Abstract
The microtubule cytoskeleton is critical for maintenance of long and long-lived neurons. The overlapping array of microtubules extends from the major site of synthesis in the cell body to the far reaches of axons and dendrites. New materials are transported from the cell body along these neuronal roads by motor proteins, and building blocks and information about the state of affairs in other parts of the cell are returned by motors moving in the opposite direction. As motor proteins walk only in one direction along microtubules, the combination of correct motor and correctly oriented microtubules is essential for moving cargoes in the right direction. In this review, we focus on how microtubule polarity is established and maintained in neurons. At first thought, it seems that figuring out how microtubules are organized in neurons should be simple. After all, microtubules are essentially sticks with a slow-growing minus end and faster-growing plus end, and arranging sticks within the constrained narrow tubes of axons and dendrites should be straightforward. It is therefore quite surprising how many mechanisms contribute to making sure they are arranged in the correct polarity. Some of these mechanisms operate to generate plus-end-out polarity of axons, and others control mixed or minus-end-out dendrites.
Graphical Abstract
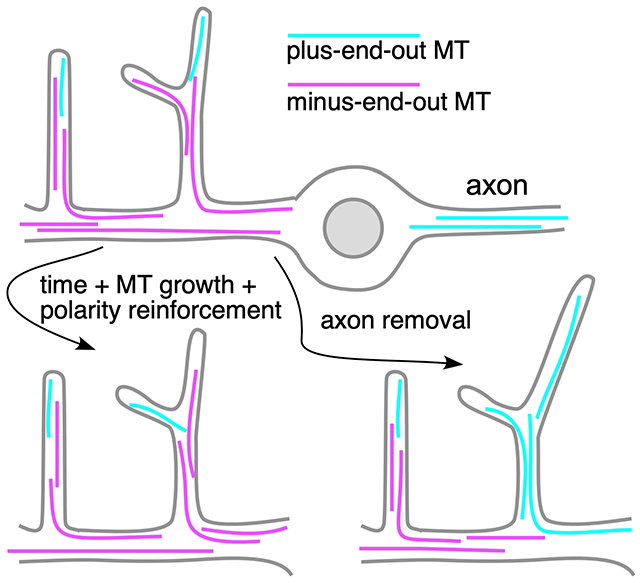
Particular about polarity
Neurons have very specific arrangements of microtubules in axons and dendrites. Every axon that has been examined, from those in mammalian cultured neurons to invertebrate neurons in vivo, has almost uniform plus-end-out polarity (1). In contrast, most dendrites have a significant proportion of minus-end-out microtubules. Dendritic microtubule polarity was first determined to be different from that of axons in primary cultures of hippocampal neurons (2). The discovery that almost half of dendritic microtubules had their minus ends distal to the cell body immediately suggested that dendrite-specific cargoes could be delivered by the minus end-directed motor dynein, but this was not confirmed in mammals until two decades later (3). In the intervening years, plus end-directed kinesin motors were the focus of polarized transport studies in dendrites and axons (4, 5). It was therefore surprising when, in the kinesin era, Drosophila dendrites were found to have almost exclusively minus-end-out microtubules (6, 7) precluding a role for kinesin as an anterograde dendritic motor in this animal. Studies in C. elegans confirmed that, like Drosophila, their dendrites have minus-end-out rather than mixed polarity microtubules (8–10). One potential reason for the discrepancy between the pure minus-end-out polarity dendritic microtubule organization in invertebrates and mixed polarity in vertebrates was that the invertebrate studies were performed in vivo, while the vertebrate analysis was done in vitro. However, this possibility was eliminated by a thorough analysis of vertebrate dendritic microtubule polarity in vivo that demonstrated mature cortical neurons maintain mixed polarity (11). It seems that mixed polarity is sufficient to establish key differences between dendrites and axons in vertebrates, and in immature Drosophila neurons (12). Perhaps pure minus-end-out dendrites are an added optimization for efficient transport that is useful in small animals. Because of their extreme take on polarity, minus-end-out dendrites have proven particularly useful for identifying strategies cells use to polarize microtubule arrays.
Growing and sliding into place
During neuronal development axons usually emerge first from the cell body, followed by dendrites. Both types of processes are initially populated by predominantly plus-end-out microtubules. In mammalian neurons this has been described very nicely for hippocampal neurons in culture. After primary cultures are plated, several short neurites initially extend, all of which have predominantly plus-end-out microtubules (11, 13). One of these begins rapid outgrowth to become the axon, and then several days later the remaining neurites begin to grow into dendrites. Minus-end-out microtubules are gradually added as the dendrites grow to reach 40-50% of the total population (11, 13). A similar series of events has been observed in Drosophila neurons in vivo, with very early stages of dendrite outgrowth characterized by predominantly plus-end-out microtubules (14) and minus-end-out microtubules added gradually as dendrites mature (12, 14). The early arrival of plus ends in newly forming neurites fits well with basic microtubule behavior observed across many cell types. Microtubules are often nucleated at a central microtubule organizing center (MTOC), typically the centrosome, and plus ends explore outwards in bouts of growth and shrinkage termed dynamic instability (15). In developing neurons, then, plus ends would be expected to grow out into new neurites (Figure 1A). Indeed, this behavior has been observed in Drosophila neurons in vivo (12, 14). This basic phenomenon of plus ends growing out to populate far regions of cells is likely why plus-end-out polarity seems to be the default organization of many types of processes. For example, oligodendrocytes grow multiple plus-end-out processes as they develop (16). Moreover, several types of neurons with only plus-end-out neurites have been described. One such cell type is ganglion cells in the sea anemone Nematostella vectensis, which have three equivalent neurites, all with plus-end-out polarity (17). In vertebrates, dorsal root ganglion cells, the major neuron type that innervates the skin to receive sensory information, also has multiple plus-end-out axons and no regions containing minus-end-out microtubules (18). In contrast, neurons with exclusively mixed or minus-end-out neurites have not been identified, although candidate dendrite-only cells like amacrine neurons have not been assessed for microtubule polarity.
Figure 1.
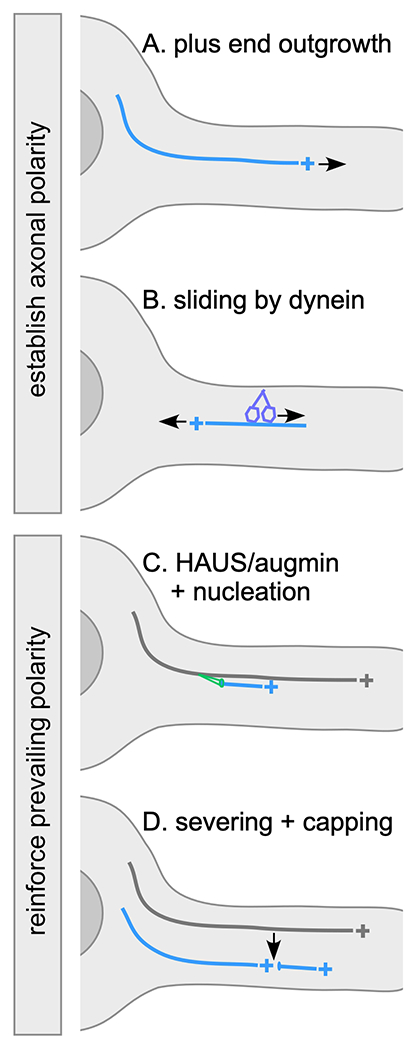
Axonal polarity control mechanisms
In all diagrams the cell body is at the left and axon is shown extending to the right. The microtubule of interest is shown in blue with plus end indicated. Older or template microtubules are shown in grey. A. Dynamic plus ends can grow out from the cell body into the axon during development and in mature neurons. Rapid plus end growth can help populate axons with plus-end-out microtubules. B. Dynein anchored to the cell cortex can slide mis-oriented microtubules out of the axon by walking towards their minus end. Plus end growth and dynein-mediated removal of microtubules can help establish initial polarity, and can also help maintain polarity over the long-term. C. The HAUS/augmin complex can recruit the gamm-tubulin ring complex to the side of existing microtubules and favors nucleation in parallel to the template. D. A microtubule severing event can generate two new correctly oriented microtubules from a parent microtubule. Minus ends are typically recognized by capping proteins including CAMSAPs when they are generated. Both severing and templated nucleation help maintain microtubule polarity by making new microtubules based on the polarity of pre-existing microtubules.
While plus end growth clearly helps populate new neurites with microtubules, microtubule sliding or transport also plays a role in driving the early stages of outgrowth (19, 20). Sliding by kinesin-1 helps initiate neurite outgrowth in Drosophila neurons (20). Later in development kinesin-1-mediated sliding is held in check by the kinesin Pavarotti in Drosophila (21) and by cortical anchoring in C. elegans (22). The role of kinesin-mediated sliding in controlling microtubule polarity is not entirely clear. In contrast, dynein has been implicated in axonal polarity in Drosophila and mammals (23, 24). It is thought to drive plus-end-out microtubules into axons, and remove minus-end-out ones (24, 25) (Figure 1B), so its sliding activity is tightly linked to control of microtubule polarity. While sliding in general is much less active as neurons mature, dynein may continue to help maintain axonal polarity. In dendrites, a tetrameric kinesin, MKLP1/CHO1 is important for establishment and maintenance of polarity, and has been proposed to slide minus-end-out microtubules into dendrites (26, 27), though direct evidence it slides microtubules in neurons is lacking. For more complete discussions of microtubule sliding in neurons, two recent reviews are excellent resources (24, 28).
Minus end maneuvers
While a role for plus end growth in helping to establish axonal microtubule polarity makes intuitive sense, a similar and surprising role for minus end growth in establishment of dendritic microtubule polarity was recently identified. At very early stages of development, Drosophila dendrites have uniformly plus-end-out microtubules (14). As dendrites extend minus ends can be seen growing slowly and persistently into dendrites (Figure 2A), and this minus end growth requires Patronin. If Patronin levels are reduced, dendrites fail to achieve normal minus-end-out polarity (14) supporting a role for minus end growth in populating dendrites with minus-end-out microtubules.
Figure 2.
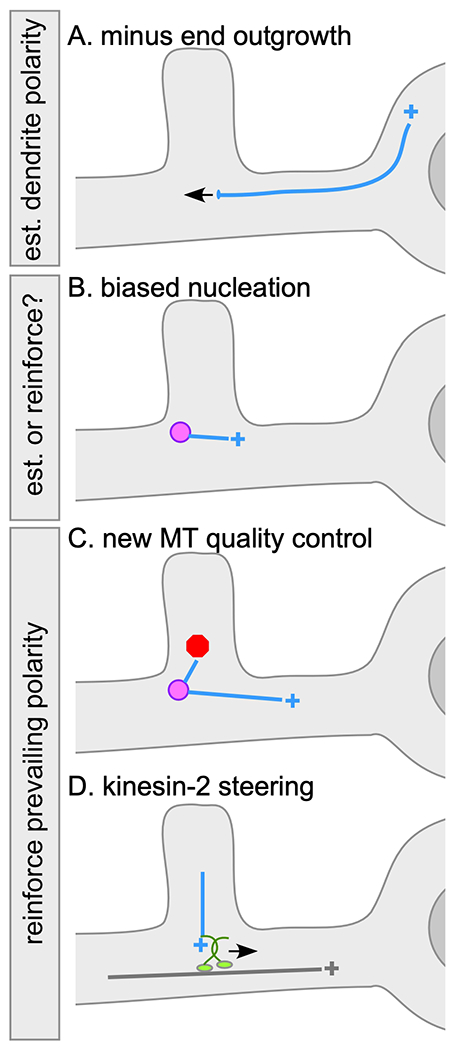
Dendritic microtubule polarity control mechanisms
In all diagrams the dendrite is at the left and cell body at right. Microtubules are drawn as in Figure 1. Endosomal nucleation sites are shown as magenta circles. A. Minus end outgrowth from the cell body into dendrites is slow and persistent, and can contribute to presence of minus-end-out microtubules in dendrites. B. In developing C. elegans neurons positioning of nucleation sites near the growing tip helps establish minus-end-out polarity (not shown). In Drosophila nucleation at dendrite branch points is biased towards the cell body. It is not clear whether this bias depends on the polarity of pre-existing microtubules or whether it is independent of them and could thus help establish minus-end-out polarity. C. New microtubules nucleated at dendrite branch points in Drosophila are more likely to succeed in growing beyond the branch point if they are oriented in the same direction as existing microtubules. Thus in a minus-end-out dendrite arbor, new microtubule growth towards the cell body is favored maintaining existing polarity. D. Kinesin-2 can be recruited to growing plus ends of microtubules and can steer the plus end along the side of a pre-existing microtubule it collides with thus reinforcing existing polarity.
Microtubule growth outwards from the cell body can help establish microtubule polarity, but as neurites lengthen new microtubule ends are generated throughout them. Many of these new ends are likely generated by severing followed by capping with minus end-binding proteins of the CAMSAP/Patronin family. Reduction of microtubule severing proteins of the AAA ATPase family in neurons results in phenotypes ranging from alterations in axon outgrowth (29) to changes in stability of distal dendrites (30), but it has been difficult to pin down when and where severing proteins act in axons or dendrites. However, minus ends capped with CAMSAP/Patronin are readily visualized in mammalian (31), C. elegans (32) and Drosophila (14), neurons and are presumably produced by severing. Microtubule severing allows for generation of new microtubules while preserving pre-existing polarity, so is a way to reinforce polarity of an array (Figure 1D).
De novo nucleation of microtubules, unlike severing, could easily lead to new microtubules being generated in random orientation. In axons, the involvement of HAUS/augmin offers a fix for this polarity problem. The HAUS/augmin complex recruits the core microtubule nucleation machinery, the γ-Tubulin Ring Complex (γ-TuRC), to the side of existing microtubules. The recruited γ-TuRC can then initiate growth of a new microtubule from the side of its parent microtubule in a process termed branching nucleation (Figure 1C). This type of nucleation preferentially initiates microtubules in similar orientation to the parent microtubule (33, 34). In cultured mammalian neurons axonal microtubule polarity is less uniformly plus-end-out when HAUS/augmin complex members are reduced, consistent with branched nucleation promoting parallel microtubule alignment (35, 36). While one might imagine that a similar mechanism could be engaged in invertebrate dendrites, where microtubules are also uniformly arranged, Drosophila with mutations in one of the augmin subunits do not exhibit changes in dendrite microtubule organization (37). Phenotypes are, however, observed when augmin mutations are combined with loss of centrosomin (37) indicating augmin does function in these cells, perhaps in parallel with other microtubule organizing mechanisms.
In the mitotic spindle, HAUS/augmin-mediated branched nucleation coexists with nucleation from the spindle pole microtubule organizing centers (MTOCs). Mini MTOCs have also been described in dendrites. Centrosomes are the classic MTOC, but many cells position γ-TuRCs on cellular membranes ranging from the nuclear envelope in muscle cells (38) to the plasma membrane in the C. elegans gonad (39). It was recently found that during dendrite outgrowth in C. elegans, Rab11 endosomes house nucleation sites near the growing tip (40). Microtubules are nucleated out from an endosomal cluster that serves as the distal dendritic MTOC in both directions-to the cell body and away from the cell body. However, the position of the endosomes in a single clump near the growth cone means that most of the growing dendrite is populated by minus-end-out microtubules and only a short region at the tip has plus-end-out ones (40). While this tip MTOC is important for specifying minus-end-out polarity during development, it seems to be inactivated in mature neurons and the nucleation site has not been determined.
In Drosophila dendrites endosomes also house nucleation sites (41). In this case, the relationship between nucleation and polarity control is not as simple as in growing C. elegans dendrites where endosome position is used to generate minus-end-out polarity. In Drosophila nucleation sites are located at branch points distributed along the dendrites (41–43) so nucleation in both directions from the mini MTOC would result in mixed polarity. So far two ways to couple nucleation and polarity in these cells have been identified. First, new microtubules do not seem to be generated equally in both directions (to and from the cell body). Instead, there is a slight bias (about 60%) of microtubules generated in the correct orientation with plus ends growing towards the cell body (44) (Figure 2B). Second, there is a quality control mechanism that favors exit of correctly oriented microtubules from the branch point into the main dendrite (44). If newly generated microtubules are generated in the same orientation as existing microtubules Trim9 and Klp61F can promote growth of the new microtubule (44). Microtubules growing in the opposite direction to existing microtubules have a high frequency of catastrophe (Figure 2C) helping to reduce the potentially disruptive impact of microtubule nucleation to minus-end-out polarity
In mammalian neurons, it is not clear whether endosomes or other membranes house dendritic nucleation sites. Although there is support for local nucleation throughout mammalian neurons (31), sites where dendritic nucleation occurs have remained elusive. Polarity regulation of nucleation would not be necessary in mammalian dendrites as they have mixed orientation microtubule arrays.
Polarity control at the growing plus end
Growth of plus ends from the cell body into neurites can clearly contribute to setting up plus-end-out polarity in short new axons, and we have already mentioned several ways that this initial short-range plus-end-out polarity could be used to template a long overlapping plus-end-out microtubule array. First, polarity could be reinforced by using severing to generate new microtubule ends without altering polarity (Figure 1D). Second, HAUS/augmin-mediated nucleation can be used to make new microtubules based on polarity of existing ones (Figure 1C). If plus end growth out from the cell body dominates during axon development, then perhaps these mechanisms are sufficient to orchestrate long-distance plus-end-out polarity.
While axonal polarity works with exuberant plus end growth, maintaining minus-end-out polarity in branched Drosophila dendrites relies on its careful control. One key control mechanism is recruitment of kinesins to the plus end to harness plus end growth to prevailing polarity. At least two different kinesins seem to act in specific contexts to coax plus ends in the “right” direction. Kinesin-2, in concert with plus end-binding proteins Apc and EB1, controls plus end growth at dendrite branch points. Microtubules entering a branch point from a distal dendrite have two exit routes: one towards the cell body and one away from the cell body. If they grow away from the cell body, they disrupt minus-end-out polarity. In normal dendrites, almost all grow towards the cell body, and this requires kinesin-2, Apc and EB1 (45) (Figure 2D). This complex seems to be engaged specifically when the incoming plus end collides with the side of an existing microtubule, and the result is that the plus end grows along the old microtubule towards its plus end (46). This type of microtubule steering by plus end-attached kinesin-2 has been reconstituted in vitro (47, 48). Essentially the plus end becomes a kinesin cargo, coupled to the cargo-binding Kap3 subunit by its interaction with Apc. The motor domain is thus free to associated with the side of the existing microtubule. The tetrameric motor kinesin-5 (Klp61F) also promotes plus end growth along parallel microtubules, but seems to function specifically on newly nucleated microtubules as they exit the branch point (see above) (44). In vitro kinesin-5 motors can act as microtubule polymerases (44, 49) so that activity is likely involved in dendrites. It works in concert with Trim9 in dendrites, and it is possible Trim9 ensures that polymerase activity is coupled to parallel growth along an existing microtubule. In mammalian neurons a Trim9 family member, Trim46, bundles parallel microtubules in the proximal axon (50) and crosslinks microtubules together in this part of the cell (51), so it is possible Drosophila Trim9 can also recognize microtubules in parallel arrangements.
Although control of plus end growth is critical for minus-end-out dendrite polarity, it may also occur in axons. Indeed, motor-mediated regulation of microtubules helps coordinate growth cone turning (52). It is therefore possible that some of the mechanisms that control plus end growth in dendrites may operate in axons, not necessarily to control polarity, but to influence other aspects of microtubule behavior.
Flipping the switch on polarity
In the sections above, one frequent theme is using the prevailing microtubule polarity to guide generation and growth of new microtubules so that polarity is constantly reinforced (Figure 1 C and D and Figure 2 C and D). However, this does not mean that once polarity is established during development it is fixed for the life of the cell. Remarkably, axon injury near the cell body can trigger complete rebuilding of the dendritic microtubule cytoskeleton in the axonal orientation. This process has been best described in Drosophila, where severing an axon close to the cell body initiates rebuilding of the dendritic microtubules. By about a day after injury, one dendrite typically loses its minus-end-out microtubule array and becomes plus-end-out (53). After this point, the plus-end-out dendrite initiates growth and takes on axonal characteristics, including pathfinding along the nerve if it encounters it (53, 54). If polarity cannot be reversed, a dendrite does not become a regenerating axon (45). In mammalian neurons, axon injury near the cell body also causes a dendrite to convert into a regenerating axon (55), and it is likely that this conversion involves rebuilding dendritic microtubules in the axonal orientation. Thus, although polarity can be maintained in individual neurites over a lifetime, this is not a fixed state. It is not understood how this dramatic reversal is orchestrated, and this remains a challenge that will put our understanding of neuronal microtubule polarity control mechanisms to the test.
The big picture of polarity
In the thirty years we have known that a critical distinction between axons and dendrites is the polarized arrangement of microtubules, we have made inroads into identifying proteins and mechanisms that contribute to polarity. One fairly early hypothesis was that the same principles used in mitosis to organize the spindle might be reused in neurons to control microtubule organization (56). From the description of polarity control players above, it should be clear that not only are the same principles used, but many of the same players, from mitotic kinesins to Patronin (originally named short spindle 4 in Drosophila) and the HAUS/augmin complex. Determining how these mitotic proteins function in post-mitotic neurons has become much more accessible over this time period with our ability to eliminate or reduce gene function in post-mitotic cells, for example using RNAi in mature cultured mammalian neurons or Drosophila neurons in vivo. However, while we have made good progress identifying players and mechanisms that operate in specific scenarios or systems, we do not yet have a coherent big picture of how the mechanisms work together or which ones are present in different neuron types. For example, endosomes have been identified as nucleation control centers that impact polarity in Drosophila and C. elegans dendrites, but it is not known where nucleation occurs in mammalian dendrites. Similarly, HAUS/augmin impacts microtubule polarity in mammalian neurons (35, 36), but polarity in a null mutant in one of the subunits is unaffected in Drosophila (37). Within a single system where multiple mechanisms have been identified, for example Drosophila dendrites, we do not understand how they work together to generate the final arrangement of microtubules. Most of the mechanisms in Drosophila act to maintain polarity by reading out existing polarity. How then does polarity change from mixed to minus-end-out during development (12) or from minus-end-out to plus-end-out after axon removal (53)? With pieces of the puzzle identified, putting them together into a more complete picture of microtubule organization across neuronal systems may now become an attainable goal.
Acknowledgements
Work on neuronal microtubule polarity in the Rolls lab is funded by the National Institutes of Health, GM085115. I am very grateful for discussions with lab members over the years, as well as with colleagues working on the neuronal cytoskeleton. Michelle Stone provided helpful comments on the manuscript, as well as contributing to many of the studies that have helped identify polarity control mechanisms.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baas PW, Lin S. Hooks and comets: The story of microtubule polarity orientation in the neuron. Developmental neurobiology. 2011;71(6):403–18. doi: 10.1002/dneu.20818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baas PW, Deitch JS, Black MM, Banker GA. Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(21):8335–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kapitein LC, Schlager MA, Kuijpers M, Wulf PS, van Spronsen M, MacKintosh FC, Hoogenraad CC. Mixed microtubules steer dynein-driven cargo transport into dendrites. Current biology : CB. 2010;20(4):290–9. doi: S0960-9822(09)02215-5 [pii] 10.1016/i.cub.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 4.Hirokawa N, Niwa S, Tanaka Y. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron. 2010;68(4):610–38. doi: 10.1016/j.neuron.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 5.Hirokawa N, Takemura R. Molecular motors and mechanisms of directional transport in neurons. Nature reviews Neuroscience. 2005;6(3):201–14. [DOI] [PubMed] [Google Scholar]
- 6.Rolls MM, Satoh D, Clyne PJ, Henner AL, Uemura T, Doe CQ. Polarity and compartmentalization of Drosophila neurons. Neural Development. 2007;2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stone MC, Roegiers F, Rolls MM. Microtubules Have Opposite Orientation in Axons and Dendrites of Drosophila Neurons. Molecular biology of the cell. 2008;19(10):4122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodwin PR, Sasaki JM, Juo P. Cyclin-dependent kinase 5 regulates the polarized trafficking of neuropeptide-containing dense-core vesicles in Caenorhabditis elegans motor neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(24):8158–72. Epub 2012/06/16. doi: 10.1523/JNEUROSCI.0251-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maniar TA, Kaplan M, Wang GJ, Shen K, Wei L, Shaw JE, Koushika SP, Bargmann CI. UNC-33 (CRMP) and ankyrin organize microtubules and localize kinesin to polarize axon-dendrite sorting. Nature neuroscience. 2012;15(1):48–56. doi: 10.1038/nn.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harterink M, Edwards SL, de Haan B, Yau KW, van den Heuvel S, Kapitein LC, Miller KG, Hoogenraad CC. Local microtubule organization promotes cargo transport in C. elegans dendrites. Journal of cell science. 2018. doi: 10.1242/jcs.223107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yau KW, Schatzle P, Tortosa E, Pages S, Holtmaat A, Kapitein LC, Hoogenraad CC. Dendrites In Vitro and In Vivo Contain Microtubules of Opposite Polarity and Axon Formation Correlates with Uniform Plus-End-Out Microtubule Orientation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2016;36(4):1071–85. doi: 10.1523/JNEUROSCI.2430-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill SE, Parmar M, Gheres KW, Guignet MA, Huang Y, Jackson FR, Rolls MM. Development of dendrite polarity in Drosophila neurons. Neural Dev. 2012;7:34. Epub 2012/11/01. doi: 10.1186/1749-8104-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baas PW, Black MM, Banker GA. Changes in microtubule polarity orientation during the development of hippocampal neurons in culture. The Journal of cell biology. 1989;109(6 Pt 1):3085–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng C, Thyagarajan P, Shorey M, Seebold DY, Weiner AT, Albertson RM, Rao KS, Sagasti A, Goetschius DJ, Rolls MM. Patronin-mediated minus end growth is required for dendritic microtubule polarity. The Journal of cell biology. 2019;218(7):2309–28. doi: 10.1083/jcb.201810155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchison T, Kirschner M. Dynamic instability of microtubule growth. Nature. 1984;312(5991):237–42. [DOI] [PubMed] [Google Scholar]
- 16.Fu MM, McAlear TS, Nguyen H, Oses-Prieto JA, Valenzuela A, Shi RD, Perrino JJ, Huang TT, Burlingame AL, Bechstedt S, Barres BA. The Golgi Outpost Protein TPPP Nucleates Microtubules and Is Critical for Myelination. Cell. 2019;179(1):132–46 e14. Epub 2019/09/17. doi: 10.1016/j.cell.2019.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stone MC, Kothe GO, Rolls MM, Jegla T. Cytoskeletal and synaptic polarity of LWamide-like+ ganglion neurons in the sea anemone Nematostella vectensis. J Exp Biol. 2020;223(Pt 21). Epub 2020/09/25. doi: 10.1242/jeb.233197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shorey M, Rao K, Stone MC, Mattie FJ, Sagasti A, Rolls MM. Microtubule organization of vertebrate sensory neurons in vivo. Developmental biology. 2021;478:1–12. Epub 2021/06/21. doi: 10.1016/j.ydbio.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmad FJ, Baas PW. Microtubules released from the neuronal centrosome are transported into the axon. Journal of cell science. 1995;108 ( Pt 8):2761–9. [DOI] [PubMed] [Google Scholar]
- 20.Lu W, Fox P, Lakonishok M, Davidson MW, Gelfand VI. Initial neurite outgrowth in Drosophila neurons is driven by Kinesin-powered microtubule sliding. Current biology : CB. 2013;23(11):1018–23. Epub 2013/05/28. doi: 10.1016/j.cub.2013.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Del Castillo U, Lu W, Winding M, Lakonishok M, Gelfand VI. Pavarotti/MKLP1 regulates microtubule sliding and neurite outgrowth in Drosophila neurons. Current biology : CB. 2015;25(2):200–5. doi: 10.1016/j.cub.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He L, Kooistra R, Das R, Oudejans E, van Leen E, Ziegler J, Portegies S, de Haan B, van Regteren Altena A, Stucchi R, Altelaar AM, Wieser S, Krieg M, Hoogenraad CC, Harterink M. Cortical anchoring of the microtubule cytoskeleton is essential for neuron polarity. eLife. 2020;9. Epub 2020/04/16. doi: 10.7554/eLife.55111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng Y, Wildonger J, Ye B, Zhang Y, Kita A, Younger SH, Zimmerman S, Jan LY, Jan YN. Dynein is required for polarized dendritic transport and uniform microtubule orientation in axons. Nature cell biology. 2008;10(10):1172–80. doi: ncb1777 [pii] 10.1038/ncb1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guha S, Patil A, Muralidharan H, Baas PW. Mini-review: Microtubule sliding in neurons. Neurosci Lett. 2021;753:135867. Epub 2021/04/05. doi: 10.1016/j.neulet.2021.135867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmad FJ, Echeverri CJ, Vallee RB, Baas PW. Cytoplasmic dynein and dynactin are required for the transport of microtubules into the axon. The Journal of cell biology. 1998;140(2):391–401. Epub 1998/02/28. doi: 10.1083/jcb.140.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharp DJ, Yu W, Ferhat L, Kuriyama R, Rueger DC, Baas PW. Identification of a microtubule-associated motor protein essential for dendritic differentiation. The Journal of cell biology. 1997;138(4):833–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu W, Cook C, Sauter C, Kuriyama R, Kaplan PL, Baas PW. Depletion of a microtubule-associated motor protein induces the loss of dendritic identity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20(15):5782–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu W, Gelfand VI. Moonlighting Motors: Kinesin, Dynein, and Cell Polarity. Trends in cell biology. 2017;27(7):505–14. Epub 2017/03/13. doi: 10.1016/j.tcb.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leo L, Yu W, D’Rozario M, Waddell EA, Marenda DR, Baird MA, Davidson MW, Zhou B, Wu B, Baker L, Sharp DJ, Baas PW. Vertebrate Fidgetin Restrains Axonal Growth by Severing Labile Domains of Microtubules. Cell reports. 2015;12(11):1723–30. doi: 10.1016/j.celrep.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stewart A, Tsubouchi A, Rolls MM, Tracey WD, Sherwood NT. Katanin p60-like1 Promotes Microtubule Growth and Terminal Dendrite Stability in the Larval Class IV Sensory Neurons of Drosophila. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(34):11631–42. Epub 2012/08/24. doi: 10.1523/JNEUROSCI.0729-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yau KW, van Beuningen SF, Cunha-Ferreira I, Cloin BM, van Battum EY, Will L, Schatzle P, Tas RP, van Krugten J, Katrukha EA, Jiang K, Wulf PS, Mikhaylova M, Harterink M, Pasterkamp RJ, Akhmanova A, Kapitein LC, Hoogenraad CC. Microtubule minus-end binding protein CAMSAP2 controls axon specification and dendrite development. Neuron. 2014;82(5):1058–73. doi: 10.1016/j.neuron.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 32.Yogev S, Cooper R, Fetter R, Horowitz M, Shen K. Microtubule Organization Determines Axonal Transport Dynamics. Neuron. 2016;92(2):449–60. doi: 10.1016/j.neuron.2016.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petry S, Groen AC, Ishihara K, Mitchison TJ, Vale RD. Branching microtubule nucleation in Xenopus egg extracts mediated by augmin and TPX2. Cell. 2013;152(4):768–77. doi: 10.1016/j.cell.2012.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alfaro-Aco R, Thawani A, Petry S. Biochemical reconstitution of branching microtubule nucleation. eLife. 2020;9. Epub 2020/01/15. doi: 10.7554/eLife.49797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cunha-Ferreira I, Chazeau A, Buijs RR, Stucchi R, Will L, Pan X, Adolfs Y, van der Meer C, Wolthuis JC, Kahn OI, Schatzle P, Altelaar M, Pasterkamp RJ, Kapitein LC, Hoogenraad CC. The HAUS Complex Is a Key Regulator of Non-centrosomal Microtubule Organization during Neuronal Development. Cell reports. 2018;24(4):791–800. doi: 10.1016/j.celrep.2018.06.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanchez-Huertas C, Freixo F, Viais R, Lacasa C, Soriano E, Luders J. Non-centrosomal nucleation mediated by augmin organizes microtubules in post-mitotic neurons and controls axonal microtubule polarity. Nat Commun. 2016;7:12187. doi: 10.1038/ncomms12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yalgin C, Ebrahimi S, Delandre C, Yoong LF, Akimoto S, Tran H, Amikura R, Spokony R, Torben-Nielsen B, White KP, Moore AW. Centrosomin represses dendrite branching by orienting microtubule nucleation. Nature neuroscience. 2015;18(10):1437–45. doi: 10.1038/nn.4099. [DOI] [PubMed] [Google Scholar]
- 38.Bugnard E, Zaal KJ, Ralston E. Reorganization of microtubule nucleation during muscle differentiation. Cell Motil Cytoskeleton. 2005;60(1):1–13. [DOI] [PubMed] [Google Scholar]
- 39.Zhou K, Rolls MM, Hall DH, Malone CJ, Hanna-Rose W. A ZYG-12-dynein interaction at the nuclear envelope defines cytoskeletal architecture in the C. elegans gonad. The Journal of cell biology. 2009;186(2):229–41. doi: jcb.200902101 [pii] 10.1083/jcb.200902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang X, Kokes M, Fetter RD, Sallee MD, Moore AW, Feldman JL, Shen K. Growth cone-localized microtubule organizing center establishes microtubule orientation in dendrites. eLife. 2020;9. Epub 2020/07/14. doi: 10.7554/eLife.56547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiner AT, Seebold DY, Torres-Gutierrez P, Folker C, Swope RD, Kothe GO, Stoltz JG, Zalenski MK, Kozlowski C, Barbera DJ, Patel MA, Thyagarajan P, Shorey M, Nye DMR, Keegan M, Behan K, Song S, Axelrod JD, Rolls MM. Endosomal Wnt signaling proteins control microtubule nucleation in dendrites. PLoS biology. 2020;18(3):e3000647. Epub 2020/03/13. doi: 10.1371/journal.pbio.3000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ori-McKenney KM, Jan LY, Jan YN. Golgi outposts shape dendrite morphology by functioning as sites of acentrosomal microtubule nucleation in neurons. Neuron. 2012;76(5):921–30. Epub 2012/12/12. doi: 10.1016/j.neuron.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen MM, McCracken CJ, Milner ES, Goetschius DJ, Weiner AT, Long MK, Michael NL, Munro S, Rolls MM. Gamma-tubulin controls neuronal microtubule polarity independently of Golgi outposts. Molecular biology of the cell. 2014;25(13):2039–50. doi: 10.1091/mbc.E13-09-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feng C, Cleary JM, Kothe GO, Stone MC, Weiner AT, Hertzler JI, Hancock WO, Rolls MM. Trim9 and Klp61F promote polymerization of new dendritic microtubules along parallel microtubules. Journal of cell science. 2021;134(11). Epub 2021/06/08. doi: 10.1242/jcs.258437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mattie FJ, Stackpole MM, Stone MC, Clippard JR, Rudnick DA, Qiu Y, Tao J, Allender DL, Parmar M, Rolls MM. Directed Microtubule Growth, +TIPs, and Kinesin-2 Are Required for Uniform Microtubule Polarity in Dendrites. Current biology : CB. 2010;20(24):2169–77. doi: S0960-9822(10)01516-2 [pii] 10.1016/i.cub.2010.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiner AT, Lanz MC, Goetschius DJ, Hancock WO, Rolls MM. Kinesin-2 and Apc function at dendrite branch points to resolve microtubule collisions. Cytoskeleton (Hoboken). 2016;73(1):35–44. doi: 10.1002/cm.21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doodhi H, Katrukha EA, Kapitein LC, Akhmanova A. Mechanical and geometrical constraints control kinesin-based microtubule guidance. Current biology : CB. 2014;24(3):322–8. doi: 10.1016/j.cub.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 48.Chen Y, Rolls MM, Hancock WO. An EB1-kinesin complex is sufficient to steer microtubule growth in vitro. Current biology : CB. 2014;24(3):316–21. doi: 10.1016/j.cub.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Y, Hancock WO. Kinesin-5 is a microtubule polymerase. Nat Commun. 2015;6:8160. doi: 10.1038/ncomms9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Beuningen SFB, Will L, Harterink M, Chazeau A, van Battum EY, Frias CP, Franker MAM, Katrukha EA, Stucchi R, Vocking K, Antunes AT, Slenders L, Doulkeridou S, Sillevis Smitt P, Altelaar AFM, Post JA, Akhmanova A, Pasterkamp RJ, Kapitein LC, de Graaff E, Hoogenraad CC. TRIM46 Controls Neuronal Polarity and Axon Specification by Driving the Formation of Parallel Microtubule Arrays. Neuron. 2015;88(6):1208–26. doi: 10.1016/j.neuron.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 51.Harterink M, Vocking K, Pan X, Soriano Jerez EM, Slenders L, Freal A, Tas RP, van de Wetering WJ, Timmer K, Motshagen J, van Beuningen SFB, Kapitein LC, Geerts WJC, Post JA, Hoogenraad CC. TRIM46 Organizes Microtubule Fasciculation in the Axon Initial Segment. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2019;39(25):4864–73. doi: 10.1523/JNEUROSCI.3105-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kahn OI, Baas PW. Microtubules and Growth Cones: Motors Drive the Turn. Trends in neurosciences. 2016;39(7):433–40. Epub 2016/05/29. doi: 10.1016/j.tins.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stone MC, Nguyen MM, Tao J, Allender DL, Rolls MM. Global up-regulation of microtubule dynamics and polarity reversal during regeneration of an axon from a dendrite. Molecular biology of the cell. 2010;21(5):767–77. doi: E09-11-0967 [pii] 10.1091/mbc.E09-11-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rao KS, Rolls MM. Two Drosophila model neurons can regenerate axons from the stump or from a converted dendrite, with feedback between the two sites. Neural Development. 2017;12(1); [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gomis-Ruth S, Wierenga CJ, Bradke F. Plasticity of polarization: changing dendrites into axons in neurons integrated in neuronal circuits. Current biology : CB. 2008;18(13):992–1000. [DOI] [PubMed] [Google Scholar]
- 56.Baas PW, Buster DW. Slow axonal transport and the genesis of neuronal morphology. Journal of neurobiology. 2004;58(1):3–17. [DOI] [PubMed] [Google Scholar]


