Abstract
This cross-sectional study of data from the Surveillance, Epidemiology and End Results database assesses temporal trends in the use of active surveillance and watchful waiting vs definitive treatment in men with low- and favorable intermediate–risk prostate cancer in the US between 2010 and 2018.
Active surveillance (AS) for prostate cancer (PC), wherein cancer is monitored for progression with intention to deliver curative treatment if the cancer progresses, is increasingly recognized as the standard of care for low- and some favorable intermediate–risk PC.1 Data to 2015 showed increasing uptake (42%) of AS use in the US.2 Using newly released national data, we assessed AS use and variables associated with it to 2018.
Methods
The cross-sectional study was approved by Vanderbilt University Medical Center’s Institutional Review Board. The deidentified data were analyzed from May 15 to September 1, 2022. The Surveillance, Epidemiology and End Results (SEER) Prostate with Watchful Waiting (WW) database was used to identify men older than 40 years with low- and favorable intermediate–risk prostate adenocarcinoma from 2010 to 2018, as defined by the National Comprehensive Cancer Network.1 The SEER-WW database contains an additional variable that indicates whether men opted for AS or WW, which differs from AS in that cancer is not monitored for progression but observed until it becomes symptomatic. Men with unknown treatment or missing clinical information were excluded.
Temporal trends and changes in treatment ages were assessed using the Cochran-Armitage test (1-sided) and linear regression analysis, respectively. Multivariable logistic regression analysis was used to assess associations with AS/WW, as opposed to definitive treatment with radical prostatectomy or radiotherapy. Sensitivity analyses including only men aged 70 years or younger, who are more likely undergo AS rather than WW,1 were performed, as well as analyses using multiple imputation, rather than exclusion as in our primary analysis, for patients with unknown or missing information. Statistical significance was defined as 2-sided P = .05.
Results
The rate of AS/WW use increased from 16.4% to 59.9% and from 7.8% to 21.8% in patients with low- and favorable intermediate–risk cancers, respectively (P < .001) (Figure). The median (IQR) age of men who underwent AS/WW decreased for low-risk PC (from 65 [IQR, 60-71] to 64 [IQR, 59-69] years; mean [SE] annual change, −0.15 [0.02] year; P < .001) and for favorable intermediate–risk PC (from 70 [IQR, 64-76] to 67 [IQR, 61-71] years; mean [SE] annual change, −0.20 [0.04] year; P < .001).
Figure. Temporal Trends in Management of Low- and Favorable Intermediate–Risk Prostate Cancer in the US, 2010-2018.
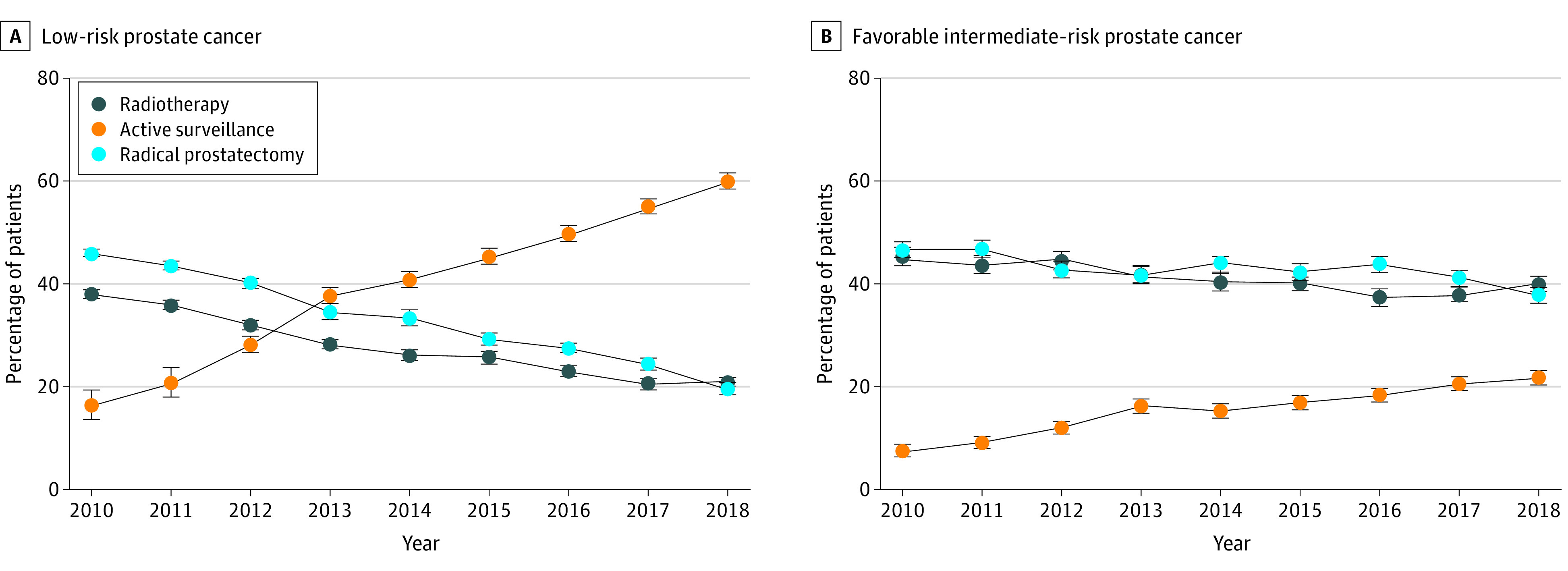
Error bars indicate 95% confidence intervals.
On multivariable analyses, higher income was associated with choosing AS/WW (Table). The number of positive biopsy cores was associated with increased odds of undergoing definitive treatment (adjusted odds ratio, 0.56 [95% CI, 0.53-0.58] for 2 positive biopsy cores in low-risk disease). Asian/Pacific Islander and Hispanic men were less likely to undergo AS/WW compared with White men, and rurality was associated with definitive treatment for low-risk disease. Sensitivity analyses of men aged 70 years or younger (Table), and the imputed data set revealed similar adjusted results.
Table. Multivariable Logistic Regression Analysis for Odds of Undergoing Active Surveillance or Watchful Waiting vs Definitive Treatment Among Men With Low- or Favorable Intermediate–Risk Prostate Cancera.
| Variable | Men with low-risk PCb | Men with favorable intermediate–risk PCc | ||||||
|---|---|---|---|---|---|---|---|---|
| All ages (n = 74 103) | Age ≤70 y (n = 63 204) | All ages (n = 31 699) | Age ≤70 y (n = 24 797) | |||||
| No. (%) | Receipt of AS/WW, aOR (95% CI) | No. (%) | Receipt of AS/WW, aOR (95% CI) | No. (%) | Receipt of AS/WW, aOR (95% CI) | No. (%) | Receipt of AS/WW, aOR (95% CI) | |
| Age, median (IQR), y | 63.0 (57.0-68.0) | 1.04 (1.04-1.04) | 61.0 (56.0-66.0) | 1.04 (1.03-1.04) | 65.0 (59.0-70.0) | 1.04 (1.04-1.05) | 63.0 (58.0-66.0) | 1.03 (1.02-1.04) |
| Race and ethnicity | ||||||||
| Asian/ Pacific Islander | 3206 (4.3) | 0.86 (0.79-0.93) | 2686 (4.2) | 0.87 (0.80-0.96) | 1567 (4.9) | 0.64 (0.55-0.74) | 1141 (4.6) | 0.63 (0.53-0.76) |
| Hispanic | 6997 (9.4) | 0.69 (0.65-0.74) | 6099 (9.6) | 0.71 (0.67-0.76) | 2394 (7.6) | 0.75 (0.66-0.85) | 1922 (7.8) | 0.78 (0.67-0.90) |
| Non-Hispanic Black | 10 841 (14.6) | 1.03 (0.98-1.09) | 9749 (15.4) | 1.08 (1.02-1.14) | 5029 (15.9) | 1.07 (0.97-1.18) | 4265 (17.2) | 1.09 (0.97-1.22) |
| Non-Hispanic White | 51 877 (70.0) | 1 [Reference] | 43 651 (69.1) | 1 [Reference] | 22 276 (70.3) | 1 [Reference] | 17 128 (69.1) | 1 [Reference] |
| Non-Hispanic other | 1182 (1.6) | 1.43 (1.26-1.64) | 1019 (1.6) | 1.52 (1.31-1.75) | 433 (1.4) | 1.26 (0.97-1.62) | 341 (1.4) | 1.50 (1.12-2.00) |
| PSA level, median (IQR), ng/mL | 5.40 (4.40-6.80) | 0.99 (0.98-1.00) | 5.30 (4.30-6.60) | 0.98 (0.97-0.99) | 6.20 (4.80-8.70) | 1.15 (1.14-1.16) | 6.00 (4.70-8.40) | 1.15 (1.14-1.16) |
| No. of cores examined | ||||||||
| ≤11 | 8157 (11.0) | 1 [Reference] | 6932 (11.0) | 1 [Reference] | 3243 (10.2) | 1 [Reference] | 2436 (9.8) | 1 [Reference] |
| 12 | 30 848 (41.6) | 1.29 (1.22-1.37) | 26 473 (41.9) | 1.29 (1.21-1.37) | 18 930 (59.7) | 1.32 (1.18-1.49) | 14 800 (59.7) | 1.27 (1.11-1.46) |
| ≥13 | 14 160 (19.1) | 1.64 (1.53-1.75) | 12 132 (19.2) | 1.63 (1.52-1.76) | 9526 (30.1) | 1.68 (1.49-1.90) | 7561 (30.5) | 1.58 (1.37-1.84) |
| Unknown | 20 938 (28.3) | 1.10 (1.03-1.18) | 17 667 (28.0) | 1.05 (0.98-1.14) | 0 | NA | 0 | NA |
| No. of positive cores | ||||||||
| 1 | 22 003 (29.7) | 1 [Reference] | 11 614 (18.4) | 1 [Reference] | 6828 (21.5) | 1 [Reference] | 5191 (20.9) | 1 [Reference] |
| 2 | 13 813 (18.6) | 0.56 (0.53-0.58) | 18 762 (29.6) | 0.55 (0.52-0.58) | 7136 (22.5) | 0.54 (0.49-0.59) | 5560 (22.4) | 0.51 (0.46-0.57) |
| 3 | 8252 (11.1) | 0.38 (0.36-0.41) | 11 679 (18.5) | 0.38 (0.35-0.40) | 5996 (18.9) | 0.34 (0.31-0.38) | 4679 (18.9) | 0.34 (0.30-0.39) |
| ≥4 | 16 273 (22.0) | 0.17 (0.16-0.18) | 7045 (11.1) | 0.16 (0.15-0.17) | 11 739 (37.0) | 0.21 (0.19-0.23) | 9367 (37.8) | 0.20 (0.18-0.22) |
| Unknown | 13 762 (18.6) | 0.34 (0.32-0.36) | 14 140 (22.4) | 0.34 (0.32-0.36) | 0 | NA | 0 | NA |
| Region | ||||||||
| West | 35 636 (48.1) | 1 [Reference] | 30 692 (48.6) | 1 [Reference] | 14 210 (44.8) | 1 [Reference] | 11 093 (44.7) | 1 [Reference] |
| Northeast | 15 282 (20.6) | 0.53 (0.51-0.56) | 12 760 (20.2) | 0.54 (0.51-0.57) | 6216 (19.6) | 0.55 (0.50-0.61) | 4730 (19.1) | 0.56 (0.50-0.62) |
| Midwest | 5617 (7.6) | 0.78 (0.72-0.83) | 4806 (7.6) | 0.74 (0.69-0.80) | 3957 (12.5) | 0.64 (0.57-0.72) | 3137 (12.7) | 0.59 (0.51-0.68) |
| South | 17 568 (23.7) | 0.54 (0.51-0.57) | 14 946 (23.6) | 0.50 (0.47-0.53) | 7316 (23.1) | 0.50 (0.45-0.56) | 5837 (23.5) | 0.48 (0.43-0.55) |
| Rural/urban countyd | ||||||||
| Urban | 67 096 (90.5) | 1 [Reference] | 57 349 (90.7) | 1 [Reference] | 28 657 (90.4) | 1 [Reference] | 22 433 (90.5) | 1 [Reference] |
| Rural | 6973 (9.4) | 0.88 (0.83-0.94) | 5823 (9.2) | 0.88 (0.82-0.95) | 3037 (9.6) | 0.96 (0.84-1.09) | 2359 (9.5) | 0.88 (0.75-1.03) |
| Unknown | 34 (0.0) | NA | 32 (0.1) | NA | 5 (0.0) | NA | 5 (0.0) | NA |
| Household income, median | ||||||||
| >$75 000 | 24 754 (33.4) | 1 [Reference] | 21 156 (33.5) | 1 [Reference] | 10 933 (34.5) | 1 [Reference] | 8485 (34.2) | 1 [Reference] |
| $60 000-$74 999 | 27 110 (36.6) | 0.87 (0.84-0.91) | 23 225 (36.7) | 0.89 (0.85-0.93) | 11 125 (35.1) | 0.86 (0.79-0.93) | 8716 (35.1) | 0.83 (0.76-0.91) |
| <$60 000 | 22 235 (30.0) | 0.70 (0.66-0.74) | 18 819 (29.8) | 0.71 (0.66-0.75) | 9640 (30.4) | 0.86 (0.77-0.95) | 7595 (30.6) | 0.84 (0.74-0.94) |
| Unknown | 4 (0.0) | NA | 4 (0.0) | NA | 1 (0.0) | NA | 1 (0.0) | NA |
Abbreviations: aOR, adjusted odds ratio; AS, active surveillance; NA, not applicable; PSA, prostate specific antigen; WW, watchful waiting.
SI conversion factor: To convert PSA from ng per mL to μg/L, multiply by 1.
Definitive treatment was defined as radiotherapy or radical prostatectomy; aORs were calculated accounting for all listed variables including year of diagnosis. Of the initial 393 972 patients with localized prostate cancer identified in the primary analyses, men with missing PSA level (n = 49 236), grade group on biopsy (n = 23 122), or T stage (n = 5036) were excluded. Men with favorable intermediate–risk PC without biopsy core information (n = 38 868) were excluded because the number of positive cores is a criterion for diagnosis. Use of AS/WW was not considered if men changed their treatment plan within 6 to 12 months for reasons other than disease progression. Men whose physicians decided not to treat due to comorbidites were excluded. Radiotherapy included external beam radiotherapy and/or brachytherapy. Percentages may not sum to 100 due to rounding.
Defined as PSA level less than 10 ng/mL, clinical stage T1C (cN0 and M0), and grade group 1.
Defined as either (1) grade group 1, PSA level between 10 and 20 ng/mL or clinical stage T2B-C, and fewer than 50% positive biopsy cores or (2) grade group 2, PSA level less than 10 ng/mL, clinical stage T1-2A (cN0 and M0), and fewer than 50% positive biopsy cores.
Rural and urban status was determined at the county level using measurements of population density, urbanization, and daily commuting according to the US Department of Agriculture. Metropolitan areas were grouped as urban and nonmetropolitan as rural.
Discussion
Use of AS increased to approximately 60% for low- and 20% for favorable intermediate–risk PC by 2018. While direct comparisons with international data are hampered by heterogeneity of AS data collection methodology, the US appears to lag behind Sweden (74% by 2014) and Australia (67% by 2016) in AS use for low-risk PC.3,4 Interestingly, in low-risk disease, 2 positive cores were associated with an almost 50% decrease in AS use. Although AS series have shown an association between cancer volume on biopsy and clinical outcomes,1 it is unclear whether the presence of a second positive core should have such an impact on AS use. This is worrisome, particularly with the increasing use of magnetic resonance imaging in biopsy, which may bias toward more positive cores and potentially higher rates of downgrading at prostatectomy.5 Unfortunately, income and race and ethnicity continue to play significant roles in PC treatment delivery.6 Study limitations include our primary analysis being limited to men with known treatment or surveillance rather than all patients with PC. Use of AS continues to increase, although uptake has been lower in men with multiple positive biopsy cores and those from minority groups, with low incomes, or who live in rural areas.
Data Sharing Statement
References
- 1.Schaeffer E, Srinivas S, Antonarakis ES, et al. NCCN guidelines insights: prostate cancer, version 1.2021. J Natl Compr Canc Netw. 2021;19(2):134-143. doi: 10.6004/jnccn.2021.0008 [DOI] [PubMed] [Google Scholar]
- 2.Mahal BA, Butler S, Franco I, et al. Use of active surveillance or watchful waiting for low-risk prostate cancer and management trends across risk groups in the United States, 2010-2015. JAMA. 2019;321(7):704-706. doi: 10.1001/jama.2018.19941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loeb S, Folkvaljon Y, Curnyn C, Robinson D, Bratt O, Stattin P. Uptake of active surveillance for very-low-risk prostate cancer in Sweden. JAMA Oncol. 2017;3(10):1393-1398. doi: 10.1001/jamaoncol.2016.3600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ong WL, Evans SM, Evans M, et al. Trends in conservative management for low-risk prostate cancer in a population-based cohort of Australian men diagnosed between 2009 and 2016. Eur Urol Oncol. 2021;4(2):319-322. doi: 10.1016/j.euo.2019.04.006 [DOI] [PubMed] [Google Scholar]
- 5.Shoag JE, Cai PY, Gross MD, et al. Impact of prebiopsy magnetic resonance imaging on biopsy and radical prostatectomy grade concordance. Cancer. 2020;126(13):2986-2990. doi: 10.1002/cncr.32821 [DOI] [PubMed] [Google Scholar]
- 6.Al Hussein Al Awamlh B, Ma X, Christos P, Hu JC, Shoag JE. Active surveillance for Black men with low-risk prostate cancer in the United States. N Engl J Med. 2019;381(26):2581-2582. doi: 10.1056/NEJMc1912868 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Sharing Statement


