A>B,C | |||
| No | 11 (40.7%) | 27 (77.1%) | 24 (68.6%) | |
| Yes | 16 (59.3%) | 8 (22.9%) | 11 (31.4%) | |
| Timepoint taper completed | NS | |||
| Admission | 1 (3.7%) | 2 (5.7%) | 2 (5.7%) | |
| Midpoint | 18 (66.7%) | 19 (54.3%) | 24 (68.6%) | |
| Dismissal | 8 (29.6%) | 14 (40.0%) | 9 (25.7%) | |
| Opioid type | NS | |||
| Nonsynthetic | 1 (3.7%) | 3 (8.6%) | 0 (0.0%) | |
| Synthetic | 26 (96.3%) | 32 (91.4%) | 35 (100.0%) | |
| Peak COWS Score | 5.000 (2.951) | 4.207 (2.664) | 3.231 (3.637) | NS |
Significance indicates P < .05 at subgroup level comparisons. COWS = Clinical Opiate Withdrawal Scale, DC = dismissal from PRC, IRLSS = International Restless Leg Study Group Severity Scale, MedWorsenRLS = serotonergic or anticholinergic medication use, MME = milligram morphine equivalent, NS = Not significant, PRC = Mayo Clinic Pain Rehabilitation Center.
After adjusting for age, sex, duration of opioid use, and exposure to alpha-2 delta ligand medications, only exposure to a baseline opioid dose > 20 MME was associated with risk of developing de novo RLS symptoms with an odds ratio of 27.2 95% confidence interval 4.4–536. Of those who developed de novo RLS symptoms, 6 (17%) had persistent symptoms at 4 weeks and 3 months after completion of the PRC and 2 (6%) did not develop RLS symptoms until 3 months after completion of the PRC. The remainder had transient symptoms during or within 4 weeks of PRC dismissal that did not persist at 3 months. The only significant differences between individuals taking > 20 MME was a higher frequency of developing de novo RLS symptoms (48% vs 17%, P = .002).
DISCUSSION
Symptoms of restless legs syndrome are relatively common in patients on chronic opioid therapy and increase in frequency during and after completion of opioid withdrawal. In our study, survey data determined that prevalence of definitive RLS increased from 27% at baseline, peaked at 41% approximately 2–4 weeks after completion of opioid taper, before returning to near baseline levels 3 months later. Importantly, 36% of patients in our cohort developed de novo symptoms of RLS during or following their opioid taper, with exposure to doses of opioids higher than 20 mg MME per day significantly increasing the risk of developing RLS and higher doses of MME being seen in those who developed de novo RLS symptoms compared with those who did not ever develop RLS symptoms—this suggests that the dose of opioids is a risk factor for the development of RLS symptoms. Further, 17% of these individuals had persistent symptoms at 4 weeks and 3 months after opioid withdrawal while the remainder had transient symptoms, suggesting some individuals are predisposed to developing de novo chronic RLS symptoms after being exposed to opioids. Our results highlight the importance of providers who supervise individuals undergoing opioid withdrawal being aware of these risks, as the development of RLS symptoms could theoretically complicate successful opioid withdrawal and abstinence in some individuals. For instance, 1 survey of individuals with chronic opioid dependence reported that the main factor influencing continuing opioid use was for the treatment of and fear of recurrence of chronic pain after withdrawal.16 Similarly, the experience of the dysesthesias from RLS could have a similar psychologic effect and induce fear of withdrawal of opioids in those who experience RLS symptoms or have in the past.
The 41% peak prevalence of RLS symptoms in our cohort was slightly lower than the approximately 50% prevalence of RLS symptoms in prior studies of opioid withdrawal.5,7 However, all participants in our study were only taking prescription opioids compared with prior studies, which evaluated RLS symptoms in individuals withdrawing from illicit opioids such as heroin and/or the misuse of prescription opioids. Additionally, these studies did not evaluate participants at multiple time points after completion of opioid withdrawal, so whether RLS symptoms persisted in these individuals is unknown. Our findings are consistent with 1 study that reported a lower risk of RLS symptoms in those taking lower doses of opioids, although MME was not reported in this study.5
Two studies have shown that the use of alpha-2 delta ligand medications such as pregabalin and gabapentin can effectively reduce RLS symptoms in patients undergoing opioid withdrawal.5,17 In our study, the use of alpha-2 delta ligands was not protective against developing de novo RLS symptoms. However, as part of the PRC treatment approach, many patients underwent concomitant dose reductions in alpha-2 delta ligands or had been on these prior to opioid tapering (rather than the medication added to address withdrawal) which limit our ability to interpret these results and also may have contributed to the development of RLS symptoms. As our study was not designed to evaluate whether addition of alpha-2 delta ligands impact RLS symptoms during opioid withdrawal, future prospective studies are needed to answer this question. Case reports have demonstrated improvement in opioid withdrawal–associated RLS symptoms with dopamine agonists, but too few participants in our cohort were taking dopamine agonists for this potential protective effect to be evaluated.18
While opioids play a central role in the treatment of refractory RLS, the mechanism by which they exert their symptomatic effect and the mechanism behind opioid withdrawal–induced RLS remains unknown. A relationship between the opioid and dopamine system has been posited to be one etiologic factor for RLS in the opioid-withdrawal setting. Chronic stimulation of the µ receptor by opioids has been shown to increase dopamine release, which eventually leads to decreased sensitivity and down regulation of dopamine receptors.19,20 Thus, the tapering of opioids may lead to a relative dopamine deficiency and evolution of RLS symptoms, and explain the resolution of RLS symptoms with addition of dopamine agonists.18,21 It appears that in certain brain areas, opioid receptor agonism suppresses γ-aminobutyric acid (GABA) release.22 GABA modulates dopamine release with lowering of GABA tone facilitating increased dopaminergic outflow in the reward pathway. Thus, removal of opioids may lead to disinhibited GABA release and resultant decreased dopaminergic signaling leading to evolution of RLS. However, proposed benefits of alpha-2 delta ligand pharmacotherapy may point to a different GABA/dopamine relationship or involvement of the glutamatergic system as has been proposed for idiopathic RLS.23 Iron status may theoretically contribute to the risk of developing opioid withdrawal–induced RLS. Opioid administration has been shown to be protective against iron deficiency–induced substantia nigra cell death in rats.2 Therefore, patients on chronic opioid therapy with underlying iron deficiency may be predisposed to develop RLS symptoms following the withdrawal of opioids, although this hypothesis requires further confirmatory testing.
Although symptoms of RLS increased in frequency during and following opioid taper, somewhat unexpectedly, the severity of insomnia progressively declined from moderately severe on PRC admission to subthreshold insomnia over the course of the study. However, an integral component of the PRC protocol is daily cognitive behavioral therapy focusing on cognitive restructuring and relaxation/mindfulness strategies which likely improve sleep latency, and maintenance as these are main tenants of cognitive behavioral therapy for insomnia.9,10 Additionally, as the ISI focuses on other aspects of sleep perception, rather than just sleep initiation and maintenance it is possible the reduction in ISI scores may be due to overall improved quality of life and coping strategies learned in PRC rather than improvement in sleep initiation or maintenance. Further, reduction in symptoms and behaviors related to pain because of participation in the PRC may also improve sleep quality. Finally, discontinuation of opioid medications, which increase sleep fragmentation and contribute to sleep-disordered breathing may also have contributed to improvements in insomnia, although this seems less likely as significant decreases in ISI scores began prior to completion of opioid withdrawal in most cases.24
Limitations of our study include, due to design of the study, the utilization of a survey to determine the presence of RLS symptoms rather than clinician interview. While the Cambridge Hopkins questionnaire is well validated with high sensitivity and specificity in the general population, it has not been studied in chronic pain populations where a survey may lead to false positives. Further, as withdrawal from opioid medications can cause general restlessness without circadian rhythmicity, this may further lower the specificity of survey-based assessment of RLS symptoms in this patient population.13 Fibromyalgia and back pain, which occurred with high frequency in patients in the current study, also have symptoms that may mimic RLS. This could potentially have led to false positives by questionnaire, further lowering its specificity for RLS in this population. However, the Cambridge Hopkins questionnaire inquires specifically about lower extremity symptoms that resolve with movement, which helps to differentiate RLS symptoms from nonspecific akathisia associated with opioid withdrawal.7 Some patient tapering off opioids may concurrently begin, change, or otherwise alter their use of other substances such as cannabinoids and/or alcohol, which could influence these findings, and we did not systematically assess this. Additionally, given the survey-based nature of our study, our results may be at risk of sampling bias since individuals without RLS symptoms may have been less likely to return completed surveys, particularly at the 4-week and 3-month timepoints and we did not confirm that participants remained opioid free at 4-week and 3-month follow ups. While prior studies have not shown differences in renal function between individuals who developed RLS during opioid withdrawal compared with those who did not, we did not systematically evaluate this.5 We were unable to routinely assess iron stores in our participants, so whether iron deficiency in individuals undergoing opioid tapering predisposes them to developing RLS requires further study. The PRC tapers patients off opioids over a shorter period than is recommended in best practice guidelines due to the intense, supportive nature of the behavioral programming.25 The RLS symptoms seen in this patient population may not reflect the experience of a slower monthly opioid taper. Finally, 12% of the initially enrolled participants dropped out of the study for various reasons, although RLS was not documented as a reason for PRC discontinuation. While it is possible these dropouts could have influenced our results, this rate is similar to what has been reported in prior studies of PRC participants, and there were no differences in RLS frequency by CH-13 questionnaire at baseline in those who did not complete the PRC (21% vs 28%) to those who did, thus making it unlikely that these significantly influenced our results.
CONCLUSIONS
Moderately severe RLS symptoms occur commonly in individuals undergoing opioid withdrawal, typically peaking within the month following drug discontinuation. In many cases symptoms are self-limited, although a minority develop persistent symptoms. Patients undergoing supervised opioid withdrawal should be periodically evaluated for the development of RLS symptoms as these have the potential to impair successful opioid withdrawal and abstinence. The role of iron stores and whether medications for the treatment of RLS such as alpha-2 delta ligands or dopamine agonists can prevent or effectively abolish RLS symptoms associated with opioid withdrawal requires further study.
DISCLOSURE STATEMENT
All authors have reviewed and approved the manuscript. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
Data availability: All relevant data have been shared and published in this article.
ABBREVIATIONS
- GABA
γ-aminobutyric acid
- IRLSS
International Restless Legs Study Group Severity Scale
- ISI
Insomnia Severity Index
- MME
morphine milligram equivalent
- PRC
Mayo Clinic Pain Rehabilitation Center
- RLS
restless legs syndrome
REFERENCES
- 1. Allen RP , Picchietti DL , Garcia-Borreguero D , et al. International Restless Legs Syndrome Study Group . Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria–history, rationale, description, and significance . Sleep Med. 2014. ; 15 ( 8 ): 860 – 873 . [DOI] [PubMed] [Google Scholar]
- 2. Sun YM , Hoang T , Neubauer JA , Walters AS . Opioids protect against substantia nigra cell degeneration under conditions of iron deprivation: a mechanism of possible relevance to the Restless Legs Syndrome (RLS) and Parkinson’s disease . J Neurol Sci. 2011. ; 304 ( 1–2 ): 93 – 101 . [DOI] [PubMed] [Google Scholar]
- 3. Herlinger K , Lingford-Hughes A . Opioid use disorder and the brain: a clinical perspective . Addiction. 2022. ; 117 ( 2 ): 495 – 505 . [DOI] [PubMed] [Google Scholar]
- 4. Park YM , Park HK , Kim L , Lee HJ , Kang SG . Acute-withdrawal restless legs syndrome following abrupt cessation of short-term tramadol . Psychiatry Investig. 2014. ; 11 ( 2 ): 204 – 206 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gupta R , Ali R , Ray R . Willis-Ekbom disease/restless legs syndrome in patients with opioid withdrawal . Sleep Med. 2018. ; 45 : 39 – 43 . [DOI] [PubMed] [Google Scholar]
- 6. Scherbaum N , Stüper B , Bonnet U , Gastpar M . Transient restless legs-like syndrome as a complication of opiate withrawal . Pharmacopsychiatry. 2003. ; 36 ( 2 ): 70 – 72 . [DOI] [PubMed] [Google Scholar]
- 7. Mackie SE , McHugh RK , McDermott K , Griffin ML , Winkelman JW , Weiss RD . Prevalence of restless legs syndrome during detoxification from alcohol and opioids . J Subst Abuse Treat. 2017. ; 73 : 35 – 39 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Allen RP , Burchell BJ , MacDonald B , Hening WA , Earley CJ . Validation of the self-completed Cambridge-Hopkins questionnaire (CH-RLSq) for ascertainment of restless legs syndrome (RLS) in a population survey . Sleep Med. 2009. ; 10 ( 10 ): 1097 – 1100 . [DOI] [PubMed] [Google Scholar]
- 9. Gilliam WP , Craner JR , Cunningham JL , et al . Longitudinal treatment outcomes for an interdisciplinary pain rehabilitation program: comparisons of subjective and objective outcomes on the basis of opioid use status . J Pain. 2018. ; 19 ( 6 ): 678 – 689 . [DOI] [PubMed] [Google Scholar]
- 10. Schumann ME , Coombes BJ , Gascho KE , et al . Pain catastrophizing and pain self-efficacy mediate interdisciplinary pain rehabilitation program outcomes at posttreatment and follow-up . Pain Med. 23 ( 4 ): 697 – 706 . [DOI] [PubMed] [Google Scholar]
- 11. Cunningham JL , Evans MM , King SM , Gehin JM , Loukianova LL . Opioid tapering in fibromyalgia patients: experience from an interdisciplinary pain rehabilitation program . Pain Med. 2016. ; 17 ( 9 ): 1676 – 1685 . [DOI] [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention . Opioid Prescribing Guideline Resources; 2022. . https://www.cdc.gov/opioids/providers/prescribing/index.html . Accessed April 27, 2022.
- 13. Tompkins DA , Bigelow GE , Harrison JA , Johnson RE , Fudala PJ , Strain EC . Concurrent validation of the Clinical Opiate Withdrawal Scale (COWS) and single-item indices against the Clinical Institute Narcotic Assessment (CINA) opioid withdrawal instrument . Drug Alcohol Depend. 2009. ; 105 ( 1–2 ): 154 – 159 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Walters AS , LeBrocq C , Dhar A , et al. International Restless Legs Syndrome Study Group . Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome . Sleep Med. 2003. ; 4 ( 2 ): 121 – 132 . [DOI] [PubMed] [Google Scholar]
- 15. Bastien CH , Vallières A , Morin CM . Validation of the Insomnia Severity Index as an outcome measure for insomnia research . Sleep Med. 2001. ; 2 ( 4 ): 297 – 307 . [DOI] [PubMed] [Google Scholar]
- 16. Weiss RD , Potter JS , Griffin ML , et al . Reasons for opioid use among patients with dependence on prescription opioids: the role of chronic pain . J Subst Abuse Treat. 2014. ; 47 ( 2 ): 140 – 145 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Freye E , Levy JV , Partecke L . Use of gabapentin for attenuation of symptoms following rapid opiate detoxification (ROD): -correlation with neurophysiological parameters- . Neurophysiol Clin. 2004. ; 34 ( 2 ): 81 – 89 . [DOI] [PubMed] [Google Scholar]
- 18. Ghosh A , Basu D . Restless legs syndrome in opioid dependent patients . Indian J Psychol Med. 2014. ; 36 ( 1 ): 85 – 87 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fields HL , Margolis EB . Understanding opioid reward . Trends Neurosci. 2015. ; 38 ( 4 ): 217 – 225 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Walker JM , Thompson LA , Frascella J , Friederich MW . Opposite effects of mu and kappa opiates on the firing-rate of dopamine cells in the substantia nigra of the rat . Eur J Pharmacol. 1987. ; 134 ( 1 ): 53 – 59 . [DOI] [PubMed] [Google Scholar]
- 21. Park EJ , Park YM . Opioid withdrawal and Restless Legs Syndrome . Chronobiol Med. 2020. ; 2 ( 4 ): 137 – 140 . [Google Scholar]
- 22. Smith HS . Introduction to Opioids . In: Smith HS , ed. Opioid Therapy in the 21st Century . 2nd ed . New York: : Oxford University Press; ; 2013. : 5 – 9 . [Google Scholar]
- 23. Allen RP , Barker PB , Horská A , Earley CJ . Thalamic glutamate/glutamine in restless legs syndrome: increased and related to disturbed sleep . Neurology. 2013. ; 80 ( 22 ): 2028 – 2034 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosen IM , Aurora RN , Kirsch DB , et al. American Academy of Sleep Medicine Board of Directors . Chronic opioid therapy and sleep: an American Academy of Sleep Medicine position statement . J Clin Sleep Med. 2019. ; 15 ( 11 ): 1671 – 1673 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. US Department of Health and Human Services . HHS Guide for Clinicians on the Appropriate Dosage Reduction or Discontinuation of Long-Term Opioid Analgesics; 2019. . https://public3.pagefreezer.com/browse/HHS.gov/16-09-2020T14:35/https://www.hhs.gov/opioids/treatment/clinicians-guide-opioid-dosage-reduction/index.html . Accessed January 29, 2023.