Abstract
Recent studies suggest that sleep disorders are present in two-thirds of patients with autoimmune encephalitis. In anti-Ma2 encephalitis, hypersomnia appears to be frequent. However, only few cases of type 1 narcolepsy have been reported to date with anti-Ma2 encephalitis. We report 2 new cases of patients with narcolepsy secondary to anti-Ma2 encephalitis. Patient 1, a 68-year-old man, had narcolepsy type 1, including sleep attacks, cataplexy, abnormal Multiple Sleep Latency Tests and hypocretin-1 deficiency (< 50 ng/L) in the cerebrospinal fluid (CSF), associated with a cerebellar syndrome. Anti-Ma2 antibodies were present in the serum and CSF and antivoltage-gated potassium channel antibodies in the serum. He benefited from a treatment with pitolisant. Patient 2, a 42-year-old man, had narcolepsy type 2, including hypersomnolence, no cataplexy, intermediate CSF levels of hypocretin-1 (138 ng/L), abnormal Multiple Sleep Latency Tests, and a limbic encephalitis presentation. Anti-Ma2 antibodies were present in the serum and CSF, and anti-Ma1 antibodies were in the CSF. For both, repeated polysomnographies were necessary to establish the precise diagnosis of central hypersomnia, emphasizing the importance of carrying out sleep investigations in a tertiary neurology center with sleep medicine expertise in patients with anti-Ma2 encephalitis.
Citation:
Brunet de Courssou J-B, Testard P, Sallansonnet-Froment M, et al. Narcolepsy secondary to anti-Ma2 encephalitis: two case reports. J Clin Sleep Med. 2023;19(4):837–841.
Keywords: auto-immune encephalitis, hypersomnia, Ma2, narcolepsy, paraneoplastic, sleep disease
INTRODUCTION
Autoimmune encephalitis is an inflammatory brain disorder related to cellular or humoral autoimmunity. Anti-Ma2 (anti-phospohprotein Ma2) antibodies are found in about 7% of autoimmune encephalitides. Recent studies suggest that sleep disorders are present in two-thirds of patient with autoimmune encephalitis.1 A central hypersomnolence disorder is reported in 20–40% of patients and remains insufficiently characterized, as most studies are carried out after the acute phase of the disease and after several lines of immunotherapy.1 This encephalitis is mostly paraneoplastic and mostly associated to testicular cancer.2,3 Symptoms at onset frequently include a limbic syndrome, gaze palsy, and sleep disorders comprising excessive daytime sleepiness.2,3 Anti-Ma2 encephalitis affects patients at a median age of 43 to 60 years old and a male predominance. We report the cases of 2 patients with excessive daytime sleepiness and diagnosed with a narcolepsy secondary to anti-Ma2 encephalitis. As both diseases are uncommon, the diagnosis may be overlooked, with the need for repeated sleep exploration.4–8
REPORT OF CASES
Patient 1
Case 1 involves a 68-year-old man with hypertension, obesity, and diabetes mellitus. He was referred for a 1-year history of severe daytime sleepiness, including sleep attacks when active. The cardiorespiratory polygraphy indicated a severe sleep apnea syndrome. Continuous positive air pressure normalized the apnea-hypopnea index but did not alleviate daytime sleepiness. A progressive cognitive decline appeared over 6 months, along with gait impairment, episodes of oblique binocular diplopia and a 4-kg weight gain. Clinical examination revealed a cerebellar syndrome and a dysexecutive syndrome.
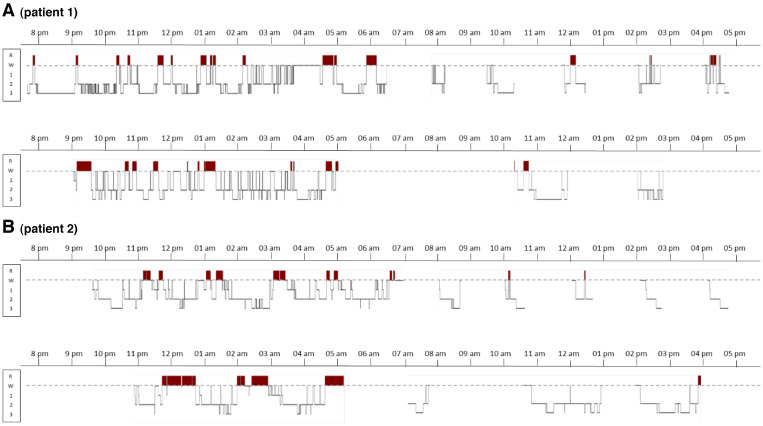
On brain magnetic resonance imaging, hyperintense FLAIR signals were located around the third ventricle and the cerebral aqueduct (Figure S1A (368.2KB, pdf) in the supplemental material). The polysomnography showed a total sleep time of 571 minutes and a residual apnea-hypopnea index of 11 events/h under continuous positive airway pressure. Multiple Sleep Latency Tests (MSLT) showed a mean sleep latency of 6.2 minutes (normal > 8 minutes) and no sleep-onset rapid eye movement period (SOREMP). There was a cerebellar hypermetabolism in the brain 18Fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) scanner. The whole body PET scan was unremarkable. Cerebrospinal fluid (CSF) analysis showed elevated protein levels of 0.86 g/L and a normal cell count. The autoantibodies screening was positive for anti-Ma2 antibodies in the serum and the CSF. Antivoltage-gated calcium channel antibodies were also detected in blood during follow-up. During the diagnostic workup, the patient reported several episodes a week of incomplete and sudden loss of muscle tone of lower limbs lasting seconds in full consciousness, which were suggestive of cataplexy, although unrelated to emotions. He had no hypnagogic hallucinations, sleep paralysis, or rapid eye movement sleep behavior disorder. Later, MSLT were repeated and residual apnea-hypopnea index was 0 events/h. The mean sleep latency was 3.7 minutes and there were 2 SOREMPs. Total sleep time was normal (534 min, Figure 1A).
Figure 1. Sleep studies during night and day showing narcolepsy in 2 patients with anti-Ma2 encephalitis.
The 48-hour procedure starts with a nighttime polysomnography stopped at 6:30 am, followed by 5 MSLT performed at 8:00 am, 10:00 am, 12:00 am, 2:00 pm, 4:00 pm (first line), followed by an 24-hour bed rest monitoring (second line). The x-axis indicates clock time and the y-axis indicates sleep stages (W = wakefulness; R = rapid eye movement sleep [REM] sleep, in red color; 1, 2, and 3 = non-REM N1, N2, and N3 sleep stages). Note the frequent sleep onset in REM periods (REM sleep appearing less than 15 min after sleep onset), whether during the night and during daytime, a feature of narcolepsy. MSLT = Multiple Sleep Latency Test.
He was eventually diagnosed with anti-Ma2 encephalitis and was treated with immunoglobulin perfusion, 2 g/kg repeated monthly. The CSF hypocretin-1 was < 50 pg/mL (normal > 200 pg/mL) when measured after the first set of immunoglobulin perfusion, confirming the comorbid diagnosis of narcolepsy type 1. The HLA DQB1*0602 genotype was absent. The patient was treated with pitolisant 36 mg/day, which improved daytime sleepiness. This drug was preferred over modafinil as it does not interact with immunosuppressant and antiepileptic drugs.
Because the patient worsened, monthly cyclophosphamide and methylprednisolone (1 g each) were added after the fourth monthly immunoglobulin perfusion and appeared to stabilize clinical status. After 6 months of this combined treatment, a fixed diplopia occurred, so that rituximab 1000 mg was added. Lower limbs cataplexy seems to have remitted, although limited walking could conceal rare episodes, and he remains on pitolisant treatment. Twenty months after diagnosis, the patient was stable and the quarterly repeated whole body 18F-FDG PET scans were normal.
Patient 2
Case 2 involves a 42-year-old man without any significant medical history. He was referred for depressive mood, anterograde memory impairment, hyposmia, 8 kg weight gain, and excessive daytime sleepiness worsening for 18 months. The patient reported sleep attacks in passive situations and no cataplexy, hypnagogic hallucination, sleep paralysis, or rapid eye movement sleep behavior disorder. There was a psychomotor slowing and deficit in anterograde memory. The brain magnetic resonance imaging showed bilateral FLAIR hyperintense signals in the medial temporal lobes (Figure S1B (368.2KB, pdf) , Figure S1C (368.2KB, pdf) ) with partial contrast enhancement. The CSF was normal apart from oligoclonal bands. The electroencephalography revealed a left temporal epileptic focus. Brain 18F-FDG PET scan showed bilateral hypermetabolism of the temporal lobes. Anti-Ma2 antibodies were found in the blood and CSF, and anti-Ma1 antibodies were found in the CSF. The screening for cancer using CT, body 18F-FDG PET scan and testicular ultrasound echography was negative.
The patient was treated with immunoglobulin perfusion 2 g/kg/month and steroids bolus. In the absence of improvement, a treatment with monthly cure of 1 g of cyclophosphamide, associated with 1g of methylprednisolone, was introduced.
The night polysomnography showed a total sleep time of 610 minutes during 24 h, close to the threshold (660 min) for central hypersomnia, and a mild sleep apnea (apnea-hypopnea index 6.3 events/h). Secondarily, the sleep tests were repeated. The 24-hour bed rest polysomnography showed a total sleep time of 505 minutes. On MSLT, the mean sleep latency was 4.3 min and there were two SOREMPs, meeting the criteria of secondary narcolepsy (Figure 1B).9,10 The HLA DQB1*0602 genotype was absent. The CSF hypocretin-1 level, measured during follow-up was 138 ng/L.10,11
The neurological symptoms and excessive daytime sleepiness improved without stimulant drugs. The epilepsy worsened, including focal seizures more than 10 times per night, requiring an antiepileptic tritherapy. After 2 years of evolution, given the persistence of several focal seizures a day, the immunosuppressor treatment was changed for rituximab with remaining difficulties in seizure control. Thirty-six months after diagnosis, the patient was stable and repeated evaluations for an underlying malignancy were negative.
DISCUSSION
Excessive daytime sleepiness is reported in one-third of patients with anti-Ma2 encephalitis.2 Other autoimmune encephalitides, associated with anti-CASPR2, LGI1, NMDAR, or IgLON5 antibodies, mainly lead to insomnia and parasomnia.1 The association of anti-Ma2 encephalitis with narcolepsy type 1 has been previously reported in 5 detailed case reports presented in Table 1.4–8 In a series of 38 patients with anti-Ma2 encephalitis, Dalmau et al2 also described cases of narcolepsy type 1. In their cohort, 2 patients had cataplexy and hypnagogic hallucinations.2 In 6 of their patients, hypocretin-1 levels in CSF were measured: it was undetectable (< 100 mg/mL) in the 4 patients with excessive daytime sleepiness and normal for the 2 nonsleepy patients.2,12 Conversely, searching for anti-Ma antibodies in 19 patients with narcolepsy, 16 with hypocretin-1 deficiency and 18 with HLA DQB1*0602 positive, was negative in all cases.12 The overall lack of known autoantibodies in primary narcolepsy has been later confirmed.13
Table 1.
Published detailed cases of narcolepsy type 1 associated with anti-Ma2 encephalitis and comparison with our 2 patients.
| Study | Present Report | ||||||
|---|---|---|---|---|---|---|---|
| Compta et al4 | Landolfi et al5 | Dauvilliers et al6 | Kritikou et al7 | Adams et al8 | Patient 1 | Patient 2 (narcolepsy type 2) | |
| Age (years) | 69 | 35 | 63 | 67 | 55 | 68 | 42 |
| Sex | Male | Male | Male | Male | Male | Male | Male |
| Auto-antibodies | Anti–Ma2 in CSF and serum. | Anti-Ma2 in serum. | Anti-Ma1 and Ma2 in CSF and serum. | Anti-Ma1 and Ma2 in CSF. | Anti-Ma1 and Ma2 in serum. | Anti–Ma2 in CSF and serum. Later, anti-VGKC in serum. | Anti-Ma2 in CSF and serum. Anti-Ma1 in CSF. |
| Hypocretin levels in CSF | 49 ng/mL | NA | Undetectable | NA | NA | < 50 ng/mL | 138 ng/mL |
| EEG findings | NA | Bitemporal electrographical seizures = | NA | Mild generalized slowing | NA | Epileptic left temporal focus. | Left temporal epileptic focus. |
| FLAIR hyperintense lesions location on MRI | Midbrain, both hippocampus and amygdala. Later, bilateral paramedian thalamus. | Bilateral medial temporal lobe. | Bilateral paramedian in the thalamus and hypothalamus. | Hypothalamus, pituitary, and mammillary bodies. | Absent | Around the third ventricle and the cerebral aqueduct. | Bilateral medial temporal lobes. |
| Mean sleep latency*** | 7 min | 9 min* | 7.2 min | NA | 2.2 min | 6.2 min (then 3.7 min) | 4.3 min |
| SOREMPs*** | 4 | 2* | 2 | Present** | 2 | 0 (then 2) | 2 |
| Accompanying features during sleep | RBD | NA | RBD, hypnagogic hallucinations | RBD | RBD, hypnagogic hallucinations | Absent | Absent |
| Cataplexy | Absent | Present | Present | Present | Present | Present | Absent |
| Associated neurological symptoms (apart from hypersomnia) | Impaired memory. Panic attacks. Apathy. Diplopia, supranuclear vertical gaze palsy. Extrapyramidal syndrome. Unsteady gait. |
Impaired memory. Anxiety. Confusion. Complex partial seizures. |
Vertical supranuclear gaze palsy and bilateral ptosis. | Impaired memory. Confusion. Apathy. Visual hallucinations. Frequent falls. |
Impaired memory. Confusion. Ophtalmoparesis, diplopia. Bradykinesia without rigidity. Unsteady gait. Tremor. Dysarthria. |
Diplopia, nystagmus. Static cerebellar syndrome with unsteady gait. |
Impaired memory. Depressive mood. Hyposmia. |
| Tumor | None diagnosed. | Intratubular germinomatous testicular carcinoma. | None detected. | Pulmonary adenocarcinoma 3 years prior. | Tonsillar squamous cell carcinoma. | None detected. | None detected. |
| Weight gain | Absent | NA | NA | NA | 10 kgs gain | 4 kgs gain | 8 kgs gain |
| HLA DQB1*0602 allele / DRB1*15 allele | Absent / Absent | Absent / Absent | NA / NA | NA / NA | NA / NA | Absent / NA | Absent / NA |
| Treatment | MP, IgIV | MP, cyclophosphamide. Right orchiectomy. |
None for autoimmunity. | MP plasma exchange. | Carcinoma treatment. MP, then cyclophosphamide | IgIV, then addition of MP and cyclophosphamide. Rituximab. | IgIV and MP. Then cyclophosphamide and MP. Currently, Rituximab. |
| Outcome | Death, 12 months after symptoms onset | Initial worsening, then mild improvement after tumor resection. | Death, 4 months after symptoms onset. | Death, 5 months after symptoms onset. | Symptoms stabilization. | Alive 20 months after diagnosis. Worsening seems slower. |
Neurological symptoms and sleepiness improved. Epilepsy worsened. |
Four of them had a concomitant RBD (not found in our patients). Hyperintense signals in the hypothalamus, where the hypocretin-producing neurons are located, was present in only 2 cases. Please note that our Patient 2 is considered to have narcolepsy type 2. *Confounding medications affecting sleep physiology are pointed out by the authors: clonidine, amitriptyline, clonazepam, valproic acid, and phenytoin. **Following 10 awakenings, during a 128-minute daytime nap (multiple sleep latency tests impossible due to confusion). ***Unless in Patient 4, from Kritikou et al7, SOREMPs and mean sleep latency are results of Multiple Sleep Latency Test (5 repetitions). CSF = cerebrospinal fluid, EEG = electroencephalogram, FLAIR = fluid attenuated inversion recovery, IgIV = intravenous immunoglobulin, MP = methylprednisolone, MRI = magnetic resonance imaging, NA = information is not available, OCB = oligoclonal band, RBD = rapid eye movement sleep behavior disorder, SOREMP = sleep-onset rapid eye movement period.
For our 2 cases, repeated sleep investigations were necessary to establish the diagnosis, emphasizing the importance of referring these patients to specialized centers.
Here, Patient 1 met the International Classification of Sleep Disorders criteria for narcolepsy type 1, in the presence of sleepiness, cataplexy, and hypocretin-1 deficiency, although his first MSLT showed only a reduced sleep onset latency but no SOREMP.4–7,12,14 The primary narcolepsy type 1 results from an autoimmune selective loss of hypocretin neurons in the hypothalamus. In the case of narcolepsy type 1 secondary to anti-Ma2 encephalitis, a single neuropathology study suggests that hypocretin neurons are destroyed by a CD8+ inflammatory-mediated response.6 In our case, the low CSF hypocretin-1 level suggests that the hypocretin neurons were indeed damaged. Patient 2 had a central disorder of hypersomnolence, which could be classified as secondary narcolepsy type 2 (2 SOREMPs on MSLT, no cataplexy, intermediate CSF hypocretin level). In his case, the level of CSF hypocretin-1 suggests that the anti-Ma2 autoimmunity has only partly damaged the hypocretin neurons or that narcolepsy is linked to another mechanism (eg, antibodies targeting hypocretin-1 receptors).
The knowledge on autoimmune encephalitis and their clinical spectrum has been growing in the last decades. These 2 cases strengthen the association between anti-Ma2 encephalitis and narcolepsy. Sleep tests including polysomnography and MSLT could be more widely used and repeated in these patients. Conversely, one may look for autoimmune encephalitis in front of narcolepsy or hypersomnia syndromes associated with focal neurological symptoms or severe cognitive deficiency.
Important notice: Patient 1 was previously described in a French continuous medical education journal with a more didactic approach concerning excessive daytime sleepiness and acquired cerebellar diseases and among 3 other cases of autoimmune vermian 18F-FDG PET-computer tomography hypermetabolism.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. Work for this study was performed at Sleep Disorders Unit, Hôpital Pitié-Salpêtrière, Paris, France. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
Author contributions: Jean-Baptiste Brunet de Courssou and Pauline Testard: acquisition of data, interpretation of data, drafting/revising the manuscript for content. Ana-Zenovia Gales, and Magali Sallansonnet-Froment, Isabelle Arnulf: acquisition of data, interpretation of data, drafting/revising the manuscript for content. Damien Ricard, Mickaël Ferrand, Dimitri Psimaras, Thibault Maillet, Patricia Depierre, Charlotte Ohlmann, Jean Capron, Marie-Laure Brechemier: acquisition of data, revision of the manuscript.
ABBREVIATIONS
- AIE
autoimmune encephalitis
- CSF
cerebrospinal fluid
- MSLT
Multiple Sleep Latency Tests
- PET
positron emission tomography
- SOREMP
sleep-onset rapid eye movement period
REFERENCES
- 1. Blattner MS , de Bruin GS , Bucelli RC , Day GS . Sleep disturbances are common in patients with autoimmune encephalitis . J Neurol. 2019. ; 266 ( 4 ): 1007 – 1015 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dalmau J , Graus F , Villarejo A , et al . Clinical analysis of anti-Ma2-associated encephalitis . Brain. 2004. ; 127 ( Pt 8 ): 1831 – 1844 . [DOI] [PubMed] [Google Scholar]
- 3. Hoffmann LA , Jarius S , Pellkofer HL , et al . Anti-Ma and anti-Ta associated paraneoplastic neurological syndromes: 22 newly diagnosed patients and review of previous cases . J Neurol Neurosurg Psychiatry. 2008. ; 79 ( 7 ): 767 – 773 . [DOI] [PubMed] [Google Scholar]
- 4. Compta Y , Iranzo A , Santamaría J , Casamitjana R , Graus F . REM sleep behavior disorder and narcoleptic features in anti-Ma2-associated encephalitis . Sleep. 2007. ; 30 ( 6 ): 767 – 769 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Landolfi JC , Nadkarni M . Paraneoplastic limbic encephalitis and possible narcolepsy in a patient with testicular cancer: case study . Neuro-oncol. 2003. ; 5 ( 3 ): 214 – 216 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dauvilliers Y , Bauer J , Rigau V , et al . Hypothalamic immunopathology in anti-Ma-associated diencephalitis with narcolepsy-cataplexy . JAMA Neurol. 2013. ; 70 ( 10 ): 1305 – 1310 . [DOI] [PubMed] [Google Scholar]
- 7. Kritikou I , Vgontzas AN , Rapp MA , Bixler EO . Anti-Ma1- and Anti-Ma2-associated encephalitis manifesting with rapid eye movement sleep disorder and narcolepsy with cataplexy: a case report . Biol Psychiatry. 2018. ; 83 ( 5 ): e39 – e40 . [DOI] [PubMed] [Google Scholar]
- 8. Adams C , McKeon A , Silber MH , Kumar R . Narcolepsy, REM sleep behavior disorder, and supranuclear gaze palsy associated with Ma1 and Ma2 antibodies and tonsillar carcinoma . Arch Neurol. 2011. ; 68 ( 4 ): 521 – 524 . [DOI] [PubMed] [Google Scholar]
- 9. Kizawa T , Hosokawa K , Nishijima T , et al . False-positive cases in multiple sleep latency test by accumulated sleep debt . Neuropsychopharmacol Rep. 2021. ; 41 ( 2 ): 192 – 198 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baumann CR , Mignot E , Lammers GJ , et al . Challenges in diagnosing narcolepsy without cataplexy: a consensus statement . Sleep. 2014. ; 37 ( 6 ): 1035 – 1042 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Andlauer O , Moore H 4th , Hong S-C , et al . Predictors of hypocretin (orexin) deficiency in narcolepsy without cataplexy . Sleep. 2012. ; 35 ( 9 ): 1247 – 55F . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Overeem S , Dalmau J , Bataller L , et al . Hypocretin-1 CSF levels in anti-Ma2 associated encephalitis . Neurology. 2004. ; 62 ( 1 ): 138 – 140 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dietmann A , Horn MP , Schinkelshoek MS , et al . Conventional autoantibodies against brain antigens are not routinely detectable in serum and CSF of narcolepsy type 1 and 2 patients . Sleep Med. 2020. ; 75 : 188 – 191 . [DOI] [PubMed] [Google Scholar]
- 14. Blumenthal DT , Salzman KL , Digre KB , Jensen RL , Dunson WA , Dalmau J . Early pathologic findings and long-term improvement in anti-Ma2-associated encephalitis . Neurology. 2006. ; 67 ( 1 ): 146 – 149 . [DOI] [PubMed] [Google Scholar]